- 1Department of Obstetrics and Gynecology, Shulan (Hangzhou) Hospital Affiliated to Zhejiang Shuren University Shulan International Medical College, Hangzhou, Zhejiang, China
- 2Department of Gynecology, Zouping People’s Hospital, Binzhou, Shandong, China
- 3Department of Obstetrics and Gynecology, Affiliated Hangzhou Xixi Hospital Zhejiang University School of Medicine, Hangzhou, Zhejiang, China
- 4Department of Obstetrics and Gynecology, Puren Hospital Affiliated to Wuhan University of Science and Technology, Wuhan, Hubei, China
Background: Cervical cancer incidence and mortality rates in the United States have substantially declined over recent decades, primarily driven by reductions in squamous cell carcinoma cases. However, the trend in recent years remains unclear. This study aimed to explore the trends in cervical cancer incidence and mortality, stratified by demographic and tumor characteristics from 1975 to 2018.
Methods: The age-adjusted incidence, incidence-based mortality, and relative survival of cervical cancer were calculated using the Surveillance, Epidemiology, and End Results (SEER)-9 database. Trend analyses with annual percent change (APC) and average annual percent change (AAPC) calculations were performed using Joinpoint Regression Software (Version 4.9.1.0, National Cancer Institute).
Results: During 1975–2018, 49,658 cervical cancer cases were diagnosed, with 17,099 recorded deaths occurring between 1995 and 2018. Squamous cell carcinoma was the most common histological type, with 34,169 cases and 11,859 deaths. Over the study period, the cervical cancer incidence rate decreased by an average of 1.9% (95% CI: −2.3% to −1.6%) per year, with the APCs decreased in recent years (−0.5% [95% CI: −1.1 to 0.1%] in 2006–2018). Squamous cell carcinoma incidence trends closely paralleled overall cervical cancer patterns, but the incidence of squamous cell carcinoma in the distant stage increased significantly (1.1% [95% CI: 0.4 to 1.8%] in 1990–2018). From 1995 to 2018, the overall cervical cancer mortality rate decreased by 1.0% (95% CI: −1.2% to −0.8%) per year. But for distant-stage squamous cell carcinoma, the mortality rate increased by 1.2% (95% CI: 0.3 to 2.1%) per year.
Conclusion: For cervical cancer cases diagnosed in the United States from 1975 to 2018, the overall incidence and mortality rates decreased significantly. However, there was an increase in the incidence and mortality of advanced-stage squamous cell carcinoma. These epidemiological patterns offer critical insights for refining cervical cancer screening protocols and developing targeted interventions for advanced-stage cases.
1 Introduction
According to the latest GLOBOCAN statistics, cervical cancer ranks as the fourth most common cancer in terms of incidence and mortality among women worldwide, following only breast cancer, colorectal cancer, and lung cancer (1). In low-income countries, cervical cancer is the second most common fatal malignant tumor (2). Evidently, cervical cancer causes a substantial burden on women’s public health globally.
Cervical carcinogenesis follows a well-defined sequence: (1) normal squamous epithelium, (2) premalignant cervical intraepithelial neoplasia (CIN1-3), and (3) invasive carcinoma (3). Human papillomavirus (HPV) infection is the predominant risk factor for the development of cervical cancer. Almost all cases of cervical cancer are associated with HPV infection, especially HPV-16 and HPV-18. Because of significant advances in understanding the infectious etiology of cervical cancer, HPV vaccines indicated the promising efficacy for cervical cancer prevention (4). The World Health Organization (WHO) launched its 90–70-90 elimination strategy in 2020: 90% HPV vaccination coverage by age 15, 70% screening with high-performance tests by age 35/45, and 90% treatment of precancerous lesions (5). While these initiatives show promise for reducing disease burden, longitudinal epidemiological trend analyses remain scarce.
The global implementation of prevention strategies has demonstrated partial success, with measurable outcomes observed in specific regions. Longitudinal analysis of GLOBOCAN 2018 data (6) revealed stabilized or declining incidence rates and mortality rates in 31 countries. Contrastingly, analysis of Puerto Rico Central Cancer Registry data (2001–2017) by Ortiz et al. (7) indicated a rising incidence specifically among women aged 35–64 years. Cervical cancer epidemiology shows strong correlation with national socioeconomic indicators, particularly healthcare expenditure and education indices. Current implementation of WHO-recommended prevention programs in low-income and developing countries fall substantially short of elimination targets (2). Effective implementation of the WHO Global Strategy requires real-time epidemiological data. For this purpose, this study collected the most dependable and comprehensive statistics from Surveillance, Epidemiology and End Results (SEER) database and carried out subgroup analyses to calculate the age-adjusted incidence and incidence-based mortality of cervical cancer according to demographic and tumor characteristics. This comprehensive analysis provides critical evidence to prevention and management of cervical cancer.
2 Methods
2.1 Data sources
Patients diagnosed with cervical cancer from the SEER-9 database between 1975 and 2018 were included in the analysis. The database is based on information from 9 high-quality registries in the U.S. and covers about 10% of the U.S. population. For each patient, demographic and tumor characteristics were extracted. The National Center for Health Statistics (NCHS) collected and maintained both national and SEER-9 mortality data (8). The SEER-9 incidence file was used to calculate age-adjusted incidence rates and relative survival rates (9). The SEER-9 incidence-based mortality file (8) provided incidence-based mortality rates (10). To ensure a maximum patient count and avoid underestimating the mortality in the earliest years, the mortality calculations were restricted to patients who were diagnosed between 1975 and 2018 and died between 1995 and 2018.
2.2 Demographic characteristics
The demographic indicators concerned in this study were race, age, family income and rural–urban distribution. All information came from medical records or death certificates. Age groups were divided into 0–19, 20–39, 40–59, 60–79, and 80+ years old, with reference to previous studies (11, 12). Family income was divided into low- and high-income groups based on $75,000.
2.3 Tumor characteristics
International Classification of Diseases for Oncology, Third Edition (ICD-O-3) was used to define cervical cancer (topography code: C53) and its histological classification (Supplementary Table 1): squamous cell carcinoma (histologic codes: 8070–8078), adenocarcinoma (8140, 8144, 8255, 8384, 8480, 8481, 8482), adenosquamous carcinoma (8560, 8570) and others. Only patients with malignant behavior (behavior code: /3) were selected. WHO grade was classified into I, II, III and unknown based on the grade code provided by SEER database and were limited to patients diagnosed between 1975 and 2017. SEER historic stage A and Combined Summary Stage classified cancer as localized, regional, distant and unknown. Since 1988, cervical cancer has been classified as stages I–IV and unknown according to the American Joint Committee on Cancer staging system (AJCC stage 3rd for 1988–2003; AJCC stage group 6th for 2004–2015; AJCC stage group 7th for 2016–2017; AJCC stage group 8th for 2018+). Beginning in 1983, patients’ tumor size was recorded in SEER database according to 4 different codes (EOD-4 for 1983–1987; EOD-10 for 1988–2003; CS for 2004–2015; Tumor Size Summary for 2016+).
2.4 Statistical analysis
SEER*Stat 8.3.9.2 was used to calculate age-adjusted incidence and incidence-based mortality rates, which were expressed per 100,000/years. All rates were adjusted to 2000 US Std Population. Patients with cancers identified only by autopsy or death certificates were excluded. Trends were quantified by annual percentage changes (APC), average annual percent change (AAPC) and 95% confidence interval (CI). This step was done by the Joinpoint program, version 4.9.0.1. The program takes trend data and fits the simplest joinpoint model that the data allow. This enables the user to test that an apparent change in trend is statistically significant. The process of selecting joinpoints involves modeling methods and model optimization. The modeling method uses the grid search method (GSM), through which all possible interval piecewise function connection points are established, and the corresponding sum of squares errors (SSE) and mean squared errors (MSE) of each possible case are calculated. The network point with minimum MSE is selected as the piecewise function connection point, and the equation parameters are fitted according to the selected connection point and interval function. Permutation test (Monte Carlo method) was used to optimize the model. The program performs permutation tests to select the number of joinpoints. Since fitting all N! possible permutations of the data would take too long, the program takes a Monte Carlo sample of these N! data sets, using a random number generator. Specify the size of the Monte Carlo sample of permuted data sets (13). A two-side p < 0.05 was considered statistically significant.
3 Results
During 1975–2018, a total of 49,658 cervical cancer cases were recorded in the SEER-9 registry (Table 1). White patients (37,140 cases [74.8%]) made up the majority of the cases. Most cervical cancer patients were diagnosed between 20 and 79 years old. The most common histological type of cervical cancer was squamous cell carcinoma (34,169 cases [68.5%]), followed by adenocarcinoma (7,317 cases [14.7%]) and adenosquamous carcinoma (1,653 cases [3.3%]). Between 1995 and 2018, there are 17,099 cervical cancer deaths. White people (12,359 deaths [72.3%]) and patients with squamous cell carcinoma (11,859 deaths [69.4%]) still predominate. The majority of deaths occurred in people over 40 years old, in metropolitan areas and in those with tumors size unknown.

Table 1. Cervical cancer incidence (1975–2018) and incidence-based mortality (1995–2018): the SEER-9 registry database.
In terms of incidence and mortality rates (Table 1), black patients had a higher incidence and mortality rate of cervical cancer than white people, reaching 12.72 and 8.23 per 100,000 person-years, respectively. Incidence and mortality rates generally increased with age, but incidence rates peaked in the 60–79 years group (14.74 per 100,000 person-years) and mortality peaks in the 80+ years group (29.05 per 100,000 person-years). Low-income groups had higher incidence and mortality rates, while non-metropolitan groups had higher incidence and lower mortality rates. According to cancer characteristics, squamous cell carcinoma had a much higher incidence (5.95 per 100,000 person-years) and mortality rate (3.09 per 100,000 person-years) than other histological types. In addition, cervical cancer patients diagnosed as AJCC stage I or larger tumor size, had higher incidence and mortality rates.
Of the 49,658 cervical cancer patients occurring during 1975–2018, 2.3% cannot obtain WHO grade, 39.7% were diagnosed before the availability of AJCC stage starting in 1988, and 19.8% were diagnosed before the availability of tumor size starting in 1983. Of the 17,099 cervical cancer deaths occurring during 1995–2018, 4.8% cannot obtain WHO grade, 23.2% were diagnosed before the availability of AJCC stage starting in 1988, and 15.0% were diagnosed before the availability of tumor size starting in 1983. In the years for which data were available, WHO grade (1975–2017) was unknown for 41.9% of cases and 40.0% of deaths, SEER stage (1975–2018) was unknown for 6.6% of cases and 7.4% of deaths, AJCC stage (1988–2018) was unknown for 9.1% of cases and 10.0% of deaths, and tumor size (1983–2018) was unknown for 50.9% of cases and 55.1% of deaths.
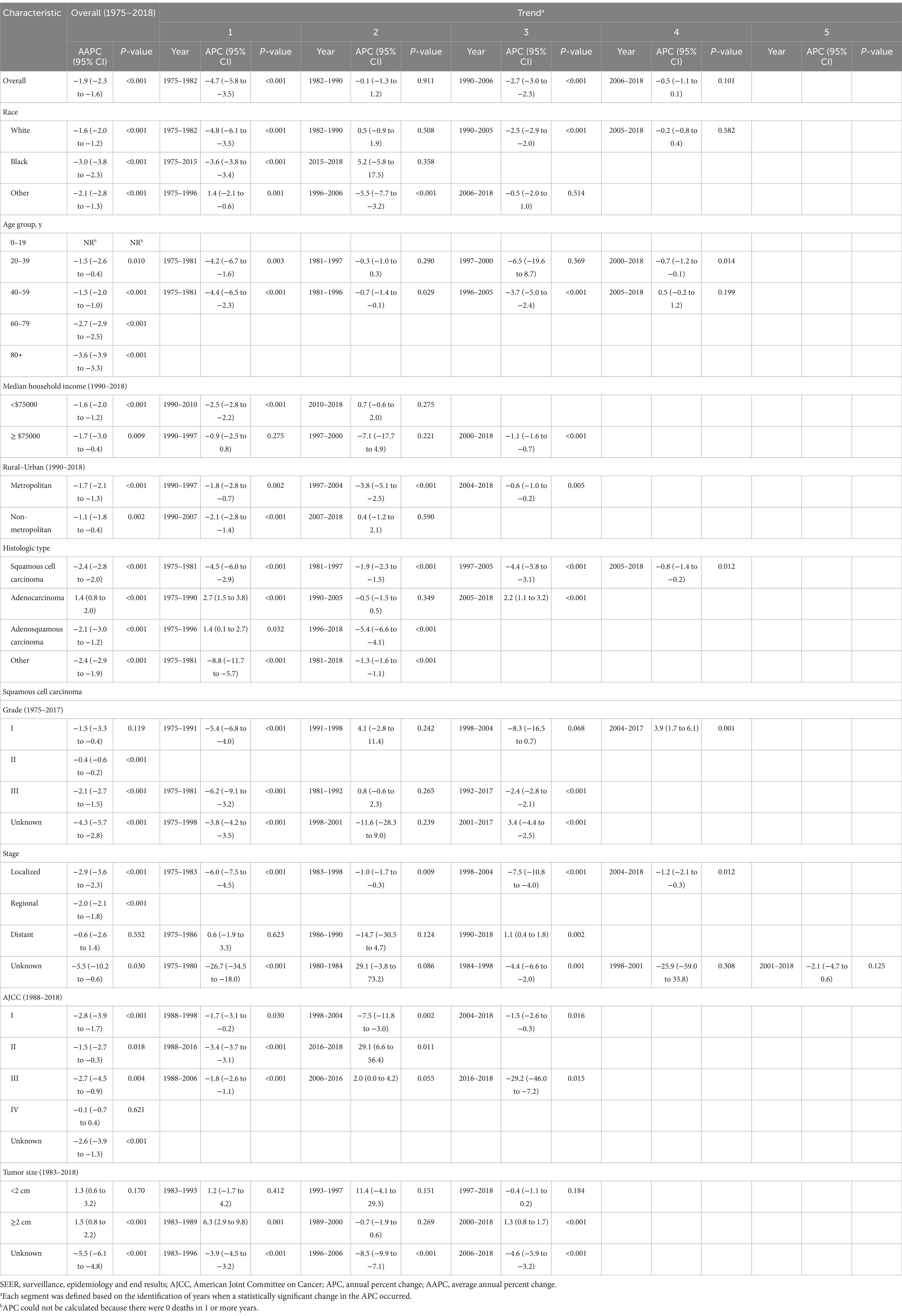
Table 2 showed the overall trend of cervical cancer incidence from 1975 to 2018 and the incidence of significant changes in the segments divided by Joinpoint. Figure 1 depicted trends in cervical cancer incidence according to histological type, tumor stage, and tumor size. Cervical cancer incidence rate declined by an average of 1.9% (95% CI, −2.3% to −1.6%) per year over the study period. Among them, the overall incidence rate decreased by −4.7% (95% CI, −5.8% to −3.5%) and −2.7% (95% CI, −3.0% to −2.3%) per year in 1975–1982 and 1990–2006, respectively. However, there was no significant downward trend (APC, −0.5% [95% CI, −1.1 to 0.1%]) between 2006 and 2018. Cervical cancer incidence decreased significantly for all race, age groups, household income and rural–urban distribution. The incidence of squamous cell carcinoma (AAPC, −2.4% [95% CI, −2.8% to −2.0%]) and adenosquamous carcinoma (AAPC, −2.1% [95% CI, −3.0% to −1.2%]) has decreased significantly, while the incidence of adenocarcinoma (AAPC, 1.4% [95% CI, 0.8 to 2.0%]) has increased significantly. SCC incidence decreased significantly for cancer with WHO grade II, III and localized, regional tumor stage. But, the incidence of grade I (2004–2017: APC, 3.9% [95% CI, 1.7 to 6.1%]) and distant metastasized (1990–2018: APC, 1.1% [95% CI, 0.4 to 1.8%]) cases had increased significantly in recent years. The incidence of AJCC stages declined significantly in all SCC, except for stage IV, where there was no significant difference. The incidence of tumor size ≥2 cm increased significantly, mainly due to a significant decrease in the incidence of unknown tumor size.
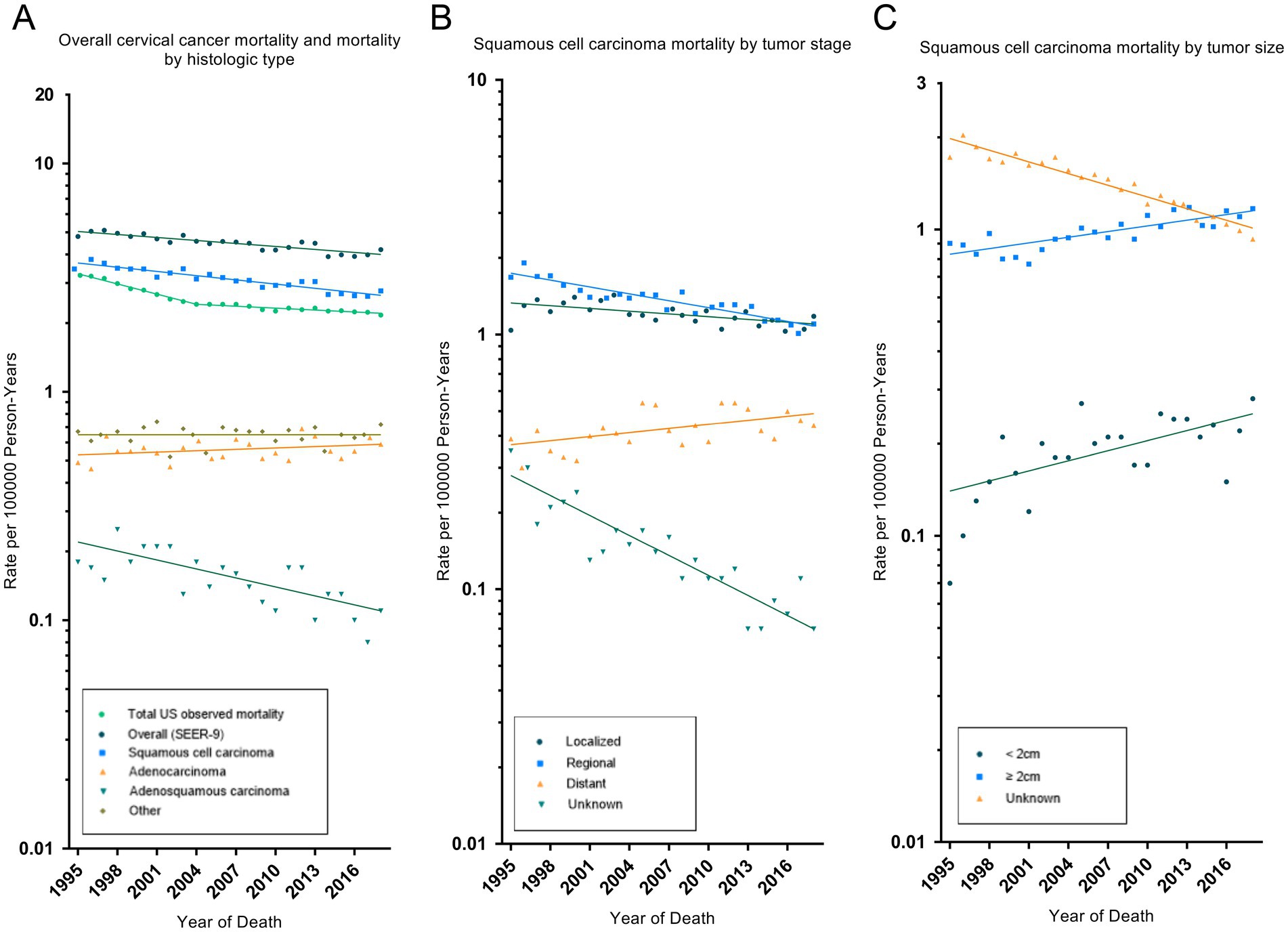
Figure 1. Trends in cervical cancer age-adjusted incidence rates. Panel (A) shows overall cervical cancer incidence and incidence by histologic type in 1975–2018. Panel (B) shows squamous cell carcinoma incidence by tumor stage in 1975–2018. Panel (C) shows squamous cell carcinoma incidence by tumor size in 1983–2018. All rates were age-adjusted to the 2000 US standard population. The dots represent annual incidence rates and the lines represent incidence trends from joinpoint analysis.
During 1995–2018, trends in cervical cancer incidence-based mortality recorded by SEER-9 were consistent with the observed total US cervical cancer mortality (Table 3 and Figure 2). The total US cervical cancer mortality rate declined by 1.7% (95% CI, −1.9% to −1.6%) per year, and its APC declined, from −3.4% (95% CI, −3.7% to −3.0%) in 1995–2004 to −0.7% (95% CI, −0.9% to −0.5%) in 2004–2018. SEER-9 incidence-based mortality decreased, on average, 1.0% (95% CI, −1.2% to −0.8%) annually from 1995 to 2018. All race and age groups had negative AAPC values, except for the rare number of deaths in the 0–19 age group. Cervical cancer mortality rate had increased significantly in low-income groups, with an AAPC of 0.8% (95% CI, 0.3 to 1.2%). By histologic type, there were significant decrease in mortality rates of SCC (AAPC, −1.4% [95% CI, −1.6% to −1.2%]) and adenosquamous carcinoma (AAPC, −2.9% [95% CI, −4.0% to −1.7%]), while adenocarcinoma mortality increased without statistically significant (AAPC, 0.4% [95% CI, −0.2 to 1.1%]). Negative APCs were observed for SCC of all WHO grade, localized, regional or AJCC II stage. However, SCC patients with distant disease had a significantly increased mortality rate (AAPC, 1.2% [95% CI, 0.3 to 2.1%]).
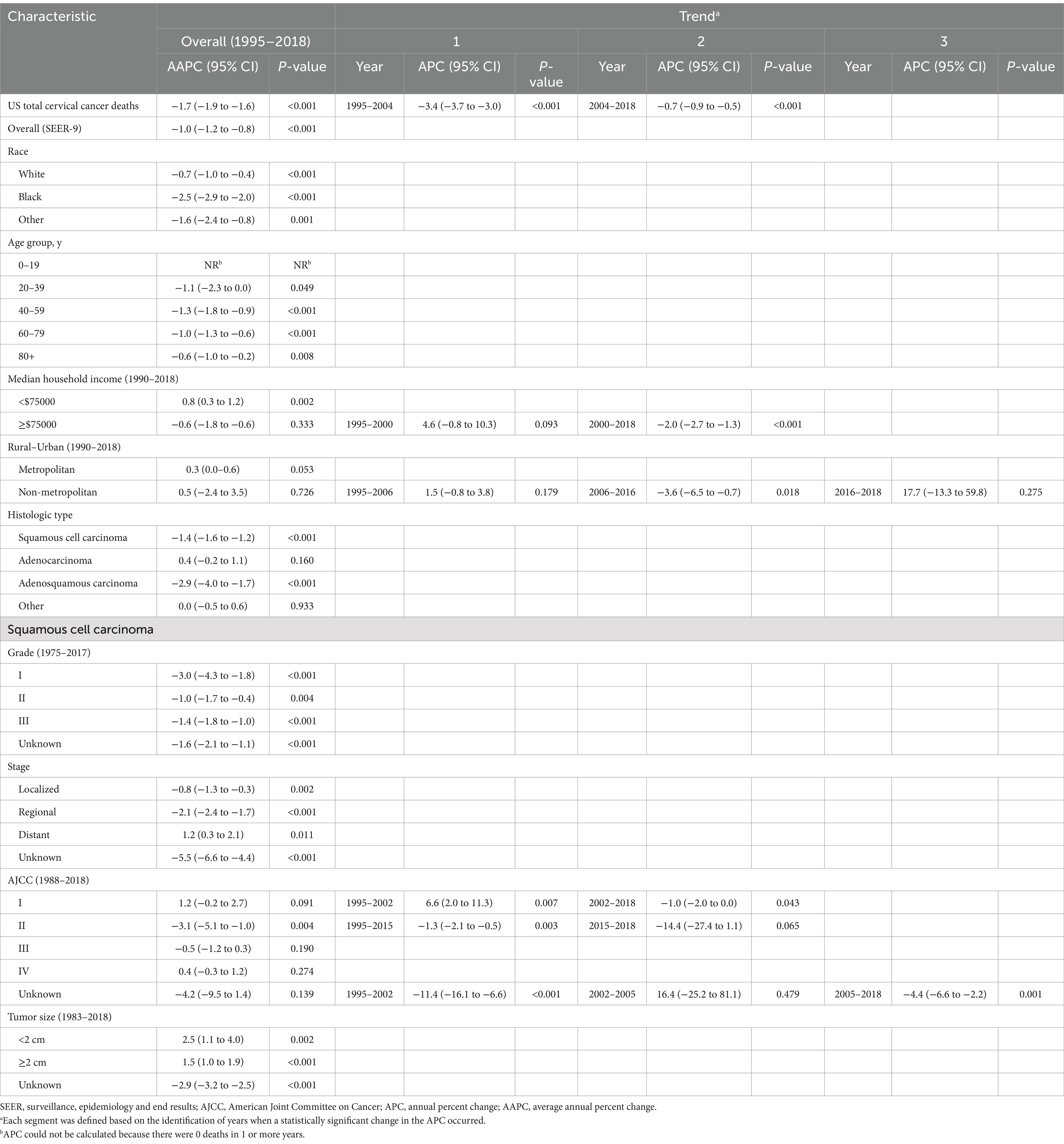
Table 3. Trends in observed cervical cancer mortality rates (Total US) and cervical cancer incidence-based mortality rates (1995–2018): the SEER-9 registry database.
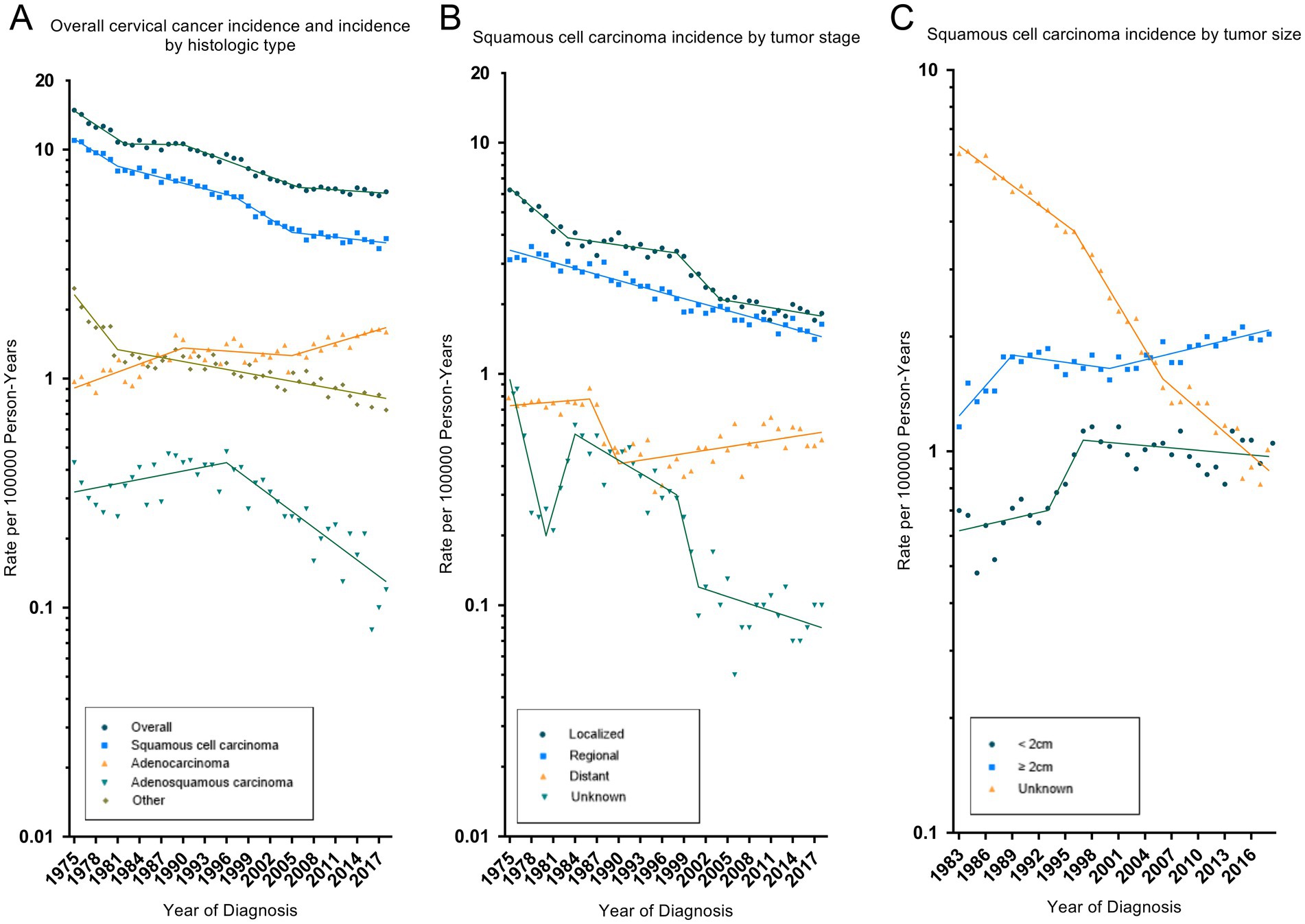
Figure 2. Trends in cervical cancer incidence-based mortality rates. Panel (A) shows observed US cervical cancer mortality (1995–2018), SEER-9 cervical cancer incidence-based mortality overall (1995–2018) and by histologic type. Panel (B) shows squamous cell carcinoma mortality by tumor stage. Panel (C) shows squamous cell carcinoma mortality by tumor size. All rates were age-adjusted to the 2000 US standard population. The dots represent annual mortality rates and the lines represent mortality trends from joinpoint analysis.
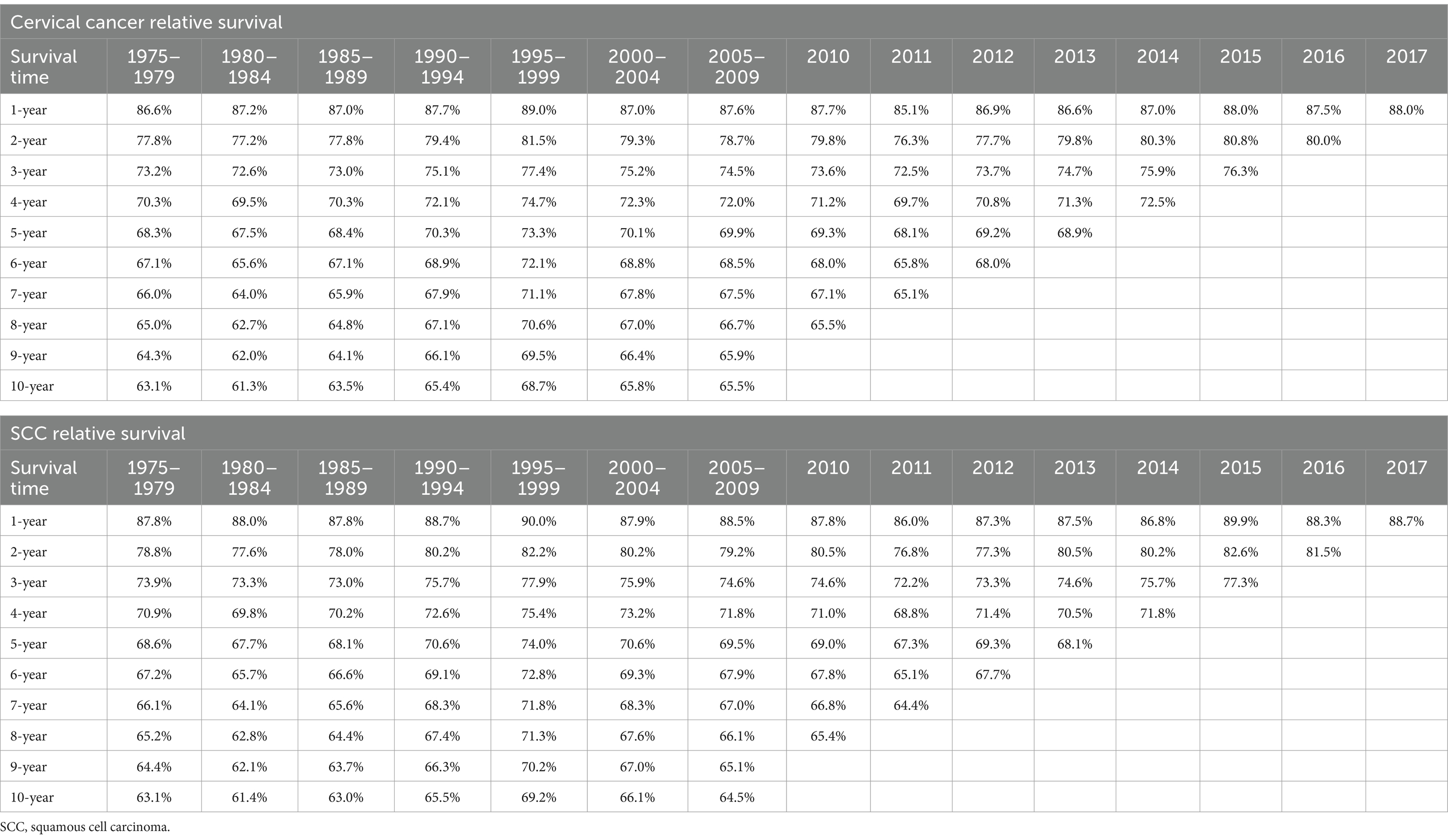
Table 4 showed the relative survival rates of cervical cancer and SCC in different years of diagnosis. All cases, deaths, incidence rates and mortality rates in the analysis were recorded in Supplementary Tables 2–17.
4 Discussion
Cervical cancer created an immense burden on global women’s health because of its high incidence and mortality. However, the current study indicated that there was still a certain gap between WHO global strategy and actual epidemiology of cervical cancer. The SEER-9 database had high-quality follow-up of the population of cancer registries in 9 U.S. states for more than 40 years. Benefiting from these data, the present study analyzed in detail the incidence and mortality trends of cervical cancer based on demographic and tumor characteristics. The main findings in our study were that the incidence of cervical cancer declined overall during the study period, but the rate of decline slowed (APC from −4.7% [95% CI: −5.8% to −3.5%] in 1975–1982 to −2.7% [95% CI: −3.0% to −2.3%] in 1990–2006 to −0.5% [95% CI: −1.1% to −0.1%] in 2006–2018); the incidence and mortality of advanced-stage SCC were significantly increasing (APC of incidence = 1.1% [95% CI: 0.4 to 1.8%] in 1990–2018; AAPC of mortality = 1.2% [95% CI: 0.3 to 2.1%] in 1995–2018). The results of the current research were consistent with the most studies reported in recent years. Many studies demonstrated that incidence in cervical cancer patients showed a stable or even decreasing temporal trend among several countries (6, 14, 15).
Accumulated evidence indicated that a variety of risk factors were associated with the occurrence of cervical cancer, including high-risk HPV infection, age, smoking, childbirth and so on (3, 16, 17). Among all of them, HPV infection was the most important risk factor for cervical cancer, especially HPV16 and HPV18, which were estimated to exist in 50–70% cases and 7–20% cases, respectively (18). Persistent infection with HPV increased the possibility of viral genome integration into the host genome through the environment of genomic instability and finally contributed to the development of cervical cancer and other cancers such as head and neck cancers (19). The recognition of the function of HPV infection as the necessary cause of cervical cancer led to the application of HPV screening (20). With the deepening of clinical research, detection of HPV has undergone a significant shift from mild cytological abnormalities, to co-testing with cytology and HPV, and lately to primary HPV screening (21). It was reported that HPV-based screening exhibited 60–70% greater protection against invasive cervical cancers than cytology (22). Therefore, HPV screening is an effective method to improve the detection rate of cervical cancer (21). This was one important reason to explain for the decreasing incidence of cervical cancer on a global scale.
Correspondingly, we conducted sub-group analyses to calculate the incidence of cervical cancer according to race, age, income, area and histological type. We found that except for the increase in the incidence of adenocarcinoma, prevalence rates were generally decreasing in all sub-groups. One possible explanation for this phenomenon could be that the value of HPV screening on adenocarcinoma was not as large as that on squamous cell carcinoma (23). Adenocarcinoma was considered to involve the deep part of the cervical canal, which makes sampling difficult and ultimately leads to missed diagnosis and misdiagnosis (24). Additional researches were needed to improve the detection rate of the patients with adenocarcinoma.
In fact, universal HPV vaccinations were another important reason for the reduction of cervical cancer. Virus-Like Particle (VLP) vaccines were first produced in 1991 and reported to be safe and immunogenic in humans in 2001 (25). After this breakthrough, the first prophylactic HPV vaccine was licensed in 2006 followed by the approval of bivalent HPV vaccine and monovalent HPV vaccine in 2007 and 2014, respectively. Numerus studies suggested that HPV vaccination should occur in females aged between 9 and 26 years old (26). On a global scale, about 24% of girls aged 9–14 years were under the protection of national HPV program in October 2016 (27). To analyze the incidence of cervical cancer in more detail, we thus conducted a sub-analysis according to the year of survey. The results suggested that there were decreasing trends in the incidence of cervical cancer in 1975–1982 and 1990–2006 while no significance differences in 1982–1990 and 2006–2018. Moreover, incidence of cervical cancer patients aged 20–39 exhibited an obvious reduction in 2000–2018. This may be partly attributed to the application of HPV vaccines. As mentioned before, the vaccinated population was mainly teenagers and young woman.
As for the mortality of cervical cancer, we found that the overall mortality decreased significantly while the mortality in patients with low-income and patients with distant metastasis presented an increase tendency. The decreased mortality could be attributed to increasing awareness, early detection, and treatment of the cervical cancer, resulting in better oncological control. It was reported that girls receiving HPV vaccination before 17 years had 88% lower cervical cancer risk and women vaccinated between the ages of 17 and 30 years exhibited half lower risk of cervical cancer (28). Conversely, the increasing mortality might be explained by the inadequate HPV vaccination in females from low-income areas and insufficient HPV screening programs.
Additionally, patients with HIV infections were more vulnerable to HPV-related diseases than those without HIV infections. Women with HIV infections suffered from a high risk of developing invasive cervical cancer, and it was a result of combination of multi-factors (29). Lower clearance of HPV virus and greater predisposition to HPV infection led to high HPV infections in women with HIV infections (30). Persistent HPV infection can enhance the effect of high-risk HPV through immune inhibition (31). One prospective study found that cervical cancer patients with HIV infection were more likely to die from the malignancy than those without HIV infections (32). Hence, it was important to carry out HPV screening and HPV vaccinations in those women with HIV infections which would be beneficial to reduce the incidence and mortality of cervical cancer.
A study on the global burden of cervical cancer (33) showed that the burden of cervical cancer was inversely associated with the socio-demographic index (SDI). Low SDI regions suffered a more severe burden of cervical cancer (the age-standardized incidence rate was 2.62 times that of high SDI regions, and the age-standardized mortality rate was 5.04 times that of high SDI regions). Sub-Saharan Africa was the region with the heaviest burden of cervical cancer globally. Implementing cervical cancer-related policies in these regions incurred high costs, and organized screening and HPV vaccination programs were always inadequate.
We had to admit there were several significant limitations in our study. Given the descriptive nature of our study, the reliability and accuracy of our findings depended on the authenticity of the data used in the present study. The SEER database does not provide information on risk factors at the individual level, lifestyle-related factors, and methods for detecting cervical cancer. Therefore, we could not precisely evaluate the association between all kinds of risk factors and incidence and mortality of cervical cancer, but can only speculate on possible explanations based on actual observed trends in incidence and mortality of cervical cancer. Additionally, there was a relatively large amount of missing data in the early recording period, especially in tumor size. The quality and completeness of the data improved over time. This would cause an over-estimation of incidence and mortality of cervical cancer in sub-group analysis based on tumor size.
5 Conclusion
For cervical cancer cases diagnosed in the United States from 1975 to 2018, the overall incidence and mortality rates decreased significantly. However, there was an increase in the incidence and mortality of advanced-stage squamous cell carcinoma. These epidemiological patterns offer critical insights for refining cervical cancer screening protocols and developing targeted interventions for advanced-stage cases.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
XC: Conceptualization, Data curation, Formal analysis, Writing – original draft. PW: Formal analysis, Writing – original draft. LC: Formal analysis, Writing – original draft. FZ: Writing – original draft. JL: Conceptualization, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
Thanks to the data providers of the SEER database.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1579446/full#supplementary-material
References
1. Sung, H, Ferlay, J, Siegel, RL, Laversanne, M, Soerjomataram, I, Jemal, A, et al. Global Cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Vu, M, Yu, J, Awolude, OA, and Chuang, L. Cervical Cancer worldwide. Curr Probl Cancer. (2018) 42:457–65. doi: 10.1016/j.currproblcancer.2018.06.003
3. Olusola, P, Banerjee, HN, Philley, JV, and Dasgupta, S. Human papilloma virus-associated cervical Cancer and health disparities. Cells. (2019) 8:622. doi: 10.3390/cells8060622
4. Sundstrom, K, and Elfstrom, KM. Advances in cervical Cancer prevention: efficacy, effectiveness, elimination? PLoS Med. (2020) 17:e1003035. doi: 10.1371/journal.pmed.1003035
5. Available online at: https://www.who.int/initiatives/cervical-cancer-elimination-initiative (Accessed June 6, 2024).
6. Lin, S, Gao, K, Gu, S, You, L, Qian, S, Tang, M, et al. Worldwide trends in cervical Cancer incidence and mortality, with predictions for the next 15 years. Cancer. (2021) 127:4030–9. doi: 10.1002/cncr.33795
7. Ortiz, AP, Ortiz-Ortiz, KJ, Colon-Lopez, V, Tortolero-Luna, G, Torres-Cintron, CR, Wu, CF, et al. Incidence of cervical Cancer in Puerto Rico, 2001-2017. JAMA Oncol. (2021) 7:456–8. doi: 10.1001/jamaoncol.2020.7488
8. National Cancer Institute, Surveillance, Epidemiology, End Results Program. Seer*Stat Database: Incidence-Based Mortality—Seer 9 Regs Research Data. Nov 2020 sub (1975-2018) <Katrina/Rita population adjustment>—linked to county attributes—Total us, 1969-2019 counties National Cancer Institute, Dccps, Surveillance Research Program. SEER database (2021).
9. National Cancer Institute, Surveillance, Epidemiology, End Results Program. Seer*Stat Database: Incidence—Seer 9 Regs Research Data. Nov 2020 sub (1975-2018) <Katrina/Rita population adjustment>—linked to county attributes—Total us, 1969-2019 counties. National Cancer Institute, Dccps, Surveillance Research Program. SEER database (2021).
10. Chu, KC, Miller, BA, Feuer, EJ, and Hankey, BF. A method for partitioning Cancer mortality trends by factors associated with diagnosis: an application to female breast Cancer. J Clin Epidemiol. (1994) 47:1451–61. doi: 10.1016/0895-4356(94)90089-2
11. Zhang, S, Li, Q, Ouyang, X, Tang, Y, Cui, J, and Yang, Z. Radiotherapy can improve overall survival in patients with lymph-node positive, high-grade neuroendocrine cervical cancer: construction of two prognostic nomograms to predict treatment outcome. Front Oncol. (2024) 14:1450382. doi: 10.3389/fonc.2024.1450382
12. Fan, X, He, W, Zhang, Q, Zhang, B, Dong, L, Li, L, et al. Evaluation and prediction analysis of 3- and 5-year relative survival rates of patients with cervical Cancer: a model-based period analysis. Cancer Control. (2024) 31:10732748241232324. doi: 10.1177/10732748241232324
13. Kim, HJ, Fay, MP, Feuer, EJ, and Midthune, DN. Permutation tests for Joinpoint regression with applications to Cancer rates. Stat Med. (2000) 19:335–51. doi: 10.1002/(SICI)1097-0258(20000215)19:3<335::AID-SIM336>3.0.CO;2-Z
14. Tran, KN, Park, Y, Kim, BW, Oh, JK, and Ki, M. Incidence and mortality of cervical Cancer in Vietnam and Korea (1999-2017). Epidemiol Health. (2020) 42:e2020075. doi: 10.4178/epih.e2020075
15. Hansen, BT, Campbell, S, and Nygard, M. Regional differences in cervical Cancer incidence and associated risk behaviors among Norwegian women: a population-based study. BMC Cancer. (2021) 21:935. doi: 10.1186/s12885-021-08614-w
16. Chelimo, C, Wouldes, TA, Cameron, LD, and Elwood, JM. Risk factors for and prevention of human papillomaviruses (Hpv), genital warts and cervical Cancer. J Infect. (2013) 66:207–17. doi: 10.1016/j.jinf.2012.10.024
17. Au, WW, Abdou-Salama, S, Sierra-Torres, CH, and Al-Hendy, A. Environmental risk factors for prevention and molecular intervention of cervical Cancer. Int J Hyg Environ Health. (2007) 210:671–8. doi: 10.1016/j.ijheh.2006.10.003
18. Stanley, M. Pathology and epidemiology of Hpv infection in females. Gynecol Oncol. (2010) 117:S5–S10. doi: 10.1016/j.ygyno.2010.01.024
19. Shanmugasundaram, S, and You, J. Targeting persistent human papillomavirus infection. Viruses. (2017) 9:8. doi: 10.3390/v9080229
20. Goodman, A. Hpv testing as a screen for cervical Cancer. BMJ. (2015) 350:h 2372. doi: 10.1136/bmj.h2372
21. Bhatla, N, and Singhal, S. Primary Hpv screening for cervical Cancer. Best Pract Res Clin Obstet Gynaecol. (2020) 65:98–108. doi: 10.1016/j.bpobgyn.2020.02.008
22. Kumari, P, Kumar, M, Reddy, CR, and Jha, B. Nitrate and phosphate regimes induced Lipidomic and biochemical changes in the intertidal macroalga Ulva lactuca (Ulvophyceae, Chlorophyta). Plant Cell Physiol. (2014) 55:52–63. doi: 10.1093/pcp/pct156
23. Gien, LT, Beauchemin, MC, and Thomas, G. Adenocarcinoma: A Unique Cervical Cancer. Gynecol Oncol. (2010) 116:140–6. doi: 10.1016/j.ygyno.2009.09.040
24. Park, KJ. Cervical adenocarcinoma: integration of Hpv status, pattern of invasion, morphology and molecular markers into classification. Histopathology. (2020) 76:112–27. doi: 10.1111/his.13995
25. Harro, CD, Pang, YY, Roden, RB, Hildesheim, A, Wang, Z, Reynolds, MJ, et al. Safety and immunogenicity trial in adult volunteers of a human papillomavirus 16 L1 virus-like particle vaccine. J Natl Cancer Inst. (2001) 93:284–92. doi: 10.1093/jnci/93.4.284
26. Ault, K, and Reisinger, K. Programmatic issues in the implementation of an Hpv vaccination program to prevent cervical Cancer. Int J Infect Dis. (2007) 11:S26–8. doi: 10.1016/S1201-9712(07)60018-6
27. Gallagher, KE, LaMontagne, DS, and Watson-Jones, D. Status of Hpv vaccine introduction and barriers to country uptake. Vaccine. (2018) 36:4761–7. doi: 10.1016/j.vaccine.2018.02.003
28. Printz, C. Lower cervical Cancer risk associated with Hpv vaccine. Cancer. (2021) 127:1171. doi: 10.1002/cncr.33577
29. Stelzle, D, Tanaka, LF, Lee, KK, Ibrahim Khalil, A, Baussano, I, Shah, ASV, et al. Estimates of the global burden of cervical Cancer associated with Hiv. Lancet Glob Health. (2021) 9:e161–9. doi: 10.1016/S2214-109X(20)30459-9
30. Liu, G, Sharma, M, Tan, N, and Barnabas, RV. Hiv-positive women have higher risk of human papilloma virus infection, precancerous lesions, and cervical Cancer. AIDS. (2018) 32:795–808. doi: 10.1097/QAD.0000000000001765
31. Humans IWGotEoCRt. Biological agents. Volume 100 B. A review of human carcinogens. IARC Monogr Eval Carcinog Risks Hum. (2012) 100:1–441.
32. Dryden-Peterson, S, Bvochora-Nsingo, M, Suneja, G, Efstathiou, JA, Grover, S, Chiyapo, S, et al. Hiv infection and survival among women with cervical Cancer. J Clin Oncol. (2016) 34:3749–57. doi: 10.1200/JCO.2016.67.9613
Keywords: cervical cancer, trends, age-adjusted incidence, incidence-based mortality, relative survival
Citation: Cheng X, Wang P, Cheng L, Zhao F and Liu J (2025) Trends in cervical cancer incidence and mortality in the United States, 1975–2018: a population-based study. Front. Med. 12:1579446. doi: 10.3389/fmed.2025.1579446
Edited by:
Redhwan Ahmed Al-Naggar, National University of Malaysia, MalaysiaReviewed by:
Florence Mbuthia, Dedan Kimathi University of Technology, KenyaBirhanu Hailu Tirkaso, Mizan Tepi University, Ethiopia
Copyright © 2025 Cheng, Wang, Cheng, Zhao and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiangang Liu, emp1Z2FuZ0AxNjMuY29t
†These authors have contributed equally to this work and share first authorship
 Xianying Cheng1†
Xianying Cheng1† Jiangang Liu
Jiangang Liu