- 1Department of Graduate Medical Education, Ocean University Medical Center, Brick Township, NJ, United States
- 2Faculty of Medicine & Surgery, University of Malta, Msida, Malta
- 3Rowan University School of Osteopathic Medicine, Stratford, NJ, United States
- 4Department of Graduate Medical Education, Tufts University School of Medicine, Boston, MA, United States
Introduction: Solitary adrenal metastasis from a primary esophageal malignancy is relatively rare. While there are case reports of aggressive treatment with esophagectomy and adrenalectomy providing long-term survival, the treatment paradigm is not well defined. Complications from esophageal adenocarcinoma and its treatment can significantly impact the patient’s quality of life and prognosis.
Case presentation: Our patient was treated with systemic therapy and, although he initially had a complete response, he later experienced local disease progression along with the development of additional metastatic sites. The patient began FOLFOX, a standard chemotherapy regimen for esophageal/gastric cancer. He experienced side effects such as fever, malaise, and constipation, which were symptomatically managed. Given these side effects, the patient’s FOLFOX regimen underwent a 25% dose reduction of the 5FU bolus. A PET/CT scan after three months showed a marked response to chemotherapy, with complete resolution of detectable disease. The patient reported fatigue, bone pain managed with Neulasta, nausea controlled with antiemetics, and neuropathy in his feet. The patient then began a new chemotherapy regimen with Taxol/Ramucirumab with dose modifications in response to side effects. Continued adjustments to the treatment and dosages were made for progressive side effects and the patient elected to receive palliative radiation to the esophagus, along with holistic supportive care. The treatment plan shifted to palliative care, focusing on quality of life, rather than curative, due to the complexities of his cancer.
Conclusion: While aggressive treatments may offer hope for a cure in select patients with isolated adrenal metastasis from esophageal cancer, the general approach should remain cautious, with systemic therapy as the first line of defense. This case highlights the need for careful selection to identify patients who may benefit from aggressive surgical treatment. Ongoing research and clinical trials are needed to better define treatment protocols and improve outcomes for this challenging patient group.
Introduction
Esophageal adenocarcinoma is a malignancy originating from the glandular tissue of the esophagus, and is characterized by its aggressive progression and poor prognosis; only 16% of patients survive 5 years after diagnosis and the median survival time is less than 1 year (1). The disease course often begins with Barrett’s esophagus, which is associated with lifestyle factors such as diet, obesity, and gastroesophageal reflux disease (2). Pathology evolves from normal histology to dysplasia and metaplasia, and eventually adenocarcinoma, with the potential to metastasize to distant organs, including the adrenal glands (3).
The treatment of esophageal adenocarcinoma is multifaceted and depends on the stage of the disease (4). Early-stage cancers may be treated with surgery alone, while advanced stages often require a combination of chemotherapy, radiation therapy, and surgery (4). Immunotherapy has also emerged as a treatment option, particularly for cases that do not respond to conventional therapies (5). The approach to treating esophageal adenocarcinoma has traditionally centered around surgery, especially in cases where the cancer is localized and resectable (6). However, for cases with distant metastasis, such as to the adrenal glands, chemotherapy, often in combination with radiation therapy, is typically employed (6). Adrenalectomy may be considered when metastasis is confined to the adrenal gland and the primary cancer is either well-controlled or resectable (6).
Complications from esophageal adenocarcinoma and its treatment can significantly impact the patient’s quality of life and prognosis (7). These may include difficulty swallowing, bleeding, pain, and blockages in the esophagus (7). This case report of solitary adrenal metastasis from esophageal adenocarcinoma underscores the rarity of such metastatic presentations and highlights the importance of a comprehensive approach to diagnosis and management (7).
Case description
A 66-year-old male veteran of the Vietnam war with a past medical history of coronary artery disease and hyperlipidemia presented with progressive dysphagia, initially to solid food and later to liquids, accompanied by odynophagia. He reported a long-standing sensation of a lump in his chest, food regurgitation, and heartburn. The patient had no history of cigarette smoking or alcohol use and no significant family history.
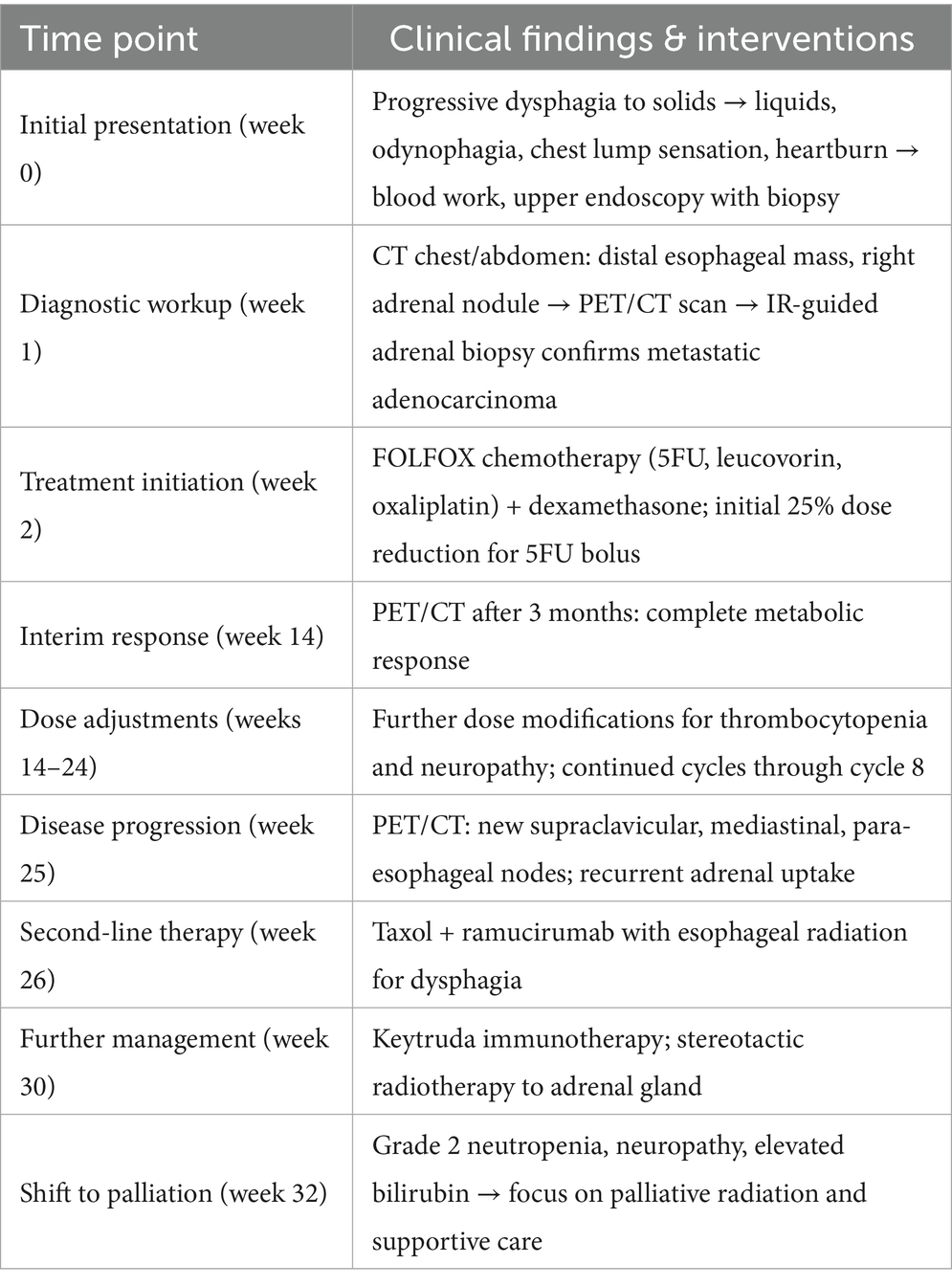
Initial assessments included a review of the patient’s medical history, blood work, and imaging (Supplementary materials). Diagnostic procedures revealed gastritis and an esophageal mass in the lower third of the esophagus, with a biopsy confirming moderately to poorly differentiated esophageal gastroesophageal junction adenocarcinoma. A colonoscopy identified tubular adenomas, and a CT scan showed abnormal lymph nodes near the distal esophagus, a mass in the right adrenal gland suggestive of possible metastasis, an enlarged prostate, and parapelvic renal cysts prompting further evaluation with a PET scan (Figure 1) and an IR-guided biopsy. Biopsy of the right adrenal gland confirmed metastatic adenocarcinoma, most consistent with a foregut/midgut primary origin (Figure 2).
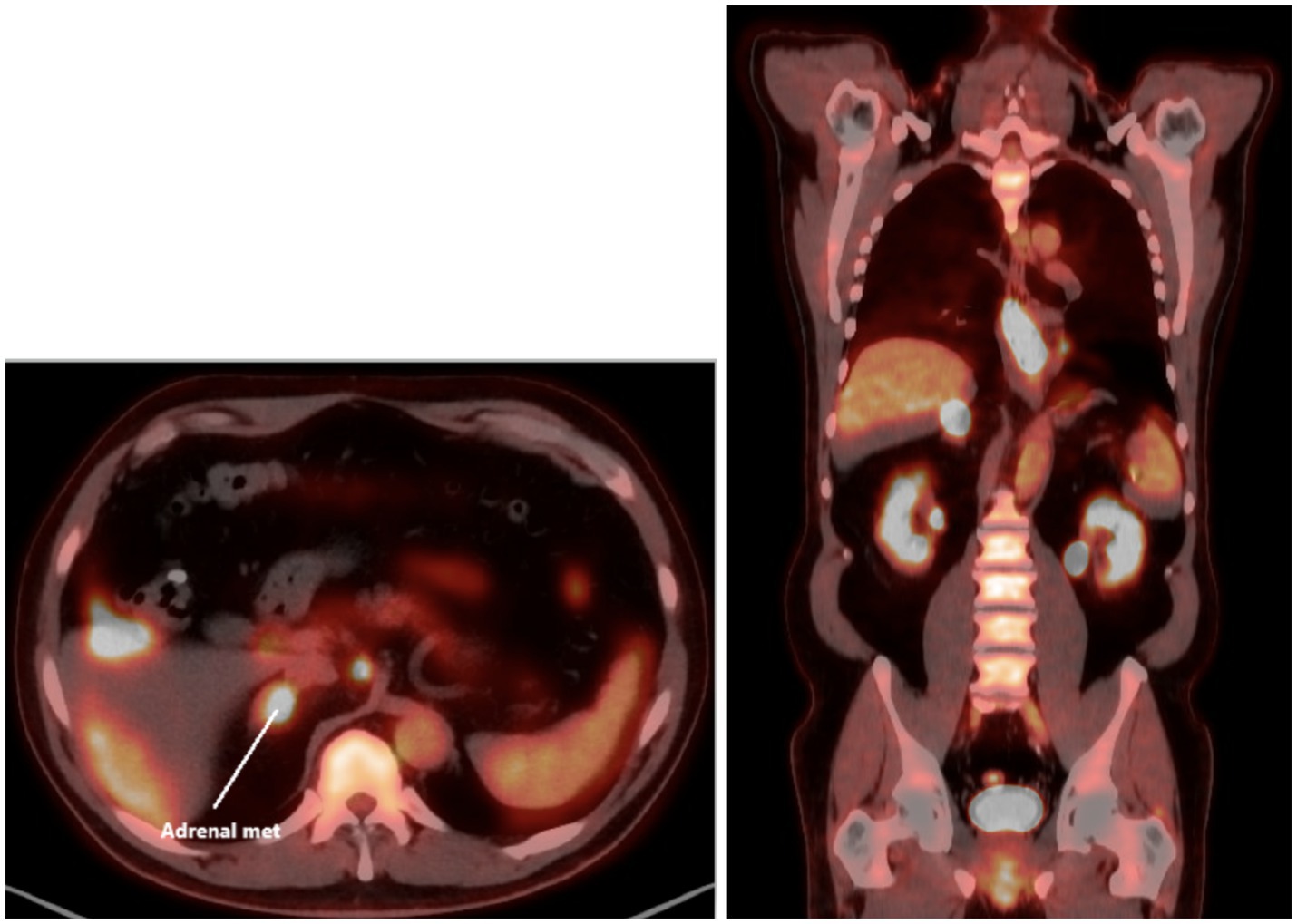
Figure 1. Adrenal metastasis on axial PET scan before treatment (left) and initial PET coronal image before systemic therapy shows esophageal and adrenal lesions (right).
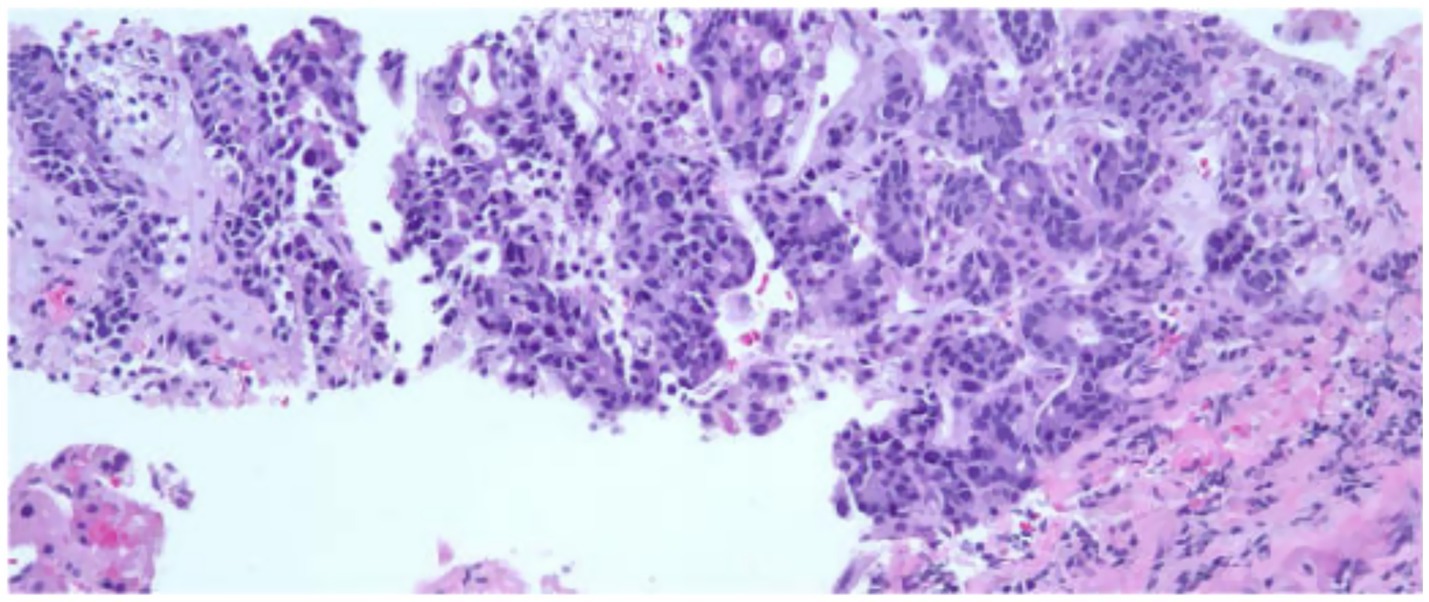
Figure 2. The adrenal gland tissue exhibits malignant glands within a desmoplastic stroma. The malignant cells exhibit pleomorphic nuclei with vesicular chromatin, prominent nucleoli and moderate amounts of amphophilic cytoplasm. Immunoperoxidase studies performed demonstrate the malignant cells to be positive for AE1/AE3, CK7, CDX2 and weakly positive for CK20. The Tumor Cells are negative for PAX8, NKX3.1, TTF-1 and GATA3. This immunohistochemical staining profile is consistent with foregut midgut primary and the pancreaticobiliary tree. Patient has a history of esophageal adenocarcinoma and this metastasis is consistent with metastatic esophageal adenocarcinoma.
The patient began FOLFOX, a standard chemotherapy regimen for esophageal/gastric cancer. The regimen included fluorouracil, leucovorin, and OXALIplatin, supplemented with dexamethasone. He experienced side effects such as fever, malaise, and constipation, which were symptomatically managed. Given these side effects, the patient’s FOLFOX regimen underwent a 25% dose reduction of the 5FU bolus. Follow-up visits before cycles 6 and 10 confirmed the patient’s ability to continue treatment, albeit with further dose reductions and modifications due to thrombocytopenia and neuropathy. A PET/CT scan after three months showed a marked response to chemotherapy, with complete resolution of detectable disease (Figure 3). The patient reported fatigue, bone pain managed with Neulasta, nausea controlled with antiemetics, and neuropathy in his feet (see Table 1).
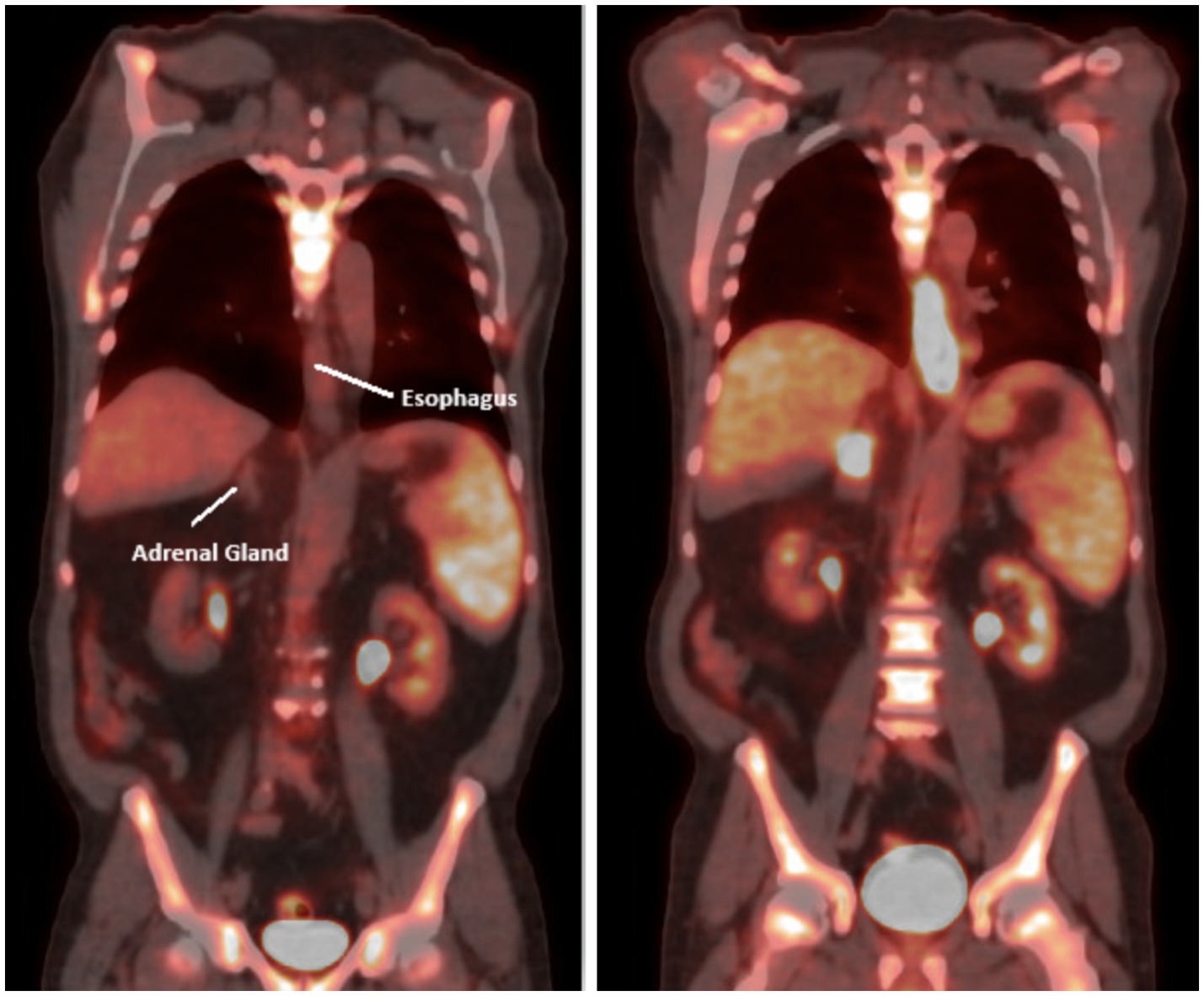
Figure 3. PET scan coronal view after 4–5 cycles of chemotherapy (left) showing interval decrease in the circumferential thickening and FDG activity associated with the distal 3rd of the thoracic esophagus and repeat PET scan after 2 additional cycles showed significant progression of disease both in the esophagus and adrenals (right).
It was decided to give an additional 2 cycles of chemotherapy due to its encouraging effect and to target undetectable cells. However, a repeat PET scan after 2 additional cycles indicated disease progression (Figure 2). The scan highlighted several areas of concern, including a right supraclavicular lymph node, focal thickening of the distal esophageal wall, and nodes in the mediastinum and paraesophageal regions, all with increased uptake indicating hypermetabolic activity. The right adrenal nodule and a retroperitoneal lymph node also showed increased uptake. The patient then began a new chemotherapy regimen with Taxol/Ramucirumab with dose modifications in response to side effects. Radiation therapy to the esophagus was also initiated to address dysphagia.
As the treatment progressed, the patient experienced grade 2 neutropenia and elevated bilirubin levels, necessitating further chemotherapy dose adjustments Oxaliplatin was switched to Abraxane due to progressive neuropathy. The treatment plan included a cycle of Keytruda, following mixed responses on PET/CT and subsequent stereotactic radiosurgery (SRS) to the right adrenal gland. Continued adjustments to the treatment and dosages were made for progressive side effects and the patient elected to receive palliative radiation to the esophagus, along with holistic supportive care. The treatment plan shifted to palliative care, focusing on quality of life, rather than curative, due to the complexities of his cancer.
Discussion
Patients with isolated adrenal metastasis from an esophageal primary cancer present a unique challenge in oncological management (8). The rarity of such cases means that there is no consensus on the optimal treatment strategy (8). Some clinicians advocate for an aggressive approach with curative intent, which may include esophagectomy and adrenalectomy (9). This is supported by a handful of case reports demonstrating long-term survival following such interventions (10–12). However, these cases are exceptional and not indicative of the general prognosis for this patient population.
The lack of a well-defined treatment paradigm leads most oncologists to favor initial systemic therapy (13). The rationale behind this approach is to control systemic disease and assess the tumor’s responsiveness to chemotherapy before considering more invasive procedures (13). FOLFOX is commonly used in this context due to its efficacy in gastrointestinal cancers (11). After 6 cycles of FOLFOX, our patient showed a complete response on PET scan, which was encouraging. The plan was to complete 8 cycles before transitioning to local therapy. However, a PET scan after 8 cycles revealed disease progression, including recurrence of adrenal metastasis and new metastatic sites. In similar cases where surgery is performed, median survival has been reported at 36 months from diagnosis (11). Adrenal metastasis were commonly identified on the following imaging modalities: CT, PET, MRI or after a postoperative follow-up from an endoscopy for the primary site (11, 12).
This progression despite systemic therapy highlights the aggressive nature of esophageal cancer and its propensity for metastasis (11). Moreover, patients with recurrent esophageal carcinoma have an average survival of 4–6 months (11). Furthermore, as seen in our patient’s complicated management, malignant adrenal gland tumors secondary to esophageal carcinoma are rare with eight cases reported between 1995 and 2014 (11) while another study reported a 3% occurrence and a 12% on autopsy (12). This metastasis was observed to favor the male gender with a median age of 63, and currently lacks a defined algorithm of care (11). It underscores the need for continuous evaluation and adjustment of treatment plans based on disease dynamics (14, 15). The case also emphasizes the importance of considering palliative care options early in the treatment process, as the goal shifts from curative to quality-of-life improvement.
Dellaportas et al. (16) and Hadlich et al. (17) described similar cases of esophageal metastasis into the adrenals, and both recommended surgery as a curative treatment for this disease. However, in the case of our patient due to the degree of metastasis and the systemic symptoms, surgical resection was not recommended and our treatment goals had to shift to maintain as much of the patients quality of life as possible. The presentation and degree of metastasis of our patient as well as the in-eligibility for surgical resection provided a unique challenge that should be further investigated. “Adrenal Incidentalomas” as Doesburg et al. (18) describes have an unknown occurrence and significance rate in the setting of esophageal cancer. While many similar case reports describe resectable tumors, the unique challenge of advanced esophageal disease with the addition of such extensive metastasis proved to be an unforeseen obstacle to treatment.
Future studies should explore chemotherapeutic agents that can target advanced esophageal cancer while also successfully targeting progressive metastasis. Future studies should also focus on the patient’s overall health and quality of life. A multidisciplinary team may be instrumental in helping patients through the challenges of systemic therapies in hopes they could tolerate a more aggressive treatment approach then current literature describes.
Conclusion
While aggressive treatments may offer hope for a cure in select patients with isolated adrenal metastasis from esophageal cancer, the general approach should remain cautious, with systemic therapy as the first line of defense. This case highlights areas where ongoing research and clinical trials are needed to better define treatment protocols and improve outcomes for this challenging patient group.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval was not required for the studies involving humans because not applicable. As this is a case report, ethical approval was not required. Written informed consent was obtained from the patient for publication of this case report and any accompanying images. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
RM: Writing – original draft, Supervision, Writing – review & editing, Data curation, Formal analysis, Conceptualization. AM: Writing – review & editing, Writing – original draft. FM: Writing – review & editing, Writing – original draft. MA: Writing – original draft, Writing – review & editing. UJ: Writing – original draft, Visualization, Writing – review & editing, Formal analysis, Data curation. JJ: Writing – review & editing, Writing – original draft. PD: Formal analysis, Writing – original draft, Writing – review & editing, Validation, Data curation.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We would like to thank Dr. Brandon Goodwin DO, for his invaluable input in the synthesis of this manuscript. We would also like to thank Futures Forward Research Institute for their support and feedback.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1623443/full#supplementary-material
References
1. Rubenstein, JH, and Shaheen, NJ. Epidemiology, diagnosis, and Management of Esophageal Adenocarcinoma. Gastroenterology. (2015) 149:302–317.e1. doi: 10.1053/j.gastro.2015.04.053
2. Stawinski, PM, Dziadkowiec, KN, Kuo, LA, Echavarria, J, and Saligram, S. Barrett’s esophagus: an updated review. Diagnostics (Basel). (2023) 13:321. doi: 10.3390/diagnostics13020321
3. Zhang, M, Hou, Z-K, Huang, Z-B, Chen, X-L, and Liu, F-B. Dietary and lifestyle factors related to gastroesophageal reflux disease: a systematic review. Ther Clin Risk Manag. (2021) 17:305–23. doi: 10.2147/TCRM.S296680
4. Acharya, R, Mahapatra, A, Verma, HK, and Bhaskar, LVKS. Unveiling therapeutic targets for esophageal Cancer: a comprehensive review. Curr Oncol. (2023) 30:9542–68. doi: 10.3390/curroncol30110691
5. Peterson, C, Denlinger, N, and Yang, Y. Recent advances and challenges in Cancer immunotherapy. Cancers (Basel). (2022) 14:3972. doi: 10.3390/cancers14163972
6. Zaborowski, AM, and Prichard, RS. Adrenalectomy for metastases. Br J Surg. (2022) 109:1030–1. doi: 10.1093/bjs/znac315
7. Xu, Q-L, Li, H, Zhu, Y-J, and Xu, G. The treatments and postoperative complications of esophageal cancer: a review. J Cardiothorac Surg. (2020) 15:163. doi: 10.1186/s13019-020-01202-2
8. Buero, A, Nardi, WS, Chimondeguy, DJ, Pankl, LG, Lyons, GA, Gonzalez Arboit, D, et al. Outcomes of surgical treatment for isolated adrenal metastasis from non-small cell lung cancer. Ecancermedicalscience. (2021) 15:1322. doi: 10.3332/ecancer.2021.1322
9. Słodkowski, M, Wroński, M, Karkocha, D, Kraj, L, Śmigielska, K, and Jachnis, A. Current approaches for the curative-intent surgical treatment of pancreatic ductal adenocarcinoma. Cancers (Basel). (2023) 15:2584. doi: 10.3390/cancers15092584
10. Gupta, V, Allen-Ayodabo, C, Davis, L, Zhao, H, Hallet, J, Mahar, AL, et al. Patient-reported symptoms for esophageal Cancer patients undergoing curative intent treatment. Ann Thorac Surg. (2020) 109:367–74. doi: 10.1016/j.athoracsur.2019.08.030
11. Kanaya, N, Noma, K, Okada, T, Maeda, N, Tanabe, S, Sakurama, K, et al. A case of long-term survival after surgical resection for solitary adrenal recurrence of esophageal squamous carcinoma. Surg Case Rep. (2017) 3:61. doi: 10.1186/s40792-017-0337-8
12. Kashyap, R, Mittal, BR, Bhattacharya, A, and Singh, B. Unusual presentation of oesophageal carcinoma with adrenal metastasis. Indian J Nucl Med. (2012) 27:181–2. doi: 10.4103/0972-3919.112725
13. Liu, S-YM, Zheng, M-M, Pan, Y, Liu, S-Y, Li, Y, and Wu, Y-L. Emerging evidence and treatment paradigm of non-small cell lung cancer. J Hematol Oncol. (2023) 16:40. doi: 10.1186/s13045-023-01436-2
14. Klein, CA, and Stoecklein, NH. Lessons from an aggressive cancer: evolutionary dynamics in esophageal carcinoma. Cancer Res. (2009) 69:5285–8. doi: 10.1158/0008-5472.CAN-08-4586
15. Gautama, MSN, Damayanti, A, and Khusnia, AF. Impact of early palliative care to improve quality of life of advanced cancer patients: a meta-analysis of randomised controlled trials. Indian J Palliat Care. (2023) 29:28. doi: 10.25259/IJPC_153_2022
16. Dellaportas, D, Lykoudis, P, Gkiokas, G, Polymeneas, G, Kondi-Pafiti, A, and Voros, D. Solitary adrenal metastasis from esophageal adenocarcinoma: a case report and review of the literature. Case Rep Med. (2011) 2011:487875. doi: 10.1155/2011/487875
17. Hadlich, E, Carbonari, APC, Assef, MS, Araki, OM, Nakao, FS, Saieg, MTA, et al. Esophageal adenocarcinoma metastasis in the left adrenal gland diagnosed by endoscopic ultrasound-guided fine needle aspiration. Endosc Ultras. (2017) 6:142–4. doi: 10.4103/2303-9027.204809
Keywords: esophageal adenocarcinoma, adrenal metastasis, systemic therapy, FOLFOX, palliative care
Citation: Machchhar R, Al Mahrizi AD, Mossolem F, Abdeen M, Jain U, Jain J and Desai P (2025) Case Report: Solitary adrenal metastasis from esophageal adenocarcinoma. Front. Med. 12:1623443. doi: 10.3389/fmed.2025.1623443
Edited by:
Hisham F. Bahmad, University of Miami, United StatesReviewed by:
Mansi Singal, ESI-PGIMSR, Medical College and Hospital, IndiaJad El Masri, American University of Beirut, Lebanon
Copyright © 2025 Machchhar, Al Mahrizi, Mossolem, Abdeen, Jain, Jain and Desai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Riddhi Machchhar, cmlkZGhpci5tYWNoY2hoYXJAaG1obi5vcmc=
 Riddhi Machchhar
Riddhi Machchhar Ahmed Dawood Al Mahrizi
Ahmed Dawood Al Mahrizi Fatima Mossolem
Fatima Mossolem Mariam Abdeen
Mariam Abdeen Ujjwala Jain1
Ujjwala Jain1