- 1Emergency Department of West China Hospital, Sichuan University, Chengdu, China
- 2Department of Emergency Medicine, West China Hospital, West China School of Nursing, Sichuan University, Chengdu, China
Purpose: To develop and validate a rapid-assessment scoring system for predicting in-hospital mortality in heat stroke (HS) patients, thereby facilitating early identification and intervention for critical cases.
Approach: We conducted a retrospective cohort analysis of HS patients admitted to emergency department (ED) of 13 hospitals in southwest of China between July 1, 2022 and December 31, 2024. Clinical parameters including demographic data, initial vital signs, and major organ function biomarkers were systematically collected. Patients were further divided into a training cohort and a validation cohort at a 7:3 ratio. The primary endpoint was all-cause in-hospital mortality. Through rigorous variable selection using Least Absolute Shrinkage and Selection Operator (LASSO) regression followed by multivariable logistic regression modeling, we developed a prognostic nomogram. Model performance was assessed via receiver operating characteristic (ROC) curve analysis, decision curve analysis (DCA), and clinical impact curve (CIC) evaluation, with comparative benchmarking against established scoring systems [Sequential Organ Failure Assessment (SOFA) and Acute Physiology and Chronic Health Evaluation II (APACHE II)].
Findings: A total of 307 patients were included in the study. 114 experienced in-hospital mortality, while 193 survived. Non-survivors exhibited significantly altered baseline values across multiple physiological domains: reduced Glasgow Coma Scale (GCS), impaired oxygenation index (OI), elevated fibrin degradation products (FDP), D-dimer, activated partial thromboplastin time (APTT), and serum creatinine (Cr) (all p < 0.0001). Through LASSO regression followed by multivariate logistic regression analysis, 27 initially significant variables were refined to four independent prognostic indicators: Cr, GCS, OI, and FDP. These predictors were subsequently integrated into a multivariate prognostic nomogram demonstrating discriminative capacity for mortality risk stratification in both training (AUC 0.811, 95% CI 0.751–0.871) and validation cohorts (AUC 0.766, 95% CI 0.706–0.826). DCA revealed superior net benefit across clinically relevant probability thresholds. The AUC of the nomogram in the entire cohort (0.794) was significantly superior to the SOFA score (0.703, DeLong’s test, p = 0.0008) and comparable to the APACHE II score (0.765, DeLong’s test, p = 0.3581).
Conclusion: We developed and validated a prognostic tool utilizing routinely available parameters in ED to predict in-hospital mortality in HS patients. This clinically implementable model demonstrates comparable accuracy to established intensive care scoring systems while offering distinct advantages in rapid bedside application, potentially enabling time-critical therapeutic decisions in emergency settings.
1 Introduction
Heat stroke (HS) is a life-threatening clinical emergency characterized by environmental heat-induced thermoregulatory failure, rapid core temperature elevation (>40°C), neurological dysfunction, and concurrent multi-organ dysfunction syndrome (MODS) (1). The escalating frequency of extreme heat events, exacerbated by anthropogenic climate change, has precipitated a concerning surge in heat-related morbidity, positioning HS as a critical public health challenge of the human society (2). Etiological classification distinguishes between classical heat stroke (CHS) and exertional heat stroke (EHS), reflecting distinct pathogenic mechanisms and population susceptibilities (3). CHS manifests as passive thermoregulatory collapse in vulnerable demographics during environmental heat extremes, whereas EHS emerges from metabolic overdrive during strenuous exertion in otherwise healthy individuals (4).
HS induces rapid multi-organ catastrophe, with pathognomonic progression to MODS within hours of onset through coordinated assault on cerebral, cardiovascular, pulmonary, hepatobiliary, renal, and gastrointestinal systems (5–7). Contemporary pathophysiological models propose a dual-pathway mechanism: Direct thermal cytotoxicity from supra-physiological core temperatures and systemic inflammatory cascade involving heat stress-induced endotoxemia, systemic inflammatory response syndrome (SIRS) (8). Experimental models demonstrate HS-induced endothelial barrier disintegration, characterized by ultrastructural damage to vascular endothelia in cardiopulmonary, hepatic, and splanchnic circulations, precipitating consumptive coagulopathy and sterile inflammation (9–13).
There have been some advances in the pathogenesis of HS. However, current therapeutic strategies for HS are still predominantly anchored in generic intensive care scoring systems, notably the Sequential Organ Failure Assessment (SOFA) (14) and Acute Physiology and Chronic Health Evaluation II (APACHEII) (15). These established frameworks exhibit critical limitations when applied to HS populations: their inability to distill disease-specific prognostic indicators from comprehensive admission datasets, while their multi-parameter acquisition protocols introduce operational complexities ill-suited to emergency workflows. SOFA and APACHE-II require complex calculations and 12–24 variables causing delays impractical for ED triage. Their non-specificity to HS pathophysiology further limits prognostic accuracy. Moreover, the absence of validated HS-specific stratification tools precludes precise quantification of baseline multi-organ injury burden. The lack of validated HS-specific stratification tools precludes precise quantification of multi-organ injury burden at baseline. Wang et al. (16) conducted a retrospective analysis of 70 HS cases, identifying procalcitonin (PCT), platelet count, and D-dimer levels as potential biomarkers for HS stratification. In a cohort study involving 151 HS patients, Chen et al. (17) established significant correlations between standard base excess (SBE) values and clinical outcomes. Concurrently, Tang et al. (18) proposed troponin I levels at ICU admission as a prognostic indicator through their analysis of 67 critical HS cases. The above studies have proved that some characteristic data can be used to predict the prognosis of patients in HS. Nevertheless, prognostic assessment tools for ED triage of HS patients remain critically underdeveloped, with current literature constrained by single-center designs and insufficient validation of rapid-assessment parameters - a critical gap impeding early identification of high-risk patients and timely intervention escalation.
The aim of this study is to identify the risk factors associated with mortality in patients with HS and to develop a clinically implementable predictive model that integrates cost-effective, readily obtainable parameters for real-time mortality risk stratification and provide emergency clinicians with a rapid decision-making tool.
2 Methods
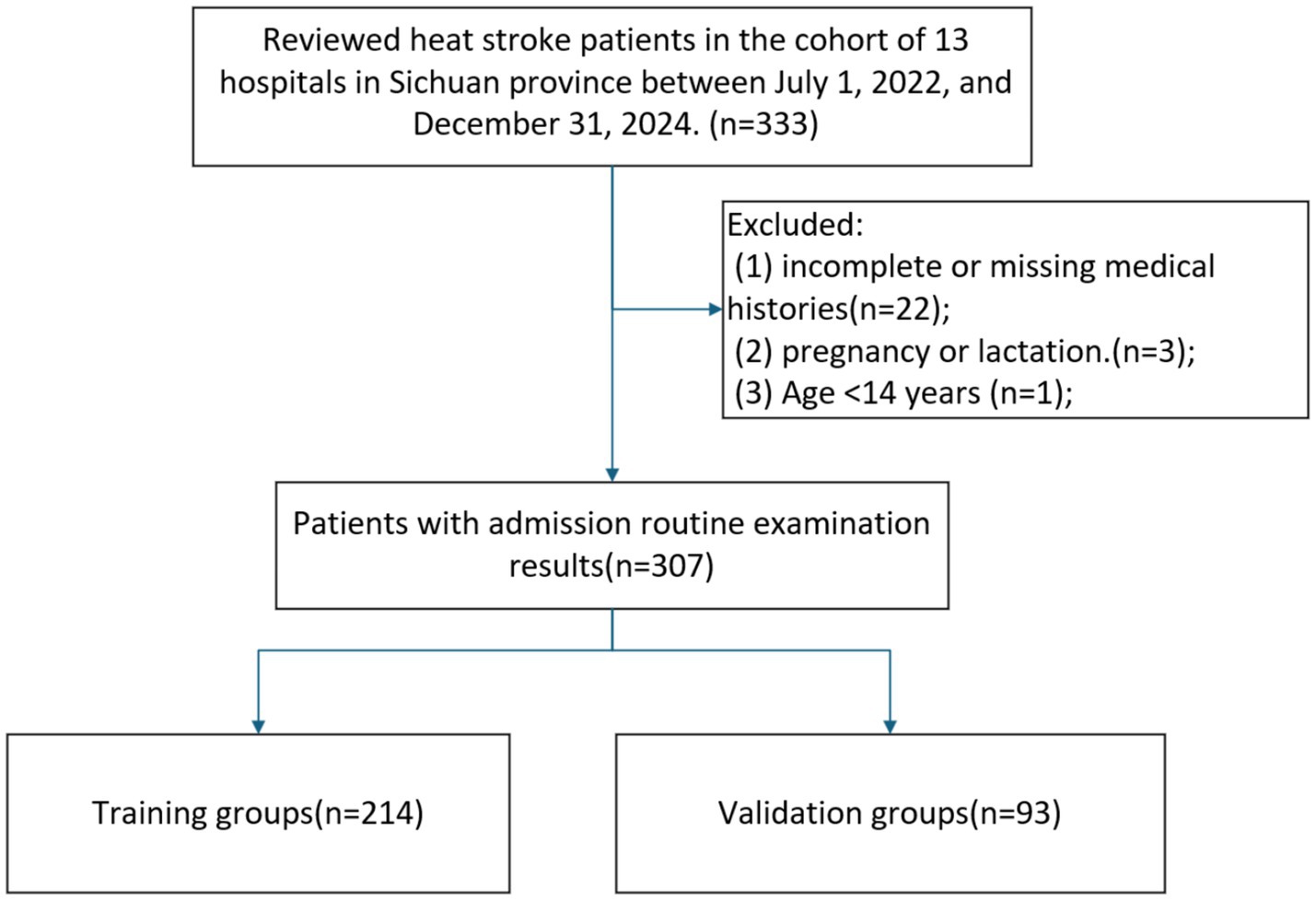
This retrospective observational study analyzed the clinical data of patients diagnosed with HS who were admitted to the ED of 13 hospitals in southwest China between July 1, 2022, and December 31, 2024. The study focused on both classic and exertional forms of HS, defined by the triad of hyperthermia, neurologic abnormalities, and recent exposure to hot weather or physical exertion (4). Patients were excluded if they met any of the following criteria: (1) Patients younger than 14 years; (2) Patients lack of medical histories and blood test; (3) Patients coexisting with other irreversible diseases affecting mortality.
For each patient, a detailed clinical profile was collected. This included medical histories, time from symptom onset to presentation (time-to-ED), physiological parameters and laboratory test results on the first day arriving at ED. Additionally, Glasgow Coma Scale (GCS), SOFA, and APACHE II scores were calculated using clinical and laboratory data. All data were retrieved from hospital electronic medical records. The primary outcome of the study was the survival status of patients at hospital discharge.
The experimental protocol was approved by the Biomedical Ethics Review Committee of West China Hospital, Sichuan University (No. 2022–1,478) in accordance with the Declaration of Helsinki. Informed consent was waived by the Biomedical Ethics Review Committee of West China Hospital, Sichuan University (No. 2022–1,478) due to the retrospective nature of this research.
2.1 Statistical analysis
All statistical analyses were performed using R software (version 4.3.1; R Foundation for Statistical Computing). Missing data handling was implemented through multiple imputation techniques utilizing the “mice” package (version 3.16.0), generating 50 complete datasets through chained equations to preserve statistical power and mitigate bias. The imputation process incorporated predictive mean matching with proper consideration of variable distributional characteristics. Final estimates were obtained using Rubin’s rules for pooling results, thereby accounting for between-imputation variance. Among all covariates, missing data proportions ranged from 5.21 to 11.40%.
The dataset underwent systematic partitioning into training and validation subsets at a 7:3 ratio, with this stratification process being executed through the implementation of a stratified resampling technique to preserve distributional parity of survival outcomes across both cohorts. Continuous variables were characterized using appropriate measures of central tendency and dispersion: normally distributed parameters were summarized as mean ± standard deviation (SD) and compared via Student’s t-test with Welch’s correction for unequal variances, while nonparametric variables were expressed as median interquartile range (IQR) [M(IQR)], and analyzed using Mann–Whitney U tests with exact permutation implementation. Categorical variables were presented as frequency counts with percentages (n, %) and assessed through Pearson’s χ2 test or Fisher’s exact test as appropriate based on expected cell frequencies.
We performed Least Absolute Shrinkage and Selection Operator (LASSO) regression with 10-fold cross-validation using the “glmnet” package, applying the one standard error (1-SE) criterion for optimal lambda selection. The selected predictors were subsequently incorporated into multivariate logistic regression models through the forced entry method utilizing the “rms” package. Results were expressed as odds ratios (ORs) with corresponding 95% confidence intervals (CIs). Model performance was compared against established prognostic scoring systems including the Sequential Organ Failure Assessment (SOFA) and Acute Physiology and Chronic Health Evaluation II (APACHE II). Discrimination capacity was evaluated via receiver operating characteristic (ROC) curve analysis using the “pROC “package, with the area under the curve (AUC) quantifying predictive accuracy.
The optimal prediction model was transformed into a clinical decision tool using nomogram construction via the “rms” package. Nomogram points reflect weighted contributions based on multivariate coefficients. Calibration accuracy was assessed through: (1) calibration-in-the-large (intercept test), (2) calibration slopes via restricted cubic splines (3 knots), and (3) graphical calibration plots with 45° reference lines. Internal validation was performed on a 30% holdout sample (stratified by outcome) using standard ROC analysis. All statistical tests were two-tailed with α = 0.05 significance threshold. The nomogram was assessed using Calibration curves, decision curve analysis (DCA), and clinical impact curves (CIC) using the “rmda “package for both the training and validation cohorts.
3 Results
3.1 Study population and univariate analysis
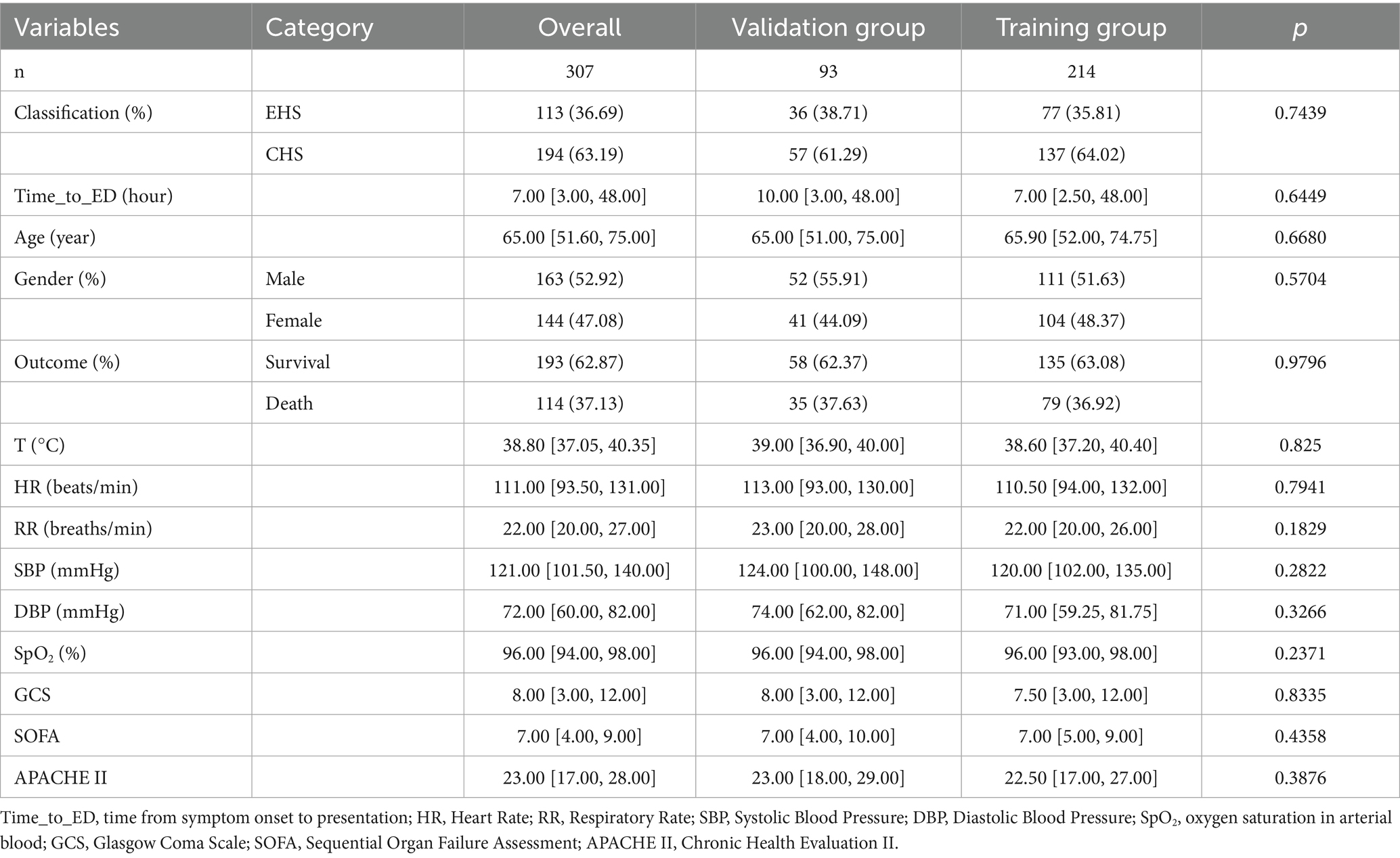
The study cohort comprised 307 HS patients meeting inclusion criteria, detailed information was shown in Figure 1. The median age of the patients was 65 years (IQR: 52–75). Among the included patients, 144 (46.91%) were female, while the majority (53.09%) were male. 114 patients (37.13%) experienced in-hospital mortality, while 193 survived. 214 patients were assigned to the training cohort and 93 to the validation cohort with a proportion of 7:3. No statistically significant differences were observed in baseline characteristics between the training and validation groups, Table 1.
In training group, non-survivor patients were older and have lower GCS scores [5.00(3.00,8.00) vs. 9.00(5.00,13.50), p < 0.0001] compared with survivor group at admission. Comparative analysis revealed significant coagulation profile disparities between survival cohorts, with non-survivors demonstrating elevated fibrin degradation products [FDP: 13.50 (9.95, 37.85) vs. 10.10 (4.72, 11.60); p < 0.0001] and D-dimer levels [7.22 (2.72, 17.73) vs. 3.88 (1.31, 6.01); p < 0.0001]. Non-survivors exhibited pronounced coagulopathic derangements characterized by prolonged activated partial thromboplastin time [APTT: 28.50 (26.60, 37.05) s vs. 27.10 (24.50, 28.95) s; p = 0.0001] and impaired oxygenation capacity [OI: 284.40 (188.86, 288.60) vs. 289.25 (288.60, 315.50); p < 0.0001]. Concurrent renal dysfunction was evidenced by elevated creatinine levels [Cr: 142.30 (102.00, 220.70) vs. 91.00 (72.00, 126.50); p < 0.0001], suggesting multiorgan failure as a terminal pathway in fatal HS cases. Besides, there was a significant difference between the non-survivor and survivors groups in heart rate, core temperature, lymphocyte count, basophil count, Cystatin C, Uric acid, BUN, direct bilirubin, aspartate aminotransferase, myoglobin, creatine kinase, lactate dehydrogenase, serum lactate, ALB, LDL, Ca, BE, PT, TT and so on (all p < 0.05; Table 1). Variables demonstrating statistically significant differences (p < 0.05) between groups in training cohort were included in subsequent data analysis detailed baseline characteristics of the patients can be found in Table 2.
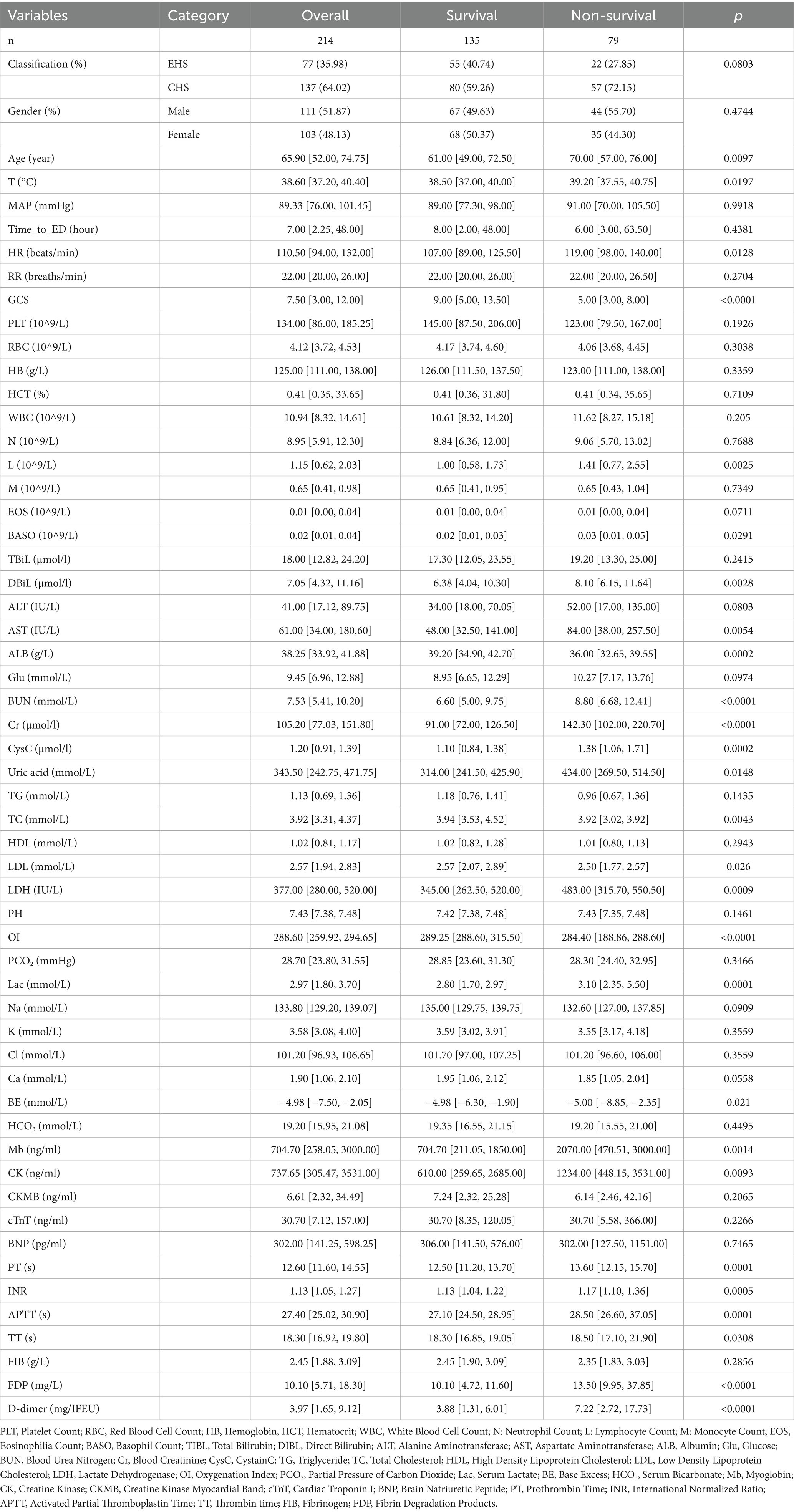
Table 2. Characteristics of the HS training cohort stratified by in-hospital mortality outcome, including additional biomarkers from univariate screening.
3.2 Nomogram variable screening
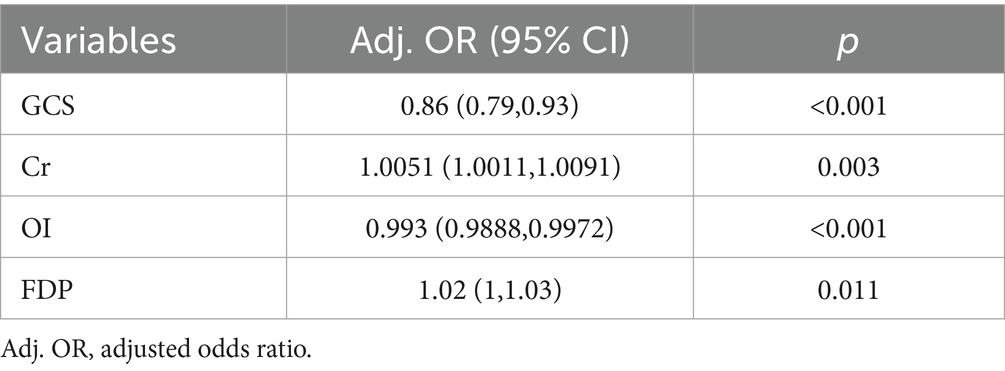
Following LASSO regression analysis, five variables (Cr, GCS, OI, cystatin C, and FDP) exhibiting non-zero coefficients were selected for model optimization based on clinical interpretability and parsimony principles. Although D-dimer and APTT showed univariate significance, LASSO regression excluded them due to high collinearity with FDP (reflecting shared coagulopathy pathways). FDP was retained for its superior specificity in diagnosing disseminated intravascular coagulation (19)—a critical HS complication—and its direct quantification of fibrinolysis, aligning with pathophysiological priority. These variables were subsequently incorporated into the final predictive model trained on the training cohort (Figure 2). After multivariable logistic regression analysis, GCS (OR: 0.8600, 95% CI: 0.7900–0.9300), Cr (1.0051, 1.0011–1.0091), OI (0.993, 0.9888–0.9972), and FDP (1.0200, 1.0000–1.0300) were identified as independent predictors of HS (Table 3).
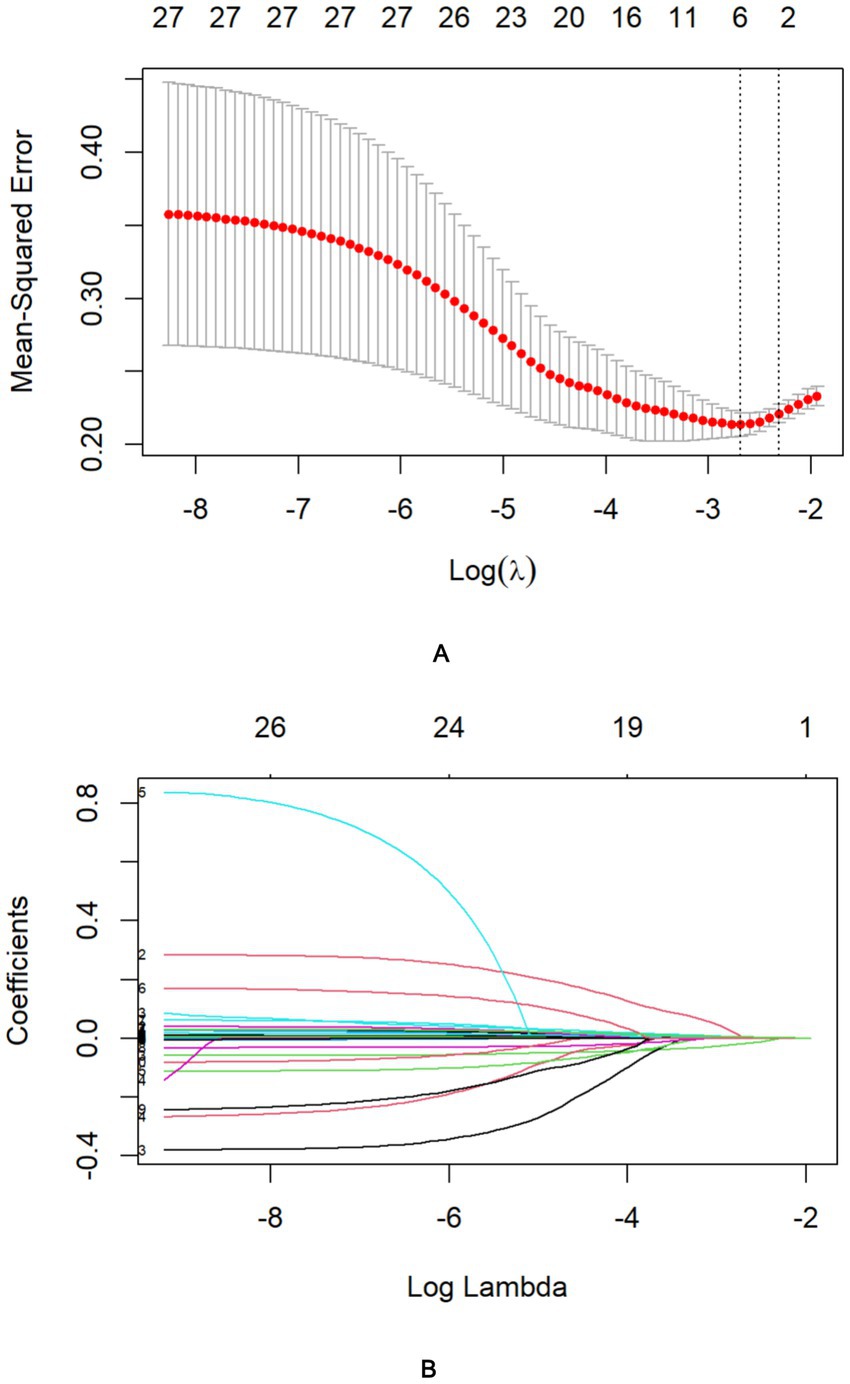
Figure 2. Identification of risk factors for in-hospital mortality in HS patients using LASSO regression with admission indices. (A) The best penalty coefficient l for Lasso regression is ascertained through 10-fold cross-validation and the one standard error (1SE) rule. (B) The LASSO regression coefficient plot for clinical features.
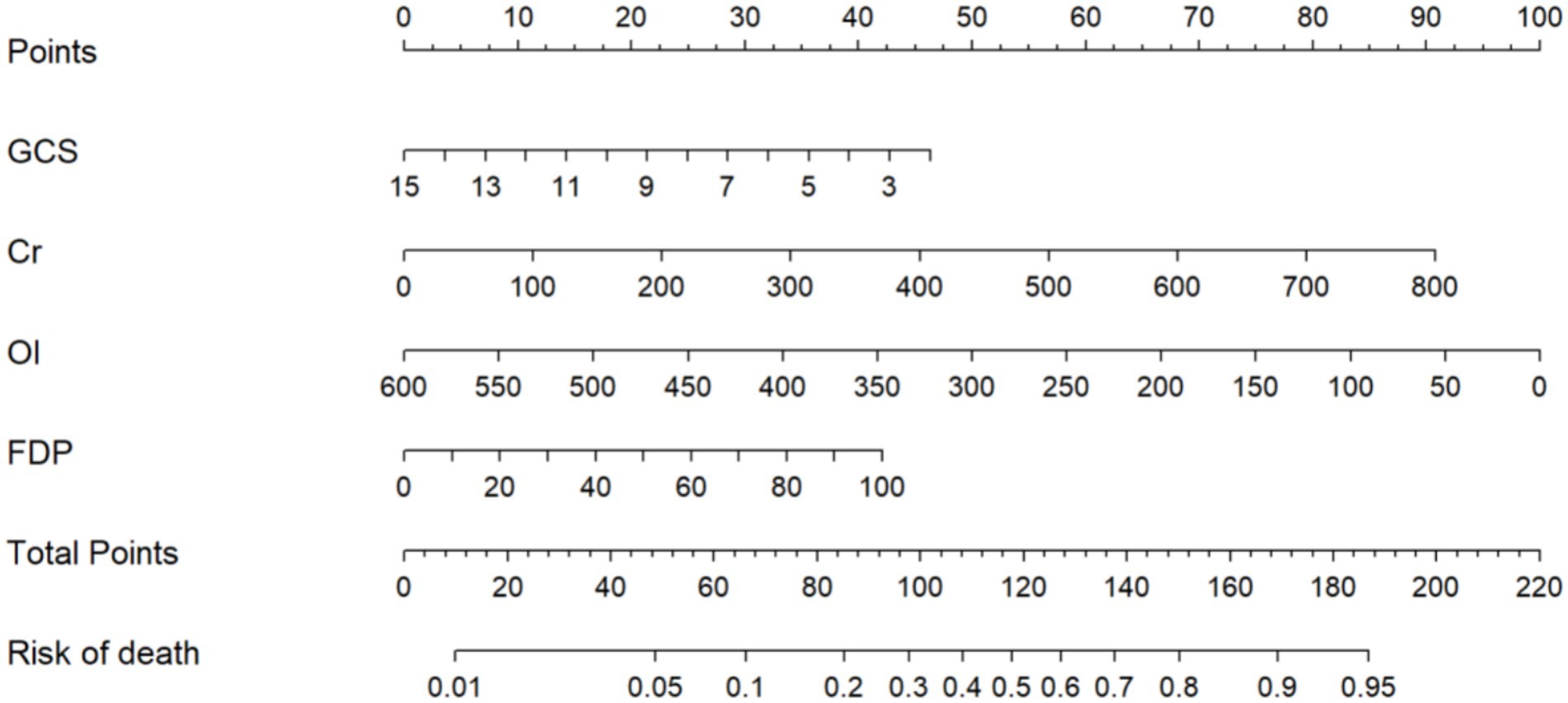
Consequently, these four prognostic determinants were integrated into a multivariate prediction algorithm, and a clinically oriented nomogram was subsequently developed to quantify individualized mortality probabilities through nonlinear transformations of independent risk predictors (Figure 3). Total scores (range: 0–220) are subsequently projected onto a mortality probability continuum at the nomogram base, enabling direct conversion of multidimensional clinical data into survival estimates. For clinical implementation, practitioners sequentially map each biomarker measurement to its corresponding point contribution via perpendicular axes intersection, then sum all component points to derive aggregate mortality risk. Clinically relevant risk strata: low (<20% mortality; <86 points), moderate (20–60%; 86–128 points), and high (>60%; >128 points)—enabling rapid decision pathways. Example calculation: Cr = 100 μmol/L (22 points), GCS = 7 (60 points), OI = 500 mmHg (36 points), FDP = 20 mg/L (18 points). Total = 136 points → Predicted mortality = 68% (high risk).
The final model achieved an AUC of 0.811 in training groups (accuracy 0.778, sensitivity 0.734, 95% CI 0.751–0.871, Figure 4A) and 0.766 in validation groups (accuracy 0.724, sensitivity 0.800, 95% CI 0.706–0.826, Figure 4B). The calibration curve is shown in Figures 4C,D suggests high consistency, demonstrating that the model’s predicted probabilities are close to the observed actual probabilities [calibration-in-the-large (Intercept = 0.09, p = 0.728), spline-based calibration slope (0.50; 95%CI:0.19–0.82)].
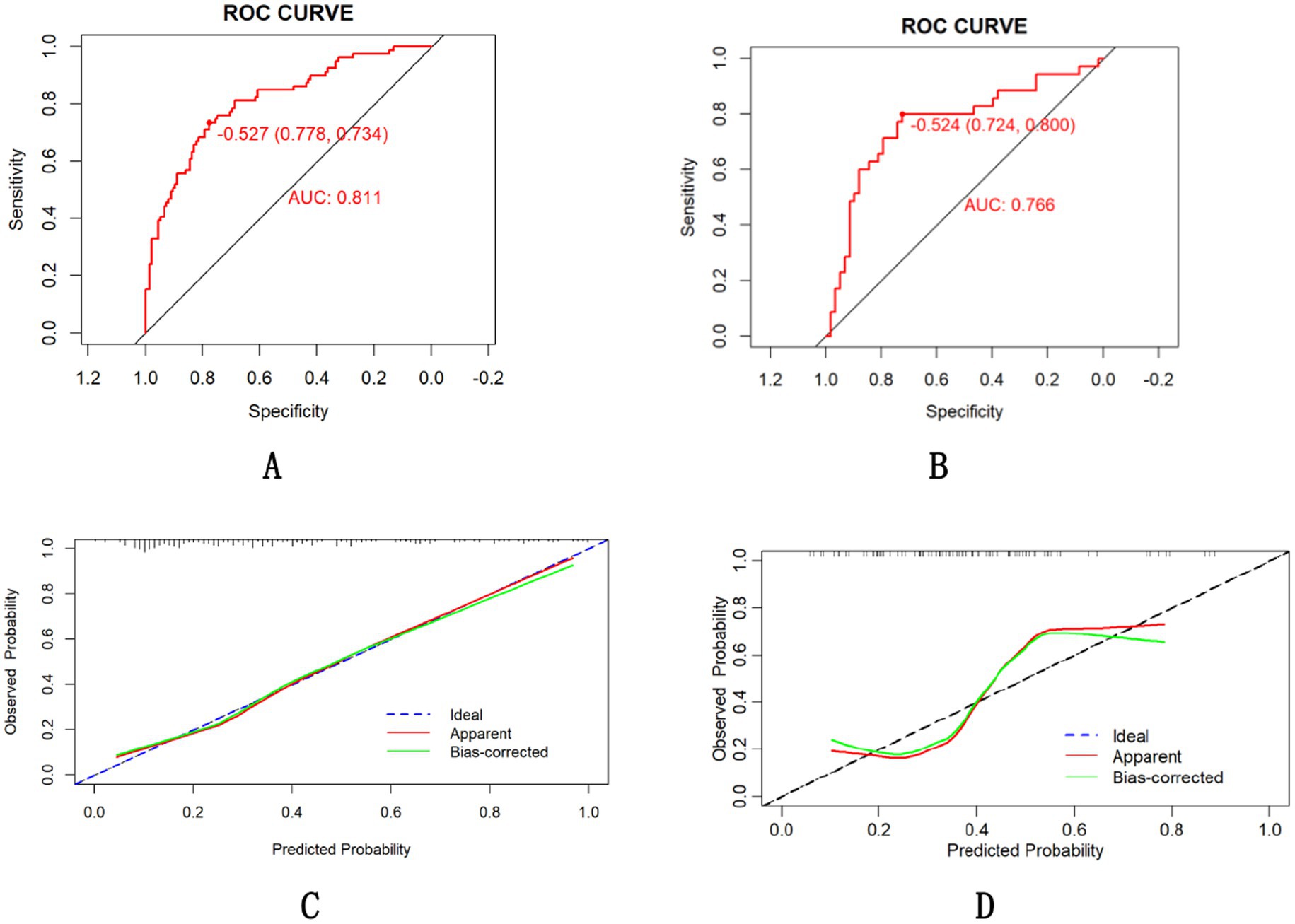
Figure 4. ROC curve of the nomogram for the training (A) and validation (B) cohorts and calibration curve in the training groups (C) and validation groups (D).
3.3 Comparison between predict model and established clinical scoring systems
Comparative analysis of the model’s predictive efficacy against established clinical scoring systems (SOFA and APACHE II) revealed statistically superior performance in mortality risk stratification. In all populations included in the study, our model achieved an AUC of 0.794, outperforming SOFA (0.703, DeLong’s test, p = 0.0008; 95% CI for AUC difference: 0.038–0.144) and non-inferior efficacy compared to APACHE II (0.765, DeLong’s test, p = 0.3581; 95% CI for AUC difference: −0.03-0.091) in all patients (Figure 5A). The tool’s robustness across HS subtypes supports broad clinical utility (Figures 5B,C).
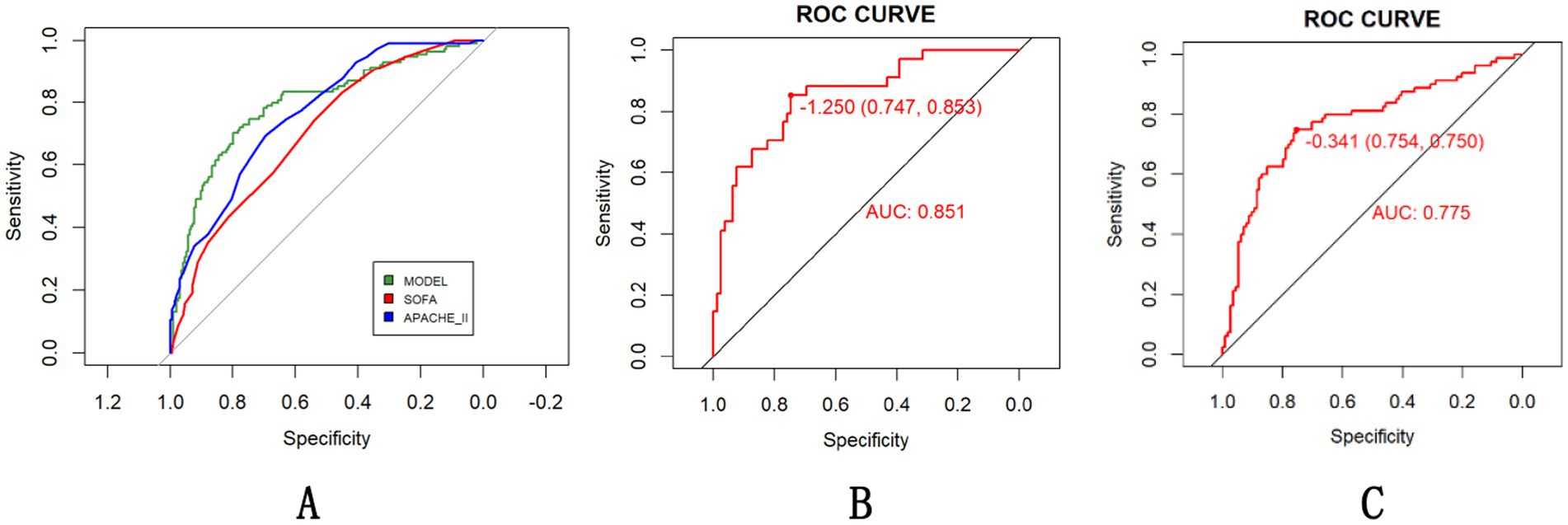
Figure 5. Evaluation of the HS prediction model compared with existing scoring systems (A) with subgroup-specific performance [(B) EHS, (C) CHS] in all population.
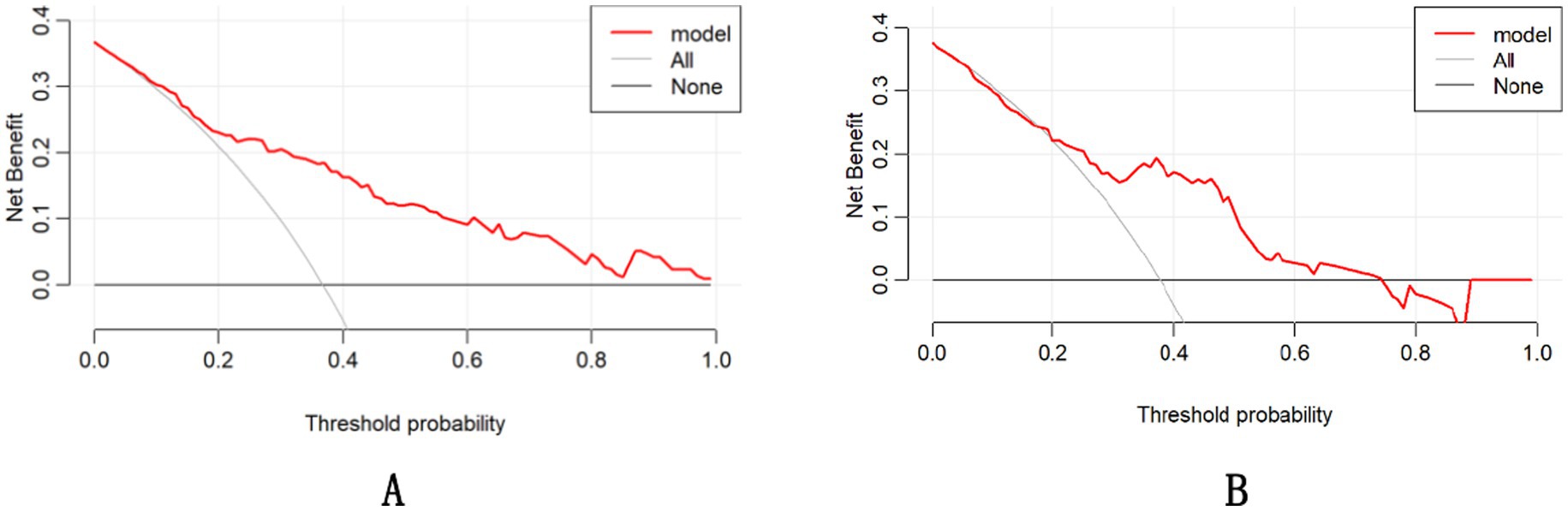
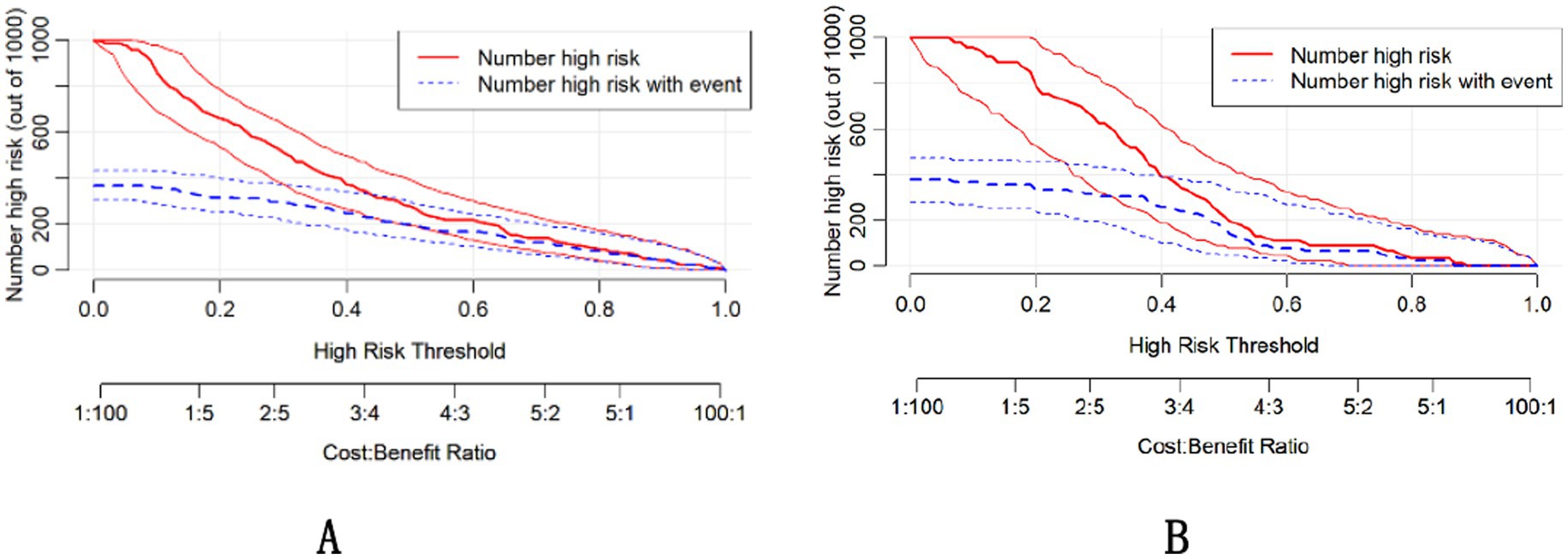
The DCA demonstrated that the nomogram had a superior overall net benefit across a wide range of practical threshold probabilities (Figures 6A,B). In addition, we plotted clinical impact curves to predict improved probability stratification for a population size of 1,000. The predicted probability coincided with the actual probability in the training and validation cohorts (Figures 7A,B).
4 Discussion
Early prognostic stratification in HS constitutes a critical clinical imperative, given its fulminant progression to multiorgan dysfunction and substantial in-hospital mortality (37% in our cohort). While conventional ICU scoring systems demonstrate epidemiological validity, they remain pathophysiologically agnostic to HS-specific mechanisms – particularly endothelial activation and thermal cytotoxicity cascades. Core temperatures at ED arrival were typically below classical diagnostic thresholds (>40°C), likely reflecting widespread pre-hospital cooling. While this limits temperature’s predictive utility in our model, it highlights the critical need for standardized pre-hospital documentation to enhance future prognostic accuracy. This investigation advances a predictive model integrating admission demographics, physiological parameters (GCS, OI), and biomarker profiles (Cr, FDP) to evaluate hospitalization risks. The resultant nomogram addresses current limitations through two critical innovations: Enhanced biological specificity via biomarkers capturing neuronal thermosensitivity (GCS), renal tubular injury (Cr), hypoxia severity (OI), and disseminated coagulopathy (FDP); Operational practicality through dimensionality reduction from 27 variables to four clinically actionable metrics. Risk stratification can be completed within 30 min using standard ED resources: immediate GCS assessment; arterial blood gas analysis for oxygenation index (OI); and routine laboratory processing of creatinine (Cr) and fibrin degradation products (FDP). Clinicians manually calculate nomogram points without specialized software during initial evaluation (Figure 8).
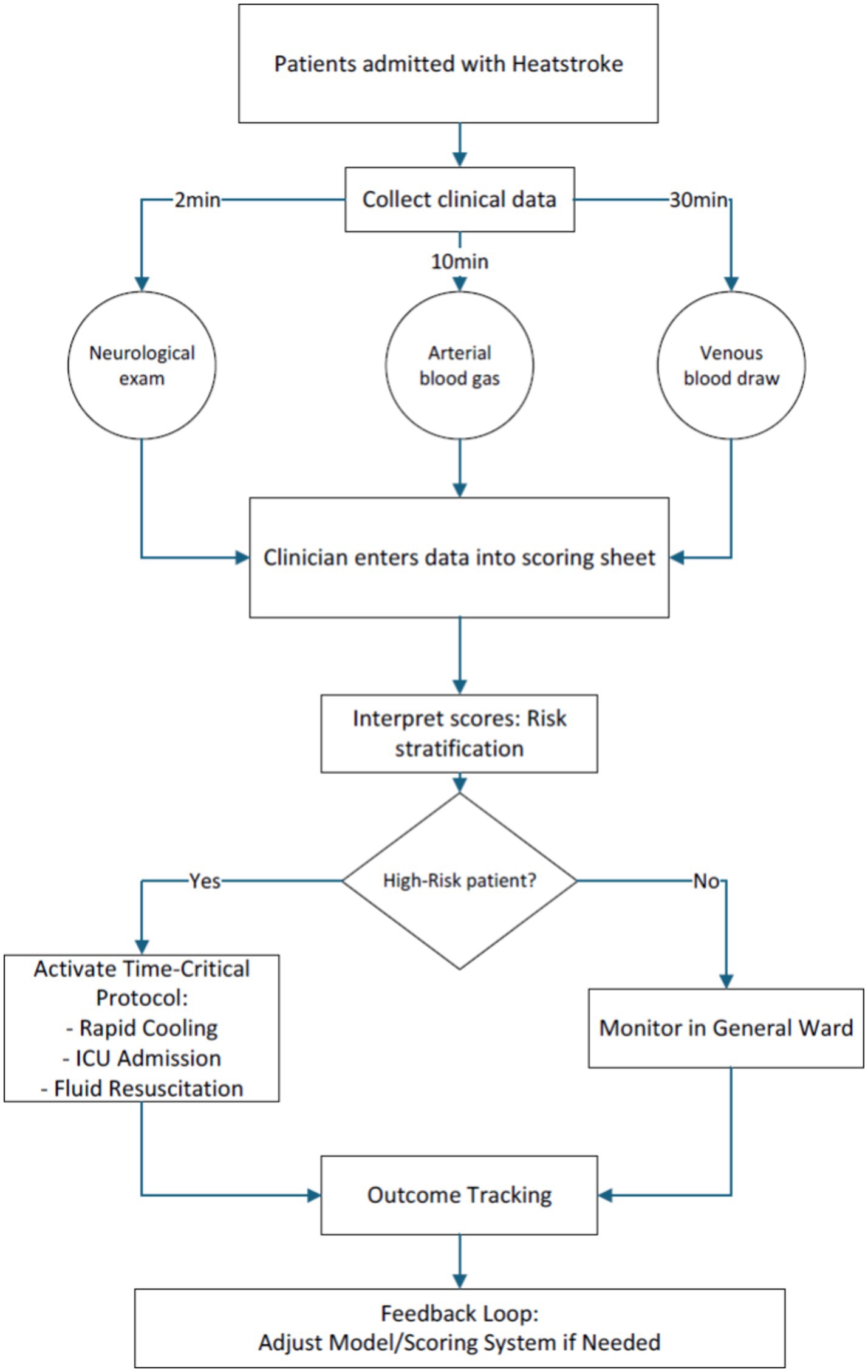
Figure 8. Standardized time-critical data collection protocol for heatstroke patients in emergency settings.
Furthermore, the developed nomogram demonstrated superior predictive accuracy compared to SOFA scores and non-inferior performance relative to APACHE II, while requiring only admission GCS assessments and routine blood biomarkers (Cr, FDP). This streamlined protocol enabled mortality risk stratification within 30 min of ED arrival - a critical temporal advantage for time-sensitive HS management. Our analysis further identifies key prognostic markers directly informing early resuscitation protocols, establishing a dual-action tool for both rapid triage and targeted intervention in HS. Unlike Wei et al.’s longitudinal model (15) and Wang et al.’s ICU-centric tool (20), our nomogram bridges critical gaps in early HS risk stratification. The 4-parameter framework balances discriminative capacity (AUC > 0.76) with operational feasibility—requiring only: Bedside GCS assessment, Arterial blood gas (OI), Routine labs (Cr, FDP). This enables mortality risk quantification within 30 min, fulfilling unmet needs for rapid ED triage during heat crises.
Our study found that the in-hospital survival outcome of HS patients was significantly correlated with GCS score. Due to the brain’s sensitivity to extremely high temperatures, central nervous system (CNS) injury is considered one of the most early and severe complications of HS. Further, neurological sequelae likely contribute significantly to post hospital mortality (1, 21). In Shi L et al.’s study (22), HS patients with neurological symptoms have a higher risk of ICU mortality (23). In the study by Yang, CNS injury caused by HS was observed that had a significant impact on the prognosis and neurological sequelae of patients, and low GCS score within 24 h of admission may be an independent risk factor for neurological sequelae. HS-induced encephalopathy emerges as an early pathological event preceding multi-organ dysfunction (22), with neurocognitive manifestations constituting the hallmark presentation of HS (2). Thus pathophysiological primacy necessitates emergency evaluation using bedside cognitive assessments enabling rapid severity stratification crucial for judgment of severe HS.
Our study also found that the Cr and OI of HS patients at admission may be related to the prognosis of patients in hospital. HS induces renal compromise through multifaceted pathophysiological mechanisms: not only via dehydration-induced hypoperfusion and reduced renal blood flow, but also through direct thermal cytotoxicity and immune-mediated inflammatory responses that provoke tubular injury (24). These synergistic mechanisms collectively drive serum creatinine alterations, potentially culminating in irreversible nephron damage and adverse patient outcomes. Clinical epidemiology reveals a 35–60% incidence of acute kidney injury (AKI) among HS patients, underscoring the critical need for early biomarkers differentiating transient functional impairment from structural renal damage (25, 26). A retrospective cohort study of 187 EHS cases in Southern China (2008–2019) identified AKI incidence of 44%, with 90-day mortality rates significantly elevated in AKI patients compared to non-AKI counterparts (27% vs. 1%, p < 0.001) (27). The impact of elevated serum creatinine on the prognosis of HS patients is considerable, as it increases mortality, especially in those requiring dialysis (28). Besides, impairment in OI may indicate respiratory dysfunction or pulmonary edema in HS patients and exerts substantial influence on in-hospital mortality outcomes. A multinational retrospective analysis encompassing >200,000 ED patients identified OI as a robust predictor of 24-h mortality (29). Notably, OI ≤ 300 mmHg stands as the gold-standard diagnostic criterion for acute lung injury (ALI) (30). Clinical epidemiology reveals ALI prevalence reaching approximately 23, and 75% of patients with ALI eventually die (22). Our study revealed a markedly impaired OI incidence of 74% among HS patients, further corroborating the critical prevalence of respiratory compromise in this population. Historically, few investigations have incorporated arterial blood gas analysis into routine diagnostic protocols for HS, potentially obscuring the recognition of ALI risk in these patients. We advocate for systematic implementation of early arterial blood gas analysis, particularly in HS patients presenting with dyspnea, to comprehensively evaluate systemic physiological status and facilitate timely intervention.
Emerging evidence identifies coagulation dysfunction as a core feature of HS. Consensus guidelines from the Thrombosis and Hemostasis have established that coagulation activation precedes endothelial injury, thrombosis, and hemorrhage, ultimately leading to death (31). FDP, a widely utilized fibrin-related biomarker, serve dual roles of diagnostic and prognostic: both as a diagnostic biomarker for disseminated intravascular coagulation (DIC) identification (19) and a predictive biomarker for venous thromboembolism (VTE) risk stratification. In addition, impaired coagulation function and endothelial injury are also strongly associated with increased risks of cardiovascular morbidity and mortality (32). This pathophysiological primacy mandates emergency coagulation panels within the golden hour of ED presentation, enabling early detection of consumptive coagulopathy critical for guiding mortality risk stratification (33).
The predictive model demonstrated superior discriminative performance to SOFA score and non-inferior prognostic accuracy compared to the APACHE II system. Besides, it is easy to obtain, requiring only the completion of serum creatinine, oxygenation index, FDP and GCS scoring. Implementation of this tool enables precision resource allocation through mortality risk stratification, particularly vital in mass casualty scenarios requiring rapid triage prioritization. In situations where clinicians lack knowledge about the severity of a patient’s condition, this model can provide accurate and prompt prognosis assessments.
Despite including a larger sample size compared to other studies, the inherent limitations and potential data bias of retrospective research cannot be completely eliminated. The retrospective design and regional focus may limit generalizability to populations with distinct climatic or demographic profiles and the observed calibration variation underscores the need for continuous model recalibration during external validation—a priority for our planned multi-regional study. First, although statistical imputation methods were employed to address missing data patterns, the restricted cohort size fundamentally constrained the statistical power for robust validation of secondary endpoints. While our findings demonstrate an expanded sample size relative to prior research, further validation through larger multicenter cohorts remains imperative before clinical implementation can be recommended. Secondly, the heterogeneous disease severity and divergent clinical presentations within our cohort precluded longitudinal tracking of dynamic clinical trajectories. Future studies should assess long-term outcomes (e.g., renal/neurological sequelae) to evaluate the model’s predictive utility beyond in-hospital mortality. Furthermore, external validation in diverse clinical settings is essential before implementing this tool in routine practice. We will prioritize prospective data collection to enhance both sample diversity and temporal resolution of pathophysiological evolution. Given rising HS incidence under escalating global temperatures, future work will prioritize external validation in multi-regional cohorts (e.g., coastal/arid zones) and develop a point-of-care digital calculator. Furthermore, while the multicentric design enhanced epidemiological generalizability, inherent inter-institutional variability in laboratory protocols and operator-dependent analytical techniques may introduce measurement bias. We will systematically address this through standard operating procedure implementation and centralized biomarker assay harmonization in further studies.
5 Conclusion
This multicenter study developed and validated a pragmatic prognostic nomogram incorporating four routinely available emergency parameters (serum creatinine, GCS, oxygenation index and fibrin degradation products) to predict in-hospital mortality in HS patients. The model demonstrated superior discriminative performance to SOFA and non-inferior prognostic accuracy compared to the APACHE II while maintaining critical advantages in rapid bedside applicability through elimination of complex physiological calculations.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Biomedical Ethics Committee of West China Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
TZ: Writing – original draft. BX: Writing – original draft. GT: Writing – review & editing. TC: Writing – review & editing. HG: Writing – review & editing. PZ: Writing – review & editing. RY: Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by National Natural Science Foundation of China (Grant no. 82472241).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Douma, MJ, Aves, T, Allan, KS, Bendall, JC, Berry, DC, Chang, WT, et al. First aid cooling techniques for heat stroke and exertional hyperthermia: a systematic review and meta-analysis. Resuscitation. (2020) 148:173–90. doi: 10.1016/j.resuscitation.2020.01.007
2. Savioli, G, Zanza, C, Longhitano, Y, Nardone, A, Varesi, A, Ceresa, IF, et al. Heat-related illness in emergency and critical care: recommendations for recognition and management with medico-legal considerations. Biomedicines. (2022) 10:2542. doi: 10.3390/biomedicines10102542
3. Bouchama, A, Abuyassin, B, Lehe, C, Laitano, O, Jay, O, O’Connor, FG, et al. Classic and exertional heatstroke. Nat Rev Dis Primers. (2022) 8:8. doi: 10.1038/s41572-021-00334-6
4. Epstein, Y, and Yanovich, R. Heatstroke. N Engl J Med. (2019) 380:2449–59. doi: 10.1056/NEJMra1810762
5. Al Mahri, S, and Bouchama, A. Heatstroke. Handb Clin Neurol. (2018) 157:531–45. doi: 10.1016/B978-0-444-64074-1.00032-X
6. Zhang, P, Tang, G, Gao, H, Zhang, T, Yang, S, Cheng, T, et al. Coexisting metabolic dysfunction-associated steatotic liver disease exacerbates in-hospital outcomes in patients with heat stroke. Front Med (Lausanne). (2024) 11:1451133. doi: 10.3389/fmed.2024.1451133
7. Gao, HG, Tang, G, Yang, S, Yang, Y, Sheng, J, and Yao, R. Construction and evaluation of a predictive model for liver injury associated with heatstroke: a multicenter retrospective study. Int J Clin Pract. (2024) 2024:6485323. doi: 10.1155/2024/6485323
8. Liu, SY, Song, JC, Mao, HD, Zhao, JB, and Song, Q. Expert consensus on the diagnosis and treatment of heat stroke in China. Mil Med Res. (2020) 7:1. doi: 10.1186/s40779-019-0229-2
9. Iba, T, Helms, J, Levi, M, and Levy, JH. Inflammation, coagulation, and cellular injury in heat-induced shock. Inflamm Res. (2023) 72:463–73. doi: 10.1007/s00011-022-01687-8
10. Chen, HS, Tong, HS, Zhao, Y, Hong, CY, Bin, JP, and Su, L. Differential expression pattern of exosome long non-coding RNAs (lncRNAs) and MicroRNAs (miRNAs) in vascular endothelial cells under heat stroke. Med Sci Monit. (2018) 24:7965–74. doi: 10.12659/MSM.909983
11. Huang, W, Mao, L, Xie, W, Cai, S, Huang, Q, Liu, Y, et al. Impact of UCP2 depletion on heat stroke-induced mitochondrial function in human umbilical vein endothelial cells. Int J Hyperth. (2022) 39:287–96. doi: 10.1080/02656736.2022.2032846
12. Wu, J, Yang, S, Wang, T, Wu, Q, Liao, X, Yao, R, et al. Comparison of immune cell profiles associated with heatstroke, sepsis, or cardiopulmonary bypass: study protocol for an exploratory, case-control study trial. Front Med (Lausanne). (2023) 10:1165786. doi: 10.3389/fmed.2023.1165786
13. Wu, J, Cheng, Z, Yang, S, Wu, Q, Yang, P, Liao, X, et al. Specific immune landscape of heatstroke distinguished from sepsis and aseptic inflammation. Int J Med Sci. (2025) 22:1450–64. doi: 10.7150/ijms.108212
14. Wu, M, Wang, C, Liu, Z, and Liu, Z. Sequential organ failure assessment score for prediction of mortality of patients with rhabdomyolysis following exertional heatstroke: a longitudinal cohort study in southern China. Front Med (Lausanne). (2021) 8:724319. doi: 10.3389/fmed.2021.724319
15. Wei, D, Gu, T, Yi, C, Tang, Y, and Liu, F. A nomogram for predicting patients with severe heatstroke. Shock. (2022) 58:95–102. doi: 10.1097/SHK.0000000000001962
16. Wang, L, Jia, H, Shen, Y, Chu, X, Chen, Z, Ren, Y, et al. Diagnostic significance of combined Calcitoninogen, platelet, and D-dimer assay in severe heatstroke: with clinical data analysis of 70 patients with severe heatstroke. Ther Hypothermia Temp Manag. (2023) 13:29–37. doi: 10.1089/ther.2022.0011
17. Chen, L, Yang, X, Zhao, J, Xu, S, Zhang, Y, and Feng, X. The association of standard base excess upon emergency admission with outcomes in patients with heat stroke. Am J Emerg Med. (2023) 72:7–15. doi: 10.1016/j.ajem.2023.06.048
18. Tang, Y, Yuan, D, Gu, T, Zhang, H, Shen, W, and Liu, F. Clinical significance of early troponin I levels on the prognosis of patients with severe heat stroke. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. (2023) 35:730–5. doi: 10.3760/cma.j.cn121430-20221028-00948
19. Ezaki, M, Wada, H, Ichikawa, Y, Ikeda, N, Shiraki, K, Yamamoto, A, et al. Plasma soluble fibrin is useful for the diagnosis of thrombotic diseases. J Clin Med. (2023) 12:2597. doi: 10.3390/jcm12072597
20. Wang, L, Fu, X, He, M, Shi, L, Wan, D, Wang, Y, et al. Risk factor analysis and nomogram for predicting in-hospital mortality in ICU patients with heat stroke: a national multicenter study. J Multidiscip Healthc. (2023) 16:3189–201. doi: 10.2147/JMDH.S428617
21. Adnan, BH. A systematic review on outcomes of patients with heatstroke and heat exhaustion. Open Access Emerg Med. (2023) 15:343–54. doi: 10.2147/OAEM.S419028
22. Yang, M, Li, Z, Zhao, Y, Zhou, F, Zhang, Y, Gao, J, et al. Outcome and risk factors associated with extent of central nervous system injury due to exertional heat stroke. Medicine (Baltimore). (2017) 96:e8417. doi: 10.1097/MD.0000000000008417
23. Shi, L, Wang, B, Wu, Q, Yang, J, Wang, L, Wan, D, et al. Heatstroke: a multicenter study in southwestern China. Front Public Health. (2024) 12:1349753. doi: 10.3389/fpubh.2024.1349753
24. Goto, H, Kinoshita, M, and Oshima, N. Heatstroke-induced acute kidney injury and the innate immune system. Front Med (Lausanne). (2023) 10:1250457. doi: 10.3389/fmed.2023.1250457
25. Abdelmoety, D, El-Bakri, NK, Almowalld, WO, Turkistani, ZA, Bugis, BH, Baseif, EA, et al. Characteristics of heat illness during hajj: a cross-sectional study. Biomed Res Int. (2018) 20:5629474. doi: 10.1155/2018/5629474
26. Yezli, S, Yassin, Y, Ghallab, S, Abdullah, M, Abuyassin, B, Vishwakarma, R, et al. Classic heat stroke in a desert climate: a systematic review of 2632 cases. J Intern Med. (2023) 294:7–20. doi: 10.1111/joim.13633
27. Wu, M, Wang, C, Liu, Z, Zhong, L, Yu, B, Cheng, B, et al. Clinical characteristics and risk factors associated with acute kidney injury inpatient with exertional heatstroke: an over 10-year intensive care survey. Front Med (Lausanne). (2021) 8:678434. doi: 10.3389/fmed.2021.678434
28. Thongprayoon, C, Qureshi, F, Petnak, T, Cheungpasitporn, W, Chewcharat, A, Cato, LD, et al. Impact of acute kidney injury on outcomes of hospitalizations for heat stroke in the United States. Diseases. (2020) 8:28. doi: 10.3390/diseases8030028
29. Candel, BGJ, de Groot, B, Nissen, SK, Thijssen, WAMH, Lameijer, H, and Kellett, J. The prediction of 24-h mortality by the respiratory rate and oxygenation index compared with National Early Warning Score in emergency department patients: an observational study. Eur J Emerg Med. (2023) 30:110–6. doi: 10.1097/MEJ.0000000000000989
30. Long, ME, Mallampalli, RK, and Horowitz, JC. Pathogenesis of pneumonia and acute lung injury. Clin Sci (Lond). (2022) 136:747–69. doi: 10.1042/CS20210879
31. Iba, T, Levi, M, Thachil, J, Helms, J, Scarlatescu, E, and Levy, JH. Communication from the scientific and standardization Committee of the International Society on thrombosis and Haemostasis on sepsis-induced coagulopathy in the management of sepsis. J Thromb Haemost. (2023) 21:145–53. doi: 10.1016/j.jtha.2022.10.022
32. Iba, T, Connors, JM, Levi, M, and Levy, JH. Heatstroke-induced coagulopathy: biomarkers, mechanistic insights, and patient management. EClinicalMedicine. (2022) 44:101276. doi: 10.1016/j.eclinm.2022.101276
Keywords: heat stroke, in-hospital mortality, emergency department, nomogram, multiorgan damage
Citation: Zhang T, Xiao B, Tang G, Cheng T, Gao H, Zhang P and Yao R (2025) Prognostic nomogram for heat stroke patients based on rapidly accessible clinical indicators. Front. Med. 12:1603374. doi: 10.3389/fmed.2025.1603374
Edited by:
Aruna Sharma, Uppsala University, SwedenReviewed by:
Ma Qiang, Southern Medical University, ChinaShuogui Xu, Second Military Medical University, China
Dengke Teng, Jilin University, China
Copyright © 2025 Zhang, Xiao, Tang, Cheng, Gao, Zhang and Yao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rong Yao, eWFvcm9uZ0B3Y2hzY3UuY24=
†These authors have contributed equally to this work
 Tianshan Zhang
Tianshan Zhang Bojie Xiao2†
Bojie Xiao2† Guo Tang
Guo Tang Tao Cheng
Tao Cheng Hongguang Gao
Hongguang Gao Ping Zhang
Ping Zhang Rong Yao
Rong Yao