- 1Department of Gastroenterology, The First Affiliated Hospital, Jiangxi Medical College, Nanchang University, Nanchang, China
- 2Department of Gastroenterology, Jiujiang City Key Laboratory of Cell Therapy, Jiujiang No. 1 People’s Hospital, Jiujiang, China
Background: Acute pancreatitis (AP) is a well-recognized digestive emergency with established clinical significance. However, current evidence regarding urban–rural distribution patterns of AP patients remains relatively limited. Through large-scale data analysis, this study aims to provide preliminary epidemiological references for this understudied area.
Methods: This 20-year retrospective cohort study (2005–2024) analyzed 12,214 acute pancreatitis (AP) cases from a tertiary medical center to investigate urban–rural disparities in etiology and clinical outcomes. Patients were stratified into urban (n = 5,002) and rural (n = 7,212) groups based on residential location. We compared demographic characteristics, etiological distributions, disease severity, complications, and hospitalization outcomes between the groups. Risk factors for moderate-to-severe AP were assessed using multivariable logistic regression, with adjustment for demographic, clinical, and temporal covariates.
Results: Urban patients exhibited a rising burden of hypertriglyceridemia-induced AP (HTG-AP; 30.6% vs. rural 26.3%, p < 0.001), surpassing biliary AP as the dominant etiology by 2023, while rural populations maintained higher biliary AP prevalence (56.4% vs. 51.7%, p < 0.001). Rural patients demonstrated prolonged symptom-to-admission intervals (median 3 vs. 2 days), elevated APACHE II scores (8 vs. 7), and increased severe AP incidence (20.7% vs. 18.3%, p < 0.01), with higher risks of infected pancreatic necrosis (5.3% vs. 4.3%) and abdominal compartment syndrome (1.7% vs. 1.1%). Multivariable analysis suggested that rural group may be associated with increased risk of moderate-to-severe AP (aOR = 1.13, p = 0.005), alongside hypertriglyceridemia (aOR = 2.06) and delayed admission (aOR = 1.01/day). Temporal trends revealed accelerated HTG-AP growth post-2020 in both groups, paralleling metabolic syndrome escalation.
Conclusion: These findings underscore the imperative for dual interventions: urban-focused metabolic risk mitigation and rural-targeted biliary disease management, informed by evolving etiological landscapes.
1 Introduction
Acute pancreatitis (AP) is one of the most common critical illnesses in the digestive system, with a global annual incidence of approximately 34 per 100,000 population (1), showing an increasing trend year by year. AP has a complex etiology with highly variable clinical outcomes. Approximately 20% of patients may progress to severe acute pancreatitis (SAP), a condition associated with multiple organ failure and mortality rates reaching 30% (2, 3). In recent years, with changes in dietary patterns and the rising prevalence of metabolic diseases, the etiological spectrum of AP has undergone significant shifts. In China, hypertriglyceridemia (HTG) has nearly surpassed or even exceeded alcohol consumption as the second most common cause of AP, following biliary etiology (4–6). However, the epidemiological characteristics and clinical outcomes of AP exhibit notable regional heterogeneity, particularly between urban and rural areas with significant disparities in healthcare resource allocation and lifestyle (7). Previous studies have suggested that the etiology, severity, and clinical outcomes of AP may be influenced by multiple factors, including demographic characteristics, underlying diseases, laboratory findings, healthcare accessibility, and health behaviors (1, 2, 8–10).
Against the backdrop of rapid urbanization in China, systematic differences exist between urban and rural residents in dietary patterns (e.g., carbohydrate-dominant diets in rural areas vs. high-fat diets in urban areas), health behaviors (e.g., alcohol consumption, physical activity levels), and healthcare accessibility (e.g., primary care capacity, referral efficiency) (11, 12). The role of urban–rural disparities in the epidemiological features and clinical outcomes of AP has attracted considerable attention. Significant differences in dietary habits, lifestyle, and healthcare resource distribution between urban and rural populations may influence the occurrence and prognosis of AP (12–14). For instance, high-fat diets and alcohol intake are major risk factors for AP, and these factors are more prevalent among urban residents (15, 16). However, rural patients may face challenges such as insufficient medical resources and delayed treatment, which could adversely affect disease management and therapeutic outcomes (17).
This study, based on a prospectively maintained large-sample AP database, presents the first longitudinal comparison over 20 years of demographic characteristics, etiological composition, and clinical outcomes between urban and rural hospitalized AP patients, aiming to provide evidence for optimizing region-specific diagnostic and therapeutic strategies.
2 Materials and methods
2.1 Data source
This study was a retrospective single-center cohort study utilizing data from a prospectively maintained AP inpatient database at the First Affiliated Hospital of Nanchang University. The database was established by trained clinicians through structured electronic medical record (EMR) extraction and independently verified by two researchers to ensure data accuracy and completeness. It encompasses comprehensive information, including demographic characteristics, laboratory findings, radiological reports, AP scoring systems (e.g., APACHE II, BISAP), clinical features, and discharge outcomes. All methods were performed in accordance with the relevant guidelines and regulations, and in compliance with the Declaration of Helsinki. The study was approved by the Medical Ethics Research Committee of the First Affiliated Hospital of Nanchang University.
2.2 Study population
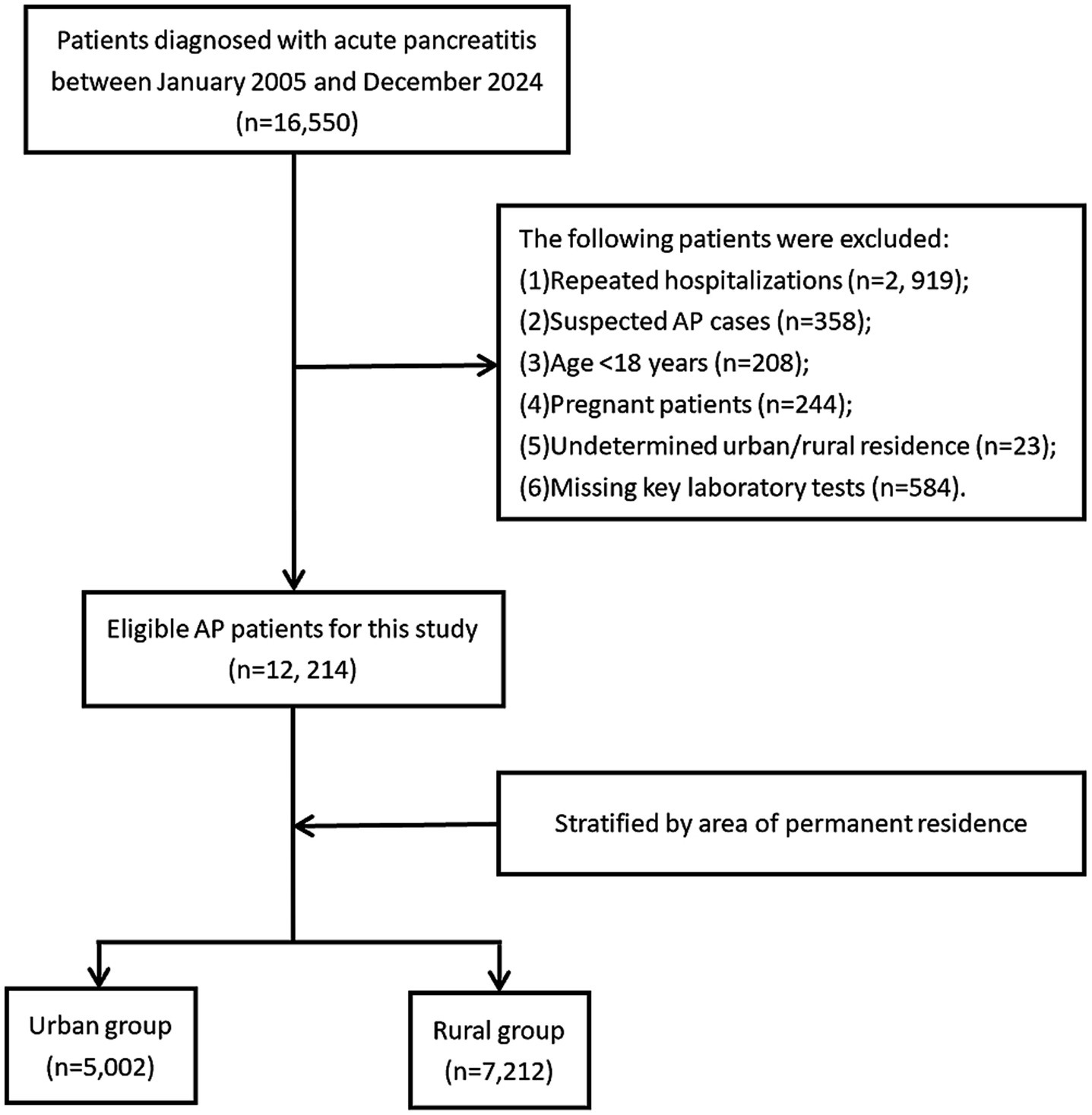
A total of 16,550 patients discharged with a diagnosis of acute pancreatitis (AP) from the First Affiliated Hospital of Nanchang University between January 2005 and December 2024 were initially identified. Hospital admission data for patients with AP were retrieved using the International Classification of Diseases, 10th Revision (ICD-10) codes K85-K85.92. AP diagnosis and severity classification followed the 2012 revised Atlanta criteria (18). Exclusion criteria included: (1): non-index admission for AP (prior AP hospitalization), (2), suspected AP cases, (3) age <18 years, (4) pregnancy, (5) undetermined urban–rural residency, and (6) missing critical laboratory parameters. After screening, 12,214 eligible AP patients were enrolled and stratified into urban (n = 5,002) and rural (n = 7,212) groups based on residential status. Figure 1 depicts the participant selection procedure.
2.3 Urban–rural classification
Patients were stratified into urban or rural groups based on residential duration and geographical criteria: urban residency was defined as permanent residence (≥6 months/year) in prefecture-level cities or municipal districts, while rural residency encompassed townships or administrative villages. Residential data were extracted from hospitalization records, which mandated patients to report detailed addresses and contact information. For migrant populations, classification prioritized actual residential address over household registration (e.g., rural-registered individuals residing long-term in urban areas were categorized as urban). Additionally, health insurance type (urban employee/resident insurance vs. new rural cooperative medical scheme) and occupation (agricultural vs. non-agricultural) were used as secondary reference criteria. Cases with indeterminate residency (e.g., temporary migrant workers lacking fixed addresses) were excluded to minimize misclassification bias. This approach ensured alignment between residential exposure and epidemiological risk profiles.
2.4 Data extraction
Data collection encompassed five domains: (1) Demographics—age, gender, residential status, smoking, and alcohol consumption; (2) Baseline disease characteristics—subclassified into etiology (biliary, HTG, alcoholic, idiopathic, drug-induced, post-surgical, traumatic, post-ERCP, autoimmune, parapapillary diverticulum of duodenum, or other), severity (mild, moderately severe, or severe AP per revised Atlanta criteria), comorbidities (hypertension, diabetes mellitus, hyperlipidemia, chronic obstructive pulmonary disease [COPD], coronary heart disease, cirrhosis, cerebral infarction, chronic renal failure), and admission clinical status (fever on admission, APACHE II score within 24 h); (3) Treatment process—including healthcare access (prior hospital transfer) and time metrics (symptom-to-admission interval); (4) Laboratory parameters—white blood cell count (×109/L) and serum amylase (U/L); (5) Clinical outcomes—stratified into short-term outcomes (in-hospital mortality, total hospital stay, ICU stay, hospitalization costs) and complications (local: infected pancreatic necrosis, abdominal compartment syndrome, enteric fistula; systemic: acute respiratory failure, acute kidney injury, circulatory failure, septic shock, heart failure, hepatic dysfunction; thrombotic: portosplenomesenteric venous thrombosis, deep vein thrombosis). This classification aligns with acute pancreatitis research frameworks, ensuring logical separation of intrinsic disease attributes from care-related factors.
2.5 Statistical analysis
All statistical analyses were performed using R software (version 4.4.1). Missing data were handled using multiple imputation with chained equations (MICE), generating 20 imputed datasets based on all analysis variables. Continuous variables were evaluated for normality using Shapiro–Wilk tests and presented as mean ± standard deviation (SD) for normally distributed data or median (interquartile range, IQR) for non-normally distributed data. Categorical variables were expressed as frequencies (percentages). Group comparisons were conducted using independent t-tests (for normally distributed continuous variables), Mann–Whitney U tests (for non-normally distributed continuous variables), or chi-square/Fisher’s exact tests (for categorical variables), as appropriate.
Continuous variables that violated linearity assumptions were categorized into clinically meaningful groups: hospitalization costs (≤11,250, 11,251-26,270, >26,270 Yuan), APACHE II scores (<8, 8–12, ≥12), and white blood cell counts (≤10, 10.1–20, >20 × 109/L). Multivariable logistic regression models were developed to identify risk factors, incorporating variables with p < 0.1 in univariate analysis or clinical significance (Supplementary Table 1). Multicollinearity was assessed using variance inflation factors (VIF < 5 for all retained variables). Model performance was evaluated by calculating area under the receiver operating characteristic curve (AUC). Temporal trends were analyzed using 5-year intervals (2005–2009 to 2020–2024). Results were presented as adjusted odds ratios with 95% confidence intervals and visualized in forest plots. All tests were two-tailed with p < 0.05 considered statistically significant.
3 Results
3.1 Demographic and clinical characteristics
This study enrolled 12,214 acute pancreatitis patients, comprising 5,002 urban cases (40.9%) and 7,212 rural cases (59.1%). Comparative analysis revealed urban group had significantly higher proportions of males, younger age, greater alcohol, and higher prevalence of hypertension, diabetes, and hyperlipidemia (all p < 0.05). Rural patients demonstrated significantly higher rates of prior hospital transfer, longer symptom-to-admission intervals, elevated APACHE II scores, and greater severe acute pancreatitis incidence (all p < 0.01). While rural patients incurred higher hospitalization costs, no significant differences existed in length of stay or mortality. Temporal trends showed rural case proportions increased markedly post-2015 (rising from 8.6% in 2005–2009 to 36.5% in 2015–2019), whereas urban cases peaked during 2020–2024. Baseline clinical characteristics of the two groups are detailed in Table 1.
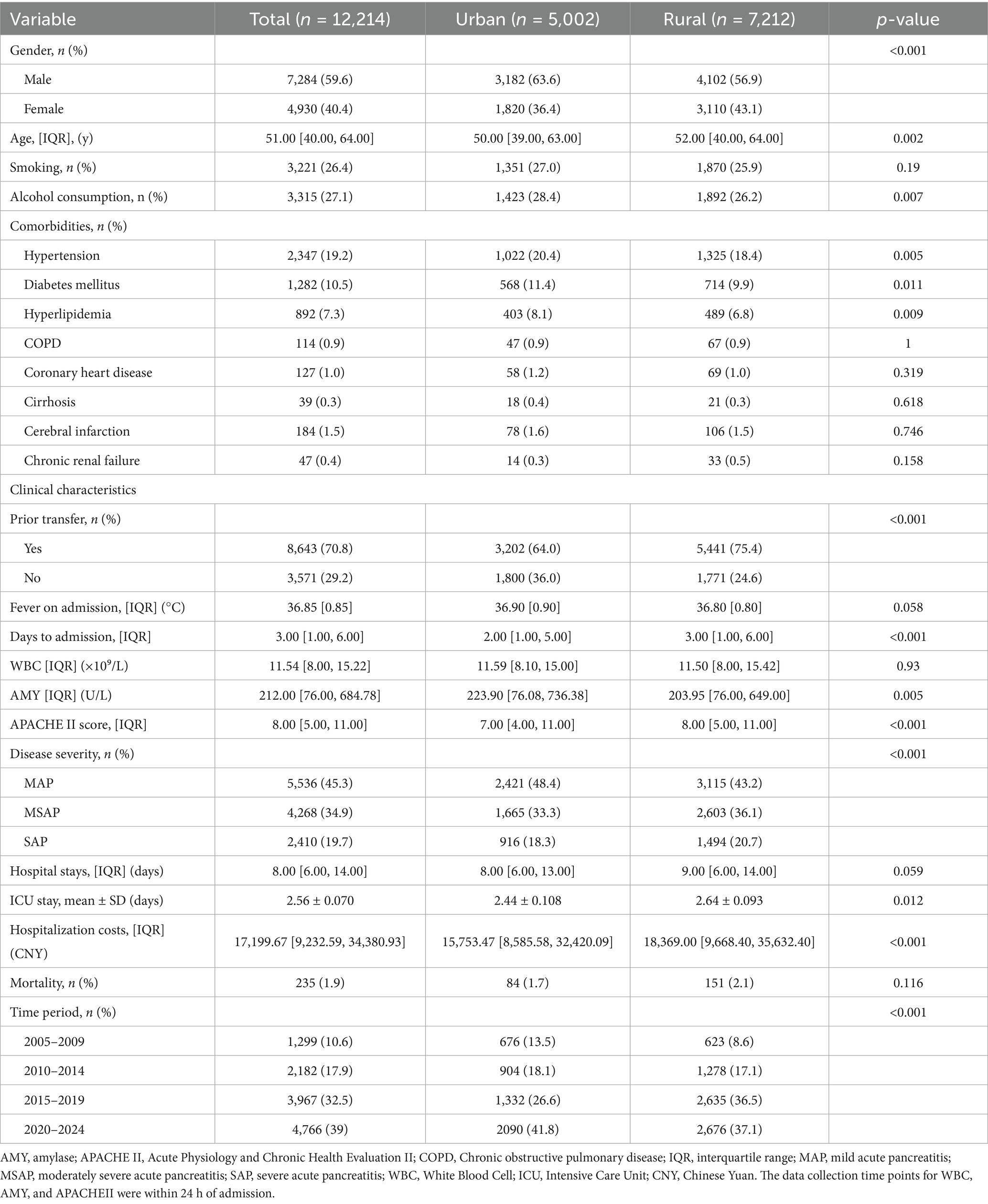
Table 1. Baseline characteristics of patients with acute pancreatitis between urban and rural groups.
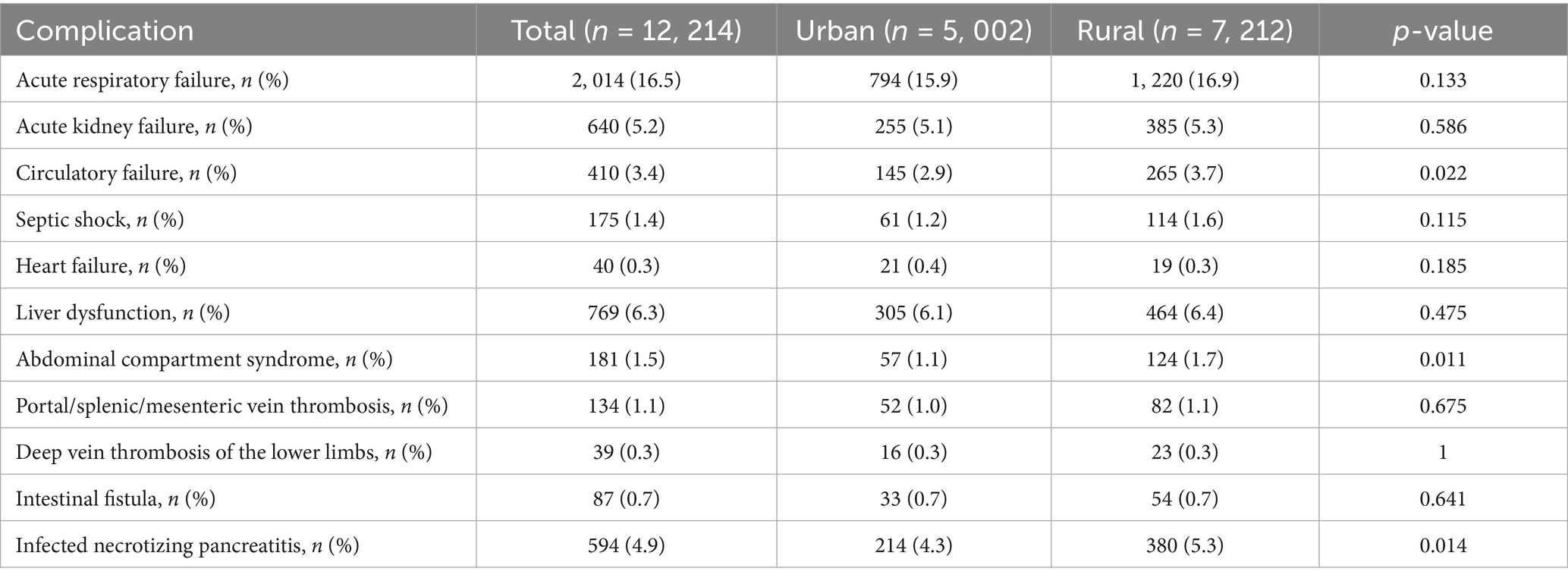
3.2 Systemic complications
As shown in Table 2, acute respiratory failure and acute kidney injury were the most common systemic complications overall, with no significant urban–rural differences (both p > 0.05). Notably, rural patients exhibited significantly higher rates of circulatory failure (3.7% vs. 2.9%, p = 0.022), abdominal compartment syndrome (1.7% vs. 1.1%, p = 0.011), and infected pancreatic necrosis (5.3% vs. 4.3%, p = 0.014). In contrast, other complications including septic shock, heart failure, and thrombotic events showed no statistically significant intergroup differences.
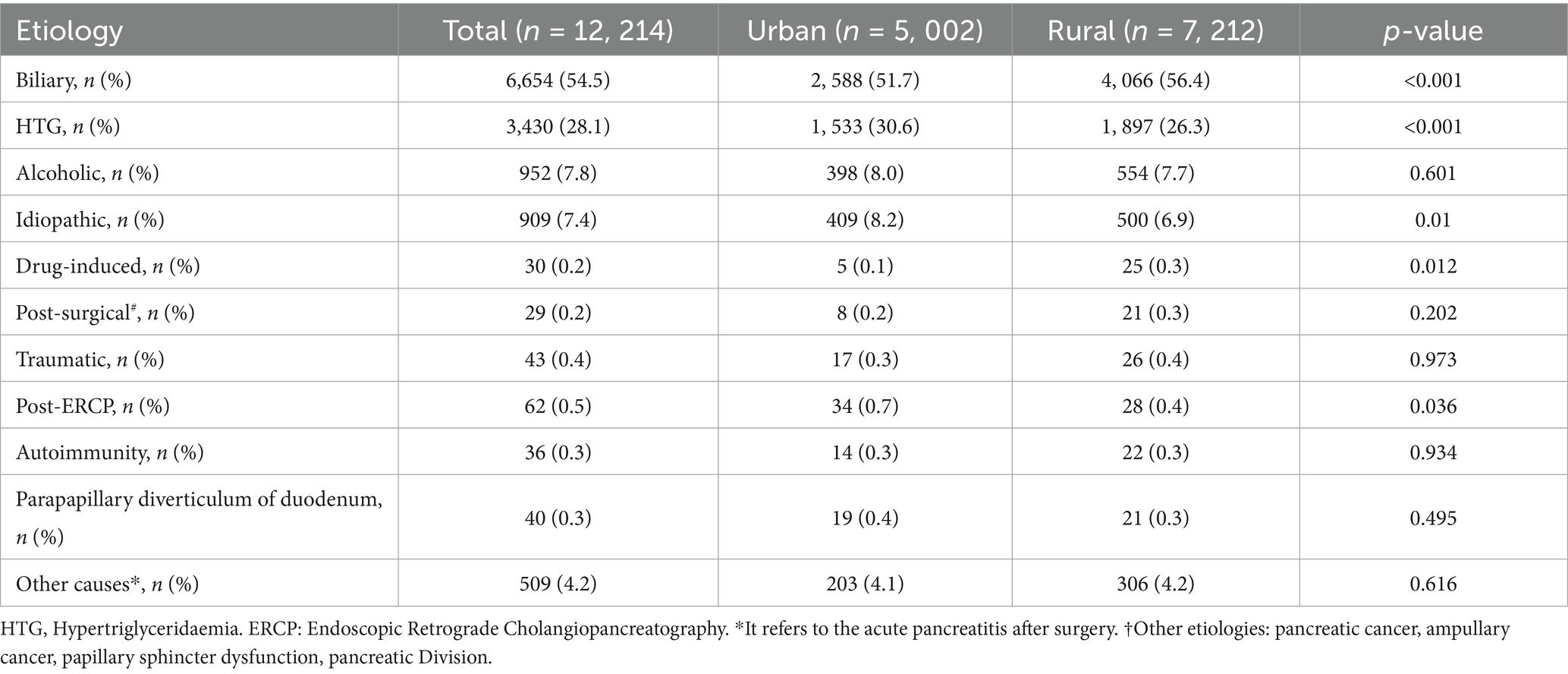
3.3 Etiology of AP
As shown in Table 3, biliary, HTG, and alcoholic etiologies constituted the top three causes of AP in both urban and rural populations. Biliary AP was the most prevalent (54.5%), with significantly higher incidence in rural patients (56.4% vs. 51.7%, p < 0.001). HTG-AP accounted for 28.1% of cases, demonstrating a higher proportion in urban areas (30.6% vs. 26.3%, p < 0.001). No significant urban–rural difference was observed in alcoholic AP, while idiopathic AP was more common in urban patients (8.2% vs. 6.9%, p = 0.010). Notably, statistically significant disparities were identified in drug-induced AP and post-ERCP AP between the two groups. Other etiologies (e.g., traumatic, autoimmune, and periampullary diverticulum-associated AP showed no significant intergroup differences).
3.4 Temporal trends in the three major etiologies of AP (2005–2024)
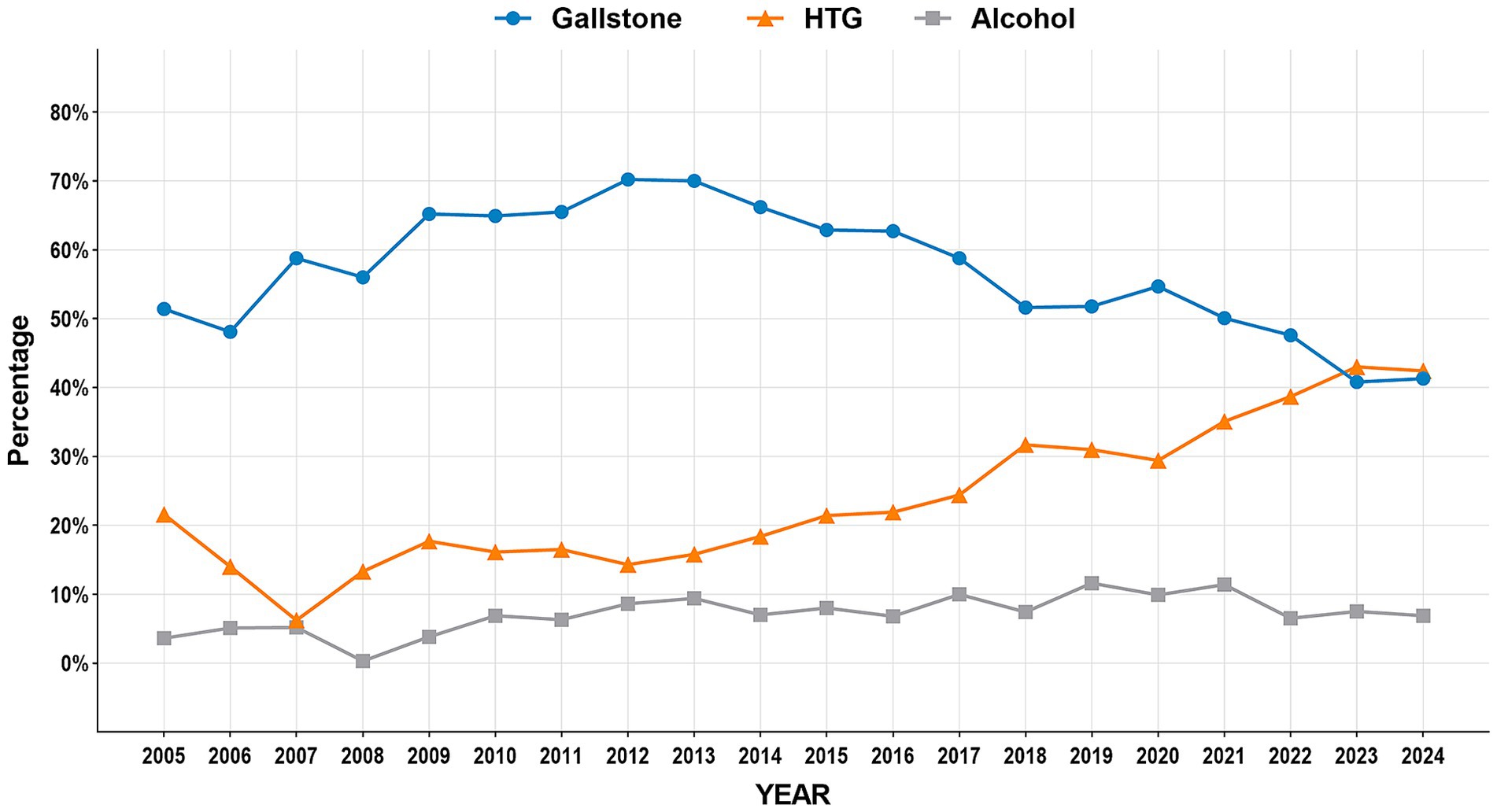
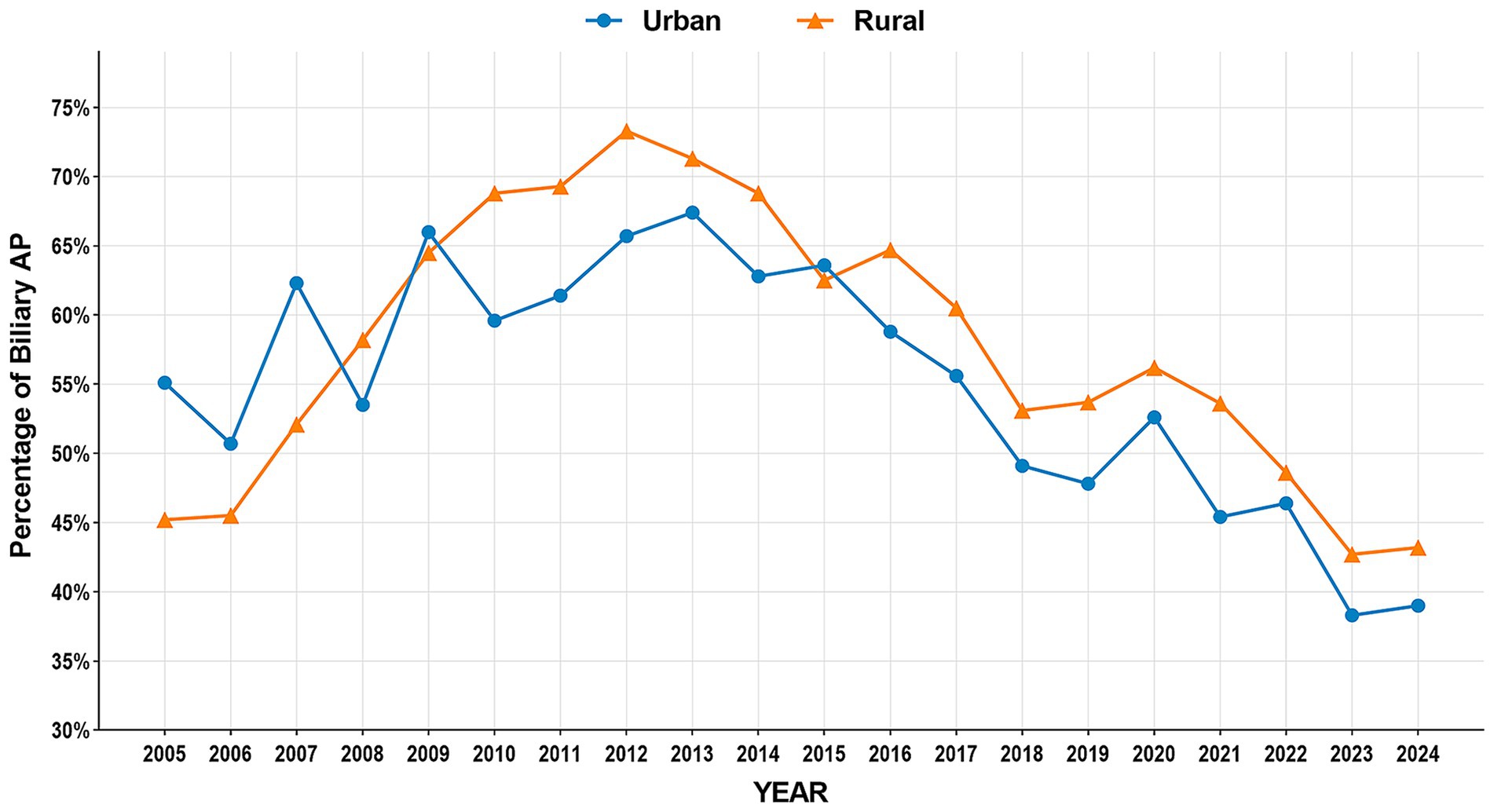
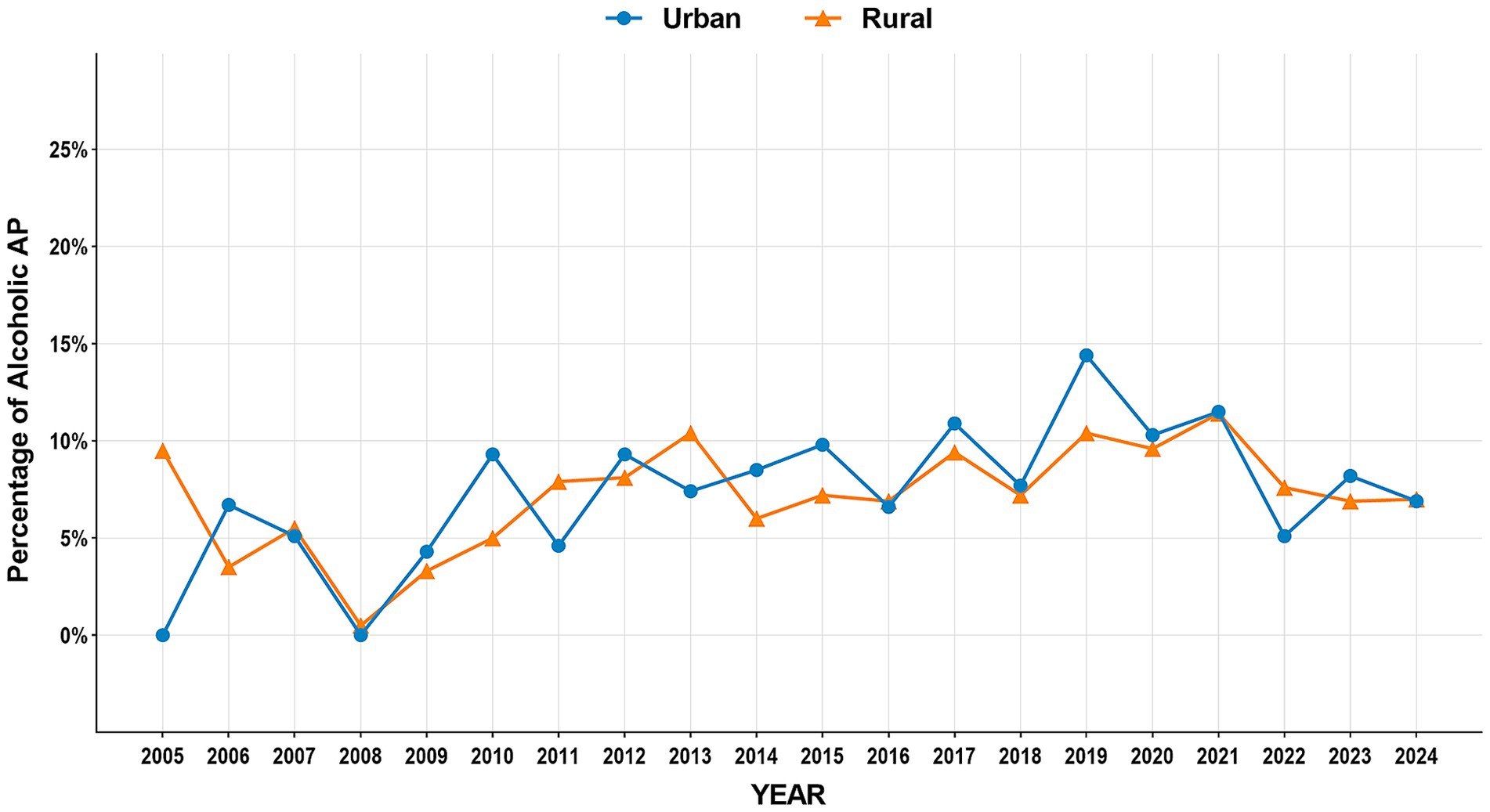
The study period witnessed significant epidemiological shifts in AP etiology (Figure 2). Biliary AP demonstrated a consistent downward trend (51.4% → 41.3%), with rural areas maintaining higher prevalence than urban areas (2024, 43.2% vs. 39.0%) (Figure 3). In contrast, HTG-AP showed remarkable growth (21.6% → 42.4%), surpassing biliary AP as the leading etiology in 2023 (43.0%). The urban group consistently demonstrated higher proportions than the rural group in most years, with both groups exhibiting a steep surge in growth rates since 2020 (Figure 4). Alcoholic AP displayed fluctuating proportions (3.6–11.6%) with diminishing urban–rural disparities (Figure 5). Importantly, the urban–rural gaps for HTG-AP and biliary AP narrowed substantially from 7.9 to 5.3% and 9.9 to 4.2%, respectively, suggesting converging epidemiological patterns.
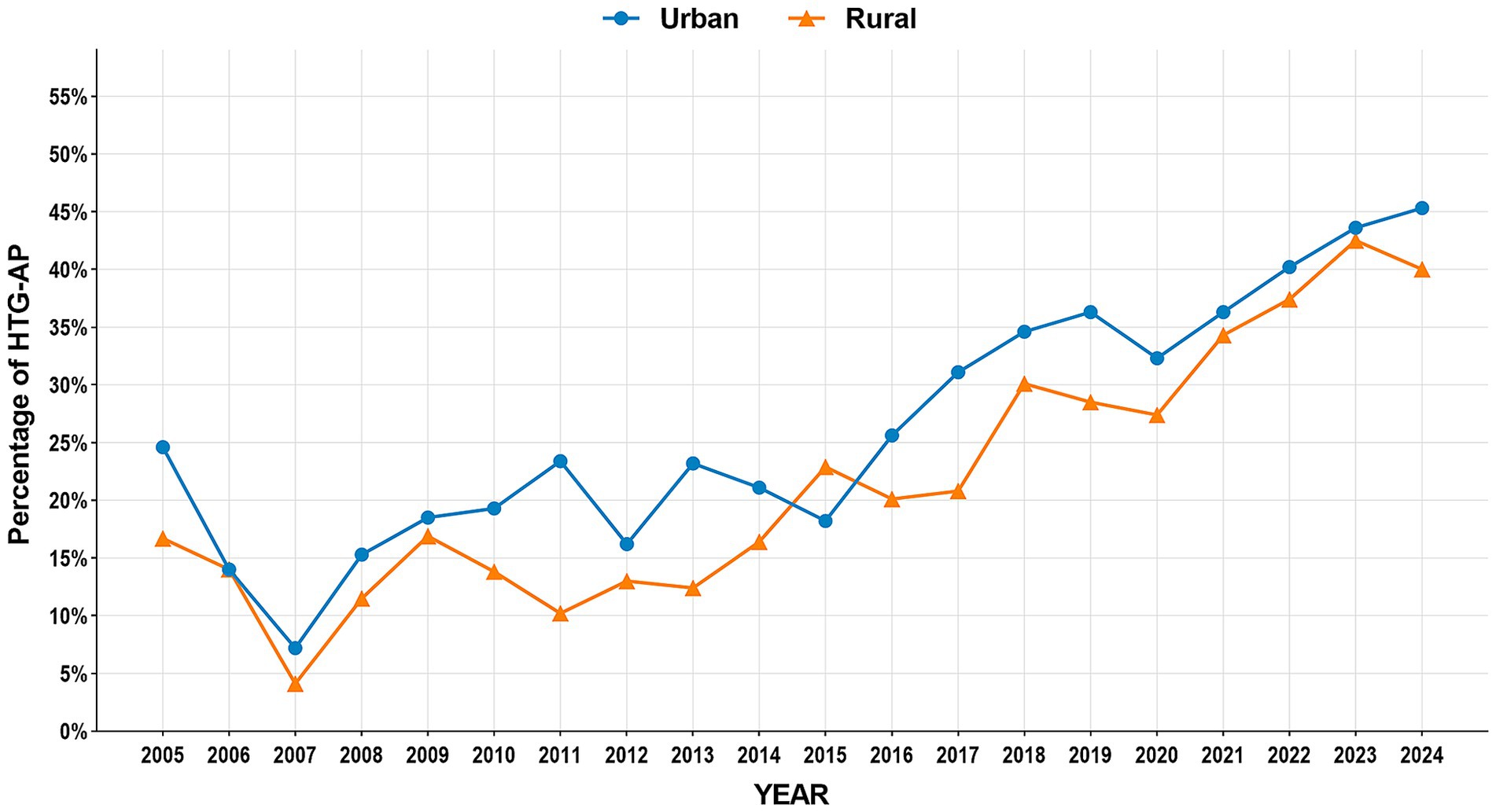
Figure 4. Annual proportion changes of hypertriglyceridemic acute pancreatitis cases: Urban vs. Rural Groups.
3.5 Multivariable logistic regression analysis of factors associated with moderate-to-severe AP
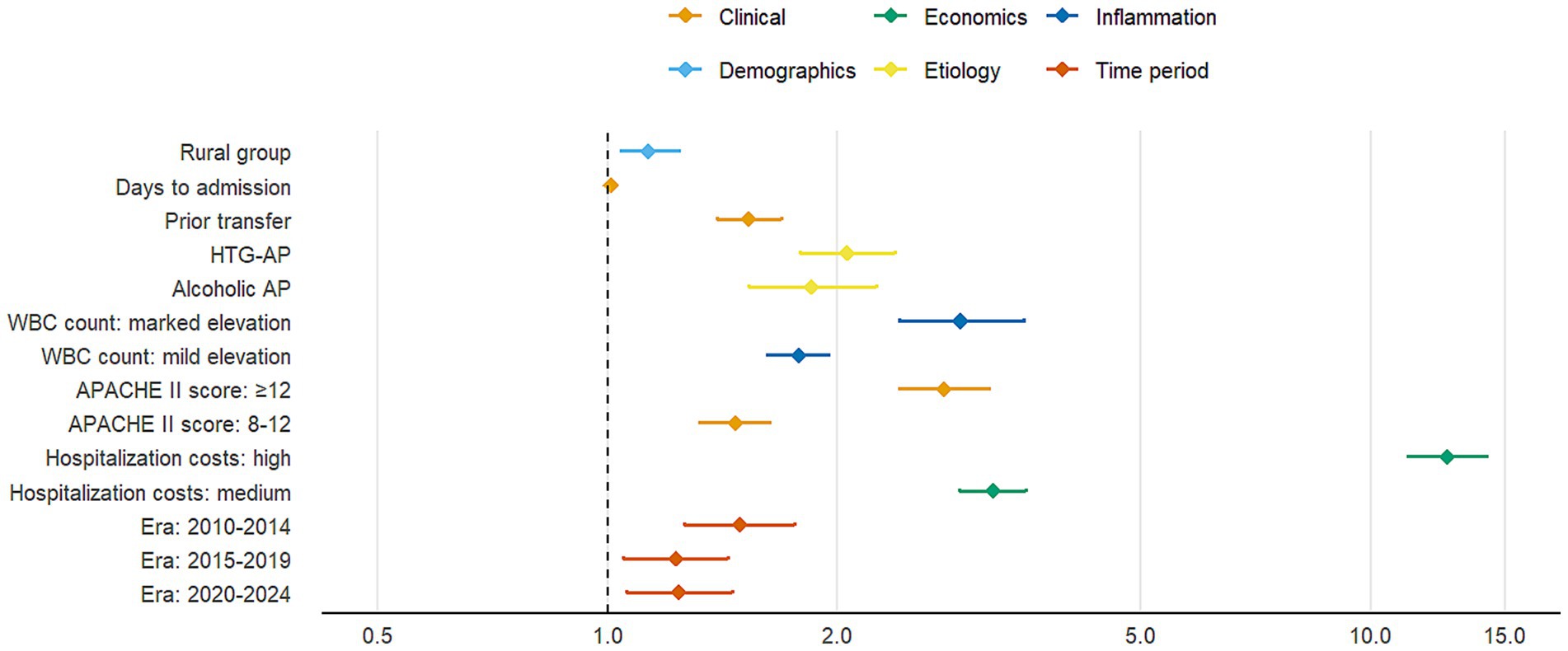
Multivariable logistic regression analysis identified significant risk factors for moderate-to-severe AP (Table 4). As shown in the fully adjusted model (Model 3), rural group was significantly associated with increased disease severity (adjusted OR = 1.13, 95% CI: 1.04–1.24, p = 0.005) compared with urban residence. Hypertriglyceridemia-induced AP (HTG-AP) demonstrated the strongest association (aOR = 2.06, 95% CI: 1.79–2.38, p < 0.001), followed by alcoholic etiology. Clinical predictors including prior hospital transfer, delayed admission, elevated white blood cell count, and higher APACHE II scores were all significantly associated with increased disease severity (all p < 0.001). Notably, patients with APACHE II scores ≥12 had 2.76-fold higher risk of severe outcomes (p < 0.001). Higher hospitalization costs were strongly correlated with disease severity (aOR = 12.6, p < 0.001), likely reflecting the more intensive treatment required for severe cases. Compared with the 2005–2009 baseline period, subsequent years showed elevated risks of severe AP.
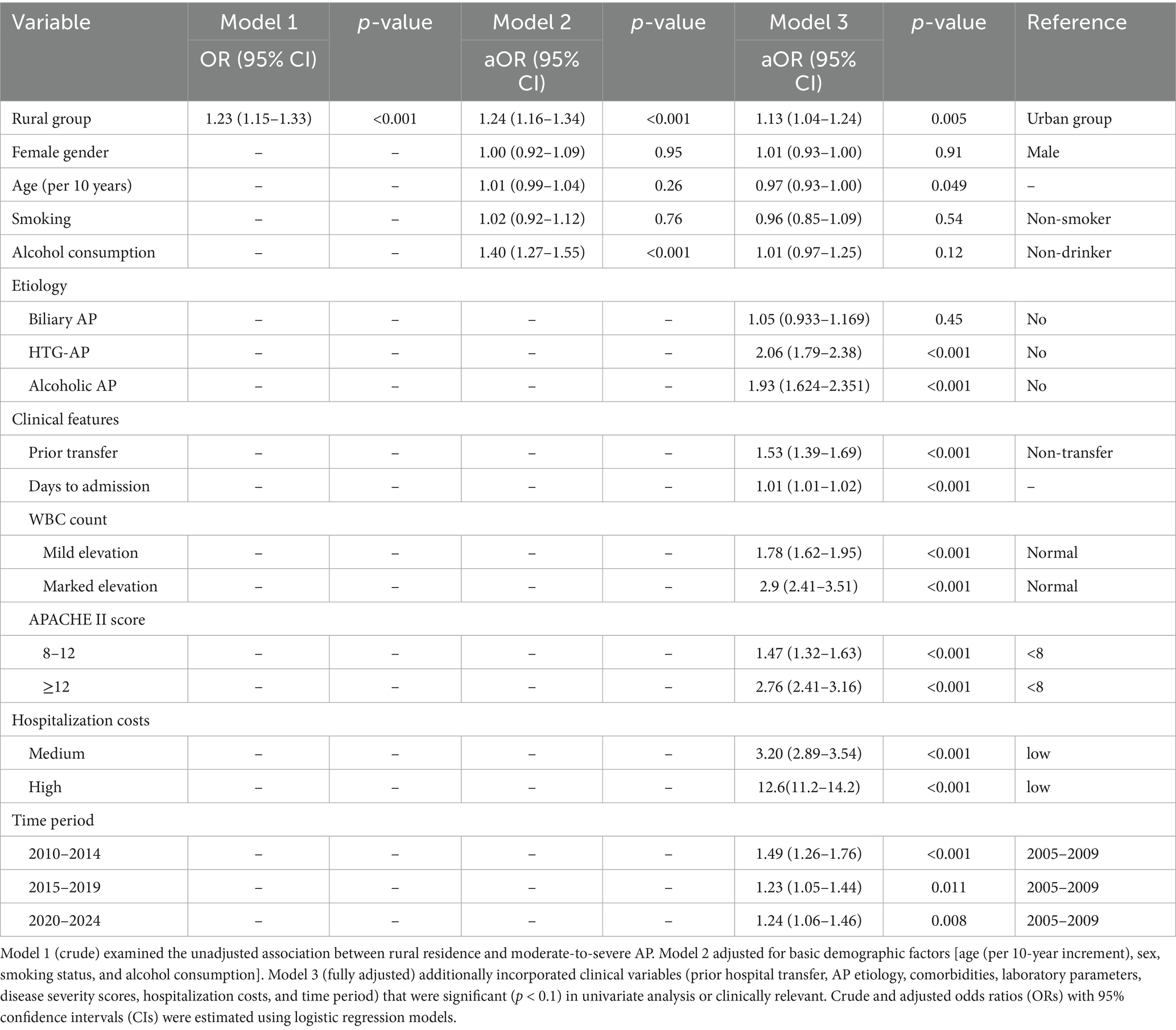
Table 4. Multivariable logistic regression analysis of factors associated with moderate-to-severe acute pancreatitis.
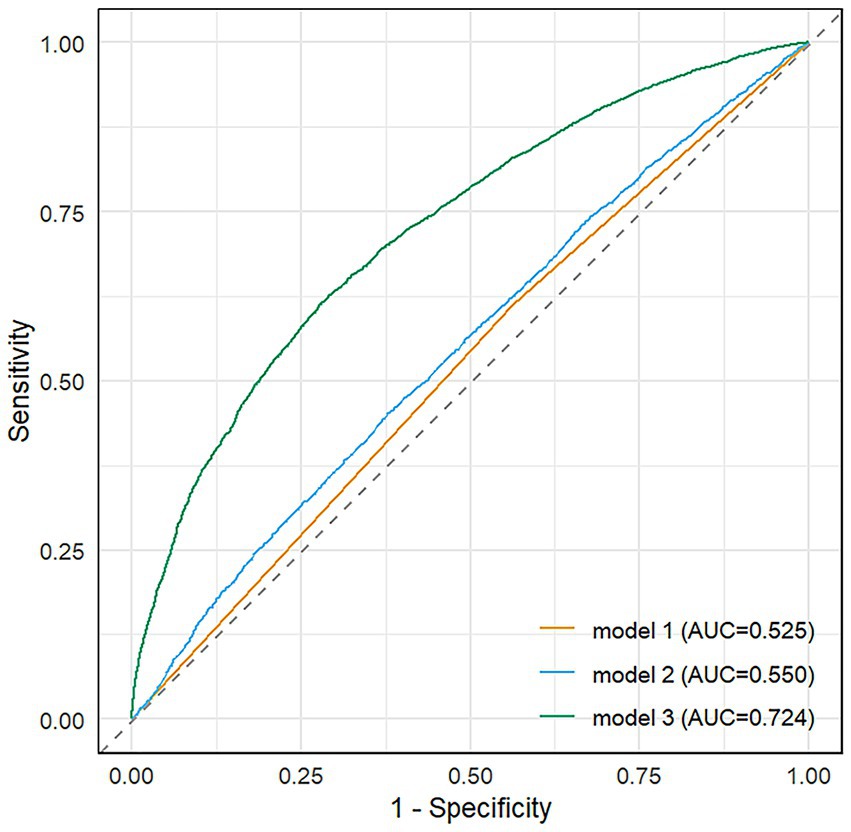
The forest plot (Figure 6) displays adjusted odds ratios with 95% confidence intervals for all variables showing statistically significant associations with disease severity (p < 0.05), organized by clinical domains. The final multivariable model (Model 3) demonstrated good discriminative capacity (AUC = 0.724; Figure 7), representing a 39.8% relative improvement over the crude model (Model 1 AUC = 0.525).
4 Discussion
This study, based on 20-year inpatient data from a single tertiary center, reveals distinct etiological, clinical, and prognostic differences between urban and rural AP patients in the region. Despite limitations in generalizability inherent to single-center data, the large sample size and extended observational period provide valuable insights into regional urban–rural healthcare disparities.
Both urban and rural populations showed similar etiological shifts in AP, characterized by decreasing biliary cases and significantly increasing HTG-AP incidence. These findings are consistent with our prior collaborative research conducted with the North China Pancreatic Center (19). HTG-AP has emerged as the second leading etiology after gallstone disease (5, 6), with its incidence persistently increasing over the past 7 years, demonstrating notable seasonality and holiday-related peaks (20). The persistently higher prevalence of biliary AP in rural patients (56.4% vs. 51.7%) may reflect insufficient screening for biliary stones and delayed health awareness despite increasing high-fat dietary intake (21, 22). Meanwhile, the narrowing urban–rural gap in HTG-AP (from 7.9 to 5.3%) highlights emerging challenges in managing metabolic risk factors in rural areas. Additionally, the higher incidence of drug-induced AP in urban patients (0.3% vs. 0.1%) may correlate with more frequent use of medications such as antineoplastic and antibiotics (23, 24), warranting enhanced clinical vigilance in pharmacotherapy.
Despite comparable mortality rates, rural patients exhibited delayed symptom-to-admission intervals (3 vs. 2 days), higher prior hospital transfer rates (75.4% vs. 64.0%), and elevated APACHE II scores (8 vs. 7), suggesting deficiencies in early AP recognition and initial management at primary care facilities (25). Multivariable analysis revealed that rural residence may be associated with an increased risk for moderate-to-severe AP (adjusted OR = 1.13, 95% CI: 1.04–1.24), likely attributable to delays in referral and insufficient prehospital emergency resources (26, 27). Notably, rural patients had higher rates of infected pancreatic necrosis (5.3% vs. 4.3%) and abdominal compartment syndrome (1.7% vs. 1.1%), potentially linked to delayed standardized fluid resuscitation and antibiotic administration (3, 28), underscoring the need to optimize acute-phase management in hierarchical healthcare systems.
Temporal trends showed a marked rise in rural AP cases after 2015 (8.6% → 36.5%), likely driven by expanded reimbursement coverage under the New Rural Cooperative Medical Scheme and improved referral practices (29, 30). The peak severity risk during 2010–2014 (aOR = 2.23) and subsequent decline to 2020–2024 (aOR = 1.75) may reflect the dissemination of AP management guidelines and minimally invasive techniques (3, 31). However, the post-2020 surge in HTG-AP across both populations raises concerns about COVID-19-related lifestyle changes (e.g., reduced physical activity, dietary shifts) accelerating metabolic pancreatitis (32, 33), a hypothesis requiring multicenter validation.
While this study benefits from its large sample and longitudinal design, limitations persist. First, as a single-center retrospective study, selection bias may distort the true urban–rural AP distribution in the general population. Second, reliance on tertiary hospital data may overestimate disease severity, particularly for rural cases due to referral bias. Third, the lack of prehospital management details and socioeconomic factors (e.g., insurance types, income) limits mechanistic exploration. Future multicenter studies incorporating primary care data and community-based surveys are needed to clarify sociodemographic determinants of urban–rural disparities and inform targeted prevention strategies.
5 Conclusion
In conclusion, our study reveals that rural patients exhibit a higher prevalence of gallstone-related AP, delayed hospital visits, and more severe clinical conditions, while HTG-AP shows a rapid upward trend in both urban and rural areas, potentially linked to dietary changes. Although rural patients demonstrate higher referral rates and complication risks, recent improvements in medical care have reduced their severe AP incidence. These findings underscore the necessity to enhance primary healthcare systems and implement targeted prevention strategies.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving humans were approved by the Medical Ethics Research Committee of the First Affiliated Hospital of Nanchang University. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because This was a retrospective analysis conducted on an anonymized database for researchers, and the First Affiliated Hospital of Nanchang University waived the informed consent.
Author contributions
XC: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Resources, Visualization, Writing – original draft, Writing – review & editing. ZL: Conceptualization, Data curation, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. JR: Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Writing – original draft. JW: Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Writing – original draft. XH: Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Writing – original draft. LX: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Writing – original draft. LL: Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Writing – original draft. XS: Data curation, Funding acquisition, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing. YZ: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Writing – review & editing. NL: Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Writing – review & editing. WH: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Supervision, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1640267/full#supplementary-material
References
1. Petrov, MS, and Yadav, D. Global epidemiology and holistic prevention of pancreatitis. Nat Rev Gastroenterol Hepatol. (2019) 16:175–84. doi: 10.1038/s41575-018-0087-5
2. Garg, PK, and Singh, VP. Organ failure due to systemic injury in acute pancreatitis. Gastroenterology. (2019) 156:2008–23. doi: 10.1053/j.gastro.2018.12.041
3. Trikudanathan, G, Yazici, C, Evans Phillips, A, and Forsmark, CE. Diagnosis and Management of Acute Pancreatitis. Gastroenterology. (2024) 167:673–88. doi: 10.1053/j.gastro.2024.02.052
4. Chinese General Practice. Expert consensus on diagnosis and treatment of hypertriglyceridemic acute pancreatitis in emergency medicine. Chin Gen Pract. (2021) 24:3781–93. doi: 10.12114/j.issn.1007-9572.2021.02.028
5. Zhang, NZH, Guo, X, and Liu, L. Meta-analysis of the etiological changes of acute pancreatitis in China over the past decade. Chin J Dig Dis Imaging (Electron Ed). (2016) 6:71–5. doi: 10.3877/cma.j.issn.2095-2015.2016.02.006
6. Zhu, Y, Pan, X, Zeng, H, He, W, Xia, L, Liu, P, et al. A study on the etiology, severity, and mortality of 3260 patients with acute pancreatitis according to the revised Atlanta classification in Jiangxi, China over an 8-year period. Pancreas. (2017) 46:504–9. doi: 10.1097/mpa.0000000000000776
7. Iannuzzi, JP, King, JA, Leong, JH, Quan, J, Windsor, JW, Tanyingoh, D, et al. Global incidence of acute pancreatitis is increasing over time: a systematic review and meta-analysis. Gastroenterology. (2022) 162:122–34. doi: 10.1053/j.gastro.2021.09.043
8. Lankisch, PG, Apte, M, and Banks, PA. Acute pancreatitis. Lancet. (2015) 386:85–96. doi: 10.1016/s0140-6736(14)60649-8
9. Fu, ZH, Zhao, ZY, Liang, YB, Cheng, DY, Luo, JM, Jiang, HX, et al. Impact of metabolic syndrome components on clinical outcomes in hypertriglyceridemia-induced acute pancreatitis. World J Gastroenterol. (2024) 30:3996–4010. doi: 10.3748/wjg.v30.i35.3996
10. Shmelev, A, Sill, AM, Horrigan, T, and Cunningham, SC. Trends and seasonality in hospitalizations for acute alcohol-related and biliary pancreatitis in the USA. Hepatobiliary Pancreat Dis Int. (2021) 20:173–81. doi: 10.1016/j.hbpd.2020.10.003
11. Yang, Y, He, D, Wei, L, Wang, S, Chen, L, Luo, M, et al. Association between diet-related knowledge, attitudes, behaviors, and self-rated health in Chinese adult residents: a population-based study. BMC Public Health. (2020) 20:720. doi: 10.1186/s12889-020-08896-y
12. Lian, Y, Gu, L, Yang, L, Wang, L, and Li, H. The reasonableness and spatial differences of the food consumption structure of urban and rural residents in China, 2015-2021. Food Secur. (2023) 12:10.3390/foods12101997. doi: 10.3390/foods12101997
13. Fan, J, Ding, L, Lu, Y, Zheng, J, Zeng, Y, and Huang, C. Epidemiology and etiology of acute pancreatitis in urban and suburban areas in Shanghai: a retrospective study. Gastroenterol Res Pract. (2018) 2018:1420590. doi: 10.1155/2018/1420590
14. Gangqiang Ding AMCS Data from. Scientific Research Report on Chinese Dietary Guidelines. (2021). Available from: https://www.chinanutri.cn/yyjkzxpt/yyjkkpzx/yytsg/zgjm/202103/P020210311486742870527.pdf
15. Charlesworth, A, Steger, A, and Crook, MA. Acute pancreatitis associated with severe hypertriglyceridaemia; a retrospective cohort study. Int J Surg. (2015) 23:23–7. doi: 10.1016/j.ijsu.2015.08.080
16. Sadr Azodi, O, Orsini, N, Andren-Sandberg, A, and Wolk, A. Effect of type of alcoholic beverage in causing acute pancreatitis. Br J Surg. (2011) 98:1609–16. doi: 10.1002/bjs.7632
17. Tian, D, Sun, L, Zhang, L, Zhang, L, Zhang, W, Li, L, et al. Large urban-rural disparity in the severity of two-week illness: updated results based on the first health service survey of Hunan Province, China. Int J Equity Health. (2016) 15:37. doi: 10.1186/s12939-016-0330-z
18. Banks, PA, Bollen, TL, Dervenis, C, Gooszen, HG, Johnson, CD, Sarr, MG, et al. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. (2013) 62:102–11. doi: 10.1136/gutjnl-2012-302779
19. Lai, T, Li, J, Zhou, Z, Rao, J, Zhu, Y, Xia, L, et al. Etiological changes and prognosis of hospitalized patients with acute pancreatitis over a 15-year period. Dig Dis Sci. (2024) 69:56–65. doi: 10.1007/s10620-023-08172-0
20. He, W, Wang, G, Yu, B, Xia, L, Zhu, Y, Liu, P, et al. Elevated hypertriglyceridemia and decreased gallstones in the etiological composition ratio of acute pancreatitis as affected by seasons and festivals: a two-center real-world study from China. Front Cell Infect Microbiol. (2022) 12:976816. doi: 10.3389/fcimb.2022.976816
21. Shelton, J, Kummerow, K, Phillips, S, Griffin, M, Holzman, MD, Nealon, W, et al. An urban-rural blight? Choledocholithiasis presentation and treatment. J Surg Res. (2012) 173:193–7. doi: 10.1016/j.jss.2011.05.031
22. Li, S, Guizzetti, L, Ma, C, Shaheen, AA, Dixon, E, Ball, C, et al. Epidemiology and outcomes of choledocholithiasis and cholangitis in the United States: trends and urban-rural variations. BMC Gastroenterol. (2023) 23:254. doi: 10.1186/s12876-023-02868-3
23. Meczker, A, Hanak, L, Parniczky, A, Szentesi, A, Eross, B, and Hegyi, P. Analysis of 1060 cases of drug-induced acute pancreatitis. Gastroenterology. (2020) 159:e8:1958–61. doi: 10.1053/j.gastro.2020.07.016
24. Li, D, Wang, H, Qin, C, Du, D, Wang, Y, Du, Q, et al. Drug-induced acute pancreatitis: a real-world pharmacovigilance study using the FDA adverse event reporting system database. Clin Pharmacol Ther. (2024) 115:535–44. doi: 10.1002/cpt.3139
25. Chen, Y, Yin, Z, and Xie, Q. Suggestions to ameliorate the inequity in urban/rural allocation of healthcare resources in China. Int J Equity Health. (2014) 13:34. doi: 10.1186/1475-9276-13-34
26. Cowan, S, and Leung, E. Rural-urban differences in wait-time to general surgical outpatient care. Can J Rural Med. (2025) 30:89–95. doi: 10.4103/cjrm.cjrm_12_24
27. Lu, S, Li, Y, Gao, H, and Zhang, Y. Difference in bypass for inpatient care and its determinants between rural and urban residents in China. Int J Equity Health. (2022) 21:132. doi: 10.1186/s12939-022-01734-0
28. Vege, SS, DiMagno, MJ, Forsmark, CE, Martel, M, and Barkun, AN. Initial medical treatment of acute pancreatitis: American Gastroenterological Association Institute technical review. Gastroenterology. (2018) 154:1103–39. doi: 10.1053/j.gastro.2018.01.031
29. Zhao, Y, Qiao, Q, Xu, X, and Bian, Y. Effectiveness of hierarchical medical system and economic growth: based on China's urban vs. rural health perspectives. Front Public Health. (2024) 12:1364584. doi: 10.3389/fpubh.2024.1364584
30. Luo, D, Deng, J, and Becker, ER. Urban-rural differences in healthcare utilization among beneficiaries in China's new cooperative medical scheme. BMC Public Health. (2021) 21:1519. doi: 10.1186/s12889-021-11573-3
31. Hines, OJ, and Pandol, SJ. Management of severe acute pancreatitis. BMJ. (2019) 367:l6227. doi: 10.1136/bmj.l6227
32. Xu, W, Li, Y, Yan, Y, Zhang, L, Zhang, J, and Yang, C. Effects of coronavirus disease 2019 lockdown on metabolic syndrome and its components among Chinese employees: a retrospective cohort study. Front Public Health. (2022) 10:885013. doi: 10.3389/fpubh.2022.885013
Keywords: acute pancreatitis, urban–rural disparities, biliary, hypertriglyceridemic, severity propensity
Citation: Cao X, Liu Z, Rao J, Wu J, Huang X, Xia L, Luo L, Shu X, Zhu Y, Lu N and He W (2025) Etiological shifts and clinical outcomes of acute pancreatitis between urban and rural areas: evidence from a 20-year retrospective database. Front. Med. 12:1640267. doi: 10.3389/fmed.2025.1640267
Edited by:
Mustafa Agah Tekindal, Izmir Kâtip Çelebi University, TürkiyeReviewed by:
Efe Kanter, Izmir Katip Celebi University, TürkiyeTutku Duman Şahan, TC Saglik Bakanligi Cesme Devlet Hastanesi, Türkiye
Dogukan Ozen, Ankara University, Türkiye
Copyright © 2025 Cao, Liu, Rao, Wu, Huang, Xia, Luo, Shu, Zhu, Lu and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenhua He, aGV3ZW5odWFAbmN1LmVkdS5jbg==
†These authors have contributed equally to this work
 Ximei Cao
Ximei Cao Zide Liu1†
Zide Liu1† Xu Shu
Xu Shu Yin Zhu
Yin Zhu Nonghua Lu
Nonghua Lu Wenhua He
Wenhua He