- Obstetrical Department, Dongyang Hospital Affiliated to Wenzhou Medical University, Dongyang, Zhejiang, China
Background: Gestational diabetes mellitus (GDM) and hypertensive disorder complicating pregnancy (HDP) share pathophysiological mechanisms that increase the risk for adverse maternal and neonatal outcomes. However, their combined impact remains underexplored.
Objective: To assess the clinical characteristics, therapeutic interventions, and maternal–fetal outcomes in pregnancies complicated by both GDM and HDP.
Methods: A retrospective study was conducted involving 82 women with GDM complicated by HDP and 118 healthy pregnant controls. Clinical parameters, mode of delivery, and pregnancy outcomes were compared between groups. Logistic regression analysis was performed to identify independent predictors of GDM complicated by HDP.
Results: Women with GDM complicated by HDP exhibited significantly higher pre-pregnancy weight, body mass index (BMI), fasting blood glucose, triglycerides, and high-density lipoprotein (HDL) cholesterol compared with healthy controls (p < 0.05). Logistic regression identified these variables as independent predictors of GDM + HDP (p < 0.001). The GDM + HDP group also had higher rates of cesarean delivery and adverse pregnancy outcomes. Individualized treatment targeting glycemic and blood pressure control significantly improved metabolic parameters and reduced the incidence of complications (p < 0.05).
Conclusion: Pre-pregnancy metabolic factors, including BMI, fasting blood glucose, triglycerides, and HDL cholesterol, are strong predictors of GDM complicated by HDP. Early identification and individualized management of these high-risk pregnancies can effectively reduce adverse maternal and neonatal outcomes.
1 Introduction
Pregnancy is a special physiological period for women (1). Compared to non-pregnant women, pregnant women (PW) exhibit significantly elevated levels of dietary fat intake, fat absorption, hepatic lipid synthesis, and circulating insulin and leptin concentrations. These physiological metabolic changes are significant for maintaining the normal growth and development of the fetus (2, 3). However, persistent abnormal increases can lead to metabolic disorders in PW, such as hypertensive disorder complicating pregnancy (HDP) and gestational diabetes mellitus (GDM). These conditions elevate the risk of adverse pregnancy outcomes, including preterm delivery, macrosomia, cesarean section, polyhydramnios, and growth restriction, posing serious threats to maternal and fetal health (4–6). In addition, the risk of GDM in the second pregnancy is about 50%, and the risk of HDP is about 30% (7). In recent years, it has been gradually found that GDM and HDP exist simultaneously in some PW, which causes the abnormal rate of fetal growth and the adverse pregnancy outcome of newborns to be higher than that of a single complication (8). GDM and HDP during pregnancy have a higher risk of adverse outcomes, which is a risk factor for adverse pregnancy outcomes (9). Clinically, HDP and GDM share similar pathological mechanisms, such as endothelial dysfunction, dyslipidemia, micro-inflammatory reactions, and disorders of the renin-angiotensin-aldosterone system. These factors elevate the risk of HDP in women with GDM (10). Attention must be given to the safety of pregnancies complicated by GDM and HDP. Currently, research primarily focuses on single conditions—GDM or HDP—and their treatment, with limited consensus on the combined impact of these conditions. This study retrospectively analyzes the clinical characteristics and treatment of PW with GDM and HDP, assesses their effects, and offers medication guidance to inform clinical interventions and enhance maternal and fetal safety.
2 Methods
2.1 Study subjects
We retrospectively analyzed clinical data from 82 singleton PW diagnosed with GDM and HDP, as well as 118 healthy singleton PW, all of whom received routine prenatal care and delivered at our hospital between January 2022 and December 2023. These participants were categorized into a GDM group combined with an HDP group and a healthy control group (HCG).
2.2 Sample size estimation
According to the sample size estimation using G*Power software, the study requires at least 42 participants in the GDM combined with HDP group and 72 participants in the healthy control group to ensure 80% statistical power at a significance level of 0.05 for detecting a significant difference in the incidence of adverse pregnancy outcomes between the two groups. To compensate for potential sample loss, a final total of 82 participants in the GDM combined with HDP group and 118 participants in the healthy control group were included, meeting the statistical requirements.
2.3 Inclusion and exclusion criteria
Inclusion criteria for the GDM combined with the HDP group:
1. Fulfilling the diagnostic criteria for both GDM and HDP, which are as follows: FPG ≥ 5.1 mmol/L, 1-h blood glucose ≥ 10.0 mmol/L, 2-h blood glucose ≥ 8.5 mmol/L at 24–28 weeks of gestation, as determined by the oral glucose tolerance test (OGTT); GDM is diagnosed based on any abnormal values (11), and systolic blood pressure (SBP) ≥ 140 mmHg and/or diastolic blood pressure (DBP) ≥ 90 mmHg for the first time after 20 weeks of gestation, with negative urine protein test (12).
2. All patients had regular checkups and were delivered in this hospital.
3. All patients provide informed consent.
Exclusion criteria:
1. History of diabetes, hypertension, or other endocrine disorders before pregnancy.
2. Presence of other pregnancy-related conditions (e.g., cholestasis of pregnancy).
3. Active infectious diseases, including hepatitis B, hepatitis C, HIV, syphilis, tuberculosis, or other systemic infections.
4. Induced labor due to fetal malformation or intrauterine fetal death.
5. Chronic kidney disease, nephrotic syndrome, or other significant organ dysfunctions.
6. Poor compliance with follow-up appointments.
Inclusion criteria for the HCG:
1. All participants had live births.
2. All had regular prenatal checkups and were delivered at this hospital.
3. No abnormalities were found during pregnancy checkups.
4. All patients provided informed consent.
Exclusion criteria:
1. Presence of combined endocrine, immune, or blood system diseases.
2. Any form of organ dysfunction.
3. History of pre-existing chronic or systemic diseases.
4. Poor compliance with regular checkups.
The current study was approved by the Ethics Committee of the Dongyang Hospital Affiliated to Wenzhou Medical University (2024-YX-323). Written informed consents from all patients were obtained for any experimental work with humans. Exclusion criteria were strictly defined to ensure homogeneous groups and avoid biases related to co-existing conditions.
2.4 Data collection and observation indices
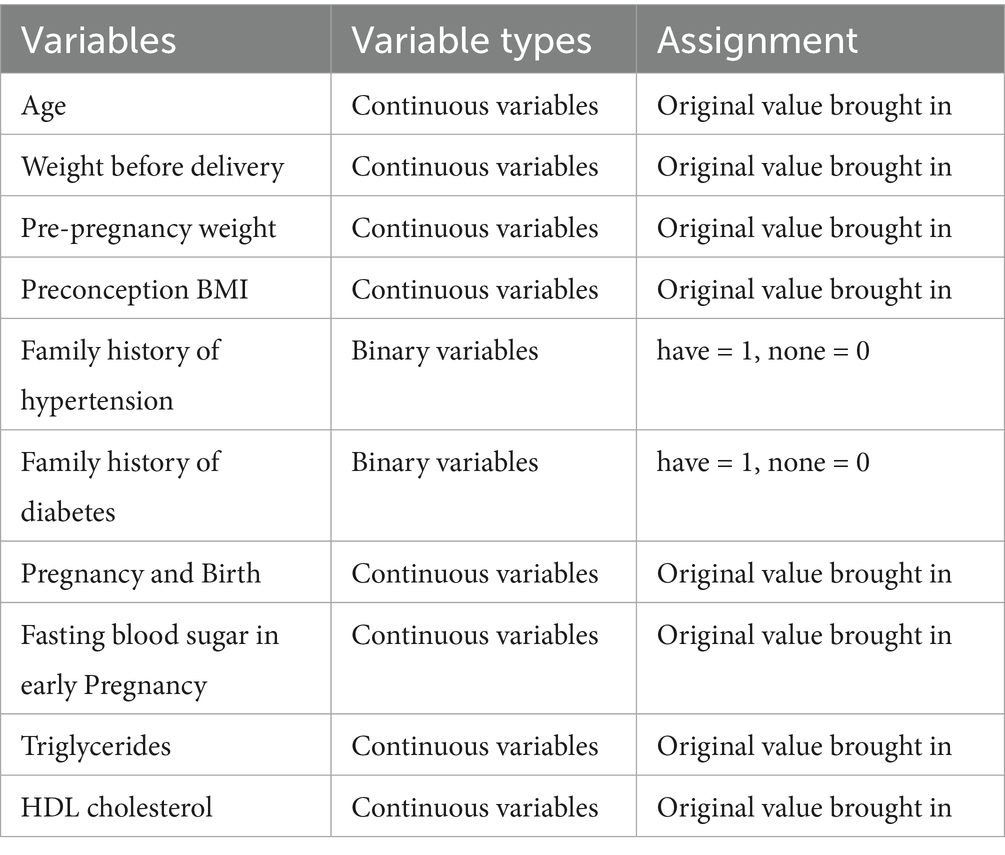
Clinical and laboratory data were retrospectively collected from the medical records of all participants under the supervision of experienced obstetricians and gynecologists. Maternal demographic and anthropometric information included age, pre-pregnancy weight, pre-delivery weight, gestational weight gain, height, and body mass index (BMI). Additional data included family history of diabetes and hypertension, parity, and educational level. Gestational weight gain was calculated as the difference between maternal weight at delivery and pre-pregnancy weight, which measures weight change during pregnancy.
Early pregnancy biochemical markers, including fasting blood glucose (FBG), postprandial blood glucose (PBG), glycosylated hemoglobin (HbA1c), total cholesterol (TC), triglycerides (TRI), high-density lipoprotein cholesterol (HDLC), and low-density lipoprotein cholesterol (LDLC), were obtained from routine prenatal laboratory assessments conducted at our hospital between 6 and 16 weeks of gestation. All PW underwent a 75-g OGTT between 24 and 28 weeks of gestation. Fasting venous blood (5 mL) was drawn in the morning after an overnight fast of at least 8 h. Blood samples were centrifuged at 3,000 rpm for 15 min, and serum was analyzed using fully automated biochemical analyzers according to standard laboratory protocols. PBG levels were measured following the same processing protocol after the participants consumed a standardized meal or glucose challenge, ensuring consistent assessment of glycemic control. Blood pressure measurements were recorded using automated sphygmomanometers to evaluate hypertension control, including systolic blood pressure (SBP) and diastolic blood pressure (DBP). Information on treatment interventions for GDM and HDP was also documented.
Delivery characteristics, including mode of delivery and postpartum hemorrhage volume, were collected. Adverse perinatal outcomes were recorded as binary variables (yes/no) and defined as follows: macrosomia (birth weight ≥ 4,000 g), low birth weight (<2,500 g), preterm delivery (<37 weeks gestation), oligohydramnios (amniotic fluid index < 5 cm), fetal growth restriction (estimated fetal weight <10th percentile for gestational age), premature rupture of membranes (rupture of membranes prior to the onset of labor), and postpartum hemorrhage (blood loss ≥500 mL for vaginal delivery or ≥1,000 mL for cesarean section).
These indices were analyzed to compare participants with GDM combined with HDP and healthy controls and assess the effects of treatment interventions on maternal and neonatal outcomes. The data collection and processing protocol ensured accuracy, consistency, and reliability for clinical and biochemical variables, minimizing potential biases due to data heterogeneity or measurement errors.
2.5 Therapeutic method
Clinical management of PW with GDM and HDP was conducted in accordance with standard clinical guidelines. Patients with GDM initially received lifestyle interventions, including dietary modification and physical activity. Insulin therapy (insulin aspart, D Novotel) was administered at doses ranging from 8 IU to 34 IU when glycemic targets were not achieved with lifestyle measures alone. For hypertension, patients were treated with labetalol hydrochloride (0.1–0.2 g, twice daily), and, when indicated, a combination of magnesium sulfate (30 mL, intravenous infusion) and nifedipine sustained-release tablets (10 mg) was administered.
Patients who received no pharmacological therapy but were managed with lifestyle interventions alone were classified as the “untreated group.” This group was distinct from the healthy control group, which included women without GDM or HDP. Treatment regimens for all patients were individualized according to clinical condition, with continuous monitoring of blood glucose and blood pressure to guide therapy. Comparisons were made between treated and untreated patients with GDM and HDP and between case and control groups to assess the impact of therapeutic interventions on maternal and perinatal outcomes.
2.6 Statistical methods
Prism 9.4.1 was used for image processing, while SPSS 23.0 was used to analyze data. The measurement data were represented by ( ) and had a normal distribution with homogeneous variance. An independent sample t-test was used to compare the two groups, and a paired t-test was applied to compare the groups among themselves. The χ2 test was used to assess intergroup comparisons for categorical data, which was reported as [n(%)]. Univariate logistic regression analyses were initially performed to explore associations between individual variables and adverse pregnancy outcomes. Variables with a p-value < 0.10 in univariate analysis or those considered clinically significant based on prior evidence were included in the multivariate logistic regression model to identify independent predictors. p-values were considered statistically significant if they were less than 0.05.
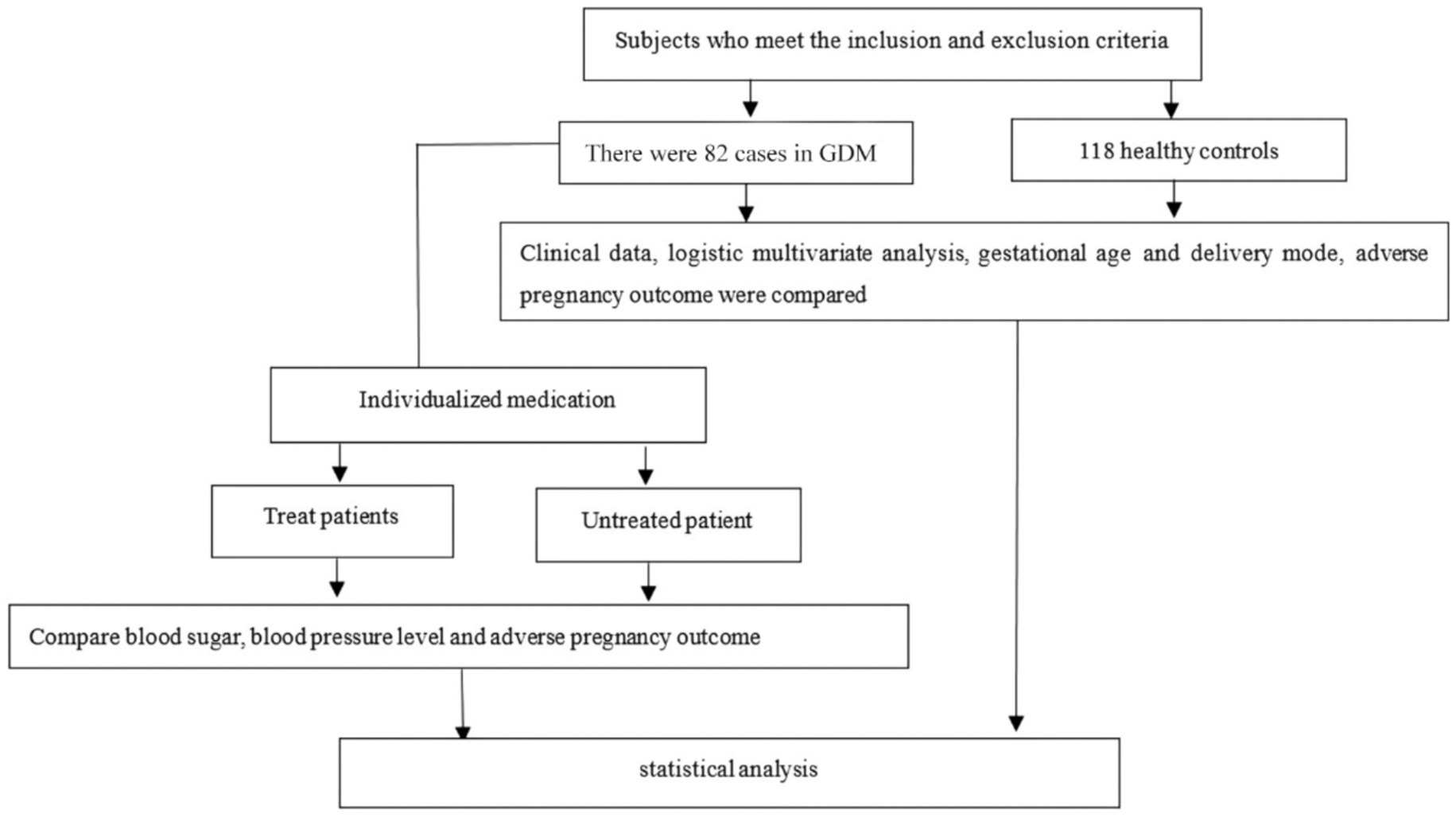
2.7 Research flow chart
The overall design and participant selection process of this study are illustrated in Figure 1. The flow chart depicts the screening, inclusion, and exclusion of participants and the final allocation into the GDM combined with HDP group and the healthy control group, providing a clear overview of the study workflow.
3 Results
3.1 Clinical data between GDM combined with HDP group and HCG
No significant differences were observed between the two groups in terms of maternal height, educational level, LDLC, TC, or gestational weight gain (p > 0.05). Compared with the HCG, women in the GDM combined with HDP group exhibited significantly higher values during the first trimester for age, pre-pregnancy weight, pre-pregnancy body mass index (BMI), family history of hypertension and diabetes, parity, FBG, triglycerides, and HDLC (p < 0.05; Table 1).
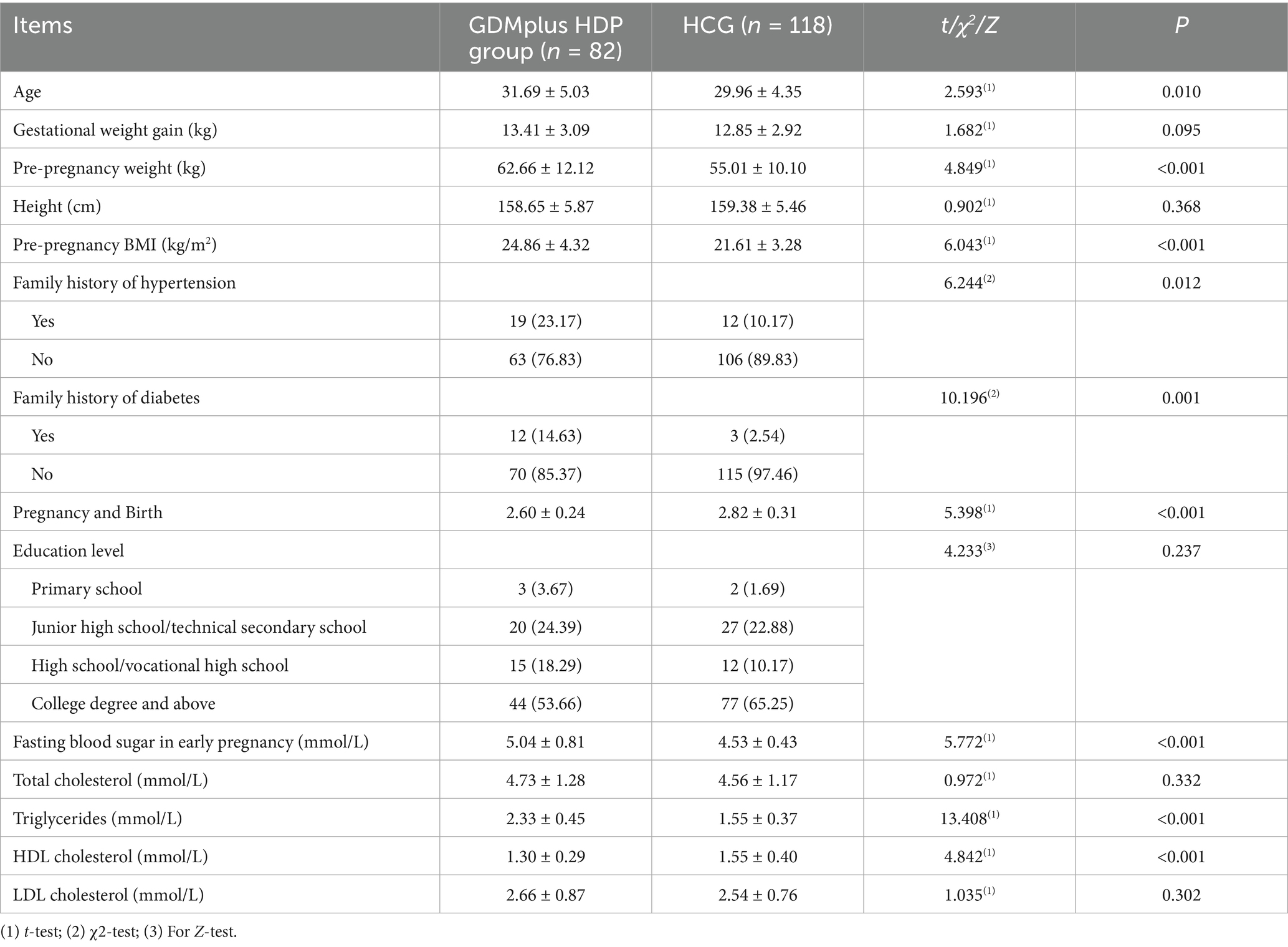
Table 1. Comparison of clinical characteristics between Women with GDM complicated by HDP and the healthy control group (HCG).
3.2 Multifactor analysis of influencing factors related to GDM complicated by HDP
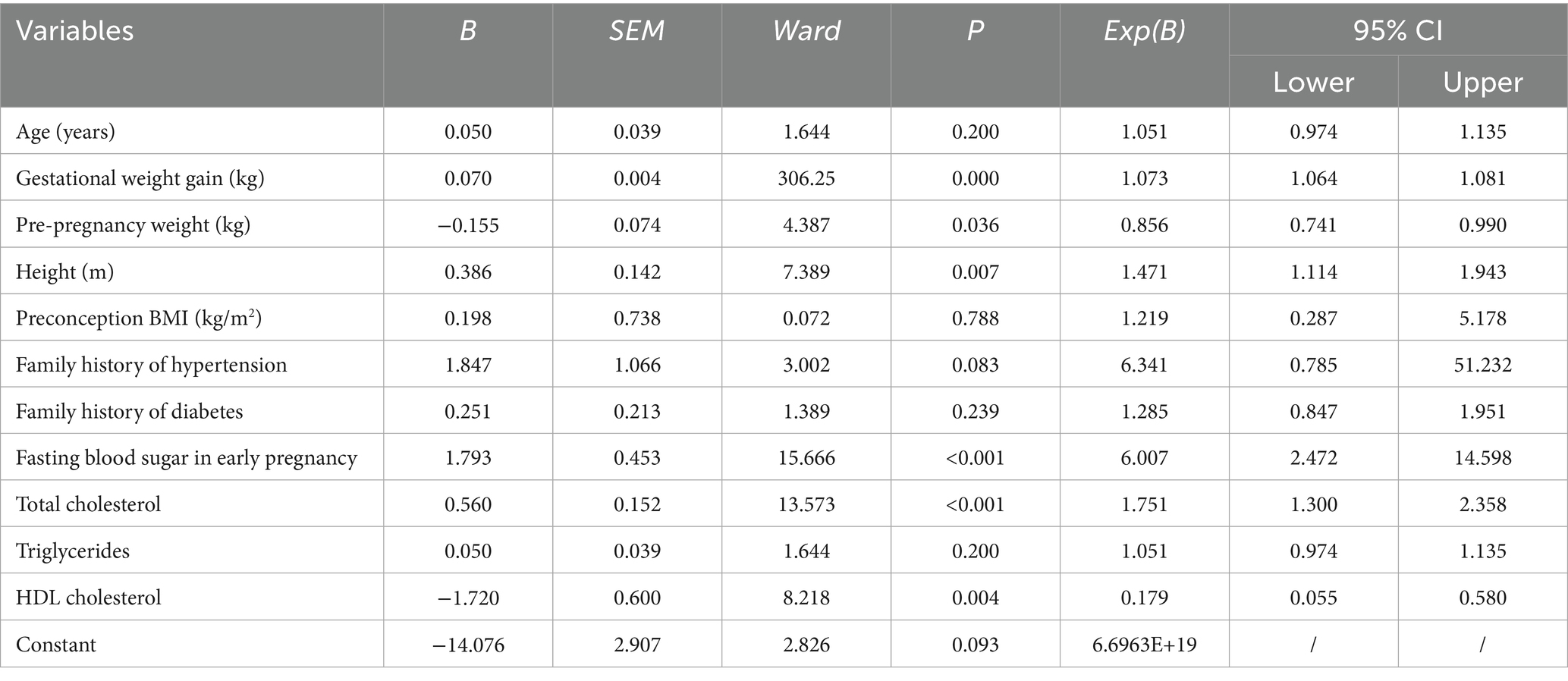
Univariate logistic regression was first performed to examine the association between each maternal characteristic and adverse pregnancy outcomes (Supplementary Table S1). Variables with p < 0.10 or considered clinically significant were then included in the multivariate logistic regression model. The multivariate analysis revealed that pre-pregnancy BMI, early pregnancy fasting blood glucose, triglycerides, high-density lipoprotein cholesterol, and pre-delivery weight were independently associated with the occurrence of GDM complicated by HDP (all p < 0.001, Table 2 and Figure 2). These results indicate that maternal anthropometric characteristics and early biochemical markers significantly predict the risk of developing GDM with HDP (Table 3).
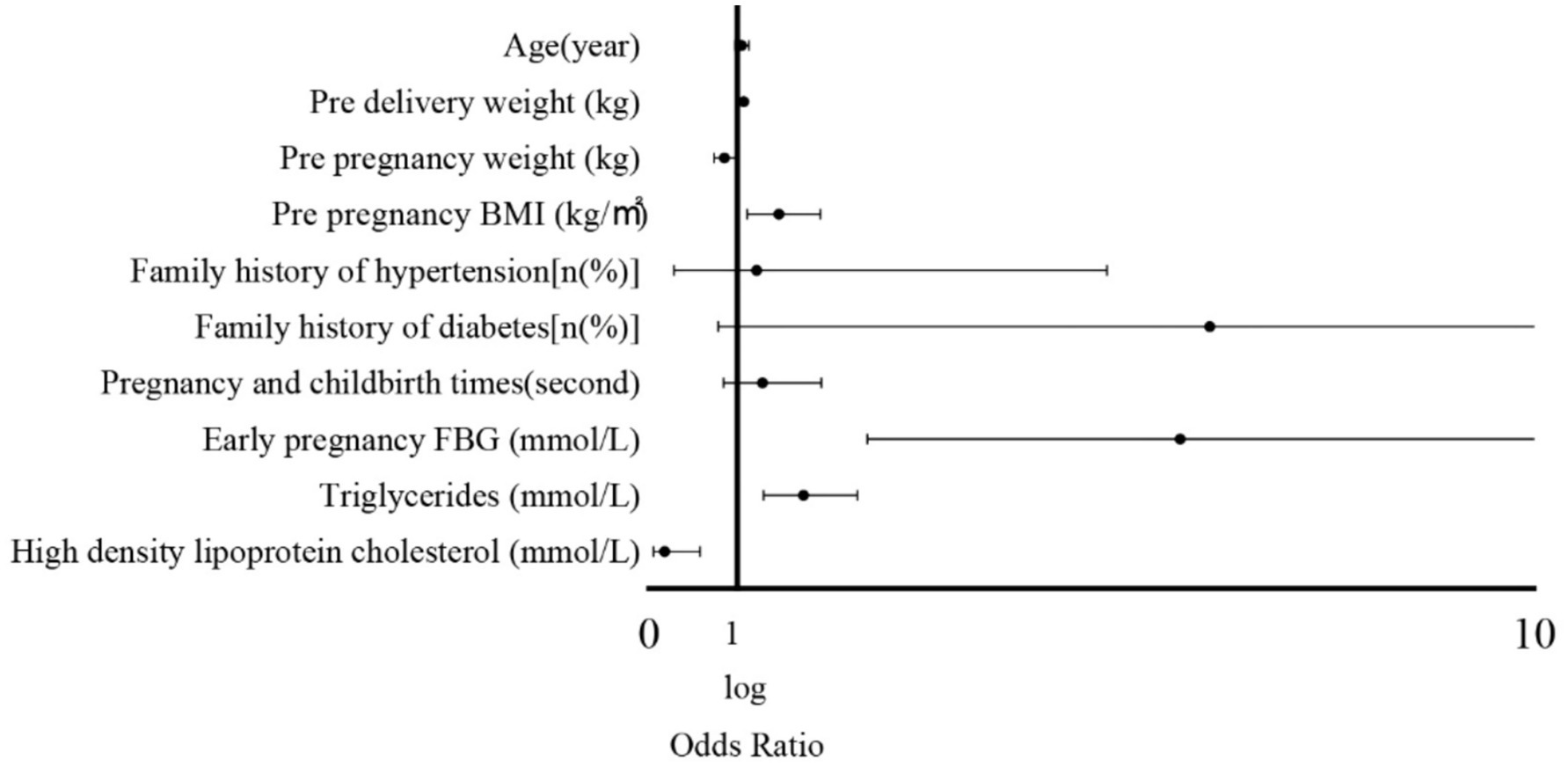
Figure 2. Forest plot of logistic regression analysis of factors influencing adverse pregnancy outcomes.
3.3 Comparison of gestational age and delivery mode in GDM combined with HDP
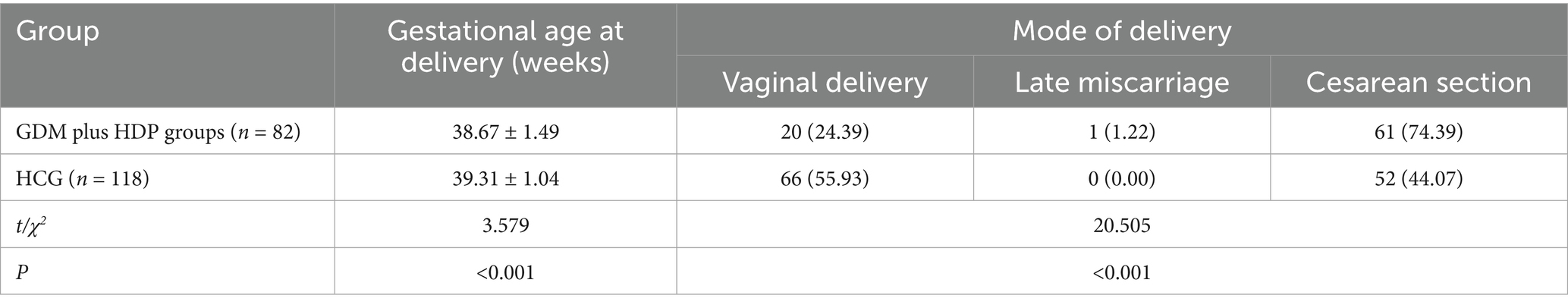
The gestational age at delivery in the GDM combined with HDP group was shorter than that in the HCG (p < 0.05). The vaginal delivery in the GDM combined with HDP group was considerably lower than that in the HCG, and the cesarean section was considerably higher than that in the HCG (p < 0.05), as depicted in Table 4.
3.4 Comparison of pregnancy outcomes between the GDM with HDP group and the HCG
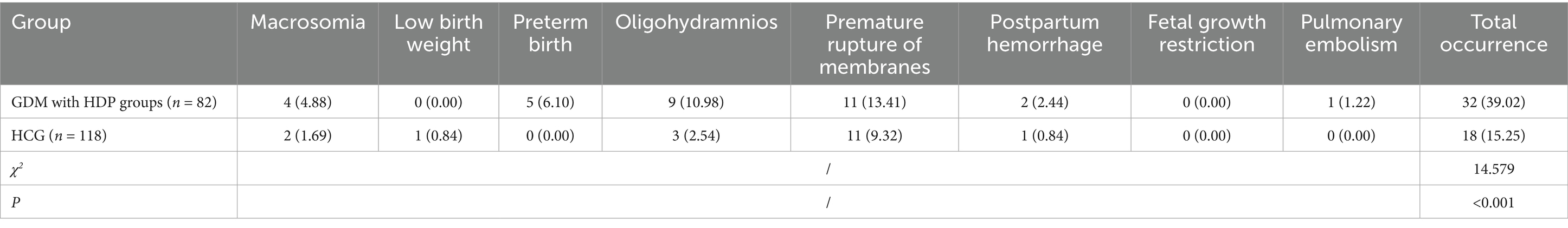
The total incidence of adverse pregnancy outcomes such as macrosomia, premature rupture of membranes, premature birth, oligohydramnios, postpartum hemorrhage, fetal growth restriction, and pulmonary embolism in the GDM with HDP group was 47.87%, which was considerably higher than that in the HCG (15.35%) (p < 0.05, Table 5).
3.5 Blood glucose, blood pressure and adverse pregnancy outcomes in individuals with GDM with HDP after medication treatment
Among the 82 patients with GDM complicated by HDP, a total of 42 patients (51.22%) received treatment, and all 42 patients underwent antihypertensive therapy. In the treated group, the HbA1c was (5.23 ± 0.57)%, FBG was (5.17 ± 0.58) mmol/L, and 2-h PBG was (6.38 ± 0.72) mmol/L. SBP was (122.38 ± 10.31) mmHg, and DBP was (82.39 ± 8.37) mmHg. In the untreated group, the HbA1c was (6.18 ± 0.85)%, FBG was (6.38 ± 0.75) mmol/L, and 2-h PBG was (8.82 ± 0.87) mmol/L. SBP was (138.98 ± 12.39) mmHg, and DBP was (98.28 ± 9.47) mmHg. The levels of 2-h PBG, FBG, HbA1c, DBP, and SBP were significantly lower in the treated patients compared with the untreated patients (p < 0.05), as shown in Figure 3.
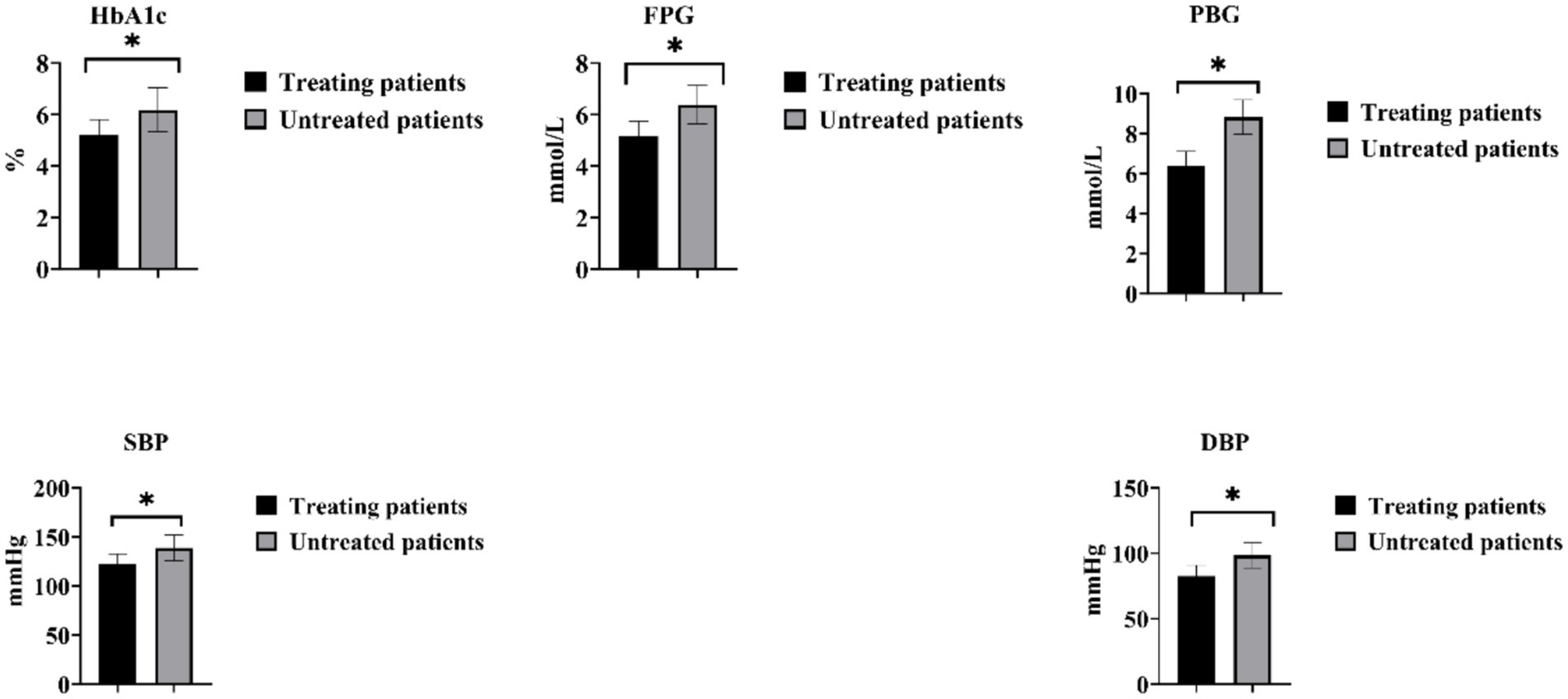
Figure 3. Comparison of blood glucose and blood pressure changes in individuals with GDM and HDP after medication treatment. *p < 0.05.
The adverse pregnancy outcome rate among untreated GDM patients with HDP was 90.00% (36/40). This included 8 cases (20.00%) of premature rupture of membranes, 5 cases (12.50%) of macrosomia, 8 cases (20.00%) of premature delivery, 8 cases (20.00%) of oligohydramnios, 3 cases (7.50%) of postpartum hemorrhage, 1 case (2.50%) of fetal growth restriction, and 3 cases (7.50%) of pulmonary embolism. In contrast, the adverse pregnancy outcome rate for GDM patients treated for HDP was 21.43% (9/42). This comprised 3 cases (7.14%) of premature rupture of membranes, 3 cases (7.14%) of oligohydramnios, 1 case (2.38%) of fetal growth restriction, 1 case (2.38%) of premature delivery, and 1 case (2.38%) of macrosomia. A significant difference was observed between the treated and untreated groups (χ2 = 23.575, p < 0.05).
4 Discussion
In this study, women with GDM complicated by HDP exhibited significantly higher pre-pregnancy BMI, fasting blood glucose, triglycerides, and HDLC compared with healthy controls. Logistic regression analysis identified pre-pregnancy BMI, early-pregnancy fasting blood glucose, triglycerides, HDLC, and pre-delivery weight as independent predictors for the development of GDM with HDP. These patients also had shorter gestational age, lower rates of vaginal delivery, higher cesarean section rates, and a substantially higher incidence of adverse pregnancy outcomes than healthy controls. Importantly, individualized treatment targeting both glycemic and hypertensive control markedly reduced adverse outcomes, demonstrating the clinical value of early and tailored intervention.
Normal pregnancy is characterized by physiological adaptations such as increased fat intake, enhanced intestinal fat absorption, and elevated hepatic lipid synthesis, contributing to progressive insulin resistance (13). Compared to non-pregnant women, PW show elevated leptin and insulin levels, leading to increased blood lipids and glucose, with lipid levels potentially doubling those of non-pregnant women (14, 15). These changes support fetal growth, maintain pregnancy, facilitate labor, and promote postpartum lactation. However, persistently elevated glucose and lipid levels may result in vascular accumulation, altered blood viscosity, endothelial dysfunction, and systemic inflammation, predisposing to GDM and HDP and increasing maternal and neonatal morbidity (16, 17). In this study, the adverse pregnancy outcome rate among women with GDM treated for HDP was 21.43%. Previous research has indicated that approximately 25% of women with GDM develop HDP (18). The similarity between these figures underscores the high-risk nature of this comorbidity. However, the substantially lower rate of adverse outcomes in our treated cohort compared with untreated patients highlights the protective effect of individualized interventions, suggesting that timely management of blood glucose and blood pressure can partially mitigate the risks associated with this well-documented complication.
Our findings are consistent with previous reports demonstrating that pre-pregnancy BMI, early-pregnancy fasting glucose, triglycerides, and HDLC are key predictors of GDM complicated by HDP (19, 20). In line with these findings, our data further emphasize that the combined burden of dyslipidemia and hyperglycemia before and during early pregnancy substantially heightens the risk of HDP among women with GDM, highlighting a shared pathophysiological pathway. Elevated BMI exacerbates insulin resistance, disrupts lipid metabolism, and promotes inflammatory mediator expression, which can damage vascular endothelium and increase the risk of adverse pregnancy outcomes (21). Similarly, early hyperglycemia reflects underlying β-cell dysfunction, which, in combination with pregnancy-induced insulin resistance, accelerates the development of GDM (22, 23). Chronic hypertriglyceridemia further impairs endothelial function, exacerbating hypertensive disorders and supporting the observed pathophysiological synergy between GDM and HDP (24–27). Moreover, this interplay may explain why women with GDM who subsequently develop HDP experience disproportionately higher rates of adverse pregnancy outcomes. These findings underscore the importance of early identification and management of metabolic risk factors before and during pregnancy. Targeted interventions addressing both glycemic and lipid abnormalities may therefore hold promise in reducing the dual burden of GDM and HDP.
The clinical consequences of this dual pathology were evident in our cohort. The GDM + HDP group demonstrated a significantly higher incidence of adverse pregnancy outcomes, including fetal growth restriction, oligohydramnios, macrosomia, premature rupture of membranes, and preterm birth. These findings align with studies highlighting the detrimental effects of hyperglycemia and hypertension on placental perfusion and uteroplacental insufficiency (28–30). The higher rate of cesarean section in this group likely reflects both iatrogenic interventions and maternal-fetal complications. These results underscore the additive burden imposed by GDM and HDP on maternal and fetal health. Early identification of women at risk and timely intervention strategies may mitigate these adverse outcomes. Our study contributes further evidence that integrated management of metabolic and vascular complications is essential for optimizing pregnancy prognosis.
A key observation of this study is the substantial reduction of adverse outcomes with individualized therapy. Targeted management of hyperglycemia and hypertension using insulin, metformin, labetalol, or nifedipine achieved a reduction in adverse outcome rates from 87.8% in untreated patients to 16.98% in those receiving therapy, highlighting the importance of a dual-targeted approach. This finding reinforces prior evidence supporting the efficacy of these interventions in high-risk pregnancies and provides quantitative support for integrated clinical management (31, 32). Importantly, these results suggest that early identification of at-risk women and tailored management strategies can mitigate the synergistic effects of GDM and HDP, providing a framework for optimizing antenatal care.
Several limitations should be acknowledged in this study. First, the single-center design and relatively small sample size may limit the generalizability of the findings. Although pharmacological treatment regimens were closely monitored, inter-individual patient variability could introduce potential bias. Second, the study did not differentiate between specific types of preterm delivery, underscoring the need for more detailed investigations in future research. Finally, the detailed data regarding the specific indications for labor induction were not available. Consequently, we could not perform subgroup analyses to determine whether medically indicated inductions influenced the observed shorter gestational age in the GDM combined with HDP group. Future studies with more comprehensive perinatal records are warranted to assess the impact of labor induction on gestational outcomes in this population.
5 Conclusion
In summary, pre-pregnancy weight, pre-pregnancy BMI, HDLC, triglycerides, and early pregnancy FBG are significantly associated with the development of GDM complicated by HDP. These patients are at an increased risk of adverse pregnancy outcomes. Individualized pharmacological management targeting blood glucose and blood pressure effectively mitigates these risks, highlighting the importance of early identification and tailored treatment strategies to improve maternal and neonatal outcomes.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethics Committee of the Dongyang Hospital Affiliated to Wenzhou Medical University (2024-YX-323). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
SD: Project administration, Conceptualization, Data curation, Writing – review & editing, Methodology, Investigation, Funding acquisition, Visualization, Resources, Software, Formal analysis. CJ: Methodology, Data curation, Supervision, Conceptualization, Investigation, Writing – original draft, Visualization, Formal analysis, Validation, Project administration. LB: Visualization, Investigation, Formal analysis, Supervision, Writing – original draft. SX: Software, Writing – review & editing, Resources, Data curation, Validation, Methodology, Supervision.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The study was supported by Medical and Health Science and Technology Plan of Zhejiang Province: 2025KY1758.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1656391/full#supplementary-material
References
1. Laposky, AD, and Pemberton, VL. Sleep-disordered breathing and pregnancy-related cardiovascular disease. J Womens Health (Larchmt). (2021) 30:194–8. doi: 10.1089/jwh.2020.8869
2. Parisi, F, Fenizia, C, Introini, A, Zavatta, A, Scaccabarozzi, C, Biasin, M, et al. The pathophysiological role of estrogens in the initial stages of pregnancy: molecular mechanisms and clinical implications for pregnancy outcome from the periconceptional period to end of the first trimester. Hum Reprod Update. (2023) 29:699–720. doi: 10.1093/humupd/dmad016
3. Sakali, AK, Papagianni, M, Bargiota, A, Rasic-Markovic, A, Macut, D, and Mastorakos, G. Environmental factors affecting pregnancy outcomes. Endocrine. (2023) 80:459–69. doi: 10.1007/s12020-023-03307-9
4. Lissaker, C, Hemmingsson, T, Kjellberg, K, Lindfors, P, and Selander, J. Occupational stress and pregnancy-related hypertension and diabetes: results from a nationwide prospective cohort. Scand J Work Environ Health. (2022) 48:239–47. doi: 10.5271/sjweh.4004
5. Sweeting, A, Wong, J, Murphy, HR, and Ross, GP. A clinical update on gestational diabetes mellitus. Endocr Rev. (2022) 43:763–93. doi: 10.1210/endrev/bnac003
6. Wu, P, Green, M, and Myers, JE. Hypertensive disorders of pregnancy. BMJ. (2023) 381:e071653. doi: 10.1136/bmj-2022-071653
7. Ijomone, OK, Osahon, IR, Okoh, COA, Akingbade, GT, and Ijomone, OM. Neurovascular dysfunctions in hypertensive disorders of pregnancy. Metab Brain Dis. (2021) 36:1109–17. doi: 10.1007/s11011-021-00710-x
8. Naz, MSG, Sheidaei, A, Azizi, F, and Tehrani, FR. Gestational diabetes mellitus and hypertensive disorder of pregnancy play as spouse-pair risk factors of diabetes and hypertension: insights from Tehran lipid and glucose study. J Diabetes Complicat. (2022) 36:108311. doi: 10.1016/j.jdiacomp.2022.108311
9. Shah, NS, Wang, MC, Kandula, NR, Carnethon, MR, Gunderson, EP, Grobman, WA, et al. Gestational diabetes and hypertensive disorders of pregnancy by maternal birthplace. Am J Prev Med. (2022) 62:e223–31. doi: 10.1016/j.amepre.2021.10.007
10. Mourad, M, Too, G, Gyamfi-Bannerman, C, and Zork, N. Hypertensive disorders of pregnancy in twin gestations complicated by gestational diabetes(). J Matern Fetal Neonatal Med. (2021) 34:720–4. doi: 10.1080/14767058.2019.1614160
11. Hod, M, Kapur, A, Sacks, DA, Hadar, E, Agarwal, M, Di Renzo, GC, et al. The International Federation of Gynecology and Obstetrics (FIGO) initiative on gestational diabetes mellitus: a pragmatic guide for diagnosis, management, and care. Int J Gynaecol Obstet. (2015) 131:S173–211. doi: 10.1016/S0020-7292(15)30033-3
12. Poon, LC, Shennan, A, Hyett, JA, Kapur, A, Hadar, E, Divakar, H, et al. The International Federation of Gynecology and Obstetrics (FIGO) initiative on pre-eclampsia: a pragmatic guide for first-trimester screening and prevention. Int J Gynaecol Obstet. (2019) 145:1–33. doi: 10.1002/ijgo.12802
13. Moore, TA, and Case, AJ. Cytokine levels throughout the perinatal period. J Matern Fetal Neonatal Med. (2022) 35:5513–9. doi: 10.1080/14767058.2021.1887121
14. Mauri, M, Calmarza, P, and Ibarretxe, D. Dyslipemias and pregnancy, an update. Clin Investig Arterioscler. (2021) 33:41–52. doi: 10.1016/j.arteri.2020.10.002
15. Jayasinghe, IU, Agampodi, TC, Dissanayake, AK, Srimantha, SM, and Agampodi, SB. Comparison of global definitions of metabolic syndrome in early pregnancy among the Rajarata pregnancy cohort participants in Sri Lanka. Sci Rep. (2022) 12:2009. doi: 10.1038/s41598-022-05919-z
16. Marschner, S, Pant, A, Henry, A, Maple-Brown, LJ, Moran, L, Cheung, NW, et al. Cardiovascular risk management following gestational diabetes and hypertensive disorders of pregnancy: a narrative review. Med J Aust. (2023) 218:484–91. doi: 10.5694/mja2.51932
17. Bittner, JMP, Gilman, SE, Zhang, C, Chen, Z, and Cheon, BK. Relationships between early-life family poverty and relative socioeconomic status with gestational diabetes, preeclampsia, and hypertensive disorders of pregnancy later in life. Ann Epidemiol. (2023) 86:8–15. doi: 10.1016/j.annepidem.2023.08.002
18. Will, JS, and Crellin, H. Gestational diabetes mellitus: update on screening, diagnosis, and management. Am Fam Physician. (2023) 108:249–58.
19. Zehravi, M, Maqbool, M, and Ara, I. Correlation between obesity, gestational diabetes mellitus, and pregnancy outcomes: an overview. Int J Adolesc Med Health. (2021) 33:339–45. doi: 10.1515/ijamh-2021-0058
20. Bastola, K, Koponen, P, Skogberg, N, Gissler, M, and Kinnunen, TI. Hypertensive disorders of pregnancy among women of migrant origin in Finland: a population-based study. Acta Obstet Gynecol Scand. (2022) 101:127–34. doi: 10.1111/aogs.14291
21. Ito, M, Kyozuka, H, Yamaguchi, T, Sugeno, M, Murata, T, Hiraiwa, T, et al. Association between gestational weight gain and risk of hypertensive disorders of pregnancy among women with obesity: a multicenter retrospective cohort study in Japan. Nutrients. (2023) 15:2428. doi: 10.3390/nu15112428
22. Santos, S, Voerman, E, Amiano, P, Barros, H, Beilin, LJ, Bergstrom, A, et al. Impact of maternal body mass index and gestational weight gain on pregnancy complications: an individual participant data meta-analysis of European, North American and Australian cohorts. BJOG. (2019) 126:984–95. doi: 10.1111/1471-0528.15661
23. Tamagawa, M, Kasuga, Y, Saisho, Y, Tanaka, Y, Hasegawa, K, Oishi, M, et al. Predictors of later insulin therapy for gestational diabetes diagnosed in early pregnancy. Endocr J. (2021) 68:1321–8. doi: 10.1507/endocrj.EJ21-0118
24. Immanuel, J, Simmons, D, Harreiter, J, Desoye, G, Corcoy, R, Adelantado, JM, et al. Metabolic phenotypes of early gestational diabetes mellitus and their association with adverse pregnancy outcomes. Diabet Med. (2021) 38:e14413. doi: 10.1111/dme.14413
25. Saucedo, R, Ortega-Camarillo, C, Ferreira-Hermosillo, A, Diaz-Velazquez, MF, Meixueiro-Calderon, C, and Valencia-Ortega, J. Role of oxidative stress and inflammation in gestational diabetes mellitus. Antioxidants (Basel). (2023) 12:1812. doi: 10.3390/antiox12101812
26. Shakhanova, A, Aukenov, N, Nurtazina, A, Kasskabayeva, A, Massabayeva, M, and Kenzhebayeva, D. Association of lipid parameters with insulin resistance in the Kazakh population. Bratisl Lek Listy. (2023) 124:604–8. doi: 10.4149/BLL_2023_094
27. Shihab, AS, Hamdi, MA, Jumaa, AM, Marbut, MM, and Jwad, SK. Dyslipidemia and other parameters in women with pregnancy induced hypertension. J Popul Ther Clin Pharmacol. (2022) 29:e116–21.
28. Cortes, YI, Conant, R, Catov, JM, Matthews, KA, Crawford, SL, Hedderson, MM, et al. Impact of nulliparity, hypertensive disorders of pregnancy, and gestational diabetes on vasomotor symptoms in midlife women. Menopause. (2020) 27:1363–70. doi: 10.1097/GME.0000000000001628
29. Soria-Contreras, DC, Perng, W, Rifas-Shiman, SL, Minguez-Alarcon, L, Hivert, MF, Shifren, J, et al. Associations of hypertensive disorders of pregnancy and gestational diabetes mellitus with menopausal symptoms at midlife in project viva. Menopause. (2022) 29:1021–7. doi: 10.1097/GME.0000000000002014
30. Martinez-Vizcaino, V, Sanabria-Martinez, G, Fernandez-Rodriguez, R, Cavero-Redondo, I, Pascual-Morena, C, Alvarez-Bueno, C, et al. Exercise during pregnancy for preventing gestational diabetes mellitus and hypertensive disorders: an umbrella review of randomised controlled trials and an updated meta-analysis. BJOG. (2023) 130:264–75. doi: 10.1111/1471-0528.17304
31. Masson, W, Barbagelata, L, Lobo, M, Berg, G, Lavalle-Cobo, A, and Nogueira, JP. Association between maternal epicardial adipose tissue, gestational diabetes mellitus, and pregnancy-related hypertensive disorders: a systematic review and meta-analysis. Arch Gynecol Obstet. (2023) 308:1057–66. doi: 10.1007/s00404-023-06933-w
Keywords: gestational diabetes mellitus, pregnancy induced hypertension, adverse pregnancy outcome, clinical characteristics, metabolic predictors
Citation: Jia C, Bo L, Xiao S and Du S (2025) Clinical features and outcomes of pregnancies complicated by coexisting gestational diabetes and hypertensive disorders. Front. Med. 12:1656391. doi: 10.3389/fmed.2025.1656391
Edited by:
Javier Diaz-Castro, University of Granada, SpainReviewed by:
Herald Midzi, Family Health International 360, ZimbabweIman Aitelhaj, University of Oradea, Romania
Gitana Ramoniene, Lithuanian University of Health Sciences, Lithuania
Copyright © 2025 Jia, Bo, Xiao and Du. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lanying Bo, MTM1NjY5ODg0NDZAMTYzLmNvbQ==; Shunlan Du, MTM3NTg5NTM2MzhAd211LmVkdS5jbg==
 Chaoying Jia
Chaoying Jia Shunlan Du
Shunlan Du