- Wuhan Pulmonary Hospital, Wuhan Institute for Tuberculosis Control, Wuhan, Hubei, China
Cat scratch disease (CSD), caused by Bartonella henselae (B. henselae), typically presents as localized swelling of lymph nodes following a scratch or bite from a cat. It is crucial to differentiate CSD from tuberculosis (TB), particularly in regions where TB is prevalent. This report describes a 56-year-old man who exhibited bilateral swelling of the cervical lymph nodes. Initially, he was suspected to have tuberculous lymphadenitis due to the granulomatous changes observed in a biopsy of the lymph nodes, typical signs of TB on a chest CT scan, and a positive result from an interferon-gamma release assay (IGRA). He was subsequently referred to our hospital for TB treatment. Testing of bronchoalveolar lavage fluid confirmed the presence of TB-DNA, indicating active pulmonary tuberculosis (PTB). However, further investigation revealed recent cat contact. This led to the identification of a B. henselae infection using metagenomic pathogen detection workflow (MetaPath™) on formalin-fixed paraffin-embedded (FFPE) histopathological sections from a cervical lymph node specimen obtained at an external hospital, which confirmed the diagnosis of CSD and ruled out TB. Through a review of the literature, we found that this represents the first documented case of concurrent active PTB and CSD-related lymphadenitis in an immunocompetent individual. It highlights the diagnostic challenges in distinguishing CSD from TB in cases of granulomatous lymphadenitis and emphasizes the need to consider CSD in patients with a history of cat exposure, showcasing the pivotal role of advanced metagenomic diagnostics in accurately diagnosing CSD.
Background
Cat scratch disease (CSD) is an infectious disease caused by B. henselae (B. henselae) (1), which belongs to the gram-negative category and is part of the Proteobacteria phylum (2). Cats Proteobacteria are the primary carriers of this pathogen, which can be transmitted to humans through scratches, bites, or licks, especially from infected young cats, with a higher prevalence in autumn and winter. CSD typically manifests as localized skin lesions and swollen lymph nodes (3). In regions endemic to tuberculosis (TB), granulomatous lymphadenitis is frequently attributed to Mycobacterium tuberculosis (MTB) (4, 5), followed by atypical mycobacterial infections, fungal infections, parasitic infections, CSD, lymphogranuloma venereum, and leprosy (6, 7). Due to the similarities in histopathological features between CSD and TB, CSD remains underrecognized, and patients may be misdiagnosed with lymphoproliferative disorders or TB (6).
This report presents the first documented instance of the coexistence of pulmonary tuberculosis (PTB) with CSD-associated cervical lymphadenitis. While the lymphadenitis was attributed to CSD rather than TB, the patient exhibited cervical lymphadenopathy, which inadvertently led to the detection of PTB.
Case presentation
On January 8, 2025, a 56-year-old man presented at an external hospital with bilateral masses in his neck, having first noticed these swellings a week prior, with the right side being more prominent. The patient reported no additional symptoms such as cough, sputum production, or fever. His medical history was unremarkable for TB, diabetes, or hypertension; however, he had a 30-year history of alcohol use and smoking. Upon examination, he was alert and oriented, with no signs of jaundice or petechiae on his skin or mucous membranes. Bilateral cervical lymphadenopathy was observed, with the largest lymph node in the right posterior cervical area measuring approximately 3 cm. These nodes had intact skin, exhibited no signs of ulceration or redness, felt firm, and were non-tender. A cervical CT scan indicated bilateral lymph node enlargement (Figure 1a), while a chest CT revealed emphysema, bullae, and a calcified patch in the left upper lobe (Figure 2a). An ultrasound of the neck confirmed an increase in both the size and number of cervical lymph nodes, with the largest measuring around 2.7 × 1.0 cm on the right side. Laboratory tests, including a complete blood count, urinalysis, and assessments of liver and kidney function, yielded normal results. The patient had no significant past medical history or history of immunocompromising conditions, and T-cell subset analysis revealed normal levels of CD4 + and CD8 + cells. Serological tests for HBV, HCV, syphilis, and HIV were negative. The ESR was recorded at 11 mm/h, hs-CRP at 0.87 mg/L, PCT at 0.06 ng/mL, and CEA at 3.30 ng/mL, while the IGRA test was positive. A biopsy taken from the right cervical lymph node on January 10, 2025, revealed multiple small abscesses within the lymph node upon microscopy. The centers of these abscesses displayed necrosis accompanied by abundant neutrophilic infiltration, surrounded by epithelioid histiocytes arranged in a palisading pattern, forming suppurative granulomatous inflammation (Figures 3a–d), which raised an initial suspicion of TB. However, TB special stains, including periodic acid-Schiff (PAS), silver methenamine, and acid-fast staining of the lymph node tissue, returned negative results, and TB-DNA was not detected. On January 17, 2025, the patient was referred to our hospital with a diagnosis of pulmonary tuberculosis and cervical tuberculous lymphadenitis, attributed to his residence in a TB-endemic area, a positive IGRA result, and characteristic imaging findings of PTB.
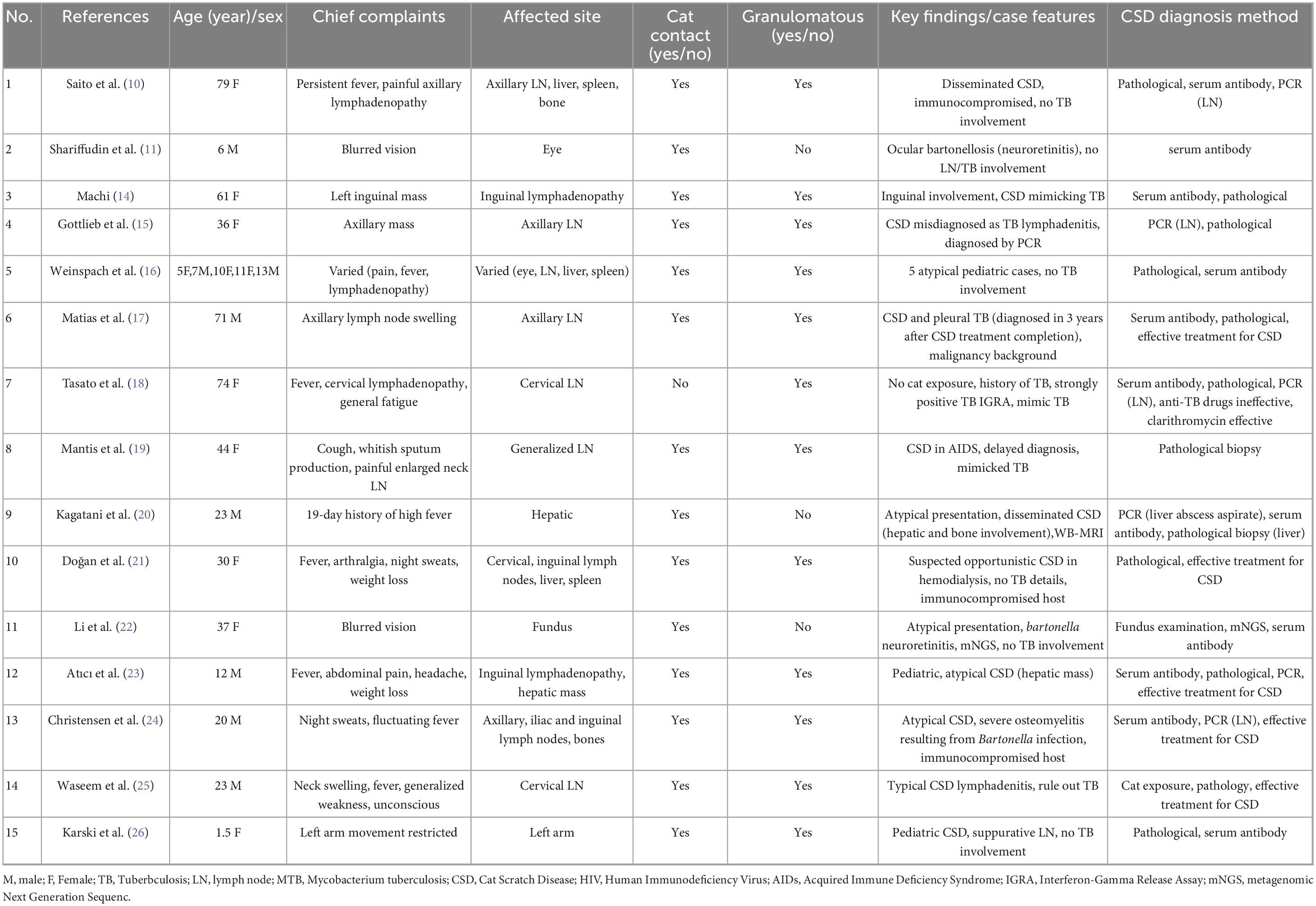
Figure 1. Resolution of CSD-associated lymphadenopathy on serial cervical CT. (a) Pre-treatment CT scan (January 8, 2025) demonstrates an enlarged right posterior cervical lymph node (red arrow). (b) Follow-up CT scan (March 26, 2025) shows the enlarged lymph node in the right posterior cervical space has disappeared (red arrow).
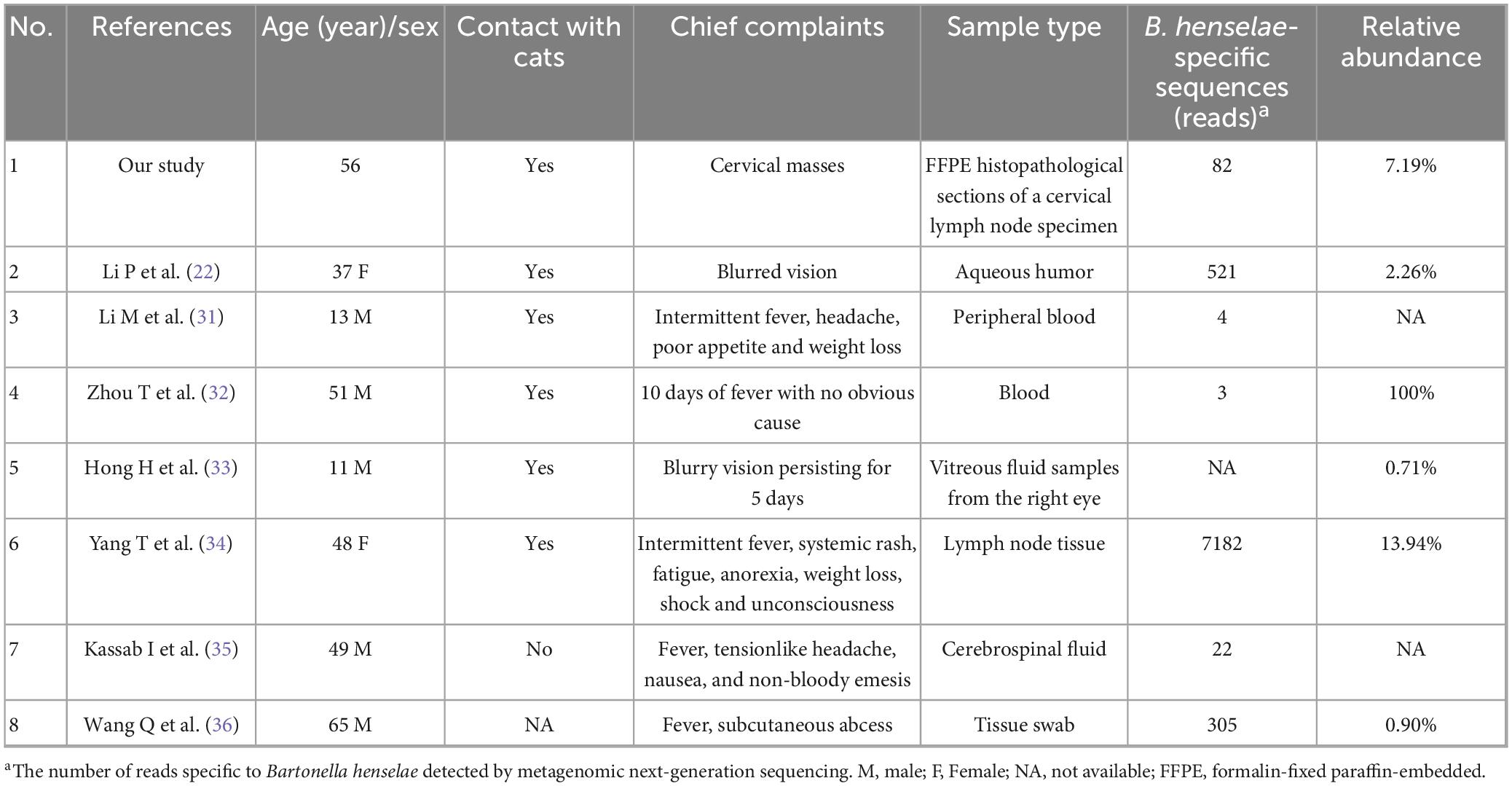
Figure 2. Pre- and post-treatment CT scans in pulmonary tuberculosis. (a) Chest CT scan on January 22, 2025. (b) Follow-up chest CT scan on March 26, 2025.
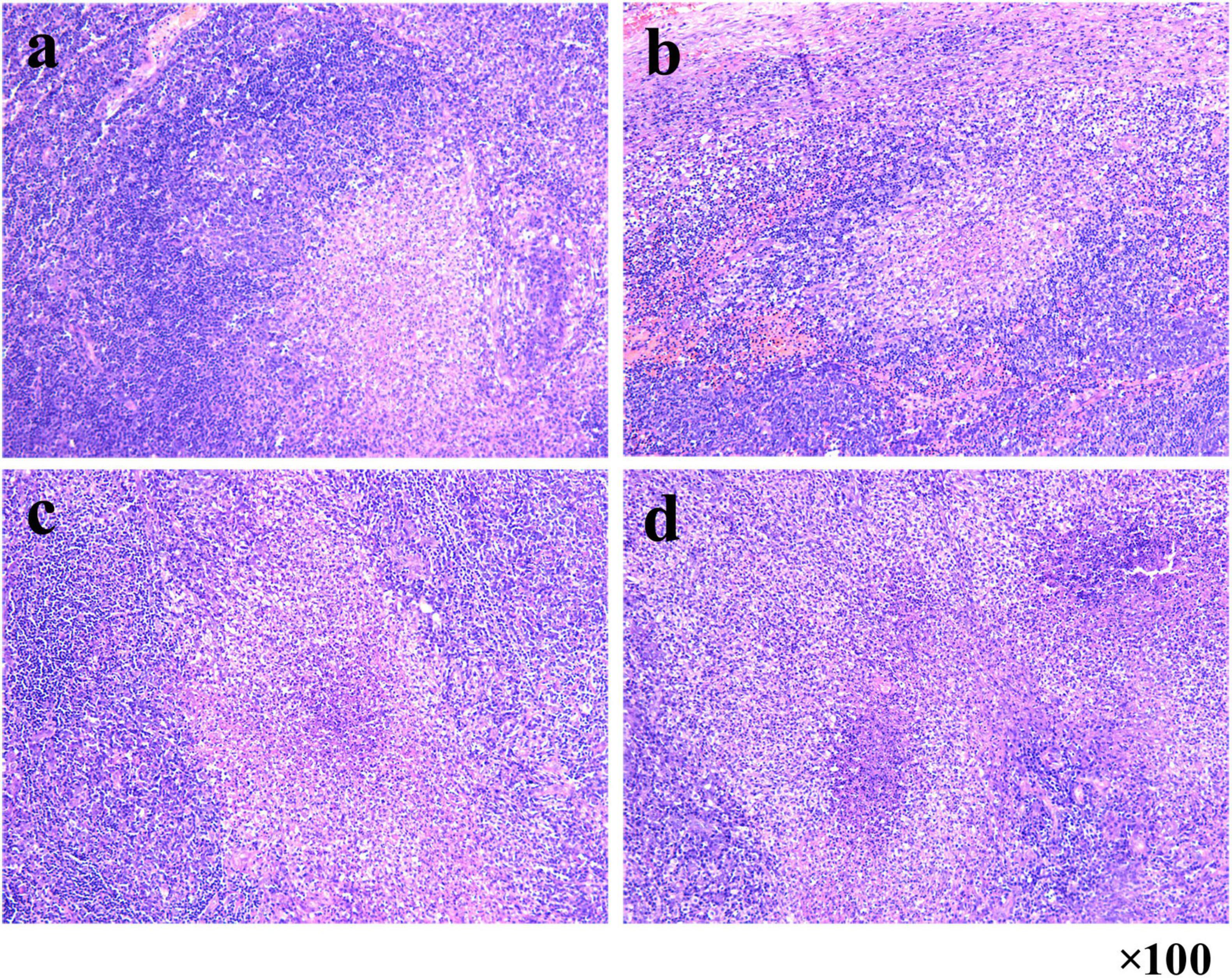
Figure 3. Suppurative granulomatous lymphadenitis in right cervical lymph node biopsy. (a–d) Microscopy reveals multiple small abscesses with central necrosis and neutrophilic infiltration, surrounded by palisaded epithelioid histiocytes (HE staining, 100 × magnification).
A bronchoscopy performed at our facility revealed no irregularities; however, the bronchoalveolar lavage fluid tested positive for TB-DNA, suggesting the presence of pulmonary tuberculosis. During a detailed history review, the patient disclosed having two cats for the past 2 years. Although there was no clear record of cat scratches, the possibility of CSD was considered due to negative results from PAS, silver methenamine, and acid-fast staining, along with the patient’s exposure to cats. Metagenomic pathogen detection workflow (MetaPath™)–a probe-enriched metagenomic next-generation sequencing (mNGS) method optimized for low-biomass specimens–successfully detected B. henselae sequences, rather than MTB, in formalin-fixed paraffin-embedded (FFPE) histopathological sections of a cervical lymph node specimen obtained externally. MetaPath™ technology of the cervical lymph node tissue detected sequences specific to B. henselae, identifying 82 sequences at a relative abundance of 7.19%, thereby confirming a diagnosis of CSD. The patient presented with lymphadenopathy, and pathological findings revealed granulomatous inflammation. The differential diagnoses include infectious etiologies such as tuberculosis, non-tuberculous mycobacterial infection, and fungal infections, as well as non-infectious conditions such as sarcoidosis and lymphoma. Based on comprehensive clinical, laboratory, radiological, pathological, etiological, and molecular evaluations, the patient final diagnoses included: (1) cervical lymphadenitis related to CSD; (2) pulmonary tuberculosis; and (3) emphysema with bullae. The patient commenced anti-tuberculosis therapy on January 18, 2025, following a regimen consisting of Isoniazid, Rifampicin, Pyrazinamide, and Ethambutol for 2 months, followed by a 4-month continuation of Isoniazid and Rifampicin. Subsequently, upon the detection of Bartonella species via MetaPath™ testing on January 23, 2025, ciprofloxacin was added for a duration of 4 weeks to address cat-scratch disease, targeting anti-Bartonella therapy. A follow-up CT scan of the chest and neck conducted on March 26 indicated partial resolution of the lesion in the left upper lung (Figure 1b) and nearly complete reduction of the enlarged lymph nodes in the right posterior cervical area (Figure 2b).
Literature review and discussion
Cat scratch disease generally resolves spontaneously in individuals with a competent immune system, initially presenting as skin lesions followed by ipsilateral regional lymphadenopathy (8). Research indicates variability in the clinical presentation of the disease. For instance, it often resolves without intervention in most children and immunocompetent individuals, while older adults or immunocompromised individuals may exhibit atypical clinical manifestations (9), including fever of unknown origin, disseminated visceral lesions, neurological complications, uveitis, bacillary angiomatosis, and infective endocarditis (10–12). The typical histopathological features of CSD in lymph nodes include granulomas with central necrosis, multinucleated giant cells, and microabscesses (6, 13–16). However, the presence of granulomatous inflammation alone does not confirm a diagnosis of CSD, as similar histological patterns can be observed in a variety of infectious and non-infectious diseases, including TB, infections from other acid-fast bacilli, fungal diseases, toxoplasmosis, lymphomas, various cancers, vasculitis, and sarcoidosis, among others (4–7).
We performed a literature review utilizing the terms “tuberculosis, cat scratch disease, granulomatous lymphadenitis” and found 15 articles that discussed case studies involving both CSD and TB. A summary comparing these cases with our current findings is presented in Table 1 (10, 11, 14–26). It is noteworthy that one case study reported the simultaneous presence of TB and CSD in the same patient (17). Matias et al. documented the onset of TB lymphadenitis 3 years after a CSD diagnosis in a patient with colon cancer who was immunocompromised (17). Bernit et al. were the first to identify a co-infection of B. quintana (not B. henselae) and TB in an HIV-positive individual suffering from generalized lymphadenitis, noting three infections occurring concurrently in a young female patient admitted for regional lymphadenitis (27). B. quintana is primarily transmitted through contamination of skin breaks by louse feces or crushed lice, with humans serving as its natural reservoir host, thus requiring no feline involvement. This pathogen chiefly causes trench fever and disseminated infections. Our case represents the first documented co-occurrence of active PTB and CSD-associated cervical lymphadenitis in an immunocompetent host, challenging the assumption that granulomatous lymphadenitis in TB-endemic regions is attributable to TB. Differentiating CSD from TB is challenging due to their similar histopathological features (necrotizing granulomas) and biases in epidemiological data. Notably, studies such as those by Gottlieb et al. and Machi et al. emphasize the misdiagnosis of CSD as TB lymphadenitis due to false-positive IGRA results or prevalence-based diagnostic presumptions (14, 15). Our case illustrates this challenge: the initial diagnosis relied on regional TB endemicity, positive IGRA results, and pulmonary CT findings, despite negative TB staining and TB DNA testing on lymph node specimens. In this instance, clinicians opted for MetaPath™ as a diagnostic approach for CSD. This contrasts sharply with the findings of Tasato et al. (18), where CSD was diagnosed only after empirical anti-TB treatment failed, underscoring the potential of advanced metagenomic diagnostics in preventing iatrogenic overtreatment.
Recognizing a history of cat exposure is crucial for diagnosing CSD, yet it is often overlooked. Notably, in 14 out of 15 analyzed cases, cat exposure was reported, although some cases lacked a clear history of scratches–similar to our patient, who was unable to recall any scratches or bites from a cat. This highlights the necessity of investigating even ambiguous instances of cat scratches or bites in suspected CSD cases, especially when lymph node samples yield negative results for specific stains (such as acid-fast and PAS) and molecular tests for MTB. Our case further illustrates that TB may coincidentally occur in patients with CSD, necessitating a comprehensive evaluation for dual pathogen infection. Immunocompromised hosts face higher risks of disseminated CSD (10, 19–21), however, our case demonstrates that dual infection can occur regardless of immune status. Among the 15 case studies reviewed, 9 documented atypical manifestations of CSD. Patients with compromised immunity, exhibit atypical manifestations, as the pathogen disseminates through lymphatic or bloodstream flow, leading to infections in organs and tissues beyond the lymph nodes. In these individuals, CSD can affect various systems, including the eyes, nervous system, musculoskeletal system, skin, breast, liver, spleen, and bones, and may also present with a range of other manifestations and syndromes, such as pneumonia, pleural effusion, erythema multiforme, hypercalcemia, glomerulonephritis, and myocarditis (10, 11, 16, 17, 19–24). The remaining 6 case reports predominantly documented fever or localized lymphadenopathy as primary presenting manifestations (14, 15, 17, 18, 25, 26). These cases further validate that the early phase of CSD infection typically begins with a papule or pustule at the site of inoculation, often accompanied by symptoms such as fever, fatigue, and nodular erythema. Concurrently, regional lymphadenopathy appears, usually unilateral, with about half of the cases involving a single lymph node, while the other half may progress to multiple lymph nodes. The disease can also lead to serious complications including cardiac issues (endocarditis), respiratory problems (pneumonia and pleural effusion), musculoskeletal conditions (osteomyelitis and paraspinal abscess), and central nervous system involvement (meningitis) (11, 16, 20, 22). Integrated with clinical features and ancillary investigations, mNGS provides a rapid and unbiased approach for detecting Bartonella species across diverse specimen types, particularly serving as a priority diagnostic tool for patients presenting with atypical manifestations (22).
The MetaPath™ platform (KingMed Center), an integrated diagnostic system that utilizes probe-based pathogen enrichment and mNGS technology, was employed for metagenomic pathogen detection from FFPE tissue sections to enhance pathogen detection rates through optimized sample processing and bioinformatics analysis. DNA was extracted following deparaffinization, enzymatic lysis, and purification, after which libraries were constructed through fragmentation, end repair, adapter ligation, and PCR amplification, followed by hybridization capture using biotinylated probes targeting over 3,000 pathogens. Sequencing was performed on an Illumina MiniSeq platform with single-end 100 bp reads, and bioinformatic analysis was conducted in the final step. A study conducted by Li M et al. demonstrated that the MetaPath™ platform achieved superior diagnostic performance for tuberculosis, with a sensitivity as high as 92.8%, significantly surpassing conventional TB detection methods such as PCR, acid-fast bacilli smear microscopy, and mycobacterial culture (28). This probe capture-based metagenomic sequencing approach addresses key limitations of traditional assays by efficiently suppressing human nucleic acid interference, tolerating ultra-low DNA inputs (<5 ng), providing rapid results, and enabling simultaneous identification of polymicrobial infections. At our tuberculosis-specialized hospital, infectious disease testing is limited to a narrow range of pathogens (29). Given that the MetaPath™ platform offers broad pathogen screening and demonstrates significant advantages for FFPE pathology specimens, and considering that the patient has such FFPE pathology samples available from an external hospital, the physician has opted to employ the MetaPath™ platform to test for the patient’s pathogens. The MetaPath™ platform played a pivotal diagnostic role in our co-infection case involving CSD-associated lymphadenitis and PTB, with the experimental methodology of the MetaPath™ platform detailed in the Supplementary file. At its core, the MetaPath™ platform comprises an optimized mNGS workflow. mNGS facilitates the simultaneous detection of tens of thousands of pathogens from limited samples, proving invaluable for diagnosing rare, atypical, and complex infections (30–32). A thorough search on PubMed using terms such as “metagenomic next-generation sequencing,” “cat scratch disease,” and “Bartonella henselae” revealed seven studies (see Table 2) that utilized mNGS on various sample types, including lymph node tissue, tissue swabs, intraocular fluid, plasma, peripheral blood, and cerebrospinal fluid, for diagnosing CSD (22, 31–36). Li et al. (31), Zhou et al. (32). reported mNGS diagnosing CSD lymphadenitis in TB-endemic regions where conventional methods yielded false negatives. Additionally, some studies by Hong et al. and Li et al. demonstrated the use of mNGS to confirm B. henselae in neuroretinitis cases that were initially negative through serological and PCR testing (22, 33). In cases of severe complications such as hemophagocytic lymphohistiocytosis or neurological issues, mNGS provided swift pathogen identification when standard tests were inconclusive (34, 35). Wang et al. further confirmed that mNGS exhibits greater sensitivity compared to culture methods in skin and soft tissue infections (36). Key advantages of mNGS include its unbiased detection of pathogens, the ability to identify difficult-to-culture organisms like Bartonella, and a rapid turnaround time of less than 48 h (22, 37). Compared to traditional PCR and serological antibody testing, MetaPath™ was chosen for its capability to simultaneously detect a wide array of known and unknown pathogens, its utilization of targeted probes to minimize host background noise, the supplementary genomic information it offers for pathogen characterization, and its compatibility with challenging sample types such as tissues and FFPE (28). Although mNGS technologies such as MetaPath™ offer significant advantages, but it’s limitations must be acknowledged. First, while probe-capture methods improve specificity, the high sensitivity of the method can lead to contamination, complicating the differentiation between colonizers, background microbes, and actual pathogens (38). Second, the associated costs are currently higher than those of conventional PCR or serology, which may restrict accessibility in resource-limited settings. Third, the requirement for specialized equipment and bioinformatics expertise further limits its widespread adoption in routine clinical practice. Thus, correlation with clinical presentation and traditional microbiological results remains essential. Additionally, as we are a pulmonary specialty hospital and do not offer serological testing for Bartonella, this patient did not undergo Bartonella IgM/IgG testing, which is a significant limitation of this case. Despite its limitations, mNGS is vital for diagnosing granulomatous lymphadenitis in areas where TB is common, as overlapping clinical features can lead to diagnostic delays. As evidenced by this case and previous literature, its early integration, particularly in patients with cat exposure, can prevent misdiagnosis, optimize therapy, and reduce iatrogenic harm.
Although granulomatous cervical lymphadenitis in TB-endemic regions is frequently attributed to TB, this case presents the first reported co-occurrence of PTB and B. henselae associated lymphadenitis revealing critical diagnostic challenges. Histopathological overlap (granulomatous inflammation) and positive IGRA initially suggested tuberculous lymphadenitis. Crucially, MetaPath™ technology detection of B. henselae sequences in lymph node tissue –triggered by a history of cat exposure, even in the absence of clear scratches and negative results from special stains (PAS, silver methenamine, acid-fast) and TB-DNA–validated the diagnosis of CSD. This incidental discovery of PTB during the evaluation of cervical lymphadenopathy highlights how granulomatous inflammation may be misdiagnosed as TB without a history of cat exposure, particularly in areas with high TB prevalence.
Conclusion
This case marks the first recorded occurrence of simultaneous active PTB and cervical lymphadenitis associated with CSD in a patient with a competent immune system. It underscores critical diagnostic challenges in TB-endemic regions, where granulomatous lymphadenitis is frequently misattributed to TB. The initial misdiagnosis of our patient highlights the histopathological similarities between TB and CSD, specifically the presence of necrotizing granulomas, as well as the limitations of relying solely on epidemiological context–such as TB-endemicity–and positive IGRA or PTB findings. A definitive diagnosis of CSD was established only after performing MetaPath™ technology on lymph node tissue, which was prompted by a history of cat contact, despite the absence of a clear cat scratch and negative results from traditional staining and TB-DNA tests. This incidental discovery of PTB during the evaluation of CSD emphasizes that granulomatous lymphadenitis may obscure non-tuberculous etiologies, particularly zoonotic infections. A comprehensive history, including cat exposure, and the early detection of B. henselae using advanced diagnostic techniques such as mNGS or MetaPath™ are essential for accurate diagnosis and appropriate treatment, thereby preventing potential iatrogenic harm and improving patient outcomes.
Data availability statement
The original contributions presented in this study are included in this article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
This study was supported by the Ethics Committee of Wuhan Pulmonary Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s)for the publication of any potentially identifiable images or data included in this article.
Author contributions
XW: Writing – original draft, Methodology, Data curation, Writing – review & editing, Investigation, Funding acquisition. SC: Writing – review & editing, Investigation, Data curation. CY: Data curation, Writing – review & editing, Investigation, Supervision, Methodology.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was supported by the Medical Research Project Fund of Wuhan Municipal Health Commission (No. WX23Q32 to XW), the Science and Technology Health Project of the Health Commission of Hubei Province (No. WJ2025M013 to XW), the Basic Applied Research Project of Wuhan Science and Technology Bureau (No. 2023020201010215 to XW).
Acknowledgments
We gratefully acknowledge all the clinical staff who contributed to the study. We also acknowledge Wuhan KingMed Center for the interpretation of pathogen detection results and the experimental technical support provided by their MetaPath™ platform.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1667171/full#supplementary-material
References
1. Bellur S, Ali A, Nguyen N, Fernandes J, Kodati S. Central retinal vein occlusion associated with bartonella henselae infection. J Ophthalmic Inflamm Infect. (2023) 13:14. doi: 10.1186/s12348-023-00334-5
2. Kamstra J, van der Meij E, de Visscher J. Cat scratch disease. Ned Tijdschr Tandheelkd. (2021) 128:21–7. doi: 10.5177/ntvt.2021.01.20049
3. Florin T, Zaoutis T, Zaoutis L. Beyond cat scratch disease: widening spectrum of bartonella henselae infection. Pediatrics. (2008) 121:e1413–25. doi: 10.1542/peds.2007-1897
4. Bezabih M, Mariam D, Selassie S. Fine needle aspiration cytology of suspected tuberculous lymphadenitis. Cytopathology. (2002) 13:284–90. doi: 10.1046/j.1365-2303.2002.00418.x
5. Fontanilla J, Barnes A, von Reyn C. Current diagnosis and management of peripheral tuberculous lymphadenitis. Clin Infect Dis. (2011) 53:555–62. doi: 10.1093/cid/cir454
6. Lamps L, Scott M. Cat-scratch disease: historic, clinical, and pathologic perspectives. Am J Clin Pathol. (2004) 121:S71–80. doi: 10.1309/jc8ym53l4e0l6pt5
7. Mahadevan M, Neeff M, Van Der Meer G, Baguley C, Wong W, Gruber M. Non-tuberculous mycobacterial head and neck infections in children: analysis of results and complications for various treatment modalities. Int J Pediatr Otorhinolaryngol. (2016) 82:102–6. doi: 10.1016/j.ijporl.2015.12.026
8. Shinall E. Cat-scratch disease: a review of the literature. Pediatr Dermatol. (1990) 7:11–8. doi: 10.1111/j.1525-1470.1990.tb01066.x
9. Windsor J. Cat-scratch disease: epidemiology, aetiology and treatment. Br J Biomed Sci. (2001) 58:101–10.
10. Saito H, Takahashi Y, Tsuge S, Nishioka R, Zoshima T, Ito K, et al. Disseminated cat-scratch disease during abatacept therapy for rheumatoid arthritis in an older patient: a case report and review of the literature. J Infect Chemother. (2025) 31:102732. doi: 10.1016/j.jiac.2025.102732
11. Shariffudin N, Min T, Adnan A, Hashim H, Teo KSS. A child with a rare presentation of ocular bartonellosis. Taiwan J Ophthalmol. (2021) 11:292–5. doi: 10.4103/tjo.tjo_29_20
12. Öcal-Demir S, Kahraman K, Bozbeyoğlu G, Esen F. Clinical presentation of cat scratch disease in pediatric patients-a single-center study. Turk Arch Pediatr. (2024) 59:574–9. doi: 10.5152/TurkArchPediatr.2024.24032
13. Dwan D, Baker C, Zhang S, Black C, Zuurbier R, diFlorio-Alexander R. Atypical cat-scratch disease: a radiology-pathology correlation. Breast J. (2020) 26:786–7. doi: 10.1111/tbj.13581
14. Machi T. [Cat scratch disease showing clinical picture resembling tuberculous lymphadenitis: a case report]. Kekkaku. (2001) 76:545–8.
15. Gottlieb T, Atkins B, Robson J. Cat scratch disease diagnosed by polymerase chain reaction in a patient with suspected tuberculous lymphadenitis. Med J Aust. (1999) 170:168–70. doi: 10.5694/j.1326-5377.1999.tb127714.x
16. Weinspach S, Tenenbaum T, Schönberger S, Schaper J, Engers R, Rueggeberg J, et al. Cat scratch disease–heterogeneous in clinical presentation: five unusual cases of an infection caused by bartonella henselae. Klin Padiatr. (2010) 222:73–8. doi: 10.1055/s-0029-1233488
17. Matias M, Marques T, Ferreira M, Ribeiro L. Cat scratch disease and lymph node tuberculosis in a colon patient with cancer. BMJ Case Rep. (2013) 2013:bcr2013010424. doi: 10.1136/bcr-2013-010424
18. Tasato D, Tateyama M, Inamine M, Hibiya K, Tamaki Y, Haranaga S, et al. [Case report: a case of cat scratch disease in elderly patient needed to differentiate tuberculous lymphadenitis]. Nihon Naika Gakkai Zasshi. (2011) 100:1969–71. doi: 10.2169/naika.100.1969
19. Mantis J, Ali Y, Junejo S. Cat-scratch disease in an aids patient presenting with generalized lymphadenopathy: an unusual presentation with delayed diagnosis. Am J Case Rep. (2018) 19:906–11. doi: 10.12659/ajcr.909325
20. Kagatani J, Asakura T, Sekine K, Watanabe H, Kawada M, Ohkusu K, et al. Clinical utility of whole body diffusion-weighted imaging in an immunocompetent adult with atypical cat scratch disease. J Infect Chemother. (2022) 28:1558–61. doi: 10.1016/j.jiac.2022.07.013
21. Doğan B, Güney İ, Ömeroğlu E. Unexplained fever in a hemodialysis patient possibly due to cat scratch disease opportunistic infection. Hemodial Int. (2025) 29:419–22. doi: 10.1111/hdi.13223
22. Li P, Qian Z, Tao Y. Application of metagenomic next-generation sequencing in the diagnosis of bartonella neuroretinitis: a case report and literature review. J Ophthalmic Inflamm Infect. (2024) 14:17. doi: 10.1186/s12348-024-00387-0
23. Atıcı S, Kadayıfcı E, Karaaslan A, Toper M, Celikel C, Soysal A, et al. Atypical presentation of cat-scratch disease in an immunocompetent child with serological and pathological evidence. Case Rep Pediatr. (2014) 2014:397437. doi: 10.1155/2014/397437
24. Christensen H, Madelung A, Nielsen A, Knudtzen F. Severe bartonella henselae bone infection in a kidney transplanted young man. BMJ Case Rep. (2022) 15:e247805. doi: 10.1136/bcr-2021-247805
25. Waseem R, Seher M, Ghazal S, Shah H, Habiba U. Cat scratch disease in a 23-year-old male-case report. Front Public Health. (2022) 10:1046666. doi: 10.3389/fpubh.2022.1046666
26. Karski J, Matuszewski Ł, Okoński M, Pietrzyk D, Karska K, Zaluski M. Cat scratch disease in a 1.5-year-old girl - case report. Ann Agric Environ Med. (2018) 25:345–8. doi: 10.26444/aaem/89547
27. Bernit E, Veit V, La Scola B, Tissot-Dupont H, Gachon J, Raoult D, et al. Bartonella quintana and mycobacterium tuberculosis coinfection in an hiv-infected patient with lymphadenitis. J Infect. (2003) 46:244–6. doi: 10.1053/jinf.2002.1040
28. Li M, Chen J, Zhang L, Chen X, Zhou J, Liu F, et al. Clinicopathological characteristics and diagnostic performance of metagenomic pathogen detection technology in mycobacterial infections among Hiv patients. Front Cell Infect Microbiol. (2025) 15:1584189. doi: 10.3389/fcimb.2025.1584189
29. Gu W, Miller S, Chiu C. clinical metagenomic next-generation sequencing for pathogen detection. Annu Rev Pathol. (2019) 14:319–38. doi: 10.1146/annurev-pathmechdis-012418-012751
30. Gu W, Deng X, Lee M, Sucu Y, Arevalo S, Stryke D, et al. Rapid pathogen detection by metagenomic next-generation sequencing of infected body fluids. Nat Med. (2021) 27:115–24. doi: 10.1038/s41591-020-1105-z
31. Li M, Yan K, Jia P, Wei E, Wang H. Metagenomic next-generation sequencing may assist diagnosis of cat-scratch disease. Front Cell Infect Microbiol. (2022) 12:946849. doi: 10.3389/fcimb.2022.946849
32. Zhou T, Zheng Y, Zhang H, Liu YA. Case report of diagnosis of cat-scratch disease using metagenomic next-generation sequencing. Front Cell Infect Microbiol. (2023) 13:1322651. doi: 10.3389/fcimb.2023.1322651
33. Hong H, Li T, Ying Y, An Q, Liu H, Liang K. Cat-scratch disease manifesting as uveitis and binocular fundus nodular lesions: a case report. BMC Ophthalmol. (2023) 23:345. doi: 10.1186/s12886-023-03063-4
34. Yang T, Mei Q, Zhang L, Chen Z, Zhu C, Fang X, et al. Hemophagocytic lymphohistiocytosis is associated with bartonella henselae infection in a patient with multiple susceptibility genes. Ann Clin Microbiol Antimicrob. (2020) 19:28. doi: 10.1186/s12941-020-00370-2
35. Kassab I, Isada C, Azar M, Sarsam N, Jiang M, Camelo-Piragua S, et al. Into the unknown: diagnosing mysterious brain lesions. Transpl Infect Dis. (2022) 24:e13829. doi: 10.1111/tid.13829
36. Wang Q, Miao Q, Pan J, Jin W, Ma Y, Zhang Y, et al. The clinical value of metagenomic next-generation sequencing in the microbiological diagnosis of skin and soft tissue infections. Int J Infect Dis. (2020) 100:414–20. doi: 10.1016/j.ijid.2020.09.007
37. Tan J, Servellita V, Stryke D, Kelly E, Streithorst J, Sumimoto N, et al. Laboratory validation of a clinical metagenomic next-generation sequencing assay for respiratory virus detection and discovery. Nat Commun. (2024) 15:9016. doi: 10.1038/s41467-024-51470-y
Keywords: cat scratch disease, pulmonary tuberculosis, granulomatous lymphadenitis, metagenomic next-generation sequencing, coinfection, bartonella henselae
Citation: Wang X, Chen S and Yang C (2025) Coexistence of cat scratch disease lymphadenitis and active pulmonary tuberculosis in an immunocompetent host – a case report with metagenomic diagnosis and literature review. Front. Med. 12:1667171. doi: 10.3389/fmed.2025.1667171
Received: 16 July 2025; Accepted: 18 September 2025;
Published: 09 October 2025.
Edited by:
Sam Donta, Falmouth Hospital, United StatesReviewed by:
Ana Afonso, NOVA University of Lisbon, PortugalBipin Kishore Prasad, Heritage Institute of Medical Sciences, India
Copyright © 2025 Wang, Chen and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chengqing Yang, MTQ3MzkxNTkzQHFxLmNvbQ==
†These authors have contributed equally to this work
 Xuan Wang
Xuan Wang Shufang Chen†
Shufang Chen† Chengqing Yang
Chengqing Yang