- 1Department of Thyroid and Breast Surgery, Cangzhou People’s Hospital, Cangzhou, China
- 2Department of Oncology, Cangzhou People’s Hospital, Cangzhou, China
- 3Department of Pathology, Cangzhou People’s Hospital, Cangzhou, China
- 4Department of Anesthesiology, Cangzhou People’s Hospital, Cangzhou, China
Introduction: Primary squamous cell carcinoma of the thyroid (PSCCT) is a rare and highly aggressive malignant tumor with a poor prognosis. Although surgery, chemotherapy and other treatment methods have been reported, the current treatment modality has not reached a consensus. This study discusses the diagnosis and treatment of a case of PSCCT with severe respiratory stenosis and endotracheal invasion and reviews the relevant literature. We report the disease of rapidly enlarging mass leading to asphyxiation to raise clinicians’ awareness of this condition.
Case presentation: We report a 76-year-old woman presenting with an enlarging right thyroid mass accompanied by severe dyspnea and hoarseness. Computed tomography (CT) scan disclosed a large solid heterogenous nodule with calcification in the right thyroid lobe and prominent adjacent lymph nodes. PSCCT was confirmed by postoperative histopathology and immunohistochemistry. Thyroidectomy with partial tracheectomy and tracheostomy was performed to relieve the patient’s dyspnea. The patient has been discharged after receiving post-operative supportive care.
Conclusion: Clinicians should pay attention to the rapidly enlarging neck mass as it may cause asphyxiation and avoid the loss of treatment opportunities.
Introduction
Primary squamous cell carcinoma of the thyroid (SCCT) is a very rare entity that accounts for <1% of all primary thyroid cancers and has extremely poor prognosis (1). 117 SCCT cases were reported in the medical literature, and nearly half of the cases (56) have been reported in Asia, with the majority reported in Japan (2). PSCCT is characterized by a highly aggressive tumor with a poor prognosis and survival usually less than 1 year (3). It usually presents as a rapidly expanding anterior neck mass with involvement of the surrounding tissues and often leads to dyspnea and hoarseness (4). The low incidence of PSCCT contributes to the complexity of understanding its pathogenesis, diagnosis, clinical presentation, and treatment strategies. Due to rapid mass growth, the diagnosis of PSCCT is usually challenging, it can easily be misdiagnosed as acute thyroiditis or other thyroid tumor (3). Furthermore, the crucial step is ruling out secondary SCC metastasis from other primary sites, as treatment and prognosis of SCC varies greatly (5). As surgery is the primary treatment, early diagnosis is critical to ensure that radical resection remains possible in cases of locally advanced disease (2, 6). Furthermore, an SCC mass can compress or even infiltrate the trachea, causing airway obstruction; tracheostomy may then be performed to relieve dyspnea if necessary (3, 5, 7). Radiotherapy, chemotherapy, and targeted therapies can also be used as adjuvant treatments, although in some cases these treatments are not sensitive to patients.
Case presentation
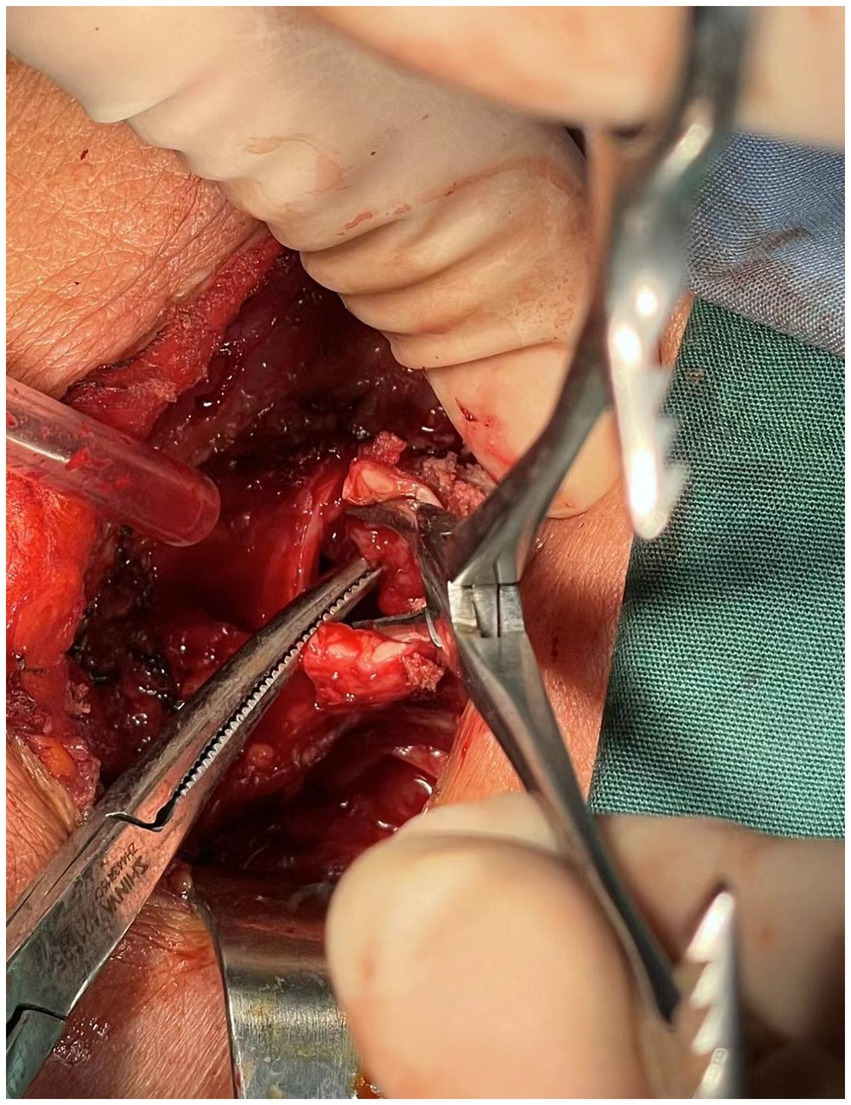
A 76-year-old female presented to the Respiratory and Critical Care Medicine Department of our hospital with a gradually enlarging neck mass for 2 months, accompanied by progressive worsening of cough, sputum production, and dyspnea. Medical history: The patient had a left upper lobe lung resection for adenocarcinoma of the lung 3 years ago. The laboratory test results were normal: TSH:3.22 μIU/mL (reference range: 0.56–5.91 μIU/L); FT3: 5.16 pmol/L (reference range: 3.28–6.47 pmol/L); Free thyroxine: 14.49 pmol/L (reference range: 7.64–16.03 pmol/L). The tumor marker CA19-9 was 37.5 U/mL (reference range, 0–27 U/mL), and the results of other markers (CEA, AFP, CA125, CA15-3, CA7-24, SCC, ProGRP, Ferrit, and calcitonin) were all within the normal range. Neck CT enhanced scan: large lesion in the right lobe of the thyroid gland, with patchy calcification within, significant compression of the trachea and lateral displacement of the trachea to the left, narrowing of the trachea (thyroid tumor invasion of the trachea, the narroworst position of the airway is 2 mm) (Figures 1, 2). No obvious metastasis to the lymph nodes is seen, and no distant metastasis is noted. Flexible fiberoptic nasolaryngoscopy examination was not performed on the patient because of the poor condition and the potential risk of inducing asphyxia. For the past 3 days, the patient’s breathing difficulty has been gradually worsening, with occasional episodes of asphyxiation. The patient was transferred to the Thyroid and Neck Surgery Department for treatment. Physical examination revealed a palpable nodule measuring approximately 5 cm in diameter in the right thyroid region, with hard texture, poor mobility, and mild tenderness. No obvious enlarged lymph nodes were palpable in the neck. After consulting with Multi-Disciplinary Treatment, a total thyroidectomy with partial tracheectomy and tracheostomy was performed under general anesthesia. Due to the patient’s airway obstruction, it was not possible to perform a routine anesthesia. The patient was placed in a semi-sitting position and maintained spontaneous breathing with an endotracheal tube while being appropriately sedated and analgesic. The tip of the endotracheal tube was inserted 4 cm below the vocal cords, but it was unable to pass through the narrowed area. In order to facilitate endotracheal intubation, we performed regional airway anesthesia before the operation. The endotracheal tube was secured in place, anesthetic induction drugs were administered, and high-frequency ventilation was used to maintain breathing. Due to tumor invasion of the trachea (Figure 3), after the entire thyroid gland was removed, an incision was made in the trachea to insert a tracheal tube, and breathing was restored after the operation. The patient was then transferred to the ward.
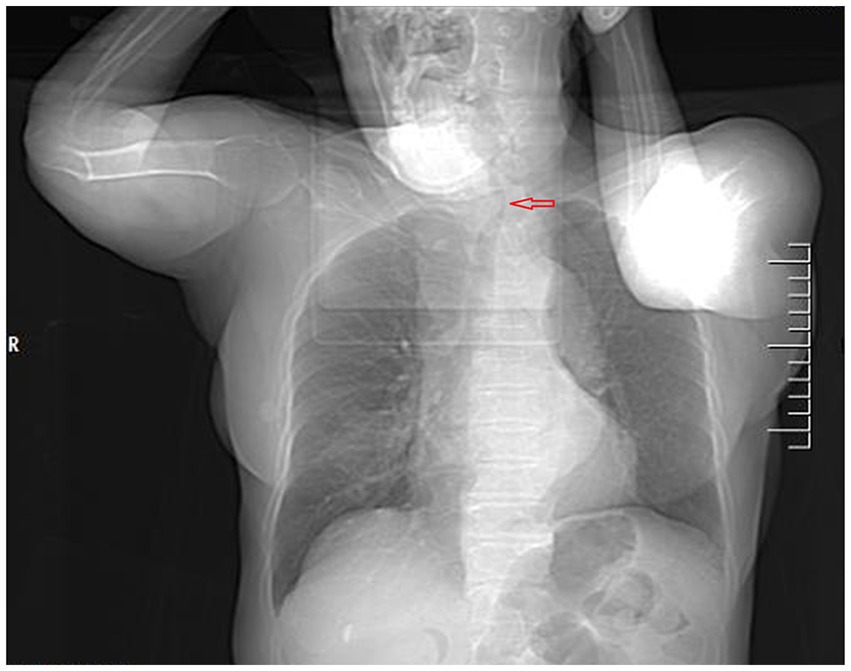
Figure 1. Computed tomography of the chest and neck revealed tracheal stenosis (arrow) and left-sided deviation (coronal planes).
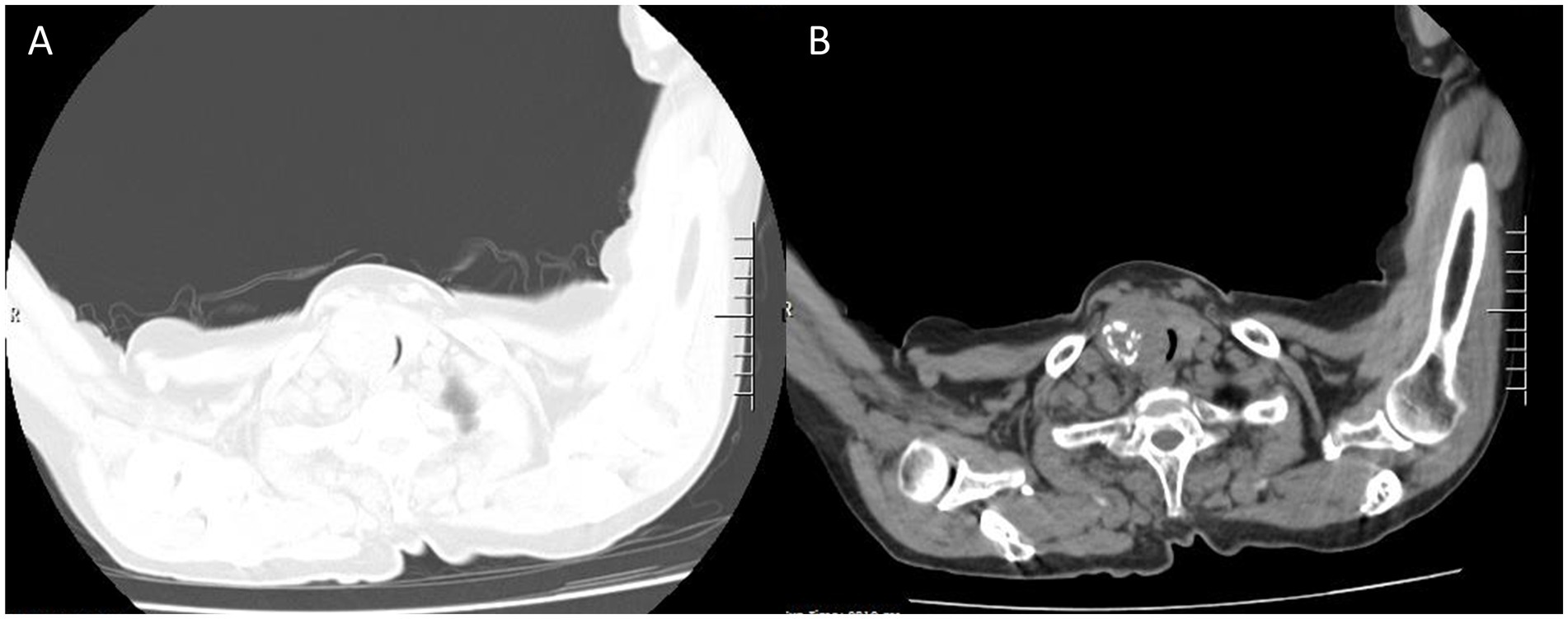
Figure 2. The same level of lung and mediastinal window CT scan show heterogeneously solid mass sized about 5.5 × 4 cm (A) in the right thyroid gland with calcification (B) and indistinct boundaries, tracheal stenosis (2 mm) and left deviation (A, lung window; B, mediastinal window).
Intraoperative frozen pathology: malignant thyroid tumor invades the tracheal muscle, right laryngeal nerve, esophageal muscle outside, and trachea. Postoperative pathology: Primary low-differentiated squamous cell carcinoma of the thyroid, size 5.5*4*2.5 cm, invading the fibrous and adipose tissue around the thyroid. Intrathoracic mass: Squamous cell carcinoma of the thyroid. Immunohistochemistry: Ki67 (40%+), TTF-1 (−), NapsinA (−), HNF1β (−), CD56 (−), CgA (−), SYN (−), CK19 (+), Calponin (−), T g (−), CK (+), Vimentin (+), Galectin-3 (+), P40 (positive), P63 (+), CK5/6 (+) (Figure 4). In order to facilitate recovery, appropriate fasting and fluid restriction measures, as well as anti-inflammatory and expectorant supportive therapies were given, and the patient’s condition stabilized. The patient was very satisfied with the relief of airway obstruction, but was not active in the postoperative adjuvant treatment. In fact, for locally advanced PSCCT, the first consideration for patients may be the quality of life, such as relieving airway obstruction and maintaining unobstructed breathing. The patient’s fear of asphyxia is greater than their concern about the impact of tumor on their own survival time.
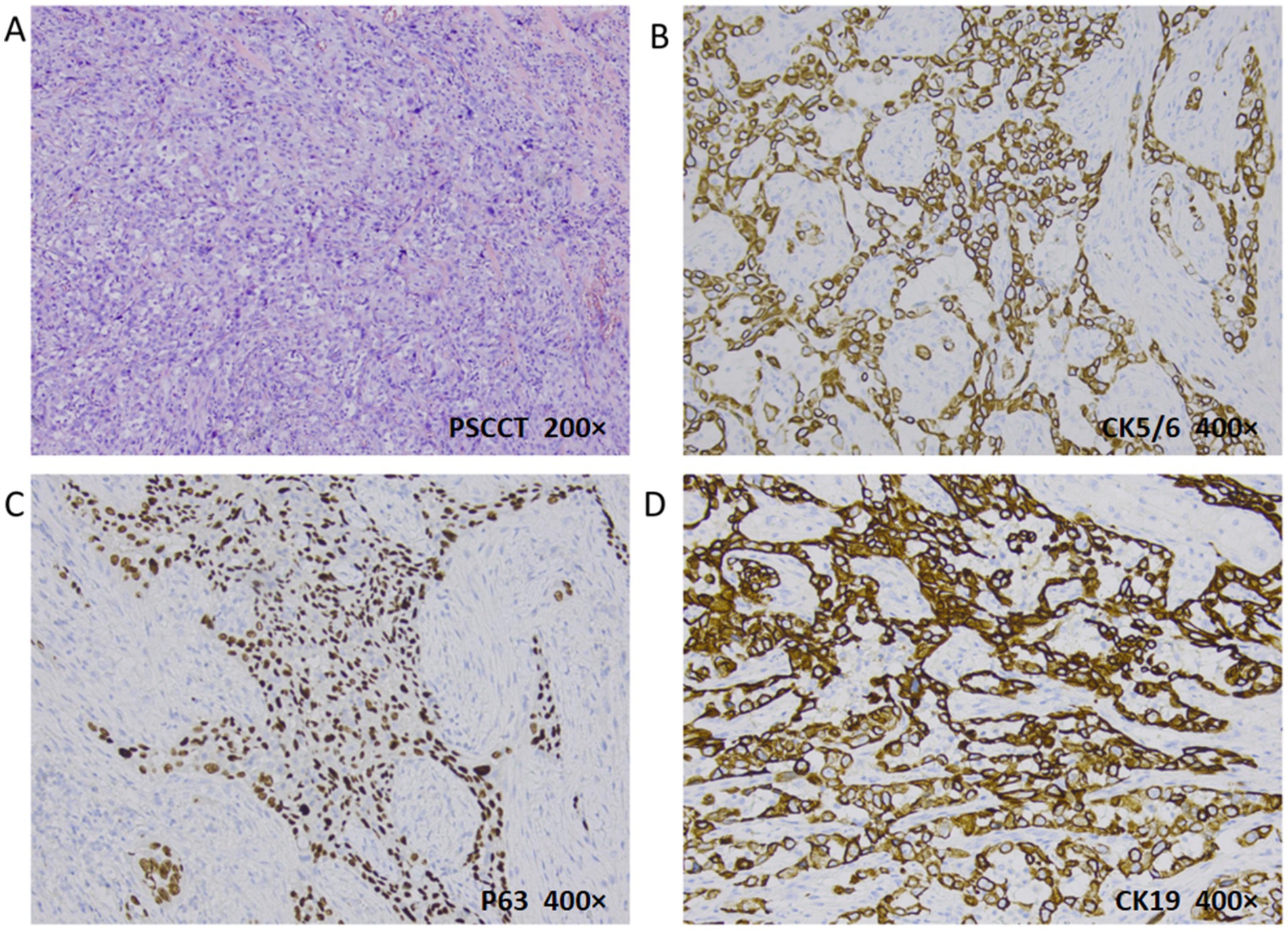
Figure 4. Histopathologic evaluation revealed PSCCT (H&E, 200×): poorly differentiated tumor cells and typical nuclear heterogeneous cells (A). PSCC immunohistochemistry: CK5/6 (B) and P63 (C) were positive (deeply membranes and nuclei stained respectively), indicating the source of squamous cells; CK19 was positive for membranes stained (D) (400×).
Timeline
The patient was hospitalized with enlarging neck mass and 3-year history of lung adenocarcinoma. The operation was performed on the 5th day after laboratory and imaging examinations and preoperative evaluations. Postoperative pathology confirmed the diagnosis as PSCCT, and the patient was discharged on the 17th day after the operation (Supplementary Figure S1).
Discussion
PSCCT is a malignant tumor with extremely rare incidence which typically has a poor prognosis. Typically arising in older adults, the mean age of diagnosis for PSCCT patients was 68 (8). The case of 76-year-old female we reported is a PSCCT coexisting with lung adenocarcinoma and is easily misdiagnosed as metastatic carcinoma of lung, which has not been previously reported in literature. Therefore, it suggests that the possibility of PSCCT should still be considered when the thyroid mass is rapidly enlarged in the presence of other primary tumors.
The cause and the origin of PSCCT are highly controversial because thyroid gland itself does not have a squamous epithelial component in its anatomy. There are three common theories: dedifferentiation of other thyroid carcinoma, such as Papillary thyroid carcinoma (PTC) and Follicular thyroid carcinoma (FTC); the residual embryonic (it assumes that during development the embryonic remnants of squamous epithelial cells (e.g., thymic epithelium, thyroglossal duct) evolve into squamous epithelial cells) and squamous epithelial metaplasia theory (PSCCT could develop from the thyroid follicular epithelium associated with chronic inflammation) (2).
As squamous cell carcinoma of thyroid is highly aggressive, the poor prognosis of patients is mainly due to failure to diagnose and early treatment. It requires a comprehensive approach involving clinical symptoms, imaging, pathology, and immunohistochemistry to confirm the diagnosis. Secondary squamous cell carcinoma of the thyroid (SSCCT) is a type of malignant tumor that metastasizes from the larynx, esophagus, lung, and other primary sites. Since SSCCT is more common than PSCCT and the treatments vary widely, it is critical to distinguish between them for accurate treatment. Immunohistochemistry plays an important role in diagnosis of PSCCT, such as PAX-8 (paired box protein 8) marker positive (5, 9). TTF-1 and CK7 positive are helpful in diagnosing PSCCT as distinct from SSCCT (2).
A study (8) has shown that PSCCT had a larger tumor size (mean size 5.35 cm) than other types of thyroid carcinoma, and with more frequent positive margins and metastasis. CT (computed tomography) can show thyroid tumors and their relationship with the surrounding tissues. CT scan of the neck, chest, and abdomen can also be used to rule out SSCCT as the primary source.
Most PSCCT patients present with neck swelling as a rapidly enlarging anterior neck mass (69.6% tumor size was >4.0 cm) (10). As the tumor grows and invades the muscles and soft tissues, patient usually complains of neck discomfort or pain (11, 12). Dyspnea, dysphagia and hoarseness are common symptoms in cases as the advanced disease invades the trachea, esophagus and the recurrent laryngeal nerve (13–16). The associated symptoms are always followed by the carcinoma, such as weight loss (2). It must be emphasized that emergency tracheostomy usually needs to be performed because most patients with advanced disease succumb to airway obstruction (9, 17, 18). For this patient, dyspnea continued to worsen and there were frequent episodes of asphyxia, relief of airway obstruction is the first step that needs to be performed.
Early detection and diagnosis are important and possible for curative surgical resection (10). However, early diagnosis is difficult due to the lack of typical clinical features, late phase tumor always developed extensive local tissue infiltrated and metastasis with poor prognosis when detected. Although R0 resection was achieved in only 18.8% of all ThySCC patients (8), surgery remains to be considered an primary effective option to reduce tumor burden and relieve symptoms of local invasion (2, 19). As study shown, whether complete tumor resection or not (R0 or R1/R2), surgery was associated with favorable prognosis (8, 20). Sometimes, tracheotomy is urgently and very necessary to relieve severe airway stenosis firstly so as to maintain the patient’s vital signs. This is because some patients admitted to the hospital due to severe airway obstruction (17, 18).
Chu (5) found that radical surgical resection of PSCCT combined with adjuvant chemoradiotherapy results in a favorable outcome. As reported, for inoperable PSCCT patients or who declining surgery, chemotherapy (carboplatin and paclitaxel, cisplatin and docetaxel, paclitaxel) and radiotherapy is effective supplementary treatment options (7, 14, 21). After comprehensively reviewing the literature on PSCCT in recent years, it is found that treatment methods mainly based on surgery can improve the survival rate (Table 1). Besides, there is no evidence that oral supplementation with exogenous thyroxine is beneficial for the prognosis of thyroid squamous cell carcinoma because the tumor cell does not have the TSH receptor (22). Anlotinib combined with Sintilimab and other BRAF and MEK inhibitors (dabrafenib and trametinib) are reported effective with initial relief of PSCCT (18, 23).
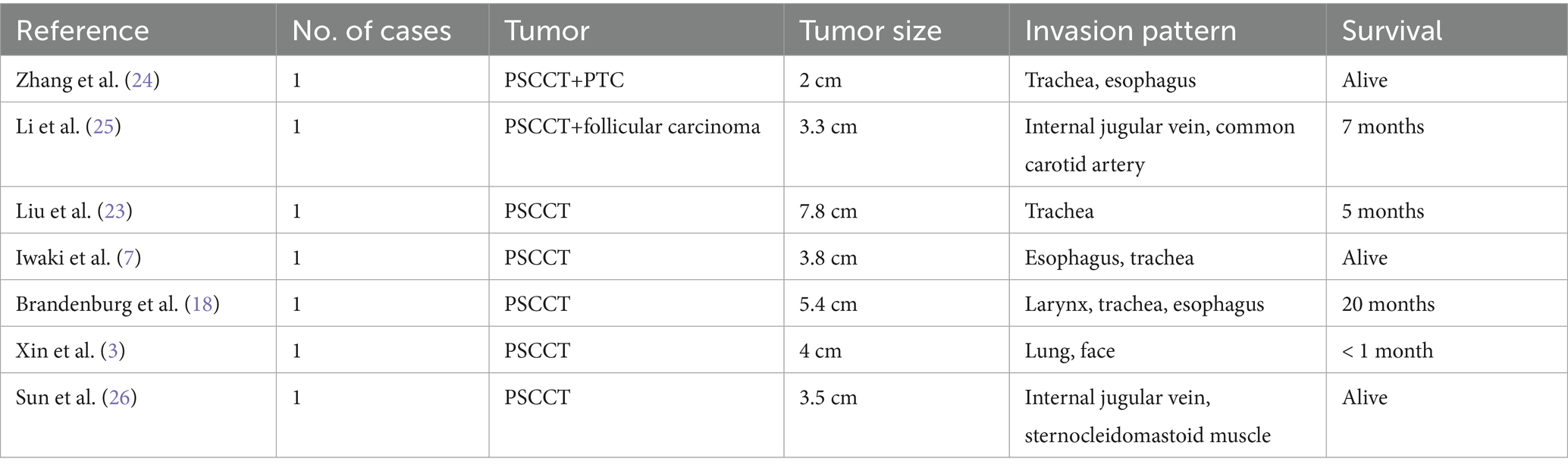
Table 1. Characteristics and survival of primary squamous cell carcinoma of the thyroid in limited studies and literature.
However, surgery and postoperative adjuvant treatment methods of PSCCT still lack a large amount of evidence-based medical support. Further research is required to explore additional treatment options and improve the prognosis for PSCCT.
Conclusion
PSCCT is a rare disease with a lack of consensus on management typically diagnosed at an advanced stage with a poor prognosis. Clinical presentation, medical history, imaging examination, and especially immunohistochemistry are essential for accurate diagnosis of PSCCT. Although surgery is the preferred option, it may not always be curative due to the high local progression and metastasis rate. The management of PSCCT requires a multi-disciplinary approach. Further research works of deeper insights into various aspects need to be done.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
This study was reviewed and approved by the ethics committee of Cangzhou People’s Hospital (K2024-109-01). Informed written consent was obtained from the patient for the publication of this case report and the accompanying images. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
XL: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Writing – original draft. SM: Formal analysis, Methodology, Writing – review & editing. YZ: Formal analysis, Writing – review & editing. ZX: Resources, Writing – review & editing. HH: Resources, Writing – review & editing. HM: Data curation, Writing – review & editing. QX: Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1631714/full#supplementary-material
SUPPLEMENTARY FIGURE S1 | A timeline of patient’s diagnosis and treatment process.
Abbreviations
SCCT, Squamous cell carcinoma of the thyroid; CT, Computed tomography; PSCCT, Primary squamous cell carcinoma of the thyroid; SSCCT, Secondary squamous cell carcinoma of the thyroid; PTC, Papillary thyroid carcinoma; FTC, Follicular thyroid carcinoma.
References
1. Sapalidis, K, Anastasiadis, I, Panteli, N, Strati, TM, Liavas, L, Poulios, C, et al. Primary squamous cell carcinoma of the thyroid gland. J Surg Case Rep. (2014) 2014:rju133. doi: 10.1093/jscr/rju133
2. Ding, W, Gao, X, and Ran, X. Progress in diagnosing and treating thyroid squamous cell carcinoma under the 5th edition of who classification. Front Endocrinol. (2024) 14:1273472. doi: 10.3389/fendo.2023.1273472
3. Xin, S, Li, W, Yuan, N, Shen, C, Zhang, D, and Chai, S. Primary squamous cell carcinoma of the thyroid: a case report. J Int Med Res. (2021) 49:4702. doi: 10.1177/03000605211004702
4. Deeken-Draisey, A, Yang, G-Y, Gao, J, and Alexiev, BA. Anaplastic thyroid carcinoma: an epidemiologic, histologic, Immunohistochemical, and molecular single-institution study. Hum Pathol. (2018) 82:140–8. doi: 10.1016/j.humpath.2018.07.027
5. Chu, MMH, Mirza, O, Bishop, PW, and Pothula, V. Primary squamous cell carcinoma of the thyroid gland successfully treated with surgical resection and adjuvant Chemoradiotherapy. BMJ Case Rep. (2021) 14:e241209. doi: 10.1136/bcr-2020-241209
6. Jin, S, Liu, X, Peng, D, Li, D, and Ye, Y-N. Differences between Cancer-specific survival of patients with anaplastic and primary squamous cell thyroid carcinoma and factors influencing prognosis: a seer database analysis. Front Endocrinol. (2022) 13:760. doi: 10.3389/fendo.2022.830760
7. Iwaki, S, Kawakita, D, Sawabe, M, Matoba, T, Takano, G, Oguri, K, et al. Long-term efficacy of weekly paclitaxel therapy in Unresectable primary squamous cell carcinoma of the thyroid. Auris Nasus Larynx. (2022) 49:1083–7. doi: 10.1016/j.anl.2021.06.005
8. Limberg, J, Ullmann, TM, Stefanova, D, Finnerty, BM, Beninato, T, Fahey, TJ, et al. Prognostic characteristics of primary squamous cell carcinoma of the thyroid: a National Cancer Database Analysis. World J Surg. (2019) 44:348–55. doi: 10.1007/s00268-019-05098-5
9. Kao, NH, Tan, CS, and Koh, AJH. The utility of immunohistochemistry in differentiating metastatic primary squamous cell carcinoma of the thyroid from a primary lung squamous cell carcinoma. Case Rep Endocrinol. (2019) 2019:1–4. doi: 10.1155/2019/8641267
10. Iqbal, A, Lee, KT, Yasinzai, AQK, Li, Z, Wali, A, Tareen, B, et al. Prognostic factors and survival outcomes in squamous cell carcinoma of the thyroid: a surveillance, epidemiology, and end results (seer) database analysis. Cureus. (2024) 16:e63326. doi: 10.7759/cureus.63326
11. Zheng, R-z, Huang, G-h, and Xu, Y-j. A primary squamous cell carcinoma of the thyroid presenting as the anaplastic thyroid carcinoma: a case report. Front Surg. (2020) 7:956. doi: 10.3389/fsurg.2020.590956
12. Othman, RT, Baizeed, AMA, and Mohammed, AA. Squamous cell carcinoma of the thyroid gland in an elderly female presenting as a rapidly enlarging thyroid mass. Int J Surg Case Rep. (2020) 70:119–22. doi: 10.1016/j.ijscr.2020.04.064
13. Lam, AK. Squamous cell carcinoma of thyroid: a unique type of Cancer in World Health Organization classification. Endocr Relat Cancer. (2020) 27:R177–92. doi: 10.1530/erc-20-0045
14. Iwamoto, YAT, Koyama, K, Ota, Y, Nakashima, K, Monobe, Y, Kaneto, H, et al. Primary squamous cell carcinoma of the thyroid with severe tracheal invasion: a case report. Eur Thyroid J. (2021) 10:548–50. doi: 10.1159/000511709
15. Torrez, M, Braunberger, RC, Yilmaz, E, and Agarwal, S. Primary squamous cell carcinoma of thyroid with a novel Braf mutation and high Pdl-1 expression: a case report with treatment implications and review of literature. Pathol Res Pract. (2020) 216:153146. doi: 10.1016/j.prp.2020.153146
16. Wang, WOQ, Meng, C, Jing, L, and Li, X. Treatment optimization and prognostic considerations for primary squamous cell carcinoma of the thyroid. Gland Surg. (2019) 8:683–90. doi: 10.21037/gs.2019.11.07
17. Ibrahim, MI, Jusoh, YR, Adam, NN, and Mohamad, I. Primary squamous cell carcinoma of the thyroid gland. Iran J Otorhinolaryngol. (2018) 30:65–8. Available at: https://pubmed.ncbi.nlm.nih.gov/29387667/
18. Brandenburg, T, Muchalla, P, Theurer, S, Schmid, KW, and Führer, D. Therapeutic effect of combined Dabrafenib and Trametinib treatment of Braf V600e-mutated primary squamous cell carcinoma of the thyroid: a case report. Eur Thyroid J. (2021) 10:511–6. doi: 10.1159/000518055
19. Yan, WCH, Li, J, Zhou, R, and Su, J. Primary squamous cell carcinoma of thyroid gland: 11 case reports and a population-based study. World J Surg Oncol. (2022) 20:352. doi: 10.1186/s12957-022-02814-9
20. Au, JK, Alonso, J, Kuan, EC, Arshi, A, and John, MA. Primary squamous cell carcinoma of the thyroid: a population-based analysis. Otolaryngol Head Neck Surg. (2017) 157:25–9. doi: 10.1177/0194599817698436
21. Hsieh, M-L, Besch, BM, Peterson, JEG, and Henson, C. Primary squamous cell carcinoma of the thyroid treated with concurrent Chemoradiation and palliative immunotherapy: a case report. J Med Case Rep. (2022) 16. doi: 10.1186/s13256-022-03596-0
22. Dong, S, Song, X-S, Chen, G, and Liu, J. Mixed primary squamous cell carcinoma, follicular carcinoma, and micropapillary carcinoma of the thyroid gland: a case report. Auris Nasus Larynx. (2016) 43:455–9. doi: 10.1016/j.anl.2015.10.011
23. Liu, Z, Yu, M, Zhao, F, and Zhu, C. Anlotinib combined with Sintilimab is win-win cooperation for primary squamous cell carcinoma of the thyroid: a case report and literature review. Front Oncol. (2023) 13:6415. doi: 10.3389/fonc.2023.976415
24. Zhang, Y, Liu, Q, Ma, D, Maimaiti, Y, and Ma, Z. The coexistence of papillary thyroid carcinoma, anaplastic thyroid carcinoma (squamous cell carcinoma subtype) and poorly differentiated thyroid carcinoma: a case report. AME Case Rep. (2024) 8:47. doi: 10.21037/acr-23-192
25. Li, J, Ma, Z, Ma, D, Maimaiti, Y, Jiang, S, and Wang, X. Cervical lymph node metastasis as the first symptom of combined anaplastic thyroid carcinoma (squamous cell carcinoma) and follicular carcinoma: a case report. BMC Endocr Disord. (2024) 24:87. doi: 10.1186/s12902-024-01617-1
Keywords: thyroid cancer, primary squamous cell carcinoma, thyroid, case report, respiratory stenosis
Citation: Liu X, Ma S, Zheng Y, Xu Z, Hao H, Ma H and Xu Q (2025) Primary thyroid squamous cell carcinoma with severe respiratory stenosis and endotracheal invasion: a case report with literature review. Front. Med. 12:1631714. doi: 10.3389/fmed.2025.1631714
Edited by:
Pabulo Henrique Rampelotto, Federal University of Rio Grande do Sul, BrazilReviewed by:
Naouar Ouattassi, Sidi Mohamed Ben Abdellah University, MoroccoBurkan Nasr, University of Aden, Yemen
Copyright © 2025 Liu, Ma, Zheng, Xu, Hao, Ma and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xueliang Liu, bHhsZGFvQDE2My5jb20=
 Xueliang Liu
Xueliang Liu Shang Ma1
Shang Ma1 Hongyan Ma
Hongyan Ma