Abstract
Objective:
The purpose of our study was to consider elderly patients with chronic heart failure (CHF) and informal caregivers as a unit of intervention and to explore the effectiveness of a program developed based on dyadic illness management theory in improving their physical and psychological health.
Methods:
In this single-center randomized controlled trial, 80 dyads of elderly CHF patients and their informal caregivers were randomly assigned (1:1) to an intervention group receiving a 3-month home-based disease management program or a control group receiving usual care. Data on primary outcome (patient QoL) and secondary outcomes (e.g., patient self-management, readmission, depression, and caregiver burden) were collected at baseline (T0), immediately post-intervention (T1), and 3 months post-intervention (T2).
Results:
At both T1 and T2, patients in the intervention group showed significantly better outcomes than controls in QoL, readmission rates, self-care behaviors, and depression incidence (all p < 0.05). Caregiver burden was consistently lower in the intervention group at both timepoints (all p < 0.05). QoL showed temporal improvements in the intervention group, with sustained enhancement at both T1 and T2 (compared to baseline) and further progression from T1 to T2. In contrast, the control group demonstrated only transient improvement at T1 (compared to baseline), followed by decline at T2. After adjusting for baseline factors, the intervention demonstrated a significant and independent effect on improving patient QoL. This effect was not only sustained but substantially strengthened from T1 (B = −6.855) to T2 (B = -25.00, both p < 0.001), indicating a cumulative benefit over time. The model for T2 accounted for the majority of the outcome variance (adjusted R2 = 0.828).
Conclusion:
The home-based, dyadically-focused disease management program significantly improved both physical and psychological health outcomes for elderly CHF patients and their caregivers. And substantially increasing improvements in patients’ QoL.
1 Introduction
Demographic aging and rising cardiovascular risks are contributing to a rising prevalence of chronic heart failure (CHF) among the elderly, where it is a leading cause of hospitalization and a critical public health challenge (1–4). As a chronic condition, CHF necessitates effective long-term management after hospitalization. International guidelines emphasize that patients with chronic heart failure require management during home care to control their condition, improve symptoms, enhance quality of life (QoL), and reduce recurrence rates and mortality (5–7). This has led to an increasing emphasis on home-based disease management, which refers to the comprehensive suite of care activities, including both patient self-management and caregiver support, conducted within the home environment (8, 9). Evidences confirms that optimized home management significantly enhances patient outcomes and may lower healthcare costs (10, 11). However, factors such as advanced age, comorbidities, and low comprehension often make treatment adherence burdensome for elderly CHF patients, leading to a generally suboptimal level of home-based disease management in China (11–14). Consequently, improving disease management capabilities in home care settings has emerged as a critical challenge requiring urgent attention.
The European Society of Cardiology highlights patient and family involvement in home-based disease management as vital for successful treatment (6), with head and neck cancer research confirming that enhanced caregiver education improves outcomes (15). Despite these benefits, family caregivers endure significant physical, psychological, and social strain (16). In China, CHF caregivers face heightened burdens due to inadequate knowledge, skills, and social support (17, 18), undermining care efficacy and emphasizing the urgent need for comprehensive training and professional support systems. In the home-based management of elderly CHF patients, informal caregivers (ICs) serve a critical role yet encounter substantial caregiving challenges. Addressing these challenges requires a dual focus: (1) meeting caregivers’ daily needs, and (2) developing targeted interventions to enhance patient and caregiver support.
Dyadic interventions, in which both the patient and family caregiver are targeted, are likely to result in better outcomes for both and are more cost-effective than interventions with a single objective (19). The theory of dyadic illness management reconceptualizes chronic disease management as an interdependent process between the patient and caregiver. This paradigm shifts the focus from the individual to the dyad, emphasizing that successful management is a bidirectional phenomenon, reliant on their collaborative assessment and health-promoting behaviors to achieve optimal mutual outcomes (20). Its core components include: Dyadic appraisal (joint evaluation of symptoms, care goals, and decision-making); Dyadic management behaviors (collaborative actions such as shared decision-making, daily health management, and emotional support); Dyadic health (an interdependent relationship encompassing both parties’ physical/mental well-being and QoL). These elements interact cyclically: appraisal influences behaviors, behaviors determine health outcomes, and health status reciprocally modifies appraisal and management. From a practical standpoint, dyadic interventions targeting both patients and family caregivers prove more cost-effective and yield better outcomes for both parties than patient-only approaches (21).
This approach, which addresses the dyad as an interdependent unit, is supported internationally by evidence of improved communication, emotional regulation, and disease management skills (22). Exploratory studies in the Chinese context further confirm benefits in discharge readiness and psychosocial outcomes for dyads facing lung cancer or heart failure (23, 24). Therefore, nursing interventions designed to target this dyadic cycle are crucial for achieving sustainable health outcomes. Despite this evidence, the application of such integrated dyadic models in CHF care for older adults in China remains limited, highlighting a significant area for development.
Nevertheless, in the context of heart failure management in China, research remains largely focused on patients or caregivers individually, with limited attention to the patient–caregiver dyad as an integrated unit, particularly among older adults. Moreover, existing self-management interventions for CHF patients predominantly consist of in-hospital health education, lacking continuity into home settings. They also fail to address the progressive decline in treatment adherence and self-management after discharge, compounded by the common absence of sustained health education beyond hospitalization (25). Therefore, this study implemented a home-based disease management intervention program grounded in dyadic illness management theory for elderly patients with CHF and their informal caregivers, aiming to examine its effects on the physical and mental health outcomes of both patient-caregiver dyads. We hypothesized that this dyadic approach would significantly improve physical and mental health outcomes for CHF patients and their informal caregivers.
2 Methods
2.1 Aim
The primary objectives of this study are to evaluate whether home-based disease management can significantly improve QoL for CHF patients, while secondary objectives aim to examine its effects on enhancing patients’ self-management behaviors, reducing readmission rates, alleviating depression, as well as reducing caregiving burden among informal caregivers.
2.2 Study design
This was a two-arm parallel randomized controlled trial (26), which was conducted in the Department of Cardiology, Jiangnan University Hospital from May 2023 to December 2024. A total of 80 dyadic pairs of elderly patients with CHF and their caregivers were randomly divided into an intervention group (n = 40 dyads) and a control group (n = 40 dyads). Given the inherent characteristics of the intervention, participant blinding was impossible. Nevertheless, blinding was strictly enforced for all outcome assessment and data handling processes to minimize bias. To this end: Outcome assessors were personnel independent of the intervention team and were not informed of the group assignments. Data collection forms used for outcome measures did not identify the study group. The principal statistician performed the final analysis on a dataset with concealed group codes. The control group was given routine nursing care including in-hospital health promotion and post-discharge follow-up, while the intervention group received routine nursing care along with a home-based disease management intervention strategy based on the dyadic illness management theory for a period of 3 months. The flow chart is shown in Figure 1.
Figure 1

Flow chart of the study. No reason indicates cases where participants did not respond to invitations (e.g., unreturned calls, no replies to messages) and no explicit reason for non-participation was obtained.
2.3 Participants
The inclusion and exclusion criteria for subjects are shown in Table 1.
Table 1
| Subjects | Criteria for inclusion | Criteria for exclusion |
|---|---|---|
| Patients |
|
|
| Informal caregivers |
|
|
Inclusion and exclusion criteria for subjects.
CHF, Chronic heart failure; NYHA, New York Heart Association.
2.4 Randomization
A computer-generated randomization sequence was created using SPSS 27.0 by independent personnel not involved in the study. Eligible patient-caregiver dyads who met the inclusion criteria and provided informed consent were sequentially assigned to their respective groups through sealed, opaque envelopes containing pre-generated allocation codes. Before the intervention was implemented, the control group and intervention group were assigned to different wards.
2.5 Intervention
Participants in the control group received routine nursing care, whereas those in the intervention group were administered home disease management programs.
2.5.1 Control group
The control group received routine nursing care, discharge instructions, and follow-up care. In-hospital health education: Health education was conducted by department doctors, using a combination of power point presentations and video playback to transmit CHF-related knowledge and disease management points to patients and their informal caregivers. At the end of the process of education, they were given the corresponding disease self-management manual. Post-discharge follow-up: Conducted by two trained research nurses through multiple modalities including structured telephone interviews, WeChat-based communication (via the instant messaging platform), and scheduled home visits. Patients were followed up once a week for the first month after discharge, and then once a month thereafter. Each follow-up visit lasted approximately 5–10 min. During follow-up visits, nurses responded to patients’ specific inquiries, conducted routine condition assessments (such as checking for worsening symptoms like edema or dyspnea, and measuring vital signs during home visits), reminded patients to take their medications, and confirmed their next outpatient appointment date. Details are shown in Figure 2.
Figure 2
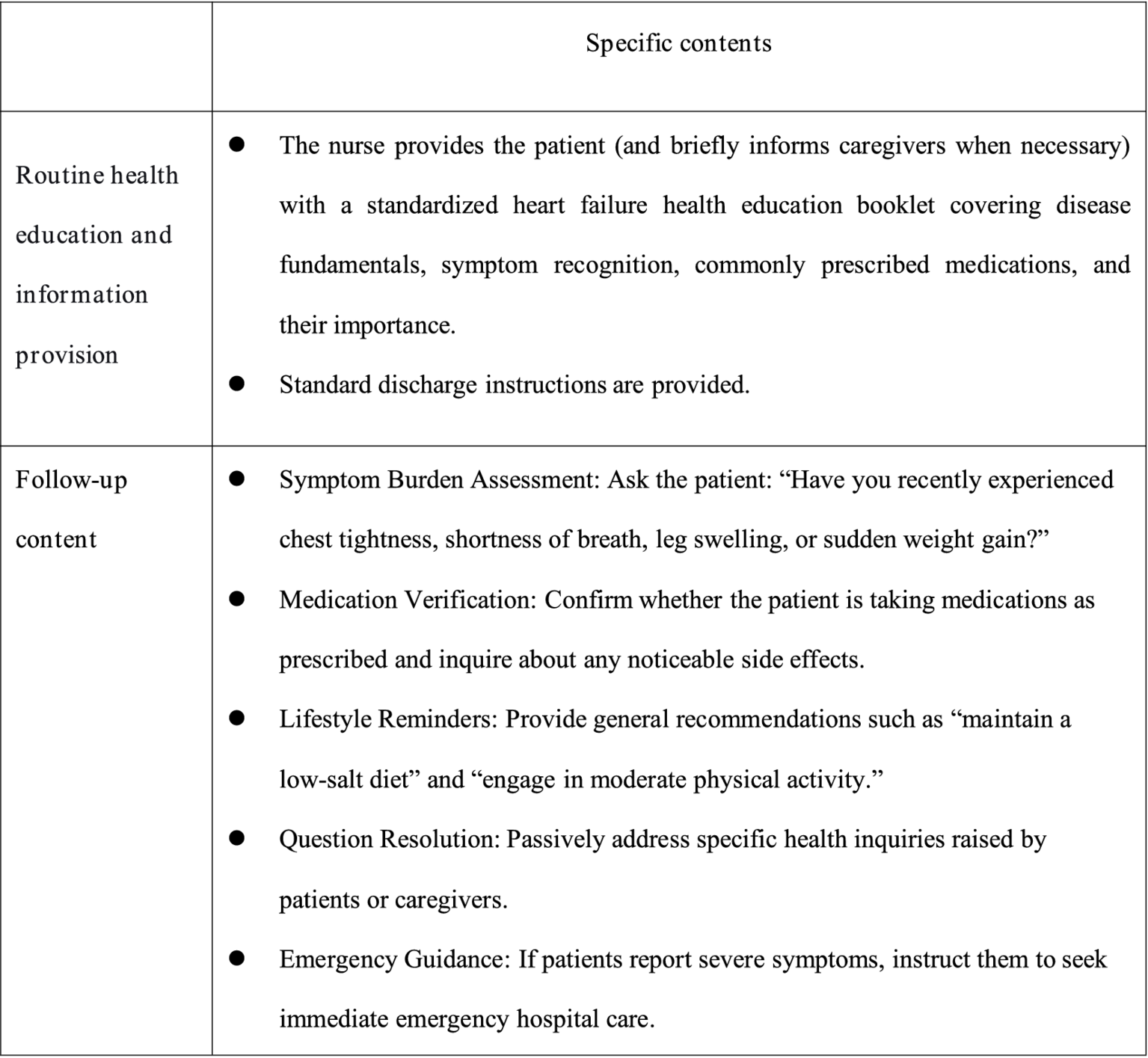
Intervention measures for the control group.
2.5.2 Intervention group
Guided by an integrated theoretical framework incorporating Dyadic Illness Management theory (20), Timing It Right theory (27), the Chronic Illness Trajectory Model (28), and relevant clinical practice guideline, this study developed a home-based disease management strategy for elderly CHF patients and their caregivers. The intervention was systematically designed to align with CHF treatment and rehabilitation phases, with detailed components presented in Figure 3. The intervention protocol comprised two sequential phases spanning 12 weeks (7 total sessions). Phase I (Weeks 0–2) delivered 2 face-to-face sessions (60 min each) during hospitalization, focusing on acute care transition and dyadic skill-building. Phase II (Weeks 2–12) implemented 5 biweekly telehealth sessions via Tencent Meeting platform (60 ± 5 min each), emphasizing chronic disease self-management.
Figure 3
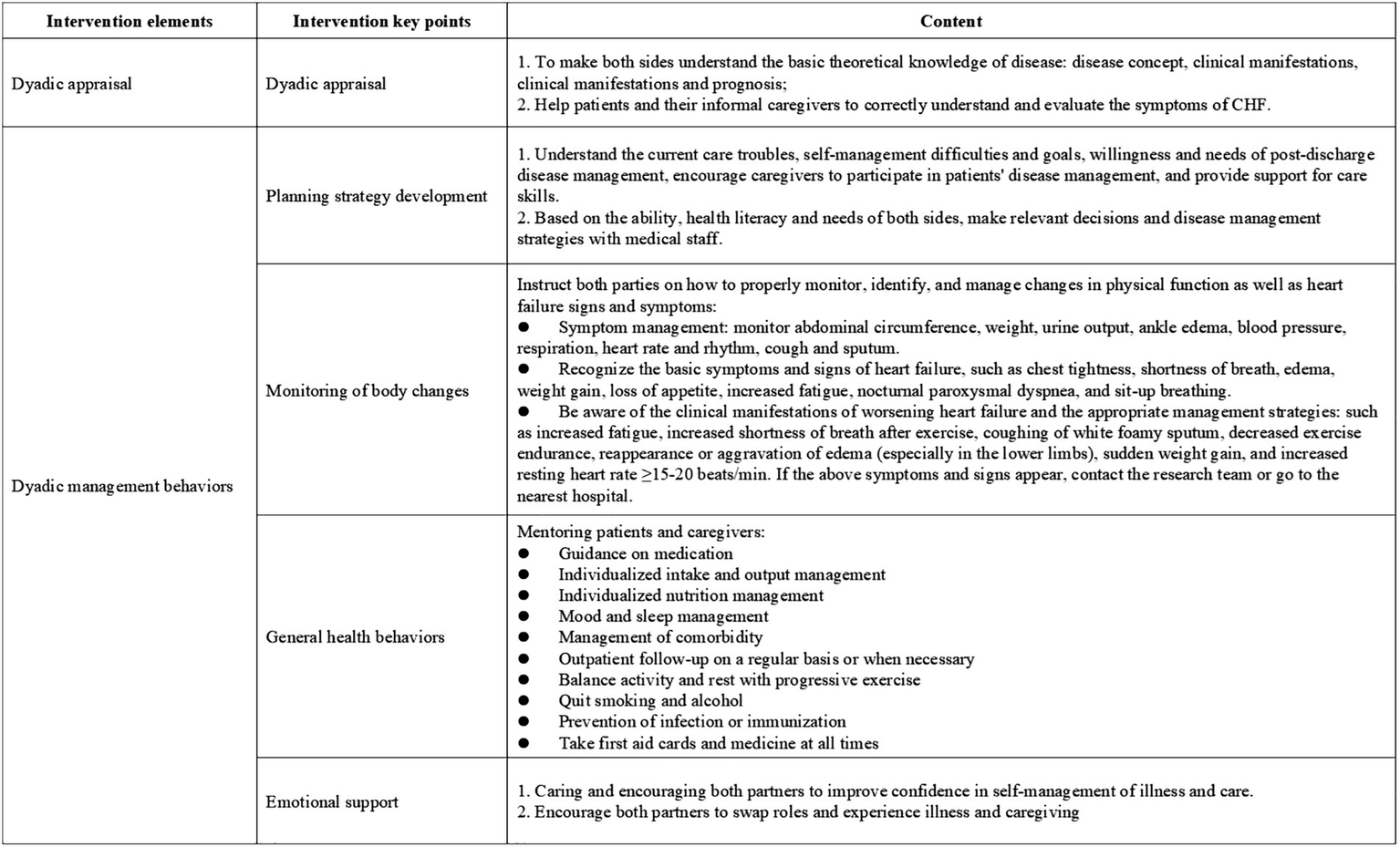
Key implementation considerations for home-based disease management.
2.6 Measurements and data collection
The primary outcome indicator of this study was the patients’ QoL. Secondary outcome measures included patients’ readmission rates, self-management behaviors, depression, and caregivers’ burden of care. The assessment instruments and the time taken to collect information are shown in Figure 4.
Figure 4

Research tools and time points for data collection. T0: Baseline (pre-intervention); T1: At the end of the intervention, T2: 3 months after the end of the intervention.
2.7 Adherence
Participant adherence is a critical factor in ensuring the validity of research data, the smooth implementation of interventions, and the reliability of conclusions. We clearly informed participants of the importance of compliance for improving personal health and the scientific validity of the study, thereby reducing the dropout rate. In addition, during the research process, we simplified the data collection process by scheduling follow-up visits at times convenient for participants and using electronic records to replace cumbersome paper forms. We also provide periodic reminders via phone calls and text messages to promptly address participants’ questions. We also offer appropriate positive incentives (such as health consultation services or gifts) to individuals who consistently participate to enhance their continued engagement.
2.8 Statistical analyses
For calculating the sample size, the sample size formula for comparing the means of two samples was used. With the specific formula (36) given by: n₁ = n₂ = 2[(Zα/2 + Zβ)2σ2]/δ2. Zα and Zβ represent the critical values from the standard normal distribution corresponding to α = 0.05 (two-tailed test) and β = 0.20, respectively. Using QoL in CHF patients as the primary outcome, based on previous literature (24), the standard deviation (σ) of the outcome measure was set at 5.01, while the expected between-group mean difference (δ) was estimated as 8.15, with a significance level of α = 0.05 and statistical power of 1-β = 0.8, the calculation showed that at least 33 patients per group were required. In order to ensure sufficient statistical power, the study increased the sample size to 40 participants per group (a total of 80 patients) and included 40 matched caregivers per group (a total of 80 caregivers), accounting for a possible 20% attrition rate.
SPSS 27.0 statistical software was used for data analysis. Continuous variables are presented as mean ± standard deviation (SD) or median with interquartile range (IQR), depending on data distribution. Analysis of differences between or within groups: independent samples t-test, paired t-test, repeated measures ANOVA, Mann–Whitney U-test, depending on the distribution of data. Categorical variables are presented as frequencies and percentages. Application of chi-square test, Fisher’s exact test and Wilcoxon rank sum test for comparison of categorical variables. To delineate the independent effect of the home-based disease management intervention on the QoL of elderly patients with CHF while controlling for potential confounders, this study employed multiple linear regression analyses. The dependent variables were QoL scores at T1 and T2. The core independent variable was the group assignment to the intervention (0 = No, 1 = Yes). Confounding variables adjusted for included demographic characteristics, disease-related features, and care-related factors. Statistical significance was set at two-sided p < 0.05.
3 Results
A total of 96 CHF patient-caregiver dyads were screened for this study. After screening, 8 dyads were excluded for failing to meet the inclusion criteria, another 5 declined to participate, and 1 was excluded for other reasons. Eventually, 80 eligible dyads were enrolled and randomly assigned to either the intervention group (n = 40 dyads) or the control group (n = 40 dyads). All enrolled dyads completed the baseline assessment. During subsequent follow-ups, 75 dyads were retained at time point T1 and 71 at time point T2. The flow of participants throughout the study process was detailed in Figure 1.
3.1 Comparison of baseline data between the two groups
The data of 36 dyads of participants in the experimental group and 35 participants in the control group were finally included in this study for statistical analysis. As shown in Tables 2, 3, there were no statistically significant differences in baseline data between the two groups.
Table 2
| Variables | CHF patients | Test value | p value | |
|---|---|---|---|---|
| Intervention group (n = 36) |
Control group (n = 35) |
|||
| Age (years) | 69.86 ± 8.58 | 71.89 ± 8.12 | 1.021a | 0.311 |
| Body mass index (kg/m2) | 25.56 ± 2.61 | 24.99 ± 3.20 | 0.821a | 0.414 |
| Gender | ||||
| Male | 30 (83.3) | 28 (80.0) | 0.132b | 0.717 |
| Female | 6 (16.7) | 7 (20.0) | ||
| Marital Status | ||||
| Unmarried | 3 (8.3) | 5 (14.3) | 0.495c | 0.871 |
| Married | 26 (72.3) | 25 (71.4) | ||
| Divorced or widowed | 7 (19.4) | 5 (14.3) | ||
| Educational level | ||||
| Primary school and below | 5 (13.9) | 7 (20.0) | 1.664d | 0.645 |
| Junior high school | 13 (36.1) | 15 (42.9) | ||
| Senior high school | 9 (25.0) | 8 (22.9) | ||
| Unior college and above | 9 (25.0) | 5 (14.3) | ||
| Monthly income (RMB) | ||||
| <5,000 | 18 (50.0) | 19 (54.3) | 0.061b | 0.806 |
| ≥5,000 | 18 (50.0) | 16 (45.7) | ||
| Residence | ||||
| Living alone | 5 (13.9) | 9 (25.7) | 1.568b | 0.211 |
| Living with family | 31 (86.1) | 26 (74.3) | ||
| Medical insurance | ||||
| Yes | 30 (83.3) | 28 (80.0) | 0.132b | 0.717 |
| No | 6 (16.7) | 7 (20.0) | ||
| NYHA functional classification | ||||
| I | 5 (13.9) | 6 (17.1) | 0.638d | 0.523 |
| II | 15 (41.7) | 16 (45.7) | ||
| III | 16 (44.4) | 13 (37.2) | ||
| EF (%) | 49.28 ± 6.49 | 50.00 ± 6.28 | 0.476a | 0.635 |
| Course of disease (years) | 0.92 (0.25, 2.92) | 0.92 (0.83,2.98) | 1.614e | 0.101 |
| Depression | ||||
| Yes | 7 (19.4) | 11 (32.4) | 1.525b | 0.217 |
| No | 29 (80.6) | 23 (67.7) | ||
| QoL (score) | 71.86 ± 6.12 | 72.91 ± 6.59 | 0.698a | 0.635 |
| Self-care behaviors (score) | 95.44 ± 6.40 | 97.00 ± 6.56 | 1.011a | 0.315 |
Baseline data comparison between the two CHF patient groups.
aIndependent sample t-test; bChi-square test; cFisher’s exact test; dWilcoxon rank sum test; eMann–Whitney U test. CHF, Chronic heart failure; EF, Ejection fraction; NYHA, New York Heart Association; QoL, Quality of life.
Table 3
| Variables | Caregiver | Test value | p value | |
|---|---|---|---|---|
| Intervention group (n = 36) |
Control group (n = 35) |
|||
| Age (years) | 61.00 (53.00,66.75) | 60.00 (50.00,66.00) | 1.112a | 0.266 |
| Gender | ||||
| Male | 13 (36.1) | 16 (45.7) | 0.677b | 0.411 |
| Female | 23 (63.9) | 19 (54.3) | ||
| Relationship with patients | ||||
| Spouse | 23 (63.9) | 18 (51.4) | 2.765c | 0.248 |
| Son or daughter | 7 (19.4) | 13 (37.2) | ||
| Other relative | 6 (16.7) | 4 (11.4) | ||
| Self-reported health | ||||
| Good | 12 (33.3) | 12 (34.3) | 0.522d | 0.617 |
| Fair | 18 (50.0) | 20 (57.1) | ||
| Bad | 6 (16.7) | 3 (8.6) | ||
| Living with patients | ||||
| Yes | 31 (86.1) | 28 (80.0) | 0.472b | 0.492 |
| No | 5 (13.9) | 7 (20.0) | ||
| Residence | ||||
| Living alone | 5 (13.9) | 9 (25.7) | 1.568b | 0.211 |
| Living with family | 31 (86.1) | 26 (74.3) | ||
| Monthly income (RMB) | ||||
| <5,000 | 24 (66.7) | 24 (68.6) | 0.029b | 0.864 |
| ≥5,000 | 12 (33.3) | 11 (31.4) | ||
| Educational Level | ||||
| Primary school and below | 5 (13.9) | 9 (20.0) | 0.258d | 0.797 |
| Junior high school | 15 (41.7) | 11 (31.4) | ||
| Senior high school | 9 (25.0) | 7 (20.0) | ||
| Unior college and above | 7 (19.4) | 10 (28.6) | ||
| Work | ||||
| Yes | 7 (19.4) | 11 (31.4) | 1.347b | 0.285 |
| No | 29 (80.6) | 24 (68.6) | ||
| Care burden (score) | 67.28 ± 4.77 | 68.20 ± 4.38 | 0.848e | 0.399 |
Comparison of baseline data between the two groups of caregivers.
aMann–Whitney U test; bChi-square test; cFisher’s exact test; dWilcoxon rank sum test; eIndependent sample t-test.
3.2 Comparing the QoL between the two patient groups
Two independent samples t-test was chosen for analysis. The results demonstrated significantly lower QoL scores in the intervention group compared to controls at both T1 and T2 timepoints (all p < 0.05). Repeated measures ANOVA was conducted to examine longitudinal changes in total QoL scores between patient groups. The results of the within-subject effect test showed that the time effect (F-time = 164.64), inter-group effect (F-intergroup = 83.33), and interaction effect (F-interaction = 87.46) of the QoL scores in the two groups of patients all showed statistically significant differences (p < 0.05). Details are shown in Table 4. The primary outcome measure of this study was QoL, which was used to evaluate the effectiveness of the intervention. We employed multivariate linear regression analysis to control for potential confounding factors and determine the impact of home-based disease management interventions on the QoL (MLHFQ scores) of elderly CHF patients at T1 and T2. A regression model is constructed using the stepwise regression method. Based on a literature review and preliminary statistical analysis of QoL in demographic and disease characteristics (Supplementary Table 1), potential confounding factors that may influence QoL were identified and incorporated into the model. The results of multiple linear regression indicated that, after controlling for baseline age, educational attainment, monthly income, gender, QoL, depression, and self-management behaviors, the intervention had a significant effect on QoL scores at T1 [B = -6.855, 95% CI (−10.200, −3.510), p < 0.001]. This indicates that, compared to the control group, the intervention in the experimental group reduced the patients’ average QoL score by 6.855 points. The adjusted R2 for the model was 0.414, meaning that the model explained 41.4% of the variance in QoL. In addition, the intervention had a significant effect on the QoL score at T2 [B = -25.00, 95% CI (−27.825, −22.175), p < 0.001]. This indicates that, compared to the control group, the intervention in the experimental group reduced the patients’ average QoL score by 25.00 points. The adjusted R2 for the model was 0.828, meaning that the model explained 82.8% of the variance in QoL. Detailed results are presented in Table 5.
Table 4
| Variables | Group | T0 | T1 | T2 | Ftime value | FIntergroup value | F Interaction value |
|---|---|---|---|---|---|---|---|
| QoL | Intervention group (n = 36) |
71.86 ± 6.12 | 53.56 ± 8.62a | 44.14 ± 5.12a,b | 164.64 | 83.33 | 87.46 |
| Control group (n = 35) |
72.91 ± 6.59 | 61.26 ± 8.11a | 69.89 ± 7.34b | – | – | – | |
| t value | 0.698 | 3.873 | 17.107 | – | – | – | |
| p value | 0.487 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Self-care behavior# | Intervention group (n = 36) |
95.44 ± 6.40 | 116.92 ± 1.67a | 107.92 ± 1.42 a,b | 89.03 | 16.95 | 34.33 |
| Control group (n = 35) |
97.00 ± 6.56 | 102.03 ± 1.69a | 99.63 ± 1.44 | – | – | – | |
| t value | 1.011 | 6.304 | 4.106 | – | – | – | |
| p value | 0.315 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Care burden | Intervention group (n = 36) |
67.28 ± 4.77 | 36.89 ± 9.06a | 43.08 ± 7.44a,b | 311.02 | 40.63 | 10.41 |
| Control group (n = 35) |
68.20 ± 4.38 | 48.80 ± 10.35a | 49.86 ± 9.42a | – | – | – | |
| t value | 0.848 | 5.165 | 3.367 | – | – | – | |
| p value | 0.399 | <0.001 | 0.001 | <0.001 | <0.001 | <0.001 |
Comparison of total scores for QoL, self-care behavior, and care burden between the two groups.
T0: pre-intervention (baseline); T1: at the end of the intervention; T2: 3 months after the end of the intervention. aCompared with T0, p < 0.05*. bCompared with T1, p < 0.05*. #Self-care behavior is measured using the SCHFI tool.
Table 5
| Variables | QoL (T2) | QoL (T3) | ||||
|---|---|---|---|---|---|---|
| B | p value | 95% confidence interval | B | p value | 95% confidence interval | |
| Received home-based disease management intervention (Yes) | −6.855 | <0.001 | −10.200, −3.510 | −25.00 | <0.001 | −27.825, −22.175 |
| QoL(T0) | 0.513 | <0.001 | 0.247, 0.779 | 0.324 | 0.005 | 0.099, 0.549 |
| Monthly income (≥ 5,000 Yuan) | −7.138 | <0.001 | −10.479, −3.796 | −3.452 | 0.017 | −6.274, −0.630 |
Multiple linear regression analysis of QoL at T1 and T2 for home-based disease management interventions.
T0: pre-intervention (baseline); T1: at the end of the intervention; T2: 3 months after the end of the intervention.
3.3 Comparing the readmission rates of the two patient groups
The chi-square test was used to statistically assess the readmissions, and the results revealed that at the finish of the intervention (T1), the intervention group’s readmission rate was significantly lower than the control groups, and the difference was statistically significant (p = 0.027). At 3 months after the end of the intervention (T2), the readmission rate of the intervention group was also significantly lower than that of the control group, and the difference was statistically significant (p = 0.033). See Table 6 for details.
Table 6
| Time | Items | Intervention group (n = 36) | Control group (n = 35) | c 2 value | p value |
|---|---|---|---|---|---|
| T1 | Readmission (Yes) | 3(8.3) | 10(28.6) | 4.860 | 0.027 |
| Depression (Yes) | 3(8.3) | 10(28.6) | 4.860 | 0.027 | |
| T2 | Readmission (Yes) | 7(19.4) | 15(42.9) | 4.549 | 0.033 |
| Depression (Yes) | 6(16.7) | 15(42.9) | 5.844 | 0.016 |
Comparison of readmission and depression between the two groups.
T1: at the end of the intervention; T2: 3 months after the end of the intervention.
3.4 Comparing the self-management behaviors of the two patient groups
The t-test for two independent samples was employed for analysis. The findings showed that patients in the intervention group had higher overall self-care behavior scores at T1 and T2 than patients in the control group at each time point, and the difference was statistically significant (p < 0.05). Repeated measures ANOVA was used to compare the differences in the total scores of self-care behaviors between the two groups of patients at different time points. The multivariate test results indicated that the time effect (F-time = 89.03), between-group effect (F-intergroup = 16.95), and interaction effect (F-interaction = 34.33) of the total self-care behavior scores in both groups showed statistically significant differences (p < 0.05). Details are presented in Table 4.
3.5 Comparing the depression of two patient groups
The results displayed that after the intervention, at T1, the incidence of depression in the intervention group was significantly lower than that in the control group (p = 0.027). At T2, the intervention group exhibited significantly lower incidence rates of depression compared to the control group (p = 0.016). Details are presented in Table 6.
3.6 Comparing the care burden of the two caregivers
Two independent samples t-test was used for analysis. The outcomes demonstrated that at T1 and T2, the total care burden scores of caregivers in the intervention group were lower than those of the control group at each time point, and the difference was statistically significant (p < 0.05). A repeated measures ANOVA was conducted to compare between-group differences in total caregiver burden scores across time points. The results of the multivariate test showed that the time effect (F-time = 311.02), the between-group effect (F- Intergroup = 40.63), and the interaction effect (F-interaction = 10.41) of the two care burden scores showed a statistically significant difference (p < 0.05), and the details are given in Table 4.
4 Discussion
The significantly lower Minnesota Living with Heart Failure Questionnaire (MLHFQ) total scores in the intervention group at both T1 and T2, coupled with a progressive improvement in their QoL, underscores the sustained benefit of the home-based, dyadically-focused management program. In addition, regression models confirmed the strong, sustained effect of the home-based intervention on QoL, which increased over time, indicating a cumulative benefit. This intervention effect persisted even as the protective influence of higher income diminished, suggesting the program may mitigate socioeconomic disparities. These results are consistent with the findings by Xiao et al. (37) in their breast cancer patient study. Foreign scholars have similarly suggested in the study that disease-specific education and guidance on dealing with symptoms are important for patients and their informal caregivers to cope with the disease and improve their QoL (38).
The efficacy of our intervention can be explained by its core mechanism: it systematically targets the dyadic relationship as the engine for sustainable health outcomes. From hospitalization through the post-discharge phase, the intervention reinforced a collaborative model where the functions of the patient and caregiver were complementary. By implementing synchronized education and rehabilitation guidance, we empowered both parties-enhancing the caregiver’s professional skills and illness coping abilities while providing the patient with more comprehensive support. This reciprocal process likely breaks the cycle of mutual distress and fosters a positive feedback loop of shared efficacy. This approach aligns with the core tenets of ideal home care as outlined by the Agency for Healthcare Research and Quality (39), which emphasizes meeting the needs of the care unit. Our study demonstrates that a paradigm shift from patient-centric to dyad-centric care is not only feasible but necessary for optimizing long-term outcomes in elderly CHF management. By addressing the patient-caregiver dyad as an integrated unit, healthcare providers can leverage this natural partnership to achieve a more profound and durable impact than by focusing on the patient alone.
The significantly lower readmission rate observed in the intervention group at both T1 and T2 can be attributed to the comprehensive, dyadically-structured intervention that transcended conventional patient education. While consistent with prior research on home-based management for chronic conditions (40) and systematic reviews affirming the value of intensive education (41, 42), our study elucidates a more nuanced mechanism: the reduction in readmission was likely mediated by the establishment of a coherent, collaborative patient-caregiver system. The efficacy of our intervention stems from its dual-focused strategy, which simultaneously augmented the capabilities of both patients and their informal caregivers. For patients, this involved enhancing self-management cognition, symptom recognition, and practical skills, fostering a more proactive understanding of their disease trajectory. For caregivers, systematic training clarified their roles and enhanced their supervisory and guidance competencies. Critically, by aligning the cognition and behaviors of both parties through a shared framework, the intervention mitigated dyadic incongruence-a state where discrepancies in illness perceptions and care goals between patients and caregivers lead to conflict and ineffective management (43). This reduction in dyadic stress and improvement in collaborative problem-solving likely served as the key mechanism preventing clinical deteriorations that typically precipitate readmission. This collaborative dyadic model breaks the cycle of readmissions by empowering the patient-caregiver unit, offering a cost-effective strategy for improving post-discharge outcomes in elderly CHF patients.
The sustained superiority of self-care behaviors in the intervention group at both T1 and T2, despite a post-intervention decline from peak levels, indicates that the dyadic intervention fostered a fundamental internalization of management skills rather than inducing only short-term compliance. This pattern of a high initial gain followed by stabilization at a level significantly above baseline aligns with Lu′s findings on the importance of skill consolidation (44), and contrasts with studies reporting a steeper decline after intervention cessation (43). In the process of home-based disease management for elderly patients with CHF, patients and informal caregivers often have cognitive differences in dimensions such as symptom recognition, cognition of self-care, medical decision-making, and confidence in self-care. This inconsistency will lead to insufficient participation of both parties in disease management, which in turn will cause the problem of reduced adherence to self-care in patients. By treating the dyad as an integrated unit and combining HF education with congruence assessment and collaborative training, our program facilitated a shared illness representation and unified action plan. This alignment likely transformed the dyad from a potential source of conflict into a cohesive, mutually reinforcing team, which is essential for maintaining complex self-care regimens over time. Furthermore, the program’s extended, follow-up provided a critical scaffold for this process. The longitudinal support allowed for the refinement and application of learned skills in the home environment, enabling dyads to proactively identify symptoms and initiate management strategies effectively, thereby preventing clinical deterioration (16). This continuous reinforcement mechanism appears to have been pivotal in translating initial behavioral gains into durable self-management capacity, suggesting that the sustainability of such interventions is contingent not only on their content but also on the duration and quality of post-instructional support.
Depression in heart failure patients are frequently underrecognized, yet they significantly impede clinical recovery and contribute to adverse long-term outcomes (45). The significantly lower incidence of depression in the intervention group at both T1 and T2 underscores the psychological benefits of a dyadic management approach. This finding aligns with prior research by Li (46) and extends it by revealing the potential mechanisms through which a structured, home-based program can mitigate depressive symptoms in elderly CHF patients. We propose that this psychological benefit was mediated by a dual-pathway mechanism. First, the intervention enhanced patients’ self-perceived health status-a known predictor of depression trajectory (47), by systematically equipping them and their caregivers with practical disease management skills. The ability to accurately recognize symptom changes and implement timely, correct coping measures fostered a sense of control and self-efficacy, directly countering feelings of helplessness and anxiety about disease progression. Second, the program strengthened the caregiver’s capacity to provide competent, scientifically grounded support. This not only improved the practical management of the illness but also significantly enhanced the patient’s psychological security by creating a reliable and effective support system (48).
The results of this study revealed that at both T1 and T2 assessment time points, the Zarit Burden Interview (ZBI) scores of informal caregivers in the intervention group were lower than those in the control group (p < 0.05). This is in line with a study conducted abroad (49). The daily unpredictability of heart failure (i.e., acute exacerbations and hospitalizations), medication burden and side effects, and patient overdependence result in a non-negligible caregiving burden for caregivers, which may contribute to caregivers becoming somewhat less engaged in the management of the patient’s disease or even abandoning the caregiving role (50). Our intervention directly counteracted these challenges by maintaining substantial, high-quality communication with caregivers. Through continuous education, value clarification, and responsive support, we facilitated their successful transition into a “skilled caregiver” role. This empowerment enabled them to perform caregiving tasks with greater competence and confidence, thereby reducing the physical and psychological strain associated with unmanaged complexities. In addition, this study, which focused on CHF patients and their caregivers as the intervention unit, significantly improved the concordance of both parties’ assessment of clinical symptoms, including shortness of breath, fatigue, pain, and edema. When patients and caregivers converged on symptom perceptions, their caregiving collaboration was more coordinated, which not only improved caregiving experience and outcomes, but also reduced the risk of negative emotions for both partners. This synergistic effect prompted patients and caregivers to engage in home heart failure management together with a more positive and optimistic attitude, thus effectively alleviating the caregiver’s burden of care.
5 Conclusion
This study developed a home-based disease management program that demonstrates multiple clinical benefits for elderly patients with CHF: it significantly improves QoL, reduces readmission rates, enhances self-management capacity, and effectively alleviates negative emotions. Notably, the program also shows remarkable efficacy in reducing caregiving burden for informal caregivers. These findings provide new practical evidence and intervention strategies for long-term management of CHF and optimization of home-based care models for chronic diseases. Disease management for CHF patients is a long-term process, during which both patients and caregivers may encounter new challenges at different stages. Continuous follow-up and timely support are crucial for sustaining intervention effects. However, due to limitations in time and human resources, this study only assessed outcomes at 3 months post-intervention. Additionally, participants were recruited from only one tertiary hospital in Jiangsu Province, which may not fully account for cultural differences and varying levels of acceptance. In future studies, we will enlarge the sample size, extend the intervention and follow-up duration, and actively explore dyadic intervention models tailored to China’s specific healthcare context, with the aim of further refining and validating our findings in subsequent research.
6 Limitation
However, due to limitations in time and human resources, this study only assessed outcomes at 3 months post-intervention. Additionally, participants were recruited from only one tertiary hospital in Jiangsu Province, which may not fully account for cultural differences and varying levels of acceptance. In future studies, we will enlarge the sample size, extend the intervention and follow-up duration, and actively explore dyadic intervention models tailored to China’s specific healthcare context, with the aim of further refining and validating our findings in subsequent research. Currently, high-quality research on dual interventions is scarce. This restricts the evidence base for translating dual disease management interventions targeting elderly patients with chronic heart failure who live at home and their informal caregivers into widespread practice. Further research is needed into the mechanisms through which dual interventions influence disease management behaviors among patients and informal caregivers.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Medical Research Ethics Committee of Affiliated Hospital of Jiangnan University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
TZ: Writing – original draft, Writing – review & editing. YH: Funding acquisition, Writing – original draft, Methodology. LZ: Writing – original draft, Formal analysis, Data curation. PZ: Writing – original draft, Data curation. XJ: Writing – review & editing, Writing – original draft, Data curation. JW: Writing – original draft, Data curation. HS: Methodology, Writing – original draft. CZ: Methodology, Visualization, Conceptualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by Jiangsu Province Hospital Association (grant no. JSYGY-3-2023-125), Jiangsu Commission of Health (grant no. ZDA2020023), and nursing research “seed” program by Affiliated Hospital of Jiangnan University (grant no. HL-25-Z03). The funders played no role in study design, data collection, analysis, interpretation, or manuscript preparation.
Acknowledgments
We are deeply grateful to the participants and their families, and we sincerely thank everyone who assisted with this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1679743/full#supplementary-material
References
1.
Khan MS Shahid I Bennis A Rakisheva A Metra M Butler J . Global epidemiology of heart failure. Nat Rev Cardiol. (2024) 21:717–34. doi: 10.1038/s41569-024-01046-6
2.
Tsao CW Aday AW Almarzooq ZI Anderson CAM Arora P Avery CL et al . Heart disease and stroke Statistics-2023 update: a report from the American Heart Association. Circulation. (2023) 147:e93–e621. doi: 10.1161/CIR.0000000000001123
3.
Wang H Li YY Chai K Zhang W Li XL Dong XG et al . Contemporary epidemiology and treatment of hospitalized heart failure patients in real clinical practice in China. Chin J Cardiol. (2019) 47:865–74. doi: 10.3760/cma.j.issn.0253-3758.2019.11.004
4.
The writing committee of the report on cardiovascular health and diseases in China . Report on cardiovascular health and diseases in China 2022: an updated summary. Chin Circ J. (2023) 38:583–612. doi: 10.3969/j.issn.1000-3614.2023.06.001
5.
Heidenreich PA Bozkurt B Aguilar D Allen LA Byun JJ Colvin MM et al . 2022 AHA/ACC/HFSA guideline for the Management of Heart Failure: executive summary: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. J Am Coll Cardiol. (2022) 79:1757–80. doi: 10.1016/j.jacc.2021.12.011
6.
McDonagh TA Metra M Adamo M Gardner RS Baumbach A Böhm M et al . 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. (2021) 42:3599–726. doi: 10.1093/eurheartj/ehab368
7.
Wang H Li Y . Chinese expert consensus 2022 on comprehensive management of patients with exacerbations of chronic heart failure. Chin J Circ. (2022) 37:215–25. doi: 10.3969/j.issn.1000-3614.2022.03.003
8.
Cherukara A Rudski L Grinman M Levine DM . Bringing heart care home: management of acute cardiovascular pathologies in the home hospital. Eur Heart J Acute Cardiovasc Care. (2025) 14:364–74. doi: 10.1093/ehjacc/zuaf053
9.
Wu ZY Yang FK Niu ME Zhao Q . Development and application of a context-awareness risk management checklist for COPD patients in home setting. J Nurs Sci. (2023) 38:97–115. doi: 10.3870/j.issn.1001-4152.2023.20.097
10.
Zhang N Li Q Chen S Wu Y Xin B Wan Q et al . Effectiveness of nurse-led electronic health interventions on illness management in patients with chronic heart failure: a systematic review and meta-analysis. Int J Nurs Stud. (2024) 150:104630. doi: 10.1016/j.ijnurstu.2023.104630
11.
Habibzadeh H Shariati A Mohammadi F Babayi S . The effect of educational intervention based on Pender's health promotion model on quality of life and health promotion in patients with heart failure: an experimental study. BMC Cardiovasc Disord. (2021) 21:478. doi: 10.1186/s12872-021-02294-x
12.
Wingham J Harding G Britten N Dalal H . Heart failure patients' attitudes, beliefs, expectations and experiences of self-management strategies: a qualitative synthesis. Chronic Illn. (2014) 10:135–54. doi: 10.1177/1742395313502993
13.
Jiang SX Li XD Zhang XN Li YT Yang WL Zang XY et al . Real-life experience of disease management at home in elderly patients with chronic heart failure. Chin Nurs Manag. (2022) 22:1061–5. doi: 10.3969/j.issn.1672-1756.2022.07.019
14.
Li JX Wang L Qu J Sun XN . General situation and influencing factors of readmission in patients with chronic heart failure. South China J Prev Med. (2024) 50:323–8. doi: 10.12183/j.scjpm.2024.0323
15.
Badr H Herbert K Chhabria K Sandulache VC Chiao EY Wagner T . Self-management intervention for head and neck cancer couples: results of a randomized pilot trial. Cancer. (2019) 125:1176–84. doi: 10.1002/cncr.31906
16.
Bidwell JT Conway C Babicheva V Lee CS . Person with heart failure and care partner dyads: current knowledge, challenges, and future directions: state-of-the-art review. J Card Fail. (2023) 29:1187–206. doi: 10.1016/j.cardfail.2023.02.017
17.
Lou Y Zhang M Zou Y Zhao L Chen Y Qiu Y . Facilitators and barriers in managing older chronic heart failure patients in community health care centers: a qualitative study of medical personnel's perspectives using the socio-ecological model. Front Health Serv. (2025) 5:1483758. doi: 10.3389/frhs.2025.1483758
18.
Yang PP Fan YY Ma MZ Du XB . Current status of research on caregiving experiences and needs of family caregivers of patients with chronic heart failure. Chin Nurs Res. (2021) 35:3469–73. doi: 10.12102/j.issn.1009-6493.2021.19.018
19.
Martire LM Schulz R Helgeson VS Small BJ Saghafi EM . Review and meta-analysis of couple-oriented interventions for chronic illness. Ann Behav Med. (2010) 40:325–42. doi: 10.1007/s12160-010-9216-2
20.
Lyons KS Lee CS . The theory of dyadic illness management. J Fam Nurs. (2018) 24:8–28. doi: 10.1177/1074840717745669
21.
Yingnan Z Shulin Z Minxia L Qiao Z Xiaoqing S . Patient and informal caregiver-centered nursing interventions for adults with heart failure: a systematic review and meta-analysis. Intensive Crit Care Nurs. (2025) 88:103943. doi: 10.1016/j.iccn.2025.103943
22.
Lambert SD Duncan LR Ellis J Schaffler JL Loban E Robinson JW et al . Acceptability and usefulness of a dyadic, tailored, web-based, psychosocial and physical activity self-management program (TEMPO): a qualitative study. J Clin Med. (2020) 9:3284. doi: 10.3390/jcm9103284
23.
Nie YX Song J Xie YQ Jin XQ . Implementation of a discharge preparation service program for elderly lung cancer patients based on the theory of dyadic illness management. J Nurs Sci. (2022) 37:20–4. doi: 10.3870/j.issn.1001-4152.2022.18.020
24.
Yu G Cui JJ . Effect of dyadic coping model on quality of life and intimacy between their spouses in patients with chronic heart failure. J Nurs (China). (2019) 26:63–7. doi: 10.16460/j.issn1008-9969.2019.16.063
25.
Son YJ Jang I . One-year trajectories of self-care behaviours and unplanned hospital readmissions among patients with heart failure: a prospective longitudinal study. J Clin Nurs. (2023) 32:6427–40. doi: 10.1111/jocn.16658
26.
Hu Y Yu L Zhu L Li L Li X Wang X et al . Effects of home disease management strategies based on the dyadic illness management theory on elderly patients with chronic heart failure and informal caregivers' physical and psychological outcomes: protocol of a randomized controlled trial. Trials. (2024) 25:667. doi: 10.1186/s13063-024-08523-w
27.
Cameron JI Gignac MA . “Timing it right”: a conceptual framework for addressing the support needs of family caregivers to stroke survivors from the hospital to the home. Patient Educ Couns. (2008) 70:305–14. doi: 10.1016/j.pec.2007.10.020
28.
Granger BB Moser D Germino B Harrell J Ekman I . Caring for patients with chronic heart failure: the trajectory model. Eur J Cardiovasc Nurs. (2006) 5:222–7. doi: 10.1016/j.ejcnurse.2006.02.001
29.
Zhu YB Hideki O Zheng J Huo YM . Evaluation of the reliability and validity of heart failure QOL (LHFQ) Chinese vision. Chin Behav Med Sci. (2004) 13:337–9. doi: 10.3760/cma.j.issn.1674-6554.2004.03.056
30.
Huang LL Xu Q Shen JH Li W . Investigation of unplanned readmission rate in 228 patients with heart failure and the establishment of logistic prediction model. Chin Cardiovasc Dis Res. (2022) 20:811–6. doi: 10.3969/j.issn.1672-5301.2022.09.006
31.
Guo JY Li Z Kang XF . Chineseization and reliability testing of the heart failure self-care index scale. Chin J Nurs. (2012) 47:653–5. doi: 10.3761/j.issn.0254-1769.2012.07.028
32.
Zigmond AS Snaith RP . The hospital anxiety and depression scale. Acta Psychiatr Scand. (1983) 67:361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x
33.
Zheng LL Wang YL Li HC . Application of hospital anxiety and depression scale in general hospital: an analysis in reliability and validity. Shanghai Arch Psychiatry. (2003) 5:264–6. doi: 10.3969/j.issn.1002-0829.2003.05.003
34.
Wang L Yang XS Hou Z Feng QL Marui E . Application and evaluation of Chinese version of zarit caregiver burden interview. Chin J Public Health. (2006) 22:970–2. doi: 10.3321/j.issn:1001-0580.2006.08.040
35.
Zarit SH Femia EE Kim K Whitlatch CJ . The structure of risk factors and outcomes for family caregivers: implications for assessment and treatment. Aging Ment Health. (2010) 14:220–31. doi: 10.1080/13607860903167861
36.
Dong F Li C Peng XX Qin HQ . Meaning, calculation methods and considerations of sample content calculation in clinical studies. Chin J Stroke. (2009) 4:854–9. doi: 10.3969/j.issn.1673-5765.2009.10.020
37.
Xiao LF . Application of hospital discharge preparation service in breast cancer patients based on binary disease management theory. Chin Evid Based Nurs. (2025) 11:789–92. doi: 10.12102/j.issn.2095-8668.2025.04.037
38.
Sedlar N Lainscak M Farkas J . Living with chronic heart failure: exploring patient, informal caregiver, and healthcare professional perceptions. Int J Environ Res Public Health. (2020) 17:2666. doi: 10.3390/ijerph17082666
39.
Brach C Borsky A . How the U.S. Agency for Healthcare Research and Quality promotes health literate health care. Stud Health Technol Inform. (2020) 269:313–23. doi: 10.3233/SHTI200046
40.
Wang LH Zhao Y Chen LY Zhang L Zhang YM . The effect of a nurse-led self-management program on outcomes of patients with chronic obstructive pulmonary disease. Clin Respir J. (2020) 14:148–57. doi: 10.1111/crj.13112
41.
Tian C Zhang J Rong J Ma W Yang H . Impact of nurse-led education on the prognosis of heart failure patients: a systematic review and meta-analysis. Int Nurs Rev. (2024) 71:180–8. doi: 10.1111/inr.12852
42.
Takeda A Martin N Taylor RS Taylor SJ . Disease management interventions for heart failure. Cochrane Database Syst Rev. (2019) 1:CD002752. doi: 10.1002/14651858
43.
Bugajski A Buck H Zeffiro V Morgan H Szalacha L Alvaro R et al . The influence of dyadic congruence and satisfaction with dyadic type on patient self-care in heart failure. Eur J Cardiovasc Nurs. (2021) 20:268–75. doi: 10.1177/1474515120960002
44.
Lu XX Luo CF Long TL . The effect of dyadic illness management-based intervention on self-care behavior consistency between chronic heart failure patients and their caregivers. Gansu Med J. (2024) 43:171–5. doi: 10.15975/j.cnki.gsyy.2024.02.030
45.
Carrillo A Belnap BH Rothenberger SD Feldman R Rollman BL Celano CM . Psychosocial predictors of health behavior adherence in heart-failure patients with comorbid depression: a secondary analysis of the hopeful heart trial. BMC Psychol. (2024) 12:328. doi: 10.1186/s40359-024-01816-4
46.
Li HM Li FM Yang MX . The effect of holistic nursing intervention on anxiety, depression and quality of life of patients with chronic heart failure. Int J Psychiatry. (2021) 48:571–4. doi: 10.13479/j.cnki.jip.2021.03.052
47.
Pang JX Liu QK Xu Y Zeng CL Ma XQ Xie H . Trajectory and predictors of depressive symptoms in disabled middle-aged and elderly adults. Chin J Mod Nurs. (2025) 31:347–53. doi: 10.3760/cma.j.cn115682-20240411-02007
48.
Dalal HM Taylor RS Wingham J Greaves CJ Jolly K Lang CC et al . A facilitated home-based cardiac rehabilitation intervention for people with heart failure and their caregivers: a research programme including the REACH-HF RCT. Southampton (UK): NIHR Journals Library (2021).
49.
Lyons KS Johnson SH Lee CS . The role of symptom appraisal, concealment and social support in optimizing dyadic mental health in heart failure. Aging Ment Health. (2021) 25:734–41. doi: 10.1080/13607863.2020.1711866
50.
Jackson JD Cotton SE Bruce Wirta S Proenca CC Zhang M Lahoz R et al . Burden of heart failure on caregivers in China: results from a cross-sectional survey. Drug Des Devel Ther. (2018) 12:1669–78. doi: 10.2147/DDDT.S148970
Summary
Keywords
chronic heart failure, disease management, dyadic health, informal caregivers, quality of life, readmission
Citation
Zhou T, Hu Y, Zhu L, Zhu P, Ji X, Wang J, Shang H and Zhao C (2025) Effects of home disease management strategies based on the dyadic illness management theory on elderly patients with chronic heart failure and informal caregivers’ physical and psychological outcomes: a randomized controlled trial. Front. Med. 12:1679743. doi: 10.3389/fmed.2025.1679743
Received
07 August 2025
Accepted
27 October 2025
Published
18 November 2025
Volume
12 - 2025
Edited by
Grazia Daniela Femminella, University of Naples Federico II, Italy
Reviewed by
Simonetta Scalvini, Scientific Clinical Institute Maugeri (ICS Maugeri), Italy
Muhammad Eid Akkawi, International Islamic University Malaysia, Malaysia
Updates
Copyright
© 2025 Zhou, Hu, Zhu, Zhu, Ji, Wang, Shang and Zhao.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chence Zhao, 329349583@qq.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.