- 1Guangzhou Panyu Bright Eye Hospital, Guangzhou, China
- 2Center of Ophthalmology, Heyou Hospital, Foshan, China
- 3Center of Ophthalmology, Zhongshan Torch Development Zone People's Hospital, Zhongshan, China
Background: Meibomian gland dysfunction (MGD) secondary to thyroid-associated ophthalmopathy (TAO) represents a significant pathogenic mechanism in dry eye disease. This study provides the first systematic review and meta-analysis of MGD indicators in TAO.
Methods: The study protocol was prospectively registered with the International Prospective Register of Systematic Reviews (PROSPERO) (Registration ID: CRD420251020327) before data extraction. Following PRISMA and MOOSE guidelines, a systematic search was conducted across PubMed, Embase, Web of Science, Scopus, Ovid Medline, and Cochrane from inception through March 27, 2025. Fourteen studies met the inclusion criteria. Key indicators included lipid layer thickness (LLT), meiboscore, meibum quality, first non-invasive tear film break-up time (NITBUT-f), average non-invasive tear film break-up time (NITBUT-avg), tear break-up time (TBUT), meibomian gland dropout area in the upper (MGDU) and lower eyelids (MGDL), and in vivo confocal microscopy (IVCM) markers (meibomian gland acinar density [MAD], meibomian gland acinar longest diameter [MALD], meibomian gland acinar shortest diameter [MASD]). Risk of bias was assessed using the AHRQ checklist or NOS. Meta-analysis was performed with Review Manager 5.4.1 and Stata 16.0. Publication bias was assessed using Egger's test and funnel plots. Fixed-effects models were used in the absence of significant heterogeneity (P > 0.10 or I2 < 50%); otherwise, random-effects models were applied.
Results: Thirteen studies (813 TAO eyes, 522 controls) were included in the meta-analysis. Quality assessment revealed moderate-to-high methodological rigor across studies. Patients with TAO exhibited significantly worse meibomian gland indicators compared to controls: shorter tear film stability (NITBUT-f, TBUT), higher LLT, increased meiboscore and greater eyelid gland dropout (MGDU, MGDL). IVCM markers indicated meibomian acinar enlargement (MALD, MASD). Significant heterogeneity was observed in several outcomes, including NITBUT-f, NITBUT-avg, meiboscore, Meibum quality, TBUT and MGDU comparisons.
Conclusions: Despite the limited number of studies and small sample sizes, TAO is linked to meibomian gland atrophy, acinar dilation, and tear film instability. Active disease is associated with more pronounced lipid layer abnormalities. Targeted evaluation and management of MGD are crucial to mitigate TAO-associated ocular surface morbidity and improve patient quality of life.
1 Background
Thyroid-associated ophthalmopathy (TAO) is a chronic inflammatory orbital disorder strongly linked to autoimmune thyroid diseases, driven by cross-reactive immune responses targeting shared antigens, such as the thyrotropin receptor (TSHR) (1, 2). Pathologically, TAO is characterized by orbital fibroblast proliferation, glycosaminoglycan deposition, and adipose tissue hyperplasia. Clinically, it manifests as ocular surface damage, progressive proptosis, eyelid retraction, extraocular muscle hypertrophy, and diplopia. Severe cases may lead to vision-threatening complications, including compressive optic neuropathy and corneal ulceration (3, 4).
Ocular surface dysfunction, common in patients with TAO, is primarily manifested as conjunctival hyperemia, dry eye disease (DED), and exposure keratitis, significantly impairing quality of life (5, 6). The pathogenesis of TAO-associated dry eye is multifactorial: autoantibodies target TSHR-expressing lacrimal glands, inducing inflammatory edema and fibrosis that disrupt reflex tear secretion (7, 8). Elevated tear inflammatory cytokines (e.g., IL-1β, IL-6, IL-8) further exacerbate ocular surface inflammation, damaging conjunctival goblet cells and accessory lacrimal glands, thus reducing mucin production and basal tear secretion (9, 10). Mechanical factors, such as proptosis-induced lagophthalmos, increase corneal exposure, while fibrotic levator palpebrae complexes contribute to incomplete blinking, accelerating tear evaporation (11). Long-term immunosuppression or radiotherapy in moderate-to-severe TAO may further compromise the epithelial barrier (12). Conventional artificial tears have limited efficacy (< 50%) in managing TAO-associated DED, highlighting the urgent need for targeted therapeutic approaches (13).
Meibomian gland dysfunction (MGD), a major cause of evaporative DED, has gained increasing recognition for its role in the pathogenesis of TAO-associated ocular surface dysfunction (14). Patients with TAO exhibit significant meibomian gland dropout, particularly in the upper eyelids, with markedly reduced acinar density and gland orifice size compared to healthy controls. This leads to insufficient lipid secretion, decreased tear film lipid layer thickness, and accelerated tear evaporation (15, 16). Elevated systemic and local proinflammatory cytokines (e.g., IL-1β, TNF-α) impair the regenerative capacity of meibomian glands and exacerbate lipid layer instability. Concurrent corneal subbasal nerve damage reduces blink frequency and disrupts neurotrophic signaling, further worsening ductal keratinization and abnormal lipid composition (17). Therefore, TAO-associated MGD necessitates further investigation into its mechanisms and potential therapeutic strategies.
Recent studies have increasingly elucidated the clinical significance, epidemiological trends, and pathophysiological mechanisms of TAO-associated MGD. To our knowledge, no systematic review has comprehensively evaluated the scope of MGD in TAO. This meta-analysis synthesizes global evidence by integrating data from multinational cohort studies, aiming to clarify the clinical manifestations, pathophysiological pathways, and correlations with disease activity in TAO-associated MGD. The findings will provide a theoretical foundation for optimizing therapeutic strategies targeting MGD.
2 Methods
2.1 Search strategy for study selection
This systematic review adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and the Meta-analysis of Observational Studies in Epidemiology (MOOSE) checklist (Supplementary Files 1 and 2) (18, 19). The study protocol was prospectively registered with the International Prospective Register of Systematic Reviews (PROSPERO; Registration ID: CRD420251020327). Two independent investigators (L.Y.L. and L.Y.R.) conducted a comprehensive search of six databases (PubMed, Embase, Web of Science, Scopus, Ovid Medline, and Cochrane Library) from inception through March 27, 2025. To capture critical emerging evidence, studies published after the search cutoff date but prior to manuscript submission were included provided they met prespecified eligibility criteria. The search used a combination of Medical Subject Headings (MeSH) terms and free-text keywords: (“meibomian glands” OR “meibomian gland dysfunction” OR “MGD”) AND (“graves ophthalmopathy” OR “thyroid eye disease” OR “thyroid-associated ophthalmopathy” OR “thyroid-associated orbitopathy” OR “graves orbitopathy” OR “graves eye disease”). Additional studies were identified through manual searches of reference lists, with full search strategies provided in the Supplementary File 3.
Eligibility criteria were defined according to the PICOS framework (20): Population – patients diagnosed with TAO based on Bartley's criteria, European Group on Graves' Orbitopathy (EUGOGO) guidelines, and the American Thyroid Association/European Thyroid Association (ATA/ETA) consensus (21, 22); Intervention – not applicable; Comparison – TAO vs. healthy controls, and active vs. inactive TAO subgroups; Outcome – meibomian gland functional indicators; Study design – English-language cross-sectional or longitudinal studies. Exclusion criteria included: (1) animal studies; (2) secondary literature (e.g., reviews, case reports); (3) studies lacking primary data; (4) non-English publications.
Citavi v5.3 (Swiss Academic Software) was utilized for reference management and duplicate removal. The screening process was carried out in two stages by two independent reviewers (L.Y.L. and L.Y.R.): (1) title/abstract screening; (2) full-text assessment against the inclusion/exclusion criteria. Discrepancies were resolved through discussion with a third investigator (L.T.L).
2.2 Data extraction
Data extraction was conducted using a predefined template that captured details such as the first author, publication year, country, ethnicity, sex, age, Clinical Activity Score (CAS), devices, study design, and meibomian gland functional indicators. Indicators eligible for meta-analysis included: lipid layer thickness (LLT), meiboscore, meibum quality, first non-invasive tear film break-up time (NITBUT-f), average non-invasive tear film break-up time (NITBUT-avg), tear break-up time (TBUT), meibomian gland dropout area of the upper (MGDU) and lower eyelids (MGDL), and in vivo confocal microscopy (IVCM) indicators (meibomian gland acinar density [MAD], meibomian gland acinar longest diameter [MALD], meibomian gland acinar shortest diameter [MASD]). According to the EUGOGO guideline (22), a CAS ≥3/7 was defined as active TAO, while CAS ≤ 2/7 indicated inactive TAO. All definitions adhered strictly to the standardized descriptions from the original studies.
Meiboscore quantified total gland loss across the upper and lower eyelids using a 0–3 grading scale for each eyelid: 0 (no loss), 1 (< 1/3 area loss), 2 (1/3–2/3 loss), and 3 (> 2/3 loss). The composite score per eye (range 0–6) was the sum of the upper and lower eyelid grades. Meibum quality was graded on a 0–3 scale: 0 (clear fluid), 1 (cloudy fluid), 2 (cloudy particulate fluid), and 3 (toothpaste-like or non-expressible) (23). LLT, NITBUT-f, and NITBUT-avg were measured objectively via non-invasive ocular surface analyzers. LLT was defined as the vertical optical thickness of the tear lipid layer, with only mean values included. NITBUT-f represented the time from the first complete blink to the initial dry spot detection, while NITBUT-avg denoted the mean of regional first-breakup times.
The TBUT test was performed by applying a fluorescein strip to the conjunctival sac. After natural blinking, the time between the last complete blink and the first corneal dry spot, observed under cobalt blue slit-lamp illumination, was recorded.
MGDU and MGDL were quantified based on infrared meibography images obtained from an automated ocular surface analyzer. The percentage of meibomian gland dropout area in the upper and lower eyelids was calculated through either manual measurement or automated analysis.
IVCM assessments (HRT III Corneal Rostock Module, Heidelberg Engineering, Germany) focused on the lower eyelids, with masked operators capturing acinar images in standardized 400 × 400 μm fields. Two independent masked evaluators selected three high-quality, non-overlapping images from the nasal, central, and temporal regions of each lower eyelid (nine images per eyelid) for quantitative analysis. Indicators included in the meta-analysis were MAD, MALD, and MASD. MAD was calculated by manually counting the number of acinar units per image using ImageJ software (National Institutes of Health, Bethesda, MD) and converting the count to acinar units per mm2. MALD and MASD were measured manually using ImageJ software's linear tool, determining the longest and shortest axes of each acinar unit and averaging all analyzed values.
Two investigators (L.Y.L. and L.Y.R.) independently performed blinded data extraction. Discrepancies were resolved through consensus with a third reviewer (L.T.L.).
2.3 Risk of bias assessment
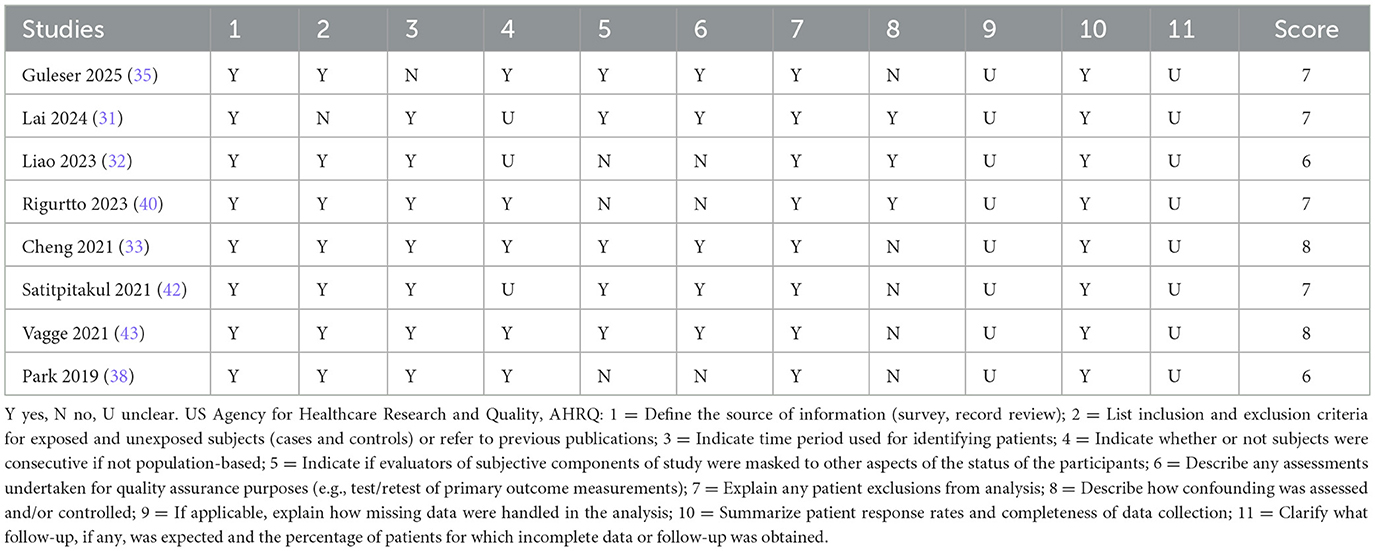
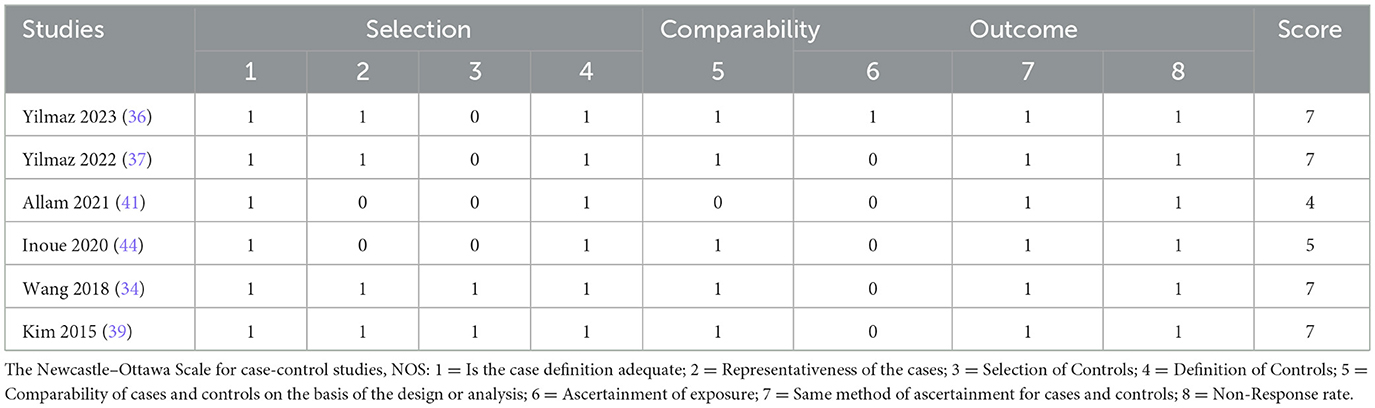
Cross-sectional studies were evaluated using the US Agency for Healthcare Research and Quality (AHRQ) checklist (11 items), which covers data sources, variable definitions, and sample representativeness (24–26). The methodological quality of case-control studies was assessed using the Newcastle-Ottawa Scale (NOS), which evaluates three domains: participant selection, group comparability, and exposure/outcome ascertainment, with a maximum score of 9. Two independent investigators (L.Y.L. and L.Y.R.) conducted the risk-of-bias assessments, resolving discrepancies through discussion with a third reviewer (L.T.L.). Studies scoring < 6 on the AHRQ checklist or < 5 on the NOS were classified as low quality with a high risk of bias and excluded from the meta-analysis to ensure robustness.
2.4 Statistical analysis
Statistical analyses were conducted using Review Manager (RevMan) v5.4.1 (Cochrane Collaboration) and Stata v16.0 (Stata Corp). Comparisons included: (1) patients with TAO vs. healthy controls; (2) active vs. inactive TAO subgroups. Continuous outcomes were expressed as mean ± standard deviation. For studies reporting medians with interquartile ranges, skewed data were first transformed using the Quantile estimation method by McGrath et al., followed by estimation of mean and SD using the methods proposed by Luo et al. and Wan et al. (27–29). Studies stratifying TAO into active/inactive subgroups were pooled using Cochrane-recommended formulas to derive combined group means and standard deviations (30).
Mean differences (MD) with 95% confidence intervals (CI) were calculated for outcomes with consistent units across studies. Heterogeneity was assessed using χ2 tests and I2 statistics: fixed-effect models were applied when P > 0.10 and I2 < 50%; random-effects models were used otherwise. Sensitivity analyses (leave-one-out method) were performed for outcomes with ≥ 3 studies. Publication bias was assessed using funnel plots for all outcomes; for analyses with ≥10 studies, Egger's test was additionally performed. Statistical significance was set at P < 0.05.
3 Results
3.1 Literature search results
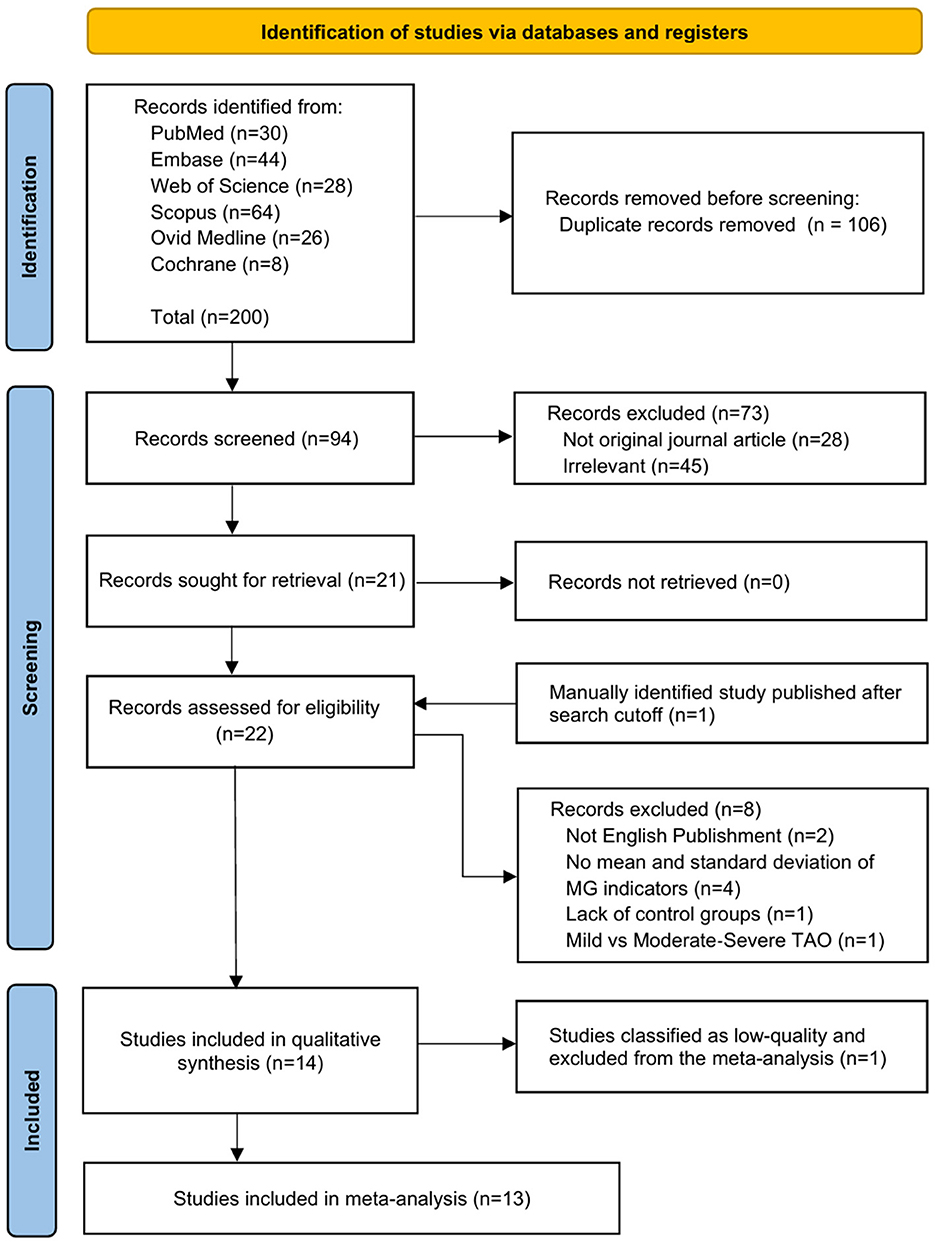
The database search initially identified 200 articles. After removing 106 duplicates, titles, abstracts, and article types of the remaining 94 articles were screened. A total of 73 articles were excluded due to irrelevance to the topic (n = 45) or ineligible publication types (n = 28). Full-text assessments of the remaining 21 articles led to the exclusion of 2 non-English publications, 4 articles that did not report mean values and standard deviations for meibomian gland indicators, 1 article lacking a control group, and 1 article missing subgroups based on eligibility criteria. Additionally, 1 article manually identified after the search date cutoff was incorporated. Consequently, 14 articles were included in the qualitative synthesis. One article was excluded due to low quality based on the quality assessment, leaving 13 articles included in the meta-analysis. The selection process adhered to PRISMA standards, as illustrated in the flow diagram (Figure 1).
3.2 Primary outcomes and qualitative synthesis
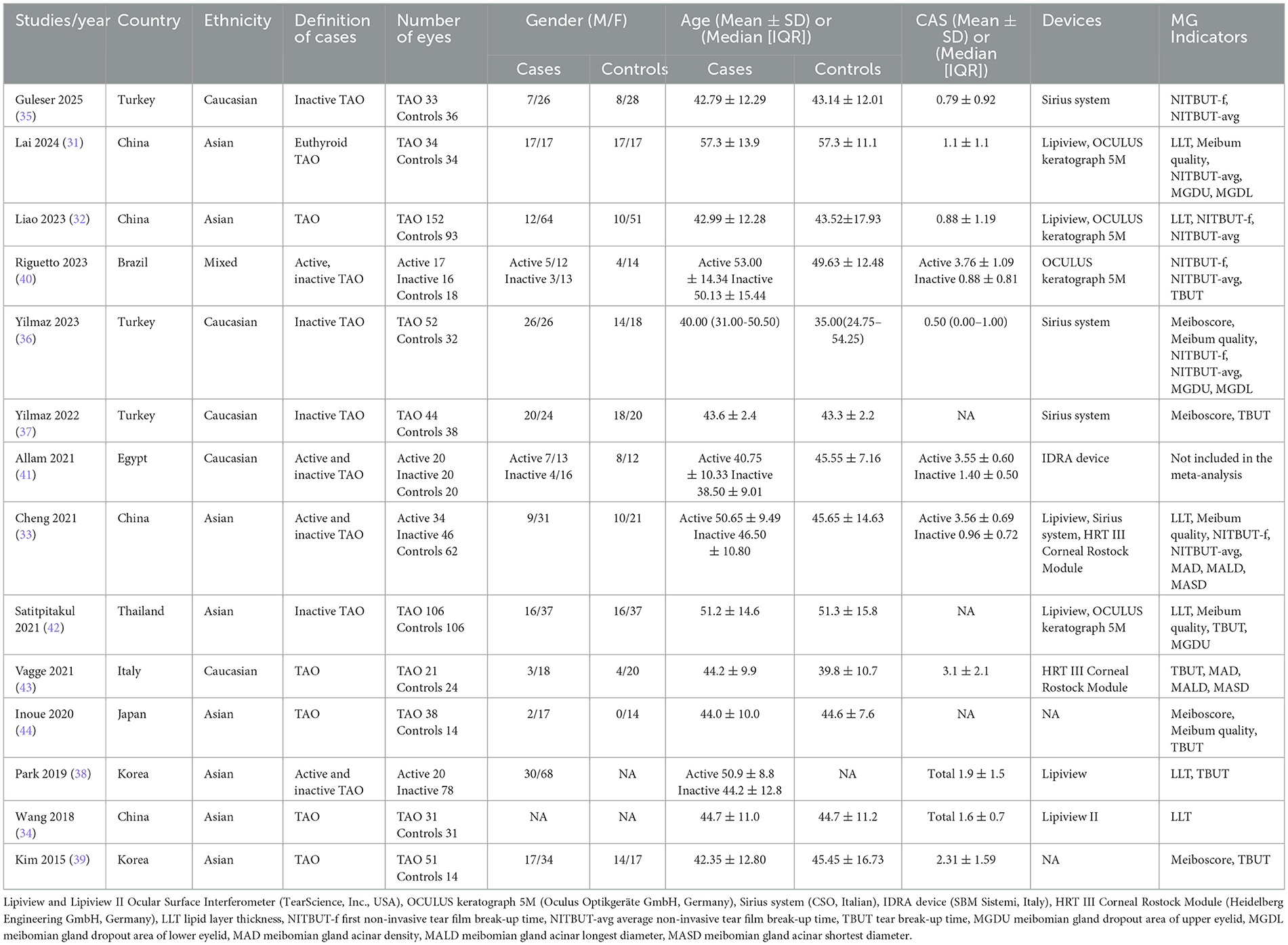
This systematic review included 14 studies (Table 1) published between 2015 and 2025, comprising 4 studies from China (31–34), 3 from Turkey (35–37), 2 from South Korea (38, 39)and 1 each from Brazil (40), Egypt (41), Thailand (42)], Italy (43), and Japan (44), with participants of Asian, Caucasian, and mixed ethnicities. The studies included 8 cross-sectional comparative and 6 retrospective case-control designs, involving 813 eyes with TAO and 522 healthy control eyes. Case definitions included active/inactive TAO and euthyroid TAO. The mean age of patients ranged from 38.5 to 57.3 years across the 14 studies, with one study reporting a median age of 40.00 years (36). Sex distribution was reported in 13 studies (301 eyes from males, 686 from females) (31–33, 35–44). CAS data were available in 11 studies (31–36, 38–41, 43), with active TAO subgroups showing mean CAS scores of 3.55–3.76, while non-stratified or inactive groups ranged from 0.79 to 3.1, with 1 study reporting a median CAS of 0.5.
Six studies reported LLT (31–34, 38, 42), four assessed meiboscore (36, 37, 39, 44), and five evaluated meibum quality (31, 33, 36, 42, 44). Tear film stability indicators included NITBUT-f (five studies) (32, 33, 35, 36, 40), NITBUT-avg (six studies) (31–33, 35, 36, 40), and TBUT (seven studies) (37–40, 42–44). MGDU and MGDL were quantified in two studies (31, 36), while two studies employed IVCM to assess MAD, MALD, and MASD (33, 43). Subjective indicators included meibum quality, TBUT, and IVCM-derived metrics (MAD, MALD, MASD), with standardized definitions and protocols for meibum quality and TBUT across all studies. Both IVCM studies utilized the HRT III Corneal Rostock Module (Heidelberg Engineering GmbH, Germany) for lower eyelid imaging, with masked analysis. Vagge et al. (43) specified imaging at 20–70 μm subepithelial depth, while Cheng et al. (33) did not report the exact depth.
Objective metrics included LLT, meiboscore, NITBUT-f, and NITBUT-avg. LLT measurements were consistently obtained using Lipiview and Lipiview II Ocular Surface Interferometers (TearScience, Inc., USA) across all six studies (31–34, 38, 42). For NITBUT assessments, four studies used the OCULUS Keratograph 5M (31, 32, 40, 42) (Oculus Optikgeräte GmbH, Germany) and the Sirius system (33, 35–37) (CSO, Italy), while one study employed the IDRA device (41) (SBM Sistemi, Italy). MGDU and MGDL were semi-objectively assessed: Lai et al. (31) used infrared meibography with the OCULUS Keratograph 5M (Oculus Optikgeräte GmbH, Germany) and manually quantified dropout areas via ImageJ software, while Yilmaz et al. (36) employed the Sirius system (CSO, Italy) for automated dropout area analysis.
3.3 Quality assessment
All included cross-sectional studies scored between 6 and 8 points on the AHRQ scale, indicating moderate to high methodological quality. The case-control studies scored between 4 and 7 points on the NOS. One study (41), scoring 4, was rated as low quality due to deficiencies in case selection and comparability and was excluded from the meta-analysis. The remaining studies demonstrated moderate to high quality (Tables 2, 3).
Publication bias was evaluated using funnel plots across all 11 meta-analyses, each containing fewer than 10 included studies. The assessment identified minimal publication bias for four specific outcomes: LLT and MGDL between TAO and controls, as well as LLT and TBUT between active TAO and inactive TAO. The studies included in four outcomes were symmetrical scatter distribution alongside the axis, indicating minimal publication bias. The remaining seven analyses employing random-effects models, where auxiliary lines were not generated. Nevertheless, studies distributed evenly and symmetrically on both sides of the axis, suggesting possible low-level publication bias (Supplementary File 4).
3.4 Results of meta-analysis
3.4.1 Meibomian gland indicators of patients with TAO vs. controls
Five studies (32, 33, 35, 36, 40) involving 587 eyes (Figure 2A and Table 4) showed no significant difference in NITBUT-f between patients with TAO and controls (MD = −3.37 s, 95% CI: −6.58 to −0.16; P = 0.04), with significant heterogeneity (I2 = 95%, P < 0.001). Subgroup analysis suggests that heterogeneity is not associated with device type. Sensitivity analysis revealed critical dependence on individual studies: exclusion of Guleser et al. (35) altered the MD to −2.17 s (95% CI: −4.97 to 0.64; P = 0.13), while omitting Liao et al. (32) resulted in MD = −2.55 s (95% CI: −7.30 to 2.20; P = 0.29), indicating model instability (Supplementary File 5). These findings highlight that the pooled result is highly sensitive to individual studies, and the conclusion should be interpreted with caution.
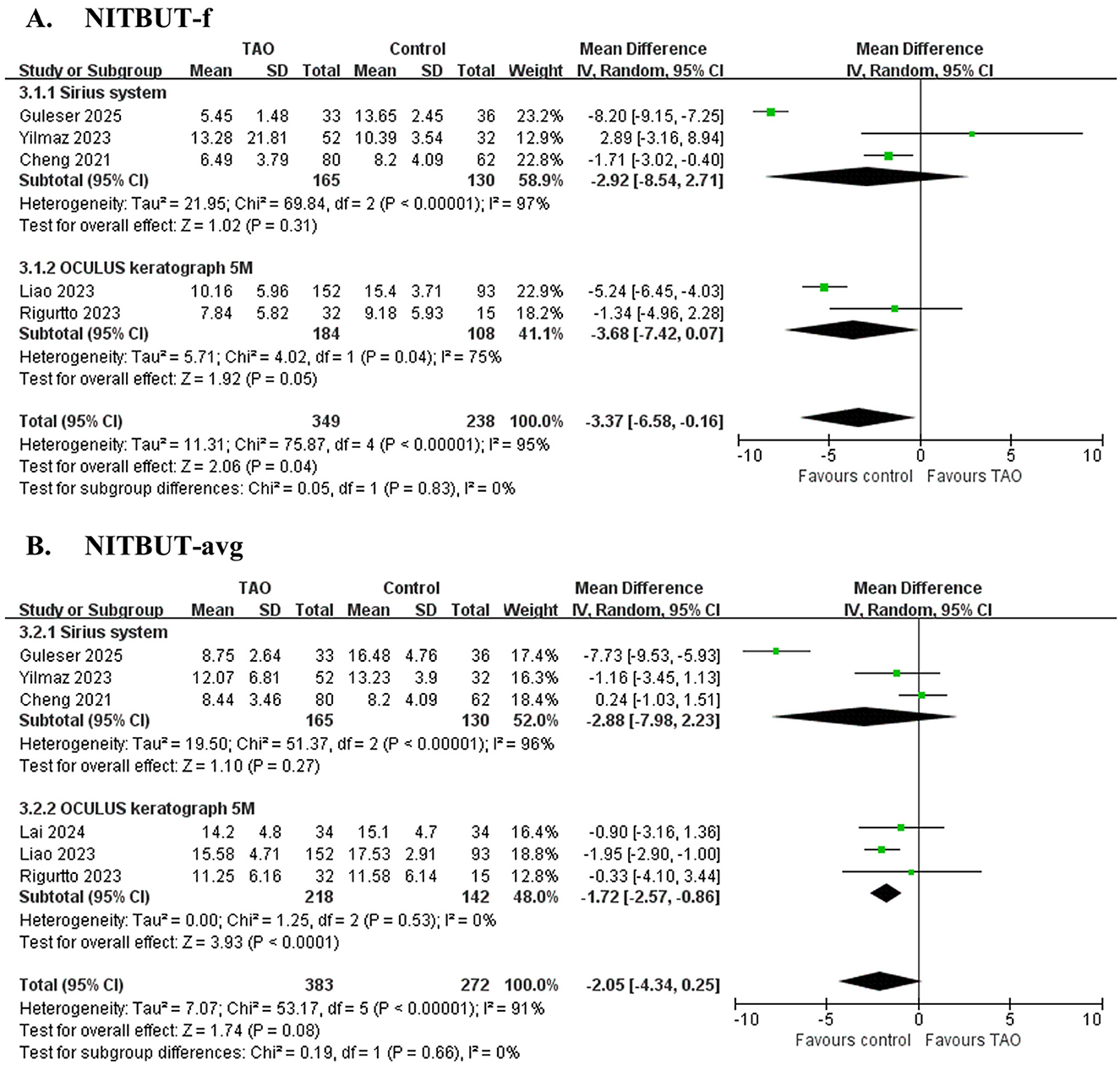
Figure 2. Forest plot of pooled difference in first non-invasive tear film break-up time (NITBUT-f), average non-invasive tear film break-up time (NITBUT-avg) between TAO and controls. (A) NITBUT-f; (B) NITBUT-avg.
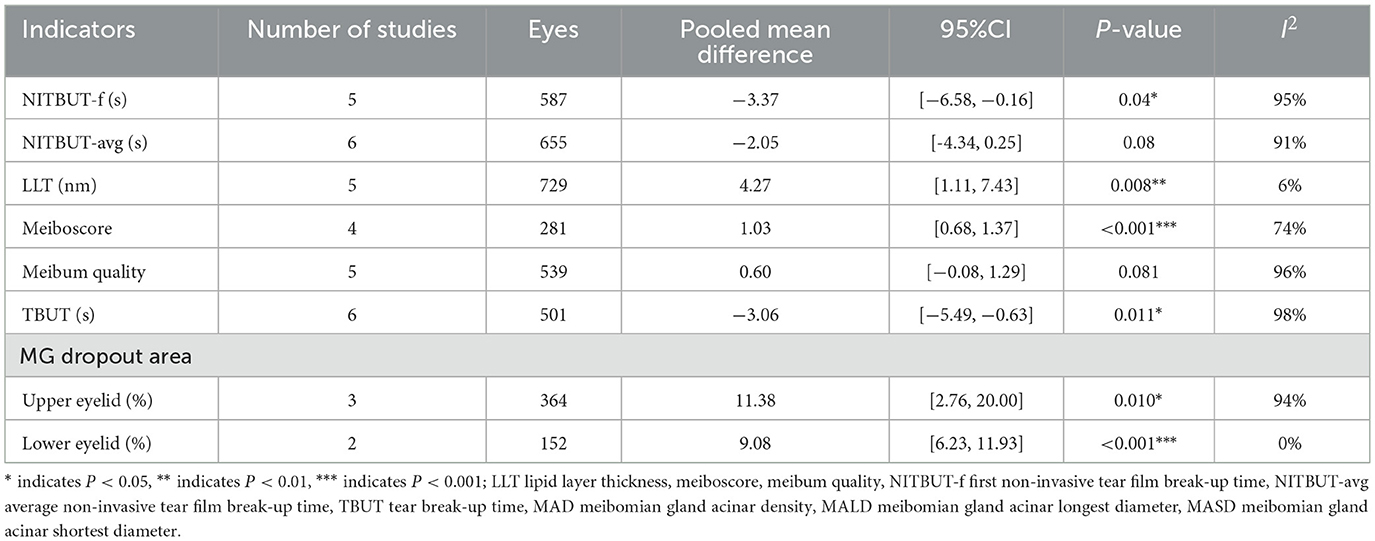
Table 4. Meta-analysis summary of differences in meibomian gland indicators between TAO and controls.
Six studies (31–33, 35, 36, 40) involving 655 eyes (Figure 2B and Table 4) demonstrated significantly shorter NITBUT-avg in patients with TAO compared to controls (MD = −2.05 s, 95% CI: −4.34 to 0.25; P = 0.08), with nonsignificant heterogeneity (I2 = 91%, P < 0.001). Subgroup analysis suggests that heterogeneity is not associated with device type. Sequential exclusion of individual studies did not alter the effect direction or statistical significance (P < 0.05), confirming the robustness of the conclusion (Supplementary File 5).
Five studies (31–34, 42) involving 729 eyes (Figure 3A and Table 4) compared LLT between patients with TAO and controls. The pooled analysis showed significantly greater LLT in patients with TAO (MD = 4.27 nm, 95% CI: 1.11 to 7.4; P = 0.008), with no significant heterogeneity across studies (I2 = 6%, P = 0.37). However, sensitivity analysis revealed substantial dependence on the study by Liao et al. Exclusion of this study removed statistical significance (adjusted MD = 2.51 nm, 95% CI: −1.32 to 6.33; P = 0.20), indicating model instability (Supplementary File 5). These findings should be interpreted with caution due to the fragility of the pooled estimate.
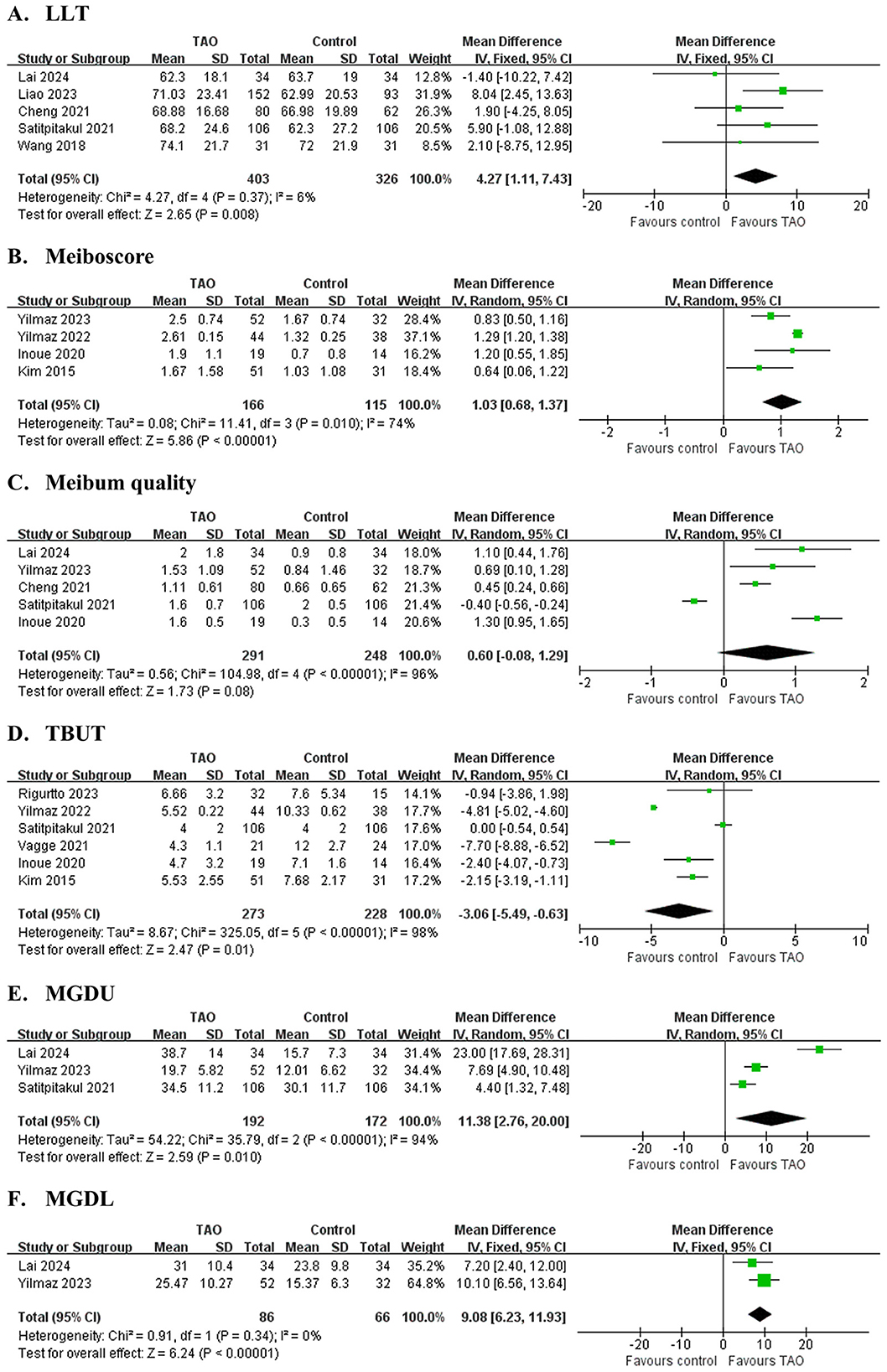
Figure 3. Forest plot of pooled difference in lipid layer thickness (LLT), meiboscore, meibum quality, tear break-up time (TBUT), meibomian glands dropout area of upper eyelid (MGDU) and lower eyelid (MGDL) between TAO and controls. (A) LLT; (B) Meiboscore; (C) Meibum quality; (D) TBUT; (E) MGDU; (F) MGDL.
Four studies (36, 37, 39, 44) involving 281 eyes (Figure 3B and Table 4) evaluated Meiboscore. Patients with TAO exhibited significantly higher Meiboscore than controls (MD = 1.03, 95% CI: 0.68 to 1.37; P < 0.001), although substantial heterogeneity was observed (I2 = 74%, P = 0.01). Sequential exclusion of individual studies did not alter the effect direction or statistical significance (P < 0.05), confirming the robustness of the conclusion.
Five studies (31, 33, 36, 42, 44) involving 539 eyes (Figure 3C and Table 4) found no significant difference in meibum quality between patients with TAO and controls (MD = 0.60, 95% CI: −0.08 to 1.29; P = 0.135), with high heterogeneity (I2 = 96%, P < 0.001). Sensitivity analysis revealed critical instability: exclusion of Satitpitakul et al. (42) shifted the MD to 0.87 (95% CI: 0.37 to 1.37; P < 0.001), reversing the statistical conclusion (Supplementary File 5). These findings suggest that the pooled result is highly sensitive to individual studies, and the conclusion should be interpreted with caution.
Six studies (37, 39, 40, 42–44) involving 501 eyes (Figure 3D and Table 4) found significantly shorter TBUT in patients with TAO compared to controls (MD = −3.06 s, 95% CI: −5.49 to −0.63; P = 0.011), with substantial heterogeneity (I2 = 98%, P < 0.001). Sensitivity analysis revealed model instability: exclusion of Yilmaz et al. (37) shifted the MD to −2.67 s (95% CI: −5.68 to 0.33; P = 0.08), while excluding Vagge et al. (43) resulted in MD = −2.10 s (95% CI:−4.80 to 0.59; P = 0.13), both eliminating statistical significance (Supplementary File 5). These findings highlight the fragility of the pooled estimate and underscore the need for cautious interpretation of the association between TAO and TBUT reduction.
Three studies (31, 36, 42) involving 364 eyes (Figure 3E and Table 4) demonstrated significantly greater meibomian gland dropout in the upper eyelid in patients with TAO compared to controls (MGDU: MD = 15.17%, 95% CI: 0.17 to 30.17; P = 0.046). However, sensitivity analysis revealed substantial dependence on the study by Yilmaz et al. (36) Exclusion of this study removed statistical significance (adjusted MD = 13.37%, 95% CI: −4.66 to 31.80; P = 0.14), indicating model instability (Supplementary File 5). These findings should be interpreted with caution due to the fragility of the pooled estimate. Two studies (31, 36) involving 152 eyes (Figure 3F and Table 4) demonstrated significantly greater meibomian gland dropout in the lower eyelid in patients with TAO compared to controls (MGDL: MD = 9.08%, 95% CI: 6.23 to 11.93; P < 0.001). Extreme heterogeneity was observed for MGDU (I2 = 96%, P < 0.001), while MGDL exhibited homogeneity (I2 = 0%, P = 0.34).
Two studies incorporated three IVCM (in vivo confocal microscopy) indicators: MAD, MASD, and MALD. Cheng et al. (33) reported statistically significant differences between TAO and controls across all three indicators: MAD (83.5 ± 34.3 vs 114.7 ± 34.9, P < 0.001), MALD (119.5 ± 28.6 vs. 58.7 ± 20.3, P < 0.001), and MASD (45.5 ± 19.9 vs 27.8 ± 9.9, P < 0.001). Similarly, Vagge et al. (43) demonstrated significant differences in MAD (24.5 ± 9.1 vs. 34.2 ± 7.5, P < 0.001), MALD (94.4 ± 21.2 vs. 64.3 ± 10.1, P < 0.001), and MASD (56.6 ± 15.3 vs. 42.2 ± 12.3, P = 0.001) between TAO and controls. Methodological variations existed between studies. Vagge et al. (43) measured MAD explicitly at 20–70 μm below the epithelial surface, whereas Cheng et al. provided no imaging depth specification. This discrepancy may introduce measurement variability. Furthermore, the limited number of available studies potentially affects statistical stability. Thus, this study did not pool IVCM data from the two studies for meta-analysis.
3.4.2 Meibomian gland indicators of patients with active TAO vs. inactive TAO
Two studies (33, 38) involving 178 eyes (Figure 4A and Table 5) compared LLT between active and inactive TAO, finding no significant difference (MD = 5.20 s, 95% CI: −1.07 to 11.47; P = 0.101), with no heterogeneity observed between studies (I2 = 0%, P = 0.34). Two studies (33, 40) involving 112 eyes (Figure 4B and Table 5) compared NITBUT avg between active and inactive TAO, finding no significant difference (MD = −0.39 s, 95% CI: −1.83 to 1.04; P = 0.594), with no heterogeneity observed between studies (I2 = 0%, P = 0.74). Two studies (38, 40) involving 130 eyes (Figure 4C and Table 5) compared TBUT between active and inactive TAO, showing no significant difference (MD = 0.72 s, 95% CI: −0.34 to 1.79; P = 0.183), with nonsignificant heterogeneity (I2 = 39%, P = 0.020).
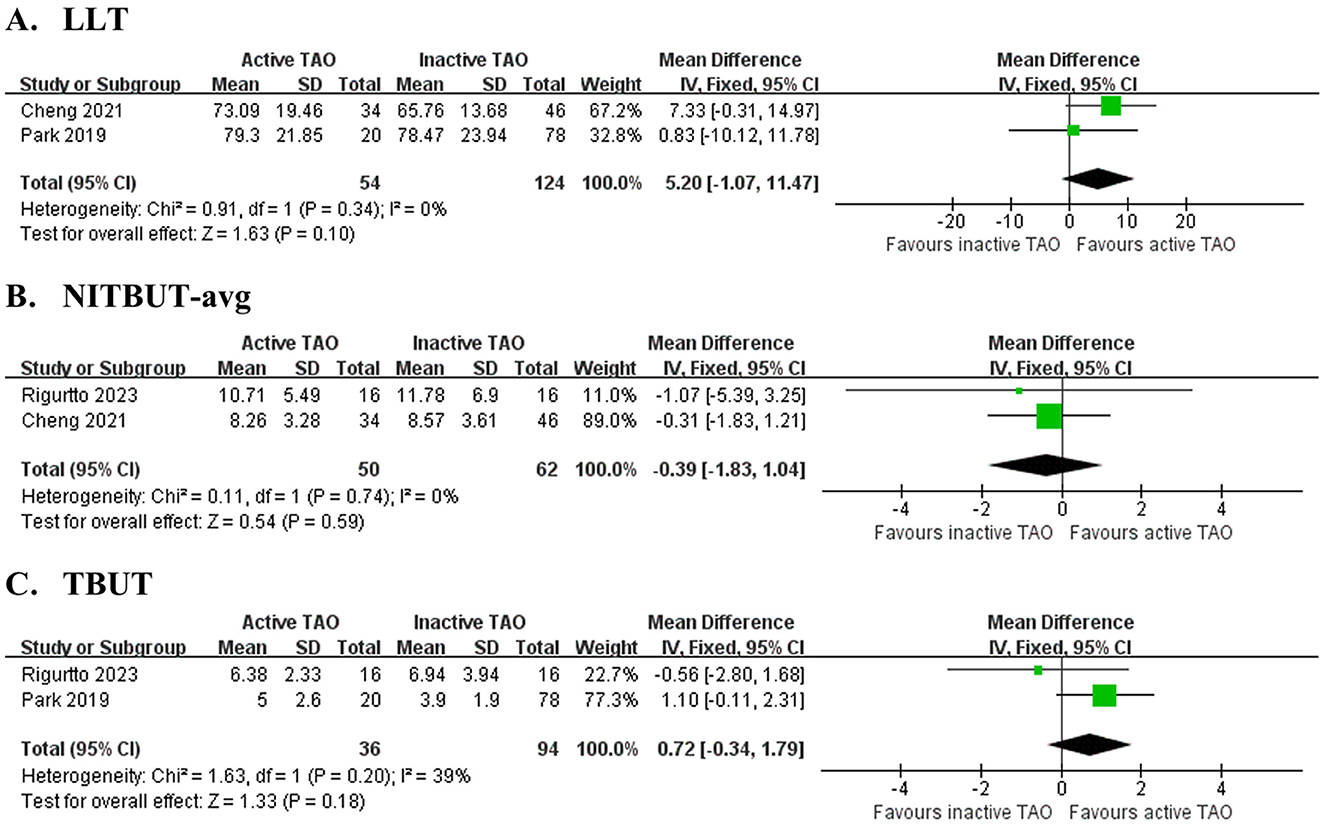
Figure 4. Forest plot of pooled difference in lipid layer thickness (LLT), average non-invasive tear film break-up time (NITBUT-avg) and tear break-up time (TBUT) between active TAO and inactive TAO. (A) LLT,; (B) NITBUT-avg; (C) TBUT.
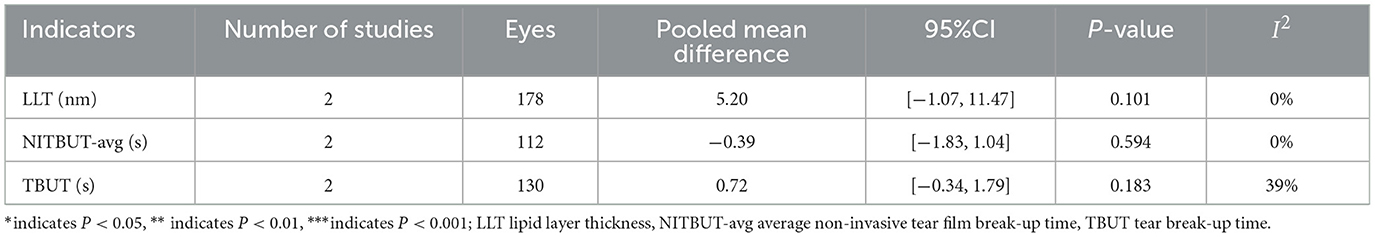
Table 5. Meta-analysis summary of differences in meibomian gland indicators between active TAO and inactive TAO.
4 Discussion
This meta-analysis systematically evaluated meibomian gland characteristics in TAO, incorporating 13 studies with 813 TAO eyes and 522 healthy control eyes. The results reveal significant structural and functional abnormalities in patients with TAO compared to controls. Structurally, TAO eyes exhibit more severe gland dropout, higher Meiboscores and higher LLT. Functionally, patients with TAO show reduced tear film stability, evidenced by shorter NITBUT-f and TBUT values. Collectively, these findings suggest that TAO accelerates MGD through a distinct pathophysiology, leading to irreversible structural damage and potential compensatory responses.
Structural changes in the meibomian glands are a key feature of TAO, including increased gland dropout and abnormal morphology. Our results demonstrate higher Meiboscores and larger gland loss areas in patients with TAO, providing direct evidence of gland atrophy and dropout (45). Notably, superior eyelid gland loss is more pronounced than inferior eyelid loss, suggesting that upper eyelids are more severely affected. This may be due to their larger exposed surface area, which increases exposure to inflammatory factors. Hwang et al. (46) proposed that ocular surface inflammation in TAO directly correlates with meibomian gland structural damage. The larger exposed area of the upper eyelids likely increases inflammatory exposure, explaining their greater involvement. Additionally, Luo et al. (47) found that orbital fat and muscle expansion in TAO mechanically compresses the eyelids, deforming meibomian ducts and obstructing lipid secretion. In vivo confocal microscopy revealed no significant difference in acinar density between patients with TAO and controls, but patients with TAO exhibited significantly enlarged acinar diameters. This may indicate compensatory hypertrophy of remaining acini after adjacent gland dropout or gland dilation caused by inflammation-induced lipid retention (33).
Meibomian gland structural damage directly impairs tear film stability. Our meta-analysis reveals an uncertain difference in the NIBUT-f between TAO patients and controls: while the pooled results showed a trend of shortening, sensitivity analysis indicated high dependence on individual studies. In contrast, the observed shortening of NITBUT-avg demonstrated greater consistency, with sensitivity analyses indicating relative stability in pooled estimates. The observed discrepancy could be partially attributed to the properties of these metrics: NITBUT-avg, by averaging multiple measurements, tends to mitigate random measurement fluctuations, whereas NITBUT-f appears more susceptible to transient perturbations such as pre-measurement blink patterns or short-term ocular surface microenvironmental changes. Furthermore, the subjective TBUT indicator also demonstrated fragility in sensitivity analysis. This may be potentially linked to methodological factors such as fluorescein interference or assessment variability, which could affect the robustness of the findings. These findings collectively suggest that impaired gland structure accelerates tear evaporation by disrupting tear film lipid layer stability (48). Mantelli et al. (49) suggested that inflammation leads to excessive tear evaporation by reducing membrane-associated mucins in the cornea and conjunctiva, as well as secretory mucins from goblet cells. Tear film stability depends not only on the lipid layer but also on the mucin layer secreted by conjunctival goblet cells. The rapid tear breakup observed in patients with TAO is likely due to defects in both layers. Notably, this study found no significant differences in NITBUT-avg or TBUT between active and inactive TAO, suggesting that tear film stability may not be influenced by disease activity. However, the limited sample size warrants further investigation to validate these findings.
LLT in patients with TAO exhibits complex patterns. Our meta-analysis demonstrated significantly greater LLT in TAO patients compared to controls. Our study found increased, rather than decreased, LLT in patients with TAO compared to controls. Although periglandular inflammation contributes to MGD, compensatory secretion from residual glands and forceful blinking due to lagophthalmos may lead to increased lipid deposition on the tear film (50, 51). Finis et al. (52) noted that meibomian glands can maintain baseline function even with over 40% gland dropout. In active TAO, significant proptosis increases eyelid tension and blink force, forcibly expelling meibum and enhancing LLT (53). Paradoxically, our study demonstrated no significant difference in LLT between active and inactive TAO groups. Additionally, our analysis showed no significant difference in meibum quality between patients with TAO and controls. While substantial meibomian gland loss in TAO would typically reduce meibum quality, Farid et al. suggest that ocular surface inflammation may alter secretion quality. However, Iqbal and Shakya (54, 55) propose that lipid metabolism in TAO-associated MGD may differ from conventional MGD. Additionally, the current meibum quality assessment may overlook subtle compositional changes in TAO, warranting molecular-level studies to confirm this hypothesis.
TAO-associated MGD involves anatomical, inflammatory, and molecular mechanisms. Anatomically, mechanical compression from orbital tissue expansion and impaired blinking due to eyelid retraction obstruct meibum excretion. Inflammatory processes include tear-mediated autoimmune reactions, where elevated pro-inflammatory cytokines drive meibomian gland fibrosis and atrophy. Notably, increased corneal Langerhans cell density distinguishes TAO from conventional MGD (56–58). Molecular studies indicate that IGF-1R signaling—central to TAO pathology—induces meibomian epithelial apoptosis via the PI3K/Akt pathway. Concurrently, oxidative stress-induced reactive oxygen species may activate the NLRP3 inflammasome, further exacerbating gland damage (59, 60). This multifactorial pathology underpins the complex and refractory nature of TAO-associated MGD.
Our findings may offer valuable clinical guidance for managing TAO. Given the high prevalence and severity of MGD in patients with TAO, conventional dry eye treatments often provide limited benefits (61). Routine comprehensive assessments should be adopted, incorporating meibomian gland imaging, meibum quality evaluation, and tear film stability measurements. Early detection and intervention for MGD, even in subclinical stages, could help delay progressive gland damage. For treatment, this study recommends combining systemic immunosuppression with specific therapies, such as thermal pulsation and intense pulsed light treatments (62, 63). This integrated approach enhances meibum excretion while controlling local inflammation, as supported by existing studies.
This study has several limitations. First, the limited number of included studies and generally small sample sizes may have reduced statistical power, making it difficult to detect subtle differences in certain indicators, which constrained the reliability of pooled estimates.
Second, Methodological differences may have introduced potential heterogeneity. Specifically, different devices such as the Sirius and OCULUS Keratograph 5M were used to measure NITBUT. Variations in measurement principles and algorithms may have compromised the comparability of results. The assessment of meibomian gland dropout also lacked standardization: some studies used manual quantification with ImageJ software, while others relied on automated analysis with the Sirius system. This discrepancy between manual and automated methods could introduce inter-observer bias and systematic error. Furthermore, meibomian gland imaging protocols were not uniform; only Vagge et al. (43) explicitly specified the imaging depth. For subjective indicators such as meibum quality and TBUT, although certain criteria were applied, inter-observer variation in assessment remains a possibility (64).
Third, there were shortcomings in patient selection and grouping. Only four studies explicitly excluded TAO patients with a pre-existing DED diagnosis or prior DED treatment, while the remaining studies did not specify such exclusions, potentially introducing population heterogeneity. In the control groups, seven studies excluded individuals with a history of DED, whereas others simply described controls as “healthy” without verification, which may have led to selection bias. Moreover, due to the predominance of cross-sectional or case-control designs, the temporal relationship between DED and TAO onset could not be determined. Variations in TAO disease duration and severity among patients may influence ocular surface and meibomian gland status (65). Future cohort studies should analyze ocular surface or meibomian gland function indicators in patients with and without DED, both before and after TAO diagnosis.
Fourth, there exists a lack of standardization in defining disease activity. Some included studies failed to categorize TAO cases into active or inactive groups based on the CAS. Furthermore, even studies analyzing active subgroups did not report specific CAS scores for each cohort. This variability in inflammation levels could confound interpretations of meibomian gland dysfunction outcomes, potentially introducing bias in result interpretation.
Finally, the inclusion of multi-regional and multi-ethnic data may introduce heterogeneity due to racial differences in eyelid anatomy and MGD epidemiology (66, 67). Future high-quality, large-scale studies are needed to validate these findings and enhance statistical power. Additionally, newly published clinical studies may be incorporated into future meta-analyses to strengthen the conclusions.
In conclusion, this meta-analysis provides systematic quantitative evidence that MGD is a common ocular manifestation in TAO. Patients with TAO exhibit significant structural damage and functional decline in meibomian glands, with a pathology characterized by chronic, progressive, and potentially irreversible changes. These findings highlight the need for clinical practice to adopt targeted MGD assessment and long-term management strategies. More specific interventions are required to effectively improve patients' long-term visual function and quality of life.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
YLu: Validation, Project administration, Conceptualization, Data curation, Supervision, Writing – review & editing, Resources, Writing – original draft, Investigation, Visualization, Formal analysis. YLi: Conceptualization, Software, Project administration, Supervision, Methodology, Visualization, Data curation, Validation, Writing – review & editing, Investigation, Formal analysis. TL: Formal analysis, Visualization, Resources, Project administration, Supervision, Methodology, Data curation, Writing – review & editing, Validation, Investigation, Conceptualization.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1709057/full#supplementary-material
References
1. Lehmann GM, Feldon SE, Smith TJ, Phipps RP. Immune mechanisms in thyroid eye disease. Thyroid. (2008) 18:959–65. doi: 10.1089/thy.2007.0407
2. Versura P, Campos EC. The ocular surface in thyroid diseases. Curr Opin Allergy Clin Immunol. (2010) 10:486–92. doi: 10.1097/ACI.0b013e32833e1749
3. Huang Y, Fang S, Li D, Zhou H, Li B, Fan X. The involvement of T cell pathogenesis in thyroid-associated ophthalmopathy. Eye. (2019) 33:176–82. doi: 10.1038/s41433-018-0279-9
4. Zhang X, Zhao Q, Li B. Current and promising therapies based on the pathogenesis of Graves' ophthalmopathy. Front Pharmacol. (2023) 14:1217253. doi: 10.3389/fphar.2023.1217253
5. Bruscolini A, Abbouda A, Locuratolo N, Restivo L, Trimboli P, Romanelli F, et al. Dry Eye Syndrome in Non-Exophthalmic Graves' Disease. Semi. Ophthalmol. (2015) 30:372–6. doi: 10.3109/08820538.2013.874491
6. Gürdal C, Saraç O, Genç I, Kirimlioglu H, Takmaz T, Can I, et al. Ocular surface and dry eye in Graves' disease. Curr Eye Res. (2011) 36:8–13. doi: 10.3109/02713683.2010.526285
7. Ugradar S, Zimmerman E, Parunakian E, Kang J, Cockerham K, Douglas RS, et al. Change in lacrimal gland volume and aqueous tear production following treatment with teprotumumab. Clin Exp Ophthalmol. (2023) 51:339–48. doi: 10.1111/ceo.14208
8. Eckstein AK, Finkenrath A, Heiligenhaus A, Renzing-Köhler K, Esser J, Krüger C, et al. Dry eye syndrome in thyroid-associated ophthalmopathy: lacrimal expression of TSH receptor suggests involvement of TSHR-specific autoantibodies. Acta ophthalmologica Scandinavica. (2004) 82:291–7. doi: 10.1111/j.1395-3907.2004.00268.x
9. Kishazi E, Dor M, Eperon S, Oberic A, Turck N, Hamedani M, et al. Differential profiling of lacrimal cytokines in patients suffering from thyroid-associated orbitopathy. Sci. Rep. (2018) 8:10792. doi: 10.1038/s41598-018-29113-2
10. Chen Q. The expression of interleukin-15 and interleukin-17 in tears and orbital tissues of Graves ophthalmopathy patients. J Cell Biochemist. (2019) 120:6299–303. doi: 10.1002/jcb.27916
11. Ma YK, Liu YY, Zhou YF, Ma BK, Sun T, Duan HY, et al. Research advances in the pathogenesis of dry eye in thyroid associated ophthalmopathy. [Zhonghua yan ke za zhi] Chin J Ophthalmol. (2025) 61:156–160. doi: 10.3760/cma.j.cn112142-20240319-00120
12. Liu X, Gao C, Liu X, Gao T. Efficacy and safety of tripterygium glycosides for Graves ophthalmopathy: a systematic review and meta-analysis. Medicine. (2019) 98:e18242. doi: 10.1097/MD.0000000000018242
13. Rana HS, Akella SS, Clabeaux CE, Skurski ZP, Aakalu VK, et al. Ocular surface disease in thyroid eye disease: a narrative review. Ocular Surface. (2022) 24:67–73. doi: 10.1016/j.jtos.2022.02.001
14. Geethika R, Mounika V, Mohamed AS, Menavath TRN, Puttagunta SB, Yalavarthy KC, et al. A simple technique of meibography for morphological and functional evaluation of meibomian glands in dry eye conditions. J Eur Orthop Surg Traumatol. (2023) 71:1420–1425. doi: 10.4103/IJO.IJO_2823_22
15. Aghaei H, Zarastvand F, Abdolalizadeh P, Karimi N, Es'haghi A, Clark JD, et al. Meibomian gland loss and dysfunction in mild versus moderate-severe thyroid eye disease. J Curr Ophthalmol. (2024) 36:145–51. doi: 10.4103/joco.joco_176_23
16. Park J, Kim J, Lee H, Park M, Baek S. Functional and structural evaluation of the meibomian gland using a LipiView interferometer in thyroid eye disease. Can J Ophthalmol. (2018) 53:373–9. doi: 10.1016/j.jcjo.2017.11.006
17. Villani E, Viola F, Sala R, Salvi M, Mapelli C, Currò N, et al. Corneal involvement in Graves' orbitopathy: an in vivo confocal study. Invest Ophthalmol Vis Sci. (2010) 51:4574–8. doi: 10.1167/iovs.10-5380
18. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
19. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. (2000) 283:2008–12. doi: 10.1001/jama.283.15.2008
20. Schardt C, Adams MB, Owens T, Keitz S, Fontelo P. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med Inform Decis Mak. (2007) 7:16. doi: 10.1186/1472-6947-7-16
21. Burch HB, Perros P, Bednarczuk T, Cooper DS, Dolman PJ, Leung AM, et al. Management of thyroid eye disease: a Consensus Statement by the American Thyroid Association and the European Thyroid Association. Eur Thyroid J. (2022) 11:e220189. doi: 10.1530/ETJ-22-0189
22. Bartalena L, Kahaly GJ, Baldeschi L, Dayan CM, Eckstein A, Marcocci C, et al. The 2021 European Group on Graves' orbitopathy (EUGOGO) clinical practice guidelines for the medical management of Graves' orbitopathy. Eur J Endocrinol. (2021) 185:G43–67. doi: 10.1530/EJE-21-0861
23. Arita R, Itoh K, Inoue K, Amano S. Noncontact infrared meibography to document age-related changes of the meibomian glands in a normal population. Ophthalmology. (2008) 115:911–5. doi: 10.1016/j.ophtha.2007.06.031
24. Lo CK, Mertz D, Loeb M. Newcastle-Ottawa Scale: comparing reviewers' to authors' assessments. BMC Med Res Methodol. (2014) 14:45. doi: 10.1186/1471-2288-14-45
25. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
26. Atkins D, Fink K, Slutsky. Better information for better health care: the evidence-based practice center program and the agency for healthcare research and quality. Ann Intern Med. (2005) 142:1035–41. doi: 10.7326/0003-4819-142-12_Part_2-200506211-00002
27. McGrath S, Katzenschlager S, Zimmer AJ, Seitel A, Steele R, Benedetti A, et al. Standard error estimation in meta-analysis of studies reporting medians. Stat Methods Med Res. (2023) 32:373–88. doi: 10.1177/09622802221139233
28. LUO D, WAN X, LIU J, et al. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res. (2018) 27:1785–805. doi: 10.1177/0962280216669183
29. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. (2014) 14:135. doi: 10.1186/1471-2288-14-135
30. Bossuyt PM, Deeks JJ, Leeflang MM, Takwoingi Y, Flemyng E, et al. Evaluating medical tests: introducing the cochrane handbook for systematic reviews of diagnostic test accuracy. Cochrane Database Syst Rev. (2023) 7:ED000163. doi: 10.1002/14651858.ED000163
31. Lai KKH, Liao X, Aljufairi FMAA, Sebastian JU, Ma A, Man Wong Y, et al. Ocular surface and meibomian gland evaluation in euthyroid Graves' ophthalmopathy. Int Ophthalmol. (2024) 44:124. doi: 10.1007/s10792-024-02919-y
32. Liao X, Lai KKH, Aljufairi FMAA, Chen W, Hu Z, Wong HYM, et al. Ocular surface changes in treatment-naive thyroid eye disease. J Clin Med. (2023) 12:3066. doi: 10.3390/jcm12093066
33. Cheng S, Yu Y, Chen J, Ye L, Wang X, Jiang F. In vivo confocal microscopy assessment of meibomian glands microstructure in patients with Graves' orbitopathy. BMC Ophthalmol. (2021), 21:261. doi: 10.1186/s12886-021-02024-z
34. Wang CY, Ho RW, Fang PC, Yu HJ, Chien CC, Hsiao CC, Kuo MT. The function and morphology of Meibomian glands in patients with thyroid eye disease: a preliminary study. BMC Ophthalmol. (2018) 18:90. doi: 10.1186/s12886-018-0763-9
35. Guleser UY, Yildiz A, Sezer H, Ozisik S, Kesim C, Hasanreisoglu M, et al. Investigation of corneal structural changes by in vivo confocal microscopy in thyroid-associated ophthalmopathy patients. Eur J. Ophthalmol. (2025) 35:1593–603. doi: 10.1177/11206721251332675
36. Yilmaz Tugan B, Özkan B, Yüksel R, Güray AB, Yüksel N. Is Corneal subbasal nerve loss associated with meibomian gland loss in inactive mild and moderate-to-severe graves' ophthalmopathy? Ophthalmic Plast Reconstr Surg. (2023) 39:174–81. doi: 10.1097/IOP.0000000000002280
37. Yilmaz Tugan B, Özkan B. Evaluation of meibomian gland loss and ocular surface changes in patients with mild and moderate-to-severe graves' ophthalmopathy. Semi Ophthalmol. (2022) 37:271–6. doi: 10.1080/08820538.2021.1937662
38. Park J, Baek S. Dry eye syndrome in thyroid eye disease patients: the role of increased incomplete blinking and Meibomian gland loss. Acta Ophthalmologica. (2019) 97:e800–6. doi: 10.1111/aos.14000
39. Kim YS, Kwak AY, Lee SY, Yoon JS, Jang SY. Meibomian gland dysfunction in Graves' orbitopathy. Can J Ophthalmol. (2015) 50:278–82. doi: 10.1016/j.jcjo.2015.05.012
40. Riguetto CM, Barbosa EB, Atihe CC, Reis F, Alves M, Zantut-Wittmann DE, et al. Ocular Surface disease related to the inflammatory and non-inflammatory phases of thyroid eye disease. Clin. Ophthalmol. (2023) 17:3465–3475. doi: 10.2147/OPTH.S430861
41. Allam IY, Lazreg S, Shafik Shaheen M, Doheim MF, Mohammed MA, et al. Ocular surface changes in patients with thyroid eye disease: an observational clinical study. Clin Ophthalmol. (2021) 15:2481–8. doi: 10.2147/OPTH.S317708
42. Satitpitakul V, Rattanaphong T, Pruksakorn V. Meibomian glands dropout in patients with inactive thyroid related orbitopathy. PloS One. (2021) 16:e0250617. doi: 10.1371/journal.pone.0250617
43. Vagge A, Bernabei F, Del Noce C, Pellegrini M, Giannaccare G, Senni C, et al. In vivo confocal microscopy morphometric analysis of meibomian glands in patients with graves ophthalmopathy. Cornea. (2021) 40:425–429. doi: 10.1097/ICO.0000000000002404
44. Inoue S, Kawashima M, Arita R, Kozaki A, Tsubota K, et al. Investigation of meibomian gland function and dry eye disease in patients with graves' ophthalmopathy. J Clin Med. (2020) 9:2814. doi: 10.3390/jcm9092814
45. Wang MTM, Power B, Xue AL, Craig JP. Blink completeness and rate in dry eye disease: an investigator-masked, prospective registry-based, cross-sectional, prognostic study. Cont Lens Anterior Eye. (2025) 48:102369. doi: 10.1016/j.clae.2025.102369
46. Hwang HS, Parfitt GJ, Brown DJ, Jester JV. Meibocyte differentiation and renewal: Insights into novel mechanisms of meibomian gland dysfunction (MGD). Exp Eye Res. (2017) 163:37–45. doi: 10.1016/j.exer.2017.02.008
47. Luo S, Djotyan GP, Joshi R, Juhasz T, Brown DJ, Jester JV. Modeling meibum secretion: alternatives for obstructive Meibomian Gland Dysfunction (MGD). Ocular Surface. (2024) 31:56–62. doi: 10.1016/j.jtos.2023.11.005
48. Alanazi MA, Almutairi A, Al-Massaad AA, Baashen MA, Almutairi MS, Alhssan A, et al. Tear film parameters in females with thyroid eye disease and diabetes: an observational study. J Pak Med Assoc. (2025) 75:48–51. doi: 10.47391/JPMA.11233
49. Mantelli F, Argüeso P. Functions of ocular surface mucins in health and disease. Curr Opin Allergy Clin Immunol. (2008) 8:477–483. doi: 10.1097/ACI.0b013e32830e6b04
50. Knop E, Knop N, Millar T, Obata H, Sullivan DA. The international workshop on meibomian gland dysfunction: report of the subcommittee on anatomy, physiology, and pathophysiology of the meibomian gland. Invest Ophthalmol Visual Sci. (2011) 52:1938–78. doi: 10.1167/iovs.10-6997c
51. Kim HM, Eom Y, Song JS. The relationship between morphology and function of the meibomian glands. Eye Contact Lens. (2018) 44:1–5. doi: 10.1097/ICL.0000000000000336
52. Finis D, Ackermann P, Pischel N, König C, Hayajneh J, Borrelli M, et al. Evaluation of meibomian gland dysfunction and local distribution of meibomian gland atrophy by non-contact infrared meibography. Curr Eye Res. (2015) 40:982–9. doi: 10.3109/02713683.2014.971929
53. Korb DR, Baron DF, Herman JP, Finnemore VM, Exford JM, Hermosa JL, et al. Tear film lipid layer thickness as a function of blinking. Cornea. (1994) 13:354–9. doi: 10.1097/00003226-199407000-00012
54. Iqbal F, Stapleton F, Masoudi S, Papas EB, Tan J. Meibomian gland shortening is associated with altered meibum composition. Invest Ophthalmol Vis Sci. (2024) 65:49. doi: 10.1167/iovs.65.8.49
55. Shakya P, Parajuli S, Sharma S. Association of meibomian gland dysfunction with serum lipid profile: a hospital-based cross-sectional study. Nepal J Ophthalmol. (2023) 15:34–38. doi: 10.3126/nepjoph.v15i1.46909
56. Wu LQ, Cheng JW, Cai JP, Le QH, Ma XY, Gao LD, et al. Observation of corneal langerhans cells by in vivo confocal microscopy in thyroid-associated ophthalmopathy. Curr Eye Res. (2016) 41:927–32. doi: 10.3109/02713683.2015.1133833
57. Qazi Y, Kheirkhah A, Blackie C, Trinidad M, Williams C, Cruzat A, et al. Clinically relevant immune-cellular metrics of inflammation in meibomian gland dysfunction. Invest Ophthalmol Visual Sci. (2018) 59:6111–23. doi: 10.1167/iovs.18-25571
58. Cai J, Zheng T, Ng TK, Xu C, Zhao Z, Li Z. Quantification of conjunctival dendritiform cells under in vivo confocal microscopy for clinical activity evaluation in thyroid eye disease. Curr Eye Res. (2025) 50:1003–9. doi: 10.1080/02713683.2025.2521865
59. Khandelwal P, Liu S, Sullivan DA. Androgen regulation of gene expression in human meibomian gland and conjunctival epithelial cells. Mol Vis. (2012) 18:1055–67. doi: 10.1161/01.STR.32.3.649
60. Jun I, Choi YJ, Kim BR, Lee HK, Seo KY, Kim TI. Activation of the mTOR pathway enhances PPARγ/SREBP-mediated lipid synthesis in human meibomian gland epithelial cells. Sci. Rep. (2024) 14:28118. doi: 10.1038/s41598-024-73969-6
61. Jie Y, Sella R, Feng J, Gomez ML, Afshari NA. Evaluation of incomplete blinking as a measurement of dry eye disease. Ocul Surf. (2019) 17:440–6. doi: 10.1016/j.jtos.2019.05.007
62. Choi M, Han SJ, Ji YW, Choi YJ, Jun I, Alotaibi MH, et al. Meibum expressibility improvement as a therapeutic target of intense pulsed light treatment in meibomian gland dysfunction and its association with tear inflammatory cytokines. Sci. Rep. (2019) 9:7648. doi: 10.1038/s41598-019-44000-0
63. Blackie CA, Murakami D, Donnenfeld E, Oliff HS. Vectored thermal pulsation as a treatment for meibomian gland dysfunction: a review spanning 15 years. Ophthalmol Ther. (2024) 13:2083–123. doi: 10.1007/s40123-024-00976-1
64. Farid M, Agrawal A, Fremgen D, Tao J, Chuyi H, Nesburn AB, et al. Age-related defects in ocular and nasal mucosal immune system and the immunopathol dry eye disease. Ocular Immunol. Inflame. (2016) 24:327–47. doi: 10.3109/09273948.2014.986581
65. Call CB, Wise RJ, Hansen MR, Carter KD, Allen RC. In vivo examination of meibomian gland morphology in patients with facial nerve palsy using infrared meibography. Ophth Plastic Reconstruct Surg. (2012) 28:396–400. doi: 10.1097/IOP.0b013e3182611641
66. Alghamdi YA, Mercado C, McClellan AL, Batawi H, Karp CL, Galor A, et al. Epidemiology of meibomian gland dysfunction in an elderly population. Cornea. (2016) 35:731–5. doi: 10.1097/ICO.0000000000000815
Keywords: thyroid-associated ophthalmopathy, meibomian gland dysfunction, dry eye, meibomian gland, meta-analysis
Citation: Lu Y, Li Y and Lin T (2025) Meibomian gland dysfunction in patients with thyroid-associated ophthalmopathy: a systematic review and meta-analysis. Front. Med. 12:1709057. doi: 10.3389/fmed.2025.1709057
Received: 19 September 2025; Accepted: 23 October 2025;
Published: 11 November 2025.
Edited by:
Xin Qi, Central South University, ChinaReviewed by:
Vicente Zanon-Moreno, University of Valencia, SpainJingsong Shi, Nanjing General Hospital of Nanjing Military Command, China
Sudi Patel, Svjetlost Clinic, Croatia
Jae Lim Chung, Share Bright Vision Eye Clinic, Republic of Korea
Copyright © 2025 Lu, Li and Lin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tulong Lin, bGludHVsb25nQG91dGxvb2suY29t
 Yanling Lu1
Yanling Lu1 Yongran Li
Yongran Li Tulong Lin
Tulong Lin