- 1School of Chinese Medicine, Hong Kong Baptist University, Hong Kong, China
- 2School of Nursing, The Hong Kong Polytechnic University, Hong Kong, China
- 3Clinical Research Center, Zhujiang Hospital, Southern Medical University, Guangzhou, China
- 4Chung Chi College, The Chinese University of Hong Kong, Hong Kong, China
- 5School of Chinese Medicine, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong, China
- 6School of Biological Sciences, Nanyang Technological University, Singapore, Singapore
Introduction: Patients recovering from long COVID often endure a spectrum of neuropsychiatric symptoms, including cognitive impairment, memory deficits, mood disturbances and sleep disorders, that significantly impact their quality of life. Acupuncture, particularly electroacupuncture, has shown promise in addressing these symptoms. Currently there is no high-quality clinical trial for acupuncture on long COVID neuropsychiatric symptoms.
Methods and analysis: In this 24 weeks, sham-controlled, patient-assessor-blinded randomized trial, 150 long COVID patients will be equally allocated to either an electroacupuncture group (EAG) or a sham control group (SCG). Each subject will receive a total of 32 intervention sessions over a 16 weeks intervention phase (two sessions each week) and will be followed up for an additional 8 weeks. Primary outcomes include changes in the Mini-Mental State Examination (MMSE) and the Chinese version of the Beck Depression Inventory (CBDI) scores. Secondary outcomes include the Insomnia Severity Index (ISI), Brief Fatigue Inventory-Taiwan (BFI-T), and the Short Form 12 (SF-12). All outcomes will be assessed at baseline and then at 4 weeks intervals during both the treatment and post-treatment periods.
Discussion: This trial aims to generate robust clinical data on the therapeutic effects of electroacupuncture for long COVID. The anticipated results will clarify electroacupuncture’s value as a therapeutic option for neuropsychiatric symptoms in long COVID patients, contributing to evidence-based practice in integrative medicine.
Introduction
Since SARS-CoV-2 first emerged in late 2019, COVID-19 has profoundly disrupted both public health and socioeconomic systems around the globe. Although the World Health Organization (WHO) officially ended the global public health emergency on 5 May 2023, chronic health issues stemming from COVID-19 continue to affect many recovering patients. These lingering symptoms, which emerge 3 months after infection, persist for a minimum of 2 months, and cannot be attributed to alternative diagnoses, are classified by the WHO as post-COVID-19 condition, or long COVID (1). Epidemiological studies from various countries report high prevalence rates of long COVID (2–9), marking it as a pressing public health issue. This condition involves a broad spectrum of multisystem complaints, predominantly severe fatigue, exertion-related malaise, shortness of breath, palpitations, brain fog, and sensory disturbances such as loss of taste or smell (10–14).
Among the various symptoms triggered by long COVID, neuropsychiatric symptoms have garnered significant attention, including cognitive impairment (15–18), persistent fatigue, anxiety, and depression (10–14). The potential mechanisms underlying long COVID remain unclear, with prevailing theories including persistent viral reservoirs (19), persistent inflammation (20), sustained autoimmune responses (21), host microbiome factors (22, 23), and endothelial dysfunction leading to subsequent blood coagulation (24). However, these theories are still in the hypothesis stage, and effective treatments remain lacking. While existing approaches such as cognitive behavioral therapy (CBT) and nasal irrigation provide some symptom relief (25–27), their efficacy is inconsistent and often associated with side effects, highlighting the urgent need to develop safe and effective non-pharmacological therapies to address the potential long-term impacts of long COVID.
Acupuncture, a widely used technique in traditional Chinese medicine (TCM), is commonly applied in the treatment of neurological and neuropsychiatric disorders. Previous studies have shown that acupuncture can regulate central nervous system activity, thereby improving emotional issues such as anxiety and depression (28, 29). Additionally, acupuncture has demonstrated significant efficacy in treating fatigue syndrome and insomnia (30–32). During the pandemic, acupuncture was employed as an adjunctive treatment in China with formal clinical guidelines (33). Collectively, these results imply that acupuncture could serve as a potential therapeutic approach in managing long COVID. Within acupuncture stimulation protocols, both manual needling and electrical stimulation at acupoints are routinely employed (34). And electroacupuncture has shown unique advantages in enhancing neural regulation and mitigating cognitive impairments associated with central nervous system disorders (35, 36). Pei (37) demonstrated that electroacupuncture substantially alleviated deficits in spatial memory. Electroacupuncture can significantly making its intervention in neuropsychiatric symptoms more targeted (38). However, there is currently no electroacupuncture research addressing neuropsychiatric symptoms in long COVID, and high-quality clinical evidence is lacking.
Building on this, the present study employs a prospective, sham-controlled clinical protocol with participants randomly allocated to receive electroacupuncture within a TCM-guided individualized treatment framework, to rigorously assess its therapeutic impact on neuropsychiatric manifestations of long COVID. The innovation of this study lies in its integration of individualized treatment concepts with randomized controlled trial design, aiming to provide a safe and effective non-pharmacological intervention option for long COVID patients and to advance the clinical application of acupuncture in managing neuropsychiatric symptoms in the post-pandemic era.
Methods and analysis
Trial design and setting
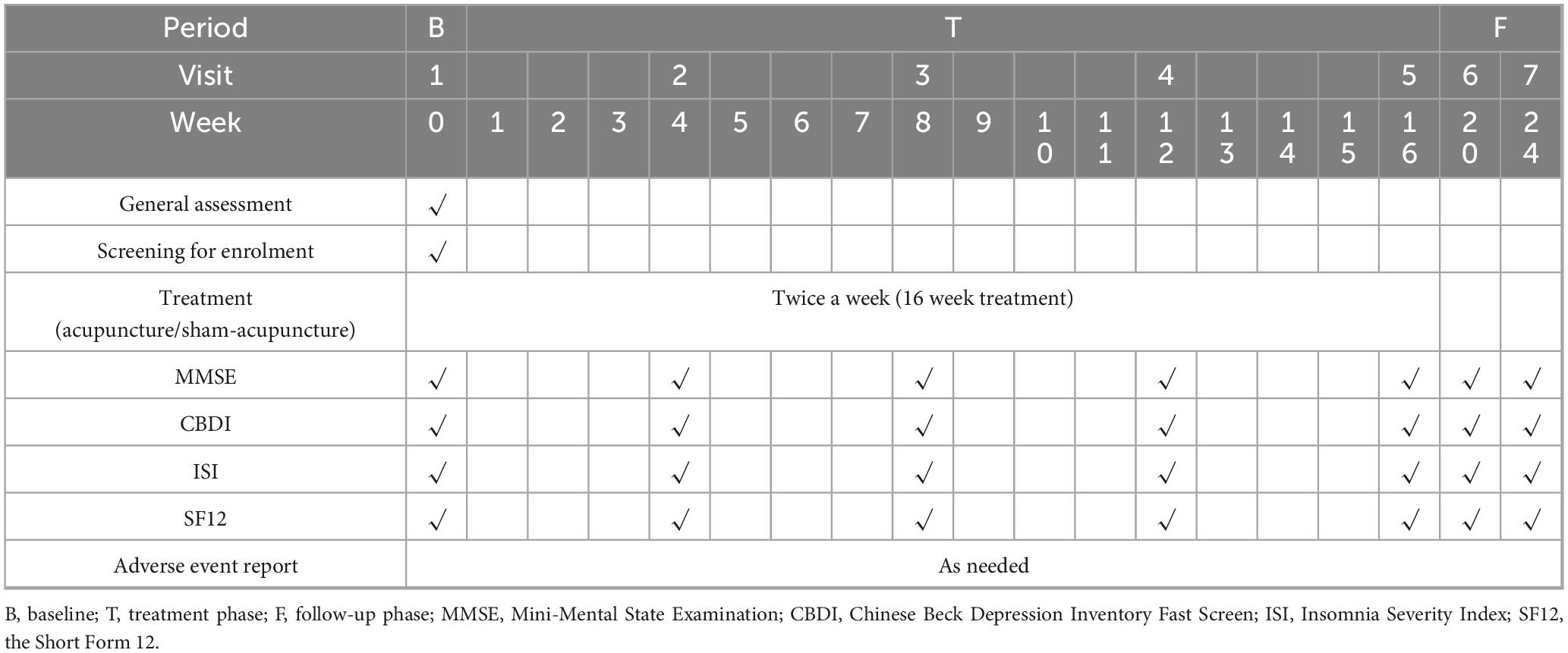
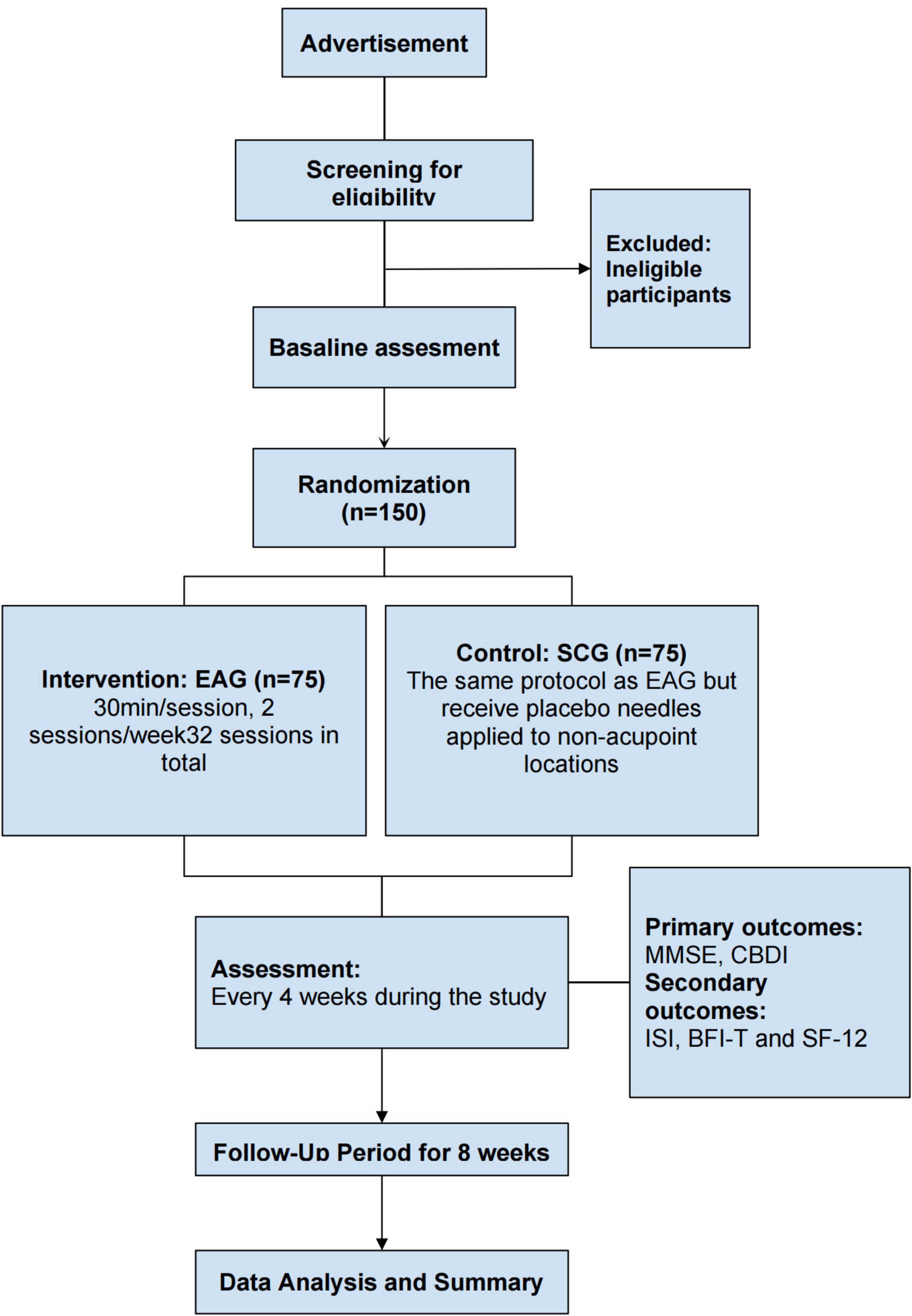
This is a prospective, patient-assessor-blinded, randomized, sham-controlled, multi-center trial designed to examine electroacupuncture’s impact on neuropsychiatric sequelae in long COVID survivors. A total of 150 eligible participants will be allocated in equal numbers to either the electroacupuncture group (EAG) or the sham control group (SCG). Each cohort will complete 32 intervention sessions over a 16 weeks period (2 sessions weekly) and will be followed up for an additional 8 weeks. The primary outcomes will be the changes in the Mini-Mental State Examination (MMSE) and the Chinese Beck Depression Inventory (CBDI) scores before and after treatment. Secondary outcomes will include changes in the Insomnia Severity Index (ISI), Brief Fatigue Inventory-Taiwanese (BFI-T), and the 12-item Short-Form Health Survey (SF-12) scores during the treatment and follow-up periods. These questionnaires (Appendix 1) will be assessed every 4 weeks during both the treatment and follow-up periods. The schedule detailing participant enrolment, intervention administration and outcome assessments are summarized in Table 1, while a flow diagram of the trial design is presented in Figure 1. This trial has been registered in clinicaltrials.gov (NCT05890508) and approved by the Research Ethics Committee of Hong Kong Baptist University (REC/21-22/0467).
Sample size
The target sample size was determined by reviewing prior trials using MMSE and BDI to assess the effectiveness of acupuncture versus sham treatment. The observed differences in MMSE and BDI scores between groups were 2.82 and 3.96 points, respectively (39, 40). Based on effect sizes of 0.76 for MMSE and 0.75 for BDI, the BDI-based between-group difference was smaller and was therefore selected for estimation of sample size to yield a more conservative result. To control the overall type I error and ensure sufficient statistical power, the following parameters were applied: a two-sided α level of 0.025 (Bonferroni correction), a power of 0.8, and an equal allocation ratio (1:1) between the acupuncture and sham acupuncture groups. Under these assumptions, the calculation yielded a requirement of 36 subjects per arm. Allowing for an anticipated 20% dropout rate, the target total sample size was adjusted to 90 cases. To increase robustness and ensure an adequate sample reserve for future subgroup analyses, we plan to recruit 150 participants (41–44). Sample size estimation was conducted using G*Power (Version 3.1).
Randomization and blinding
Participants will be randomized in a 1:1 ratio to either the EAG or SCG. Those allocated to the active treatment arm will undergo standardized electroacupuncture sessions, while the control arm will receive an equivalent sham procedure. A computer-generated simple randomization sequence will be created prior to the study, without stratification or block assignment. The randomization procedure will be conducted by the research unit of the School of Chinese Medicine, Hong Kong Baptist University. Allocation codes will be placed in sequentially numbered, opaque, tamper-evident envelopes and stored in a locked cabinet, with sole key custody maintained by the Principal Investigator (PI). All participants, outcome assessors, statisticians, and all research staff involved in patient contact-except the acupuncturists delivering the treatments, will remain blinded to treatment assignment. To assess the success of blinding, the James Blinding Index will be used at the end of the study. Emergency unblinding is allowed if serious adverse events arise and immediate measures are required to ensure participant safety. Any request for emergency unblinding must be submitted to the PI by co-investigator. Appropriate medical interventions will be provided promptly, and the reasons for unblinding will be documented in detail, including the date and signature of the responsible investigator. The participant involved will be withdrawn from the study.
Participants
Participants will be enrolled through two primary channels: (1) newspaper advertisements inviting interested individuals to contact the research team via telephone; or (2) referrals from clinics affiliated with the Hong Kong Hospital Authority. The relevant clinical teams will first contact potential participants, after which the research staff will conduct eligibility screenings. During the initial consultation, a physician (co-investigator) will conduct a detailed clinical history review and comprehensive examination. the PI or a designated co-investigator will furnish potential participants with detailed information regarding the study objectives, methodology, and any risks associated with acupuncture. Written informed consent (Appendix 2: Participant Consent Form) will be obtained prior to participation. Participants will also be informed of their right to withdraw at any point during the study. A total of 150 long COVID patients with neuropsychiatric symptoms will be recruited. The study will be conducted across six Chinese Medicine Research and Clinical Centers of Hong Kong Baptist University. Eligibility criteria are detailed in Table 2.
Participant retention and follow-up
This is a 24 weeks clinical trial, which subjects will need to take 32 sessions of acupuncture treatment and seven regular visits (treatment and follow-up). To bolster participant adherence, we will employ a three-pronged strategy. First, during the informed-consent interview, the research team will review the study timeline, outline possible adverse effects, clarify participant obligations, and provide continuous support and reassurance. Second, a two-week run-in period will screen out ineligible or poorly compliant volunteers before randomization. Third, a dedicated email address (cmcs@hkbu.edu.hk) and a direct telephone hotline will be activated to ensure prompt, proactive communication and address any participant queries throughout the trial.
Handling of withdraw and dropout
Participants who meet withdrawal criteria or voluntarily withdraw from the study will have their reasons documented whenever possible. Data collected before withdrawal will be retained for analysis under the intention-to-treat principle unless consent is specifically withdrawn for data usage. Efforts will be made to minimize dropout through proactive follow-ups, flexible scheduling, and continuous communication via the designated email and hotline. Adverse events (AEs) or concerns will be addressed promptly, and any serious issues will be reported to the PI and the ethics committee. Withdrawals and dropouts will be transparently reported in the final study results to ensure data integrity and scientific rigor.
Interventions
Following baseline assessment, participants randomized to the EAG will receive electroacupuncture stimulation from a registered Chinese Medicine practitioner with more than 5 years of Chinese medicine university education and more than 5 years of clinical experience. A systematic review (45) identified GV20 (Baihui), EX-HN1 (Sishencong), EX-HN3 (Yintang), SP6 (Sanyinjiao), ST36 (Zusanli), ST40 (Fenglong), and LR3 (Taichong) as the most frequently used acupuncture points for cognitive impairment. Accordingly, the rationale for choosing the acupoints is as follows: the acupoints on the head such as GV20 (Baihui) and EX-HN1 (Sishencong) have the local effectof regulating the brain function; the other acupoints on the lower limb, such as LR3 (Taichong) and ST36 (Zusanli), have the effect of soothing liver and strengthening spleenis (46). All acupoint locations adhere to WHO standard definitions (47), specific details of acupoints are summarized in Table 3 and Appendix 3.
• EAG: electroacupuncture will be performed using single-use, sterile acupuncture needles with a diameter of 0.20 mm. Needles will be inserted to a depth of 1–3 cm, based on the thickness of local tissue, in accordance with traditional TCM standards. The acupoints are subjected to 2–5 Hz electroacupuncture from an electroacupuncture device (Hwato, Electronic Acupuncture Treatment Instrument, Suzhou Medical Appliance Factory, Jiangsu, China) at an intensity (5–10 mA) that can produce a muscle twitch acceptable to the participant. Each participant allocated to the EAG will undergo 30 min electroacupuncture sessions twice weekly throughout the four-month intervention period (48).
• SCG: for the sham-control arm, Streitberger’s non-invasive acupuncture needles (Gauge 8 × 1.2/0.30 × 30 mm) will be positioned at non-acupoint sites approximately 0.5 cun from the corresponding acupoints, replicating the same insertion procedure as the acupuncture group (49). Additionally, the needles will be connected to inactive output ports on the electroacupuncture device. The stimulator will emit only the same beeping sound and flashing light continuously, thus producing a “pseudo stimulation” setup that maintains blinding integrity without delivering a therapeutic effect. This sham procedure has been previously validated for both its credibility and its ability to maintain participant blinding (50–54).
Outcomes measurement
Primary outcome
• Mini-Mental State Examination (MMSE)
Cognitive function will be evaluated using the Chinese version of MMSE scale (45), which assesses five domains: orientation (up to 10 points), memory (6 points), attention and calculation (5 points), language (8 points), and visuoconstructional ability (1 point). Total scores range from 0 to 30, with 21–24 indicating mild impairment, 10–20 moderate, and below 10 severe. Assessments will be performed at baseline, at weeks 4, 8, 12, and 16 (end of the intervention), and again 2 months after completion of treatment.
• Chinese Beck Depression Inventory (CBDI)
Depressive symptoms will be measured using the CBDI, a validated self-reported questionnaire comprising 21 items (maximum total score = 63) (55), with score ranges of 14–19 indicating mild, 20–28 moderate, and above 29 severe depression. Assessments will occur at baseline, at week 4, 8, 12, 16 (end of treatment) and again two months post-intervention.
Secondary outcome
Secondary endpoints include the score of ISI (56), BFI-T Form (57), and SF12 (58). All scales are assessed at baseline, at four-week intervals during the 16-week treatment phase (week 4, 8, 12, 16) and again at the 8-week post-treatment follow-up (Table 1). All adverse events will be prospectively recorded, with documentation of their intensity (mild, moderate or severe), duration, outcome, and potential relationship to the study.
Data management
To ensure impartiality, separate investigators, comprising the data collector, data manager, statistician and outcome assessor, will remain blinded throughout the trial. At week 0 (baseline), participants’ demographic characteristics, symptom severity, medical history, psychological status, and quality of life will be captured via standardized paper questionnaires and electronic surveys. All data will be securely housed on a dedicated network under the supervision of an independent data manager. Two researchers will independently export and assemble the dataset, then perform a cross-verification to confirm completeness and accuracy, any discrepancies will be resolved by a third reviewer referencing the original source documents.
Data processing and analysis
Descriptive metrics, namely recruitment rates, attrition rates, and treatment adherence, will be reported as count and percentage. All efficacy and safety endpoints will be analyzed under a modified intention-to-treat (ITT) framework. Statistical analyses will be conducted using SPSS for Windows (version 27.0). Statistical significance is defined as a two- sided P-value < 0.05. Baseline continuous variables will be expressed as mean ± SD (or median and interquartile range, as appropriate), and categorical variables as frequencies and percentages. Between-group comparisons of normally distributed continuous outcomes will use independent-samples t-test, while non-parametric continuous variables will be assessed with Mann-Whitney U test. Categorical data will be evaluated using chi-squared test or Fisher’s exact test. Group differences at weeks 16 and 24 will be tested via analysis of covariance (ANCOVA), adjusting for baseline values. Within-group changes over time will be examined with pair t-test for parametric data and Wilcoxon signed-rank test for non-parametric data. Based on the ITT, missing efficacy data will be imputed by last observation carried forward.
Data and Safety Monitoring Board (DSMB)
Data and Safety Monitoring Board will be established to oversee trial conduct and data integrity. The DSMB will convene at predetermined intervals to evaluate adherence to ethical and safety guidelines, verify the completeness and accuracy of collected data, and review overall study progress. It will also adjudicate adverse event reports and retain the authority to recommend suspension or early termination of the trial if participant safety or data validity is compromised.
Adverse events
Throughout the trial, the treating acupuncturist will systematically document all AEs. Using a standardized reporting form, each episode’s timing, characteristics, intensity, and presumed cause will be captured. Before each session, the acupuncturist will also actively solicit any delayed reactions since the prior visit. Immediate on-site management will be provided for all events, and participants experiencing serious adverse events will be withdrawn from the study.
Discussion
This work outlines a single-center, parallel-group trial employing randomization and sham control to rigorously assess the safety and therapeutic efficacy of electroacupuncture for neuropsychiatric manifestations of long COVID. The trial employs strict randomization and blinding procedures to ensure scientific rigor and reliability. The primary and secondary outcomes were chosen to assess multiple dimensions, including cognition, emotion, sleep, and fatigue, allowing for a comprehensive evaluation of the intervention’s potential benefits.
Scientific basis for acupoint selection
The selection of acupoints plays a crucial role in electroacupuncture treatment. This study selected key acupoints, including GV20 (Baihui), SP6 (Sanyinjiao), and ST36 (Zusanli), based on systematic reviews and expert consensus. Head acupoints are intended to regulate central nervous system functions, while lower limb acupoints emphasize overall balance and meridian flow. The sham control group involves non-acupoint stimulation and mock electrical stimulation to minimize non-specific effects, ensuring comparability between intervention and control groups.
Multidimensional outcome evaluation
This study combines subjective and objective assessment tools to comprehensively record patient outcomes. Primary outcomes include the MMSE and the CBDI, which reflect changes in cognition and emotion. Secondary outcomes, such as the ISI, BFI-T, and SF-12, further capture changes in sleep quality, fatigue severity, and overall health-related quality of life. The selection and application of these tools ensure sensitivity and accuracy in evaluating outcomes.
Relevance to clinical practice
Neuropsychiatric symptoms of long COVID significantly affect patients’ quality of life and social participation. However, there is a lack of research on non-pharmacological interventions for these symptoms. This study explores the potential of electroacupuncture as a personalized treatment approach to improve these symptoms. Electroacupuncture is widely used in traditional Chinese medicine. If its efficacy and safety are demonstrated, this study will provide evidence-based support for managing neuropsychiatric symptoms in long COVID. This could enhance clinical acupuncture practices and integrate traditional medicine into modern health management.
Potential limitations
This trial has certain limitations. First, the treatment schedule and frequency might pose challenges to patient adherence. Second, as a single-center study, the characteristics of participants may reflect only the local population, limiting external generalizability. Additionally, the sham stimulation may not fully eliminate psychological effects, requiring careful consideration during data analysis.
Strengths and limitations of this study
• This study is a rigorously designed, randomized, patient-assessor-blinded, sham-controlled trial, providing high-level evidence on the efficacy and safety of electroacupuncture for neuropsychiatric symptoms in long COVID patients.
• It employs validated outcome measures ensuring reliable assessment of cognitive, emotional, and sleep-related changes.
• The standardized electroacupuncture protocol, guided by expert consensus and evidence from systematic reviews, ensures consistent intervention across participants and centers.
• The study is limited to long COVID patients in Hong Kong, potentially reducing its generalizability to populations with different healthcare systems or cultural contexts.
• The sham acupuncture design, although validated, may not perfectly mimic the physiological and psychological effects of true acupuncture, potentially introducing bias.
Ethics statement
The studies involving humans were approved by the Research Ethics Committee of Hong Kong Baptist University (REC/21-22/0467). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
D-jW: Writing – original draft, Formal analysis, Project administration, Data curation, Methodology, Investigation, Conceptualization. C-wC: Project administration, Data curation, Writing – original draft, Investigation. WC: Investigation, Project administration, Formal analysis, Writing – original draft. W-fY: Validation, Conceptualization, Writing – review & editing. P-hC: Validation, Conceptualization, Writing – review & editing. CL: Conceptualization, Writing – review & editing. H-yC: Writing – review & editing, Conceptualization. SZ: Writing – review & editing, Conceptualization. LZ: Resources, Funding acquisition, Conceptualization, Writing – review & editing, Supervision, Project administration, Validation, Formal analysis, Methodology, Visualization.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by Health and Medical Research Fund (No. 20211471).
Acknowledgments
We would like to thank all the participants who agreed to be involved in this project. Furthermore, all treating practitioner who are responsible for providing participants in the interventions and the Clinical Department of Hong Kong Baptist University for providing the venue.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1620288/full#supplementary-material
Abbreviations
EAG, electroacupuncture group; SCG, sham control group; MMSE, the Mini-Mental State Examination; CBDI, the Chinese version of the Beck Depression Inventory; ISI, the Insomnia Severity Index; BFI-T, Brief Fatigue Inventory-Taiwan; SF-12, the Short Form 12; WHO, World Health Organization; CBT, cognitive behavioral therapy; PI, Principal Investigator; AEs, adverse events; ITT: intention-to-treat; DSMB, Data and Safety Monitoring Board.
References
1. World Health Organization. Post COVID-19 Condition (Long COVID). (2024). Available online at: https://www.who.int/europe/news-room/fact-sheets/item/post-covid-19-condition (accessed November 18, 2024).
3. Davis H, Assaf G, McCorkell L, Wei H, Low J, Reem Y, et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. eClinicalMedicine. (2021) 38:101019. doi: 10.1016/j.eclinm.2021.101019
4. Carvalho-Schneider C, Laurent E, Lemaignen A, Beaufils E, Bourbao-Tournois C, Laribi S, et al. Follow-up of adults with noncritical COVID-19 two months after symptom onset. Clin Microbiol Infect. (2021) 27:258–63. doi: 10.1016/j.cmi.2020.09.052
5. Moreno-Pérez O, Merino E, Leon-Ramirez J, Andres M, Ramos J, Arenas-Jiménez J, et al. Post-acute COVID-19 syndrome. Incidence and risk factors: a Mediterranean cohort study. J Infect. (2021) 82:378. doi: 10.1016/j.jinf.2021.01.004
6. Halpin S, McIvor C, Whyatt G, Adams A, Harvey O, McLean L, et al. Postdischarge symptoms and rehabilitation needs in survivors of COVID-19 infection: a cross-sectional evaluation. J Med Virol. (2021) 93:1013–22. doi: 10.1002/jmv.26368
7. Ballering A, Zon S, Hartman TC, Rosmalen JGM. Persistence of somatic symptoms after COVID-19 in the Netherlands: an observational cohort study. Lancet. (2022) 400:452. doi: 10.1016/S0140-6736(22)01214-4
8. Arnold D, Hamilton F, Milne A, Morley A, Viner J, Attwood M, et al. Patient outcomes after hospitalisation with COVID-19 and implications for follow-up: results from a prospective UK cohort. Thorax. (2020) 76:399. doi: 10.1136/thoraxjnl-2020-216086
9. Günster C, Busse R, Spoden M, Rombey T, Schillinger G, Hoffmann W, et al. 6-month mortality and readmissions of hospitalized COVID-19 patients: a nationwide cohort study of 8,679 patients in Germany. PLoS One. (2021) 16:e0255427. doi: 10.1371/journal.pone.0255427
10. Crook H, Raza S, Nowell J, Young M, Edison P. Long covid—mechanisms, risk factors, and management. BMJ. (2021) 374:n1648. doi: 10.1136/bmj.n1648
11. Bowe B, Xie Y, Al-Aly Z. Postacute sequelae of COVID-19 at 2 years. Nat Med. (2023) 29:2347. doi: 10.1038/s41591-023-02521-2
12. Huang C, Huang L, Wang Y, Li X, Ren L, Gu X, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. (2023) 401:e21. doi: 10.1016/S0140-6736(23)00810-3
13. Huang L, Li X, Gu X, Zhang H, Ren L, Guo L, et al. Health outcomes in people 2 years after surviving hospitalisation with COVID-19: a longitudinal cohort study. Lancet Respiratory Med. (2022) 10:863. doi: 10.1016/S2213-2600(22)00126-6
14. Zhang H, Huang C, Gu X, Wang Y, Li X, Liu M, et al. 3-year outcomes of discharged survivors of COVID-19 following the SARS-CoV-2 omicron (B.1.1.529) wave in 2022 in China: a longitudinal cohort study. Lancet Respiratory Med. (2024) 12:55–66. doi: 10.1016/S2213-2600(23)00387-9
15. Zhao S, Martin E, Reuken P, Scholcz A, Ganse-Dumrath A, Srowig A, et al. Long COVID is associated with severe cognitive slowing: a multicentre cross-sectional study. eClinicalMedicine. (2024) 68:102434. doi: 10.1016/j.eclinm.2024.102434
16. Liu Y, Chen Y, Wang Q, Wang L, Jiang L, Yang Y, et al. One-year trajectory of cognitive changes in older survivors of COVID-19 in Wuhan, China: a longitudinal cohort study. JAMA Neurol. (2022) 79:509. doi: 10.1001/jamaneurol.2022.0461
17. Cheetham N, Penfold R, Giunchiglia V, Bowyer V, Sudre C, Canas L, et al. The effects of COVID-19 on cognitive performance in a community-based cohort: a COVID symptom study biobank prospective cohort study. eClinicalMedicine. (2023) 62:102086. doi: 10.1016/j.eclinm.2023.102086
18. Hampshire A, Azor A, Atchison C, Trender W, Hellyer P, Giunchiglia V, et al. Cognition and memory after Covid-19 in a large community sample. N Engl J Med. (2024) 390:806. doi: 10.1056/NEJMoa2311330
19. Proal A, VanElzakker M. Long COVID or post-acute sequelae of COVID-19 (PASC): an overview of biological factors that may contribute to persistent symptoms. Front Microbiol. (2021) 12:698169. doi: 10.3389/fmicb.2021.698169
20. Morrow A, Sykes R, McIntosh A, Kamdar A, Bagot C, Bayes H, et al. A multisystem, cardio-renal investigation of post-COVID-19 illness. Nat Med. (2022) 28:1303–13. doi: 10.1038/s41591-022-01837-9
21. Mehandru S, Merad M. Pathological sequelae of long-haul COVID. Nat Immunol. (2022) 23:194–202. doi: 10.1038/s41590-021-01104-y
22. Liu Q, Mak J, Su Q, Yeoh Y, Lui G, Ng S, et al. Gut microbiota dynamics in a prospective cohort of patients with post-acute COVID-19 syndrome. Gut. (2022) 71:544–52. doi: 10.1136/gutjnl-2021-325989
23. Sherif Z, Gomez C, Connors T, Henrich T, Reeves W. RECOVER mechanistic pathway task force. Pathogenic mechanisms of post-acute sequelae of SARS-CoV-2 infection (PASC). Rosen CJ, Zaidi M, eds. eLife. (2023) 12:e86002. doi: 10.7554/eLife.86002
24. Osiaevi I, Schulze A, Evers G, Harmening K, Vink H, Kümpers P, et al. Persistent capillary rarefication in long COVID syndrome. Angiogenesis. (2023) 26:53–61. doi: 10.1007/s10456-022-09850-9
25. National Institute for Health and Care Excellence (NICE). COVID-19 Rapid Guideline: Managing the Long-Term Effects of COVID-19. (2024). Available online at: http://www.ncbi.nlm.nih.gov/books/NBK567261/ (accessed November 20, 2024).
26. Suran M. VA finds nirmatrelvir associated with lower risk of long COVID. JAMA. (2022) 328:2386. doi: 10.1001/jama.2022.20051
27. Vaira L, Hopkins C, Petrocelli M, Lechien J, Cutrupi S, Salzano G, et al. Efficacy of corticosteroid therapy in the treatment of long- lasting olfactory disorders in COVID-19 patients. Rhinology. (2021) 59:21–5. doi: 10.4193/Rhin20.515
28. Yang N, Lin L, Li Y, Li H, Cao Y, Tan C, et al. Potential mechanisms and clinical effectiveness of acupuncture in depression. Curr Neuropharmacol. (2022) 20:738. doi: 10.2174/1570159X19666210609162809
29. Smith C, Armour M, Lee M, Wang L, Hay P. Acupuncture for depression. Cochrane Database Syst Rev. (2018) 2018:CD004046. doi: 10.1002/14651858.CD004046.pub4
30. Fang Y, Yue B, Ma H, Yuan Y. Acupuncture and moxibustion for chronic fatigue syndrome: a systematic review and network meta-analysis. Medicine. (2022) 101:e29310. doi: 10.1097/MD.0000000000029310
31. Kim S, Lee S, Kim J, van den Noort M, Bosch P, Won T, et al. Efficacy of acupuncture for insomnia: a systematic review and meta-analysis. Am J Chin Med. (2021) 49:1135–50. doi: 10.1142/S0192415X21500543
32. Zhao F, Spencer S, Kennedy G, Zheng Z, Conduit R, Zhang W, et al. Acupuncture for primary insomnia: effectiveness, safety, mechanisms and recommendations for clinical practice. Sleep Med Rev. (2024) 74:101892. doi: 10.1016/j.smrv.2023.101892
33. Liu W, Guo S, Wang F, Hao Y. Understanding of guidance for acupuncture and moxibustion interventions on COVID-19 (Second edition) issued by CAAM. World J Acupuncture Moxibustion. (2020) 30:1. doi: 10.1016/j.wjam.2020.03.005
34. Evans R, McAuley H, Harrison E, Shikotra A, Singapuri A, Sereno M, et al. Physical, cognitive, and mental health impacts of COVID-19 after hospitalisation (PHOSP-COVID): a UK multicentre, prospective cohort study. Lancet Respiratory Med. (2021) 9:1275. doi: 10.1016/S2213-2600(21)00383-0
35. Iadecola C, Anrather J, Kamel H. Effects of COVID-19 on the nervous system. Cell. (2020) 183:16. doi: 10.1016/j.cell.2020.08.028
36. Cheuk D, Yeung W, Chung K, Wong V. Acupuncture for insomnia. Cochrane Database Syst Rev. (2012) 2012:CD005472. doi: 10.1002/14651858.CD005472.pub3
37. Lin C, Chen M, Yu S, Ju M. Chronic electrical stimulation of four acupuncture points on rat diabetic neuropathy. In: Proceedings of the 2005 IEEE Engineering in Medicine and Biology 27th Annual Conference. Piscataway, NJ: IEEE (2005). p. 4271–4. doi: 10.1109/IEMBS.2005.1615408
38. Chen X, Lü W. A comparative study on the local effects of manual acupuncture and electroacupuncture in the acupoint area. J Clin Acupuncture Moxibustion. (2022) 7:6–9. doi: 10.19917/j.cnki.1005-0779.022125
39. Tan T, Wang D, Huang J. Modulatory effects of acupuncture on brain networks in mild cognitive impairment patients. Neural Regeneration Res. (2017) 12:250. doi: 10.4103/1673-5374.200808
40. Yang W, Yu X, Zhao N. Clinical observation of kidney-tonifying and mind-calming acupuncture therapy in the treatment of perimenopausal insomnia. J Acupunct Tuina Sci. (2024) 22:48–57. doi: 10.1007/s11726-023-1415-z
41. Brookes S, Whitley E, Peters T, Mulheran P, Egger M, Davey Smith G. Subgroup analyses in randomised controlled trials: quantifying the risks of false-positives and false-negatives. Health Technol Assess. (2001) 5:1–56. doi: 10.3310/hta5330
42. Brookes S, Whitely E, Egger M, Smith G, Mulheran P, Peters T. Subgroup analyses in randomized trials: risks of subgroup-specific analyses; power and sample size for the interaction test. J Clin Epidemiol. (2004) 57:229–36. doi: 10.1016/j.jclinepi.2003.08.009
43. Zahouani I, Desmeules F, Perreault K, Campeau-Lecours A, Best K, Beaulieu-Bonneau S, et al. Physical and cognitive impairments in people suffering from long COVID: protocol for a longitudinal population-based cohort study. BMJ Open. (2023) 13:e064054. doi: 10.1136/bmjopen-2022-064054
44. Eliason S. Maximum Likelihood Estimation: Logic and Practice. Thousand Oaks, CA: SAGE Publications (1993).
45. He W, Li M, Han X, Zhang W. Acupuncture for mild cognitive impairment and dementia: an overview of systematic reviews. Front Aging Neurosci. (2021) 13:647629. doi: 10.3389/fnagi.2021.647629
46. Han Z, Zhang Y, Wang P, Tang Q, Zhang K. Is acupuncture effective in the treatment of COVID-19 related symptoms? Based on bioinformatics/network topology strategy. Brief Bioinform. (2021) 22:bbab110. doi: 10.1093/bib/bbab110
47. World Health Organization. WHO Standard Acupuncture Point Locations in the Western Pacific Region. Geneva: World Health Organization (2008).
48. Zhang B, Zhang K, Tang Q, Sun K, Han Z. Acupuncture for breathlessness in COVID-19: a protocol for systematic review and meta-analysis. Medicine. (2020) 99:e20701. doi: 10.1097/MD.0000000000020701
49. Zhang Q, Xu X, Sun S, Cao F, Li J, Qi X, et al. Efficacy of acupuncture and moxibustion in adjuvant treatment of patients with novel coronavirus disease 2019 (COVID-19): a protocol for systematic review and meta analysis. Medicine. (2020) 99:e21039. doi: 10.1097/MD.0000000000021039
50. Jia H, Han Z, Zhang K, Tang Q, Sun K, Huang H, et al. Acupuncture and related interventions for anxiety in coronavirus disease 2019: a protocol for systematic review and meta-analysis. Medicine. (2020) 99:e21317. doi: 10.1097/MD.0000000000021317
51. Xie C, Wen X, Jiang L, Xie M, Fu WB. Validity of the “Streitberger” needle in a Chinese population with acupuncture: a randomized, single-blinded, and crossover pilot study. Evid Based Compl Alternative Med. (2013) 2013:251603. doi: 10.1155/2013/251603
52. Streitberger K, Kleinhenz J. Introducing a placebo needle into acupuncture research. Lancet. (1998) 352:364–5. doi: 10.1016/S0140-6736(97)10471-8
53. Harris R, Zubieta J, Scott D, Napadow V, Gracely R, Clauw D. Traditional Chinese acupuncture and placebo (Sham) Acupuncture are differentiated by their effects on μ-opioid receptors (MORs). Neuroimage. (2009) 47:1077–85. doi: 10.1016/j.neuroimage.2009.05.083
54. Zeng D, Yan X, Deng H, Li J, Xiao J, Yuan J, et al. Placebo response varies between different types of sham acupuncture: a randomized double-blind trial in neck pain patients. Eur J Pain. (2022) 26:1006–20. doi: 10.1002/ejp.1924
55. Su X, Wang L, Li J, Zhang N, Wang L, Shi G, et al. Acupuncture therapy for cognitive impairment: a delphi expert consensus survey. Front Aging Neurosci. (2020) 12:596081. doi: 10.3389/fnagi.2020.596081
56. White A, Filshie J, Cummings T. Clinical trials of acupuncture: consensus recommendations for optimal treatment, sham controls and blinding. Compl Therapies Med. (2001) 9:237–45. doi: 10.1054/ctim.2001.0489
57. Sun Y, Liu Y, Liu B, Zhou K, Yue Z, Zhang W, et al. Efficacy of acupuncture for chronic prostatitis/chronic pelvic pain syndrome : a randomized trial. Ann Intern Med. (2021) 174:1357–66. doi: 10.7326/M21-1814
58. Tu J, Yang J, Shi G, Yu Z, Li J, Lin L, et al. Efficacy of intensive acupuncture versus sham acupuncture in knee osteoarthritis: a randomized controlled trial. Arthritis Rheumatol. (2021) 73:448–58. doi: 10.1002/art.41584
Keywords: long COVID, electroacupuncture, neuropsychiatric symptoms, randomized clinical trial, protocol
Citation: Wei D-j, Chow C-w, Cheung WYH, Yeung W-f, Cao P-h, Liong C, Chen H-y, Zhang S and Zhong LLD (2025) Electro-acupuncture for long COVID neuropsychiatric symptoms: study protocol for a prospective, randomized sham-controlled, patient-assessor-blinded clinical trial. Front. Med. 12:1620288. doi: 10.3389/fmed.2025.1620288
Received: 29 April 2025; Accepted: 21 August 2025;
Published: 04 September 2025.
Edited by:
Iván Pérez-Neri, National Institute of Rehabilitation Luis Guillermo Ibarra Ibarra, MexicoReviewed by:
Qinhui Fu, Shanghai University of Traditional Chinese Medicine, ChinaRenato García González, National Institute of Rehabilitation Luis Guillermo Ibarra Ibarra, Mexico
Copyright © 2025 Wei, Chow, Cheung, Yeung, Cao, Liong, Chen, Zhang and Zhong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Linda L. D. Zhong, bGluZGEuemhvbmdAbnR1LmVkdS5zZw==
 Dong-jue Wei1
Dong-jue Wei1 Wing-fai Yeung
Wing-fai Yeung Pei-hua Cao
Pei-hua Cao Hai-yong Chen
Hai-yong Chen Shipping Zhang
Shipping Zhang Linda L. D. Zhong
Linda L. D. Zhong