- 1Department of Anesthesiology, Shaoxing People’s Hospital, Zhejiang University, Shaoxing, China
- 2Department of Anesthesiology, Shulan (Hangzhou) Hospital, Shulan International Medical College, Zhejiang Shuren University, Hangzhou, China
- 3Department of Clinical Medicine, Wannan Medical College, Wuhu, China
- 4Karolinska Institutet at Danderyds Hospital (KIDS), Stockholm, Sweden
Objectives: The urine concentration of metabolic end products increases in response to low habitual water intake or acute dehydration. We examined the impact of concentrated urine on surgical outcomes with special attention given to plasma creatinine levels.
Methods: A prospective observational study was conducted involving 921 patients scheduled for non-cardiac major surgery. The degree of urine concentration was quantified prior to the surgery using a composite index (Fluid Retention Index, FRI) that reflects renal water conservation. Arterial pressure was monitored every 5 min during the operations. A perioperative increase in plasma creatinine of >50% or ≥26.5 μmol/L was designated as acute kidney injury (AKI).
Results: The average operating time averaged 2.9 ± 1.3 h (mean ± SD) during which the mean arterial pressure was 5.2 ± 13.1 mmHg lower than the preoperative reading. Concentrated urine (FRI > 4.0) was present in just 7% of the patients, signifying that dehydration was infrequent. Univariate analysis showed that these patients still had extended gastrointestinal recovery time (p < 0.001), larger hemorrhages (5% vs. 1% > 500 mL; p = 0.047), and a heightened occurrence of fever (28% vs. 17%; p < 0.03). Multivariate analysis showed an extended gastrointestinal recovery time and smaller urine output despite receiving more crystalloid fluid (all correlations p < 0.001). Gradually higher FRI was associated with lower MAPs at baseline (p < 0.024). Postoperative AKI developed in only 1% of the patients, which made the study underpowered to detect a statistically significant relationship between concentrated urine and AKI (odds ratio 0.988, 95% confidence interval 0.980–0.996; p = 0.43).
Conclusion: Patients with concentrated urine before surgery had a lower urine output during surgery and a longer postoperative period of food intolerance and more often fever than patients with dilute urine. The occurrence of postoperative AKI was very low, which was probably due to the generally good hydration status.
1 Introduction
Postoperative complications are costly in terms of suffering and economy, but preventive measures require good understanding of the conditions that promote their development. “Concentrated urine” is rarely considered to influence the incidence of surgical complications. This trait indicates that the urinary concentrations of metabolic end products are high because of renal water conservation, which might be due to acute dehydration but, more commonly, to low daily water consumption (1–3). Only a few small studies suggest that concentrated urine impacts surgical complications. For example, 60% of those with concentrated urine who underwent hip fracture surgery had at least one postoperative complication, while the incidence was 30% in the others.
Acute kidney injury (AKI) is a postoperative complication of special interest as concentrated urine brings the patient closer to the renal threshold for creatinine excretion (4). In a study of 642 surgical patients from four countries, concentrated urine before surgery was associated with low urine flow and increased incidents of postoperative AKI (5). This clinical syndrome is common after major surgery (usually 10%) and associated with higher long-term morbidity and mortality (6). AKI denotes an abrupt decrease of kidney function that might be due to specific kidney diseases and non-specific conditions such as ischemia and toxic injury, and well as to extrarenal pathology (7). The diagnosis is based on an acute elevation of plasma creatinine (+50% or ≥26.5 μmol/L) and/or on persistent oliguria (7, 8). Plasma creatinine typically hits its highest point on the first or second postoperative day, whereafter normalization usually (but not always) occurs (9). Aside from plasma creatinine, no sufficiently powered study has yet compared the effects of concentrated urine on the course of anesthesia and surgery.
The aim of the present study was to evaluate the relationship between concentrated urine measured prior to non-cardiac elective surgery and postoperative complications, with a particular focus on plasma creatinine. Concentrated urine was assessed using the Fluid Retention Index (FRI) scale, which is a summary measure of four biomarkers that have been used to quantify renal water conservation in dietary studies (2, 3), sports medicine (10–12), and surgery (4, 5, 13). The robust FRI scale integrates these biomarkers into a single value, which is an approach so far employed in approximately 15 studies in geriatric care and surgery (14–20).
The study hypothesis was that concentrated urine poses a risk of postoperative morbidity, including the development of AKI.
2 Methods
2.1 Study design and setting
We conducted a single-center, prospective clinical study at Shaoxing People’s Hospital, which is university hospital in the People’s Republic of China.
2.2 Patients
The study included 921 patients who underwent elective open and laparoscopic surgeries, the majority of which was performed due to a cancer diagnosis. The criteria for enrolment included being in the ASA Class I–II, aged between 18 and 100 years, and having a body mass index of 18–30 kg/m2. Those with urogenital diseases or severe cardiac, lung, renal, or hepatic disease were excluded. Hence, patients with pre-existing kidney disease were excluded. The types of studied surgeries are listed in Supplementary File 1. Patients were recruited during two periods, from June 2018 and 1 year forward, and between February and June, 2023. The first 126 patients have been reported previously (20) after which the included measurements were adopted as a routine. This presentation follows the STROBE checklist.
2.3 Procedures
The patients did not ingest solid food from midnight. They arrived at the operating theatre between 7 a.m. and 9 a.m. None were given premedication. General anesthesia was induced with midazolam 50 μg/kg, propofol 1.5 mg/kg, cis-atracurium 0.15 mg/kg, and sufentanil 5 μg/kg. This was followed by endotracheal intubation and mechanical ventilation. The anesthesia was maintained with propofol 6 mg/kg/h and 1.5 MAC of sevoflurane. Additional cis-atracurium 0.05 mg/kg and sufentanil 2 μg/kg was given as required.
Intravenous (i.v.) fluid therapy consisted in lactated Ringer’s solution (Pharmacia-Baxter, Shanghai, China) and 6% hydroxyethyl starch 130/0.4 (Voluven®; Fresenius Kabi Deutschland GmbH, Bad Homburg, Germany) but were not given according to a strict protocol. Voluven® was initiated during the induction of anesthesia with the purpose of maintaining stable hemodynamics. Blood products were given at the clinicians’ discretion.
2.4 Data collection
Monitoring consisted of pulse oximetry, heart rate, invasive arterial pressure, and electrocardiography. A catheter was placed in the radial artery before induction of anesthesia and the mean arterial pressure (MAP) recorded every 5 min from the preoperative phase until the end of the surgery. The data was stored on a DoCare Anesthesia Clinical Information System (Medical System, Shuzhou, China).
The “patient mean MAP” refers to the mean of the MAP measurements in a single patient but reported separately for measurements made before and after anesthesia was induced. We identified instances of hypotension (MAP < 60 mmHg) and noted the total duration of hypotension in each patient. According to a systematic review 60 mmHg is the most frequently used threshold for hypotension during surgery (21). Ephedrine 5–10 mg was given i.v. if a patient experienced both hypotension and bradycardia. Continuous infusions of catecholamines were not used.
Blood and urine samples were collected prior to anesthesia induction. Additional blood samples were gathered on the first postoperative day, and for 96% of the patients up to 9 days post-surgery. These samples were evaluated for plasma creatinine and C-reactive protein levels in the hospital’s Clinical Chemistry Laboratory. Additional blood analyses, including protein, albumin, liver enzymes, and immunoglobulins, are available in Supplementary File 2.
Blood loss was determined by measuring the blood volume in the suction tubes and weighing the sponges. Urine volume was measured and sampled from the start of anesthesia until discharge from the postoperative care unit, using an indwelling catheter. The first portion of the collected urine was tested for color, gravity, osmolality, and creatinine. Color was assessed using a UrineHydration chart (en.wikipedia.org/wiki/File:Urine_Hydration.chart.jpg) (1).
2.5 Fluid Retention Index (FRI)
The Fluid Retention Index (FRI) was used to quantify the urine concentration before the surgeries. This scale was constructed in 2013 and has been applied in approximately 15 studies in sports medicine, surgery, and geriatric care. The FRI is a composite index of renal water conservation based on four urinary biomarkers: urine-specific gravity, creatinine, osmolality, and urine color. These variables represent metabolic waste products that are excreted at a constant rate regardless of variations in urine flow.
The FRI concept is based on the curvilinear relationship between urine-specific gravity and the change in body weight found during progressive dehydration in 7 published studies in sports medicine and further validated in volunteers performing recreational sports activities (12). Urine-specific gravity, urine osmolality, and urine creatinine also distinguish between volunteers who have different daily consumptions of water (2, 3). There are strong inter-correlations between the four biomarkers; correlation coefficients range from 0.71 to 0.84 (12), and this correlation strength was consistent when re-tested with 300 hospital workers (1).
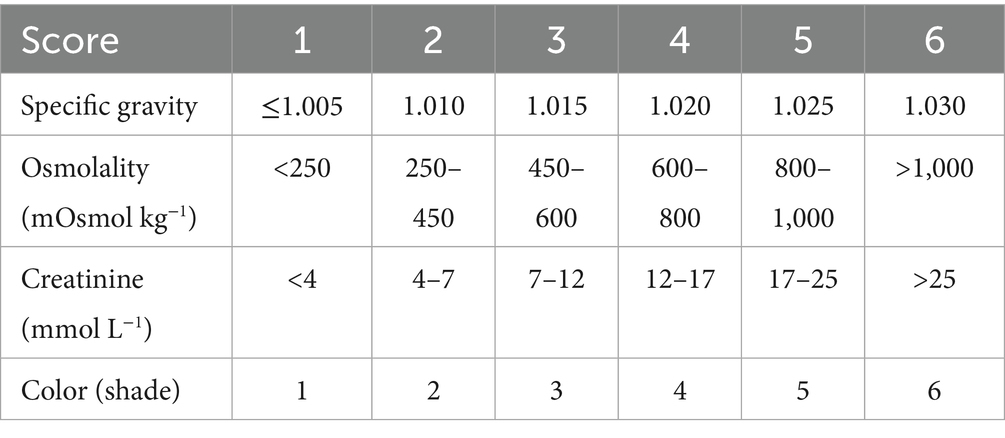
The FRI scale is constructed so that each of the four biomarkers is assigned a score ranging from 1 to 6, with the average score representing the final FRI value (Table 1). The boundaries for the FRI scores were decided based on the relationships between the four indices of dehydration as obtained in 57 subjects aged 17–69 years (12) and in 256 patients admitted to hospital for acute geriatric care (14). Despite the strong correlations, using a composite index provides less sensitivity to outliers than individual biomarkers, which can occasionally be affected by diet and medication.
FRI > 4.0 indicates a 3% body weight reduction due to dehydration after physical activity (10–12). This cut-off was used to differentiate between properly hydrated patients and those who are dehydrated.
Patients were not managed in any particular way to account for their preoperative hydration status as the result was not known to the anesthesiology team.
2.6 Outcome measures
Data on postoperative complications (other than AKI) were extracted from the medical records and thus represent those routinely tracked by the hospital. These complications included respiratory infection, would infection, fever, reoperation, pain, need for intensive care, and length or hospital stay. Pain was quantified using a visual analogue scale graded from 0 to 10 where 10 was the worst possible pain intensity; the number of occasions where a patient scored >4.0 were counted. The definitions used for the International Surgical Outcomes Study (ISOS, see http://isos.org.uk) were used. We also recorded the gastrointestinal recovery time (i.e., end of paralytic ileus determined by the return of bowel sounds) and when the oral food intolerance time ended.
2.7 Acute kidney injury
Acute kidney injury (AKI) was diagnosed based on an increase of the plasma creatinine concentration by ≥ 50% or an increase by ≥ 26.5 μmol/L on the first or second postoperative day (6–8). AKI can also be diagnosed based on low urine output (<0.5 mL/kg/h for >6 h) but was not used here as oliguria is less clearly associated with poor outcomes than AKI diagnosed by plasma creatinine (8).
2.8 Statistics
Data showing a normal distribution are presented as the mean ± standard deviation. Changes were studied by the paired t test. Differences between groups were assessed using one-way analysis of variance (ANOVA) followed by the Scheffé post hoc test (if >2 groups).
Data with a skewed distribution are presented as the median and the 25th–75th percentile, and differences were evaluated using the Mann–Whitney U test (for 2 groups) or the Kruskal-Wallis test (for ≥3 groups) followed by the pairwise post hoc test in SPSS version 30.0.0 for Mac (IBM Corp., Armonk, NY). The Hodges-Lehmann estimate was used to report the 95% confidence interval (CI) for the differences between the data in two groups where the distribution was skewed.
For categorical data, the chi-square test was used, with squared z-values determining statistical differences between sub-groups.
Univariate and stepwise multiple linear and logistic regression (Hosmer-Lemeshow goodness-of fit) were used to identify demographic and surgical variables that correlated significantly with the outcome measures. p < 0.05 was considered statistically significant.
The power analysis focused on determining if a high FRI score before surgery was statistically more common among patients who developed postoperative AKI. Our pooled data study from four countries revealed high FRI scores in 24% of the patients, and AKI developed in 6.1% (5). The required number of patients would then be 875, which provided 90% statistical power at the p < 0.01 significance level.
3 Results
3.1 Baseline data
The analysis involved 921 patients (47% females) with an average age of 61 ± 12 years, body weight of 60 ± 9 kg, and body mass index (BMI) of 22.8 ± 2.8 kg/m2. Of these patients, 9% were over 75 years old, and 19% had a BMI greater than 25 kg/m2. Data were complete except for AKI which was available in 773 patients.
The average FRI was 2.3 ± 1.1, with only 64 patients (7%) scoring >4.0 as evidence of dehydration (Figure 1). The basic data classified by surgery type is presented in Supplementary Table S1.
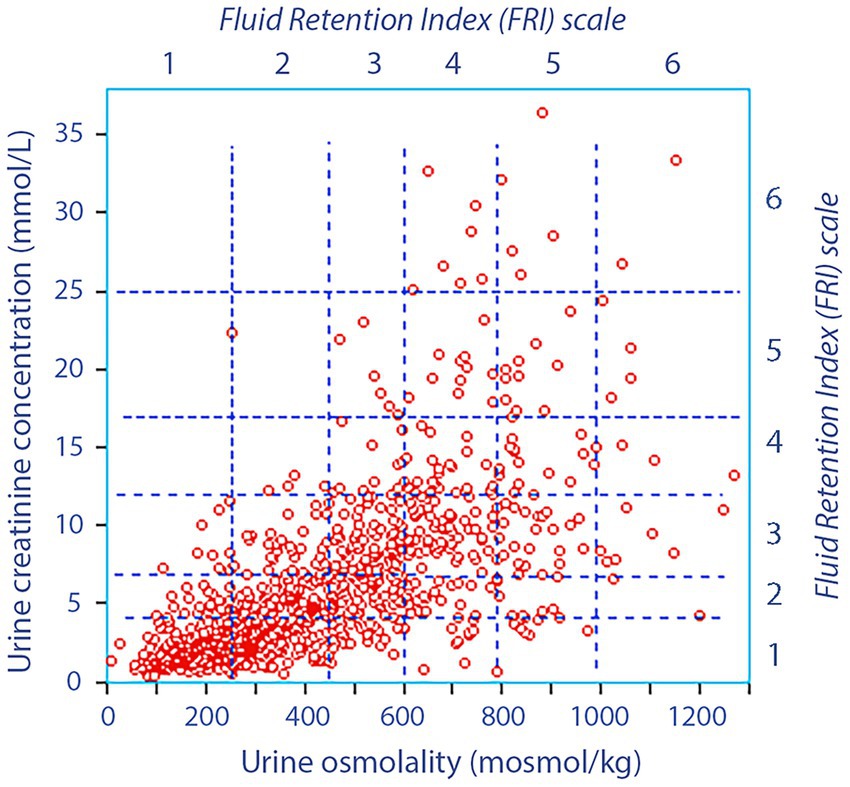
Figure 1. Urine osmolality versus the urine creatinine concentration before surgery in 921 patients. The vertical hatched lines indicate the FRI scores with respect to urine osmolality and the horizontal lines the divisions with respect to urine creatinine. Hence, each square gives one FRI score for urine creatinine and another score for urine creatinine, and the final FRI value is the mean of four scores (the other two being urine color and urine-specific gravity).
3.2 Perioperative data
The surgery lasted for 2.9 ± 1.3 h, with 63% of the procedures carried out laparoscopically or thoracoscopically. The Ringer’s solution given amounted to 1,998 ± 724 mL and 93% of the patients received 500 mL of Voluven.
The patient mean MAP during surgery (87 ± 10 mmHg) was only slightly lower than before the surgery (92 ± 10 mmHg: paired t test p < 0.001). The mean change was −5.2 ± 13.1 mmHg, the greatest reductions occurring in those having a high MAP at baseline (Figure 2A).
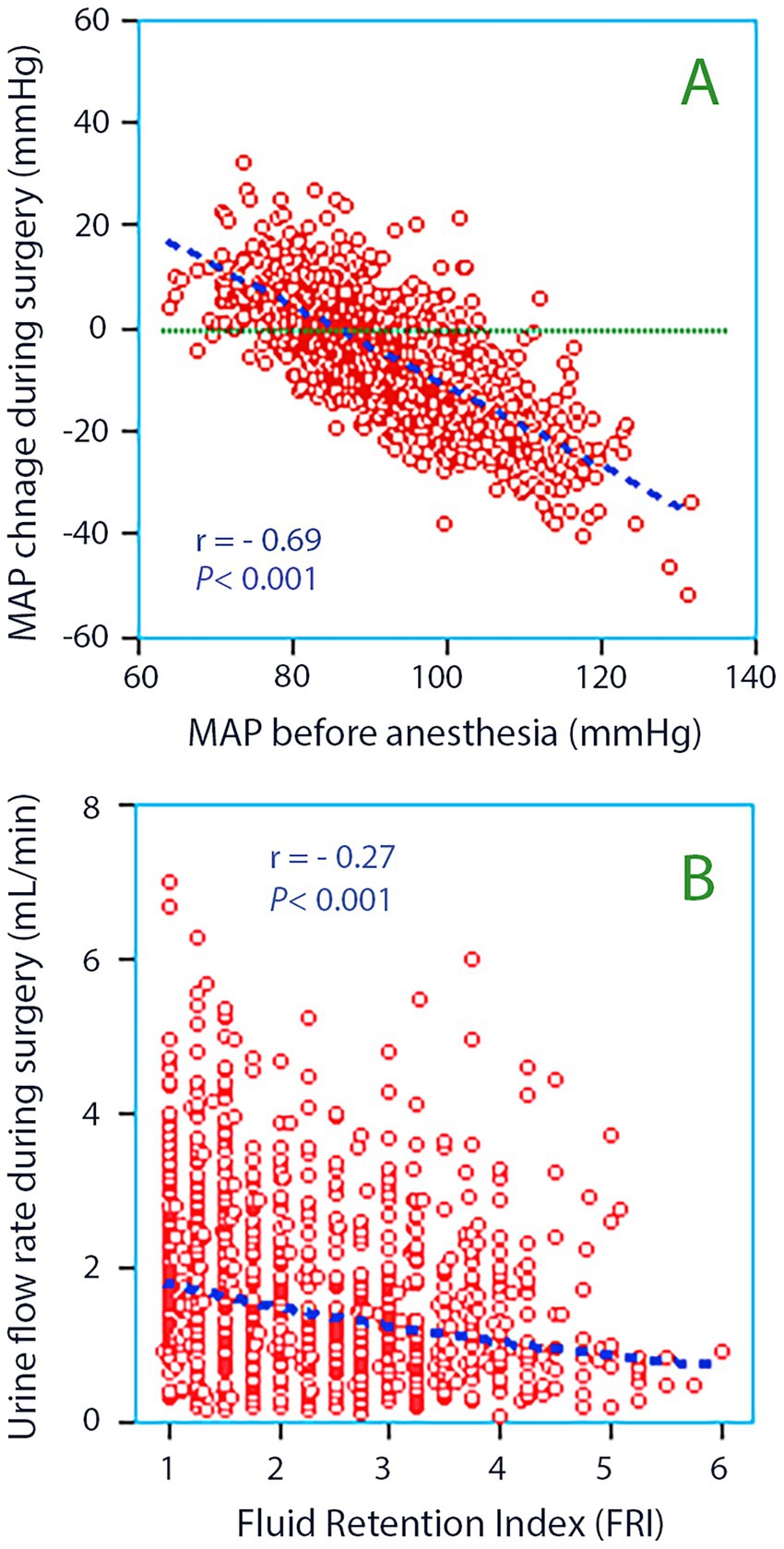
Figure 2. (A) The mean arterial pressure (MAP) measured before anesthesia was induced versus the change that occurred during anesthesia and surgery. The latter was obtained as the mean of all invasive measurements taken every 5 min throughout each surgery. Simple linear regression was used for the statistics. (B) Fluid Retention Index (FRI) versus the urine flow rate during surgery. Whether the kidneys are pre-set to retain or excrete fluid influences the urine flow.
Hypotension (<60 mmHg) occurred in 122 patients (13.3%); one fifth (26 patients) showed more than two 5-min recordings of hypotension. MAP during hypotension was 54 ± 7 mmHg and the total hypotension time was 70 min of a total of 162,096 min (0.043%).
The median blood loss was 80 mL (25th–75th percentile limits, 50–148 mL), and the urine output was 350 mL (200–580), corresponding to a flow of 1.3 mL/min (0.8–2.1).
The plasma concentration of C-reactive protein was 1.2 μg/L (0.5–3.2) before the surgery and 51 μg/L (33–89) postoperatively. Blood products were given to 147 patients.
After the surgery, paralytic ileus persisted for 1.5 (0.3–2.0) days and the time of food intolerance was 2.0 days (0.3–4.0). Post-surgery, 18% of the patients experienced fever, 21% contracted a respiratory infection, and 10% developed a wound infection.
Ten patients were reoperated (1.1%). Intensive care was needed for 25 patients (2.7%). The length of the hospital stay for the patients needing intensive care was 14.0 ± 5.4 days while being 9.0 ± 4.2 days for the others (p < 0.001).
The outcomes in the sub-groups are displayed in Supplementary Table S2. No significant difference was observed in the perioperative change in plasma creatinine between them.
3.3 High FRI, univariate analyses
Patients with FRI > 4.0 were younger and received more Ringer’s solution but still had lower urinary flow than the other patients (Figure 2B). They were more often males, had lower MAP at baseline, experienced larger surgical hemorrhages, prolonged paralytic ileus, and food intolerance post-surgery. These patients also had a higher rate of fever and wound infection compared to patients with FRI ≤ 4.0 (Table 2). Hypotensive events were not more prevalent among those with high FRI; it was even associated with unchanged MAP during the surgical period relative to the preoperative records.
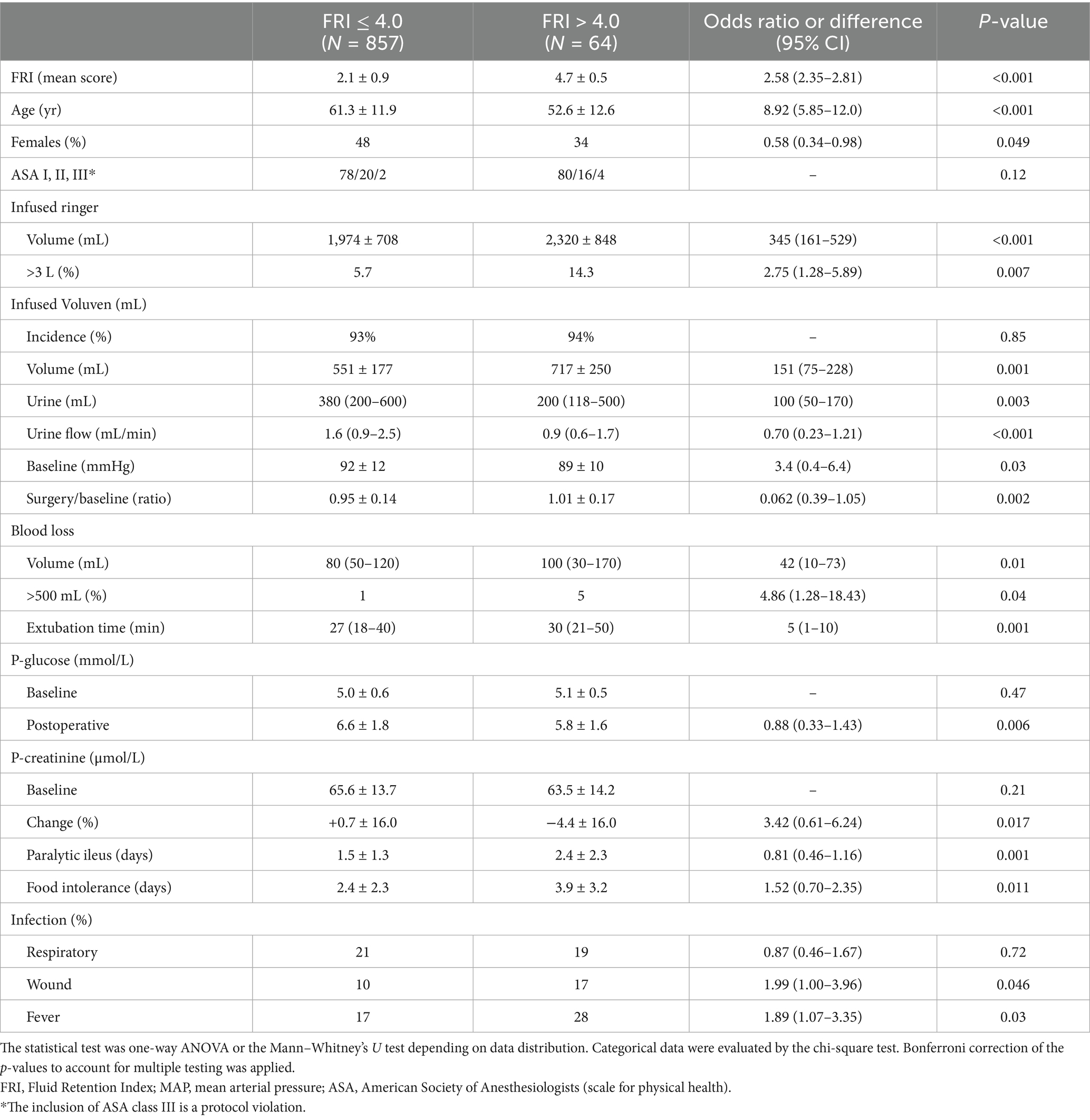
Table 2. Differences between patients having a Fluid Retention Index (FRI) of >4.0 on admission to hospital as evidence of strong renal water conservation before the surgery.
3.4 High FRI, multivariate analyses
The multivariate analyses confirmed that FRI > 4.0 became less common with age and that an association existed with longer food intolerance times. FRI > 4.0 was also independently associated with lower urine output despite receiving a larger amount of Ringer’s solution during the surgery (all associations, p < 0.001; Table 3, top).
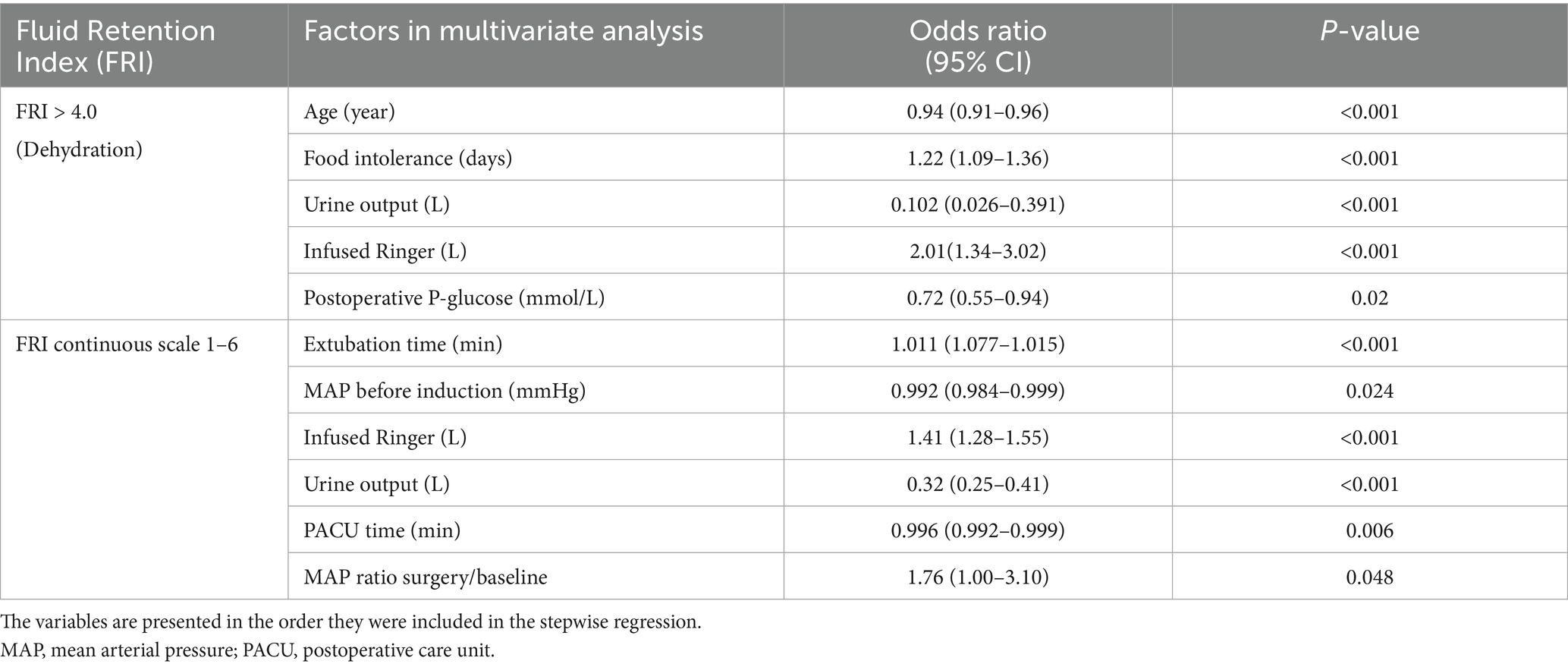
Table 3. Multiple logistic and linear regression analysis of the relationship between the Fluid Retention Index (FRI) and the perioperative variables.
When FRI was expressed on a continuous scale, multivariate analysis also identified that higher FRI was associated with lower MAP at baseline while the decrease during surgery was smaller (Table 3, bottom).
3.5 Postoperative AKI
An increase of the plasma creatinine concentration of ≥50% from the preoperative value occurred in four patients (0.5%) on the first or second day following surgery (Figure 3A). Two of them had Stage 1 AKI, one had Stage 2, and one had Stage 3 AKI. However, three out of these four had very low preoperative levels (Figure 3B) and none of the increases surpassed the normal range (<120 μmol/L).
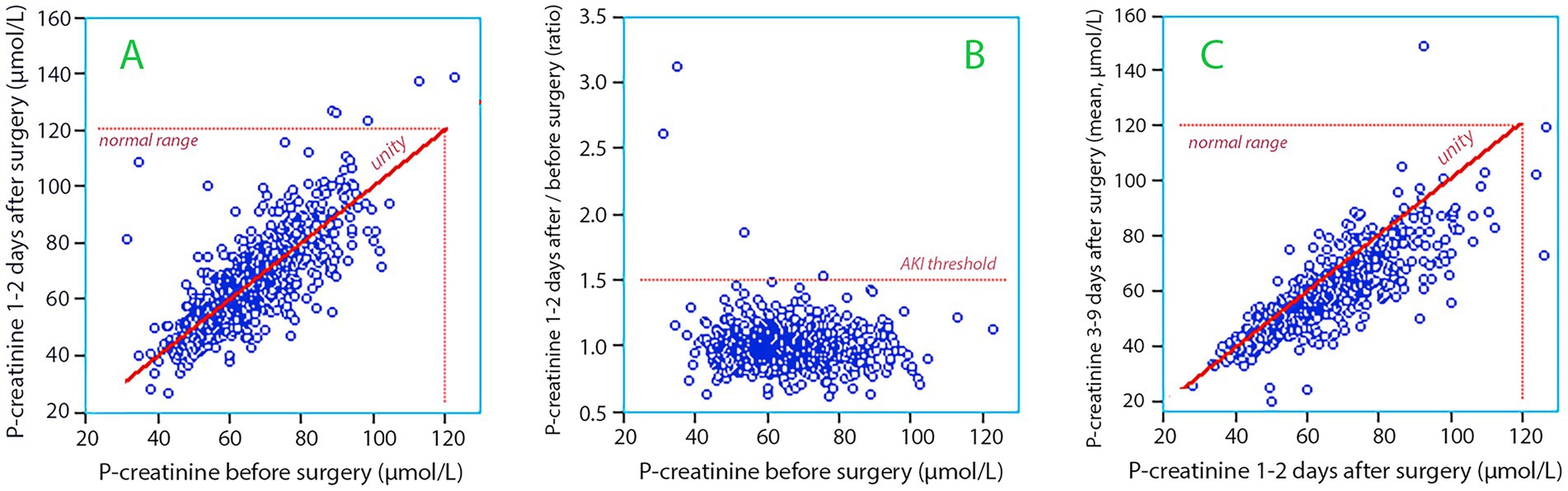
Figure 3. (A) The plasma creatinine concentration measured before surgery versus the concentration measured 1–2 days postoperatively. (B) Same data as in subplot A but the postoperative concentration is expressed as a ratio. Three of the four patients who fulfilled the criterion for Stage 1 of Acute Kidney Injury (AKI) had low plasma creatinine before the surgery (31, 34, and 54 μmol/L). (C) The plasma creatinine concentration measured 1–2 days after surgery versus the mean values of all measurements made 3–9 days postoperatively.
Five additional patients developed an increase in plasma creatinine ≥ 26.5 μmol/L (0.5%) which also classified them as AKI. Two of these patients marginally surpassed the normal range at 122 and 126 μmol/L.
All AKI patients belonged to the FRI ≤ 4.0 group, but the difference from the patients in the FRI > 4.0 group was still not statistically significant (chi-square p = 0.43 and p = 0.93 chi-square with Yates’s continuity correction; odds ratio 0.988, 95% CI 0.980–0.996). The 9 patients with AKI had a higher MAP before anesthesia was induced (102.7 ± 8.5 vs. 92.0 ± 11.8 mmHg; p < 0.01) and a greater decrease during the induction (−15.1 ± 6.2 vs. -5.1 ± 13.1 mmHg; p < 0.04) but MAP during surgery did not differ significantly from the other patients.
Between Days 3 and 9, plasma creatinine levels decreased in most patients (Figure 3C). During this period, 0.4% had elevations in plasma creatinine ≥ 50%. Only one patient was discharged with a plasma creatinine >120 μmol/L.
3.6 Outcomes, multivariate analyses
Binary multivariate regression confirmed that postoperative AKI was more likely in patients with a high MAP at baseline. An increased risk of AKI was also found in those who developed postoperative respiratory infection.
The other analyses per postoperative complication shown in Table 4 further support the association between FRI and food intolerance, paralytic ileus, and postoperative fever.
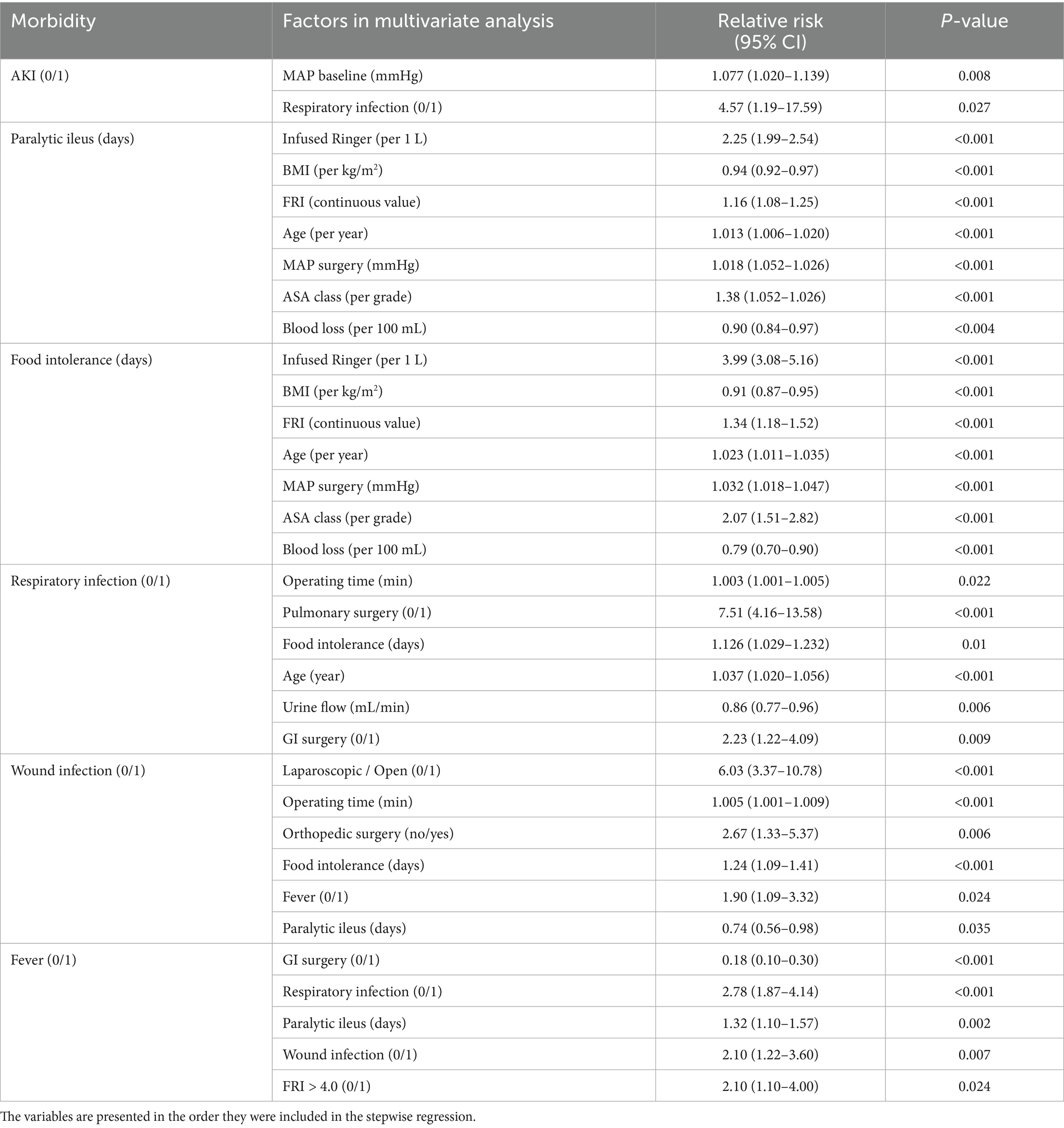
Table 4. Multiple linear and logistic regression analysis of the relationship between the demographic and surgical variables shown in Table 1 and the studied outcomes.
Respiratory infection and wound infection were both associated with lengthy surgery. Respiratory infection was most common after pulmonary and gastrointestinal surgery while wound infection occurred after open surgery and orthopedic surgery.
Fever occurred with both types of infections and but was also twice as common in patients with preoperative FRI > 4.0 compared to those having a lower FRI.
More frequent occurrence of high pain scores (>4.0) was statistically associated with fever [relative risk (RR), 1.16 (95% CI 1.05–1.29)].
Wound infection was the only outcome significantly associated with reoperation [RR 14.5 (95% CI 4.0–52.2)].
Pulmonary surgery [RR 23.0 (95% CI 5.38–98.3)] and advanced age [1.24 per year (1.15–1.33)] were strongly associated a postoperative admission to intensive care. A large surgical hemorrhage [1.65 per liter (1.15–1.33)] were also over-represented among those who were referred to intensive care.
4 Discussion
Concentrated urine (FRI > 4.0) prior to surgery was associated with increased incidence of fever and with low urine output despite greater need for crystalloid fluid. There was also prolonged postoperative duration of paralytic ileus and food intolerance. Blood loss was slightly greater than that in other patients, a finding previously noted (17), and hemorrhage of clinical importance (>500 mL) was more common. Multivariate analysis confirmed that young age, a longer food intolerance time, and lower urine output despite greater need for crystalloid fluid were significantly associated with concentrated urine, while FRI expressed on a continuous scale also identified an association with lower MAP at baseline. The analysis per postoperative complication shown in Table 4 further supports the association between FRI and food intolerance, paralytic ileus, and postoperative fever.
Concentrated urine was still rare (7%) and AKI had a much lower incidence (1%) than in previous studies of major surgery; this skewed distribution made the study underpowered to confirm the hypothesized correlation between concentrated urine and AKI (4, 5). High MAP at baseline appeared to be the main factor that increased the risk of AKI; having 100 mmHg instead of 75 mmHg before the surgery would statistically increase the risk of developing AKI by almost 30%. However, when interpreting this result, one must keep the very low incidence of AKI in mind.
Several variables affirmed that patients with a high FRI in were dehydrated. They exhibited a lower MAP prior to the initiation of anesthesia and needed more intravenous fluid during surgery, which still resulted in less urine being excreted. Interestingly, high FRI also seemed to offer certain advantages, including a milder hyperglycemic response to the surgery. Yet only 7% of the cohort exhibited concentrated urine, suggesting that hydration was usually adequate when surgery was initiated.
Overall, the hemodynamics was stable and the urine flow adequate. Only 2.8% of the patients showed hypotension during more than 2 recordings. Moreover, after adjusting for body weight, the urine flow rate was 20% higher than in our previous study conducted across four countries (5). MAP is known to be closely associated with urine output during surgery (22) and, therefore, the high urine flow might be due to that MAP during the surgery was only slightly lower than before anesthesia was induced.
These three variables (hypotensive events, low urine output, and low mean MAP) increase the risk of a postoperative elevation of plasma creatinine and of AKI (5, 8, 19, 20, 23, 24). Urine flow <0.5 mL/kg/h during >6 h even serves as an independent criterion for the diagnosis (7). The good urine flow, well maintained hemodynamics, and only brief events of hypotension probably contributed, together with the good preoperative hydration, to the low incidence of AKI in the present study.
Elevations of plasma creatinine of ≥ 50% varies greatly after major surgery, from 2% (24), 6% (5), 8% (13), 12% (25), 20% (26), and up to 59% (27). These occurrences bear a risk of higher long-term morbidity and mortality (8, 25, 28, 29). The diagnosis does not require that the elevation is sustained, but in one study 10% of the patients who developed Stage 1 AKI showed a decrease of the glomerular filtration rate by 30% at 3 months after surgery, while the incidence was only 2% in those without AKI (9).
The ultimate cause of postoperative AKI has not been established, but the syndrome is considered to be multifactorial. Decreased kidney perfusion is a frequently suspected cause in the perioperative setting (7). Current research focus on finding new biomarkers of renal injury (30) and avoidance of arterial hypotension (31). Established preventive measures include identification of risk factors (metabolic syndrome, hypertension etc.), avoidance of nephrotoxins, as well as hemodynamic and intravascular volume optimization (6–8, 32).
In a large cohort study by Myles et al. (33) the incidence fell from 8.6 to 5.0% when liberal rather than restrictive fluid therapy was applied, which shows that adequate fluid therapy is important. Marques et al. (34) reported that an intraoperative fluid administration rate of <5 mL/kg/h, which is considered to be adjacent to “restrictive,” was strongly and independently associated with the development of postoperative AKI in bladder cancer patients. Furrer et al. (35) also found a higher occurrence of AKI when less crystalloid fluid was administered during bladder cancer surgery, but both rates averaged <5 mL/kg/h. The amount of fluid administered in the present study was clearly higher and can be placed in-between the liberal and restrictive fluid programs used in the study by Myles et al. (33). With regard to the fluid balance, we believe that the fairly liberal intravenous fluid administration despite small hemorrhage volumes, and possibly the use of a colloid fluid, contributed to the low incidence of AKI in our study.
Ellis et al. (36) have reported that patients who were dehydrated or mildly dehydrated had an increased risk of postoperative AKI after kidney tumor surgery. However, surgery on the kidney might be a special case with regard to the development of AKI. On the other hand, our previous study involving 642 patients across four countries showed that increasingly concentrated urine before general non-cardiac surgery correlated with gradually increasing plasma creatinine up to 25% postoperatively (5). The lack of a statistically significant relationship between FRI and AKI in the present study differs from the previous result and could be due to the low incidence of both variables, which gave the statistical analysis very low power. Statistical links between FRI and other postoperative complications were found, but these complications had a much higher incidence than AKI (Table 2).
A recent finding is that the incidence of AKI after cardiac surgery, which is a high-risk procedure, decreased from 31.7 to 26.9% after a 30-h infusion of amino acids (37) which has recently been confirmed in a meta-analysis (38). Loading with amino acids has several effects on renal physiology, including a recruitment of a “renal function reserve” which increases the glomerular filtration rate in those having a normal kidney function (39). Another effect is that an amino acid mixture increases the diuresis (40). Certain amino acids, such as glycine, even induce osmotic diuresis even in relatively modest amounts (41).
Intravenous fluid is administered to elective surgical patients under the assumption that all of them arrive at the hospital with a similar fluid status, but this may not be the case. Measuring the urine concentration by using FRI, or an individual metabolic end product, may help the clinician to evaluate if the daily intake of water is adequate or not (2, 3). However, the preoperative hydration status has not yet been acknowledged to play a role in the development of postoperative complications, including AKI.
The FRI scale is a tool used quantify concentrated urine. This scale was initially designed to detect dehydration following sports activities (12), but it can also be employed to approximate regular water consumption. Higher water intake decreases the FRI value quite slowly (days) as the regulating hormone, vasopressin, then operates in a low and narrow range (2). In a volunteer study, an increase of the daily intake of water by 32% had not decreased the urine concentration by 15–20% until 1 week later (3). Therefore, FRI in the morning urine is a stable indicator of the 24-h intake of water, although the accuracy decreases transiently in spot urine after ingestion of water (42). The kidneys increase the FRI value much faster in response to surgical stimuli, such as arterial hypotension and peritoneal stretching, due to a more dramatic vasopressin response (18, 20).
The FRI scale has also been applied in other healthcare settings, and patients have typically showed higher values than found here. FRI > 4.0 occurred in 16% of patients admitted to acute geriatric care and correlated with a statistically higher mortality rate within 30 days (14). It should be emphasized that the FRI should be assessed just before surgery starts to be independent of surgical stress. In our compilation of eight surgical patient studies, the average FRI was 3.0 (5), which is 30% higher than observed in this research. Pre-surgical patients with concentrated urine exhibited a 20% lower stroke volume than others (43). Notably, an elevated FRI tends to delay gastrointestinal recuperation post-surgery (19). Yet, the most compelling correlation discovered in previous work is the one between FRI and postoperative creatinine (5, 16). The present study has, to our knowledge, the lowest reported incidence of postoperative AKI in any published major cohort of surgical patients.
Concentrated urine is of great interest as predictor postoperative complications because it can be manipulated before surgery. The hydration status can be graded by measuring urine creatinine, urine osmolality, or even the FRI on the preoperative visit to the hospital before surgery. Additional intake of water could then be prescribed to patients with strong renal water conservation, although this type of prevention has not yet been attempted. Original data from a diet study suggests that, in those presenting with high urine creatinine (>12 mmol/L), an increase of the daily water intake by 700 mL decreases the concentration by 25% or more within 4 days (3). This volume corresponds to one additional glass of water to every meal.
Crystalloid fluid is considered the standard of care for intravascular volume expansion to prevent or treat AKI. This rationale is based on lack of clear evidence that colloids are superior (7). However, there is suspicion that specific colloids, such as Voluven, may cause AKI (44). The use of Voluven® in the present study was not part of the study protocol and was administered to nearly all patients (93%). This fluid is widely used in China where this study was performed. The low incidence of postoperative AKI we found contradicts the concern that Voluven® jeopardizes renal safety, which has resulted in regulatory limitations of its use in Europe and the US. In addition, three meta-analyses (45–47), two randomized trials (48, 49), and one recent retrospective case–control study of 11,000 paired Chinese patients (50) report no association between the use of Voluven® and postoperative AKI. If anything, our study suggest that Voluven prevents AKI rather than promoting it because the incidence is, to the best of our knowledge, the lowest ever reported. However, this proposal must be confirmed by a randomized trial.
4.1 Limitations
To our knowledge, this is the largest prospective study of AKI that has been performed. Interestingly, it shows that the occurrence of AKI can be very low despite lengthy surgery, and we have suggested factors that we believe contributed to this result. A few of the AKI diagnoses can even be questioned. Three patients who were diagnosed with AKI had very low plasma creatinine levels before the study but normal values for the cohort at the postoperative follow-up (Figure 3B). This opens the possibility that sample dilution caused erroneous starting values.
Preoperative plasma creatinine and C-reactive protein could be measured 1 day before the surgery or just before induction of anesthesia and postoperative sampling was performed in the first morning after the surgery (72%) or delayed to the second day (28%). However, only minor changes in plasma creatinine occur between these days; elevations are even firmly established already within 6 h after the end of surgery (19) and decreases take place quite slowly, as shown in the present study.
The observational design of the study makes conclusions about mechanistic effects debatable. The statistical analyses demonstrate associations, which interpretation can cause dilemmas. For example, patients who received larger volumes of fluid may have been perceived as dehydrated by the anesthesiology team. Therefore, complications during the postoperative follow-up in patients with high FRI could potentially be the result of the larger amount of crystalloid fluid given to them (51). However, in this case, the multivariate analysis identified both FRI and the amount of infused fluid as being independent predictors of the food intolerance time (Table 4).
The length of hospital stay surpassed what is common in European and American hospitals but reflect local practices and the predominance of lengthy cancer surgery.
Surgery was lengthy but ASA Class III patients and those with kidney failure avoided, which limits generalizability. However, the study still comprised patients with hypertension and the metabolic syndrome, which are known risk groups for postoperative AKI (6–8, 35).
5 Conclusion
Concentrated urine compatible with low habitual intake of was uncommon (7%) before 921 surgical operations averaging 2.9 h in duration. Patients with concentrated urine were more likely to have low urine output during surgery despite larger volumes of administered intravenous fluid. They also had a longer period of food intolerance after the surgery and a higher occurrence of fever. The incidence of AKI was very low (1%), which we credited to the adequate preoperative hydration, maintenance of normal arterial pressure during surgery, and proper urine flow. The extremely low incidences of both concentrated urine and AKI rendered the study underpowered to demonstrate an association between these two variables.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The procedures followed the Declaration of Helsinki and received approval from the Ethics Committee of the Shaoxing People’s Hospital, Zhejiang, People´s Republic of China (Chairperson Yu Qian) on February 28, 2018, which was followed by an extension dated August 1, 2022. The study was registered in the Chinese Clinical Trial Registry ChiCTR1800016510 and extended as ChiCTR2300068126. All patients provided written informed consent pre-study, and their identities were kept anonymous during data analysis.
Author contributions
KX: Investigation, Writing – original draft, Writing – review & editing. YL: Funding acquisition, Investigation, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing. WZ: Investigation, Writing – original draft, Writing – review & editing. RHe: Investigation, Writing – original draft, Writing – review & editing. RHa: Conceptualization, Data curation, Formal analysis, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. YL was funded by Hangzhou Medical and Health Science and Technology Project (Grant Nos. B20210683 and B202330394); Zhejiang Traditional Chinese Medicine Science and Technology Program (Grant No. 2023ZL593); RHa was funded by Mats Kleberg Foundation, Stockholm (https://matsklebergsstiftelse.se). Funders had no role in the project.
Acknowledgments
We thank Dr. Jinquan Qian and Dr. Shuangyan Hu, Department of Anesthesiology at Shaoxing People’s Hospital, Zhejiang University, and Dr. Shuyuan Gan, Department of Anesthesiology at the First Affiliated Hospital, College of Medicine, Zhejiang University in Hangzhou, China, for assistance with the collection of data. Dr. Shuangyan Hu also helped with the Ethics applications.
Conflict of interest
RHa received the Albus Award 2023 grant from Grifols for a study of 20% albumin as infusion fluid during surgery.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1662177/full#supplementary-material
SUPPLEMENTARY FILE 1 | Surgical details on the subgroups (Table S1) and their outcomes (Table S2).
SUPPLEMENTARY FILE 2 | All blood and urine chemistry (Page 1), arterial pressures (Page 2), and postoperative complications (Page 3).
References
1. Hahn, RG, Grankvist, N, and Krizhanovskii, C. Urinary analysis of fluid retention in the general population: a cross–sectional study. PLoS One. (2016) 11:e0164152. doi: 10.1371/journal.pone.0164152
2. Perrier, E, Vergne, S, Klein, A, Poupin, M, Rondeau, P, Le Bellegro, L, et al. Hydration biomarkers in free–living adults with different levels of habitual fluid consumption. Br J Nutr. (2013) 109:1678–87. doi: 10.1017/S0007114512003601
3. Hahn, RG. Effects of diet, habitual water intake and increased hydration on body fluid volumes and urinary analysis of renal fluid retention in healthy volunteers. Eur J Nutr. (2021) 60:691–702. doi: 10.1007/s00394-020-02275-4
4. Engel, D, Löffel, LM, Wuethrich, PY, and Hahn, RG. Preoperative concentrated urine increases the incidence of plasma creatinine elevation after major surgery. Front Med (Lausanne). (2021) 8:699969
5. Hahn, RG, Weinberg, L, Li, Y, Bahlmann, H, Bellomo, R, and Wuethrich, PY. Concentrated urine, low urine flow, and postoperative elevation of plasma creatinine: a retrospective analysis of pooled data. PLoS One. (2023) 18:e0290071. doi: 10.1371/journal.pone.0290071
6. Prowle, JR, Forni, LG, Bell, M, Chew, MS, Edwards, M, Grams, ME, et al. Postoperative acute kidney injury in adult non-cardiac surgery: joint consensus report of the acute disease quality initiative and PeriOperative quality initiative. Nat Rev Nephrol. (2021) 17:605–18. doi: 10.1038/s41581-021-00418-2
7. Kidney Disease Improving Global Outcomes (KDIGO): KDIGO clinical practice guideline for acute kidney injury. Available online at: https://kdigo.org/guidelines/acute-kidney-injury/ (Accessed January 20, 2025).
8. Zarbock, A, Koyner, JL, Hoste, EAJ, and Kellum, JA. Update on perioperative acute kidney injury. Anesth Analg. (2018) 127:1236–45. doi: 10.1213/ANE.0000000000003741
9. Grams, ME, Sang, Y, Coresh, J, Ballew, SH, Matsushita, K, Levey, AS, et al. Candidate surrogate end points for ESRD after AKI. J Am Soc Nephrol. (2016) 27:2851–9. doi: 10.1681/ASN.2015070829
10. Casa, DJ, Armstrong, LE, Hillman, SK, Montain, SJ, Reiff, RV, Rich, BS, et al. National athletic trainers' association position statement: fluid replacement for athletes. J Athl Train. (2000) 35:212–24.
11. Popowski, LA, Oppliger, RA, Patrick Lambert, G, Johnson, RF, Kim Johnson, A, and Gisolf, CV. Blood and urinary measures of hydration status during progressive acute dehydration. Med Sci Sports Exerc. (2001) 33:747–53. doi: 10.1097/00005768-200105000-00011
12. Hahn, RG, and Waldréus, N. An aggregate urine analysis tool to detect acute dehydration. Int J Sport Nutr Exerc Metab. (2013) 23:303–11.23994895. doi: 10.1123/ijsnem.23.4.303
13. Ylinenvaara, SI, Elisson, O, Berg, K, Zdolsek, JH, Krook, H, and Hahn, RG. Preoperative urine–specific gravity and the incidence of complications after hip fracture surgery. A prospective, observational study. Eur J Anaesthesiol. (2014) 31:85–90. doi: 10.1097/01.EJA.0000435057.72303.0e
14. Johnson, P, Waldréus, N, Hahn, RG, Stenström, H, and Sjöstrand, F. Fluid retention index predicts the 30-day mortality in geriatric care. Scand J Clin Lab Invest. (2015) 75:444–51. doi: 10.3109/00365513.2015.1039057
15. Hahn, RG. Renal injury during hip fracture surgery: an exploratory study. Anaesthesiol Intensive Ther. (2015) 47:284–90. doi: 10.5603/AIT.a2015.0029
16. Hahn, RG, Li, Y, and He, R. Fluid retention is alleviated by crystalloid but not by colloid fluid after induction of general anesthesia: an open–labeled clinical trial. J Anesth Clin Res. (2016) 7:1. doi: 10.4172/2155-6148.1000597
17. Hahn, RG, Bahlmann, H, and Nilsson, L. Preoperative fluid retention increases blood loss during major abdominal surgery. Perioper Med. (2017) 6:12. doi: 10.1186/s13741-017-0068-1
18. Hahn, RG. Renal water conservation determines the increase in body weight after surgery; a randomized controlled trial. Saudi J Anaesth. (2017) 11:144–51. doi: 10.4103/1658-354X.20318
19. Löffel, LM, Engel, D, Beilstein, CM, Hahn, RG, Furrer, MA, and Wuethrich, PY. Dehydration before major urological surgery and the perioperative pattern of plasma creatinine: a prospective cohort series. J Clin Med. (2021) 10:5817. doi: 10.3390/jcm10245817
20. Li, Y, He, R, Hu, S, and Hahn, RG. Renal water conservation and plasma creatinine in colorectal cancer surgery; a single–group clinical study. Front Med. (2022) 9:837414. doi: 10.3389/fmed.2022.837414
21. Weinberg, L, Ying Li, S, Louis, M, Karp, J, Poci, N, Car, BS, et al. Reported definitions of intraoperative hypotension in adults undergoing non-cardiac surgery under general anesthesia: a review. BMC Anesthesiol. (2022) 22:69. doi: 10.1186/s12871-022-01605-9
22. Hahn, RG. Arterial pressure and the rate of elimination of crystalloid fluid. Anesth Analg. (2017) 124:1824–33. doi: 10.1213/ANE.0000000000002075
23. Sun, LY, Wijeysundera, DN, Tait, GA, and Beattie, WS. Association of intraoperative hypotension with acute kidney injury after elective noncardiac surgery. Anesthesiology. (2015) 23:515–23. doi: 10.1097/ALN.000000000000765
24. Salmasi, V, Maheshwari, K, Yang, D, Mascha, EJ, Singh, A, Sessler, DI, et al. Relationship between intraoperative hypotension, defined by either reduction from baseline or absolute thresholds, and acute kidney and myocardial injury after noncardiac surgery: a retrospective cohort analysis. Anesthesiology. (2017) 126:47–65. doi: 10.1097/ALN.000000000001432
25. Kork, F, Balzer, F, Spies, CD, Wernecke, KD, Ginde, AA, Jankowski, J, et al. Minor postoperative increases of creatinine are associated with higher mortality and longer hospital length of stay in surgical patients. Anesthesiology. (2015) 123:1301–11. doi: 10.1097/ALN.0000000000000891
26. Beilstein, CM, Buehler, OD, Furrer, MA, Martig, L, Burkhard, FC, Wuethrich, PY, et al. Impact of early postoperative creatinine increase on mid–term renal function after cystectomy. Int J Urol. (2022) 29:713–23. doi: 10.1111/iju.14879
27. Tholén, M, Lannemyr, L, Møller–Sørensen, H, and Ricksten, SE. Serum creatinine is an unreliable marker of renal function in patients undergoing heart transplantation. Acta Anaesthesiol Scand. (2024) 68:619–25. doi: 10.1111/aas.14397
28. Romagnoli, S, Ricci, Z, and Ronco, C. Perioperative acute kidney injury: prevention, early recognition, and supportive measures. Nephron. (2018) 140:105–10. doi: 10.1159/000490500
29. Boyer, N, Eldridge, J, Prowle, JR, Forni, LG, and AKI, P. Postoperative acute kidney injury. Clin J Am Soc Nephrol. (2022) 17:1535–45. doi: 10.2215/CJN.16541221
30. Mårtensson, J, Martling, CR, and Bell, M. Novel biomarkers of acute kidney injury and failure: clinical applicability. Br J Anaesth. (2012) 109:843–50. doi: 10.1093/bja/aes357
31. Saugel, B, Sander, M, Katzer, C, Hahn, C, Koch, C, Leicht, D, et al. Association of intraoperative hypotension and cumulative norepinephrine dose with postoperative acute kidney injury in patients having noncardiac surgery: a retrospective cohort analysis. Br J Anaesth. (2025) 134:54–62. doi: 10.1016/j.bja.2024.11.005
32. Yu, X, and Feng, Z. Analysis of risk factors for perioperative acute kidney injury and management strategies. Front Med (Lausanne). (2021) 8:751793. doi: 10.3389/fmed.2021.751793
33. Myles, PS, Bellomo, R, Corcoran, T, Forbes, A, Peyton, P, Story, D, et al. Restrictive versus liberal fluid therapy for major abdominal surgery. N Engl J Med. (2018) 378:2263–74. doi: 10.1056/NEJMoa1801601
34. Marques, M, Tezier, M, Tourret, M, Cazenave, L, Brun, C, Duong, LN, et al. Risk factors for postoperative acute kidney injury after radical cystectomy for bladder cancer in the era of ERAS protocols: a retrospective observational study. PLoS One. (2024) 19:e0309549. doi: 10.1371/journal.pone.0309549
35. Furrer, MA, Schneider, MP, Löffel, LM, Burkhard, FC, and Wuethrich, PY. Impact of intra-operative fluid and noradrenaline administration on early postoperative renal function after cystectomy and urinary diversion: a retrospective observational cohort study. Eur J Anaesthesiol. (2018) 35:641–9. doi: 10.1097/EJA.0000000000000808
36. Ellis, RJ, Del Vecchio, SJ, Kalma, B, Lim Ng, K, Morais, C, Francis, RS, et al. Association between preoperative hydration status and acute kidney injury in patients managed surgically for kidney tumours. Int Urol Nephrol. (2018) 50:1211–7. doi: 10.1007/s11255-018-1901-2
37. Landoni, G, Monaco, F, Ti, LK, Baiardo Redaelli, M, Bradic, N, Comis, M, et al. A randomized trial of intravenous amino acids for kidney protection. N Engl J Med. (2024) 391:687–98. doi: 10.1056/NEJMoa2403769
38. Pruna, A, Losiggio, R, Landoni, G, Kotani, Y, Redaelli, MB, Veneziano, M, et al. Amino acid infusion for perioperative functional renal protection: a meta-analysis. J Cardiothorac Vasc Anesth. (2024) 38:3076–85. doi: 10.1053/j.jvca.2024.08.033
39. Losiggio, R, Redaelli, MB, Pruna, A, Landoni, G, and Bellomo, R. The renal effects of amino acids infusion. Signa Vitae. (2024) 20:1–4. doi: 10.22514/sv2024.079
40. Pu, H, Doig, GS, Heighes, PT, Allingstrup, MJ, Wang, A, Brereton, J, et al. Intravenous amino acid therapy for kidney protection in cardiac surgery patients: a pilot randomized controlled trial. J Thorac Cardiovasc Surg. (2019) 157:2356–66. doi: 10.1016/j.jtcvs.2018.11.097
41. Hahn, RG. Amino acid concentrations in serum and urine after intravenous infusion of 1.5% glycine in prostatectomy patients. Prostate. (1992) 21:173–81. doi: 10.1002/pros.2990210302
42. Hahn, RG. Quantifying the daily intake of water from morning and spot urine samples. BMC Nutr. (2023) 9:3. doi: 10.1186/s40795-022-00660-2
43. Li, Y, He, R, Ying, X, and Hahn, RG. Dehydration, hemodynamics and fluid volume optimization after induction of general anesthesia. Clinics. (2014) 69:809–16. doi: 10.6061/clinics/2014(12)04
44. Reinhart, K, Perner, A, Sprung, CL, Jaeschke, R, Schortgen, F, Johan Groeneveld, AB, et al. Consensus statement of the ESICM task force on colloid volume therapy in critically ill patients. Intensive Care Med. (2012) 38:368–83. doi: 10.1007/s00134-012-2472-9
45. Martin, C, Jacob, M, Vicaut, E, Guldet, B, van Aken, H, and Kurz, A. Effect of waxy maize-derived hydroxyethyl starch 130/0.4 on renal function in surgical patients. Anesthesiology. (2013) 118:387–94. doi: 10.1097/ALN.0b013e31827e5569
46. van der Linden, P, James, M, Mythen, M, and Weiskopf, RB. Safety of modern starches used during surgery. Anesth Analg. (2013) 116:35–48. doi: 10.1213/ANE.0b013e31827175da
47. Gilles, MA, Habicher, M, Jhanji, S, Sander, M, Mythen, M, Hamilton, M, et al. Incidence of postoperative death and acute kidney injury associated with i.v. 6% hydroxyethyl starch use: systematic review and meta-analysis. Br J Anaesth. (2014) 112:25–34. doi: 10.1093/bja/aet303
48. Joosten, A, Delaporte, A, Mortier, J, Ickx, B, Van Obbergh, L, Vincent, JL, et al. Long-term impact of crystalloid versus colloid solutions on renal function and disability-free survival after major abdominal surgery. Anesthesiology. (2019) 130:227–36. doi: 10.1097/ALN.0000000000002501
49. Kabon, B, Sessler, DI, and Kurz, A. Effect of intraoperative goal-directed balanced crystalloid versus colloid administration on major postoperative morbidity: a randomized trial. Anesthesiology. (2019) 130:728–44. doi: 10.1097/ALN.0000000000002601
50. Yang, MJ, Chen, N, Ye, CY, Li, Q, Luo, H, Wu, JH, et al. Association between hydroxyethyl starch 130/0.4 administration during noncardiac surgery and postoperative acute kidney injury: a propensity score-matched analysis of a large cohort in China. J Clin Anesth. (2024) 96:111493. doi: 10.1016/j.jclinane.2024.111493
Keywords: acute kidney injury, surgery, blood creatinine, hydroxyethyl starch, hypotension, postoperative complications, urine creatinine
Citation: Xie K, Li Y, Zou W, He R and Hahn RG (2025) Effects of concentrated urine on complications and plasma creatinine: a prospective study in elective non-cardiac surgery patients. Front. Med. 12:1662177. doi: 10.3389/fmed.2025.1662177
Edited by:
Sepiso Kenias Masenga, Mulungushi University, ZambiaReviewed by:
Katongo Hope Mutengo, University of Zambia, ZambiaLukundo Siame, Mulungushi University, Zambia
Copyright © 2025 Xie, Li, Zou, He and Hahn. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Robert G. Hahn, cm9iZXJ0LmhhaG5Aa2kuc2U=
†These authors share first authorship
‡ORCID: Robert G. Hahn, orcid.org/0000-0002-1528-3803
 Kai Xie
Kai Xie Yuhong Li
Yuhong Li Wenjie Zou3
Wenjie Zou3 Rui He
Rui He Robert G. Hahn
Robert G. Hahn