- ICMR-Regional Medical Research Centre, Bhubaneswar, Odisha, India
Introduction: Osteoarthritis is a leading cause of chronic pain and reduced mobility, especially in weight-bearing joints such as the knees and hips. While physical limitations associated with knee and/or hip osteoarthritis (KHOA) are well-documented, increasing attention is being paid to its impact on sleep disturbances and overall sleep quality. Understanding the extent and nature of these sleep-related issues is essential for the holistic management of knee and/or hip osteoarthritis. This study aims to synthesize current evidence on sleep disturbances and sleep quality in individuals diagnosed with knee and/or hip osteoarthritis.
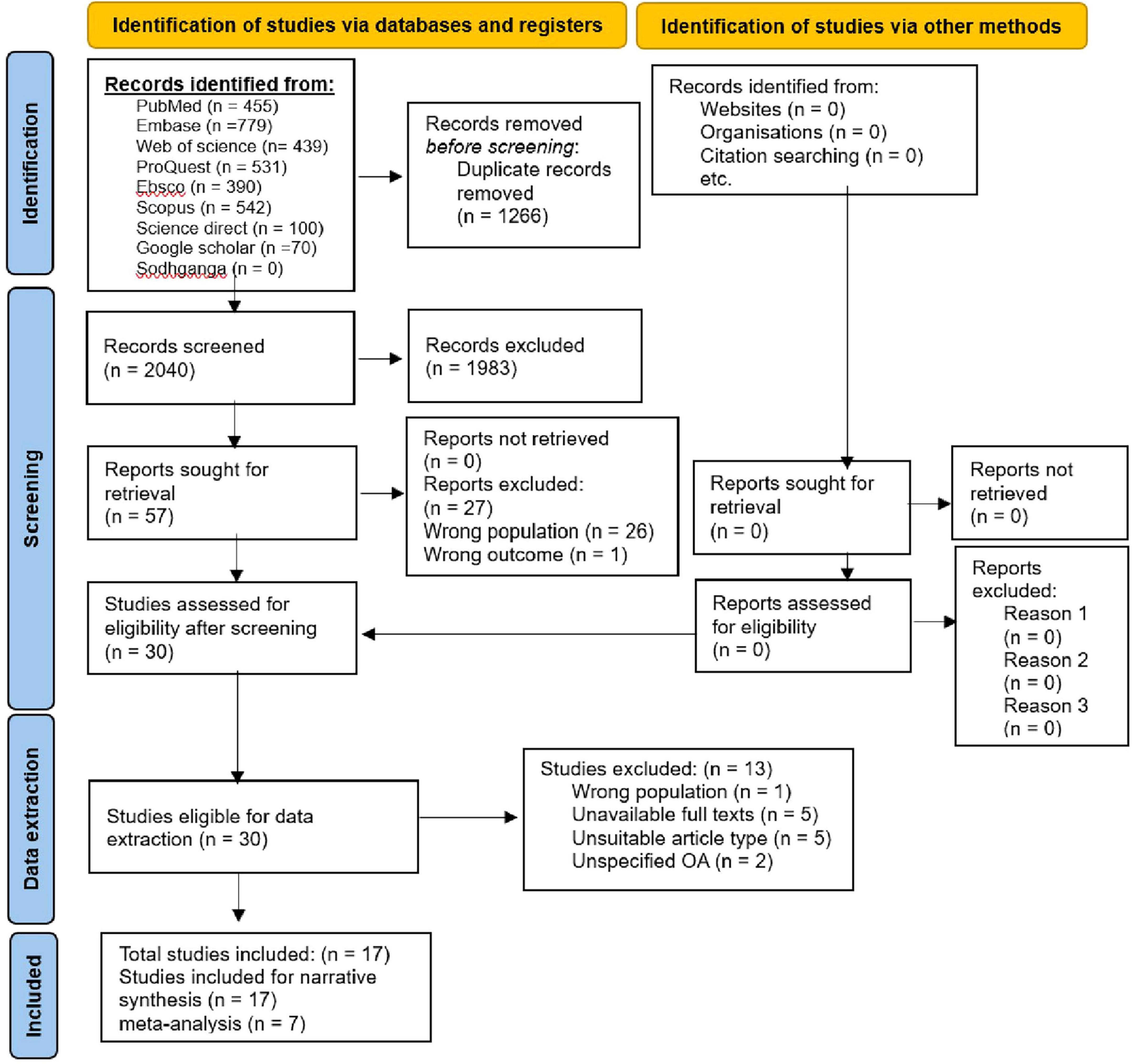
Methods: A systematic review and meta-analysis were conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Comprehensive searches were performed across multiple electronic databases, including Medline via PubMed, EMBASE, CINAHL via EBSCO, Scopus, Web of Science, and ScienceDirect. Additionally, gray literature was sourced through Google Scholar, ProQuest, and Sodhaganga. Studies focused on sleep quality, disturbances, and related factors among individuals with KHOA were screened. Finally, 17 articles were included in the final analysis.
Results: Depression and elevated pain levels emerged as prominent contributors to sleep disturbances in individuals with KHOA. The meta-analysis revealed a pooled effect size of 8.53 (95% CI: 7.18–9.87) in Pittsburgh Sleep Quality Index (PSQI) scores, indicating significantly poorer sleep quality among patients with knee and/or hip osteoarthritis compared to healthy controls (p < 0.0001). However, there was substantial heterogeneity across studies (I2 = 94.96%).
Conclusion: This study highlights that individuals with knee and/or hip osteoarthritis experience significantly impaired sleep quality, primarily driven by pain and psychological factors. The findings underscore the need for integrated clinical approaches that address not only the physical symptoms of OA but also its broader impact on sleep and mental health.
Systematic review registration: [https://www.crd.york.ac.uk/prospero/], identifier [CRD42024547589].
1 Introduction
Osteoarthritis (OA) contributes to persistent pain and reduced mobility, particularly affecting weight-bearing joints, such as the knees and hips (1–3). It is characterized by cartilage degeneration, leading to joint stiffness, pain, and functional limitations (1). Disrupted sleep is a frequently reported comorbidity in individuals with OA, significantly impacting their quality of life (3, 4) by altering the body’s homeostasis (5). Sleep disturbances are directly proportional to worse pain outcomes (6) and act as a catalyst for pain induction and the creation of new illnesses, such as memory deficit and cognitive impairments, etc., (7). Studies suggest a reciprocal relationship between sleep and OA, where pain can disrupt sleep, and poor sleep quality can exacerbate pain perception (8). The current factsheet evidences a 113% increase in OA cases from 1990 to 2019, accounting for about 528 million cases globally, of which 73% of the population belongs to the more than 55 years age group (9), with 365 million knee OA sufferers, followed by hip (10, 11). There is a future prediction of a rise in OA until 2050, which will increase the global health burden (12). OA, along with sleep disturbances, results in a double health burden with functional limitations, psychosocial dysfunction, and increased healthcare utilization, imposing significant economic cost to society (13, 14).
Understanding the relationship between sleep disturbances/quality and knee and/or hip osteoarthritis (KHOA) in individuals with KHOA is crucial for developing effective management strategies. There is a lack of evidence regarding sleep assessment in knee and hip OA till now (15). Secondly, most studies merged different classifications of arthritis pain into a unified category against personalized effects on sleep. Thirdly, there is a lack of current publications focusing on specific joint affections instead of symptomatic OA (16). To date, there is no systematic review on the prevalence and assessment of sleep disturbances and sleep quality in individuals diagnosed with knee and hip OA, which is a matter of concern carrying the utmost significance. This systematic review assesses the existing scientific literature on this topic comprehensively and offers crucial insights to inform clinical practice and guide future research efforts in improving sleep for individuals with KHOA.
1.1 Objectives
• To estimate the extent of sleep disturbances in individuals with KHOA.
• To identify factors influencing sleep disturbances in individuals KHOA.
• To assess various aspects of sleep quality, including type, pattern, duration, onset time, and efficiency.
2 Materials and methods
Adhering to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (17, 18), this systematic review and meta-analysis were performed to evaluate and consolidate information on sleep issues and quality in KHOA (PRISMA 2020 checklist in Supplementary Table 1). Before completing the main search, the protocol was documented and registered in the PROSPERO database under the registration number CRD42024547589.
2.1 Study selection criteria
Inclusion criteria:
• Studies investigating sleep disturbances or various aspects of sleep quality in patients with KHOA
• Availability of full-text articles
• Articles published in English within the past 20 years (2004–2024)
Exclusion criteria:
• Case reports, case series, reviews (including literature, scoping, systematic, and umbrella reviews), editorials, conference proceedings, policy briefs, letters, reports, synopses, and policy or program documents that do not provide evidence-based information on sleep issues and quality in KHOA patients
• Studies focusing on osteoarthritis in joints other than the knee or hip
• Studies that do not evaluate sleep disturbances, sleep quality, or interventions aimed at improving sleep in KHOA patients
2.2 Eligibility criteria
Population:
• Individuals of all age groups
• Clinically diagnosed KHOA as confirmed by a physician
• Self-reported KHOA patients with validated radiological or computed tomography (CT) scan findings
Exposure:
• Contributors to sleep disturbances such as pain, medication usage, psychological factors, and physical activity levels
• Strategies and interventions for improving sleep quality in individuals with KHOA
Outcomes:
• Prevalence of sleep disturbances concerning KHOA as primary outcomes
• Factors associated with sleep issues in individuals with KHOA
• Variations in sleep quality, including sleep onset, duration, efficiency, latency, types, and patterns
2.3 Search strategy
A systematic search was performed across electronic databases, including Medline via PubMed, CINAHL via EBSCO, EMBASE, Scopus, ScienceDirect, and Web of Science. The structure of the search strategy was prepared in accordance with the research question’s requirements and the specific formats required by the databases. Additionally, gray literature was explored using Google Scholar, ProQuest, and Sodhganga.
“Sleep disorders,” “sleep deprivation,” “sleep disturbance,” “sleep variation,” “sleep quality,” “sleep quantity,” “sleep wake disorders,” “circadian rhythm,” “sleep disorders, circadian rhythm,” “osteoarthritis,” “knee osteoarthritis,” and “hip osteoarthritis” have been applied as search terms. The comprehensive search strategy for the above databases is available in Supplementary Table 1.
2.4 Study screening and selection
Once the data and references were collected, they were converted into multiple formats, such as CSV and RIS. The studies were then imported into the Rayyan QCRI software to identify and remove duplicates. The screening process was performed in two stages: first, an initial title and abstract screening, followed by a full-text review.
During the title-abstract screening, three independent reviewers evaluated the studies based on predefined inclusion and exclusion criteria. Any conflicts were resolved through discussion with a third reviewer. Full-text articles that satisfy the inclusion criteria were then thoroughly assessed by two impartial reviewers. Disagreements arose regarding the eligibility of certain studies, prompting a third consulting reviewer to intervene and facilitate a resolution.
A record of excluded articles from the full-text screening was maintained, along with documented reasons for exclusion based on eligibility criteria. Relevant studies were categorized according to study design, osteoarthritis, and sleep components, and then systematically organized into a tabulated excel sheet.
2.5 Data extraction
A pre-prepared pilot standard coding form containing the study type, study setting, study period, sample size, publication year, authors, osteoarthritis assessment criteria/tool, and sleep-related judgment criteria, was utilized for significant data extraction from included studies for evidence synthesis concerning the impact of KHOA on sleep including the overall objective. The literature screening process and results are provided through the PRISMA flowchart (Figure 1).
The data extraction sheet included study characteristics, participant characteristics, KHOA assessment tools, sleep assessment methods, outcomes related to sleep disturbances and sleep quality, main findings and conclusions.
2.6 Study of quality appraisal to assess risk of bias
Quality appraisal was carried out independently by two reviewers. The risk of bias was assessed in accordance with the Joanna Briggs Institute (JBI) guidelines. (19)
2.7 Statistical method
A narrative synthesis was conducted in accordance with the Synthesis Without Meta-Analysis (SWiM) guidelines (20) to examine sleep disturbances related to KHOA. Data have been analyzed using RStudio (version 2024 12.0 + 467) software. Both non-continuous and continuous variables were reported with 95% confidence intervals (CIs), and forest plots were generated. Heterogeneity across studies was evaluated using I2 statistics and the Q-test. When the P-value was ≥ 0.10 and I2 < 50%, indicating homogeneity, a fixed-effects model was applied. Conversely, when the P-value was < 0.10 and I2 ≥ 50%, suggesting significant heterogeneity, a random-effects model was utilized. A P-value less than 0.05 is considered statistically significant. A sensitivity analysis was conducted to determine the extent of variation in the pooled effect size across various studies by systematically removing individual studies. Subgroup analysis could not be performed due to limited data for comparisons and the presence of extreme heterogeneity.
2.8 Risk of bias assessment
After applying the JBI critical appraisal tool, studies were classified as low quality if they achieved a score below 50%, moderate quality if they achieved a score between 50% and 70%, and high quality if they scored above 70%.
3 Results
A total of 3,306 relevant articles were retrieved. Following deduplication, 2,040 articles were screened. After the title-abstract review, 30 articles met the inclusion criteria. Following a full-text review, 13 articles were excluded, including five due to unavailability of full texts, five with unsuitable article types, and three that did not specify the type of osteoarthritis or focused on conditions other than knee or hip osteoarthritis. Ultimately, 17 articles were included in this study.
3.1 Study characteristics
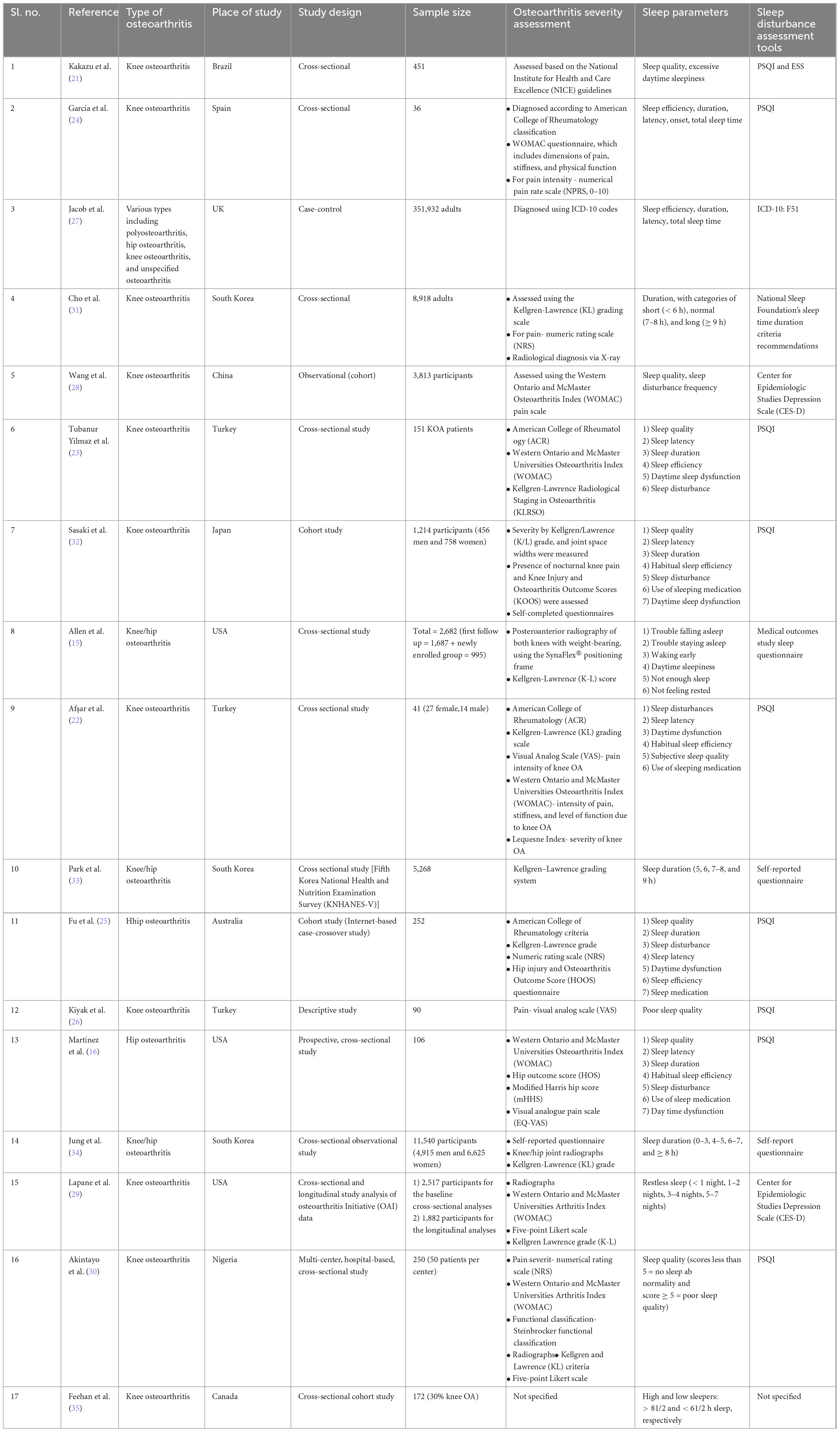
The study characteristics demonstrated that all articles fell within the medium to high quality range. The included studies consisted of one case-control study, two cohort studies, and 14 cross-sectional studies. The studies covered cases from Brazil, Spain, China, the United Kingdom, Japan, Australia, Nigeria, and Canada (each with one study), South Korea (three studies), the United States, and Turkey (each with three studies).
Knee and/or hip osteoarthritis diagnosis and severity were determined using various criteria, including the National Institute for Health and Care Excellence (NICE) guidelines (21), American College of Rheumatology classification (22–26), International Classification of Diseases- Tenth revision (ICD-10) codes (27), and the Western Ontario and McMaster Osteoarthritis Index (WOMAC) questionnaire (16, 22–24, 28–30), which assesses pain, stiffness, and physical function. Additional measures included the Numerical Pain Rating Scale (NPRS, 0–10) for pain intensity (24, 25, 30, 31), Kellgren/Lawrence (K/L) grading for severity (15, 22, 23, 25, 29, 31–34), Knee Injury and Osteoarthritis Outcome Score (KOOS) (32), Lequesne Index for knee OA severity, Visual Analog Scale (VAS) (22, 26) for knee OA pain intensity, Hip Outcome Score (HOS) (16), modified Harris Hip Score (mHHS) (16), Steinbrocker functional classification, Hip Injury and Osteoarthritis Outcome Score (HOOS) questionnaire (25), and radiographic assessments.
The Pittsburgh Sleep Quality Index (PSQI) is a widely accepted and frequently used questionnaire for assessing sleep quality, completed subjectively by patients. A higher PSQI score indicates poorer sleep quality. Sleep disturbance assessment relied on the PSQI questionnaire in nine studies (16, 21–26, 30, 32), the Epworth Sleepiness Scale (ESS) in one study (21), the National Sleep Foundation’s sleep duration criteria in one study (31), the Medical Outcomes Study sleep questionnaire in one study (15), the ICD-10 disease code in one study (27), other self-assessment questionnaires in two studies (33, 34), and the Center for Epidemiologic Studies Depression Scale (CES-D) in two studies (28, 29). One study did not specify the sleep assessment tool used. The PSQI provides a global sleep score in addition to six sub-scores assessing sleep disturbance, sleep latency, sleep duration, subjective sleep quality, habitual sleep efficiency, use of sleep medications, and daytime somnolence, with individual domain scores from 0 to 3. Higher scores indicate poorer performance in that category.
Osteoarthritis diagnosis and sleep assessment tools were not specified clearly in one study by Feehan et al. (35).
Seven studies reported sleep disturbances or poor sleep quality among KHOA patients using various measures. The occurrence of sleep disturbances is higher in individuals with OA. All studies found a significant association between sleep quality and KHOA, indicating that greater KHOA severity is linked to poorer sleep quality. Four studies explicitly stated in their results that more than 55% of KHOA patients had a PSQI score of ≥ 5. Six studies identified additional influencing factors, including anxiety, depression, high pain scores, greater KHOA severity, and comorbidities. Regarding sleep disorders, one study reported that 20% of patients had obstructive sleep apnea (OSA), while another study found that 71% experienced a recent sleep problem, 56% reported having insomnia, and 56% reported inadequate sleep.
3.2 Quality appraisal to assess the risk of bias
The assessment was conducted using the critical appraisal tools from the JBI (19), as required (Supplementary Table 2). The quality assessment of the included articles revealed that cross-sectional studies received scores ranging from 5 to 8 out of 8, indicating moderate to high quality. Cohort studies received scores ranging from 8 to 11 out of 11, reflecting high-quality research. Similarly, case-control studies attained a perfect score of 10 out of 10, confirming their high quality. Table 1 represents the characteristics of the included studies of this systematic review.
3.3 Meta-analysis
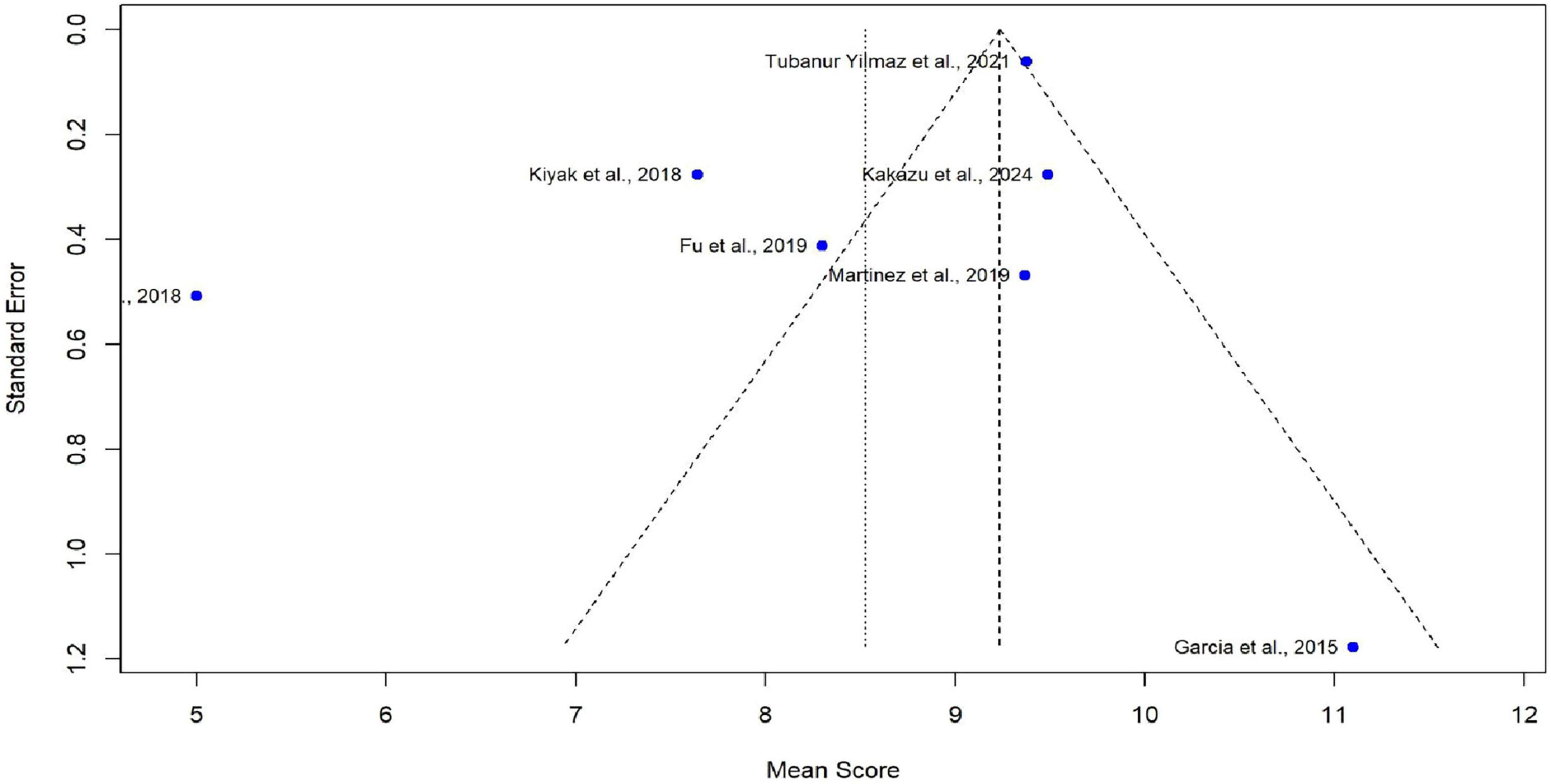
Meta-analysis was conducted using seven studies that employed a common sleep assessment tool, with PSQI score as the outcome measure for KHOA patients, reported in terms of mean and standard deviation (Figures 2–4). The pooled mean PSQI score among individuals with KHOA was 8.53, which is above the standard cutoff of 5, indicating poor sleep quality. In other words, the average PSQI score among KHOA patients was 8.53, which exceeds the established threshold of 5 for poor sleep quality. So, we can be 95% confident that the true effect size lies within the interval 7.18–9.87, indicating a statistically significant overall effect. The relatively narrow confidence interval suggests a precise estimate with a low likelihood of results occurring by chance. Heterogeneity analysis revealed substantial variability across studies, with an I2 of 94.96%, indicating extremely high heterogeneity. A p-value < 0.0001 and H2 = 39.31 further confirm considerable heterogeneity. The estimated level of variance (τ2 = 3.0317) across studies justified the application of the random-effects model in the meta-analysis. The forest plot showed that Afsar et al. (22) had the lowest mean score (5.00), while Alburquerque-García et al. (24) had the highest (11.10). The funnel plot indicated an outlier in the lower-right region Alburquerque-García et al. (24), suggesting potential true heterogeneity due to variations in study populations. A leave-one-out sensitivity analysis was performed to evaluate the robustness of a meta-analysis systematically and to identify whether a single study had a disproportionate influence on the overall results. Ggplot showed that removing Afşar et al. (22), Alburquerque-García et al. (24) significantly impacted the effect size, increasing it to approximately 9.0 or decreasing it to around 8.2, respectively, highlighting heterogeneity in the overall estimate. This high heterogeneity (I2 = 94.96%) makes interpretation of the pooled estimate more challenging. Several factors may explain this variability. First, differences in study populations could have contributed; some studies included younger patients, while others had older or more severe cases, which can affect sleep patterns. Second, cultural and regional differences in sleep habits and healthcare access may have influenced the reporting of PSQI. Third, although all studies used PSQI, the application of the tool may have varied (for example, in cut-off thresholds, versions used, or interviewer versus self-administered assessments). Fourth, differences in study design and sample size may also contribute to variability. Finally, co-morbidities such as depression, obesity, or medication use were inconsistently reported across studies and might explain variations in sleep quality.
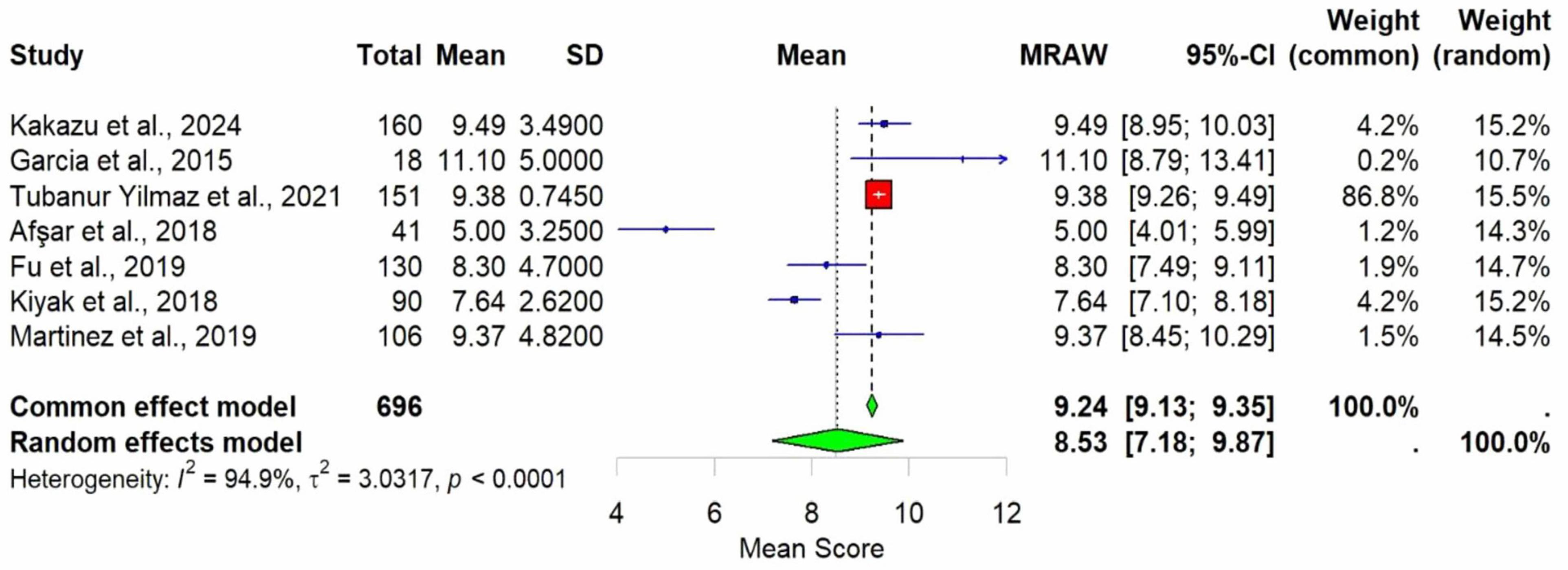
Figure 2. Forest plot of poor sleep among KHOAa patients based on PSQIb scores. Mean is used as an effect size.
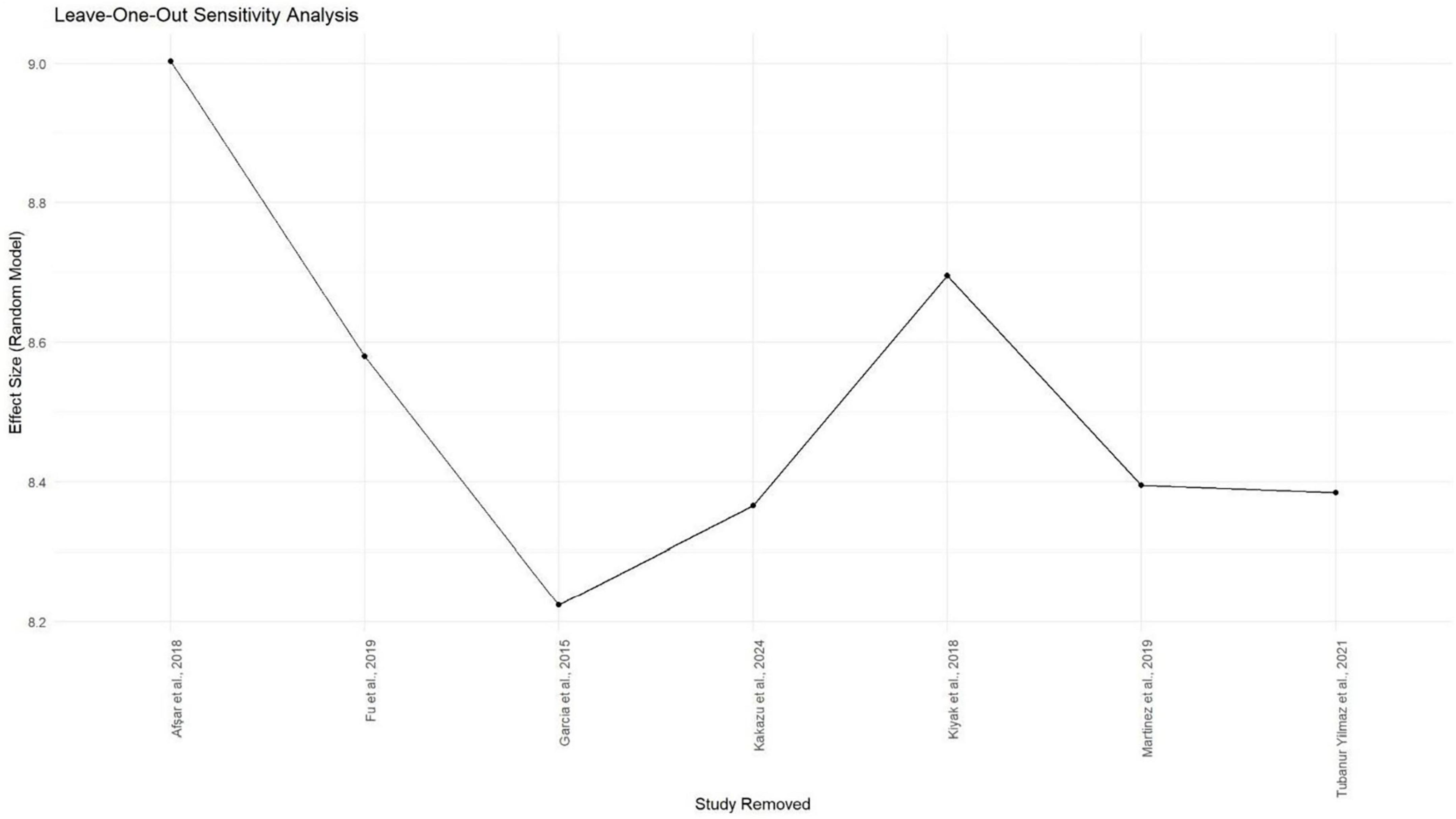
Figure 4. Gg plot depicting the impact of individual studies on the PSQIa mean score effect size through the random effect model.
Even though subgroup analysis was not feasible due to the small number of studies, a narrative interpretation suggests that both clinical and methodological diversity are likely sources of heterogeneity. This indicates that while the pooled effect supports an overall trend of poor sleep in KHOA patients, caution is warranted in generalizing the exact magnitude of the effect. These findings underscore the need for future research with more standardized study designs and better control for confounding variables, which will be critical to refining our understanding of sleep problems in KHOA and guiding targeted clinical interventions.
4 Discussion
As previously defined, this study aims to provide a comprehensive understanding of the prevalence and characteristics of sleep disturbances and sleep quality in KHOA individuals, as well as the impact of KHOA on sleep patterns. It also examines the contributing factors and sleep assessment tools used in these evaluations. The findings indicate that KHOA individuals have significantly poorer sleep quality and a higher risk of sleep disturbances compared to the healthy population. Based on PSQI scores, they demonstrate lower subjective sleep quality, longer sleep latency, shorter sleep duration, and a higher prevalence of sleep disorders. Nevertheless, no significant differences were observed in the use of sleep medications, sleep efficiency, daytime dysfunction, or reliance on sleeping aids. Additionally, KHOA patients have a higher likelihood of experiencing unhealthy sleep durations, including both insufficient and excessive sleep, compared to healthy individuals.
Our systematic review confirms previous findings that patients with OA have a higher likelihood of experiencing sleep disturbances (36, 37). Recognizing the gap in sleep data within knee OA trials, the authors stressed the need for formal sleep assessments to evaluate their impact on treatment outcomes, leading to this systematic review (8). Evidence suggests that individuals with KHOA are prone to daytime lethargy and sleepiness, largely attributed to poor sleep quality, nocturnal pain, and other contributing factors (16, 38). However, we found no considerable difference in daytime dysfunction between KHOA individuals and the healthy population.
Findings from the PSQI in these studies revealed considerable variability in total sleep duration among KHOA patients, with a pronounced tendency toward insufficient sleep rather than excessive sleep (16, 25).
Pain, inflammation, depression, and anxiety are frequently linked to short sleep duration and reduced sleep quality in KHOA patients (39). Research revealed that targeted interventions, including cognitive-behavioral therapy, effective pain management, and mind-body practices like yoga, can help alleviate sleep disturbances in OA patients by addressing these key contributing factors (40–42).
Martinez et al. (16) reported that 20% of patients with symptomatic hip osteoarthritis had a history of OSA, highlighting a clinically significant overlap likely due to shared risk factors and effects on pain and function (16). Evidence from previous studies suggests that OSA is considerably more common among OA patients than among the general population, occurring in 66% of OA patients compared to 17% of the general population (43, 44). Both conditions are strongly associated with obesity, aging, and systemic inflammation, likely contributing to their co-occurrence. Hip OA pain disrupts sleep architecture, resulting in delayed sleep onset, reduced sleep efficiency, and daytime fatigue, while poor sleep further amplifies pain sensitivity, creating a self-perpetuating cycle. OSA-induced intermittent hypoxia elevates inflammatory mediators, including IL-6 and TNF-alpha, which sensitize nociceptive pathways and exacerbate functional impairment such as reduced physical activity, increased fatigue, and poorer WOMAC scores, an independent predictor of poor sleep. Screening for OSA enables targeted interventions, such as continuous positive airway pressure (CPAP) therapy, which can improve sleep, alleviate pain, and enhance postoperative recovery, underscoring the value of a holistic, multidisciplinary approach to OA management (45).
Furthermore, a bidirectional relationship exists between KHOA and sleep disturbances, where poor sleep can result in the onset or worsening of KHOA symptoms (29). This cyclical interaction is influenced by pain, inflammation, psychological distress, and neurobiological factors. KHOA-related joint pain often reduces physical activity, further disrupting sleep patterns. Nocturnal pain, inflammation, psychological distress, and coexisting sleep disorders contribute to fragmented and poor-quality sleep. Inadequate sleep increases pain sensitivity, promotes inflammation, accelerates cartilage degeneration, and disrupts metabolism, ultimately driving OA progression. As OA symptoms further disrupt sleep, the resulting sleep disturbances, in turn, exacerbate OA, creating a self-perpetuating cycle of disease worsening.
In a few of the included studies, BMI was either not reported or, when available (27), its potential impact on sleep quality was not assessed. Although obesity was occasionally identified as a comorbidity, it was generally mentioned only superficially and not examined in relation to BMI as a contributing factor to impaired sleep. Furthermore, in OA, excessive mechanical loading and activity-induced synovitis during daily activities contribute to knee pain, which in turn adversely affects sleep quality.
4.1 Strength and limitations
This is the inaugural systematic review focused on mapping the existing literature and assessing sleep appraisal in KHOA, a critical and significant area of concern, by analyzing data from 17 studies. Our review confirms a significant positive correlation between sleep disturbances/quality and osteoarthritis concerning KHOA. The first limitation is that only studies focusing on knee and/or hip OA are included to assess their impact on sleep quality, while other types of osteoarthritis are excluded. Additionally, the search is restricted to studies published within the last 20 years, potentially omitting relevant findings from earlier years. Furthermore, limiting the analysis to English-language studies may exclude valuable insights available in other languages. Further research should explore sleep-specific interventions for individuals with KHOA and their potential effect on disease activity and overall quality of life. Many of the studies used self-reported questionnaires, such as the PSQI, ESS, and CES-D, with very few employing objective measures like polysomnography or other formal sleep studies. This is particularly important because self-reported data are inherently subject to bias and may not accurately reflect actual sleep patterns or disturbances.
5 Conclusion and recommendation
This review evidences the current status of sleep, addressing the gaps in sleep assessment among KHOA individuals, thereby finding a better way to combat the bifold struggle with sleep dysfunction and most popular, degenerative joint disorder OA, knee and hip specific to people in declining years. Pain, inflammation, comorbidities, depression, and anxiety were contributing factors for substandard sleep in KHOA individuals. Our meta-analysis confirms the significant association between lower sleep quality and KHOA individuals compared to the healthy population. This included poor sleep quality, longer sleep latency, and a higher risk of unhealthy sleep duration, such as insufficient sleep or excessive sleep. This underscores the need for targeted interventions to manage pain and mental health in order to improve sleep outcomes. Therefore, national and clinical guidelines for osteoarthritis management should incorporate routine screening for sleep disturbances, including assessments of insomnia and sleep quality, as a standard component of care. Globally, sleep health should be systematically integrated into chronic disease care models through ongoing national health programs to enhance patient outcomes, improve quality of life, and ensure more comprehensive, person-centered care. In the future, additional large-scale prospective studies are essential to further support patient management and treatment.
Data availability statement
The original contributions presented in this study are included in this article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
AP: Conceptualization, Methodology, Software, Validation, Formal analysis, Resources, Data curation, Writing – original draft, Writing – review & editing, Supervision. PS: Conceptualization, Methodology, Validation, Formal analysis, Data curation, Writing – original draft, Writing – review & editing. SM: Methodology, Data curation, Writing – review & editing. RD: Methodology, Data curation, Writing – review & editing. JS: Methodology, Data curation, Writing – review & editing. SP: Software, Resources, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that Generative AI was used in the creation of this manuscript. Generative AI technology was used solely for language editing. The authors reviewed and verified all AI-assisted outputs to ensure accuracy and originality.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1653047/full#supplementary-material
References
1. Safiri S, Kolahi A, Smith E, Hill C, Bettampadi D, Mansournia M, et al. Global, regional and national burden of osteoarthritis 1990-2017: a systematic analysis of the Global burden of disease study 2017. Ann Rheum Dis. (2020) 79:819–28. doi: 10.1136/annrheumdis-2019-216515
2. Ni J, Zhou W, Cen H, Chen G, Huang J, Yin K, et al. Evidence for causal effects of sleep disturbances on risk for osteoarthritis: a univariable and multivariable Mendelian randomization study. Osteoarthritis Cartilage. (2022) 30:443–50. doi: 10.1016/j.joca.2021.11.021
3. Conaghan P, Birrel F, Porcheret M, Doherty M, Dziedzic K, Bernstein I, et al. Osteoarthritis. London: National Clinical Guideline Centre (2014). p. 1–498.
4. Duo L, Yu X, Hu R, Duan X, Zhou J, Wang K. Sleep disorders in chronic pain and its neurochemical mechanisms: a narrative review. Front Psychiatry. (2023) 14:1157790. doi: 10.3389/fpsyt.2023.1157790
5. Frange C, Hachul H, Hirotsu C, Tufik S, Andersen M. Temporal analysis of chronic musculoskeletal pain and sleep in postmenopausal women. J Clin Sleep Med. (2019) 15:223–34. doi: 10.5664/jcsm.7622
6. Saconi B, Polomano R, Compton P, McPhillips M, Kuna S, Sawyer A. The influence of sleep disturbances and sleep disorders on pain outcomes among veterans: a systematic scoping review. Sleep Med Rev. (2021) 56:101411. doi: 10.1016/j.smrv.2020.101411
7. Rodrigues T, Shigaeff N. Sleep disorders and attention: a systematic review. Arq Neuropsiquiatr. (2022) 80:530–8. doi: 10.1590/0004-282X-ANP-2021-0182
8. Feda J, Miller T, Young J, Neilson B, Rhon D. Measures of sleep are not routinely captured in trials assessing treatment outcomes in knee osteoarthritis - a scoping systematic review and call to action. Osteoarthr Cartil Open. (2023) 5:100400. doi: 10.1016/j.ocarto.2023.100400
9. IHME. VizHub - GBD Results. (2024). Available online at: https://vizhub.healthdata.org/gbd-results/ (accessed May 17, 2024).
10. World Health Organization. Osteoarthritis. (2024). Available online at: https://www.who.int/news-room/fact-sheets/detail/osteoarthritis (accessed May 17, 2024)
11. Long H, Liu Q, Yin H, Wang K, Diao N, Zhang Y, et al. Prevalence trends of site-specific osteoarthritis from 1990 to 2019: findings from the Global burden of disease study 2019. Arthritis Rheumatol. (2022) 74:1172–83. doi: 10.1002/art.42089
12. Steinmetz J, Culbreth G, Haile L, Rafferty Q, Lo J, Fukutaki K, et al. Global, regional, and national burden of osteoarthritis, 1990–2020 and projections to 2050: a systematic analysis for the Global burden of disease study 2021. Lancet Rheumatol. (2023) 5:e508–22. doi: 10.1016/S2665-9913(23)00163-7
13. Neogi T. The epidemiology and impact of pain in osteoarthritis. Osteoarthritis Cartilage. (2013) 21:1145–53. doi: 10.1016/j.joca.2013.03.018
14. Parmelee P, Tighe C, Dautovich N. Sleep disturbance in osteoarthritis: linkages with pain, disability, and depressive symptoms. Arthritis Care Res. (2025) 67:358–65. doi: 10.1002/acr.22459
15. Allen K, Renner J, Devellis B, Helmick C, Jordan J. Osteoarthritis and sleep: the Johnston county osteoarthritis project. J Rheumatol. (2024) 35:1102–7.
16. Martinez R, Reddy N, Mulligan E, Hynan L, Wells J. Sleep quality and nocturnal pain in patients with hip osteoarthritis. Medicine. (2019) 98:e17464. doi: 10.1097/MD.0000000000017464
17. Page M, McKenzie J, Bossuyt P, Boutron I, Hoffmann T, Mulrow C, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
18. PRISMA. PRISMA 2020 checklist — PRISMA statement. (2025). Available online at: https://www.prisma-statement.org/prisma-2020-checklist (accessed March 6, 2025)
19. JBI. JBI Critical Appraisal Tools | JBI. (2025). Available online at: https://jbi.global/critical-appraisal-tools (accessed February 4, 2025).
20. Campbell M, McKenzie J, Sowden A, Katikireddi S, Brennan S, Ellis S, et al. Synthesis without meta-analysis (SWiM) in systematic reviews: reporting guideline. BMJ. (2020) 368:l6890. doi: 10.1136/bmj.l6890
21. Kakazu V, Pinto R, Dokkedal-Silva V, Fernandes G, Araujo C, Pires G, et al. Are sleep quality, daytime sleepiness, and depression associated with knee pain? A cross-sectional study in older adults. Sleep Sci. (2024) 18:e91–6. doi: 10.1055/s-0044-1787528
22. Afsar I, Bölük H, Özen S. Relationship Among the Symptom Severity of Knee Osteoarthritis, Quality of Life and Sleep Quality Diz Osteoartriti Semptom Şiddeti, Yaşam Kalitesi ve Uyku Kalitesi Arasındaki Ýlişki [Relationship between the symptom severity of knee osteoarthritis, quality of life and sleep quality]. Ankara Med J. (2018) 18:519–46. doi: 10.17098/amj.497329 Turkish.
23. Mercan Başpinar M, Yilmaz T, Güneş Arslan N, Yilmaz Yalçinkaya E, Basat O. FİZİKSEL TIP VE REHABİLİTASYON BİLİMLERİ DERGİSİ is there an effect of pain and radiological stage on quality of life and sleep in patients with osteoarthritis? J Phys Med Rehabil Sci. (2021) 24:97–106. doi: 10.31609/jpmrs.2020-77436
24. Alburquerque-García A, Rodrigues-de-Souza D, Fernández-de-las-Peñas C, Alburquerque-Sendín F. Association between muscle trigger points, ongoing pain, function, and sleep quality in elderly women with bilateral painful knee osteoarthritis. J Manipulative Physiol Ther. (2015) 38:262–8. doi: 10.1016/j.jmpt.2014.10.018
25. Fu K, Makovey J, Metcalf B, Bennell K, Zhang Y, Asher R, et al. Sleep quality and fatigue are associated with pain exacerbations of hip osteoarthritis: an internet-based case-crossover study. J Rheumatol. (2019) 46:1524–30. doi: 10.3899/jrheum.181406
26. Kuralay C, Kiyak E. Sleep quality and factors affecting patients with knee osteoarthritis. Int J Caring Sci. (2018) 11:1141.
27. Jacob L, Smith L, Konrad M, Kostev K. Association between sleep disorders and osteoarthritis: a case-control study of 351,932 adults in the UK. J Sleep Res. (2021) 30:e13367. doi: 10.1111/jsr.13367
28. Wang Y, Li X, Zhang Y, Ma Y, Xu S, Shuai Z, et al. Association of sleep disturbance with catastrophizing and knee pain: data from the osteoarthritis initiative. Arthritis Care Res. (2023) 75:2134–41. doi: 10.1002/acr.25127
29. Lapane K, Shridharmurthy D, Harkey M, Driban J, Dubé C, Liu S. The relationship between restless sleep and symptoms of the knee: data from the Osteoarthritis Initiative. Clin Rheumatol. (2021) 40:2167–75. doi: 10.1007/s10067-020-05531-4
30. Akintayo R, Yerima A, Uhunmwangho C, Olaosebikan H, Akpabio A. Tossing and turning with degenerative arthropathy: an assessment of poor sleep quality in knee osteoarthritis. Reumatologia. (2019) 57:207–13. doi: 10.5114/reum.2019.87615
31. Cho Y, Jung B, Lee Y, Kim M, Kim E, Sung W, et al. Association between sleep duration and osteoarthritis and their prevalence in Koreans: a cross-sectional study. PLoS One. (2020) 15:e0230481. doi: 10.1371/journal.pone.0230481
32. Sasaki E, Tsuda E, Yamamoto Y, Maeda S, Inoue R, Chiba D, et al. Nocturnal knee pain increases with the severity of knee osteoarthritis, disturbing patient sleep quality. Arthritis Care Res. (2014) 66:1027–32. doi: 10.1002/acr.22258
33. Park H, Kwon Y, Kim H, Lee Y. Relationship between sleep duration and osteoarthritis in middle-aged and older women: a nationwide population-based study. J Clin Med. (2019) 8:356. doi: 10.3390/jcm8030356
34. Jung J, Seok H, Choi S, Bae J, Lee S, Lee M, et al. The association between osteoarthritis and sleep duration in Koreans: a nationwide cross-sectional observational study. Clin Rheumatol. (2018) 37:1653–9. doi: 10.1007/s10067-018-4040-3
35. Feehan L, Lu N, Xie H, Li L. Twenty-four hour activity and sleep profiles for adults living with arthritis: habits matter. Arthritis Care Res. (2020) 72:1678–86. doi: 10.1002/acr.24424
36. Ho K, Ferreira P, Pinheiro M, Aquino Silva D, Miller C, Grunstein R, et al. Sleep interventions for osteoarthritis and spinal pain: a systematic review and meta-analysis of randomized controlled trials. Osteoarthritis Cartilage. (2019) 27:196–218. doi: 10.1016/j.joca.2018.09.014
37. Sánchez Romero E, Martínez-Pozas O, García-González M, de-Pedro M, González-Álvarez M, Esteban-González P, et al. Association between sleep disorders and sleep quality in patients with temporomandibular joint osteoarthritis: a systematic review. Biomedicines. (2022) 10:2143. doi: 10.3390/biomedicines10092143
38. Fertelli T, Tuncay F. Fatigue in individuals with knee osteoarthritis: its relationship with sleep quality, pain and depression. Pak J Med Sci. (2019) 35:1040–4. doi: 10.12669/pjms.35.4.383
39. Schepman P, Thakkar S, Robinson R, Malhotra D, Emir B, Beck C. Moderate to severe osteoarthritis pain and its impact on patients in the United States: a national survey. J Pain Res. (2021) 14:2313–26. doi: 10.2147/JPR.S310368
40. Taibi D, Vitiello MV. A pilot study of gentle yoga for sleep disturbance in women with osteoarthritis. Sleep Med. (2011) 12:512–7. doi: 10.1016/j.sleep.2010.09.016
41. Smith M, Finan P, Buenaver L, Robinson M, Haque U, Quain A, et al. Cognitive-behavioral therapy for insomnia in knee osteoarthritis: a randomized, double-blind, active placebo-controlled clinical trial. Arthritis Rheumatol. (2015) 67:1221–33. doi: 10.1002/art.39048
42. Kosinski M, Janagap C, Gajria K, Schein J, Freedman J. Pain relief and pain-related sleep disturbance with extended-release tramadol in patients with osteoarthritis. Curr Med Res Opin. (2007) 23:1615–26. doi: 10.1185/030079907x199493
43. Yang Z, Lv T, Jin L, Lv X, Zhu X, Wang X, et al. The relationship between obstructive sleep apnea and osteoarthritis: evidence from an observational and Mendelian randomization study. Front Neurol. (2024) 15:1425327. doi: 10.3389/fneur.2024.1425327
44. Luo X, Chen M, Xu J. Exploring the role of aging in the relationship between obstructive sleep apnea syndrome and osteoarthritis: insights from NHANES data. Front Med. (2024) 11:1486807. doi: 10.3389/fmed.2024.1486807
Keywords: sleep disturbances, sleep quality, osteoarthritis knee, osteoarthritis hip, systematic review
Citation: Panigrahi A, Sahu P, Mohanty SS, Dandsena RS, Sahani JI and Pati S (2025) Sleep disturbances and sleep quality among individuals diagnosed with osteoarthritis: a systematic review and meta-analysis. Front. Med. 12:1653047. doi: 10.3389/fmed.2025.1653047
Received: 30 June 2025; Accepted: 31 October 2025;
Published: 19 November 2025.
Edited by:
Antonella Fioravanti, ISMH (International Society of Medical Hydrology and Climatology), United KingdomReviewed by:
Jihyun Song, The University of Utah, United StatesAdriana Barni Truccolo, Universidade Estadual do Rio Grande do Sul, Brazil
Copyright © 2025 Panigrahi, Sahu, Mohanty, Dandsena, Sahani and Pati. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ansuman Panigrahi, ZHIuYW5zdW1hbjNAZ21haWwuY29t
†These authors have contributed equally to this work and share first authorship
 Ansuman Panigrahi
Ansuman Panigrahi Priyanka Sahu†
Priyanka Sahu† Swati Sambita Mohanty
Swati Sambita Mohanty Rutuparna Sibani Dandsena
Rutuparna Sibani Dandsena Sanghamitra Pati
Sanghamitra Pati