- 1Department of General Surgery, Affiliated Hospital of Hebei University, Baoding, China
- 2Department of Dermatology, Affiliated Hospital of Hebei University, Baoding, China
Background: Primary Anorectal malignant melanoma (pARMM) is an exceedingly rare and aggressive malignancy, accounting for approximately 1% of anorectal cancers. It originates from melanocytes in the anorectal mucosa and lacks distinctive clinical features, leading to frequent misdiagnosis and advanced presentation.
Methods: A retrospective analysis was conducted on 9 patients (1 male, 8 females; median age 59 years) with histopathologically and immunohistochemically confirmed ARMM who underwent surgical resection (Wide Local Excision, WLE = 4; Abdominoperineal Resection, APR = 5) and had complete follow-up data (median 19 months, up to May 2025). Diagnostic methods included clinical evaluation, digital rectal exam (DRE), colonoscopy, imaging (CT), histopathology, and immunohistochemistry (IHC). Treatment approaches and outcomes were analyzed.
Results: Common presenting symptoms were hematochezia (44.4%), tenesmus (22.2%), altered bowel habits, anal mass protrusion, or were asymptomatic (11.1% each). DRE revealed exophytic (n = 6) or polypoid (n = 3) masses. Colonoscopy showed lesions near the dentate line; only 33.3% had obvious pigmentation. IHC positivity: HMB-45/Melan-A 66.7%, S-100 55.6%. Pathological R0 resection was achieved in all patients. During follow-up, 3 patients (33.3%) developed distant metastases (lung, liver), 2 of whom died. Six patients remained disease-free.
Conclusion: Primary Anorectal malignant melanoma (pARMM) often presents with symptoms mimicking common benign anorectal conditions, leading to frequent diagnostic errors. Definitive diagnosis requires histopathological examination, with immunohistochemical markers (HMB-45 and Melan-A positivity) providing critical confirmation. While surgical resection remains the primary treatment, a growing expert consensus supports wide local excision with adequate margins (≥1 cm) as sufficient management. Emerging evidence indicates comparable survival outcomes to more radical procedures in appropriately selected patients.
Introduction
Malignant melanoma originates from melanocytes predominantly located in the skin. However, melanocytes also reside in mucosal sites, including the gastrointestinal tract, where they may give rise to primary mucosal melanomas (1, 2). Among these, primary anorectal malignant melanoma (pARMM) represents an exceptionally rare entity. pARMM accounts for only 0.4–1.6% of all melanomas and approximately 1% of anorectal malignancies (3–5). It exhibits a striking demographic predilection, predominantly affecting elderly females (male-to-female ratio ≈1:4) (6). Over 50% of patients present with metastatic disease at diagnosis due to delayed detection (7), critically contributing to its poor prognosis (5-year survival <20%) (8). A definitive diagnosis of pARMM necessitates rigorous exclusion of metastatic melanoma from cutaneous, ocular, or other primary sites through comprehensive dermatological examination, imaging, and histopathological correlation (9).
The pathogenesis of pARMM remains incompletely elucidated. Current evidence supports a neural crest origin, wherein precursor melanocytes migrate to the rectal mucosa during embryogenesis (10). Chronic mechanical irritation from fecal transit, coupled with local inflammatory mediators (e.g., COX-2/PGE2 upregulation), is postulated to drive malignant transformation (11). Molecular analyses have identified key driver mutations in: KIT loss-of-function variants (~15–40%), BRAF V600E (~10–25%), and NRAS Q61 mutations (~5–15%) (12, 13). These genetic aberrations constitutively activate the MAPK/ERK signaling pathway, thereby driving melanomagenesis.
pARMM lacks pathognomonic clinical features and frequently mimics benign conditions such as hemorrhoids, leading to a high misdiagnosis rate of 60–70% (12). Common presenting symptoms include hematochezia (60–80%), protrusion of an anal mass (40–60%), and tenesmus or altered bowel habits (20–30%) (14). Notably, 30–40% of cases are amelanotic (15), frequently evading recognition during standard endoscopy. Consequently, immunohistochemistry (IHC) is indispensable for definitive diagnosis, demonstrating positivity for HMB-45/Melan-A (specificity >95%) and S-100 (sensitivity >97% but lower specificity) (16).
Surgical resection remains the cornerstone of management for pARMM; however, the optimal surgical approach is debated. Wide Local Excision (WLE) preserves sphincter function but carries a risk of margin positivity (17), whereas Abdominoperineal Resection (APR) improves local control at the cost of necessitating a permanent colostomy (18). Given the lack of significant survival difference between WLE and APR (HR 1.12, 95% CI 0.87–1.44) shown in recent meta-analyses (19–21), clinical consensus is shifting toward organ-preserving strategies. The role of adjuvant immunotherapy (specifically anti-PD-1/CTLA-4 agents) remains investigative (22).
Due to the rarity of pARMM and persistent controversies surrounding its surgical management, this study aimed to analyze our institutional experience. We conducted a retrospective analysis of nine histologically confirmed pARMM cases treated at our institution to summarize diagnostic and therapeutic experiences, accompanied by a brief literature review. This report aims to provide insights for clinicians managing similar cases.
Materials and methods
Patient selection criteria
Patient selection was based on stringent inclusion and exclusion criteria. For inclusion, patients were required to meet all of the following conditions: (1) a histopathologically and immunohistochemically confirmed diagnosis of pARMM; (2) availability of complete clinical data, including medical history, physical examination findings, imaging studies, and laboratory results; (3) treatment with surgical resection (either wide local excision [WLE] or abdominoperineal resection [APR]) followed by regular postoperative follow-up; and (4) initial diagnostic workup involving systemic surveillance and multidisciplinary consultations (specifically dermatology and ophthalmology) to definitively exclude cutaneous and extracutaneous primary lesions. Patients were excluded if they met any of the following criteria: (1) concurrent diagnosis of another malignancy; (2) confirmed distant metastasis prior to surgical intervention; (3) incomplete clinical data or loss to follow-up; or (4) any prior history of cutaneous or mucosal melanoma.
Patient characteristics
Nine patients met the inclusion criteria (Table 1), comprising 8 females (88.9%) and 1 male (11.1%). The cohort’s age range was 47–70 years (median 59 years), with symptom duration ranging from 20 to 185 days (median 75 days) before diagnosis. Tumor diameters measured 0.5–3.0 cm (mean 2.4 cm). As mandated by exclusion criterion 4, no patient had a prior melanoma history.
Diagnostic methods
Clinical presentation was heterogeneous: hematochezia was the most common symptom (44.4%, n = 4), followed by tenesmus (22.2%, n = 2), while altered bowel habits, anal mass protrusion, and asymptomatic presentation each occurred in 11.1% (n = 1) of cases.
Digital rectal examination revealed exophytic, cauliflower-like masses in six patients and polypoid masses in three.
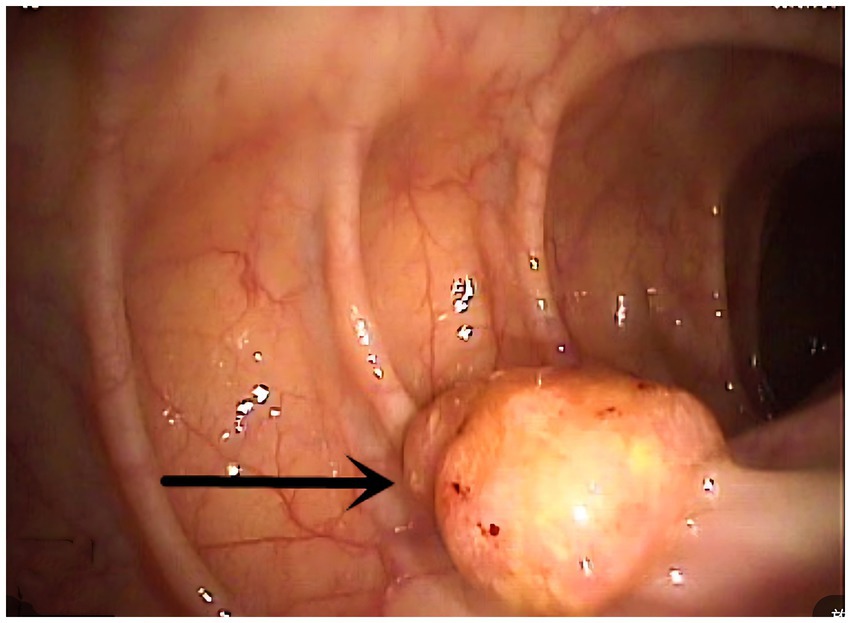
Endoscopic evaluation demonstrated that all tumors involved the dentate line region, appearing as sessile protrusions with surface erosion/ulceration, friability, and bleeding tendency (Figure 1). Pigmentation was observed in only three cases (33.3%), absent in the majority (n = 6). Esophagogastroduodenoscopy (EGD) was performed only when upper GI symptoms were present, per institutional protocol.
Contrast-enhanced abdominal CT uniformly showed focal rectal wall thickening with heterogeneous enhancement, with no distant metastasis detected.
Cutaneous and Extracutaneous Primary Exclusion Cutaneous and extracutaneous primary exclusion were systematically confirmed through dermatological and ophthalmological evaluations for all patients. No primary cutaneous or ocular melanomas were identified.
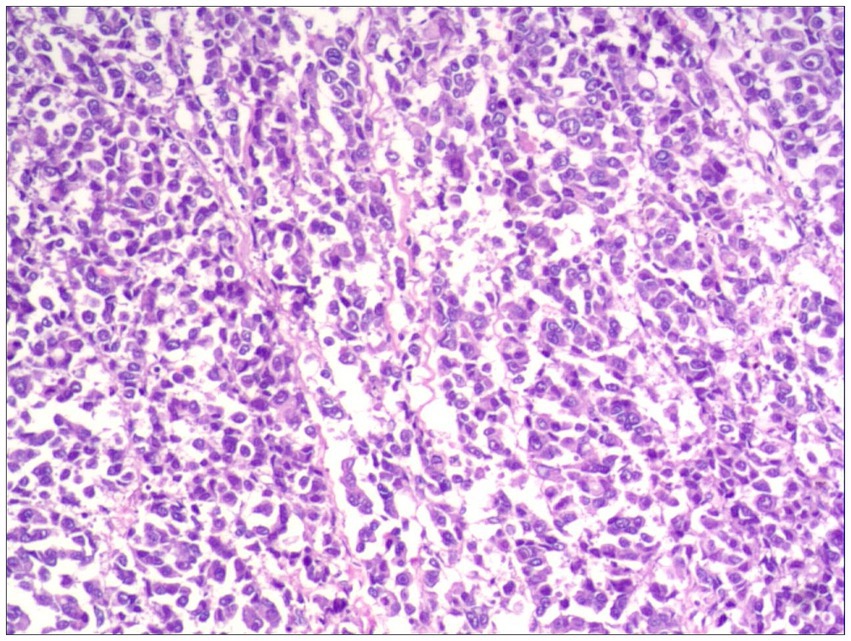
Histopathological examination confirmed R0 resection in all cases, featuring epithelioid or spindled neoplastic cells with nuclear pleomorphism (enlargement, hyperchromasia, prominent nucleoli) and abundant eosinophilic to granular cytoplasm. Tumors exhibited infiltrative growth patterns into smooth muscle and fibrous septa, with associated focal melanin deposition and adjacent mucosal necrosis (Figure 2).
Immunohistochemistry demonstrated HMB-45/Melan-A positivity in 66.7% (6/9) and S-100 positivity in 55.6% (5/9) of cases (Table 2)—rates notably lower than typical literature values. Representative immunohistochemical staining patterns were documented: Melan-A positivity showing nested/cord-like brown staining (Supplementary Figure S1), HMB-45 demonstrating patchy/dot-like positivity in a purple cellular background (Supplementary Figure S2), and S-100 exhibiting diffuse/patchy brown staining (Supplementary Figure S3).
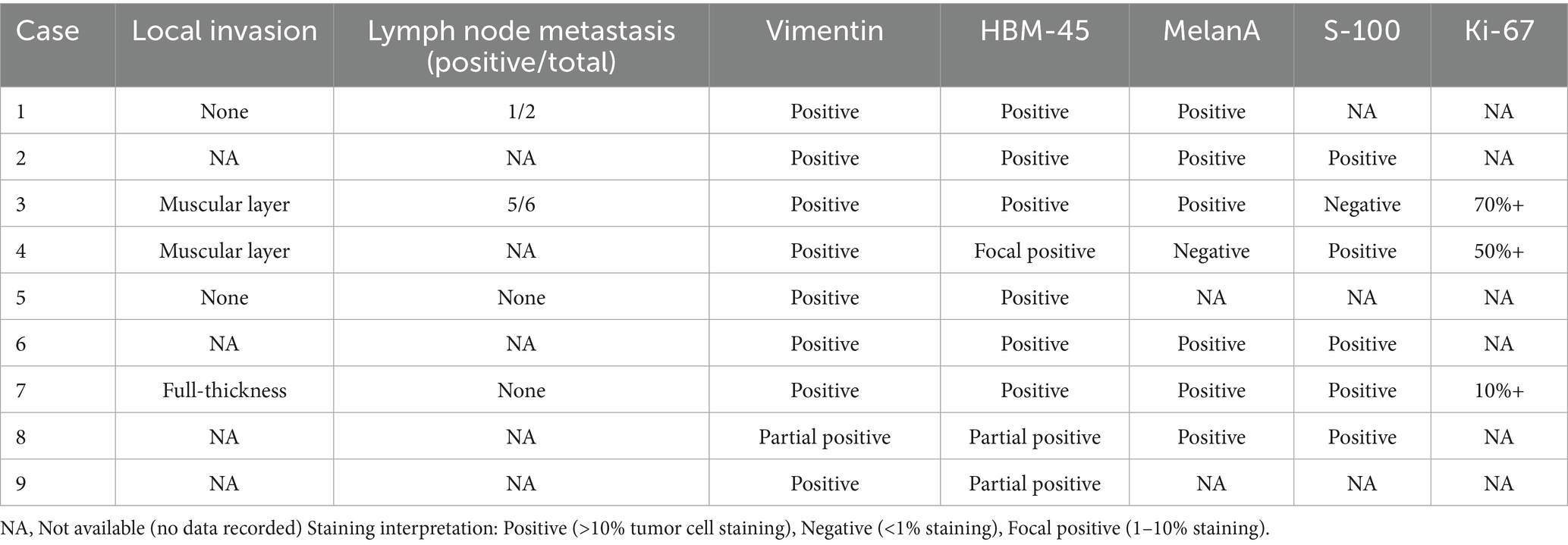
Table 2. Lymph node metastasis and local tissue invasion and immunohistochemical results in nine cases.
Treatment modalities consisted primarily of surgical intervention. Wide local excision (WLE) was performed in four patients, while abdominoperineal resection (APR) was undertaken in five. Surgical approach selection was individualized, integrating tumor characteristics (location relative to the dentate line, diameter, and depth of invasion/T-stage), nodal status (suspicion of metastasis on imaging), and patient preference regarding sphincter preservation. WLE was typically offered for smaller tumors (≤2.5 cm diameter) with superficial invasion (T1/T2) and no evidence of nodal involvement. Conversely, APR was generally indicated for larger tumors (>2.5 cm), deeply invasive lesions (T3/T4), or cases with radiologically suspicious lymph nodes. Adjuvant therapy was administered to only one patient due to high-risk histopathological features (ulceration, high mitotic rate). This patient received a regimen comprising dacarbazine (systemic chemotherapy), temozolomide (for systemic disease control, not prophylactic intent), and sorafenib (initiated based on molecular testing confirming a BRAF V600E mutation).
Results
Surgical and pathological outcomes
Surgico-pathological findings are summarized in Table 2. Among the five patients undergoing abdominoperineal resection (APR), four presented with advanced disease: two exhibited lymph node metastasis (pN1), and two demonstrated deep tumor invasion into the perirectal fat (pT3). In the four patients treated with wide local excision (WLE), tumors were confined to the submucosal layer (pT1).
Survival outcomes
With a median follow-up of 19 months (range: 12–30 months), systemic recurrence occurred in three patients (33.3%), involving the lung (n = 1), liver (n = 1), and peritoneum (n = 1). Disease progression resulted in two deaths. The 12-month and 24-month overall survival rates were 88.9 and 77.8%, respectively. Six patients (66.7%) remained disease-free at the last follow-up assessment.
Discussion
Our case series of 9 primary anorectal malignant melanoma (pARMM) patients reinforces established characteristics of this rare malignancy while highlighting clinically relevant nuances. The pronounced female predominance (88.9%, 8/9) exceeds the reported male-to-female ratio of ≈1:4 (23), suggesting a more skewed distribution (1:8) than previously documented. Although potentially influenced by limited sample size, this finding warrants investigation into undefined gender-specific biological or environmental factors in pARMM pathogenesis. All cases demonstrated de novo primary origins, pathologically confirmed by mucosal melanocytic infiltration and supported by multidisciplinary exclusion of extracutaneous primaries.
A critical diagnostic observation was the high proportion of amelanotic lesions (66.7%, 6/9), exceeding literature estimates (30–40%) (15). This likely contributed to frequent misdiagnosis as benign conditions (e.g., hemorrhoids) and the prolonged median symptom duration (75 days). Notably, IHC marker sensitivity was lower than expected: HMB-45/Melan-A (66.7%) and S-100 (55.6%) fell below reported rates (>95 and >97%, respectively) (16, 24). This discrepancy underscores the need for expanded IHC panels (e.g., SOX10, MITF) and expert pathology review, particularly for amelanotic lesions.
Regarding management, our experience supports sphincter-preserving surgery when oncologically appropriate. APR was reserved for larger/deeply invasive tumors (n = 5), while WLE (n = 4) achieved local control in selected superficial cases. Notably, no recurrences occurred in the WLE cohort during follow-up (median 19 months), aligning with meta-analyses showing comparable survival between approaches (19–21). Both disease-specific deaths occurred in the APR group; however, this cohort inherently harbored higher-risk features (nodal metastasis: 2/5; deep invasion: 3/5). This reinforces that tumor biology and staging—rather than surgical extent—drive prognosis, and WLE remains viable for early-stage tumors without compromising intermediate-term outcomes.
Study limitations include its retrospective design, small cohort size (reflecting disease rarity), median follow-up of 19 months (insufficient for long-term survival assessment), and absence of molecular profiling (e.g., KIT/BRAF/NRAS), which could elucidate biological heterogeneity.
Conclusion
In summary, pARMM lacks pathognomonic clinical manifestations, and ancillary diagnostic tools rarely establish a definitive preoperative diagnosis, frequently leading to misdiagnosis or delayed detection. Given its aggressive biological behavior and poor prognosis, early endoscopic biopsy of suspicious anorectal lesions is critical to improve diagnostic accuracy. Surgical resection remains the cornerstone of management, with strategies tailored to individual patient and tumor characteristics and prioritizing quality of life (QoL) preservation. Adjuvant therapies may be considered postoperatively, though evidence supporting survival benefit remains limited. Clinicians must maintain a high index of suspicion for pARMM to minimize diagnostic delays or errors and optimize treatment outcomes.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Affiliated Hospital of Hebei University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
Author contributions
XR: Conceptualization, Investigation, Data curation, Supervision, Validation, Methodology, Writing – review & editing, Resources, Funding acquisition, Formal analysis, Project administration, Writing – original draft. XJ: Writing – review & editing, Supervision, Methodology, Writing – original draft. TX: Formal analysis, Writing – original draft, Project administration, Conceptualization, Writing – review & editing. LL: Formal analysis, Writing – review & editing, Methodology, Supervision, Writing – original draft. QW: Writing – review & editing, Writing – original draft, Methodology, Investigation, Supervision. XS: Conceptualization, Methodology, Data curation, Writing – review & editing, Writing – original draft. MZ: Writing – original draft, Writing – review & editing, Investigation, Methodology, Conceptualization, Project administration, Supervision, Data curation.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by Youth Research Fund of Affiliated Hospital of Hebei University, grant number 2021Q024.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1614614/full#supplementary-material
References
1. Weinstock, MA. Epidemiology and prognosis of anorectal melanoma. Gastroenterology. (1993) 104:174–8. doi: 10.1016/0016-5085(93)90849-8
2. Blecker, D, Abraham, S, and Furth, EE. Melanoma in the gastrointestinal trac. Am J Gastroenterol. (1999) 94:3427–33.
3. Wu, J, Chen, H, Li, H, Tang, Y, Yang, L, Cao, S, et al. Antidepressant potential of chlorogenic acid - enriched extract from Eucommia ul- moides Oliver bark with neuron protection and promotion of serotonin release through enhancing synapsin I expression. Molecules. (2016) 21:260. doi: 10.3390/molecules21030260
4. Moore, WD. Recurrent melanosis of the rectum, after previous removal from the verge of the anus, in a man aged sixty-five. Lancet. (1857) 1:290.
5. Falch, C, Stojadinovic, A, Hann-von-Weyhern, C, Protic, M, Nissan, A, Faries, MB, et al. Anorectal malignant melanoma: extensive 45- year review and proposal for a novel staging classification. J Am Coll Surg. (2013) 217:324–35. doi: 10.1016/j.jamcollsurg.2013.02.031
6. Carcoforo, P, Raiji, MT, Palini, GM, Pedriali, M, Maestroni, U, Soliani, G, et al. Primary anorectal melanoma: an update. J Cancer. (2012) 3:449–53. doi: 10.7150/jca.5187
7. Meguerditchian, AN, Meterissian, SH, and Dunn, KB. Anorectal melanoma diagnosis and treatment. Dis Colon Rectum. (2011) 54:638–44. doi: 10.1007/DCR.0b013e31820c9b1b
8. Brady, MS, Kavolius, JP, and Quan, SH. Anorectal melanoma a 64-year experience at Memorial Sloan-Kettering Cancer Center. Dis Colon Rectum. (1995) 38:146–51. doi: 10.1007/BF02052442
9. Amaris, MA, Kallas, HE, Gonzalo, DH, and Orlando, FA. Gastric and colonic metastases of malignant melanoma diagnosed during endoscopic evaluation of symptomatic anemia presenting as angina: a case report. Front Med (Lausanne). (2023) 10:1268973. doi: 10.3389/fmed.2023.1268973
10. Schoneveld, M, Dev, K, and Vandw, N. Intussusception of the small intestine caused by a primary melanoma. Case Rep Gastroenterol. (2012) 6:15–9. doi: 10.1159/000335882
11. Malaguarnera, G, Madeddu, R, and Catania, VE. Anorectal mucosal melanoma. Oncotarget. (2018) 9:8785800. doi: 10.18632/oncotarget.23835
12. Paolino, G, Didona, D, Macrì, G, Calvieri, S, and Mercuri, SR. Anorectal melanoma. Noncutaneous Melanoma. Brisbane City, Australia: Exon Publications (2018).
13. Row, D, and Weiser, MR. Anorectal melanoma. Clin Colon Rectal Surg. (2009) 22:120–6. doi: 10.1055/s-0029-1223844
14. Pham, BV, Kang, JH, Phan, HH, Cho, MS, and Kim, NK. Malignant melanoma of anorectum: two case reports. Ann Coloproctol. (2021) 37:65–70. doi: 10.3393/ac.2020.01.07.1
15. Guan, X, and Ning, J. A rare case of primary anorectal malignant melanoma. Asian J Surg. (2023) 46:2746–7. doi: 10.1016/j.asjsur.2023.01.018
16. Ankeny, JS, Labadie, B, Luke, J, Hsueh, E, Messina, J, and Zager, JS. Review of diagnostic, prognostic, and predictive biomarkers in melanoma. Clin Exp Metastasis. (2018) 35:487–93. doi: 10.1007/s10585-018-9892-z
17. Nusrath, S, Thammineedi, SR, Patnaik, SC, Raju, KVVN, Pawar, S, Goel, V, et al. Anorectal malignant melanoma-defining the optimal surgical treatment and prognostic factors. Indian J Surg Oncol. (2018) 9:519–23. doi: 10.1007/s13193-018-0791-1
18. Matsuda, A, Miyashita, M, Matsumoto, S, Takahashi, G, Matsutani, T, Yamada, T, et al. Abdominoperineal resection provides better local control but equivalent overall survival to local excision of anorectal malignant melanoma: a systematic review. Ann Surg. (2015) 261:670–7. doi: 10.1097/SLA.0000000000000862
19. Jutten, E, Kruijff, S, Francken, AB, Lutke Holzik, MF, van Leeuwen, BL, van Westreenen, HL, et al. Surgical treatment of anorectal melanoma: a systematic review and meta-analysis. BJS Open. (2021) 5:107. doi: 10.1093/bjsopen/zrab107
20. Temperley, HC, O’Sullivan, NJ, Keyes, A, Kavanagh, DO, Larkin, JO, Mehigan, BJ, et al. Optimal surgical management strategy for treatment of primary anorectal malignant melanoma - a systematic review and meta-analysis. Langenbeck’s Arch Surg. (2022) 407:3193–200. doi: 10.1007/s00423-022-02715-1
21. Fadel, MG, Mohamed, HS, Weir, J, Hayes, AJ, Larkin, J, and Smith, MJ. Surgical management of primary anorectal melanoma: is less more? J Gastrointest Cancer. (2024) 55:714–22. doi: 10.1007/s12029-023-01009-z
22. Song, EJ, Scolyer, RA, and Damian, DL. Primary oesophageal melanoma- a case report. World J Surg Oncol. (2014) 12:77. doi: 10.1186/1477-7819-12-77
23. Chang, AE, Karnell, LH, and Menck, HR. The National Cancer Data Base report on cutaneous and noncutaneous melanoma: a summary of 84,836 cases from the past decade the American College of Surgeons Commission on Cancer and the American Cancer Society Cancer (1998) 83:1664–78.
Keywords: primary anorectal malignant, melanoma, wide local excision, misdiagnosis, case series
Citation: Ren X, Jin X, Xie T, Liu L, Wang Q, Sun X and Zhang M (2025) Case Report: Surgical management and prognostic factors in primary anorectal melanoma: a retrospective analysis of nine cases. Front. Med. 12:1614614. doi: 10.3389/fmed.2025.1614614
Edited by:
Frank A. Orlando, University of Florida, United StatesReviewed by:
Rahul Gupta, Synergy Institute of Medical Sciences, IndiaHenrique Kallas, UF Health Shands Hospital, United States
Copyright © 2025 Ren, Jin, Xie, Liu, Wang, Sun and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Meng Zhang, cGZrem0xOTkxQDE2My5jb20=
 Xiangxiang Ren
Xiangxiang Ren Xiaoshi Jin1
Xiaoshi Jin1 Tianhao Xie
Tianhao Xie Meng Zhang
Meng Zhang