- Department of Cardiovascular Surgery, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
Objective: Advanced heart failure in children sometimes requires mechanical circulatory support as a bridge to transplantation, with extracorporeal membrane oxygenation (ECMO) remaining a critical option despite its associated risks. The pediatric Sequential Organ Failure Assessment (pSOFA) may have potential in evaluating prognosis in ECMO-bridged candidates.
Methods: 188 Children underwent orthotopic heart transplantation in Union hospital, Tongji Medical College, Huazhong University of Science and Technology, between January 2018 and April 2025 were studied retrospectively, with 24 received ECMO assistance as a bridge to transplant. Patients were divided into two groups according to outcomes while discharged. Serial pediatric Sequential Organ Failure Assessment and other medical data during bridging were collected for comparison.
Results: 66.7% of the 24 patients survived to discharge, with mortality linked to younger age (p = 0.034), higher pre-ECMO pSOFA scores (p = 0.019), and congenital heart disease. ECMO cannulation was mostly peripheral (66.7%), with left heart decompression in 87.5%. External cardiopulmonary resuscitation (50% of cases) increased mortality risk (p = 0.027). The death group had higher peak/trough/average pSOFA scores, reinforcing its predictive value. Non-survivors had more complications (ECMO reuse, septic shock, neurological issues) after heart transplant. pSOFA trends distinguished outcomes: survivors showed declining scores (p = 0.006), and average pSOFA ≤8 predicted better survival (p = 0.003). ECPR patients had worse baselines but might recover with optimized management. Findings support pSOFA-guided risk stratification in ECMO-bridged HTx.
Conclusion: Continuous pSOFA monitoring effectively risk-stratifies ECMO-bridged pediatric transplant candidates, identifying high-risk patients after transplant. Planned ECMO initiation yields better outcomes than ECPR. These findings warrant prospective validation to optimize bridging strategies.
1 Introduction
Advanced heart failure (ADHF) is one of the leading causes of death in children with cardiovascular diseases. For children who do not respond to guideline-directed medical therapy, heart transplantation (HTx) remains the primary treatment. However, according to the Pediatric Heart Transplant Society (PHTS), the severe shortage of donor hearts leads to approximately 17% of children dying while waiting, with about 20.2–26% experiencing progressive hemodynamic deterioration requiring temporary mechanical circulatory support (tMCS) as a bridge to heart replacement therapy, including extracorporeal membrane oxygenation (ECMO)(4.7–5.7%) and ventricular assist device (VAD) (10–20.2%) (1, 2). Due to limitations in VAD size and domestic development in China, most children (especially those with low body weight) are not suitable for VAD. Union Hospital, Tongji Medical College, Huazhong University of Science and Technology is the largest institution for pediatric HTx nationwide, with 227 children undergoing HTx (including 2 re-transplant) since 2009, none of whom had used VAD as bridge to HTx. Therefore, although the use of VAD in children is increasing internationally, and studies have shown that the prognosis of VAD bridging is comparable to or better than ECMO (3, 4), and some even recommend using ECMO as a bridge-to-decision to transition to VAD (4), ECMO remains an important option for pediatric emergency bridging in short future, especially in China, due to its rapid establishment, biventricular support capability and fewer body weight restrictions.
However, compared to adult patients, pediatric populations, especially low-weight children, face unique clinical challenges, including the complexity and technical demands of cannulating, precise anticoagulation management, a higher proportion of neurological complications (10–20%) and infection risks due to immature immune systems (5–8). These factors make perioperative ECMO management a critical factor affecting transplant outcomes in these children. Multiple studies have shown that the transplant prognosis of children bridged with ECMO is significantly worse than that of children transplanted directly (3, 9), and children implanted under external cardiopulmonary resuscitation (ECPR) face the additional challenge of impact of cardiac arrest and low hemoperfusion. When ECMO initiation seems to be unavoidable, determining the optimal implantation timing, optimizing recipient status during bridging, and early identification of risk factors may improve outcomes for these children.
The pediatric sequential organ failure assessment (pSOFA) score was proposed in 2017 by Matics et al. (10) as an adaptation of the adult SOFA score, adjusted for age to create a pediatric version, covering respiratory, circulating, hepatic, renal, coagulating and neurological systems. Originally used to evaluate organ status in septic shock, its comprehensive system coverage and continuous scoring have led many to apply it to assess organ function and prognosis in other critically ill children (11–14), and it has gained increasing popularity. Currently, pSOFA has not been used to evaluate the status of children supported by ECMO, especially those bridged to HTx. This study retrospectively reviewed children who underwent HTx at our center, using pSOFA to assess their organ function before and during ECMO bridging, in order to guide ECMO management and prognosis prediction.
2 Materials and methods
2.1 Research objectives and study population
This research was designed to compare the peri-bridge and peri-heart transplantation clinical characteristics of ECMO-bridged pediatric candidates with different outcomes, and to explore whether pSOFA has the ability to differentiate between different groups. We screened 188 patients under 18 years of age who underwent HTx at Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, from January 2018 to April 2025, identifying 26 children who received veno-arterial ECMO (VA-ECMO) for bridging preoperatively. Two children who underwent ECMO bridging for retransplantation due to primary graft dysfunction (PGD) were excluded, leaving 24 cases included. Cases before 2018 were not considered for analyzing because of missing data and possible heterogeneity of medical protocols, as well as no application of ECMO for bridging. Children discharged in a moribund state with parents refusing further medical care were considered dead in hospital. The children were divided into two groups based on their living status while discharged, and the clinical characteristics of the two groups during the perioperative period, especially during ECMO support, were compared, which included pre-ECMO echocardiography results, blood test data, ECMO cannulating protocol and complications, as well as transplant surgery duration, donor characteristics, postoperative support, complications, hospital stay, and costs. pSOFA scores were collected once before ECMO and daily after ECMO implantation till the day before transplant. Few children underwent right heart catheterization for pulmonary artery pressure (PAP) measurement, which therefore was not included in this research. The study was approved by the Ethics Committee of Union Hospital, and all data were collected from the hospital’s medical record system.
2.2 ECMO implantation indications
The decision to implant ECMO was made jointly by cardiovascular surgeons, cardiac ICU physicians, and perfusionists. Inability to maintain circulation under standard cardiopulmonary resuscitation was an absolute indication for ECMO implantation, known as ECPR. However, if the patient’s circulation could barely be maintained with vasoactive drugs, whether to implant ECMO was controversial. Most centers relied on the comprehensive judgment of multiple physicians based on experiences to determine the need for ECMO. At our center, the primary indications for planned ECMO implantation included progressive oliguria, liver injury, severe arrhythmias, and gradually increasing doses of inotropic drugs, but there was no absolute cutoff value.
2.3 ECMO implantation and transplant protocol
Among the 24 children, 4 were transferred from other hospitals on ECMO. We obtained their ECMO implantation timing and methods, as well as whether they were implanted under ECPR, from medical records, but pre-implantation test results were unavailable. The remaining children underwent ECMO implantation at our center, with some receiving left heart decompression to alleviate left ventricular preload and pulmonary edema. For centrally cannulated children, venous cannulas were placed in the right atrium, with or without left atrial cannulation, and arterial cannulas were placed in the aorta. For peripherally cannulated children, most were cannulated via the right carotid artery and jugular vein, with 2 children cannulated via the right femoral vessels, with or without atrial septal shunt devices (aperture 4–10 mm). One child underwent a hybrid cannulation method with aortic cannulation and femoral vein/superior vena cava cannulation. All children were intubated before ECMO implantation and failed to wean from ECMO before transplantation. The surgery were all performed using the bicaval orthotopic heart transplantation method. Donor hearts were evaluated and collected by the cardiac surgeons in our department, and postoperative management was performed by the same team.
2.4 General ECMO management
All children initially received heparin anticoagulation with monitoring of coagulation function, aiming to maintain ACT at 180–200 s and APTT at 1.5–2 times the normal value. One child was switched to argatroban due to significant heparin induced thrombocytopenia (HIT), and another was switched to argatroban combined with low-molecular-weight heparin due to rapidly developing ECMO thrombosis despite high-dose heparin in the first 24 h. MAP was maintained at 50–70 mmHg according to age, with dopamine, dobutamine, epinephrine, captopril, or nitroprusside used for blood pressure control as needed. Aortic valve opening was observed with echocardiography in all children during ECMO support. Tracheal extubation was performed in children with relatively stable neurological and pulmonary conditions. Children who could not be extubated received intermittent sedation with dexmedetomidine and fentanyl, with neurological function assessed during sedation pauses.
2.5 Statistical methods
Measurement data are expressed as median (IQR) and analyzed using the Mann–Whitney U test while categorical variables are expressed as percentages (%)and analyzed using the chi-square test or Fisher’s exact test. Survival analysis was performed using the Breslow test and Kaplan–Meier survival curves. Statistical significance was defined as p < 0.05. All statistical analyses and figure plotting were performed using GraphPad Prism 10.3.1.
3 Results
3.1 Clinical characteristics during perioprative period
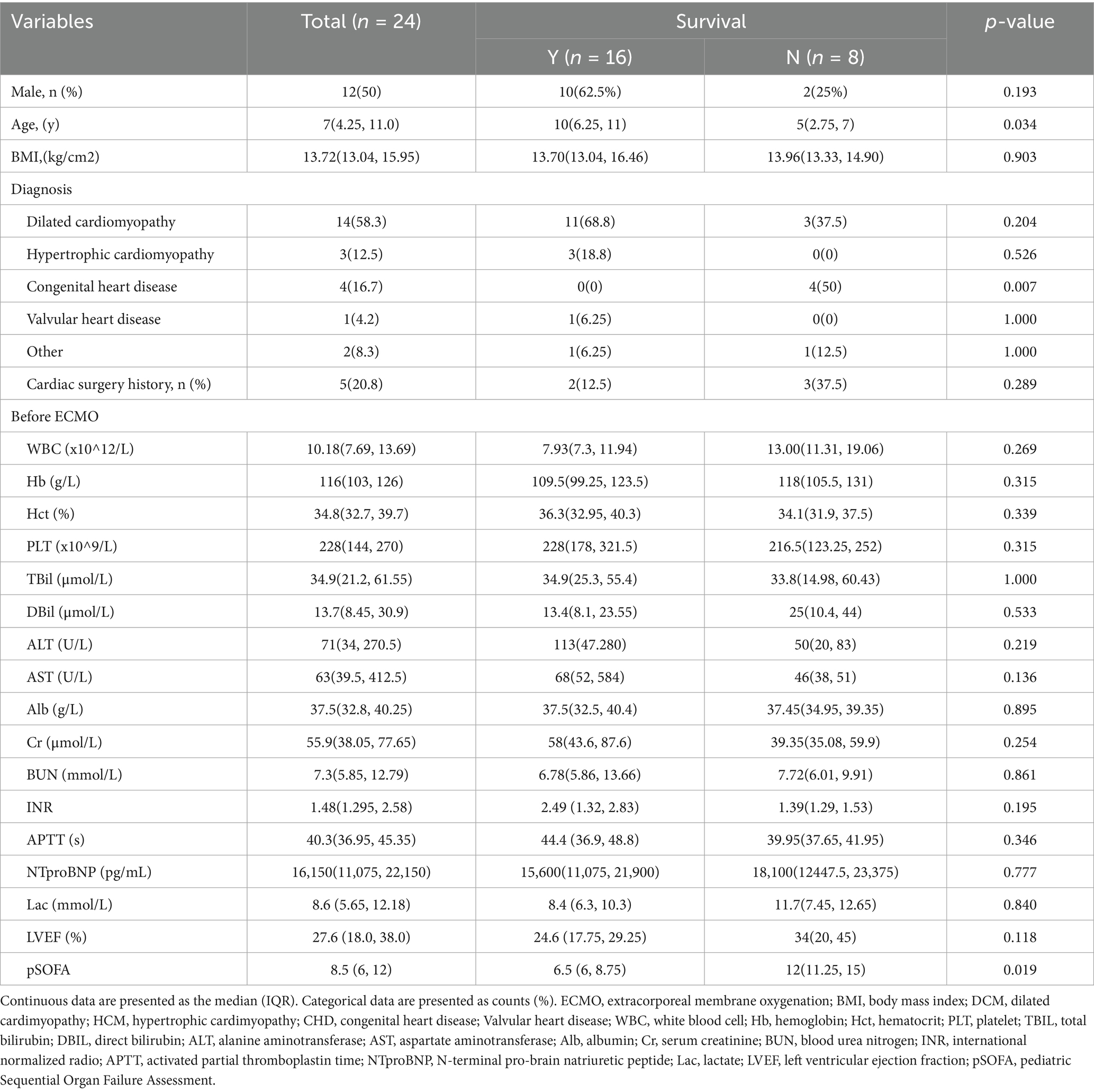
This study included 24 children under 18 years of age who underwent VA-ECMO bridging for orthotopic heart transplantation at our center from January 2018 to April 2025, all of whom were assessed as INTERMACS level 1 or 2. Children who received retransplantation during hospitalization were excluded (Figure 1). Sixteen children (66.7%) were alive while discharged, and eight (33.3%) were not. Among them, 12 were male (50%), with the youngest aged 2 years and the oldest 14 years, the lowest weight 10 kg, and the highest weight 55 kg. The median BMI was 13.72 (13.04, 15.95) kg/cm2. The predominant cause of ADHF was cardiomyopathy (70.8%), including dilated cardiomyopathy (DCM) in 14 children (58.3%) and hypertrophic cardiomyopathy (HCM) in 3 (12.5%), followed by congenital heart disease (CHD) in 4 (16.7%). The longest ECMO support duration was 32 days, and the shortest was 1 day. Most underwent echocardiography and necessary blood tests before ECMO implantation, with no significant differences in baseline characteristics. However, data showed the death group had relatively younger age (p = 0.034) and higher pSOFA score before ECMO initiation (p = 0.019) compared to the survival group (Table 1) with an overall median score of 8.5 (6, 12). Besides, heart congenital heart disease appeared to be a risk factor for death after ECMO-bridged HTx.
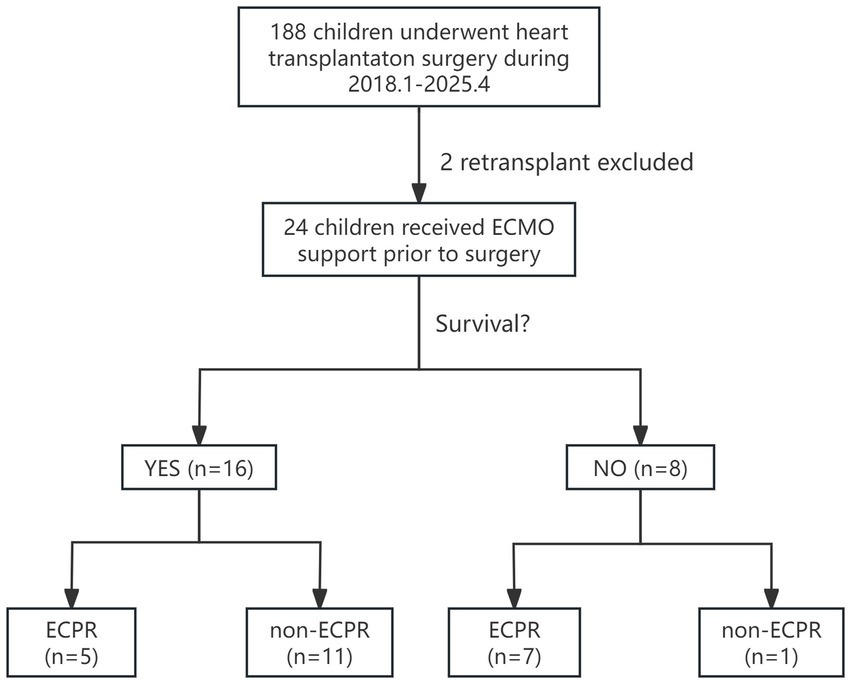
Figure 1. Enrollment, grouping, and in-hospital outcomes follow-up for children who received ECMO as a bridge to heart transplant. ECMO, extracorporeal membrane oxygenation; ECPR, extracorporeal membrane oxygenation.
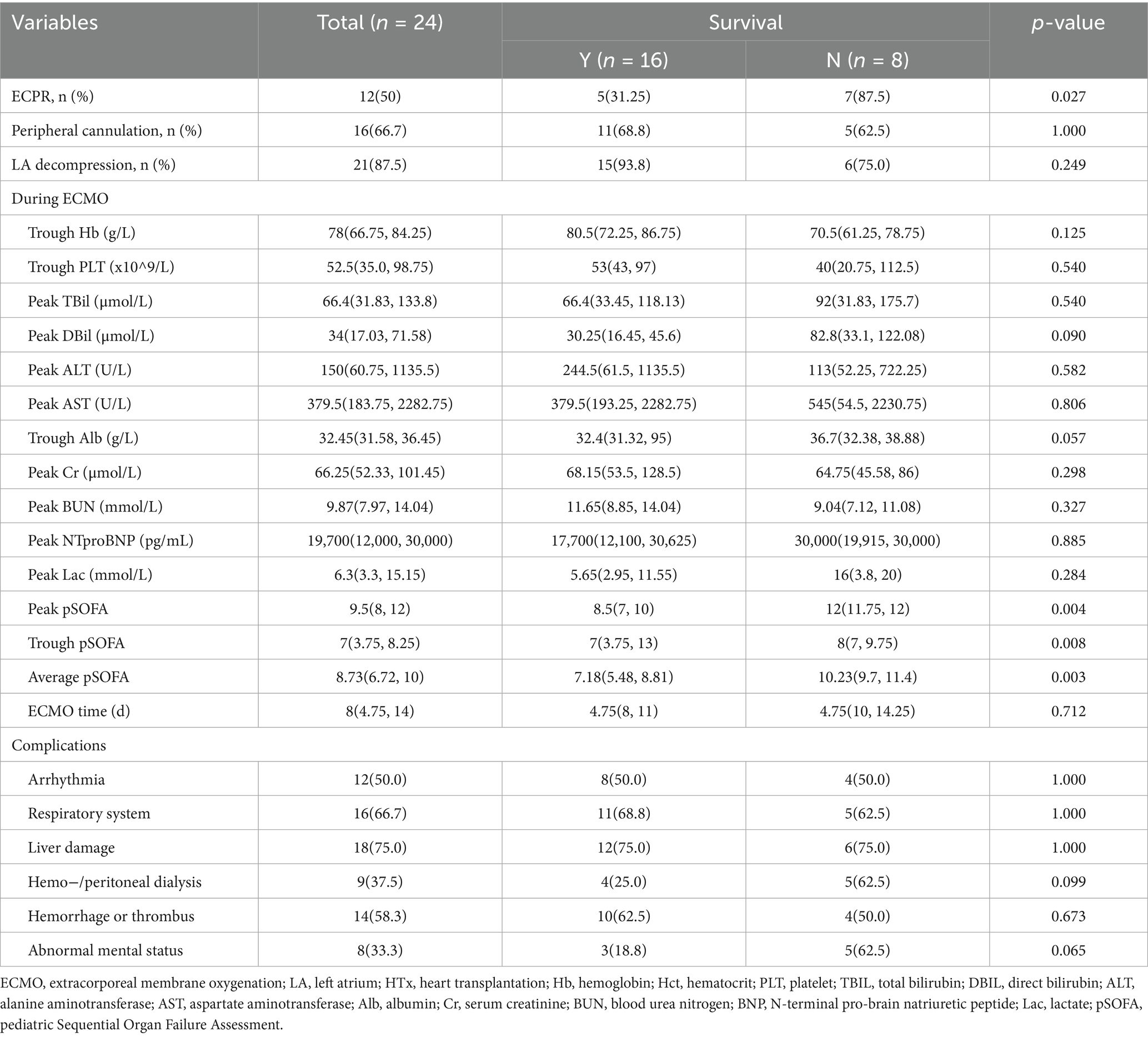
Characteristics of the 24 children during ECMO bridging were showed in Table 2, none of whom were successfully weaned from ECMO before transplantation. Right jugular vessels for ECMO cannulation were firstly used at our center in 2021. Before that, central cannulating was widely used for most children, while femoral vessels were typically used for teenagers and adults. Among the 24 children, 16 (66.7%) underwent peripheral cannulation, and 21 (87.5%) received left heart decompression, including left atrial cannulation in 8 (33.3%) and atrial septostomy in 13 (54.2%). Half of them were implanted under ECPR with data indicating ECPR as a risk factor for in-hospital death (p = 0.027). Comparing the incidence of pulmonary edema and lung infection between the two decompression methods, atrial septostomy was slightly inferior to direct cannulating in left heart (p = 0.056), with 3 children who did not undergo left heart decompression were excluded from comparison due to the small sample size. There were no significant differences between the two groups in the worst values of blood test results during bridging and ECMO support duration [median ECMO duration: 8 (4.75, 14)days]. The incidence of arrhythmias, pulmonary complications, renal complications (requiring dialysis), hemorrhage/thrombosis and neurological complications (including delayed awakening, seizures, delirium, and amorphous type)during bridging also showed no notable differences. However, the death group had a significantly higher peak, trough and average pSOFA value, which showed greater potential for outcomes prediction than single indicators.
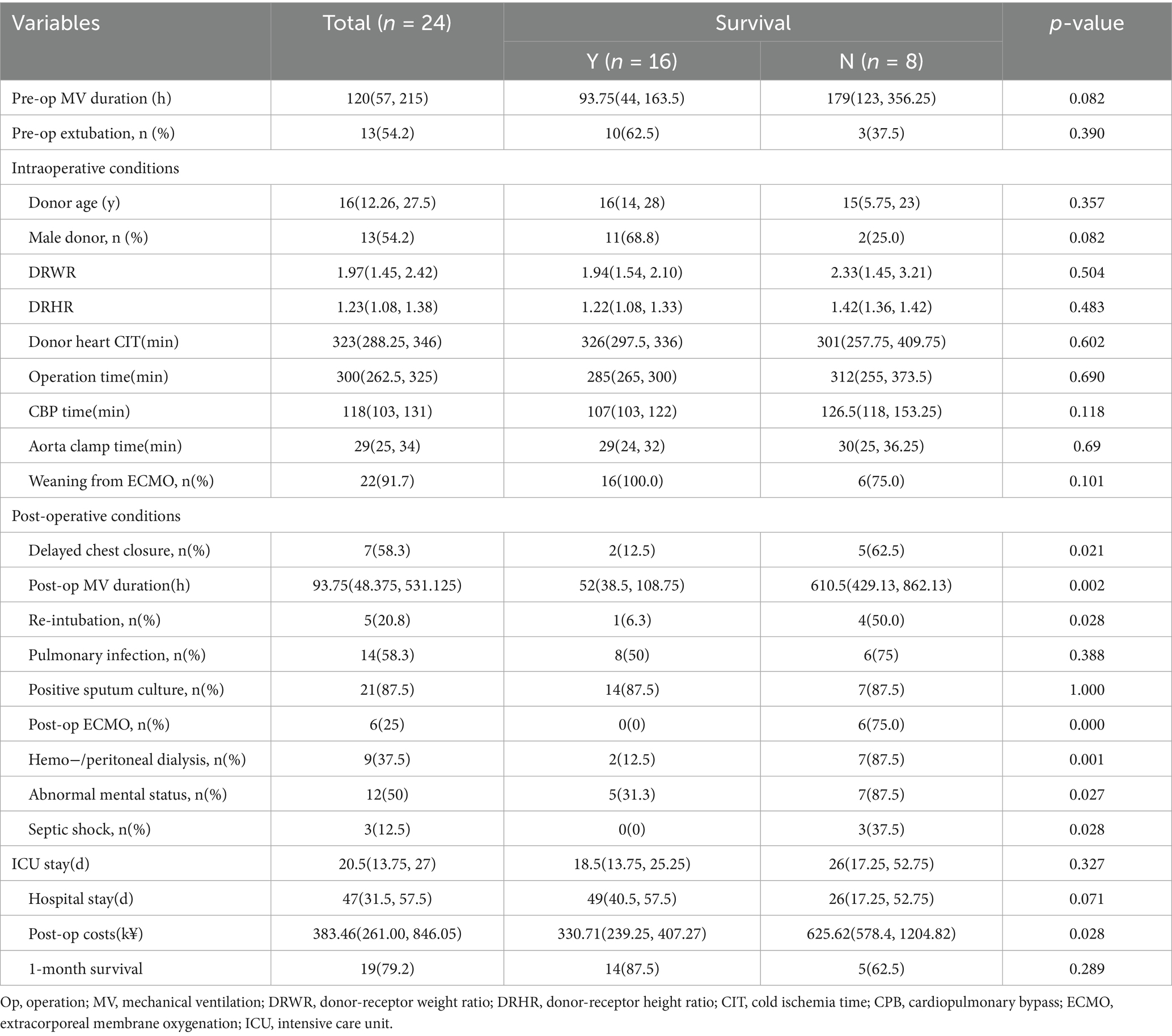
Table 3 showed datas of peri-HTx period. All children were mechanically ventilated before transplantation with a median mechanical ventilation time of 120 (58,215) hours, and 21 children (87.5%) were extubated before the surgery, with no significant difference between the two groups. The median donor age was 16 (12.26, 27.5) years, with 13 (54.2%) being male. The median donor-recipient weight ratio (DRWR) was 1.97 (1.45, 2.42), and the median donor-recipient height ratio (DRHR) was 1.23 (1.08, 1.38), which slightly exceeded the guideline-recommended range of 0.7–1.2 (15) due to donor scarcity. The median cold ischemia time (CIT) for donor hearts was 323 (288.25, 346) minutes, with the median surgical time 300 (262.5, 325) minutes, the median cardiopulmonary bypass (CPB) time 118 (103,131) minutes and the median aortic cross-clamp time 29 (25, 34) minutes. ECMO was weaned intraoperatively in 22 children (91.7%), with no statistically differences between the two groups. But the death group fared worse in many aspects after HTx, including higher delayed chest closure (p = 0.021) rate, reintubation rate (p = 0.028), ECMO reuse rate (p < 0.001), dialysis rate (p = 0.001), septic shock rate (p = 0.028) and neurological complication rate (p = 0.027), as well as longer mechanical ventilation time (p = 0.002) and post operative costs (p = 0.028).
3.2 Application of pSOFA score for outcome predicting
Daily pSOFA scores were recorded during bridging until the day before transplantation, 20 children (83.3%) receiving donor hearts within 2 weeks. pSOFA scores of the 2 groups from day 0 (before ECMO) to day 9 (datas were insufficient for calculating after day 9) were plotted against bridging time (Figure 2A), Addressed as pSOFA0-9, revealing a general downward trend in survival group, with significant differences of daily average pSOFA compared to the death group (p = 0.006) throughout bridging duration. For ECPR patients, the trend also showed statistically difference after day 6 (Figure 2B), which suggests that patients who have better prognosis after HTx may already have signs during a short term of ECMO bridging no matter whether they were bridged under ECPR. With 8.73 being the median value of pSOFA score through out bridging, we choose 8 as a cutoff to divide the 24 children into two groups based on their average pSOFA scores (mpSOFA) throughout bridging duration (mpSOFA≤8 vs. mpSOFA>8). Kaplan–Meier curves showed significantly better postoperative survival in children with lower mpSOFA (p = 0.003), indicating that pSOFA scores during ECMO bridging have great potential in predicting transplant prognosis (Figure 2C).
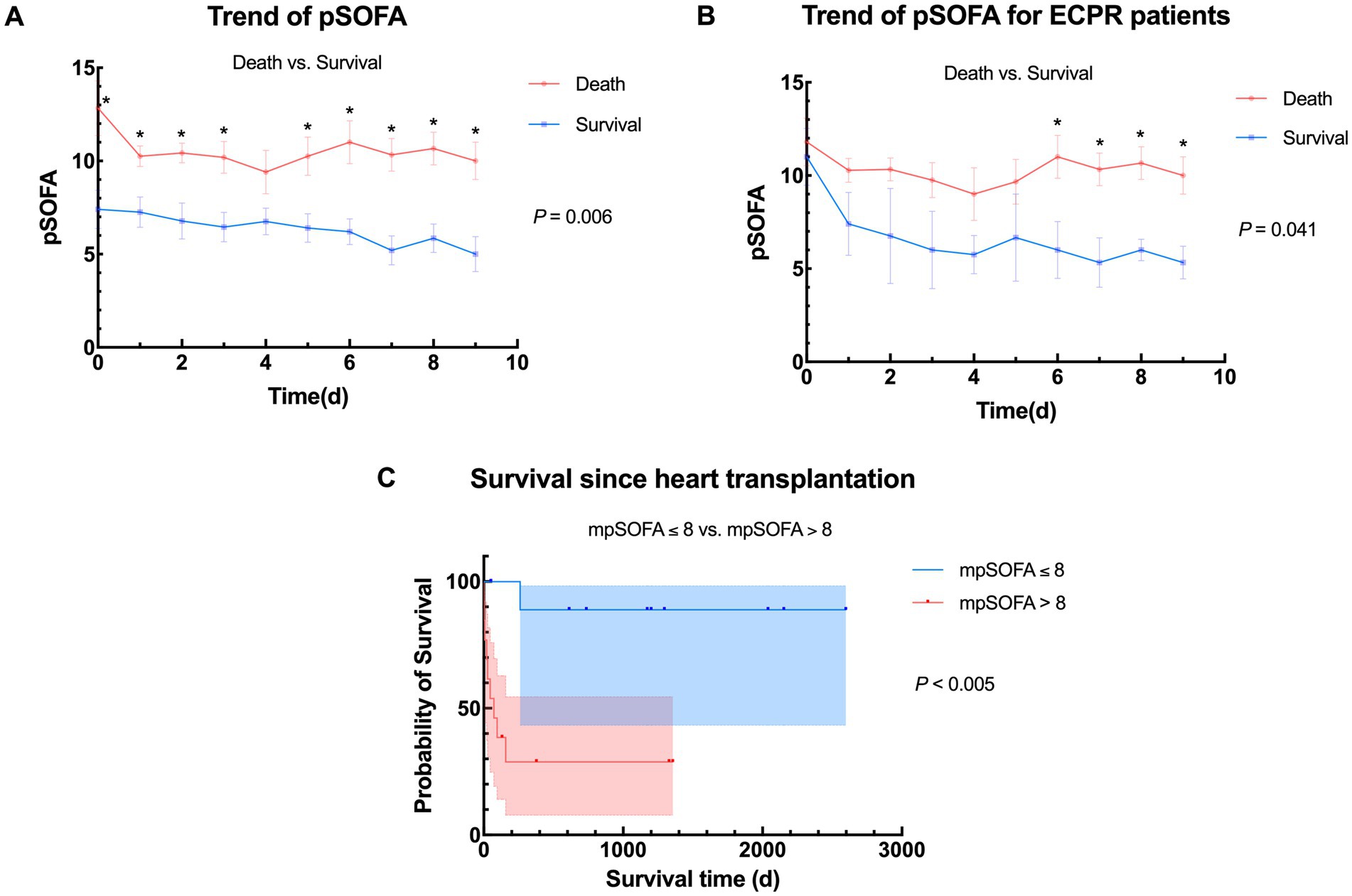
Figure 2. Trends of pSOFA and survival between different populations. (A) Overall trend of pSOFA between death group and survival group. Average daily pSOFA were significantly different (p = 0.006); (B) Trend of pSOFA between death group and survival group in ECPR patients. Average daily pSOFA were significantly different (p = 0.041); (C) Comparison of survival between different stratification according to average pSOFA during bridging (mpSOFA ≤ 8 vs. mpSOFA > 8, n = 11 vs. 13), pSOFA, pediatric Sequential Organ Failure Assessment; mpSOFA, average pediatric Sequential Organ Failure Assessment; ECPR, extracorporeal membrane oxygenation; ECMO, extracorporeal membrane oxygenation.
3.3 pSOFA in comparison of whether ECPR or not
While it is well known that ECMO-bridged children have worse prognosis than non-ECMO children, consistent with our center’s experience (p < 0.001) (Figure 3A), patients undergoing ECPR also shows poorer outcomes according to Figure 3B, as was expected. Supplementary Tables 1–3 demonstrates the differences when grouped by ECPR or not, also showing poorer pre-ECMO indicators and worse outcomes. Interestingly, children with planned ECMO implantation had lower preoperative left ventricular ejection fraction (LVEF) (p = 0.011). This may be because the non-ECPR group included more children with DCM, while other types of ADHF may not primarily present with reduced LVEF. Alternatively, lower LVEF may prompt doctors to be more vigilant and initiate mechanical support more aggressively.
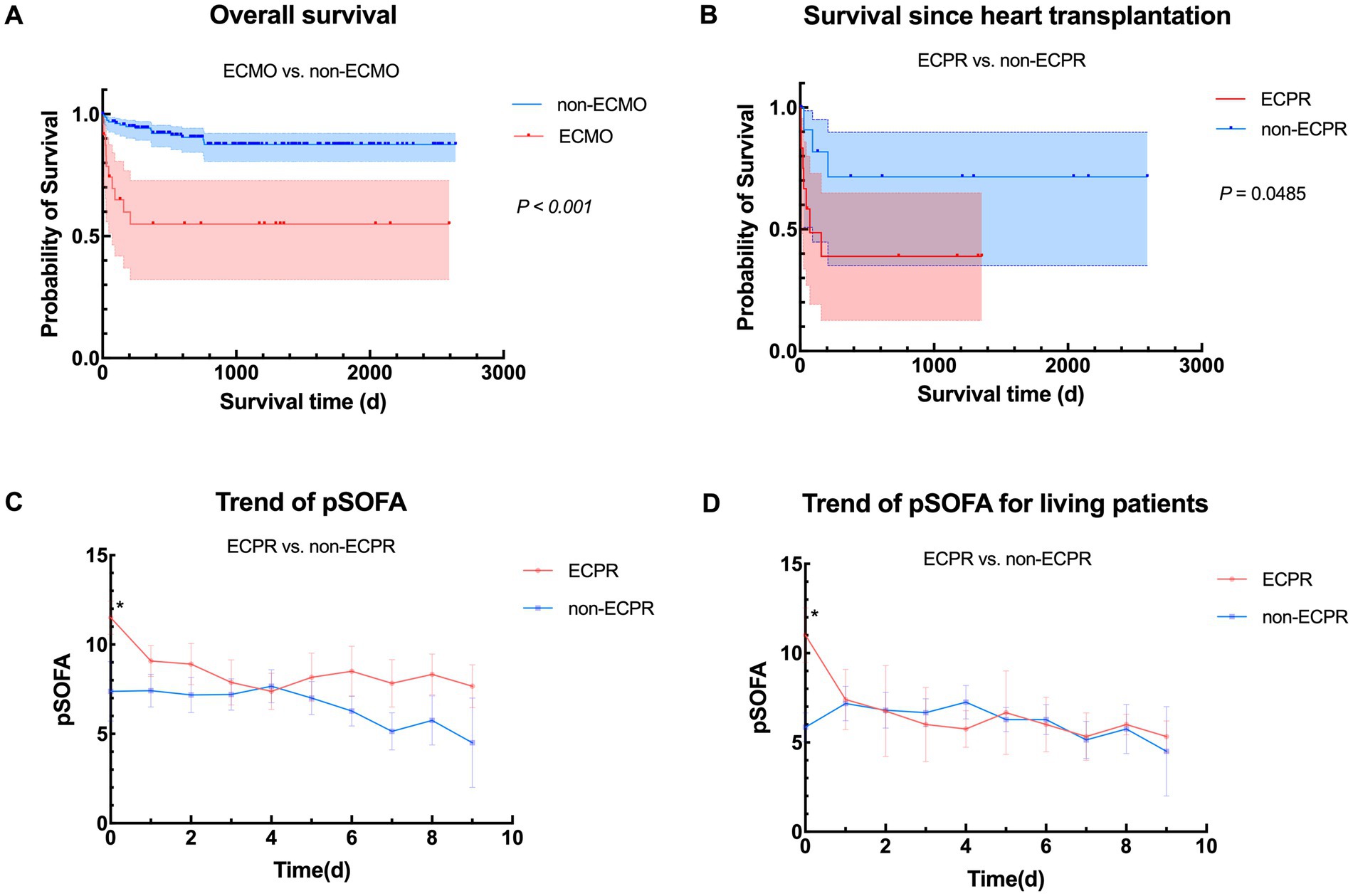
Figure 3. Comparison between ECPR and non-ECPR. (A) Overall survival in heart transplant children with or without ECMO support from January 2018 to April 2025 (n = 24 vs. 162); (B) Comparison of survival between different ECMO implanting status (ECPR vs. non-ECPR, n = 12 vs. 12); (C) Overall trend of pSOFA between ECPR group and non-ECPR group. pSOFA before ECMO initiation (pSOFA0) were significantly different (p = 0.020); (D) Trend of pSOFA between ECPR group and non-ECPR group in living patients. pSOFA before ECMO initiation were significantly different (p = 0.028). ECMO, veno-arterial extracorporeal membrane oxygenation; pSOFA, pediatric Sequential Organ Failure Assessment; ECPR, extracorporeal cardiopulmonary resuscitation.
To further investigate if pSOFA score may also work in telling patients under ECPR from those were not, we plotted Figures 3C,D, which turned out that only pSOFA0 showed notable differences in overall and the living population, implying that although being a risk factor, the reversible adverse effects of ECPR may gradually alleviated during ECMO support due to improved circulation and optimized management, even approaching non-ECPR patients. To sum up, ECPR patients are inferior to non-ECPR patients in terms of their status before ECMO bridging, late recovery and outcomes. However, if management strategy is well modified during ECMO bridging and key organ function is maintained under the guidance of serial scoring, the damage caused by heart arrest and low-flow status may be made up.
4 Discussion
This study summarizes the perioperative characteristics of pediatric heart transplant candidates bridged with ECMO for heart transplantation and introduces pSOFA scoring during ECMO support, which suggests that mpSOFA>8 and ECPR may predict poorer outcomes, highlighting the potential of pSOFA application and significance of organ protection during ECMO bridging, maybe also the advantages of planned ECMO implantation. We are the first to validate pSOFA’s predictive potential in pediatric ECMO-bridged heart transplantation, extending its application to mechanical support and organ transplantation.
Besides pSOFA, the Pediatric Risk of Mortality (PRISM) (16) and Pediatric Logistic Organ Dysfunction-2 (PELOD-2) (17) scores are also widely used pediatric scoring systems. PRISM predicts mortality based on the worst indicators within 24 h of PICU admission, while PELOD-2 assesses organ failure at specific time points (day 1, 2, 5, 8, 12, 16, 18, and PICU discharge) across five systems (neurological, cardiovascular, respiratory, platelet, and renal) (17). In contrast, pSOFA focuses on daily continuous monitoring of organ function changes. ADHF in children leads to systemic hypoperfusion, affecting multiple organs and vice versa. Organs may maintain delicate balance for extended periods or deteriorate rapidly, but donor heart availability is unpredictable. For such children with prolonged but unstable conditions, PRISM’s one-time scoring may be insufficient, and even modified versions PRISM-III (18) also lack comprehensive and continuous monitoring.
What’s more, in cardiac dysfunction, especially right heart failure, liver injury (e.g., hepatomegaly, elevated bilirubin) may emerge early and correlate closely with prognosis. Bilirubin levels twice the normal limit are even considered a relative contraindication for HTx (15, 19–21). pSOFA incorporates bilirubin monitoring, and its daily scoring enables physicians to detect trends more clearly, identify risk factors earlier, and prioritize organ protection, especially for liver. By categorizing children during ECMO support into high-risk (mpSOFA>8) and low-risk (mpSOFA≤8) groups, with the former having significantly worse prognoses, pSOFA scoring may guide targeted organ support, optimize transplant waiting lists, inform decisions on ECMO weaning or transition to VAD, adjust postoperative management, and help parents establish realistic expectations early.
The pSOFA score was originally designed for critically ill children with infections and did not account for mechanical support (10). In this study, ECMO improved circulatory function, potentially leading to underestimation of cardiovascular scores based on mean arterial pressure (MAP) and vasoactive drug requirements. Additionally, ECMO, which provides continuous non-pulsatile flow, may further reduce pulse pressure and potentially affect other organs (22, 23). Future studies could refine scoring weights based on MCS-specific considerations.
Due to incontinuous and incomplete pre-ECMO medical records, we only performed single-time pSOFA scoring before ECMO implantation, limiting its utility in guiding implantation timing. But it has highlighted the importance of avoiding ECPR in children awaiting transplantation. ECMO indications remain somewhat subjective and ambiguous (7), and concerns about hemolysis and anticoagulation challenges often lead to hesitation and delayed implantation. This study shows that ECPR children had higher pre-implantation lactate levels and pSOFA0 scores, suggesting that earlier ECMO initiation may be warranted when organ function shows trend of deterioration. For ADHF children, initiating continuous pSOFA monitoring upon admission could provide objective data to guide ICU transfer or MCS decisions.
As a single-center retrospective study with a small sample size, this research lacks multivariate regression statistics, pre-ECMO organ function assessments and does not fully explore systemic effects of ECMO, leading to limitations especially for patients with even more complicated post-transplant physiology. Future multi-center prospective studies incorporating mechanical support characteristics, lactate levels, nutritional status, and growth indicators could refine scoring systems, improve risk stratification, enhance high-risk patient monitoring, and optimize perioperative management in pediatric heart transplantation.
5 Conclusion
In summary, our study demonstrated that continuous pSOFA monitoring may effectively risk-stratifies ECMO-bridged pediatric transplant candidates, identifying high-risk patients (mpSOFA>8), and planned ECMO initiation yields better outcomes than ECPR. These findings warrant prospective validation to optimize bridging strategies.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethics Committee of Union Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
W-zL: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Validation, Writing – original draft. X-mZ: Data curation, Software, Validation, Writing – review & editing. WS: Resources, Writing – review & editing. CZ: Resources, Writing – review & editing. G-hW: Resources, Writing – review & editing. J-wS: Conceptualization, Project administration, Resources, Supervision, Validation, Writing – review & editing. N-gD: Funding acquisition, Project administration, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The study was funded by the National Key Research and Development Program of China (No: 2023YFC2706205 and No. 2023YFC2706200).
Acknowledgments
We would like to thank Qian Xu for advising and language polishing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1631616/full#supplementary-material
References
1. Edelson, JB, Huang, Y, Griffis, H, Huang, J, Mascio, CE, Chen, JM, et al. The influence of mechanical circulatory support on post-transplant outcomes in pediatric patients: a multicenter study from the International Society for Heart and Lung Transplantation (ISHLT) registry. J Heart Lung Transplant. (2021) 40:1443–53. doi: 10.1016/j.healun.2021.06.003
2. Butts, RJ, Toombs, L, Kirklin, JK, Schumacher, KR, Conway, J, West, SC, et al. Waitlist outcomes for pediatric heart transplantation in the current era: an analysis of the pediatric heart transplant Society database. Circulation. (2024) 150:362–73. doi: 10.1161/circulationaha.123.068189
3. Chen, JM, Richmond, ME, Charette, K, Takayama, H, Williams, M, Gilmore, L, et al. A decade of pediatric mechanical circulatory support before and after cardiac transplantation. J Thorac Cardiovasc Surg. (2012) 143:344–51. doi: 10.1016/j.jtcvs.2011.10.072
4. Velleca, A, Shullo, MA, Dhital, K, Azeka, E, Colvin, M, DePasquale, E, et al. The International Society for Heart and Lung Transplantation (ISHLT) guidelines for the care of heart transplant recipients. J Heart Lung Transplant. (2023) 42:e1–e141. doi: 10.1016/j.healun.2022.10.015
5. Jensen, AR, Davis, C, and Gray, BW. Cannulation and decannulation techniques for neonatal ECMO. Semin Fetal Neonatal Med. (2022) 27:101404. doi: 10.1016/j.siny.2022.101404
6. Gajkowski, EF, Herrera, G, Hatton, L, Velia Antonini, M, Vercaemst, L, and Cooley, E. ELSO guidelines for adult and pediatric extracorporeal membrane oxygenation circuits. ASAIO J. (2022) 68:133–52. doi: 10.1097/mat.0000000000001630
7. Brown, G, Moynihan, KM, Deatrick, KB, Hoskote, A, Sandhu, HS, Aganga, D, et al. Extracorporeal life support organization (ELSO): guidelines for pediatric cardiac failure. ASAIO J. (2021) 67:463–75. doi: 10.1097/mat.0000000000001431
8. Hong, SJ, De Souza, BJ, Penberthy, KK, Hwang, L, Procaccini, DE, Kheir, JN, et al. Plasma brain-related biomarkers and potential therapeutic targets in pediatric ECMO. Neurotherapeutics. (2025) 22:e00521. doi: 10.1016/j.neurot.2024.e00521
9. Yin, MY, Wever-Pinzon, O, Mehra, MR, Selzman, CH, Toll, AE, Cherikh, WS, et al. Post-transplant outcome in patients bridged to transplant with temporary mechanical circulatory support devices. J Heart Lung Transplant. (2019) 38:858–69. doi: 10.1016/j.healun.2019.04.003
10. Matics, TJ, and Sanchez-Pinto, LN. Adaptation and validation of a pediatric sequential organ failure assessment score and Evaluation of the Sepsis-3 definitions in critically ill children. JAMA Pediatr. (2017) 171:e172352. doi: 10.1001/jamapediatrics.2017.2352
11. Agrwal, S, Saxena, R, Jha, M, and Jhamb, U. Comparison of pSOFA with PRISM III and PIM 2 as predictors of outcome in a tertiary care pediatric ICU: a prospective cross-sectional study. Indian J Crit Care Med. (2024) 28:796–801. doi: 10.5005/jp-journals-10071-24772
12. Jyotsna, K, Kumar, R, Sharan, S, Kishore, S, and Prakash, J. The various scoring systems in pediatric intensive care units: a prospective observational study. Cureus. (2023) 15:e39679. doi: 10.7759/cureus.39679
13. Akhondi-Asl, A, Geva, A, Burns, JP, and Mehta, NM. Dynamic prediction of mortality using longitudinally measured pediatric sequential organ failure assessment scores: a joint modeling approach. Pediatr Crit Care Med. (2024) 25:443–51. doi: 10.1097/pcc.0000000000003457
14. Schlapbach, LJ, Weiss, SL, Bembea, MM, Carcillo, JA, Leclerc, F, Leteurtre, S, et al. Scoring Systems for Organ Dysfunction and Multiple Organ Dysfunction: the PODIUM consensus conference. Pediatrics. (2022) 149:S23–s31. doi: 10.1542/peds.2021-052888D
15. Peled, Y, Ducharme, A, Kittleson, M, Bansal, N, Stehlik, J, Amdani, S, et al. International SOCIETY for heart and lung transplantation guidelines for the EVALUATION and care of cardiac transplant CANDIDATES-2024. J Heart Lung Transplant. (2024) 43:1529–1628.e54. doi: 10.1016/j.healun.2024.05.010
16. Pollack, MM, Ruttimann, UE, and Getson, PR. Pediatric risk of mortality (PRISM) score. Crit Care Med. (1988) 16:1110–6. doi: 10.1097/00003246-198811000-00006
17. Leteurtre, S, Duhamel, A, Salleron, J, Grandbastien, B, Lacroix, J, and Leclerc, F. PELOD-2: an update of the pediatric logistic organ dysfunction score. Crit Care Med. (2013) 41:1761–73. doi: 10.1097/CCM.0b013e31828a2bbd
18. Pollack, MM, Patel, KM, and Ruttimann, UE. PRISM III: an updated pediatric risk of mortality score. Crit Care Med. (1996) 24:743–52. doi: 10.1097/00003246-199605000-00004
19. Shinagawa, H, Inomata, T, Koitabashi, T, Nakano, H, Takeuchi, I, Naruke, T, et al. Prognostic significance of increased serum bilirubin levels coincident with cardiac decompensation in chronic heart failure. Circ J. (2008) 72:364–9. doi: 10.1253/circj.72.364
20. Poelzl, G, Ess, M, Mussner-Seeber, C, Pachinger, O, Frick, M, and Ulmer, H. Liver dysfunction in chronic heart failure: prevalence, characteristics and prognostic significance. Eur J Clin Investig. (2012) 42:153–63. doi: 10.1111/j.1365-2362.2011.02573.x
21. Liang, W, He, X, Wu, D, Xue, R, Dong, B, Owusu-Agyeman, M, et al. Prognostic implication of liver function tests in heart failure with preserved ejection fraction without chronic hepatic diseases: insight from TOPCAT trial. Front Cardiovasc Med. (2021) 8:618816. doi: 10.3389/fcvm.2021.618816
22. Kanagarajan, D, Heinsar, S, Gandini, L, Suen, JY, Dau, VT, Pauls, J, et al. Preclinical studies on pulsatile Veno-arterial extracorporeal membrane oxygenation: a systematic review. ASAIO J. (2023) 69:e167–80. doi: 10.1097/mat.0000000000001922
Keywords: ECMO, advanced heart failure, pediatric heart transplant, pSOFA, bridge-to-transplant
Citation: Li W-z, Zhou X-m, Su W, Zhou C, Wang G-h, Shi J-w and Dong N-g (2025) Pediatric sequential organ failure assessment for predicting outcomes in ECMO-bridged pediatric heart transplant recipients: experience from the largest pediatric heart transplant center in China. Front. Med. 12:1631616. doi: 10.3389/fmed.2025.1631616
Edited by:
Danica Momcicevic, University Clinical Centre of the Republic of Srpska, Bosnia and HerzegovinaReviewed by:
Ivan Golub Palibrk, University of Belgrade, SerbiaMilka Jandric, University Clinical Center of the Republic of Srpska, Bosnia and Herzegovina
Copyright © 2025 Li, Zhou, Su, Zhou, Wang, Shi and Dong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nian-guo Dong, ZG9uZ25pYW5ndW9AaG90bWFpbC5jb20=; Jia-wei Shi, c2hpamlhd2VpQDIxY24uY29t
†These authors have contributed equally to this work and share first authorship
 Wang-zi Li
Wang-zi Li Xian-ming Zhou†
Xian-ming Zhou† Cheng Zhou
Cheng Zhou Jia-wei Shi
Jia-wei Shi Nian-guo Dong
Nian-guo Dong