- 1Department of Radiology, The Third Affiliated Hospital of Kunming Medical University (Yunnan Cancer Hospital, Yunnan Cancer Center), Kunming, Yunnan, China
- 2The First College of Clinical Medicine, Gansu University of Chinese Medicine, Lanzhou, Gansu, China
- 3920th Hospital of Joint Logistics Support Force, Kunming, Yunnan, China
- 4The Second Hospital of Hebei Medical University, Shijiazhuang, Hebei, China
- 5Department of Gynecology, The Third Affiliated Hospital of Kunming Medical University (Yunnan Cancer Hospital, Yunnan Cancer Center), Kunming, Yunnan, China
Purpose: This meta-analysis evaluates and compares the diagnostic accuracy of [18F]FDG PET/CT and [18F]FDG PET/MRI in detecting breast cancer recurrence.
Methods: A search was conducted across PubMed, Web of Science, and Embase databases up to June 10, 2025, to identify studies evaluating the diagnostic performance of [18F]FDG PET/CT and/or [18F]FDG PET/MRI in breast cancer recurrence. Sensitivity and specificity were calculated using the DerSimonian and Laird method with Freeman-Tukey double arcsine transformation. The Quality Assessment for Studies of Diagnostic Accuracy-2 (QUADAS-2) guidelines were employed to perform the quality evaluation.
Results: Seventeen studies involving 1,450 patients were included. At the lesion level, the sensitivity of [18F]FDG PET/CT was 0.97 (95% CI: 0.91–1.00), with a specificity of 0.79 (95% CI: 0.58–0.94). [18F]FDG PET/MRI showed a sensitivity of 0.95 (95% CI: 0.91–0.99) and specificity of 0.87 (95% CI:0.75–0.95). Both modalities demonstrated similar sensitivity (p = 0.71) and specificity (p = 0.66). At the patient level, the sensitivity of [18F]FDG PET/CT was 0.93 (95% CI: 0.88–0.96), with a specificity of 0.87 (95% CI: 0.80–0.93). [18F]FDG PET/MRI showed a sensitivity of 0.99 (95% CI: 0.94–1.00) and specificity of 0.98 (95% CI, 0.90–1.00). Both modalities demonstrated similar sensitivity (p = 0.07) and specificity (p = 0.06).
Conclusion: [18F]FDG PET/CT and [18F]FDG PET/MRI exhibit comparable sensitivity and specificity in detecting breast cancer recurrence at both the lesion and patient levels. However, high heterogeneity warrants further head-to-head studies to strengthen the evidence and provide more comprehensive insights.
1 Introduction
Among the top causes of female mortality, breast cancer remains a major global health concern (1, 2). According to projections by the American Cancer Society, approximately 313,510 individuals in the United States are expected to be diagnosed with breast cancer in 2024, while an estimated 42,780 are anticipated to succumb to the disease (3). In the 10 years following initial treatment, at least 35% of breast cancer patients experience local or distant recurrence (4). Breast cancer frequently recurs locally and metastasizes distantly, leading to poor prognosis and high mortality. Therefore, it is essential for patients to get a timely diagnosis of local recurrences and distant metastases.
Breast cancer recurrence is generally evaluated with a physical exam, as well as a mammogram, computed tomography (CT), magnetic resonance imaging (MRI), and bone scintigram. Despite detecting masses and lymph node abnormalities, physical examinations lack sensitivity to detect small metastases and early recurrences, complicating accurate staging (5). Both CT and MRI are susceptible to false positives due to postoperative fibrosis and scarring, and their resolution may be insufficient for small lesions (6). Although bone scans are highly sensitive for detecting bone metastases but they lack specificity, which can result in misdiagnoses due to non-cancerous conditions (7). These methods frequently struggle to distinguish between postoperative changes and true pathology, especially in early or small recurrences.
To address these limitations, recent advancements in multimodal and molecular imaging have introduced new approaches for detecting breast cancer recurrence and metastasis. [18F] Fluorodeoxyglucose positron emission tomography/CT ([18F]FDG PET/CT) and [18F]FDG PET/MRI are rapidly advancing molecular imaging technologies widely used for detecting and localizing breast cancer recurrence. [18F]FDG is a radiotracer that determines cellular metabolism by exploiting in vivo metabolic properties, which enables it to detect tumors and abnormal tissues (8). [18F]FDG PET/CT combines positron emission tomography and computed tomography, offering precise metabolic data and high-resolution anatomical images, crucial for identifying and analyzing recurrent lesions (9). [18F]FDG PET/MRI integrates positron emission tomography with magnetic resonance imaging, providing both metabolic and functional information, potentially advantageous for studying the biological traits of recurrent lesions (10). In addition, PET/MR excels in soft tissue contrast, making it more sensitive to vascular and soft tissue diseases (11). However, the relative diagnostic performance of these technologies remain debated.
In this study, articles with [18F]FDG PET/CT and [18F]FDG PET/MRI results have been evaluated and compared to detect recurrent breast cancer. A direct comparison in this context has not been thoroughly explored, making this study an important contribution to the understanding of imaging techniques in breast cancer recurrence evaluation.
2 Methods
This meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses for Diagnostic Test Accuracy (PRISMA-DTA) guidelines for diagnostic test accuracy (12). PROSPERO network (ID CRD42024556069) prospectively registered the protocol.
2.1 Search strategy
A comprehensive search was performed across PubMed, Web of Science, and Embase databases to identify relevant articles, covering studies published up to June 10, 2025. The search queries included the following key terms: “Breast Neoplasms,” “Positron-Emission Tomography,” and “Recurrence.” Supplementary Table S1 provides details on the search strategy. To locate any further relevant studies, we manually searched the references of the retrieved articles.
2.2 Inclusion and exclusion criteria
In accordance with the PICOS (participants, intervention, control, outcomes, study design) framework, we included the following elements in our study: (1) Participants (P): Patients with suspected recurrence in breast cancer; (2) Intervention (I): Studies employing [18F]FDG PET/CT; (3) Comparisons (C): Studies comparing with [18F]FDG PET/MRI; (4) Outcomes (O): Studies reporting on sensitivity and specificity; and (5) Study design (S): Retrospective or prospective studies.
Studies were deemed eligible for exclusion from the meta-analysis if they satisfied the following criteria: (1) Case report, abstract, letter, review, meta-analysis; (2) Irrelevant titles and abstracts; (3) Unable to retrieve data on diagnosis-related metrics, including True-positive (TP), False-positive (FP), False-negative (FN), and True-negative (TN); (4) Non-English publication; (5) Non-suspected recurrence patients; (6) Studies employing other radiotracers (non-FDG).
2.3 Quality assessment
To assess the quality of methodologies in the included studies, this meta-analysis followed the Quality Assessment for Studies of Diagnostic Accuracy-2 (QUADAS-2) guidelines, which are specifically designed for evaluating systematic reviews of diagnostic accuracy studies (13), consisting of four critical domains: (1) patient selection; (2) index test; (3) reference standard; and (4) flow and timing. According to QUADAS, the risk of bias in each study was classified as “high risk,” “low risk,” or “unclear risk”.
Two independent authors assessed all included studies, with any discrepancies settled by consulting an additional author to reach an agreement.
2.4 Data extraction
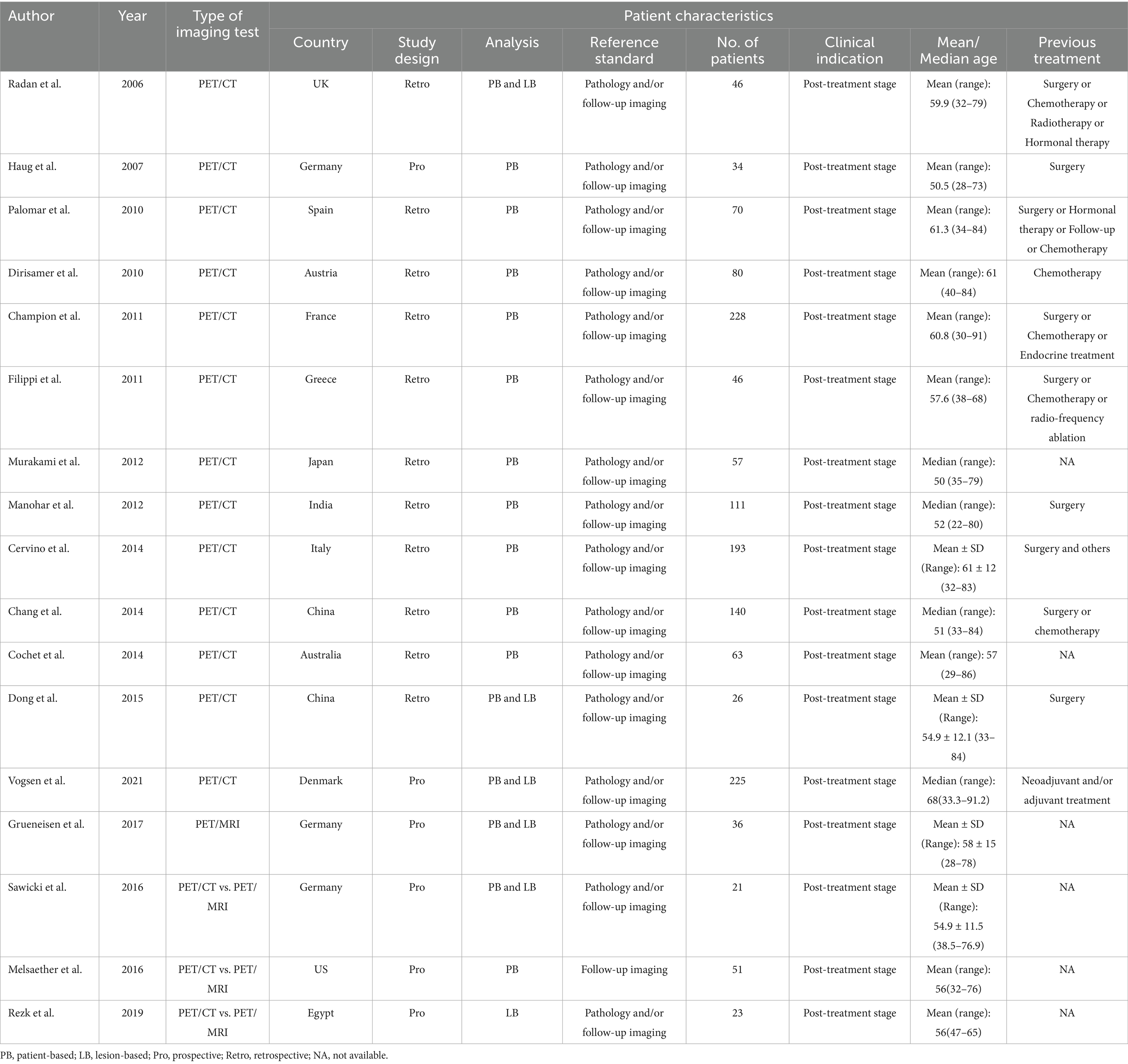
The data extracted from the selected studies included as follows: the author, publication year, type of imaging test (PET/CT or PET/MRI), country of the study, study design (prospective or retrospective), analysis type (patient-based or lesion-based), reference standard (pathology and/or follow-up imaging), number of patients, clinical indication stage, mean or median age of patients, previous treatment (such as surgery, chemotherapy, radiotherapy, hormonal therapy, etc.), as well as the diagnostic outcomes including TP, FP, TN, and FN.
Two reviewers independently carried out the data extraction from each study. Disagreements were resolved by discussion, achieving consensus to guarantee precision in the data extraction process.
2.5 Outcome measures
In this meta-analysis, the primary outcome measures centered on the diagnostic performance of [18F]FDG PET/CT and [18F]FDG PET/MRI in detecting breast cancer recurrence. The diagnostic performance of these imaging modalities was evaluated through their sensitivities and specificities. Sensitivity was defined as the proportion of TP imaging results to the total number of TP and FN results. Specificity was defined as the proportion of TN results to the total number of TN and FP results.
2.6 Statistical analysis
The present study utilized the recommended standard method for conducting a diagnostic meta-analysis (14). The DerSimonian and Laird method was employed to evaluate specificity and sensitivity, followed by transformation using the Freeman-Tukey double arcsine transformation (15). Confidence intervals were calculated using the Jackson method (16). Heterogeneity within and between groups was assessed with the Cochrane Q and I2 statistics (17). In cases of significant heterogeneity (p < 0.10 or I2 > 50%), a sensitivity analysis was conducted by sequentially excluding studies to reassess sensitivity or specificity (18).
To evaluate publication bias, funnel plots were used alongside Egger’s test. For heterogeneity tests, p < 0.10 was considered significant, while for all other statistical tests, a significance level of p < 0.05 was applied. All statistical analyses and graphical representations were performed using R software version 4.4.0.
3 Results
3.1 Study selection
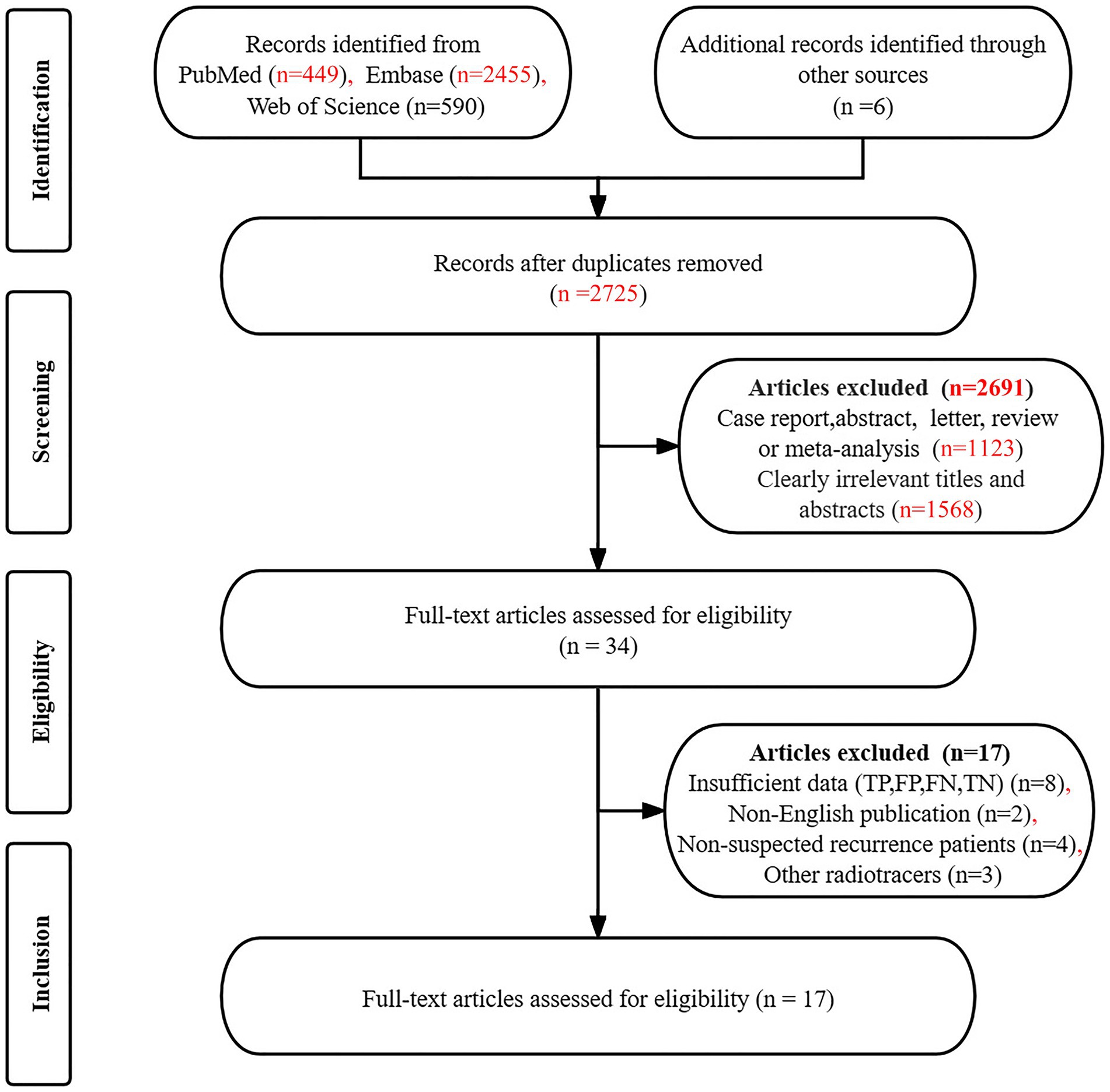
The initial search yielded 3,500 articles, from which 775 duplicates were excluded. Additionally, 2,725 articles did not meet the established inclusion criteria, leaving 34 articles for further consideration. After a detailed reading of the complete texts of the remaining articles, an additional 17 were excluded for the following reasons: unavailable data on TP, TN, FP, and FN (n = 8), non-English publications (n = 2), studies involving non-suspected recurrence patients (n = 4), and the use of other radiotracers (n = 3). Consequently, 17 articles (19–35) were included in the final analysis, which assessed the diagnostic effectiveness of [18F]FDG PET/CT (n = 16) (19–32, 34, 35) and [18F]FDG PET/MRI (n = 4) (31–34), including 3 articles (31, 32, 34) that allowed for head-to-head comparison. Figure 1 provides details of the article selection process based on the PRISMA flow diagram.
3.2 Study description and quality assessment
This analysis incorporated 17 eligible studies, encompassing a total of 1,450 patients who were being evaluated for suspected recurrence of breast cancer, with ages ranging from 22 to 91.2 years. Of these studies, 11 (65%) were retrospective in nature (19, 21–30), while 6 (35%) were prospective studies (20, 31–35). In terms of analysis methods, 10 studies (59%) performed patient-based analyses (19, 20, 22–24, 26, 27, 30, 31, 33), whereas 7 studies (41%) utilized lesion-based analyses (21, 25, 28, 29, 32, 34, 35). The reference standards applied included pathology and/or imaging follow-up in 16 articles (94%) (19–30, 32–35), with one article (5%) relying solely on imaging follow-up (31). All patients included in these studies were in the post-treatment stage of breast cancer. The previous treatments they had undergone varied and included types such as surgery and chemotherapy. Table 1 summarizes the basic information and patient characteristics of the included studies.
Based on the QUADAS-2 tool, the risk of bias in all studies is illustrated in Figure 2. In assessing the risk of bias related to patient selection, index testing, and reference standards, the majority of the articles were deemed low risk, with no articles classified as high risk. Regarding the flow and timing aspect, 6 studies (35%) (27, 28, 31–33, 35) were judged to be at “high risk” due to a time interval exceeding 3 months. Overall, no significant quality issues were identified in the included studies.
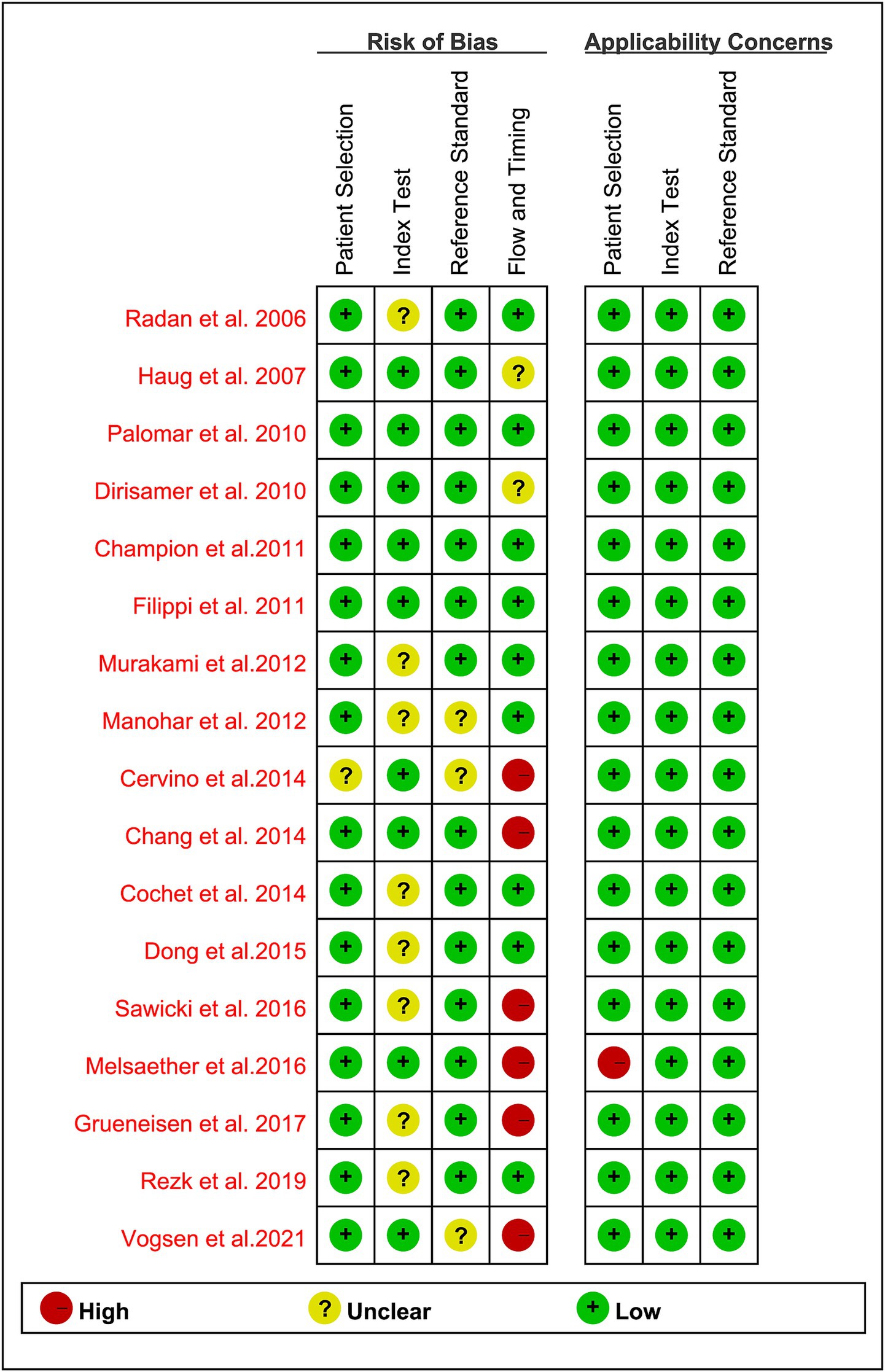
Figure 2. Summary of risk of bias and applicability concerns of all included studies according to QUADAS-2 tool.
3.3 Lesion-based comparison of the sensitivity of [18F]FDG PET/CT and [18F]FDG PET/MRI in breast cancer recurrence
This meta-analysis assesses the diagnostic efficacy of [18F]FDG PET/CT and [18F]FDG PET/MRI in detecting recurrence of breast cancer at the lesion level. The analysis incorporates data from six studies (19, 30, 32–35), encompassing a total of 377 patients and 1,313 lesions. Specifically, five studies (19, 30, 32, 34, 35) involving 341 patients and 829 lesions employed [18F]FDG PET/CT, whereas three studies (32–34) with 80 patients and 484 lesions utilized [18F]FDG PET/MRI.
The pooled sensitivity for [18F]FDG PET/CT was 0.97 (95% CI: 0.91–1.00). In comparison, [18F]FDG PET/MRI demonstrated a pooled sensitivity of 0.95 (95% CI: 0.91–0.99). The comparative analysis of pooled sensitivity between [18F]FDG PET/CT and [18F]FDG PET/MRI revealed no statistically significant difference (p = 0.71) (Figure 3).
![Forest plot comparing two imaging techniques: [¹⁸F]FDG PET/CT with a total weight of 62.4% and [¹⁸F]FDG PET/MRI with 37.6%. Results indicate a combined IV, Random, 95% CI of 0.96 [0.91; 0.99]. Heterogeneity is significant, with Chi-squared and I-squared values provided for each subgroup.](https://www.frontiersin.org/files/Articles/1602415/fmed-12-1602415-HTML/image_m/fmed-12-1602415-g003.jpg)
Figure 3. Lesion-based forest plot illustrating the sensitivity of [18F]FDG PET/CT and [18F]FDG PET/MRI for detecting breast cancer recurrence.
For lesion-based [18F]FDG PET/CT, heterogeneity had an I2 of 86.5%, with stable results in the leave-one-out sensitivity analysis (Supplementary Figure S1).
3.4 Lesion-based comparing the specificity of [18F]FDG PET/CT and [18F]FDG PET/MRI in breast cancer recurrence
In the detection of recurrence for breast cancer, at the lesion level, the overall specificity of [18F]FDG PET/CT was 0.79 (95% CI: 0.58–0.94), compared to a pooled specificity of 0.87 (95% CI:0.75–0.95) for [18F]FDG PET/MRI. The total specificity of [18F]FDG PET/CT and [18F]FDG PET/MRI showed no statistical difference (p = 0.44) (Figure 4).
![Forest plot comparing imaging studies using [18F]FDG PET/CT and PET/MRI. Data includes events, totals, and weights for studies by Radan, Dong, Vogsen, Sawicki, Rezk, and Grueneisen from 2006 to 2021. The plot shows pooled estimates with confidence intervals, heterogeneity metrics (Tau-squared, Chi-squared, degrees of freedom, P-values), and I-squared values. PET/CT has a combined effect size of 0.79, while PET/MRI is 0.87. Overall effect size for both modalities is 0.82. Horizontal lines and diamonds indicate confidence intervals and pooled estimates.](https://www.frontiersin.org/files/Articles/1602415/fmed-12-1602415-HTML/image_m/fmed-12-1602415-g004.jpg)
Figure 4. Lesion-based forest plot illustrating the specificity of [18F]FDG PET/CT and [18F]FDG PET/MRI for detecting breast cancer recurrence.
For [18F]FDG PET/CT, the lesion-based specificity exhibited I2 values of 70%. The leave-one-out sensitivity analysis revealed that the results remained stable (Supplementary Figure S2).
3.5 Patient-based comparison of the sensitivity of [18F]FDG PET/CT and [18F]FDG PET/MRI in breast cancer recurrence
This meta-analysis evaluates the diagnostic efficacy of [18F]FDG PET/CT and [18F]FDG PET/MRI for the detection of breast cancer recurrence at the patient level. The analysis synthesizes data from 16 studies (19–33, 35), which collectively included 1,427 patients. Specifically, 15 studies (19–32, 35) involving 1,391 patients utilized [18F]FDG PET/CT, while three studies (31–33) with 108 patients employed [18F]FDG PET/MRI.
In diagnosing recurrence in breast cancer at the patient level, [18F]FDG PET/CT demonstrated a pooled sensitivity of 0.93 (95% CI: 0.88–0.96), while [18F]FDG PET/MRI showed an overall sensitivity of 0.99 (95% CI: 0.94–1.00). Statistical analysis revealed no significant difference in the overall sensitivity between [18F]FDG PET/CT and [18F]FDG PET/MRI (p = 0.07) (Figure 5).
![Forest plot comparing studies using [18F]FDG PET/CT and PET/MRI imaging techniques. It shows individual study results and combined effect sizes with 95% confidence intervals. PET/CT has a total of 713 events and PET/MRI has 59. Overall total events are 772 with a combined effect size of 0.94, indicating good effectiveness. Confidence intervals vary among studies, with heterogeneity indicated by I-squared values: 64.9% for PET/CT and 0% for PET/MRI. The diamond shapes represent pooled effect estimates.](https://www.frontiersin.org/files/Articles/1602415/fmed-12-1602415-HTML/image_m/fmed-12-1602415-g005.jpg)
Figure 5. Patient-based forest plot illustrating the sensitivity of [18F]FDG PET/CT and [18F]FDG PET/MRI for detecting breast cancer recurrence.
In patient-based [18F]FDG PET/CT analyses, heterogeneity was quantified with an I2 value of 64.9%, and the results remained consistent upon conducting a leave-one-out sensitivity analysis (Supplementary Figure S3).
3.6 Patient-based comparison of the specificity of [18F]FDG PET/CT and [18F]FDG PET/MRI in breast cancer recurrence
In the context of diagnosing breast cancer recurrence at the patient level, the aggregated specificity of [18F]FDG PET/CT was determined to be 0.87 (95% CI: 0.80–0.93). In comparison, [18F]FDG PET/MRI demonstrated an overall specificity of 0.98 (95% CI: 0.90–1.00). Statistical analysis indicated no significant difference in specificity between the two imaging modalities (p = 0.06) (Figure 6).
![Forest plot comparing imaging techniques \([^{18}F]\text{FDG PET/CT}\) and \([^{18}F]\text{FDG PET/MRI}\) across multiple studies. The plot displays individual study weights, event totals, and confidence intervals. \([^{18}F]\text{FDG PET/CT}\) has a total sample size of 539, with a summary effect of 0.87 [0.80; 0.93]. \([^{18}F]\text{FDG PET/MRI}\) has 49 participants, with a summary effect of 0.98 [0.90; 1.00]. The combined total effect across both imaging modalities is 0.89 [0.83; 0.94]. The plot includes heterogeneity statistics.](https://www.frontiersin.org/files/Articles/1602415/fmed-12-1602415-HTML/image_m/fmed-12-1602415-g006.jpg)
Figure 6. Patient-based forest plot illustrating the head-to-head comparison of the specificity of [18F]FDG PET/CT and [18F]FDG PET/MRI for detecting breast cancer recurrence.
In analyses of patient-based [18F]FDG PET/CT, heterogeneity was quantified with an I2 value of 76.6%. The results demonstrated robustness, as evidenced by their consistency in a leave-one-out sensitivity analysis (Supplementary Figure S4).
3.7 Publication bias
The funnel plot and Egger’s test demonstrated an absence of evidence for publication bias regarding the sensitivity and specificity of [18F]FDG PET/CT on both lesion-based and patient-based analyses, respectively (all p > 0.50) (Supplementary Figures S5–S8).
4 Discussion
Current guidelines from prominent oncology organizations, including the American Society of Clinical Oncology, the National Comprehensive Cancer Network, and the European Society for Medical Oncology, advise that follow-up care for breast cancer patients who are asymptomatic should primarily consist of regular physical examinations and annual mammographic screenings. The use of additional laboratory or imaging tests on a routine basis is not recommended within these guidelines (36–38). However, for breast cancer patients with a suspected recurrence, there is currently no standardized follow-up protocol in place, making additional radiologic imaging crucial, particularly when recurrence is suggested by elevated tumor markers or concerning symptoms.
A prospective study evaluated [18F]FDG PET/CT in 100 patients with suspected breast cancer recurrence compared to thoracoabdominal contrast-enhanced CTs (ceCT) and bone scans (39). The findings revealed that 22% of patients were diagnosed with distant recurrence, 19% had local recurrence only, and 59% exhibited no evidence of recurrence. The diagnostic accuracy for identifying distant recurrence was assessed using the area under the ROC curve, with [18F]FDG PET/CT achieving an AUC of 0.99, compared to 0.84 for ceCT and 0.86 for the combination of ceCT and bone scans (39). Another study reported that [18F]FDG PET/CT exhibits a high positive predictive value of 0.97 and an accuracy ranging from 0.83 to 0.86 in detecting suspected recurrent condition (24, 40). Additionally, other clinical guidelines suggest that [18F]FDG PET/CT can be particularly useful in identifying the site of recurrence when conventional imaging methods yield inconclusive results (41). In contrast, another meta-analysis has demonstrated that [18F]FDG PET/MRI offers robust diagnostic accuracy, with a sensitivity of 0.94 and specificity of 0.90 for nodal staging, as well as a sensitivity of 0.98 and specificity of 0.96 for distant staging (42). Preliminary data suggest that PET/MRI could be a viable alternative to PET/CT for current clinical applications (43). In conclusion, both [18F]FDG PET/CT and [18F]FDG PET/MRI perform well in detecting breast cancer recurrence. However, a systematic comparison is still lacking, leaving the question of which modality is superior unresolved. This meta-analysis seeks to fill a gap in the literature by comparing the diagnostic sensitivity and specificity of [18F]FDG PET/CT and [18F]FDG PET/MRI in breast cancer recurrence.
The meta-analytic outcomes of this systematic review demonstrate that there is no statistically significant difference in overall sensitivity between [18F]FDG PET/CT and [18F]FDG PET/MRI for the detection of breast cancer recurrence at both the lesion level (p = 0.71) and the patient level (p = 0.07). Similarly, the overall specificity of the two imaging modalities does not differ significantly at the lesion level (p = 0.44) or the patient level (p = 0.06). These findings indicate that both imaging techniques provide comparable diagnostic performance in clinical settings. These findings collectively indicate that both imaging techniques demonstrate equivalent diagnostic accuracy in the detection of recurrent breast cancer within clinical practice.
The diagnostic equivalency observed can be attributed to the common use of [18F]FDG, a glucose analog that preferentially accumulates in metabolically active tumor cells (44). While PET/MRI provides enhanced soft tissue contrast, especially in breast and pelvic areas, the diagnostic efficacy is predominantly determined by the metabolic imaging aspect (45, 46). Consequently, the PET-based assessment of glucose metabolism is likely the primary determinant of sensitivity and specificity in both imaging modalities.
In addition, at the lesion-based analysis, a study assessing the performance of these imaging modalities revealed that [18F]FDG PET/MRI correctly identified a greater proportion of lesions, achieving a sensitivity of 98.5% compared to 94.8% for [18F]FDG PET/CT (32). This finding suggests that PET/MRI may be more effective in detecting smaller or less conspicuous lesions that could be overlooked by PET/CT (32). Furthermore, patient-based analyses indicate that [18F]FDG PET/MRI offers advantages over PET/CT. A meta-analysis comparing these modalities found that [18F]FDG PET/MRI exhibited higher sensitivity for detecting distant metastases in breast cancer patients, with a sensitivity of 1.00 compared to 0.96 for PET/CT (47).
To date, no comprehensive meta-analysis or extensive comparative study has specifically investigated the influence of recurrence site on the relative diagnostic efficacy of [18F]FDG PET/CT versus PET/MRI. This omission constitutes a significant gap in the existing literature, as elucidating lesion-specific diagnostic advantages could facilitate the development of more personalized and anatomically targeted imaging strategies for patients with suspected recurrence. In response to this gap, the current study is pioneering in its examination of the impact of recurrence site on imaging modality performance through stratified subgroup analyses, providing novel insights into how anatomical context may affect diagnostic sensitivity and specificity. In our meta-analysis, both analytical methodologies produced consistent results, demonstrating no statistically significant difference in sensitivity or specificity between [18F]FDG PET/CT and PET/MRI at either the lesion level or the patient level. This internal consistency enhances confidence in the robustness of our findings and indicates that the diagnostic equivalence between these modalities is maintained irrespective of the level of analytical granularity. However, future research should clearly differentiate between these analytical levels and, where feasible, employ hierarchical modeling approaches that account for the clustering of lesions within patients.
Furthermore, this meta-analysis offers a thorough assessment of the diagnostic effectiveness of [18F]FDG PET/CT and [18F]FDG PET/MRI for detecting breast cancer recurrence, filling critical gaps from earlier research. Xiao et al. (48) conducted a systematic review and meta-analysis, demonstrating the high sensitivity (0.90) and specificity (0.81) of [18F]FDG PET/CT in detecting breast cancer recurrence. While their findings highlighted the diagnostic value of [18F]FDG PET/CT, they did not compare it with PET/MRI, leaving unresolved the question of which modality performs better in this context. Our study builds upon their work by providing the first head-to-head comparison between PET/CT and PET/MRI, offering a clearer, evidence-based understanding of the diagnostic capabilities of both modalities in breast cancer recurrence.
In contrast, Dan et al. (49) focused on the diagnostic performance of PET/MRI in breast cancer, including initial diagnosis, lymph node involvement, and bone metastasis. While their study demonstrated superior sensitivity (0.95) and specificity (0.94) of PET/MRI for breast lesions and metastases, a direct comparison between PET/MRI and PET/CT was not conducted, leaving an important gap in understanding their relative performance.
In comparing the diagnostic efficacy of [18F]FDG PET/CT and [18F]FDG PET/MRI in breast cancer recurrence, both modalities demonstrated similar sensitivity and specificity, indicating comparable performance in detecting recurrent disease. Nonetheless, each option has its unique benefits and drawbacks. Several studies have demonstrated that PET/CT is a cost-effective and widely available option, including one emphasizing its utility for monitoring metastatic breast cancer treatment responses (50). PET/CT also offers quicker scan times, reducing patient discomfort and rendering it a viable option in numerous clinical practice. A PET/MRI scan, on the other hand, enhances the contrast of soft tissue while reducing radiation exposure, which is especially important for younger or more vulnerable patients (51). In light of these factors, while PET/CT appears to be more economical and accessible, PET/MRI’s enhanced diagnostic performance and safety profile in specific cases cannot be ignored. The complementary strengths of these modalities suggest that combining them into a hybrid diagnostic approach may improve overall diagnostic accuracy. Patients’ clinical circumstances and diagnostic needs should ultimately determine whether PET/CT or PET/MRI is appropriate.
Interpreting the results of this meta-analysis requires consideration of certain limitations. First, the heterogeneity among the included studies may have influenced the pooled sensitivities and specificities of [18F]FDG PET/CT and [18F]FDG PET/MRI. In order to identify potential sources of heterogeneity and evaluate the robustness of our findings, we conducted a leave-one-out sensitivity analysis. The results demonstrated stability across all iterations, with no individual study exerting a disproportionate influence on the aggregated sensitivity or specificity estimates. This consistency indicates that our meta-analytic conclusions are robust and not significantly impacted by any outlier study. Moreover, the imbalance in studies between [18F]FDG PET/CT (16 studies) and [18F]FDG PET/MRI (4 studies) may introduce bias, with PET/CT’s larger sample size potentially affecting statistical power and confidence intervals for PET/MRI. We conducted subgroup analyses by anatomical regions and sample sizes to address this, but the disparity still limits our analysis’s robustness. Future research should aim for more balanced and larger sample sizes for both imaging methods to ensure more reliable results. Another key limitation is the reliance on retrospective studies (9 out of 16), which are susceptible to selection and verification biases, potentially impacting our findings. Although prospective cohort studies would be preferable, the unpredictable nature of breast cancer recurrence makes them impractical. Thus, we used retrospective data despite its limitations. Recognizing these biases is crucial, and future research should prioritize prospective, multi-center cohort studies to minimize biases and improve result generalizability. The absence of standardized imaging protocols across studies is a limitation, as variations in MRI sequences and PET methods may lead to inconsistent diagnostic performance. A universally accepted protocol for imaging breast cancer recurrence is lacking. Developing such standardized protocols should be a research priority to enhance result consistency and enable reliable comparisons between imaging modalities. Lastly, while we assessed publication bias using a funnel plot and Egger’s test, it’s crucial to recognize that studies favoring PET/MRI may dominate the literature, potentially inflating its perceived diagnostic efficacy. Research on breast cancer recurrence often emphasizes imaging techniques like PET/MRI, leading to a higher likelihood of publishing positive results. This bias can misrepresent PET/MRI’s true effectiveness, as studies with negative or inconclusive outcomes may be underreported. Future research should aim to include both positive and negative findings to provide a more accurate evaluation of PET/MRI’s clinical value in detecting breast cancer recurrence.
To overcome the discussed limitations, we suggest larger, balanced studies, standardized imaging protocols, detailed site comparisons, and reducing publication bias. These measures are crucial for enhancing the diagnostic accuracy of [18F]FDG PET/CT and [18F]FDG PET/MRI in detecting breast cancer recurrence, thereby improving oncology clinical decisions.
5 Conclusion
The findings of our meta-analysis suggest that [18F]FDG PET/CT and [18F]FDG PET/MRI exhibit comparable sensitivity and specificity in diagnosing breast cancer recurrence at both the lesion and patient levels. In a clinical setting, both of these imaging techniques have their respective strengths and limitations, and physicians should take these into account when making the most suitable choice for patients. Considering that the number of articles on [18F]FDG PET/MRI in diagnosing recurrence of breast cancer is fewer compared to [18F]FDG PET/CT, more head-to-head researches are needed to expand this field to obtain more robust and comprehensive results.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
SL: Project administration, Formal analysis, Data curation, Writing – review & editing, Writing – original draft. YX: Writing – original draft. WD: Data curation, Writing – review & editing. XM: Writing – original draft. ZL: Writing – review & editing, Methodology. LZ: Resources, Methodology, Writing – review & editing. YZ: Resources, Writing – review & editing, Supervision, Investigation. CA: Project administration, Funding acquisition, Resources, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the National Natural Science Foundation of China (NO. 82160524); Yunnan Fundamental Research Kunming Medical University Projects (grant NO. 202501AY070001-110); Yunnan Fundamental Research Projects (202401AT070006); and Major Science and Technology Projects in Yunnan Province (202201AY070001-148); Yunnan Provincial Health Commission’s Training Program for Medical Discipline Leaders (D-2024012); the Applied Basic Research Foundation of Yunnan Province Science and Technology Department (NO. 202301AT070246).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1602415/full#supplementary-material
References
1. Arnold, M, Morgan, E, Rumgay, H, Mafra, A, Singh, D, Laversanne, M, et al. Current and future burden of breast cancer: global statistics for 2020 and 2040. Breast. (2022) 66:15–23. doi: 10.1016/j.breast.2022.08.010
2. Shelton, J, Zotow, E, Smith, L, Johnson, SA, Thomson, CS, Ahmad, A, et al. 25 year trends in cancer incidence and mortality among adults aged 35-69 years in the UK, 1993-2018: retrospective secondary analysis. BMJ. (2024) 384:e076962. doi: 10.1136/bmj-2023-076962
3. Siegel, RL, Giaquinto, AN, and Jemal, A. Cancer statistics, 2024. CA Cancer J Clin. (2024) 74:12–49. doi: 10.3322/caac.21820
4. Jackson, KM, Millen, JC, Handy, N, Orozco, JIJ, Stern, SL, Fancher, CE, et al. Breast conservation project: clinical outcomes of extreme oncoplastic breast-conserving therapy versus mastectomy for large and multiple lesions. Ann Surg Oncol. (2024) 31:7582–93. doi: 10.1245/s10434-024-15799-4
5. Menes, TS, Coster, D, Coster, D, and Shenhar-Tsarfaty, S. Contribution of clinical breast exam to cancer detection in women participating in a modern screening program. BMC Womens Health. (2021) 21:368. doi: 10.1186/s12905-021-01507-x
6. Lother, D, Robert, M, Elwood, E, Smith, S, Tunariu, N, Johnston, SRD, et al. Imaging in metastatic breast cancer, CT, PET/CT, MRI, WB-DWI, CCA: review and new perspectives. Cancer Imaging. (2023) 23:53. doi: 10.1186/s40644-023-00557-8
7. Miyaji, N, Miwa, K, Tokiwa, A, Ichikawa, H, Terauchi, T, Koizumi, M, et al. Phantom and clinical evaluation of bone SPECT/CT image reconstruction with XSPECT algorithm. EJNMMI Res. (2020) 10:71. doi: 10.1186/s13550-020-00659-5
8. Kwiecinski, J. (18)F-fluorodeoxyglucose and (18)F-sodium fluoride for imaging atherosclerotic plaque activity. J Nucl Cardiol. (2022) 29:1710–2. doi: 10.1007/s12350-022-02947-0
9. Dong, Z, Wang, GY, Dai, DY, Qin, GJ, Tang, LL, Xu, C, et al. Prognostic value of pre-treatment [(18)F] FDG PET/CT in recurrent nasopharyngeal carcinoma without distant metastasis. BMC Cancer. (2024) 24:466. doi: 10.1186/s12885-024-12189-7
10. Lo, HZ, Choy, KT, and Kong, JCH. FDG-PET/MRI in colorectal cancer care: an updated systematic review. Abdom Radiol. (2024) 50:49–63. doi: 10.1007/s00261-024-04460-z
11. Fukushima, K, Ito, H, and Takeishi, Y. Comprehensive assessment of molecular function, tissue characterization, and hemodynamic performance by non-invasive hybrid imaging: potential role of cardiac PETMR. J Cardiol. (2023) 82:286–92. doi: 10.1016/j.jjcc.2023.06.004
12. Page, MJ, Mckenzie, JE, Bossuyt, PM, Boutron, I, Hoffmann, TC, Mulrow, CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
13. Whiting, PF, Rutjes, AW, Westwood, ME, Mallett, S, Deeks, JJ, Reitsma, JB, et al. Quadas-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. (2011) 155:529–36. doi: 10.7326/0003-4819-155-8-201110180-00009
14. Jones, CM, Ashrafian, H, Skapinakis, P, Arora, S, Darzi, A, Dimopoulos, K, et al. Diagnostic accuracy meta-analysis: a review of the basic principles of interpretation and application. Int J Cardiol. (2010) 140:138–44. doi: 10.1016/j.ijcard.2009.05.063
15. George, BJ, and Aban, IB. An application of meta-analysis based on DerSimonian and Laird method. J Nucl Cardiol. (2016) 23:690–2. doi: 10.1007/s12350-015-0249-6
16. Jackson, D, Turner, R, Rhodes, K, and Viechtbauer, W. Methods for calculating confidence and credible intervals for the residual between-study variance in random effects meta-regression models. BMC Med Res Methodol. (2014) 14:103. doi: 10.1186/1471-2288-14-103
17. Lin, L. Comparison of four heterogeneity measures for meta-analysis. J Eval Clin Pract. (2020) 26:376–84. doi: 10.1111/jep.13159
18. Patsopoulos, NA, Evangelou, E, and Ioannidis, JP. Sensitivity of between-study heterogeneity in meta-analysis: proposed metrics and empirical evaluation. Int J Epidemiol. (2008) 37:1148–57. doi: 10.1093/ije/dyn065
19. Radan, L, Ben-Haim, S, Bar-Shalom, R, Guralnik, L, and Israel, O. The role of FDG-PET/CT in suspected recurrence of breast cancer. Cancer. (2006) 107:2545–51. doi: 10.1002/cncr.22292
20. Haug, AR, Schmidt, GP, Klingenstein, A, Heinemann, V, Stieber, P, Priebe, M, et al. F-18-fluoro-2-deoxyglucose positron emission tomography/computed tomography in the follow-up of breast cancer with elevated levels of tumor markers. J Comput Assist Tomogr. (2007) 31:629–34. doi: 10.1097/01.rct.0000284394.83696.42
21. Dirisamer, A, Halpern, BS, Flöry, D, Wolf, F, Beheshti, M, Mayerhoefer, ME, et al. Integrated contrast-enhanced diagnostic whole-body PET/CT as a first-line restaging modality in patients with suspected metastatic recurrence of breast cancer. Eur J Radiol. (2010) 73:294–9. doi: 10.1016/j.ejrad.2008.10.031
22. Palomar Muñoz, A, García Vicente, AM, Talavera Rubio, MP, Pilkington Woll, JP, Poblete García, VM, Bellón Guardia, ME, et al. Diagnostic and therapeutic impact of 18F-FDG-PET/CT in patients with suspected breast cancer recurrence. Rev Esp Med Nucl. (2010) 29:100–8. doi: 10.1016/j.remn.2010.02.004
23. Champion, L, Brain, E, Giraudet, AL, le Stanc, E, Wartski, M, Edeline, V, et al. Breast cancer recurrence diagnosis suspected on tumor marker rising: value of whole-body 18FDG-PET/CT imaging and impact on patient management. Cancer. (2011) 117:1621–9. doi: 10.1002/cncr.25727
24. Filippi, V, Malamitsi, J, Vlachou, F, Laspas, F, Georgiou, E, Prassopoulos, V, et al. The impact of FDG-PET/CT On the management of breast cancer patients with elevated tumor markers and negative or equivocal conventional imaging modalities. Nucl Med Commun. (2011) 32:85–90. doi: 10.1097/MNM.0b013e328341c898
25. Manohar, K, Mittal, BR, Senthil, R, Kashyap, R, Bhattacharya, A, and Singh, G. Clinical utility of F-18 FDG PET/CT in recurrent breast carcinoma. Nucl Med Commun. (2012) 33:591–6. doi: 10.1097/MNM.0b013e3283516716
26. Murakami, R, Kumita, S, Yoshida, T, Ishihara, K, Kiriyama, T, Hakozaki, K, et al. FDG-PET/CT in the diagnosis of recurrent breast cancer. Acta Radiol. (2012) 53:12–6. doi: 10.1258/ar.2011.110245
27. Cervino, AR, Saibene, T, Michieletto, S, Ghiotto, C, Bozza, F, Saladini, G, et al. Correlation between cancer antigen 15.3 value and qualitative and semiquantitative parameters of positron emission tomography/computed tomography in breast cancer patients. Curr Radiopharm. (2014) 7:20–8. doi: 10.2174/1874471007666140515111134
28. Chang, HT, Hu, C, Chiu, YL, Peng, NJ, and Liu, RS. Role of 2-[18F] fluoro-2-deoxy-D-glucose-positron emission tomography/computed tomography in the post-therapy surveillance of breast cancer. PLoS One. (2014) 9:e115127. doi: 10.1371/journal.pone.0115127
29. Cochet, A, David, S, Moodie, K, Drummond, E, Dutu, G, MacManus, M, et al. The utility of 18 F-FDG PET/CT for suspected recurrent breast cancer: impact and prognostic stratification. Cancer Imaging. (2014) 14:13. doi: 10.1186/1470-7330-14-13
30. Dong, Y, Hou, H, Wang, C, Li, J, Yao, Q, Amer, S, et al. The diagnostic value of 18F-FDG PET/CT in association with serum tumor marker assays in breast cancer recurrence and metastasis. Biomed Res Int. (2015) 2015:489021. doi: 10.1155/2015/489021
31. Melsaether, AN, Raad, RA, Pujara, AC, Ponzo, FD, Pysarenko, KM, Jhaveri, K, et al. Comparison of whole-body (18)F FDG PET/MR imaging and whole-body (18)F FDG PET/CT in terms of lesion detection and radiation dose in patients with breast cancer. Radiology. (2016) 281:193–202. doi: 10.1148/radiol.2016151155
32. Sawicki, LM, Grueneisen, J, Schaarschmidt, BM, Buchbender, C, Nagarajah, J, Umutlu, L, et al. Evaluation of 18F-FDG PET/MRI, 18F-FDG PET/CT, MRI, and CT in whole-body staging of recurrent breast cancer. Eur J Radiol. (2016) 85:459–65. doi: 10.1016/j.ejrad.2015.12.010
33. Grueneisen, J, Sawicki, LM, Wetter, A, Kirchner, J, Kinner, S, Aktas, B, et al. Evaluation of PET and MR datasets in integrated 18F-FDG PET/MRI: a comparison of different MR sequences for whole-body restaging of breast cancer patients. Eur J Radiol. (2017) 89:14–9. doi: 10.1016/j.ejrad.2016.12.019
34. Rezk, M, Nasr, I, Ali, I, and Abdelhamed, H. Comparative study between (18)F FDG-PET/CT and whole body MRI DWIBS in assessment of recurrent breast cancer (prospective, comparative, cross-sectional study design). Indian J Nucl Med. (2019) 34:1–9. doi: 10.4103/ijnm.IJNM_121_18
35. Vogsen, M, Jensen, JD, Gerke, O, Jylling, AMB, Asmussen, JT, Christensen, IY, et al. Benefits and harms of implementing [(18)F]FDG-PET/CT for diagnosing recurrent breast cancer: a prospective clinical study. EJNMMI Res. (2021) 11:93. doi: 10.1186/s13550-021-00833-3
36. Mosele, MF, Westphalen, CB, Stenzinger, A, Barlesi, F, Bayle, A, Bièche, I, et al. Recommendations for the use of next-generation sequencing (NGS) for patients with advanced cancer in 2024: a report from the ESMO precision medicine working group. Ann Oncol. (2024) 35:588–606. doi: 10.1016/j.annonc.2024.04.005
37. Freedman, RA, Caswell-Jin, JL, Hassett, M, Somerfield, MR, and Giordano, SHOptimal Adjuvant Chemotherapy and Targeted Therapy for Early Breast Cancer Guideline Expert Panel. Optimal adjuvant chemotherapy and targeted therapy for early breast cancer-cyclin-dependent kinase 4 and 6 inhibitors: ASCO guideline rapid recommendation update. J Clin Oncol. (2024) 42:2233–5. doi: 10.1200/JCO.24.00886
38. Gradishar, WJ, Moran, MS, Abraham, J, Abramson, V, Aft, R, Agnese, D, et al. Breast cancer, version 3.2024, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw. (2024) 22:331–57. doi: 10.6004/jnccn.2024.0035
39. Hildebrandt, MG, Gerke, O, Baun, C, Falch, K, Hansen, JA, Farahani, ZA, et al. [18F]Fluorodeoxyglucose (FDG)-positron emission tomography (PET)/computed tomography (CT) in suspected recurrent breast cancer: a prospective comparative study of dual-time-point FDG-PET/CT, contrast-enhanced CT, and bone scintigraphy. J Clin Oncol. (2016) 34:1889–97. doi: 10.1200/JCO.2015.63.5185
40. Evangelista, L, Cervino, AR, Ghiotto, C, al-Nahhas, A, Rubello, D, and Muzzio, PC. Tumor marker-guided pet in breast cancer patients – a recipe for a perfect wedding: a systematic literature review and meta-analysis. Clin Nucl Med. (2012) 37:467–74. doi: 10.1097/RLU.0b013e31824850b0
41. Cardoso, F, Harbeck, N, Fallowfield, L, Kyriakides, S, and Senkus, EESMO Guidelines Working Group. Locally recurrent or metastatic breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. (2012) 23:vii11–9. doi: 10.1093/annonc/mds232
42. Lin, CY, Lin, CL, and Kao, CH. Staging/restaging performance of F18-fluorodeoxyglucose positron emission tomography/magnetic resonance imaging in breast cancer: a review and meta-analysis. Eur J Radiol. (2018) 107:158–65. doi: 10.1016/j.ejrad.2018.09.003
43. Incoronato, M, Grimaldi, AM, Cavaliere, C, Inglese, M, Mirabelli, P, Monti, S, et al. Relationship between functional imaging and immunohistochemical markers and prediction of breast cancer subtype: a PET/MRI study. Eur J Nucl Med Mol Imaging. (2018) 45:1680–93. doi: 10.1007/s00259-018-4010-7
44. Kaanders, J, Bussink, J, Aarntzen, E, Braam, P, Rütten, H, van der Maazen, R, et al. [18F]FDG-PET-based personalized radiotherapy dose prescription. Semin Radiat Oncol. (2023) 33:287–97. doi: 10.1016/j.semradonc.2023.03.006
45. Baid, U, Talbar, S, Rane, S, Gupta, S, Thakur, MH, Moiyadi, A, et al. A novel approach for fully automatic intra-tumor segmentation with 3D U-net architecture for gliomas. Front Comput Neurosci. (2020) 14:10. doi: 10.3389/fncom.2020.00010
46. Zhang, C, Liang, Z, Liu, W, Zeng, X, and Mo, Y. Comparison of whole-body 18F-FDG PET/CT and PET/MRI for distant metastases in patients with malignant tumors: a meta-analysis. BMC Cancer. (2023) 23:37. doi: 10.1186/s12885-022-10493-8
47. Shen, F, Liu, Q, Wang, Y, Chen, C, and Ma, H. Comparison of [18F] FDG PET/CT and [18F]FDG PET/MRI in the detection of distant metastases in breast cancer: a meta-analysis. Clin Breast Cancer. (2025) 25:e113–e123.e4. doi: 10.1016/j.clbc.2024.09.015
48. Xiao, Y, Wang, L, Jiang, X, She, W, He, L, and Hu, G. Diagnostic efficacy of 18F-FDG-PET or PET/CT in breast cancer with suspected recurrence: a systematic review and meta-analysis. Nucl Med Commun. (2016) 37:1180–8. doi: 10.1097/MNM.0000000000000573
49. Ruan, D, and Sun, L. Diagnostic performance of PET/MRI in breast cancer: a systematic review and Bayesian bivariate meta-analysis. Clin Breast Cancer. (2023) 23:108–24. doi: 10.1016/j.clbc.2022.11.010
50. Naghavi-Behzad, M, Gerke, O, Kodahl, AR, Vogsen, M, Asmussen, JT, Weber, W, et al. Cost-effectiveness of 2-[(18)F]FDG-PET/CT versus CE-CT for response monitoring in patients with metastatic breast cancer: a register-based comparative study. Sci Rep. (2023) 13:16315. doi: 10.1038/s41598-023-43446-7
Keywords: breast cancer, [18F]FDG PET/CT, [18F]FDG PET/MRI, recurrence, meta-analysis
Citation: Liu S, Xie Y, Ding W, Ma X, Li Z, Zhang L, Zhang Y and Ai C (2025) Comparing the diagnostic efficacy of [18F]FDG PET/CT and [18F]FDG PET/MRI in breast cancer recurrence: a systematic review and meta-analysis. Front. Med. 12:1602415. doi: 10.3389/fmed.2025.1602415
Edited by:
Miguel Angel Morcillo, Centro de Investigaciones Energéticas, Medioambientales y Tecnológicas, SpainReviewed by:
Carmelo Caldarella, Fondazione Policlinico Universitario A. Gemelli IRCCS, ItalySusovan Jana, National Institute of Mental Health (NIH), United States
Copyright © 2025 Liu, Xie, Ding, Ma, Li, Zhang, Zhang and Ai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lei Zhang, emhhbmdsZWk4MzExMTlAMTYzLmNvbQ==; Ya Zhang, c2ludnllQDE2My5jb20=; Conghui Ai, YWlqaWEyMzExOWFpQDE2My5jb20=
†These authors have contributed equally to this work
 Shiqing Liu
Shiqing Liu Yu Xie1†
Yu Xie1† Xiaopeng Ma
Xiaopeng Ma Ya Zhang
Ya Zhang Conghui Ai
Conghui Ai