- 1The Daffodil Centre, University of Sydney, a joint venture with Cancer Council NSW, Sydney, NSW, Australia
- 2Melanoma Institute Australia, The University of Sydney, Sydney, NSW, Australia
- 3The Centre for Genetics Education, Health Education and Training Institute (HETI) NSW Health, Sydney, NSW, Australia
- 4Community and Primary Health Care, Faculty of Medicine, The University of Sydney, Sydney, NSW, Australia
- 5Leeder Centre for Health Policy, Economics and Data, Faculty of Medicine and Health, The University of Sydney, Sydney, NSW, Australia
- 6Australian Genomics, Melbourne, VIC, Australia
- 7School of Clinical Medicine, Faculty of Health and Medicine, UNSW, Sydney, NSW, Australia
- 8School of Health Sciences, The Faculty of Medicine and Health, The University of Sydney, Sydney, NSW, Australia
- 9The Royal Australian College of General Practitioners Ltd (RACGP), East Melbourne, VIC, Australia
- 10Academic Education, Sydney Medical School, The University of Sydney, Sydney, NSW, Australia
- 11Specialty of Genomic Medicine, Faculty of Medicine and Health, The University of Sydney, Sydney, NSW, Australia
- 12Department of Clinical Genetics, Sydney Children’s Hospital Network, Sydney, NSW, Australia
Introduction: Improving clinical capacity for genomics in primary care promises to lead to better health, but genomics uptake in the sector is slow and patchy. This review aimed to identify the attitudes of primary care practitioners (PCPs) and the education needs and enablers in applying genomics to inform priorities in education and implementation.
Methods: Searches were conducted across Medline, Scopus, CINAHL, Embase, and Cochrane CENTRAL until November 2023. Barriers and enablers were mapped to the Theoretical Domains Framework and the Genomic Medicine Integrative Research Framework.
Results: A total of 52 studies were included, and the most frequently mapped domains from the Theoretical Domains Framework were ‘Knowledge’ (65.4% of papers), ‘Environmental context and resources’ (40.4%), ‘Skills’ (38.5%), and ‘Social/professional role and identity’ (32.7%). Four key implications were identified: knowledge as a major barrier and enabler, education to build capacity, uncertainty about the role of PCPs, and additional needs beyond education alone.
Discussion: While PCPs are optimistic about genomics, long-standing barriers to delivery in primary care remain. Multifaceted, evidence-based education strategies, including interactive components to change behaviour, will help to address barriers. Clarifying the role of PCPs, referral pathways, and collaboration with tertiary genetics services will further build capacity for genomics delivery in primary care.
1 Introduction
Primary care practitioners (PCPs) are increasingly at the forefront of genomics and are in a unique position to enable the widespread application of precision medicine in the community. Recent rapid advances in genomics have led to cheaper and faster genomic testing and screening, and the emergence of new treatments (1), including targeted therapies for cancer, gene therapies, and tailored medication prescribing guided by pharmacogenomics. Clinical trials are also underway for the use of polygenic scores to provide risk-tailored prevention or early detection of common conditions such as heart disease and cancer, as well as for population-based screening for genetic conditions, with the potential to reduce unnecessary interventions and improve healthcare at scale (1, 2).
Improving clinical capacity for genomics in primary healthcare promises to lead to better health, through earlier diagnosis, more targeted risk management, and early intervention (3). Primary healthcare supports first-contact, person-focused care and serves as a strategic entry point to the health system (4). This includes family physicians, general practitioners, nurse practitioners, and physician assistants.
Despite considerable development of genomics education resources for health professionals in the last decade, there has been a relatively slow uptake of genomics into primary care, with many practitioners reporting inadequate capacity, capabilities, training, and support to enable genomics to be embedded into their practice (5, 6). In addition, the rapid pace of genomic advancements has the potential to outstrip updates provided by existing education resources, presenting additional challenges in engaging PCPs in genomics education. Internationally, strategies to support primary care professionals in the delivery of genomics medicine have been proposed (7, 8), but there remains a lack of evidence on the most effective education approaches and key priorities in genomics education and implementation in this sector.
We conducted a scoping review to present a cohesive overview of the attitudes of PCPs to genomics and education needs and enablers in applying genomics in primary healthcare to better understand how to build capacity through education and inform implementation. We defined ‘enablers’ as any factors facilitating the successful implementation of education, such as tailored resources addressing stakeholder needs. Moreover, we have defined primary care practitioners as those that align with the WHO definition of primary care, providing first contact, accessible, continuous, comprehensive, coordinated care that is person-focussed (4). Specifically, as this study is funded through an Australian Medical Research Futures Fund project aimed at finding genomic solutions for general practitioners, we tried to align the definition of primary care practitioner as closely to the Australian system as possible in our search strategy.
This scoping review had two key objectives:
1. To understand the attitudes of PCPs, in particular GPs, toward genomic practice in the context of genomics education and how these can be addressed; and.
2. To examine the evidence on genomics education in primary care to identify what works and the needs of PCPs.
2 Materials and methods
The review has been reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Extension for Scoping Review (PRISMA-ScR) guidelines (9).
2.1 Criteria
A detailed search strategy and eligibility criteria for screening of studies were developed in collaboration with the authors and a clinical librarian at the University of Sydney. Studies were included if they met the following criteria:
• Included genomics education and/or resources (excluding out-of-scope topics, i.e., non-genetic newborn bloodspot screening and tumour testing in tertiary setting)
• Reported genomics education needs, gaps, and enablers
• Based on primary care settings involving primary care professionals (e.g., general practitioners, primary care nurses, or equivalent roles in primary care settings outside Australia, such as family physicians and physician assistants)
• Published between 2011 and 2023 and
• Full text was available in English.
Studies were excluded if primary care professional roles and responses were not clearly differentiated from professionals in other health sectors, such as tertiary care, in the data analysis. For example, if a study interviewed PCPs and surgeons and included all their responses mixed without differentiation, these were excluded. This was to ensure that we only had responses purely from primary care professionals, so that relevant barriers and enablers could be attributed to evidence from primary care practitioners. This means we deliberately excluded studies performed in the tertiary care sector due to the different health system issues in this field, and also there is a relative abundance of studies looking at mainstreaming in tertiary care (10). We excluded protocols, conference abstracts, commentaries, letters, editorials, or perspectives and studies not available in English.
2.2 Information sources and search
Searches were conducted across five databases, Medline, Scopus, CINAHL, Embase, and Cochrane CENTRAL, to capture all relevant literature published on the research topic from the genomic era, January 2011 until November 2023. Search strategies and terms used across the different databases are available in Supplementary Table 1.
2.3 Selection of sources of evidence
All studies were analysed for relevance to the objectives using the eligibility criteria. All studies were initially uploaded to Endnote, a citation and reference management tool, where duplicates were removed from the library. Covidence, a systematic review management software, was used to screen studies to be included in the scoping review. The studies captured via the database searches were uploaded to Covidence to commence screening studies based on the relevancy of the abstract and title. This stage was completed by two authors (NS and KD), with a subset of articles (10%) reviewed by both to ensure screening reliability before commencing the full set of studies. A second screening was automatically completed at this stage by Covidence to verify that all duplicate studies were excluded before the authors (NS and KD) independently commenced screening the select studies based on their full texts. Consensus on the inclusion of studies was met through weekly discussions throughout the screening process; any conflicts were resolved by discussions with senior authors in the team (ASM and AS) until fully agreed.
2.4 Data charting process and items
Data were extracted, and studies were charted in a table format by two authors (NS and KD), documenting article details (title, author, year of publication, and country of publication), study summary (aims, methods, and results), study details (participants, specialty within genomics, type of intervention, and key outcome measures), and outcomes (needs, gaps, barriers, and facilitators). Studies were categorised according to their key focus, either attitudes of PCPs toward practicing genomics or educational interventions (genomics).
2.5 Synthesis of results
The selection, screening, and synthesis of studies in this review was completed in 12 months. Studies were further coded to two frameworks and discussed in regular meetings over the following 6 months to provide a structured approach for deductive analysis (barriers and enablers) and inductive analysis to determine implications as follows:
A. Behavioural Domains using the Theoretical Domains Framework (TDF): The TDF has been applied across a broad range of healthcare settings and behaviours to categorize barriers and enablers, including in genomics uptake (11). This framework provides a comprehensive structure to understand determinants of behaviour change relevant to the education delivery of genomics in primary care. Each of the 14 domains of TDF was defined by the study team, with examples in the context of this review available in Supplementary Table S3. These domains were mapped initially by two authors (NS and KD) and further correlated by a third author to ensure consistency (AM). Barriers and enablers were further coded for each article according to attitudes-based or educational intervention-focused studies in line with the aims of the review. Any discrepancies were discussed among five co-authors (NS, KD, ASM, AKS, and ALM) at regular research meetings. A frequency analysis of domains was completed by one author (NS) to further guide understanding of the key genomics-related gaps and needs of PCPs prevalent in the literature (included in Supplementary Table 3 with TDF domain definitions).
B. The Genomic Medicine Integrative Research Framework (GMIR): GMIR (12) is a conceptual framework to help design measures for integrating genomics into clinical practice. TDF barriers, enablers, and needs were coded into the GMIR to capture contextual factors, educational interventions, processes, and outcomes to guide further analysis (12) (included in Supplementary Tables 4, 5). Findings within the GMIR were checked and discussed amongst authors, including the impact of genomics education approaches and how they play out in the real world of primary care. A discussion was conducted at regular research meetings (NS, KD, ASM, AKS, and ALM) and at two additional meetings with all authors whose range of academic backgrounds include epidemiology, clinical genetics, implementation science, genomics education, health professional education, and primary care. This enabled the determination of implications for building capacity for genomics in this setting. This scoping review provided a high-level map of existing literature and knowledge, and so a critical appraisal of individual studies was not conducted.
3 Results
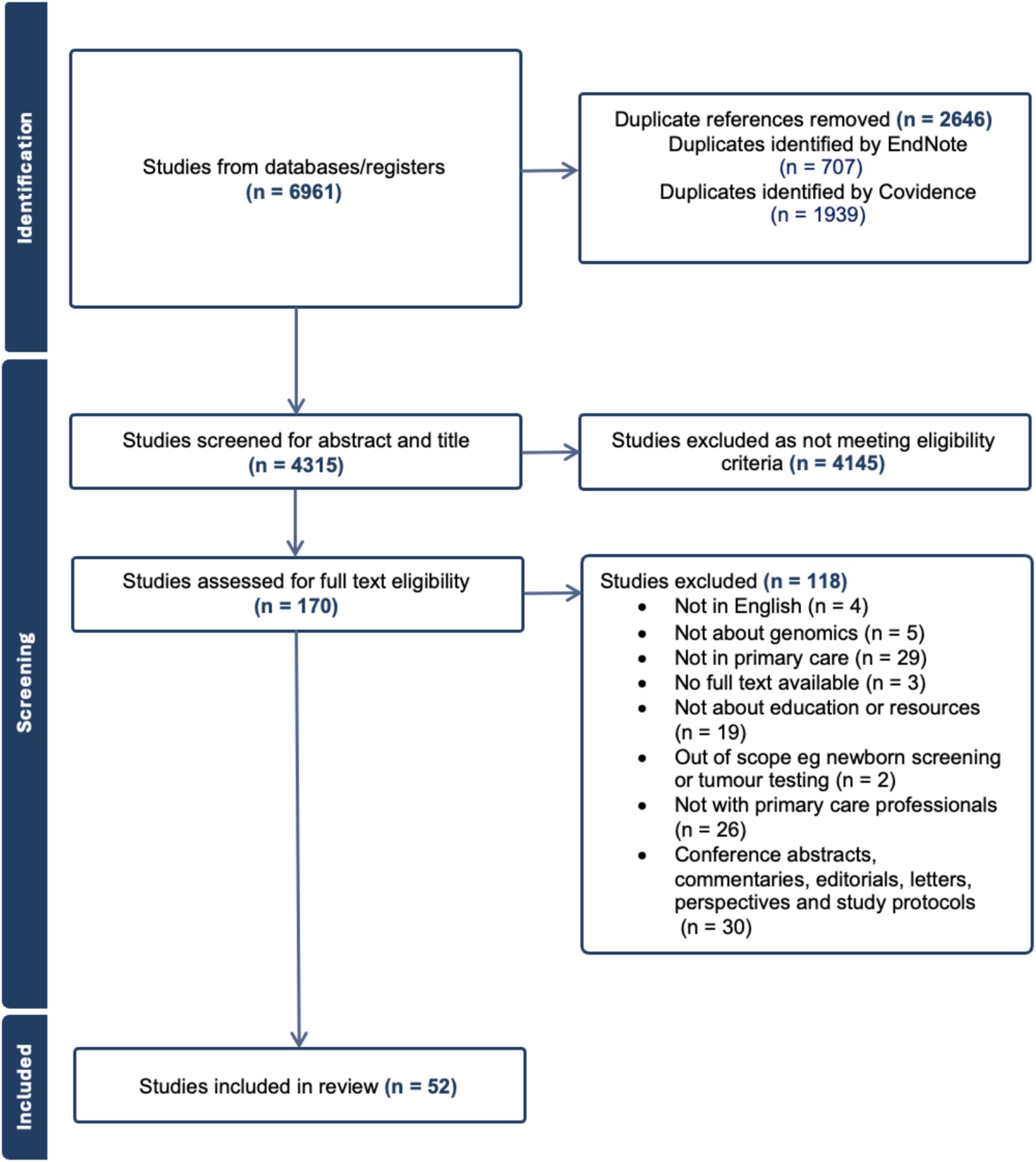
After all duplicates were removed, a total of 4,315 studies were screened for abstract and title (Figure 1). After excluding studies that did not meet the eligibility criteria, a total of 170 papers were assessed in full text, and 52 were included in the review, with 33 focused on attitudes of PCPs and 19 on educational interventions (genomics).
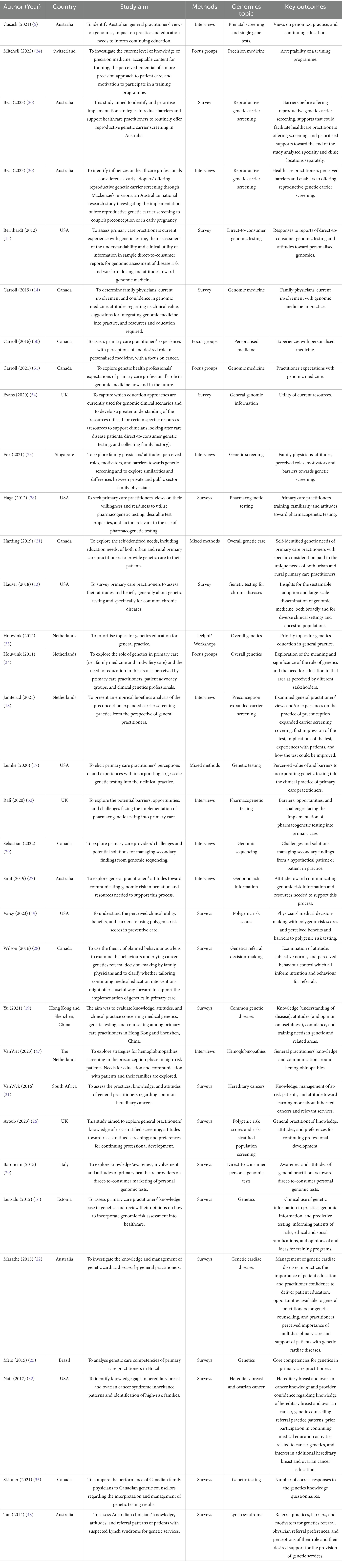
Demographic information and study characteristics of all included studies are shown in Supplementary Table 2. All included studies with other extracted data items are shown in Table 1 (those with a focus on attitudes and views of primary care practitioners) and Table 2 (those with a focus on educational interventions for primary care practitioners).
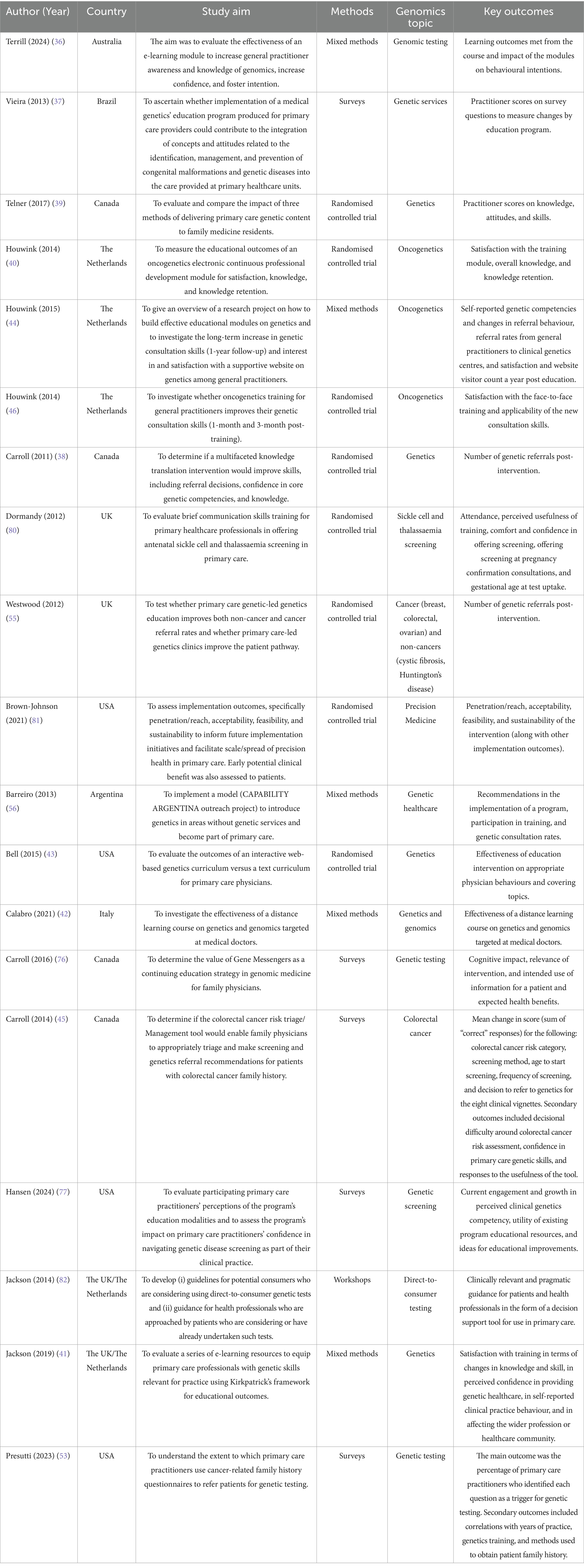
Table 2. All included studies with a focus on educational interventions for primary care practitioners.
Attitudes of PCPs were optimistic about the potential for genomics to improve clinical care (13–19) and as an area of responsibility for primary care (5, 20, 21). However, most reported low skill and knowledge (13, 19, 22, 23), in particular in referral pathways and dealing with complexities of genomics (5, 24, 25), lack of confidence, especially in counselling and interpreting genomic results (5, 14, 17, 22, 26–29), and poorly defined roles (24). There was strong interest amongst PCPs in genomics education (21, 30–32).
Barriers, enablers, and needs were most frequently mapped to the TDF domain ‘Knowledge’ in 34/52 (65.4%) of articles, followed by ‘Environmental context and resources’ in 21 papers (40.4%). Barriers and enablers were also frequently categorised into skills (38.5%), social/professional role and identity (32.7%), beliefs about capabilities (17.3%), memory, attention, and decision processes (13.5%), optimism (11.5%), intentions (9.6%), and beliefs about consequences (7.7%). Mapping to other TDF domains occurred on one occasion or not at all. A summary of the barriers, enablers, and needs for the key TDF domains, categorised according to attitudes-focused or educational intervention-focused studies, is included in Table 3. All 14 TDF domains were relevant in this scoping review, and the remaining domains are included in Supplementary Tables 3–5.
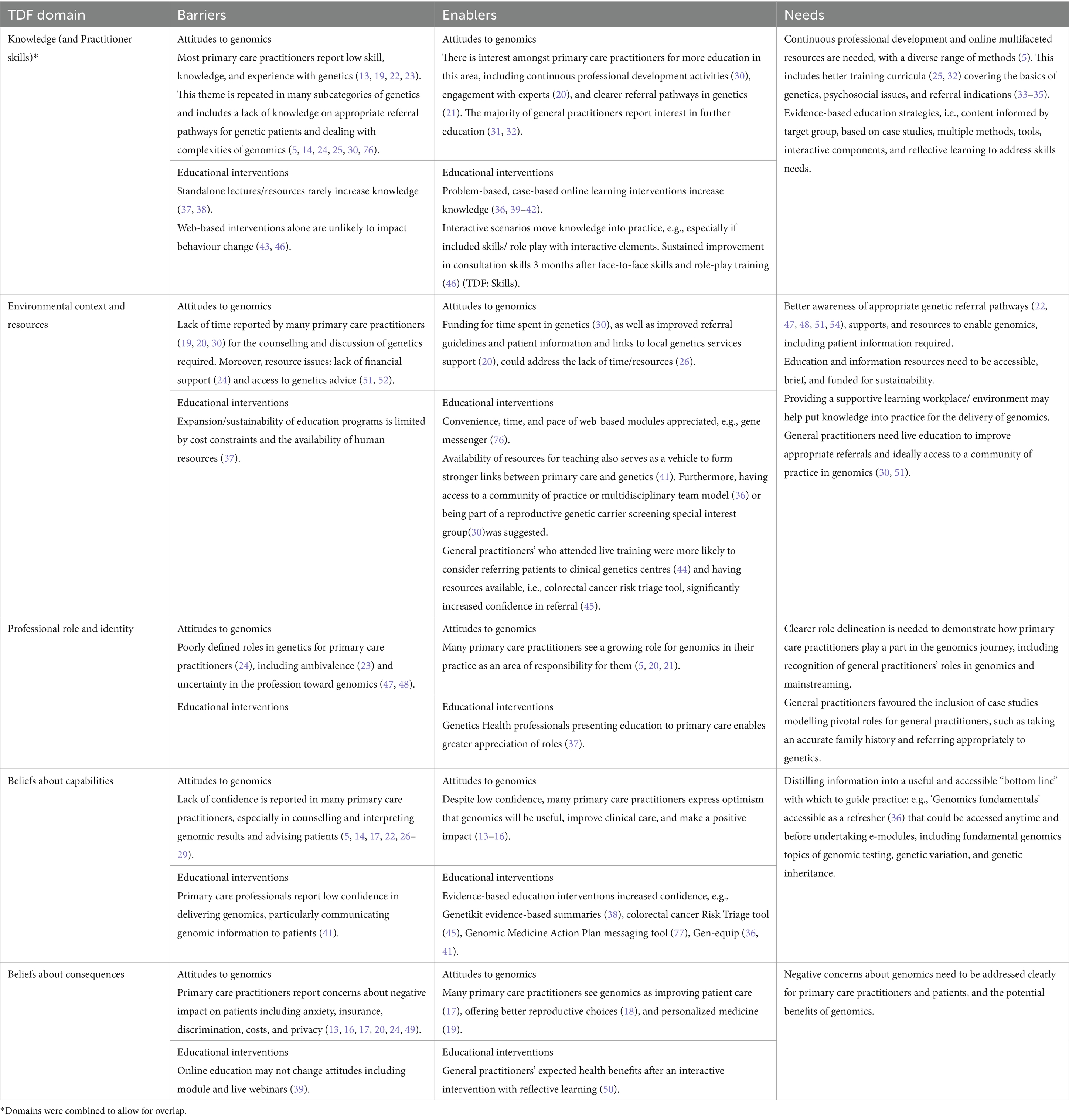
Table 3. Summary of key barriers, enablers, and needs in primary care for five common TDF domains [according to attitudes to genomics or educational interventions].
3.1 Implications for building capacity for genomics in primary care
Four key implications were identified from the data analysis of included studies as follows and summarised in Figure 2.
3.1.1 Knowledge as a major barrier and enabler to genomics in primary care
Limited knowledge about genomics and lack of experience in genomics was a frequently reported barrier (TDF: Knowledge) to delivering genomics in primary care, amplified by the complexities of genomics (5, 14, 24, 25, 30). This was linked closely to the barrier of PCP’s low confidence and perceived ability to perform genomics in practice, such as counselling families and explaining genomic results (5, 14, 17, 27–29) (TDF: Beliefs about capabilities). Key enablers included PCP’s optimism that genomics will be useful, improve clinical care, and make a positive impact (13–16) and the high interest in further education (20, 31, 32). Other enablers included engagement with experts (20).
The need for continuous professional development (CPD), accredited and multifaceted in approach (using multiple modalities, e.g., online, face-to-face, workshops, and modules), was identified to meet the range of PCP’s needs and preferences for delivery (5). These include better training curricula (25, 32) covering the basics of genetics, psychosocial issues, referral indications (33–35), and useful ‘bottom-line’ information accessible anytime to help build confidence (36).
3.1.2 Education as an approach to building capacity
There were two overarching barriers to educational interventions for building capacity for genomics. The first included the low impact of standalone lectures and resources, with two articles reporting knowledge was not retained after lecture series (37) and evidence-based summaries (38) (TDF: Knowledge). Implementing education informed by evidence-based strategies to increase confidence and knowledge (TDF: Knowledge) was a key enabler. For example, several studies reported that active problem-based, case-based online learning interventions were effective evidence-based strategies, increasing genomic knowledge for PCPs (36, 39–42).
The second barrier identified was the limited evidence of behaviour change despite PCPs participating in evidence-based educational interventions (40) (TDF: Knowledge). Significant impact on applying knowledge, for example, key counselling behaviours, was not achieved following participation in an e-module (40) and a web-based genetic curriculum (43). Improvement in self-reported genetic competencies and referral behaviour at 1-year follow-up was reported by PCPs who completed comprehensive oncogenetic training (44) (a module, live education, and a website), though clinical genetics centres reported no significant change in referral numbers 1 year after the training.
Despite this, PCPs did frequently report an intention to implement support for genomic testing in practice following such interventions (36, 44, 45), and behaviour change was achieved in a small number of studies that included an interactive education component. Sustained improvement in consultation skills was reported by PCPs 3 months following interactive face-to-face skills and role-play training (46). An e-learning tool that provided evidence-based summaries of new genetic tests with primary care recommendations, while not improving knowledge, increased confidence and changed practice with participants choosing to continue to receive the resource (46). Poor access was reported by some as a deterrent to participating in education, with convenience, time, and pace of web-based modules as recognised enablers (39).
3.1.3 Uncertainty about the role of GPs in genomics
Poorly defined roles in genetics for PCPs (24) were seen as barriers to the delivery of genomics, including ambivalence (23) and uncertainty in the profession toward genomics (47, 48) (TDF: Professional Role and Identity). Enablers included that some PCPs see a growing role for genomics in their practice as an area of responsibility for them (5, 20, 21). Strategies to clarify the role of the GP in genomics, such as reflecting the PCP’s role in activities provided by professional bodies and training curricula to provide baseline knowledge (36), were also reported. Genetic health professionals presenting education to primary care may enable a greater appreciation of roles (37).
Concerns about the negative consequences of genomic testing on patients, including anxiety, insurance, discrimination, costs, and privacy (13, 16, 17, 20, 24, 49), were also key barriers (TDF: Beliefs about consequences). A key enabler to increase confidence and reduce concerns similarly includes evidence-based interactive education. For example, after an interactive intervention with reflective learning, GPs reported expecting health benefits for their patients from genomics (50).
3.1.4 Major needs identified beyond education alone
While effective education was highlighted as a major need, lack of time for the counselling and discussion of genetics required (19, 20, 30) and lack of financial support (24) and resources in terms of access to genetics advice and services (51, 52) were also reported as barriers to delivering genomics in primary care (TDF: Environmental context and resources). Cost constraints and the availability of human resources were barriers to the expansion and sustainability of education and services (TDF: Environmental context and resources). Enablers included funding for time for GPs to spend on genetic services (30) and establishing links to local genetics services support (20, 26).
Appropriate referral of patients to genetic services remains a key role for PCPs. Barriers to appropriate referral were attributed to a lack of awareness of indications for referral (32, 34, 53) and uncertainty about their role (47, 48), with many requesting referral guidelines and education (14, 21, 36) (TDF: Knowledge; TDF: Environmental context and resources). In one study (44), education increased intention to refer but not appropriate genetics referrals. The impact of education on appropriate referral was otherwise not reported. Enablers included having clear referral pathways in genetics (21) and accessible resources (54), including risk assessment tools. For example, the CRC Risk Triage tool was found to significantly increase confidence in referral (45). Additional enablers include primary-care genetics-led education, as GPs who attended a genetic counsellor-led practice-based seminar, which included referral access details and guidelines, increased appropriate referral of patients at high genetic risk of developing cancer (55). No significant changes were found for non-cancer referrals. The delivery of knowledge as a cycle rather than a one-off event was recommended for impact (55, 56), and other potential solutions included providing access to a community of practice or multidisciplinary team model (36) and being part of a genomics team (51).
4 Discussion
This review synthesises current attitudes to and educational interventions for genomics in primary care, identifying barriers and enablers associated with building capacity for delivery. Most studies in this review focused on aspects related to TDF domains of knowledge, environmental context and resources, professional role and identity, beliefs about capabilities, and beliefs about consequences, reflecting priority areas for PCPs. We identified four key implications (themes) associated with barriers and enablers that include knowledge as a major barrier and enabler to genomics in primary care, education as an approach to building capacity, uncertainty about the role of GPs in genomics, and major needs identified beyond education alone. These have implications for resource development, including investing in evidence-based education, alternate modes of delivery, and creating pathways and links to genetic services support. Considering the many barriers and enablers identified, it is imperative to continue to further explore and develop strategies that effectively build capacity.
When we compare our findings to a previous systematic review of genetics in primary care from almost a decade ago (6), many similar themes arise, even though only one article (34) overlaps with this 2015 review. Barriers most frequently mentioned in the systematic review by primary-care providers included a lack of knowledge (most frequently cited) about genetics and genetic risk assessment, concern for patient anxiety, a lack of access to genetics, and a lack of time—which are much the same as the barriers identified here. It is striking how similar their findings and concerns were, including the risk of genetic discrimination and harm and the lack of referral guidelines for access to genetics services, even for articles written before the genomics era.
The similarities, despite the passing of 10 years of additional genomic education, programs, and efforts to improve uptake into primary care internationally, reflect that there are systemic issues beyond education alone and that maybe a new approach is required. Moreover, as these efforts to integrate genomics and promise ‘precision’ or ‘personalized’ medicine continue, there is evidence of ambivalence and scepticism in the primary care sector, as these promises often fail to deliver (57) and may actually worsen existing inequities. Instead of more promises and programs to deliver this, there is increasing evidence that undertaking co-design (58) in partnerships with consumers and PCPs is needed (59), and incorporating more genetic skills experiences into primary care training may be required (60). Moreover, addressing the many social challenges, such as ethical and legal aspects of genomics, public acceptance, and costs, is required to enable systemic change and improve uptake in the sector (61).
In the coming decade, it could be argued that the role of the PCP is even greater in genomics, with access to more testing and guidelines and the consequent growing importance of identifying patients who would benefit from further genetic evaluation (62). Moreover, there is evidence of the important role PCPs have, as consumers value their involvement in the genomic testing process (63) and trust their PCPs to provide genomic advice and information (64). However, our findings report PCP’s ongoing concern about their role in genomics and a lack of access to genetics expertise and services, possibly reflecting the lack of effective interventions to address these longstanding problems and the rapidly changing new applications of genomics.
Genomic ‘mainstreaming’ has been promoted in many areas as a potential solution by integrating genomics into non-genetics healthcare practices such as in nursing, subspecialist physicians (10), and primary care. In the mainstreaming literature, including cancer mainstreaming literature, similar barriers have also been identified in secondary and tertiary care sectors to the uptake of genomics, including low genomic literacy and knowledge (65) and the lack of strong evidence on the type of educational interventions that lead to effective behaviour change. Some additional non-education interventions identified to impact mainstreaming include family history and referral tools, as well as embedding of genetics staff (e.g., genetic counsellors) into non-genetics areas. This can be challenging in primary care, where referral criteria and tools are very location-specific, and the low number of genetics services compared to PCPs makes it very difficult to scale up an embedded clinical service.
While genomics is a rapidly growing field with many new applications in primary care, it is helpful to compare our findings to the broader general literature on PCP education and behaviour change. A recent systematic review of reviews on primary care practitioner behaviour change using TDF has demonstrated very similar findings, with knowledge being the most frequently identified barrier and enabler identified in the primary care literature in general (66). Poor knowledge was identified as leading to uncertainty, low confidence, and poor awareness amongst PCPs. Despite this, there is literature pointing toward little apparent change in practice behaviour, even with targeted education in genetics and genomics from our study (67). This highlights the importance of evidence-based educational interventions and blended learning approaches (68) that deal with behaviour change beyond knowledge improvement.
In addition, the time and workload required to change behaviours, combined with poor resourcing and lack of time to upskill, and follow guidelines, is a major barrier identified in the primary care literature in general (69, 70). While we have focussed on just one area—genomics—a similar theme is emerging across the field, with major implications for primary care training, practice, and the way the primary care sector can adapt and change to the evolving evidence in medicine. For example, including skills-based genomics education in the training program curriculum for physicians specialising in primary care could address many of the shortfalls in the knowledge that are so common across the sector. Moreover, the time and resourcing issue speaks to a broader problem in the sector of short consultations (69) and low renumeration for time-intensive tasks such as counselling and discussing complex interventions such as genomics with patients. This is potentially compounded for discussions with patients from a non-English speaking, rural/remote, or socioeconomically disadvantaged background, where genomics may be a low priority (71). Any new interventions to address genomics uptake in primary care must also be implemented in the context of a time- and resource-poor clinician seeking quick answers to help manage their patients and broader systemic issues such as equity and training.
In the primary care literature, important social influence enablers were patient-centred care and collaboration with specialists, which is similar to the idea of a ‘community of practice’ raised as an environmental context influence enabler in our review. These are groups with a shared concern, set of problems, and regular interactions to address this and have been shown to improve primary care outcomes (72). Other models of interdisciplinary care, such as genomic multidisciplinary teams where clinical geneticists and genetic counsellors partner with non-genetics professionals to handle genomic cases and facilitate mainstreaming, are also worth considering (73, 74). Both approaches are worth exploring to enable PCPs to facilitate genomics in primary care, with genetics support and patient-centred collaborations to improve outcomes.
A strength of this review is the use of a comprehensive search strategy across multiple databases to understand the education needs, gaps, and enablers for building capacity in genomics within primary care. However, in our initial search, studies were excluded if they did not discuss genomics in the context of education, As a result, we may not have adequately captured literature related to relevant issues explored in genomics.
Moreover, we limited our definition of primary care practitioners to closely align with the Australian context of general practitioners, which excluded some physicians that are considered part of primary care in other jurisdictions, such as internal medicine and paediatric physicians. These specialties are already well represented in other research on genomic mainstreaming needs, mainly in the tertiary sector (10), but we acknowledge that this limits some of the findings to the family physician context.
Only a minority of studies in the genomics education and primary care literature utilised any theory-based frameworks or implementation science, such as using the TDF. Such use of theories is helpful for consistency across the literature and in devising interventions to address the barriers, which seem to be common across the primary care literature as a whole rather than specific to genetics itself. A potential area of future study would be the types of interventions best suited to implementing genomics beyond conventional education alone; for example, audit and feedback have been used to enable prescribing behaviour change (75), and the emergence of artificial intelligence/virtual reality-based learning tools are worth exploring further.
Although PCPs report optimism about the benefits of genomics and interest in genomics education, longstanding entrenched barriers to the delivery of genomics in primary care remain. Ensuring that education strategies are multifaceted and evidence-based and include interactive components to change behaviour will help address these barriers. Clarifying the role of the GP in training curricula, resourcing for genomics, providing clearer referral pathways, and establishing links to local genetics services support could be expected to further help the delivery of genomics in primary care. Moreover, the emergence of AI tools in practice management software and education, as well as the role of the genetic counsellor in primary care, are worth exploring in future studies. Such strategies call for close collaboration between primary care and tertiary-based genetics services to facilitate education, and even a community of practice for GPs in genomics, as a key step toward building capacity.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
On behalf of the PRECISE project team
Kristi J. Jones, Sydney Children’s Hospitals Network; Robyn Jamieson, Sydney Children’s Hospitals Network, Children’s Medical Research Institute; Nicole Rankin, The University of Melbourne; Stephen Barnett, General Practice, The University of Wollongong, Medcast; Kirsten Boggs, Murdoch Children’s Research Institute; Edwina Middleton, Centre for Genetics Education, HETI, NSW Health; Emma Bonser, Genetic Alliance Australia; Jan Mumford, Consumer Advocate; Anthony Brown, Health Consumers NSW; Caitlin Forwood, Clinical Genetics, Northern Sydney Local Health District; Alexandra Williams, HealthPathways, Nepean Blue Mountains Primary Health Network; Fi Lam, General Practice; Nick Rosser, Nepean Blue Mountains Health Pathways; Kate Baker-Marges, General Practitioner, Nepean Blue Mountains HealthPathways; Mehrnoush Bonakdar Tehrani, Postdoctoral Fellow, PRECISE, University of Sydney; Janette Hayward, Genetic Counsellor, PRECISE, Sydney Children’s Hospitals Network.
Author contributions
KD: Conceptualization, Methodology, Formal analysis, Investigation, Supervision, Writing – original draft, Writing – review & editing. NS: Conceptualization, Methodology, Formal analysis, Investigation, Writing – original draft, Writing – review & editing. AS: Conceptualization, Methodology, Writing – original draft, Writing – review & editing. ALM: Methodology, Formal analysis, Writing – review & editing. JS: Conceptualization, Methodology, Writing – review & editing. AC: Conceptualization, Methodology, Writing – review & editing. MM: Conceptualization, Methodology, Writing – review & editing. CB: Conceptualization, Methodology, Writing – review & editing. BT: Conceptualization, Methodology, Writing – review & editing. LM: Conceptualization, Methodology, Writing – review & editing. DW: Conceptualization, Methodology, Writing – review & editing. SS: Conceptualization, Methodology, Writing – review & editing. AlM: Conceptualization, Formal Analysis, Funding acquisition, Investigation, Methodology, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The study is the first phase of The PRECISE (Practitioner Readiness, Education, Capabilities, with Implementation Science and Evaluation) genomics project funded by the Australian Government’s Medical Research Futures Fund (MRFF #2024995). JS is the recipient of a Cancer Institute NSW Career Development Fellowship (#2022/CDF1154). AC is supported by a NHMRC Investigator Grant (#2008454).
Acknowledgments
The authors are grateful to The University of Sydney Faculty Liaison Librarians for their assistance with preliminary search strategies and to the PRECISE project investigators (in addition to authors) for conceptualisation and support.
Conflict of interest
DW was employed by The Royal Australian College of General Practitioners Ltd (RACGP).
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1577958/full#supplementary-material
References
1. O'Shea, R, Ma, AS, Jamieson, RV, and Rankin, NM. Precision medicine in Australia: now is the time to get it right. Med J Aust. (2022) 217:559–63. doi: 10.5694/mja2.51777
2. Hayward, J, Bishop, M, Rafi, I, and Davison, V. Genomics in routine clinical care: what does this mean for primary care? Br J Gen Pract. (2017) 67:58–9. doi: 10.3399/bjgp17X688945
3. Parliment, A. The new frontier - delivering better health for all Australians: Parliment of Australia ; (2021) Available online at:https://www.aph.gov.au/Parliamentary_Business/Committees/House/Health_Aged_Care_and_Sport/Newdrugs/Report.
4. WHO. Primary care: World Health Organisation; (2023). Available online at:https://www.who.int/health-topics/primary-health-care#tab=tab_1.
5. Cusack, MB, Hickerton, C, Nisselle, A, McClaren, B, Terrill, B, Gaff, C, et al. General practitioners' views on genomics, practice and education: a qualitative interview study. Aust J Gen Pract. (2021) 50:747–52. doi: 10.31128/AJGP-05-20-5448
6. Mikat-Stevens, NA, Larson, IA, and Tarini, BA. Primary-care providers’ perceived barriers to integration of genetics services: a systematic review of the literature. Genet Med. (2015) 17:169–76. doi: 10.1038/gim.2014.101
7. Larson, EA, and Wilke, RA. Integration of genomics in primary care. Am J Med. (2015) 128:1251. doi: 10.1016/j.amjmed.2015.05.011
8. Hayward, J, Evans, W, Miller, E, and Rafi, I. Embedding genomics across the NHS: a primary care perspective. Future Healthcare J. (2023) 10:263–9. doi: 10.7861/fhj.2023-0116
9. Tricco, AC, Lillie, E, Zarin, W, O'Brien, KK, Colquhoun, H, Levac, D, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. (2018) 169:467–73. doi: 10.7326/M18-0850
10. White, S, Jacobs, C, and Phillips, J. Mainstreaming genetics and genomics: a systematic review of the barriers and facilitators for nurses and physicians in secondary and tertiary care. Genet Med. (2020) 22:1149–55. doi: 10.1038/s41436-020-0785-6
11. Atkins, L, Francis, J, Islam, R, O’Connor, D, Patey, A, Ivers, N, et al. A guide to using the theoretical domains framework of behaviour change to investigate implementation problems. Implement Sci. (2017) 12:1–18. doi: 10.1186/s13012-017-0605-9
12. Horowitz, CR, Orlando, LA, Slavotinek, AM, Peterson, J, Angelo, F, Biesecker, B, et al. The genomic medicine integrative research framework: a conceptual framework for conducting genomic medicine research. Am J Hum Genet. (2019) 104:1088–96. doi: 10.1016/j.ajhg.2019.04.006
13. Hauser, D, Obeng, AO, Fei, K, Ramos, MA, and Horowitz, CR. Views of primary care providers on testing patients for genetic risks for common chronic diseases. Health Aff (Millwood). (2018) 37:793–800. doi: 10.1377/hlthaff.2017.1548
14. Carroll, JC, Allanson, J, Morrison, S, Miller, FA, Wilson, BJ, Permaul, JA, et al. Informing integration of genomic medicine into primary care: An assessment of current practice, attitudes, and desired resources. Front Genet. (2019) 10:1189. doi: 10.3389/fgene.2019.01189
15. Bernhardt, BA, Zayac, C, Gordon, ES, Wawak, L, Pyeritz, RE, and Gollust, SE. Incorporating direct-to-consumer genomic information into patient care: attitudes and experiences of primary care physicians. Per Med. (2012) 9:683–92. doi: 10.2217/pme.12.80
16. Leitsalu, L, Hercher, L, and Metspalu, A. Giving and withholding of information following genomic screening: challenges identified in a study of primary care physicians in Estonia. J Genet Couns. (2012) 21:591–604. doi: 10.1007/s10897-011-9424-3
17. Lemke, AA, Amendola, LM, Kuchta, K, Dunnenberger, HM, Thompson, J, Johnson, C, et al. Primary care physician experiences with integrated population-scale genetic testing: a mixed-methods assessment. J Pers Med. (2020) 10:165. doi: 10.3390/jpm10040165
18. Morberg Jamterud, S, Snoek, A, van Langen, IM, Verkerk, M, and Zeiler, K. Qualitative study of GPs' views and experiences of population-based preconception expanded carrier screening in the Netherlands: bioethical perspectives. BMJ Open. (2021) 11:e056869. doi: 10.1136/bmjopen-2021-056869
19. Yu, MWC, Fung, JLF, Ng, APP, Li, Z, Lan, W, Chung, CCY, et al. Preparing genomic revolution: attitudes, clinical practice, and training needs in delivering genetic counseling in primary care in Hong Kong and Shenzhen, China. Mol Genet Genomic Med. (2021) 9:e1702. doi: 10.1002/mgg3.1702
20. Best, S, Long, JC, Fehlberg, Z, Theodorou, T, Hatem, S, Archibald, A, et al. The more you do it, the easier it gets: using behaviour change theory to support health care professionals offering reproductive genetic carrier screening. Eur J Hum Genet. (2023) 31:430–44. doi: 10.1038/s41431-022-01224-5
21. Harding, B, Webber, C, Ruhland, L, Dalgarno, N, Armour, C, Birtwhistle, R, et al. Bridging the gap in genetics: a progressive model for primary to specialist care. BMC Med Educ. (2019) 19:195. doi: 10.1186/s12909-019-1622-y
22. Marathe, JA, Woodroffe, J, Ogden, K, and Hughes, C. General Practitioners' knowledge and use of genetic counselling in managing patients with genetic cardiac disease in non-specialised settings. J Community Genet. (2015) 6:375–82. doi: 10.1007/s12687-015-0229-1
23. Fok, RW, Ong, CSB, Lie, D, Ishak, D, Fung, SM, Tang, WE, et al. How practice setting affects family physicians' views on genetic screening: a qualitative study. BMC Fam Pract. (2021) 22:141. doi: 10.1186/s12875-021-01492-y
24. Mitchell, S, Jaccard, E, Schmitz, FM, von Kanel, E, Collombet, P, Cornuz, J, et al. Investigating acceptability of a training programme in precision medicine for frontline healthcare professionals: a mixed methods study. BMC Med Educ. (2022) 22:556. doi: 10.1186/s12909-022-03613-2
25. Melo, DG, de Paula, PK, de Araujo, RS, da Silva de Avo, LR, Germano, CM, and Demarzo, MM. Genetics in primary health care and the National Policy on Comprehensive Care for People with rare diseases in Brazil: opportunities and challenges for professional education. J Community Genet. (2015) 6:231–40. doi: 10.1007/s12687-015-0224-6
26. Ayoub, A, Lapointe, J, Nabi, H, and Pashayan, N. Risk-stratified breast Cancer screening incorporating a polygenic risk score: a survey of UK general Practitioners' knowledge and attitudes. Genes (Basel). (2023) 14:732. doi: 10.3390/genes14030732
27. Smit, AK, Newson, AJ, Keogh, L, Best, M, Dunlop, K, Vuong, K, et al. GP attitudes to and expectations for providing personal genomic risk information to the public: a qualitative study. BJGP Open. (2019) 3. doi: 10.3399/bjgpopen18X101633
28. Wilson, BJ, Islam, R, Francis, JJ, Grimshaw, JM, Permaul, JA, Allanson, JE, et al. Supporting genetics in primary care: investigating how theory can inform professional education. Eur J Hum Genet. (2016) 24:1541–6. doi: 10.1038/ejhg.2016.68
29. Baroncini, A, Calabrese, O, Colotto, M, Pelo, E, Torricelli, F, and Boccia, S. Knowledge and attitude of general pratictioners towards direct-to-consumer genomic tests: a survey conducted in Italy. Epidemiol Biostat Public Health. (2015) 12. doi: 10.2427/11613
30. Best, S, Long, JC, Fehlberg, Z, Archibald, AD, and Braithwaite, J. Supporting healthcare professionals to offer reproductive genetic carrier screening: a behaviour change theory approach. Aust J Prim Health. (2023) 29:480–9. doi: 10.1071/PY23022
31. Van Wyk, C, Wessels, TM, Kromberg, JG, and Krause, A. Knowledge regarding basic concepts of hereditary cancers, and the available genetic counselling and testing services: a survey of general practitioners in Johannesburg, South Africa. S Afr Med J. (2016) 106:268–71. doi: 10.7196/SAMJ.2016.v106i3.10162
32. Nair, N, Bellcross, C, Haddad, L, Martin, M, Matthews, R, Gabram-Mendola, S, et al. Georgia primary care Providers' knowledge of hereditary breast and ovarian Cancer syndrome. J Cancer Educ. (2017) 32:119–24. doi: 10.1007/s13187-015-0950-9
33. Houwink, EJ, Henneman, L, Westerneng, M, van Luijk, SJ, Cornel, MC, Dinant, JG, et al. Prioritization of future genetics education for general practitioners: a Delphi study. Genet Med. (2012) 14:323–9. doi: 10.1038/gim.2011.15
34. Houwink, EJ, van Luijk, SJ, Henneman, L, van der Vleuten, C, Jan Dinant, G, and Cornel, MC. Genetic educational needs and the role of genetics in primary care: a focus group study with multiple perspectives. BMC Fam Pract. (2011) 12:1–9. doi: 10.1186/1471-2296-12-5
35. Skinner, SJ, Clay, AT, McCarron, MCE, and Liskowich, S. Interpretation and management of genetic test results by Canadian family physicians: a multiple choice survey of performance. J Community Genet. (2021) 12:479–84. doi: 10.1007/s12687-021-00511-w
36. Terrill, BN, Pearce, A, Chau, A, and Young, M-A. Navigating genomic testing: evaluation of an e-learning module with general practitioners. Focus Health Profes Educ. (2024) 25:37–50. doi: 10.11157/fohpe.v25i1.630
37. Vieira, TA, Giugliani, C, da Silva, LP, Faccini, LS, Loguercio Leite, JC, Artigalas, OA, et al. Inclusion of medical genetics in primary health care: report of a pilot project in Brazil. J Community Genet. (2013) 4:137–45. doi: 10.1007/s12687-012-0110-4
38. Carroll, JC, Wilson, BJ, Allanson, J, Grimshaw, J, Blaine, SM, Meschino, WS, et al. GenetiKit: a randomized controlled trial to enhance delivery of genetics services by family physicians. Fam Pract. (2011) 28:615–23. doi: 10.1093/fampra/cmr040
39. Telner, D, Carroll, JC, Regehr, G, Tabak, D, Semotiuk, K, and Freeman, R. Teaching primary care genetics: a randomized controlled trial comparison. Fam Med. (2017) 49:443–50.
40. Houwink, EJ, van Teeffelen, SR, Muijtjens, AM, Henneman, L, Jacobi, F, van Luijk, SJ, et al. Sustained effects of online genetics education: a randomized controlled trial on oncogenetics. Eur J Hum Genet. (2014) 22:310–6. doi: 10.1038/ejhg.2013.163
41. Jackson, L, O'Connor, A, Paneque, M, Curtisova, V, Lunt, PW, Pourova, RK, et al. The gen-equip project: evaluation and impact of genetics e-learning resources for primary care in six European languages. Genet Med. (2019) 21:718–26. doi: 10.1038/s41436-018-0132-3
42. Calabro, GE, Tognetto, A, Mazzaccara, A, Barbina, D, Carbone, P, Guerrera, D, et al. Capacity building of health professionals on genetics and genomics practice: evaluation of the effectiveness of a distance learning training course for Italian physicians. Front Genet. (2021) 12:626685. doi: 10.3389/fgene.2021.626685
43. Bell, RA, McDermott, H, Fancher, TL, Green, MJ, Day, FC, and Wilkes, MS. Impact of a randomized controlled educational trial to improve physician practice behaviors around screening for inherited breast cancer. J Gen Intern Med. (2015) 30:334–41. doi: 10.1007/s11606-014-3113-5
44. Houwink, EJ, Muijtjens, AM, van Teeffelen, SR, Henneman, L, Rethans, JJ, Jacobi, F, et al. Effect of comprehensive oncogenetics training interventions for general practitioners, evaluated at multiple performance levels. PLoS One. (2015) 10:e0122648. doi: 10.1371/journal.pone.0122648
45. Carroll, JC, Blaine, S, Permaul, J, Dicks, E, Warner, E, Esplen, MJ, et al. Efficacy of an educational intervention on family physicians' risk assessment and management of colorectal cancer. J Community Genet. (2014) 5:303–11. doi: 10.1007/s12687-014-0185-1
46. Houwink, EJ, Muijtjens, AM, van Teeffelen, SR, Henneman, L, Rethans, JJ, van der Jagt, LE, et al. Effectiveness of oncogenetics training on general practitioners' consultation skills: a randomized controlled trial. Genet Med. (2014) 16:45–52. doi: 10.1038/gim.2013.69
47. van Vliet, ME, Kerkhoffs, JH, Harteveld, CL, and Houwink, EJF. Hemoglobinopathy screening in primary care in the Netherlands: exploring the problems and needs of patients and general practitioners. Eur J Hum Genet. (2023) 31:417–23. doi: 10.1038/s41431-022-01156-0
48. Tan, YY, Spurdle, AB, and Obermair, A. Knowledge, attitudes and referral patterns of lynch syndrome: a survey of clinicians in Australia. J Pers Med. (2014) 4:218–44. doi: 10.3390/jpm4020218
49. Vassy, JL, Kerman, BJ, Harris, EJ, Lemke, AA, Clayman, ML, Antwi, AA, et al. Perceived benefits and barriers to implementing precision preventive care: results of a national physician survey. Eur J Hum Genet. (2023) 31:1309–16. doi: 10.1038/s41431-023-01318-8
50. Carroll, JC, Makuwaza, T, Manca, DP, Sopcak, N, Permaul, JA, O’Brien, MA, et al. Primary care providers’ experiences with and perceptions of personalized genomic medicine. Can Fam Physician. (2016) 62:e626–35.
51. Carroll, JC, Morrison, S, Miller, FA, Wilson, BJ, Permaul, JA, and Allanson, J. Anticipating the primary care role in genomic medicine: expectations of genetics health professionals. J Community Genet. (2021) 12:559–68. doi: 10.1007/s12687-021-00544-1
52. Rafi, I, Crinson, I, Dawes, M, Rafi, D, Pirmohamed, M, and Walter, FM. The implementation of pharmacogenomics into UK general practice: a qualitative study exploring barriers, challenges and opportunities. J Community Genet. (2020) 11:269–77. doi: 10.1007/s12687-020-00468-2
53. Presutti, RJ, Pujalte, GGA, Woodruff, A, Agarwal, A, Robinson, CN, Reese, RL, et al. Do physicians know when to refer patients for genetic testing? J Genet Couns. (2023) 33:786–792. doi: 10.1002/jgc4.1787
54. Evans, WRH, Tranter, J, Rafi, I, Hayward, J, and Qureshi, N. How genomic information is accessed in clinical practice: an electronic survey of UK general practitioners. J Community Genet. (2020) 11:377–86. doi: 10.1007/s12687-020-00457-5
55. Westwood, G, Pickering, R, Latter, S, Little, P, Gerard, K, Lucassen, A, et al. A primary care specialist genetics service: a cluster-randomised factorial trial. Br J Gen Pract. (2012) 62:e191–7. doi: 10.3399/bjgp12X630089
56. Barreiro, CZ, Bidondo, MP, Garrido, JA, Deurloo, J, Acevedo, E, Luna, A, et al. CHACO outreach project: the development of a primary health care-based medical genetic service in an Argentinean province. J Community Genet. (2013) 4:321–34. doi: 10.1007/s12687-013-0157-x
57. Pot, M, Spalletta, O, and Green, S. Precision medicine in primary care: how GPs envision “old” and “new” forms of personalization. Soc Sci Med. (2024) 358:117259. doi: 10.1016/j.socscimed.2024.117259
58. Frost, A, Kelly, A, Bishop, M, Bogue, D, Copson, E, Gompertz, L, et al. Genotes–a ‘just-in-time’genomics education resource co-designed with clinicians. BMC Med Educ. (2024) 24:1378. doi: 10.1186/s12909-024-06059-w
59. Evans, W, Meslin, EM, Kai, J, and Qureshi, N. Precision medicine—are we there yet? A narrative review of precision medicine’s applicability in primary care. J Personal Med. (2024) 14:418. doi: 10.3390/jpm14040418
60. Falah, N, Umer, A, Warnick, E, Vallejo, M, and Lefeber, T. Genetics education in primary care residency training: satisfaction and current barriers. BMC Primary Care. (2022) 23:156. doi: 10.1186/s12875-022-01765-0
61. Mai, C-W, Sridhar, SB, Karattuthodi, MS, Ganesan, PM, Shareef, J, Lee, EL, et al. Scoping review of enablers and challenges of implementing pharmacogenomics testing in the primary care settings. BMJ Open. (2024) 14:e087064. doi: 10.1136/bmjopen-2024-087064
62. Hull, LE, Gold, NB, and Armstrong, KA. Revisiting the roles of primary care clinicians in genetic medicine. JAMA. (2020) 324:1607–8. doi: 10.1001/jama.2020.18745
63. Puryear, L, Downs, N, Nevedal, A, Lewis, ET, Ormond, KE, Bregendahl, M, et al. Patient and provider perspectives on the development of personalized medicine: a mixed-methods approach. J Community Genet. (2018) 9:283–91. doi: 10.1007/s12687-017-0349-x
64. Miller, FA, Carroll, JC, Wilson, BJ, Bytautas, JP, Allanson, J, Cappelli, M, et al. The primary care physician role in cancer genetics: a qualitative study of patient experience. Fam Pract. (2010) 27:563–9. doi: 10.1093/fampra/cmq035
65. Morrow, A, Chan, P, Tucker, KM, and Taylor, N. The design, implementation, and effectiveness of intervention strategies aimed at improving genetic referral practices: a systematic review of the literature. Genet Med. (2021) 23:2239–49. doi: 10.1038/s41436-021-01272-0
66. Mather, M, Pettigrew, LM, and Navaratnam, S. Barriers and facilitators to clinical behaviour change by primary care practitioners: a theory-informed systematic review of reviews using the theoretical domains framework and behaviour change wheel. Syst Rev. (2022) 11:180. doi: 10.1186/s13643-022-02030-2
67. Paneque, M, Turchetti, D, Jackson, L, Lunt, P, Houwink, E, and Skirton, H. A systematic review of interventions to provide genetics education for primary care. BMC Fam Pract. (2016) 17:89. doi: 10.1186/s12875-016-0483-2
68. Lyu, X, and Li, S. Professional medical education approaches: mobilizing evidence for clinicians. Front Med. (2023) 10:1071545. doi: 10.3389/fmed.2023.1071545
69. Fiscella, K, and Epstein, RM. So much to do, so little time: care for the socially disadvantaged and the 15-minute visit. Arch Intern Med. (2008) 168:1843–52. doi: 10.1001/archinte.168.17.1843
70. Holmér, S, Nedlund, A-C, Thomas, K, and Krevers, B. How health care professionals handle limited resources in primary care – an interview study. BMC Health Serv Res. (2023) 23:6. doi: 10.1186/s12913-022-08996-y
71. Best, S, Vidic, N, An, K, Collins, F, and White, SM. A systematic review of geographical inequities for accessing clinical genomic and genetic services for non-cancer related rare disease. Eur J Hum Genet. (2022) 30:645–52. doi: 10.1038/s41431-021-01022-5
72. Noar, AP, Jeffery, HE, Subbiah Ponniah, H, and Jaffer, U. The aims and effectiveness of communities of practice in healthcare: a systematic review. PLoS One. (2023) 18:e0292343. doi: 10.1371/journal.pone.0292343
73. Ma, A, O’Shea, R, Wedd, L, Wong, C, Jamieson, RV, and Rankin, N. What is the power of a genomic multidisciplinary team approach? A systematic review of implementation and sustainability. Eur J Hum Genet. (2024) 32:381–91. doi: 10.1038/s41431-024-01555-5
74. Ma, A, Newing, TP, O'Shea, R, Gokoolparsadh, A, Murdoch, E, Hayward, J, et al. Genomic multidisciplinary teams: a model for navigating genetic mainstreaming and precision medicine. J Paediatr Child Health. (2024) 60:118–24. doi: 10.1111/jpc.16547
75. Alderson, SL, Bald, A, Carder, P, Farrin, A, and Foy, R. Establishing a primary care audit and feedback implementation laboratory: a consensus study. Implement. Sci. Commun. (2021) 2:3. doi: 10.1186/s43058-020-00103-8
76. Carroll, JC, Grad, R, Allanson, JE, Pluye, P, Permaul, JA, Pimlott, N, et al. The gene messenger impact project: An innovative genetics continuing education strategy for primary care providers. J Contin Educ Heal Prof. (2016) 36:178–85. doi: 10.1097/CEH.0000000000000079
77. Hansen, CA, Reiter, AW, and Wildin, RS. Growth in perceived clinical genetics competency among primary care providers participating in genomic population health screening. J Community Genet. (2024) 15:33–7. doi: 10.1007/s12687-023-00675-7
78. Haga, SB, Burke, W, Ginsburg, GS, Mills, R, and Agans, R. Primary care physicians\u0027 knowledge of and experience with pharmacogenetic testing. Clin Genet. (2012) 82:388–94. doi: 10.1111/j.1399-0004.2012.01908.x
79. Sebastian, A, Carroll, JC, Vanstone, M, Clausen, M, Kodida, R, and Reble, E. Challenges and practical solutions for managing secondary genomic findings in primary care. Eur J Med Genet. (2022) 65:104384. doi: 10.1016/j.ejmg.2021.104384
80. Dormandy, E, Reid, E, Tsianakas, V, O\u0027Neil, B, Gill, E, and Marteau, TM. Offering antenatal sickle cell and thalassaemia screening in primary care: A pre-post evaluation of a brief type of communication skills training. Patient Educ Couns. (2012) 89:129–33. doi: 10.1016/j.pec.2012.05.004
81. Brown-Johnson, CG, Safaeinili, N, Baratta, J, Palaniappan, L, Mahoney, M, Rosas, LG, et al. Implementation outcomes of Humanwide: integrated precision health in team-based family practice primary care. BMC Fam Pract. (2021) 22:28. doi: 10.1186/s12875-021-01373-4
Keywords: genomics, genomics education, education strategies, primary care, general practitioner
Citation: Dunlop KLA, Singh N, Smit AK, Morrow AL, Steinberg J, Cust AE, Makeham M, Bonner C, Terrill B, Monrouxe LV, Wilkinson D, Sawleshwarkar S and Ma AS (2025) Building capacity for genomics in primary care: a scoping review of practitioner attitudes, education needs, and enablers. Front. Med. 12:1577958. doi: 10.3389/fmed.2025.1577958
Edited by:
Xiaoling Xuei, Indiana University School of Medicine, United StatesReviewed by:
Kathleen Holt, University of Rochester, United StatesLucinda Freeman, University of Technology Sydney, Australia
Copyright © 2025 Dunlop, Singh, Smit, Morrow, Steinberg, Cust, Makeham, Bonner, Terrill, Monrouxe, Wilkinson, Sawleshwarkar and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kate L. A. Dunlop, a2F0ZS5kdW5sb3BAc3lkbmV5LmVkdS5hdQ==
†These authors share first authorship
 Kate L. A. Dunlop1,2*†
Kate L. A. Dunlop1,2*† Nehal Singh
Nehal Singh Anne E. Cust
Anne E. Cust Meredith Makeham
Meredith Makeham Lynn V. Monrouxe
Lynn V. Monrouxe Shailendra Sawleshwarkar
Shailendra Sawleshwarkar Alan S. Ma
Alan S. Ma