- 1Department of Pharmacy, The Affiliated People's Hospital of Ningbo University, Ningbo, China
- 2WillingMed Technology (Beijing) Co., Ltd., Beijing, China
- 3Department of Intensive Care Unit, The Affiliated People's Hospital of Ningbo University, Ningbo, China
Background: Talaromycosis, caused by Talaromyces marneffei (T. marneffei), has become more common in HIV-negative and immunocompetent patients. The fungus colonizes the body through dormant spores, causing opportunistic infections. Early diagnosis is challenging. This study aims to analyze the clinical features, diagnosis, treatment, and prognosis of T. marneffei infections.
Methods: Patients diagnosed with T. marneffei infection or colonization at the People’s Hospital of Ningbo University between August 2022 and July 2024 were included. Demographic characteristics, clinical data, diagnostic approaches, and treatment outcomes were analyzed.
Results: Seven patients were diagnosed with T. marneffei infection, and three with colonization. Productive cough and fever were the predominant symptoms in all patients. Nodules, cavitary lesions, and pleural effusions on chest imaging were observed exclusively in infected patients. The positivity rates for metagenomic next-generation sequencing (mNGS) and conventional microbiological testing were 100 and 10%, respectively. Of the seven infected patients, three had a single infection with T. marneffei, and four had co-infection with T. marneffei and Mycobacterium avium complex. All patients were treated with monotherapy or combination therapy using voriconazole. All but one recovered.
Conclusion: Early diagnosis and combination therapy are critical for T. marneffei infection. mNGS complements traditional methods, facilitating accurate diagnosis and guiding targeted treatment.
1 Introduction
Talaromyces marneffei (T. marneffei) is an opportunistic, temperature-dependent, dimorphic fungus commonly found in East and Southeast Asia (1). It was originally isolated from a bamboo rat in Vietnam (2). Talaromycosis can affect the skin, respiratory and digestive tracts, and reticuloendothelial system, resulting in disseminated infection (3). The respiratory system is often the first to be affected by the fungus (4). If left undiagnosed and untreated, it can significantly increase patient mortality.
It is an important opportunistic infection in HIV-infected patients living in endemic areas, but in recent years, an increasing number of T. marneffei infections have been observed in patients who are not HIV-infected or who are immunocompetent (5, 6). The definitive diagnosis of T. marneffei infection is based on isolation of the fungus through microscopy and culture, although these tests are time-consuming and have a low positive rate (7). Rapid and accurate diagnosis of T. marneffei remains a top priority for effective treatment and improved patient outcomes. In recent years, metagenomic next-generation sequencing (mNGS) has emerged as a rapid diagnostic adjunct for T. marneffei, though evidence remains largely confined to case reports.
Infections with fungi such as T. marneffei are usually caused by inhalation of dormant spores. Subsequent host or temperature-dependent signals trigger dimorphic transitions, enabling pathogenic growth (8). To improve early clinical recognition, we analyzed the epidemiological and clinical features of 10 T. marneffei infection or colonization cases diagnosed at a tertiary hospital in southern China.
2 Materials and methods
2.1 Patient enrolment and study design
In this study, 404 samples with suspected lung infection in the Department of Critical Care Medicine of the People’s Hospital of Ningbo University from August 2022 to July 2024 were screened for mNGS testing. The clinical records, laboratory test results, imaging results, and clinical treatment of 10 patients with T. marneffei positive and HIV-negative were reviewed. The inclusion criteria were as follows: (1) suspected pulmonary infection, defined by focal or diffuse infiltrative lesions on chest X-ray or computed tomography, combined with at least one of the following: (a) fever > 37°C; (b) cough, sputum production, hypoxia, or worsening of existing respiratory symptoms; (c) leukocytosis; (d) clinical signs of lung consolidation or crackles; (2) positive results for T. marneffii obtained using bronchoalveolar lavage fluid (BALF) mNGS; (3) HIV-negative status; (4) Conventional microbial testing (CMT, including culture, G/GM test, T-spot and PCR) performed simultaneously. The exclusion criteria were: (1) HIV-positive patients; (2) Absence of simultaneous CMT; (3) missing case information. The primary endpoint was the time to discharge.
2.2 Conventional microbial testing and diagnostic criteria for Talaromycosis
All patients were tested with BALF samples for culture and mNGS assays. Sputum and throat swab samples were collected for selected CMT based on the patient’s clinical presentation, including smear, culture, G/GM testing, GeneXpert, and respiratory PCR targeting Mycoplasma pneumoniae, Chlamydia pneumoniae, SARS-CoV-2, influenza A virus, influenza B virus, Legionella pneumophila, CMV, and Epstein–Barr virus.
Diagnostic criteria for Talaromycosis are based on previous guidelines and studies (9, 10). The criteria are as follows: (1) presence of fever or respiratory/gastrointestinal abnormalities, papulonecrotic skin lesions, lymphadenopathy, splenomegaly, hepatomegaly, or anemia; (2) Computed tomography (CT) revealing evidence of fungal infection; (3) exclusion of other fungal infections; (4) regression of relevant symptoms and indicators following antifungal therapy targeting T. marneffei; (5) identification of pathogens by culture or microscopic examination of clinical samples. A final clinical diagnosis is made if either (1, 5), or (1)–(4), are met. When the presence of T. marneffei was confirmed by BALF mNGS alone, other pathogens were excluded, antifungal therapy was effective, and these patients were also considered to have confirmed Talaromycosis in the analysis (11).
2.3 Nucleic acid extraction, library construction, and sequencing
BALF samples were collected according to standard operating procedures. Immediately after collection, the samples were sent to WillingMed Technology (Beijing) Co., Ltd. for mNGS testing. Samples were subjected to nucleic acid extraction based on previous standards (12, 13). Libraries were prepared with the KAPA DNA HyperPrep Kit (KK8504, KAPA, Kapa Biosystems, Wilmington, MA, United States). Pooled libraries were sequenced on the MGISEQ-200 platform using a 50 bp single-ended sequencing kit (MGI Technology), with at least 20 million sequencing reads per sample, as described in. Quality control was performed after high-quality sequencing data had been obtained. Bowtie2 v2.4.3 was used to screen and remove data consistent with the GRCh37 (hg19) sequence of the human reference genome (14). Kraken2 v2.1.0 was used to compare the remaining sequences with existing microbial nucleic acid sequences in the database to classify and identify microorganisms (15). In pathogen identification, a threshold called RPTM (readings per ten million) is used. Bacteria and fungi with RPTM ≥ 20, viruses with RPTM ≥ 3, and specific pathogens (including Cryptococcus, Mycobacteria, and T. marneffei) with RPTM ≥ 1 are considered positive (16).
2.4 Statistical analysis
Statistical analysis was performed using Prism 9 (GraphPad, La Jolla, CA). Data are presented as median (range). The Wilcoxon-Mann–Whitney test was used for group comparisons, and the chi-square test was used for categorical data. A p-value of less than 0.05 was considered statistically significant.
3 Results
3.1 Baseline characteristics of participants
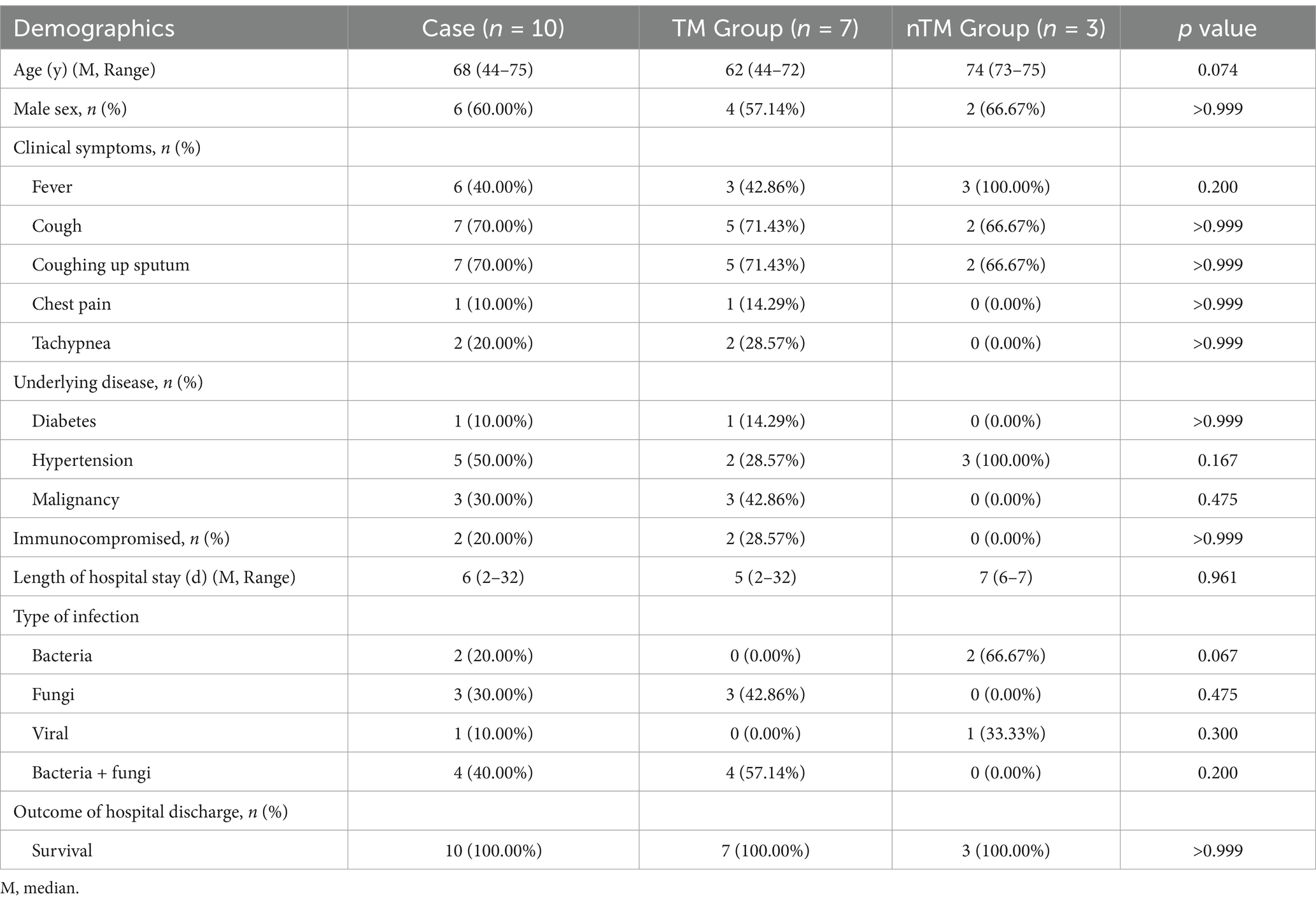
A retrospective analysis was performed on 10 HIV-negative patients who were mNGS-positive for T. marneffei, all residing in Ningbo, Zhejiang Province, China, an area not typically endemic for this fungus. The study population consisted of six men and four women, with a median age of 68 years. The most common clinical symptoms were cough and productive cough (70%, 7/10). Fever was present in 60% (6/10) of patients, and two patients experienced shortness of breath. Chest pain occurred in only one patient. Hypertension was present in 50% of patients, and malignancy was present in 30% (3/10). Only 20% (2/10) of patients were immunocompromised. The median hospital stay was 6 days, and all patients survived to discharge (Table 1).
According to the final clinical diagnosis, the most common type of infection in all patients was fungal-bacterial coinfection, followed by fungal infections alone. In the nTM group, two patients had bacterial infections, and one had a viral infection.
3.2 Laboratory examination results and imaging findings
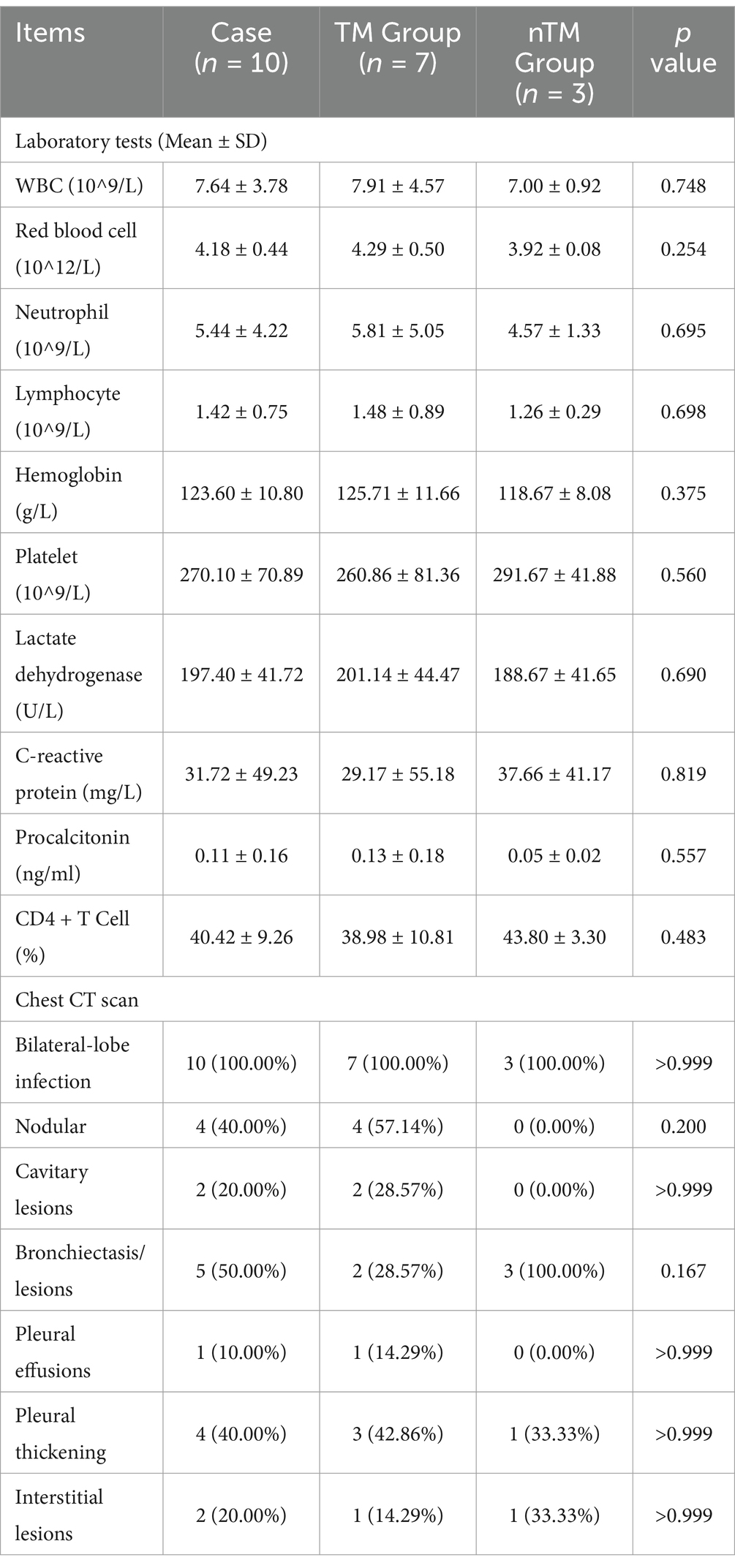
Evaluation of the complete blood count results showed elevated C-reactive protein levels in all patients, while other indices were within the normal range. Although the TM group had lower C-reactive protein levels and higher procalcitonin levels than the nTM group, there was no statistically significant difference in laboratory findings between the two groups (Table 2).
Chest imaging in all patients showed bilateral lung lobe involvement (Figure 1). Bronchiectasis or lesions were present in 50% of patients. Both nodules and pleural thickening were observed in 40%, while cavitary and interstitial lesions were seen in 20%. Only one patient exhibited a pleural effusion. Imaging features such as nodules, cavitary lesions, and pleural effusions were observed only in the TM group.
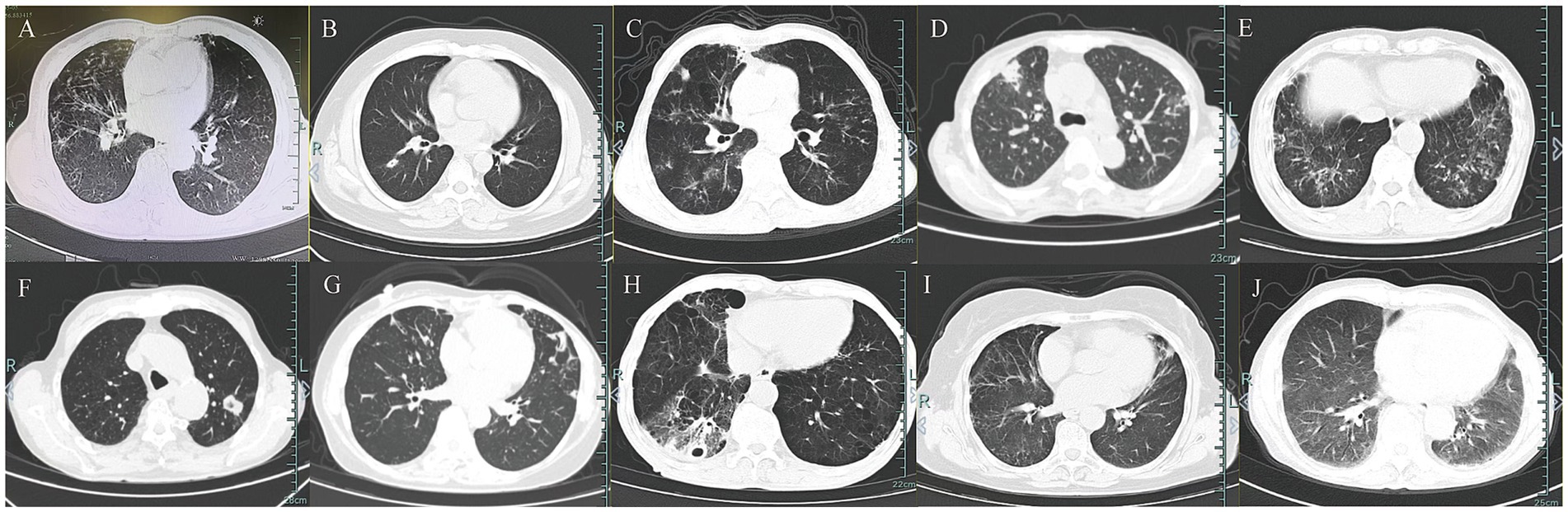
Figure 1. Chest imaging features in 10 patients. (A–G) Chest CT in patients with T. marneffei infection. (H–J) Chest CT in patients with non-T. marneffei infection.
3.3 mNGS results and clinical judgments
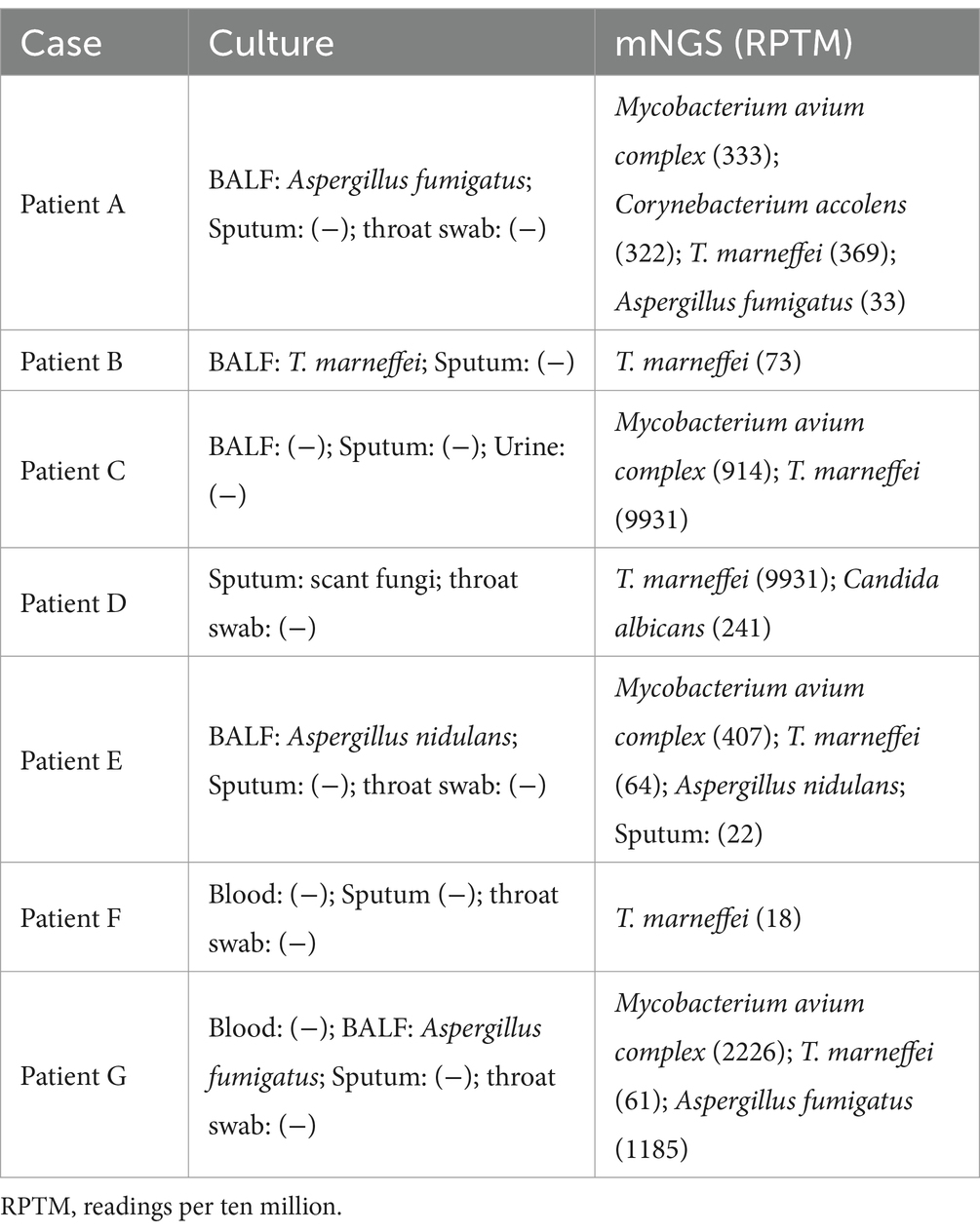
In 10 cases, mNGS detection results were positive for T. marneffei. Of these, 3 patients were diagnosed with non-T. marneffei infections (nTM group), with the main pathogens being Streptococcus pneumoniae, Haemophilus influenzae, and Epstein–Barr virus (Figure 2A). Seven patients were clinically diagnosed with T. marneffei infection (TM group). Among these 7 patients, only 1 had T. marneffei detected by BALF culture (Table 3). Four of these patients were diagnosed with co-infection of T. marneffei and Mycobacterium avium complex (MAC). Three patients were diagnosed with T. marneffei infection alone. In infected patients, mNGS detected more T. marneffei sequences compared to uninfected patients (Supplementary Figure 1). However, the difference in the number of sequences between the two groups was not statistically significant due to the small sample size (Figure 2B).
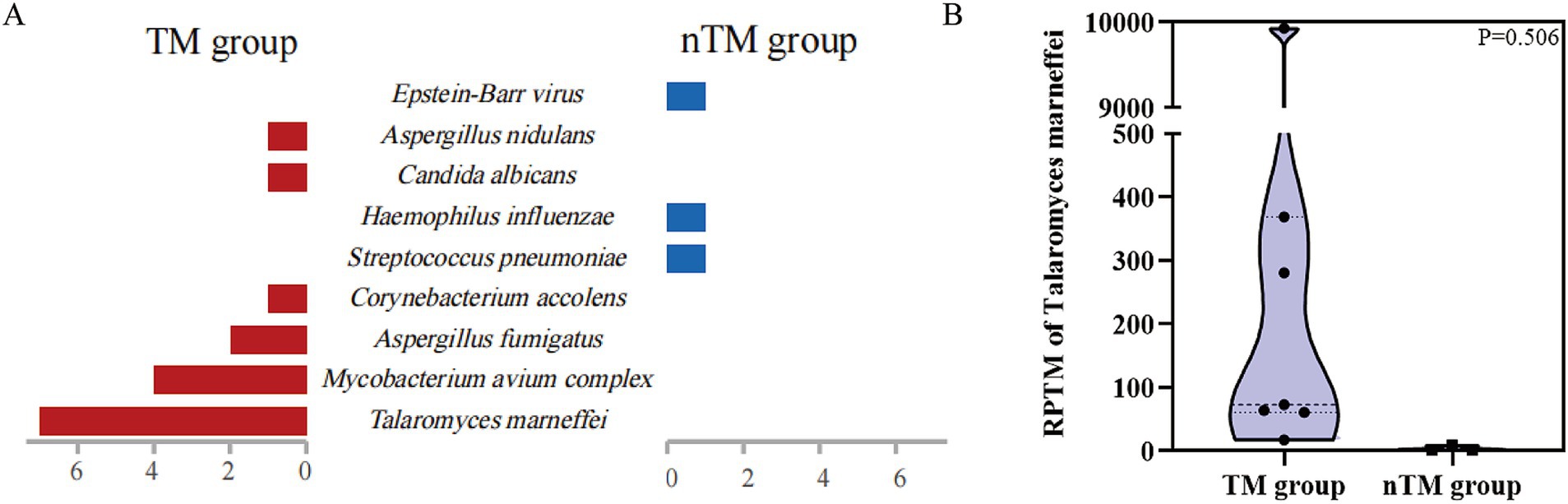
Figure 2. mNGS results based on clinical adjudication. (A) Clinical pathogens detected by mNGS in 10 patients. (B) Differences in the quantity of T. marneffei sequences detected by mNGS between T. marneffei infected and non-T. marneffei infected groups.
3.4 Diagnosis, treatment, and clinical outcomes of Talaromyces marneffei infection
Lung disease was present in patients diagnosed with T. marneffei infection. Notably, none of the patients were diagnosed with a fungal infection at the time of admission. Therefore, they did not receive antifungal therapy before the mNGS results were available. Before diagnosis, 86% (6/7) of patients were treated with at least one antimicrobial agent. These agents were empirically used to treat bacterial infections. After the diagnosis of T. marneffei infection, 57% (4/7) of patients received combination therapy. 29% (2/7) of patients received antifungal monotherapy. Overall, all but one patient received voriconazole directly for T. marneffei infection and were discharged after improvement (Table 4).
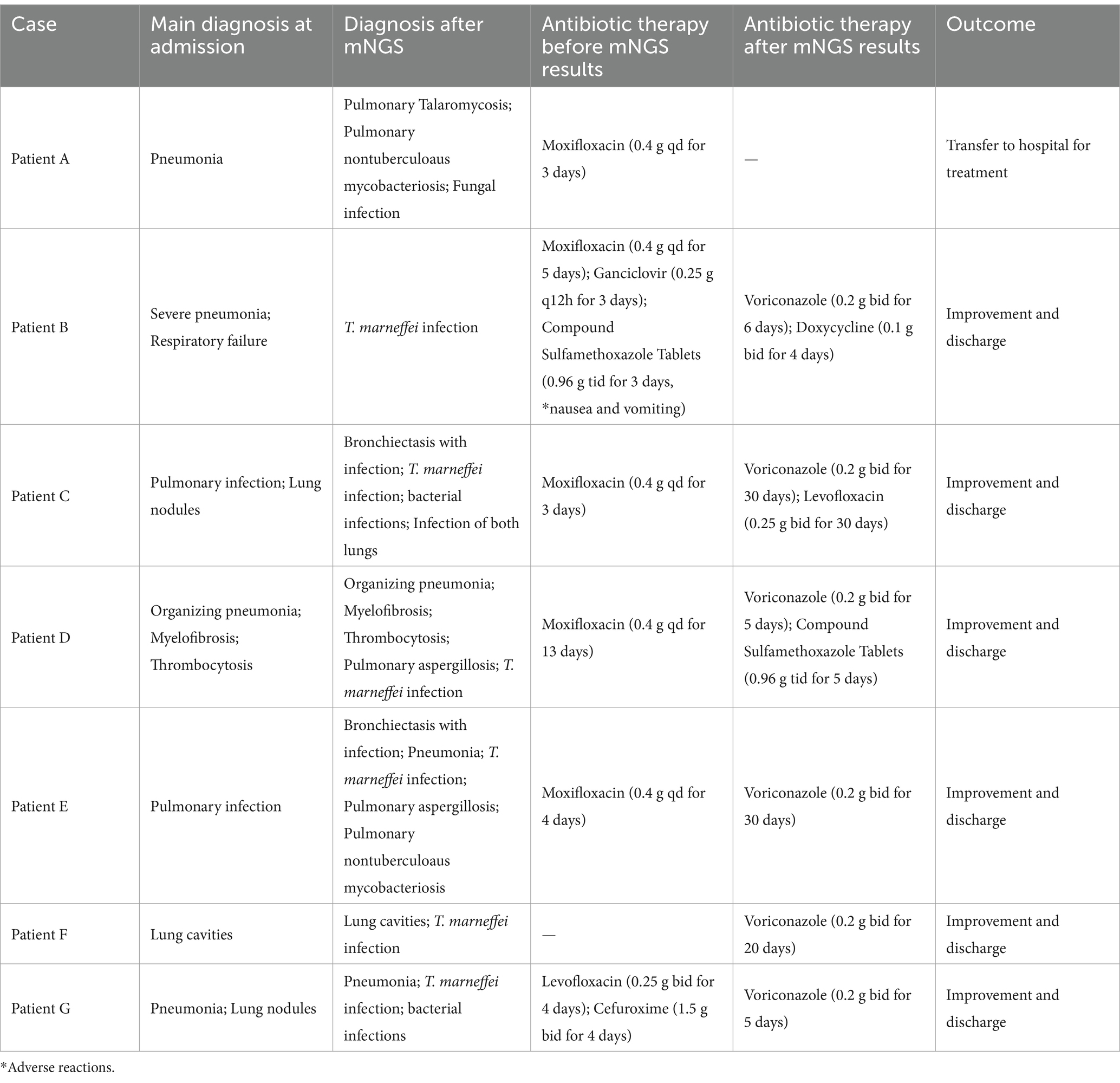
Table 4. Treatment course and outcomes of patients clinically diagnosed with T. marneffei infection.
4 Discussion
Talaromyces marneffei is the third most common opportunistic infection among HIV-positive individuals, following tuberculosis and cryptococcosis (17). However, in recent years, an increasing number of HIV-negative patients with poor cell-mediated immunity have also been diagnosed with T. marneffei infection (18). T. marneffei is widely distributed in temperate tropical regions and can be isolated from various types of bamboo rats (7). In our study, one patient had a history of capturing bamboo rats prior to admission, which provided a vector for the patient’s infection. Severe disseminated T. marneffei infection is common in immunosuppressed individuals, and the severity of the disease depends on the degree of immunosuppression (19, 20). In our study, all patients were discharged after receiving targeted therapy, possibly because they were HIV-negative and the majority were immunocompetent (21). Of the 7 patients infected with T. Marneffei, 3 had malignancies (hematological, prostate, and breast cancers), and the immunocompromise caused by these conditions made the patients more susceptible to invasive T. Marneffei infections and even death (22, 23). One infected patient had diabetes, which can weaken the immune system. Previous studies have also shown that diabetes is a potential risk factor for opportunistic fungal infections (24). In contrast, all patients with non- T. marneffei infections had a history of hypertension, and some evidence suggests that hypertension contributes to bacterial and viral infections (25). Asexual spores of T. marneffei are transmitted from the respiratory tract to the whole body by inhalation or through the affected skin (4). Therefore, respiratory lesions due to T. marneffei infection are the most common manifestation. In our study, most patients with T. marneffei infection exhibited symptoms similar to those previously reported, such as productive cough and fever (26, 27). However, none of the patients showed typical features of diarrhea, weight loss, lymphadenopathy, or hepatosplenomegaly. A previous study in Thailand found that non-HIV patients were less likely to develop features like hepatosplenomegaly (28). Our study also shows the lack of specificity of early T. marneffei infection in non-HIV patients.
Respiratory tract infections involving T. marneffei are often misdiagnosed as tuberculosis or nontuberculous mycobacterial disease (4). This is due to the patient’s physical signs and imaging findings being similar to those of tuberculosis (11). In our study, compared with patients with non- T. marneffei infections, those infected with T. marneffei showed characteristic nodules, cavitary lesions, and pleural effusions on imaging, which were similar to those of tuberculosis and therefore difficult to distinguish based on imaging alone. Therefore, the diagnosis of T. marneffei infection must be made by testing for pathogenic bacteria.
Concomitant infections with T. marneffei and other opportunistic pathogens is common (29). Previous case studies have shown that co-infection with Mycobacterium tuberculosis or nontuberculous mycobacteria (NTM) can lead to sepsis (29, 30). In our study, 3 of the 7 patients in the infection group had a single infection with T. marneffei, while the remaining 4 were co-infected with T. marneffei and MAC. This may be due to the rise of NTM infections in southern China in recent years, with MAC being the most common (31). Co-infection can significantly increase the refractoriness of the disease, especially when both T. marneffei and MAC are intracellular pathogens (32). In our study, all infected patients were initially misdiagnosed with a bacterial infection and were treated empirically with antibacterial therapy. Traditional tests for T. marneffei typically include biopsy, culture, and microscopy, but these tests are often time-consuming and may delay treatment (33). Although serological tests and PCR are widely used, they detect only a narrow range of pathogens. When T. marneffei is co-infected with multiple pathogens, conventional testing methods may delay timely treatment, leading to disease progression. In our study, only 1 of the 7 patients diagnosed with T. marneffei infection had both BALF culture and mNGS tests performed, while the remaining 6 patients were diagnosed by mNGS. Although mNGS has clear advantages in detecting intracellular pathogen infections with a low positive rate in conventional cultures, the identification of detected pathogens still requires support from clinical information and imaging evidence (34).
Because the symptoms of early T. marneffei infection were atypical, fungal infection was not considered during the early stage of admission for any of the patients. T. marneffei is highly susceptible to antifungal therapy, and amphotericin B and itraconazole are recommended for HIV-positive T. marneffei infections (4). Previous studies have shown that voriconazole and caspofungin are used for antifungal prophylaxis in patients (35). Voriconazole is a second-generation triazole antifungal drug effective against T. marneffei and is often used in patients who cannot tolerate amphotericin B (36). In our study, all but one patient were transferred to the hospital and discharged with improvement after receiving monotherapy with voriconazole or combination therapy with multiple agents. Although our study yielded satisfactory results in the treatment of T. marneffei with voriconazole, it is important to monitor the gradual development of resistance to voriconazole in T. marneffei, especially with the increasing trend of drug-resistant fungi in large-scale clinical practice (37).
There are some limitations to this study. First, our sample size is small, which limits the study’s generalizability. Second, a single-center design may introduce bias in the results. Given the scarcity of data on this topic, we believe that the current study can help inform clinicians in diagnosing and treating T. marneffei.
5 Conclusion
Cases of infection or colonization caused by T. marneffei are unique, and they are easily confused with bacterial infections. As a complement to traditional laboratory cultures and imaging tests, mNGS may therefore be useful for accurate diagnosis and effective antimicrobial therapy in the management of fungal infections.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary material.
Ethics statement
The studies involving humans were approved by the Ethics Committee of the People’s Hospital Affiliated to Ningbo University (approval no. 2024-058). The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because due to the retrospective nature of the study, the Ethics Committee waived the requirement for patient consents. The patients were anonymized, and their information was nonidentifiable.
Author contributions
AW: Conceptualization, Data curation, Writing – original draft, Writing – review & editing. WG: Investigation, Writing – review & editing. YG: Methodology, Writing – review & editing. CZ: Data curation, Writing – review & editing. YX: Data curation, Investigation, Writing – review & editing. XZ: Methodology, Writing – review & editing. HW: Funding acquisition, Project administration, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was funded by the Medical Science and Technology Project of Zhejiang Province (2025KY1479) and 2024 Annual Yinzhou District First Batch of Science and Technology Projects (2024AS022).
Acknowledgments
We would like to acknowledge the patient reported in this article.
Conflict of interest
WG, YG, and XZ were employed by WillingMed Technology (Beijing) Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1579522/full#supplementary-material
References
1. Chakrabarti, A, and Slavin, MA. Endemic fungal infections in the Asia-Pacific region. Med Mycol. (2011) 49:337–44. doi: 10.3109/13693786.2010.551426
2. Yao, Z, Pan, Z, Li, G, Liao, Z, Yu, Z, Zhan, L, et al. Talaromycosis from Wuhan: two-case report and literature review. Front Cell Infect Microbiol. (2024) 14:1347677. doi: 10.3389/fcimb.2024.1347677
3. Qiu, Y, Zhang, J, Liu, G, Zhong, X, Deng, J, He, Z, et al. Retrospective analysis of 14 cases of disseminated Penicillium marneffei infection with osteolytic lesions. BMC Infect Dis. (2015) 15:47. doi: 10.1186/s12879-015-0782-6
4. Qiu, Y, Zhang, JQ, Pan, ML, Zeng, W, Tang, SD, and Tan, CM. Determinants of prognosis in Talaromyces marneffei infections with respiratory system lesions. Chin Med J. (2019) 132:1909–18. doi: 10.1097/CM9.0000000000000345
5. Chan, JF, Lau, SK, Yuen, KY, and Woo, PC. Talaromyces (Penicillium) marneffei infection in non-HIV-infected patients. Emerg Microbes Infect. (2016) 5:e19. doi: 10.1038/emi.2016.18
6. Qiu, Y, Liao, H, Zhang, J, Zhong, X, Tan, C, and Lu, D. Differences in clinical characteristics and prognosis of Penicilliosis among HIV-negative patients with or without underlying disease in southern China: a retrospective study. BMC Infect Dis. (2015) 15:525. doi: 10.1186/s12879-015-1243-y
7. Zhang, J, Zhang, D, Du, J, Zhou, Y, Cai, Y, Sun, R, et al. Rapid diagnosis of Talaromyces marneffei infection assisted by metagenomic next-generation sequencing in a HIV-negative patient. IDCases. (2021) 23:e01055. doi: 10.1016/j.idcr.2021.e01055
8. Boyce, KJ, and Andrianopoulos, A. Fungal dimorphism: the switch from hyphae to yeast is a specialized morphogenetic adaptation allowing colonization of a host. FEMS Microbiol Rev. (2015) 39:797–811. doi: 10.1093/femsre/fuv035
9. AIDS and Hepatitis C Professional Group SoID, Chinese Medical Association, Chinese Center for Disease Control and Prevention. Chinese guidelines for diagnosis and treatment of HIV/AIDS (2021st edition). Zhonghua Nei Ke Za Zhi. (2021) 60:1106–28. doi: 10.3760/cma.j.cn112138-20211006-00676
10. Mao, Y, Shen, H, Yang, C, Jia, Q, Li, J, Chen, Y, et al. Clinical performance of metagenomic next-generation sequencing for the rapid diagnosis of talaromycosis in HIV-infected patients. Front Cell Infect Microbiol. (2022) 12:962441. doi: 10.3389/fcimb.2022.962441
11. Pan, M, Fang, G, Zheng, F, Lin, F, Zeng, W, Qiu, Y, et al. Clinical characteristics of tracheobronchial Talaromyces marneffei infection in non-HIV-infected patients in South China. Ann Med. (2023) 55:2276310. doi: 10.1080/07853890.2023.2276310
12. Xu, X, Zheng, Y, Zhang, X, Zhang, C, Gai, W, and Yang, Z. Utility of metagenomic next-generation sequencing for diagnosis of infectious diseases in critically ill immunocompromised pediatric patients. Infect Drug Resist. (2024) 17:3579–91. doi: 10.2147/IDR.S472129
13. Chen, H, Zheng, Y, Zhang, X, Liu, S, Yin, Y, Guo, Y, et al. Clinical evaluation of cell-free and cellular metagenomic next-generation sequencing of infected body fluids. J Adv Res. (2023) 55:119–29. doi: 10.1016/j.jare.2023.02.018
14. Langmead, B, and Salzberg, SL. Fast gapped-read alignment with bowtie 2. Nat Methods. (2012) 9:357–9. doi: 10.1038/nmeth.1923
15. Wood, DE, Lu, J, and Langmead, B. Improved metagenomic analysis with kraken 2. Genome Biol. (2019) 20:1–13. doi: 10.1186/s13059-019-1891-0
16. Li, J, Pan, D, Guo, Y, Zhang, B, Lu, X, Deng, C, et al. Clinical application value of simultaneous plasma and bronchoalveolar lavage fluid metagenomic next generation sequencing in patients with pneumonia-derived sepsis. BMC Infect Dis. (2024) 24:1393. doi: 10.1186/s12879-024-10292-5
17. Wang, Y, Mo, X, Zhang, J, Yan, Z, Fang, Y, Deng, W, et al. Clinical features of Talaromyces marneffei infection in HIV-positive and HIV-negative individuals: a retrospective study in southern China. Med Mycol. (2023) 61:myad083. doi: 10.1093/mmy/myad083
18. Liu, Y, Guo, H, Yuan, W, Zou, Y, Qian, Z, Mei, X, et al. HIV-negative case of Talaromyces marneffei pulmonary infection with liver cirrhosis in China: a case report and literature review. Infect Drug Resist. (2024) 17:1333–43. doi: 10.2147/IDR.S451880
19. Castro-Lainez, MT, Sierra-Hoffman, M, LLompart-Zeno, J, Adams, R, Howell, A, Hoffman-Roberts, H, et al. Talaromyces marneffei infection in a non-HIV non-endemic population. IDCases. (2018) 12:21–4. doi: 10.1016/j.idcr.2018.02.013
20. Boccalini, S, Panatto, D, Mennini, FS, Marcellusi, A, Bini, C, Amicizia, D, et al. Health technology assessment (HTA) of the introduction of additional cohorts for anti-meningococcal vaccination with quadrivalent conjugate vaccines in Italy. J Prev Med Hyg. (2021) 62:E1–e128. doi: 10.15167/2421-4248/jpmh2021.62.1s1
21. Boccalini, S, Pariani, E, Calabrò, GE, Dew, C, Panatto, D, Amicizia, D, et al. Health technology assessment (HTA) of the introduction of influenza vaccination for Italian children with Fluenz tetra(®). J Prev Med Hyg. (2021) 62:E1–e118. doi: 10.15167/2421-4248/jpmh2021.62.2s1
22. He, H, Cai, L, Lin, Y, Zheng, F, Liao, W, Xue, X, et al. Advances in the understanding of talaromycosis in HIV-negative patients (especially in children and patients with hematological malignancies): a comprehensive review. Med Mycol. (2024) 62:myae094. doi: 10.1093/mmy/myae094
23. Singh, MK, Borson, S, Lei, V, Molloy, R, Weng, B, and Sutjita, M. Rare cases of Talaromyces pneumonia in individuals with underlying cancer and no travel to endemic areas. IDCases. (2023) 33:e01831. doi: 10.1016/j.idcr.2023.e01831
24. Chen, M, Houbraken, J, Pan, W, Zhang, C, Peng, H, Wu, L, et al. Pulmonary fungus ball caused by Penicillium capsulatum in a patient with type 2 diabetes: a case report. BMC Infect Dis. (2013) 13:496. doi: 10.1186/1471-2334-13-496
25. Guzik, TJ, Nosalski, R, Maffia, P, and Drummond, GR. Immune and inflammatory mechanisms in hypertension. Nat Rev Cardiol. (2024) 21:396–416. doi: 10.1038/s41569-023-00964-1
26. Li, D, Liang, H, Zhu, Y, Chang, Q, Pan, P, and Zhang, Y. Clinical characteristics, laboratory findings, and prognosis in patients with Talaromyces marneffei infection across various immune statuses. Front Med. (2022) 9:841674. doi: 10.3389/fmed.2022.841674
27. Li, Y, Lin, Z, Shi, X, Mo, L, Li, W, Mo, W, et al. Retrospective analysis of 15 cases of Penicillium marneffei infection in HIV-positive and HIV-negative patients. Microb Pathog. (2017) 105:321–5. doi: 10.1016/j.micpath.2017.01.026
28. Kawila, R, Chaiwarith, R, and Supparatpinyo, K. Clinical and laboratory characteristics of Penicilliosis marneffei among patients with and without HIV infection in northern Thailand: a retrospective study. BMC Infect Dis. (2013) 13:464. doi: 10.1186/1471-2334-13-464
29. Qiu, Y, Huang, J, Li, Y, Zeng, W, Pan, M, Cen, J, et al. Talaromyces marneffei and nontuberculous mycobacteria co-infection in HIV-negative patients. Sci Rep. (2021) 11:16177. doi: 10.1038/s41598-021-95686-0
30. Hatakeyama, S, Yamashita, T, Sakai, T, and Kamei, K. Case report: disseminated Talaromyces (Penicillium) marneffei and Mycobacterium tuberculosis coinfection in a Japanese patient with acquired immunodeficiency syndrome. Am J Trop Med Hyg. (2017) 97:38–41. doi: 10.4269/ajtmh.16-1004
31. Wang, DM, Liu, H, Zheng, YL, Xu, YH, and Liao, Y. Epidemiology of nontuberculous mycobacteria in tuberculosis suspects, southwest of China, 2017-2022. Front Cell Infect Microbiol. (2023) 13:1282902. doi: 10.3389/fcimb.2023.1282902
32. Frumento, D, and Ţălu, Ş. Treatment with directly acting antivirals (DAAs) in HCV mono-infected and HIV-HCV co-infected patients. Microbes Infect Dis. (2024). doi: 10.21608/mid.2024.303563.2069
33. Mo, W, Deng, Z, and Li, S. Clinical blood routine and bone marrow smear manifestations of disseminated Penicilliosis marneffei. Chin Med J. (2002) 115:1892–4. doi: 10.3760/cma.j.issn.0366-6999.2002.12.127
34. Simner, PJ, Miller, S, and Carroll, KC. Understanding the promises and hurdles of metagenomic next-generation sequencing as a diagnostic tool for infectious diseases. Clin Infect Dis. (2018) 66:778–88. doi: 10.1093/cid/cix881
35. Guo, P, Chen, W, Chen, S, Chen, M, Hu, F, Chen, X, et al. The delayed clearance of Talaromyces marneffei in blood culture may be associated with higher MIC of voriconazole after antifungal therapy among AIDS patients with talaromycosis. PLoS Negl Trop Dis. (2023) 17:e0011201. doi: 10.1371/journal.pntd.0011201
36. Huang, W, Li, T, Zhou, C, Wei, F, Cao, C, and Jiang, J. Voriconazole versus amphotericin B as induction therapy for Talaromycosis in HIV/AIDS patients: a retrospective study. Mycopathologia. (2021) 186:269–76. doi: 10.1007/s11046-021-00533-5
37. Lei, HL, Li, LH, Chen, WS, Song, WN, He, Y, Hu, FY, et al. Susceptibility profile of echinocandins, azoles and amphotericin B against yeast phase of Talaromyces marneffei isolated from HIV-infected patients in Guangdong, China. Eur J Clin Microbiol Infect Dis. (2018) 37:1099–102. doi: 10.1007/s10096-018-3222-x
Keywords: Talaromyces marneffei , metagenomic next-generation sequencing, infection, clinical characteristics, treatment
Citation: Wu A, Gai W, Guo Y, Zhou C, Xu Y, Zhang X and Wang H (2025) Clinical features of Talaromyces marneffei infection and colonization in HIV-negative patients: the role of mNGS in diagnosis. Front. Med. 12:1579522. doi: 10.3389/fmed.2025.1579522
Edited by:
Franklin Wang-Ngai Chow, Hong Kong Polytechnic University, Hong Kong SAR, ChinaCopyright © 2025 Wu, Gai, Guo, Zhou, Xu, Zhang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huajun Wang, d2hqMjY5Njk2QDE2My5jb20=
 Aixiang Wu1
Aixiang Wu1 Yuxin Guo
Yuxin Guo Xiaojing Zhang
Xiaojing Zhang Huajun Wang
Huajun Wang