- 1Department of Health Services Research, Management and Policy, University of Florida, Gainesville, FL, United States
- 2Department of Community Health and Family Medicine, University of Florida, Gainesville, FL, United States
Background: Normal weight obesity (NWO) – a normal body mass index (BMI) with high body fat percentage (BF%) – has been linked to increased cardiometabolic risk. This study examined whether NWO is associated with systemic inflammation.
Methods: Using 2017–2018 NHANES data, we categorized adult respondents aged 18–59 with BMI ≥ 18.5 into four groups:
1. Reference: Normal BMI (18.5–24.9) with normal BF% (< 25% males/ < 35% females)
2. NWO: Normal BMI with high BF% (≥ 25% males/ ≥ 35% females)
3. Elevated BMI (≥ 25) with normal BF%
4. Elevated BMI with high BF%
Survey-weighted logistic regression examined associations with elevated hs-CRP (> 3.0 mg/L), adjusting for age and race/ethnicity. Sex-stratified analyses were also conducted.
Results: Inflammation prevalence was 32.7% overall, highest among individuals with elevated BMI and high BF% (43.6%). Compared to the reference group, individuals with NWO had over 3-fold increased odds of inflammation [AOR 3.34 (95% CI: 1.83, 6.08)]; individuals with elevated BMI and high BF% had over 6-fold increased odds [AOR 6.19 (95% CI: 3.66, 10.50)]. Elevated BMI with normal BF% was not significantly associated with inflammation.
In sex-stratified analyses, NWO was associated with inflammation in both males [AOR 4.44 (95% CI: 1.62, 12.10)] and females [AOR 2.78 (95% CI: 1.40, 5.52)]. Elevated BMI and high BF% was also associated with inflammation in both sexes.
Conclusion: In this cross-sectional study, NWO was associated with inflammation, although causality cannot be inferred. Reliance on BMI alone may misclassify cardiometabolic risk therefore BF% should be considered in clinical assessments.
Introduction
Chronic low-grade inflammation has been linked to the development and progression of cardiometabolic diseases, including hypertension, atherosclerosis, coronary artery disease, and type 2 diabetes, as well as cancer (1–15). High-sensitivity C-reactive protein (hs-CRP) is a well-established blood test used to measure inflammation, and elevated hs-CRP levels have been associated with an increased risk of future cardiovascular events, as well as all-cause, cardiovascular-related, and cancer-related mortality (16–21).
Obesity is a driver of both inflammation and chronic and cardiometabolic conditions (22, 23), and a growing body of evidence suggests that assessing body fat percentage (BF%), rather than relying solely on body mass index (BMI), may be more relevant in determining health risks and mortality (24, 25). Although the gold standard definition of obesity is an excess of body fat (26), BMI is the most commonly used metric in clinical practice. Thresholds for overweight and obesity are defined by BMI, and current guidelines rely on BMI thresholds to determine screening and interventions for a variety of weight-related comorbidities, such as prediabetes and type 2 diabetes (27). The USPSTF also recommends clinicians refer adults with a BMI of 30 or higher to intensive, multicomponent behavioral interventions (28).
While BMI has long been the primary metric in clinical weight management, it is a poor surrogate marker of actual adiposity or body fat percentage (29). Approximately 30 million Americans have a high BF% despite having a normal BMI (30). This condition, referred to as normal weight obesity (NWO) (31) is associated with significant health risks. Goodpaster et al. found that greater visceral fat among normal weight males was associated with more than double the odds of metabolic syndrome (24). More recent studies have shown that a significant proportion of individuals with NWO have prediabetes, undiagnosed diabetes, hypertension, or metabolic dysfunction-associated steatotic liver disease (32–34). Lower lean body mass in relation to body fat is being increasingly recognized as a critical contributor to cardiometabolic risk, more so than obesity itself (35–37). Thus, assessing patients based solely on BMI may misclassify a substantial number of those who are at high risk (25, 38).
Incorporating BF% measurements may provide additional, important insights into the risk of inflammation and related cardiometabolic risk compared to relying on BMI alone. Understanding the association between different body composition profiles and inflammation may reveal important limitations in the current reliance on BMI-based classifications of obesity and inform more targeted screening and prevention strategies. This study examined whether having NWO is associated with elevated inflammation in a nationally representative sample of U.S. adults from the National Health and Nutrition Examination Survey (NHANES).
Methods
Data source
We conducted a cross-sectional analysis using data from the 2017 to 2018 NHANES, a large, nationally representative survey of the non-institutionalized U.S. population administered by the Centers for Disease Control and Prevention (CDC) (39). More information about the NHANES methodology and protocols is available on the CDC website (40). NHANES participants answer questions about their health and undergo a standardized medical examination including blood tests for biomarkers and body measurements. The 2017–2018 cycle was selected as the most recent cycle to include measurements from whole-body dual-energy X-ray absorptiometry (DXA). This study was conducted using publicly available and deidentified data. As such, it did not involve human subjects research and did not require Institutional Review Board review. The study adheres to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines for reporting observational research (41).
Study population
The study population consisted of adult respondents from the 2017 to 2018 NHANES who were eligible for DXA, which was conducted only among individuals aged 18–59 years. NHANES further restricted DXA eligibility to non-pregnant participants with no radiographic contrast use in the past 7 days, and with self-reported weight ≤ 450 pounds and height ≤ 6 feet 5 inches (42).
Individuals with BMI < 18.5 were excluded to reduce potential confounding from undernutrition and to align with the study’s aim to assess inflammation risk across different body composition groups in those with clinically defined healthy or elevated BMI, with the term “elevated BMI” used in this study to refer to BMI ≥ 25. Participants were included if they had non-missing data for BMI, total body BF%, and high-sensitivity C-reactive protein (hs-CRP). Figure 1 (flow diagram) depicts the study population selection. The final unweighted sample size was 2,255 individuals, representing a weighted population of 114,132,307 U.S. adults.
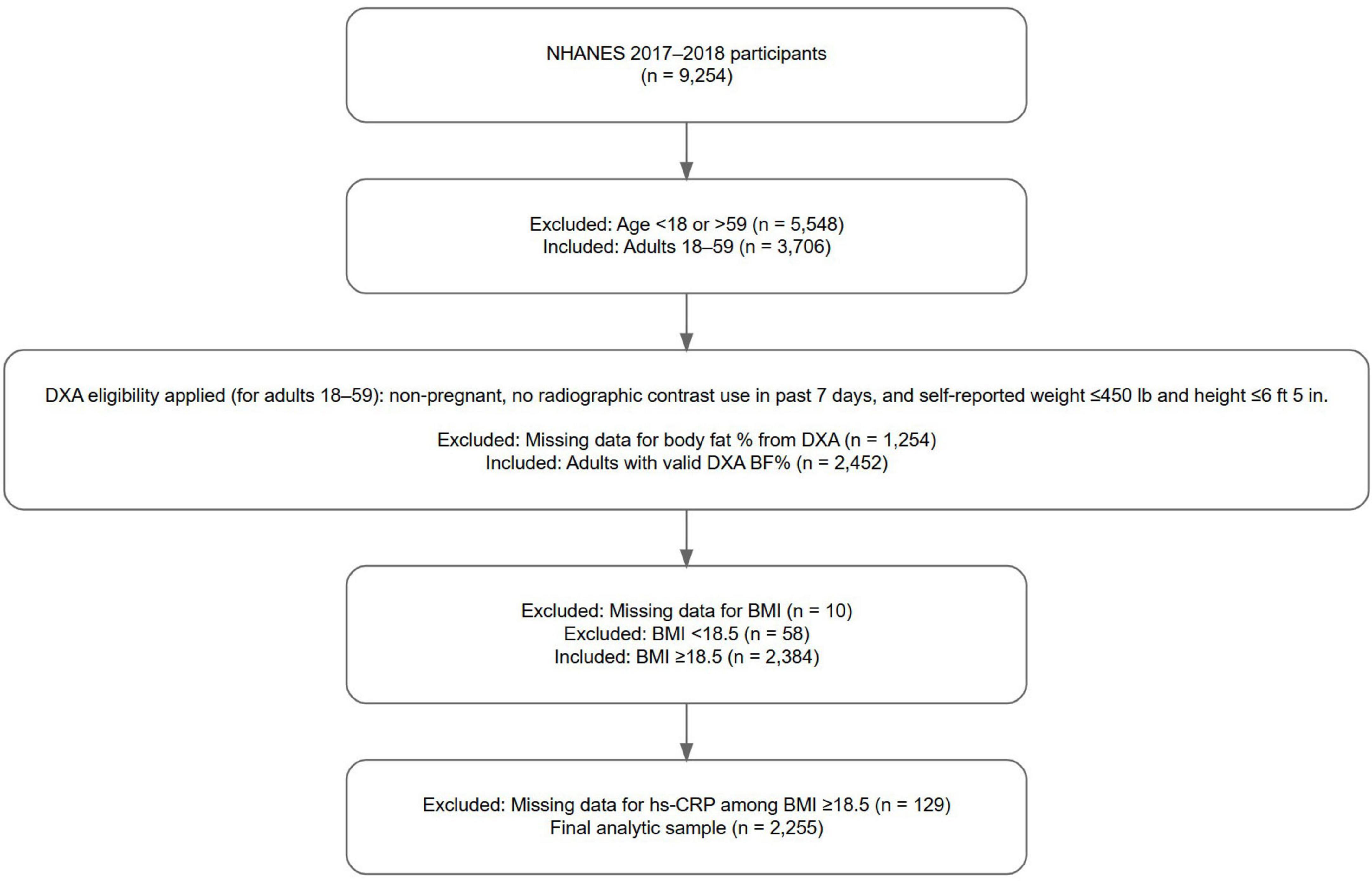
Figure 1. Selection procedure for the study population. Participants were drawn from the 2017 to 2018 National Health and Nutrition Examination Survey (NHANES). Inclusion criteria required participants to have non-missing data for body mass index (BMI), body fat percentage as measured by whole body dual-energy X-ray absorptiometry (DXA), and high-sensitivity C-reactive protein (hs-CRP), with BMI ≥ 18.5. NHANES DXA eligibility criteria included non-pregnant participants aged 18–59 years, with no radiographic contrast use in the past 7 days, and self-reported weight ≤ 450 pounds and height ≤ 6 feet 5 inches. The final unweighted sample size was 2,255 individuals, representing a weighted population of 114,132,307 U.S. adults.
Study outcome
The primary outcome measure of this study was the presence of systemic inflammation, defined as hs-CRP levels greater than 3.0 mg/L. This threshold corresponds to the high-risk category for major coronary events as defined by the American Heart Association, which classifies hs-CRP levels into three categories: low risk (< 1.0 mg/L), average risk (1.0–3.0 mg/L), and high risk (> 3.0 mg/L) (19). These cut-points represent approximate tertiles of the distribution of hs-CRP levels in the adult population, with the high-risk category associated with an approximately 2-fold increase in the relative risk of major coronary events compared to the low-risk category (19). The threshold of 3.0 mg/L is also consistent with that used in previous studies (16, 20, 43–48).
Independent variable
We assessed the association between body composition group, defined by categories of BMI and BF%, and the odds of elevated inflammation. Individuals with a normal BMI and a normal BF% served as the reference group. BF% was measured using whole body dual-energy X-ray absorptiometry (DXA), the gold standard for assessing body composition (49, 50).
Normal BMI was defined as 18.5–24.9 kg/m2 and elevated BMI as ≥ 25 kg/m2. Normal BF% was defined as < 25% in males and < 35% in females, and high BF% as ≥ 25% in males and ≥ 35% in females. The thresholds for BF% were selected based on the American Association of Clinical Endocrinologists/American College of Endocrinology Obesity Task Force 1998 position statement on the prevention, diagnosis, and treatment of obesity, and those used in prior studies (51–53). Individuals were categorized into four mutually exclusive groups:
1. Reference Group: Normal BMI with a normal BF%.
2. Group with Normal Weight Obesity: Normal BMI with a high BF%.
3. Elevated BMI with a normal BF%.
4. Elevated BMI with a high BF%.
Statistical analysis
We conducted survey-weighted analyses accounting for the NHANES complex sampling design, using the appropriate weight, strata, and cluster variables for our study population. Descriptive statistics were calculated to compare participant characteristics across body composition groups. Modified Rao-Scott Chi-Square tests of independence, with variance estimated using Taylor Series Linearization, were used to assess differences between these groups.
Unadjusted and adjusted survey-weighted logistic regression models were fitted to examine the association between body composition group and the odds of elevated hs-CRP (> 3.0 mg/L). Models were fitted for the overall study population, as well as separately for males and females, to account for sex-based differences in body composition (54). It should be noted that some sex-stratified subgroups, such as males with normal weight obesity and females with elevated BMI and normal BF%, had small unweighted sample sizes; therefore, the precision of these estimates (as reflected in their confidence intervals) should be interpreted with caution. The adjusted models controlled for age and race/ethnicity. Given our aim was to compare how different body composition profiles defined by BMI and BF% thresholds are associated with inflammation in a manner that reflects clinical practice, where classification based on these thresholds does not vary according to comorbidities, we limited adjustment to key demographic variables (age and race/ethnicity), which were treated as background confounders. Because our objective was to evaluate potential misclassification of inflammatory risk when relying on BMI alone, rather than to estimate the independent association of NWO with inflammation, other factors such as lifestyle behaviors and comorbidities were not adjusted for, as these may act as mediators on the pathway between body composition and inflammation. Adjusting for them could constitute overadjustment and obscure the associations we aimed to describe between different body composition profiles and inflammation in the general adult U.S. population.
As a sensitivity analysis, we also fitted all models again after excluding individuals with hs-CRP levels > 10 mg/L (n = 153) to account for the potential influence of acute inflammation that may have led to substantially elevated hs-CRP unrelated to adiposity. The results of this sensitivity analysis are presented in Supplementary Appendix Table 1 and were compared to the main analyses to assess the robustness of the findings.
All analyses were conducted using R version 4.4.1 (2024-06-14, ucrt) and RStudio 2024.12.1 (Posit Software, PBC) (55, 56). Survey-weighted logistic regression models were fitted using the survey package with svydesign() and svy(glm) to incorporate the NHANES strata, cluster, and weight variables, providing nationally representative estimates for the U.S population. All p-values were two-sided, with p < 0.05 considered statistically significant. Due to the use of design-adjusted degrees of freedom in survey-weighted analyses, p-values are based on the t-distribution and may differ slightly from confidence intervals derived using normal (z) approximations. This may result in marginal discrepancies between p-values and 95% CIs.
Results
The study sample included 2,255 (unweighted) individuals, representing 114,132,307 (weighted) non-institutionalized U.S. adults, of whom 1,077 (weighted, 56,004,781) were male and 1,178 (weighted, 58,127,527) were female. Table 1 presents the distribution of participant characteristics across the four body composition groups. Overall, 9.2% of the population had NWO, representing 30.5% of all individuals classified as having a normal BMI.
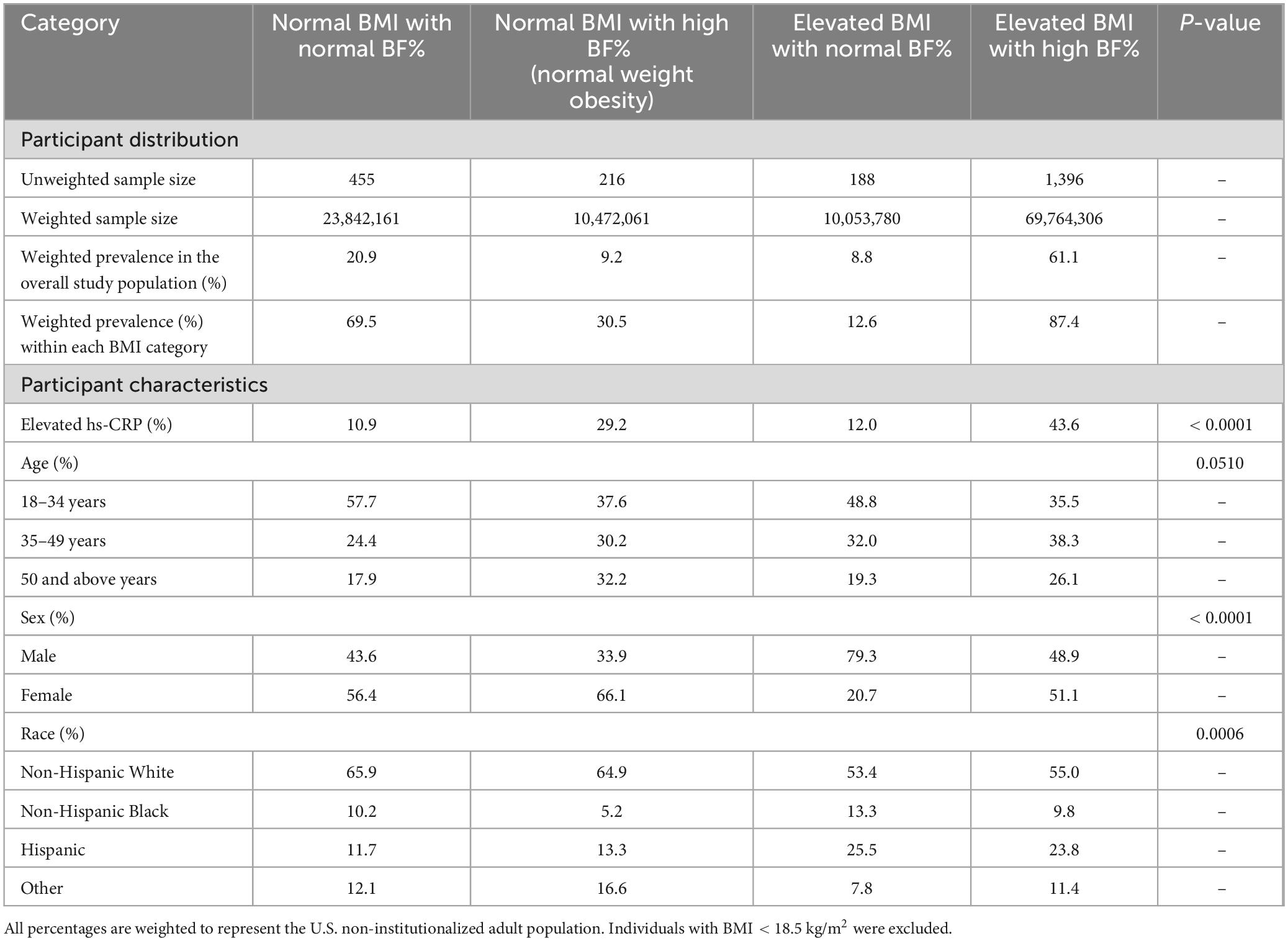
Table 1. Distribution and characteristics of U.S. adults aged 18–59 years by body composition group based on body mass index (BMI) and body fat percentage, National Health and Nutrition Examination Survey (NHANES) 2017–2018.
The weighted prevalence of elevated hs-CRP (> 3.0 mg/L) was 32.7% in the overall study population, 24.7% in males, and 40.3% in females. The weighted prevalence of elevated hs-CRP was highest among individuals classified as having an elevated BMI with a high BF%, with 43.6% of this group classified as having elevated hs-CRP. This was followed by individuals classified as having normal weight obesity, among whom 29.2% had elevated hs-CRP levels.
There were no statistically significant differences in age distribution across the groups (Table 1). However, significant differences were observed in sex and race/ethnicity. A greater proportion of individuals with an elevated BMI and a normal BF% were male, while a greater proportion of individuals with NWO were female. Non-Hispanic White individuals were more prevalent in both groups with a normal BMI, including the group with NWO, whereas Hispanic individuals were more prevalent in both groups with elevated BMI.
The results of survey-weighted logistic regression presented in Table 2 show that, among the overall study population, and after adjusting for age and race/ethnicity, individuals with NWO had more than 3-fold increased odds of inflammation compared to the reference group (individuals with a normal BMI and a normal BF%), with an adjusted odds ratio (AOR) of 3.34 (95% CI: 1.83, 6.08). Individuals with an elevated BMI and a high BF% had more than 6-fold increased odds (AOR 6.19; 95% CI: 3.66, 10.50), while individuals with an elevated BMI and a normal BF% did not have significantly increased odds of inflammation compared to the reference group.
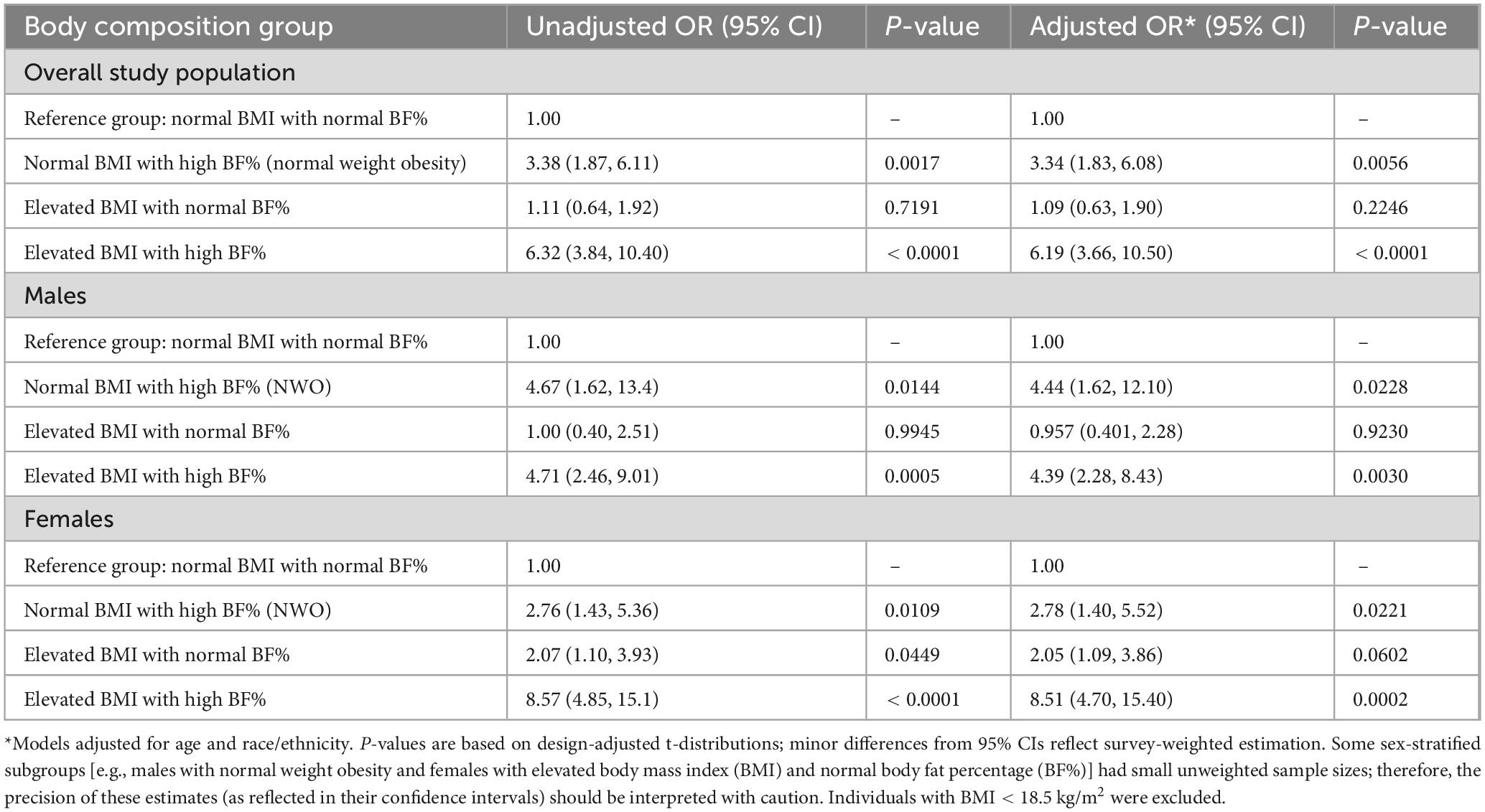
Table 2. Association between body composition and odds of elevated high-sensitivity C-reactive protein (hs-CRP) levels among U.S. adults aged 18–59 years: unadjusted and adjusted odds ratios from survey-weighted logistic regression, overall and stratified by sex, National Health and Nutrition Examination Survey (NHANES) 2017–2018.
Also shown in Table 2 are the results of the sex-stratified analyses. In the sex-stratified models which adjusted for age and race/ethnicity, compared to the reference group, having NWO was associated with inflammation in both sexes, with a stronger association in males (AOR 4.44; 95% CI: 1.62, 12.10) than in females (AOR 2.78; 95% CI: 1.40, 5.52). Having an elevated BMI with a high BF% was also associated with inflammation in both sexes, and the association was stronger in females (AOR 8.51; 95% CI: 4.70, 15.40) than in males (AOR 4.39; 95% CI: 2.28, 8.43). In contrast, having an elevated BMI with a normal BF% was not significantly associated with inflammation in males, females, or in the overall study population.
The results of the sensitivity analysis, in which all models were refitted after excluding individuals with hs-CRP levels > 10 mg/L (n = 153) to account for the potential influence of acute inflammation, confirm the robustness of the main findings, showing no meaningful differences in the magnitude or direction of odds ratios or in the statistical significance of the associations across the overall and sex-specific models.
Discussion
This nationally representative analysis of U.S. adults reveals a clear link between systemic inflammation and a high BF%, even among individuals with a normal BMI. In the overall study population, individuals with NWO had more than three times higher odds of inflammation compared to those with a normal BMI and a normal BF%, even after adjusting for age and race/ethnicity. Among individuals with a normal BF%, there was no significant difference in odds of inflammation between those with a normal BMI and those with an elevated BMI. Notably, nearly one-third (30.5%) of individuals with a normal BMI had NWO, representing 9.2% of the overall study population. These findings add to evidence that relying on BMI alone may misclassify cardiometabolic risk for a significant proportion of the population by overlooking individuals with excess body fat despite a normal BMI (25, 38).
When stratified by sex and adjusted for age and race/ethnicity, both males with NWO and males with an elevated BMI and a high BF% had similarly increased odds of inflammation compared with the reference group (males with a normal BMI and a normal BF%). Meanwhile, the increased odds of inflammation among females with an elevated BMI and a high BF% were markedly higher than they were for males with this body composition profile, when comparing both with their respective reference groups. A possible hypothesis is that once BMI and BF% exceed a certain threshold there could be a synergistic effect that is more deleterious for females than for males, although this cannot be confirmed given the cross-sectional nature of the study. It may also suggest that any lower propensity for inflammation observed among females at a normal BMI could be lost once the elevated BMI threshold is surpassed. One possible explanation for these findings is sex-specific variation in the association of inflammation with excess adiposity. A prior study that examined the association between BMI and hs-CRP in 119 adults observed that the pro-inflammatory effect associated with increases in BMI was greater in females than in males (57). In other studies that looked specifically at body fat, Cartier et al. observed a significantly steeper slope in the association between CRP and both visceral and subcutaneous adipose tissue in females compared to males (58), while Schorr et al. reported that visceral adipose tissue was more strongly associated with cardiometabolic risk markers in females, while intramyocellular lipids were more strongly associated with risk markers in males (59). Although our study did not assess fat distribution, our findings, interpreted in the context of these prior studies, may suggest potential sex-based variations in the association of adiposity with inflammation. This may have implications for clinicians deciding which patients to query about the possible downstream effects of inflammation such as heart disease and cancer. It is important to note that although the difference in the odds ratios in the sex-stratified analyses suggested that the association between body composition and inflammation may differ by sex, these comparisons were not derived from a formal sex × body composition interaction test. Given the overlapping confidence intervals, these findings should be interpreted as suggestive rather than conclusive and warrant confirmation in further research studies designed to evaluate potential sex-specific differences in the association of NWO with inflammation between males and females.
To our knowledge, this is the first nationally representative study of U.S. adults showing that NWO is associated with significantly higher odds of inflammation as measured by elevated hs-CRP levels, a widely used and clinically relevant biomarker of inflammation. This finding is supported by a recent systematic review of studies on NWO and inflammatory markers which found that NWO was associated with high levels of CRP and IL6 (60). For example, a prior study by De Lorenzo et al., included in the aforementioned systematic review, reported that having NWO was associated with significantly higher levels of interleukin and TNF-alpha (61). Although individuals with a normal BMI are typically considered as lower risk for chronic and cardiometabolic conditions, our findings indicate that those with a high BF% have significantly increased odds of systemic inflammation, which evidence shows plays an important role in the development and progression of multiple chronic diseases, including hypertension, atherosclerosis, coronary artery disease, type 2 diabetes, and cancer (1–15).
Our study contributes to a growing body of literature showing that excess body fat, even at a normal BMI, is associated with increased cardiometabolic risk (24, 30–38). Routine assessment of body composition could help identify individuals with NWO who may otherwise be missed by BMI-based screening alone. The recent 2025 statement from the American College of Cardiology (ACC) on inflammation and cardiovascular disease recommends hs-CRP measurement as part of CVD primary prevention, reinforcing the clinical relevance of inflammation as a modifiable risk factor (62). Our findings underscore the importance of identifying inflammation in individuals with normal BMI but excess adiposity and support calls to improve how body composition is assessed in clinical practice. While DXA is the gold standard, its cost and use of ionizing radiation limit its routine use (49, 50). Bioelectrical impedance analysis (BIA) is a low-cost alternative that may be more feasible for widespread implementation in primary care (63, 64). As such technologies become more accessible, incorporating BF% assessment into routine health screenings could enhance early identification of high-risk individuals and support more targeted prevention strategies, including early lifestyle interventions to reduce inflammation and cardiometabolic risk.
This study has several strengths, including the use of a nationally representative sample of U.S. adults, with data on BF% measured using DXA (a gold standard for body composition assessment) and inflammation measured using hs-CRP, a clinically relevant and widely used biomarker. Our categorization of individuals into four groups based on BMI and BF% enabled us to assess the joint association of these metrics as combined predictors of the odds of having systemic inflammation, as well as to identify and quantify associations with inflammation across distinct BMI-BF% phenotypes. Given the cross-sectional nature of the study, it is important to emphasize that these findings are associations rather than causal effects. While BMI and BF% were modeled as a composite variable, preventing direct comparisons between their individual associations with inflammation, our findings clearly demonstrate that BF% adds clinically meaningful information beyond BMI alone, thus underscoring the value of including BF% alongside BMI to more accurately assess the likelihood of inflammation and subsequent cardiometabolic and chronic disease risk in clinical settings.
Limitations of our study include its cross-sectional design, which prevents inference about causality or temporality, and reliance on a single hs-CRP measurement, which may not reflect long-term inflammatory status, as hs-CRP levels can fluctuate and may spike due to acute infections or injuries (65, 66). However, the results of a sensitivity analysis excluding individuals with hs-CRP levels > 10 mg/L confirmed the robustness of the main findings. Another limitation of this study is that the sample was restricted to adults aged 18–59 years due to NHANES DXA eligibility criteria; therefore, the findings apply only to non-elderly adults. We recommend that future research examine the association between NWO and inflammation in older adults as data on body composition becomes available, since the relationship may differ or be even more pronounced with aging, for example, in the context of sarcopenic obesity. Additionally, NHANES DXA eligibility criteria excluded those with self-reported weight > 450 pounds or height > 6 feet 5 inches, meaning that extremely high-BMI individuals were not represented. This could slightly bias the “elevated BMI and high BF%” group toward less extreme obesity. Although individuals exceeding these thresholds represent a very small fraction of the U.S. population, it is important to note that their inflammation levels might be even higher. In addition, although models adjusted for age and race/ethnicity, other potential confounders such as lifestyle behaviors and comorbidities were not included, as these variables may act as mediators on the pathway between body composition and inflammation. Therefore, the reported associations reflect relationships between BMI- and BF%-defined body composition profiles and inflammation as they exist in the general U.S. adult population, rather than associations independent of these factors. Thus, the potential for residual confounding cannot be excluded.
In summary, NWO is associated with markedly elevated odds of inflammation in U.S. adults, although causality cannot be inferred due to the cross-sectional design of the study. Compared with individuals who had both a normal BMI and a normal BF%, those with NWO had significantly higher odds of inflammation, while those with an elevated BMI but a normal BF% did not exhibit increased odds in the overall study population. These findings highlight the limitations of relying on BMI as a standalone measure of body composition and underscore the importance of incorporating BF% as part of a more comprehensive assessment to better evaluate chronic disease risk in the clinical setting. We recommend that clinicians interpret BMI cautiously and consider body fat percentage assessment, when feasible, to better identify patients at increased cardiometabolic and inflammatory risk.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: https://wwwn.cdc.gov/nchs/nhanes/default.aspx.
Author contributions
RL-G: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. FO: Conceptualization, Writing – original draft, Writing – review & editing. AS: Conceptualization, Writing – original draft, Writing – review & editing. AJ: Conceptualization, Writing – original draft, Writing – review & editing. KS: Writing – original draft, Writing – review & editing. AMM: Writing – original draft, Writing – review & editing. DN: Writing – original draft, Writing – review & editing. ES: Writing – original draft, Writing – review & editing. AGM: Conceptualization, Methodology, Project administration, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1695935/full#supplementary-material
Abbreviations
AOR, adjusted odds ratio; BF%, body fat percentage; BMI, body mass index; DXA, dual-energy X-ray absorptiometry; hs-CRP, high-sensitivity C-reactive protein; NHANES, National Health and Nutrition Examination Survey; NWO, normal weight obesity; USPSTF, United States Preventive Services Taskforce.
References
1. Barbu E, Popescu M, Popescu A, Balanescu S. Inflammation as a precursor of atherothrombosis, diabetes and early vascular aging. Int J Mol Sci. (2022). 23:963. doi: 10.3390/ijms23020963
2. Chen L, Deng H, Cui H, Fang J, Zuo Z, Deng J, et al. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget (2017) 9:7204–18. doi: 10.18632/oncotarget.23208
3. Fernandes Q, Inchakalody V, Bedhiafi T, Mestiri S, Taib N, Uddin S, et al. Chronic inflammation and cancer; the two sides of a coin. Life Sci. (2024) 338:122390. doi: 10.1016/j.lfs.2023.122390
4. Ferrucci L, Fabbri E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol. (2018) 15:505–22. doi: 10.1038/s41569-018-0064-2
5. Furman D, Campisi J, Verdin E, Carrera-Bastos P, Targ S, Franceschi C, et al. Chronic inflammation in the etiology of disease across the life span. Nat Med. (2019) 25:1822–32. doi: 10.1038/s41591-019-0675-0
6. Galkina E, Ley K. Immune and inflammatory mechanisms of atherosclerosis (*). Annu Rev Immunol. (2009) 27:165–97. doi: 10.1146/annurev.immunol.021908.132620
7. Henein M, Vancheri S, Longo G, Vancheri F. The role of inflammation in cardiovascular disease. Int J Mol Sci. (2022) 23:12906. doi: 10.3390/ijms232112906
8. Hibino S, Kawazoe T, Kasahara H, Itoh S, Ishimoto T, Sakata-Yanagimoto M, et al. Inflammation-induced tumorigenesis and metastasis. Int J Mol Sci. (2021) 22:5421. doi: 10.3390/ijms22115421
9. Kalogeropoulos A, Georgiopoulou V, Psaty B, Rodondi N, Smith A, Harrison D, et al. Inflammatory markers and incident heart failure risk in older adults: the health ABC (Health, aging, and body composition) study. J Am Coll Cardiol. (2010) 55:2129–37. doi: 10.1016/j.jacc.2009.12.045
10. Kong P, Cui Z, Huang X, Zhang D, Guo R, Han M. Inflammation and atherosclerosis: signaling pathways and therapeutic intervention. Signal Transduct Target Ther. (2022) 7:1–24. doi: 10.1038/s41392-022-00955-7
11. Multhoff G, Molls M, Radons J. Chronic inflammation in cancer development. Front Immunol. (2012) 2:98. doi: 10.3389/fimmu.2011.00098
12. Rios F, de Ciuceis C, Georgiopoulos G, Lazaridis A, Nosalski R, Pavlidis G, et al. Mechanisms of vascular inflammation and potential therapeutic targets: a position paper from the ESH working group on small arteries. Hypertension. (2024) 81:1218–32. doi: 10.1161/HYPERTENSIONAHA.123.22483
13. Savoia C, Schiffrin E. Vascular inflammation in hypertension and diabetes: molecular mechanisms and therapeutic interventions. Clin Sci. (2007) 112:375–84. doi: 10.1042/CS20060247
14. Tsalamandris S, Antonopoulos A, Oikonomou E, Papamikroulis G, Vogiatzi G, Papaioannou S, et al. The role of inflammation in diabetes: current concepts and future perspectives. Eur Cardiol. (2019) 14:50–9. doi: 10.15420/ecr.2018.33.1
15. Wen Y, Zhu Y, Zhang C, Yang X, Gao Y, Li M, et al. Chronic inflammation, cancer development and immunotherapy. Front Pharmacol. (2022) 13:1040163. doi: 10.3389/fphar.2022.1040163
16. Bassuk S, Rifai N, Ridker P. High-sensitivity C-reactive protein: clinical importance. Curr Probl Cardiol. (2004) 29:439–93. doi: 10.1016/j.cpcardiol.2004.03.004
17. Burger P, Pradhan A, Dorresteijn J, Koudstaal S, Teraa M, de Borst G, et al. C-reactive protein and risk of cardiovascular events and mortality in patients with various cardiovascular disease locations. Am J Cardiol. (2023) 197:13–23. doi: 10.1016/j.amjcard.2023.03.025
18. Li Y, Zhong X, Cheng G, Zhao C, Zhang L, Hong Y, et al. Hs-CRP and all-cause, cardiovascular, and cancer mortality risk: a meta-analysis. Atherosclerosis. (2017) 259:75–82. doi: 10.1016/j.atherosclerosis.2017.02.003
19. Pearson T, Mensah G, Alexander R, Anderson J, Cannon R, Criqui M, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for disease control and prevention and the American heart association. Circulation. (2003) 107:499–511. doi: 10.1161/01.cir.0000052939.59093.45
20. Ridker PM. A test in context: high-sensitivity C-reactive protein. J Am Coll Cardiol. (2016) 67:712–23. doi: 10.1016/j.jacc.2015.11.037
21. Zhang J, Ji C, Zhai X, Tong H, Hu J. Frontiers and hotspots evolution in anti-inflammatory studies for coronary heart disease: a bibliometric analysis of 1990-2022. Front Cardiovasc Med. (2023) 10:1038738. doi: 10.3389/fcvm.2023.1038738
22. Powell-Wiley T, Poirier P, Burke L, Després J, Gordon-Larsen P, Lavie C, et al. Obesity and cardiovascular disease: a scientific statement from the American heart association. Circulation. (2021) 143:e984–1010. doi: 10.1161/CIR.0000000000000973
23. Da Costa L, Arora P, García-Bailo B, Karmali M, El-Sohemy A, Badawi A. The association between obesity, cardiometabolic disease biomarkers, and innate immunity-related inflammation in Canadian adults. Diabetes Metab Syndr Obes. (2012) 5:347–55. doi: 10.2147/DMSO.S35115
24. Goodpaster B, Krishnaswami S, Harris T, Katsiaras A, Kritchevsky S, Simonsick E, et al. Obesity, regional body fat distribution, and the metabolic syndrome in older men and women. Arch Intern Med. (2005) 165:777–83. doi: 10.1001/archinte.165.7.777
25. Mainous A, Yin L, Wu V, Sharma P, Jenkins B, Saguil A, et al. Body mass index vs body fat percentage as a predictor of mortality in adults aged 20-49 years. Ann Fam Med. (2025) 23:337–43. doi: 10.1370/afm.240330
26. World Health Organization. Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Committee. World Health Organ Tech Rep Ser. (1995) 854:1–452.
27. Us Preventive Services Task Force, Davidson KW, Barry MJ, Mangione CM, Cabana M, Caughey AB, et al. Screening for prediabetes and type 2 diabetes: us preventive services task force recommendation statement. JAMA. (2021) 326:736–43. doi: 10.1001/jama.2021.12531
28. Us Preventive Services Task Force, Curry SJ, Krist AH, Owens DK, Barry MJ, Caughey AB, et al. Behavioral weight loss interventions to prevent obesity-related morbidity and mortality in adults: us preventive services task force recommendation statement. JAMA. (2018) 320:1163–71. doi: 10.1001/jama.2018.13022
29. Prentice A, Jebb S. Beyond body mass index. Obes Rev. (2001) 2:141–7. doi: 10.1046/j.1467-789x.2001.00031.x
30. Romero-Corral A, Somers V, Sierra-Johnson J, Korenfeld Y, Boarin S, Korinek J, et al. Normal weight obesity: a risk factor for cardiometabolic dysregulation and cardiovascular mortality. Eur Heart J. (2010) 31:737–46. doi: 10.1093/eurheartj/ehp487
31. Oliveros E, Somers V, Sochor O, Goel K, Lopez-Jimenez F. The concept of normal weight obesity. Prog Cardiovasc Dis. (2014) 56:426–33. doi: 10.1016/j.pcad.2013.10.003
32. Mainous A, Tanner R, Jo A, Anton S. Prevalence of prediabetes and abdominal obesity among healthy-weight adults: 18-year trend. Ann Fam Med. (2016) 14:304–10. doi: 10.1370/afm.1946
33. Mainous A, Rooks B, Medley J, Dickmann S. Body composition among adults at a healthy body mass index and association with undetected non-alcoholic fatty liver. Int J Obes. (2022) 46:1403–5. doi: 10.1038/s41366-022-01124-0
34. Wee C, Hamel M, Huang A, Davis R, Mittleman M, McCarthy E. Obesity and undiagnosed diabetes in the U.S. Diabetes Care. (2008) 31:1813–5. doi: 10.2337/dc07-1867
35. Ponti F, Plazzi A, Guglielmi G, Marchesini G, Bazzocchi A. Body composition, dual-energy X-ray absorptiometry and obesity: the paradigm of fat (re)distribution. BJR Case Rep. (2019) 5:20170078. doi: 10.1259/bjrcr.20170078
36. Prado C, Wells J, Smith S, Stephan B, Siervo M. Sarcopenic obesity: a critical appraisal of the current evidence. Clin Nutr. (2012) 31:583–601. doi: 10.1016/j.clnu.2012.06.010
37. Sakuma K, Yamaguchi A. Sarcopenic obesity and endocrinal adaptation with age. Int J Endocrinol. (2013) 2013:204164. doi: 10.1155/2013/204164
38. Gómez-Ambrosi J, Silva C, Galofré J, Escalada J, Santos S, Millán D, et al. Body mass index classification misses subjects with increased cardiometabolic risk factors related to elevated adiposity. Int J Obes. (2012) 36:286–94. doi: 10.1038/ijo.2011.100
39. Centers for Disease Control and Prevention. NHANES - About the National Health and Nutrition Examination Survey. (2024). Available online at: https://www.cdc.gov/nchs/nhanes/about_nhanes.htm (accessed November 2, 2024)
40. Centers for Disease Control and Prevention. NHANES Survey Methods and Analytic Guidelines. (2024). Available online at: https://wwwn.cdc.gov/nchs/nhanes/analyticguidelines.aspx (accessed November 2, 2024).
41. von Elm E, Altman D, Egger M, Pocock S, Gøtzsche P, Vandenbroucke J, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. (2007) 335:806–8. doi: 10.1136/bmj.39335.541782.AD
42. Centers for Disease Control and Prevention. (2020). Available online at: https://wwwn.cdc.gov/Nchs/Data/Nhanes/Public/2017/DataFiles/DXX_J.htm (accessed April 7, 2025)
43. Alvarez Garcia B, Ruiz C, Chacon P, Sabin J, Matas M. High-sensitivity C-reactive protein in high-grade carotid stenosis: risk marker for unstable carotid plaque. J Vasc Surg. (2003) 38:1018–24. doi: 10.1016/s0741-5214(03)00709-2
44. Graf J, Scherzer R, Grunfeld C, Imboden J. Levels of C-reactive protein associated with high and very high cardiovascular risk are prevalent in patients with rheumatoid arthritis. PLoS One. (2009) 4:e6242. doi: 10.1371/journal.pone.0006242
45. Mainous A, Orlando F, Yin L, Sharma P, Wu V, Saguil A. Inflammation and poverty as individual and combined predictors of 15-year mortality risk in middle aged and older adults in the US. Front Med. (2024) 10:1261083. doi: 10.3389/fmed.2023.1261083
46. Martínez-Quintana E, Alcántara-Castellano M, García-Suárez M, Rodríguez-González F. C-reactive protein and long-term prognosis in adult patients with congenital heart disease. J Clin Med. (2024) 13:2199. doi: 10.3390/jcm13082199
47. Ridker PM. C-reactive protein, inflammation, and cardiovascular disease: clinical update. Tex Heart Inst J. (2005) 32:384–6.
48. Ridker P, Cook N. Clinical usefulness of very high and very low levels of C-reactive protein across the full range of Framingham risk scores. Circulation. (2004) 109:1955–9. doi: 10.1161/01.CIR.0000125690.80303.A8
49. Ng B, Hinton B, Fan B, Kanaya A, Shepherd J. Clinical anthropometrics and body composition from 3D whole-body surface scans. Eur J Clin Nutr. (2016) 70:1265–70. doi: 10.1038/ejcn.2016.109
50. Ritter S, Staub K, Eppenberger P. Associations between relative body fat and areal body surface roughness characteristics in 3D photonic body scans-a proof of feasibility. Int J Obes. (2021) 45:906–13. doi: 10.1038/s41366-021-00758-w
51. Dickey R, Bray GA. AACE/ACE position statement on the prevention, diagnosis, and treatment of obesity. Endocrine Pract. (1998) 4:300.
52. Jo A, Mainous A. Informational value of percent body fat with body mass index for the risk of abnormal blood glucose: a nationally representative cross-sectional study. BMJ Open. (2018) 8:e019200. doi: 10.1136/bmjopen-2017-019200
53. Potter A, Chin G, Looney D, Friedl K. Defining overweight and obesity by percent body fat instead of body mass index. J Clin Endocrinol Metab. (2025) 110:e1103–7. doi: 10.1210/clinem/dgae341
54. Bredella M. Sex differences in body composition. Adv Exp Med Biol. (2017) 1043:9–27. doi: 10.1007/978-3-319-70178-3_2
55. R Core Team. R: A Language and Environment for Statistical Computing. Vienna: R Core Statistical Computing.(2024)
56. RStudio Team. RStudio: Integrated development environment for R. Boston, MA: RStudio Team (2025).
57. Liqiang S, Fang-Hui L, Minghui Q, Haichun C. Threshold effect and sex characteristics of the relationship between chronic inflammation and BMI. BMC Endocr Disord. (2023) 23:175. doi: 10.1186/s12902-023-01396-1
58. Cartier A, Côté M, Lemieux I, Pérusse L, Tremblay A, Bouchard C, et al. Sex differences in inflammatory markers: what is the contribution of visceral adiposity? Am J Clin Nutr. (2009) 89:1307–14. doi: 10.3945/ajcn.2008.27030
59. Schorr M, Dichtel L, Gerweck A, Valera R, Torriani M, Miller K, et al. Sex differences in body composition and association with cardiometabolic risk. Biol Sex Differ. (2018) 9:28. doi: 10.1186/s13293-018-0189-3
60. Mohammadian Khonsari N, Baygi F, Tabatabaei-Malazy O, Mohammadpoor Nami S, Ehsani A, Asadi S, et al. Association of normal weight obesity phenotype with inflammatory markers: a systematic review and meta-analysis. Front Immunol. (2023) 14:1044178. doi: 10.3389/fimmu.2023.1044178
61. De Lorenzo A, Del Gobbo V, Premrov M, Bigioni M, Galvano F, Di Renzo L. Normal-weight obese syndrome: early inflammation? Am J Clin Nutr. (2007) 85:40–5. doi: 10.1093/ajcn/85.1.40
62. Mensah G, Arnold N, Prabhu S, Ridker P, Welty F. Inflammation and cardiovascular disease: 2025 ACC scientific statement: a report of the american college of cardiology. J Am Coll Cardiol. (2025). doi: 10.1016/j.jacc.2025.08.047 [Epub ahead of print].
63. Branco M, Mateus C, Capelas M, Pimenta N, Santos T, Mäkitie A, et al. Bioelectrical impedance analysis (BIA) for the assessment of body composition in oncology: a scoping review. Nutrients. (2023) 15:4792. doi: 10.3390/nu15224792
64. Lee S, Gallagher D. Assessment methods in human body composition. Curr Opin Clin Nutr Metab Care. (2008) 11:566–72. doi: 10.1097/MCO.0b013e32830b5f23
65. Mendall M, Patel P, Ballam L, Strachan D, Northfield TC. C-reactive protein and its relation to cardiovascular risk factors: a population based cross sectional study. BMJ. (1996) 312:1061–5. doi: 10.1136/bmj.312.7038.1061
Keywords: body composition, body fat percentage, body mass index, inflammation, CRP - C-reactive protein, screening, cardiometabolic health, normal weight obesity
Citation: Liu-Galvin R, Orlando FA, Saguil AA, Jo A, Smith KB, Miller AM, Nelson DS, Sanders EC and Mainous AG III (2025) More evidence of the health risks of normal weight obesity: the association with systemic inflammation. Front. Med. 12:1695935. doi: 10.3389/fmed.2025.1695935
Received: 30 August 2025; Accepted: 28 October 2025;
Published: 18 November 2025.
Edited by:
Sofia Sieczkowska, Independent Researcher, Juiz de Fora, BrazilReviewed by:
Marcos Ferreira, State University of Campinas, BrazilMohamed Hany, Alexandria University, Egypt
Copyright © 2025 Liu-Galvin, Orlando, Saguil, Jo, Smith, Miller, Nelson, Sanders and Mainous. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rachel Liu-Galvin, cmFjaGVsLmdhbHZpbkB1ZmwuZWR1
 Rachel Liu-Galvin
Rachel Liu-Galvin Frank A. Orlando
Frank A. Orlando Aaron A. Saguil2
Aaron A. Saguil2 Ara Jo
Ara Jo Andrew M. Miller
Andrew M. Miller Danielle S. Nelson
Danielle S. Nelson Arch G. Mainous III
Arch G. Mainous III