- Teaching and Research Section of Clinical Nursing, The Second Xiangya Hospital, Central South University, Changsha, China
Background: The growing application of assisted reproductive technology (ART) has enabled more women with polycystic ovary syndrome (PCOS) to achieve pregnancy. However, the causal association between PCOS and reproductive outcomes remains uncertain. This study conducted a meta-analysis of cohort studies to explore the association between PCOS and adverse pregnancy and perinatal outcomes.
Methods: A comprehensive search was carried out in PubMed, Web of Science, Embase, and the Cochrane Library to identify studies published prior to March 22, 2025. Cohort studies evaluating differences in adverse pregnancy and perinatal outcomes between women with PCOS and those without PCOS undergoing ART were included. Meta-analysis was conducted using R 4.3.2 and STATA 12.0 to estimate risk ratios (RRs) and 95% confidence intervals (CIs) for the association between PCOS and adverse outcomes. Study heterogeneity was assessed through Cochran’s Q test, I2 statistics, and 95% prediction intervals (PIs). Additionally, subgroup analysis, sensitivity analysis, and publication bias evaluation were performed to ensure the reliability and validity of the results.
Results: This meta-analysis included 18 cohort studies, comprising 16,365 women with PCOS and 111,503 controls. Women with PCOS undergoing ART were found to have significantly higher clinical pregnancy rate (RR = 1.158, 95% CI: 1.004–1.335; 95% PI: 0.751–1.785) and live birth rate (RR = 1.084, 95% CI: 1.027–1.144; 95% PI: 0.827–1.361) compared to those without PCOS. However, these patients also exhibited an increased risk of miscarriage (RR = 1.301, 95% CI: 1.181–1.433; 95% PI: 0.917–1.957), gestational diabetes mellitus (GDM), hypertensive disorders of pregnancy (HDP), gestational hypertension, preterm premature rupture of membranes (PPROM), preterm birth (PTB) (RR = 1.259, 95% CI: 1.152–1.376; 95% PI: 1.143–1.387), and very preterm birth (VPTB), while showing a reduced risk of cesarean delivery (RR = 0.898, 95% CI: 0.810–0.994; 95% PI: 0.717–1.124). No significant differences were identified between PCOS and control groups regarding the risks of low birth weight, very low birth weight, macrosomia, small for gestational age, very small for gestational age, large for gestational age, or fetal malformation (all p > 0.05). Subgroup analysis of patients undergoing frozen embryo transfer (FET) yielded consistent results.
Conclusion: PCOS may affect pregnancy and perinatal outcomes in women undergoing ART, with an increased risk of miscarriage, GDM, HDP, gestational hypertension, PPROM, PTB, and VPTB. These results underscore the importance of tailored reproductive strategies and specialized perinatal management for women affected by PCOS.
1 Introduction
Polycystic ovary syndrome (PCOS), a prevalent endocrine disorder, affects an estimated 5–20% of women of reproductive age globally, making it one of the leading causes of female infertility (1). This complex disorder is marked by irregular ovarian function, an imbalance in androgen levels, and the presence of cyst-like structures in the ovaries (2). Beyond its reproductive implications, this syndrome is associated with metabolic disturbances and psychological comorbidities, exerting a multifaceted impact across the lifespan (3). Infertility in women with PCOS is frequently attributed to anovulation (4), often necessitating the use of assisted reproductive technologies (ART) to achieve pregnancy. Techniques such as in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI) have demonstrated efficacy in improving fertility outcomes for affected individuals (4, 5). However, the underlying pathophysiological complexities of PCOS may predispose patients to heightened risks during pregnancy and childbirth, with potential adverse effects on maternal health and neonatal outcomes.
Existing evidence indicates that women with PCOS are at an elevated risk of adverse pregnancy and perinatal outcomes, including preterm birth (PTB), miscarriage, gestational hypertension, and gestational diabetes mellitus (GDM) (6–8), irrespective of whether conception occurs naturally or through ART. Recent investigations further suggested that frozen embryo transfer (FET) cycles in PCOS patients were associated with an increased likelihood of neonatal complications, such as PTB (9). A multicenter randomized controlled trial involving 1,508 infertile women with PCOS after their first IVF cycle demonstrated that FET was associated with a significantly higher live birth rate compared with fresh embryo transfer (10). However, the heterogeneous nature of PCOS, coupled with its metabolic and hormonal complexities, renders its impact on pregnancy outcomes following IVF contentious. For instance, Sterling et al. (11) identified an increased risk of PTB and large for gestational age (LGA) among women with PCOS undergoing fresh embryo transfer, while no significant differences were observed for preterm premature rupture of membranes (PPROM). However, Qiu et al. (12) reported no differences in neonatal birth weight but noted a higher incidence of very preterm birth (VPTB) and PPROM in PCOS patients undergoing FET. These conflicting findings underscore the need for a meta-analysis to clarify the association between PCOS and adverse maternal and neonatal outcomes in ART-conceived pregnancies.
While previous meta-analyses have explored the association between PCOS and adverse pregnancy or perinatal outcomes (13–15), the inclusion of case–control and cross-sectional studies has limited the ability to establish a clear causal relationship. Therefore, we performed a meta-analysis focused exclusively on cohort studies to evaluate the risks of maternal and neonatal complications in women with PCOS undergoing ART, with a specific emphasis on comparing outcomes between FET and fresh embryo transfer cycles. Importantly, data for this analysis were extracted directly from logistic regression models reported in the included studies, enhancing the reliability and precision of the findings. This study aimed to provide robust evidence to better elucidate the causal relationship between PCOS and adverse reproductive outcomes following ART.
2 Materials and methods
2.1 Study design
This systematic review and meta-analysis was conducted in accordance with the Preferred Reporting Item for Systematic Reviews and Meta-analysis (PRISMA) guidelines (16). The study protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO) under the identifier CRD420251079585.
2.2 Search strategy
To identify high-quality studies, a systematic search was conducted across 4 major electronic databases, including Web of Science, PubMed, Embase, and the Cochrane Library, from their inception to March 22, 2025. The search strategy incorporated a combination of terms, encompassing (“polycystic ovary syndrome” OR “PCOS” OR “polycystic ovarian syndrome” OR “sclerocystic ovary syndrome”) AND (“preterm birth” OR “low birth weight” OR “macrosomia” OR “small for gestational age” OR “large for gestational age” OR “gestational diabetes mellitus” OR “hypertensive disorders of pregnancy” OR “cesarean delivery” OR “preterm premature rupture of membranes” OR “malformations” OR “clinical pregnancy rate” OR “miscarriage” OR “live birth rate” OR “pregnancy outcomes” OR “obstetric outcomes” OR “reproductive outcomes” OR “fertility outcomes”) AND (“cohort study” OR “cohort studies” OR “retrospective” OR “prospective”). Detailed search methodologies tailored to each database were outlined in Supplementary File 1. No restrictions on language were applied during the search process. Additionally, reference lists of relevant original studies and review articles were manually screened to identify any additional eligible studies.
2.3 Inclusion and exclusion criteria
Eligible studies were identified according to the following inclusion criteria: (1) the use of a cohort design, either prospective or retrospective; (2) the exposed population consisted of women diagnosed with PCOS who underwent ART; (3) the comparison group included women without PCOS who also underwent ART; (4) the study provided risk estimates, such as risk ratios (RRs) or odds ratios (ORs), accompanied by 95% confidence intervals (CIs), evaluating the relationship between PCOS and adverse pregnancy or perinatal outcomes; (5) no restrictions were applied to the language of the study. Studies were excluded if they met any of the following criteria: (1) employed a case–control or cross-sectional design; (2) analyzed a mixed population without distinguishing outcomes from natural conception versus ART; (3) failed to provide data on relevant outcomes; (4) case reports, conference abstracts, reviews, animal studies, editorials, or commentaries.
2.4 Data extraction and quality assessment
Following the predefined eligibility criteria, two reviewers independently evaluated the titles, abstracts, and full texts to determine suitability for inclusion. Data extracted from the eligible studies included the following variables: name of the first author, year of publication, study location, research design, sample size, selection of the controls, maternal age and body mass index (BMI) for PCOS patients and controls, type of ART utilized, adjusted confounding factors, and outcomes included in the meta-analysis. The methodological quality of the included cohort studies was appraised using the Newcastle-Ottawa Scale (NOS) (17), which evaluates studies across three domains: selection of participants, comparability of groups, and assessment of exposure. Each domain contains specific criteria scored on a scale of one or two points, depending on the degree to which standards are met. Studies were categorized according to their NOS scores as low quality (0–3 points), moderate quality (4–6 points), or high quality (7–9 points) (18).
2.5 Statistical analysis
To examine the association between PCOS and adverse pregnancy or perinatal outcomes, RRs with 95% CIs were calculated. Heterogeneity among studies was assessed using Cochran’s Q test, complemented by the I2 and Tau2 statistics, as well as the 95% prediction interval (PI) (19, 20). When the data demonstrated homogeneity (p < 0.10 or I2 > 50%), a random-effects model, utilizing the DerSimonian-Laird method, was applied to estimate the association. In contrast, when no significant heterogeneity was detected, the Mantel–Haenszel fixed-effects model was used (21). To ensure the reliability of the findings, sensitivity analyses were performed by systematically excluding individual studies. Additionally, subgroup analyses were conducted for categories with at least two studies to explore the influence of ART type on pooled RR estimates. Publication bias was assessed using Begg’s (22) and Egger’s (23) tests. A two-sided p-value of less than 0.05 was considered statistically significant. All statistical analyses were conducted using STATA (version 12.0) and R (version 4.3.2).
3 Results
3.1 Search results
A systematic database search initially identified 9,351 articles for potential inclusion. Following the removal of 3,180 duplicate records using EndNote X9 software and the exclusion of 6,055 studies based on a preliminary screening of titles and abstracts, 116 articles were retained for detailed evaluation. After applying the inclusion and exclusion criteria, 98 studies were excluded from the final meta-analysis. Of these, 39 studies were removed because not all patients received ART conception, and 32 were excluded due to the absence of reported RRs or ORs with corresponding 95% CIs for adverse pregnancy or perinatal outcomes. Additionally, 8 studies were excluded due to their case–control or cross-sectional design, and 5 were removed as overlapping cohorts. A further 10 studies were excluded for failing to meet the definition of the PCOS group, and 4 lacked an appropriate control group. Ultimately, 18 studies met the eligibility criteria and were included in the meta-analysis (9, 11, 12, 24–38) (Figure 1).
3.2 Characteristics and quality assessment of the included studies
The characteristics of the included studies were summarized in Table 1. To ensure relevance and timeliness, only research published from 2009 onwards was eligible for inclusion in the meta-analysis. A total of 18 retrospective cohort studies were analyzed, comprising 16,365 participants diagnosed with PCOS and 111,503 individuals in the control group. The maternal outcomes assessed in the meta-analysis included clinical pregnancy rate, miscarriage, GDM, hypertensive disorders of pregnancy (HDP), gestational hypertension, PPROM, and cesarean delivery. Fetal outcomes comprised live birth rate, PTB, VPTB, low birth weight (LBW), very low birth weight (VLBW), macrosomia, small for gestational age (SGA), very small for gestational age (VSGA), LGA, and fetal malformation. According to the World Health Organization (WHO), PTB was defined as delivery before 37 weeks of gestation, while VPTB referred to delivery before 32 weeks. LBW and VLBW were categorized as birth weights below 2,500 g and 1,500 g, respectively, whereas macrosomia was defined as a birth weight exceeding 4,000 g. SGA and VSGA were classified as birth weights below the 10th and 3rd percentiles, respectively (39), while LGA was defined as a birth weight above the 90th percentile. All studies included in the meta-analysis were deemed to be of high methodological quality, as they provided comprehensive descriptions of their study designs (Supplemental File 2).
3.3 Meta-analysis of adverse maternal outcomes
3.3.1 Clinical pregnancy rate and miscarriage
The meta-analysis of 10 studies investigating the clinical pregnancy rate in women with PCOS undergoing ART revealed a pooled RR of 1.158 (95% CI: 1.004–1.335; 95% PI: 0.751–1.785), indicating a modest increase in clinical pregnancy rates compared with women without PCOS. Notably, substantial heterogeneity was detected across the included studies (I2 = 84.4%, Tau2 = 0.0313) (Table 2; Figure 2A). Subgroup analysis revealed that the association between PCOS and higher clinical pregnancy rate persisted in women receiving FET (RR = 1.204, 95% CI: 1.018–1.423; 95% PI: 0.760–1.906), though significant heterogeneity was still present (I2 = 68.9%, Tau2 = 0.0246). However, whereas no significant association was observed among fresh/frozen embryo transfer patients (RR = 1.015, 95% CI: 0.993–1.037; 95% PI: 0.882–1.168) (Table 2; Supplementary Figure S1A,B).
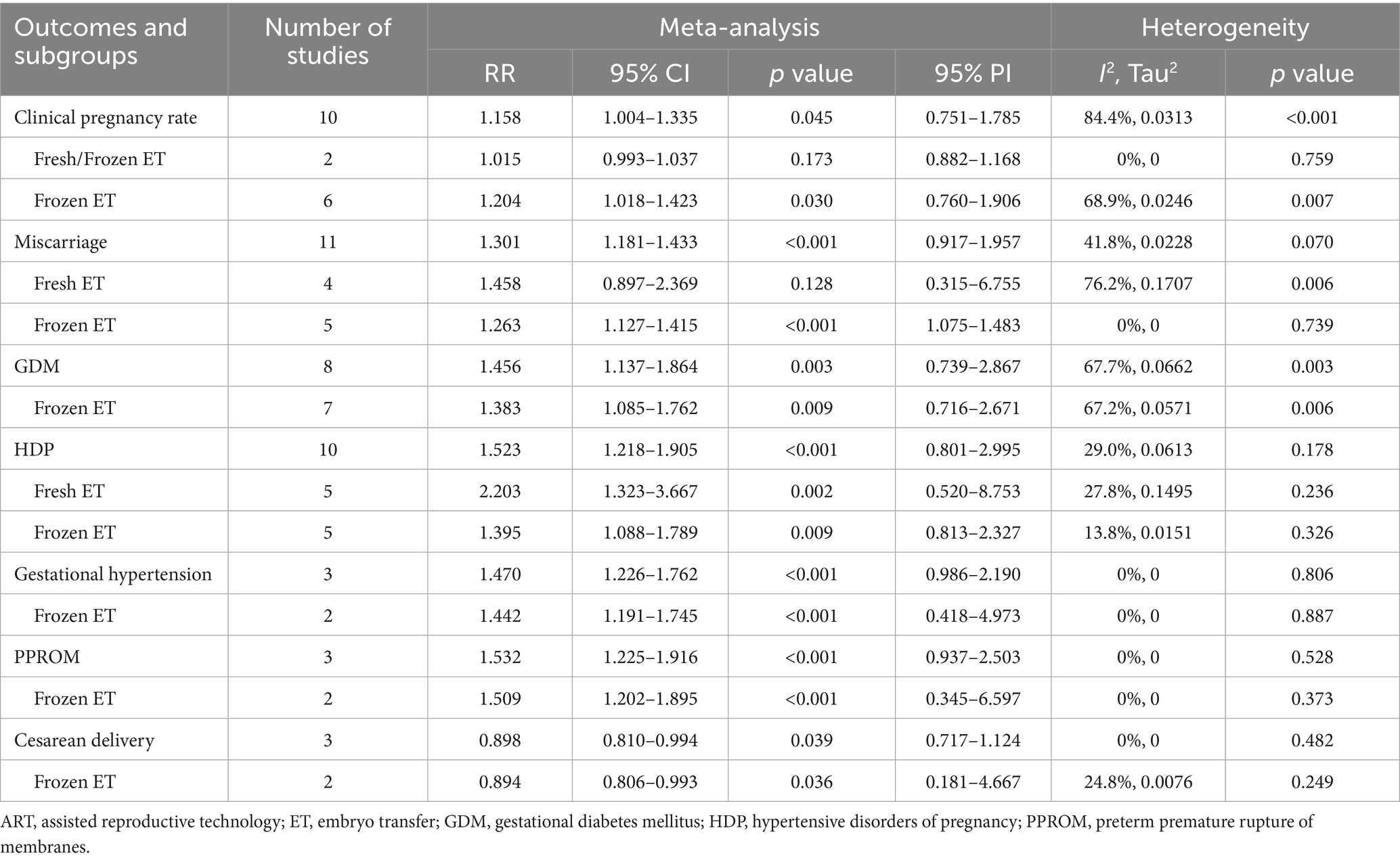
Table 2. Pooled effect and subgroup analysis of the association between polycystic ovary syndrome and adverse maternal outcomes in women who had undergone ART.
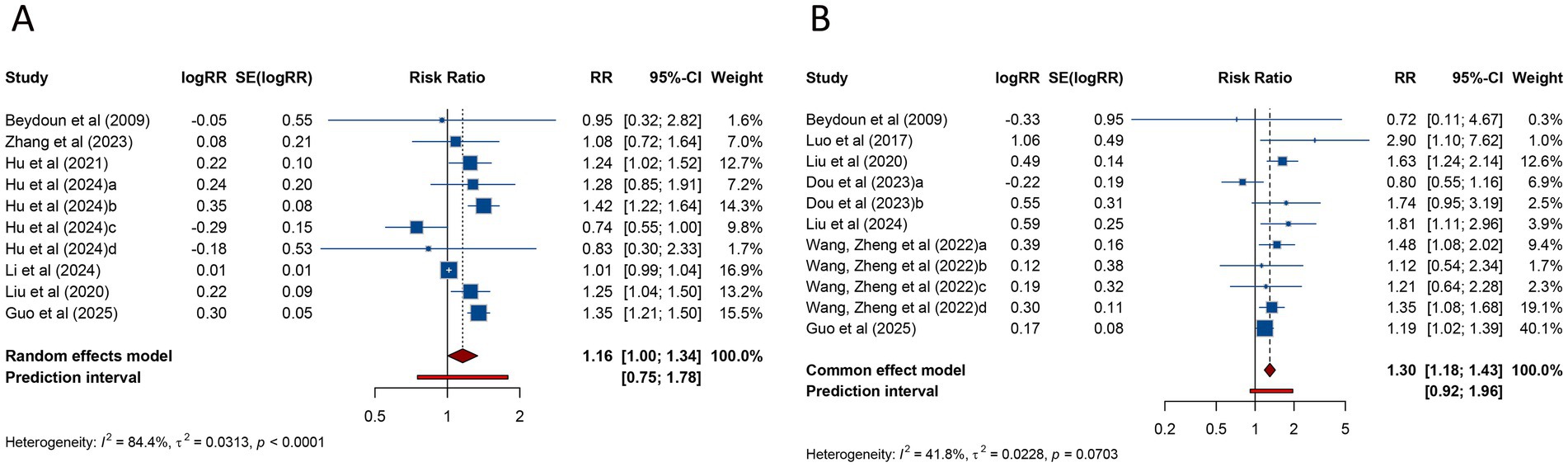
Figure 2. Forest plots of the association between polycystic ovary syndrome and clinical pregnancy rate (A) and miscarriage (B) in women undergoing assisted reproductive technology.
Regarding miscarriage, a meta-analysis of 11 studies indicated that PCOS was associated with a significantly higher risk of miscarriage (RR = 1.301, 95% CI: 1.181–1.433; 95% PI: 0.917–1.957), with no significant heterogeneity (I2 = 41.8%, Tau2 = 0.0228) (Table 2; Figure 2B). Subgroup analysis revealed that the heightened miscarriage risk was significant in patients undergoing FET (RR = 1.263, 95% CI: 1.127–1.415; 95% PI: 1.075–1.483), whereas no such association was found in those receiving fresh embryo transfer (RR = 1.458, 95% CI: 0.897–2.369; 95% PI: 0.315–6.755) (Table 2; Supplementary Figure S1C,D).
3.3.2 GDM, HDP, and gestational hypertension
Eight studies assessed the risk of GDM in women with PCOS undergoing ART. The combined analysis demonstrated that PCOS patients had a significantly increased risk of GDM compared to those without PCOS (RR = 1.456, 95% CI: 1.137–1.864; 95% PI: 0.739–2.867), accompanied by notable heterogeneity across studies (I2 = 67.7%, Tau2 = 0.0662) (Table 2; Figure 3A). Subgroup analysis showed that this elevated risk remained significant in patients undergoing FET (RR = 1.383, 95% CI: 1.085–1.762; 95% PI: 0.716–2.671) (Table 2; Supplementary Figure S2A).
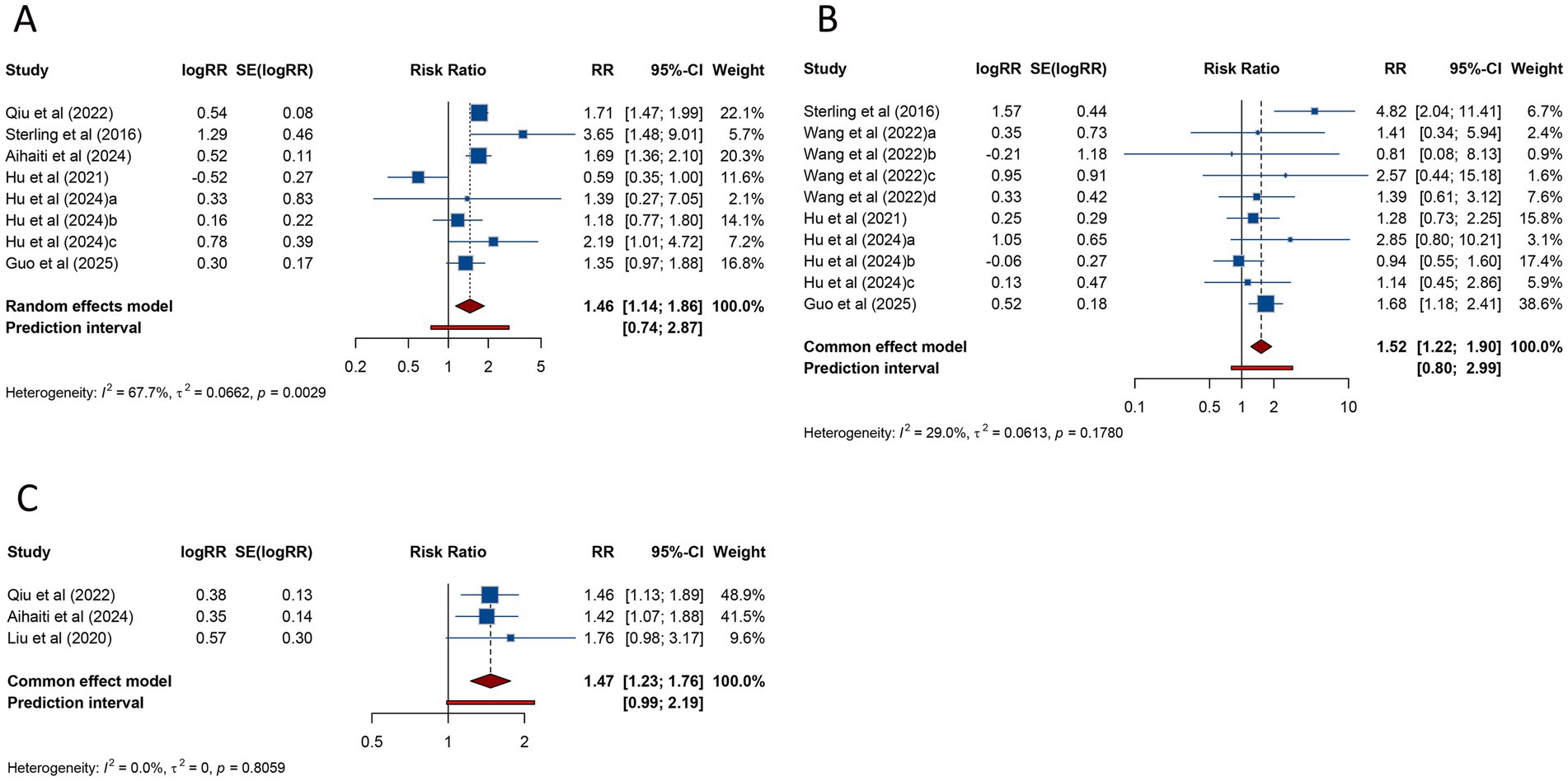
Figure 3. Forest plots of the association between polycystic ovary syndrome and gestational diabetes mellitus (A), hypertensive disorders of pregnancy (B), and gestational hypertension (C) in women undergoing assisted reproductive technology.
HDP was examined in 10 studies, with pooled data indicating a significantly higher risk in women with PCOS compared to controls (RR = 1.523, 95% CI: 1.218–1.905; 95% PI: 0.801–2.995). Unlike the findings for GDM, no significant heterogeneity was observed (I2 = 29.0%, Tau2 = 0.0613) (Table 2; Figure 3B). Subgroup analysis confirmed that this association persisted in women undergoing either fresh embryo transfer (RR = 2.203, 95% CI: 1.323–3.667; 95% PI: 0.520–8.753) or FET (RR = 1.395, 95% CI: 1.088–1.789; 95% PI: 0.813–2.327) (Table 2; Supplementary Figure S2B,C).
Three studies specifically addressed gestational hypertension, showing a significantly increased risk in women with PCOS (RR = 1.470, 95% CI: 1.226–1.762; 95% PI: 0.986–2.190), with no evidence of heterogeneity (I2 = 0%, Tau2 = 0) (Table 2; Figure 3C). This elevated risk was also observed in the subgroup of patients undergoing FET (RR = 1.442, 95% CI: 1.191–1.745; 95% PI: 0.418–4.973) (Table 2; Supplementary Figure S2D).
3.3.3 PPROM and cesarean delivery
Three studies assessed the risk of PPROM in women with PCOS undergoing ART. The pooled analysis identified a significantly elevated risk of PPROM in patients with PCOS compared to those without the condition (RR = 1.532, 95% CI: 1.225–1.916; 95% PI: 0.937–2.503), with no evidence of substantial heterogeneity (I2 = 0%, Tau2 = 0) (Table 2; Figure 4A). Subgroup analysis further revealed that the increased risk of PPROM persisted among women undergoing FET (RR = 1.509, 95% CI: 1.202–1.895; 95% PI: 0.345–6.597) (Table 2; Supplementary Figure S3A).
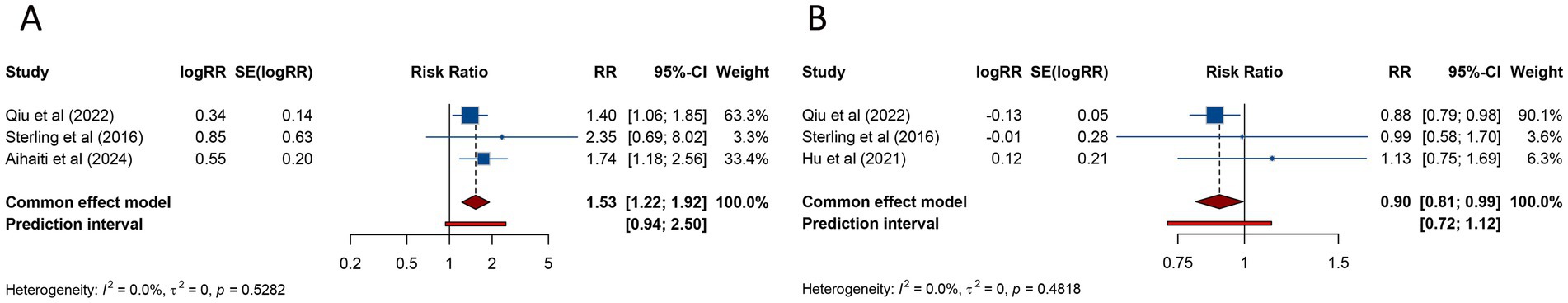
Figure 4. Forest plots of the association between polycystic ovary syndrome and preterm premature rupture of membranes (A) and cesarean delivery (B) in women undergoing assisted reproductive technology.
Cesarean delivery was investigated in 3 studies, with pooled findings showing a significantly reduced likelihood of cesarean delivery among women with PCOS (RR = 0.898, 95% CI: 0.810–0.994; 95% PI: 0.717–1.124). No substantial heterogeneity was detected in the analysis (I2 = 0%, Tau2 = 0) (Table 2; Figure 4B). Subgroup analysis also demonstrated that the lower risk of cesarean delivery persisted in women who underwent FET (RR = 0.894, 95% CI: 0.806–0.993; 95% PI: 0.181–4.667) (Table 2; Supplementary Figure S3B).
3.4 Meta-analysis of adverse fetal outcomes
3.4.1 Live birth rate, PTB, and VPTB
The meta-analysis of 13 studies evaluating live birth rate in women with PCOS undergoing ART revealed a pooled RR of 1.084 (95% CI: 1.027–1.144; 95% PI: 0.827–1.361), suggesting a modest increase in live birth rate compared to women without PCOS. No significant heterogeneity was observed (I2 = 48.8%, Tau2 = 0.0108) (Table 3; Figure 5A). Subgroup analysis indicated that the association between PCOS and higher live birth rate was significant in patients undergoing FET (RR = 1.171, 95% CI: 1.092–1.256; 95% PI: 0.918–1.421), but not in those receiving fresh embryo transfer or combined fresh/frozen embryo transfer (all p > 0.05) (Table 3; Supplementary Figure S4A–C).

Table 3. Pooled effect and subgroup analysis of the association between polycystic ovary syndrome and adverse fetal outcomes in women who had undergone ART.
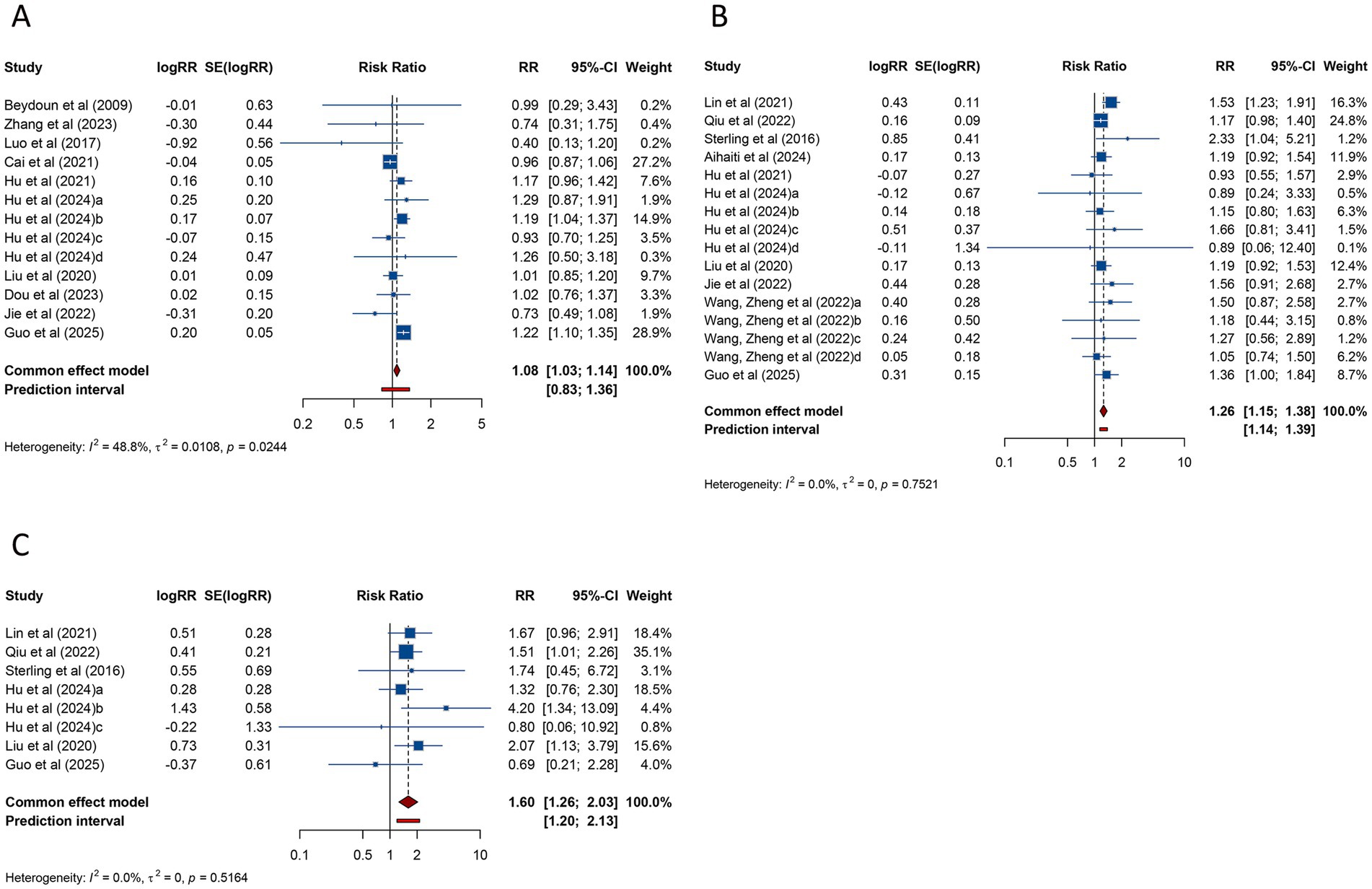
Figure 5. Forest plots of the association between polycystic ovary syndrome and live birth rate (A), preterm birth (B), and very preterm birth (C) in women undergoing assisted reproductive technology.
PTB, analyzed across 16 studies, was found to be significantly more frequent in women with PCOS undergoing ART (RR = 1.259, 95% CI: 1.152–1.376; 95% PI: 1.143–1.387), with no evidence of heterogeneity (I2 = 0%, Tau2 = 0) (Table 3; Figure 5B). Subgroup analysis revealed that the increased risk of PTB was significant in patients undergoing FET (RR = 1.259, 95% CI: 1.144–1.385; 95% PI: 1.133–1.399) but not in those receiving fresh embryo transfer (RR = 1.485, 95% CI: 0.797–2.769; 95% PI: 0.003–708.499) (Table 3; Supplementary Figure S5A,B).
The risk of VPTB was examined in 8 studies, with pooled results showing a significantly higher incidence in women with PCOS (RR = 1.597, 95% CI: 1.258–2.027; 95% PI: 1.198–2.130). No heterogeneity was detected (I2 = 0%, Tau2 = 0) (Table 3; Figure 5C). Subgroup analysis confirmed that this elevated risk was consistent across fresh embryo transfer (RR = 2.013, 95% CI: 1.160–3.493; 95% PI: 0.056–71.796) or FET (RR = 1.514, 95% CI: 1.162–1.973; 95% PI: 0.980–2.343) (Table 3; Supplementary Figure S5C,D).
3.4.2 LBW, VLBW, and macrosomia
The risk of LBW was assessed in 10 studies, with pooled analysis showing no significant association between PCOS and LBW (RR = 1.082, 95% CI: 0.942–1.242; 95% PI: 0.631–1.696), although heterogeneity was not significant (I2 = 38.2%, Tau2 = 0.0362) (Table 3; Figure 6A). Subgroup analysis focusing on FET similarly found no evidence of a significant association (RR = 1.075, 95% CI: 0.935–1.236; 95% PI: 0.588–1.756) (Table 3; Supplementary Figure S6A).
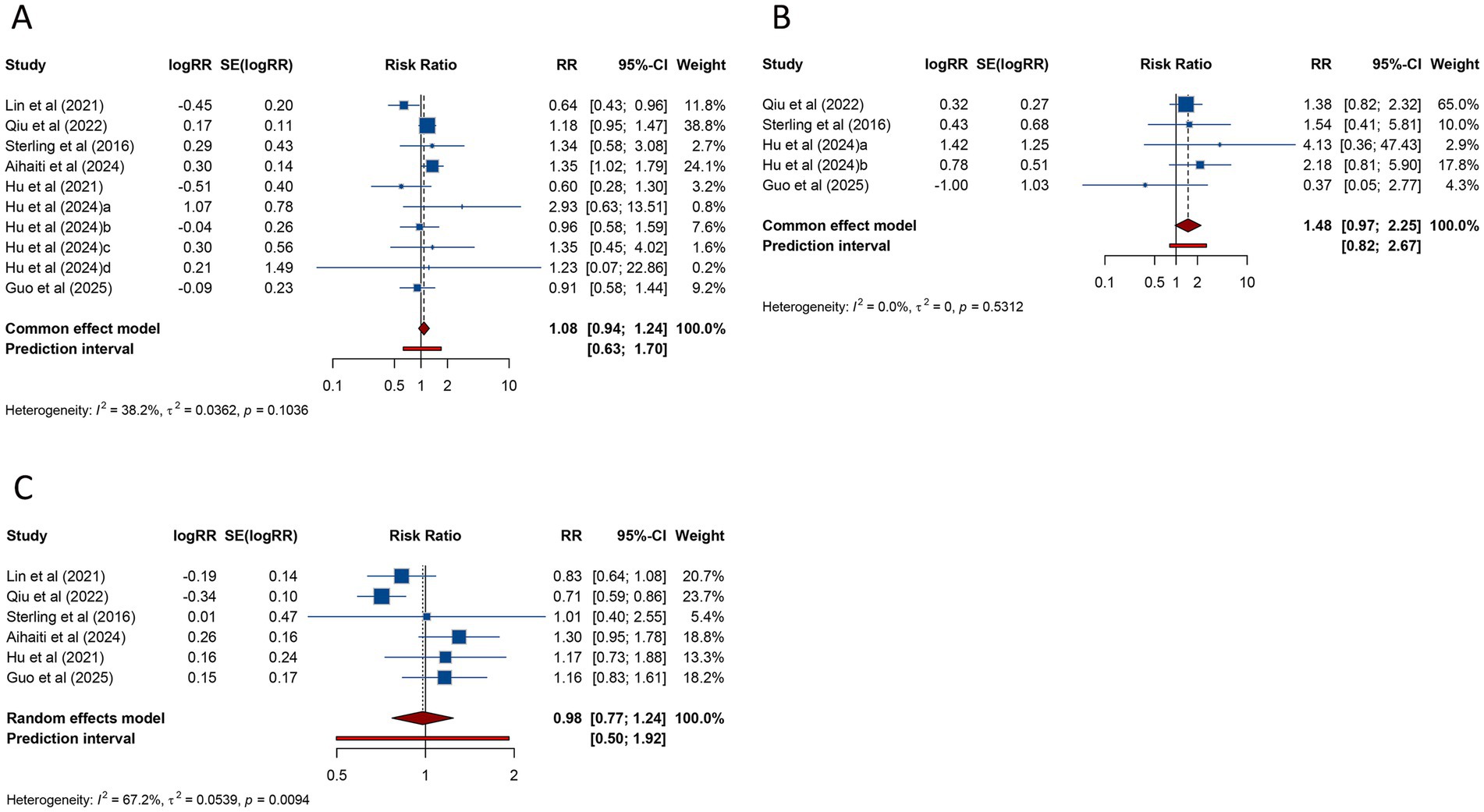
Figure 6. Forest plots of the association between polycystic ovary syndrome and low birth weight (A), very low birth weight (B), and macrosomia (C) in women undergoing assisted reproductive technology.
VLBW was investigated in 5 studies, and the pooled findings indicated no substantial increase in risk for women with PCOS undergoing ART (RR = 1.476, 95% CI: 0.971–2.245; 95% PI: 0.815–2.674) (Table 3; Figure 6B). Subgroup analysis for FET also demonstrated no significant association (RR = 1.470, 95% CI: 0.945–2.286; 95% PI: 0.604–3.617) (Table 3; Supplementary Figure S6B).
Macrosomia, assessed in 6 studies, showed no significant association with PCOS (RR = 0.979, 95% CI: 0.770–1.243; 95% PI: 0.499–1.921), with significant heterogeneity (I2 = 67.2%, Tau2 = 0.0539) (Table 3; Figure 6C). Subgroup analysis for FET similarly showed no significant association (RR = 0.979, 95% CI: 0.757–1.266; 95% PI: 0.452–2.124) (Table 3; Supplementary Figure S6C).
3.4.3 SGA, VSGA, LGA, and fetal malformation
The relationship between PCOS and SGA was investigated in 8 studies, with pooled data indicating no significant association (RR = 0.973, 95% CI: 0.835–1.134; 95% PI: 0.645–1.543) and an absence of heterogeneity (I2 = 29.7%, Tau2 = 0.0234) (Table 3; Figure 7A). Subgroup analysis of FET yielded comparable results (RR = 0.973, 95% CI: 0.833–1.136; 95% PI: 0.597–1.689) (Table 3; Supplementary Figure S7A).
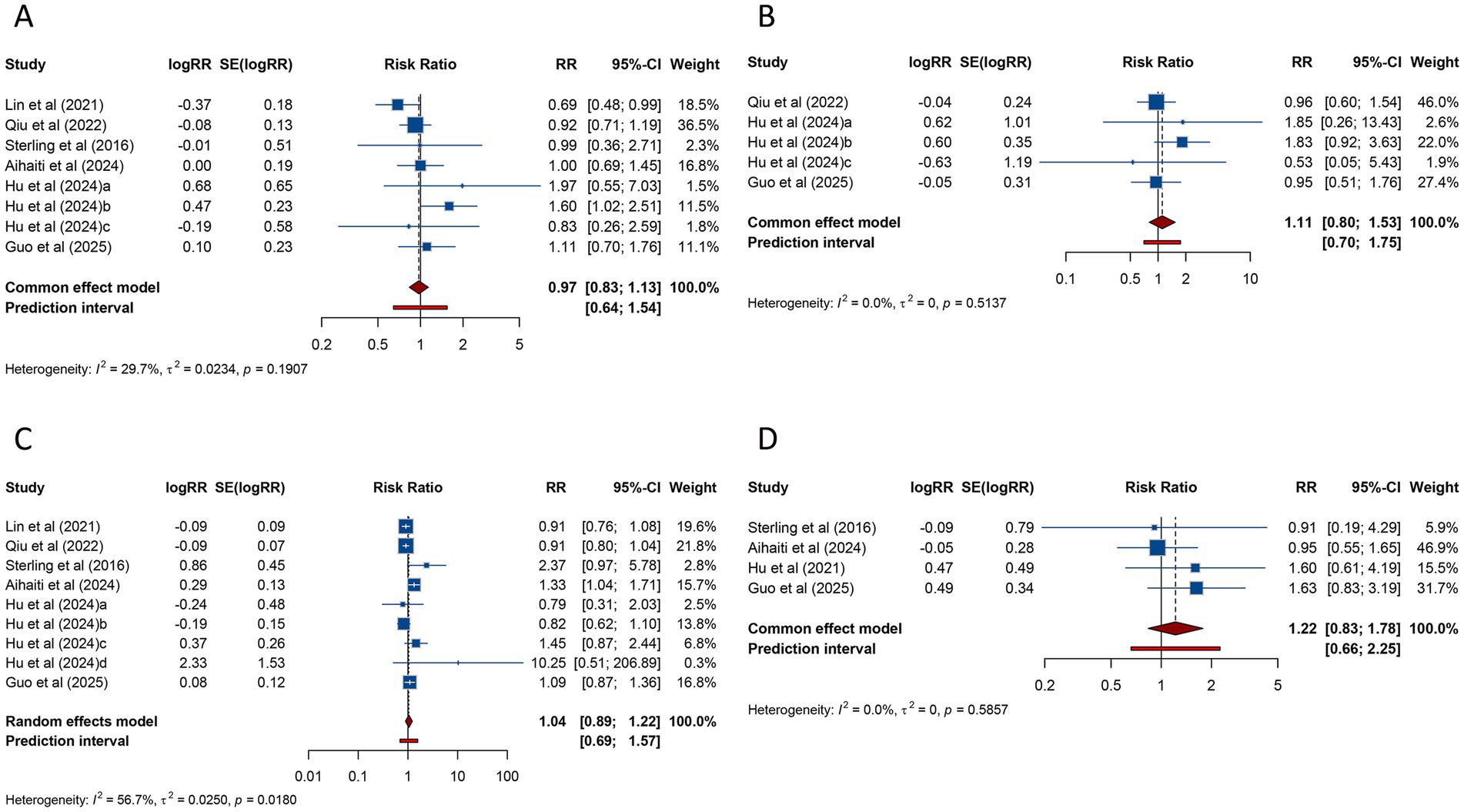
Figure 7. Forest plots of the association between polycystic ovary syndrome and small for gestational age (A), very small for gestational age (B), large for gestational age (C), and fetal malformation (D) in women undergoing assisted reproductive technology.
VSGA was analyzed in 5 studies, which similarly found no significant association between PCOS and VSGA (RR = 1.110, 95% CI: 0.805–1.532; 95% PI: 0.704–1.752), with heterogeneity remaining negligible (I2 = 0%, Tau2 = 0) (Table 3; Figure 7B). Consistent results were obtained among women receiving FET (p < 0.05) (Table 3; Supplementary Figure S7B).
Nine studies examined LGA among women undergoing ART. Pooled analysis demonstrated no significant link between PCOS and LGA (RR = 1.044, 95% CI: 0.892–1.221; 95% PI: 0.693–1.571) (Table 3; Figure 7C), and subgroup analysis focusing on FET showed consistent trends (RR = 1.015, 95% CI: 0.877–1.174; 95% PI: 0.702–1.466) (Table 3; Supplementary Figure S7C).
Four studies reported fetal malformation. The meta-analysis revealed no significant association between PCOS and fetal malformation (RR = 1.218, 95% CI: 0.835–1.778; 95% PI: 0.659–2.251), with no significant heterogeneity (I2 = 0%, Tau2 = 0) (Table 3; Figure 7D). Subgroup analysis for FET showed similar findings (RR = 1.241, 95% CI: 0.840–1.833; 95% PI: 0.527–2.921) (Table 3; Supplementary Figure S7D).
3.5 Sensitivity analysis and publication bias
Sensitivity analyses and tests for publication bias were conducted solely for maternal and fetal outcomes that included 10 or more studies. To evaluate the robustness of the results, a leave-one-out method was applied, which demonstrated that excluding any individual study had no notable impact on the overall conclusions regarding miscarriage, HDP, and PTB. These findings highlight the stability and reliability of our results (Supplementary Figure S8). Publication bias was examined using Begg’s and Egger’s tests, neither of which identified significant bias among the included studies (all p > 0.05). Corresponding funnel plots were presented in Supplementary Figure S9.
4 Discussion
This study utilized cohort studies to explore the potential causal associations between PCOS and adverse pregnancy and perinatal outcomes among women undergoing ART. Our meta-analysis revealed that compared with non-PCOS patients undergoing ART, women with PCOS who had undergone ART showed higher clinical pregnancy rate and live birth rate. Nonetheless, they were also found to have significantly elevated risks of miscarriage, GDM, HDP, gestational hypertension, PPROM, PTB, and VPTB. Conversely, the likelihood of cesarean delivery was lower in PCOS patients. No significant differences were identified between PCOS and non-PCOS groups in the risks of LBW, VLBW, macrosomia, SGA, VSGA, LGA, or fetal malformation. Further subgroup analyses demonstrated consistent statistical significance among women who conceived through FET.
The clinical pregnancy rate and live birth rate, which integrate outcomes from both fresh embryo transfer and FET cycles, offer a robust measure of the overall effectiveness of IVF/ICSI procedures (40, 41). Research by Liu et al. reported that women with PCOS had significantly greater numbers of oocytes retrieved and fertilized. This enhanced ovarian response facilitates the collection of more oocytes and improves fertilization potential, allowing for better embryo selection and ultimately contributing to higher pregnancy rates (33). The ovaries of women with PCOS harbor a higher follicular reserve compared to those without the condition (42), and biomarkers such as elevated serum anti-Müllerian hormone (AMH) levels and increased antral follicle counts (AFC) remain consistently high, even beyond the age of 35 years (43–45). This abundant ovarian reserve provides the foundation for generating a sufficient number of embryos, which underpins the observed improvements in clinical pregnancy rate and live birth rate. However, subgroup analyses in this study revealed that the observed improvements in clinical pregnancy rate and live birth rate were confined to patients undergoing FET. No statistically significant differences were observed among those undergoing fresh embryo transfer or mixed (fresh/frozen) embryo transfer. These results suggest potential advantages of FET for women with PCOS. By decoupling ovarian stimulation from embryo transfer, the FET approach offers an opportunity to mitigate the risks associated with ovarian hyperstimulation syndrome (OHSS) and allows time for the endometrium to recover (46), fostering a more receptive environment for implantation. Additionally, FET circumvents the adverse impact of elevated estrogen levels on endometrial receptivity, a common occurrence during fresh embryo transfer cycles (47). Women with PCOS, due to their heightened ovarian sensitivity to gonadotropins, are more prone to excessive estrogen production during fresh cycles (48, 49), which may impair endometrial receptivity and compromise embryo implantation and pregnancy maintenance (50).
Several meta-analyses have systematically explored the reproductive outcomes of IVF and ICSI in women with PCOS, consistently demonstrating a higher risk of miscarriage compared with women without PCOS (8, 13, 51). Retrospective data from 2,357 women with PCOS who conceived via IVF revealed a significantly increased incidence of late miscarriage (26). Notably, even after accounting for chromosomal abnormalities in embryos, the miscarriage rate among PCOS patients remained substantially higher than that of the control group (31). Our pooled analysis corroborated these findings, identifying an elevated risk of miscarriage in women with PCOS undergoing ART compared with non-PCOS counterparts. Recent research suggests that the elevated miscarriage risk associated with PCOS may be linked to factors such as hyperandrogenism and insulin resistance, which can interfere with mitochondrial function and disrupt the balance between oxidative stress and antioxidant defense mechanisms in the uterus during pregnancy (52). However, it is important to note that these findings are based on animal studies, and their applicability to human cases has yet to be confirmed. In our subgroup analysis, we observed that women with PCOS faced a markedly higher miscarriage risk following FET, whereas no similar increase was identified after fresh embryo transfer. These findings highlight the potential role of FET as a contributing factor to miscarriage in patients with PCOS. The FET procedure involves freezing and thawing embryos, which could potentially affect embryo viability and contribute to the observed rise in miscarriage risk. Furthermore, although FET is often associated with a more natural hormonal environment for endometrial preparation, the underlying pathological alterations in the endometrium of women with PCOS, such as chronic inflammation and abnormal angiogenesis (53, 54), may persist, thereby limiting the potential benefits of FET.
PTB remains a major contributor to neonatal mortality and morbidity (55). Numerous studies have consistently shown that women PCOS face a substantially higher risk of PTB and VPTB following ART (11, 12, 28). Our findings further support this association. Chronic low-grade inflammation and hyperandrogenism, characteristic of PCOS, are hypothesized to compromise placental development and perfusion (56), leading to placental dysfunction and a subsequent increase in PTB risk. An interaction between PCOS and ART appears to further compound the risk of PTB, as many women with PCOS rely on ART to conceive. Importantly, pregnancies achieved through ART are independently associated with an increased likelihood of PTB, even in singleton gestations (57). A retrospective cohort study by Naver et al. (58) similarly identified an increased incidence of PTB in women with PCOS compared with the general population, based on logistic regression analyses adjusted for maternal age, BMI, and parity. However, as this study included pregnancies conceived both naturally and through ART, it was unable to fully disentangle the contribution of ART to the observed PTB risk. Our subgroup analysis demonstrated that PCOS patients undergoing FET had a significantly higher risk of PTB, whereas those undergoing fresh embryo transfer did not exhibit a comparable increase. This discrepancy may be attributed to the use of high doses of estrogen and progesterone during endometrial preparation for FET, which could disrupt endometrial angiogenesis and immune regulation (59), thereby contributing to the elevated PTB risk. Further investigation is needed to clarify the mechanisms underlying these subgroup findings and to better understand the interplay between PCOS, ART, and PTB.
GDM, HDP, and gestational hypertension are complex, pregnancy-specific conditions involving multiple organ systems. Our meta-analysis revealed that women with PCOS had a substantially elevated risk of developing these complications, irrespective of whether conception occurred via FET or fresh embryo transfer. The underlying pathophysiology of PCOS is most commonly attributed to insulin resistance and hyperinsulinemia, with many women exhibiting insulin resistance independent of their BMI (60). During pregnancy, the inability to adequately compensate for this resistance leads to impaired glucose metabolism and intolerance (61). In the context of pregnancy, the additive effects of placental hormones exacerbate pre-existing insulin resistance (62), resulting in hyperglycemia and contributing to the increased prevalence of GDM in women with PCOS. Additionally, a recent meta-analysis encompassing both fresh and frozen embryo transfer cycles confirmed a heightened risk of pregnancy-induced hypertension among women with PCOS (51). Beyond the syndrome itself, hyperandrogenism may play an independent role in the development of hypertensive disorders (63). Elevated androgen levels, a hallmark of PCOS, have been implicated in vascular remodeling, including thickening of the carotid intima-media, which predisposes to hypertension (64). Other contributing factors warrant further exploration, including dyslipidemia, particularly elevated low-density lipoprotein (LDL) cholesterol levels, and persistent hyperinsulinemia. These factors may activate pro-inflammatory pathways, impair endothelial function, diminish vascular reactivity, and promote subclinical atherosclerosis (65).
Our analysis identified an elevated risk of PPROM among PCOS patients undergoing ART, while the likelihood of cesarean delivery was notably reduced. Comparable results were observed in the subgroup of patients who underwent FET. However, due to the limited number of available studies, we were unable to perform a subgroup analysis for those undergoing fresh embryo transfer. Further research is needed to further validate and support these findings. In addition, our meta-analysis found no significant differences in the risks of LBW, VLBW, macrosomia, SGA, VSGA, or LGA between PCOS and non-PCOS patients following ART. These results suggest that maternal PCOS may not exert a substantial influence on fetal or neonatal weight outcomes. Similarly, no significant difference in the risk of fetal malformation was observed between the two groups. Notably, as the majority of included studies focused on pregnancies achieved through FET, a clearer understanding of the associations between PCOS and fetal or neonatal weight, as well as fetal malformation, in fresh embryo transfer populations remains an important area for future investigation.
Our meta-analysis included only cohort studies, excluding case–control and cross-sectional studies, thereby providing robust evidence to clarify the causal association between PCOS and adverse pregnancy and perinatal outcomes. Furthermore, we prioritized the extraction of RRs and 95% CIs that had been adjusted for confounding factors in the included studies, which helped to minimize the influence of potential confounders on the final results. Additionally, we conducted subgroup analyses for each outcome based on the mode of ART, further exploring the impact of FET and fresh embryo transfer on the outcomes. However, this study has several limitations. First, the majority of the studies included in the final analysis were conducted in China, which limited the feasibility of performing subgroup analysis based on ethnicity and constrained the generalizability of our findings to the broader population. Second, only a limited number of studies have investigated the association between PCOS and adverse pregnancy and perinatal outcomes, such as miscarriage, HDP, and live birth rate, in patients undergoing fresh embryo transfer. This paucity of evidence has constrained our ability to perform subgroup analyses for fresh embryo transfer. Consequently, further research is urgently needed to better understand the differences in the associations between PCOS and adverse outcomes in the context of FET versus fresh embryo transfer. Third, the included studies varied in their adjustment for potential confounders, with some studies providing multivariable analyses that adjusted for factors such as maternal age and BMI, while others lacked adjustments for critical variables. This variability in study-level covariates may have contributed to the heterogeneity observed in certain outcomes and underscores the need for caution when interpreting the pooled estimates. Additionally, residual confounding remains a concern, as not all included studies adjusted for all critical covariates. These unmeasured confounders may partially explain the observed associations. Nevertheless, heterogeneity and sensitivity analyses indicated that most findings in our study were robust, with low heterogeneity and consistent reliability.
5 Conclusion
In conclusion, this study suggested that women with PCOS undergoing ART may have a higher clinical pregnancy rate and live birth rate compared with women without PCOS. However, these patients also appear to face notably increased risks of miscarriage, GDM, HDP, gestational hypertension, PPROM, PTB, and VPTB. Conversely, the risk of cesarean delivery might be lower in the PCOS group. No significant differences were observed between the PCOS and control groups regarding the risks of LBW, VLBW, macrosomia, SGA, VSGA, LGA, or fetal malformation. Similar findings were observed among patients undergoing FET. Further investigation is required to delineate the differential impact of PCOS on adverse outcomes in the context of FET versus fresh embryo transfer.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
NX: Formal analysis, Data curation, Software, Methodology, Writing – original draft, Investigation. WZ: Validation, Supervision, Methodology, Conceptualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1656389/full#supplementary-material
References
1. Lizneva, D, Suturina, L, Walker, W, Brakta, S, Gavrilova-Jordan, L, and Azziz, R. Criteria, prevalence, and phenotypes of polycystic ovary syndrome. Fertil Steril. (2016) 106:6–15. doi: 10.1016/j.fertnstert.2016.05.003
2. Azziz, R, Carmina, E, Dewailly, D, Diamanti-Kandarakis, E, Escobar-Morreale, HF, Futterweit, W, et al. The androgen excess and PCOS society criteria for the polycystic ovary syndrome: the complete task force report. Fertil Steril. (2009) 91:456–88. doi: 10.1016/j.fertnstert.2008.06.035
3. Joham, AE, Norman, RJ, Stener-Victorin, E, Legro, RS, Franks, S, Moran, LJ, et al. Polycystic ovary syndrome. Lancet Diabetes Endocrinol. (2022) 10:668–80. doi: 10.1016/s2213-8587(22)00163-2
4. Balen, AH, Morley, LC, Misso, M, Franks, S, Legro, RS, Wijeyaratne, CN, et al. The management of anovulatory infertility in women with polycystic ovary syndrome: an analysis of the evidence to support the development of global WHO guidance. Hum Reprod Update. (2016) 22:687–708. doi: 10.1093/humupd/dmw025
5. Della Corte, L, Foreste, V, Barra, F, Gustavino, C, Alessandri, F, Centurioni, MG, et al. Current and experimental drug therapy for the treatment of polycystic ovarian syndrome. Expert Opin Investig Drugs. (2020) 29:819–30. doi: 10.1080/13543784.2020.1781815
6. Boomsma, CM, Eijkemans, MJ, Hughes, EG, Visser, GH, Fauser, BC, and Macklon, NS. A meta-analysis of pregnancy outcomes in women with polycystic ovary syndrome. Hum Reprod Update. (2006) 12:673–83. doi: 10.1093/humupd/dml036
7. Kjerulff, LE, Sanchez-Ramos, L, and Duffy, D. Pregnancy outcomes in women with polycystic ovary syndrome: a metaanalysis. Am J Obstet Gynecol. (2011) 204:e1-6:558. doi: 10.1016/j.ajog.2011.03.021
8. Bahri Khomami, M, Joham, AE, Boyle, JA, Piltonen, T, Silagy, M, Arora, C, et al. Increased maternal pregnancy complications in polycystic ovary syndrome appear to be independent of obesity-a systematic review, meta-analysis, and meta-regression. Obes Rev. (2019) 20:659–74. doi: 10.1111/obr.12829
9. Lin, J, Guo, H, Wang, B, Chen, Q, and Zhu, Q. Neonatal outcomes in women with polycystic ovary syndrome after frozen-thawed embryo transfer. Fertil Steril. (2021) 115:447–54. doi: 10.1016/j.fertnstert.2020.08.1435
10. Chen, ZJ, Shi, Y, Sun, Y, Zhang, B, Liang, X, Cao, Y, et al. Fresh versus frozen embryos for infertility in the polycystic ovary syndrome. N Engl J Med. (2016) 375:523–33. doi: 10.1056/NEJMoa1513873
11. Sterling, L, Liu, J, Okun, N, Sakhuja, A, Sierra, S, and Greenblatt, E. Pregnancy outcomes in women with polycystic ovary syndrome undergoing in vitro fertilization. Fertil Steril. (2016) 105:791–7.e2. doi: 10.1016/j.fertnstert.2015.11.019
12. Qiu, M, Qu, J, Tian, Y, and Wang, Y. The influence of polycystic ovarian syndrome on obstetric and neonatal outcomes after frozen-thawed embryo transfer. Reprod Biomed Online. (2022) 45:745–53. doi: 10.1016/j.rbmo.2022.05.024
13. Bahri Khomami, M, Shorakae, S, Hashemi, S, Harrison, CL, Piltonen, TT, Romualdi, D, et al. Systematic review and meta-analysis of pregnancy outcomes in women with polycystic ovary syndrome. Nat Commun. (2024) 15:5591. doi: 10.1038/s41467-024-49749-1
14. Ban, M, Sun, Y, Chen, X, Zhou, X, Zhang, Y, and Cui, L. Association between maternal polycystic ovarian syndrome undergoing assisted reproductive technology and pregnancy complications and neonatal outcomes: a systematic review and meta-analysis. J Ovarian Res. (2024) 17:6. doi: 10.1186/s13048-023-01331-x
15. Bahri Khomami, M, Hashemi, S, Shorakae, S, Harrison, CL, Piltonen, TT, Romualdi, D, et al. Systematic review and meta-analysis of birth outcomes in women with polycystic ovary syndrome. Nat Commun. (2024) 15:5592. doi: 10.1038/s41467-024-49752-6
16. Moher, D, Liberati, A, Tetzlaff, J, and Altman, DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. (2009) 339:b2535. doi: 10.1136/bmj.b2535
17. Wells, G, Shea, B, and O'Connell, J. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa Health Res Inst Web Site. (2014):7.
18. Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
19. Bowden, J, Tierney, JF, Copas, AJ, and Burdett, S. Quantifying, displaying and accounting for heterogeneity in the meta-analysis of RCTs using standard and generalised Q statistics. BMC Med Res Methodol. (2011) 11:41. doi: 10.1186/1471-2288-11-41
20. IntHout, J, Ioannidis, JP, Rovers, MM, and Goeman, JJ. Plea for routinely presenting prediction intervals in meta-analysis. BMJ Open. (2016) 6:e010247. doi: 10.1136/bmjopen-2015-010247
21. Higgins, JP, and Thompson, SG. Quantifying heterogeneity in a meta-analysis. Stat Med. (2002) 21:1539–58. doi: 10.1002/sim.1186
22. Begg, CB, and Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics. (1994) 50:1088–101. doi: 10.2307/2533446
23. Egger, M, Davey Smith, G, Schneider, M, and Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629-34. doi: 10.1136/bmj.315.7109.629
24. Aihaiti, R, Shen, Z, Wu, X, and Niu, Z. Pregnancy complications and birth outcomes in women with polycystic ovary syndrome undergoing frozen embryo transfer. Fertil Steril. (2024) 122:1055–62. doi: 10.1016/j.fertnstert.2024.07.017
25. Beydoun, HA, Stadtmauer, L, Zhao, Y, Russell, H, Matson, DO, and Oehninger, S. Impact of polycystic ovary syndrome on selected indicators of in vitro fertilization and intracytoplasmic sperm injection treatment success. J Womens Health (Larchmt). (2009) 18:717–23. doi: 10.1089/jwh.2008.1149
26. Cai, H, Mol, BW, Gordts, S, Wang, H, Wang, T, Li, N, et al. Early and late pregnancy loss in women with polycystic ovary syndrome undergoing IVF/ICSI treatment: a retrospective cohort analysis of 21 820 pregnancies. BJOG. (2021) 128:1160–9. doi: 10.1111/1471-0528.16590
27. Dou, Q, Ma, LY, Li, PF, Xu, XT, Yu, G, Zhang, D, et al. The influence of polycystic ovary syndrome on abortion rate after in vitro fertilization/intracytoplasmic sperm injection fresh cycle pregnancy. Sci Rep. (2023) 13:5978. doi: 10.1038/s41598-023-32988-5
28. Guo, Y, Yang, J, Chen, H, Zhou, Y, Yang, Y, Wang, B, et al. Enhancing understanding of endometrial function in patients with PCOS: clinical and immunological insights. J Ovarian Res. (2025) 18:52. doi: 10.1186/s13048-025-01638-x
29. Hu, S, Xu, B, Long, R, and Jin, L. The effect of polycystic ovary syndrome without hyperandrogenism on pregnancy-related outcomes: a retrospective cohort study. BJOG. (2021) 128:1003–10. doi: 10.1111/1471-0528.16557
30. Hu, X, Yan, E, Peng, W, Zhou, Y, Jin, L, and Qian, K. Higher pre-pregnancy body mass index was associated with adverse pregnancy and perinatal outcomes in women with polycystic ovary syndrome after a freeze-all strategy: a historical cohort study. Acta Obstet Gynecol Scand. (2024) 103:884–96. doi: 10.1111/aogs.14771
31. Jie, HY, Zhou, X, Zhao, MP, Hu, M, Mai, QY, and Zhou, CQ. Pregnancy outcomes in patients with polycystic ovary syndrome who conceived after single thawed blastocyst transfer: a propensity score-matched study. BMC Pregnancy Childbirth. (2022) 22:718. doi: 10.1186/s12884-022-05011-4
32. Li, H, Xu, L, Niu, Y, Zhu, X, Gao, X, and Ma, T. The effects of fresh embryo transfer and frozen-thawed embryo transfer on the perinatal outcomes of single fetuses from mothers with PCOS. PLoS One. (2024) 19:e0312003. doi: 10.1371/journal.pone.0312003
33. Liu, S, Mo, M, Xiao, S, Li, L, Hu, X, Hong, L, et al. Pregnancy outcomes of women with polycystic ovary syndrome for the first in vitro fertilization treatment: a retrospective cohort study with 7678 patients. Front Endocrinol (Lausanne). (2020) 11:575337. doi: 10.3389/fendo.2020.575337
34. Liu, S, Zhou, X, Jie, H, Zheng, Z, Cai, B, Mai, Q, et al. Higher cumulative live birth rate but also higher late miscarriage risk in non-obese women with polycystic ovary syndrome undergoing the first IVF/ICSI cycle. Int J Women's Health. (2024) 16:289–98. doi: 10.2147/ijwh.S445021
35. Luo, L, Gu, F, Jie, H, Ding, C, Zhao, Q, Wang, Q, et al. Early miscarriage rate in lean polycystic ovary syndrome women after euploid embryo transfer - a matched-pair study. Reprod Biomed Online. (2017) 35:576–82. doi: 10.1016/j.rbmo.2017.07.010
36. Wang, Q, Wang, H, Li, P, Li, X, Wang, Z, Yan, L, et al. Association of polycystic ovary syndrome phenotypes with adverse pregnancy outcomes after in-vitro fertilization/intracytoplasmic sperm injection. Front Endocrinol (Lausanne). (2022) 13:889029. doi: 10.3389/fendo.2022.889029
37. Wang, Q, Zheng, Y, Li, P, Zhang, G, Gao, S, Wang, Z, et al. Increased risk of abortion after frozen-thawed embryo transfer in women with polycystic ovary syndrome phenotypes A and D. Sci Rep. (2022) 12:14852. doi: 10.1038/s41598-022-18704-9
38. Zhang, X, Lian, F, and Liu, D. Comparison of IVF/ICSI outcomes in advanced reproductive age patients with polycystic ovary syndrome and advanced reproductive age normal controls: a retrospective cohort study. BMC Pregnancy Childbirth. (2023) 23:440. doi: 10.1186/s12884-023-05732-0
39. Dai, L, Deng, C, Li, Y, Zhu, J, Mu, Y, Deng, Y, et al. Birth weight reference percentiles for Chinese. PLoS One. (2014) 9:e104779. doi: 10.1371/journal.pone.0104779
40. Wu, X, Zhou, WJ, Xu, BF, Chen, Q, Xia, L, Zhao, S, et al. Association between transferred embryos and multiple pregnancy/live birth rate in frozen embryo transfer cycles: a retrospective study. Front Endocrinol (Lausanne). (2022) 13:1073164. doi: 10.3389/fendo.2022.1073164
41. Jiang, L, Sun, Y, Pan, P, Li, L, Yang, D, Huang, J, et al. Live birth rate per fresh embryo transfer and cumulative live birth rate in patients with PCOS under the POSEIDON classification: a retrospective study. Front Endocrinol (Lausanne). (2024) 15:1348771. doi: 10.3389/fendo.2024.1348771
42. Webber, LJ, Stubbs, S, Stark, J, Trew, GH, Margara, R, Hardy, K, et al. Formation and early development of follicles in the polycystic ovary. Lancet. (2003) 362:1017–21. doi: 10.1016/s0140-6736(03)14410-8
43. Cui, Y, Shi, Y, Cui, L, Han, T, Gao, X, and Chen, ZJ. Age-specific serum antimüllerian hormone levels in women with and without polycystic ovary syndrome. Fertil Steril. (2014) 102:230–6.e2. doi: 10.1016/j.fertnstert.2014.03.032
44. Mai, Z, Liu, M, Pan, P, Li, L, Huang, J, Chen, X, et al. Comparison of cumulative live birth rate between aged PCOS women and controls in IVF/ICSI cycles. Front Endocrinol (Lausanne). (2021) 12:724333. doi: 10.3389/fendo.2021.724333
45. Hudecova, M, Holte, J, Olovsson, M, and Sundström Poromaa, I. Long-term follow-up of patients with polycystic ovary syndrome: reproductive outcome and ovarian reserve. Hum Reprod. (2009) 24:1176–83. doi: 10.1093/humrep/den482
46. Zech, J, Brandao, A, Zech, M, Lugger, K, Neururer, S, Ulmer, H, et al. Elective frozen-thawed embryo transfer (FET) in women at risk for ovarian hyperstimulation syndrome. Reprod Biol. (2018) 18:46–52. doi: 10.1016/j.repbio.2017.12.004
47. Adeviye Erşahin, A, Acet, M, Erşahin, SS, and Dokuzeylül Güngör, N. Frozen embryo transfer prevents the detrimental effect of high estrogen on endometrium receptivity. J Turk-Germ Gynecol Assoc. (2017) 18:38–42. doi: 10.4274/jtgga.2016.0186
48. Silva, MSB, Desroziers, E, Hessler, S, Prescott, M, Coyle, C, Herbison, AE, et al. Activation of arcuate nucleus GABA neurons promotes luteinizing hormone secretion and reproductive dysfunction: implications for polycystic ovary syndrome. EBioMedicine. (2019) 44:582–96. doi: 10.1016/j.ebiom.2019.05.065
49. Bildik, G, Akin, N, Seyhan, A, Esmaeilian, Y, Yakin, K, Keles, I, et al. Luteal granulosa cells from natural cycles are more capable of maintaining their viability, steroidogenic activity and LH receptor expression than those of stimulated IVF cycles. Hum Reprod. (2019) 34:345–55. doi: 10.1093/humrep/dey353
50. Kalakota, NR, George, LC, Morelli, SS, Douglas, NC, and Babwah, AV. Towards an improved understanding of the effects of elevated progesterone levels on human endometrial receptivity and oocyte/embryo quality during assisted reproductive technologies. Cells. (2022) 11:11. doi: 10.3390/cells11091405
51. Sha, T, Wang, X, Cheng, W, and Yan, Y. A meta-analysis of pregnancy-related outcomes and complications in women with polycystic ovary syndrome undergoing IVF. Reprod Biomed Online. (2019) 39:281–93. doi: 10.1016/j.rbmo.2019.03.203
52. Hu, M, Zhang, Y, Guo, X, Jia, W, Liu, G, Zhang, J, et al. Hyperandrogenism and insulin resistance induce gravid uterine defects in association with mitochondrial dysfunction and aberrant reactive oxygen species production. Am J Physiol Endocrinol Metab. (2019) 316:E794–e809. doi: 10.1152/ajpendo.00359.2018
53. Dabravolski, SA, Nikiforov, NG, Eid, AH, Nedosugova, LV, Starodubova, AV, Popkova, TV, et al. Mitochondrial dysfunction and chronic inflammation in polycystic ovary syndrome. Int J Mol Sci. (2021) 22:22. doi: 10.3390/ijms22083923
54. Di Pietro, M, Pascuali, N, Parborell, F, and Abramovich, D. Ovarian angiogenesis in polycystic ovary syndrome. Reproduction. (2018) 155:R199–r209. doi: 10.1530/rep-17-0597
55. Di Renzo, GC, Tosto, V, and Giardina, I. The biological basis and prevention of preterm birth. Best Pract Res Clin Obstet Gynaecol. (2018) 52:13–22. doi: 10.1016/j.bpobgyn.2018.01.022
56. Vannuccini, S, Clifton, VL, Fraser, IS, Taylor, HS, Critchley, H, Giudice, LC, et al. Infertility and reproductive disorders: impact of hormonal and inflammatory mechanisms on pregnancy outcome. Hum Reprod Update. (2016) 22:104–15. doi: 10.1093/humupd/dmv044
57. McDonald, SD, Han, Z, Mulla, S, Murphy, KE, Beyene, J, and Ohlsson, A. Preterm birth and low birth weight among in vitro fertilization singletons: a systematic review and meta-analyses. Eur J Obstet Gynecol Reprod Biol. (2009) 146:138–48. doi: 10.1016/j.ejogrb.2009.05.035
58. Naver, KV, Grinsted, J, Larsen, SO, Hedley, PL, Jørgensen, FS, Christiansen, M, et al. Increased risk of preterm delivery and pre-eclampsia in women with polycystic ovary syndrome and hyperandrogenaemia. BJOG. (2014) 121:575–81. doi: 10.1111/1471-0528.12558
59. Conrad, KP, Graham, GM, Chi, YY, Zhai, X, Li, M, Williams, RS, et al. Potential influence of the corpus luteum on circulating reproductive and volume regulatory hormones, angiogenic and immunoregulatory factors in pregnant women. Am J Physiol Endocrinol Metab. (2019) 317:E677–85. doi: 10.1152/ajpendo.00225.2019
60. Azziz, R, Carmina, E, Chen, Z, Dunaif, A, Laven, JS, Legro, RS, et al. Polycystic ovary syndrome. Nat Rev Dis Primers. (2016) 2:16057. doi: 10.1038/nrdp.2016.57
61. Tieu, J, McPhee, AJ, Crowther, CA, Middleton, P, and Shepherd, E. Screening for gestational diabetes mellitus based on different risk profiles and settings for improving maternal and infant health. Cochrane Database Syst Rev. (2017) 8:Cd007222. doi: 10.1002/14651858.CD007222.pub4
62. Toulis, KA, Goulis, DG, Kolibianakis, EM, Venetis, CA, Tarlatzis, BC, and Papadimas, I. Risk of gestational diabetes mellitus in women with polycystic ovary syndrome: a systematic review and a meta-analysis. Fertil Steril. (2009) 92:667–77. doi: 10.1016/j.fertnstert.2008.06.045
63. Mumm, H, Jensen, DM, Sørensen, JA, Andersen, LL, Ravn, P, Andersen, M, et al. Hyperandrogenism and phenotypes of polycystic ovary syndrome are not associated with differences in obstetric outcomes. Acta Obstet Gynecol Scand. (2015) 94:204–11. doi: 10.1111/aogs.12545
64. Vryonidou, A, Papatheodorou, A, Tavridou, A, Terzi, T, Loi, V, Vatalas, IA, et al. Association of hyperandrogenemic and metabolic phenotype with carotid intima-media thickness in young women with polycystic ovary syndrome. J Clin Endocrinol Metab. (2005) 90:2740–6. doi: 10.1210/jc.2004-2363
Keywords: polycystic ovary syndrome, pregnancy outcomes, assisted reproductive technology, miscarriage, preterm birth, meta-analysis
Citation: Xie N and Zhao W (2025) Adverse pregnancy and perinatal outcomes in women with polycystic ovary syndrome undergoing assisted reproductive technology: a systematic review and meta-analysis. Front. Med. 12:1656389. doi: 10.3389/fmed.2025.1656389
Edited by:
Jing He, Guangzhou Medical University, ChinaReviewed by:
Sanja Medenica, Clinical Center of Montenegro, MontenegroIrene Iavarone, University of Campania Luigi Vanvitelli, Italy
Copyright © 2025 Xie and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenwen Zhao, end3MTUxMTExMzg1MDNAMTYzLmNvbQ==
 Nian Xie
Nian Xie Wenwen Zhao*
Wenwen Zhao*
