- 1Chongqing Medical University, Chongqing, China
- 2Department of Pathology, The Chongqing General Hospital, Chongqing, China
Background: Idiopathic myointimal hyperplasia of the mesenteric veins (IMHMV) is a rare and poorly understood disease, typically affecting the rectosigmoid colon of young, otherwise healthy men. Clinically, it is often mistaken for inflammatory bowel disease, as biopsies show ischemic mucosal changes without classic inflammatory features. Surgical resection is both diagnostic and curative, although the etiology of IMHMV remains unclear.
Case presentation: We report the case of a female patient with IMHMV involving the right hemicolon, concomitant with Clostridium difficile infection. Her symptoms persisted despite targeted treatment for C. difficile. She subsequently underwent a laparoscopic right hemicolectomy, which revealed mesenteric vein occlusion due to myointimal hyperplasia, confirmed by elastin staining and desmin immunohistochemistry. Histopathological examination established the diagnosis of IMHMV. The patient recovered well postoperatively, with no recurrence observed during follow-up.
Conclusion: This is the first documented case of IMHMV involving the right hemicolon and complicated by Clostridium difficile infection. In addition, we reviewed 82 previously reported cases from 1991 to 2024, highlighting the clinical, imaging, and pathological characteristics of IMHMV. Recognition of this rare entity is essential to avoid unnecessary pharmacotherapy, prevent misdiagnosis as inflammatory bowel disease, and facilitate timely surgical management.
1 Introduction
Idiopathic Myointimal Hyperplasia of the Mesenteric Veins (IMHMV) is a rare and poorly understood condition that presents a diagnostic challenge for clinicians and pathologists. It is classified as an ischemic bowel disease characterized by venous occlusion due to smooth muscle proliferation in the tunica intima of the mesenteric veins, without the presence of a thrombus (1). IMHMV is often misdiagnosed as inflammatory bowel disease (IBD) (Supplementary Table S1), as definitive diagnosis of bowel ischemia and venous thrombotic disease relies on pathological changes that are not distinguishable through preoperative radiological or clinical assessments. Consequently, a definitive diagnosis can only be achieved postoperatively, since biopsies are unable to differentiate ischemic abnormalities from those associated with known IBD manifestations (2). Since the first case was reported in the United States in 1991, approximately 81 cases of IMHMV have been documented in the literature, with the present case representing the 82nd reported instance. Majority of these cases ultimately end up with different degrees of bowel resection (3).
Idiopathic myointimal hyperplasia of the mesenteric veins mostly involves the thickening of small and medium-sized mesenteric veins with the hallmark manifestation of intimal smooth muscle proliferation resulting in luminal occlusion and mucosal ischemic changes (2). Pathologists may miss the diagnosis unless elastin stains are performed, as the affected veins can easily be mistaken for arteries. The etiology of IMHMV remains unclear. One hypothesis suggests that the disease may result from an arteriovenous fistula. Another Hypothesis proposes that IMHMV represents the end stage of ‘phlebitis,’ as lesions resembling IMHMV have been observed in cases of lymphocytic, granulomatous, and necrotizing phlebitis (4, 5).
We herein describe the case of a female patient with idiopathic myointimal hyperplasia of the mesenteric veins (IMHMV) at right colon with Clostridium difficile infection (CDI).
2 Case presentation
A 68-year-old female patient, with a history of chronic abdominal pain, was admitted to the hospital with chief complaint of severe abdominal pain for the past 2 months. The abdominal pain initially started in the left hypochondrium as a vague discomfort, but later migrated and localized as a persistent distending pain in the lower abdomen, accompanied by vomiting, bloody stools, and low-grade fever. There was history of loss of appetite. She has consumed only porridge in the past 2 months. There was no history of joint pain, skin lesions, chest pain, cough, or contact with contaminated water. One month prior, she was admitted with a diagnosis of hypertensive heart disease, grade 3 hypertension, abnormal coagulation function, ischemic enteropathy, and chronic atrophic gastritis. She has previously undergone resection of a lung abscess. She self-reports an allergy to penicillins and abdominal pain after taking aspirin. No significant family history.
The physical examination revealed tenderness in the upper middle abdomen, left flank, and lower abdomen, more prominent in the upper middle and lower abdomen. No rebound tenderness or palpable masses were noted. Normal bowel sounds. The following Laboratory findings were reported: CRP = 20.51 mg/L, Hg = 106 g/L, D-Dimer = 0.78 mg/L, VIII factor activity = 267.90%, TP = 63.7 g/L, ALB = 31.8 g/L, positive fecal occult blood test, and Clostridium difficile antigen test.
A colonoscopy revealed congested and inflamed mucosa in the colon (Figures 1D–I). Salt and pepper appearance was seen in the region. The transverse colon exhibited a whitish appearance with loss of vasculature. Biopsy findings revealed a non-specific ulcer.
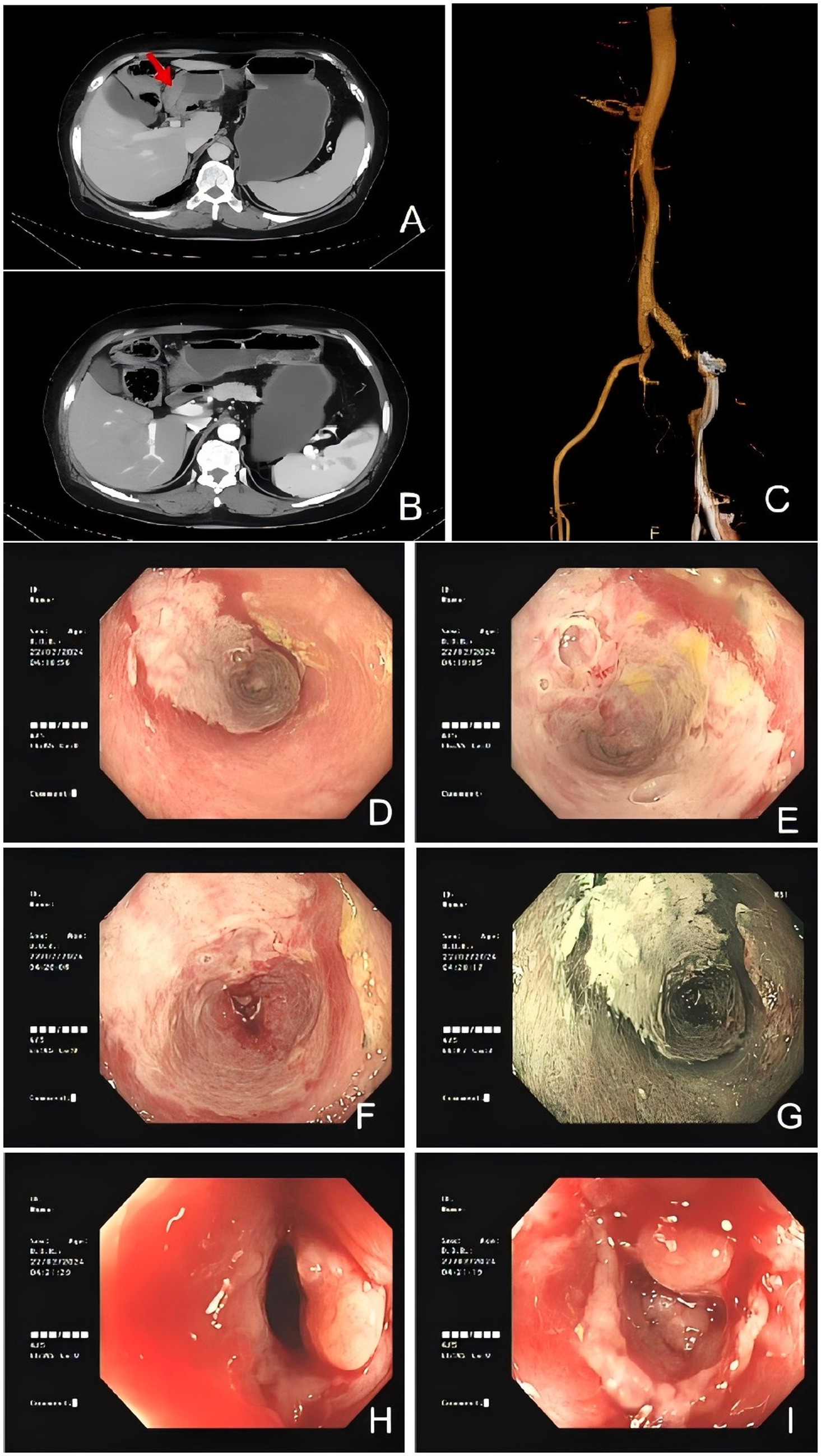
Figure 1. Colonic imaging and computed tomography suggested IMHMV. (A,B) Axial CT images showing thickened colonic wall with edema (red arrowhead) and no mesenteric artery stenosis. (C) Abdominal CTA revealed mixed plaques at the origin of the abdominal aorta and bilateral common iliac arteries, suggesting the possibility of mesenteric microthrombosis. Flexible sigmoidoscopy images demonstrated severely inflamed mucosa (D–J) with significant luminal narrowing (H,I).
Computed tomography angiography (CTA) of the abdomen showed mixed plaques in the abdominal aorta and at the origin of the bilateral common iliac arteries (Figure 1C). Computed tomography (CT) scan of the abdomen results showed a swollen colon wall with diminished enhancement and surrounding exudate that was suggestive of ischemic bowel disease (Figures 1A,B). Based on the presentation and investigative findings, she was diagnosed with a suspected case of ischemic bowel disease and was started on scopolamine hydrobromide for spasm and pain relief, fasting, omeprazole for gastric protection, and atorvastatin calcium tablets to stabilize plaques. The patient’s abdominal pain slightly subsided, but intermittent abdominal pain persisted, primarily around the navel, with slight relief after activity or oxygen inhalation. The stools were yellow and pasty. Papaverine was additionally used to relieve spasm and improve intestinal blood supply, while mesalazine was administered to repair the intestinal mucosa. Metronidazole tablets were added to treat the infection, but the patient experienced nausea and acid reflux after administration. Additionally, no pseudomembranous enteritis was found in the colonoscopy, and there was no mucus or pseudomembranes in the stool. Metronidazole was discontinued after 1 week. A conservative management approach was followed, but the symptoms did not resolve. A decision was made to proceed with a laparoscopic right hemicolectomy.
Postoperative pathology revealed the removal of 1 cm of ileum and 48 cm of colon. A local intestinal stricture was present 41 cm from the ileal resection margin, with a length of approximately 2 cm. The intestinal mucosa at this site was grayish-brown with granular hyperplasia. An ulcer was observed 28 cm from the ileocecal valve, adjacent to the stricture, measuring 10 × 3 cm. At this site, the intestinal mucosal folds were absent and the intestinal wall was hardened. The remaining proximal colon exhibited multiple scattered ulcers with a maximum diameter of 1–3 cm. The mesenteric fat at the lesion site was hyperplastic and encircled the intestinal wall, with the stricture being most prominent. Three lymph nodes were found around the intestine, with a maximum diameter of 0.2–0.3 cm (Figure 2).
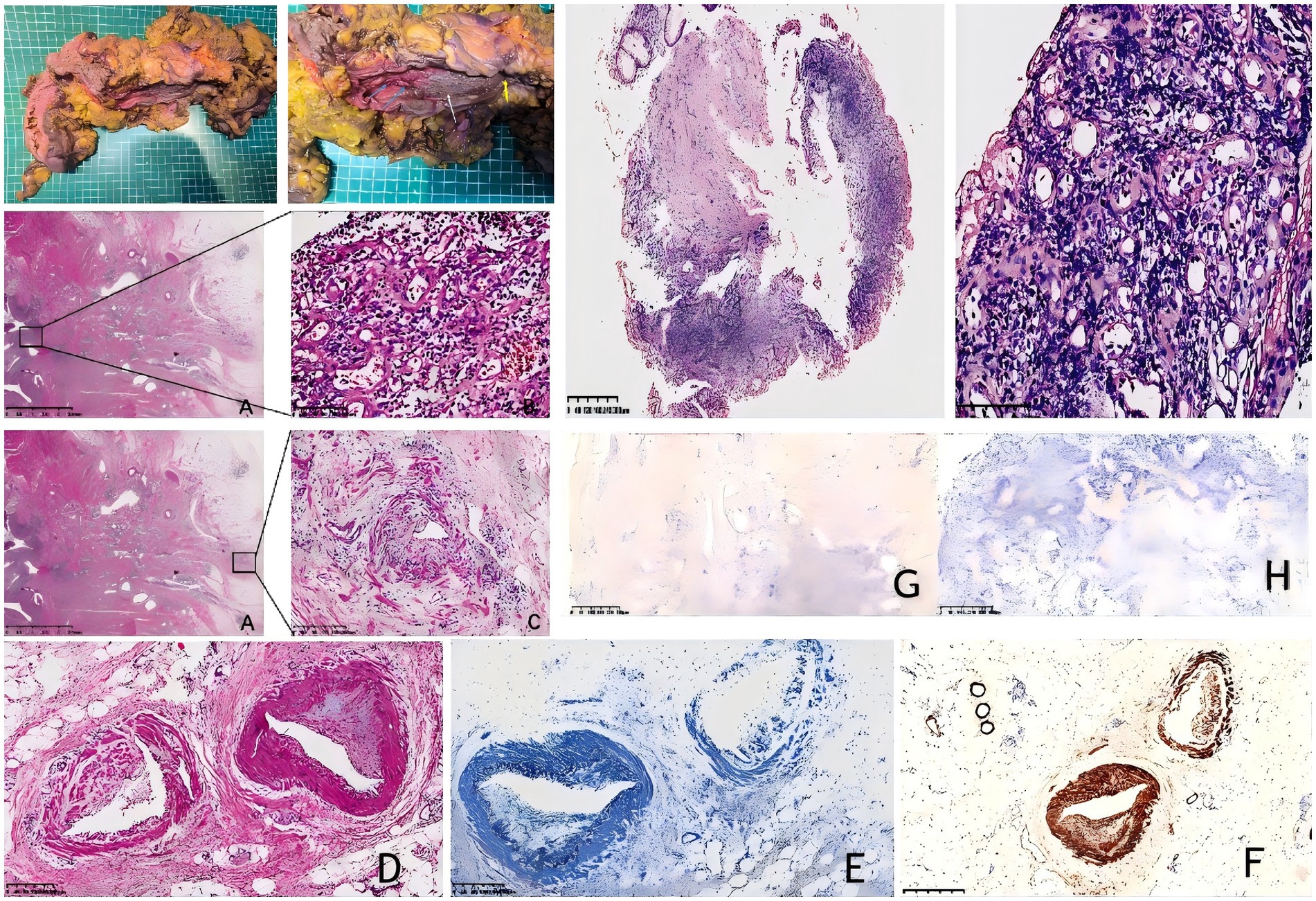
Figure 2. (A–D) Hematoxylin–eosin (H&E) staining of the resected surgical specimen (original magnification ×200); (E) elastic fiber staining (200x magnification); (F) immunostaining for smooth muscle actin shows the endoluminal nature of the venous proliferation. (200x magnification); (G) EBER in situ hybridization (ISH) test. (200x magnification); (H) CMV staining is negative. (I&J) Surgical resection specimen from IMHMV. Large ulcer (white arrow); scattered ulcers (blue arrow); stenosis (yellow arrow); (K–M) Hematoxylin–eosin (H&E) staining of the mucosal biopsy (original magnification ×200) shows ischemic-type changes, including epithelial sloughing, ulceration, and atrophy, with dense neutrophilic infiltration and prominent capillary proliferation but without perivascular hyalinization. The muscularis mucosae is thickened with associated fibrosis.
2.1 Integrated histopathology description
Histopathological examination revealed that the intestinal mucosa and submucosa were replaced by fibrous tissue, with prominent proliferation of small- to medium-sized veins, some extending from the lamina propria to the serosa. The mucosal villi were absent at the stenotic segments, while the crypt architecture was largely preserved, without significant distortion or branching. Inflammatory infiltrates were present but limited, predominantly composed of neutrophils, with minimal lymphoplasmacytic component. Notably, there was no basal plasmacytosis, crypt abscesses, or Paneth cell metaplasia, features that typically indicate chronic inflammatory bowel disease, supporting the exclusion of IBD. The hyperplastic veins showed extensive intimal proliferation, resulting in slit-like lumina, with thickened walls and protruding endothelial cells. Capillaries in the affected areas also exhibited slit-like lumina and thickened walls. No histopathological features characteristic of Clostridium difficile infection, such as pseudomembranes, mucosal necrosis, or prominent exudates, were observed.
Immunohistochemical staining with Desmin confirmed that the smooth muscle in the venous intima was proliferating in a semicircular pattern. Concurrently, Verhoeff-Van Gieson (VVG) elastic fiber staining demonstrated noticeable intimal thickening in the arteriole-type vessels. No vasculitis was present; EBER RNA and CMV expression were negative, excluding EBV colitis and CMV colitis (Figure 2). No evidence of thrombosis or malignancy was observed. The final diagnosis was idiopathic myointimal hyperplasia of the mesenteric veins (IMHMV). Follow-up over 1 year after surgery, she was doing well, without issue.
We reviewed the patient’s previous biopsy specimens and noted dense neutrophilic infiltration of the mucosal layer with prominent capillary proliferation but without perivascular hyalinization. The muscularis mucosae was thickened with associated fibrosis and lacked features of chronic active inflammation, such as lymphoplasmacytic infiltrates, basement membrane disruption, or crypt abscesses. Although these findings were not diagnostic, they did not support inflammatory bowel disease or Clostridium difficile infection (Figures 2K–M).
3 Discussion
Idiopathic myointimal hyperplasia is a rare cause of intestinal ischemia. Compared to other inflammatory conditions of the gastrointestinal tract, IMHMV is not caused by arterial thromboembolism, venous thrombus or vasculitis, and its etiology remains poorly understood.
The most common symptoms reported in previous cases include abdominal pain, perforation, and hematochezia (Supplementary Table S1). The patient was admitted with chief complaint of severe abdominal pain. Patients with C. difficile enteritis generally present with diarrhea, crampy abdominal pain, and leukocytosis (6). Given that the patient presented with Clostridium difficile infection (CDI) and the clinical manifestations of IMHMV and pseudomembranous colitis caused by CDI were similar, the histological findings were distinctly different. The former often results in mucosal changes due to non-specific ischemic injury, rather than a toxin-mediated inflammatory process, with the absence of pseudomembrane formation and interstitial necrosis (7). CDI may lead to microvascular dysfunction and thrombosis of the superior mesenteric artery (8, 9) and affect the development of ischemic bowel disease (10). Ischemic colitis may be a complication of CDI (11). As a rare cause of ischemic bowel disease, CDI may offer insight into the pathogenesis of IMHMV. This case is notable as the first reported instance of IMHMV combined with CDI. CT imaging of our patient revealed focal colonic wall thickening and submucosal edema with preserved mesenteric arterial patency. However, the characteristic radiographic findings of IMHMV described in previous reports—enlarged and tortuous pericolonic vessels with rich, dilated peripheral veins—were not observed in this case (Figure 1). This absence may reflect the early stage and limited extent of venous involvement in our patient, as well as differences in imaging protocol compared with prior studies (Table 1).
Our patient has hypertension and hypertensive heart disease, consistent with previous reports noting cardiovascular risk factors in many IMHMV cases (4, 12–14). IMHMV is characterized by myointimal hyperplasia leading to mesenteric vein occlusion. Experimental studies have shown that elevated blood pressure can induce intimal thickening and endothelial changes in small vessels (15–26), which may suggest a potential role of hypertension in the pathogenesis of IMHMV. Further research is warranted to clarify this association.
The absence of concrete histopathological criteria for a definitive diagnosis of IMHMV makes biopsy-based diagnosis challenging for pathologists, leading to the early initiation of treatment with anti-inflammatory drugs rather than surgery, which, at present, remains the only effective treatment for this condition and has been reported to be completely curative. However, only six cases of IMHMV have been diagnosed preoperatively (27–29). Arteriolized capillary, subendothelial fibrin deposits, and perivascular hyalinization are the most recent specific pathological features that facilitate the identification of IMHMV in mucosal biopsy samples (1). Biopsy specimens from this patient were reviewed to summarize findings indicating the absence of the muscular layer in the intestinal wall, a gap-like appearance of the intestinal wall due to capillary hyperplasia, thickening of the wall, and prominent endothelial cells. Notably, while the mesenteric vein exhibited characteristic smooth muscle proliferation in the intima (consistent with IMHMV pathology), the arterial changes presented a diagnostic paradox: although these vessels retained their intimal architecture, significant non-myointimal thickening was observed, which differed fundamentally from the venous pathology. we describe these as vessels with arterial-type wall thickening of uncertain significance, confirmed by immunohistochemistry to be non-myointimal in nature (Figure 2). This histologic feature has not been documented in prior IMHMV cases and warrants particular attention, as it may represent (1) a previously unrecognized disease variant, (2) concurrent vascular pathology, or (3) a broader disease spectrum or secondary vascular remodeling, given that small-vessel involvement of this type has not been reported in the existing literature. The presence of definitive venous pathology meets the current diagnostic criteria for IMHMV, while the observed alterations in other small vessels may indicate secondary vascular remodeling, a coexisting process, or a previously unrecognized disease spectrum that requires further investigation.
Currently, all cases are diagnosed based on pathological results, and the lack of methods for early diagnosis means that the diagnosis of IMHMV is often delayed. The clinical manifestations and endoscopic findings are nonspecific and closely resemble those of IBD and IMP. Similar imaging manifestations have also been observed, with IMP showing more similarity in this regard. Below, we summarize the differential diagnosis of IMHMV, IBD and IMP (Table 2). In particular, IBD, with nearly 53.1% of IMHMV cases being diagnosed as IBD before surgery (Supplementary Table S2), will be subdivided into the two subtypes of IBD, namely UC and CD, to differentiate them from IMHMV. This approach can enhance clinicians’ understanding of the disease and improve the diagnostic rate.
4 Conclusion
This case report describes the first documented instance of Idiopathic Myointimal Hyperplasia of the Mesenteric Veins (IMHMV) with right colonic involvement complicated by Clostridium difficile infection in a female patient, and also provides the inaugural description of small vessels exhibiting arterial-type wall thickening of uncertain significance in IMHMV.
Histopathological analysis demonstrated a paradoxical coexistence of characteristic venous myointimal hyperplasia and a subset of small vessels showing arterial-type wall thickening that distinctly lacked Desmin-positive smooth muscle cell proliferation—an essential feature of the venous lesions—thus creating a diagnostic paradox given the established venocentric pathology of IMHMV. Clinicians should maintain a high index of suspicion for IMHMV when encountering venocentric myointimal hyperplasia in biopsy specimens from elderly patients with suspected inflammatory bowel disease or distal colorectal ischemia, with the caveat that concurrent arterial-type wall thickening of uncertain significance should prompt thorough clinicopathological correlation to exclude alternative vasculopathies.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving humans were approved by Medical Ethics Committee of Chongqing People’s Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The human samples used in this study were acquired from a by- product of routine care or industry. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article. Written informed consent was obtained from the participants for the publication of this case report.
Author contributions
WW: Conceptualization, Data curation, Formal analysis, Writing – original draft, Writing – review & editing. XD: Investigation, Resources, Writing – review & editing. XJ: Formal analysis, Visualization, Writing – review & editing. MY: Methodology, Supervision, Writing – review & editing. XT: Investigation, Validation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. XT is currently receiving grants (2022TIAD-KPX0243 and 2023MSXM034) from Organization Technological Innovation and Application Development of Chongqing. XJ is currently receiving a grant (2024MSXM162) from the project of the Chongqing Municipal Health Commission.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1674469/full#supplementary-material
Abbreviations
IMHMV, Idiopathic myointimal hyperplasia of mesenteric veins; CDI, Clostridium difficile infection; IBD, Inflammatory bowel disease; UC, Ulcerative colitis; CD, Crohn’s disease; IC, Ischemic colitis; IMP, idiopathic mesenteric phlebosclerosis; CT, computed tomography; CTA, computed tomography angiography.
References
1. Yamada, K, Hiraki, M, Tanaka, T, Mori, D, Tanaka, F, Manabe, T, et al. A case of idiopathic myointimal hyperplasia of the mesenteric veins presenting with small bowel obstruction. Surg Case Rep. (2021) 7:17. doi: 10.1186/s40792-020-01100-8
2. Song, SJ, and Shroff, SG. Idiopathic Myointimal hyperplasia of mesenteric veins of the ileum and Colon in a patient with Crohn’s disease: a case report and brief review of the literature. Case Rep Pathol. (2017) 2017:1–6. doi: 10.1155/2017/6793031
3. Yantiss, RK, Cui, I, Panarelli, NC, and Jessurun, J. Idiopathic myointimal hyperplasia of mesenteric veins: an uncommon cause of ischemic colitis with distinct mucosal features. Am J Surg Pathol. (2017) 41:1657–65. doi: 10.1097/PAS.0000000000000905
4. Yun, SJ, Nam, DH, Kim, J, Ryu, JK, and Lee, SH. The radiologic diagnosis of idiopathic myointimal hyperplasia of mesenteric veins with a novel presentation: case report and literature review. Clin Imaging. (2016) 40:870–4. doi: 10.1016/j.clinimag.2015.12.017
5. Abu-Alfa, AK, Ayer, U, and West, AB. Mucosal biopsy findings and venous abnormalities in idiopathic myointimal hyperplasia of the mesenteric veins. Am J Surg Pathol. (1996) 20:1271–8. doi: 10.1097/00000478-199610000-00014
6. Killeen, S, Martin, ST, Hyland, J, O’ Connell, PR, and Winter, DC. Clostridium difficile enteritis: a new role for an old foe. Surgeon. (2014) 12:256–62. doi: 10.1016/j.surge.2014.01.008
7. Bramdev, A. Pseudomembranous colitis. A clinicopathological review of 7 cases. S Afr Med J. (1989) 76:221–3.
8. Kurose, I, Pothoulakis, C, LaMont, JT, Anderson, DC, Paulson, JC, Miyasaka, M, et al. Clostridium difficile toxin a-induced microvascular dysfunction. Role of histamine. J Clin Invest. (1994) 94:1919–26. doi: 10.1172/JCI117542
9. Mastroianni, A, Vangeli, V, Mauro, MV, Manfredi, R, and Greco, S. Upper mesenteric artery thrombosis as a complication of Clostridium difficile infection. Am J Emerg Med. (2023) 70:181–2. doi: 10.1016/j.ajem.2023.02.002
10. Adejumo, AC, Akanbi, O, and Pani, L. Among inpatients, ischemic bowel disease predisposes to Clostridium difficile infection with concomitant higher mortality and worse outcomes. Eur J Gastroenterol Hepatol. (2019) 31:109–15. doi: 10.1097/MEG.0000000000001273
11. Ionescu, EM, Curte, A-M, Olteanu, AO, Preda, CM, Tieranu, I, Klimko, A, et al. Rare clinical association between Clostridioides difficile infection and ischemic colitis: case report and literature review. Medicina (Kaunas). (2021) 57:705. doi: 10.3390/medicina57070705
12. Sahara, K, Yamada, R, Fujiwara, T, Koizumi, K, Horiguchi, S, Hishima, T, et al. Idiopathic myointimal hyperplasia of mesenteric veins: rare case of ischemic colitis mimicking inflammatory bowel disease. Dig Endosc. (2015) 27:768–71. doi: 10.1111/den.12470
13. García-Castellanos, R, López, R, de Vega, VM, Ojanguren, I, Piñol, M, Boix, J, et al. Idiopathic myointimal hyperplasia of mesenteric veins and pneumatosis intestinalis: a previously unreported association. J Crohns Colitis. (2011) 5:239–44. doi: 10.1016/j.crohns.2010.12.003
14. Bryant, J. Unexpected sudden death during propranolol therapy in a patient with mild mesenteric venous myointimal hyperplasia. J Forensic Sci. (1998) 43:905–7. doi: 10.1520/JFS14328J
15. Chobanian, AV. 1989 Corcoran lecture: adaptive and maladaptive responses of the arterial wall to hypertension. Hypertension. (1990) 15:666–74. doi: 10.1161/01.hyp.15.6.666 1989 Corcoran lecture: adaptive and maladaptive responses of the arterial wall to hypertension
16. Bobik, A, and Campbell, JH. Vascular derived growth factors: cell biology, pathophysiology, and pharmacology. Pharmacol Rev. (1993) 45:1–42. doi: 10.1016/S0031-6997(25)00451-X
17. Chobanian, AV. The influence of hypertension and other hemodynamic factors in atherogenesis. Prog Cardiovasc Dis. (1983) 26:177–96. doi: 10.1016/0033-0620(83)90005-1
18. Goldby, FS, and Beilin, LJ. How an acute rise in arterial pressure damages arterioles. Electron microscopic changes during angiotensin infusion. Cardiovasc Res. (1972) 6:569–84. doi: 10.1093/cvr/6.5.569
19. Schwartz, SM, Heimark, RL, and Majesky, MW. Developmental mechanisms underlying pathology of arteries. Physiol Rev. (1990) 70:1177–209. doi: 10.1152/physrev.1990.70.4.1177
20. Robertson, AL, and Khairallah, PA. Arterial endothelial permeability and vascular disease. The “trap door” effect. Exp Mol Pathol. (1973) 18:241–60. doi: 10.1016/0014-4800(73)90022-1
21. Pulmonary Hypertension | Pulmonary Medicine | JAMA | JAMA Network, (2012). Available online at: https://jamanetwork.com/journals/jama/article-abstract/1367450 (Accessed February 19, 2025)
22. Tuder, RM, Marecki, JC, Richter, A, Fijalkowska, I, and Flores, S. Pathology of pulmonary hypertension. Clin Chest Med. (2007) 28:23–42. doi: 10.1016/j.ccm.2006.11.010
23. Leopold, JA. Pulmonary venous Remodeling in pulmonary hypertension: the veins take Center stage. Circulation. (2018) 137:1811–3. doi: 10.1161/CIRCULATIONAHA.118.033013
24. Wijeratne, DT, Lajkosz, K, Brogly, SB, Lougheed, MD, Jiang, L, Housin, A, et al. Increasing incidence and prevalence of World Health Organization groups 1 to 4 pulmonary hypertension: a population-based cohort study in Ontario, Canada. Circ Cardiovasc Qual Outcomes. (2018) 11:e003973. doi: 10.1161/CIRCOUTCOMES.117.003973
25. Humbert, M, Kovacs, G, Hoeper, MM, Badagliacca, R, Berger, RMF, Brida, M, et al. 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. (2022) 43:3618–731. doi: 10.1093/eurheartj/ehac237
26. Fayyaz, AU, Edwards, WD, Maleszewski, JJ, Konik, EA, DuBrock, HM, Borlaug, BA, et al. Global pulmonary vascular Remodeling in pulmonary hypertension associated with heart failure and preserved or reduced ejection fraction. Circulation. (2018) 137:1796–810. doi: 10.1161/CIRCULATIONAHA.117.031608
27. Kawasaki, K, Kawatoko, S, Torisu, T, Mizuuchi, Y, Iura, T, Ohtani, H, et al. Idiopathic myointimal hyperplasia of mesenteric veins depicted by barium enema examination, and conventional and magnifying colonoscopy. Clin J Gastroenterol. (2022) 15:734–9. doi: 10.1007/s12328-022-01647-z
28. Kim, S-W, Ho Park, S, Hyoung Park, S, Sik Yoon, Y, and Kim, J. Idiopathic Myointimal hyperplasia of the mesenteric veins is a peculiar venous ischemia that may be diagnosed before surgery. Dis Colon Rectum. (2022) 65:e707–17. doi: 10.1097/DCR.0000000000002072
29. López Morales, P, González Valverde, FM, Giménez Francés, C, Pastor Quirante, F, and Albarracín Marín-Blázquez, A. Idiopathic myointimal hyperplasia of the mesenteric veins, an uncommon cause of intestinal ischemia. Rev Esp Enferm Dig. (2022) 114:368–9. doi: 10.17235/reed.2022.8654/2022
30. Shah, YB, Lee, D, and Khaddash, TS. Endovascular approach in the management of idiopathic myointimal hyperplasia of the inferior mesenteric vein. CVIR Endovasc. (2021) 4:88. doi: 10.1186/s42155-021-00272-0
31. Wong, R, Westerveld, D, Yeo, H, Jessurun, J, and Jesudian, A. Ischemic colitis from idiopathic Myointimal hyperplasia of the mesenteric veins in a post-liver transplant patient. ACG Case Rep J. (2021) 8:e00692. doi: 10.14309/crj.0000000000000692
32. Xie, H, and Xu, X. Radiological and clinical findings of idiopathic myointimal hyperplasia of mesenteric veins: case report. Medicine (Baltimore). (2021) 100:e27574. doi: 10.1097/MD.0000000000027574
33. Al Ansari, A, Ahmed, S, Mansour, E, and Abass, MA. Idiopathic myointimal hyperplasia of the mesenteric veins. J Surg Case Rep. (2021) 2021:rjaa453. doi: 10.1093/jscr/rjaa453
34. Fang, S, Song, Y, Zhang, C, and Wang, L. Efficacy and safety of vedolizumab for pediatrics with inflammatory bowel disease: a systematic review. BMC Pediatr. (2022) 22:175. doi: 10.1186/s12887-022-03229-x
35. Almumtin, A, Al Sulais, E, and Elhag, MA. Idiopathic Myointimal hyperplasia of mesenteric veins (IMHMV) with two spontaneous bowel perforations: a case report and literature review. Int J Surg Case Rep. (2021) 83:106022. doi: 10.1016/j.ijscr.2021.106022
36. Kelly Wu, W, Tombazzi, CR, Howe, CF, Kendall, MA, Walton, DB, Washington, MK, et al. Idiopathic Myointimal hyperplasia of the mesenteric veins: a rare imitator of inflammatory bowel disease. Am Surg. (2023) 89:1141–3. doi: 10.1177/0003134820973390
37. Martin, FC, Yang, LS, Fehily, SR, D’Souza, B, Lim, A, and McKelvie, PA. Idiopathic myointimal hyperplasia of the mesenteric veins: case report and review of the literature. JGH Open. (2020) 4:345–50. doi: 10.1002/jgh3.12297
38. Chudy-Onwugaje, K, Ali, O, and Umoren, M. Idiopathic Myointimal hyperplasia of the mesenteric veins of the Colon. Clin Gastroenterol Hepatol. (2020) 18:A19–20. doi: 10.1016/j.cgh.2019.07.030
39. Anderson, B, Smyrk, TC, Graham, RP, Lightner, A, and Sweetser, S. Idiopathic myointimal hyperplasia is a distinct cause of chronic colon ischaemia. Color Dis. (2019) 21:1073–8. doi: 10.1111/codi.14685
40. Louie, CY, DiMaio, MA, Charville, GW, Berry, GJ, and Longacre, TA. Gastrointestinal tract vasculopathy: clinicopathology and description of a possible “new entity” with protean features. Am J Surg Pathol. (2018) 42:866–76. doi: 10.1097/PAS.0000000000001060
41. Gonai, T, Toya, Y, Nakamura, S, Kawasaki, K, Yanai, S, Fujita, Y, et al. Gastrointestinal: idiopathic myointimal hyperplasia of mesenteric veins. J Gastroenterol Hepatol. (2018) 33:1939. doi: 10.1111/jgh.14384
42. Yang, KH, Kwon, TH, Park, KS, Kim, ES, Cho, KB, Baek, SK, et al. Idiopathic Myointimal hyperplasia of mesenteric veins. Korean J Gastroenterol. (2016) 67:54–7. doi: 10.4166/kjg.2016.67.1.54
43. Patel, AD, Schneider, Y, Saumoy, M, Maltz, C, Yeo, H, Jessurun, J, et al. Idiopathic Myointimal hyperplasia of the mesenteric veins. ACG Case Rep J. (2016) 3:e84. doi: 10.14309/crj.2016.57
44. Guadagno, E, Del Basso De Caro, M, Del Prete, E, D’Armiento, FP, and Campione, S. Coexistence of multiple ileal neuroendocrine tumors and idiopathic myointimal hyperplasia of mesenteric veins: coincidence or consequence? Case report and review of literature. Int J Surg Pathol. (2016) 24:627–30. doi: 10.1177/1066896916642289
45. Costa, MN, Saiote, J, Pinheiro, MJ, Duarte, P, Bentes, T, Oliveira, MF, et al. Segmental colitis caused by idiopathic myointimal hyperplasia of mesenteric veins. Rev Esp Enferm Dig. (2016) 108:821–6. doi: 10.17235/reed.2016.4051/2015
46. Cauchois, A, Desfourneaux, V, Kammerer-Jacquet, S-F, Bouguen, G, Rioux-Leclercq, N, and Henno, S. A case of idiopathic myointimal hyperplasia of mesenteric veins. Ann Pathol. (2016) 36:415–9. doi: 10.1016/j.annpat.2016.09.002
47. Wangensteen, KJ, Fogt, F, Kann, BR, and Osterman, MT. Idiopathic Myointimal hyperplasia of the mesenteric veins diagnosed preoperatively. J Clin Gastroenterol. (2015) 49:491–4. doi: 10.1097/MCG.0000000000000290
48. Abbott, S, Hewett, P, Cooper, J, and Ruszkiewicz, A. Idiopathic myointimal hyperplasia of the mesenteric veins: a rare differential to be considered in idiopathic colitis. ANZ J Surg. (2018) 88:242–3. doi: 10.1111/ans.13210
49. Laskaratos, F-M, Hamilton, M, Novelli, M, Shepherd, N, Jones, G, Lawrence, C, et al. A rare cause of abdominal pain, diarrhoea and GI bleeding. Idiopathic myointimal hyperplasia of the mesenteric veins (IMHMV). Gut. (2015) 64:214–350. doi: 10.1136/gutjnl-2014-308319
50. Zijlstra, M, Tjhie-Wensing, JWMA, van Dijk, MAAM, and Wegdam, JA. Idiopathic myointimal hyperplasia of mesenteric veins: an unusual cause of diarrhoea. Ned Tijdschr Geneeskd. (2014) 158:A7752
51. Korenblit, J, Burkart, A, Frankel, R, Klinge, M, Greenbau, L, Goldstein, S, et al. Refractory pancolitis: a novel presentation of idiopathic myointimal hyperplasia of mesenteric veins. Gastroenterol Hepatol (N Y). (2012) 8:696–700.
52. Feo, L, Cheeyandira, A, and Schaffzin, DM. Idiopathic myointimal hyperplasia of mesenteric veins in the elderly. Int J Color Dis. (2013) 28:433–4. doi: 10.1007/s00384-012-1480-0
53. Lanitis, S, Kontovounisios, C, and Karaliotas, C. An extremely rare small bowel lesion associated with refractory ascites. Idiopathic myointimal hyperplasia of mesenteric veins of the small bowel associated with appendiceal mucocoele and pseudomyxoma peritonei. Gastroenterology. (2012) 142:e5–7. doi: 10.1053/j.gastro.2011.11.052
54. Chiang, C-K, Lee, C-L, Huang, C-S, Huang, S-H, and Wu, C-H. A rare cause of ischemic proctosigmoiditis: idiopathic myointimal hyperplasia of mesenteric veins. Endoscopy. (2012) 44:E54–5. doi: 10.1055/s-0031-1291529
55. Kao, PC, Vecchio, JA, Hyman, NH, West, AB, and Blaszyk, H. Idiopathic myointimal hyperplasia of mesenteric veins: a rare mimic of idiopathic inflammatory bowel disease. J Clin Gastroenterol. (2005) 39:704–8. doi: 10.1097/00004836-200509000-00011
56. Savoie, LM, and Abrams, AV. Refractory proctosigmoiditis caused by myointimal hyperplasia of mesenteric veins: report of a case. Dis Colon Rectum. (1999) 42:1093–6. doi: 10.1007/BF02236711
57. Genta, RM, and Haggitt, RC. Idiopathic myointimal hyperplasia of mesenteric veins. Gastroenterology. (1991) 101:533–9. doi: 10.1016/0016-5085(91)90035-j
58. Li, H, Shu, H, Zhang, H, Cui, M, Gao, Y, and Tian, F. Idiopathic Myointimal hyperplasia of the mesenteric veins: a case report and scoping review of previously reported cases from clinical features to treatment. Front Med (Lausanne). (2022) 9:855335. doi: 10.3389/fmed.2022.855335
59. Shi, Q, Chen, D, Wu, H, Wang, Y, Liu, S, and Sun, Q. Chin J Inflamm Bowel Dis. (2022) 6:275–7. doi: 10.3760/cma.j.cn101480-20211203-00098
60. Morimura, F, Edo, H, Niwa, T, Sugiura, H, Suyama, Y, Okazaki, S, et al. Idiopathic myointimal hyperplasia of mesenteric veins: radiological evaluation using CT angiography. BJR|Case Reports. (2024) 10:uaad009. doi: 10.1093/bjrcr/uaad009
61. Bhatt, H, Moreira, RK, Shawki, SF, and Rumer, KK. Atypical presentation of a rare disorder; idiopathic myointimal hyperplasia of mesenteric veins (IMHMV): report of two cases. Int J Surg Case Rep. (2023) 111:108839. doi: 10.1016/j.ijscr.2023.108839
Keywords: idiopathic myointimal hyperplasia of mesenteric veins, Clostridium difficile infection, inflammatory bowel disease, idiopathic mesenteric phlebosclerosis, vessels with arterial-type wall thickening of uncertain significance
Citation: Wang W, Deng X, Jiang X, Yang M and Tang X (2025) Case Report: Idiopathic myointimal hyperplasia of mesenteric veins mimicking inflammatory bowel disease: a case report with literature review. Front. Med. 12:1674469. doi: 10.3389/fmed.2025.1674469
Edited by:
Monjur Ahmed, Thomas Jefferson University, United StatesReviewed by:
Sudhakar Ramamoorthy, All India Institute of Medical Sciences, Mangalagiri, IndiaKristen Rumer, Mayo Clinic, United States
Copyright © 2025 Wang, Deng, Jiang, Yang and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xuefeng Tang, dHhmYXR5QDE2My5jb20=
 Wei Wang
Wei Wang Xue Deng1,2
Xue Deng1,2 Xin Jiang
Xin Jiang Xuefeng Tang
Xuefeng Tang