- 1Department of Reproductive Medicine, Wuxi Affiliated Hospital of Nanjing University of Chinese Medicine, Wuxi, China
- 2Institute of Traditional Chinese Medicine, Wuxi Affiliated Hospital of Nanjing University of Chinese Medicine, Wuxi, China
- 3School of Medicine, Jiangnan University, Wuxi, China
Objective: To predict potential herbal medicines targeting polycystic ovary syndrome (PCOS)-related infertility using an in silico reverse network pharmacology approach and identify core herbal candidates.
Methods: This computational study began by collecting disease targets for PCOS and infertility from multiple public databases. Common targets were identified, and active compounds associated with these targets were retrieved from the Uniprot and TCMSP databases. These compounds were subsequently filtered using PubChem and SwissADME based on pharmacokinetic properties and mapped to corresponding herbs via TCMSP. Herbal properties (nature, flavor, meridian tropism) were statistically analyzed. A core network of targets-compounds-herbs was constructed using Cytoscape 3.8.0, and a subnetwork was generated from nodes with a Degree > 20. Finally, Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analyses were performed on the targets of the core herbal combination to elucidate potential mechanisms.
Results: A total of 2,500 common targets for PCOS and infertility, 1,545 active compounds, and 488 corresponding herbs were identified. Analysis of herbal properties revealed a predominance of Warm and Pungent medicines, followed by Cold, Bitter, Neutral, and Sweet medicines. A core herbal combination consisting of Ephedra sinica (Mahuang), Magnolia officinalis (Houpo), Bupleurum chinense (Chaihu), Chrysanthemum morifolium (Juhua), Angelica dahurica (Baizhi), and Morus alba (Sangye) was identified through frequency statistics, association rules, and cluster analysis. GO and KEGG enrichment analyses of the core combination’s targets highlighted mechanisms involving oxidative stress, inflammatory responses, and endocrine regulation, including the TNF and PI3K-Akt signaling pathways.
Conclusion: This study successfully employed reverse network pharmacology to predict a core herbal combination for treating PCOS-related infertility. The findings, while requiring experimental validation, offer novel insights for developing therapeutic strategies and provide a foundation for future clinical management.
1 Introduction
PCOS is a complex endocrine and metabolic disorder characterized by reproductive dysfunction and systemic metabolic disturbances, presenting high clinical heterogeneity across a woman’s lifespan from adolescence to menopause (1). Epidemiological studies indicate that PCOS predominantly affects women of reproductive age, with the highest prevalence observed between puberty and 40 years (2). Concurrently, infertility rates have risen globally; for instance, China experienced an increase from 12 to 18% between 2007 and 2020 (3). Notably, PCOS-associated infertility accounts for approximately 70% of anovulatory infertility cases, primarily driven by pathophysiological factors such as hyperandrogenism, insulin resistance, and chronic inflammation (4). Over the past decade, the incidence of PCOS in China has surged by 65%, which has further exacerbated its societal and healthcare burden (5).
Current clinical interventions for PCOS-related infertility are centered on ovulation induction (e.g., clomiphene citrate) and assisted reproductive technologies (ART). However, these approaches face significant limitations, including variable ovarian response rates, increased risks of multiple pregnancies, and substantial financial costs (6). In contrast, traditional Chinese medicine (TCM) demonstrates unique advantages in improving the ovarian microenvironment, regulating menstrual cycles, and enhancing pregnancy outcomes through its multi-target, systemic modulatory effects (7). Despite these benefits, the lack of standardized herbal protocols and insufficient mechanistic clarity has hindered its widespread clinical adoption and integration into mainstream medicine.
Network pharmacology, which integrates systems biology, bioinformatics, and data mining, has emerged as a powerful tool for elucidating the “multi-component, multi-target” mechanisms of herbal therapies (8). Unlike conventional methods that start with known herbs to identify their potential targets, reverse network pharmacology begins with disease-associated targets to trace back to bioactive compounds and their corresponding herbs. This “disease-target-drug” approach aligns well with TCM’s holistic philosophy of treating complex syndromes and offers a robust framework for discovering and optimizing herbal formulas.
Here, we employed a reverse network pharmacology strategy to predict herb-target interactions for PCOS-related infertility, analyze the properties of the identified herbs, and screen for core herbal combinations. This study aims to bridge the gap between traditional TCM theory and modern pharmacology, providing evidence-based, hypothesis-generating insights that could inform future therapeutic strategies for PCOS management.
2 Materials and methods
2.1 Acquisition of common targets for PCOS and infertility
The keyword “Polycystic Ovary Syndrome” was used to search the Genecards database (9) (v5.15),1 OMIM database (10) (Online Mendelian Inheritance in Man),2 PharmGKB database (11) (Pharmacogenomics Knowledgebase),3 DisGeNet database (12) (v7.0),4 and DrugBank database (13) (v5.1.10).5 Similarly, the keyword “Female infertility” was queried in the Genecards, OMIM, PharmGKB, DisGeNet, and TTD databases (14) (Therapeutic Target Database).6 All database searches were conducted in October 2023. After removing duplicate entries from each search, the intersection of PCOS and infertility targets was extracted. A Venn diagram was generated to visualize the overlapping targets.
2.2 Reverse identification of herbal compounds from targets
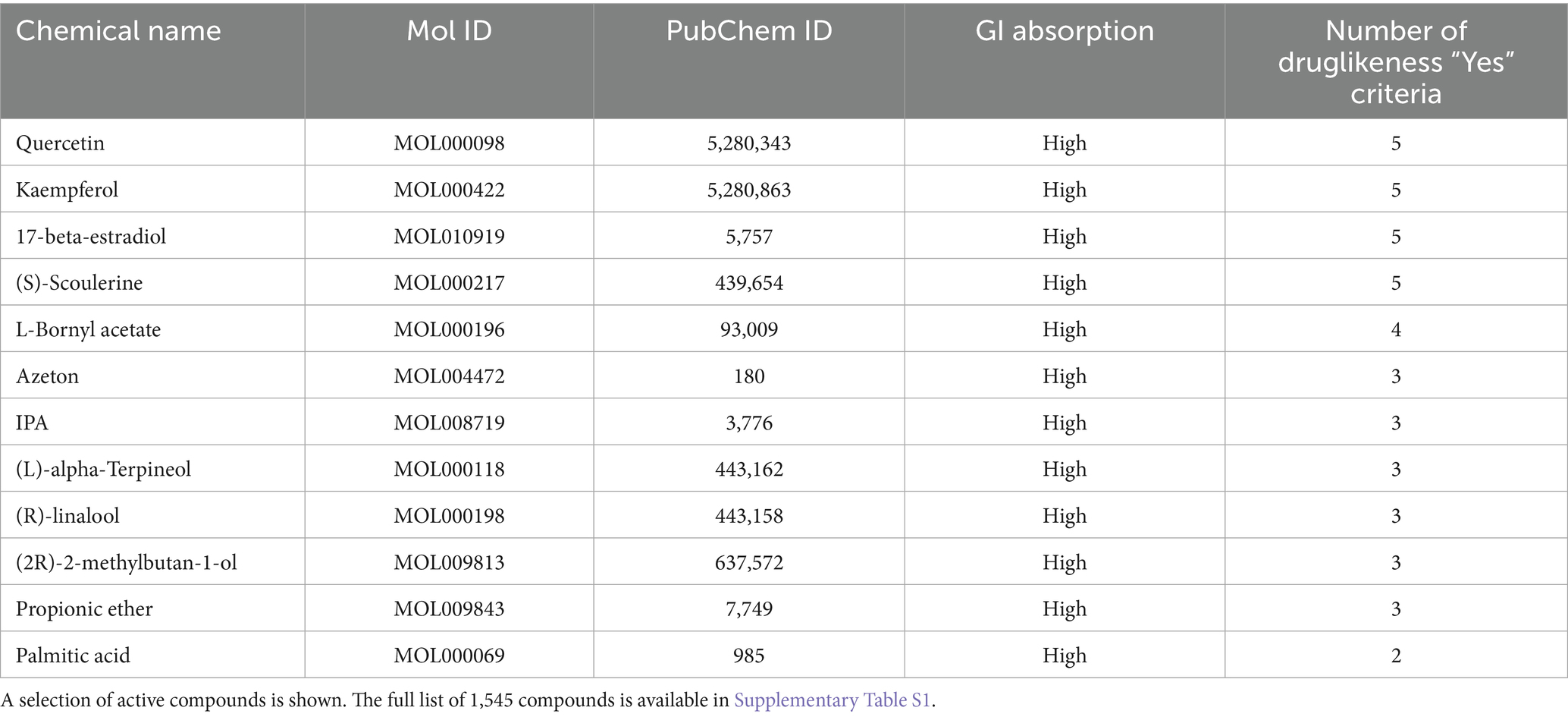
The list of common intersection targets was submitted to the Uniprot database (https://www.uniprot.org/, release 2023_04) to convert gene names into standardized protein names. The TCMSP database (Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform)7 was then used to retrieve all compounds associated with these target proteins. The SMILES (Simplified Molecular Input Line Entry System) identifiers of the retrieved compounds were obtained from the PubChem database.8 Active compounds were screened using the SwissADME web tool9 based on two criteria: “GI absorption = High” and “Druglikeness ≥ 2 Yes.” These widely accepted criteria were chosen to enrich for compounds with favorable oral bioavailability and pharmacokinetic properties, thereby increasing their potential as therapeutic agents.
2.3 Reverse mapping of herbs from active compounds
Herbs corresponding to the screened active compounds were identified using the TCMSP database. A comprehensive target-compound-herb network was constructed and visualized using Cytoscape software (v3.8.0). To identify the most influential components within this network, nodes (targets, compounds, and herbs) with a Degree value > 20 were defined as core components. The Degree > 20 threshold is a commonly used metric in network pharmacology to identify highly connected “hub” nodes that are considered functionally significant within the network. A subnetwork consisting of these core targets, compounds, and herbs was then extracted and visualized.
2.4 Analysis of herbal properties
The frequency, nature (Four Natures), flavor (Five Flavors), meridian tropism, and therapeutic categories of the herbs associated with the active compounds were statistically analyzed using Microsoft Excel. Herbs with a usage frequency > 10 were classified as core candidates for further analysis. Association rule mining was performed on these core herbs using IBM SPSS Modeler 18.0 software, with thresholds set at support > 1 and confidence > 60% to identify significant co-occurrence patterns. Finally, hierarchical cluster analysis of the core herbs was conducted with IBM SPSS Statistics 26.0 to identify distinct medication patterns and herbal clusters.
2.5 Screening of core herbal combinations and enrichment analysis
A core herbal combination was formulated based on a synthesis of the results from the frequency statistics, association rule analysis, and cluster analysis. The targets associated with this core herbal combination were retrieved from the TCMSP database. These targets were then intersected with the previously identified 2,500 common PCOS-infertility targets. To elucidate the underlying biological mechanisms of the core combination, Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analyses were performed on the final set of overlapping targets. The analysis was conducted using the DAVID (Database for Annotation, Visualization and Integrated Discovery) platform, and terms with an adjusted p-value (FDR) < 0.05 were considered statistically significant.
3 Results
3.1 Common targets of PCOS and infertility
From the five databases, we retrieved 5,476 PCOS-related targets and 5,210 infertility-related targets from Genecards; 1,310 PCOS targets and 8 infertility targets from OMIM; 165 PCOS targets and 226 infertility targets from PharmGKB; 988 PCOS targets and 37 infertility targets from DisGeNet; and 32 PCOS targets from DrugBank. The TTD database provided an additional 9 infertility-related targets. After removing all duplicate entries, a total of 6,488 unique PCOS-related targets and 5,291 unique infertility-related targets were retained. The intersection of these two sets identified 2,500 shared targets, which were considered the common therapeutic targets for PCOS-related infertility (Figure 1).
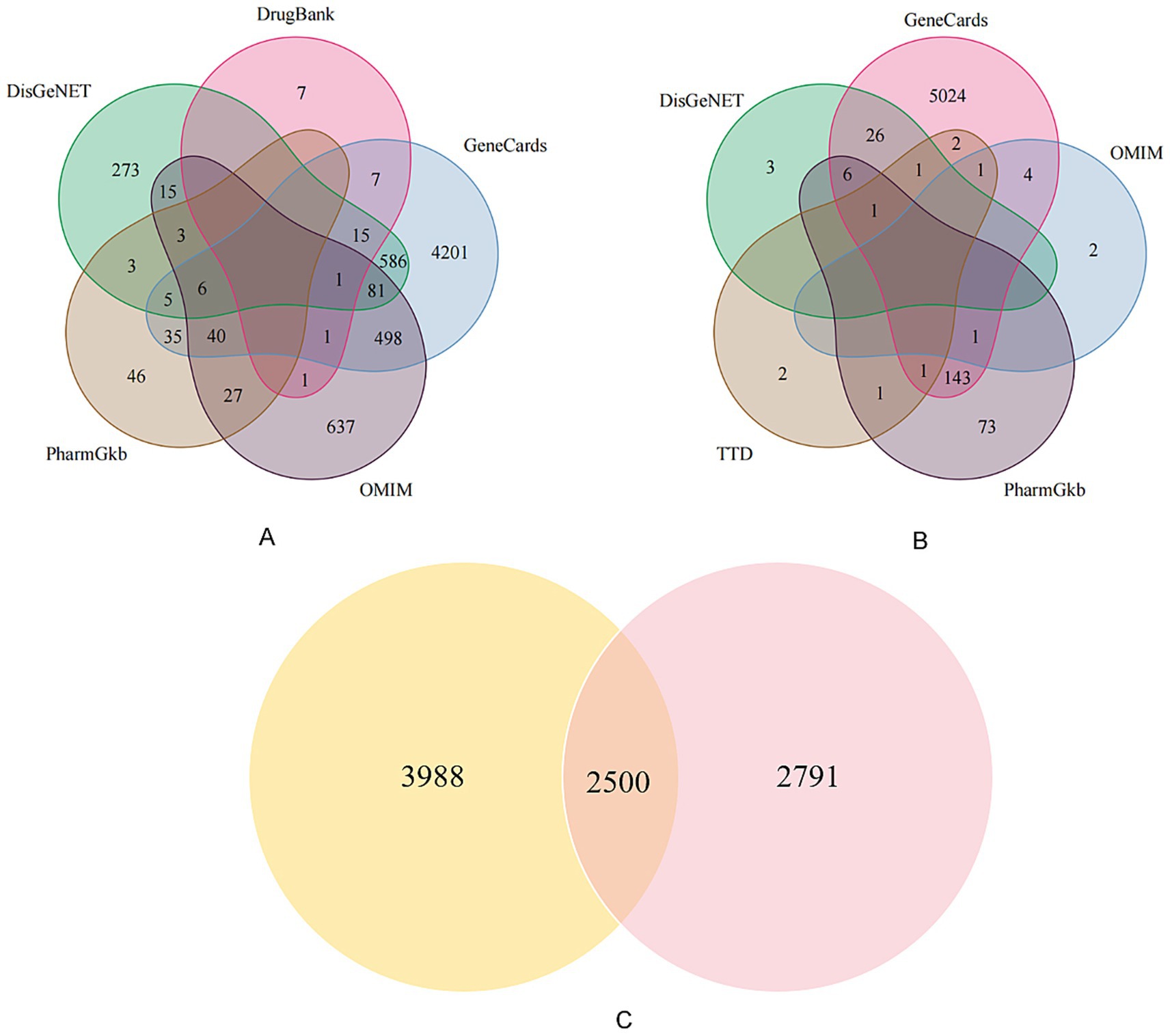
Figure 1. Venn diagrams of disease-related targets. This figure illustrates the process of identifying common targets. (A) Venn diagram showing the distribution of 6,488 unique PCOS-related targets collected from five databases: DrugBank, GeneCards, DisGeNET, PharmGkb, and OMIM. (B) Venn diagram showing the distribution of 5,291 unique infertility-related targets from five databases: GeneCards, OMIM, DisGeNET, TTD, and PharmGkb. (C) Venn diagram showing the 2,500 overlapping targets common to both PCOS and infertility, which formed the basis for subsequent analysis.
3.2 Active compounds associated with PCOS-related infertility
The 2,500 common targets were reverse-mapped through the TCMSP database, resulting in the identification of 25,540 associated compounds. These compounds were then subjected to pharmacokinetic screening using PubChem and SwissADME with the criteria of “GI absorption = High” and “Druglikeness ≥ 2 Yes.” This filtering process yielded 1,545 active compounds predicted to have therapeutic potential for PCOS-related infertility (Table 1).
3.3 Herbal medicines associated with PCOS-related infertility
The 1,545 active compounds were traced back to their herbal sources, corresponding to 488 unique herbs and a total of 180 targets. A preliminary target-compound-herb network was constructed using Cytoscape 3.8.0, which comprised 2,213 nodes and 15,465 edges. An analysis of the network topology revealed the top 10 herbs with the highest number of associated targets: Pueraria lobata (Gegen, 143 targets), Morus alba (Sangye, 136 targets), Oroxylum indicum (Muhudie, 135 targets), Zanthoxylum bungeanum (Huajiao, 126 targets), Carthamus tinctorius (Honghua, 121 targets), Eriobotrya japonica (Pipaye, 120 targets), Ginkgo biloba (Yinxingye, 120 targets), Syzygium aromaticum (Dingxiang, 119 targets), Astragalus membranaceus (Huangqi, 118 targets), and Ephedra sinica (Mahuang, 118 targets). To focus on the most significant interactions, nodes with a Degree > 20 (representing 40 targets, 79 compounds, and 89 herbs) were selected to reconstruct a refined, core target-compound-herb network (Figure 2).
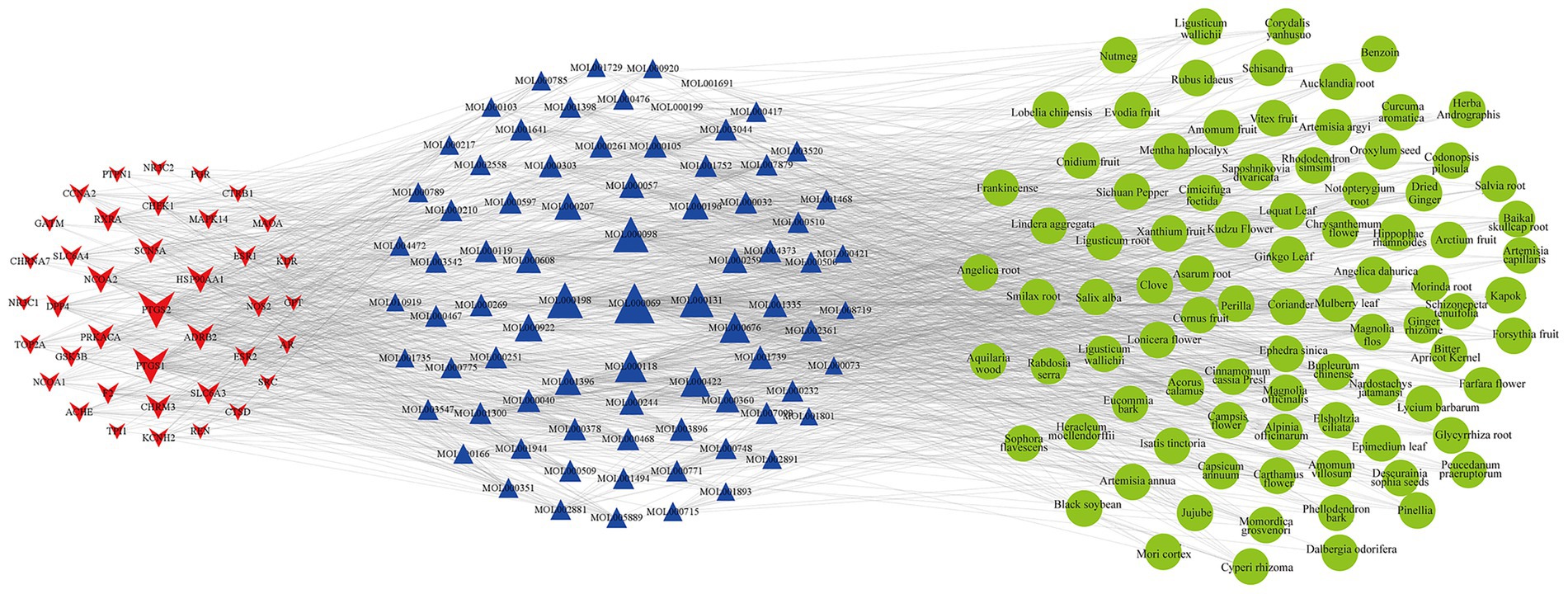
Figure 2. The core target-compound-herb network for PCOS-related infertility. This network illustrates the key interactions among disease targets, active compounds, and herbal medicines. Red star-shaped nodes represent the core PCOS-infertility targets, blue triangle nodes represent the core active compounds, and green circular nodes represent the core herbs. The size of each node is proportional to its Degree value, indicating its connectedness within the network. Larger nodes represent components with a higher number of connections, suggesting greater importance in the therapeutic mechanism.
3.4 Herbal property analysis
3.4.1 Frequency analysis of herbs
Among the 488 herbs identified, 472 were validated against the Chinese Pharmacopoeia (2015) and Chinese Herbal Medicine, while 16 were excluded due to non-standardization. These herbs appeared a total of 6,365 times across the 1,545 compounds. High-frequency herbs (appearing >50 times) included Ephedra sinica (Mahuang, 91), Glycyrrhiza uralensis (Gancao, 71), Cinnamomum cassia (Guizhi, 64), Bupleurum chinense (Chaihu, 63), Perilla frutescens (Zisu, 59), Coriandrum sativum (Yansui, 59), Chrysanthemum morifolium (Juhua, 59), Morus alba (Sangye, 58), Ginkgo biloba (Yinxingye, 57), Eriobotrya japonica (Pipaye, 57), and Asarum heterotropoides (Xixin, 52). A total of 41 herbs exhibited frequencies >30 (Table 2).
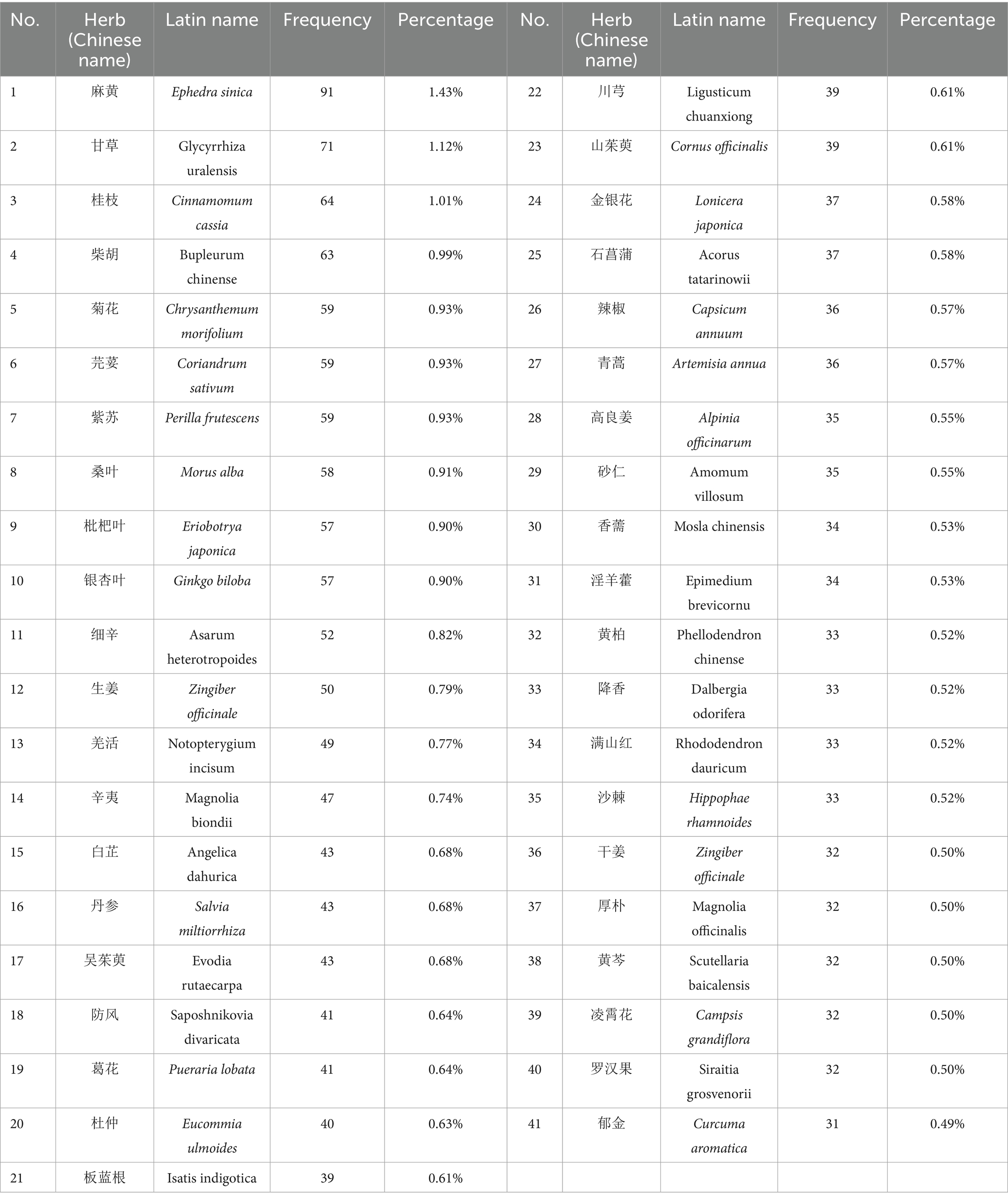
Table 2. Statistical results of 41 Chinese herbal medicines with frequency >30 for PCOS-related infertility.
3.4.2 Four natures, five flavors, and meridian tropism
Four Natures Analysis: The dominant medicinal properties were Warm (2,103 occurrences, 33.04%), Cold (1,325, 20.82%), and Neutral (1,072, 16.84%), followed by Cool, Slightly Cold, Slightly Warm, Hot, Very Hot, and Extremely Cold (Figure 3A).
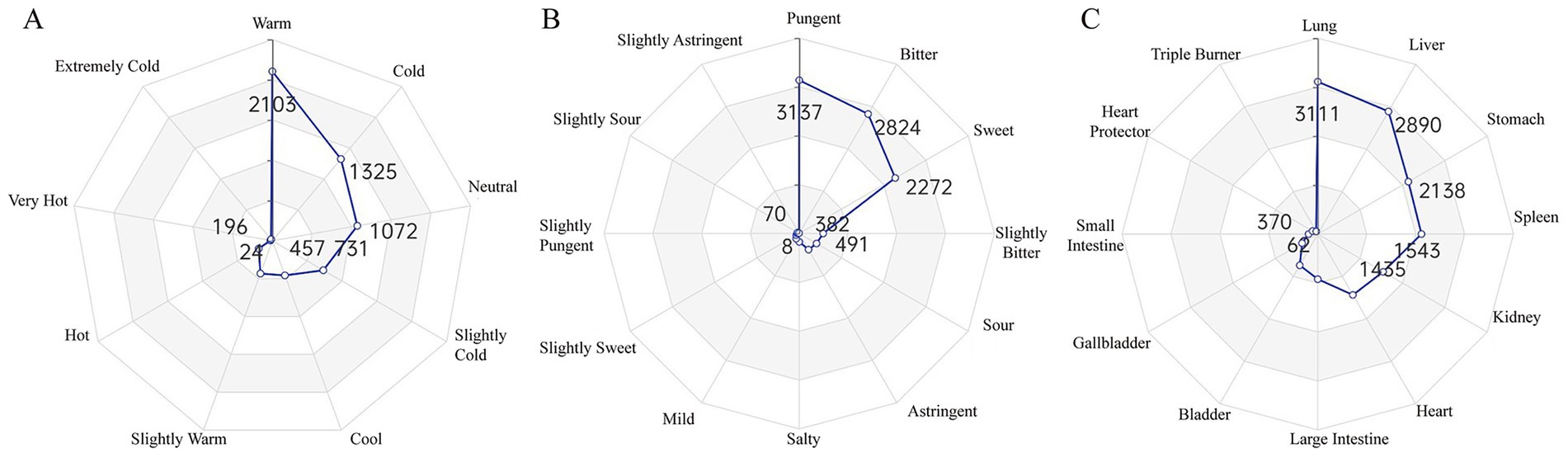
Figure 3. Analysis of herbal properties based on traditional chinese medicine theory. These radar charts quantify the TCM properties of the identified herbs. (A) Distribution of the Four Natures, showing a predominance of Warm and Cold properties. (B) Distribution of the Five Flavors, with Pungent, Bitter, and Sweet being the most common. (C) Distribution of Meridian Tropism, indicating that the herbs primarily act on the Lung, Liver, and Stomach meridians. The numbers on the axes represent the frequency of occurrence for each property.
Five Flavors Analysis: Pungent (3,137, 31.45%), Bitter (2,824, 28.31%), and Sweet (2,272, 22.78%) were the predominant flavors, with minor contributions from Slightly Bitter, Sour, Astringent, Salty, Mild, Slightly Sweet, Slightly Pungent, Slightly Sour, and Slightly Astringent (Figure 3B).
Meridian Tropism Analysis: The most frequently targeted meridians were the Lung meridian (3,111, 19.90%), Liver meridian (2,890, 18.48%), and Stomach meridian (2,138, 13.67%), followed by the Spleen (1,543, 9.87%), Kidney (1,543, 9.87%), and Heart meridians (1,435, 9.18%) (Figure 3C).
3.4.3 Herbal categories
The identified herbs were classified into various therapeutic categories. The most prominent categories included Relieving Exterior Symptoms (15.08%), Clearing Heat (12.36%), and Tonifying Deficiency (5.55%). Subcategories further specified their actions, such as Dispelling Wind-Cold (a subcategory of Relieving Exterior), Clearing Heat and Toxins (a subcategory of Clearing Heat), and Suppressing Cough and Asthma (7.69%; Figure 4).
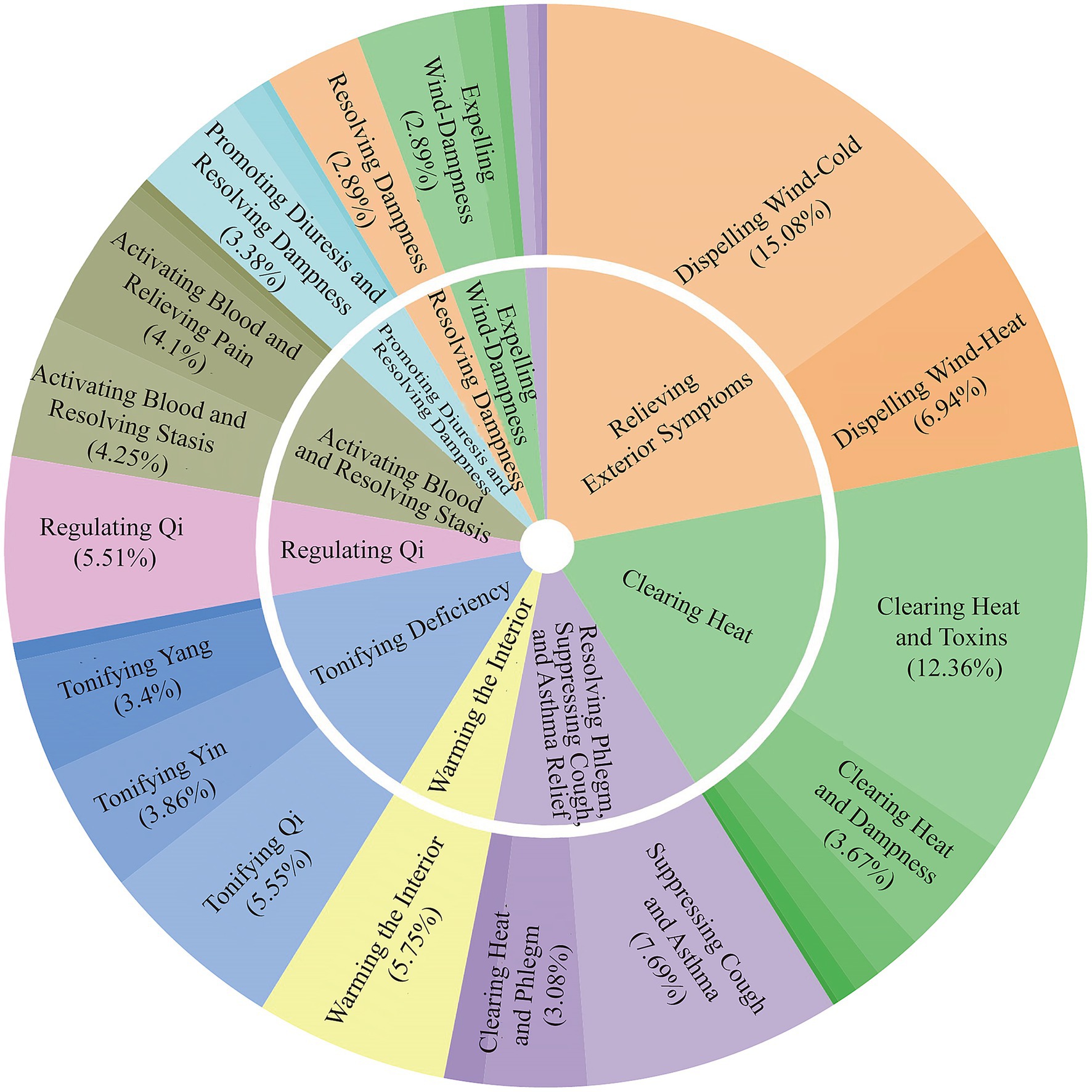
Figure 4. Sunburst chart of herbal therapeutic categories. This chart displays the hierarchical classification of the 488 herbs based on their primary therapeutic functions in TCM. The inner ring shows the main categories, while the outer ring provides more specific subcategories. The size of each segment corresponds to the percentage of herbs in that category, highlighting functions like Relieving Exterior Symptoms and Clearing Heat.
3.4.4 Association rule analysis
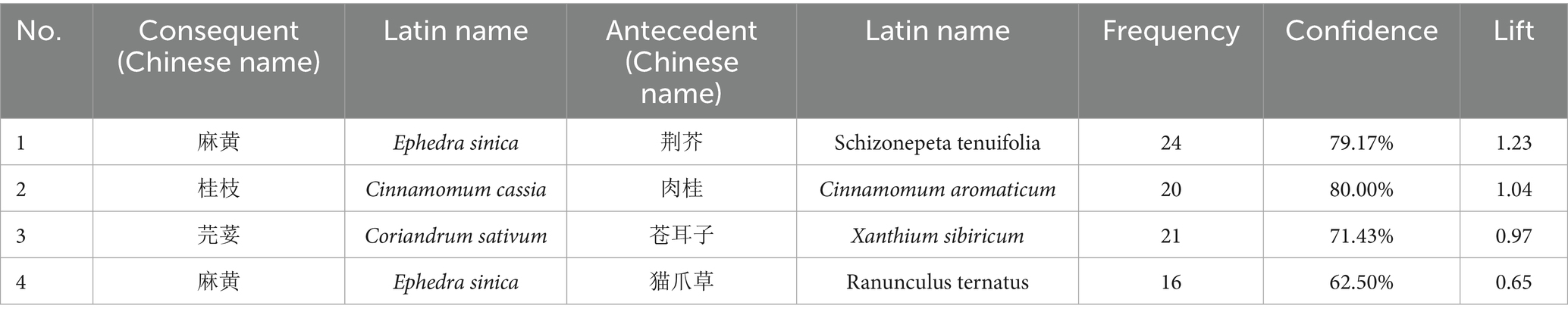
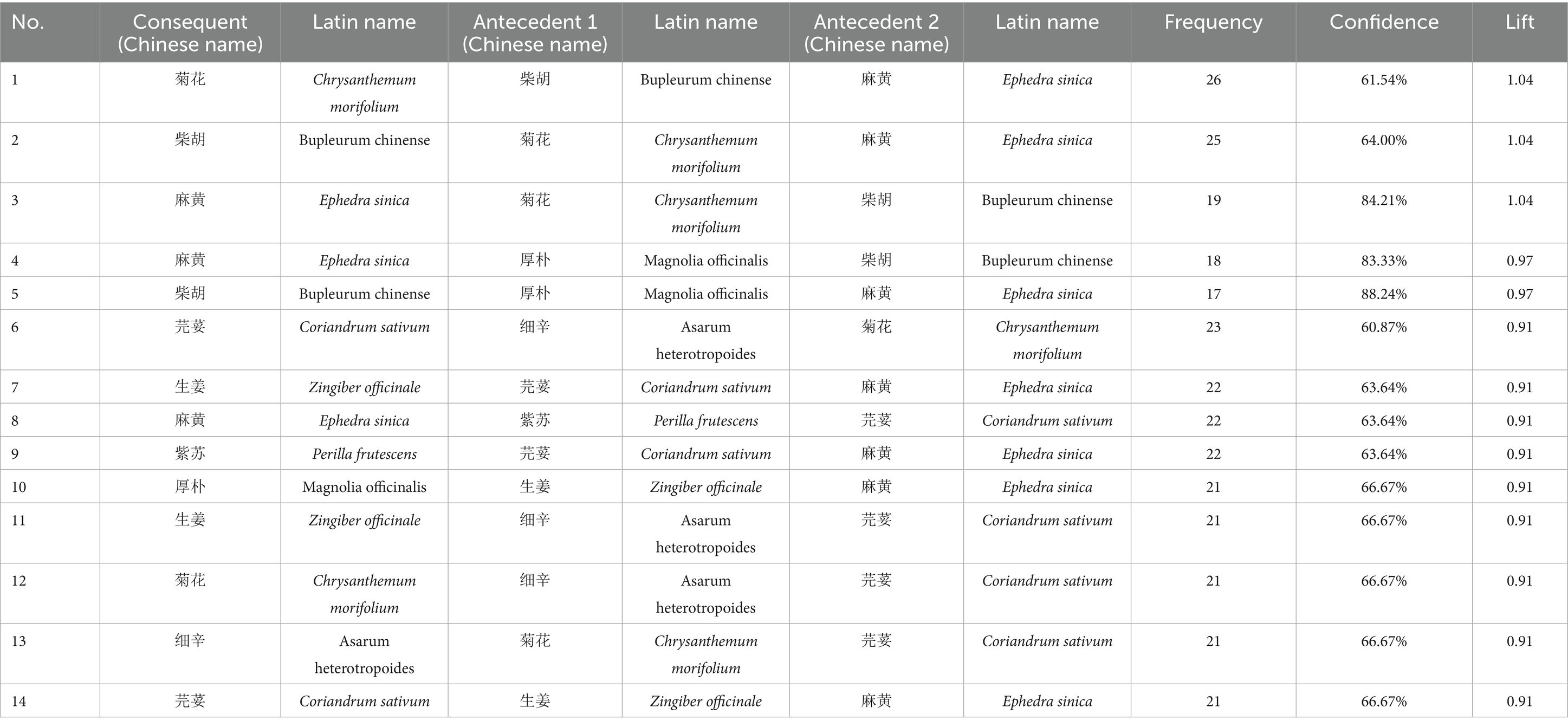
A complex network analysis of 247 herbs (frequency ≥10) was performed using IBM SPSS Modeler 18.0, with thresholds set at “strong link = 19, weak link = 10.” This analysis revealed key herbal associations. For instance, a strong association was found where the presence of Bupleurum chinense (Chaihu) and Ephedra sinica (Mahuang) predicted the co-occurrence of Chrysanthemum morifolium (Juhua) (26 occurrences). The detailed binary and ternary association rules are presented in Tables 3, 4, and a visualization of the network is shown in Figure 5.
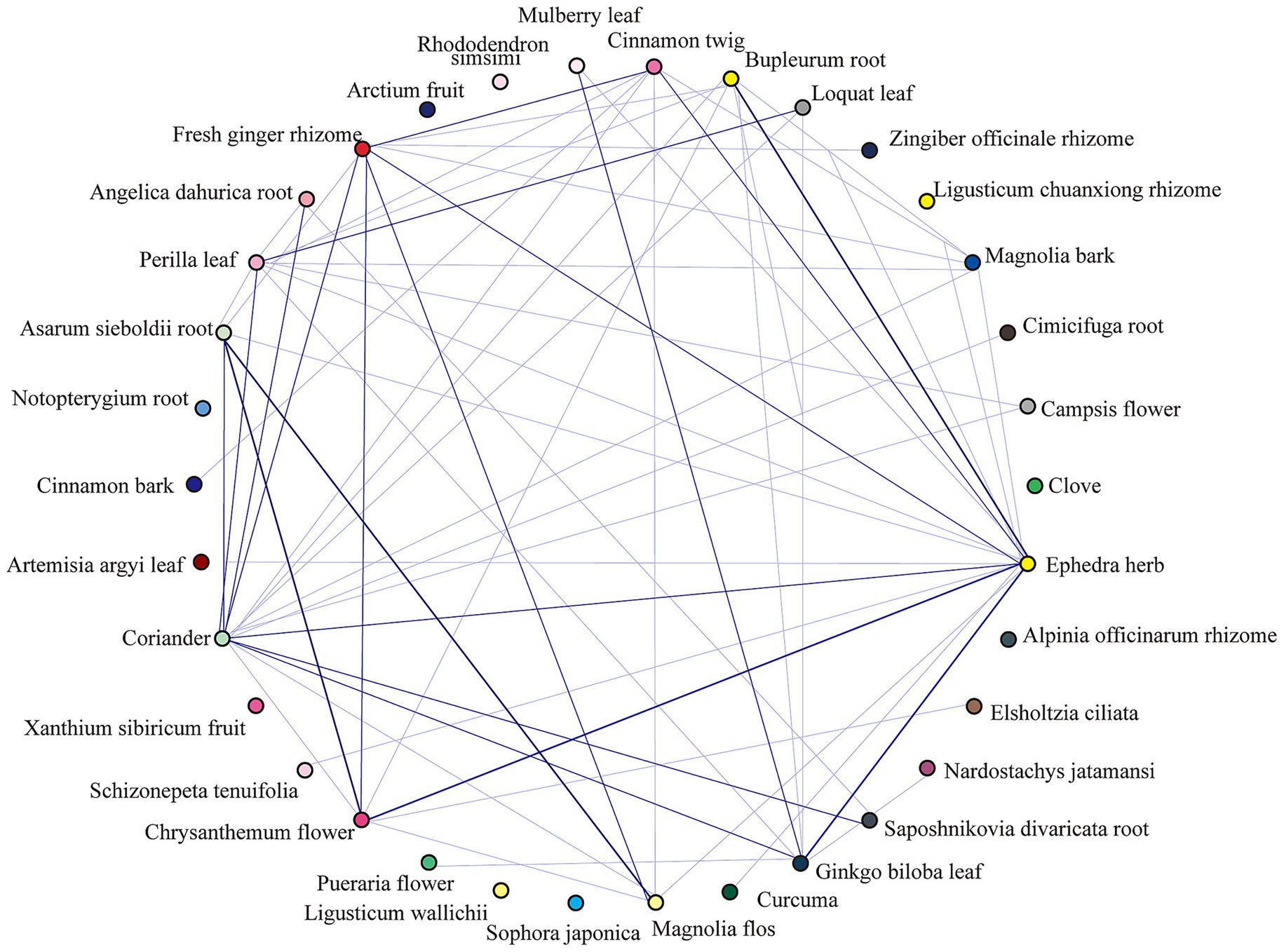
Figure 5. Chord diagram of top 30 herbal associations. This chord diagram visualizes the co-occurrence relationships among the top 30 most frequent herbs identified for PCOS-related infertility. Each herb is represented by an arc on the circumference. The connecting bands (chords) illustrate the strength of the association between pairs of herbs, with wider bands indicating a stronger co-occurrence based on the association rule analysis. This visualization highlights key herbal pairings, such as the strong relationship between Ephedra sinica, Bupleurum chinense, and Chrysanthemum morifolium.
3.5 Network pharmacological analysis of core herbal combination
3.5.1 Screening of core herbal combination
Based on a comprehensive assessment of the frequency statistics, association rules, and cluster analysis, a core herbal combination was identified: “Ephedra sinica (Mahuang)–Magnolia officinalis (Houpo)–Bupleurum chinense (Chaihu)–Chrysanthemum morifolium (Juhua)–Angelica dahurica (Baizhi)–Morus alba (Sangye).” These six herbs contributed 23, 2, 17, 20, 23, and 29 active compounds, respectively. After removing duplicates, this combination yielded 55 unique active compounds, which corresponded to 242 potential targets. The intersection of these 242 targets with the 2,500 common PCOS-infertility targets resulted in 146 shared targets for the core combination (Figure 6).
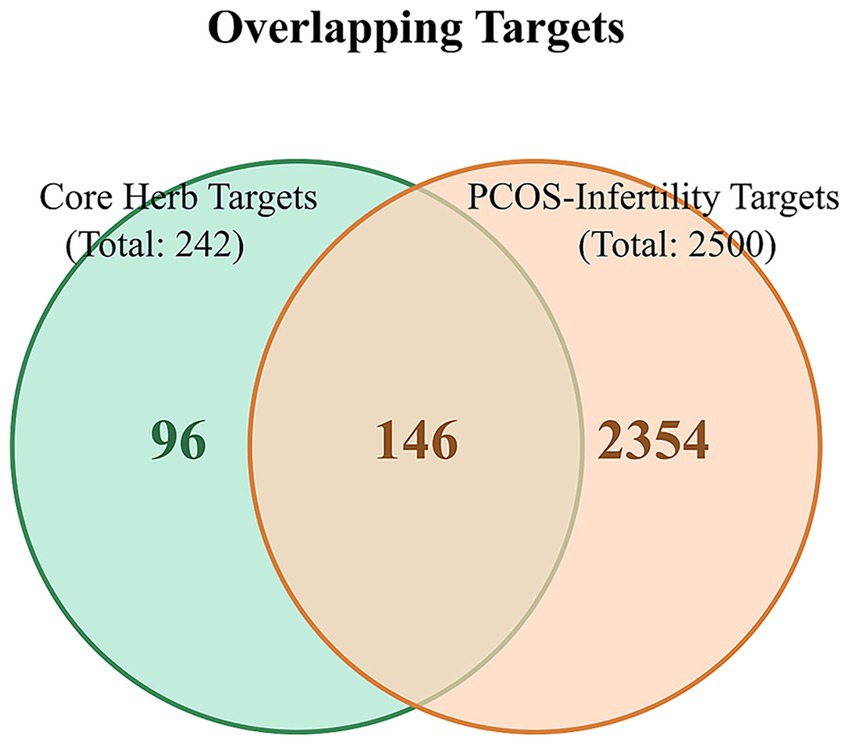
Figure 6. Venn diagram of overlapping targets between the core herbal combination and PCOS-infertility. The light green circle represents the 242 targets associated with the six core herbs. The light orange circle represents the 2,500 common targets of PCOS and infertility. The overlapping region indicates the 146 targets that are modulated by the core herbal combination and are also directly relevant to the pathophysiology of PCOS-related infertility.
3.5.2 GO and KEGG enrichment analysis
GO Analysis: The 146 shared targets were subjected to GO enrichment analysis. The most significantly enriched biological processes (BP) included cellular response to chemical stress, response to lipopolysaccharide, and response to oxidative stress. Enriched cellular components (CC) primarily involved membrane raft, cyclin-dependent protein kinase holoenzyme complex, and serine/threonine protein kinase complex. For molecular functions (MF), key enriched terms included DNA-binding transcription factor binding, nuclear receptor activity, and steroid hormone receptor activity (Figure 7).
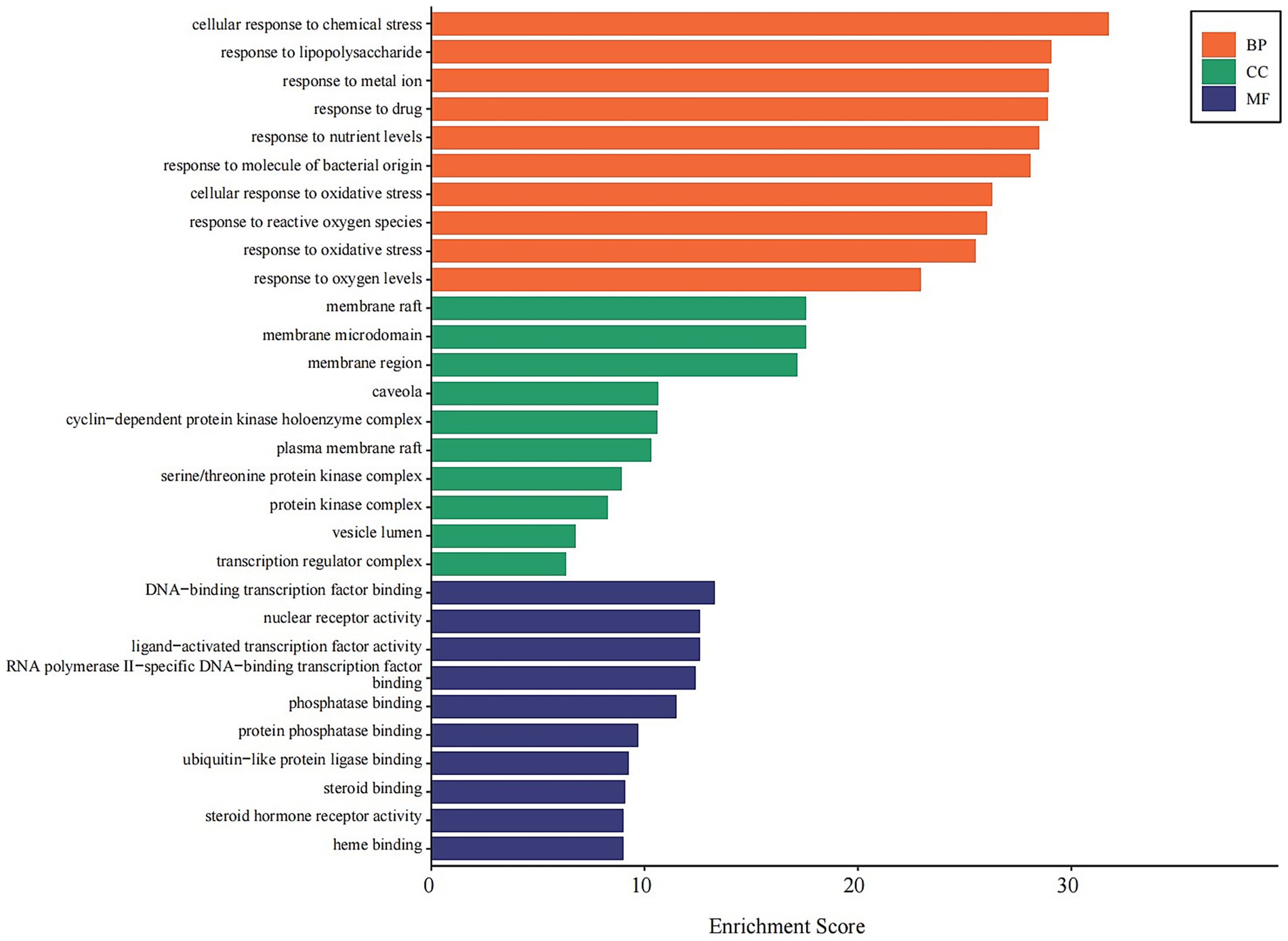
Figure 7. GO enrichment analysis of core combination targets. This bar chart displays the top 10 significantly enriched Gene Ontology (GO) terms for the 146 core targets, categorized by Biological Process (BP, orange), Cellular Component (CC, green), and Molecular Function (MF, blue). The x-axis represents the enrichment score, indicating the significance of each term. The analysis highlights the roles of these targets in stress responses, membrane signaling, and transcriptional regulation. Enriched terms with an adjusted p-value < 0.05 were considered significant.
KEGG Analysis: KEGG pathway enrichment analysis revealed that the 146 targets were significantly involved in pathways critical to human diseases and cellular processes. Key pathways included endocrine resistance, TNF signaling pathway, PI3K-Akt signaling pathway, apoptosis, and cellular senescence (Figure 8).
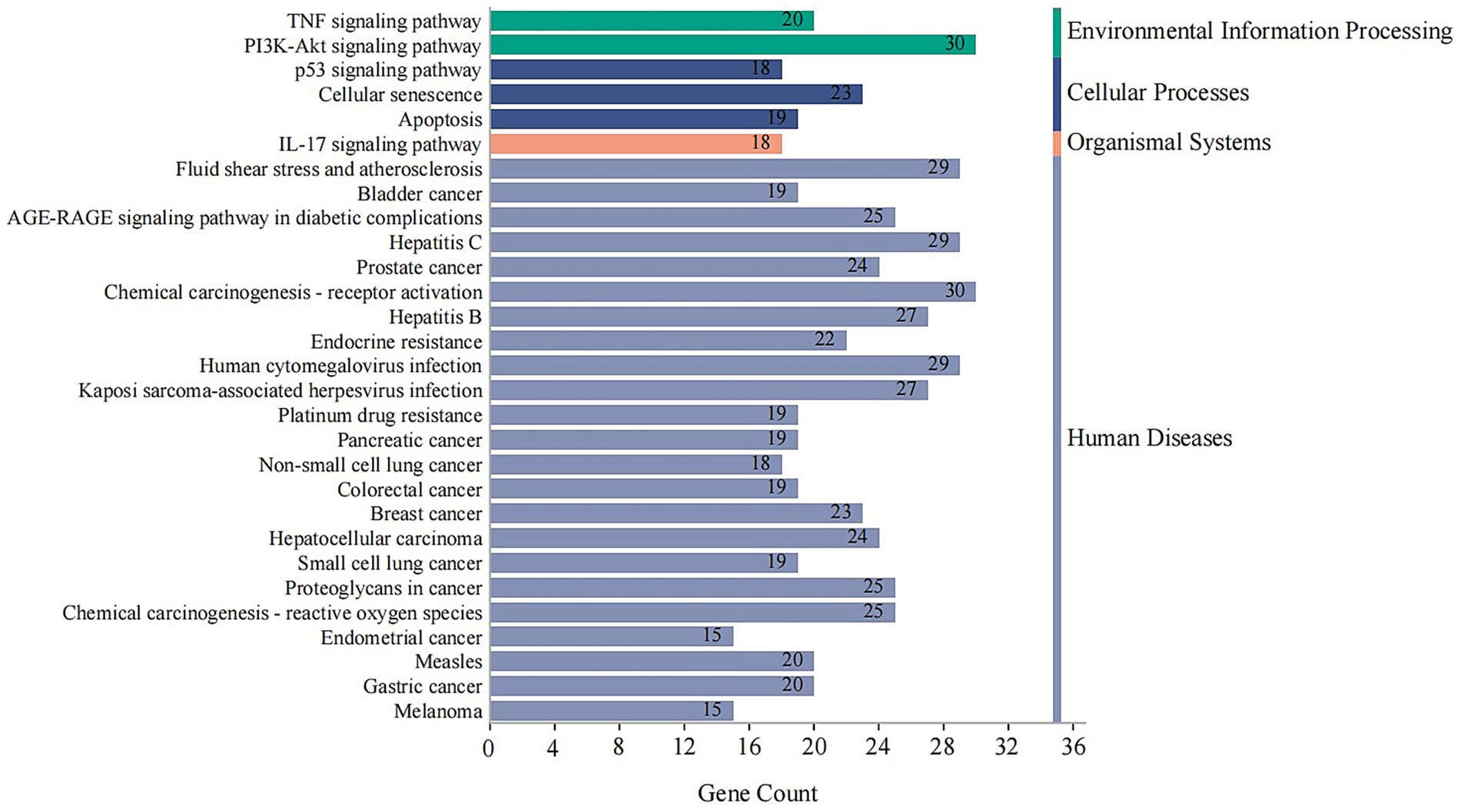
Figure 8. KEGG pathway enrichment analysis of core combination targets. This bar chart shows the top significantly enriched KEGG pathways for the 146 core targets. The x-axis represents the number of genes (Gene Count) enriched in each pathway, and the bars are colored according to their KEGG classification (e.g., Human Diseases, Cellular Processes). The analysis points to key pathways like TNF and PI3K-Akt signaling, which are central to inflammation, endocrine function, and cell survival. Enriched pathways with an adjusted p-value < 0.05 were considered significant.
4 Discussion
Polycystic ovary syndrome (PCOS) is a multifactorial disorder influenced by a complex interplay of genetic, environmental, metabolic, and endocrine factors. A growing body of evidence highlights the critical role of inflammatory mechanisms in its pathogenesis, particularly oxidative stress and chronic low-grade inflammation (15). Elevated levels of inflammatory markers such as IL-6, IL-17, and TNF-α are commonly observed in PCOS patients (16, 17) and are closely associated with follicular developmental arrest, insulin resistance, and metabolic syndrome (18). This creates a vicious cycle that exacerbates the clinical manifestations of the disorder.
4.1 PCOS-infertility related targets
Our intersection analysis identified 2,500 shared targets between PCOS and infertility. From these, the TCMSP database and Cytoscape analysis helped pinpoint 40 core targets related to active herbal compounds. Among these, PTGS1 and PTGS2 are known to exacerbate chronic inflammation by promoting PGE2 synthesis, which stimulates androgen secretion from theca cells, thus aggravating insulin resistance and metabolic dysregulation (19). Sex hormone receptors (ESR1, ESR2, PGR, and AR) are increasingly recognized for their dual roles in both reproductive function and the modulation of inflammation and oxidative stress (20–23). Clinical evidence shows that DPP-4 inhibitors can reverse polycystic ovarian morphology and reduce serum androgen levels in women with PCOS (24). Additionally, dysfunction of the ADRB2 gene contributes to energy metabolism disturbances by impairing fatty acid release, and its polymorphisms have been linked to PCOS susceptibility (25). These identified core targets predominantly mediate processes of inflammation, oxidative stress, immune dysregulation, and metabolic dysfunction, aligning with the known pathophysiology of PCOS.
4.2 Prediction of PCOS-infertility related compounds
Among the identified active compounds, quercetin, kaempferol, and palmitic acid ranked highest by Degree value. Quercetin, a flavonoid with well-documented antioxidant, anti-inflammatory, and immunomodulatory properties, is widely investigated in gynecology (26). In PCOS models, it has been shown to suppress the Ox-LDL/TLR-4/NF-κB pathway, downregulating ovarian IL-1β, IL-6, and TNF-α expression (27), while also improving levels of SOD, CAT, and MDA to restore ovarian function (26). It also inhibits androgen receptor (AR) expression, thereby lowering androgen levels (28). Recent studies highlight its dual action on both ovarian tissue and the pituitary-ovarian axis to promote folliculogenesis, marking it as a promising therapeutic candidate for PCOS (29). Kaempferol, another flavonol, exhibits potent free radical scavenging and antioxidant effects, showing therapeutic potential for oxidative stress-related conditions (30). It has been found to reduce body weight, fasting glucose, and insulin resistance in PCOS rats by modulating hypothalamic inflammation and energy balance (31). Conversely, palmitic acid, a prevalent saturated fatty acid, is often elevated in the serum of PCOS patients (32) and is known to induce inflammation (33), oxidative stress (34), mitochondrial dysfunction, and insulin resistance in vitro (35, 36). The identification of these compounds aligns with the pathophysiology of PCOS, as they primarily target oxidative stress, chronic inflammation, and metabolic dysregulation.
4.3 Prediction of PCOS-infertility related herbs
The 488 herbs identified in this study were predominantly Warm and Pungent, followed by Cold, Bitter, Neutral, and Sweet medicines. This distribution reflects TCM’s therapeutic principles: Pungent-Warm herbs are used to resolve phlegm-dampness, Bitter-Cold herbs to clear heat-toxicity, and Sweet-Neutral herbs to tonify visceral functions. A clinical study of 234 PCOS patients revealed that the most prevalent TCM syndrome was spleen deficiency with phlegm-dampness (44.4%), followed by kidney deficiency with liver stagnation (36.3%) and phlegm-stasis interaction (12.0%) (37), which is consistent with our findings on herbal flavor distribution. The meridian tropism analysis emphasized the Lung, Liver, Stomach, Spleen, and Kidney meridians, aligning with therapeutic strategies to regulate qi, resolve phlegm, clear heat, and tonify organs. The top five herbs screened by Degree value (>20), namely Ephedra sinica, Glycyrrhiza uralensis, Cinnamomum cassia, Bupleurum chinense, and Perilla frutescens, may be considered priority candidates for treating PCOS-related infertility.
4.4 Core herbal combination for PCOS-related infertility
Through a comprehensive analysis of frequency statistics, association rules, and cluster analysis, we identified a core herbal combination: “Ephedra sinica (Mahuang)–Magnolia officinalis (Houpo)–Bupleurum chinense (Chaihu)–Chrysanthemum morifolium (Juhua)–Angelica dahurica (Baizhi)–Morus alba (Sangye).”
The rationale for this combination can be understood through TCM theory. Classical texts suggest a strong link between lung function and menstruation. For example, a passage in The Yellow Emperor’s Inner Classic implies that when lung qi is obstructed, its descending function is impaired, which can disrupt the connection to the uterus and lead to amenorrhea (38). Modern lifestyle factors, such as excessive consumption of cold and sweet foods, can damage yang qi and lead to internal phlegm-dampness. Sedentary habits and lack of exercise can further impair the diffusion of lung qi, causing phlegm-dampness obstruction, which blocks uterine collaterals and leads to menstrual disorders and infertility. This aligns with the TCM axiom, “The spleen is the source of phlegm production, while the lungs store the phlegm.” Therefore, regulating lung-spleen function may improve polycystic ovarian morphology in PCOS patients.
The core formula employs Ephedra sinica, Bupleurum chinense, and Angelica dahurica to diffuse lung qi and release the exterior; Magnolia officinalis to dry dampness and strengthen the spleen; and Chrysanthemum morifolium and Morus alba to clear heat and drain fire. Interestingly, the classical formula Wuji San, used for accumulations of “qi, blood, phlegm, fluid, and food,” contains Ephedra sinica, Magnolia officinalis, and Angelica dahurica. Research suggests Wuji San may regulate inflammatory factors, thereby improving ovulation and pregnancy rates in PCOS patients (39).
The compatibility of this formula can be analyzed from a TCM perspective. Ephedra acts as the monarch herb, powerfully diffusing lung qi to open blockages. It is assisted by Bupleurum, which soothes liver qi, and Angelica dahurica, which dispels wind-dampness, together addressing both the upper (lung) and middle (liver/spleen) jiao. Magnolia officinalis serves as the minister herb, drying dampness and promoting qi circulation to resolve the phlegm-dampness pathology. Chrysanthemum and Morus alba act as assistant and envoy herbs, clearing heat that may arise from stagnation and guiding the formula’s actions downward. From a safety perspective, the inclusion of heat-clearing herbs like Chrysanthemum and Morus alba helps to balance the warming and drying nature of Ephedra and Magnolia officinalis, reducing the risk of depleting yin or generating excess heat. However, the use of Ephedra requires caution due to its cardiovascular and central nervous system stimulant effects; its dosage and the patient’s condition must be carefully monitored in any future clinical application. This predicted synergy and balancing mechanism provides a strong rationale for its validation in animal models.
Bupleurum chinense, with its exterior-releasing and liver-soothing properties, is widely used for gynecological disorders, especially those related to emotional stress and liver qi stagnation (40, 41). For instance, Xiaoyao San, which features Bupleurum, may enhance endometrial receptivity in PCOS by regulating VEGF signaling (42). Similarly, historical texts note that Morus alba (mulberry leaf) and Chrysanthemum morifolium (chrysanthemum) are key herbs for treating uterine bleeding caused by liver heat (43), a condition that can manifest in PCOS. Modern TCM practitioners like Professor Shen Shaogong have successfully used modified versions of formulas containing these herbs to regulate yin-yang balance in PCOS patients (44).
Modern pharmacological studies lend further support to this combination. Ephedrine from Ephedra sinica has anti-inflammatory effects and may improve metabolic rate and circulation, countering insulin resistance (45). Magnolol from Magnolia officinalis demonstrates potent antioxidant and anti-inflammatory activity and improves insulin resistance (46–48). Active compounds in Bupleurum chinense modulate pathways like PI3K-AKT to improve glucose metabolism (49). Components of Morus alba (50), Angelica dahurica (51), and Chrysanthemum morifolium (52) have also been shown to improve glucose tolerance, insulin secretion, and lipid metabolism while fighting oxidative stress.
Our study’s findings are consistent with prior network pharmacology research on PCOS, yet offer a unique perspective due to the reverse methodology. For example, a study on the Guizhi Fulingwan formula identified targets related to inflammation and apoptosis, such as AKT1, TNF, and IL6 (53), which overlap with our findings. Another study on the Zishen Yutai Pill highlighted pathways in cancer and PI3K-Akt signaling (54). While these studies confirm the importance of inflammation and metabolic regulation, our reverse approach started from a broader disease-target landscape, leading to the identification of a novel herbal combination that emphasizes the role of the lung system in PCOS, a connection less explored in conventional network pharmacology analyses.
The GO enrichment analysis highlighted oxidative stress-related processes, while KEGG analysis pointed to endocrine resistance, TNF signaling, and PI3K-Akt signaling pathways. These findings collectively suggest that the core herbs treat PCOS-related infertility by ameliorating oxidative stress and endocrine dysfunction, which aligns perfectly with the functions of the identified PCOS-infertility targets.
4.5 Limitations
This study has several limitations that should be acknowledged. First, as a purely computational investigation, its findings are hypothesis-generating and lack experimental or clinical validation. Future studies involving cell culture experiments, animal models (e.g., letrozole-induced PCOS rats), and eventually clinical trials are necessary to verify the efficacy and safety of the predicted herbal combination. Second, the reliance on public databases for targets and compounds introduces potential biases, as some herbs or compounds may be underrepresented or incompletely annotated. The data does not account for tissue-specific gene expression or pharmacodynamics, which could influence therapeutic outcomes. Third, the statistical analysis of herbal properties, while informative, has inherent limitations. Our classification of herbs into therapeutic categories was based on their primary functions as documented in standardized texts, which does not account for their multiple effects or their specific roles (e.g., monarch, minister) within a formula. This could introduce bias, as suggested by the reviewer. For instance, high-frequency herbs with broad applications, such as licorice, might influence the distribution across categories. Future studies could employ weighted statistical methods and incorporate multi-center prescription data to refine these findings. The suggestion to use a Sankey diagram to visualize channel-efficacy flow is excellent and represents a valuable direction for future, more granular analyses. Fourth, the screening criteria for active compounds (e.g., high GI absorption, druglikeness) might have inadvertently excluded potentially effective natural metabolites that do not meet these stringent pharmaceutical filters but could still exert biological effects. Fifth, this study did not address crucial clinical considerations such as optimal dosage, potential herb-drug interactions, or toxicity of the proposed herbs. Finally, PCOS is a highly heterogeneous syndrome with multiple phenotypes (e.g., lean vs. obese PCOS) and diverse TCM syndromic patterns. Our analysis treated PCOS as a single entity and did not differentiate between these subtypes, which may require tailored therapeutic approaches.
5 Conclusion
This reverse network pharmacology study identified 2,500 shared targets and 1,545 active compounds relevant to PCOS-related infertility, culminating in the prediction of a core herbal combination: “Ephedra sinica–Magnolia officinalis–Bupleurum chinense–Chrysanthemum morifolium–Angelica dahurica–Morus alba.” These herbs are predicted to synergistically target oxidative stress, inflammation, and endocrine dysregulation. While further experimental validation is essential, these findings offer a novel integrative strategy for PCOS management and provide a theoretical foundation for future clinical practice and mechanistic research.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
YW: Writing – original draft, Investigation, Software. FJ: Writing – review & editing, Data curation. JH: Methodology, Writing – review & editing. ZS: Supervision, Writing – review & editing. HH: Conceptualization, Writing – review & editing. YZ: Funding acquisition, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by Wuxi Municipal Health Commission Scientific Research Project (Q202367, Q202405, Z202412) and Natural Science Foundation project of Nanjing University of Chinese Medicine (XZR2024311).
Acknowledgments
We are grateful to all staff professionals and participants.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1614165/full#supplementary-material
Footnotes
References
1. Teede, HJ, Tay, CT, Laven, J, Dokras, A, Moran, LJ, Piltonen, TT, et al. Recommendations from the 2023 international evidence-based guideline for the assessment and Management of Polycystic Ovary Syndrome. Hum Reprod. (2023) 38:1655–79. doi: 10.1093/humrep/dead156
2. Liu, L, Shao, T, and Fang, Y. Analysis of the incidence trend of polycystic ovary syndrome in China from 1990 to 2019. Chinese J Preventive Med. (2023) 24:259–63. doi: 10.16506/j.1009-6639.2023.03.016
3. Qiao, J, Wang, Y, Li, X, Jiang, F, Zhang, Y, Ma, J, et al. A lancet commission on 70 years of women's reproductive, maternal, newborn, child, and adolescent health in China. Lancet. (2021) 397:2497–536. doi: 10.1016/S0140-6736(20)32708-2
4. Yao, K, Bian, C, and Zhao, X. Association of polycystic ovary syndrome with metabolic syndrome and gestational diabetes: aggravated complication of pregnancy. Exp Ther Med. (2017) 14:1271–6. doi: 10.3892/etm.2017.4642
5. Yang, R, Li, Q, Zhou, Z, Qian, W, Zhang, J, Wu, Z, et al. Changes in the prevalence of polycystic ovary syndrome in China over the past decade. Lancet Regional Health - Western Pacific. (2022) 25:100494. doi: 10.1016/j.lanwpc.2022.100494
6. Santoro, N, Eisenberg, E, Trussell, JC, Craig, LTB, Gracia, C, Huang, H, et al. Fertility-related quality of life from two RCT cohorts with infertility: unexplained infertility and polycystic ovary syndrome. Hum Reprod. (2016) 31:2268–79. doi: 10.1093/humrep/dew175
7. Chen, Q, Zhang, S, and Shen, M. Effects of Tiaojing Yunyu formula on hemodynamic parameters of ovarian and uterine tissues in patients with ovulatory disorder infertility. Chinese J Info Traditional Chinese Med. (2003) 10:6–9. doi: 10.3969/j.issn.1005-5304.2003.12.005
8. Wang, C, and Ma, X. Exploring the targets and signaling pathways of Angelica sinensis-Cuscuta chinensis in treating polycystic ovary syndrome based on network pharmacology. J China Medical University. (2021) 50:51–6. doi: 10.12007/j.issn.0258-4646.2021.01.010
9. Safran, M, Dalah, I, and Alexander, J. GeneCards Version 3: the human gene integrator. Database 2010. (2010) 2010:baq020. doi: 10.1093/database/baq020
10. Amberger, JS, Bocchini, CA, Scott, AF, and Hamosh, A. OMIM.Org: leveraging knowledge across phenotype-gene relationships. Nucleic Acids Res. (2019) 47:D1038–43. doi: 10.1093/nar/gky1151
11. Stevens, T, Sangkuhl, K, Brown, JT, Altman, RB, and Klein, TE. PharmGKB summary: methylphenidate pathway, pharmacokinetics/pharmacodynamics. Pharmacogenet Genomics. (2019) 29:136–54. doi: 10.1097/FPC.0000000000000376
12. Pinero, J, Queralt-Rosinach, N, Bravo, A, Deu-Pons, J, Bauer-Mehren, A, Baron, M, et al. DisGeNET: a discovery platform for the dynamical exploration of human diseases and their genes. Database. (2015) 2015:bav028. doi: 10.1093/database/bav028
13. Knox, C, Wilson, M, Klinger, CM, Franklin, M, Oler, E, Wilson, A, et al. DrugBank 6.0: the DrugBank knowledgebase for 2024. Nucleic Acids Res. (2024) 52:D1265–75. doi: 10.1093/nar/gkad976
14. Zhou, Y, Zhang, Y, Zhao, D, Yu, X, Shen, X, Zhou, Y, et al. TTD: therapeutic target database describing target druggability information. Nucleic Acids Res. (2024) 52:D1465–77. doi: 10.1093/nar/gkad751
15. Gao, H, Qian, B, and Ni, Y. Research progress on the pathogenesis of polycystic ovary syndrome. J Sichuan University (Medical Sci Edition). (2024) 55:1049–54. doi: 10.12182/20240760208
16. Cavalcante, R, Leao, L, and Tavares, A. Fat distribution and its correlation with insulin resistance, androgen markers, and Proinflammatory cytokines in polycystic ovary syndrome. Horm Metab Res. (2024) 57:25–32. doi: 10.1055/a-2386-9281
17. Chen, H, Zhang, Y, Li, S, Tao, Y, Gao, R, Xu, W, et al. The association between genetically predicted systemic inflammatory regulators and polycystic ovary syndrome: a Mendelian randomization study. Front Endocrinol (Lausanne). (2021) 12:731569. doi: 10.3389/fendo.2021.731569
18. Li, X, Gao, B, Gao, B, Li, X, and Xia, X. Transcriptome profiling reveals dysregulation of inflammatory and protein synthesis genes in PCOS. Sci Rep. (2024) 14:16596. doi: 10.1038/s41598-024-67461-4
19. Banaszewska, B, Ozegowska, K, Polska, M, Pawelczyk, L, Chang, RJ, and Duleba, AJ. Ibuprofen reduces testosterone level in women with polycystic ovary syndrome. J Endocrine Society. (2022) 6:bvac128. doi: 10.1210/jendso/bvac128
20. Dey, A, Dhadhal, S, Maharjan, R, Nagar, PS, and Nampoothiri, L. Partially purified non-polar phytocomponents from Aloe barbadensis mill. gel restores metabolic and reproductive comorbidities in letrozole-induced polycystic ovary syndrome rodent model- an "in-vivo" study. J Ethnopharmacol. (2022) 291:115161. doi: 10.1016/j.jep.2022.115161
21. Elghareeb, MM, Elshopakey, GE, Elkhooly, TA, Salama, B, Samy, A, Bazer, FW, et al. Estradiol and zinc-doped nano hydroxyapatite as therapeutic agents in the prevention of osteoporosis; oxidative stress status, inflammation, bone turnover, bone mineral density, and histological alterations in ovariectomized rats. Front Physiol. (2022) 13:989487. doi: 10.3389/fphys.2022.989487
22. Park, CJ, Lin, PC, Zhou, S, Barakat, R, Bashir, ST, Choi, JM, et al. Progesterone receptor serves the ovary as a trigger of ovulation and a terminator of inflammation. Cell Rep. (2020) 31:107496. doi: 10.1016/j.celrep.2020.03.060
23. Traish, A, Bolanos, J, and Nair, S. Do androgens modulate the pathophysiological pathways of inflammation? Appraising the contemporary evidence. J Clin Med.(2019) 7:549.doi: 10.3390/jcm7120549
24. Jensterle, M, Janez, A, Fliers, E, DeVries, JH, Vrtacnik-Bokal, E, and Siegelaar, SE. The role of glucagon-like peptide-1 in reproduction: from physiology to therapeutic perspective. Hum Reprod Update. (2019) 25:504–17. doi: 10.1093/humupd/dmz019
25. Da, FA, Assis, I, and Salum, K. Genetic variants in DBC1, SIRT1, UCP2 and ADRB2 as potential biomarkers for severe obesity and metabolic complications. Front Genet. (2024) 15:1363417. doi: 10.3389/fgene.2024.1363417
26. Jian, X, Shi, C, Luo, W, Zhou, L, Jiang, L, and Liu, K. Therapeutic effects and molecular mechanisms of quercetin in gynecological disorders. Biomed Pharmacother. (2024) 173:116418. doi: 10.1016/j.biopha.2024.116418
27. Wang, Z, Zhai, D, Zhang, D, Bai, L, Yao, R, Yu, J, et al. Quercetin decreases insulin resistance in a polycystic ovary syndrome rat model by improving inflammatory microenvironment. Reprod Sci. (2017) 24:682–90. doi: 10.1177/1933719116667218
28. Zheng, S, Chen, Y, Ma, M, and Li, M. Mechanism of quercetin on the improvement of ovulation disorder and regulation of ovarian CNP/NPR2 in PCOS model rats. J Formos Med Assoc. (2022) 121:1081–92. doi: 10.1016/j.jfma.2021.08.015
29. Shah, M, Shrivastva, VK, Mir, MA, Shah, MZ u h, Sheikh, WM, Ganie, MA, et al. Effect of quercetin on steroidogenesis and folliculogenesis in ovary of mice with experimentally-induced polycystic ovarian syndrome. Front Endocrinol (Lausanne). (2023) 14:1153289. doi: 10.3389/fendo.2023.1153289
30. Hussain, MS, Altamimi, A, Afzal, M, Altamimi, ASA, Almalki, WH, Kazmi, I, et al. Kaempferol: paving the path for advanced treatments in aging-related diseases. Exp Gerontol. (2024) 188:112389. doi: 10.1016/j.exger.2024.112389
31. Bu, N, Jamil, A, Hussain, L, Alshammari, A, Albekairi, TH, Alharbi, M, et al. Phytochemical-based study of Ethanolic extract of Saraca asoca in Letrozole-induced polycystic ovarian syndrome in female adult rats. ACS Omega. (2023) 8:42586–97. doi: 10.1021/acsomega.3c05274
32. Zhang, Z, Hong, Y, Chen, M, Tan, N, Liu, S, Nie, X, et al. Serum metabolomics reveals metabolic profiling for women with hyperandrogenism and insulin resistance in polycystic ovary syndrome. Metabolomics. (2020) 16:20. doi: 10.1007/s11306-020-1642-y
33. Tu, TH, Kim, H, Yang, S, Kim, JK, and Kim, JG. Linoleic acid rescues microglia inflammation triggered by saturated fatty acid. Biochem Biophys Res Commun. (2019) 513:201–6. doi: 10.1016/j.bbrc.2019.03.047
34. Marwarha, G, Claycombe, K, and Schommer, J. Palmitate-induced endoplasmic reticulum stress and subsequent C/EBPalpha homologous protein activation attenuates leptin and insulin-like growth factor 1 expression in the brain. Cell Signal. (2016) 28:1789–805. doi: 10.1016/j.cellsig.2016.08.012
35. Vesga-Jimenez, DJ, Martin, C, and Barreto, GE. Fatty acids: an insight into the pathogenesis of neurodegenerative diseases and therapeutic potential. Int J Mol Sci. (2022) 23:20. doi: 10.3390/ijms23052577
36. Carta, G, Murru, E, Banni, S, and Manca, C. Palmitic acid: physiological role, metabolism and nutritional implications. Front Physiol. (2017) 8:902. doi: 10.3389/fphys.2017.00902
37. Sun, Y, Jiang, Y, and Jiang, W. Correlation between TCM syndrome types and lifestyle in patients with polycystic ovary syndrome. J Guangzhou University Traditional Chinese Med. (2024) 41:2882–90. doi: 10.13359/j.cnki.gzxbtcm.2024.11.009
38. Wang, B. Huangdi Neijing (yellow emperor's inner canon). Beijing: Scientific and Technical Documentation Press (1996).
39. Zhang, Y, Wang, Y, and Liu, B. Clinical study of Wuji powder combined with clomiphene in the treatment of infertility with phlegm-dampness type polycystic ovary syndrome. J Nanjing University of Traditional Chinese Med. (2020) 36:444–8. doi: 10.14148/j.issn.1672-0482.2020.0444
40. Le, P, Tang, N, and Zhou, M. Clinical observation of modified Dachaihu decoction combined with letrozole in the treatment of phlegm-heat stasis type polycystic ovary syndrome. Sichuan J Traditional Chinese Med. (2024) 42:175–8.
41. Sun, N, Yin, P, and Zhang, Z. Research progress of Xiaochaihu decoction in the treatment of infertility. World Chinese Med. (2024) 19:124–7. doi: 10.3969/j.issn.1673-7202.2024.01.024
42. Wang, Q, He, Y, and Li, Z. Exploring the mechanism of Xiaoyao powder in regulating endometrial receptivity in polycystic ovary syndrome based on network pharmacology and experimental verification. J Hunan University of Chinese Med. (2023) 43:69–79.
43. He, Y, Luo, G, and Li, L. Discussion on the application of mulberry leaf in gynecological diseases. Global Traditional Chinese Med. (2023) 16:284–7.
44. Lu, K, Huang, J, and Lin, H. Collection of academic thoughts on contemporary famous TCM gynecologists in the diagnosis and treatment of polycystic ovary syndrome. Lishizhen Med Materia Medica Res. (2024) 35:1994–7.
45. Ma, Y, Su, J, and Li, Q. Research progress on clinical application and mechanism of Ephedra. Chinese J Drug Abuse Prevention Treatment. (2024) 30:75–9. doi: 10.15900/j.cnki.zylf1995.2024.01.019
46. Zhang, X, Zuo, D, and Hu, N. Research progress on chemical constituents and pharmacological effects of Magnolia officinalis. Info Traditional Chinese Med. (2023) 40:85–9. doi: 10.19656/j.cnki.1002-2406.20230216
47. Cao, X, Gu, S, and Wang, J. Pharmacodynamic study of honokiol on glucose and lipid metabolism in type 2 diabetic KK/Upj-AY mice. Traditional Chinese Drug Res Clin Pharmacol. (2019) 30:806–11. doi: 10.19378/j.issn.1003-9783.2019.07.009
48. Jiao, H, Peng, X, and Peng, X. Effects of honokiol on insulin resistance in diabetic rats and its mechanism. Northwest Pharmaceutical J. (2022) 37:45–50.
49. Wang, H, Zhou, Q, and Li, B. Research progress on chemical constituents and pharmacological effects of Bupleurum. Jilin J Traditional Chinese Med. (2024) 44:96–100. doi: 10.13463/j.cnki.jlzyy.2024.01.022
50. Xu, W, Lu, C, and Xiao, X. Research progress on bioactive substances and comprehensive utilization of mulberry leaves. J Food Saf Qual. (2024) 15:219–28. doi: 10.19812/j.cnki.jfsq11-5956/ts.20240619001
51. Zou, J, Su, W, and Pan, Y. Research progress on chemical constituents and pharmacological effects of Angelica dahurica and prediction analysis of its quality markers (Q-marker). Modernization of Traditional Chinese Med Materia Medica-World Sci Technol. (2023) 25:2535–48. doi: 10.11842/wst.20220524003
52. Zhang, Y, and Jin, L. Effects of aqueous extract of wild chrysanthemum on development and metabolic function of insulin pathway mutant Drosophila. Acta Chin Med Pharmacol. (2019) 47:24–7. doi: 10.19664/j.cnki.1002-2392.190105
53. Ye, Y, Zhou, W, Ren, Y, Lu, J, Chen, A, Jin, R, et al. The ameliorating effects of Guizhi Fuling wan combined with rosiglitazone in a rat ovarian model of polycystic ovary syndrome by the PI3K/AKT/NF-κB and Nrf2/HO-1 pathways. Gynecol Endocrinol. (2023) 39:2254848. doi: 10.1080/09513590.2023.2254848
Keywords: polycystic ovary syndrome, infertility, reverse network pharmacology, herbal formula prediction, traditional Chinese medicine
Citation: Wang Y, Jia F, Hu J, Shi Z, Huang H and Zhou Y (2025) A reverse network pharmacology and bioinformatics-based approach to exploring medication patterns for polycystic ovary syndrome-related infertility. Front. Med. 12:1614165. doi: 10.3389/fmed.2025.1614165
Edited by:
Amany Abdel-Rahman Mohamed, Zagazig University, EgyptReviewed by:
Su Liu, Shenzhen Zhongshan Urological Hospital, ChinaXiangping Peng, Waseda University, Japan
Copyright © 2025 Wang, Jia, Hu, Shi, Huang and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haixia Huang, aHVhbmdoYWl4aWE3NzkwQDE2My5jb20=; Yahong Zhou, eWh6aG91dHdvQDE2My5jb20=
†These authors have contributed equally to this work
 Yueyan Wang1†
Yueyan Wang1† Haixia Huang
Haixia Huang