Abstract
Background:
Prakriti or constitutional typology is the foundation of personalized health care in Ayurveda. Traditionally, Ayurvedic clinicians have assessed Prakriti in a primarily experience-based and often subjective manner. However, in the past few decades attempts to develop objective tools have been made by researchers from multidisciplinary domains. This review aimed to identify existing Ayurvedic Prakriti assessment tools and evaluate their scientific rigor.
Methods:
Aligned with the SANRA framework, our narrative review incorporated systematic elements. A Boolean search in PubMed, Scopus, and Cochrane in November 2024 using (“Prakriti”) AND (“Ayurveda” OR “Ayurvedic”) yielded 635 articles, together with 12 additional articles from citations search. Ninety four studies met the inclusion criteria. Prakriti assessment tools were quantified and evaluated using Scale Development and Validation Framework by Boateng et al., alongside custom set of study quality indicators to assess their methodological rigor.
Results:
Between 1987 and 2024, 64 unique Prakriti assessment tools (PATs) were identified, each using one or more methods to perform data collection and decision-making tasks. Variations in the selection and application of these methods resulted in the development of diverse methodological frameworks for Prakriti assessment. Of the 64 PATs identified, only 20 PATs underwent any form of validation and among them, just two PATs, the CCRAS-PAS software and ACPI scale met seven of the nine recommended criteria. Most tools lacked dimensionality testing, test–retest reliability, contextual validity and were not tested across diverse populations, indicating a high risk of developer-bias. Additionally, 32 categories of measurable correlates to Prakriti have been studied across 94 studies, but only five of them were studied using validated tools.
Conclusion:
Much progress has been made in developing methodology and integrating technology for creating Prakriti assessment tools along with attempts to identifying measurable correlates to Prakriti that could potentially serve as Prakriti biomarkers. Currently no tool fully meets the evaluation criteria of the Scale Development and Validation framework, except CCRAS-PAS and ACPI that show partial readiness and can be refined. Further work is needed to establish Prakriti as a clinically validated measurable construct and to integrate Ayurveda into the domain of personalized health care.
1 Introduction
Ayurveda translates to “Knowledge of life.” It is the traditional knowledge system from India that offers a comprehensive understanding of life, health, longevity, along with the therapeutic aspects. The earliest texts of Ayurveda had attained a high level of systematization by 500 BCE, though the tradition of Ayurveda dates back much earlier (1). The use of Ayurveda is still very prominent in India (2), with a growing global academic interest. Academic training programs in Ayurveda are now offered by dedicated Ayurveda institutions as well as established universities across Europe, United States, Canada, Australia (3). A recent study showed that Ayurveda was preferred for its “natural approach” and “fewer side effects” by patients in the Organization for Economic Cooperation and Development (OECD) countries for managing non-communicable diseases (NCDs) (4).
Personalized treatment is an integral part of Ayurvedic clinical practice (5) which involves implementing multiple therapeutic approaches to treat different people with the same diagnosis. With its culturally-sensitive and holistic approach, Ayurveda also offers better affordability and accessibility (6).
Recent advances in personalized medicine has made it possible to predict disease susceptibility and make early detection through genetic, genomic, and other individual-level profiling (7). It allows physicians to personalize preventive, promotive, and therapeutic strategies using approaches like pharmacogenetics (8) with promising applications in family medicine and primary care. However, personalized medical care remains inaccessible for much of the global population (8). Given the rising burden of diseases (9) and the urgent need for affordable and, accessible primary care, traditional systems like Ayurveda could play a pivotal role.
The Ayurvedic concept of Prakriti is at the core of understanding health, disease, and therapeutic intervention for personalized treatment across the clinical care continuum. While all individuals are composed of the same elemental constituents, Ayurveda emphasizes variation in their configuration, expressed through Deha Prakriti (physical constitution) (10–13) and Manasika Prakriti (mental constitution) (14). This aligns with modern scientific understanding that, despite shared biomolecular and cellular components, individuals differ in genetic, epigenetic, metabolic, and other profiles.
Traditionally, Prakriti was assessed through clinical methods such as Trividha Pariksha (15), using observation, palpation and interrogation; Astavidha Pariksha (16) using pulse, urine, feces, tongue, sound, touch, eyes, physique and other methods. While these methods are foundational, they were inherently subjective, limiting their reproducibility and broader clinical applicability.
Early assessments were typically conducted by a single physician, relying heavily on their individual judgment. This approach lacked clarity regarding the domains and data points that informed the Physician’s final Prakriti classification, making it highly subjective. Over time, efforts to address this subjectivity have led to the adoption of more objective approaches, including the use of pulse assessment, a questionnaire or digital interfaces that standardize data entry, and more advanced computational techniques such as Machine Learning and Computer Vision. Collectively, these developments show a progressive shift from highly subjective to increasingly structured and objective methods, laying the groundwork for the diverse Prakriti assessment tools that exist today.
1.1 Theoretical construct of Prakriti as a measurable clinical parameter
Prakriti in Ayurveda involves constitutional phenotyping, defined as an individual’s inherent psychophysical constitution established at the time of conception (12, 13). Prakriti is considered to remain unchanged (13, 17, 133) across the lifespan and influences an individual’s physical features, physiological responses, psychological tendencies, behavioral patterns, disease susceptibility, and response to medical interventions. The classical texts of Ayurveda have provided 200–250 characteristics. For example, body build, frequency of hunger, skin complexion, sleep patterns, voice characteristics, tolerance to temperature, taste preference, mental temperament, encompassing physical, physiological, psychological and behavioral traits (18). Therefore, Prakriti represents a multidimensional construct comprising both observable traits and latent dimensions that require systematic exploration. Much like the constructs of psychology and personality used in Behavioral medicine, Prakriti also requires measurement through structured instruments.
The physical constitution reflects a unique configuration of the three doshas (Tridosha) of Vata, Pitta, and Kapha (134) resulting in seven primary types. The mental constitution represents the configuration of the three mental attributes (Trigunas) i.e., Sattva, Rajas, and Tamas that are classified into 16 types (14). While both the physical and mental constitution influence psychological traits, the mental constitution also influences moral disposition and spiritual inclinations. Ayurveda conceptualizes health as a dynamic balance between the physical and mental domains, and the root cause of disease is due to the disturbances in the doshas, caused by various factors including the mind. Therefore, though physical and mental constitution are described as conceptually distinct in the classical texts, they exert a combined influence on the mind of the individual. Among the two, the physical constitution has been more widely studied due to its measurable physical attributes.
The classification of physical constitution helps in assessing disease predisposition, customizing diet and lifestyle recommendations, and guiding therapeutic choices, highlighting its alignment with personalized medicine. Its conceptual foundation in Tridosha theory, pool of observable traits, its independence from transient states like disease (Vikriti) allows a trait-based assessment. The stable nature and multidomain expression of an individual’s physical constitution meet key criteria for clinical scale development, with the potential to be translated as a clinically measurable parameter.
However, difficulties in relation to Prakriti tool development exist such as absence of standardized trait definitions, contextual variability (age, geography, season) (10, 132), and the confounding influence of Vikriti (disease), which can obscure baseline traits. For example, the influence of hypothyroidism on the voice characteristics (19).
Over the past three decades, efforts have been made to create a scoring system based on the available 200–250 characteristic traits to make Prakriti a measurable parameter. For example, the physical traits like height and build of an individual have been measured using anthropometric methods such as body mass index (20, 21), image analysis for hair characteristics and facial features (18, 22); physiological functions like bowel health have been measured using microbiome analysis (23–27); psychological traits have been measured using validated scales such as the Big Five Inventory (BFI) to assess personality (28, 29). Additionally, researchers have also attempted to correlate Prakriti classifications with various genetic and genomic factors (30–33), which has led to the advancement of research related to Prakriti assessment.
In this background, several Prakriti assessment tools (PATs) have been developed using various methods. A PAT has two core functions to be performed, data collection and decision-making. Different methods have been used to perform these tasks in a PAT that brings out the variability between the PATs. As highlighted by Bhalerao and Patwardhan, the methodologies and tools used for Prakriti assessment have several issues such as conceptual ambiguities and lack of methodological clarity (34). Recognizing Prakriti as a measurable clinical parameter allows its evaluation through frameworks like that of Boateng et al. (35), which emphasize clear domain definition and identification, structured item analysis and reduction for a rigorous scale development and empirical validation. Positioning Prakriti within such a framework supports its transition from classical diagnostic insight to a reliable, evidence-based tool for personalized care.
Therefore, our study aims to quantify the different tools used for Prakriti assessment and evaluate the validity of these tools. To our knowledge, this is the first review to systematically evaluate PATs using a tool development and validation framework. This represents a first step toward bridging the gap between Prakriti as a foundational Ayurvedic concept and its translation to a clinically measurable parameter.
2 Methodology
This is a narrative review, adhering to the SANRA framework—a scale for the quality assessment of narrative review articles (36) while using elements of the PRISMA flow diagram designed for systematic reviews (37) to present the study selection process. The methodology evolved through multiple stages: formulating review questions, conducting the literature search, selecting articles for the review, charting and data synthesis, and data analysis.
We framed the narrative synthesis based on two questions:
-
What are the different tools available for Prakriti assessment?
-
Do the currently available Prakriti assessment tools adequately meet established standards for tool development and validation?
2.1 Inclusion criteria
-
• Types of research articles included:
-
o Studies focusing on the development, validation, or comparison of assessment tools for the assessment of Prakriti.
-
o Quasi-experimental or experimental clinical studies, scoping review, narrative review, and systematic reviews that involve the assessment of Prakriti were also included.
-
o Original research articles published in journals or conference proceedings.
-
o Abstract-only articles providing relevant details on Prakriti assessment methods and mentioning specific assessment tools.
-
• Language:
-
o Only studies published in English were included in the review.
-
• Population:
-
o Studies conducted on human participants of any age, gender, or ethnicity where Prakriti assessment was performed were eligible.
-
• Quality criteria for article and journal selection:
-
o Compliance with the article reporting guidelines was the basis for inclusion of articles, irrespective of whether the article was published in a non-indexed or indexed journal.
2.2 Exclusion criteria
-
• Relevance:
-
o Studies that lacked identifiable Prakriti assessment tools or methods were excluded.
-
o Articles with insufficient data to contribute to the data synthesis for the review were excluded.
-
• Type of research articles:
-
o Reviews, opinion pieces, commentaries, and book chapters that did not look at Prakriti assessment and did not contribute to the data synthesis of the review were excluded.
-
• Type of study subjects:
-
o Research conducted on animal models or in vitro studies.
-
• Quality criteria:
-
o Articles from gray journals, i.e., journals not indexed in reputed databases such as Scopus, PubMed, or Cochrane Library; journals not published by respectable publishers such as Springer, Nature, Elsevier; journals that made unverifiable claims about its impact factor or editorial board were excluded.
-
o Articles or thesis that have not undergone rigorous peer-review process and not published in the indexed journals such as Scopus, PubMed, or Cochrane Library were also excluded.
2.3 Search strategy
The search was conducted in November 2024. The search strategy aimed to identify published studies exploring the use of tools or methods for Prakriti assessment. Various keyword combinations were tested across three databases—Scopus, PubMed, and Cochrane Library. The study used Boolean operators to refine searches, with no time restriction and the final combination being:
(“Prakriti”) AND (“Ayurveda” OR “Ayurvedic”)
2.4 Study selection process
All identified citations were exported as CSV files, with or without abstracts. A total of 635 records were retrieved through database searches, and 12 papers were added to the pool based on citation search. One hundred and thirty-nine duplicates were identified using pivot tables in Microsoft Excel, which were excluded from the analysis. Of the remaining 508 records screened for eligibility, 350 were excluded based on the exclusion criteria, 61 due to insufficient details, 2 could not be retrieved, and 1 was a retracted study. This brought the total number of included reports to 94 published between 1987 and 2024. We have presented the results using a PRISMA flow diagram (see Figure 1).
Figure 1

PRISMA flow diagram.
2.5 Charting, data synthesis and analysis
A single reviewer reviewed each selected paper. We addressed the single reviewer bias to some extent by using a holistic scoring rubric, to make the data extraction objective (see Supplementary Appendix 1). Any doubts or concerns raised by the reviewer were clarified by the co-authors of this paper.
The data from 94 papers were charted and synthesized to answer the two study objectives. To address the first review question, we looked for identifiable Prakriti assessment tools (PATs). An identifiable tool would have a unique name as given by a researcher or research group like “AyuSoft software” (38) or “Mysore Tridosha Scale” (39). In the absence of a unique name, a name was coined by our study team, based on the method employed by the tool, followed by the researcher’s name and the year it was developed or name of the research group. For example, as part of the CSIR consortium project, a software tool was developed but not formally named; therefore, the term “CSIR software” (33) was coined for this PAT.
For the purpose of this review, each PAT was systematically analyzed with respect to the methods used for (i) data collection and (ii) decision-making. This enabled us to identify the range of approaches adopted for gathering information and the mechanisms through which final Prakriti classifications were derived. Particular attention was given to whether the final classification depended on the physician’s interpretation of collected data or whether it was determined autonomously. This distinction allowed us to examine variations in the degree of subjectivity versus automation across PATs.
Addressing the second review question which to establish the validity and reliability of Prakriti assessment tools, was particularly challenging due to the lack of an established standard for evaluating Prakriti assessment methods and tools. To address this methodological gap, we conducted a targeted literature search to identify the most appropriate framework for evaluation. The search yielded six relevant sources, comprising one book (40) and five journal articles (35, 41–44) focused on scale development, validity, and reliability testing. Among these, the “Scale Development and Validation Framework” by Boateng et al. (35), was selected for this study. We have used the original figure developed by the authors, which provides an overview of the three phases, and nine steps of scale development and validation has been provided (see Figure 2). This framework is frequently cited and emphasizes best practices for developing and validating scales in health, social, and behavioral research. It offers a structured evaluative approach with specific estimates suitable for critically appraising tools designed to measure complex phenomena or constructs like Prakriti.
Figure 2
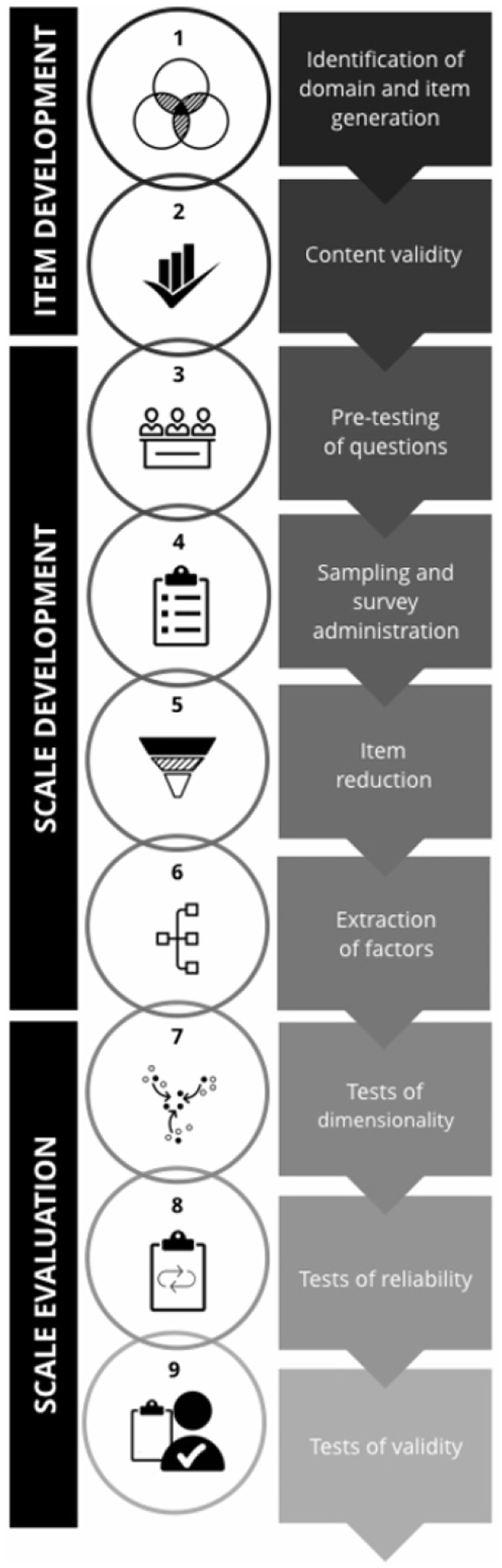
An overview of the three phases and nine steps of scale development and validation. Reprinted from Boateng et al. (35), licensed under CC BY-NC 4.0.
We evaluated the tools and their adherence to the prescribed methodological steps in the framework using the rubric. Each PAT was qualitatively evaluated against the nine steps of the Boateng framework using a scoring rubric (Supplementary Appendix 1); fulfillment of at least one specific analysis or procedure per step (e.g., expert panel review for content validity, EFA/CFA for dimensionality) was considered sufficient to score that step as achieved. If no evidence was reported for a given step, the tool received a score of 0 for that step, whereas tools that provided partial evidence (e.g., only expert panel review without CVR/CVI for content validity) received 1 point, ensuring consistent scoring while accommodating methodological variation across studies.
This approach enabled us to perform a structured, comparative analysis of methodological rigor across tools, providing insight into their robustness. However, it must be noted that the scoring was done purely based on what has been reported in the available literature.
Given the inherent complexity of assessing Prakriti, which is influenced by diverse factors, it was recognized that evaluating the study quality, particularly in terms of sample size was critical for appraising the robustness of assessment tools used in the study. Special emphasis was placed on five key factors considered to influence Prakriti: ethnicity (Jati), family lineage (Kula), age (Vaya), geographic location (Desha), and season (Kala) (10). We also examined the range of study designs employed across the 94 included studies. Collectively, the sample size, the five influencing factors and the type of study design were called as “Study Quality Indicators” for the purpose of this evaluation.
In addition to these, we categorized the measurable correlates that have been studied in relation to Prakriti, including genomic, genetic, physiological, biochemical parameters etc., using various PATs.
We conducted a descriptive analysis of the synthesized data using Pivot Tables in Microsoft Excel and the results have been presented in the following section.
3 Results
We have presented the results through five sub-sections. The first sub-section lists all the identified PATs and classifies them based on technology integration. The second sub-section presents the evaluation of the tool development and validation based on the Boateng et al., framework. The third sub-section shows the analysis using study quality indicators and the fourth sub-section examines the extent to which these tools have been used to investigate measurable correlates to Prakriti.
3.1 Prakriti assessment tools across 94 studies
We identified and cataloged 64 unique Prakriti assessment tools (PATs) (see Table 1).
Table 1
| No. | Year | Tool name |
|---|---|---|
| 1 | 1987 | Q (88) |
| 2 | 2005 | CA (30) |
| 3 | 2008 | CSIR software (33) |
| 4 | 2010 | Q (53) |
| 5 | 2011 | Mysore Tridosha scale software (39) |
| 6 | 2011 | Q (89) |
| 7 | 2011 | SAQ (90) |
| 8 | 2010 | AyuSoft software (38) |
| 9 | 2012 | CCRAS Q (91) |
| 10 | 2012 | CPA and CA of hand (55) |
| 11 | 2012 | Prototype Prakriti Analysis Tool (PPAT) (92) |
| 12 | 2012 | SAQ (93) |
| 13 | 2012 | Survey Q (94) |
| 14 | 2012 | TNMC Prakriti 2004 Q (95) |
| 15 | 2013 | SAQ (96) |
| 16 | 2013 | SAQ-ABC (97) |
| 17 | 2013 | TSSC SAQ-Tridosha State Scale for Children (47) |
| 18 | 2014 | ACPI scale- Ayurveda Child Personality Inventory (46) |
| 19 | 2014 | Sushruta Prakriti Inventory (SPI-Q & SPI-C) (50) |
| 20 | 2015 | SRQ (QDAV-R) (29) |
| 21 | 2015 | Survey Q (98) |
| 22 | 2016 | ML & Q (99) |
| 23 | 2017 | CCRAS-PAS software (45, 100) |
| 24 | 2017 | ML-Gathered Data (101) |
| 25 | 2017 | SRQ (102) |
| 26 | 2018 | Q (103) |
| 27 | 2018 | Q (104) |
| 28 | 2018 | Q (54) |
| 29 | 2018 | Q (105) |
| 30 | 2019 | Mathew IAS rating Scale (48) |
| 31 | 2019 | ML & CA (106) |
| 32 | 2019 | Q (107) |
| 33 | 2019 | Q (23) |
| 34 | 2019 | Q (52) |
| 35 | 2019 | Q (108) |
| 36 | 2019 | SAQ (109) |
| 37 | 2019 | SAQ Prakruti Dosha Mind Body Quiz and Vikruti Subdosha Questionnaire (28) |
| 38 | 2020 | ML & Q (49) |
| 39 | 2020 | Q (110) |
| 40 | 2020 | RGB analysis (111) |
| 41 | 2020 | SAQ (112) |
| 42 | 2020 | ML & SAQ (113) |
| 43 | 2021 | Portable Radial Pulse [VPK] Signal Acquisition and Recording System (114) |
| 44 | 2021 | Prakriti Q modified for T2DM (115) |
| 45 | 2021 | PRAS-IPA software (22) |
| 46 | 2021 | SAQ (116) |
| 47 | 2021 | SAQ (117) |
| 48 | 2021 | VIYETT Ayurvedic-Constitution Q (20) |
| 49 | 2022 | CPA & Q (118) |
| 50 | 2022 | Q (119) |
| 51 | 2022 | SAQ (51) |
| 52 | 2022 | SAQ (120) |
| 53 | 2023 | CV, ML & CPA (121) |
| 54 | 2023 | ML-Gathered Data (122) |
| 55 | 2023 | Prakriti Assessment Tool (PAT) (123) |
| 56 | 2023 | Q (124) |
| 57 | 2023 | ML & Q (125) |
| 58 | 2023 | SAQ (126) |
| 59 | 2024 | CPA method (127) |
| 60 | 2024 | CV, ML & CA (18) |
| 61 | 2024 | ML & CA (128) |
| 62 | 2024 | ML-Smart device data (129) |
| 63 | 2024 | Q (130) |
| 64 | 2024 | CPA & SRQ (131) |
Year-wise list of unique PATs.
3.1.1 Diverse methodological frameworks adopted by PATs
Initially, we wanted to identify and quantify the tools used for Prakriti assessment, but we found that every PAT varied in terms of the methods they used for data collection, and decision-making, resulting in diverse methodological frameworks being used for Prakrit assessment (see Figure 3). The heterogeneity between tools was evident not only at the level of frameworks but also within each functional domain. In data collection, tools differed in the domains assessed (e.g., physical, physiological, psychological traits) and in the specific data points used for Prakriti evaluation. In decision-making, the degree of subjectivity versus objectivity varied according to the methods employed. For instance, some studies relied on clinical assessments performed by multiple physicians, while others used algorithmic approaches that reduced or eliminated physician involvement.
Figure 3
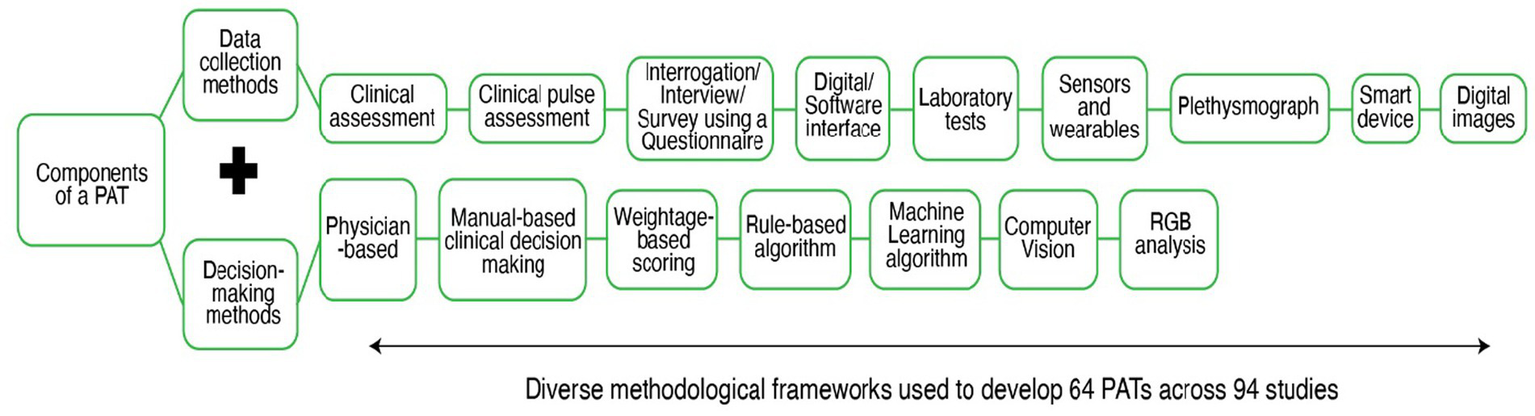
Data collection and Decision-making methods used across 64 PATs.
3.1.2 Trends related to technology integration of PATs
Out of the 64 PATs, there were 41 Questionnaires and 4 software. Other PATs involved the use of different methodological frameworks with varying degrees of Physician involvement and technology use. Figure 4 presents the percentage classification of the tools based on the nature of technological support they require. It must also be noted that, while the tech-supported tools and hybrid tools cannot be used without the support of a hardware (computers, sensors, plethysmograph etc.) and/or software (web-based or offline applications, MATLAB etc.), the non-tech tools could be used manually to perform the Prakriti assessment. Out of 64 tools, we found 44 non-tech tools; 11 tech-supported tools and 9 hybrid tools (tech + non-tech). We also found that though newer techniques like Machine Learning (ML) and Computer Vision (CV) have been used for Prakriti assessment, they still exist as frameworks and have not been developed into usable tools.
Figure 4
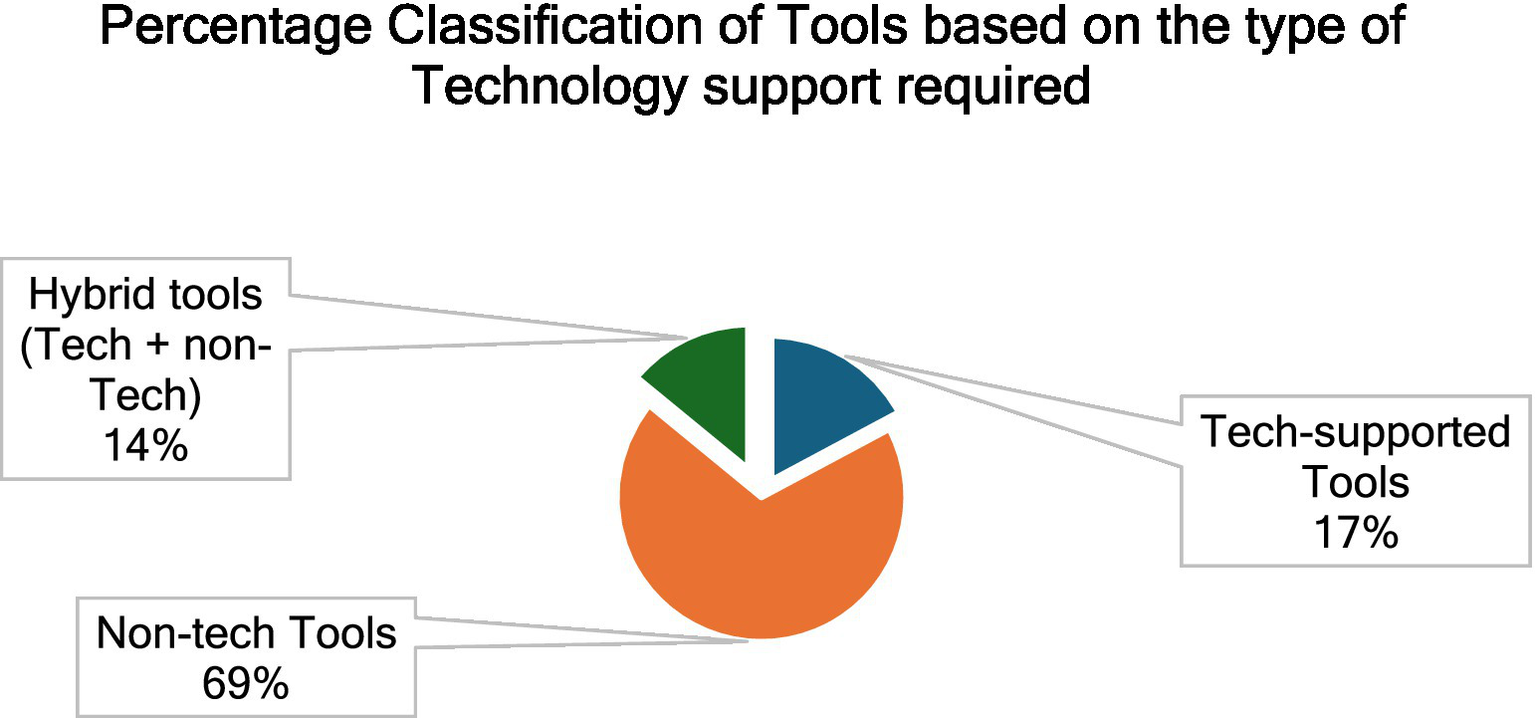
Percentage classification of the tools based on the type of technological support required.
Due to the variability in the involvement of Physician(s) and technology use, the PATs either served as a Decision support system (DSS) or an autonomous Decision-making system (DMS). While most PATs operated as autonomous DMS, only one PAT, AyuSoft, functioned as a DSS. The AyuSoft software enabled structured data collection, and the decision-making depended on physician-defined weightages.
3.2 Tool evaluation based on the scale development and validation framework
We evaluated all the identified PATs using a holistic scoring rubric (Supplementary Appendix 1) based on the identified Framework by Boateng et al. (35), and found that out of 64, only 20 PATs have undergone some form of validity and reliability testing using standard methods. The result of this analysis is given in Figure 5.
Figure 5
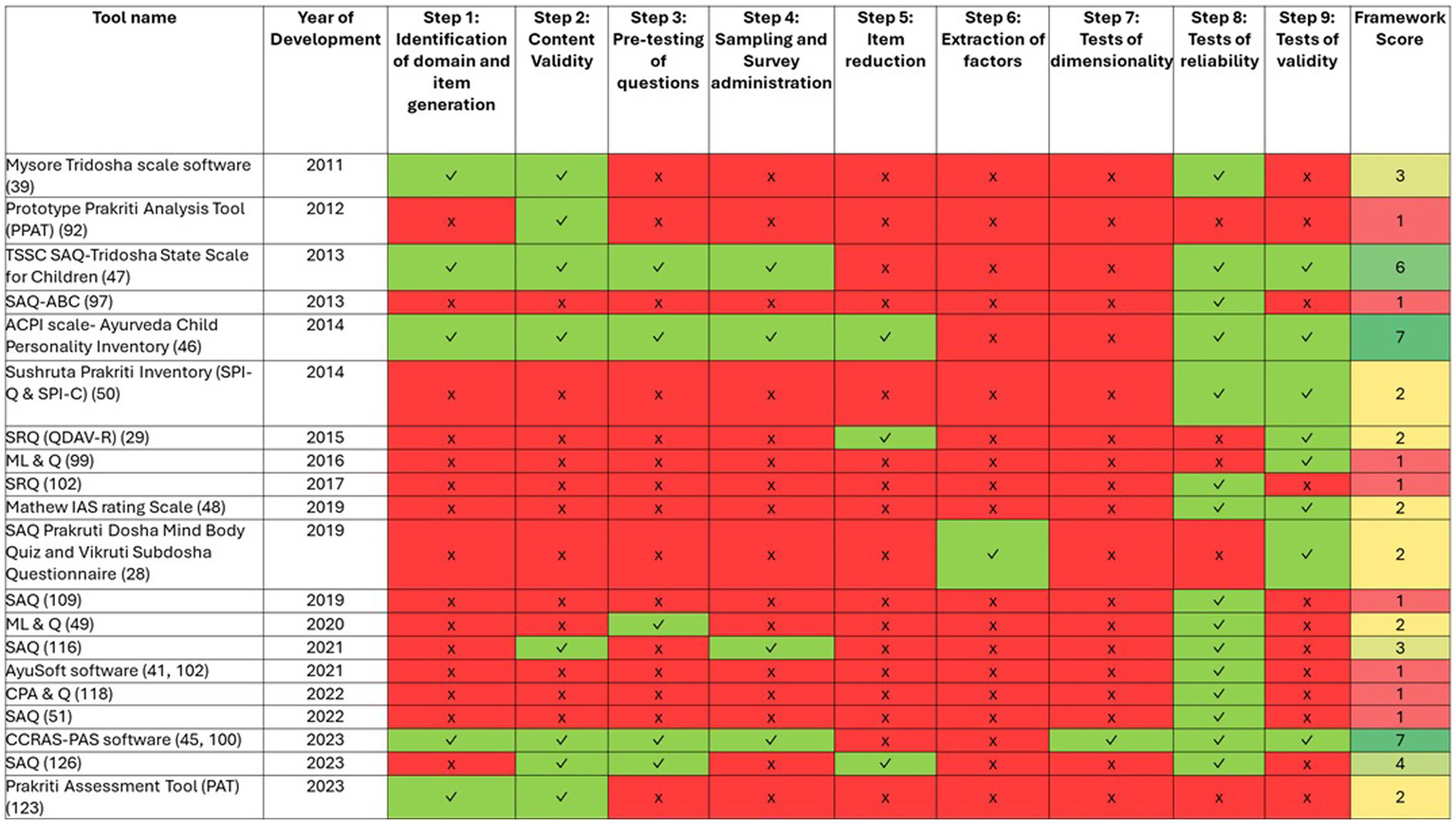
Validation of Prakriti Assessment Tools (PATs) against scale development and evaluation criteria (1987–2024). Each row represents a PAT, and each column represents one of the nine evaluation criteria. Green cells (✓) indicate the criterion was fulfilled, red cells (✗) indicate it was not fulfilled. The numbers in the rightmost column indicate the total count of criteria fulfilled for each tool, where shades of green color indicate PATs scoring >4; shades of yellow indicate PATs scoring 2–4 and red indicate PATs scoring <2.
We found that no tool looked at all 9 steps spanning the three phases listed in the framework. Two tools, the CCRAS-PAS software (45) and the Ayurveda Child Personality Inventory (ACPI scale) (46) fulfilled seven out of nine steps defined in the evaluation framework and one tool, the TSSC-SAQ (47) fulfilled 6 of the 9 steps.
Tools scoring below 5 of 9 often skipped early phases of the item and scale development and relied on inadequate validity and reliability testing. Many tools scoring ≤3 omitted the first two phases of item and scale development. Among the 20 PATs, most focused on assessing the physical constitution, only one tool- the Mathew IAS rating Scale (48) assessed mental constitution.
Nineteen out of twenty tools were tested by their developers, except the widely used AyuSoft software, which was externally tested for internal consistency along with Intra-class correlation coefficient (ICC) for inter-rater reliability.
Overall, both reliability and validity testing were conducted only for five PATs; 13 had either reliability or validity testing conducted, while two had neither conducted. Reliability assessments were largely limited to Cronbach’s alpha, Cohen’s kappa, and inter-rater reliability, with test–retest reliability performed for only two of the 20 PATs. In terms of validity, content validity, face validity, criterion validity, and construct validity were most applied, while predictive and discriminant validity were rarely used.
Figure 5 summarizes the year-wise development of various PATs in relation to the framework’s scale development and validation criteria. While progress is evident, the heatmap illustrates the inconsistent sequential adherence to the criteria. It also highlights considerable and persistent gaps in validation that have remained unaddressed over the years.
3.3 Evaluation of the studies that employed the 20 unique PATs using study quality indicators
We standardized the evaluation of the studies that used the 20 unique PATs with respect to their sample size, type of study design and the five influencing factors (Age, Location, Season, Ethnicity and Family history) of Prakriti. This led to a comparative ranking of the Prakriti assessment tools (see Table 2):
Table 2
| No. | Tool name | Year of development | Framework score | Study sample size | Type of study design | Vaya (No. of age range sections) | Desha (No. of locations) | Jati (ethnicity) | Kula (family history) | Kala (season) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | CCRAS-PAS software | 2017 | 7 | 500 | Observational cross-sectional | 3.5 | 7 | No info | No info | No info |
| 2 | ACPI scale- Ayurveda Child Personality Inventory | 2014 | 7 | 230 | Observational cross-sectional | 0.5 | 1 | No info | No info | No info |
| 3 | TSSC SAQ- Tridosha State Scale for Children | 2013 | 6 | 108 | Observational cross-sectional | 0.3 | 1 | No info | No info | No info |
| 4 | SAQ (126) | 2023 | 4 | 210 | Observational cross-sectional | 1.3 | 1 | No info | No info | No info |
| 5 | SAQ (116) | 2021 | 3 | 250 | Observational cross-sectional | 0.8 | 1 | No info | No info | No info |
| 6 | Mysore Tridosha scale (Software) | 2011 | 3 | 1,548 | Observational cross-sectional | 2.6 | 1 | No info | No info | No info |
| 7 | Mathew IAS rating Scale | 2019 | 2 | 293 | Observational cross-sectional | 2.5 | 1 | No info | No info | No info |
| 8 | Prakriti Assessment Tool (PAT) | 2023 | 2 | 0* | Observational cross-sectional | 0* | 0* | No info | No info | No info |
| 9 | ML & Q (113) | 2020 | 2 | 405 | Observational cross-sectional | 3.3 | 1 | No info | No info | No info |
| 10 | SAQ Prakruti Dosha Mind Body Quiz and Vikruti Subdosha Questionnaire | 2019 | 2 | 101 | Exploratory cross-sectional | 4.2 | 2 | No info | No info | No info |
| 11 | SRQ (QDAV-R) | 2015 | 2 | 173 | Comparative study | 3.3 | 1 | No info | No info | No info |
| 12 | Sushruta Prakriti Inventory (SPI-Q & SPI-C) | 2014 | 2 | 120 | Observational cross-sectional | 4.9 | 1 | No info | No info | No info |
| 13 | ML & Q (99) | 2016 | 1 | 67 | Observational cross-sectional | ** | 1 | No info | No info | No info |
| 14 | AyuSoft software (38, 135) | 2010 | 1 | 112 | Observational cross-sectional | 2 | 3 | No info | No info | No info |
| 15 | CPA & Q (118) | 2022 | 1 | 50 | Experimental study | ** | 1 | No info | No info | No info |
| 16 | Prototype Prakriti Analysis Tool (PPAT) | 2012 | 1 | 26 | Observational cross-sectional | 0.7 | 1 | No info | No info | No info |
| 17 | SAQ (51) | 2022 | 1 | 76 | Observational cross-sectional | 4.9 | 1 | No info | No info | No info |
| 18 | SAQ (109) | 2019 | 1 | 428 | Observational cross-sectional | 1.7 | 1 | No info | No info | No info |
| 19 | SAQ-ABC | 2013 | 1 | 20 | Observational cross-sectional | 0.2 | 1 | No info | No info | No info |
| 20 | SRQ (102) | 2017 | 1 | 19 | Observational cross-sectional | 3.4 | 1 | No info | No info | No info |
Comparative ranking of PATs using “Study Quality Indicators.”
*The study only involved Ayurvedic Physicians for rating the items to be included in the scale and did not involve any subjects.
**The age range of the subjects was not reported in the study.
None of the 20 PATs considered or factored all five influencing factors of Prakriti- ethnicity (Jati), family lineage (Kula), age (Vaya), geographic location (Desha), and season (Kala).
Eighteen PATs were developed for use in adult population, whereas 2 PATs- the ACPI scale and TSSC-SAQ were developed for Prakriti assessment in children.
As for age range, both CCRAS-PAS software and Q (49) reported an age range of 18 or 20 to 60 years, respectively, which covers more than 3 sections of age, when the age range between 0 to 60 is distributed across 5 sections of 12 years each. While some other tools like SAQ Prakruti Dosha Mind Body Quiz and Vikruti Subdosha Questionnaire (28), Sushruta Prakriti Inventory (SPI-Q & SPI-C) (50), and SAQ (51) considered a wider age range, their sample size was found to be very less.
With regards to the type of study design employed for performing the validity and reliability testing of tools, 17 tools reported an observational cross-sectional study design, and 1 tool each reported an experimental design, exploratory study design and a comparative study design (see Table 3). Among the 20 PATs, no tool was developed using a longitudinal study design and only one tool, the Sushruta Prakriti Inventory (SPI-Q & SPI-C) was used in a prospective matched controlled trial. Six other PATs-Q (52), CCRAS Q, TNMC Prakriti 2004 Q, Q (53), Q (54), and CPA and CA of hand (52–57) have been used in randomized clinical trials (RCTs). But these tools were not previously validated, and two of these tools were validated within the RCT with a very limited study sample. The details related to this analysis can be found in Supplementary Appendix 2.
Table 3
| Tool name | Framework score | Categories of measurable correlates |
|---|---|---|
| CCRAS Q† | – | Hematological, Genetic |
| CSIR software† | – | Genomic, Biochemical, Hematological, Genetic, Microbiome (Gut), Physiological |
| ML-Smart device data (129)† | – | Sleep Quality |
| Prakriti Q modified for T2DM (115)† | – | Biochemical, Genetic |
| PRAS-IPA software† | – | RGB scores |
| Q (107)† | – | Anthropometry, Biochemical |
| Q (23)† | – | Microbiome (Gut, oral and skin), Genomic |
| Q (103)† | – | Genetic |
| Q (89)† | – | Pharmacogenomic |
| Q (104)† | – | Karyotyping |
| Q (52)† | – | Hematological, Genetic |
| Q (108)† | – | Rate of disease incidence, Morbidity rate, Type of morbidity |
| Q (105)† | – | Anthropometry, Biochemical, Metabolomics |
| Q (110)† | – | Biochemical |
| RGB analysis (111)† | – | Hue Saturation Value space (HSV), RGB scores |
| SAQ (112)† | – | Anthropometry, Physiological, Neuropsychological tests |
| SAQ (120)† | – | Jatharagni |
| SAQ (117)† | – | Physiological |
| SAQ (93)† | – | Physiological, Hematological |
| SAQ (90)† | – | Stress Handling Capacity, Physiological, Anthropometry |
| SAQ (96)† | – | Skin type |
| TNMC Prakriti 2004 Q† | – | Physiological, Anthropometry, Biochemical, Microbiome (Gut and oral) |
| VIYETT Ayurvedic-Constitution Q† | – | Anthropometry |
| AyuSoft software* | – | Biochemical, Inflammatory markers, Lifestyle variables, Immunophenotyping, Genomic, Genetic, Epigenetic, Pharmacogenomic |
| 1* | Anthropometry, Place of birth | |
| SAQ (109) | 1 | Anthropometry, Biochemical, Physiological, Genetic |
| SAQ Prakruti Dosha Mind Body Quiz and Vikruti Subdosha Questionnaire | 2 | Psychological states in terms of Vikruti |
| SRQ (QDAV-R) | 2 | Western personality constructs |
| CCRAS-PAS software | 7 | Pharmacodynamic, Cognitive and behavioral assessment scores, Genomic, Inflammatory markers |
List of PATs, their framework score and categories of measurable correlates to Prakriti studied using the tools.
†Insufficient data related to tool validation.
*AyuSoft was used in 8 studies, primarily to explore objective correlates. Only one study conducted partial reliability testing; the rest lacked validation per the framework. Therefore, this tool has a score of both 0 and 1.
3.4 Investigating measurable correlates as biomarkers of Prakriti using different PATs
Here, we have identified 32 categories of measurable correlates that have been investigated in relation to Prakriti between 1987 and 2024. We have created a catalog of these 32 categories and the specific biomarkers that have been measured under each of these categories in Supplementary Appendix 3. In Table 3, we have listed the PATs which have been used in studying these measurable correlates to Prakriti. However, only 4 of the 32 categories of measurable correlates studied using the CCRAS-PAS software could be considered methodologically valid, owing to its high evaluation score. Tools like the CSIR software have been used to investigate extreme Prakriti types with genomic (33, 58–60), biochemical (33), physiological (61) and microbiome markers (24–26). Concurrently, other studies used tools such as AyuSoft (32, 62–66) to explore similar associations. But these tools were not previously validated. Also, all the identified measurable correlates have been studied independently and not integrated into the PATs. These findings highlight a critical gap in the current body of research on Prakriti.
Overall, the review found that of the 64 unique PATs identified, only 20 had undergone any form of validation or reliability testing. Among these, only two tools, the CCRAS-PAS software and the ACPI scale demonstrated notable methodological rigor as per the framework. While the CCRAS-PAS software was validated in adult populations, the ACPI scale was specifically developed and tested for use in children.
4 Discussion
This review highlights an overall progression in Prakriti assessment from highly subjective, physician-dependent approaches toward more structured and data-driven decision-making systems. Early attempts to reduce subjectivity included consensus between multiple physicians, structured questionnaires with predefined scoring systems, followed by use of digital interfaces that standardized data entry and software applications along with standard operating procedures that introduced greater consistency in decision-making. Parallelly, sensors, wearables, and laboratory-based investigations have been used to explore measurable correlates of Prakriti, and pilot applications of Machine Learning and Computer Vision demonstrate enhanced objectivity and potential for scalability. However, our findings indicate that despite these advancements, most tools remain under-validated, with limited testing across diverse populations, thereby constraining their generalizability and scalability. Figure 6 illustrates a mind map of Prakriti assessment as per the overall findings of this review.
Figure 6
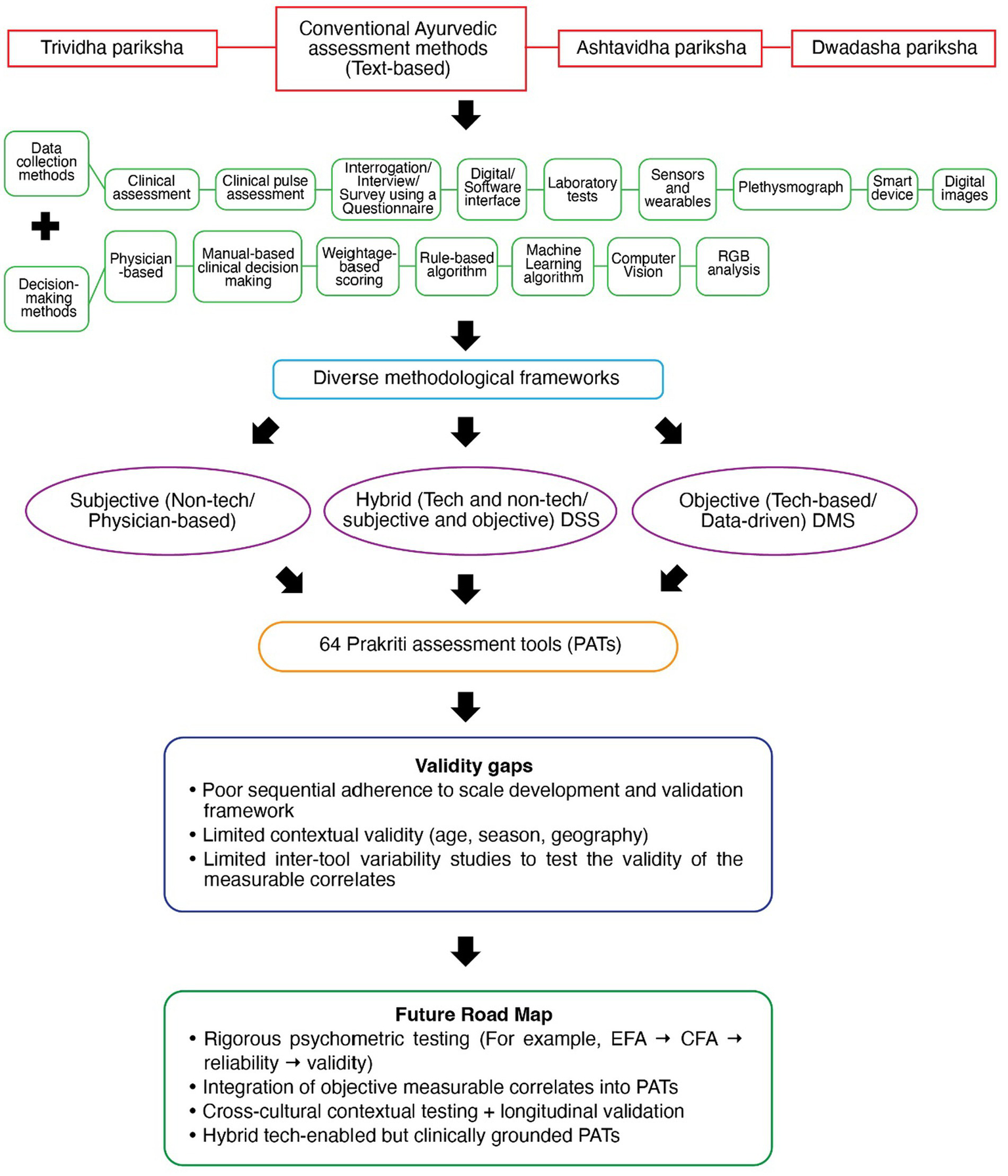
Mind map of Prakriti assessment: From conventional Ayurvedic assessments to development of PATs.
With specific reference to tool development, the identified PATs were generally developed based on textual descriptions that broadly outline only three types of Prakriti. These characteristics are not exhaustive and may not be sensitive enough to capture the full spectrum of Prakriti variations. On the other hand, the robustness of a PAT would depend upon a critical set of data heads and data points, that have been explored and factored from an exhaustive list. This exercise has not been performed, which is a critical gap. Technology-based assessments is an emerging area of research that enhances assessment methodology (67). Leveraging advanced technologies like generative Artificial Intelligence (AI) and ML offer the potential to identify latent dimensions of the Prakriti construct that are not explicitly described in classical Ayurvedic texts. This could pave the way for the development of more robust PATs.
As per the Validation and Evaluation framework, the phase I of tool development, requires the construct of the parameter being measured to be clearly defined within a theoretical or conceptual framework. The absence of such an approach has several implications. Apart from literature review, the conceptual and theoretical frameworks offer different dimensions on the topic of study that are essential to guide methodological decisions and elucidate critical insights (68). It is fundamental to scale development and improves the rigor and coherence of the scale (35). Secondly, the content validity of the scale is established at this stage. If the domains are not identified and clearly defined within a theoretical or conceptual framework, the items will not fully represent the construct’s domain, impacting the overall validity of the scale at the very beginning (69).
This phase also requires the generation of a comprehensive item pool using both deductive and inductive approaches. Importantly, item development must extend beyond the researchers’ subjective conceptualization to capture the full breadth of the target construct. As recommended by Kline (70) and Schinka et al. (71), the initial item pool should ideally be at least twice the length of the intended final scale.
Some researchers have attempted to provide a molecular framework for Prakriti with respect to stratified medicine (72), drug discovery and advancement for personalized care (73) and precision and integrative medicine (5). However, these frameworks are not suitable for a scale or tool development itself. There are two theories fundamental to any scale or tool development- the Classical Test Theory (CTT) and Item Response Theory (IRT) (74) that will ensure deriving functionally valid items specific to the construct of the domain of interest (35).
Under Phase II, critical steps like item reduction and extraction of factors that involve the use of standard methods such as item difficulty index, item discrimination test, inter-item and item-total correlations, distractor efficiency analysis and deleting or imputing missing cases were missing or not clearly explained. The item difficulty step is crucial to make sure only consistent items are included in the scale. Reynolds et al., has particularly highlighted the challenge of choosing the right procedures to ensure proper item-selection decisions that improves the tool’s overall validity (75). The type of responses like binary response or multiple-choice response or categorical items will also determine the choice of these procedures (40).
Under Phase III, dimensionality testing is crucial as it enables the accurate mapping of factors that specifically contribute to each construct, minimizing overlap and enhancing construct clarity. It is also very important to do the dimensionality testing at a different time point with the same sample or on a new sample (76). Performing dimensionality testing using statistical methods can bring about both conceptual clarity and empirical validation of tools measuring complex constructs (77).
Another major concern with respect to Dimensionality testing was the use of Confirmatory Factor Analysis (CFA) without first conducting Exploratory Factor Analysis (EFA), while developing the CCRAS-PAS software. While EFA is used to identify the underlying factor structure, CFA is used to test and confirm hypothesized models based on theory (78). Bypassing EFA step while performing sampling in phase II implies that the latent structure of the questionnaire was not adequately explored before confirming it which can lead to construct underrepresentation (79). This will also impact the subsequent steps of phase III- dimensionality, validity and reliability testing. Therefore, though the CFA under Dimensionality testing has been performed for CCRAS-PAS software, it has been done in the absence of EFA, suggesting that the dimensionality testing may not accurately represent the underlying construct.
Overall, the absence of a structured and sequential approach, beginning with identifying data heads and data points within a theoretical framework, followed by exhaustive item generation, rigorous item reduction to derive a critical set of items, and subsequently progressing through, EFA, CFA, dimensionality testing, and other validation steps has not yet been systematically undertaken. Implementing these steps would be essential to ensure that the reliability and validity testing of PATs is carried out rigorously. Kyriazos et al. (80) emphasize that the scale development and standardization process must be both sequential and iterative to ensure greater reliability and validity. Morgado et al. (81) identified and highlighted limitations in the scale development process which when overlooked or not understood adequately limit the future applicability of the scale and also hinder its generalizability. Although many PATs have reported validity and/or reliability testing, these were often conducted without the preceding foundational steps, which limits the robustness and interpretability of their findings. It is also important to note that while reliability testing is necessary, it is not sufficient on its own as it is a sample-dependent and context-dependent process (82). On the other hand, validity testing is a continuous process that needs to be performed throughout the scale development and evaluation process. For example, Boateng et al. (35) recommend validation across all three phases: content validity during item development (Phase 1), internal structure testing using EFA and CFA during scale development (Phase 2), and criterion and construct validity during scale evaluation (Phase 3).
In addition to tool evaluation, we also evaluated the studies that employed these tools. We incorporated study quality indicators, based on sample size adequacy which is very crucial and has a significant impact on research outcomes (83). With regards to sample size- three tools stood out. The CCRAS-PAS software considered a sample size of 500, but it has taken these samples from 7 different locations, limiting the number of study samples per location. Including 7 locations could have proven beneficial if the influence of location was factored in along with a larger sample size. Another ML-based tool, ML & Q (49) reported a higher overall sample size (n = 405), however the testing sample was only 81. This may be too low for the multiclass classification to get statistically significant values of precision and recall. Also, there is limited information on demographic diversity that would affect the model generalizability, when applying to diverse populations. A recommended testing sample is around 20–30 samples per category or domain (84). In this case, there are 7 categories, therefore about 140–210 samples in the test set would be required. Though the Mysore Tridosha Scale (39) was tested on the largest sample size (n = 1,548), it was limited in scope due to its primary focus on psychological expressions of doshas, and did not look at the other domains like physical features, behavioral traits etc.
With respect to the types of study designs, most of the tools (n = 17) were developed using an observational cross-sectional study design which is acceptable but none of these tools were additionally tested using controlled studies, implying lack of clinical validation. Clinical trials are fundamental to evidence-based practice that validates and informs clinically relevant research, requiring periodic testing and updating (85). The paucity of controlled studies poses a major constraint on the predictive capacity and clinical relevance of the PATs. To translate Prakriti into a measurable parameter in personalized medical care, systematic tool development and validation must occur before and alongside their use in trials.
Collectively, the study quality indicators set the tone for establishing contextual validity and wider applicability (across different cultures, ecology, language etc.) of the PATs. At present, even the PATs with the highest Framework scores have not accounted for key influencing factors of Prakriti, thereby limiting their generalizability. But even before pursuing multiple contexts and scalability, a PAT must first establish validity and reliability within at least one clearly defined context. This requires a methodical approach- from establishing a conceptual framework, generating context-sensitive items, scale development, and evaluation ensuring both relevance and repeatability. Frongillo et al. describe validity and “cross-context equivalence” of measures and discuss the methods to establish them (86).
While the scientific validation of Prakriti assessment is still a work under progress, studies continue to explore associations of Prakriti with genomic, biochemical, microbiome and other markers using different PATs. Over the past two decades, significant strides have been made to identify objective measurable correlates as biomarkers of Prakriti, especially by the Ayurgenomics study initiated by CSIR-TRISUTRA consortium (87). The heterogeneity of findings across these studies hinders the identification of a definitive biomarker(s) for Prakriti classification.
Multiple PATs have been involved in studying the measurable correlates to Prakriti, but no replication of studies have been performed using same correlates and different PATs. Such inter-tool variability studies would help establish the methodological robustness of the PATs and also the validity of these measurable correlates.
Though, currently there are no established biomarker(s) for Prakriti classification, there are some domains more prominently studied than the others such as Biochemical, Anthropometry, Genomic, Physiological, Genetic, Microbiome, Inflammatory markers and Hematological parameters. We propose a framework for prioritizing the existing domains. We could look at four criteria: (i) Replicability: Same biomarkers must be studied in multiple, independent cohorts, using different PATs. Currently, only biochemical parameters like lipid profile and blood glucose and anthropometric parameters like height, weight, BMI alone have been replicated in multiple independent cohorts, which have not shown any promising links to the tridoshas. Other domians need to be replicated too; (ii) Biological plausibility: links to dosha theory [e.g., PGM1 gene with Pitta phenotype (32)]; however most of the studies that explored measurable correlates to prakriti have only considered extreme constitution types, limiting its applicability to all constitution types, as a majority of population would belong to dual constitution types; (iii) Integration capacity: ability to combine with digital PATs (e.g., genomic or metabolomic data linked to questionnaires/software); (iv) Clinical translational potential: feasibility of testing in real-world settings (e.g., microbiome profiling is becoming cheaper and more accessible).
Based on the current evidence, Prakriti seems to represent a polygenic, systems-level phenotype. Therefore, rather than a single biomarker, a multi-omic, integrative approach combining genetic, microbiome, immunological and metabolomic parameters alongside validated PATs appears most promising. While it may not be feasible to have too many tests as a part of Prakriti assessment, future studies should prioritize biomarkers that are replicable across populations, clinically feasible, and theoretically aligned with Ayurvedic concepts of Prakriti.
5 Limitations
This study has certain limitations, including the exclusion of non-English language journals and Indian databases. Secondly, as this was not a systematic review, the methodology was not pre-registered in a review registry such as PROSPERO before initiating the review. Finally, in the absence of a dual review, we may not have eliminated bias completely.
6 Conclusion
Despite the proliferation of numerous Prakriti assessment tools, inadequate adherence to standardized protocols for development, validation and reliability testing leaves major gaps in methodological rigor and robustness of available tools. This hampers both the effective utilization of technology and the progression of Ayurvedic research. Moving forward, the adoption of structured approaches for tool development and validation, including rigorous item development, dimensionality, validation, and reliability testing is essential. This can then be followed by cross-cultural validation studies and non-developer testing through multi-center and cross-context trials (India and diaspora populations). Furthermore, integrating advanced technologies and incorporating measurable correlates within the Prakriti assessment tools will make it more robust, clinically relevant and suitable for integration into mainstream personalized health care.
Statements
Author contributions
AV: Investigation, Visualization, Data curation, Resources, Methodology, Conceptualization, Funding acquisition, Writing – original draft, Writing – review & editing, Formal analysis. LJ: Resources, Writing – review & editing, Formal analysis, Validation, Visualization, Methodology, Supervision. SP: Writing – review & editing, Methodology, Formal analysis, Visualization, Data curation. SG: Supervision, Writing – review & editing, Validation, Methodology. KS: Supervision, Methodology, Validation, Writing – review & editing. CK: Validation, Writing – review & editing, Supervision. SR: Validation, Supervision, Writing – review & editing. RP: Conceptualization, Writing – review & editing, Supervision, Funding acquisition, Resources, Project administration, Validation, Visualization, Methodology.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Adani Indology Research Scholarship Program. Grant/Funding ID: Not applicable. The Article Processing Charge (APC) was supported by the Provost Office, Amrita Vishwa Vidyapeetham.
Acknowledgments
We thank Amrita Vishwa Vidyapeetham for providing institutional support and resources to facilitate this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1656249/full#supplementary-material
References
1.
Meulenbeld G . The history of Indian medical literature, vol. 1–5. Groningen: E. Frosten (1999).
2.
PIB Delhi. Ministry of Statistics & Programme Implementation . (2024). RESULTS OF SURVEY ON AYUSH (July 2022 to June 2023) released. Available online at: https://www.pib.gov.in/PressReleaseIframePage.aspx?PRID=2025076
3.
Acharya R . Annexe of Ayurveda in G-20 countries: a brief insight. J Drug Res Ayurvedic Sci. (2023) 8:309. doi: 10.4103/jdras.jdras_344_23
4.
Egwumba P Wang H Nellums L Bains M Chattopadhyay K . Ayurveda for managing noncommunicable diseases in organisation for economic cooperation and development nations: A qualitative systematic review. Health Sci Rep. (2025) 8:e70624. doi: 10.1002/hsr2.70624
5.
Mukerji M . Ayurgenomics-based frameworks in precision and integrative medicine: translational opportunities. Camb Prisms Precis Med. (2023) 1:e29. doi: 10.1017/pcm.2023.15
6.
Patwardhan B Wieland LS Aginam O Chuthaputti A Ghelman R Ghods R et al . Evidence-based traditional medicine for transforming global health and well-being. J Ayurveda Integr Med. (2023) 14:100790. doi: 10.1016/j.jaim.2023.100790
7.
Elemento O . The future of precision medicine: towards a more predictive personalized medicine. Emerg Top Life Sci. (2020) 4:175–7. doi: 10.1042/ETLS20190197
8.
Amaro-Álvarez L Cordero-Ramos J Calleja-Hernández MÁ . Exploring the impact of pharmacogenetics on personalized medicine: a systematic review. Farm Hosp. (2024) 48:299–309. doi: 10.1016/j.farma.2023.12.004
9.
Institute for Health Metrics and Evaluation . Global burden of disease: compare. (2020). Available online at: https://vizhub.healthdata.org/gbd-compare/
10.
Charaka In: SharmaRKDashB, editors. Charaka Samhita, Śārīrasthāna 3/4–13: Text with English translation and critical exposition based on Chakrapani Datta’s Ayurveda Dipika. Varanasi: Chaukhambha Sanskrit Series Office (2014)
11.
Sushruta . Sushruta Samhita, Śārīrasthāna 4/63–68 In: The Sushruta Samhita with English translation. Varanasi: Chaukhambha Orientalia (1998)
12.
Vagbhata . Ashtanga Sangraha of Vagbhata, Sūtrasthāna 1/13–15 In: Ashtanga Sangraha of Vagbhata. Varanasi: Chaukhambha Orientalia (2012)
13.
Vagbhata . Ashtanga Hridaya of Vagbhata, Sūtrasthāna 1/7–10 In: Ashtanga Hridaya of Vagbhata. Varanasi: Chaukhambha Krishnadas Academy (2012)
14.
Sharma P . Charaka Samhita: Text with English translation. Shārirasthanam 4/36–40. 2nd ed. Varanasi: Chaukhambha Orientalia (2017).
15.
Vagbhata . Ashtanga Hridayam, Sutra Sthana 1/22 In: VaidyaHP, editor. Ashtanga Hridayam. 1st ed. Varanasi: Krishnadas Academy (2000)
16.
Yogaratnakara . Roganidana Adhyaya. In: ShastriL. (ed and trans) Yogaratnakara with Vidyotini Hindi commentary. Reprint edition. Varanasi: Chaukhambha Sanskrit Sansthan; (2017), 5.
17.
Rasavaisheshika In: ShastriAD, editor. Rasavaisheshika sutra with commentary. Varanasi: Chaukhambha Bharati Academy (2009)
18.
Suguna GC Veerabhadrappa ST . Identification and classification of Prakriti of human using facial features. IAES Int J Artif Intell IJ-AI. (2024) 13:2093. doi: 10.11591/ijai.v13.i2.pp2093-2101
19.
Junuzović-Žunić L Ibrahimagić A Altumbabić S . Voice characteristics in patients with thyroid disorders. Eurasian J Med. (2019) 51:101–5. doi: 10.5152/eurasianjmed.2018.18331
20.
Konjengbam H Leona Devi Y Meitei SY . Correlation of body composition parameters and anthropometric somatotypes with Prakriti body types among the Meitei adults of Manipur. India Ann Hum Biol. (2021) 48:160–5. doi: 10.1080/03014460.2021.1919205
21.
Singh S Agrawal NK Singh G Gehlot S Singh SK Singh R . Clinical prediction of type 2 diabetes mellitus (T2DM) via anthropometric and biochemical variations in Prakriti. Dent Discourse. (2022) 10:15. doi: 10.3390/diseases10010015
22.
Garima S Singh BM . Co-relation of Prakriti of an infant with skin color RGB values of facial photograph and standardization of reference standards of Prakriti color representor. J Nat Remedies. (2021) 21:141. doi: 10.18311/jnr/2021/25862
23.
Chaudhari D Dhotre D Agarwal D Gondhali A Nagarkar A Lad V et al . Understanding the association between the human gut, oral and skin microbiome and the Ayurvedic concept of prakriti. J Biosci. (2019) 44:112. Available at: http://link.springer.com/10.1007/s12038-019-9939-6
24.
Chauhan NS Pandey R Mondal AK Gupta S Verma MK Jain S et al . Western Indian rural gut microbial diversity in extreme Prakriti endo-phenotypes reveals signature microbes. Front Microbiol. (2018) 9:118. doi: 10.3389/fmicb.2018.00118
25.
Mobeen F Sharma V Prakash T . Comparative gut microbiome analysis of the Prakriti and Sasang systems reveals functional level similarities in constitutionally similar classes. 3 Biotech. (2020) 10:379. doi: 10.1007/s13205-020-02376-1
26.
Mobeen F Sharma V Prakash T . Functional signature analysis of extreme Prakriti endo-phenotypes in gut microbiome of western Indian rural population. Bioinformation. (2019) 15:490–505. doi: 10.6026/97320630015490
27.
Shalini TV Jnana A Sriranjini SJ Tanwar AS Brand A Murali TS et al . Exploring the signature gut and oral microbiome in individuals of specific Ayurveda prakriti. J Biosci. (2021) 46:54. Available at: https://link.springer.com/10.1007/s12038-021-00182-2
28.
Mills PJ Peterson CT Wilson KL Pung MA Patel S Weiss L et al . Relationships among classifications of ayurvedic medicine diagnostics for imbalances and western measures of psychological states: an exploratory study. J Ayurveda Integr Med. (2019) 10:198–202. doi: 10.1016/j.jaim.2018.02.001
29.
Delle Fave A Negri L Ram Manohar P Morandi A Bassi M . The Ayurveda concept of Prakŗti and the Western construct of personality: a comparative pilot study. Eur J Integr Med. (2015) 7:396–408. doi: 10.1016/j.eujim.2014.09.133
30.
Bhushan P Kalpana J Arvind C . Classification of human population based on HLA gene polymorphism and the concept of Prakriti in Ayurveda. J Altern Complement Med. (2005) 11:349–53. doi: 10.1089/acm.2005.11.349
31.
Aggarwal S Negi S Jha P Singh PK Stobdan T Pasha MAQ et al . EGLN1 involvement in high-altitude adaptation revealed through genetic analysis of extreme constitution types defined in Ayurveda. Proc Natl Acad Sci. (2010) 107:18961–6. doi: 10.1073/pnas.1006108107
32.
Govindaraj P Nizamuddin S Sharath A Jyothi V Rotti H Raval R et al . Genome-wide analysis correlates Ayurveda Prakriti. Sci Rep. (2015) 5:15786. doi: 10.1038/srep15786
33.
Prasher B Negi S Aggarwal S Mandal AK Sethi TP Deshmukh SR et al . Whole genome expression and biochemical correlates of extreme constitutional types defined in Ayurveda. J Transl Med. (2008) 6:48. doi: 10.1186/1479-5876-6-48
34.
Bhalerao S Patwardhan K . Prakriti-based research: good reporting practices. J Ayurveda Integr Med. (2016) 7:69–72. doi: 10.1016/j.jaim.2015.08.002
35.
Boateng GO Neilands TB Frongillo EA Melgar-Quiñonez HR Young SL . Best practices for developing and validating scales for health, social, and behavioral research: a primer. Front Public Health. (2018) 6:149. doi: 10.3389/fpubh.2018.00149
36.
Baethge C Goldbeck-Wood S Mertens S . SANRA—a scale for the quality assessment of narrative review articles. Res Integr Peer Rev. (2019) 4:5. doi: 10.1186/s41073-019-0064-8
37.
Haddaway N Page M Pritchard C McGuinness L . An R package and shiny app for producing PRISMA 2020-compliant flow diagrams, with interactivity for optimised digital transparency and open synthesis. Campbell Syst Rev. (2018) 18:e 1230. doi: 10.1002/cl2.1230
38.
C-DAC . AyuSoft: Ayurveda decision support system. Pune: C-DAC: Centre for Development of advanced computing (C-DAC).
39.
Shilpa S Venkatesha Murthy C . Development and standardization of Mysore Tridosha scale. AYU Int Q J Res Ayurveda. (2011) 32:308. doi: 10.4103/0974-8520.93905
40.
DeVellis R . Scale development: Theory and applications. 4th ed. Thousand Oaks, CA: SAGE publications (2016).
41.
Zhou Y . A mixed methods model of scale development and validation analysis. Meas Interdiscip Res Perspect. (2019) 17:38–47. doi: 10.1080/15366367.2018.1479088
42.
Lamm K Lamm A Edgar D . Scale development and validation: methodology and recommendations. J Int Agric Ext Educ. (2020) 27:24–35. doi: 10.5191/jiaee.2020.27224
43.
Badenes-Ribera L Silver NC Pedroli E . Editorial: Scale development and score validation. Front Psychol. (2020) 11:799. doi: 10.3389/fpsyg.2020.00799
44.
Carpenter S . Ten steps in scale development and reporting: a guide for researchers. Commun Methods Meas. (2017) 12:25–44. doi: 10.1080/19312458.2017.1396583
45.
Singh R Ota S Bharti Srikanth N Dhiman KS Vyas N et al . Development of standardized Prakriti assessment tool: an overview of ongoing CCRAS initiatives. J Res Ayurvedic Sci. (2017) 1:165–208. doi: 10.5005/jp-journals-10064-0019
46.
Nagendra H Jagan A Suchitra S . Development and initial standardization of Ayurveda child personality inventory. J Ayurveda Integr Med. (2014) 5:205. doi: 10.4103/0975-9476.146562
47.
Suchitra S Nagendra H . A self-rating scale to measure tridoṣas in children. Anc Sci Life. (2013) 33:85. doi: 10.4103/0257-7941.139042
48.
Jain T Sharma R Singh A Mehta K . Evaluation of levels of Gunas in Indian athletes using Prakriti concept. Indian J Public Health Res Dev. (2019) 10:168. doi: 10.5958/0976-5506.2019.00280.8
49.
Madaan V Goyal A . Predicting ayurveda-based constituent balancing in human body using machine learning methods. IEEE Access. (2020) 8:65060–70. doi: 10.1109/ACCESS.2020.2985717
50.
Ramakrishna B Kishore K V V Nagaratna R Nagendra H . Standardization of Sushrutha Prakriti inventory- SPI an Ayurveda based personality assessment tool with scientific methods. J Ayurveda Holist Med. (2015) 2:1–8. doi: 10.70066/jahm.v2i9.212
51.
Edwards MT Streiner DL . Development of a reliable dosha self-assessment questionnaire. Explore. (2022) 18:573–8. doi: 10.1016/j.explore.2021.09.003
52.
Mangalasseri P Roy S Surendran E Jayadevan C Kumar AM Chandran S . A cross-sectional study on the role of stress in hyperglycemia and the effect of Mahatiktaka Kashaya (an Ayurvedic formulation) in its management. AYU Int Q J Res Ayurveda. (2019) 40:114. doi: 10.4103/ayu.AYU_200_19
53.
Patki P Sharma R . Double-blind, placebo-controlled clinical evaluation of an Ayurvedic formulation (GlucoCare capsules) in non-insulin dependent diabetes mellitus. J Ayurveda Integr Med. (2010) 1:45. doi: 10.4103/0975-9476.59827
54.
Kessler CS Morandi A Kumar A Dhiman KS Gupta S Icke K et al . Reliability of ayurvedic diagnosis for knee osteoarthritis patients: a nested diagnostic study within a randomized controlled trial. J Altern Complement Med. (2019) 25:910–9. doi: 10.1089/acm.2018.0273
55.
Kurande V Waagepetersen R Toft E Prasad R Raturi L . Repeatability of pulse diagnosis and body constitution diagnosis in traditional Indian Ayurveda medicine. Glob Adv Health Med. (2012) 1:36–42. doi: 10.7453/gahmj.2012.1.5.011
56.
Karunagoda K Perera PK Senanayake H . Efficacy of two Ayurveda drug regimens for uterine fibroids, a randomized, single blind, three-arm, clinical trial- study protocol. Int J Pharm Sci Res. (2019) 10:1366–71. doi: 10.13040/IJPSR.0975-8232.10(3).1366-71
57.
Munshi R Karande-Patil S Kumbhar D Deshmukh A Hingorani L . A randomized, controlled, comparative, proof-of-concept study to evaluate the efficacy and safety of Nisha-Amalaki capsules in prediabetic patients for preventing progression to diabetes. J Ayurveda Integr Med. (2023) 14:100806. doi: 10.1016/j.jaim.2023.100806
58.
Indian Genome Variation Consortium Aggarwal S Gheware A Agrawal A Ghosh S Prasher B et al . Combined genetic effects of EGLN1 and VWF modulate thrombotic outcome in hypoxia revealed by Ayurgenomics approach. J Transl Med. (2015) 13:184. doi: 10.1186/s12967-015-0542-9
59.
Chakraborty S . Baseline cell proliferation rates and response to UV differ in lymphoblastoid cell lines derived from healthy individuals of extreme constitution types. Cell Cycle. (2021) 20:903–13. doi: 10.1080/15384101.2021.1909884
60.
Abbas T Chaturvedi G Prakrithi P Pathak AK Kutum R Dakle P et al . Whole exome sequencing in healthy individuals of extreme constitution types reveals differential disease risk: a novel approach towards predictive medicine. J Pers Med. (2022) 12:489. doi: 10.3390/jpm12030489
61.
Rani R Rengarajan P Sethi T Khuntia BK Kumar A Punera DS et al . Heart rate variability during head-up tilt shows inter-individual differences among healthy individuals of extreme Prakriti types. Physiol Rep. (2022) 10:e15435. doi: 10.14814/phy2.15435
62.
Mahalle N Kulkarni M Pendse N Naik S . Association of constitutional type of Ayurveda with cardiovascular risk factors, inflammatory markers and insulin resistance. J Ayurveda Integr Med. (2012) 3:150. doi: 10.4103/0975-9476.100186
63.
Satyamoorthy K Guruprasad K Nayak J Kabekkodu S Kukreja H Mallya S et al . Immunophenotyping of normal individuals classified on the basis of human dosha prakriti. J Ayurveda Integr Med. (2014) 5:43. doi: 10.4103/0975-9476.128857
64.
Satyamoorthy K Valiathan MV Anchan S Bellampalli R Bhale S Bharadwaj R et al . Determinants of prakriti, the human constitution types of Indian traditional medicine and its correlation with contemporary science. J Ayurveda Integr Med. (2014) 5:166. doi: 10.4103/0975-9476.140478
65.
Rotti H Mallya S Kabekkodu SP Chakrabarty S Bhale S Bharadwaj R et al . DNA methylation analysis of phenotype specific stratified Indian population. J Transl Med. (2015) 13:151. doi: 10.1186/s12967-015-0506-0
66.
Thaker SJ Gandhe PP Godbole CJ Bendkhale SR Mali NB Thatte UM et al . A prospective study to assess the association between genotype, phenotype and Prakriti in individuals on phenytoin monotherapy. J Ayurveda Integr Med. (2017) 8:37–41. doi: 10.1016/j.jaim.2016.12.001
67.
Goldhammer F Scherer R Greiff S . Editorial: advancements in technology-based assessment: emerging item formats, test designs, and data sources. Front Psychol. (2020) 10:3047. doi: 10.3389/fpsyg.2019.03047
68.
Luft JA Jeong S Idsardi R Gardner G . Literature reviews, theoretical frameworks, and conceptual frameworks: an introduction for new biology education researchers. NehmR, editor. CBE—Life Sci Educ (2022) 21:rm33.
69.
Haynes SN Richard DCS Kubany ES . Content validity in psychological assessment: a functional approach to concepts and methods. Psychol Assess. (1995) 7:238–47. doi: 10.1037/1040-3590.7.3.238
70.
Kline P . A handbook of psychological testing. 2nd ed. London: Routledge: Taylor & Francis Group (1993).
71.
Schinka J Velicer W Weiner I . Handbook of psychology, Vol. 2, research methods in psychology. Hoboken, NJ: John Wiley & Sons, Inc. (2012).
72.
Prasher B Varma B Kumar A Khuntia BK Pandey R Narang A et al . Ayurgenomics for stratified medicine: TRISUTRA consortium initiative across ethnically and geographically diverse Indian populations. J Ethnopharmacol. (2017) 197:274–93. doi: 10.1016/j.jep.2016.07.063
73.
Huang Z Chavda VP Bezbaruah R Uversky VN Sucharitha P Patel AB et al . An ayurgenomics approach: prakriti-based drug discovery and development for personalized care. Front Pharmacol. (2022) 13:866827. doi: 10.3389/fphar.2022.1080753
74.
Fan X . Item response theory and classical test theory: an empirical comparison of their item/person statistics. Educ Psychol Meas. (1998) 58:357–81. doi: 10.1177/0013164498058003001
75.
Reynolds CR Altmann RA Allen DN . Item analysis: methods for fitting the right items to the right test In: ReynoldsCRAltmannRAAllenDN, editors. Mastering modern psychological testing: Theory and methods. Cham: Springer International Publishing (2021). 263–89.
76.
Brown T . Confirmatory factor analysis for applied research. New York, NY: Guildford Press (2014).
77.
Torres Irribarra D Arneson AE . The challenge of defining and interpreting dimensionality in educational and psychological assessments. Measurement. (2023) 221:113430. doi: 10.1016/j.measurement.2023.113430
78.
Suhr D . Exploratory or confirmatory factor analysis?Cary, NC: SAS Institute (2006).
79.
Chen YH Li IY Chason W . The SAGE encyclopedia of educational research, measurement, and evaluation.
80.
Kyriazos TA Stalikas A . Applied psychometrics: the steps of scale development and standardization process. Psychology. (2018) 9:2531–60. doi: 10.4236/psych.2018.911145
81.
Morgado FFR Meireles JFF Neves CM Amaral ACS Ferreira MEC . Scale development: ten main limitations and recommendations to improve future research practices. Psicol Reflex Crit. (2017) 30:3. doi: 10.1186/s41155-016-0057-1
82.
Kline P . A handbook of test construction (psychology revivals): Introduction to psychometric design. London: Routledge (2015). 274 p.
83.
Faber J Fonseca LM . How sample size influences research outcomes. Dent Press J Orthod. (2014) 19:27–9. doi: 10.1590/2176-9451.19.4.027-029.ebo
84.
James G Witten D Hastie T . An introduction to statistical learning with applications in RSpringer (2023).
85.
Sackett DL . Evidence-based medicine. Semin Perinatol. (1997) 21:3–5. doi: 10.1016/S0146-0005(97)80013-4
86.
Frongillo EA Baranowski T Subar AF Tooze JA Kirkpatrick SI . Establishing validity and cross-context equivalence of measures and indicators. J Acad Nutr Diet. (2019) 119:1817–30. doi: 10.1016/j.jand.2018.09.005
87.
Prasher B Sachidanandan C Mukerji M Agarwal A . Ayurgenomics: Bringing age-old wisdom to the healthcare of the future. Council of Scientific & industrial research (CSIR). Available online at: https://www.csir.res.in/csir-success-stories/ayurgenomics-bringing-age-old-wisdom-healthcare-future
88.
Venkatraghavan S Sundaresan T Rajagopalan V Srinivasan K . Constitutional study of cancer patients – its prognostic and therapeutic scope. Anc Sci Life. (1987) 7:110–5. PMID:
89.
Ghodke Y Joshi K Patwardhan B . Traditional medicine to modern pharmacogenomics: Ayurveda Prakriti type and CYP2C19 gene polymorphism associated with the metabolic variability. Evid Based Complement Alternat Med. (2011) 2011:249528. doi: 10.1093/ecam/nep206
90.
Tripathi PK Patwardhan K Singh G . The basic cardiovascular responses to postural changes, exercise, and cold Pressor test: do they vary in accordance with the dual constitutional types of Ayurveda?Evid Based Complement Alternat Med. (2011) 2011:251850. doi: 10.1155/2011/251850
91.
Juyal RC Negi S Wakhode P Bhat S Bhat B Thelma BK . Potential of ayurgenomics approach in complex trait research: leads from a pilot study on rheumatoid arthritis. PLoS One. (2012) 7:e45752. doi: 10.1371/journal.pone.0045752
92.
Rastogi S Chiappelli F . Development and validation of a prototype Prakriti analysis tool (PPAT): inferences from a pilot study. AYU Int Q J Res Ayurveda. (2012) 33:209. doi: 10.4103/0974-8520.105240
93.
Tiwari S Tiwari S Gehlot S Singh G . Effect of walking (aerobic isotonic exercise) on physiological variants with special reference to Prameha (diabetes mellitus) as per Prakriti. AYU Int Q J Res Ayurveda. (2012) 33:44. doi: 10.4103/0974-8520.100308
94.
Rohit S Rohit G Hetal A Pk P . Prevalence of diabetes mellitus in Saurashtra region of Gujarat: a survey. Int J Res Ayurveda Pharm. (2012) 3:169–74.
95.
Bhalerao S Deshpande T Thatte U . Prakriti (Ayurvedic concept of constitution) and variations in platelet aggregation. BMC Complement Altern Med. (2012) 12:248. doi: 10.1186/1472-6882-12-248
96.
Umarkar SV Vyas DM Sathe KD Kulkarni SB . Case series study of different predominant Deha Prakriti with special reference to Fitzpatrick skin type classification. Int J Res Ayurveda Pharm. (2013) 4:797–9. doi: 10.7897/2277-4343.04603
97.
Kurande V Bilgrau AE Waagepetersen R Toft E Prasad R . Interrater reliability of diagnostic methods in traditional Indian Ayurvedic medicine. Evid Based Complement Alternat Med. (2013) 2013:1–12. doi: 10.1155/2013/658275
98.
Sharma R Prajapati P . Rising risk of type 2 diabetes among inhabitants of Jamnagar, Gujarat: a cross-sectional survey. AYU Int Q J Res Ayurveda. (2015) 36:10. doi: 10.4103/0974-8520.169014
99.
Junaid Farooque MM Aref M Khan MI Mohammed S . Data mining application in classification scheme of human subjects according to Ayurvedic Prakruti – temperament. Indian J Sci Technol. (2016) 9:1–4. doi: 10.17485/ijst/2016/v9i13/84658
100.
Singh R Sharma L Ota S Gupta B Singhal R Rana R et al . Development of a standardized assessment scale for assessing Prakriti (psychosomatic constitution). AYU Int Q J Res Ayurveda. (2022) 43:109–29. doi: 10.4103/ayu.ayu_239_22
101.
Tiwari P Kutum R Sethi T Shrivastava A Girase B Aggarwal S et al . Recapitulation of Ayurveda constitution types by machine learning of phenotypic traits. PLoS One. (2017) 12:e0185380. doi: 10.1371/journal.pone.0185380
102.
Dunlap C Hanes D Elder C Nygaard C Zwickey H . Reliability of self-reported constitutional questionnaires in Ayurveda diagnosis. J Ayurveda Integr Med. (2017) 8:257–62. doi: 10.1016/j.jaim.2017.04.011
103.
Gupta A Ali A Tewari P Agrawal N Patel R Byadgi P . Association of Kaphaja and Kapha-Pittaja Prakriti and methylenetetrahydrofolate reductase C677T allele with type 2 diabetes. AYU Int Q J Res Ayurveda. (2018) 39:146. doi: 10.4103/ayu.AYU_230_18
104.
Kardam L Rai S Singh KN Singh R . Genetic association of infertility in vataj prakriti female patients with reproductive age group. Int J Green Pharmacy. (2018) 12:212–5. doi: 10.22377/ijgp.v12i03.1954
105.
Shirolkar A Chakraborty S Mandal T Dabur R . Plasma metabolomics reveal the correlation of metabolic pathways and Prakritis of humans. J Ayurveda Integr Med. (2018) 9:113–22. doi: 10.1016/j.jaim.2017.05.002
106.
Madaan V Gayal A . An adaptive neuro fuzzy inference system for predicting Ayurvedic Dosha In: 4th international conference on information systems and computer networks (ISCON). Mathura: IEEE (2019). 335–9.
107.
Banerjee S Chattopadhyay K Biswas TK Chattopadhyay B . Comparative analysis of the biochemical parameters among the three Prakriti individuals having type 2 diabetes mellitus. (2019)
108.
Niraj S Sangeeta G . Affiliation among infantile age, morbidity and prakriti (physical constitution): a longitudinal preliminary study. J Nat Remedies. (2019) 19:43–8. doi: 10.18311/jnr/2019/23286
109.
Tripathi K Gehlot S . Development, validation and confirmation of an archetype tool to evaluate Prakriti. J Nat Remedies. (2019) 19:206–13. doi: 10.18311/jnr/2019/23788
110.
Sivapuram MS Srivastava V Kaur N Anand A Nagarathna R Patil S et al . Ayurveda body–mind constitutional types and role of yoga intervention among type 2 diabetes mellitus population of Chandigarh and Panchkula regions. Ann Neurosci. (2020) 27:214–23. doi: 10.1177/09727531211000040
111.
Prasad S Prasad R Prasad V Prasad RS . Identification of ‘Manas’ (states of mind): simulation studies in biophotonics In: 2020 international conference on e-health and bioengineering (EHB). Iasi, Romania: IEEE (2020). 1–4.
112.
Bhanushali D Tyagi R N L(RN) Anand A . Effect of mindfulness meditation protocol in subjects with various psychometric characteristics at high altitude. Brain Behav. (2020) 10:e01604. doi: 10.1002/brb3.1604
113.
Madaan V Goyal A . Analysis and synthesis of a human Prakriti identification system based on soft computing techniques. Recent Adv Comput Sci Commun. (2020) 13:1126–35. doi: 10.2174/2213275912666190207144831
114.
Joshi S Bajaj P . Design & Development of portable Vata, Pitta & Kapha [VPK] pulse detector to find Prakriti of an individual using artificial neural network In: 2021 6th international conference for convergence in technology (I2CT). Maharashtra: IEEE (2021). 1–6.
115.
Banerjee S Biswas TK Chattopadhyay K Arzoo SH Chattopadhyay B . An approach to screen genotoxic-susceptible diabetic population of various Prakriti groups for personalized disease management. J Altern Complement Med. (2021) 27:80–7. doi: 10.1089/acm.2020.0001
116.
Mangampadath A Sudhikumar KB . Development of a clinically useful tool for Prakriti assessment. Int J Ayurvedic Med. (2021) 12:599–609. doi: 10.47552/ijam.v12i3.2042
117.
Patil A Pande B Sorte S Borade N . Comparison of cardiovascular autonomic function status in normal healthy individuals of various Ayurveda Prakritis. J Pharm Negative Results. (2021) 12:49–53.
118.
Rao S Rao KR . A reliability test for the body constitution diagnosis using wrist pulse analysis based on Ayurveda. Indian J Tradit Knowl. (2022) 21:730–6.
119.
Chinnaiah MC Dubey S Janardhan N Pathi V K N M A . Analysis of Pitta imbalance in young Indian adult using machine learning algorithm In: 2022 2nd international conference on intelligent technologies (CONIT). Hubli: IEEE (2022). 1–5.
120.
Kuttikrishnan M Sridhar R Varghese E . Jatharagni and Prakriti of young Indian adult population: a descriptive cross-sectional study. J Ayurveda Integr Med. (2022) 13:100438. doi: 10.1016/j.jaim.2021.04.008
121.
Deshmukh M Khemchandani M . Illness diagnosis based on Ayurveda (vat, Pitta, Kapha Dosha) using machine learning In: 2023 6th international conference on advances in science and technology (ICAST). Mumbai: IEEE (2023). 213–6.
122.
Khatua D Sekh AA Kutum R Mukherji M Prasher B Kar S . Classification of Ayurveda constitution types: a deep learning approach. Soft Comput. (2023) 27:5309–17. doi: 10.1007/s00500-023-07942-2
123.
Doddoli G Doddoli S Shete S Verma A . Development and validation of rapid Prakriti analysis tool for a standard clinical approach. Tradit Med Mod Med. (2023) 6:43–52. doi: 10.1142/S2575900023500106
124.
Dua P Seth S Pandey RM Maulik S . Indian system of medicine used concurrently with standard conventional medicine improves quality of life in patients of cardio vascular diseases (C.V.D). Indian J Tradit Knowl. (2023) 22:314–21. doi: 10.56042/ijtk.v22i2.36672
125.
Jamdaade KM Patil HY . Prakruti Nishchitikaran of human body using supervised machine learning approach In: Data management, analytics and innovation lecture notes in networks and systems 662. Singapore: Springer Nature Singapore (2023). 187–200.
126.
Roushan R Singhal R Barla M Gaur M . Development and validation of a self-assessment tool to determine Prakriti, the human constitution types of Indian traditional medicine. Indian J Health Sci Biomed Res KLEU. (2023) 16:376. doi: 10.4103/kleuhsj.kleuhsj_170_22
127.
Khuntia BK Rathore S Sharma V Rani A Agarwal A Rengarajan K et al . Comparison of questionnaire and clinician-based assessment of Prakriti/body constitution in young adults: an observational study. Int J Commun Med Public Health. (2024) 11:4326–34. doi: 10.18203/2394-6040.ijcmph20243293
128.
Bhosale A Girase P Waghmare A Barve S Chikmurge D . Predicting human body Prakriti with Ayurvedic Tridosha features using ensemble learning models In: 2024 international conference on intelligent algorithms for computational intelligence systems (IACIS). Hassan: IEEE (2024). 1–7.
129.
Piplani Y Mehra PS . An unsupervised learning model for Ayurvedic Prakriti determination using smart device data In: 2024 OPJU international technology conference (OTCON) on smart computing for innovation and advancement in industry 40. Raigarh: IEEE (2024). 1–6.
130.
Tawalare K Wakde S Tawalare KA Bhamkar S Bagde R Saoji A et al . Estimation of prakriti (body constitution) in women of eastern Maharashtra tribal belt of Central India featuring early and premature menopause. J Fam Med Prim Care. (2024) 13:1665–9. doi: 10.4103/jfmpc.jfmpc_376_23
131.
Mathew AA Shanmugasundaram V . Single-electrode triboelectric nanogenerator device framework for wrist pulse signal (Nadi) analysis and disease detection. IEEE Access. (2024) 12:93531–45. doi: 10.1109/ACCESS.2024.3423401
132
Charaka . Charaka Samhita, Indriya Sthana 1/5: Text with English Translation and Critical Exposition Based on Chakrapani Datta’s Ayurveda Dipika. In: SharmaRKDashB, editors. Varanasi: Chaukhambha Sanskrit Series Office; (2014).
133
Vagbhata . Ashtanga Hridaya of Vagbhata, Śārīrasthāna 3/87–91. In: Ashtanga Hridaya of Vagbhata. Varanasi: Chaukhambha Krishnadas Academy; (2012).
134.
Sharma PV . Charaka Samhita: Text with English Translation. Vimānasthānam 8/95. 2nd ed.Varanasi: Chaukhambha Orientalia; (2017).
135
Bhargav H Jasti N More P Kumar V Chikkanna U Kishore Kumar R et al . Correlation of prakriti diagnosis using AyuSoft prakriti diagnostic tool with clinician rating in patients with psychiatric disorders. J Ayurveda Integr Med [Internet]. (2021) Apr [cited 2025 Jun 11];12:365–8. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0975947621000115
Summary
Keywords
Prakriti, Ayurveda, body constitutional typology, personalized health care, technology integration, measurable correlates
Citation
Venkatesh A, Johansson L, Sivanandan PV, Gopakumar SP, Sankaranarayanan K, Kessler CS, Ravani S and Puthiyedath R (2025) Prakriti (constitutional typology) in Ayurveda: a critical review of Prakriti assessment tools and their scientific validity. Front. Med. 12:1656249. doi: 10.3389/fmed.2025.1656249
Received
29 June 2025
Accepted
26 September 2025
Published
06 November 2025
Volume
12 - 2025
Edited by
Chandra Kant Katiyar, Emami, India
Reviewed by
Avvinish Annant Narine, National Institute of Ayurveda, India; Abhimanyu Kumar, Centre for Ayurveda Education, Innovation and Technology (CAYEIT), India
Updates
Copyright
© 2025 Venkatesh, Johansson, Sivanandan, Gopakumar, Sankaranarayanan, Kessler, Ravani and Puthiyedath.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rammanohar Puthiyedath, rammanohar@ay.amrita.edu
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.