- 1Department of Respiratory Medicine, Chongqing Emergency Medical Center, Chongqing University Central Hospital, Chongqing, China
- 2Department of Neurology, Chongqing Emergency Medical Center, Chongqing University Central Hospital, Chongqing, China
- 3Department of Central Sterile Supply, Chongqing Emergency Medical Center, Chongqing University Central Hospital, Chongqing, China
Background: The ratio of neutrophil percentage to albumin (NPAR) has been recognized as an inflammatory indicator for predicting the prognosis of various diseases. Nevertheless, no research has explored the relationship between NPAR and prognosis in patients who develop community acquired pneumonia (CAP) during long-term and systemic glucocorticoids therapy. Therefore, this study aims to investigate the association between NPAR on admission and mortality in the aforementioned patients.
Method: The data of this study were extracted from the Dryad database. An analysis was conducted data from patients diagnosed with CAP who had received either oral or intravenous glucocorticoids before hospital admission. Patients were categorized into three groups based on their NPAR levels upon admission. Kaplan-Meier survival curves, multivariable Cox regression models, restricted cubic spline curves, and subgroup analyses were performed to evaluate the association between the NPAR and 30-day as well as 90-day mortality in these patients, respectively. Sensitivity analysis were performed to verify the stability of the results.
Results: Among the 570 patients diagnosed with CAP incorporated into the study, the 30-day and 90-day mortality were 21.9% and 24.9%, respectively. The study revealed that the NPAR exhibited a significantly positive correlation with mortality. Multivariable Cox regression analyses, after adjustment for all possible confounders, indicated that a higher NPAR level was correlated with an elevated risk of 30-day mortality (HR: 1.21, 95% CI: 1.14–1.28). Compared with patients in tertile 1, those in tertile 2 and tertile 3 exhibited a notably increased risk of 30-day mortality (HR: 1. 83, 95% CI: 1. 38–2. 43; HR: 3. 19, 95% CI: 2. 72–4. 2, respectively). Analogous findings were also observed for 90-day mortality. Kaplan-Meier survival curves showed that the highest tertile had the lowest survival rates for 30-day and 90-day mortality. Additionally, subgroup analysis revealed no interactions and demonstrated robust results across different subgroups. A linear relationship was observed between NPAR and mortality.
Conclusion: Higher level of NPAR was significantly associated with an increased risk of 30-day and 90-day mortality in patients with community acquired pneumonia receiving systemic glucocorticoids therapy.
1 Introduction
Community-acquired pneumonia (CAP) is a common condition that results in high morbidity and mortality worldwide, imposing substantial healthcare burdens (1, 2). Due to underlying conditions including connective tissue diseases (3), chronic lung diseases (4), nephrotic syndrome or chronic glomerulonephritis (5), hematological disorders (6), etc., a subset of patients requires long-term use of systemic glucocorticoid therapy. The risk of pulmonary infections, including CAP, has significantly increased in this population (7–10). Furthermore, studies have also indicated that these patients often exhibit increased disease severity and higher mortality rates upon developing CAP compared to those not using systemic steroids (11). Thus, reliable scoring systems or indicators are needed to guide clinical decisions and evaluate the prognosis of these pneumonia patients.
At present, both the CURB-65 (confusion, urea nitrogen, respiratory rate, blood pressure, age ≥ 65 years) and pneumonia severity index (PSI) serve as assessment tools for the severity of illness and prognosis of patients with CAP in clinical practice (12, 13). Although these scores have certain application value, they have limitations when applied to specific populations. Both prognostic scoring systems are primarily used for immunocompetent populations; however, their prognostic accuracy diminishes in immunosuppressed populations (10, 14), such as glucocorticoid-treated patients who develop CAP. Certain inflammatory biomarkers, including leukocyte count, procalcitonin (PCT), and C-reactive protein (CRP), have also been utilized to evaluate the prognosis of pneumonia (15). Nevertheless, the levels of these biomarkers might be affected by the immunosuppressive effects of glucocorticoids (16). Consequently, it is crucial to explore novel and effective prognostic markers to evaluate the mortality risk in patients with pneumonia receiving glucocorticoids therapy.
The neutrophil percentage-to-albumin ratio (NPAR), a novel inflammatory biomarker, integrates the neutrophil percentage and albumin and exhibits a substantial correlation with inflammatory reaction and nutritional status of the body. Previous researches have indicated that NPAR was significantly associated with the prognosis of various diseases, including those aged 80 years or older with CAP (17), sepsis (18), cardiovascular diseases (19), stroke (20), acute kidney injury (21), etc. Although NPAR has been linked to a certain predictive value for the prognosis of patients with different diseases, its role in predicting mortality among pneumonia patients receiving glucocorticoids remains unclear. Therefore, the present study investigates the correlation between NPAR and mortality among CAP patients treated with long-term and systemic glucocorticoids, aiming to identify potential prognostic indicators to enhance clinical management for this high-risk population.
2 Materials and methods
2.1 Data source
The data were obtained from the Dryad Digital Repository,1 which allows unrestricted use of the data for other researchers. The dataset containing 716 pneumonia patients who received oral or intravenous glucocorticoids treatment was firstly provided by Li et al. (8). These patients were diagnosed with pneumonia upon admission or during hospitalization at six secondary and tertiary academic hospitals in China between January 2013 and December 2017 (8).
2.2 Study population
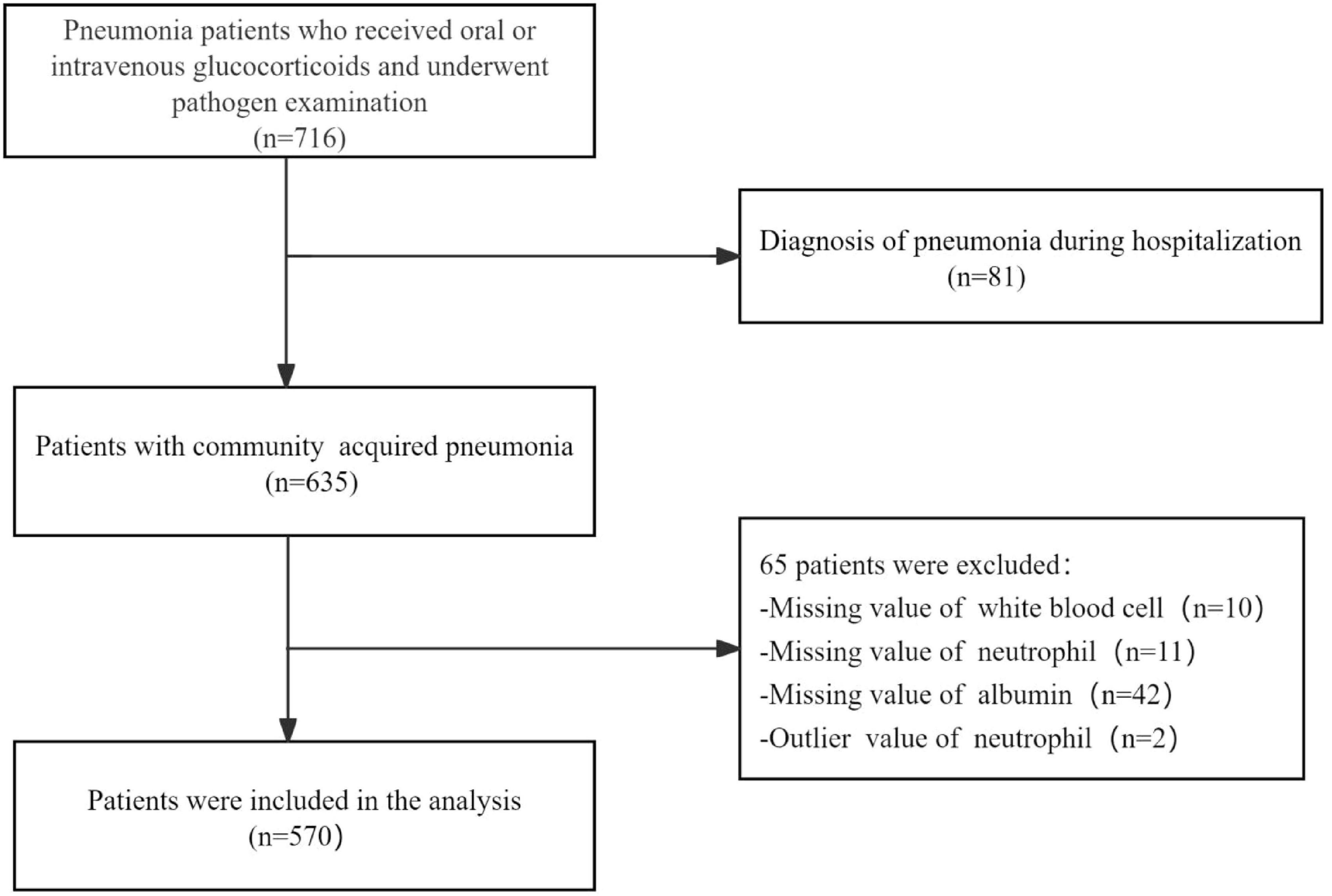
This study included patients aged 16 years or older who had received oral or intravenous glucocorticoids therapy before admission and were hospitalized due to CAP. The diagnosis of CAP was performed in accordance with the guideline of the American Thoracic Society and Infectious Disease Society of America (22). Exclusion criteria were as follows: (1) inability to provide informed consent; (2) hospital-acquired pneumonia; (3) missing values for white blood cell, neutrophil, and albumin; (4) Outlier values of neutrophil. Ultimately, 570 patients were included in the study for subsequent analysis (Figure 1).
2.3 Data extraction
The covariates in this study were selected based on previous research, including: (1) demographic data, such as age and gender; (2) underlying diseases, which comprised connective tissue diseases (CTD), chronic obstructive pulmonary disease (COPD), interstitial lung disease (ILD), hematological disorders, and idiopathic interstitial pneumonia (IIP), nephrotic syndrome or chronic renal failure (CRF), liver failure, cirrhosis, nephrotic syndrome, congestive heart disease, or tumor, etc; (3) pneumonia severity scoring systems, including CURB-65 (confusion, urea nitrogen, respiratory rate, blood pressure, age ≥ 65 years) and the Pneumonia Severity Index (PSI); (4) laboratory data, which included white blood cell (WBC), neutrophil, lymphocyte, hemoglobin, platelet, albumin, lactate dehydrogenase (LDH), blood urea nitrogen (BUN), serum creatinine, procalcitonin (PCT), and the total pathogenic positive rate; (5) therapeutic interventions, such as use of high - dose glucocorticoids, cumulative methylprednisolone dosages, oxygen inhalation, intensive care unit (ICU) admission, mechanical ventilation, intubation, extracorporeal membrane oxygenation (ECMO), continuous veno-venous hemofiltration (CVVH), and vasoactive drugs; (6) survival status at 30 days and 90 days post-admission. All the laboratory tests mentioned above were collected within 24 h of admission.
The neutrophil percentage was delineated as the proportion of neutrophil in the total white blood cell count. The NPAR was calculated via the formula: (Neutrophil percentage × 100)/Albumin (g/dL), utilizing the identical blood samples collected upon admission. High-dose glucocorticoids was defined as the administration of 30 mg/day or more of prednisolone, or an equivalent glucocorticoids within the 30 days preceding admission (23). Persistent lymphocytopenia was characterized as a peripheral blood lymphocyte count below 1 × 109/L persisting for more than 7 days (24).
2.4 Statistical analysis
Normally distributed continuous variables were expressed as mean ± standard deviation (SD), and skewedly distributed continuous variables were described using median with interquartile range (IQR). While categorical variables were delineated by frequencies with percentages. To eliminate the dimensional differences of NPAR, it was z-score transformed before analysis.
Upon reviewing previous literature, patients were categorized into tertiles according to NPAR levels upon admission [tertile 1 (T1): NPAR < 0.218; tertile 2 (T2): NPAR ≥ 0.218, <0.275; tertile 3 (T3): NPAR ≥ 0.275], and T1 group served as the reference group. Trend tests across tertiles were conducted using median values. Comparisons between the three groups were conducted using ANOVA test or Kruskal-Wallis for continuous variables, and χχ2 test for categorical variables. Variables with a missing rate exceeding 25% were excluded from this analysis. Multiple imputation with five replications was utilized to tackle other missing data. Survival rates between groups were estimated by Kaplan-Meier curves and compared by log-rank test. Univariate Cox regression analyses were utilized to assess the correlation between prognostic factors and 30-day and 90-day mortality. Multivariate analyses were conducted via Cox proportional hazards models to evaluate the independent correlation between the NPAR and mortality. Variables were incorporated into the multivariate Cox proportional hazard models in accordance with clinical experience, the results of univariate regression and an alteration in their effective estimate exceeding 10%. Three models were constructed to control for confounding factors. Model I was adjusted for age and gender. Model II was based on Model I, incorporating the CURB-65, PSI, COPD, and persistent lymphocytopenia. Model III was based on Model II, with the addition of intubation, ICU admission, ventilation, and vasoactive drugs. The outcomes were presented in the form of hazard ratios (HR) accompanied by 95% confidence intervals (CI). Restricted cubic splines were used to observe and analyze the relationship between NPAR and mortality. Additionally, subgroup analyses and interactions were conducted using Cox regression models based on age, gender, COPD, CURB-65, persistent lymphocytopenia, mechanical ventilation, and vasoactive drugs. The results were visualized through forest plots. Furthermore, since chronic renal failure, liver failure, cirrhosis, nephrotic syndrome, congestive heart disease, and tumor may affect albumin levels, we excluded these subset of patients and performed sensitivity analyses.
Statistical analyses were carried out using R software (The R Foundation)2 and Free Statistics software version 2.2. All tests were two-tailed, and P-values < 0.05 were considered statistically significant.
3 Results
3.1 Baseline characteristics of patients
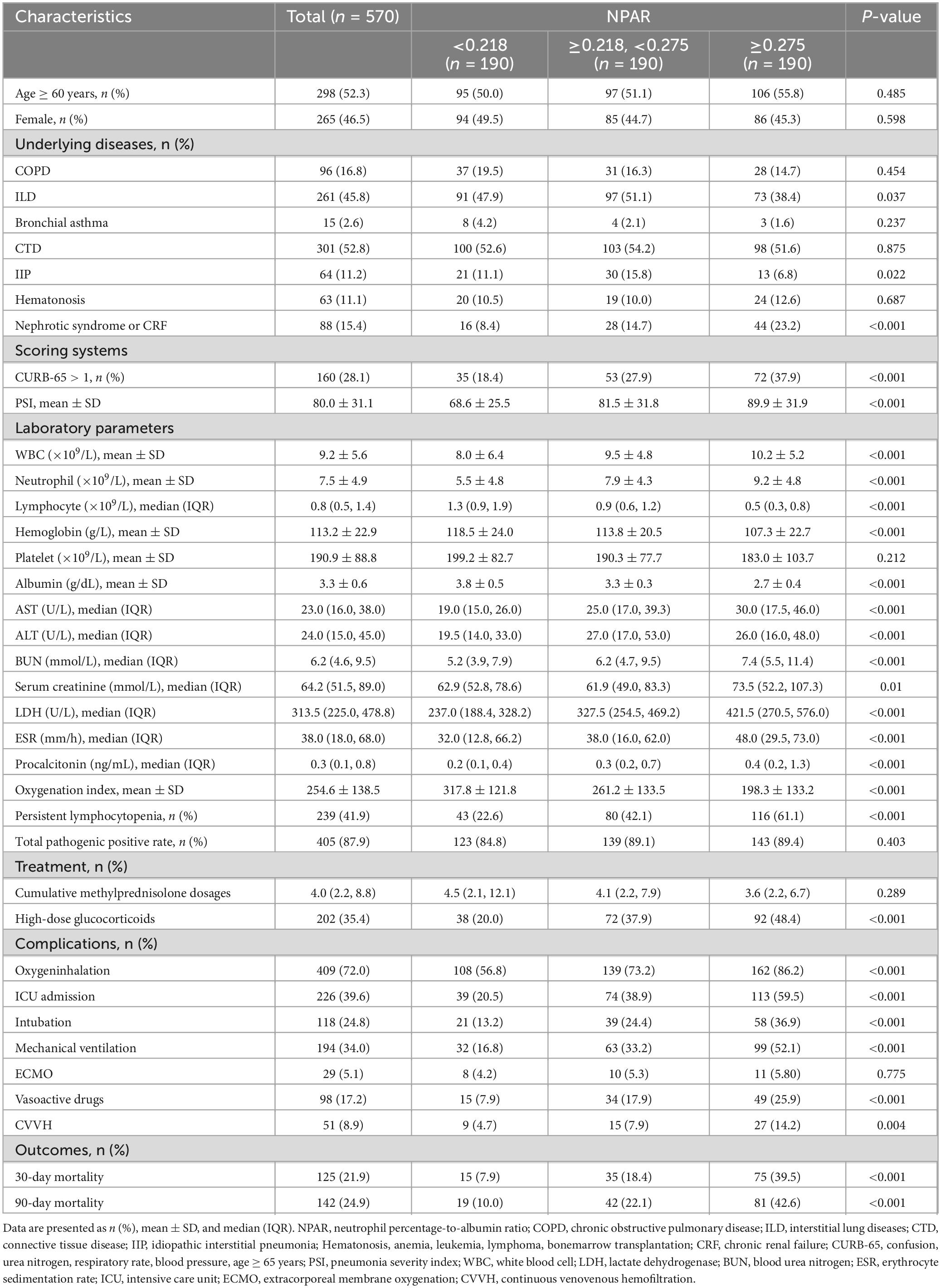
A total of 570 participants, who received glucocorticoids therapy and subsequently developed community acquired pneumonia, were ultimately included in this study (Figure 1). The baseline characteristics of the pneumonia patients stratified according to tertiles of NPAR, are summarized in Table 1. Each group included 190 patients. Among the study population, 298 (52.3%) were aged 60 years or older, and 265 (46.5%) were female. The most prevalent underlying diseases among those receiving glucocorticoid therapy were CTD (52.8%), ILD (45.8%), and COPD (16.8%). Patients in the highest NPAR tertile exhibited higher CURB-65 and PSI scores, and may need to be admitted to the ICU for treatment compared to those in the other tertiles. As NPAR increased, WBC, neutrophils, LDH, BUN, creatinine, and procalcitonin levels increased, whereas their oxygenation index, hemoglobin and albumin levels decreased. Furthermore, patients with a higher NPAR also experienced persistent lymphocytopenia, along with increased use of oxygen inhalation, intubation, ventilators, vasoactive drugs, and CVVH compared to those in the other tertiles. The 30-day and 90-day mortality rates were documented as 21.9% and 24.9%, respectively.
3.2 Kaplan–Meier curves
The Kaplan-Meier survival curves illustrating the 30-day and 90-day mortality rates classified by the NPAR tertiles were presented in Figure 2. A notable discrepancy was observed among each group. Throughout the follow-up period, the 30-day survival rate in group T3 was significantly lower than those in groups T1 and T2 (log-rank test: P < 0.0001) (Figure 2A). Similar trends were found in 90-day survival rate (log-rank test: P < 0.0001) (Figure 2B).
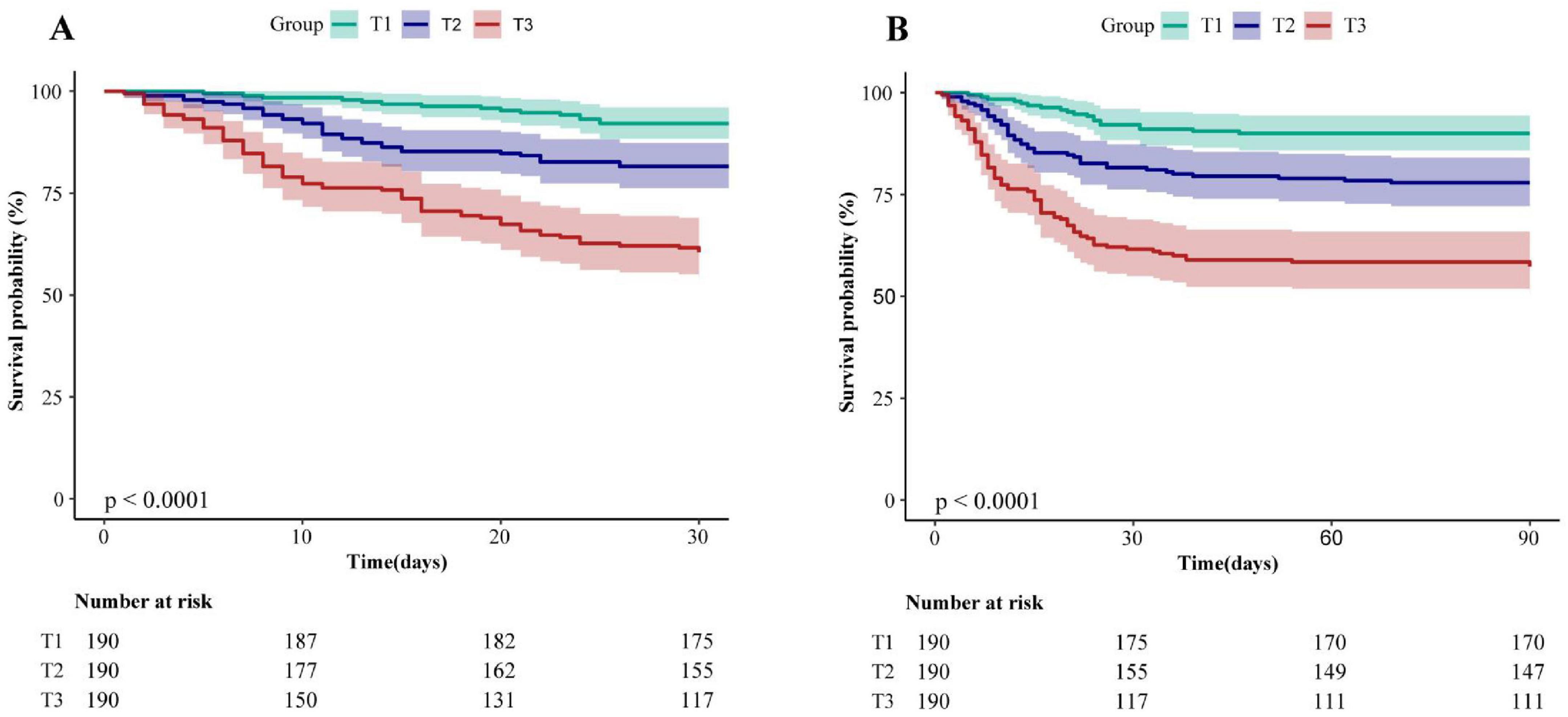
Figure 2. Kaplan–Meier analysis of 30-day (A) and 90-day (B) mortality for patients with CAP receiving glucocorticoids therapy (log-rank test, P < 0.0001). NPAR, neutrophil percentage-to-albumin ratio; T, Tertile; T1: <0.218, T2: ≥0.218, <0.275, T3: ≥0.275.
3.3 Multivariable COX regression analysis
To demonstrate whether the NPAR admission level was associated with 30-day and 90-day mortality in patients with CAP receiving systemic glucocorticoids therapy, univariate Cox regression analyses were conducted and are detailed in Supplementary Table 1. A positive correlation was observed between the NPAR (z-score) and mortality (P < 0.001). Subsequently, we further employed three multivariate Cox regression models to evaluate the independent effect of NPAR on 30-day and 90-day mortality (Table 2). When evaluated as a continuous variable, we found that each SD increment of NPAR in unadjusted model, the risk of 30-day mortality was increased by 47% (HR: 1.47; 95% CI: 1.4–1.54). Even after adjusting for all potential confounders in model III, this association still persisted strongly, and the risk of 30-day mortality elevated by 21% (HR: 1.21, 95% CI: 1.14–1.28, P < 0.001). To ensure the robustness of the results, we also analyzed the NPAR as a categorical variable according to its tertiles, using the tertile 1 group (T1) as a reference. For the outcome of 30-day mortality, we found that higher NPAR was related to increased risk of mortality. The HRs (95% CI) values of the middle-tertile (T2) and the highest tertile (T3) were 2.52 (1.92–3.3) and 6.26 (4.88–8.02), respectively, when compared with the reference group (T1). Trend testing confirmed a significant association between increasing NPAR levels and mortality (P < 0.001). This association remained statistically even after adjusted for relevant covariates. After adjustment for age, gender in model I, an increasing trend was also observed in the highest NPAR tertile (HR: 6.16, 95% CI: 4.81–7.9, P < 0.001). After further adjustment for potential confounders (age, gender, CURB-65, PSI, COPD, persistent lymphocytopenia) in model II, the upward trend remained statistically significant in the high tertile (HR: 3.52, 95% CI: 2.72–4.57, P < 0.001). In model III, the fully adjusted HRs (95% CI) were 1.83 (95% CI: 1.38–2.43) and 3.19 (95% CI: 2.42–4.2) for tertile 2 and tertile 3, respectively. Furthermore, similar results were also observed for the association between NPAR and 90-day mortality (Table 2).
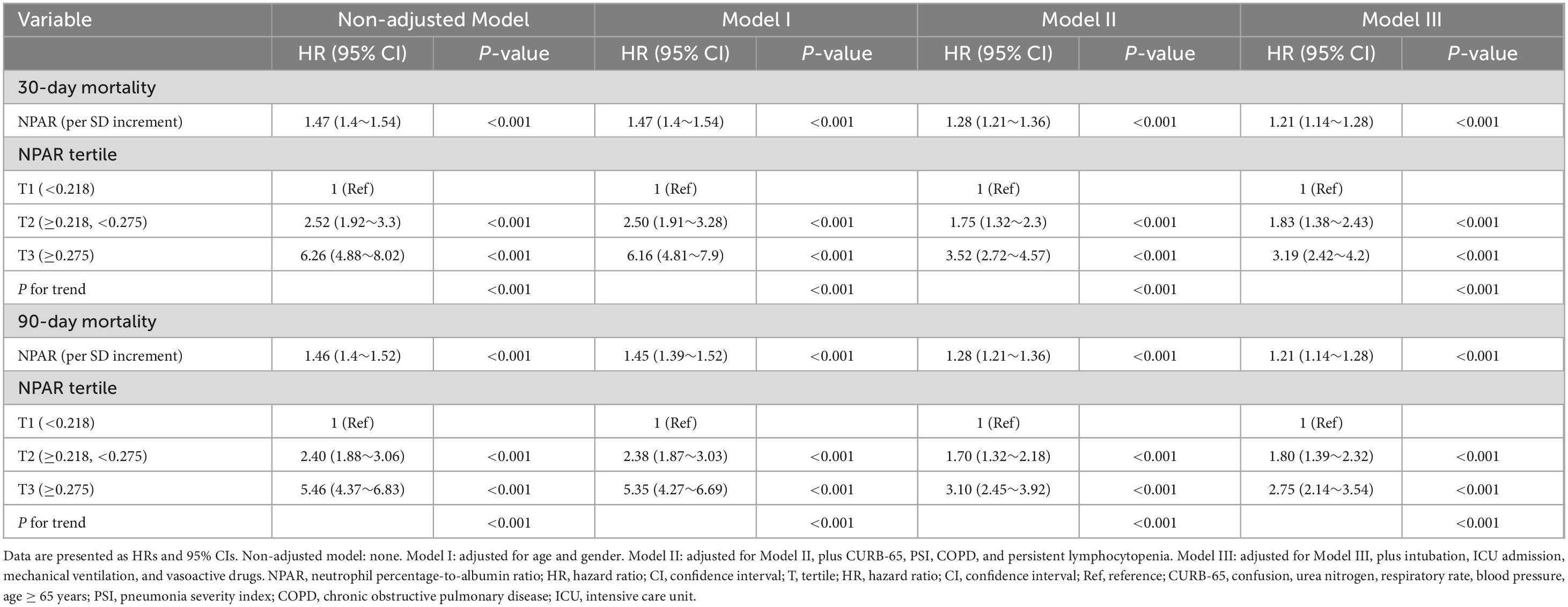
Table 2. Multivariable Cox regression to assess the association of NPAR with 30-day and 90-day mortality.
3.4 Restricted cubic spline analysis
Furthermore, adjusting for confounders according to the Cox regression model III, restricted cubic spline analysis confirmed a linear association between NPAR and mortality (Supplementary Figure) (P for non-linearity > 0.05). The risk of mortality exhibited an upward tend with the rise in NPAR levels.
3.5 Subgroup analysis
To further verify the robustness of the results, subgroup analyses were conducted according to the following stratification variables: age, gender, COPD, CURB-65, persistent lymphocytopenia, mechanical ventilation, vasoactive drugs. The trend of the effect size was consistent in all subgroups (Figures 3A, B). No interactions were identified within all subgroups (all P for interaction > 0.05).
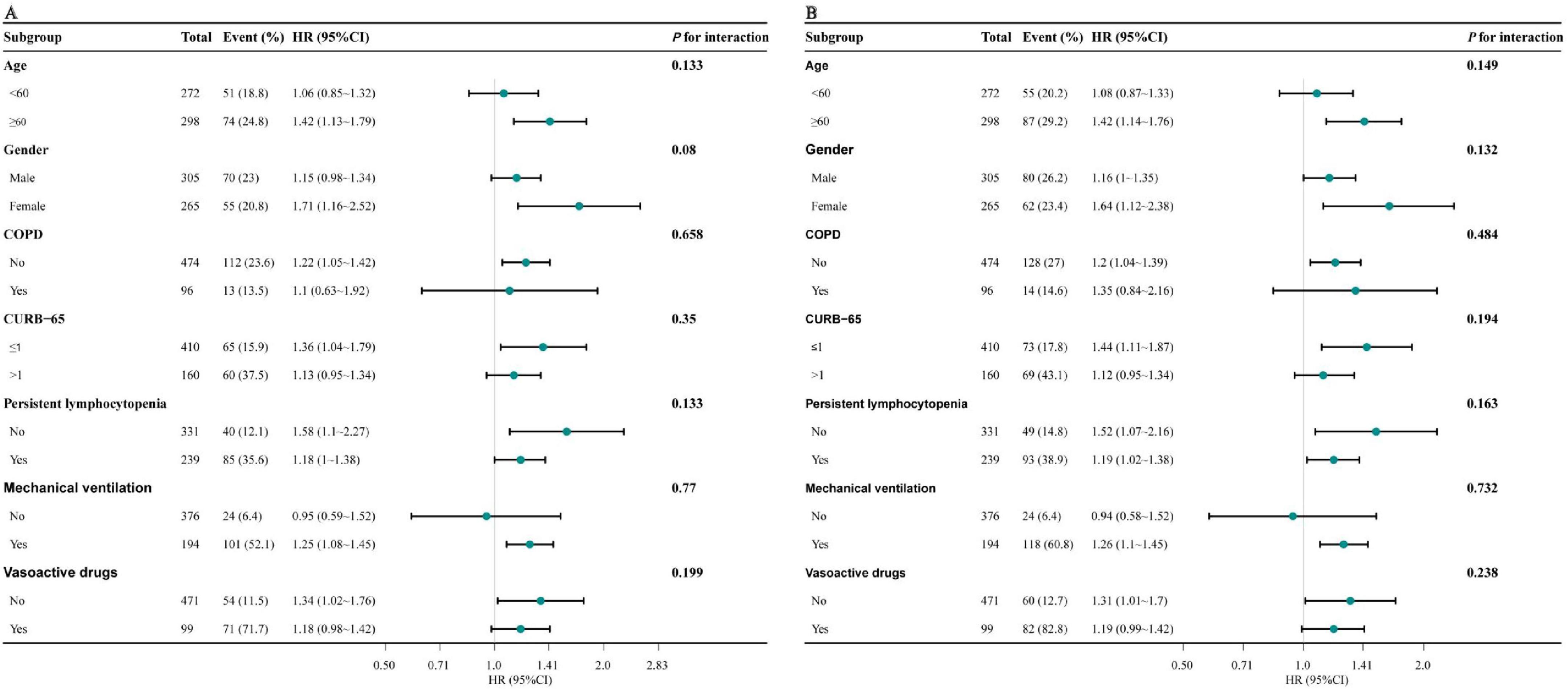
Figure 3. Forest plot for subgroup analysis of the association between NPAR and 30-day (A) and 90-day mortality (B). Models adjusted for age, sex, CURB-65, PSI, COPD, persistent lymphocytopenia, intubation, ICU admission, mechanical ventilation, and vasoactive drugs. NPAR, neutrophil percentage-to-albumin ratio; HR, hazard ratio; CI, confidence interval; COPD, chronic obstructive pulmonary disease; CURB-65, confusion, urea nitrogen, respiratory rate, blood pressure, age ≥ 65 years; PSI, pneumonia severity index; COPD, chronic obstructive pulmonary disease; ICU, intensive care unit.
3.6 Sensitivity analysis
To verify the stability of the results, we performed sensitivity analysis. After exclusion of 133 patients with chronic renal failure, liver failure, cirrhosis, nephrotic syndrome, congestive heart disease, or tumor at the baseline, the sensitivity analysis confirmed our findings that the NPAR was significantly associated with 30-day and 90-day mortality among patients with community-acquired pneumonia receiving systemic glucocorticoids therapy (Supplementary Table 2).
4 Discussion
In this retrospective cohort study, we revealed the association between higher NPAR levels and an increased risk of 30-day and 90-day mortality in patients who developed community-acquired pneumonia after receiving glucocorticoids therapy. The restricted cubic spline curve visually demonstrates this. Kaplan-Meier survival curves indicated that a higher NPAR was significantly associated with an increased risk of 30-day and 90-day mortality compared to lower NPAR values. Subgroup analysis found no significant interaction across different subgroups. The finding suggest that NPAR could serve as a potential biomarker for predicting mortality risk in clinical practice.
Prolonged use of systemic glucocorticoids at high doses may lead to lymphocytopenia and inhibit macrophage function in patients (25), thereby resulting in severe immunosuppression and consequently increasing the incidence of opportunistic infections, including CAP (7, 8, 10, 26). The study indicated that the incidence of CAP was notably increased in individuals receiving systemic glucocorticoids (equivalent to a daily dose of >20 mg prednisone), and was approximately threefold higher than in those not using systemic glucocorticoids. Agustí C et al. discovered that the overall mortality rate among patients undergoing on long-term oral glucocorticoids treatment was as high as 45% upon developing pulmonary infection, rising to 93% if mechanical ventilation was required (10).
Inflammatory responses play a pivotal influence in the development and progression of CAP (27). The neutrophil percentage, a crucial indicator of the body’s inflammatory response, is often significantly increased in pneumonia patients as a result of the immune system’s activation to counteract pathogens (28, 29). However, in patients receiving long-term glucocorticoids, this response is frequently dysregulated (30). Although neutrophil percentage is relatively high, their ability to eliminate pathogens is impaired due to the immune-suppressive effects of glucocorticoids (31). This “ineffective inflammation” can result in persistent pulmonary infections, thereby increasing the risk of severe complications and death. Albumin, a crucial protein with the body, has been employed as a biochemical marker for nutritional status, immune function, and systemic inflammatory state (29, 32). Inflammation leads to a decrease in serum albumin levels by suppressing hepatic albumin synthesis, increasing the production of inflammatory factors and impairing the vascular endothelium to prompt albumin leakage from blood vessels (33). Consequently, hypoalbuminemia is common in patients with CAP, especially those with severe CAP. Furthermore, in patients treated with long-term and systemic glucocorticoids, hypoalbuminemia may be exacerbated by several factors, including the promotion of protein catabolism by glucocorticoids (34), the reduction of hepatic albumin synthesis (35), and the increase in urinary protein excretion (36). Numerous studies have demonstrated that low albumin levels are correlated with mortality (37). The NPAR, a novel biomarker combining the neutrophil percentage and serum albumin, which is closely associated with the inflammatory response and nutritional status (38). In addition to long-term use of glucocorticoids, we found that the main reason for the decrease of albumin in the high NPAR group was an increased systemic inflammatory burden in the present study. Therefore, hypoalbuminemia, along with elevated NPAR, was significantly associated with increased mortality.
Several previous studies have demonstrated that NPAR was an effective marker for predicting mortality across populations. For instance, Maside Ari et al. reported that NPAR could potentially serve as a biomarker for predicting disease severity and mortality risk among pneumonia patients aged over 80 years (17). Another investigation has revealed that elevated NPAR levels were correlated with an augmented risk of 30-day and 90-day mortality among patients suffering from severe sepsis or septic shock (18). Similarly, in pneumonia patients with stroke (20) or traumatic spinal cord injury (39), NPAR has been demonstrated to be correlated with mortality. Consistent with prior findings, the present study also revealed that a higher NPAR was associated with an increased risk of mortality, particularly in the highest NPAR tertile, which had a significantly higher risk for 30-day mortality (HR: 3.19, 95% CI: 2.42–4.2) and 90-day mortality (HR: 2.75, 95% CI: 2.14–3.54). Additionally, a linear relationship was observed between NPAR levels and both 30-day and 90-day mortality.
Given the restricted predictive ability of inflammatory markers including leukocytosis, CRP, and PCT for mortality (15), certain studies have explored other measurable laboratory markers associated with prognosis in CAP patients treated with prolonged glucocorticoids. A study conducted by Xia et al. (40) revealed that the BUN/ALB ratio served as a prognostic indicator for unfavorable 30-day outcomes in these patients. Similarly, Bai et al. (41) demonstrated that an elevated level of the systemic immune inflammatory Index (SII) was an independent predictor for 90-day mortality in ILD patients complicated with pneumonia. Furthermore, Cheng et al. (42) proposed that LDH served as a prognostic indicator for 90-day mortality in CTD patients with pneumonia. In this study, we focused on a relatively high-proportion cohort of patients with COPD, ILD, bronchial asthma, CTD, IIP, hematological diseases, nephrotic syndrome, chronic glomerulonephritis, or other indications for glucocorticoids therapy. Furthermore, the association between NPAR and inflammation was more direct, rendering it more valuable for comprehensive evaluation in patients with CAP after receiving glucocorticoids therapy. Simultaneously, a notable correlation was identified between the NPAR and 30-day as well as 90-day mortality in this study population, even subsequent to the adjustment for multiple confounding factors. To our knowledge, there are currently no other studies on the association between NPAR and the prognosis of patients who developed community-acquired pneumonia after receiving glucocorticoids therapy. Our study, which focuses on this particular population, addresses a gap in the existing literature. Unlike complex scoring systems for assessing CAP severity, such as CURB-65 and PSI, which necessitate multiple clinical and laboratory parameters, NPAR consists of the neutrophils percentage and serum albumin, routinely measured in blood tests. For patients with CAP, even in the absence of imaging examinations, NPAR could still function as an effective marker for rapid risk assessments. Recent research indicates that NPAR may not only serve as a prognostic marker but also act as a potential indicator for guiding therapeutic strategies (43). Consequently, the early detection of elevated levels of the NPAR in CAP patients after receiving glucocorticoids treatment can help identify high-risk patients and assist clinicians in making precise decisions to improve outcomes.
The present study inevitably had several limitations. First, this study was a retrospective cohort study which might have introduced some bias. Second, NPAR was calculated from data obtained from the first blood tests upon admission. Consequently, dynamic observation of NPAR was not feasible. Random errors caused by using only the first blood result might be unavoidable. Third, due to the limitations in the original dataset, we were unable to collect detailed information about the patients’ dietary habits and did not analyze the effects of dietary habits on the association of NPAR with mortality. Fourth, our study lacked data regarding the prevalence of pneumonic infiltrations, which significantly affected the assessment of disease’s clinical progression and prognosis. Fifth, although we have made efforts to control possible confounders by using multivariate models, there still existed confounding factors that might potentially influence the results. Nevertheless, the findings require validation through large-scale prospective studies.
5 Conclusion
The present study revealed that an elevated NPAR was significantly associated with 30-day and 90-day mortality in patients who developed CAP during treatment with oral or intravenous glucocorticoids. NPAR is expected to serve as an important independent predictor for this specific population.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary material.
Ethics statement
The studies involving humans were approved by the Ethics Committee of China-Japan Friendship Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
JG: Conceptualization, Data curation, Formal analysis, Writing –original draft. CJ: Data curation, Visualization, Writing –original draft. JR: Supervision, Writing – original draft, Writing – review & editing. LT: Conceptualization, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We express our sincere gratitude to Lijuan Li et al. for providing the data and sharing it in DYARD. We gratefully thank the Free Statistics team for providing technical assistance and valuable tools for data analysis and visualization.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1689323/full#supplementary-material
Footnotes
References
1. Aliberti S, Dela Cruz C, Amati F, Sotgiu G, Restrepo M. Community-acquired pneumonia. Lancet. (2021) 398:906–19. doi: 10.1016/S0140-6736(21)00630-9
2. Torres A, Cilloniz C, Niederman M, Menéndez R, Chalmers J, Wunderink R, et al. Pneumonia. Nat Rev Dis Prim. (2021) 7:25. doi: 10.1038/s41572-021-00259-0
3. Papiris S, Manali, Kolilekas L, Kagouridis K, Maniati M, Filippatos G, et al. Acute respiratory events in connective tissue disorders. Respiration. (2016) 91:181–201. doi: 10.1159/000444535
4. Scholl T, Kiser T, Vondracek S. Evaluation of systemic corticosteroids in patients with an acute exacerbation of COPD and a diagnosis of pneumonia. Chronic Obstr Pulm Dis. (2018) 5:57–65. doi: 10.15326/jcopdf.5.1.2017.0157
5. Lv J, Wong M, Hladunewich M, Jha V, Hooi L, Monaghan H, et al. Effect of oral methylprednisolone on decline in kidney function or kidney failure in patients with IgA nephropathy: the TESTING randomized clinical trial. JAMA. (2022) 327:1888–98. doi: 10.1001/jama.2022.5368
6. Certan M, Garcia Garrido H, Wong G, Heijmans J, Grobusch M, Goorhuis A. Incidence and predictors of community-acquired pneumonia in patients with hematological cancers between 2016 and 2019. Clin Infect Dis. (2022) 75:1046–53. doi: 10.1093/cid/ciac005
7. Reichel F, Tesch F, Berger S, Seifert M, Koschel D, Schmitt J, et al. Epidemiology and risk factors of community-acquired pneumonia in patients with different causes of immunosuppression. Infection. (2024) 52:2475–86. doi: 10.1007/s15010-024-02314-w
8. Li L, Hsu S, Gu X, Jiang S, Shang L, Sun G, et al. Aetiology and prognostic risk factors of mortality in patients with pneumonia receiving glucocorticoids alone or glucocorticoids and other immunosuppressants: a retrospective cohort study. BMJ Open. (2020) 10:e037419. doi: 10.1136/bmjopen-2020-037419
9. Guo H, Ye Y, Cao R, Liu Z, He Q. Association between the cumulative dose of glucocorticoids before the development of pneumonia and death in patients receiving long-term glucocorticoids: a secondary analysis based on a Chinese cohort study. Front Med. (2023) 10:1175855. doi: 10.3389/fmed.2023.1175855
10. Agustí C, Rañó A, Filella X, González J, Moreno A, Xaubet A, et al. Pulmonary infiltrates in patients receiving long-term glucocorticoid treatment: etiology, prognostic factors, and associated inflammatory response. Chest. (2003) 123:488–98. doi: 10.1378/chest.123.2.488
11. Di Pasquale M, Sotgiu G, Gramegna A, Radovanovic D, Terraneo S, Reyes L, et al. Prevalence and etiology of community-acquired pneumonia in immunocompromised patients. Clin Infect Dis. (2019) 68:1482–93. doi: 10.1093/cid/ciy723
12. Chalmers J, Singanayagam A, Akram A, Mandal P, Short P, Choudhury G, et al. Severity assessment tools for predicting mortality in hospitalised patients with community-acquired pneumonia. Systematic review and meta-analysis. Thorax. (2010) 65:878–83. doi: 10.1136/thx.2009.133280
13. Loke Y, Kwok C, Niruban A, Myint P. Value of severity scales in predicting mortality from community-acquired pneumonia: systematic review and meta-analysis. Thorax. (2010) 65:884–90. doi: 10.1136/thx.2009.134072
14. Gonzalez C, Johnson T, Rolston K, Merriman K, Warneke C, Evans S. Predicting pneumonia mortality using CURB-65, PSI, and patient characteristics in patients presenting to the emergency department of a comprehensive cancer center. Cancer Med. (2014) 3:962–70. doi: 10.1002/cam4.240
15. Sungurlu S, Balk R. The role of biomarkers in the diagnosis and management of pneumonia. Infect Dis Clin North Am. (2024) 38:35–49. doi: 10.1016/j.idc.2023.12.005
16. Kutz A, Grolimund E, Christ-Crain M, Thomann R, Falconnier C, Hoess C, et al. Pre-analytic factors and initial biomarker levels in community-acquired pneumonia patients. BMC Anesthesiol. (2014) 14:102. doi: 10.1186/1471-2253-14-102
17. Ari M, Haykir Solay A, Ozdemir T, Yildiz M, Mentes O, Tuten O, et al. Neutrophil percentage-to-albumin ratio as a prognostic marker in pneumonia patients aged 80 and above in intensive care. J Clin Med. (2025) 14:3033. doi: 10.3390/jcm14093033
18. Gong Y, Li D, Cheng B, Ying B, Wang B. Increased neutrophil percentage-to-albumin ratio is associated with all-cause mortality in patients with severe sepsis or septic shock. Epidemiol Infect. (2020) 148:e87. doi: 10.1017/S0950268820000771
19. Kurkiewicz K, Gąsior M, Szyguła-Jurkiewicz B. Markers of malnutrition, inflammation, and tissue remodeling are associated with 1-year outcomes in patients with advanced heart failure. Pol Arch Intern Med. (2023) 133:16411. doi: 10.20452/pamw.16411
20. Lu Y, Mao B, Wang M, Wan S. Neutrophil percentage-to-albumin ratio as a prognostic marker for mortality in ischemic stroke patients. Int J Med Sci. (2025) 22:2663–75. doi: 10.7150/ijms.108493
21. Wang B, Li D, Cheng B, Ying B, Gong Y. The neutrophil percentage-to-albumin ratio is associated with all-cause mortality in critically ill patients with acute kidney injury. Biomed Res Int. (2020) 2020:5687672. doi: 10.1155/2020/5687672
22. Metlay J, Waterer G, Long A, Anzueto A, Brozek J, Crothers K, et al. Diagnosis and treatment of adults with community-acquired pneumonia. an official clinical practice guideline of the American thoracic society and infectious diseases society of America. Am J Respir Crit Care Med. (2019) 200:e45–67. doi: 10.1164/rccm.201908-1581ST
23. Mann B, Chung K. Blood neutrophil activation markers in severe asthma: lack of inhibition by prednisolone therapy. Respir Res. (2006) 7:59. doi: 10.1186/1465-9921-7-59
24. Jiang J, Du H, Su Y, Li X, Zhang J, Chen M, et al. Nonviral infection-related lymphocytopenia for the prediction of adult sepsis and its persistence indicates a higher mortality. Medicine. (2019) 98:e16535. doi: 10.1097/MD.0000000000016535
25. Coutinho A, Chapman K. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol Cell Endocrinol. (2011) 335:2–13. doi: 10.1016/j.mce.2010.04.005
26. Elsouri K, Arboleda V, Basbous L, Heiser S, Collins D, Ragusa P, et al. Glucocorticoid use in rheumatoid arthritis patients and the onset of pneumonia: a systematic review and meta-analysis. J Osteopath Med. (2023) 123:179–86. doi: 10.1515/jom-2022-0177
27. Rombauts A, Abelenda-Alonso G, Cuervo G, Gudiol C, Carratalà J. Role of the inflammatory response in community-acquired pneumonia: clinical implications. Expert Rev Anti Infect Ther. (2022) 20:1261–74. doi: 10.1080/14787210.2021.1834848
28. Castanheira F, Kubes P. Neutrophils and NETs in modulating acute and chronic inflammation. Blood. (2019) 133:2178–85. doi: 10.1182/blood-2018-11-844530
29. Eckart A, Struja T, Kutz A, Baumgartner A, Baumgartner T, Zurfluh S, et al. Relationship of nutritional status, inflammation, and serum albumin levels during acute illness: a prospective study. Am J Med. (2020) 133:713–22.e7. doi: 10.1016/j.amjmed.2019.10.031
30. Raess N, Schuetz P, Cesana-Nigro N, Winzeler B, Urwyler S, Schaedelin S, et al. Influence of prednisone on inflammatory biomarkers in community-acquired pneumonia: secondary analysis of a randomized trial. J Clin Pharmacol. (2021) 61:1406–14. doi: 10.1002/jcph.1914
31. Yang J, Yang L, Luo R, Xu J. Corticosteroid administration for viral pneumonia: COVID-19 and beyond. Clin Microbiol Infect. (2020) 26:1171–7. doi: 10.1016/j.cmi.2020.06.020
32. Chen L, Lu X, Zhu C. Prognostic value of albumin-red cell distribution width score in patients with severe community-acquired pneumonia. Ann Palliat Med. (2020) 9:759–65. doi: 10.21037/apm.2020.04.22
33. Turcato G, Zaboli A, Lucente F, Filippi L, Maggi M, Brigiari G, et al. Sepsis-induced coagulopathy and hypoalbuminemia: endothelial damage as common pathway and clinical implications on mortality and transfusion risk. J Clin Med. (2025) 14:4483. doi: 10.3390/jcm14134483
34. Montón C, Ewig S, Torres A, El-Ebiary M, Filella X, Rañó A, et al. Role of glucocorticoids on inflammatory response in nonimmunosuppressed patients with pneumonia: a pilot study. Eur Respir J. (1999) 14:218–20. doi: 10.1034/j.1399-3003.1999.14a37.x
35. Shi Y, Chen R, Sun H, Xu K, Li Z, Wang M, et al. Prognostic analysis of concurrent Pneumocystis jirovecii pneumonia in patients with systemic lupus erythematosus: a retrospective study. BMC Infect Dis. (2024) 24:874. doi: 10.1186/s12879-024-09757-4
36. Clement L, Avila-Casado C, Macé C, Soria E, Bakker W, Kersten S, et al. Podocyte-secreted angiopoietin-like-4 mediates proteinuria in glucocorticoid-sensitive nephrotic syndrome. Nat Med. (2011) 17:117–22. doi: 10.1038/nm.2261
37. Miyazaki H, Nagata N, Akagi T, Takeda S, Harada T, Ushijima S, et al. Comprehensive analysis of prognostic factors in hospitalized patients with pneumonia occurring outside hospital: serum albumin is not less important than pneumonia severity assessment scale. J Infect Chemother. (2018) 24:602–9. doi: 10.1016/j.jiac.2018.03.006
38. Gao S, Yu F, Han Y. Association between neutrophil percentage-to-albumin ratio and anemia risk: a population-based study. Sci Rep. (2025) 15:16649. doi: 10.1038/s41598-025-98708-3
39. Wang C, Yu X, Wang T, Ding M, Ran L, Wang L, et al. Association between neutrophil percentage-to-albumin ratio and pneumonia in patients with traumatic spinal cord injury. Spinal Cord. (2023) 61:106–10. doi: 10.1038/s41393-022-00844-4
40. Xia B, Song B, Zhang J, Zhu T, Hu H. Prognostic value of blood urea nitrogen-to-serum albumin ratio for mortality of pneumonia in patients receiving glucocorticoids: secondary analysis based on a retrospective cohort study. J Infect Chemother. (2022) 28:767–73. doi: 10.1016/j.jiac.2022.02.015
41. Bai W, Wang Y, Li F. Effect of novel inflammatory biomarkers on adverse outcomes in patients with interstitial lung disease and pneumonia: a multicenter retrospective cohort study. Comb Chem High Throughput Screen. (2025) 28:798–807. doi: 10.2174/0113862073286907240308095907
42. Cheng X, Liu L, Tian Y, Lin Y. Serum lactate dehydrogenase as a prognostic marker for 90-day mortality in connective tissue disease patients receiving glucocorticoids and hospitalized with pneumonia: a cohort study. Sci Rep. (2025) 15:16806. doi: 10.1038/s41598-025-01721-9
Keywords: neutrophil percentage-to-albumin ratio, glucocorticoids, mortality, community acquired pneumonia, Dryad database
Citation: Guo J, Jiang C, Ran J and Tang L (2025) Association between neutrophil percentage-to-albumin ratio and mortality in patients with community acquired pneumonia receiving systemic glucocorticoids: a retrospective cohort study. Front. Med. 12:1689323. doi: 10.3389/fmed.2025.1689323
Received: 20 August 2025; Accepted: 30 September 2025;
Published: 13 October 2025.
Edited by:
Guoyun Chen, University of Tennessee Health Science Center (UTHSC), United StatesReviewed by:
Seher Satar, Turkish Thoracic Society, Turks and Caicos IslandsKamile Yucel, KTO Karatay University, Türkiye
Copyright © 2025 Guo, Jiang, Ran and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li Tang, dGFuZ2xpMTYyMl95YXlhQDE2My5jb20=; Juanyou Ran, cmp5NzQxODUyQDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
 Juan Guo1†
Juan Guo1† Li Tang
Li Tang