- Department of Special Inspection, The Affiliated People’s Hospital of Ningbo University, Ningbo, China
Background: Coronary heart disease (CHD) remains a leading cause of mortality worldwide, highlighting the need for early and accurate diagnosis of myocardial ischemia to improve patient outcomes. Dynamic electrocardiography (ECG) has become a critical diagnostic tool due to its capacity for continuous cardiac electrical activity monitoring. However, existing studies show considerable variation in its diagnostic performance. To establish higher-level evidence, this study systematically evaluates the diagnostic accuracy of dynamic ECG for myocardial ischemic episodes in patients with CHD through a comprehensive systematic review and meta-analysis.
Methods: A comprehensive literature search was conducted through May 2025 using PubMed, Embase, Web of Science, Cochrane Library, China National Knowledge Infrastructure, and Wanfang databases. Study quality was assessed using the QUADAS-2 instrument. Diagnostic performance was evaluated using sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR), diagnostic odds ratio (DOR), and the area under the receiver operating characteristic curve (AUC).
Results: The meta-analysis included 24 studies comprising 3,509 participants. The pooled diagnostic performance of dynamic ECG for myocardial ischemia showed a sensitivity of 0.75 (95% CI: 0.70–0.80), specificity of 0.70 (95% CI: 0.64–0.75), PLR of 2.50 (95% CI: 1.99–3.13), NLR of 0.36 (95% CI: 0.28–0.45), DOR of 6.64 (95% CI: 4.55–9.69), and an AUC of 0.79 (95% CI: 0.75–0.82). Subgroup analyses indicated higher diagnostic accuracy in studies involving confirmed CHD cases and those using coronary angiography as the reference standard.
Conclusion: Dynamic ECG exhibits moderate diagnostic value for detecting myocardial ischemia in patients with CHD. Its clinical application should be integrated with complementary diagnostic approaches. Further high-quality research is necessary to confirm its diagnostic utility and refine implementation protocols.
Systematic review registration: INPLASY202560026.
Introduction
Coronary heart disease (CHD) is a pathological condition characterized by coronary artery stenosis or occlusion caused by atherosclerosis. Acute cardiovascular events triggered by CHD remain the leading cause of mortality worldwide (1). According to the 2023 Report on Cardiovascular Health and Diseases in China, the number of patients with CHD has reached 11.39 million. The disease burden continues to grow, driven by an aging population and the increasing prevalence of metabolic disorders (2). Myocardial ischemia—the central clinical manifestation of CHD—results from an imbalance between coronary blood supply and myocardial oxygen demand, leading to insufficient oxygen delivery to cardiac tissue (3). Without timely intervention, transient ischemia may quickly evolve into acute coronary syndrome, significantly elevating the risk of sudden cardiac death (4). Accordingly, the development of reliable early diagnostic tools for myocardial ischemia is critical to improving outcomes and optimizing healthcare resource allocation.
Among current diagnostic modalities, dynamic electrocardiography (ECG) is widely employed for out-of-hospital myocardial ischemia monitoring due to its non-invasive nature and accessibility (5). Compared to conventional resting ECG, which captures only brief electrical changes, dynamic ECG enhances the detection of transient ST-T segment abnormalities through continuous 24–72-h recording, making it particularly effective for outpatient populations experiencing paroxysmal chest pain (6). Additionally, its ability to monitor cardiac activity in real time during daily life facilitates the identification of associations between myocardial workload and ischemic episodes, supporting personalized treatment approaches (7).
However, the diagnostic performance of dynamic ECG for myocardial ischemia remains controversial. Several studies have demonstrated substantial variability in sensitivity and specificity, largely attributable to: (1) bias in baseline characteristics of study populations (e.g., age distribution, comorbid conditions such as diabetes); (2) lack of standardized diagnostic thresholds (e.g., differing criteria for ST-segment depression ≥0.1 mV or ≥1 mm, lasting ≥1 min); (3) technical inconsistencies (lead configuration, motion artifact recognition); and (4) divergent reference standards (coronary angiography, myocardial perfusion imaging, or intravascular ultrasound) (8). These methodological inconsistencies hinder clinical interpretation and limit the development of evidence-based guidelines.
To address these limitations and provide a comprehensive assessment of diagnostic performance, this systematic review and meta-analysis aims to synthesize available evidence on dynamic ECG for detecting myocardial ischemic episodes in patients with CHD. By pooling results across studies, we evaluate its diagnostic accuracy, explore sources of heterogeneity, and identify key factors influencing performance.
Methods
Data sources, search strategy, and selection criteria
This systematic review and meta-analysis was conducted in accordance with the updated 2020 Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (9). Our study was registered in INPLASY platform (number: INPLASY202560026). We included studies that evaluated the diagnostic performance of dynamic ECG monitoring for myocardial ischemia in patients with CHD, regardless of language. A globally comprehensive search was conducted through May 2025 across both international (PubMed, Embase, Web of Science, Cochrane Library) and Chinese (China National Knowledge Infrastructure, Wanfang) databases to minimize geographic bias. The search strategy combined terms covering dynamic ECG, coronary heart disease, myocardial ischemia, and diagnosis (detailed in Supplementary File 1). We explicitly included non-Chinese language studies and applied no regional restrictions. Additionally, we manually reviewed reference lists of relevant articles and reviews to identify additional eligible studies not captured in the database search.
Two reviewers independently conducted title/abstract screening and full-text evaluation using a standardized form. Discrepancies were first resolved through structured discussion, with explicit documentation of reasoning. For studies consensus was not reached after two rounds of discussion, a third senior reviewer, arbitrated by re-evaluating the original study against inclusion criteria and providing a final decision, which was documented in a conflict resolution log. Inclusion criteria were: (1) population: patients with confirmed or suspected CHD; (2) diagnostic tool: 12-lead dynamic ECG monitoring; (3) outcome: reported diagnostic data for myocardial ischemia with a complete contingency table (true positives, false positives, true negatives, false negatives); and (4) no restriction on study design. Studies were excluded if they: (1) included <10 participants; (2) lacked extractable diagnostic accuracy data; (3) involved non-human participants; (4) provided insufficient methodological detail for quality assessment; or (5) employed non-standard ECG configurations (<12 leads or modified placements).
Data collection and quality assessment
A dual verification process was used: (1) independent re-extraction after a 24-h interval; (2) cross-checking numerical data with original sources; and (3) resolving discrepancies through documented consensus meetings. For data extraction discrepancies, unresolved disagreements after discussion were escalated to the third senior reviewer, who verified the original study data and made a final determination, with all decisions recorded in a data extraction audit trail. Extracted information included: first author’s surname, publication year, geographical region, sample size, mean age, male proportion, clinical status, reference standard, diagnostic performance metrics (true positives, false positives, true negatives, false negatives). For QUADAS-2 quality assessment, two reviewers independently rated each domain (patient selection, index test, reference standard, flow/timing) as “low,” “high,” or “unclear” risk of bias (10). Disagreements in 15% of domain ratings were first addressed through discussion. Unresolved cases were reviewed by the third senior reviewer, who re-evaluated the study methodology against QUADAS-2 criteria, provided a final rating, and documented the rationale in a quality assessment log.
Statistical analysis
This study employed true positive, false positive, true negative, and false negative data to derive key diagnostic metrics including sensitivity (detection capability of true positives), specificity (capacity to correctly exclude non-cases), positive likelihood ratio (PLR), negative likelihood ratio (NLR), diagnostic odds ratio (DOR), and the area under the receiver operating characteristic curve (AUC). Pooled estimates were generated using bivariate generalized linear mixed models with random effects to account for between-study variability (11, 12). Heterogeneity was assessed using the I2 statistic and Cochran’s Q-test, with I2 ≥ 50.0% or Q-test p < 0.10 indicating significant heterogeneity (13, 14). Prespecified subgroup analyses were performed based on disease status (confirmed vs. suspected CHD) and reference standard methodology. Publication bias was evaluated through funnel plots and Deeks’ asymmetry test (15). All meta-analytic outcomes were interpreted through two-tailed statistical testing with an α-level threshold of 0.05. The complete analytical workflow was executed using STATA version 14.0 (StataCorp LP, College Station, TX, United States), ensuring methodological reproducibility through standardized scripting protocols.
Results
Literature search
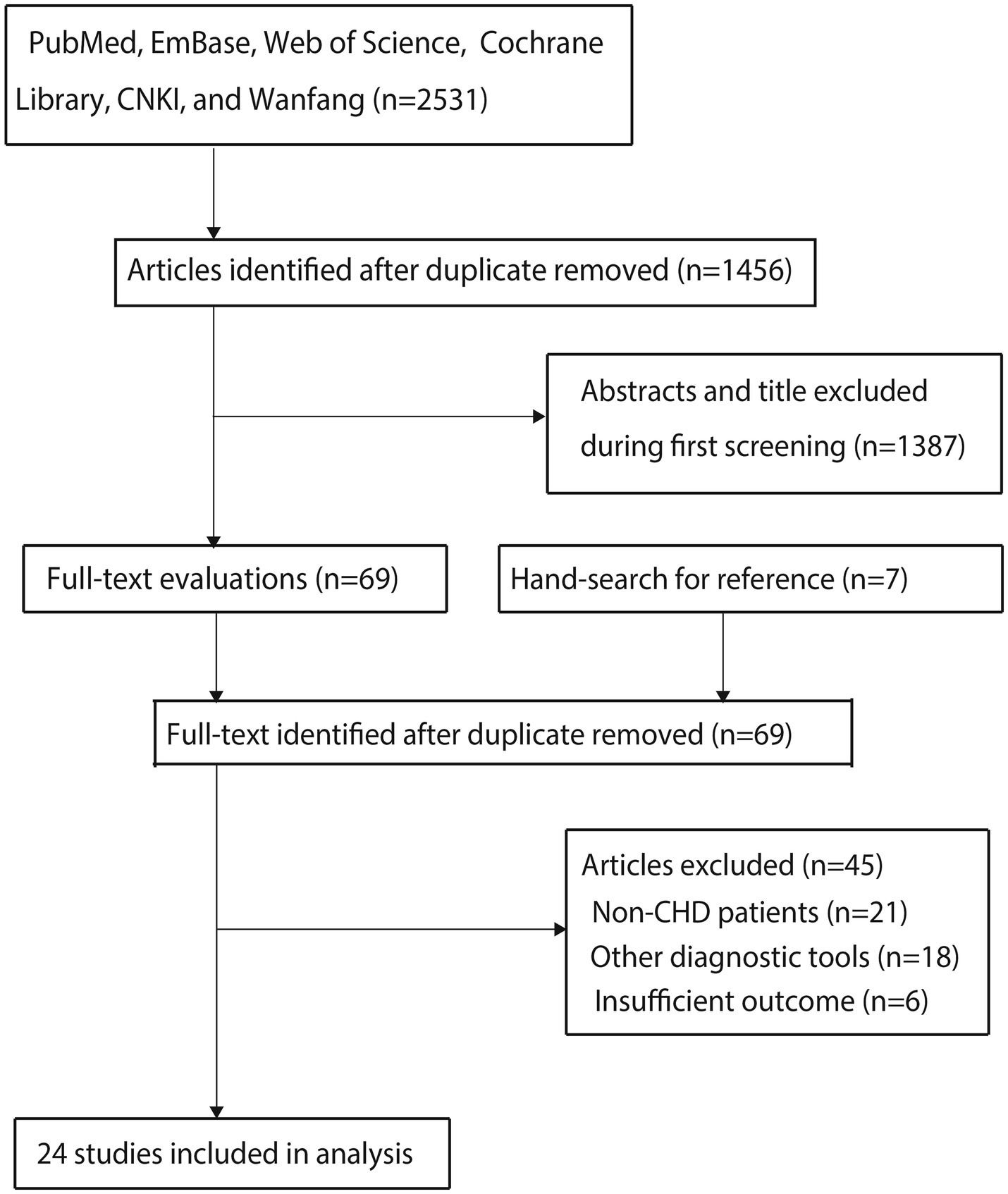
The electronic database search initially identified 2,531 studies, with 1,456 retained after duplicate removal. Title and abstract screening excluded 1,387 studies, leaving 69 for full-text evaluation. Following detailed assessment, 45 studies were excluded due to: (1) non-CHD populations (n = 21); (2) alternative diagnostic tools (n = 18); and (3) insufficient outcome reporting (n = 6). Ultimately, 24 studies met the inclusion criteria for meta-analysis (16–39). A manual reference search identified seven additional potentially relevant studies, all of which had already been captured in the original electronic search. The complete study selection process is illustrated in Figure 1.
Study characteristics
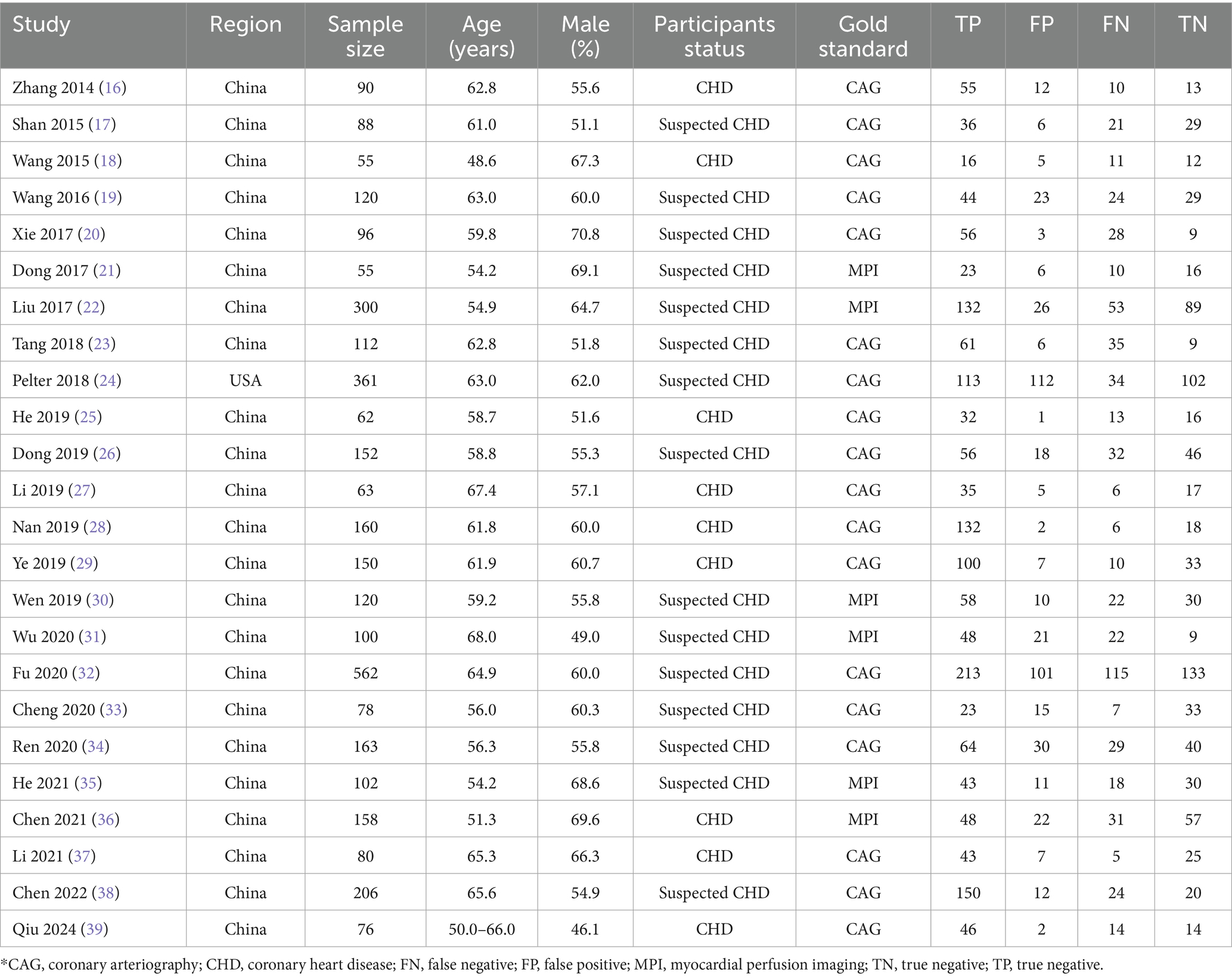
Table 1 summarizes the baseline characteristics of the included studies and participants. The 24 studies involved 3,509 participants, with 23 conducted in China and one in the United States. The mean age of enrolled participants ranged from 48.6 to 68.0 years, with male representation ranging from 46.1 to 70.8%. Nine studies exclusively included confirmed CHD participants, while the remaining 15 included those with suspected CHD. Coronary angiography (CAG) was used as the reference standard in 18 studies, while myocardial perfusion imaging (MPI) was employed in six. Methodological quality assessment, detailed in Table 2, indicated moderate to high quality across all studies, with overall high methodological rigor.
Sensitivity and specificity
Figure 2 presents the pooled diagnostic performance of dynamic ECG in detecting myocardial ischemia among participants with CHD, demonstrating a sensitivity of 0.75 (95% CI: 0.70–0.80) and specificity of 0.70 (95% CI: 0.64–0.75). Significant heterogeneity was observed for both metrics (I2 ≥ 50%, p < 0.10). Subgroup analyses showed higher sensitivity and specificity in studies involving confirmed CHD participants compared to those with suspected CHD, with statistically significant between-subgroup differences. Diagnostic performance was also higher when CAG was used as the reference standard, although no significant subgroup difference in specificity was observed (Table 3).
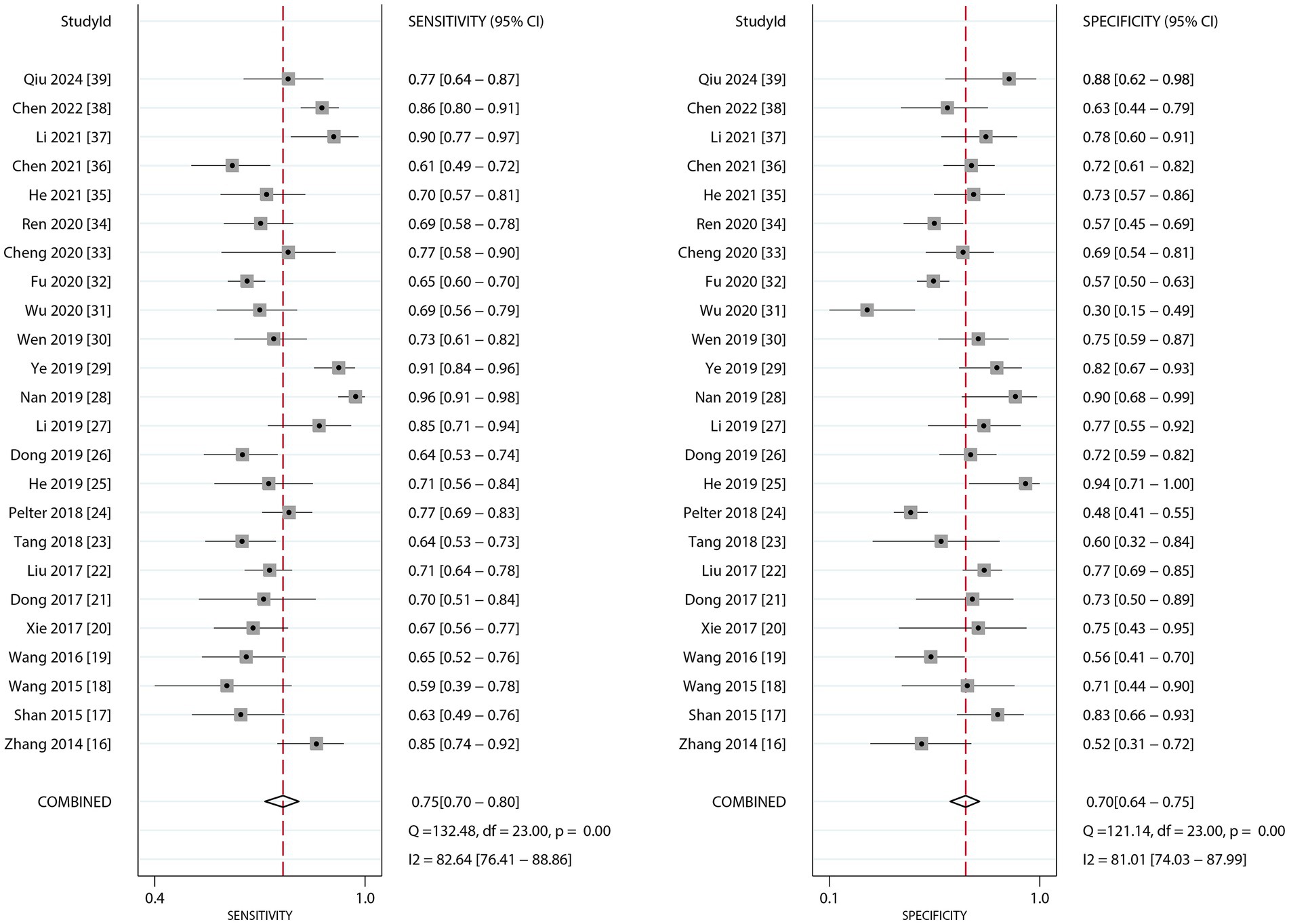
Figure 2. The summary sensitivity and specificity of dynamic ECG for detecting myocardial ischemia among CHD patients.
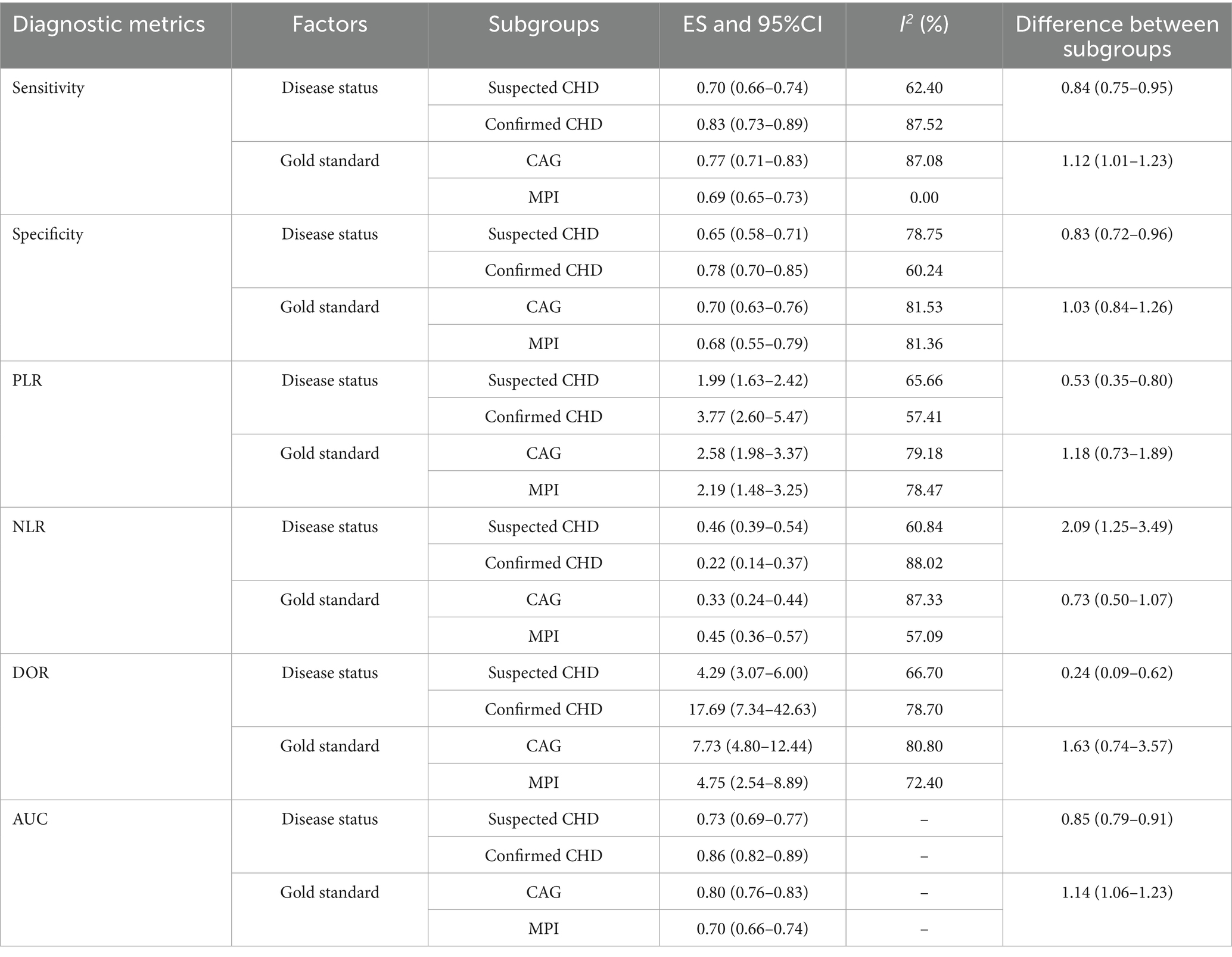
Table 3. Subgroup analyses for the diagnostic performance of dynamic electrocardiography for detecting myocardial ischemic attack in coronary heart disease.
PLR and NLR
Figure 3 presents the pooled results for PLR and NLR of dynamic ECG in diagnosing myocardial ischemia among participants with CHD. The pooled PLR was 2.50 (95% CI: 1.99–3.13), and the NLR was 0.36 (95% CI: 0.28–0.45). Significant heterogeneity was found for both metrics (I2 ≥ 50%, p < 0.10). Subgroup analyses revealed higher PLR values in studies involving confirmed participants with CHD and those using CAG as the reference standard. Statistically significant subgroup differences were observed for participant status (p < 0.05), but not for reference standard methodology. Conversely, NLR values were lower in confirmed CHD participants and CAG-based studies, with significant differences by participant status (p < 0.01) but not across reference standards (Table 3).
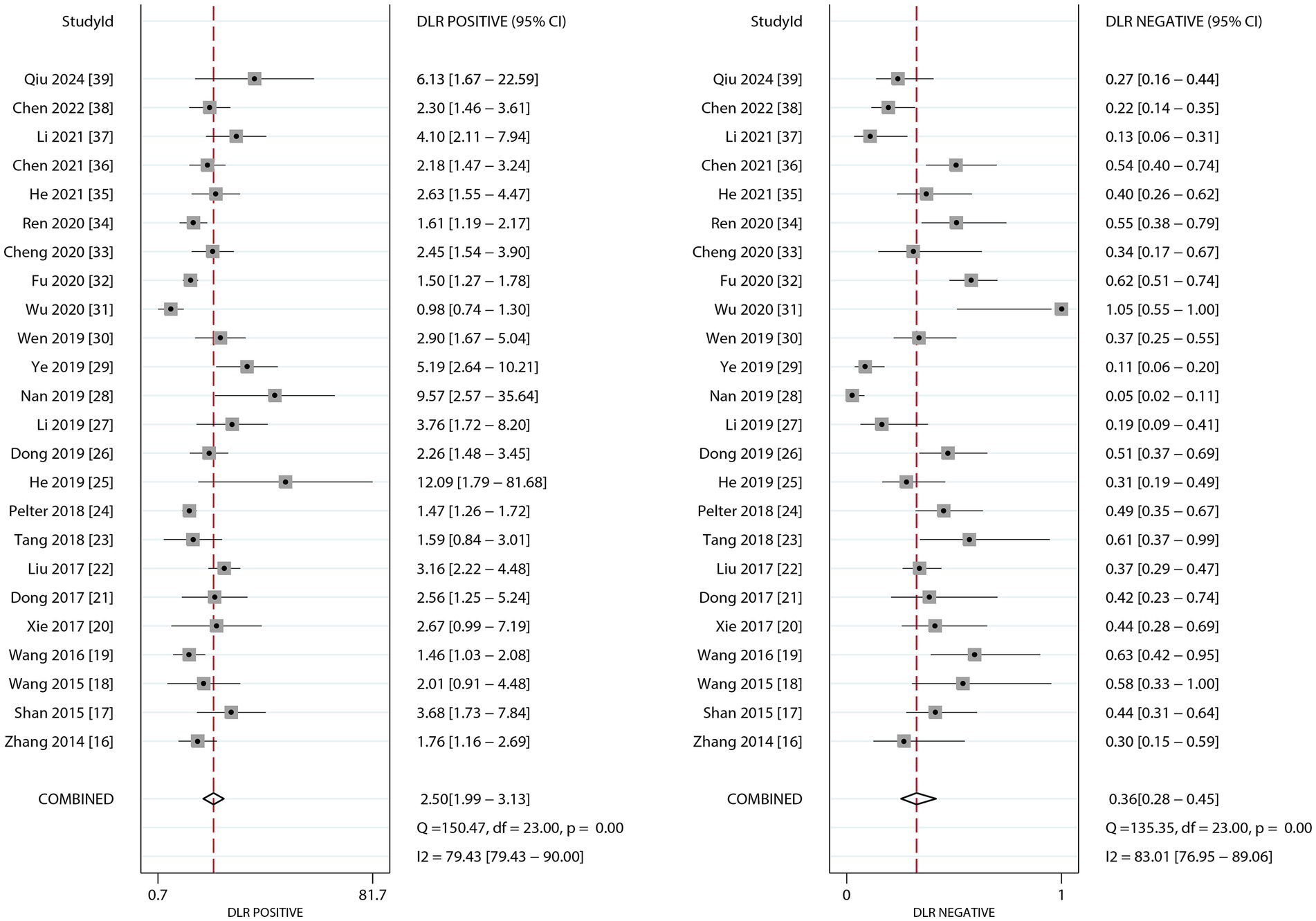
Figure 3. The summary PLR and DLR of dynamic ECG for detecting myocardial ischemia among CHD patients.
DOR
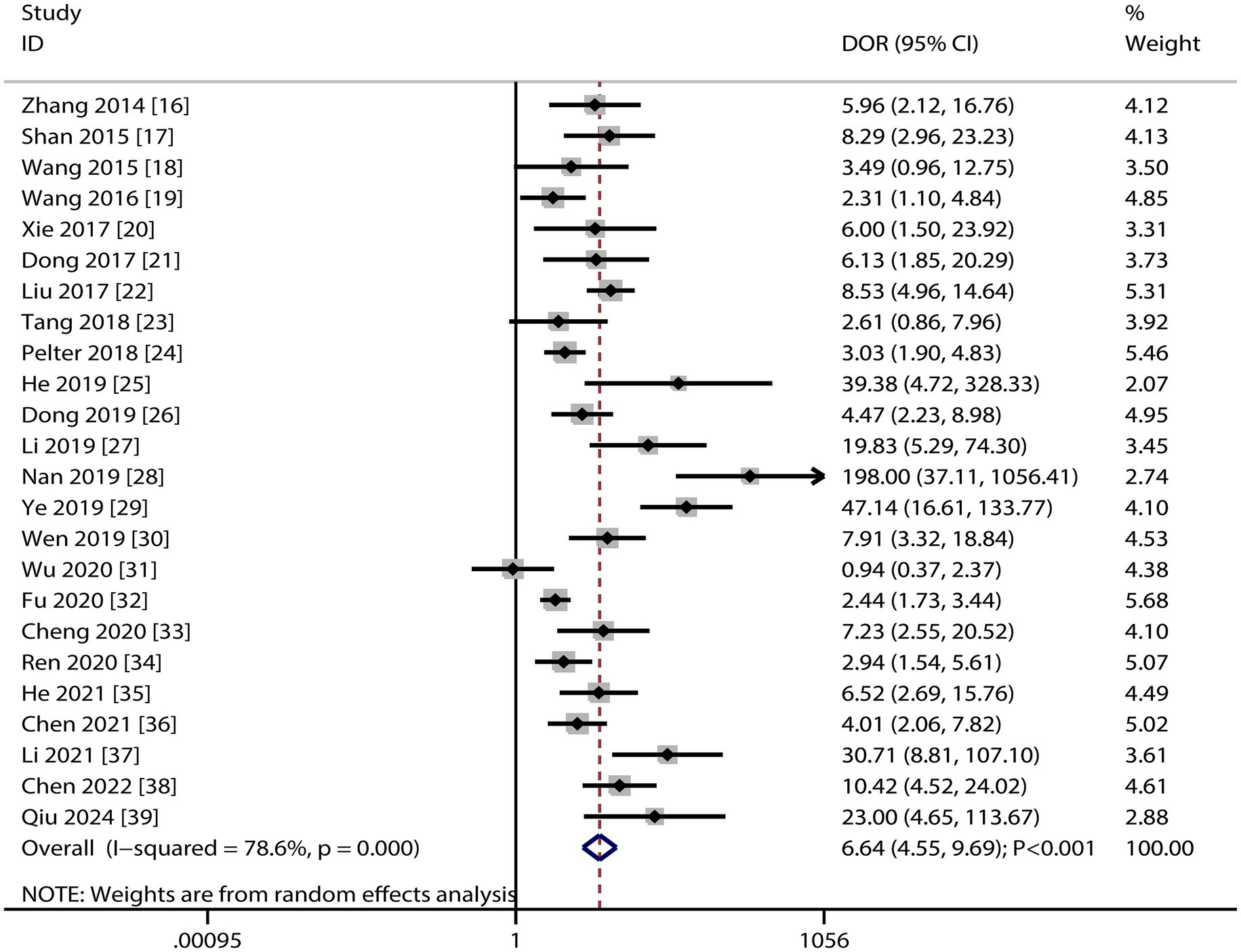
Figure 4 presents the pooled DOR results for dynamic ECG in detecting myocardial ischemia among participants with CHD, showing a DOR of 6.64 (95% CI: 4.55–9.69). Significant heterogeneity was observed in the DOR estimates (I2 ≥ 50%, p < 0.10). Subgroup analyses demonstrated elevated DOR values in studies involving confirmed CHD participants and those using CAG as the reference standard. Statistically significant between-subgroup differences were found for participant status (p < 0.05), while no significant variation was observed across reference standard subgroups (Table 3).
AUC
Figure 5 presents the pooled AUC results for dynamic ECG in detecting myocardial ischemia among participants with CHD, demonstrating an AUC of 0.79 (95% CI: 0.75–0.82). Subgroup analyses revealed significantly higher AUC values in studies involving confirmed CHD participants and those using CAG as the reference standard, with statistically significant between-subgroup differences (p < 0.05), as shown in Table 3.
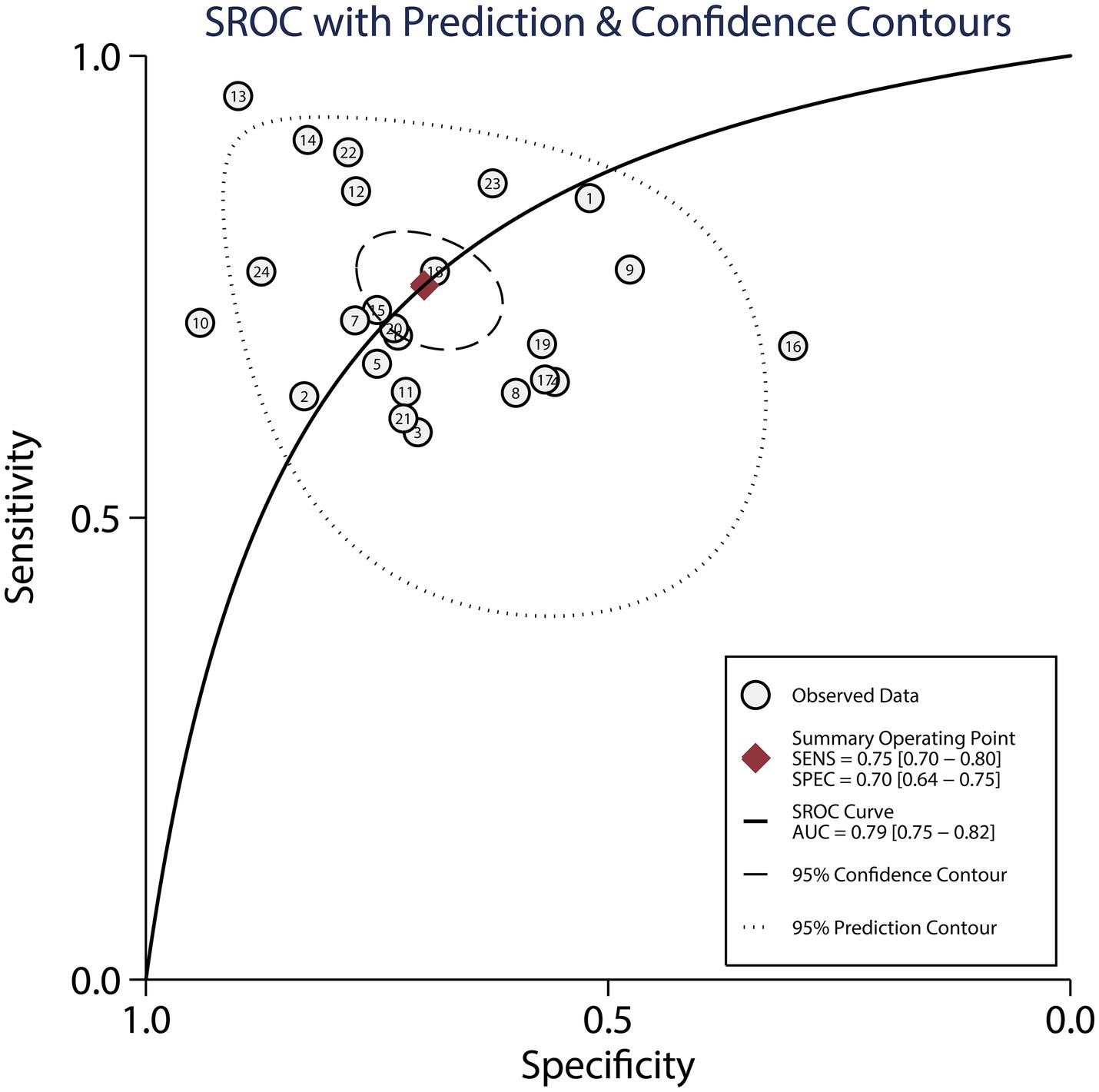
Figure 5. The summary area under the receiver operating characteristic curve of dynamic ECG for detecting myocardial ischemia among CHD patients.
Publication bias
Visual inspection of the funnel plot could not rule out potential publication bias (Figure 6). Deeks’ asymmetry test indicated significant publication bias in the diagnostic performance of dynamic ECG for myocardial ischemia detection (p = 0.02). We conducted a trim-fill analysis to adjust for this bias, After adjusting potential publication bias using the trim and fill method, the pooled diagnostic metrics remained consistent. These adjusted values confirm that while publication bias may modestly overestimate accuracy, the overall pattern of moderate diagnostic utility remains unchanged.
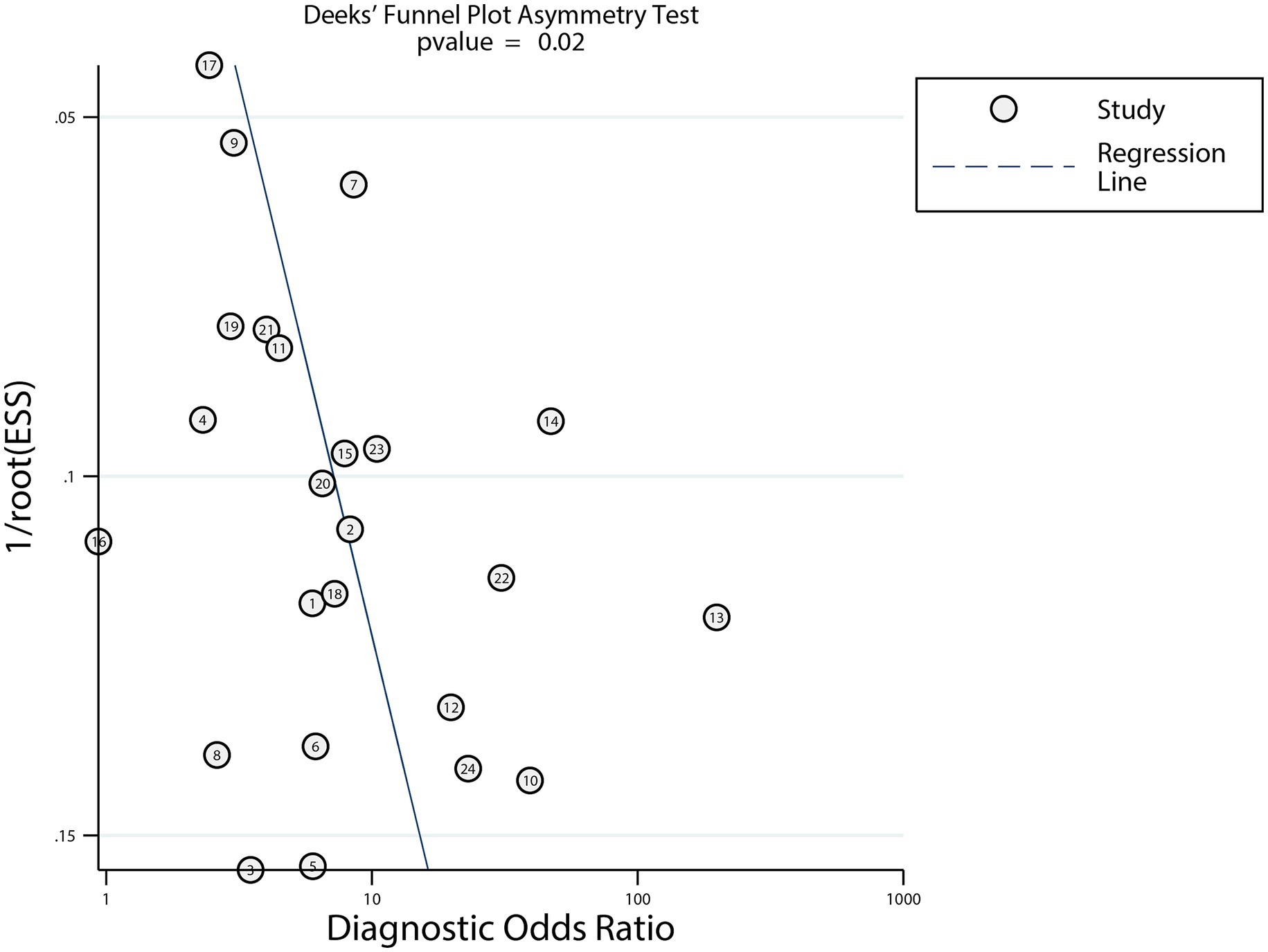
Figure 6. Funnel plots with Deeks’ asymmetry tests of dynamic ECG for detecting myocardial ischemia among CHD patients.
Discussion
This systematic review and meta-analysis provides a comprehensive evaluation of dynamic ECG for detecting myocardial ischemia in participants with CHD. The pooled diagnostic estimates—sensitivity, 0.75; specificity, 0.70; PLR, 2.50; NLR, 0.36; DOR, 6.64 and AUC, 0.79—indicate moderate diagnostic utility. While clinically relevant, these values fall short of ideal diagnostic thresholds, highlighting the need for complementary diagnostic tools (40). A PLR of 2.50 suggests that a positive dynamic ECG increases the post-test probability of myocardial ischemia by approximately 30% in moderate-prevalence populations, while an NLR of 0.36 decreases the probability by 40–50%. These findings support the use of dynamic ECG as a triage tool rather than a definitive diagnostic method, consistent with its established role in ambulatory monitoring of transient ischemic episodes (40).
The high heterogeneity (I2 > 80% for sensitivity and specificity) is multifactorial, with unreported methodological details emerging as a critical challenge. First, population heterogeneity—including differences in age, sex distribution (46.1–70.8% male), and comorbidities such as diabetes—may have contributed to inconsistent ischemic patterns, as diabetes alters ST-segment morphology through autonomic dysfunction (41). Second, variation in ischemic threshold definitions (e.g., ST-segment depression criteria: ≥0.1 mV vs. ≥1 mm; duration: ≥1 min vs. transient episodes) introduces diagnostic inconsistency, as minor differences in cutoff values can significantly affect sensitivity and specificity trade-offs (42). Third, differences in lead configurations and artifact discrimination algorithms impact signal fidelity, particularly during patient movement—a known limitation of ambulatory monitoring. Finally, use of different reference standards introduces spectrum bias: CAG directly visualizes coronary stenosis, while MPI evaluates functional ischemia, capturing distinct pathophysiological processes.
Subgroup analyses demonstrated enhanced diagnostic performance in confirmed participants with CHD (vs. suspected cases) and CAG-based studies. The superior performance in confirmed CHD cohorts likely reflects greater atherosclerotic burden, allowing dynamic ECG to more reliably detect ischemia-induced repolarization abnormalities (43). In contrast, participants with suspected CHD may exhibit non-ischemic ST-T changes resulting from conditions such as microvascular dysfunction or electrolyte imbalance, reducing specificity. The improved performance in CAG-based studies underscores the value of anatomical correlation, as transient ECG changes may not align with perfusion defects seen in MPI—particularly in cases of balanced multivessel disease (44, 45). However, the absence of significant specificity differences across reference standards indicates ongoing challenges in distinguishing true ischemic events from physiological confounders.
Significant publication bias (Deeks’ test p = 0.02) suggests that smaller studies with less favorable diagnostic performance may have been underreported, a common issue in diagnostic meta-analyses (46). Several factors likely contribute: (1) researchers and journals may be more inclined to publish studies with “positive” findings, while studies with non-significant or lower accuracy are less likely to be submitted or accepted. This is particularly relevant for dynamic ECG research, where institutional or commercial interests in validating diagnostic tools may influence publication trends; (2) our trim-fill analysis imputed five hypothetical missing studies, which slightly reduced pooled metrics but preserved the conclusion of moderate diagnostic utility. This suggests the overestimation due to bias is modest rather than transformative; and (3) while we cannot access unpublished data, we can infer their potential characteristics: smaller sample sizes, higher risk of bias, or populations with lower disease prevalence—factors known to reduce diagnostic metric precision. Inclusion of such studies would likely widen confidence intervals but not negate the core finding that dynamic ECG has clinical utility for ischemia detection.
A notable limitation of this meta-analysis is the overrepresentation of Chinese studies (23/24), which may restrict the extrapolability of conclusions to other populations. Several factors may explain the paucity of international studies meeting our criteria: (1) Clinical practice variations: Dynamic ECG utilization patterns differ globally—while it is widely adopted as a first-line ambulatory monitoring tool in China for CHD patients, international guidelines often prioritize stress testing or coronary CT angiography for ischemia detection, potentially reducing the number of dedicated dynamic ECG diagnostic studies; and (2) Data reporting standards: International guideline focus on dynamic ECG for arrhythmia detection rather than myocardial ischemia, or lack complete diagnostic data required for meta-analysis. Thus, while our findings provide valuable evidence for Chinese clinical practice, extrapolation to other regions should be cautious. Future studies should prioritize multi-center, international collaborations to include diverse ethnicities, healthcare systems, and clinical practice patterns, thereby enhancing the generalizability of dynamic ECG’s diagnostic performance data. Moreover, the lack of standardized reporting of ST-segment depression thresholds and duration criteria in mostly included studies. Threshold variations are well-known to drive sensitivity-specificity trade-offs in diagnostic testing. Without this data, we cannot quantify their contribution to heterogeneity, highlighting a major gap in dynamic ECG research: the absence of consensus on reporting diagnostic criteria.
Conclusion
Despite limitations—including significant geographic bias (23 of 24 studies from China) and methodological heterogeneity—these findings reinforce the utility of dynamic ECG in non-invasive ischemia monitoring. The consistency between the single U. S. study and pooled Chinese results provides preliminary support for generalizability, but future international studies are needed to confirm these findings across diverse populations. Its strength lies in capturing transient episodes during routine activity, offering a preferable alternative to stress testing in older adults and patients with frailty. Clinicians should interpret results within the broader clinical context. Future research should prioritize standardization of ischemic criteria, adoption of advanced signal-processing technologies, and validation across diverse populations. Integrating dynamic ECG with high-sensitivity troponin assays or coronary CT angiography may enhance diagnostic accuracy, particularly in emergency department evaluations for chest pain.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
WL: Methodology, Data curation, Conceptualization, Validation, Writing – original draft, Investigation, Formal analysis, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1646417/full#supplementary-material
References
1. Roth, GA, Mensah, GA, Johnson, CO, Addolorato, G, Ammirati, E, Baddour, LM, et al. Global burden of cardiovascular diseases and risk factors, 1990-2019: update from the GBD 2019 study. J Am Coll Cardiol. (2020) 76:2982–3021. doi: 10.1016/j.jacc.2020.11.010
2. Liu, MB, Wang, ZW, Fan, J, and Hu, SS. China cardiovascular health and disease report 2023. Key points of China cardiovascular health and disease report 2023. Chin Cardiovasc Res. (2024) 22:577–93.
3. Yang, YC, Dou, Y, Wang, ZW, Yin, RH, Pan, CJ, Duan, SF, et al. Prediction of myocardial ischemia in coronary heart disease patients using a CCTA-based radiomic nomogram. Front Cardiovasc Med. (2023) 10:1024773. doi: 10.3389/fcvm.2023.1024773
4. La Grutta, L, Toia, P, Maffei, E, Cademartiri, F, Lagalla, R, and Midiri, M. Infarct characterization using CT. Cardiovasc Diagn Ther. (2017) 7:171–88. doi: 10.21037/cdt.2017.03.18
5. Verrier, RL, Nearing, BD, and D'Avila, A. Spectrum of clinical applications of interlead ECG heterogeneity assessment: from myocardial ischemia detection to sudden cardiac death risk stratification. Ann Noninvasive Electrocardiol. (2021) 26:e12894. doi: 10.1111/anec.12894
6. Drew, BJ, Dempsey, ED, Joo, TH, Sommargren, CE, Glancy, JP, Benedict, K, et al. Pre-hospital synthesized 12-lead ECG ischemia monitoring with trans-telephonic transmission in acute coronary syndromes: pilot study results of the ST SMART trial. J Electrocardiol. (2004) 37:214–21. doi: 10.1016/j.jelectrocard.2004.08.060
7. Benson, LC, Räisänen, AM, Volkova, VG, Pasanen, K, and Emery, CA. Workload a-WEAR-ness: monitoring workload in team sports with wearable technology. A scoping review. J Orthop Sports Phys Ther. (2020) 50:549–63. doi: 10.2519/jospt.2020.9753
8. Hashimoto, K, and Harada, N. Recent progress of Holter-based late potential for predicting serious cardiac events and its implications and future challenges. J Electrocardiol. (2023) 81:136–41. doi: 10.1016/j.jelectrocard.2023.08.018
9. Page, MJ, McKenzie, JE, Bossuyt, PM, Boutron, I, Hoffmann, TC, Mulrow, CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
10. Whiting, PF, Rutjes, AW, Westwood, ME, Mallett, S, Deeks, JJ, Reitsma, JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. (2011) 155:529–36. doi: 10.7326/0003-4819-155-8-201110180-00009
11. DerSimonian, R, and Laird, N. Meta-analysis in clinical trials. Control Clin Trials. (1986) 7:177–88. doi: 10.1016/0197-2456(86)90046-2
12. Walter, SD. Properties of the summary receiver operating characteristic (SROC) curve for diagnostic test data. Stat Med. (2002) 21:1237–56. doi: 10.1002/sim.1099
13. Deeks, JJ, Higgins, JPT, and Altman, DG. Analyzing data and undertaking meta-analyses In: J Higgins and S Green, editors. Cochrane handbook for systematic reviews of interventions 5.0.1. Oxford, UK: The Cochrane Collaboration (2008)
14. Higgins, JP, Thompson, SG, Deeks, JJ, and Altman, DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
15. Deeks, JJ, Macaskill, P, and Irwig, L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J Clin Epidemiol. (2005) 58:882–93. doi: 10.1016/j.jclinepi.2005.01.016
16. Zhang, Y, Song, BL, and Bai, MY. Comparison of the diagnostic value of dynamic electrocardiogram, treadmill exercise test and routine electrocardiogram in asymptomatic myocardial ischemia. China J Coal Ind Med. (2014) 17:1932–4.
17. Shan, ZQ. Clinical significance of dynamic electrocardiogram QTc interval in the diagnosis of myocardial ischemia in patients with suspected coronary heart disease. Med Recap. (2015) 21:317–9.
18. Wang, YB, Tang, SQ, Xu, G, Wei, X, and Zhan, J. Study on the diagnostic effect and clinical value of 12-lead dynamic electrocardiogram and treadmill exercise test in patients with coronary heart disease with myocardial ischemia. J Pract Clin Med. (2015) 19:127–9.
19. Wang, SF. The value of treadmill exercise test and 12-lead dynamic electrocardiogram in the diagnosis of myocardial ischemic coronary heart disease. J Mod Integr Tradit Chin West Med. (2016) 25:1346–8.
20. Xie, YJ, and Wang, C. Clinical value of exercise stress electrocardiogram and dynamic electrocardiogram in the diagnosis of occult coronary heart disease. J Integr Tradit Chin West Med. (2017) 26:3404–5.
21. Dong, XB, Wang, Y, and Yu, XY. Comparative analysis of dynamic electrocardiogram and CT first-pass myocardial perfusion imaging in the diagnosis of myocardial ischemia in coronary heart disease. China Med Herald. (2017) 14:110–3.
22. Liu, JH, and Jun, J. Application of coronary artery CT angiography combined with dynamic electrocardiogram in the diagnosis of myocardial ischemia in patients with coronary heart disease. Chin J CT MRI. (2017) 15:61–3.
23. Tang, J, and Zhong, CJ. Comparative study of 12-lead dynamic electrocardiogram and treadmill exercise test in the diagnosis of myocardial ischemic coronary heart disease. J Cardio-Cerebro Dis Integ Tradit Chin West Med. (2018) 6:2546–8.
24. Pelter, MM, Xu, Y, Fidler, R, Xiao, R, Mortara, DW, and Xiao, H. Evaluation of ECG algorithms designed to improve detect of transient myocardial ischemia to minimize false alarms in patients with suspected acute coronary syndrome. J Electrocardiol. (2018) 51:288–95. doi: 10.1016/j.jelectrocard.2017.10.005
25. He, HB. Application of stress dynamic CT myocardial perfusion imaging combined with dynamic electrocardiogram QTc interval in the diagnosis of myocardial ischemia in coronary heart disease. Cent South J Med Sci. (2019) 47:479–82.
26. Dong, N. The value of dynamic electrocardiogram QTc interval in predicting myocardial ischemic attack in patients with coronary heart disease. J Cardio-Cerebro Dis Integ Tradit Chin West Med. (2019) 17:1884–7.
27. Li, LL, and Wang, QY. Comparative analysis of ambulatory electrocardiogram and coronary artery CT angiography in patients with asymptomatic myocardial ischemia. Chin Magaz CT MRI. (2019) 17:18–20.
28. Nan, CP, and Zhang, FR. Diagnostic efficacy of dynamic electrocardiogram and routine electrocardiogram in myocardial ischemic attack of coronary heart disease. J Prev Med Peoples Liberat Army. (2019) 37:61–2.
29. Ye, HR, and Qiu, DD. Early diagnostic value of 24-hour dynamic electrocardiogram in middle-aged and elderly patients with coronary heart disease complicated with asymptomatic myocardial ischemia. China Med Innov. (2019) 16:123–6.
30. Wen, C, Yuan, JX, and Wang, WH. The value of dynamic electrocardiogram combined with MSCT in the diagnosis of asymptomatic myocardial ischemia. Chin Magaz CT MRI. (2019) 17:69–72.
31. Wu, RF. Diagnostic value of coronary artery CT angiography combined with dynamic electrocardiogram in myocardial ischemia of coronary heart disease. Chin Med Clinic. (2020) 20:1094–5.
32. Fu, HN, Hua, PD, Wei, XF, and Lv, SZ. The value of cardiopulmonary exercise test, exercise treadmill test and dynamic electrocardiogram in the evaluation of myocardial ischemia. Chin J Evid Based Cardiovasc Med. (2020) 6:1348–51.
33. Cheng, HL. Clinical comparative analysis of 12-lead dynamic electrocardiogram and treadmill exercise test in the diagnosis of myocardial ischemia in patients with coronary heart disease. Shanxi Med J. (2020) 49:16–8.
34. Ren, L, and Luo, WG. The value of dynamic electrocardiogram in the diagnosis of myocardial ischemia in patients with coronary heart disease. J Cardio-Cerebro Dis Integ Tradit Chin West Med. (2020) 18:611–3.
35. He, Y, Zhong, J, and Yang, XJ. Diagnostic value of dynamic electrocardiogram combined with CT first-pass perfusion imaging in patients with coronary heart disease with myocardial ischemia. Chin J CT MRI. (2021) 19:77–9.
36. Chen, P, Wu, T, Peng, QH, Fan, N, Shi, Y, Wang, H, et al. Application value between dynamic electrocardiogram and MSCT myocardial perfusion imaging in the diagnosis of myocardial ischemia in coronary heart disease. Ann Palliat Med. (2021) 10:10720–5. doi: 10.21037/apm-21-2481
37. Li, X, Lai, S, and Liang, J. A comparative analysis of the effects of treadmill exercise test and ambulatory electrocardiogram in diagnosing asymptomatic myocardial ischemia in coronary heart disease. J China Prescipt Drug. (2021) 19:150–1.
38. Chen, Z, Tan, H, Liu, X, and Tang, M. Application of 24 h dynamic electrocardiography in the diagnosis of asymptomatic myocardial ischemia with arrhythmia in elderly patients with coronary heart disease. Emerg Med Int. (2022) 2022:1–5. doi: 10.1155/2022/3228023
39. Qiu, J, Liu, J, Hong, D, and Zheng, M. The application of echocardiography combined with ambulatory electrocardiography in the diagnosis of asymptomatic myocardial ischemia. J Mod Electrophysiol. (2024) 31:48–50. doi: 10.1155/2024/9861978
40. Liu, Y, Ping, J, Qiu, L, Sun, C, and Chen, M. Comparative analysis of ischemic changes in electrocardiogram and coronary angiography results: a retrospective study. Medicine. (2021) 100:e26007. doi: 10.1097/MD.0000000000026007
41. Rana, BS, Band, MM, Ogston, S, Morris, AD, Pringle, SD, and Struthers, AD. Relation of QT interval dispersion to the number of different cardiac abnormalities in diabetes mellitus. Am J Cardiol. (2002) 90:483–7. doi: 10.1016/S0002-9149(02)02518-3
42. Ruan, W. Meta-analysis of dynamic electrocardiography in the diagnosis of myocardial ischemic attack of coronary heart disease. Comput Math Methods Med. (2022) 2022:1–11. doi: 10.1155/2022/3472413
43. Kang, R, Li, Y, Gao, C, Li, J, Zhang, C, and Wang, J. Discussion on repolarization reserve between patients with coronary heart disease and normal controls. Comput Math Methods Med. (2022) 2022:1–8. doi: 10.1155/2022/7944969
44. Marques, J, Pereira, L, Messias, A, Fonseca, N, Cotovio, P, Ferreira, A, et al. The burden of coronary heart disease in simultaneous pancreas-kidney transplantation: coronary angiography as a diagnostic method for all? - a retrospective study. J Bras Nefrol. (2022) 44:522–6. doi: 10.1590/2175-8239-jbn-2021-0156
45. Rammos, A, Bechlioulis, A, Kekiopoulou, A, Kekiopoulos, P, Katsouras, CS, and Sioka, C. Myocardial perfusion imaging and C-reactive protein in myocardial ischemia: a retrospective single-center study. Life. (2024) 14:261. doi: 10.3390/life14020261
Keywords: coronary heart disease, myocardial ischemia, dynamic electrocardiography, diagnostic performance, meta-analysis
Citation: Lv W (2025) Diagnostic performance of dynamic electrocardiography in the diagnosis of myocardial ischemic attack in coronary heart disease: a systematic review and meta-analysis. Front. Med. 12:1646417. doi: 10.3389/fmed.2025.1646417
Edited by:
Nikolai Klymiuk, Technical University of Munich, GermanyReviewed by:
Yan Li, Beijing University of Chinese Medicine, ChinaEmma Cerracchio, Ospedale Sacro Cuore di Gesù, Italy
Copyright © 2025 Lv. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenming Lv, MTg3MTg1NjEwQHFxLmNvbQ==
 Wenming Lv
Wenming Lv