- 1Department of Orthopedics and Traumatology, Tai’an Traditional Chinese Medicine Hospital, Tai’an, Shandong, China
- 2Department of Emergency, Jinan Central Hospital, Jinan, Shandong, China
Background: Piroxicam is a widely used antipyretic and analgesic. Due to an increasing number of adverse event (AE) reports, effective pharmacovigilance is essential for evaluating its benefit-risk profile.
Methods: We assessed the safety profile of piroxicam through disproportionality analysis based on all AE reports involving the drug in the FDA Adverse Event Reporting System (FAERS) from 2004 till 2024. Signal detection was performed using the reporting odds ratio, proportional reporting ratio, multi-item gamma Poisson shrinker, and Bayesian confidence propagation neural network methods. The Weibull distribution was applied to model time-to-onset of AEs. Analyses were stratified by age and sex, and subgroup patterns were examined. AE outcomes were categorized accordingly.
Results: Our findings confirmed known label-reported AEs, such as hypersensitivity, urticaria, gastric ulcers, and gastrointestinal hemorrhage. Additionally, several potentially less well-known AEs were identified, such as acute generalized exanthematous pustulosis, blister formation, and urinary retention. Subgroup analyses revealed significant variations in AE patterns across different age groups and sexes. The majority of AEs occurred during the early stages of treatment, highlighting the importance of vigilant monitoring of AEs especially during initial dosing.
Conclusion: This real-world study reinforces established safety concerns related to piroxicam while identifying potentially less well-known safety signals. These findings offer valuable insights for clinicians aiming to optimize patient safety during piroxicam therapy.
1 Introduction
Piroxicam is a non-steroidal anti-inflammatory drug (NSAID) belonging to the oxicam class, commonly prescribed for managing acute pain and chronic inflammatory diseases such as osteoarthritis and rheumatoid arthritis due to its analgesic, anti-inflammatory, and antipyretic properties (1). Arthritis, particularly its osteoarthritis and rheumatoid arthritis forms, presents a significant public health challenge, affecting the quality of life and daily functioning (2, 3). Chronic pain associated with arthritis can lead to physical limitations and psychological distress, including depression and anxiety (4). The social burden of arthritis-induced pain is substantial, with implications that extend beyond individual discomfort.
Piroxicam exerts its effects by inhibiting cyclooxygenase enzymes (COX-1 and COX-2), thereby reducing in the synthesis of prostaglandins key mediators of pain and inflammation (5). A notable advantage of piroxicam is its long half-life, which allows once-daily dosing, thereby improving patient compliance compared with other NSAIDs requiring multiple doses throughout the day (6). It has also demonstrated efficacy in providing pre-emptive analgesia, significantly reducing postoperative pain and minimizing the need for additional analgesics (7). However, its safety profile warrants careful consideration. Common side effects include gastrointestinal disturbances such as nausea, vomiting, dyspepsia, and more severe complications like gastric ulcers or bleeding due to COX-1 inhibition (8). Long-term use of piroxicam has also been linked to an increased risk of cardiovascular events, particularly in patients with pre-existing heart diseases (9).
The FDA Adverse Event Reporting System (FAERS) is a database created by the U.S. Food and Drug Administration (FDA) to collect and evaluate reports on drug-related adverse events (AEs) (10). This vital pharmacovigilance tool thus aids the U.S. FDA in recognizing possible drug-related safety issues. By analyzing FAERS data, this study assessed the real-world safety of piroxicam, offering healthcare professionals crucial information for making informed prescribing decisions.
2 Materials and methods
2.1 Data sources, management, and study design
This research used raw data sourced from the FAERS database–a non-mandatory, publicly accessible reporting system that primarily receives submissions from consumers, pharmacists, physicians, and various other stakeholders. The analysis included all reports of AEs in the original ASCII data packet format, wherein piroxicam was recognized as the main drug of concern, covering the timeframe from the first quarter of 2004 through the fourth quarter of 2024.
In terms of data management, this process primarily focused on the removal of duplicate reports and the standardization of AE terminology. The approach for addressing duplicate reports followed the procedures recommended by the FDA. For reports sharing the same case identifier (CASEID), the report with the most recent FDA receipt date (FDA_DT) was preserved. In cases where the CASEID and FDA_DT values were identical, reports possessing the largest unique identifier (PRIMARYID) were retained. In addition, the MedDRA dictionary 27.1 was used to standardize AE terminology, which included preferred terms (PTs) and the corresponding system organ classifications (SOC), so as to improve the reliability of subsequent statistical analysis. A comprehensive flowchart outlining the study design has been presented in Figure 1.
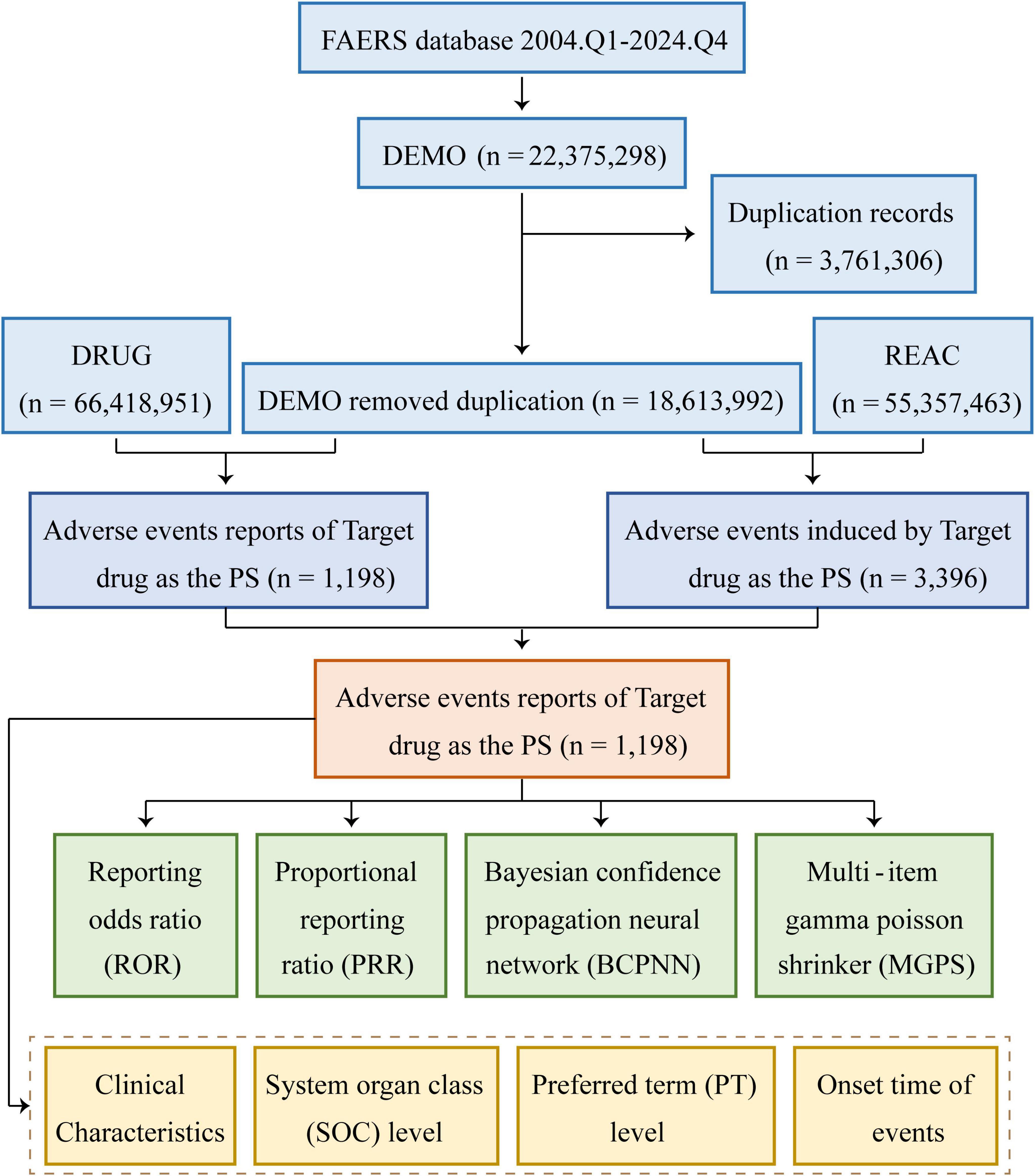
Figure 1. Flowchart demonstrating the AE analysis process for piroxicam using the FAERS database. DEMO, demographics; REAC, reaction; PS, primary suspect.
2.2 Statistical analysis
A descriptive analysis was performed to outline the characteristics of AE reports linked to piroxicam. Four methods for disproportionate analysis were thereby applied to identify signals indicating potential adverse reactions to piroxicam. These methods included the reporting odds ratio (ROR) (11), proportional reporting ratio (PRR) (12), Bayesian confidence propagation neural network (BCPNN) (13), and multi-item gamma Poisson shrinker (MGPS) (14). An AE was deemed a potential reaction if it surpassed the positive threshold in at least one of these methods (10). Supplementary Table 1 shows the detailed two-by-two contingency tables, whereas Supplementary Table 2 lists the formulas and thresholds used for various disproportionate analyses.
The onset timing of AEs connected to piroxicam was characterized based on the period between the occurrence of reported AEs [as sourced from the DEMO (Demonstration) file] and the initiation of Piroxicam treatment [derived from the THER file (Therapeutic Period File)]. In order to simulate the temporal fluctuations in the occurrence rate of AEs, Weibull distribution was employed. The Weibull distribution was selected primarily due to its superior flexibility in characterizing time-to-event data–a critical advantage over the exponential and log-normal distributions–especially in capturing the dynamic hazard patterns of drug-induced AEs. All analyses were conducted using SAS software version 9.4.
3 Results
3.1 Clinical characteristics
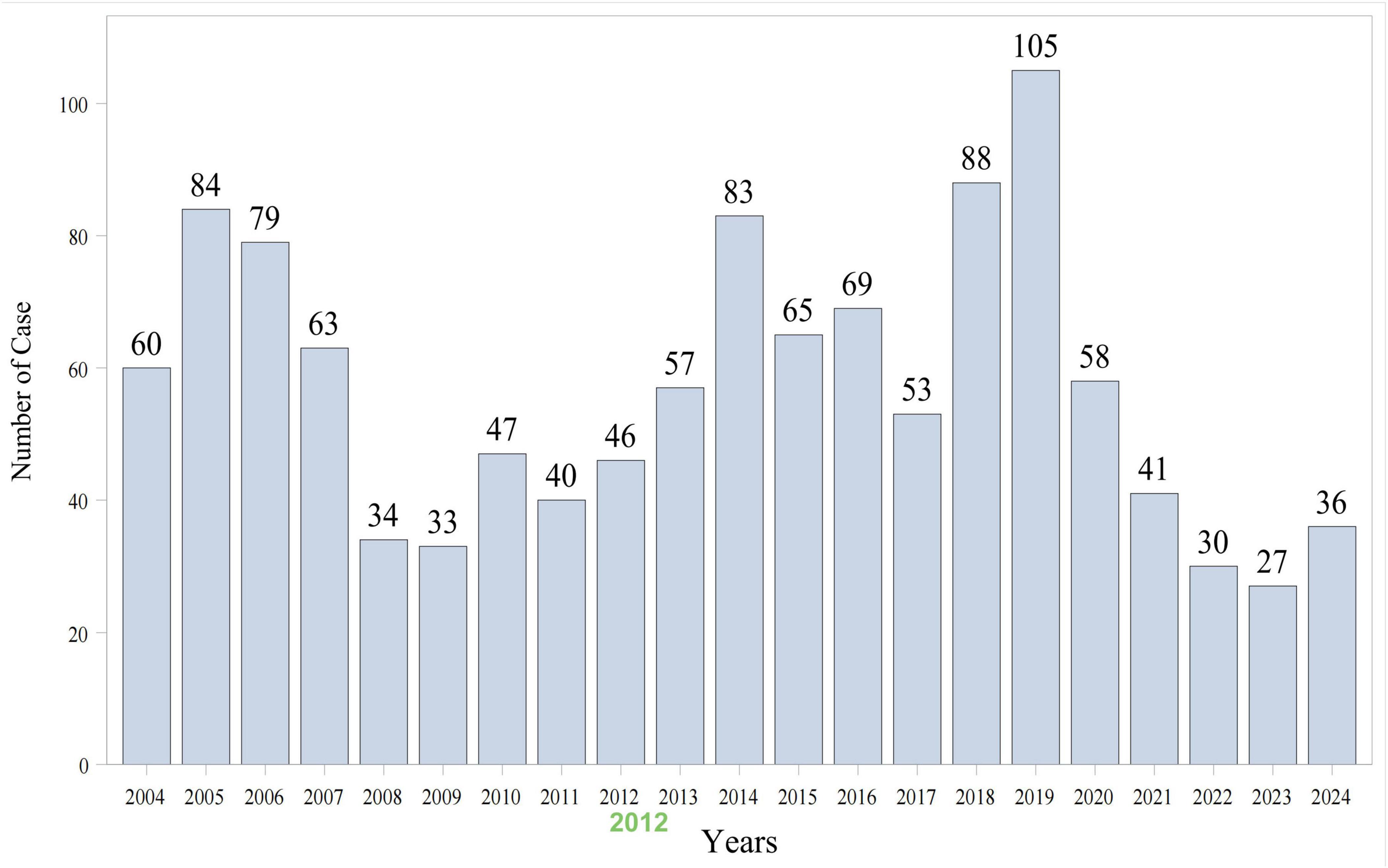
As shown in Figure 1, data from 84 quarters (Q1 2004 to Q4 2024) included 18,613,992 patients and 55,357,463 AEs. Piroxicam was listed as the primary suspect drug in 1,198 cases, corresponding to 3,396 AEs. Table 1 presents the clinical characteristics of piroxicam-related events. Of these cases, 60% (n = 715) were female patients, while 32% (n = 382) were male patients. The majority were aged ≥65 years (28%, n = 340), followed by those aged 45–64 years (26%, n = 307). Most reports were submitted by physicians (35%, n = 418), followed by consumers (25%, n = 301) and other healthcare professionals (19%, n = 224). Geographically, the majority of reports originated from the United States (43%, n = 510), France (15%, n = 174), and Brazil (8%, n = 91). In terms of outcomes, 29% (n = 353) of cases were hospitalized or had prolonged hospital stay. AE reporting showed a fluctuating pattern over the years, peaking in 2018 (7%, n = 88) and 2019 (9%, n = 105) (Figure 2).
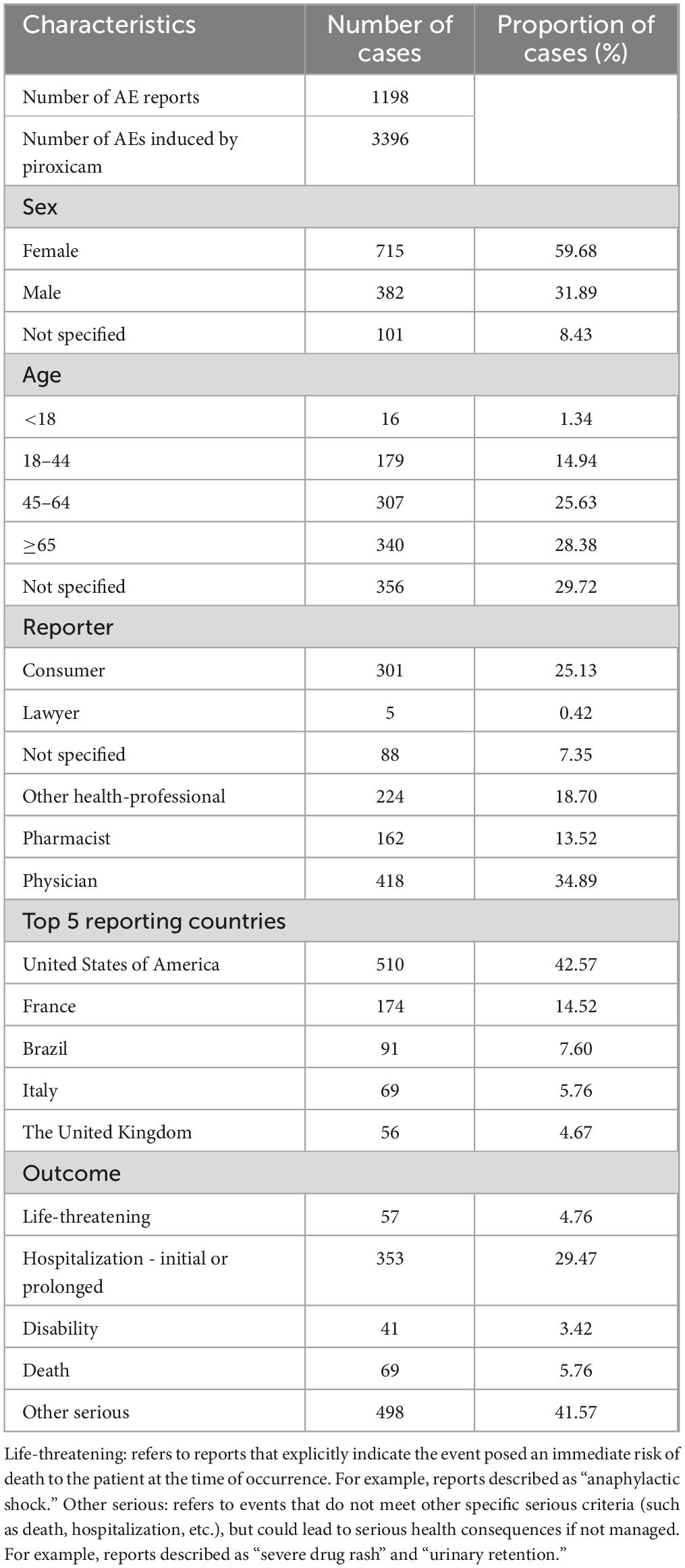
Table 1. Clinical characteristics of AE reports related to piroxicam from the FAERS database (Q1 2004–Q4 2024).
3.2 Distribution of AEs at the SOC level
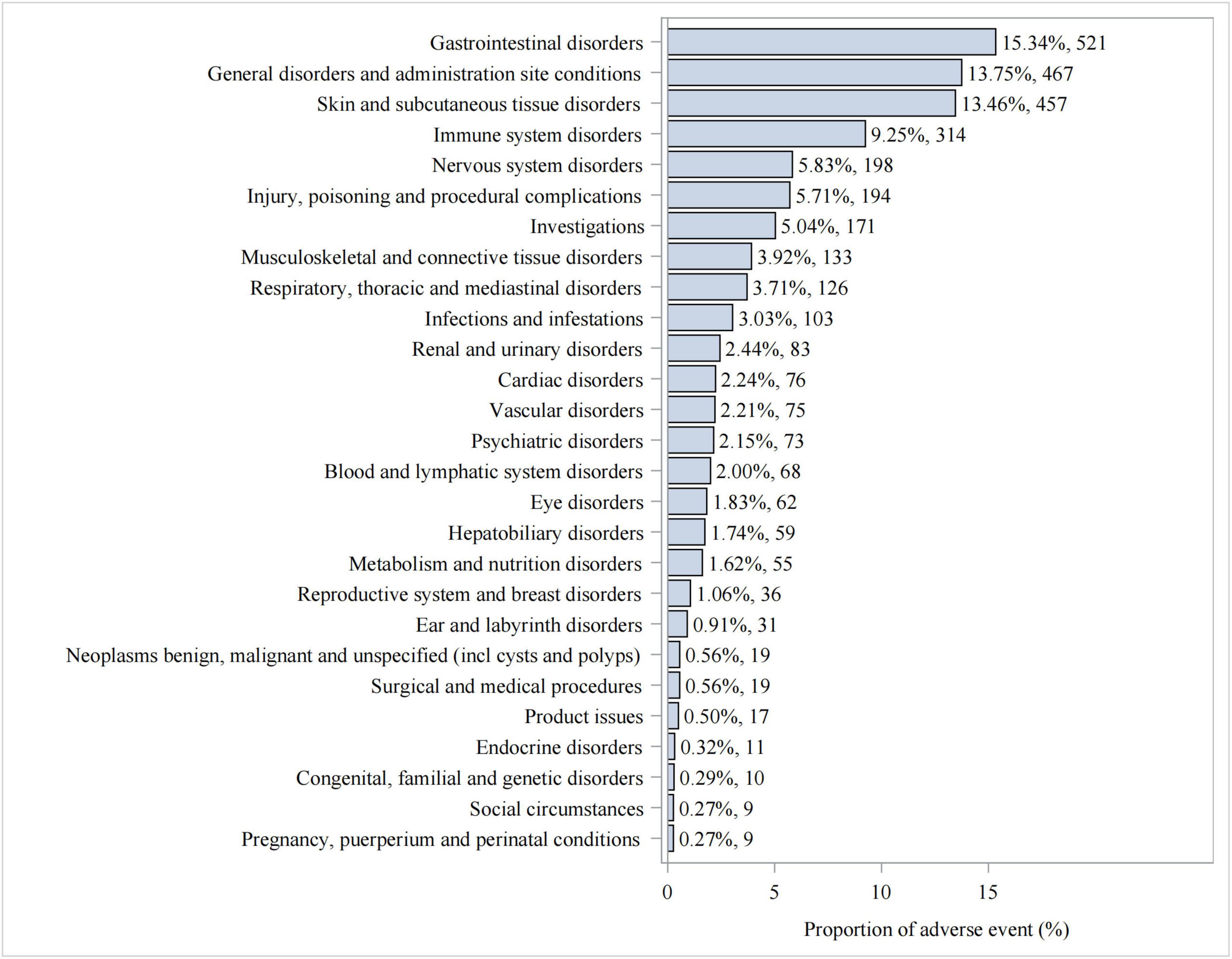
We statistically identified 27 system organ classes associated with piroxicam-induced AEs, suggesting the wide-ranging effects of piroxicam. As shown in Table 2, the strongest signal emerged in immune system disorders (SOC: 10021428, n = 314), while gastrointestinal disorders (SOC: 10017947, n = 521) were the most frequently reported. Significant AEs were also noted in skin and subcutaneous tissue disorders (SOC: 10040785, n = 457) and ear and labyrinth disorders (SOC: 10013993, n = 31). Additionally, hepatobiliary disorders (SOC: 10019805, n = 59); endocrine disorders (SOC: 10014698, n = 11); and respiratory, thoracic, and mediastinal disorders (SOC: 10038738, n = 126) were noteworthy. The latter, in particular, represents a less well-known AE not documented in the current piroxicam label information. The distribution of AEs at the SOC level is presented in Figure 3.
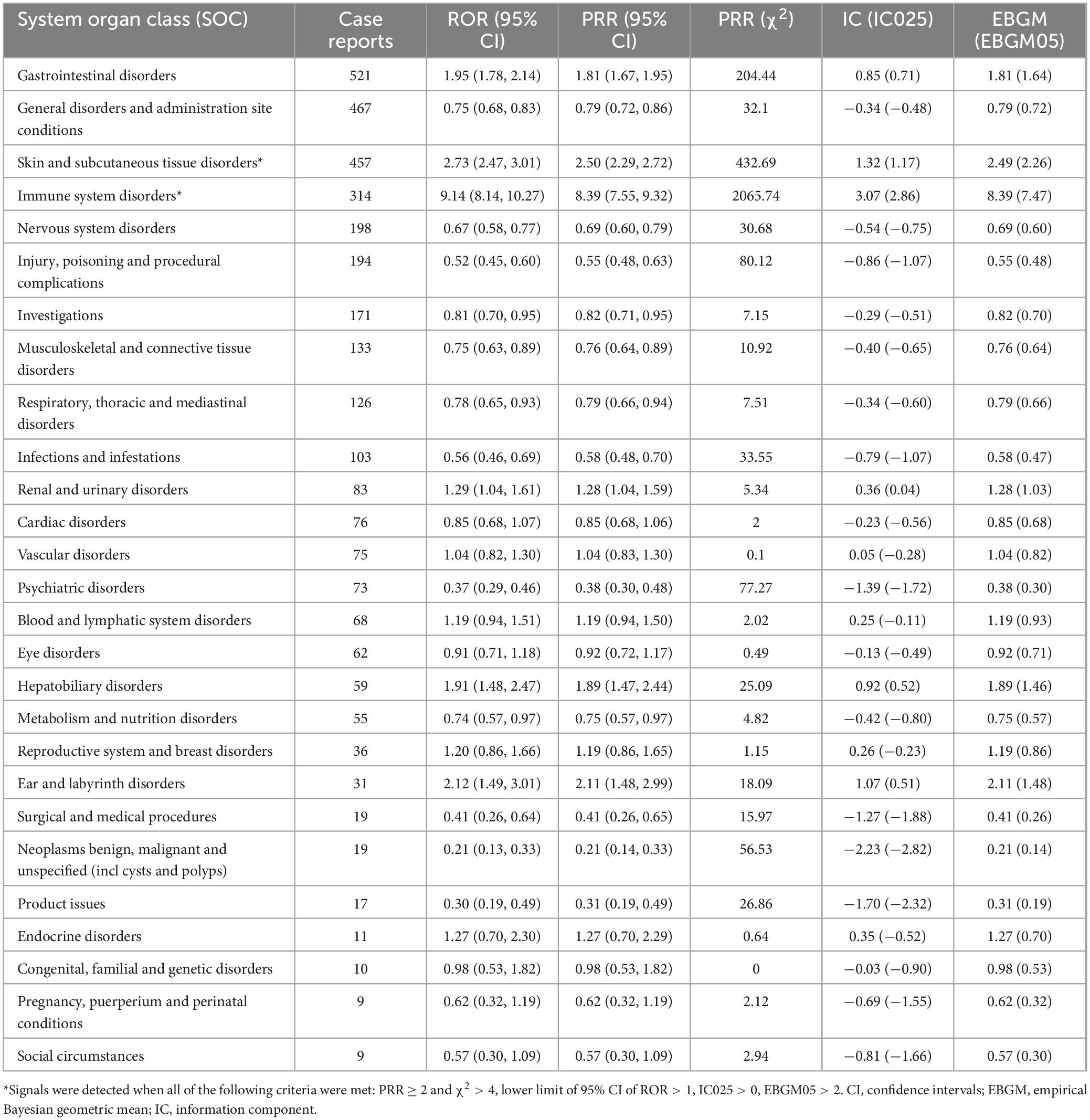
Table 2. Signal intensity of piroxicam-related AEs at the system organ classification (SOC) level in the FAERS database.
3.3 Distribution of AEs at the PT level
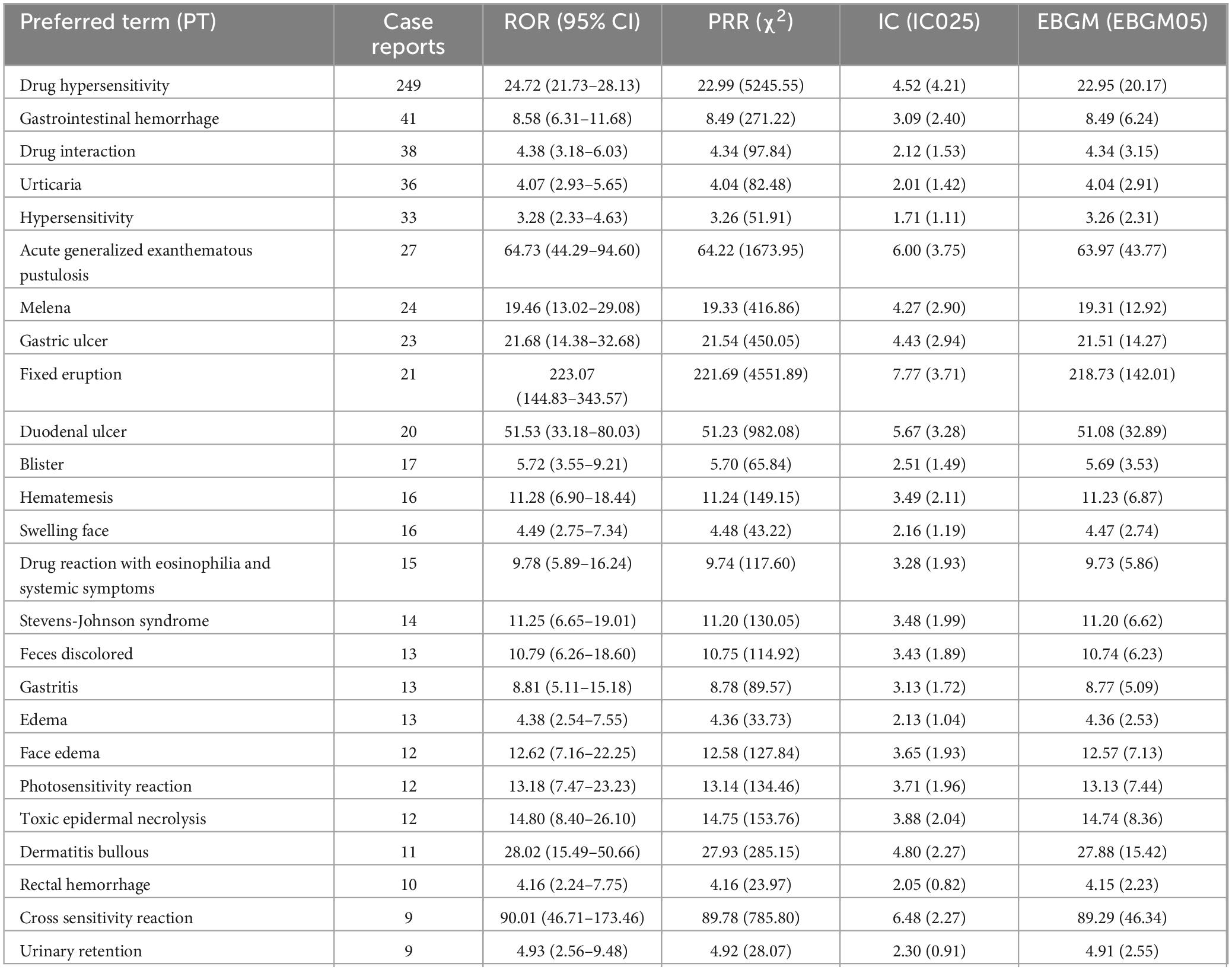
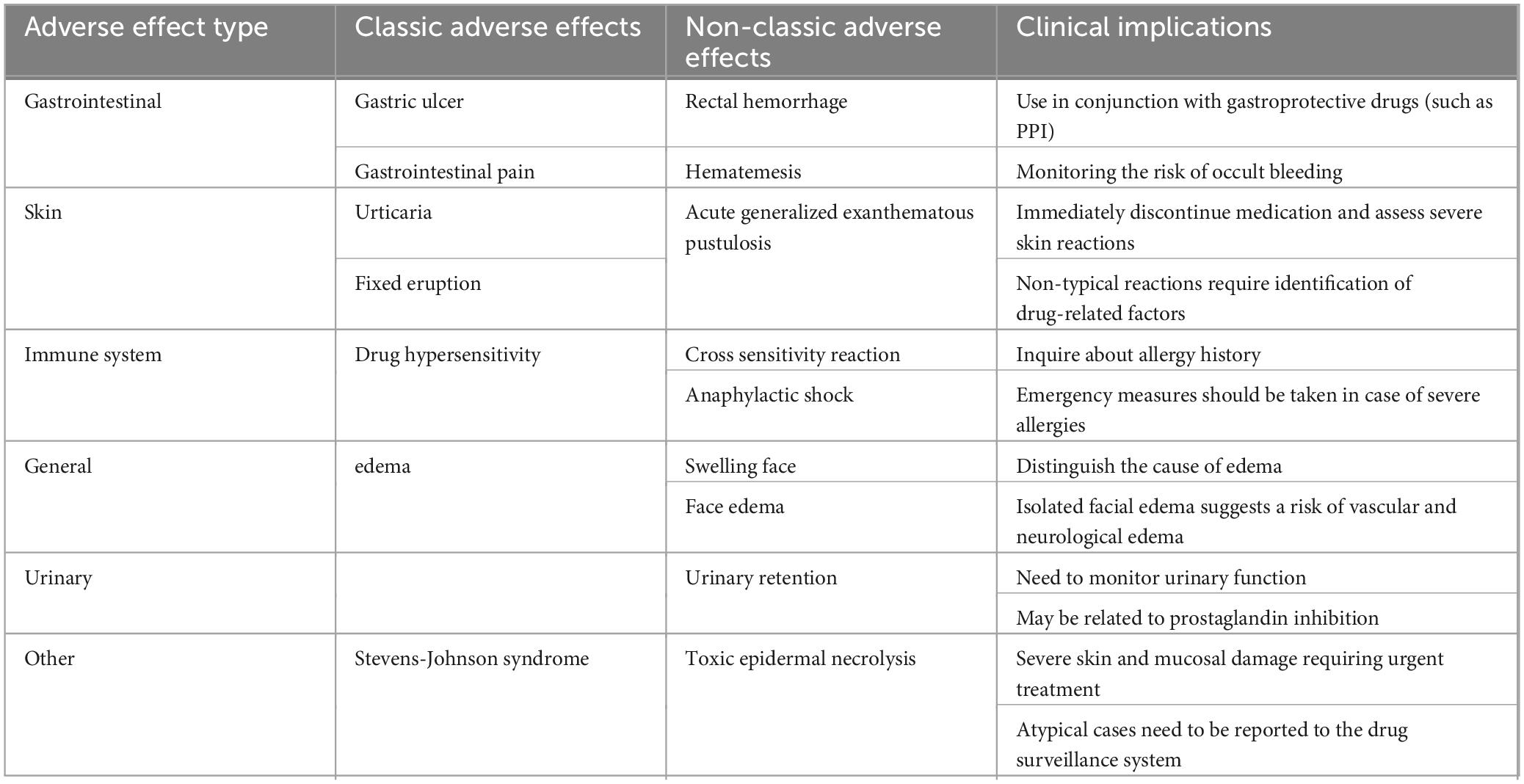
Table 3 lists the 25 most commonly reported PTs. Known side effects such as hypersensitivity, gastric ulcer, urticaria, edema, and eosinophilia were frequently reported. Severe or rare adverse reactions included gastrointestinal hemorrhage, Stevens–Johnson syndrome, and photosensitivity. Unlabeled AEs such as acute generalized exanthematous pustulosis (AGEP), blister formation, and urinary retention also appeared, suggesting potential new safety concerns. A comprehensive list of all reported PT-level AEs is provided in Supplementary Table 3. Table 4 outlines the clinical significance of typical and atypical AEs of piroxicam.
3.4 Onset time of AEs
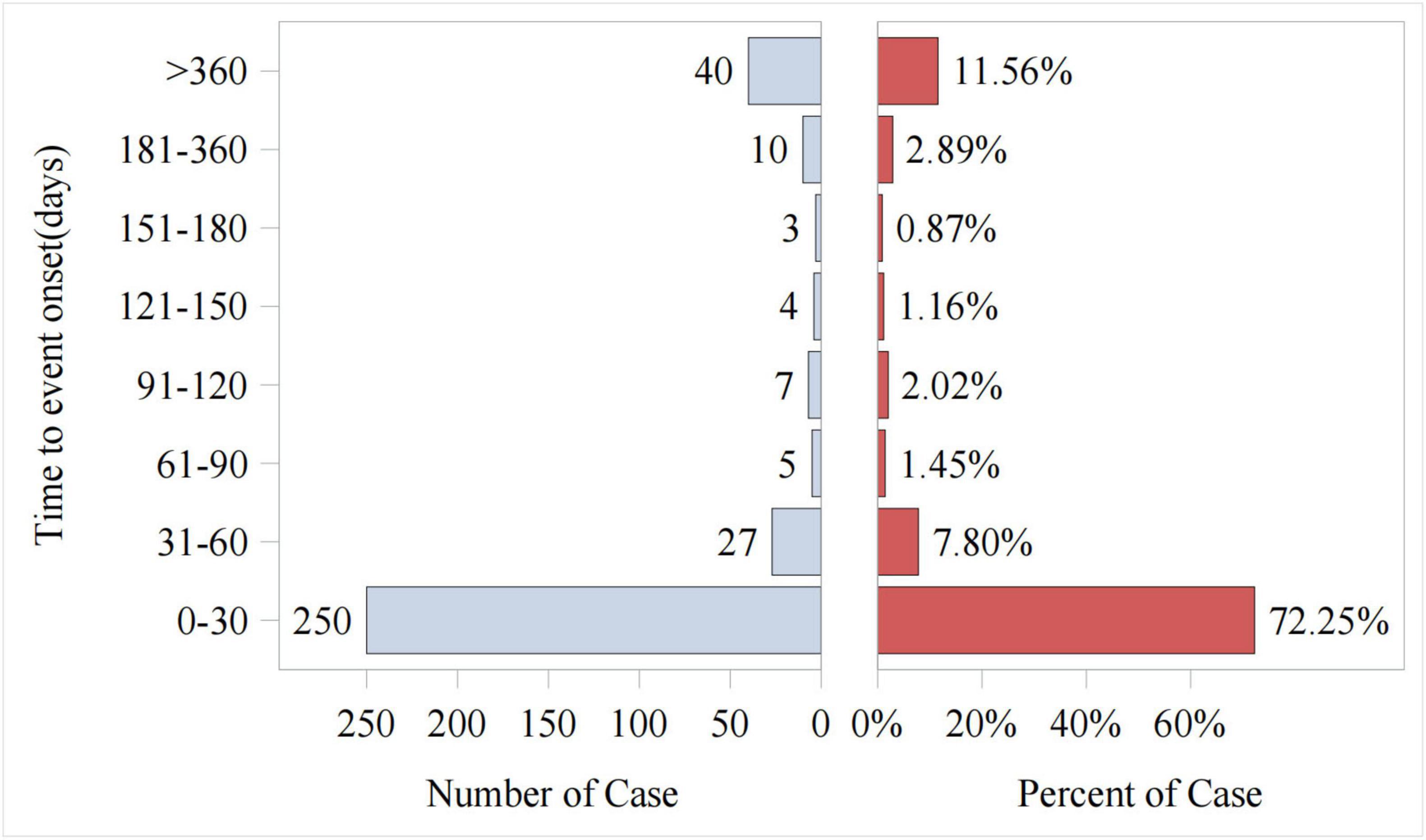
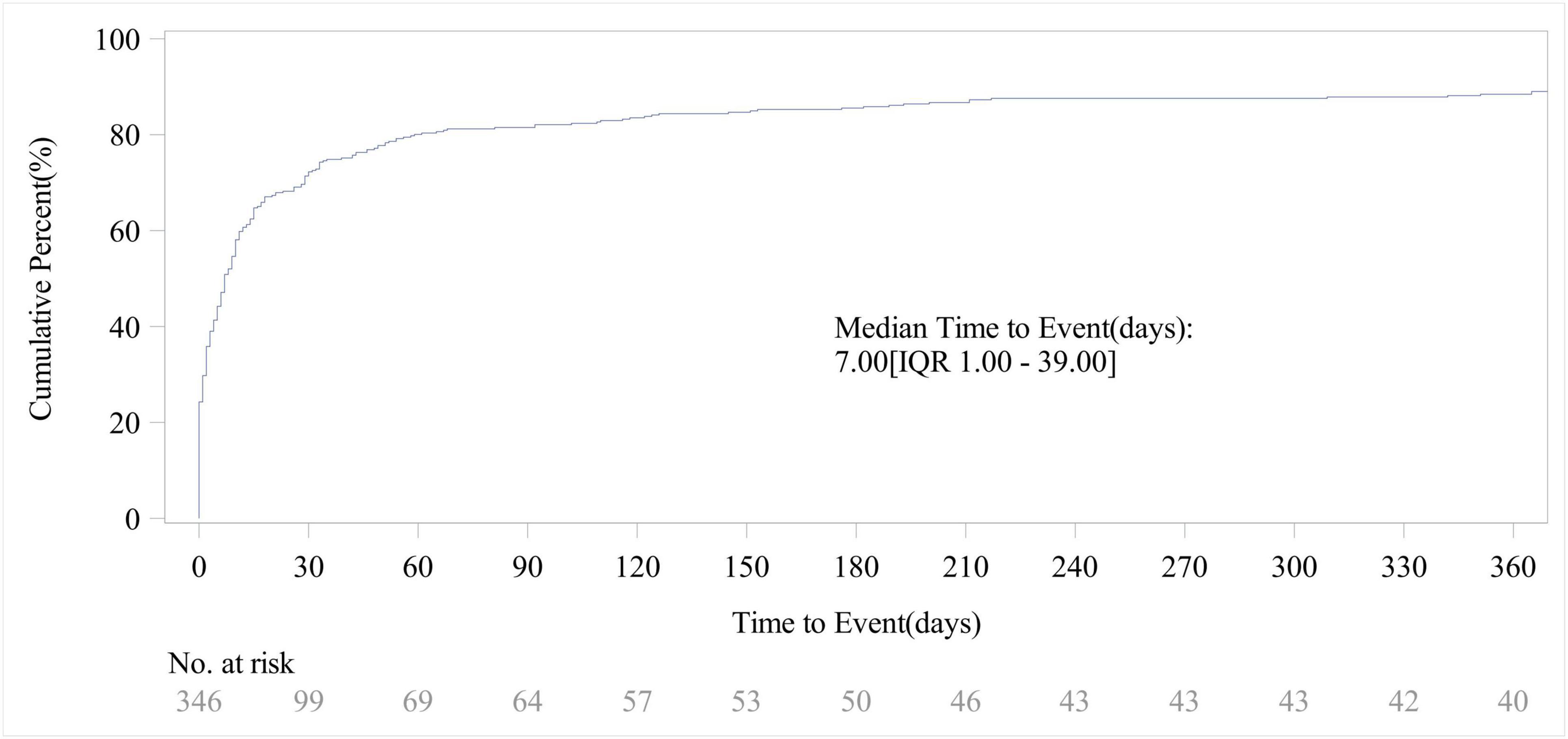
Out of the total reports on piroxicam-related AEs, 346 included data on time to onset. As shown in Figure 4, the vast majority of AEs occurred within the first month of piroxicam use (72%, n = 250). The cumulative incidence curve for AEs (Figure 5) revealed a median AE onset time of 7 days [interquartile range (IQR) 1–39 days], the shape parameter is 0.4 (95% confidence interval [CI] 0.37–0.44) and the scale parameter is 79 (95% CI 57–109), emphasizing the critical importance of early monitoring during therapy.
3.5 Subgroup analysis
Subgroup analysis of AEs associated with piroxicam revealed distinct patterns based on sex and age. Among the 10 most common AEs that met the criteria for a positive signal, female-specific AEs included pruritus, hypersensitivity, erythema, weight loss, and anemia. In contrast, male-specific AEs included melena, AGEP, duodenal ulcer, acute kidney injury (AKI), and fixed drug eruption (Supplementary Figure 1). In patients aged >65 years, frequently reported AEs with positive signals included drug hypersensitivity, gastrointestinal hemorrhage, AKI, melena, and anemia. In the 45–64 years age group, the most common AEs were drug hypersensitivity, rash, urticaria, gastrointestinal hemorrhage, and duodenal ulcer. Among patients aged 18–44 years, pruritus, AGEP, and rash were most prevalent. Notably, two AEs, eye pain and blurred vision, were reported in patients under 18 years (Supplementary Figure 2). The median time to AE onset was 7 days for both sexes, with no significant difference in cumulative incidence curves (Supplementary Figure 3).
3.6 Outcome analysis
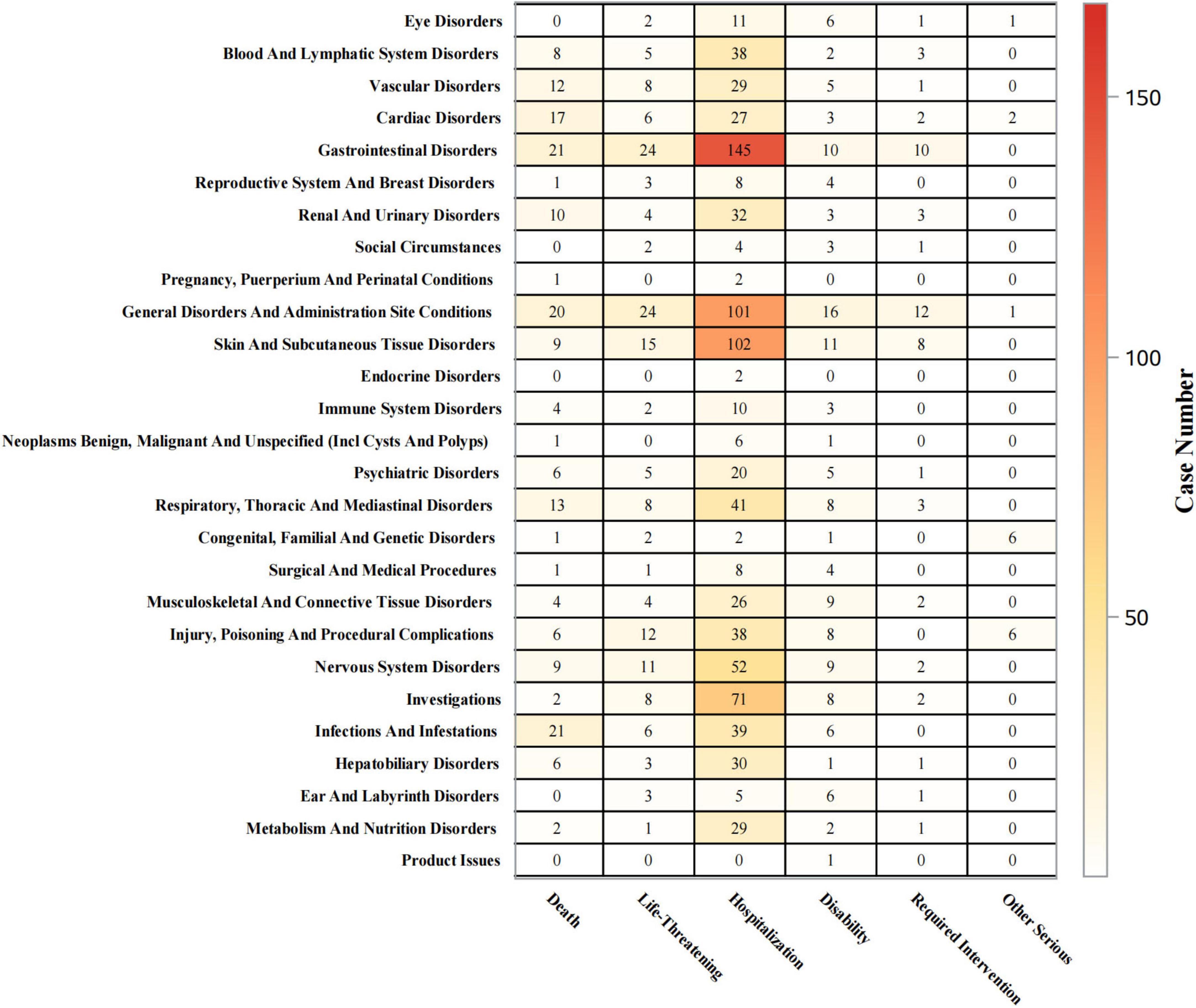
Outcomes of piroxicam-related AEs were statistically analyzed at both SOC and PT levels. As shown in Figure 6, for gastrointestinal disorders, hospitalizations occurred in 69% (n = 145) of cases, life-threatening events in 11% (n = 24), and deaths in 10% (n = 21). For skin and subcutaneous tissue disorders, the hospitalization rate was 70% (n = 102), with life-threatening events and deaths accounting for 10% (n = 15), and 6% (n = 9) of cases, respectively. Immune system disorders led to hospitalizations in 53% (n = 10) of cases, life-threatening events in 11% (n = 2), and deaths in 21% (n = 4).
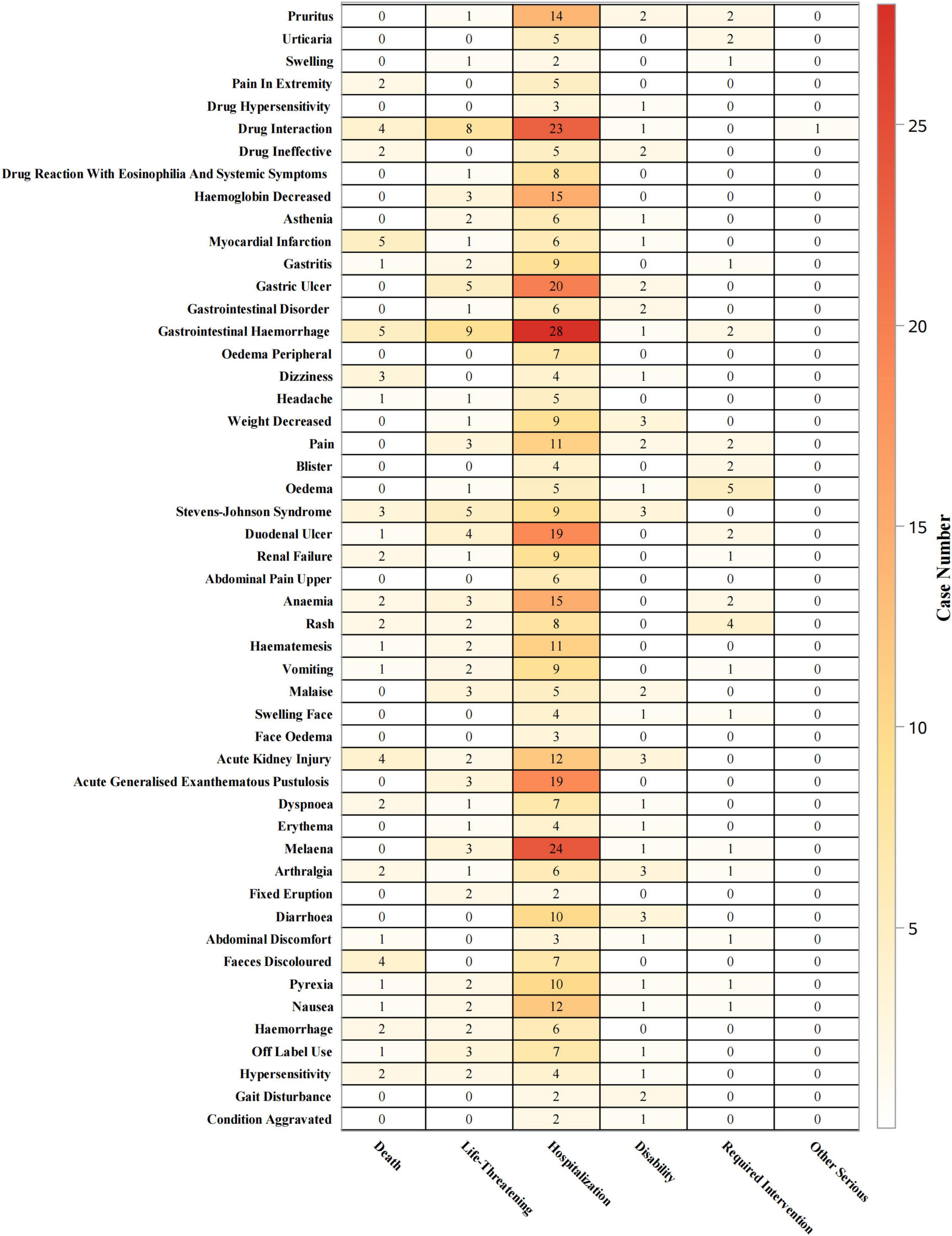
At the PT level (Figure 7), gastrointestinal hemorrhage was associated with hospitalizations in 62% (n = 28) of cases, life-threatening events in 20% (n = 9), and deaths in 11% (n = 5). Gastric ulcer led to hospitalizations in 74% (n = 20) of cases, life-threatening events in 19% (n = 5), and no deaths. Pruritus led to hospitalizations in 74% (n = 14), with one life-threatening case (5%) and no deaths. AKI was associated with hospitalizations in 57% (n = 12) of cases, life-threatening events in 10% (n = 2), and deaths in 19% (n = 4). Stevens-Johnson syndrome led to hospitalizations in 45% (n = 9) of cases, life-threatening events in 25% (n = 5), and deaths in 15% (n = 3). AGEP resulted in hospitalizations in 86% (n = 19) of cases, life-threatening events in 14% (n = 3), and no deaths.
4 Discussion
For medications such as piroxicam, continuous post-marketing surveillance and prompt reporting of suspected adverse events are vital for evaluating their benefit risk profiles in real-world settings, which are crucial for clinical decision-making (15). This study provides a comprehensive analysis of AEs linked to piroxicam between Q1 2004 and Q4 2024 using data from the FAERS database. This study confirms well-documented AEs–such as hypersensitivity, gastric ulcer, urticaria, and severe adverse reactions, including gastrointestinal hemorrhage and Stevens-Johnson syndrome–and identifies potentially less well-known signals, including AGEP, blister formation, and urinary retention. These findings underscore the importance of vigilant monitoring during both the early and long-term phases of treatment to mitigate serious outcomes on time.
Piroxicam’s gastrointestinal, hepatic, and renal toxicities are primarily attributed to non-selective COX inhibition and oxidative stress (16). Gastrointestinal toxicity, particularly gastric ulceration, arises due to COX-1 inhibition in the gastric mucosa, which suppresses prostaglandin (PG) synthesis (e.g., PGE2), compromising mucosal integrity via acid suppression, mucus production, and mucosal blood flow regulation (17). This effect is compounded by oxidative stress (e.g., elevated Malondialdehyde, depleted Glutathione, and reduced Catalase activity) and mitochondrial dysfunction-driven apoptosis (via caspase-3 upregulation) (18). Hepatic toxicity is characterized by oxidative damage during hepatic metabolism, resulting in glutathione depletion, reduced antioxidant enzyme activity, lipid peroxidation (Malondialdehyde elevation), and histopathological necrosis (19). Renal injury is linked to COX-1-mediated dysregulation of PG-dependent renal hemodynamics overproduction of reactive oxygen species, which activate caspase-3-mediated apoptotic pathways, leading to AKI or chronic kidney injury (20).
This study identified several AEs not currently listed on piroxicam’s label, including AGEP, blister, and urinary retention. These findings possibly originate from distinct pathophysiological mechanisms. AGEP, for instance, is likely driven by a T cell-mediated delayed hypersensitivity reaction (21), where piroxicam may act as a hapten triggering immune activation, leading to neutrophil infiltration and pustule formation (22). Blister formation may reflect epidermal cell damage or epidermal-dermal separation (23), potentially mediated by keratinocyte apoptosis or antibody-driven inflammation. These conditions may be underdiagnosed due to their rarity or misattribution to other causes in clinical trials. Urinary retention, another unlisted AE, may result from COX inhibition leading to decreased PG synthesis. PGs like PGE2 normally promote the relaxation of bladder muscles (24); their reduction can void function. This effect is particularly concerning in elderly males or individuals with pre-existing urological conditions. Early clinical trials may have underrepresented these populations. These findings suggest the need for increased dermal monitoring during dosing due to the risk of progression to severe skin reactions. In addition, it is advisable to assess prostate function and residual bladder volume in at-risk patients before initiating piroxicam.
The time-to-onset analysis revealed that most piroxicam-related AEs occurred within the first month of therapy with a median onset of 7 days. This highlights the need for close clinical observation during the early stages of treatment to enable timely detection and intervention for emerging adverse reactions, thus potentially improving patient outcomes and safety.
The subgroup analysis reveals significant demographic variations in adverse drug reactions associated with piroxicam. Females exhibit a higher susceptibility to hypersensitivity reactions, likely driven by estrogen-mediated immune bias and sex-specific differences in drug metabolism (25). Conversely, males are more prone to gastrointestinal ulcers and AKI, potentially due to androgen- associated mucosal vulnerability and enhanced suppression of COX-1 (26). Age-stratified data indicate that older adults (≥65 years) disproportionately experience bleeding and renal complications, possibly due to age-related pharmacokinetic alterations and polypharmacy (27). Middle-aged adults (45–64 years) show concurrent risks of allergic rash and gastrointestinal ulceration, reflecting sustained immune reactivity and frequent NSAID use. Younger adults (18–44 years) mainly experience dermatologic reactions such as rashes and AGEP, likely a consequence of robust immune system responses. Notably, in children under 18, rare but concerning adverse events such as eye pain and blurred vision suggest potential vulnerability to developing neural or ocular structures. These findings highlight the need for tailored risk mitigation strategies. Women and younger patients may benefit from early allergy surveillance, while older adults and men may require gastrointestinal and renal protective measures, including prophylactic use of acid-suppressing drugs. Elderly patients, especially those with a history of ulcers or renal impairment, should be screened for latent bleeding tendencies or declining renal function. Ultimately, these data reinforce that a one-size-fits-all dosing approach is inadequate–demographic characteristics must guide piroxicam prescribing decisions.
It is noteworthy that only 1,198 reports were submitted for piroxicam, a drug that has been on the market for many years. We posit that this relatively low number of reports may be attributed to two primary factors. First, the evolution of NSAIDs. Over the past two decades, numerous novel NSAIDs with improved safety profiles have been introduced. Concurrently, well-documented gastrointestinal and cardiovascular safety concerns associated with piroxicam have prompted regulatory warnings, leading to a decline in physician prescribing. Second, underreporting of adverse events. Healthcare providers, patients, and pharmacists may exhibit limited awareness of the FAERS, encounter challenges in attributing adverse events in polypharmacy settings, or perceive mild adverse events as clinically insignificant and therefore not warranting reporting. The events reported in this study may predominantly be severe or rare cases, which, while highlighting significant safety signals, could also underestimate the true frequency and diversity of AEs associated with piroxicam. Future research should integrate additional data sources–such as large-scale observational studies–to provide a more comprehensive assessment of piroxicam’s safety profile and enhance the generalizability of findings.
Like all studies based on the FAERS database, this analysis is subject to inherent limitations. First, FAERS relies on spontaneous, voluntary reporting from multiple sources, often resulting in incomplete, inconsistent, or inaccurate data, which may introduce bias in the analysis. Known AEs associated with piroxicam are more likely to be reported compared to less well-known ones. The Weber effect also plays a role, where the relative change in the perception of an AE’s significance affects reporting. Minor changes in the occurrence of AEs might not be reported if they are not perceived as significant enough relative to the baseline. Media influence can also have a profound impact. Second, critical confounding variables–such as dosage, treatment duration, patient comorbidities, concomitant medications, and other variables potentially influencing AEs–are frequently missing or poorly documented, limiting the ability to control for influencing factors. Third, a fundamental limitation of using the FAERS database is the lack of a denominator. Without knowing the total number of patients exposed to piroxicam, we cannot calculate the absolute risk of any AE. While our disproportionality analysis may show a strong signal, the actual risk to an individual patient could be very low. This lack of absolute risk information limits the clinical utility of our findings. Fourth, the high proportion of “not specified” values for age and sex is a major source of potential bias, especially in subgroup analyses. Certain AEs might be more prevalent in specific age brackets or among a particular sex, but without complete data, we may under- or over-estimate these differences. Fifth, although the method for removing duplicates in the FAERS database is standard, it is imperfect. There is a possibility that some duplicates may remain in our dataset, which can inflate the reported frequencies of AEs and lead to over estimation of the drug’s risk. Sixth, due to the inability to completely eliminate unrecognized deletions in FAERS, the median onset time of 7 days may still slightly underestimate the true value. Finally, the disproportionality analysis conducted here could identify statistical associations but failed to establish causality between piroxicam and the reported AEs. It is crucial to note that the observed signals might be confounded by various factors. For instance, confounding by indication could be at play, where the reason for prescribing piroxicam (such as the patient’s underlying condition) might be the actual cause of the AEs rather than the drug itself. Co-medications are another potential confounder; patients taking piroxicam often use other drugs simultaneously, and the interactions between these drugs could lead to the reported AEs. Underlying diseases can also contribute to this confounding. It serves to generate hypotheses rather than quantify the absolute risk or prove cause-effect relationships. Nevertheless, despite these limitations, the large and diverse pool of international cases in FAERS offers valuable insights and enables the preliminary identification of potential safety signals related to piroxicam.
5 Conclusion
This study employed the FAERS database to conduct a thorough and systematic evaluation examination of AEs associated with piroxicam from 2004 through 2024. The analysis confirmed several known safety concerns while uncovering previously unlisted but clinically significant AEs–including AGEP, urinary retention, and blistering. These findings underscore important safety considerations for piroxicam’s clinical use and emphasize the significance of vigilant patient monitoring throughout treatment. Given the drug’s risk profile, sustained pharmacovigilance is essential to ensure the safe administration of piroxicam in routine healthcare practice.
Data availability statement
The original contributions presented in this study are included in this article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
YW: Conceptualization, Data curation, Methodology, Writing – original draft. QK: Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
This study was performed using the FDA Adverse Event Reporting System (FAERS) source that was provided by the FDA. The information, results, or interpretation of the current study do not represent any opinion of the FDA.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1687088/full#supplementary-material
References
1. Brogden R, Heel R, Speight T, Avery G. Piroxicam: a review of its pharmacological properties and therapeutic efficacy. Drugs. (1981) 22:165–87. doi: 10.2165/00003495-198122030-00001
2. Wilk M, Zimba O, Haugeberg G, Korkosz M. Pain catastrophizing in rheumatic diseases: prevalence, origin, and implications. Rheumatol Int. (2024) 44:985–1002. doi: 10.1007/s00296-024-05583-8
3. Zhou Y, Luo X, Li P, Liu X, Li J, Su L, et al. The burden of rheumatoid arthritis in China from 1990 to 2019 and projections to 2030. Public Health. (2025) 242:71–8. doi: 10.1016/j.puhe.2025.02.033
4. Moreira V, Signorelli F, Hattori W, Dionisio V. Association between psychological factors and physical performance in individuals with knee osteoarthritis: A cross-sectional study. J Bodyw Mov Ther. (2025) 42:274–82. doi: 10.1016/j.jbmt.2024.12.030
5. Khalil N, Ahmed E, Tharwat T, Mahmoud Z. NSAIDs between past and present; a long journey towards an ideal COX-2 inhibitor lead. RSC Adv. (2024) 14:30647–61. doi: 10.1039/d4ra04686b
6. Richardson C, Blocka K, Ross S, Verbeeck R. Effects of age and sex on piroxicam disposition. Clin Pharmacol Ther. (1985) 37:13–8. doi: 10.1038/clpt.1985.4
7. Muthuluri T, Chandrupatla S, Rajan R, Reddy V, Jhawar D, Potturi A. Pre-emptive analgesia efficacy of piroxicam versus tramadol in oral surgery. J Dent Anesth Pain Med. (2022) 22:443–50. doi: 10.17245/jdapm.2022.22.6.443
8. Arellano F, Yood M, Wentworth C, Oliveria S, Rivero E, Verma A, et al. Use of cyclo-oxygenase 2 inhibitors (COX-2) and prescription non-steroidal anti-inflammatory drugs (NSAIDS) in UK and USA populations. Implications for COX-2 cardiovascular profile. Pharmacoepidemiol Drug Saf. (2006) 15:861–72. doi: 10.1002/pds.1343
9. McGettigan P, Henry D. Cardiovascular risk and inhibition of cyclooxygenase: a systematic review of the observational studies of selective and nonselective inhibitors of cyclooxygenase 2. JAMA. (2006) 296:1633–44. doi: 10.1001/jama.296.13.jrv60011
10. Xu X, Guo Q, Li Y, Zhai C, Mao Y, Zhang Y, et al. Assessing the real-world safety of regadenoson for myocardial perfusion imaging: insights from a comprehensive analysis of FAERS data. J Clin Med. (2025) 14:1860. doi: 10.3390/jcm14061860
11. Rothman K, Lanes S, Sacks S. The reporting odds ratio and its advantages over the proportional reporting ratio. Pharmacoepidemiol Drug Saf. (2004) 13:519–23. doi: 10.1002/pds.1001
12. Evans S, Waller P, Davis S. Use of proportional reporting ratios (PRRs) for signal generation from spontaneous adverse drug reaction reports. Pharmacoepidemiol Drug Saf. (2001) 10:483–6. doi: 10.1002/pds.677
13. Ang P, Chen Z, Chan C, Tai B. Data mining spontaneous adverse drug event reports for safety signals in Singapore – a comparison of three different disproportionality measures. Expert Opin Drug Saf. (2016) 15:583–90. doi: 10.1517/14740338.2016.1167184
14. Rivkees S, Szarfman A. Dissimilar hepatotoxicity profiles of propylthiouracil and methimazole in children. J Clin Endocrinol Metab. (2010) 95:3260–7. doi: 10.1210/jc.2009-2546
15. Zhao B, Zhang X, Chen M, Wang Y. A real-world data analysis of acetylsalicylic acid in FDA adverse event reporting system (FAERS) database. Expert Opin Drug Metab Toxicol. (2023) 19:381–7. doi: 10.1080/17425255.2023.2235267
16. Yunita M, Plumeriastuti H, Agustono B, Fahlefi M, Fadilla A, Pangastutie R, et al. The protective effects of garlic (Allium sativum var. Solo Garlic) extract mitigates piroxicam-induced liver damage and oxidative stress in rats. Open Vet J. (2025) 15:1349–57. doi: 10.5455/OVJ.2025.v15.i3.26
17. Takeuchi K, Amagase K. Roles of cyclooxygenase, prostaglandin E2 and EP receptors in mucosal protection and ulcer healing in the gastrointestinal tract. Curr Pharm Des. (2018) 24:2002–11. doi: 10.2174/1381612824666180629111227
18. Abdeen A, Aboubakr M, Elgazzar D, Abdo M, Abdelkader A, Ibrahim S, et al. Rosuvastatin attenuates piroxicam-mediated gastric ulceration and hepato-renal toxicity in rats. Biomed Pharmacother. (2019) 110:895–905. doi: 10.1016/j.biopha.2018.11.004
19. Badawi M. Histological study of the protective role of ginger on piroxicam-induced liver toxicity in mice. J Chin Med Assoc. (2019) 82:11–8. doi: 10.1016/j.jcma.2018.06.006
20. Ebaid H, Dkhil M, Danfour M, Tohamy A, Gabry M. Piroxicam-induced hepatic and renal histopathological changes in mice. Libyan J Med. (2007) 2:82–9. doi: 10.4176/070130
21. Hadavand M, Kaffenberger B, Cartron A, Trinidad J. Clinical presentation and management of atypical and recalcitrant acute generalized Exanthematous pustulosis. J Am Acad Dermatol. (2022) 87:632–9. doi: 10.1016/j.jaad.2020.09.024
22. Fernando S. Acute generalised exanthematous pustulosis. Australas J Dermatol. (2012) 53:87–92. doi: 10.1111/j.1440-0960.2011.00845.x
23. Yang M, Wu H, Zhao M, Chang C, Lu Q. The pathogenesis of bullous skin diseases. J Transl Autoimmun. (2019) 2:100014. doi: 10.1016/j.jtauto.2019.100014
24. Sellers D, Chess-Williams R, Michel M. Modulation of lower urinary tract smooth muscle contraction and relaxation by the urothelium. Naunyn Schmiedebergs Arch Pharmacol. (2018) 391:675–94. doi: 10.1007/s00210-018-1510-8
25. Trenti A, Tedesco S, Boscaro C, Trevisi L, Bolego C, Cignarella A. Estrogen, angiogenesis, immunity and cell metabolism: solving the puzzle. Int J Mol Sci. (2018) 19:859. doi: 10.3390/ijms19030859
26. Lin X, Wang W. Analysis of high risk factors for chronic atrophic gastritis. Saudi J Gastroenterol. (2023) 29:127–34. doi: 10.4103/sjg.sjg_383_22
Keywords: piroxicam, FAERS, disproportionality analysis, adverse event, real-world safety
Citation: Wang Y and Kong Q (2025) A real-world data analysis of piroxicam in the FDA Adverse Event Reporting System (FAERS) database. Front. Med. 12:1687088. doi: 10.3389/fmed.2025.1687088
Received: 16 August 2025; Accepted: 29 September 2025;
Published: 10 October 2025.
Edited by:
Shameer Khader, Sanofi, FranceReviewed by:
Frits Lekkerkerker, Consultant, Amsterdam, NetherlandsXiaoming He, Mead Johnson, United States
Copyright © 2025 Wang and Kong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiuhong Kong, a3FobGx4QDE2My5jb20=
 Yanhe Wang1
Yanhe Wang1 Qiuhong Kong
Qiuhong Kong