- 1Department of Cell Biology and Histology, School of Medicine and Nursing, University of the Basque Country UPV/EHU, Leioa, Spain
- 2BEGIKER Ophthalmology Research Group, IIS Biobizkaia, Barakaldo, Spain
- 3Department of Ophthalmology, University Hospital of Cruces, Barakaldo, Spain
- 4R&D&I Department, ICQO Instituto Clínico-Quirúrgico de Oftalmología, Bilbao, Spain
Objectives: Persistent epithelial defects (PEDs) and chronic corneal ulcers are lesions resistant to treatment for over 2 weeks, risking inadequate healing, reduced sensitivity, and corneal lysis or perforation. This study evaluates the regenerative potential of functionalized gelatin-based hydrogels for treating rabbit corneal wounds as a non-surgical alternative.
Methods: Thirty female New Zealand white rabbits underwent anterior stromal keratectomy and were assigned to five groups: control (0.2% HA artificial tears) and four hydrogel treatment groups. Hydrogels included non-functionalized gelatin-RFP (H) and functionalized versions with infliximab (H-Ab), autologous serum (H-AS), and human amniotic membrane extracts (H-HAMe). Crosslinking was performed in situ with blue light. Corneas were evaluated at 7 and 21 days for re-epithelialization, fibrosis, and inflammation using histology, qPCR and immunohistochemistry, focusing on markers of proliferation (Ki67), differentiation (CK3), stemness (PAX6, p63, CD44), adhesion (integrin β4), and fibrosis (α-SMA).
Results: All treatments supported re-epithelialization by day 7 and restored barrier function (ZO-1), with H-AS achieving the fastest closure. Expression of the adhesion marker integrin β4 improved over time across all groups. Hydrogel formulations promoted limbal activation (PAX6, CD44), with H-AS and H-HAMe showing elevated p63 expression at day 7. All hydrogels reduced fibrosis (α-SMA), though extracellular matrix organization varied. H-Ab and H-HAMe reduced inflammation (IL-1β), while H-AS showed minimal irritability.
Conclusion: Functionalized gelatin-RFP hydrogels promote re-epithelialization, reduce fibrosis and inflammation, and restore ocular integrity, offering a promising solution for corneal wound repair.
1 Introduction
The human cornea is a transparent, avascular tissue that accounts for approximately two-thirds of the eye’s total refractive power. Despite its critical function, it is particularly vulnerable to environmental insults such as trauma, infections, and chemical injuries (1). Damage to the corneal epithelium can result in persistent epithelial defects (PEDs) and corneal ulcers, which ulcers represent significant therapeutic challenges in ophthalmological practice, frequently resulting in severe complications such as scarring, corneal perforation and irreversible visual loss (2, 3). While standard therapies—such as lubricants, antibiotics, and eye drops enriched with specific bioactive compounds—can offer symptomatic relief, they often fail to adequately promote tissue regeneration, particularly in severe or chronic cases.
Recent advances in regenerative medicine have transformed the therapeutic landscape, particularly through the integration of biomaterials with biologically active and blood-derived agents. These strategies have opened new avenues for promoting corneal healing and restoring visual function (4–8). Hydrogels have emerged as promising platforms for corneal repair, offering biocompatible, tunable scaffolds capable of delivering therapeutic agents in a sustained and localized manner (9). Both natural and synthetic hydrogels—including gelatin (10–12), collagen (13, 14), chitosan (15, 16), polyethylene glycol (PEG) (17, 18), and hyaluronic acid (HA) (13, 19)—have been explored for their potential to support epithelial regeneration.
Among the most extensively studied biological therapies are blood-derived products, which contain a complex array of growth factors and proteins at concentrations similar to those found in natural tear fluid. These constituents are vital for promoting epithelial repair and maintaining ocular surface homeostasis. Autologous serum (AS) eye drops—pioneered as the first blood-derived treatment for ocular surface diseases—deliver more than lubrication; they provide essential bioactive molecules such as vitamin A, epidermal growth factor (EGF), and fibronectin, all of which support epithelial proliferation and differentiation (20–22). In parallel, plasma rich in growth factors (PRGF) eye drops have demonstrated effectiveness in conditions such as neurotrophic keratitis, dry eye disease, and post-surgical corneal wound healing, including after photorefractive keratectomy and epithelial debridement (23–31).
The therapeutic potential of the human amniotic membrane (AM) and its derivatives is also well established. Acting as both a structural scaffold and a reservoir of bioactive factors, AM supports epithelial cell proliferation and migration while exhibiting anti-inflammatory, immunomodulatory, antibacterial, anti-scarring, hemocompatible, and angio-modulatory properties (32–37). Like AS and PRGF, AM-derived products deliver a rich combination of cytokines and growth factors that suppress inflammation and promote corneal healing. Nevertheless, their broader clinical application has been hindered by variability in biological composition and the lack of effective sustained delivery mechanisms.
Combining these biological agents with hydrogel systems represents a promising strategy for overcoming these limitations. Namely, the incorporation of anti-inflammatory molecules into hydrogels has been shown to enhance corneal healing outcomes by mitigating inflammation and preventing ulcer formation (38). Infliximab, a monoclonal antibody targeting tumor necrosis factor alpha (TNF-α), has demonstrated protective effects in dry eye disease and corneal neovascularization, and is currently being investigated for use in treating corneal melt (39–42).
In our previous work, we developed a photo-crosslinkable hydrogel composed of 5% gelatin and 0.01% riboflavin phosphate (RFP), crosslinked using blue light. This system was thoroughly characterized in vitro and ex vivo for applications in corneal tissue engineering, demonstrating favorable gelation kinetics, mechanical resilience, cytocompatibility, and the ability to support epithelial cell integration and migration (43). Building upon these foundational results, the present study extends our research into an in vivo context, employing a rabbit stromal keratectomy model to evaluate the therapeutic efficacy of gelatin–riboflavin hydrogels functionalized with autologous serum (AS), human amniotic membrane extract (HAMe), or infliximab. The in vivo model allows for a more comprehensive assessment of ocular healing, closely mimicking clinical conditions and enabling the evaluation of both early and sustained responses to treatment. Specifically, we monitored ocular morbidity, wound healing progression, gene expression profiles, and immunohistochemical analyses to investigate the modulation of inflammation, fibrosis, and re-epithelialization. This study provides a systematic comparison that simultaneously evaluates three distinct bioactive agents usually used in clinical ophthalmology (autologous serum, infliximab, and HAMe) integrated into the same hydrogel platform. The idea is to contribute to the advancement of ocular regenerative medicine through an innovative approach and a comprehensive comparative methodology.
2 Materials and methods
2.1 Hydrogel synthesis
Porcine skin gelatin (10% w/v, Sigma Aldrich, St. Louis, MO, United States) was crosslinked with riboflavin phosphate (RFP, 0.02% w/v; Sigma Aldrich) to synthesize hydrogels. Infliximab-loaded hydrogels were prepared by mixing infliximab (2 mg/mL, Merck KGaA, Darmstadt, Germany) with gelatin-RFP stock to obtain final concentrations of 1 mg/mL infliximab, 5% w/v gelatin, and 0.01% w/v RFP. Hydrogels with autologous serum (AS) or HAMe were prepared by combining gelatin-RFP stock with AS or HAMe extracts (1,1 dilution), resulting in 50% of AS or 50% HAMe, 5% w/v gelatin, and 0.01% w/v RFP. All hydrogels were adjusted to pH 7 with 1 M NaOH, filtered (0.22 μm, MILLEX® filters, Merck KGaA), and stored at 4 °C.
To prevent photodegradation, hydrogel solutions were shielded from light by storing them in amber glass containers or covering the vessels with aluminum foil during both the mixing process and storage. Once the wounds were filled with the hydrogels, they were crosslinked for 2 mins under blue light using the Led.C curing lamp (Woodpecker Medical Instrument Co., Guilin, Guangxi, China), which operates within a wavelength range of 420–480 nm and a light intensity of 1,000–1,200 mW/cm2. To ensure consistent exposure and reproducibility, the lamp was maintained at a fixed distance of 10 cm from the animals.
Detailed in vitro physicochemical characterizations of the gelatin–riboflavin hydrogel, including rheology, optical transparency, swelling behavior, and release kinetics, were comprehensively reported previously (43).
2.2 Human amniotic membrane extract preparation
Placentas were obtained with informed consent, following the Declaration of Helsinki and Ethics Committee approval (University Hospital of Cruces). The amnion was manually separated from the chorion and the HAM was sectioned into distal, medial, and proximal regions relative to the placenta. Sections were placed in sterile DMEM (Lonza Bioscience, Basel, Switzerland) supplemented with 1.25 μg/mL amphotericin B (Gibco, Paisley, United Kingdom), 50 μg/mL penicillin–streptomycin (Sigma Aldrich), and 50 μg/mL neomycin (Gibco), cut into 4–4.5 cm pieces, washed, and stored at −80 °C until use. To obtain HAM extracts, HAM fragments were frozen in liquid nitrogen for 5 min, powdered in a pre-cooled mortar, weighed, and resuspended in PBS with 5 mL/g protease inhibitor (P8340, Sigma Aldrich). Samples were sonicated for 20 min at 90% amplitude (Bandelin Sonoplus, Sigma Aldrich) using a 1.5 mm probe, centrifuged (3,000 g, 10 min, 4 °C), and supernatants were collected. The solution was filtered (0.22 μm MILLEX® filters), aliquoted into 0.2 mL tubes, and stored at −80 °C.
2.3 Animals
Thirty 2-kg female New Zealand white rabbits were used for in vivo studies. Experiments adhered to protocols approved by the Animal Research Ethics Committee of the University of the Basque Country (UPV/EHU) and complied with the Tenets of the Declaration of Helsinki and the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. All procedures were conducted at the UPV/EHU animal facility (Sgiker).
2.4 Extraction of rabbit autologous serum
After locally anesthetizing the rabbit with lidocaine, the blood was collected by central ear artery venipuncture into Vacutainer® tubes containing a polymer gel for serum separation (BD, Franklin Lakes, NJ, United States).
Blood was allowed to clot for 2 h at room temperature and centrifuged at 1,000 g for 15 min. The collected supernatants, which constituted the AS, were filtered (0.22 μm MILLEX® filters) and stored at −80 °C until their use.
2.5 Surgical procedure
Thirty rabbits were divided into five groups (n = 6 each): a control group treated with 0.2% HA artificial tears (Dr. Gerhard Mann, Chem.-pharm. Fabrik GmbH, Berlin, Germany) and four groups treated with gelatin-RFP hydrogels: non-functionalized (H) or functionalized with infliximab (H-Ab), autologous serum (H-AS), or human amniotic membrane extract (H-HAMe). The four groups treated with hydrogel also received 0.2% HA artificial tears.
The rabbits were further divided into three surgery groups (n = 10 each). Surgery was performed first on the right eye, followed by a 1-week washout period before repeating the procedure on the left eye. A pilot study involving 10 eyes established experimental conditions.
In each surgery round, the right eyes of the 10 rabbits were operated on first. These eyes were allocated into the treatment and control groups (two eyes per treatment) and monitored over 7 days. After this period, a 7-day washout interval was observed to ensure clearance of any residual medication. On day 14 (counting from the right-eye surgery), the left eyes of the same rabbits were operated on. Treatments for the left eyes were assigned randomly, and both eyes of a given rabbit did not necessarily receive the same treatment. The left eyes were monitored for an additional 7 days. On day 21 of the cycle (relative to the right-eye surgery), all animals were euthanized, and both corneas were collected. At that time, right eyes had been followed for 21-days after right-eye surgery whereas left eyes had been followed for only 7-days after left-eye surgery. This procedure was repeated for two additional rounds, resulting in a total of 30 rabbits and 60 eyes across all experiments. Experiments 1, 3, and 5 corresponded to right eyes; Experiments 2, 4, and 6 corresponded to left eyes. An outline of the experimental development is shown in Figure 1 while the complete experimental design can be found in Supplementary Table S1.
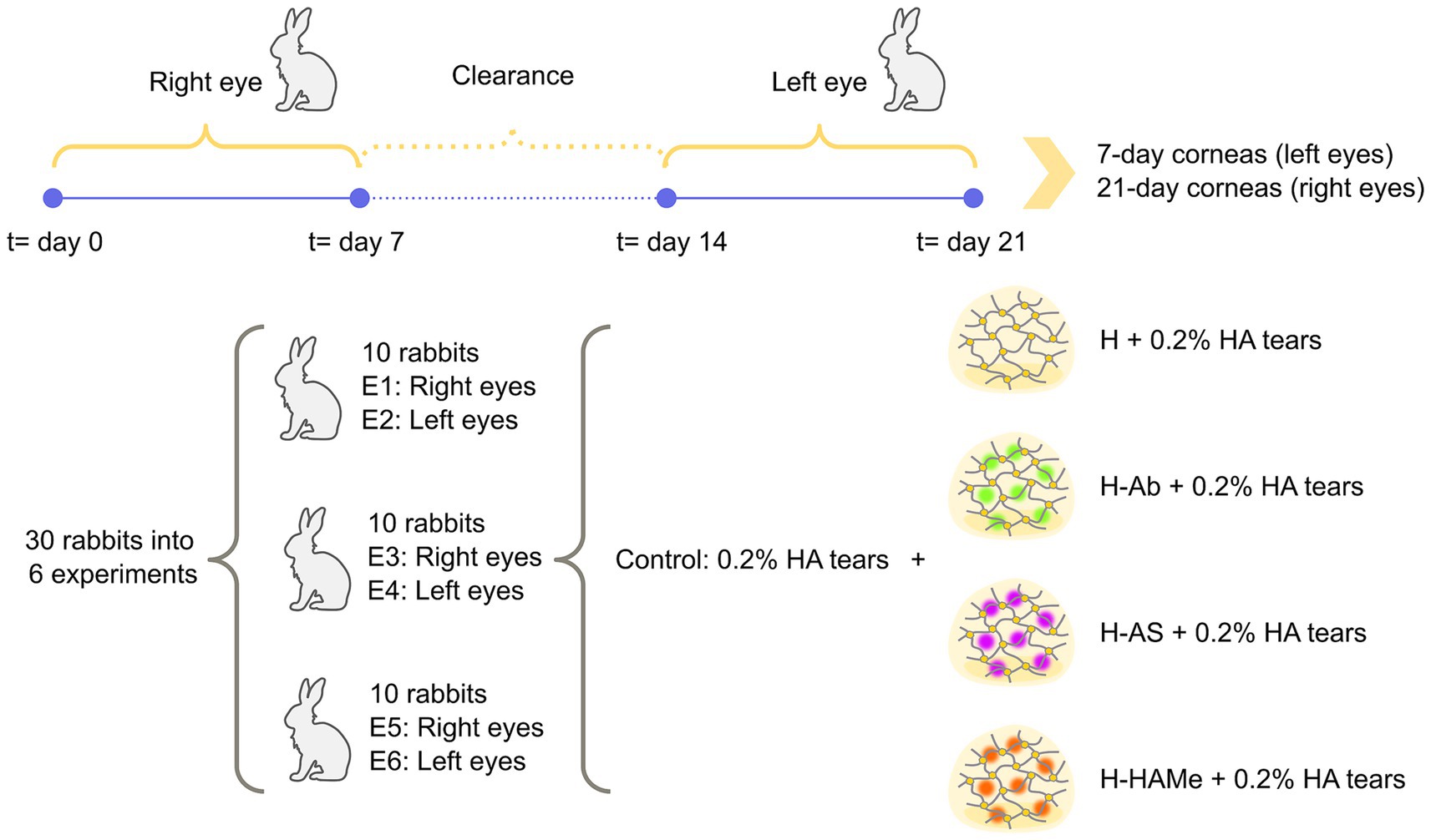
Figure 1. Outline of experimental procedure. 30 female New Zealand white rabbits were divided into 5 groups: 4 study groups (H, H-Ab, H-AS, H-HAMe) and a control group, and 6 experiments. Odd experiments (E01/E03/E05) used right eyes and even experiments (E02/E04/E06) used left eyes. Control eyes received surgery and 0.2% HA artificial tears 4 times daily until wound closure, also given to hydrogel-treated corneas. Surgery was first done on right eyes (2 per group), treated, and evaluated for 7 days. After a 1-week clearance, the procedure was repeated on left eyes. Both corneas from each rabbit were collected on day 21 for histology and inflammation analysis.
Anesthesia was administered intramuscularly using 1 mL/kg ketamine (Ketolar® 50 mg/mL, Pfizer, Spain) and 0.3 mL/kg xylazine (Xylagesic 20 mg/mL, Laboratorios Calier, Spain). After applying double anesthesia eye drops (oxybuprocaine and tetracaine, Alcon Healthcare, Spain), a 6.5 mm corneal Hessburg-Barron trephine (Katena, CorzaMedical, United States) created an anterior stromal keratectomy (3/4 turn, 187 μm depth), followed by defect excision with a crescent blade. The defect was filled with 50 μL preheated hydrogel at 34 °C, crosslinked in situ with blue light (Led.C curing lamp, Woodpecker Medical Instrument Co, China) for 2 min and protected by partial tarsorrhaphy for 72 h. Postoperative care included 0.05 mg/kg subcutaneous buprenorphine (Buprecare®, Ecuphar, Spain) every 12 h for 72 h, Tobrex® (Tobramycin, Novartis, Spain) antibiotic eye drops (2 × daily) and HA artificial tears (4 × daily) until closure. Eyes were assessed daily for 7 days. Euthanasia was performed using 2 mL/kg ketamine, 0.6 mL/kg xylazine, and intravenous saturated potassium chloride, with death confirmed by rigor mortis.
2.6 Clinical evaluation of eye injuries
Ocular morbidity was evaluated from days 3 to 7 using the Draize ocular toxicity test via slit-lamp examination. This test scores corneal opacity, iritis, conjunctival redness, edema, and discharge, with total scores (0–110) classified per Kay and Calandra criteria (44).
Iris and conjunctiva were examined first, followed by corneal assessment using 5 μL of 2% fluorescein Colicursí® Fluotest eye drops (Novartis) under blue light. Photographs were taken on day 0 and from day 3 on until wound closure. The fluorescein-stained area was quantified with ImageJ software. On day 7 (left eyes) or day 21 (right eyes), corneas were dissected and prepared for further histology, immunostaining or gene expression analysis.
2.7 RNA extraction, retrotransciption, cDNA preamplification and real-time PCR
RNA was extracted from corneal tissues using a bullet blender (Next Advanced, NY, United States) with zirconium oxide beads and Trizol (Thermo Fisher Scientific, Waltham, MA, United States) following the manufacturer’s protocol. Extracted RNA was treated with DNase I (1 unit/μl; Zymo Research, Irvine, CA, United States) for 15 min at room temperature, followed by inactivation with EDTA (2.5 μL, 25 mM) and heating at 65 °C for 10 min.
Reverse transcription of 1 μg RNA per sample was performed using the iScriptTM cDNA Synthesis Kit (Bio-Rad, Hercules, CA, United States). cDNA pre-amplification was carried out with the Multiplex PCR Kit (Qiagen, Hilden, Germany) under thermal conditions of 95 °C for 15 min, 14 amplification cycles, and a 30-min extension at 60 °C, including in the pre-amplification mix the primers to be used later.
qPCR was performed using a CFX96 Real-Time System (Bio-Rad) with SYBR® Green dye. Reactions (20 μL) included 1 μL pre-amplified cDNA, 250 nM gene-specific primers, and iQ SYBR Green Supermix (Bio-Rad). The protocol comprised polymerase activation (95 °C, 3 min), 40 cycles of denaturation (95 °C, 30 s), annealing (gene-specific temperatures, 30 s), and elongation (72 °C, 30 s). Melting curves (65–95 °C in 0.5 °C increments over 60 cycles) were generated post-amplification, followed by a 4 °C hold. Primer details are in Table 1.
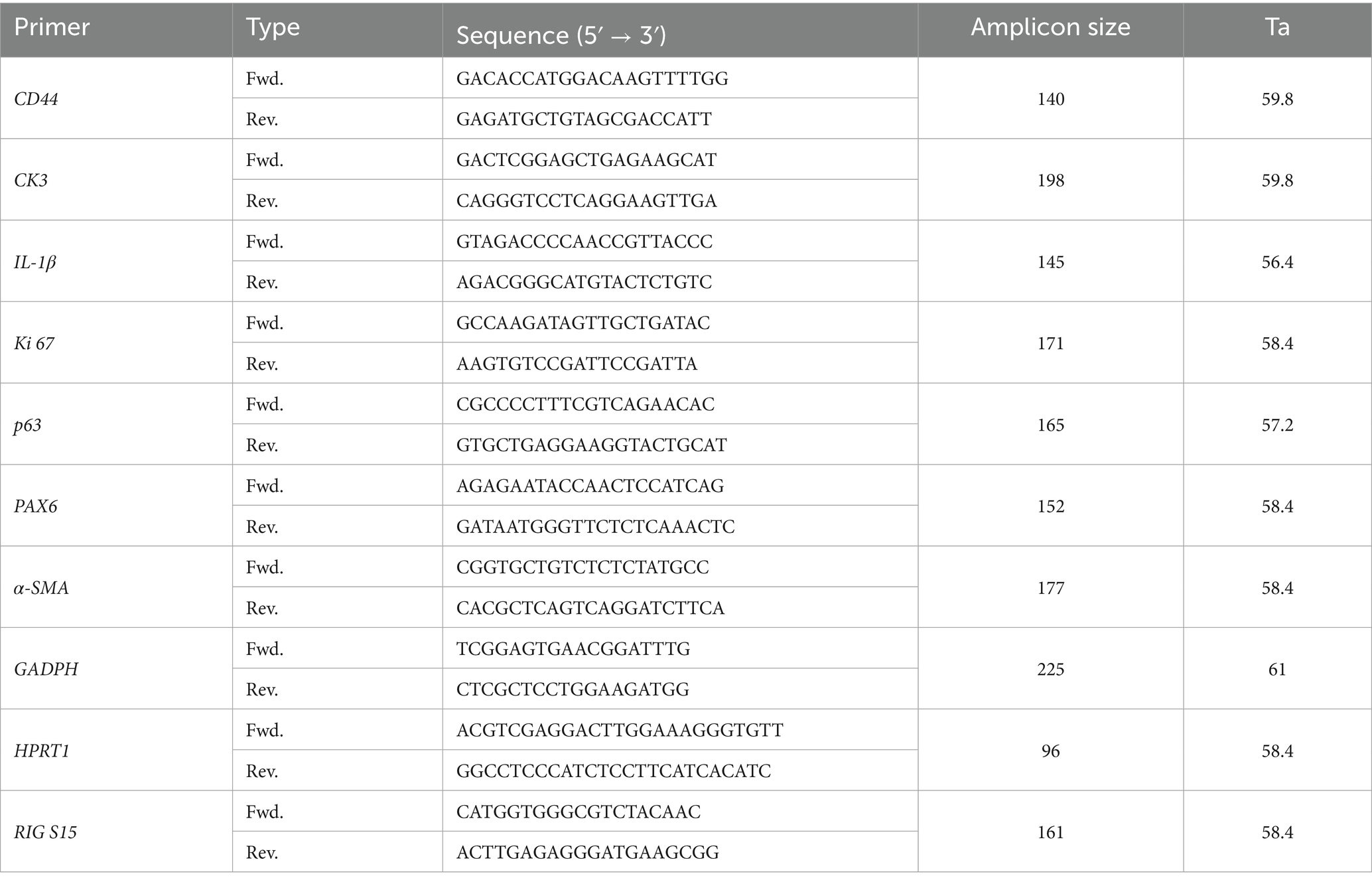
Table 1. Sequences, amplicon sizes and annealing temperatures (Ta) of the primers used in in vivo assays.
Negative and RT-minus controls were included. Samples at 7 and 21 days were analyzed with 3 and 2 biological replicates, respectively, and 3 technical replicates each. Detection ranges were assessed with 5-fold serial dilutions (1:5 to 1:625), achieving over 99% PCR efficiency and R2 above 0.98 for all primers. Expression was normalized using GAPDH, HPRT1, and RIG/S15 as reference genes, and data were analyzed via Bio-Rad CFX Maestro software.
2.8 Histological examination
Frozen corneal sections of 10 μm thickness were obtained from OCT-embedded tissues using a CM 3050S cryostat (Leica, Wetzlar, Germany). Following sectioning, the samples were fixed in 4% paraformaldehyde (PFA), stained with H&E, mounted with Dibutylphthalate polystyrene xylene (DPX) under coverslips and observed with an Olympus BX50 microscope (Olympus, Tokyo, Japan).
2.9 Immunohistochemistry
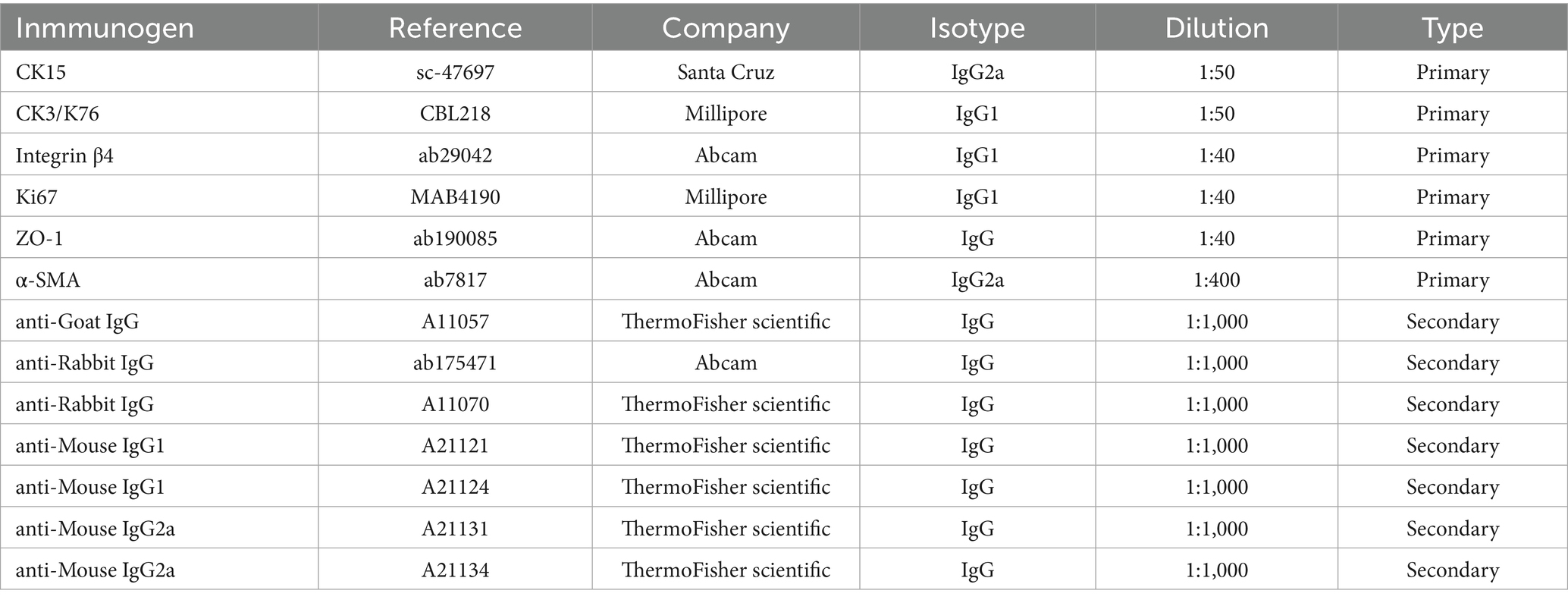
Frozen 10 μm slides were thawed and rinsed twice with PBS (5 min each). Sections were permeabilized with 0.1% Triton TX-100 (Sigma Aldrich) in PBS (PBST) (10 min, twice), blocked with 10% normal goat serum (Thermo Fisher Scientific) (10 min), and incubated overnight at 4°C with primary antibodies (Table 2) in blocking solution (0.1% BSA, 5% FBS in PBST).
After PBS (10 min, once) and PBST washes (15 min twice), samples were stained with secondary antibodies (1,1,000 in blocking solution) for 1.5 h at room temperature in the dark, washed again, and counterstained with 4 mg/mL Hoechst 33342 (Thermo Fisher Scientific) (15 min, room temperature). Final PBS washes (5 min, twice) preceded mounting with Fluoromount G (SouthernBiotech; Birmingham, United Kingdom). Slides imaged with a Nikon Ti-U fluorescence microscope (Nikon, Tokyo, Japan).
2.10 Analysis and statistics
Data normality was assessed using Shapiro-Wilks tests, confirming nonparametric distribution (Supplementary Tables S2, S3). A Kruskal-Wallis test followed by Dunn’s multiple comparison test was applied in all the results, with significance set at p < 0.05. Statistical analyses were conducted using R software (R Foundation for Statistical Computing, Vienna, Austria) and GraphPad Prism 8 (San Diego, CA, United States).
3 Results
3.1 In vivo re-epithelialization in a rabbit animal model
Corneal wounds were assessed daily post-tarsorrhaphy removal (day 3). By this time, median wound areas across groups reduced to ≤ 20% (Figures 2A,C). The control group achieved the most rapid closure considering all groups, with 60% of wounds fully closed by day 5 (Figures 2B–D), though 20% remained unhealed by day 7 due to reopening or plateauing (Figures 2A–C).
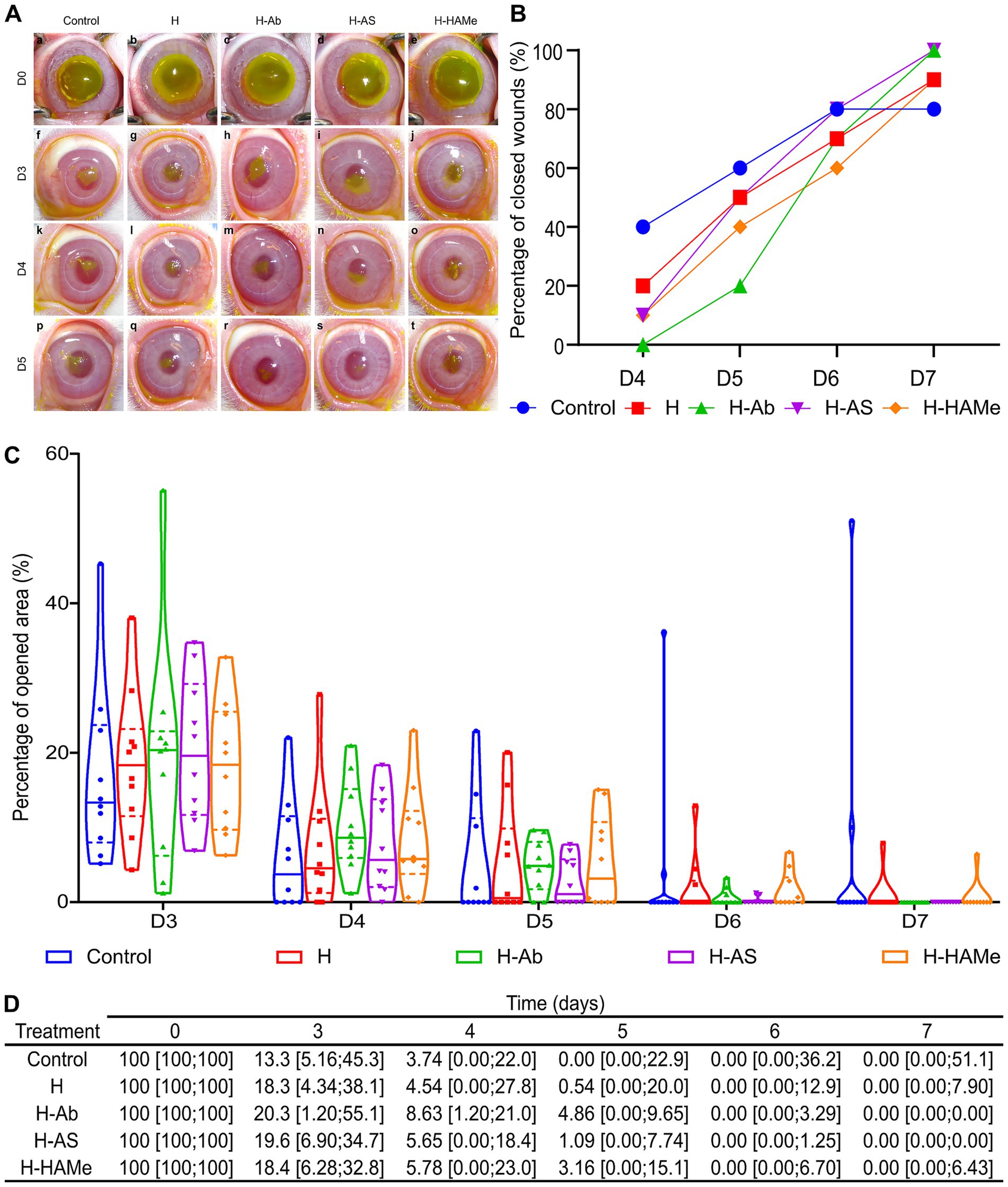
Figure 2. (A) Progression of epithelial defects in rabbit eyes treated with artificial tears (Control) or hydrogel variants (H, H-Ab, H-AS, H-HAMe) from day 3 (D3) to day 5 (D5), assessed via fluorescein staining. (B) The percentage of wounds closed per day from day 4 (D4) to day 7 (D7). (C) Percentage of the surgical wound area remaining open from day 3 (D3) to day 7 (D7), with median (solid line) and interquartile range (dashed line) shown in violin plots. (D) Re-epithelialization progress in rabbit eyes treated with hydrogel variants (H, H-Ab, H-AS or H-HAMe) or control, shown as median wound area (%) and minimum and maximum values [min; max]. No significant differences were observed between treatments and samples over time.
Hydrogel treatments facilitated steady healing. H-AS promoted the fastest closure among the hydrogel treatments, with a median wound area of 1.09% [0.00; 7.74] by day 5 and complete healing by day 7 (Figures 2B–D). H-Ab-treated corneas exhibited slower early closure, with 4.86% [0.00; 9.65] open on day 4, but all were healed by day 7. H and H-HAMe treatments showed similar trends, achieving 90% closure by day 7, though 10% remained unhealed (Figures 2B–D). Biological variability likely explained the absence of significant differences between groups.
Histological analysis confirmed epithelial regrowth across all treatments. Cryoprocessing compromised tissue structure preservation, particularly in the anterior stroma. At 7 days, control corneas showed epithelial thickening and abundance of stromal myofibroblast, progressing to stromal edema and increased myofibroblasts number by 21 days (Figures 3a,f). H-treated corneas displayed epithelial thickening and wound-edge myofibroblasts at 7 days, followed by epithelial thinning and increased myofibroblasts at 21 days (Figures 3b,g). All corneas treated with functionalized hydrogels exhibited a more compact epithelium and robust epithelial-stromal adhesion at 21 days. Epithelial detachment shown at 7 days in H-AS and H-HAMe was likely due to hydrogel loss during tissue processing (Figures 3d,i). By day 21, corneas treated with H-AS exhibited a well-organized extracellular matrix and an absence of myofibroblasts (Figures 3d,j), whereas those treated with H-Ab and H-HAMe showed mild matrix disorganization. Corneas treated with H-Ab displayed a reduced presence of myofibroblasts (Figures 3c,h), while those treated with H-HAMe showed a higher presence of myofibroblasts at the wound margins (Figures 3e,j).
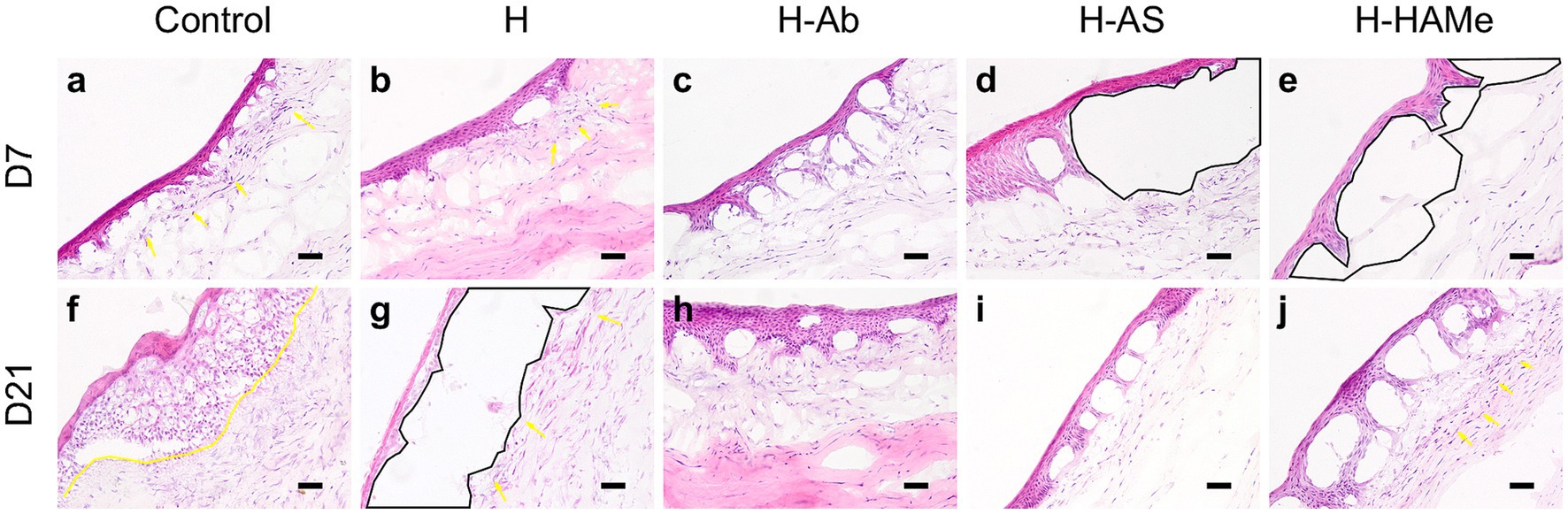
Figure 3. Hematoxylin-Eosin staining of central cornea sections from rabbit eyes post re-epithelialization. Samples treated with artificial tears (Control) or hydrogels (H, H-Ab, H-AS, H-HAMe) were analyzed at 7 (a–e) and 21 days (f–j) post-surgery. Yellow arrows and highlighted regions indicate myofibroblast infiltration, while black areas denote epithelial detachment. Images at 20 × magnification; scale bars = 50 μm.
3.2 Epithelial cell differentiation, proliferation and stemness
ZO-1, a marker of corneal barrier integrity, was consistently detected by immunohistochemistry in the superficial epithelial layers within the wound area across all groups and time points, demonstrating the restoration of epithelial barrier function (Figures 4a–j). In parallel, cell proliferation was evaluated. The highest proliferative activity was observed in the epithelia of untreated controls at both time points (Figures 4a,f). Ki67-positive cells, indicative of cellular proliferation, were most abundant at day 7 in the control group, with a substantial decrease in all treatment groups by day 21 (Figures 4a–j). Consistently, at the RNA level, Ki67 transcripts were markedly reduced in all hydrogel-treated groups by day 21, with H-AS and H-HAMe showing statistically significant decreases (p = 0.0031 and p = 0.0008, respectively) compared to controls (Figures 5A,D).
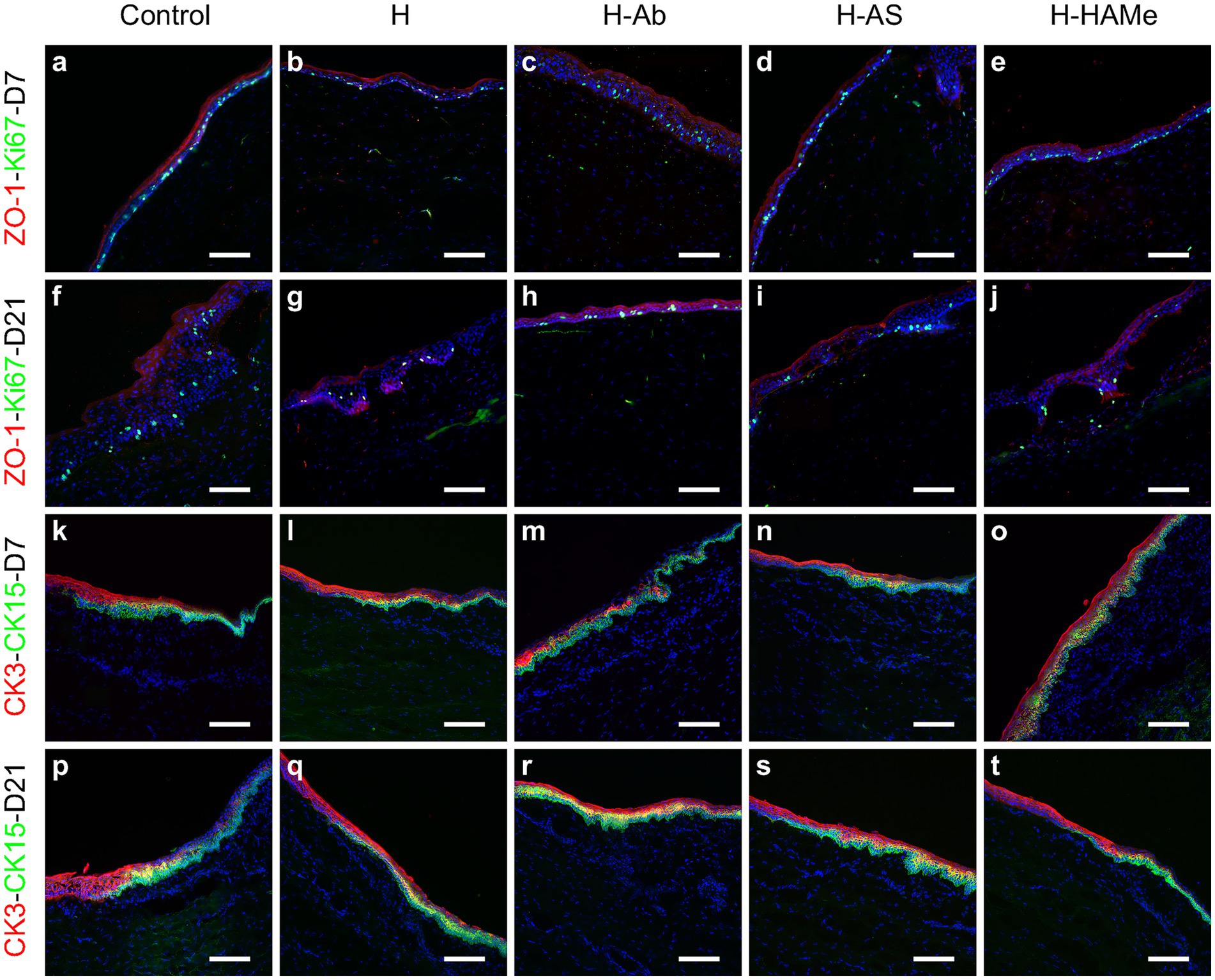
Figure 4. Protein expression of ZO-1 (red), Ki67 (green) (a–j) in the central cornea and CK3 (red), CK15 (green) in the limbus (k–t) of rabbit corneas treated with artificial tears (Control) or hydrogels (H, H-Ab, H-AS, H-HAMe). Samples analyzed at 7 (a–e,k–o) and 21 days (f–j,p–t) post-surgery. Images at 20 × magnification; scale bars correspond to 100 μm.
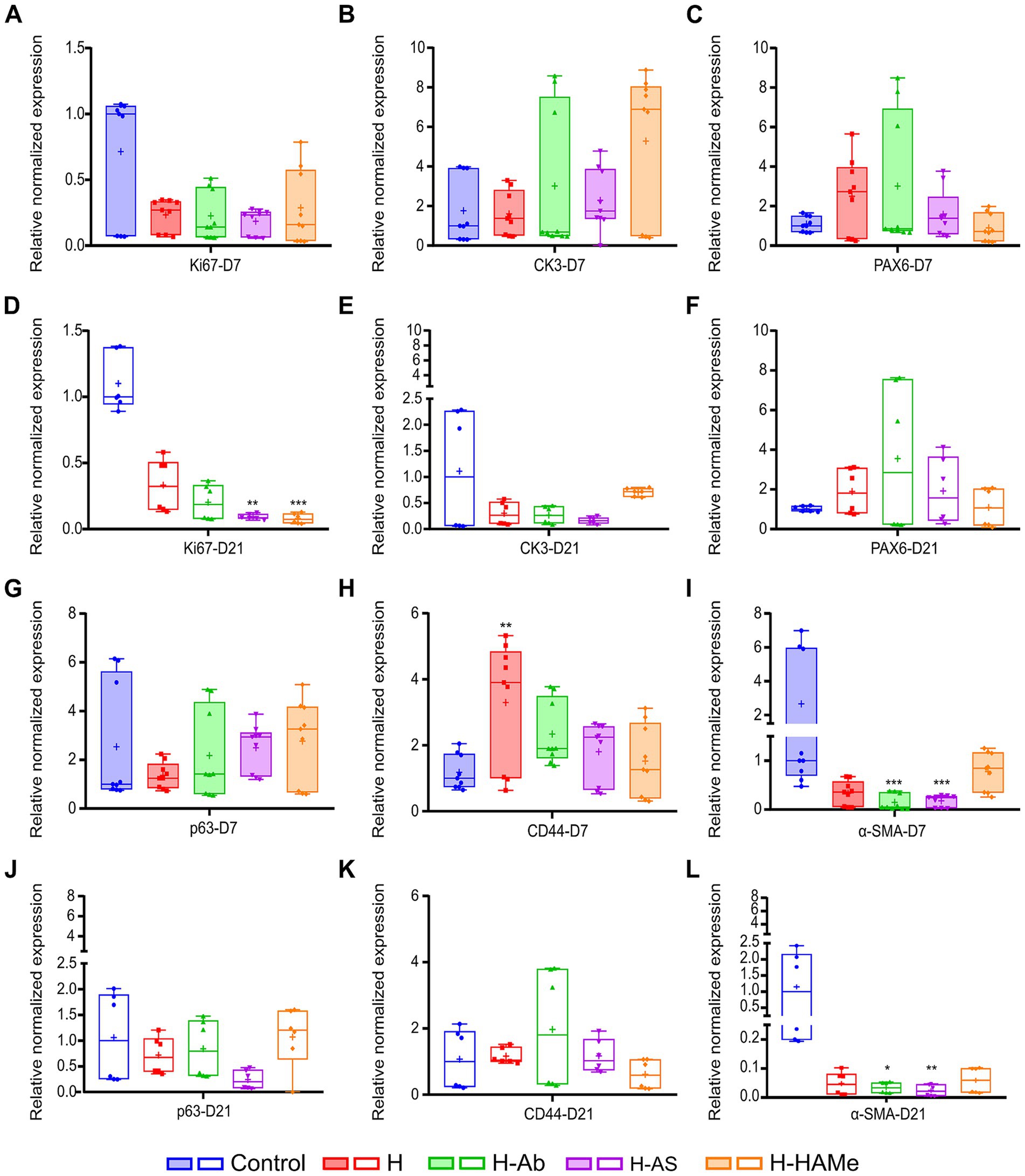
Figure 5. Relative normalized gene expression (ΔΔCq) of Ki67, CK3, PAX6, p63, CD44 and α-SMA in rabbit corneas 7 days (A–C,G–I) and 21 days (D–F,J–L) post-surgery. “+” sign is used to denote the mean value of each box plot. Significant differences (∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001) are indicated relative to the control treatment for each time point.
The CK3 differentiation protein marker was centrally expressed in all samples and decreased toward the limbus (Figures 4k–t). CK15 protein labeling, a potential limbal stem cell marker, was restricted to the limbus and co-localized with CK3 in the peripheral cornea. CK15 expression increased by 21 days, indicating limbal activation (Figures 4p–t).
qPCR analysis revealed no significant differences in RNA expression at 7 or 21 days for the differentiated epithelial cells’ marker CK3 (Figures 5B,E), though H-HAMe had the highest median levels at 7 days, showing 6.89 fold change expression with respect to all the other treatments (Figure 5B). By day 21, CK3 expression had notably declined in all hydrogel-treated corneas (Figure 5E). H-HAMe reached levels similar to the control, while CK3 levels in corneas treated with H, H-Ab, and H-AS were markedly lower than those in the control group (Figures 5B,E).
Limbal stem/progenitor markers PAX6 and p63 showed no significant differences from controls at RNA expression levels (Figures 5C,F,G,J). Still, PAX6 transcript levels remained above control levels at day 21 in all H-treated groups, suggesting a promotion of limbal stemness (Figure 5F). p63 expression peaked at day 7 in H-AS and H-HAMe, but decreased by day 21, while remaining stable in H and H-Ab (Figures 5G,J). Gene expression levels of CD44, a transmembrane receptor protein that mediates cell adhesion, migration and stem cell retention in the niche, were elevated in all hydrogel-treated corneas, with H significantly exceeding control levels at 7 days (p = 0.0035, Kruskal-Wallis test followed by Dunn’s multiple comparisons) (Figure 5H). By day 21, all hydrogel-treated corneas showed levels equivalent to the control (Figure 5K).
3.3 Cell-substrate adhesion and fibrosis
Integrin β4 protein labeling, a cell-basement membrane adhesion molecule, showed discontinuity at 7 days but increased across all groups by day 21 (Figures 6a–j).
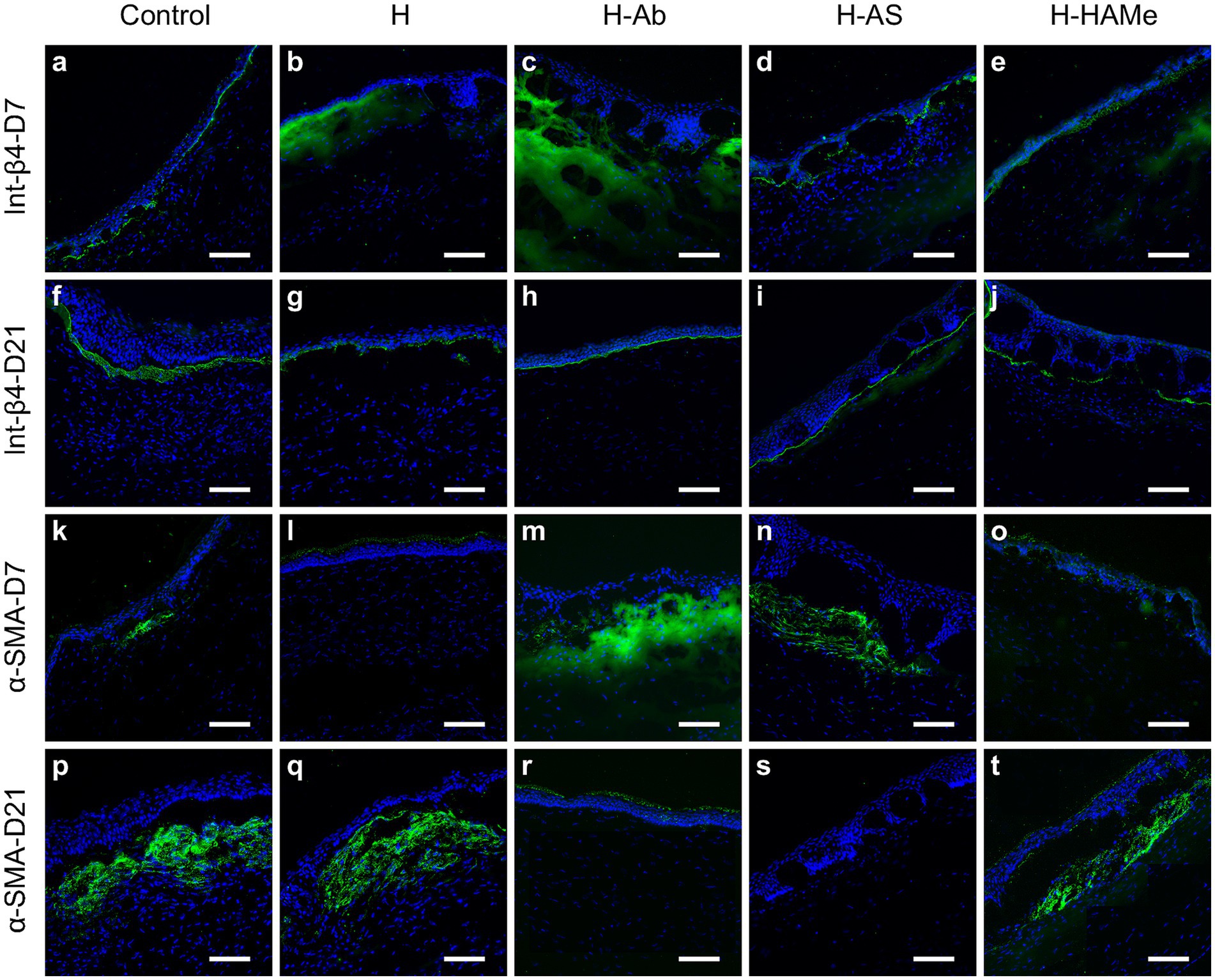
Figure 6. Protein expression of Integrin-β4 (a–j) and α-SMA (k–t) in the central cornea of rabbit corneas treated with artificial tears (Control) or hydrogels (H, H-Ab, H-AS, H-HAMe). Samples analyzed at 7 (a–e,k–o) and 21 days (f–j,p–t) post-surgery. Images at 20 × magnification; scale bars correspond to 100 μm.
Immunohistochemical analysis of α-SMA, a marker of myofibroblast differentiation and fibrosis, revealed subepithelial α-SMA staining at day 7, with increased expression by day 21 in the control, H, and H-HAMe groups—evidence of progressive myofibroblast formation (Figures 6k–t). In contrast, H-Ab exhibited minimal staining, and H-AS showed no detectable α-SMA signal in the stroma (Figures 6r,s). At the RNA level, α-SMA expression was significantly reduced in H-Ab and H-AS groups at both day 7 (p = 0.0004) and day 21 (p = 0.0315 for H-Ab, p = 0.0012 for H-AS) compared to controls (Figures 5I,L). The H group at days 7 and 21 and the H-HAMe group at day 21 also showed reduced α-SMA levels, though these differences were not statistically significant (Figure 5L).
3.4 Eye irritation and inflammation
The Draize test showed minimal irritation across treatments by day 3, primarily due to surgery-induced redness, chemosis, or discharge (Figure 7A). H-AS exhibited the least irritant response among the tested formulations. Severe corneal opacity in one H-Ab-treated animal was excluded as an outlier. Symptoms improved by day 4, with control, H, and H-AS groups reaching non-irritative scores (0.6–2.5 points). H-Ab and H-HAMe achieved non-irritative levels by day 5. H-AS facilitated the fastest recovery, with irritation nearly resolved by day 6 (0.2 ± 0.63), and all groups scored 0 by day 7. No treatment exceeded 15 points, staying below the mild irritation threshold.

Figure 7. (A) Draize test scores from day 3 (D3) to day 7 (D7). Scores 2.5–15 (dotted line at y = 15) indicate minimal irritation; values above 15 are classified as mild or higher irritation. No significant differences were observed between samples. Relative normalized gene expression (ΔΔCq) of IL-1β in rabbit corneas 7 days (B) and 21 days (C) post-surgery. “+” sign is used to denote the mean value of each box plot. Significant differences (∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001) are indicated relative to the control treatment for each time point.
IL-1β levels measured by qPCR showed no significant differences at day 7 (Figure 7B). H-Ab reduced IL-1β expression by half, while H-HAMe showed increased levels compared to controls. By day 21, all hydrogel treatments led to a reduction in IL-1β levels, with significant decreases in H-HAMe (ΔΔCq: 0.05; p < 0.0001, Kruskal-Wallis test followed by Dunn’s multiple comparisons) and notable reductions in H-Ab (ΔΔCq: 0.18), and H (ΔΔCq: 0.17), indicating effective inflammation control (Figure 7C).
4 Discussion
In the context of tissue healing, replacement and regeneration are distinct concepts. Regeneration enables damaged tissue to fully restore affected parts to their original state by promoting new growth. Replacement refers to the healing of severely damaged area through the deposition of new abnormal tissue, it involves patching rather than restoring and it commonly implies scarring. The functionalized hydrogel aimed to promote stromal repair and tissue regeneration by facilitating the release of bioactive compounds. This non-surgical strategy sought to enable epithelial recovery, gradual stromal regeneration, and restoration of corneal transparency.
During the early experimental phase (days 3–5), the control group exhibited larger areas of epithelial closure compared to the hydrogel-treated groups, although some control corneas failed to fully close and occasionally reopened. Tarsorrhaphy appeared to accelerate epithelial defect closure. Additionally, the absence of the hydrogel as a physical barrier in the control group allowed for unhindered epithelial migration (45–47). Epithelial cells in the control group were able to migrate freely over the wound bed without encountering any physical barrier, unlike the hydrogel-treated corneas where the presence of the hydrogel could transiently modulate or slow cell migration. However, in some control cases this rapid closure appeared to be incomplete or unstable, resulting in partial wound reopening during the observation period. In contrast, although epithelial closure in the hydrogel-treated groups progressed more gradually, the histological images suggest a possible enhancement of epithelial–stromal adhesion in the hydrogel-treated corneas. This may reflect the hydrogel’s ability to provide a provisional matrix that supports more robust epithelial anchorage, potentially contributing to longer-term wound stability despite the slower initial closure rate.
Epithelial hyperplasia was observed in all corneas, likely representing a compensatory initial remodeling response to stromal loss to maintain corneal thickness and curvature (48). Proliferative activity, as indicated by increased numbers of Ki67-positive cells and elevated Ki67 gene expression, was higher in all treatments by day 7, and especially in the control group.
All hydrogel treatments achieved complete re-epithelialization. H-AS demonstrated the fastest closure among hydrogel-treated groups from day 5, reflecting blood products’ epitheliothropic-enhancing effects (25–27, 49–52). H-HAMe also facilitated rapid closure, highlighting the pro-regenerative properties of amniotic membrane (AM) products (53–55). H-Ab exhibited slower initial closure and fewer cases closed by day 5 but achieved full re-epithelialization alongside H-AS. Infliximab, known for its anti-inflammatory properties, has been shown to support epithelial repair without compromising cell viability or phenotype (56, 57). The anti-inflammatory effects of Infliximab may permit autonomous epithelial repair in H-Ab treated corneas, leading to similar closure outcomes for H-Ab, H-AS, and H-HAMe.
Consistent ZO-1 labeling across groups at 7 and 21 days indicated a functional epithelium and prioritized barrier restoration during healing (52, 58, 59). Similarly, integrin β4, crucial for hemidesmosome formation and epithelial stability (60), showed discontinuous labeling at the wound site by day 7, with irregular staining in controls and gaps in hydrogel-treated corneas. By day 21, stronger integrin β4 staining indicated improved epithelial attachment and extracellular matrix integration.
CK15, a corneal epithelial progenitor marker (61, 62), was elevated in all corneas at day 21, indicative of limbal activation following injury. Although RNA analysis using whole sclerocorneal samples did not show statistically significant differences in PAX6 or p63 expression—likely due to dilution effects—a trend toward increased p63 was noted at day 7 in H-AS and H-HAMe-treated corneas. Given that p63, particularly the ΔNp63 isoform, acts as a master regulator of epithelial stemness and self-renewal (63, 64), this early upregulation likely reflects activation of limbal epithelial stem/progenitor cells (LESCs) by growth factors present in HAMe and AS. This interpretation is in line with previous reports demonstrating that sPRGF and platelet-rich plasma formulations preserve stem/progenitor potential and enhance epithelial repair (25, 65, 66).
Correspondingly, AM-derived treatments have been shown to enhance LESC proliferation and stem cell marker expression while suppressing differentiation markers (67–69). The observed trends suggest that, in the presence of these bioactive agents, early stem cell activation precedes later differentiation, consistent with enhanced regenerative capacity.
At day 21, H, H-Ab, and H-AS treated groups displayed increased PAX6 and decreased CK3 expression. PAX6, a pivotal regulator of LESC fate and differentiation, is essential for maintaining the limbal niche and preventing transdifferentiation into non-corneal lineages (70, 71). Its upregulation in our hydrogel groups, as supported by in vitro data showing promotion of holoclone formation and suppression of differentiation markers (71), indicates preservation of stemness, likely mediated by the hydrogels’ modulation of inflammation and serving as a regenerative scaffold.
CD44, a transmembrane glycoprotein and HA receptor, is crucial for LESC adhesion, migration, and interaction within the niche, essential for maintaining the undifferentiated state and regenerative potential of LESCs (72, 73). Hydrogel-treated corneas exhibited generally elevated CD44 expression, with a statistically significant increase in the H group at day 7. Through CD44-HA interactions, hydrogels likely support sustained regenerative signaling, cytoskeletal remodeling, and epithelial repair (74–76). The use of 0.2% HA across all groups could have masked differences; however, the enhanced retention and local concentration of HA within the hydrogel vehicle may have reinforced CD44 activation in the H group, contributing to improved healing dynamics (77).
In summary, sequential elevation of p63 (early) and subsequent increases in PAX6 across hydrogel-treated groups, especially with H-AS and H-HAMe supplementation, reflect coordinated limbal activation and niche support. Notably, the hydrogels may also act as reservoirs for topically administered hyaluronic acid (HA), prolonging its residence time on the ocular surface and enhancing its biological effects. This HA retention could underlie the increased expression of CD44, a principal HA receptor, and may also contribute to the upregulation of PAX6. In contrast, the marked increase in p63 expression observed at day 7, particularly in the H-AS and H-HAMe groups, is more likely attributable to the presence of growth factors delivered via these specific hydrogel formulations. These findings suggest that the bioactive properties of hydrogels, including extended HA retention and localized delivery of growth factors, facilitate a mechanistic pathway of stem cell activation, niche preservation, and regenerative support in the injured cornea.
This study examined myofibroblastic responses in control and hydrogel-treated corneas after stromal injury, finding reduced α-SMA expression in hydrogel-treated samples. While an initial fibrotic response aids healing, excess myofibroblast-derived extracellular matrix can cause stromal opacity, requiring keratocyte-mediated reabsorption for transparency restoration. Hydrogel-treated corneas had significantly lower α-SMA levels at day 7 and 21, with H-Ab and H-AS showing the greatest reductions. Prior research demonstrated undiluted sPRGF eye drops reduce α-SMA expression, minimizing haze and scarring (25, 26). Gelatin in hydrogels likely influenced fibrosis, as GelMA hydrogels inhibit myofibroblast differentiation, prevent fibrosis, and maintain corneal properties (78). Increased GelMA hydrogel stiffness was associated with higher fibrosis-related gene expression and myofibroblast activity (79).
Immunofluorescence revealed α-SMA in the stroma and epithelium, indicating epithelial-mesenchymal transition (EMT) during corneal repair, consistent with prior studies, though the mechanisms remain unclear (80–82). Treatments for corneal scars often include corticosteroids (e.g., prednisolone, dexamethasone) to reduce inflammation and fibrosis, with severe cases requiring lenses or transplants (83–86). Hydrogel therapies demonstrated promise in reducing fibrosis and promoting stromal regeneration (87–90). While no treatment fully prevented fibrosis, hydrogels significantly reduced α-SMA expression, highlighting their potential to modulate early responses and reduce corneal opacification.
Draize scale assessments identified H-AS as the least irritating treatment, with H-Ab and H-HAMe causing mild irritation through days 3–5 but becoming non-irritating by day 6. Despite initial irritability, H-Ab reduced inflammation, as evidenced by IL-1β gene expression decreasing from a normalized median of 1 to 0.1 by day 21. Infliximab, a TNF-α inhibitor, has previously demonstrated efficacy in lowering IL-1β levels, inflammation, and fibrosis in corneal injuries (39). H-HAMe exhibited the strongest anti-inflammatory effect at day 21, likely due to HAMe-derived growth factors modulating cytokines by reducing TNF-α and IL-6 while increasing IL-10, promoting healing and reducing fibrosis and infection risk (91). HAM and its derivatives reduce corneal inflammation through IL-1ra and IL-10 secretion by amniotic epithelial cells (92). HAMe eye drops promote epithelial healing and limit conjunctivalization and vascularization in limbal stem cell deficiency (65), while HC-HA/PTX3 matrix component effectively suppresses inflammation, angiogenesis, and scarring (93).
H-Ab and H-HAMe hydrogels, which most effectively reduced IL-1β expression, initially caused greater clinical inflammation (e.g., chemosis and redness) as assessed by the Draize scale. In contrast, H-AS exhibited less redness and chemosis despite higher IL-1β expression at day 21. AS, enriched in factors that aid corneal repair, is effective in inflammatory conditions like severe dry eye disease (DED). Blood-derived drops, including AS and platelet products, surpass artificial tears due to regenerative properties (24, 94–99), though their efficacy varies with donor health, as elevated pro-inflammatory cytokines are observed in rheumatoid arthritis and Sjögren’s syndrome patients (100–102). To ensure consistency, rabbit sera were collected pre-surgery to avoid disease-related variations.
Considering the overall in vivo response, both functionalized and non-functionalized hydrogels enhanced wound healing by promoting re-epithelialization and significantly reducing the inflammatory and fibrotic response. In particular, H-AS facilitated the fastest epithelial closure, with reduced fibrosis and minimal irritability on the Draize scale. HAMe-functionalized hydrogels showed better anti-inflammatory effects but delayed epithelial closure and caused greater irritability. H-Ab effectively reduced IL-1β and α-SMA, demonstrating strong anti-inflammatory and antifibrotic properties, though epithelial closure was slower. Despite its higher initial irritability, it remained minimally irritating and achieved complete healing.
Among the limitations of the study, immunohistochemistry was applied qualitatively to support the qPCR findings, as image-based quantification proved technically challenging in this context. In addition, corneal transparency was assessed qualitatively through slit-lamp biomicroscopy performed by experienced ophthalmologists, combined with histological evaluation of fixed corneal sections. However, we acknowledge that a quantitative assessment of transparency—such as optical coherence tomography (OCT) or in vivo confocal microscopy—could have provided further insights into stromal remodeling and fibrotic changes.
Taken together, these findings highlight the therapeutic potential of functionalized hydrogels as bioactive platforms for corneal regeneration. This study set out to address the critical need for effective, biocompatible therapies that enhance corneal wound healing while minimizing inflammation and fibrosis—key challenges in the treatment of persistent epithelial defects and stromal injuries. By integrating hydrogel technology with clinically relevant bioactive components—including autologous serum, amniotic membrane extract, and infliximab—this work offers a novel comparative evaluation of advanced ophthalmic formulations within a controlled in vivo model.
The results demonstrate that each biofunctionalized hydrogel exerted beneficial effects on epithelial regeneration by promoting stemness and modulating fibrotic and inflammatory markers, albeit with differing kinetics and tissue responses. Importantly, our intention was also to emphasize that the hydrogel platform can be functionalized with different bioactive agents, thereby offering conceptual flexibility to tailor treatments to individual clinical needs and pathological contexts. These findings not only validate the regenerative potential of these formulations but also underscore the importance of selecting and combining functional components strategically to match specific therapeutic goals. This comparative approach, which is rarely explored in preclinical corneal research, provides new insights into the distinct roles of widely used ophthalmic biologics when integrated into a common hydrogel platform. Ultimately, the study advances the field of corneal regenerative medicine by establishing a foundational framework for the rational design and selection of multifunctional non-surgical biomaterial-based therapies.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving humans were approved by the Ethics Committee of the University Hospital of Cruces. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. The animal study was approved by Animal Research Ethics Committee of the University of the Basque Country (UPV/EHU). The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
CR-V: Data curation, Methodology, Writing – original draft, Formal analysis, Investigation, Writing – review & editing. JE: Conceptualization, Investigation, Writing – review & editing. VF: Writing – review & editing, Investigation. MR-A: Writing – review & editing, Investigation, Formal analysis, Validation, Visualization. JA: Writing – review & editing, Supervision. NA: Writing – review & editing, Funding acquisition, Supervision, Project administration.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research study was supported by grants from the Department of Heath of the Basque Government (2023111027 and IT524-22). CR-V was supported by a fellowship from the University of the Basque Country UPV/EHU.
Acknowledgments
We gratefully acknowledge the technical support with animal care provided by SGIker (UPV/EHU) and the statistical analysis provided by “Bioinformatics, Biostatistics, and Information Systems” (Bio2SI) platform of IIS Biobizkaia.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1667446/full#supplementary-material
References
1. Meek, K, and Knupp, C. Corneal structure and transparency. Prog Retin Eye Res. (2015) 49:1–16. doi: 10.1016/j.preteyeres.2015.07.001
2. Vaidyanathan, U, Hopping, GC, Liu, HY, Somani, AN, Ronquillo, YC, Hoopes, PC, et al. Persistent corneal epithelial defects: a review article. Med Hypotheses Discov Innov Ophthalmol. (2019) 8:163–76.
3. Wilson, SE, and Goshe, JM. Prevention and treatment of persistent epithelial defects after common refractive surgery procedures. J Refract Surg. (2024) 40:117–24. doi: 10.3928/1081597X-20240102-01
4. Anitua, E, De la Sen-Corcuera, B, Orive, G, Sánchez-Ávila, RM, Heredia, P, Muruzabal, F, et al. Progress in the use of plasma rich in growth factors in ophthalmology: from ocular surface to ocular fundus. Expert Opin Biol Ther. (2021) 22:31–45. doi: 10.1080/14712598.2021.1945030
5. Meng, S, Hu, H, Qiao, Y, Wang, F, Zhang, BN, Sun, D, et al. A versatile hydrogel with antibacterial and sequential drug-releasing capability for the programmable healing of infectious keratitis. ACS Nano. (2023) 17:24055–69. doi: 10.1021/acsnano.3c09034
6. Rafat, M, Jabbarvand, M, Sharma, N, Xeroudaki, M, Tabe, S, Omrani, R, et al. Bioengineered corneal tissue for minimally invasive vision restoration in advanced keratoconus in two clinical cohorts. Nat Biotechnol. (2023) 41:70–81. doi: 10.1038/s41587-022-01408-w
7. Di Girolamo, N. Biologicals and biomaterials for corneal regeneration and vision restoration in limbal stem cell deficiency. Adv Mater. (2024) 36:2401763. doi: 10.1002/adma.202401763
8. Huang, J, Jiang, T, Qie, J, Cheng, X, Wang, Y, Ye, Y, et al. Biologically inspired bioactive hydrogels for scarless corneal repair. Sci Adv. (2024) 10:eadt1643. doi: 10.1126/sciadv.adt1643
9. Hori, K, Sotozono, C, Hamuro, J, Yamasaki, K, Kimura, Y, Ozeki, M, et al. Controlled-release of epidermal growth factor from cationized gelatin hydrogel enhances corneal epithelial wound healing. J Control Release. (2006) 118:169–76. doi: 10.1016/j.jconrel.2006.12.011
10. Sani, ES, Kheirkhah, A, Rana, D, Shirzaei Sani, E, Sun, Z, Foulsham, W, et al. Sutureless repair of corneal injuries using naturally derived bioadhesive hydrogels. Sci Adv. (2019) 5:eaav1281. doi: 10.1126/sciadv.aav1281
11. Sharifi, S, Islam, MM, Sharifi, H, Islam, R, Koza, D, Reyes-Ortega, F, et al. Tuning gelatin-based hydrogel towards bioadhesive ocular tissue engineering applications. Bioact Mater. (2021) 6:3947–61. doi: 10.1016/j.bioactmat.2021.03.042
12. Li, M, Wei, R, Liu, C, Fang, H, Yang, W, Wang, Y, et al. A “T.E.S.T.” hydrogel bioadhesive assisted by corneal cross-linking for in situ sutureless corneal repair. Bioact Mater. (2023) 25:333–46. doi: 10.1016/j.bioactmat.2023.02.006
13. Chen, F, Le, P, Fernandes-Cunha, GM, Heilshorn, SC, and Myung, D. Bioorthogonally crosslinked hyaluronatecollagen hydrogel for suture-free corneal defect repair. Biomaterials. (2020) 255:120176. doi: 10.1016/j.biomaterials.2020.120176
14. McTiernan, CD, Simpson, FC, Haagdorens, M, Samarawickrama, C, Hunter, D, Buznyk, O, et al. LiQD cornea: pro-regeneration collagen mimetics as patches and alternatives to corneal transplantation. Sci Adv. (2020) 6:eaba2187. doi: 10.1126/sciadv.aba2187
15. Feng, L, Liu, R, Zhang, X, Li, J, Zhu, L, Li, Z, et al. Thermo-gelling dendronized chitosans as biomimetic scaffolds for corneal tissue engineering. ACS Appl Mater Interfaces. (2021) 13:49369–79. doi: 10.1021/acsami.1c16087
16. Tang, Q, Lu, B, He, J, Chen, X, Fu, Q, Han, H, et al. Exosomes-loaded thermosensitive hydrogels for corneal epithelium and stroma regeneration. Biomaterials. (2022) 280:121320. doi: 10.1016/j.biomaterials.2021.121320
17. Lee, YP, Liu, HY, Lin, PC, Lee, YH, Yu, LR, Hsieh, CC, et al. Facile fabrication of superporous and biocompatible hydrogel scaffolds for artificial corneal periphery. Colloids Surf B Biointerfaces. (2019) 175:26–35. doi: 10.1016/j.colsurfb.2018.11.013
18. He, B, Wang, J, Xie, M, Xu, M, Zhang, Y, Hao, H, et al. 3D printed biomimetic epithelium/stroma bilayer hydrogel implant for corneal regeneration. Bioact Mater. (2022) 17:234–47. doi: 10.1016/j.bioactmat.2022.01.034
19. Fernandes-Cunha, GM, Jeong, SH, Logan, CM, Le, P, Mundy, D, Chen, F, et al. Supramolecular host-guest hyaluronic acid hydrogels enhance corneal wound healing through dynamic spatiotemporal effects. Ocul Surf. (2023) 23:148–61. doi: 10.1016/j.jtos.2021.09.002
20. Tsubota, K, Goto, E, Shimmura, S, and Shimazaki, J. Treatment of persistent corneal epithelial defect by autologous serum application. Ophthalmology. (1999) 106:1984–9. doi: 10.1016/S0161-6420(99)90412-8
21. Geerling, G, MacLennan, S, and Hartwig, D. Autologous serum eye drops for ocular surface disorders. Br J Ophthalmol. (2004) 88:1467–74. doi: 10.1136/bjo.2004.044347
22. Jeng, BH, and Dupps, WJ. Autologous serum 50% eyedrops in the treatment of persistent corneal epithelial defects. Cornea. (2009) 28:104–1108. doi: 10.1097/ICO.0b013e3181a2a7f6
23. López-Plandolit, S, Morales, MC, Freire, V, Etxebarria, J, and Duran, JA. Plasma rich in growth factors as a therapeutic agent for persistent corneal epithelial defects. Cornea. (2010) 29:843–8. doi: 10.1097/ICO.0b013e3181a81820
24. López-Plandolit, S, Morales, MC, Freire, V, Grau, AE, and Duran, JA. Efficacy of plasma rich in growth factors for the treatment of dry eye. Cornea. (2011) 30:1312. doi: 10.1097/ICO.0b013e31820d86d6
25. Etxebarria, J, Sanz-Lázaro, S, Hernáez-Moya, R, Freire, V, Durán, JA, Morales, MC, et al. Serum from plasma rich in growth factors regenerates rabbit corneas by promoting cell proliferation, migration, differentiation, adhesion and limbal stemness. Acta Ophthalmol. (2017) 95:e693–705. doi: 10.1111/aos.13371
26. Anitua, E, Muruzabal, F, Alcalde, I, Merayo-Lloves, J, and Orive, G. Plasma rich in growth factors (PRGF-Endoret) stimulates corneal wound healing and reduces haze formation after PRK surgery. Exp Eye Res. (2013) 115:153–61. doi: 10.1016/j.exer.2013.07.007
27. Freire, V, Andollo, N, Etxebarria, J, Durán, JA, and Morales, MC. In vitro effects of three blood derivatives on human corneal epithelial cells. Cornea. (2012) 53:5571–8. doi: 10.1167/iovs.11-7340
28. Sánchez-Ávila, RM, Merayo-Lloves, J, Fernández, ML, Rodríguez-Gutiérrez, LA, Rodríguez-Calvo, PP, Fernández-Vega, A, et al. Plasma rich in growth factors eye drops to treat secondary ocular surface disorders in patients with glaucoma. Int Med Case Rep J. (2018) 11:97–103. doi: 10.2147/IMCRJ.S153918
29. Sánchez-Ávila, RM, Merayo-Lloves, J, Fernández, ML, Rodríguez-Gutiérrez, LA, Jurado, N, Muruzabal, F, et al. Plasma rich in growth factors for the treatment of dry eye after LASIK surgery. Ophthalmic Res. (2018) 60:80–6. doi: 10.1159/000487951
30. Sánchez-Ávila, RM, Merayo-Lloves, J, Riestra, AC, Fernández-Vega, L, Anitua, E, Begoña, L, et al. Treatment of patients with neurotrophic keratitis stages 2 and 3 with plasma rich in growth factors (PRGF-Endoret) eye-drops. Int Ophthalmol. (2018) 38:1193–204. doi: 10.1007/s10792-017-0582-7
31. Sánchez-Ávila, RM, Uribe-Badillo, EE, Fernández-Vega, J, Muruzabal, F, Jurado, N, Alfonso-Bartolozzi, B, et al. Plasma rich in growth factors versus mitomycin c in photorefractive keratectomy. Medicine. (2021) 100:e24139. doi: 10.1097/MD.0000000000024139
32. Kubo, M, Sonoda, Y, Muramatsu, R, and Usui, M. Immunogenicity of human amniotic membrane in experimental xenotransplantation. Invest Ophthalmol Vis Sci. (2001) 42:1539–46.
33. Garfias, Y, Zaga-Clavellina, V, Vadillo-Ortega, F, Osorio, M, and Jiménez-Martínez, MC. Amniotic membrane is an immunosuppressor of peripheral blood mononuclear cells. Immunol Investig. (2011) 40:183–96. doi: 10.3109/08820139.2010.532266
34. Yazdanpanah, G, Shen, X, Nguyen, T, Anwar, KN, Jeon, O, Jiang, Y, et al. A light-curable and tunable extracellular matrix hydrogel for in situ suture-free corneal repair. Adv Funct Mater. (2022) 32:2113383. doi: 10.1002/adfm.202113383
35. Niknejad, H, Yazdanpanah, G, and Ahmadiani, A. Induction of apoptosis, stimulation of cell-cycle arrest and inhibition of angiogenesis make human amnion-derived cells promising sources for cell therapy of cancer. Cell Tissue Res. (2016) 363:599–608. doi: 10.1007/s00441-016-2364-3
36. Kakavand, M, Yazdanpanah, G, Ahmadiani, A, and Niknejad, H. Blood compatibility of human amniotic membrane compared with heparin-coated ePTFE for vascular tissue engineering. J Tissue Eng Regen Med. (2017) 11:1701–9. doi: 10.1002/term.2064
37. Basasoro, A, Mendicute, J, Rezola, M, Burgos, J, Fernández, M, Esporrín-Ubieto, D, et al. The influence of amniotic membrane proteins on corneal regeneration when delivered directly or using hydrogel platforms. Front Med Lausanne. (2025) 12:1498319. doi: 10.3389/fmed.2025.1498319
38. Zhou, C, Robert, M, Kapoulea, V, Robert, MC, Lei, F, Stagner, AM, et al. Sustained subconjunctival delivery of infliximab protects the cornea and retina following alkali burn to the eye. Invest Ophthalmol Vis Sci. (2017) 58:96–105. doi: 10.1167/iovs.16-20339
39. Ferrari, G, Bignami, F, Giacomini, C, Franchini, S, and Rama, P. Safety and efficacy of topical infliximab in a mouse model of ocular surface scarring. Invest Ophthalmol Vis Sci. (2013) 54:1680–8. doi: 10.1167/iovs.12-10782
40. Li, Z, Choi, W, Oh, HJ, and Yoon, KC. Effectiveness of topical infliximab in a mouse model of experimental dry eye. Cornea. (2012) 31:25–31. doi: 10.1097/ICO.0b013e31826a80ea
41. Kim, JW, and Chung, SK. The effect of topical infliximab on corneal neovascularization in rabbits. Cornea. (2013) 32:185–90. doi: 10.1097/ICO.0b013e318271cc2a
42. Robert, MC. Topical infliximab for sterile corneal melt; ClinicalTrials.Gov identifier: NCT02987686. ClinicalTrials.Gov. (2024). Available online at: https://clinicaltrials.gov/study/NCT02987686 (Acessed February 17, 2025).
43. Romo-Valera, C, Appel, EA, Etxebarria, J, Arluzea, J, and Andollo, N. In vitro evaluation of gelatin-based hydrogels as potential fillers for corneal wounds. Biomacromolecules. (2025) 26:3344–55. doi: 10.1021/acs.biomac.4c01759
44. Kay, J, and Calandra, J. Interpretation of eye irritation tests. J Soc Cosmet Sci. (1962) 13:281–9.
45. Na, KS, Fernandes-Cunha, GM, Varela, IB, Lee, HJ, Seo, YA, and Myung, D. Effect of mesenchymal stromal cells encapsulated within polyethylene glycol-collagen hydrogels formed in situ on alkali-burned corneas in an ex vivo organ culture model. Cytotherapy. (2021) 23:500–9. doi: 10.1016/j.jcyt.2021.02.001
46. Chun, YH, Park, SK, Kim, EJ, Lee, HJ, Kim, H, Koh, WG, et al. In vivo biocompatibility evaluation of in situ-forming polyethylene glycol-collagen hydrogels in corneal defects. Sci Rep. (2021) 11:23913. doi: 10.1038/s41598-021-03270-3
47. Zhang, W, Lan, X, Zhu, J, Zhang, C, Huang, Y, Mo, K, et al. Healing ability of central corneal epithelium in rabbit ocular surface injury models. Transl Vis Sci Technol. (2022) 11:28. doi: 10.1167/tvst.11.6.28
48. Hwang, ES, Schallhorn, JM, and Randleman, JB. Utility of regional epithelial thickness measurements in corneal evaluations. Surv Ophthalmol. (2020) 65:187–204. doi: 10.1016/j.survophthal.2019.09.003
49. Liu, L, Hartwig, D, Harloff, S, Herminghaus, P, Wedel, T, Kasper, K, et al. Corneal epitheliotrophic capacity of three different blood-derived preparations. Invest Ophthalmol Vis Sci. (2006) 47:2438–44. doi: 10.1167/iovs.05-0876
50. Akyol-Salman, I. Effects of autologous serum eye drops on corneal wound healing after superficial keratectomy in rabbits. Cornea. (2006) 25:1178–81. doi: 10.1097/01.ico.0000208817.40237.8c
51. Kiristioglu, MO, Baykara, M, Yavas, O, Kupeli, ZA, and Ozyigit, MO. The effect of platelet-rich plasma and sodium alginate hydrogel on corneal wound healing after corneal alkali burns in rats with computer-assisted anterior segment optical coherence tomography image analysis. Exp Eye Res. (2024) 247:110044. doi: 10.1016/j.exer.2024.110044
52. Suárez-Barrio, C, Etxebarria, J, Hernáez-Moya, R, del Val-Alonso, M, Rodriguez-Astigarraga, M, Urkaregi, A, et al. Hyaluronic acid combined with serum rich in growth factors in corneal epithelial defects. Int J Mol Sci. (2019) 20:1655. doi: 10.3390/ijms20071655
53. Choi, JA, Jin, HJ, Jung, S, Yang, E, Choi, JS, Chung, SH, et al. Effects of amniotic membrane suspension in human corneal wound healing in vitro. Mol Vis. (2009) 5:2230–8.
54. Dudok, DV, Nagdee, I, Cheung, K, Liu, H, Vedovelli, L, Ghinelli, E, et al. Effects of amniotic membrane extract on primary human corneal epithelial and limbal cells. Clin Experiment Ophthalmol. (2015) 43:443–8. doi: 10.1111/ceo.12480
55. Hu, S, Wang, Z, Jin, C, Chen, Q, Fang, Y, Jin, J, et al. Human amniotic epithelial cell-derived extracellular vesicles provide an extracellular matrix-based microenvironment for corneal injury repair. J Tissue Eng. (2022) 13:20417314221122123. doi: 10.1177/20417314221122123
56. Uchida, M, Kamoi, K, Ando, N, Wei, C, Karube, H, Ohno-Matsui, K, et al. Safety of infliximab for the eye under human T-cell leukemia virus type 1 infectious conditions in vitro. Front Microbiol. (2019) 18:2148. doi: 10.3389/fmicb.2019.02148
57. Duijvestein, M, Molendijk, I, Roelofs, H, Vos, ACW, Verhaar, AP, Reinders, MEJ, et al. Mesenchymal stromal cell function is not affected by drugs used in the treatment of inflammatory bowel disease. Cytotherapy. (2011) 13:1066–73. doi: 10.3109/14653249.2011.597379
58. Movahedan, A, Afsharkhamseh, N, Sagha, HM, Shah, JR, Milani, BY, Milani, FY, et al. Loss of Notch1 disrupts the barrier repair in the corneal epithelium. PLoS One. (2013) 8:113. doi: 10.1371/journal.pone.0069113
59. An, S, Anwar, K, Ashraf, M, Lee, H, Jung, R, Koganti, R, et al. Wound-healing effects of mesenchymal stromal cell Secretome in the cornea and the role of exosomes. Pharmaceutics. (2023) 15:1486. doi: 10.3390/pharmaceutics15051486
60. Lauweryns, B, den Oord, JJV, Volpes, R, Foets, B, and Missotten, L. Distribution of very late activation integrins in the human cornea: an immunohistochemical study using monoclonal antibodies. Invest Ophthalmol Vis Sci. (1991) 32:2079–85.
61. Secker, GA, and Daniels, JT. Limbal epithelial stem cells of the cornea In: Lisa Girard, editor. Harvard University, Massachusetts, USA: Harvard Stem Cell Institute. StemBook : The Stem Cell Research Community (2009).
62. Schlötzer-Schrehardt, U, and Kruse, FE. Identification and characterization of limbal stem cells. Exp Eye Res. (2005) 81:247–64. doi: 10.1016/j.exer.2005.02.016
63. Li, Y, Giovannini, S, Wang, T, Fang, J, Li, P, Shao, C, et al. p63: a crucial player in epithelial stemness regulation. Oncogene. (2023) 42:3371–84. doi: 10.1038/s41388-023-02859-4
64. Di Iorio, E, Barbaro, V, Ruzza, A, Ponzin, D, Pellegrini, G, and De Luca, M. Isoforms of DeltaNp63 and the migration of ocular limbal cells in human corneal regeneration. Proc Natl Acad Sci USA. (2005) 102:9523–8. doi: 10.1073/pnas.0503437102
65. Li, H, Usas, A, Poddar, M, Chen, C-W, Thompson, S, Ahani, B, et al. Platelet-rich plasma promotes the proliferation of human muscle derived progenitor cells and maintains their stemness. PLoS One. (2013) 8:e64923. doi: 10.1371/journal.pone.0064923
66. Andia, I, and Abate, M. Platelet-rich plasma in the treatment of skeletal muscle injuries. Expert Opin Biol Ther. (2015) 15:987–99. doi: 10.1517/14712598.2015.1038234
67. Asl, NS, Nejat, F, Mohammadi, P, Nekoukar, A, Hesam, S, Ebrahimi, M, et al. Amniotic membrane extract eye drop promotes limbal stem cell proliferation and corneal epithelium healing. Cell J. (2019) 20:459–68. doi: 10.22074/cellj.2019.5423
68. Baradaran-Rafii, A, Asl, NS, Ebrahimi, M, Jabbehdari, S, Bamdad, S, Roshandel, D, et al. The role of amniotic membrane extract eye drop (AMEED) in in vivo cultivation of limbal stem cells. Ocul Surf. (2018) 16:146–53. doi: 10.1016/j.jtos.2017.11.001
69. Lai, JY, and Luo, LJ. Effect of riboflavin concentration on the development of photo-crosslinked amniotic membranes for cultivation of limbal epithelial cells. RSC Adv. (2015) 5:3425–34. doi: 10.1039/C4RA11980K
70. Polisetti, N, Schlunck, G, and Reinhard, T. PAX6 expression patterns in the adult human Limbal stem cell niche. Cells. (2023) 12:400. doi: 10.3390/cells12030400
71. Chen, SY, Cheng, AMS, Zhang, Y, Zhu, YT, He, H, Mahabole, M, et al. Pax 6 controls neural crest potential of Limbal niche cells to support self-renewal of Limbal epithelial stem cells. Sci Rep. (2019) 9:9763. doi: 10.1038/s41598-019-45100-7
72. Williams, K, Motiani, K, Giridhar, PV, and Kasper, S. CD44 integrates signaling in normal stem cell, cancer stem cell and (pre)metastatic niches. Exp Biol Med. (2013) 238:324–38. doi: 10.1177/1535370213480714
73. Li, S, Sun, H, Chen, L, and Fu, Y. Targeting limbal epithelial stem cells: master conductors of corneal epithelial regeneration from the bench to multilevel theranostics. J Transl Med. (2024) 22:794. doi: 10.1186/s12967-024-05603-y
74. Knudson, W, and Peterson, RS. Chapter 5-the Hyaluronan receptor: CD44 In: Hari G. Garg and Charles A. Hales, editors. Massachusetts USA: Harvard Medical School, Pulmonary and Critical Care Unit. Chemistry and biology of Hyaluronan (2004). 83–123.
75. Zhu, SN. Expression of adhesion molecule CD44 on human corneas. Br J Ophthalmol. (1997) 81:80–4. doi: 10.1136/bjo.81.1.80
76. Zhong, J, Deng, Y, Tian, B, Wang, B, Sun, Y, Huang, H, et al. Hyaluronate acid-dependent protection and enhanced corneal wound healing against oxidative damage in corneal epithelial cells. J Ophthalmol. (2016) 2016:1–10. doi: 10.1155/2016/6538051
77. Gomes, JA, Amankwah, R, Powell-Richards, A, and Dua, HS. Sodium hyaluronate (hyaluronic acid) promotes migration of human corneal epithelial cells in vitro. Br J Ophthalmol. (2004) 88:821–5. doi: 10.1136/bjo.2003.027573
78. Chen, Y, Dong, L, Kong, B, Huang, Y, Zhong, S, Connon, C, et al. Effects of gelatin methacrylate hydrogel on corneal repair and regeneration in rats. Transl Vis Sci Technol. (2021) 10:25. doi: 10.1167/tvst.10.14.25
79. Ibañez, RI, Do Amaral, RJFC, Reis, RL, Marques, AP, Murphy, CM, O'Brien, FJ, et al. 3D-printed gelatin methacrylate scaffolds with controlled architecture and stiffness modulate the fibroblast phenotype towards dermal regeneration. Polymers. (2021) 13:2510. doi: 10.3390/polym13152510
80. Saika, S, Shirai, K, Yamanaka, O, Miyazaki, K, Okada, Y, Kitano, A, et al. Loss of osteopontin perturbs the epithelial-mesenchymal transition in an injured mouse lens epithelium. Lab Investig. (2007) 87:130–8. doi: 10.1038/labinvest.3700508
81. Zheng, D, Song, T, Zhongliu, X, Wu, M, Liang, J, Liu, Y, et al. Downregulation of transforming growth factor-β type II receptor prohibit epithelial-to-mesenchymal transition in lens epithelium. Mol Vis. (2012) 18:1238–46.
82. Guo, R, Meng, Q, Guo, H, Xiao, L, Yang, X, Cui, Y, et al. TGF-β2 induces epithelial-mesenchymal transition in cultured human lens epithelial cells through activation of the PI3K/Akt/mTOR signaling pathway. Mol Med Rep. (2016) 13:1105–10. doi: 10.3892/mmr.2015.4645
83. Kwok, SS, Shih, KC, Bu, Y, Lo, AC, Chan, TC, Lai, JS, et al. Systematic review on therapeutic strategies to minimize corneal stromal scarring after injury. Eye Contact Lens. (2019) 45:347–55. doi: 10.1097/ICL.0000000000000584
84. Gaballa, SA, Kompella, UB, Elgarhy, O, Alqahtani, AM, Pierscionek, B, Alany, RG, et al. Corticosteroids in ophthalmology: drug delivery innovations, pharmacology, clinical applications, and future perspectives. Drug Deliv Transl Res. (2021) 11:866–93. doi: 10.1007/s13346-020-00843-z
85. Aung, YY, and McLeod, A. Contact lens management of irregular corneas after traumatic aphakia: a pediatric case series. Contact Lens Anterior Eye. (2015) 38:382–8. doi: 10.1016/j.clae.2015.03.015
86. Maharana, P, Sahay, P, Singhal, D, Garg, I, Titiyal, JS, and Sharma, N. Component corneal surgery: An update. Indian J Ophthalmol. (2017) 65:658–72. doi: 10.4103/ijo.IJO_582_17
87. Chameettachal, S, Prasad, D, Parekh, Y, Basu, S, Singh, V, Bokara, KK, et al. Prevention of corneal myofibroblastic differentiation in vitro using a biomimetic ECM hydrogel for corneal tissue regeneration. ACS Appl Bio Mater. (2021) 4:533–44. doi: 10.1021/acsabm.0c01112
88. Chameettachal, S, Venuganti, A, Parekh, Y, Prasad, D, Joshi, VP, Vashishtha, A, et al. Human cornea-derived extracellular matrix hydrogel for prevention of post-traumatic corneal scarring: a translational approach. Acta Biomater. (2023) 171:289–307. doi: 10.1016/j.actbio.2023.09.002
89. Liu, Y, and Hong, J. Msc-laden in situ–forming hydrogel for preventing corneal stromal opacity. Cornea. (2024) 43:609–26. doi: 10.1097/ICO.0000000000003475
90. Huang, J, Jiang, T, Li, J, Qie, J, Cheng, X, Wang, Y, et al. Biomimetic corneal stroma for Scarless corneal wound healing via structural restoration and microenvironment modulation. Adv Healthc Mater. (2024) 13:e2302889. doi: 10.1002/adhm.202302889
91. Murri, MS, Moshirfar, M, Birdsong, OC, Ronquillo, YC, Ding, Y, and Hoopes, PC. Amniotic membrane extract and eye drops: a review of literature and clinical application. Clin Ophthalmol. (2018) 12:1105–12. doi: 10.2147/OPTH.S165553
92. Kamiya, K, Wang, M, Uchida, S, Amano, S, Oshika, T, Sakuragawa, N, et al. Topical application of culture supernatant from human amniotic epithelial cells suppresses inflammatory reactions in cornea. Exp Eye Res. (2005) 80:671–9. doi: 10.1016/j.exer.2004.11.018
93. Tseng, SCG. HC-HA/PTX3 purified from amniotic membrane as novel regenerative matrix: insight into relationship between inflammation and regeneration. Invest Ophthalmol Vis Sci. (2016) 57:ORSFh1-8. doi: 10.1167/iovs.15-17637
94. Celebi, ARC, Ulusoy, C, and Mirza, GE. The efficacy of autologous serum eye drops for severe dry eye syndrome: a randomized double-blind crossover study. Graefes Arch Clin Exp Ophthalmol. (2014) 252:619–26. doi: 10.1007/s00417-014-2599-1
95. Ali, TK, Gibbons, A, Cartes, C, Zarei-Ghanavati, S, Gomaa, M, Gonzalez, I, et al. Use of autologous serum tears for the treatment of ocular surface disease from patients with systemic autoimmune diseases. Am J Ophthalmol. (2018) 189:65–70. doi: 10.1016/j.ajo.2018.02.009
96. Shtein, RM, Shen, JF, Kuo, AN, Hammersmith, KM, Li, JY, and Weikert, MP. Autologous serum-based eye drops for treatment of ocular surface disease: a report by the American Academy of ophthalmology. Ophthalmology. (2020) 127:128–33. doi: 10.1016/j.ophtha.2019.08.018
97. Alio, JL, Colecha, JR, Pastor, S, Rodriguez, A, and Artola, A. Symptomatic dry eye treatment with autologous platelet-rich plasma. Ophthalmic Res. (2007) 39:124–9. doi: 10.1159/000100933
98. Alio, JL, Rodriguez, AE, Ferreira-Oliveira, R, Wróbel-Dudzińska, D, and Abdelghany, AA. Treatment of dry eye disease with autologous platelet-rich plasma: a prospective, interventional, non-randomized study. Ophthalmol Ther. (2017) 6:285–93. doi: 10.1007/s40123-017-0100-z
99. Garcia-Conca, V, Abad-Collado, M, Hueso-Abancens, JR, Mengual-Verdú, E, Piñero, DP, Aguirre-Balsalobre, F, et al. Efficacy and safety of treatment of hyposecretory dry eye with platelet-rich plasma. Acta Ophthalmol. (2019) 97:e170–8. doi: 10.1111/aos.13907
100. Oranskiy, SP, Yeliseyeva, LN, Tsanaeva, AV, and Zaytseva, NV. Body composition and serum levels of adiponectin, vascular endothelial growth factor, and interleukin-6 in patients with rheumatoid arthritis. Croat Med J. (2012) 53:350–6. doi: 10.3325/cmj.2012.53.350
101. Hwang, J, Chung, SH, Jeon, S, Kwok, SK, Park, SH, and Kim, MS. Comparison of clinical efficacies of autologous serum eye drops in patients with primary and secondary Sjögren syndrome. Cornea. (2014) 33:663–7. doi: 10.1097/ICO.0000000000000147
Keywords: gelatin, riboflavin-phosphate, functionalized-hydrogels, corneal wound healing, persistent epithelial defects, corneal regeneration
Citation: Romo-Valera C, Etxebarria J, Freire V, Rodriguez-Astigarraga M, Arluzea J and Andollo N (2025) Comparison of the regenerative potential of different functionalized gelatin-based hydrogels as fillers of rabbit corneal wounds. Front. Med. 12:1667446. doi: 10.3389/fmed.2025.1667446
Edited by:
Weihua Yang, Southern Medical University, ChinaReviewed by:
Yi Dong, Tianjin Eye Hospital, ChinaMarcela Aldrovani Rodrigues, University of Franca, Brazil
Jiaoman Wang, Jinan University, China
Copyright © 2025 Romo-Valera, Etxebarria, Freire, Rodriguez-Astigarraga, Arluzea and Andollo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Noelia Andollo, bm9lbGlhLmFuZG9sbG9AZWh1LmV1cw==
 Cristina Romo-Valera
Cristina Romo-Valera Jaime Etxebarria1,2,3
Jaime Etxebarria1,2,3