- Department of Infection Control, Second Affiliated Hospital of Zhejiang University School of Medicine, Hangzhou, Zhejiang, China
Background: Chlorhexidine (CHX) and povidone-iodine (PVI) are the most commonly used antiseptic agents for preoperative skin preparation to prevent surgical site infections (SSIs). This meta-analysis aimed to determine the superior agent between them for SSI prevention.
Methods: We conducted a systematic review and meta-analysis in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. A comprehensive search of electronic databases (PubMed, Web of Science, Embase, and Cochrane Central Register of Controlled Trials) was performed from inception to 1 May 2025, to identify relevant randomized controlled trials (RCTs). Heterogeneity was assessed using the chi-squared (Q) test and the I2 statistic. A random-effects model was applied when significant heterogeneity was present. The robustness of the findings was evaluated using trial sequential analysis (TSA) with a random-effects model. All statistical analyses were performed using Review Manager.
Results: A total of 32 high-quality RCTs, involving 29,748 participants, were included. The pooled analysis using a random-effects model demonstrated that CHX was significantly more effective than PVI in preventing SSIs (RR = 0.83, 95% CI 0.72–0.95, p = 0.009). Subgroup analysis by wound classification revealed that CHX was superior to PVI in clean-contaminated surgeries (11 RCTs; RR = 0.75, 95% CI 0.62–0.92, p = 0.004), but no significant difference was observed in clean surgeries (20 RCTs; RR = 0.90, 95% CI 0.67–1.20, p = 0.46). Further stratification by SSI type indicated that CHX significantly reduced the risk of superficial incisional SSIs (18 RCTs; RR = 0.82, 95% CI 0.69–0.98, p = 0.03), but not deep incisional SSIs (16 RCTs; RR = 0.95, 95% CI 0.76–1.18, p = 0.63) or organ-space SSIs (11 RCTs; RR = 1.13, 95% CI 0.89–1.42, p = 0.32). Additionally, CHX was associated with a significantly lower risk of bacterial decolonization (RR = 0.38, 95% CI 0.26–0.57, p < 0.001) and febrile episodes (RR = 0.57, 95% CI 0.35–0.92, p = 0.02) compared to PVI. The TSA confirmed the robustness of these findings, indicating that the cumulative evidence was sufficient and conclusive.
Conclusion: CHX-based antiseptics are more effective than PVI-based ones in preventing overall SSIs, particularly in clean-contaminated procedures. The superiority of CHX is primarily evident in reducing superficial incisional SSIs, with no significant advantage observed for deep incisional or organ-space SSIs.
Introduction
Surgical site infections (SSIs) are among the most common healthcare-associated infections (HAIs). Approximately 11% of patients undergoing general surgery will develop surgical incision sites 30 days after surgery (1). They are associated with longer post-operative hospital stays, additional surgical procedures, and higher mortality (2). The World Health Organization (WHO) suggests that preoperative skin antisepsis is one of the most critical factors for postoperative SSIs (3).
Povidone iodine (PVI) is a preeminent antiseptic measure in surgery that does not induce resistance or cross-resistance to antibiotics and is economically reasonable. Chlorhexidine (CHX), a broad-spectrum antimicrobial agent that damages bacterial cytoplasmic membranes without causing bacterial resistance, is a possible alternative antiseptic agent (4). Some clinical practice guidelines recommend the use of antiseptic skin solutions containing CHX gluconate and iodophor to prevent SSIs; however, there is a lack of consensus regarding the most effective agent (3, 5). A meta-analysis of high-quality randomized controlled trials (RCTs) did not show any benefit of alcoholic chlorhexidine skin preparation, which is more expensive than other readily available alternatives (6). However, some other meta-analyses revealed that chlorhexidine was superior to PVI in preventing postoperative SSIs (7, 8). Furthermore, some high-quality RCTs have been implemented recently, providing strong evidence of controversy regarding the most effective agent.
Considering the contradiction of the results and the publication of large, randomized trials in recent years, this justifies the need for updated meta-analyses. This meta-analysis aimed to evaluate the effects of chlorhexidine and PVI on SSI prevention.
Materials and methods
This systematic review and meta-analysis adhered to the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines (9).
Search strategy
A systematic literature search was conducted by two investigators (SY and ZL) in collaboration with an experienced medical librarian (LW). We searched the electronic databases of PubMed, Web of Science, Embase, and the Cochrane Central Register of Controlled Trials from their inception to 1 May 2025. The search strategy used a combination of keywords and subject headings related to “chlorhexidine,” “povidone-iodine,” and “randomized controlled trial.” The full search syntax for all databases is provided in Supplementary Table 1. The search was restricted to English-language publications. To ensure comprehensive coverage, we also manually screened the reference lists of all included studies and relevant review articles. All identified records were imported into EndNote X9 (Clarivate Analytics), and duplicates were removed electronically.
Inclusion criteria and exclusion criteria
Two investigators independently screened the titles and abstracts of all identified records for eligibility. Subsequently, the full texts of potentially relevant studies were retrieved and assessed independently against the predefined inclusion criteria. Any discrepancies between the reviewers were resolved through consensus or through consultation with a third investigator (FW). The inclusion criteria were as follows:
Patients: Preoperative skin antisepsis in adult patients (>18y);
Intervention: The CHX-containing solution was reoperative skin antiseptic around the surgical site, whether alcoholic or aqueous.
Control: A PVI-containing solution was used for skin disinfection around the surgical site, whether alcoholic or aqueous.
Outcomes: Reported outcomes of interest, total SSIs, superficial incisional infection, deep incisional infection, and organ space infection.
Study design: All RCTs.
The exclusion criteria were: (1) non-surgical or pediatric (<18 years) populations, or patients with chlorhexidine/povidone-iodine allergy; (2) use of non-comparator antiseptics or combination regimens; (3) absence of a control group, animal/in vitro studies, or insufficient outcome data for RR calculation; (4) studies on indwelling catheters or blood sampling procedures; and (5) gray literature, including conference abstracts, reviews, editorials, and case reports.
Data extraction
Data were systematically extracted from the included studies using a standardized form. The extracted information included: first author, publication year, participant sex and age, sample size, surgical type, wound classification, follow-up duration, details of the intervention and comparator groups, and primary/secondary outcomes. When multiple similar studies were published by the same author or group, only the most recent publication was included to avoid data overlap. For trials with more than two intervention arms, only data relevant to the chlorhexidine and povidone–iodine groups were extracted. The primary outcome was the incidence of postoperative surgical site infections (SSIs). Secondary outcomes included bacterial decolonization and episodes of fever. When critical data were missing or unclear, the corresponding authors were contacted for clarification.
Quality assessment
The quality of all RCTs was assessed using the Cochrane evaluation criteria, which included the following domains: random sequence generation, allocation concealment, performance bias, detection bias, attrition bias, reporting bias, and other sources of bias. There are three levels of bias: low, unclear, or high (10).
Data synthesis and statistical analysis
A meta-analysis was performed to pool the relative risk of each study. Chi-squared-based Q test and I2 were used to evaluate the heterogeneity within the studies. The random-effects meta-analysis model was used when the heterogeneity was statistically significant (I2 > 50%, p < 0.05) (11). Significant heterogeneity was assessed using subgroup, sensitivity, and descriptive analyses. Leave-one-out sensitivity analyses were performed by removing a single study each time to assess whether the results of this meta-analysis were robust. Publication bias was explored using the funnel plot method by graphing the effect size of the trials on the horizontal axis and the number of participants in each trial on the vertical axis. Asymmetry in the funnel plot suggested publication bias.
Trial sequential analysis (TSA) was performed to evaluate the statistical power of the current sample size using TSA software (12).1 The heterogeneity-adjusted required information size was calculated using a two-sided conventional boundary with a 5% type I error rate and an 80% statistical power.
The meta-analysis was conducted using Review Manager (v5.3), and leave-one-out analysis was conducted using STATA software (v15.0, College Station, TX, United States).
TSA was conducted with TSA 0.9.5.10 Beta software (Copenhagen Trial Unit, Centre for Clinical Intervention Research, Copenhagen).
Results
Identification of studies
The search strategy yielded a total of 1703 abstracts from four English databases, while a manual search of the references cited in other available articles and previous reviews yielded an additional 37 abstracts. After removing duplicates and screening the abstracts, 114 studies were included. After the full-text articles were assessed for eligibility, 82 studies were excluded: 32 studies were for non-chlorhexidine products or other iodine-containing products, 19 studies had no primary outcome, 8 studies were for pediatric patients, 14 studies were for reviews, and 9 studies were for protocols. Finally, 32 studies were included in the quantitative synthesis (Figure 1).
Characteristics of the involved studies
The baseline characteristics of the 32 included studies, comprising a total of 29,748 participants, are summarized in Table 1. Of these, 14,473 (48.6%) patients were preoperatively prepared with chlorhexidine (CHX), while 15,275 (51.4%) received povidone-iodine (PVI). According to wound classification, 8 studies involved clean wounds and 17 involved clean-contaminated wounds. One study by the PREP-IT Investigators reported outcomes separately for closed and open fractures. The follow-up duration across the studies varied widely, ranging from 14 days to 3 years. The methodological quality assessment is presented in Figure 2, which illustrates the distribution of risk-of-bias judgments for all evaluated domains.
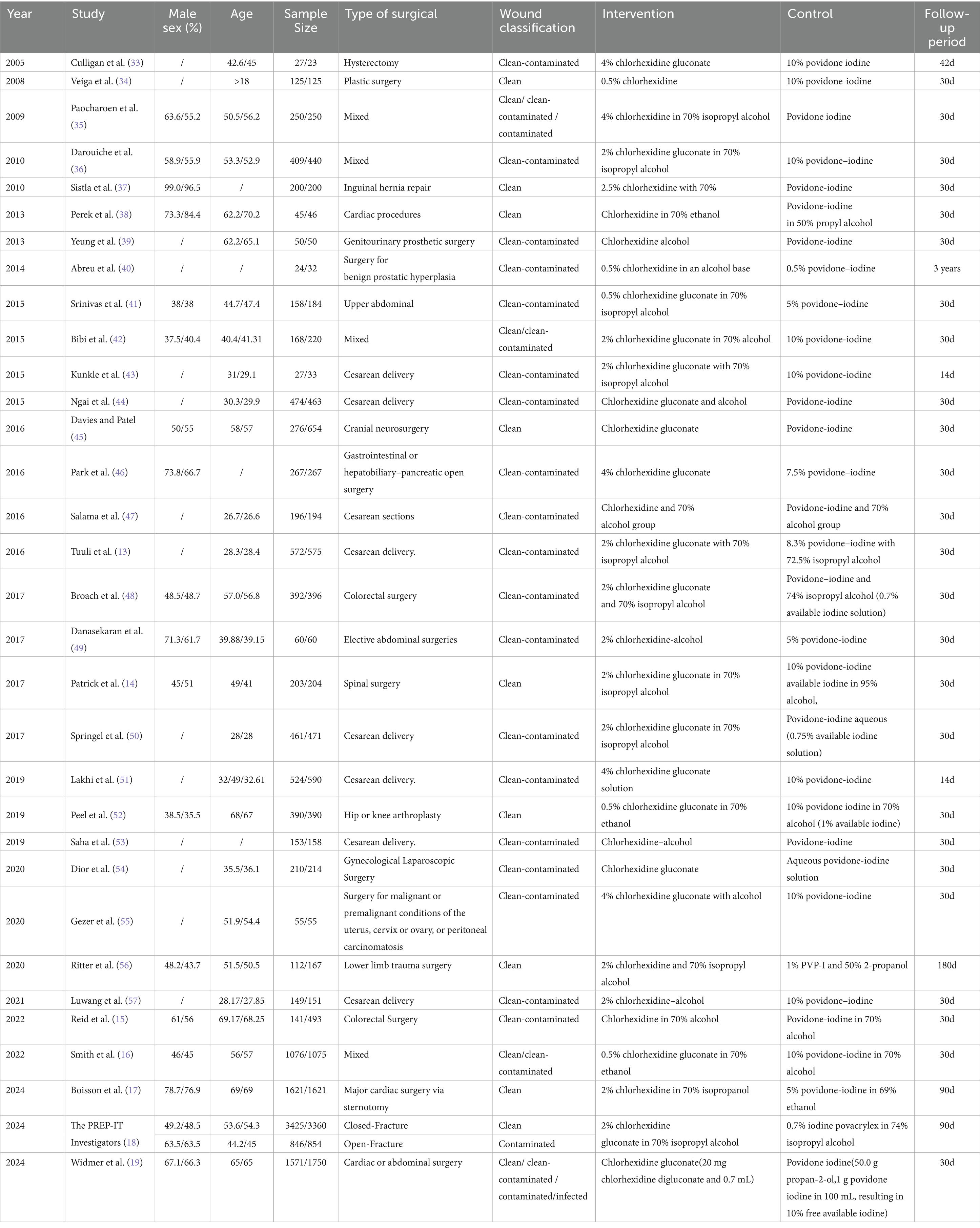
Table 1. Characteristics of RCT compare chlorhexidine-containing solutions with povidone-iodine solutions for skin disinfection to prevent SSIs.
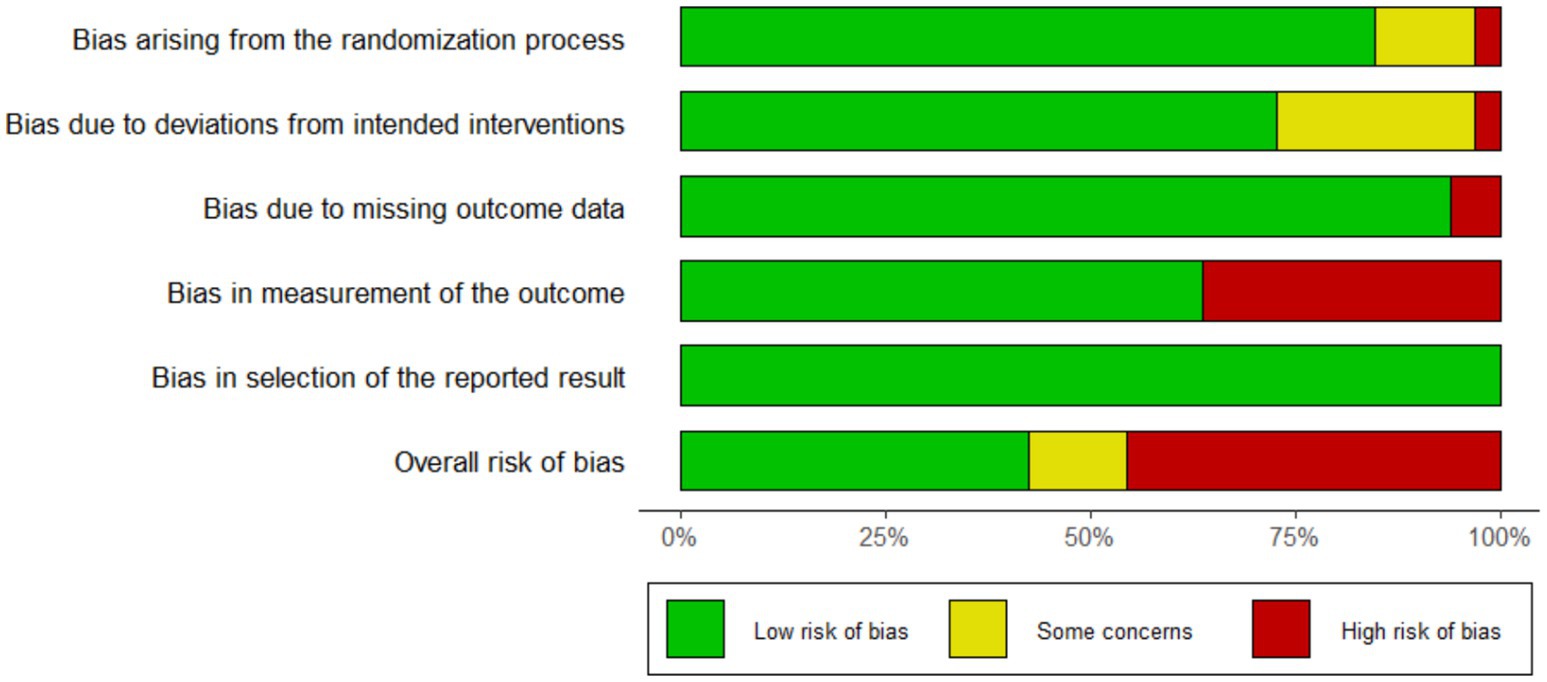
Figure 2. Risk of bias graph: review authors’ judgments about each risk of bias item presented as percentages across all included studies.
Pooled surgical site infection rate
A total of 32 studies provided data comparing the incidence of surgical site infections (SSIs) between chlorhexidine (CHX) and povidone-iodine (PVI). Among the 29,748 patients included in the analysis, 1,900 (6.4%) developed an SSI. The incidence was lower in the CHX group (867/14, 473, 6.0%) compared to the PVI group (1,033/15, 275, 6.6%). The pooled random-effects meta-analysis demonstrated a superior effect of CHX over PVI in preventing SSIs (RR = 0.83, 95% CI 0.72–0.95, p = 0.009; Figure 3). Considerable heterogeneity was observed among the studies (I2 = 49%, p = 0.001). However, the funnel plot appeared symmetrical, indicating a low likelihood of publication bias (Figure 4). Furthermore, the leave-one-out sensitivity analysis confirmed the robustness of the pooled result.
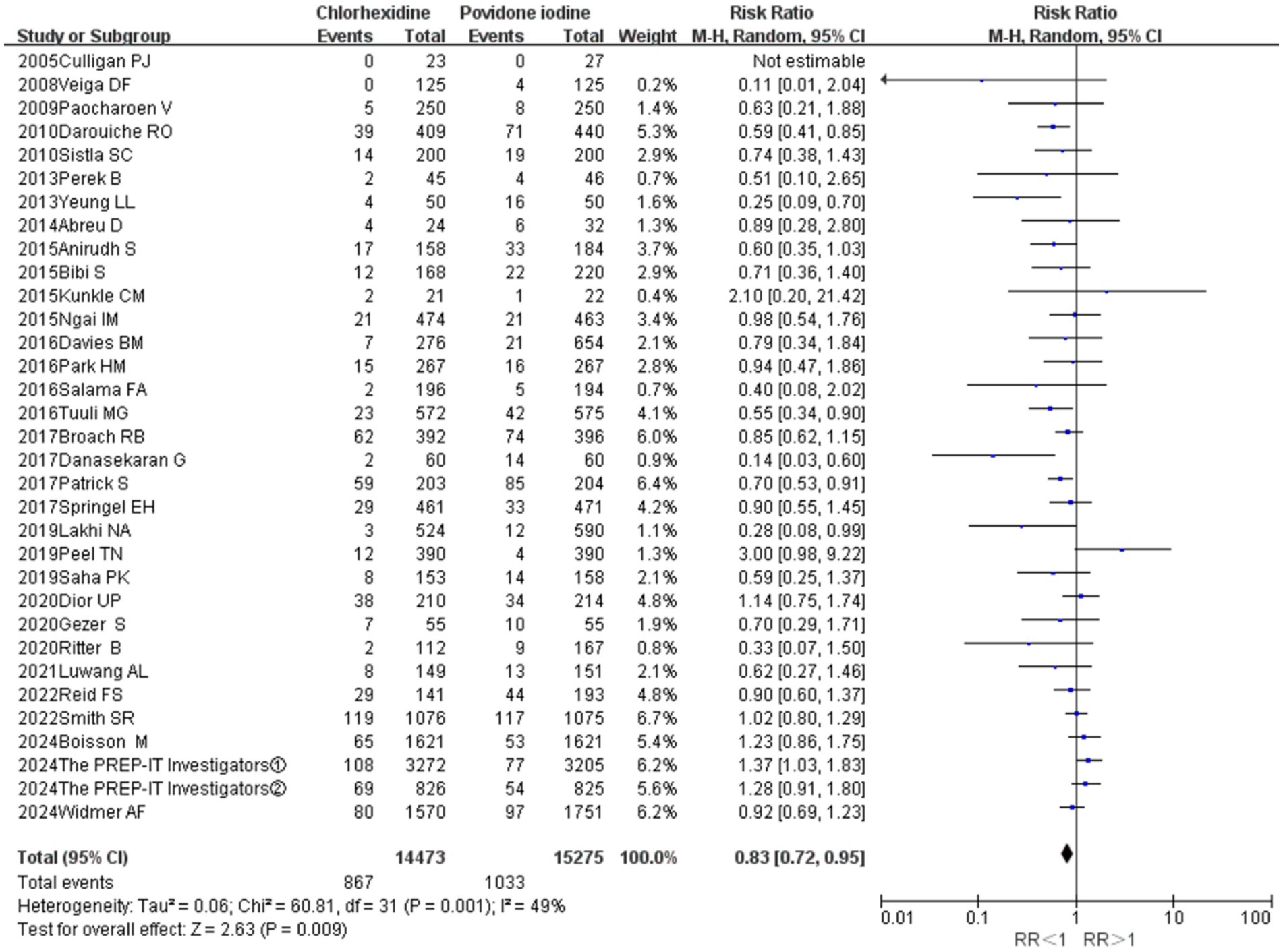
Figure 3. Meta-analysis forest plot: chlorhexidine compared with povidone-iodine solution for the prevention of surgical site infection.
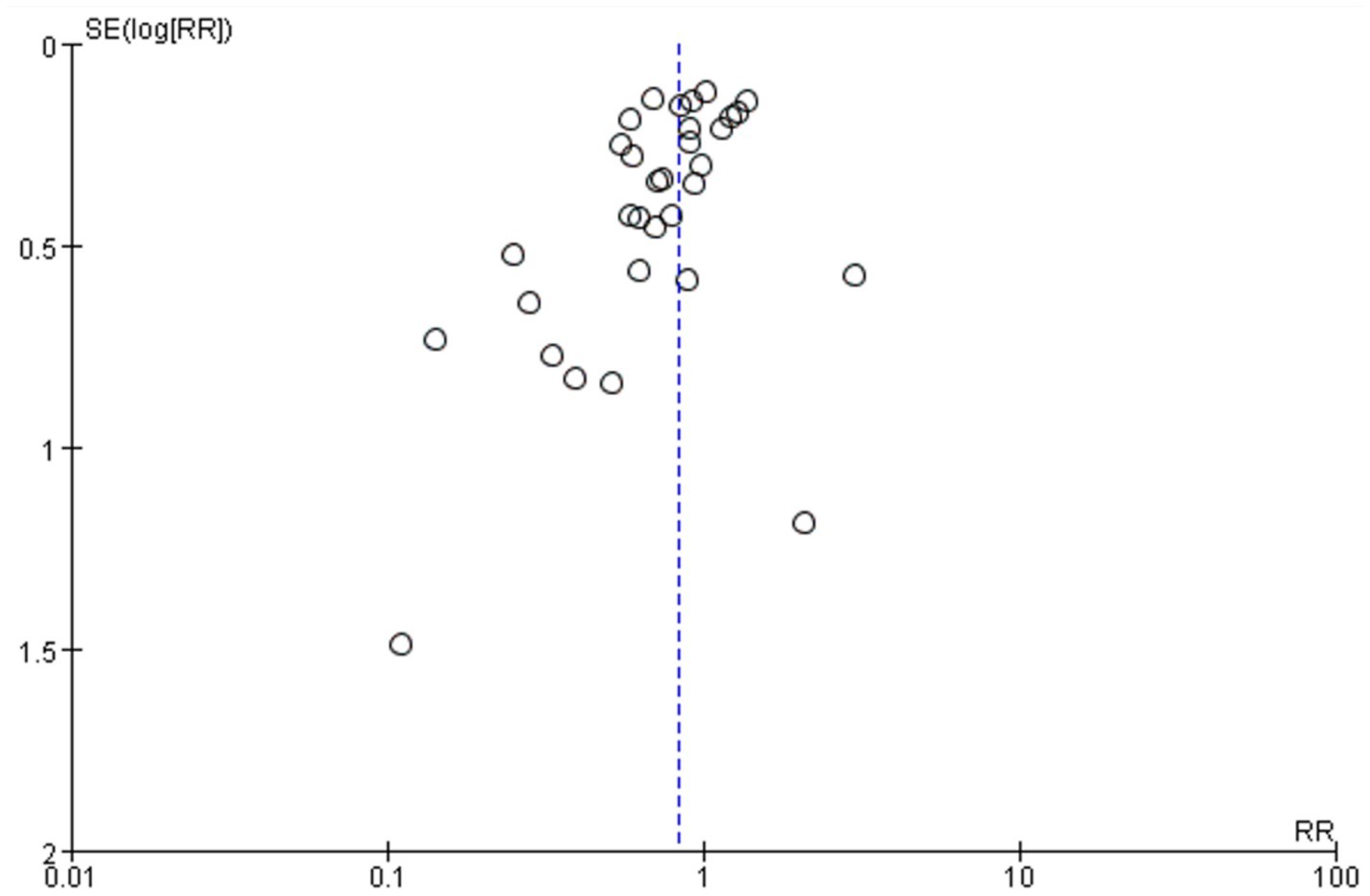
Figure 4. Meta-analysis funnel plot: chlorhexidine compared with povidone-iodine solution for the prevention of surgical site infection.
Wound classification
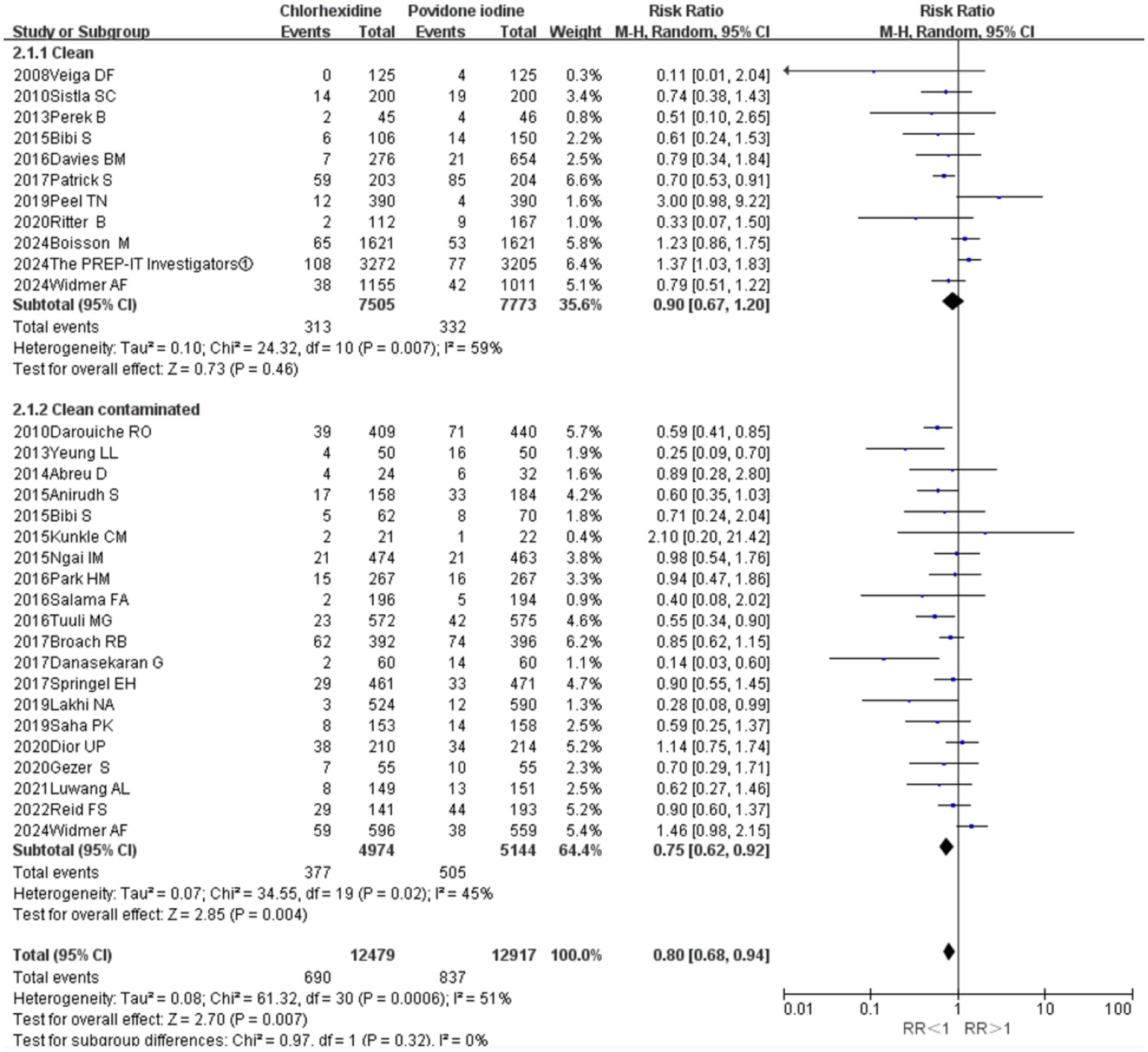
Stratified analysis by wound classification revealed that CHX was significantly superior to PVI in clean-contaminated surgeries (11 RCTs; RR = 0.75, 95% CI 0.62–0.92, p = 0.004; I2 = 45%; Figure 5). In contrast, no significant difference in SSI incidence was observed between the two antiseptics in clean surgeries (20 RCTs; RR = 0.90, 95% CI 0.67–1.20, p = 0.46; I2 = 59%; Figure 5).
Classification of surgical site infections
Preventing superficial incisional SSIs (18 RCTs; RR = 0.82, 95% CI 0.69–0.98, p = 0.03; Figure 6). In contrast, no significant differences were observed between the two antiseptics for the prevention of deep incisional SSIs (16 RCTs; RR = 0.95, 95% CI 0.76–1.18, p = 0.63) or organ-space SSIs (11 RCTs; RR = 1.13, 95% CI 0.89–1.42, p = 0.32; Figure 6). The analyses for deep incisional (I2 = 0%) and organ-space SSIs (I2 = 16%) showed negligible heterogeneity, while the analysis for superficial SSIs indicated low to moderate heterogeneity (I2 = 39%).
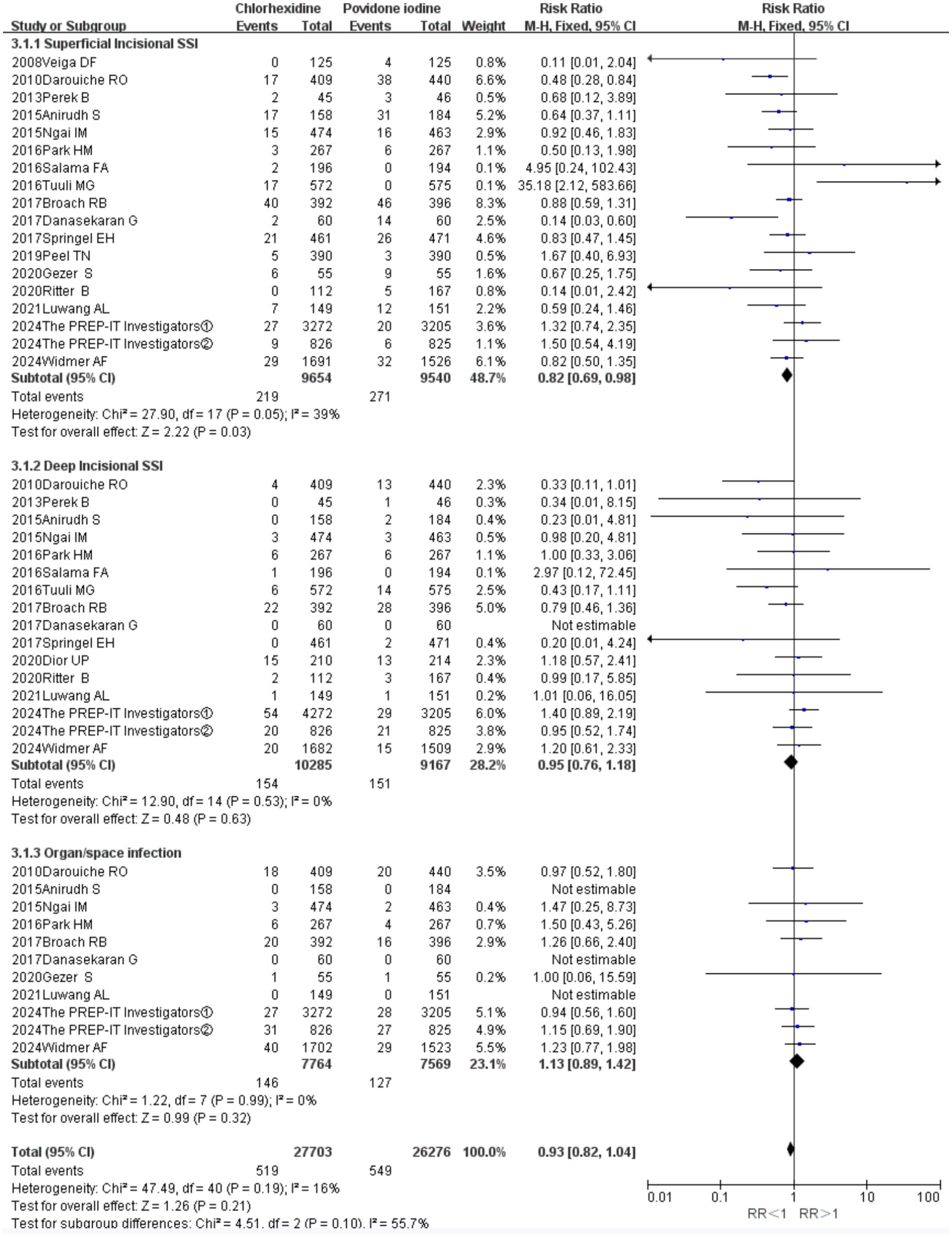
Figure 6. Forest plot of subgroup analysis: superficial incisional SSIs versus deep incisional SSIs versus organ-space SSIs.
Diverse formulations
The meta-analysis demonstrated a superior effect of CHX over alcohol-based PVI (RR = 0.88, 95% CI 0.78–1.00, p = 0.040; Figure 7) and aqueous PVI (RR = 0.61, 95% CI 0.49–0.77, p = 0.001) in preventing SSIs. Meta-analysis of 14 RCTs revealed CHX was associated with a reduced risk of preventing SSIs when compared to 10% PVI (RR = 0.80, 95% CI 0.70–0.92, p = 0.001). In contrast, no significant differences were observed between CHX and 5% PVI for the prevention of deep incisional SSIs.
Type of surgical
The fixed-effects pooled analysis revealed that CHX-containing solution was superior to PVI in the prevention of postoperative surgical site infection in cesarean delivery (eight RCTs, RR = 0.69, 95% CI 0.54–0.89, p = 0.004; Supplementary Figure 1). Potential heterogeneity was not observed in the included studies (I2 = 0%, p = 0.46). There were no significant differences in rates of SSIs between chlorhexidine and PVI in the prevention of gastrointestinal surgeries (three RCTs, RR = 0.87, 95% CI 0.69–1.11, p = 0.26), orthopedic surgery (five RCTs, RR = 1.09, 95% CI 0.93–1.29, p = 0.29), and cardiac procedures (three RCTs, RR = 1.02, 95% CI 0.82–1.27, p = 0.87).
Bacterial decolonization and fever episodes
Meta-analysis of 11 RCTs revealed chlorhexidine was associated with a reduced risk of bacterial decolonization when compared to PI (RR = 0.38, 95% CI 0.26–0.57, p = 0.001; Supplementary Figure 2). Furthermore, there were also statistical differences between chlorhexidine and PVI in the incidence of fever episodes (RR = 0.57, 95% CI 0.35–0.92, p = 0.02; Supplementary Figure 3).
Trial sequential analysis
TSA showed that 44.14% (28,097 out of 63,660 patients) of the heterogeneity-adjusted information size required was accrued. We also found that the cumulative Z-curve crossed the trial sequential monitoring boundary, providing robust evidence of chlorhexidine compared to povidone-iodine solution for the prevention of surgical site infection based on the sample size (Figure 8).
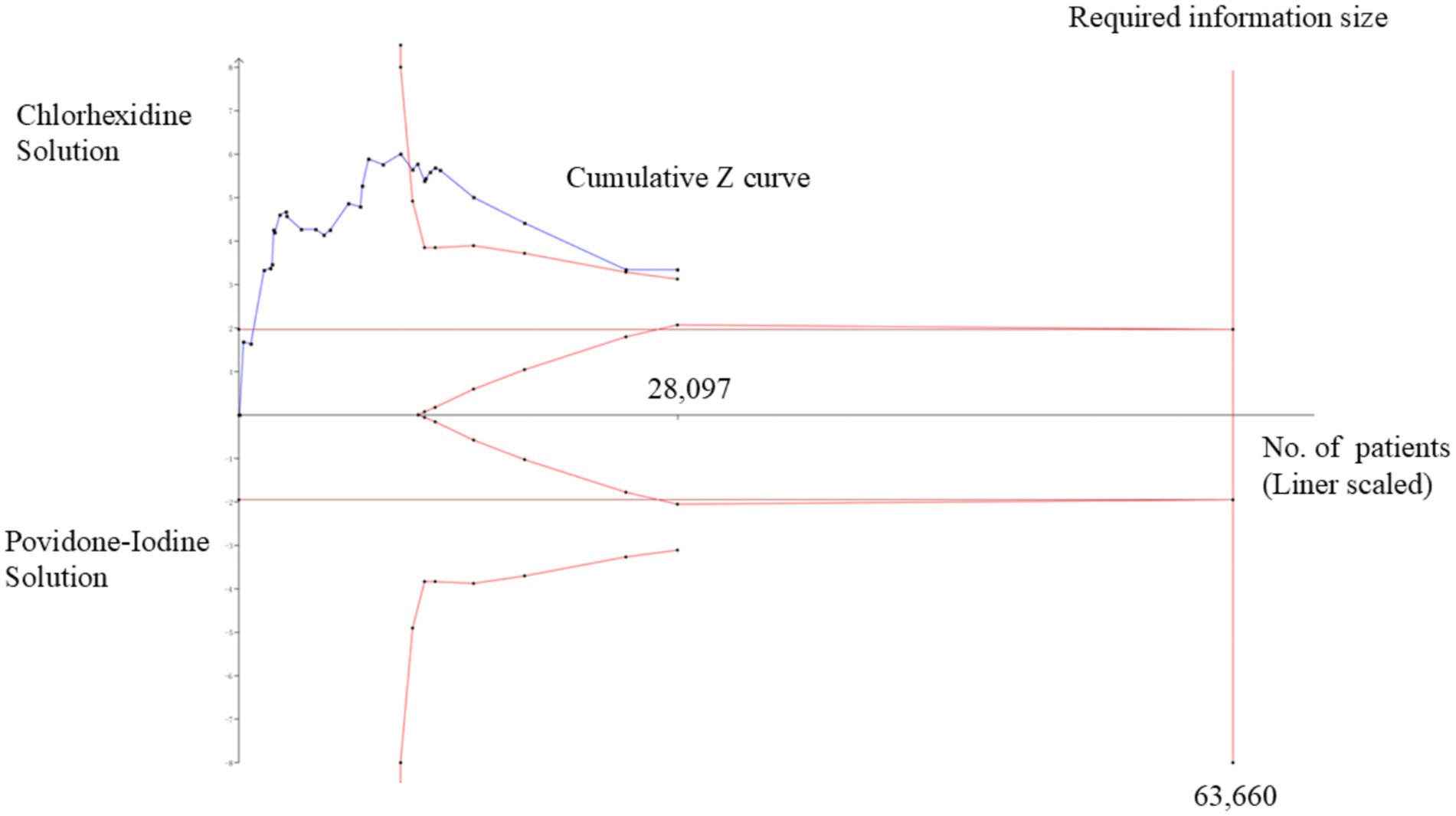
Figure 8. Trial sequential analysis (TSA) of pooled surgical site infections (SSIs) rate. Heterogeneity-adjusted required information size of 63,660 participants, calculated with a two-sided conventional boundary with a 5% type I error rate and an 80% statistical power.
Discussion
Preoperative skin antisepsis with chlorhexidine was associated with a lower risk of surgical site infections (SSIs) overall compared to povidone-iodine. This beneficial effect was primarily driven by a significant reduction in SSI risk following clean-contaminated surgeries and in the incidence of superficial incisional SSIs. In contrast, no significant differences between the antiseptics were observed for the prevention of deep incisional or organ-space SSIs.
This meta-analysis confirms that chlorhexidine (CHX) is superior to povidone-iodine (PVI) in preventing overall surgical site infection (SSIs), thereby reinforcing the findings of a prior meta-analysis by Chen et al. (2020) (7). Notably, the present review strengthens the existing evidence by exclusively including randomized controlled trials (RCTs), whereas the earlier work incorporated observational designs that may introduce bias. Furthermore, our study provides novel insights by demonstrating that the superiority of CHX is specifically significant in preventing superficial incisional SSIs and in clean-contaminated surgeries—associations not previously detailed in the literature. Our findings align with the conclusions of Privitera et al. (8), which offered moderate-quality evidence supporting CHX. While that analysis was limited by the number of available RCTs, our updated synthesis includes a substantially larger body of evidence from high-quality RCTs. Contrary to these conclusions, a 2022 meta-analysis from the National Institute of Health Research Unit on Global Surgery found no significant differences between alcoholic CHX and aqueous PVI (13–16). This discrepancy may be partly explained by their comparison not including alcoholic PVI formulations, potentially influencing the pooled effect. Regarding safety, although our review did not perform a quantitative analysis of adverse skin reactions due to inconsistent reporting definitions, the existing literature cited in these prior reviews suggests that CHX is generally well-tolerated with a low incidence of hypersensitivity (8).
Several high-quality RCTs have been reported in the last 2 years. An RCT published in Intensive Care Med (17) found skin disinfection at the surgical site using CHX-alcohol was not superior to PVI-alcohol in reducing surgical site infection rates among patients requiring sternotomy for major heart or aortic surgery. This finding differs from previously published results, and the sample size was relatively large. Two other RCTs published in NEJM (18) and JAMA (19) were also included in this meta-analysis. A previous study reported the results in patients with closed extremities and open fractures. The latest two studies in 2024 and 2025 (20) were excluded from our meta-analysis because of the lack of SSI rates and study design (a prospective observational analysis).
Povidone-iodine is a microbicide skin preparation with broad-spectrum antiseptic properties and local tolerability, which can rapidly penetrate microorganisms and attack their nucleotides, fatty acids, and thiol groups, while inhibiting microbial protein synthesis by oxidizing thiol groups (21). In clean surgery, we found no difference between CHX and PVI. CHX is a cationic chlorinated biguanide that precipitates in the bacterial cell membranes and cytoplasmic components. CHX resists neutralization by organic materials, is active over a wider pH (5–8) than iodine compounds, has a more prolonged bactericidal action, and has a lesser incidence of skin sensitization (22, 23). Macias JH (24) demonstrated a longer reduction of colony-forming units by cell-bound CHX. Furthermore, CHX remains activated in the presence of organic fluids, such as blood or pus, in contrast to iodophores, which become inactivated (25). Our study proved that the advantage of CHX over PVI is obvious in the prevention of superficial incisional SSIs. Chlorhexidine-alcohol is a newer skin preparation agent commonly composed of 2% chlorhexidine gluconate and 70% isopropyl alcohol (26). Although it is more expensive than PVI, it is reported to have a more rapid onset of action than PI and persistent activity in the presence of body fluids. Literature on the most appropriate concentration of chlorhexidine is sparse. In a randomized trial of 100 patients, Casey compared 2% chlorhexidine with 0.5% chlorhexidine. CHX 2% significantly reduced the number of microorganisms on the skin but did not reduce the incidence of SSIs (27).
However, the study outcomes of CHX-alcohol combinations are often attributed to CHX alone. The rate of incorrect attribution among the articles that we assessed ranged from 29% for catheters to 43% for surgery (28). Alcohol, which is a conventional antiseptic, may play a critical role in this process. WHO guidelines recommend the use of chlorhexidine alcohol-based antiseptic solutions for surgical site skin preparation (3). A recent meta-analysis found good evidence favoring CHG-alcohol (C-Alc) combinations over aqueous PVI, the most commonly tested alternative, in all three areas of skin antisepsis (28). Randomized controlled trials involving alcoholic and aqueous chlorhexidine are required. Given the increased risk and the hypothesis that the improvement in SSI rate in other trials is due to the addition of alcohol, a three-armed RCT compared povidone-iodine in an alcohol base (PI-Alc) to povidone-iodine in an aqueous base (PI-Aq) alongside a non-inferiority trial and found PI-Alc to be non-inferior to C-Alc and not superior to PI-Aq. Furthermore, the risks of surgical fire, chemical burns, anaphylaxis, and cost should be considered when choosing an appropriate skin preparation (15). However, considering the limited data, we could not perform a subgroup analysis. Furthermore, SSI prevention is a multi-factorial endeavor. While skin antisepsis plays an important role, other evidence-based measures—such as appropriate antibiotic prophylaxis, glycemic control, maintenance of normothermia, and adherence to sterile technique—are also critical components within comprehensive SSI prevention bundles.
The subgroup analysis in this study revealed a critical and nuanced finding: the relative efficacy of antiseptics is highly dependent on the formulation of PVP-I. While the overall analysis suggested that CHX might be superior to PVP-I, the picture became more complex when disaggregated into carrier solution. First, consistent with most previous meta-analyses, this study found that CHX was significantly superior to aqueous PVP-I in preventing surgical site infections (29). This is likely attributable to the excellent residual activity of CHX, which provides prolonged antimicrobial effects. In contrast, the iodine activity of aqueous PVP-I gradually diminishes after drying and is more easily inactivated by organic matter such as blood or serous secretions (30, 31). Alcohol-based PVP-I, however, combines the rapid bactericidal capacity of alcohol with the broad-spectrum antimicrobial activity and sustained action of iodine. The alcohol carrier not only rapidly kills microorganisms itself but also enhances the penetration of iodine compounds into deeper skin structures (32). This combination of rapid initial kill and persistent residual antimicrobial action may render alcohol-based PVP-I a highly powerful antiseptic option in specific clinical scenarios.
Several limitations inherent in this meta-analysis warrant acknowledgment. First, the pooled results are derived from a heterogeneous mix of surgical procedures (e.g., plastic, spinal, and prosthetic surgeries), and the effect of CHX may differ among them. Our ability to perform subgroup analyses was constrained by a lack of reporting on other prevalent surgery types. Second, variations in CHX concentration, application technique, and contact duration across studies could have influenced the observed effect on SSI prevention. Furthermore, our analysis primarily contrasted the active agents (CHX vs. PVI) and could not fully disentangle the confounding effect of different solvents (alcohol vs. aqueous), potentially reducing the reliability of the findings. The exclusion of non-English studies may have introduced language and publication biases. Our analysis was also limited to clean and clean-contaminated wounds due to the absence of data on more contaminated wound classes. Moreover, the exclusion of pediatric populations means that the results are not generalizable to patients under 18 years of age. Finally, the highly variable SSI monitoring periods (ranging from 14 days to 3 years) represent a major source of bias, potentially leading to an underestimation of infection risk and hindering direct comparisons. Although a sensitivity analysis suggested robustness, this bias cannot be fully eliminated, urging caution in interpreting the summary results.
Conclusion
This meta-analysis demonstrated that CHX-containing solutions were more effective than PVI-containing solutions in preventing postoperative SSIs, particularly in clean-contaminated surgeries. As for the classification of SSIs, the advantage of CHX versus PVI is obvious in preventing superficial incisional SSIs, but not in deep incisional SSIs and organ-space SSIs. SSI prevention is a multi-factorial endeavor. While skin antisepsis plays an important role, other evidence-based measures—such as appropriate antibiotic prophylaxis, glycemic control, maintenance of normothermia, and adherence to sterile technique—are also critical components within comprehensive SSI prevention bundles.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author/s.
Author contributions
SY: Formal analysis, Investigation, Methodology, Software, Visualization, Writing – original draft. ZL: Formal analysis, Investigation, Software, Visualization, Writing – review & editing. FW: Formal analysis, Software, Visualization, Writing – review & editing. LS: Formal analysis, Methodology, Writing – review & editing. YH: Formal analysis, Writing – review & editing. CW: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1641815/full#supplementary-material
Footnotes
References
1. Gillespie, BM, Harbeck, E, Rattray, M, Liang, R, Walker, R, Latimer, S, et al. Worldwide incidence of surgical site infections in general surgical patients: a systematic review and meta-analysis of 488,594 patients. Int J Surg. (2021) 95:106136. doi: 10.1016/j.ijsu.2021.106136
2. Seidelman, JL, Mantyh, CR, and Anderson, DJ. Surgical site infection prevention: a review. JAMA. (2023) 329:244–52. doi: 10.1001/jama.2022.24075
3. Allegranzi, B, Bischoff, P, de Jonge, S, Kubilay, NZ, Zayed, B, Gomes, SM, et al. New WHO recommendations on preoperative measures for surgical site infection prevention: an evidence-based global perspective. Lancet Infect Dis. (2016) 16:e276–87. doi: 10.1016/S1473-3099(16)30398-X
4. Kuroki, M, Short, AC, and Coombs, LA. Chlorhexidine gluconate treatment adherence among nurses and patients to reduce central line-associated bloodstream infections. Clin J Oncol Nurs. (2025) 29:E37–e46. doi: 10.1188/25.CJON.E37-E46
5. Calderwood, MS, Anderson, DJ, Bratzler, DW, Dellinger, EP, Garcia-Houchins, S, Maragakis, LL, et al. Strategies to prevent surgical site infections in acute-care hospitals: 2022 update. Infect Control Hosp Epidemiol. (2023) 44:695–720. doi: 10.1017/ice.2023.67
6. National Institute of Health Research Unit on Global Surgery. Alcoholic chlorhexidine skin preparation or triclosan-coated sutures to reduce surgical site infection: a systematic review and meta-analysis of high-quality randomised controlled trials. Lancet Infect Dis. (2022) 22:1242–51. doi: 10.1016/S1473-3099(22)00133-5
7. Chen, S, Chen, JW, Guo, B, and Xu, CC. Preoperative antisepsis with chlorhexidine versus povidone-iodine for the prevention of surgical site infection: a systematic review and Meta-analysis. World J Surg. (2020) 44:1412–24. doi: 10.1007/s00268-020-05384-7
8. Privitera, GP, Costa, AL, Brusaferro, S, Chirletti, P, Crosasso, P, Massimetti, G, et al. Skin antisepsis with chlorhexidine versus iodine for the prevention of surgical site infection: a systematic review and meta-analysis. Am J Infect Control. (2017) 45:180–9. doi: 10.1016/j.ajic.2016.09.017
9. Moher, D, Shamseer, L, Clarke, M, Ghersi, D, Liberati, A, Petticrew, M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. (2015) 4:1. doi: 10.1186/2046-4053-4-1
10. Higgins, JP, Altman, DG, Gøtzsche, PC, Jüni, P, Moher, D, Oxman, AD, et al. The Cochrane collaboration's tool for assessing risk of bias in randomised trials. BMJ (Clin Res Ed). (2011) 343:d5928. doi: 10.1136/bmj.d5928
11. Higgins, JP, and Thompson, SG. Quantifying heterogeneity in a meta-analysis. Stat Med. (2002) 21:1539–58. doi: 10.1002/sim.1186
12. Wetterslev, J, Thorlund, K, Brok, J, and Gluud, C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta-analysis. J Clin Epidemiol. (2008) 61:64–75. doi: 10.1016/j.jclinepi.2007.03.013
13. Tuuli, MG, Liu, J, Stout, MJ, Martin, S, Cahill, AG, Odibo, AO, et al. A randomized trial comparing skin antiseptic agents at cesarean delivery. N Engl J Med. (2016) 374:647–55. doi: 10.1056/NEJMoa1511048
14. Patrick, S, McDowell, A, Lee, A, Frau, A, Martin, U, Gardner, E, et al. Antisepsis of the skin before spinal surgery with povidone iodine-alcohol followed by chlorhexidine gluconate-alcohol versus povidone iodine-alcohol applied twice for the prevention of contamination of the wound by bacteria: a randomised controlled trial. Bone Joint J. (2017) 99-b:1354–65. doi: 10.1302/0301-620X.99B10.BJJ-2017-0291.R1
15. Reid, FS, Stephensen, B, Carroll, R, Lott, N, Attia, JR, and Smith, SR. Antiseptic skin preparation agents to prevent surgical site infection in colorectal surgery: a 3-armed randomized controlled trial. Dis Colon Rectum. (2022) 65:1391–6. doi: 10.1097/DCR.0000000000002171
16. Smith, SR, Gani, J, Carroll, R, Lott, N, Hampton, J, Oldmeadow, C, et al. Antiseptic skin agents to prevent surgical site infection after incisional surgery: a randomized, three-armed combined non-inferiority and superiority clinical trial (NEWSkin prep study). Ann Surg. (2022) 275:842–8. doi: 10.1097/SLA.0000000000005244
17. Boisson, M, Allain, G, Roussel, JC, d'Ostrevy, N, Burbassi, S, Demondion, P, et al. Chlorhexidine-alcohol compared with povidone-iodine-alcohol skin antisepsis protocols in major cardiac surgery: a randomized clinical trial. Intensive Care Med. (2024) 50:2114–24. doi: 10.1007/s00134-024-07693-0
18. Sprague, S, Slobogean, G, Wells, JL, O’Hara, NN, Thabane, L, Mullins, CD, et al. Skin antisepsis before surgical fixation of extremity fractures. N Engl J Med. (2024) 390:409–20. doi: 10.1056/NEJMoa2307679
19. Widmer, AF, Atkinson, A, Kuster, SP, Wolfensberger, A, Klimke, S, Sommerstein, R, et al. Povidone iodine vs chlorhexidine gluconate in alcohol for preoperative skin antisepsis: a randomized clinical trial. JAMA. (2024) 332:541–9. doi: 10.1001/jama.2024.8531
20. Da Costa, A, Ovache, L, Chellali, S, Guichard, JB, Romeyer, C, Yvorel, C, et al. Preoperative skin antiseptics for the prevention of cardiac implantable electronic device infections: chlorhexidine-alcohol versus povidone-iodine-alcohol. J Intervent Cardiac Electrophysiol. (2025) 68:1563–72. doi: 10.1007/s10840-025-02049-0
21. Fleischer, W, and Reimer, K. Povidone-iodine in antisepsis – state of the art. Dermatology (Basel, Switzerland). (1997) 195:3–9. doi: 10.1159/000246022
22. Furukawa, K, Ogawa, R, Norose, Y, and Tajiri, T. A new surgical handwashing and hand antisepsis from scrubbing to rubbing. J Nippon Med Sch. (2004) 71:190–7. doi: 10.1272/jnms.71.190
23. Bibbo, C, Patel, DV, Gehrmann, RM, and Lin, SS. Chlorhexidine provides superior skin decontamination in foot and ankle surgery: a prospective randomized study. Clin Orthop Relat Res. (2005) 438:204–8. doi: 10.1097/01.blo.0000167832.47464.22
24. Macias, JH, Arreguin, V, Munoz, JM, Alvarez, JA, Mosqueda, JL, and Macias, AE. Chlorhexidine is a better antiseptic than povidone iodine and sodium hypochlorite because of its substantive effect. Am J Infect Control. (2013) 41:634–7. doi: 10.1016/j.ajic.2012.10.002
25. Lim, KS, and Kam, PC. Chlorhexidine--pharmacology and clinical applications. Anaesth Intensive Care. (2008) 36:502–12. doi: 10.1177/0310057X0803600404
26. Mangram, AJ, Horan, TC, Pearson, ML, Silver, LC, and Jarvis, WRGuideline for Prevention of Surgical Site Infection. Centers for Disease Control and Prevention (CDC) hospital infection control practices advisory committee. Am J Infect Control. (1999) 27:97–134. doi: 10.1016/S0196-6553(99)70088-X
27. Casey, A, Itrakjy, A, Birkett, C, Clethro, A, Bonser, R, Graham, T, et al. A comparison of the efficacy of 70% v/v isopropyl alcohol with either 0.5% w/v or 2% w/v chlorhexidine gluconate for skin preparation before harvest of the long saphenous vein used in coronary artery bypass grafting. Am J Infect Control. (2015) 43:816–20. doi: 10.1016/j.ajic.2015.03.034
28. Maiwald, M, and Chan, ES. The forgotten role of alcohol: a systematic review and meta-analysis of the clinical efficacy and perceived role of chlorhexidine in skin antisepsis. PLoS One. (2012) 7:e44277. doi: 10.1371/journal.pone.0044277
29. Peristeri, DV, Nour, HM, Ahsan, A, Abogabal, S, Singh, KK, and Sajid, MS. Alcohol-containing versus aqueous-based solutions for skin preparation in abdominal surgery: a systematic review and meta-analysis. J Surg Res. (2023) 291:734–41. doi: 10.1016/j.jss.2023.06.011
30. Lai, NM, Lai, NA, O'Riordan, E, Chaiyakunapruk, N, Taylor, JE, and Tan, K. Skin antisepsis for reducing central venous catheter-related infections. Cochrane Database Syst Rev. (2016) 7:Cd010140. doi: 10.1002/14651858.CD010140.pub2
31. Sidhwa, F, and Itani, KM. Skin preparation before surgery: options and evidence. Surg Infect. (2015) 16:14–23. doi: 10.1089/sur.2015.010
32. Jalalzadeh, H, Groenen, H, Buis, DR, Dreissen, YE, Goosen, JH, Ijpma, FF, et al. Efficacy of different preoperative skin antiseptics on the incidence of surgical site infections: a systematic review, GRADE assessment, and network meta-analysis. Lancet Microbe. (2022) 3:e762–71. doi: 10.1016/S2666-5247(22)00187-2
33. Culligan, PJ, Kubik, K, Murphy, M, Blackwell, L, and Snyder, J. A randomized trial that compared povidone iodine and chlorhexidine as antiseptics for vaginal hysterectomy. Am J Obstet Gynecol. (2005) 192:422–5. doi: 10.1016/j.ajog.2004.08.010
34. Veiga, DF, Damasceno, CAV, Veiga-Filho, J, Figueiras, RG, Vieira, RB, Florenzano, FH, et al. Povidone iodine versus chlorhexidine in skin antisepsis before elective plastic surgery procedures: a randomized controlled trial. Plast Reconstr Surg. (2008) 122:170e–1e. doi: 10.1097/PRS.0b013e318186cd7f
35. Paocharoen, V, Mingmalairak, C, and Apisarnthanarak, A. Comparison of surgical wound infection after preoperative skin preparation with 4% chlorhexidine [correction of chlohexidine] and povidone iodine: a prospective randomized trial. J Med Assoc Thailand. (2009) 92:898–902.
36. Darouiche, RO, Wall, MJ Jr, Itani, KM, Otterson, MF, Webb, AL, Carrick, MM, et al. Chlorhexidine-alcohol versus povidone-iodine for surgical-site antisepsis. N Engl J Med. (2010) 362:18–26. doi: 10.1056/NEJMoa0810988
37. Sistla, SC, Prabhu, G, Sistla, S, and Sadasivan, J. Minimizing wound contamination in a 'clean' surgery: comparison of chlorhexidine-ethanol and povidone-iodine. Chemotherapy. (2010) 56:261–7. doi: 10.1159/000319901
38. Perek, B, Lipski, A, Stefaniak, S, and Jemielity, M. Comparative analysis of the antiseptic effectiveness of two commercially available skin disinfectants in cardiac surgery - a preliminary report. Polish J Thoracic Cardiovasc Surg. (2013) 10:177–81.
39. Yeung, LL, Grewal, S, Bullock, A, Lai, HH, and Brandes, SB. A comparison of chlorhexidine-alcohol versus povidone-iodine for eliminating skin flora before genitourinary prosthetic surgery: a randomized controlled trial. J Urol. (2013) 189:136–40. doi: 10.1016/j.juro.2012.08.086
40. Abreu, D, Campos, E, Seija, V, Arroyo, C, Suarez, R, Rotemberg, P, et al. Surgical site infection in surgery for benign prostatic hyperplasia: comparison of two skin antiseptics and risk factors. Surg Infect. (2014) 15:763–7. doi: 10.1089/sur.2013.174
41. Srinivas, A, Kaman, L, Raj, P, Gautam, V, Dahiya, D, Singh, G, et al. Comparison of the efficacy of chlorhexidine gluconate versus povidone iodine as preoperative skin preparation for the prevention of surgical site infections in clean-contaminated upper abdominal surgeries. Surg Today. (2015) 45:1378–84. doi: 10.1007/s00595-014-1078-y
42. Bibi, S, Shah, SA, Qureshi, S, Siddiqui, TR, Soomro, IA, Ahmed, W, et al. Is chlorhexidine-gluconate superior than povidone-iodine in preventing surgical site infections? A multicenter study. J Pak Med Assoc. (2015) 65:1197–201.
43. Kunkle, CM, Marchan, J, Safadi, S, Whitman, S, and Chmait, RH. Chlorhexidine gluconate versus povidone iodine at cesarean delivery: a randomized controlled trial. J Matern Fetal Neonatal Med. (2015) 28:573–7. doi: 10.3109/14767058.2014.926884
44. Ngai, IM, Van Arsdale, A, Govindappagari, S, Judge, NE, Neto, NK, Bernstein, J, et al. Skin preparation for prevention of surgical site infection after cesarean delivery: a randomized controlled trial. Obstet Gynecol. (2015) 126:1251–7. doi: 10.1097/AOG.0000000000001118
45. Davies, BM, and Patel, HC. Does chlorhexidine and povidone-iodine preoperative antisepsis reduce surgical site infection in cranial neurosurgery? Ann R Coll Surg Engl. (2016) 98:405–8. doi: 10.1308/rcsann.2016.0143
46. Park, HM, Han, SS, Lee, EC, Lee, SD, Yoon, HM, Eom, BW, et al. Randomized clinical trial of preoperative skin antisepsis with chlorhexidine gluconate or povidone-iodine. Br J Surg. (2017) 104:e145–50. doi: 10.1002/bjs.10395
47. Salama, FA, Yehia, AH, Wahba, KA, and Abdelmoniem, RM. Efficacy and safety of chlorhexidine versus povidone-iodine skin antisepsis in reducing surgical site infection in cesarean sections: a randomized, controlled clinical trial. Evid Based Womens Health J. (2016) 6:32–6. doi: 10.1097/01.EBX.0000479690.07770.cb
48. Broach, RB, Paulson, EC, Scott, C, and Mahmoud, NN. Randomized controlled trial of two alcohol-based preparations for surgical site antisepsis in colorectal surgery. Ann Surg. (2017) 266:946–51. doi: 10.1097/SLA.0000000000002189
49. Danasekaran, G, Rasu, S, and Palani, M. A study of comparative evaluation of preoperative skin preparation with chlorhexidinealcohol versus povidone-iodine in prevention of surgical site infections. J Evid Based Med Healthcare. (2017) 4:41.
50. Springel, EH, Wang, XY, Sarfoh, VM, Stetzer, BP, Weight, SA, and Mercer, BM. A randomized open-label controlled trial of chlorhexidine-alcohol vs povidone-iodine for cesarean antisepsis: the CAPICA trial. Am J Obstet Gynecol. (2017) 217:463.e461–8. doi: 10.1016/j.ajog.2017.05.060
51. Lakhi, NA, Tricorico, G, Osipova, Y, and Moretti, ML. Vaginal cleansing with chlorhexidine gluconate or povidone-iodine prior to cesarean delivery: a randomized comparator-controlled trial. Am J Obstet Gynecol. (2019) 1:2–9. doi: 10.1016/j.ajogmf.2019.03.004
52. Peel, TN, Dowsey, MM, Buising, KL, Cheng, AC, and Choong, PFM. Chlorhexidine-alcohol versus iodine-alcohol for surgical site skin preparation in an elective arthroplasty (ACAISA) study: a cluster randomized controlled trial. Clin Microbiol Infect. (2019) 25:1239–45. doi: 10.1016/j.cmi.2019.06.016
53. Saha, PK, Luwang, AL, Rohilla, M, Sikka, P, Saha, L, and Gautam, V. Chlorhexidine-alcohol versus povidone-iodine as preoperative skin antisepsis for prevention of surgical site infection in caesarean section. BJOG. (2019) 126:162
54. Dior, UP, Kathurusinghe, S, Cheng, C, Reddington, C, Daley, AJ, Ang, C, et al. Effect of surgical skin antisepsis on surgical site infections in patients undergoing gynecological laparoscopic surgery: a double-blind randomized clinical trial. JAMA Surg. (2020) 155:807–15. doi: 10.1001/jamasurg.2020.1953
55. Gezer, S, Yalvaç, HM, Güngör, K, and Yücesoy, İ. Povidone-iodine vs chlorhexidine alcohol for skin preparation in malignant and premalignant gynaecologic diseases: a randomized controlled study. Eur J Obstet Gynecol Reprod Biol. (2020) 244:45–50. doi: 10.1016/j.ejogrb.2019.10.035
56. Ritter, B, Herlyn, PKE, Mittlmeier, T, and Herlyn, A. Preoperative skin antisepsis using chlorhexidine may reduce surgical wound infections in lower limb trauma surgery when compared to povidone-iodine - a prospective randomized trial. Am J Infect Control. (2020) 48:167–72. doi: 10.1016/j.ajic.2019.08.008
57. Luwang, AL, Saha, PK, Rohilla, M, Sikka, P, Saha, L, and Gautam, V. Chlorhexidine-alcohol versus povidone-iodine as preoperative skin antisepsis for prevention of surgical site infection in cesarean delivery-a pilot randomized control trial. Trials. (2021) 22:540. doi: 10.1186/s13063-021-05490-4
Keywords: chlorhexidine, povidone-iodine, surgical site infection, meta-analysis, randomized controlled trials
Citation: Yang S, Li Z, Wu F, Sun L, He Y and Wang C (2025) Chlorhexidine versus povidone-iodine for surgical site infection prevention: an updated meta-analysis and trial sequential analysis of randomized controlled trials. Front. Med. 12:1641815. doi: 10.3389/fmed.2025.1641815
Edited by:
Xiaoping Mu, Guangxi Academy of Medical Sciences, ChinaReviewed by:
Wei Xiaodong, People’s Hospital of Guangxi Zhuang Autonomous Region, ChinaEbru Melek Benligül, Tınaztepe University, Türkiye
Copyright © 2025 Yang, Li, Wu, Sun, He and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Changxian Wang, MjMyMTE4OEB6anUuZWR1LmNu
 Shengyi Yang
Shengyi Yang Zhenwei Li
Zhenwei Li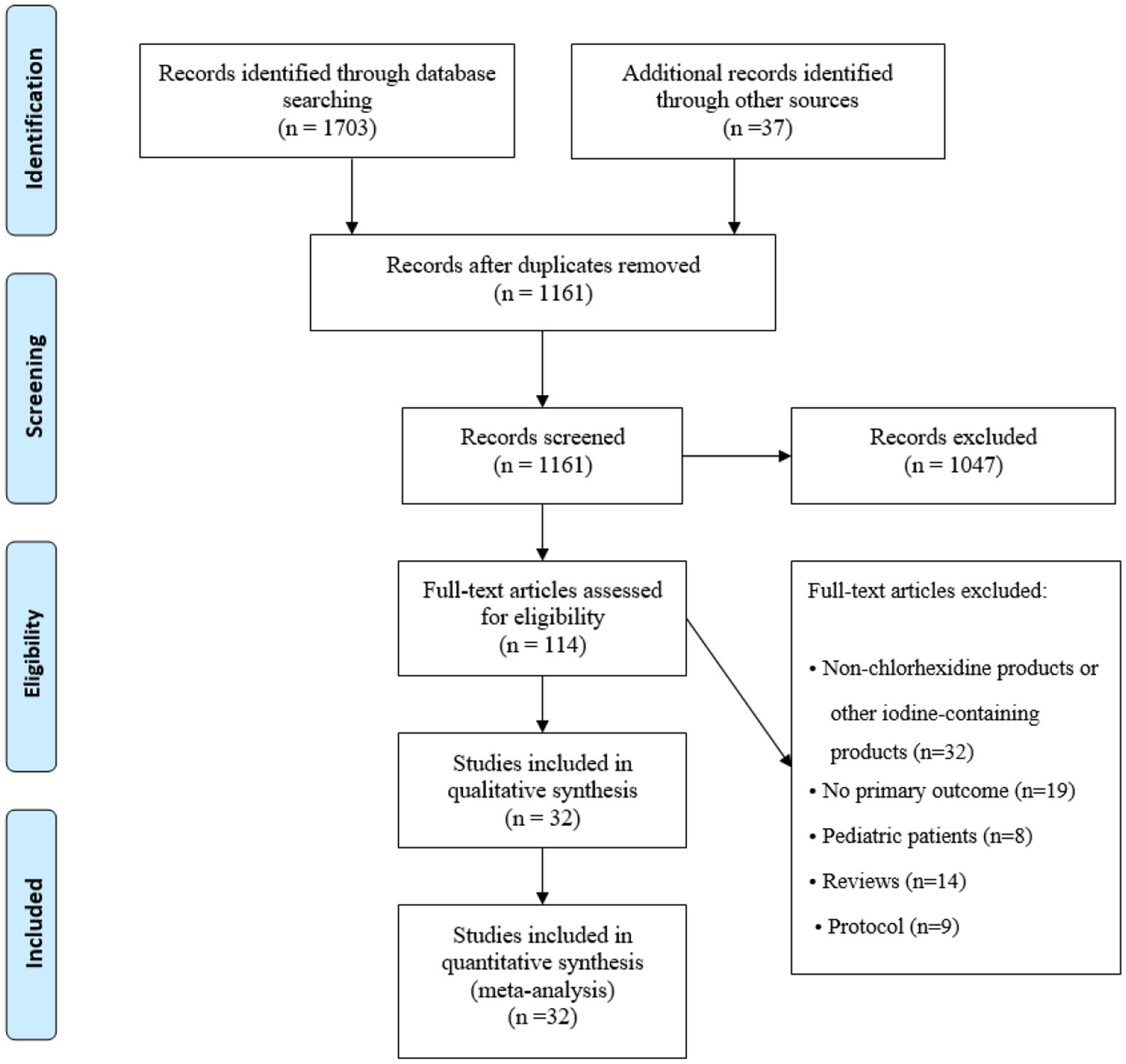
![Forest plot illustrating risk ratios for different subgroups comparing experimental treatments to CHX control. The plot includes studies on alcohol-based PVI, aqueous PVI, 10% PVI, and 5% PVI versus CHX, each with risk ratios and confidence intervals. Subtotals for each subgroup and the overall effect are shown, indicating a general favor towards experimental treatments. Risk ratios, sample sizes, and heterogeneity statistics are presented, demonstrating varying levels of significance across subgroups. The overall risk ratio for all subgroups combined is 0.81 [0.75, 0.88].](https://www.frontiersin.org/files/Articles/1641815/fmed-12-1641815-HTML/image_m/fmed-12-1641815-g007.jpg)