- 1Department of Pharmacy, The Third Hospital of Changsha (Changsha Hospital Affiliated to Hunan University), Changsha, Hunan, China
- 2Hunan Provincial Key Laboratory of Anti-Resistance Microbial Drugs, Changsha, Hunan, China
- 3Beijing Key Laboratory of Traditional Chinese Medicine Basic Research on Prevention and Treatment for Major Diseases, Experimental Research Center, China Academy of Chinese Medical Sciences, Beijing, China
Background: Alarelin acetate, a synthetic gonadotropin-releasing hormone (GnRH) analogue, is widely used to manage endometriosis and hormone-sensitive malignancies. Although its safety profile is generally favorable, we report the first documented case of severe hepatotoxicity associated with alarelin acetate administration.
Case summary: A 37-year-old female participant in a phase I clinical trial developed acute hepatocellular injury following subcutaneous administration of alarelin acetate (150 μg/day). The Roussel Uclaf Causality Assessment Method (RUCAM) yielded a score of 6, indicating a “highly probable” causal relationship between the drug and liver injury. Hepatic enzyme levels normalized within 18 days after drug discontinuation and initiation of hepatoprotective therapy (glycyrrhizin and polyene phosphatidylcholine). Pharmacogenomic profiling identified specific genetic variations that may be associated with alarelin acetate-related hepatotoxicity, including a homozygous NUDT15 variant (*3/*3 diplotype) and human leukocyte antigen (HLA) risk alleles (HLA-DRB1*15:01, HLA-DQB1*06:01).
Conclusion: This novel case highlights the risk of alarelin acetate-related hepatotoxicity. Pharmacogenomic profiling indicated that its hepatotoxicity may be related to gene polymorphisms; however, further research or larger-scale studies are needed to validate these associations.
Introduction
Alarelin acetate (AA), a synthetic nonapeptide gonadotropin-releasing hormone (GnRH) analogue, induces reversible hypogonadism through pituitary desensitization, making it effective for endometriosis and hormone-dependent cancers (1). While its labeled adverse effects (e.g., vasomotor symptoms and genitourinary atrophy) reflect hypoestrogenic states, hepatotoxicity has not been documented in the current literature.
Drug-induced liver injury (DILI) represents a growing public health concern, with an estimated annual incidence of 23.8/100,000 cases in China, exceeding global rates (2, 3). DILI manifests clinically as acute, subacute, or chronic liver injury, typically characterized by elevated levels of liver function markers, including serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), lactate dehydrogenase (LDH), alkaline phosphatase (ALP), γ-glutamyltransferase (GGT), and total bilirubin (TBil). The diagnostic criteria for DILI include the following: (1) ALT ≥5 × ULN, (2) ALP ≥ 2 × ULN (with concurrent GGT elevation), or (3) ALT ≥3 × ULN and TBil ≥2 × ULN (4). The pathogenesis of DILI is complex, and the known risk factors can be grouped into two categories: drug-related and host-related (5). Emerging evidence implicates genetic polymorphisms in drug metabolism enzymes and human leukocyte antigen (HLA) alleles as key determinants of DILI susceptibility.
Pharmacogenomics (PGx) is an interdisciplinary field integrating genetics, genomics, and pharmacogenetics (6). It focuses on the relationship between human genomic information and drug response and uses genomic information to explain the reasons for the differences in the response of different individuals to the same drug (7). Genes associated with drug response include drug-metabolizing enzymes, drug transporters, and specific HLA alleles (8). Drugs are typically metabolized in the liver through phase I and/or phase II reactions and are catalyzed by drug-metabolizing enzymes in order to form water-soluble metabolites before excretion.
DILI initiation frequently involves drug metabolism pathways, particularly those mediated by cytochrome P450 enzymes (CYP450) and UDP-glucuronosyltransferases (UGTs) (9). Polymorphisms in genes encoding drug-metabolizing enzymes, such as UGTs, glutathione S-transferases (GSTs), and N-acetyltransferases (NATs), are associated with the risk of DILI (10, 11). The HLA complex represents the most polymorphic system in humans, comprising highly conserved major histocompatibility complex (MHC) molecules that are critical for immune function (12). Except for monozygotic twins, HLA genotypes are unique to each individual. The HLA genes are located on the short arm of chromosome 6 and are classified into class I, II, and III regions based on their gene structure and function (13). HLA class I and II molecules primarily present antigens to T cells, while class III genes encode immune-related proteins, such as complement components, cytokines, and heat shock proteins. Experts in drug metabolism and toxicology suggest that HLA molecules present drug-derived antigens and are involved in the pathogenesis of DILI (14).
Herein, we report a case of DILI following treatment with alarelin acetate in a previously healthy individual. To the best of our knowledge, this is the first documented case of alarelin acetate-induced DILI. Furthermore, we sought to identify novel rare or low-frequency (minor allele frequency (MAF) < 5%) single-nucleotide polymorphisms (SNPs) that may potentially contributing to susceptibility to DILI associated with AA.
Participants and methods
Study design
The study protocol was approved by the Changsha Third Hospital Ethics Committee (NO. LZ-BARL-PK-01) and was registered on ClinicalTrials.gov (NCT2023LP02263). All participants self-identified as Han Chinese and provided written informed consent.
DILI causality assessment for alarelin acetate
The Roussel Uclaf Causality Assessment Method (RUCAM) was used to determine the causal relationship between liver injury and alarelin acetate (15).
Pharmacogenomic analysis
Genomic DNA was extracted from peripheral blood using the EasyPure Blood Genomic DNA Kit (TransGen Biotech) and quantified using agarose gel electrophoresis.
Library preparation involved enzymatic fragmentation, end repair, A-tailing, adapter ligation, and PCR amplification. The resulting libraries were then hybridized with custom RNA probes designed to capture genomic regions relevant to PGx studies. Following hybridization, target fragments were enriched using streptavidin-coated magnetic beads. After the quality control assessment, high-throughput sequencing was performed. PGx-relevant variants were identified using GATK (version 3.8) analysis.
The probe panel captured 4,453 PGx-related loci, covering a span of 24.44 kb. This allows for a comprehensive genomic characterization. Sequencing parameters were optimized to ensure that critical loci achieved sufficient coverage, with read depths consistently ranging from 10× to 20×, thereby enhancing data reliability and accuracy.
HLA high-resolution genotyping
Genomic DNA extraction and quantification were performed as described above.
Library preparation involved identical enzymatic fragmentation, end repair, A-tailing, adapter ligation, and PCR amplification. The libraries were hybridized with DNA probes that specifically targeted all exons and critical intronic regions of 22 HLA genes (Table 1), covering approximately 300 kb of genomic sequence. Streptavidin-coated magnetic beads were used for target enrichment. The post-enrichment libraries underwent quality control before high-throughput sequencing. HLA genotyping was determined through specialized bioinformatics analysis of the sequencing data.
Information on the reagents used in the pharmacogenomic analysis and HLA high-resolution genotyping experiments is provided in Supplementary Table 1.
Case report
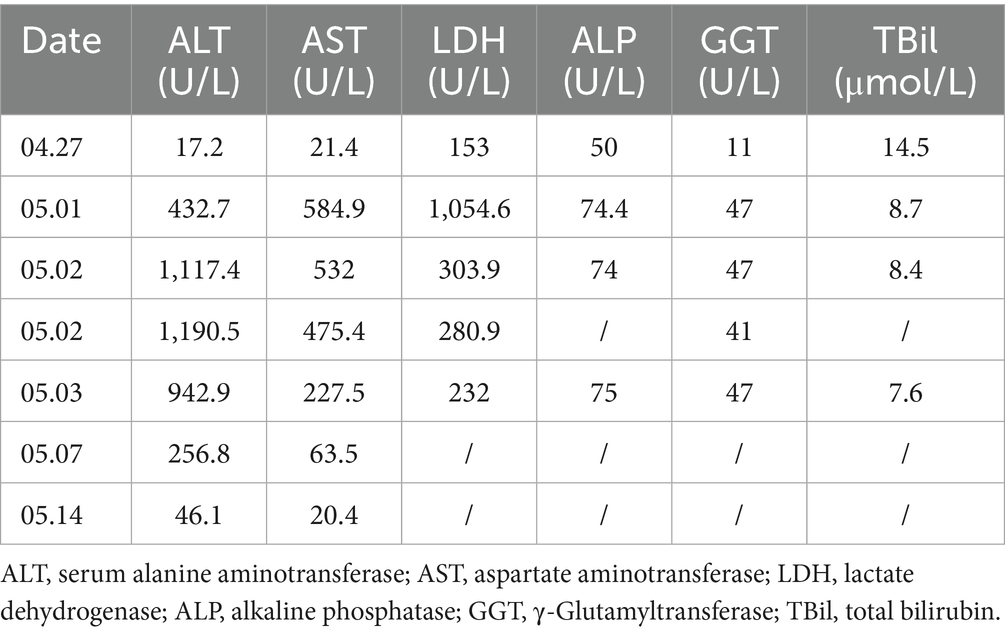
A 37-year-old woman participated in a phase I clinical trial titled “A single-center, non-randomized, open-label, positive drug-controlled study evaluating the safety, tolerability, pharmacokinetics and pharmacodynamics of a single subcutaneous injection of alanyl acetate in healthy Chinese adult female subjects” at Changsha Third Hospital on 26 April 2024. The participant met the inclusion criteria, had no significant past medical history or drug allergies, no history of substance abuse, and had not used any concomitant medications in the 4 weeks prior to enrollment. No specific negative behaviors (smoking, alcohol use, or recreational drug use) were reported. Laboratory results were within the normal range, and the liver function test (conducted on 27 April 2024) showed the following: ALT 17.2 U/L, AST 21.4 U/L, LDH 153 U/L, and TBil 14.5 μmol/L.
The participant was randomly assigned to the reference group and received alarelin acetate (BBCA Pharmaceutical Co., Ltd., China). Subcutaneous administration (150 μg/day) began on 30 April 2024 and continued for 14 consecutive days. On 1 May 2024, the laboratory test revealed elevated enzyme levels: ALT 432.7 U/L, AST 584.9 U/L, and LDH 1,054.6 U/L; however, the participant remained asymptomatic. A repeat test on 2 May 2024 showed further progression, with ALT 1,117.4 U/L, AST 532.0 U/L, and LDH 303.9 U/L. The investigator concluded that the participant developed significant transaminitis (ALT: 17.2 → 432.7 → 1,117.4 U/L and AST: 21.4 → 584.9 → 532.0 U/L), fulfilling Hy’s Law criteria for severe DILI (ALT ≥ 5 × ULN). Biochemical resolution followed apparent first-order kinetics (estimated ALT t1/2 = 3.2 days) after drug discontinuation. Due to safety concerns, the principal investigator withdrew the participant from the trial.
An immediate hepatology consultation confirmed a diagnosis of DILI. Treatment with compound glycyrrhizin tablets and polyene phosphatidylcholine capsules was initiated. Follow-up liver function tests indicated a progressive improvement in the participant’s condition:
• 3 May 2024: ALT 942.9 U/L and AST 227.5 U/L (significant decrease).
• 7 May 2024: ALT 256.8 U/L and AST 63.5 U/L (further decrease).
• 14 May 2024: ALT 46.1 U/L (near normalization) and AST 20.4 U/L (normal) (Table 2).
The participant remained asymptomatic throughout the 18-day follow-up period, and the adverse event (AE) resolved.
PGx and HLA gene analyses
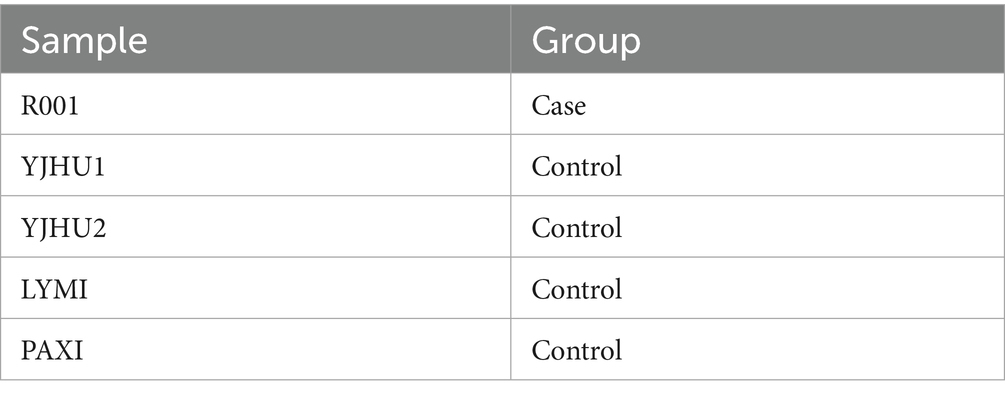
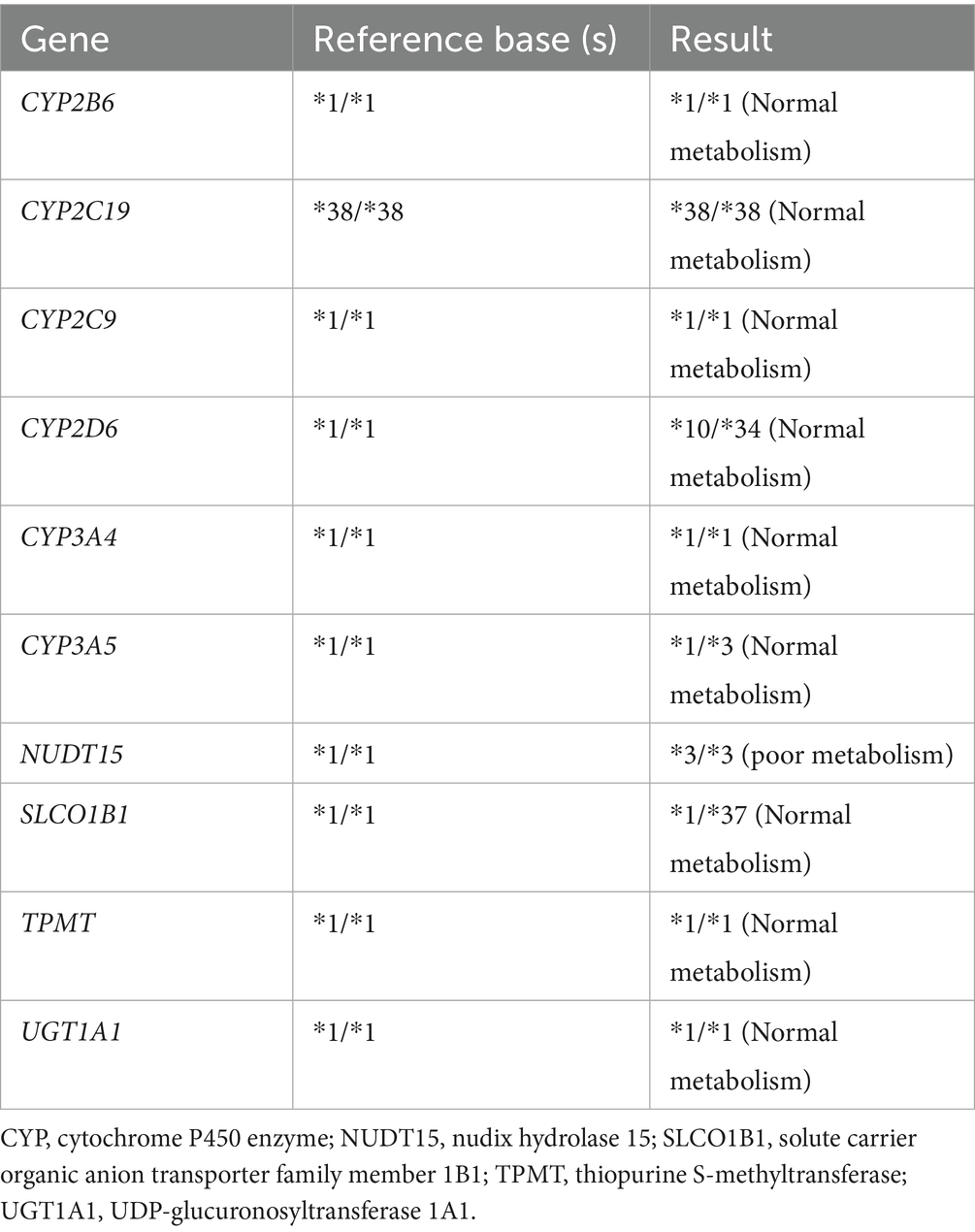
In the study, we analyzed one participant who developed acute liver injury (case) and four unaffected participants (controls) (Table 3). To investigate the genetic basis of the hepatotoxicity, we performed comprehensive PGx and HLA genotyping (Figure 1A). The pharmacogenomic analysis of 4,343 loci across all five samples revealed 2,044 variants in the index case (R001) (Supplementary file 1), including 18 high-frequency variants in the Chinese population (Supplementary file 2). The control variant counts were 1,986 (YJHU1), 2,056 (YJHU2), 1,981 (LYMI), and 2,018 (PAXI) (Figure 1B; Supplementary file 1). Figure 1C displays the distribution of the low-frequency variants (MAF < 5%). The comparative analysis identified 148 unique loci in R001 compared to the controls (Figure 2A; Supplementary file 3). The haplotype analysis of 10 pharmacogenes influencing drug metabolism (CYP2B6, CYP2C19, CYP2C9, CYP2D6, CYP3A4, CYP3A5, NUDT15, SLCO1B1, TPMT, and UGT1A1) revealed that R001 carried a NUDT15 *3/*3 diplotype, indicating a slow metabolizer status (Table 4).
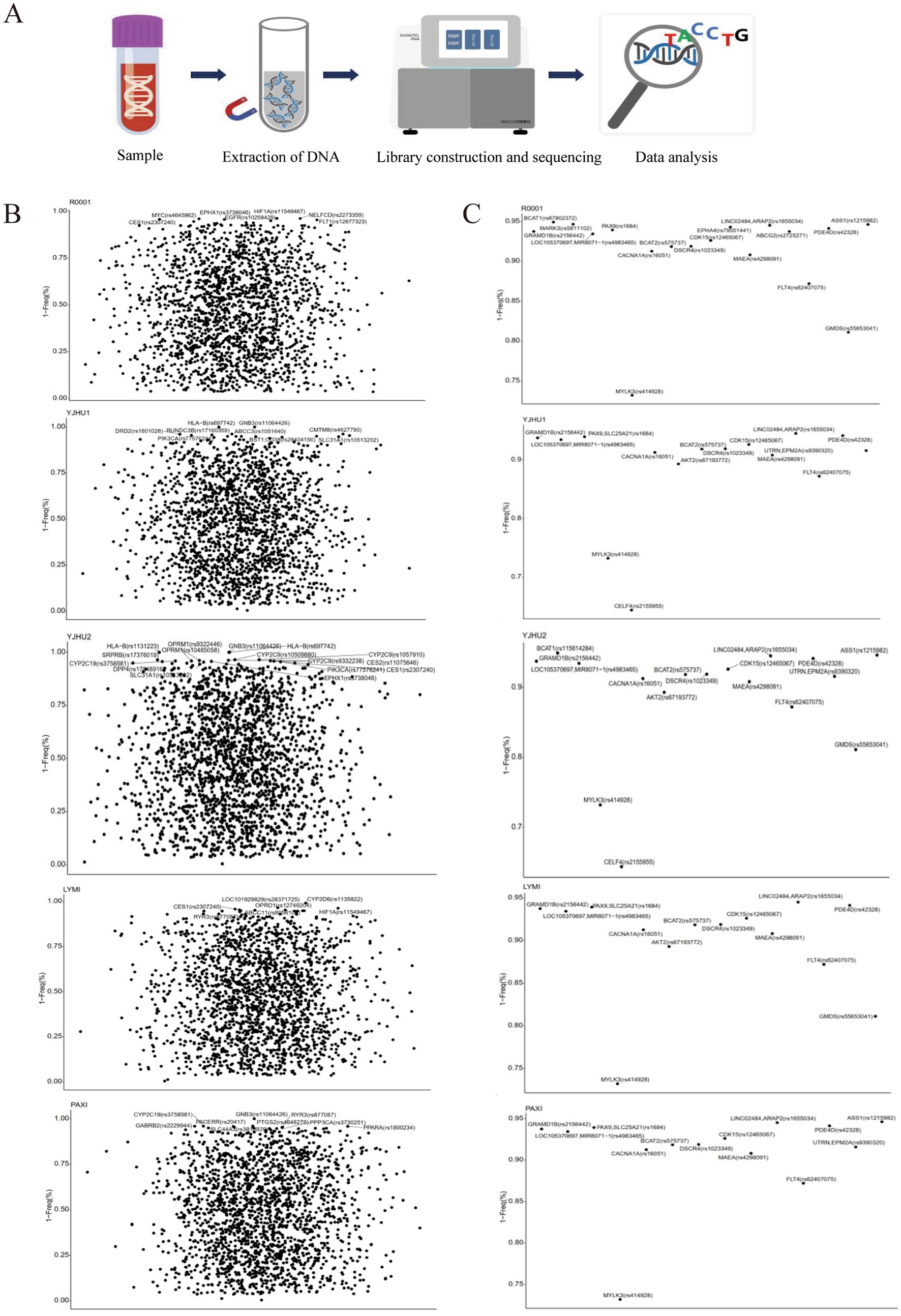
Figure 1. (A) Flowchart of the pharmacogenomic analysis and HLA gene analysis. (B) The possibility of drug-gene involvement in alarelin acetate-induced liver injury. Loci with a gene frequency of less than 5% are annotated. (C) The possibility that alarelin acetate-induced liver injury is associated with highly variable drug-gene loci specific to the Chinese population.
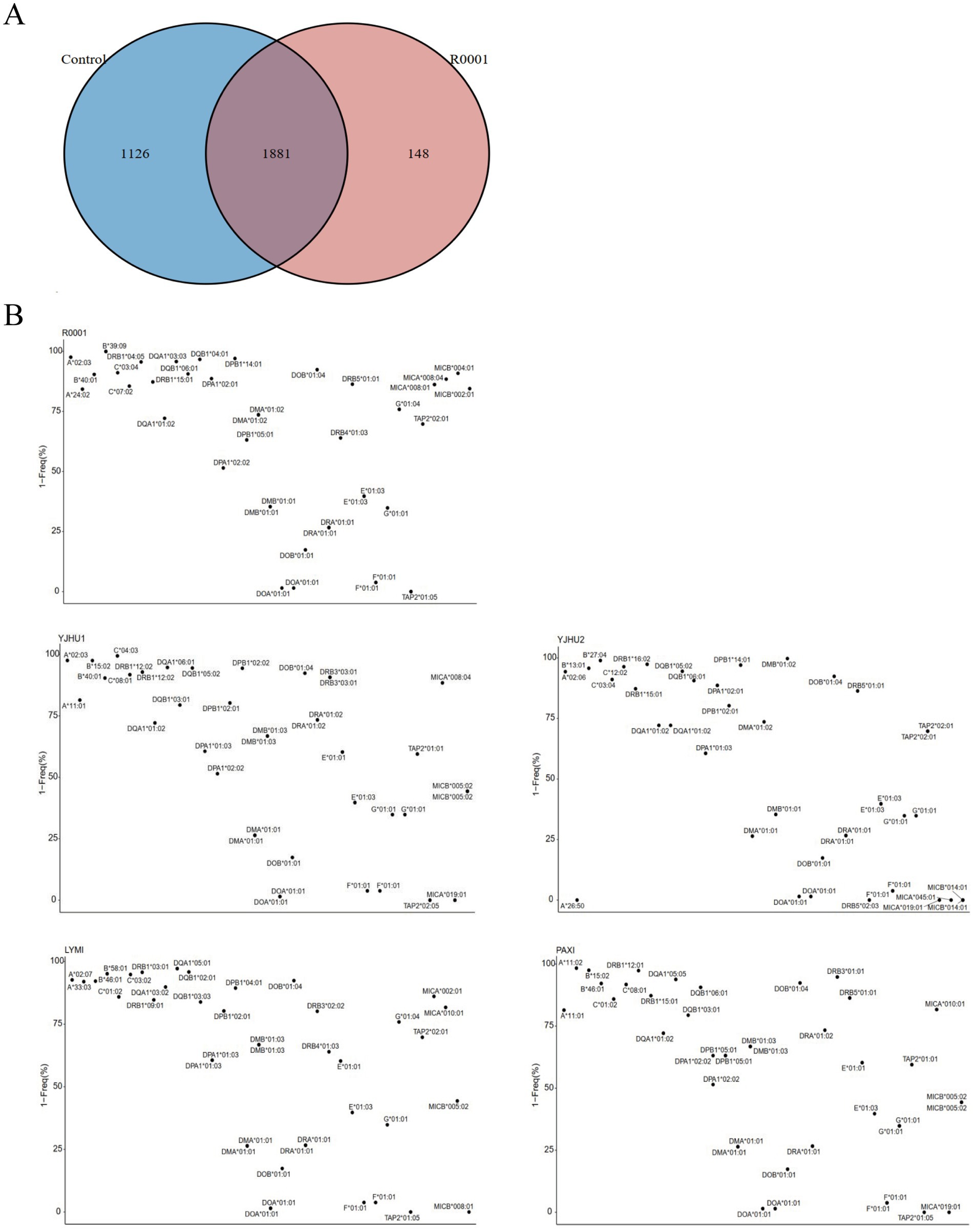
Figure 2. (A) Venn diagram of the variation site differences between the control sample and case sample. (B) The possibility that alarelin acetate-induced liver injury is related to HLA genes.
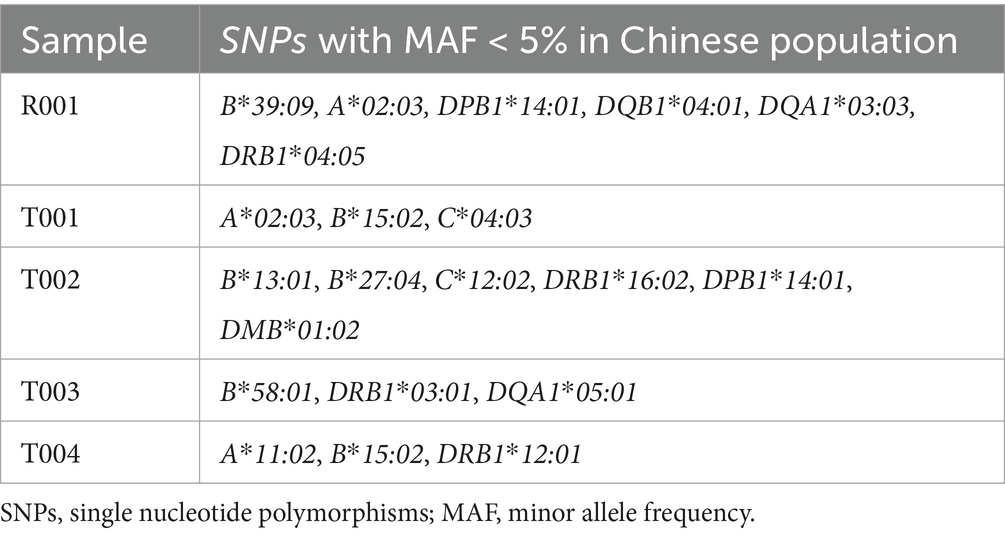
High-resolution HLA genotyping was completed for all participants (Supplementary file 4). The case participant (R001) exhibited 40 distinct HLA alleles (Supplementary file 5). The analysis of rare HLA alleles (MAF < 5% in the Chinese population) revealed the following: R001 had HLA-A*02:03, HLA-B*39:09, HLA-DPB1*14:01, HLA-DQB1*04:01, HLA-DQA1*03:03, and HLA-DRB1*04:05. In the control group, YJHU1 had HLA-A*02:03, HLA-B*15:02, and HLA-C*04:03; YJHU2 had HLA-B*13:01, HLA-B*27:04, HLA-C*12:02, HLA-DRB1*16:02, HLA-DPB1*14:01, and HLA-DMB*01:02; LYMI had HLA-B*58:01, HLA-DRB1*03:01, and HLA-DQA1*05:01; and PAXI had HLA-A*11:02, HLA-B*15:02, and HLA-DRB1*12:01 (Figure 2B; Table 5).
Discussion
As a synthetic GnRH analogue, alarelin acetate is widely used in the treatment of endometriosis and hormone-sensitive tumors. However, alarelin acetate-induced DILI is extremely rare, and the mechanism is unknown. This study is the first to report a clinical case of alarelin acetate-associated DILI with an in-depth genetic analysis. This case expands the cognitive scope of the safety of GnRH analogues, suggesting that alarelin acetate may mediate hepatotoxicity through HLA-restricted immune mechanisms. Our study revealed three possible key mechanisms: metabolic pathway-related gene variants (CYP-UGT pathway), a NUDT15 slow metabolism-related phenotype, and an abnormal immune response mediated by HLA polymorphism. Together, these factors formed the basis for triple susceptibility in this case.
The polymorphism of drug-metabolizing enzyme genes is the core factor leading to inter-individual differences observed in drug responses. In this study, we found that the case samples carried multiple low-frequency genetic variants (MAF < 5%), including MYC (rs4645962 T > C), EPHA4 (rs79551441 -> T), and FLT1 (rs12877323 T > G). These variants may increase liver sensitivity to injury by altering pharmacokinetic profiles. Notably, MARK3 (rs5811102 -> T), EPHA4 (rs79551441 -> T), and ABCG2 (rs2725271 C > T) belonged to the 532 high-frequency variant loci identified in the Chinese population (16). These results indicate that ethnic-specific genetic backgrounds may play an important role in the occurrence of DILI.
A number of studies have confirmed that variants in the genes of drug transporters and metabolic enzymes are closely related to the risk of DILI. Several loci detected in this case have been previously reported to be associated with the occurrence of DILI: ABCB1 (rs2032582 A > C) affects the hepatobiliary transport of multiple drugs (17), CYP2C8 (rs11572078 -> A) and CYP2E1 (rs2070676 -> C) are involved in drug oxidative metabolism (18), and IL6 (rs2069840 C > G) regulates the inflammatory response (19). In contrast, multiple variants in the UGT1A9 cluster (rs11568319 -> G, rs12052787 -> T, and others) affect glucuronic acid binding, a key detoxification pathway in phase II drug metabolism (20). The synergistic variation of these metabolic pathway genes may significantly reduce the liver’s ability to process drugs and their active metabolites, leading to the accumulation of toxic substances.
The presence of the NUDT15*3/*3 polymorphism (rs116855232) in this case requires further discussion. NUDT15, as a nucleotide pyrohydrolase, plays a key role in the metabolism of thiopurines, such as azathioprine (21). The hypometabolic phenotype of this gene is more common in Asian populations and leads to abnormal accumulation of the active metabolite 6-thioguanine nucleotide, increasing the risk of myelosuppression (22). Notably, the NUDT15*3/*3 genotype was found to be associated with azathioprine-induced liver injury (23). However, alarelin acetate is a peptide drug whose metabolism is theoretically independent of the NUDT15 pathway. Our findings provide new insights into the role of NUDT15 in the hepatotoxicity of non-thiopurines.
The polymorphism of the HLA system plays a decisive role in the drug-specific immune response (24–27). This study found that the patient carried multiple HLA alleles associated with adverse drug reactions, forming a unique immune susceptibility background (Table 6). These alleles show clear patterns associated with different organ damage, especially in the skin and liver. The skin, another common target organ, shows different characteristics of the HLA association. HLA-A*24:02, HLA-B*40:01, HLA-DPA1*02:02, and HLA-DRB1*04:05 carried by this patient are associated with severe skin reactions, such as antipsychotic-induced drug reaction with eosinophilia and systemic symptoms (DRESS) and Stevens–Johnson syndrome (SJS) (28–31). HLA-DQA1*03:03 and HLA-DQB1*04:01 have also been reported to be associated with drug-induced cutaneous adverse reactions (32). The liver, a major metabolic organ, exhibits significant gene-drug specificity in HLA-related drug-induced damage. For example, liver injury caused by amoxicillin-clavulanic acid is associated with the HLA-DRB1*15:01 and HLA-DQB1*06:01 haplotypes (33, 34). Liver injury caused by antituberculosis drugs is associated with the HLA-DQA1*01:02 haplotype (35). In addition, HLA-B*39:09 is related to clozapine-induced agranulocytosis (36, 37). HLA-DPA1*02:01 is related to a negative antibody after measles vaccination (38). By altering the structure of the antigen-binding groove, these alleles affect the recognition efficiency of the drug–antigen complex with the T-cell receptor, resulting in pathological immune activation.
Although this study provides important genetic insights into alarelin acetate-associated DILI, there are still some limitations: (a) sample size limitation: It is difficult to distinguish pathogenic mutations from benign polymorphisms in single-case reports, and it is necessary to expand the sample size in order to verify key genetic markers. (b) Lack of functional validation: the actual ability of HLA molecules to present the alarelin acetate antigen or elicit a T-cell response was not confirmed. (c) Insufficient integration of multi-omics data: There was a lack of synergistic analysis of epigenetic regulation, transcriptomic data, and proteomic information. (d) Insufficient population diversity: The study was based on an Asian population, and the results may not apply to other ethnic groups.
Conclusion
In the study, we incidentally discovered and reported that alarelin acetate may induce the occurrence of DILI. We were the first to elucidate the possible causes of alarelin acetate-associated DILI by genetic analysis; however, larger clinical studies are needed to further confirm this hypothesis. In addition, this pharmacovigilance report implies the value of preemptive PGx/HLA screening in clinical trials.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The study protocol was approved by Changsha Third Hospital Ethics Committee (No. LZ-BARL-PK-01) and registered at ClinicalTrials.gov (NCT2023LP02263). All participants were self-reported as Han Chinese and provided written informed consent. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
FY: Methodology, Writing – review & editing, Writing – original draft, Funding acquisition. PZ: Writing – original draft, Data curation. ML: Writing – original draft, Methodology. YL: Methodology, Writing – original draft. BX: Supervision, Writing – original draft. XL: Funding acquisition, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Hunan Provincial Key Laboratory of Anti-Resistance Microbial Drugs (No. 2023TP1013), the Project of Changsha Municipal Health Commission (No. KJ-B2023064).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1634101/full#supplementary-material
SUPPLEMENTARY FILE 1 | The pharmacogenomics results of 4,343 locus in 5 samples was assessed, 2,044 (R001), 1,986 (YJHU1), 2,056 (YJHU2), 1,981 (LYMI) and 2,018 (PAXI) pharmacogenomic mutations were detected.
SUPPLEMENTARY FILE 2 | 18 high-frequency genes in the Chinese population were found in R001.
SUPPLEMENTARY FILE 3 | 148 unique loci were found in R001.
SUPPLEMENTARY FILE 4 | The genotyping of 22 HLA genes in 5 samples.
SUPPLEMENTARY FILE 5 | Forty HLA alleles were detected in R001 by HLA genotyping.
References
1. Wei, S, Chen, S, Gong, Z, Ouyang, X, An, L, Xie, K, et al. Alarelin active immunization influences expression levels of GnRHR, FSHR and LHR proteins in the ovary and enhances follicular development in ewes. Anim Sci J. (2013) 84:466–75. doi: 10.1111/asj.12030
2. Mao, YM. Standardize the diagnosis and treatment of drug-induced liver injury, and strengthen clinical and translational research. Zhonghua Gan Zang Bing Za Zhi. (2023) 31:337–8. doi: 10.3760/cma.j.cn501113-20230419-00176
3. SW, M, Liu, CH, and Liu, XY. Chinese guideline for diagnosis and management of drug-induced liver injury (2023 version). Zhonghua Gan Zang Bing Za Zhi. (2023) 31:355–84. doi: 10.3760/cma.j.cn501113-20230419-00176-1
4. Mao, Y, Ma, S, Liu, C, Liu, X, Su, M, Li, D, et al. Chinese guideline for the diagnosis and treatment of drug-induced liver injury: an update. Hepatol Int. (2024) 18:384–419. doi: 10.1007/s12072-023-10633-7
5. Li, X, Gao, P, and Niu, J. Metabolic comorbidities and risk of development and severity of drug-induced liver injury. Biomed Res Int. (2019) 2019:8764093. doi: 10.1155/2019/8764093
6. Caraballo, PJ, Sutton, JA, Giri, J, Wright, JA, Nicholson, WT, Kullo, IJ, et al. Integrating pharmacogenomics into the electronic health record by implementing genomic indicators. J Am Med Inform Assoc. (2020) 27:154–8. doi: 10.1093/jamia/ocz177
7. Del Toro-Pagán, NM, Matos, A, Bardolia, C, Michaud, V, Turgeon, J, and Amin, NS. Pharmacist assessment of drug-gene interactions and drug-induced phenoconversion in major depressive disorder: a case report. BMC Psychiatry. (2022) 22:46. doi: 10.1186/s12888-021-03659-4
8. Olloquequi, J, Castro-Santos, P, and Díaz-Peña, R. Pharmacogenetic variation and its clinical relevance in a Latin American rural population. Int J Mol Sci. (2022) 23:11758. doi: 10.3390/ijms231911758
9. Zuo, HL, Huang, HY, Lin, YCD, Cai, XX, Kong, XJ, Luo, DL, et al. Enzyme activity of natural products on cytochrome P450. Molecules. (2022) 27:27(2). doi: 10.3390/molecules27020515
10. Jarrar, Y, and Lee, SJ. The functionality of UDP-glucuronosyltransferase genetic variants and their association with drug responses and human diseases. J Pers Med. (2021) 11:554. doi: 10.3390/jpm11060554
11. Boondam, Y, Songvut, P, Tantisira, MH, Tapechum, S, Tilokskulchai, K, and Pakaprot, N. Inverted U-shaped response of a standardized extract of Centella asiatica (ECa 233) on memory enhancement. Sci Rep. (2019) 9:8404. doi: 10.1038/s41598-019-44867-z
12. Mei, Z, Huang, J, Qiao, B, and Lam, AKY. Immune checkpoint pathways in immunotherapy for head and neck squamous cell carcinoma. Int J Oral Sci. (2020) 12:16. doi: 10.1038/s41368-020-0084-8
13. Song, C, Wang, L, Li, Q, Liao, B, Qiao, W, Li, Q, et al. Generation of individualized immunocompatible endothelial cells from HLA-I-matched human pluripotent stem cells. Stem Cell Res Ther. (2022) 13:48. doi: 10.1186/s13287-022-02720-7
14. Stephens, C, Lucena, MI, and Andrade, RJ. Genetic risk factors in the development of idiosyncratic drug-induced liver injury. Expert Opin Drug Metab Toxicol. (2021) 17:153–69. doi: 10.1080/17425255.2021.1854726
15. Fu, L, Qian, Y, Shang, Z, Sun, X, Kong, X, and Gao, Y. Antibiotics enhancing drug-induced liver injury assessed for causality using Roussel Uclaf causality assessment method: emerging role of gut microbiota dysbiosis. Front Med (Lausanne). (2022) 9:972518. doi: 10.3389/fmed.2022.972518
16. Wang, L-Y, Yu, B, Peng, Y, Mou, K, Zhan, Y, Wang, YM, et al. The pharmacogenomic landscape in the Chinese: an analytics of pharmacogenetic variants in 206,640 individuals. Innov (Camb). (2025) 6:100773. doi: 10.1016/j.xinn.2024.100773
17. Xiong, X, Li, L, and Wang, B. Research progress on gene polymorphism of drug-induced liver injury. Chin J Hosp Pharm. (2020) 40, 827–830. doi: 10.13286/j.1001-5213.2020.07.23
18. Ren, Y, Pan, C, and Shen, S. Research progress on gene polymorphisms associated with drug-induced liver injury. Chin J Hosp Pharm. (2019) 35, 3257–3260. doi: 10.13699/j.cnki.1001-6821.2019.24.023
19. Jiao, H, Zhang, M, Li, W, Wang, X, Zhang, H, Chen, P, et al. Meta-analysis of the association between gene polymorphisms of IL-4, IL-6, IL-10, TNF-α and drug-induced liver injury analysis. Chin J Hosp Pharm. (2020) 40, 1462–1470. doi: 10.13286/j.1001-5213.2020.13.12
20. Bernard, O, and Guillemette, C. The main role of UGT1A9 in the hepatic metabolism of mycophenolic acid and the effects of naturally occurring variants. Drug Metab Dispos. (2004) 32:775–8. doi: 10.1124/dmd.32.8.775
21. Suiter, CC, Moriyama, T, Matreyek, KA, Yang, W, Scaletti, ER, Nishii, R, et al. Massively parallel variant characterization identifies NUDT15 alleles associated with thiopurine toxicity. Proc Natl Acad Sci USA. (2020) 117:5394–401. doi: 10.1073/pnas.1915680117
22. Zhang, F, Amat, G, Tang, Y, Chen, R, Tian, X, Hu, W, et al. NUDT15 genetic variants in Chinese Han, Uighur, Kirghiz, and Dai nationalities. Front Pediatr. (2022) 10:832363. doi: 10.3389/fped.2022.832363
23. Wang, X, and Wang, W. Association between polymorphism of NUDT15 gene and hepatotoxicity induced by 6-MP in children with acute lymphoblastic leukemia. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. (2021) 38:1258–61. doi: 10.3760/cma.j.cn511374-20200615-00538
24. Elzagallaai, AA, Abuzgaia, AM, and Rieder, MJ. A comprehensive update on the human leukocyte antigen and idiosyncratic adverse drug reactions. Expert Opin Drug Metab Toxicol. (2025) 21:551–62. doi: 10.1080/17425255.2025.2455388
25. Petry, N, Forest, K, and Wilke, RA. The expanding role of HLA gene tests for predicting drug side effects. Am J Med Sci. (2024) 367:14–20. doi: 10.1016/j.amjms.2023.10.004
26. Chang, CJ, Chen, CB, Hung, SI, Ji, C, and Chung, WH. Pharmacogenetic testing for prevention of severe cutaneous adverse drug reactions. Front Pharmacol. (2020) 11:969. doi: 10.3389/fphar.2020.00969
27. Mosedale, M, and Watkins, PB. Understanding idiosyncratic toxicity: lessons learned from drug-induced liver injury. J Med Chem. (2020) 63:6436–61. doi: 10.1021/acs.jmedchem.9b01297
28. Ramírez, E, Bellón, T, Tong, HY, Borobia, AM, de Abajo, FJ, Lerma, V, et al. Significant HLA class I type associations with aromatic antiepileptic drug (AED)-induced SJS/TEN are different from those found for the same AED-induced DRESS in the Spanish population. Pharmacol Res. (2017) 115:168–78. doi: 10.1016/j.phrs.2016.11.027
29. Mori, F, Saretta, F, Riscassi, S, Caimmi, S, Bottau, P, Liotti, L, et al. Risk factors for drug hypersensitivity reactions in children. Ital J Pediatr. (2024) 50:127. doi: 10.1186/s13052-024-01694-x
30. Spina Tensini, T, de Paola, L, Boldt, ABW, Von Glehn, C d QC, Bettinotti, M, and Silvado, CES. HLA alleles and antiseizure medication-induced cutaneous reactions in Brazil: a case-control study. HLA. (2023) 102:269–77. doi: 10.1111/tan.15045
31. Jiang, M, Yang, F, Zhang, L, Xu, D, Jia, Y, Cheng, Y, et al. Unique motif shared by HLA-B*59:01 and HLA-B*55:02 is associated with methazolamide-induced Stevens-Johnson syndrome and toxic epidermal necrolysis in Han Chinese. J Eur Acad Dermatol Venereol. (2022) 36:873–80. doi: 10.1111/jdv.17980
32. Koomdee, N, Pratoomwun, J, Jantararoungtong, T, Theeramoke, V, Tassaneeyakul, W, Klaewsongkram, J, et al. Association of HLA-A and HLA-B alleles with lamotrigine-induced cutaneous adverse drug reactions in the Thai population. Front Pharmacol. (2017) 8:879. doi: 10.3389/fphar.2017.00879
33. Daly, AK. Genetics of drug-induced liver injury: current knowledge and future prospects. Clin Transl Sci. (2023) 16:37–42. doi: 10.1111/cts.13424
34. Kaliyaperumal, K, Grove, JI, Delahay, RM, Griffiths, WJH, Duckworth, A, and Aithal, GP. Pharmacogenomics of drug-induced liver injury (DILI): molecular biology to clinical applications. J Hepatol. (2018) 69:948–57. doi: 10.1016/j.jhep.2018.05.013
35. Katran, ZY, Bulut, İ, Babalik, A, and Keren, M. Management of type 1 immediate hypersensitivity reactions to antituberculosis drug: succesful desensitization. Allergy Asthma Clin Immunol. (2022) 18:97. doi: 10.1186/s13223-022-00737-4
36. Islam, F, Men, X, Yoshida, K, Zai, CC, and Müller, DJ. Pharmacogenetics-guided advances in antipsychotic treatment. Clin Pharmacol Ther. (2021) 110:582–8. doi: 10.1002/cpt.2339
37. Hernandez, M, Cullell, N, Cendros, M, Serra-Llovich, A, and Arranz, MJ. Clinical utility and implementation of pharmacogenomics for the personalisation of antipsychotic treatments. Pharmaceutics. (2024) 16:244. doi: 10.3390/pharmaceutics16020244
38. Neidhart, S, Vlad, B, Hilty, M, Högelin, KA, Ziegler, M, Berenjeno-Correa, E, et al. HLA associations of intrathecal IgG production against specific viruses in multiple sclerosis. Ann Neurol. (2024) 95:1112–26. doi: 10.1002/ana.26921
Glossary
GnRH - gonadotropin-releasing hormone
SNPs - single-nucleotide polymorphisms
DILI - drug-induced liver injury
HLA - human leukocyte antigen
ALT - alanine aminotransferase
AST - aspartate aminotransferase
LDH - lactate dehydrogenase
ALP - alkaline phosphatase
GGTγ - Glutamyltransferase
TBil - total bilirubin
PGx - pharmacogenomic
CYP450 - cytochrome P450 proteins
UGTs - UDP-glucuronosyltransferases
GST - glutathione S-transferases
NAT - N-acetyltransferase
MAF - minor allele frequency
RUCAM - Roussel Uclaf Causality Assessment Method
NUDT15 - NUDIX-type 15
DRESS - drug reaction with eosinophilia and systemic symptoms
SJS - Stevens–Johnson syndrome
TEN - toxic epidermal necrolysis
MPE - maculopapular exanthema
Keywords: alarelin acetate, drug-induced liver injury, pharmacogenomics, human leukocyte antigen, single nucleotide polymorphism
Citation: Yuan F, Zhang P, Liu M, Li Y, Xu B and Li X (2025) Pharmacogenomic analysis of alarelin acetate-induced hepatotoxicity: a case report and literature review. Front. Med. 12:1634101. doi: 10.3389/fmed.2025.1634101
Edited by:
Ankit P. Laddha, University of Connecticut, United StatesReviewed by:
Parbeen Singh, University of Connecticut, United StatesEsienanwan Efiong, Federal University Lafia Faculty of Sciences, Nigeria
Copyright © 2025 Yuan, Zhang, Liu, Li, Xu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xin Li, WGluLWxpQGNzc2RzeXkuY29t
 Fang Yuan
Fang Yuan Ping Zhang1
Ping Zhang1 Xin Li
Xin Li