- 1Host-Microbe Interactomics, Animal Sciences, Wageningen University, Wageningen, Netherlands
- 2NIZO Food Research B. V., Ede, Netherlands
In S. suis the ComX-inducing peptide (XIP) pheromone regulates ComR-dependent transcriptional activation of comX (or sigX) the regulator of the late competence regulon. The aims of this study were to identify the ComR-regulated genes and in S. suis using genome-wide transcriptomics and identify their function based on orthology and the construction of specific knockout mutants. The ComX regulon we identified, includes all homologs of the “transformasome” a type 4-like pilus DNA binding and transport apparatus identified in Streptococcus pneumoniae, Streptococcus mutans, and Streptococcus thermophilus. A conserved CIN-box (YTACGAAYW), predicted to be bound by ComX, was found in the promoters of operons encoding genes involved in expression of the transformasome. Mutants lacking the major pilin gene comYC were not transformable demonstrating that the DNA uptake pilus is indeed required for competence development in S. suis. Competence was a transient state with the comX regulon shut down after ~15 min even when transcription of comX had not returned to basal levels, indicating other mechanisms control the exit from competence. The ComX regulon also included genes involved in DNA repair including cinA which we showed to be required for high efficiency transformation. In contrast to S. pneumoniae and S. mutans the ComX regulon of S. suis did not include endA which converts the transforming DNA into ssDNA, or ssbA, which protects the transforming ssDNA from degradation. EndA appeared to be essential in S. suis so we could not generate mutants and confirm its role in DNA transformation. Finally, we identified a putative homolog of fratricin, and a putative bacteriocin gene cluster, that were also part of the CIN-box regulon and thus may play a role in DNA release from non-competent cells, enabling gene transfer between S. suis pherotypes or S. suis and other species. S. suis mutants of oppA, the binding subunit of the general oligopeptide transporter were not transformable, suggesting that it is required for the import of XIP.
Introduction
The process of natural competence for DNA transformation in specific habitats or “natural competence” has been established as an important mechanism impacting on bacterial evolution and speciation. A nutritional benefit of natural competence has also been proposed through the provision of DNA nucleotides generated by degradation of one DNA strand during transport into the cytoplasm. The genes associated with natural competence are widely distributed throughout the bacterial kingdom, although experimental evidence for natural competence is limited to only a few genera.
Most streptococcal species belonging to the Streptococcus mutans, Streptococcus thermophilus, and Streptococcus gordonii phylogenetic groups possess conserved genetic components of the competence machinery (Johnston et al., 2014), and natural competence has been experimentally demonstrated in around 16 species of Streptococcus (Håvarstein et al., 1995; Fontaine et al., 2010; Mashburn-Warren et al., 2010; Morrison et al., 2013; Zaccaria et al., 2014). In streptococci competence is induced by an alternative sigma factor, ComX or SigX, which regulates expression of the late competence genes encoding functions in DNA uptake and recombination. Two main types of pheromone regulatory systems control the proximal regulatory switch for comX expression. The first is exemplified by Streptococcus pneumoniae, which uses a two-component system to sense and respond to a competence stimulating peptide (CSP) by inducing ComX. This alternative sigma factor controls the late competence regulon via interaction with the Com box motifs (also known as CIN-Box motifs) in promoter DNA sequences and interaction with RNA polymerase. The late competence regulon includes operons encoding genes for assembly of the type 4-like pilus, a DNA uptake system (Laurenceau et al., 2013) generating a single stranded DNA (ssDNA) molecule, and DNA recombination and repair enzymes that promote formation of the recombination synapse, heteroduplex formation and strand exchange between homologous DNAs (Campbell et al., 1998; Luo and Morrison, 2003; Peterson et al., 2004).
A second pheromone regulatory system for induction of competence was discovered in S. mutans, S. thermophilus, and S. pyogenes. In these species, ComR, an Rgg family transcriptional activator, positively regulates expression of comX and comS, through allosteric interaction with a processed form of the pheromone encoded by comS (Mashburn-Warren et al., 2010, 2012; Gardan et al., 2013; Zaccaria et al., 2014). The mature pheromone peptide induces competence from outside the bacteria but its mechanism of export is unknown. In S. mutans and S. thermophilus, the import of the mature pheromone is dependent on Opp, a general peptide transporter (Gardan et al., 2009; Guo et al., 2014).
The Opp (or Ami) peptide transport system is essential for competence development of S. mutans and S. thermophilus (Gardan et al., 2009, 2013; Mashburn-Warren et al., 2010; Fleuchot et al., 2011), and it appears to be responsible for the internalization of the XIP. The Opp transporters consist of two transmembrane hydrophobic pore-forming domains (OppB and OppC) and two ATP-binding proteins (OppD and OppF), that hydrolyse ATP to provide the energy required for peptide transport (Higgins, 2001). In addition to these conserved proteins, the Opp operon encodes a ligand-binding protein (OppA) that is responsible for recognizing and binding extracellular peptides, thus conferring specificity to the transport system.
In streptococci, competence is a transient physiological bacterial state (Seaton et al., 2011, 2015; Desai et al., 2012; Federle and Morrison, 2012; Guo et al., 2014) and the mechanisms mediating shut-down have only been partially elucidated in some species (Boutry et al., 2012; Tian et al., 2013; Weng et al., 2013; Dong et al., 2014; Wahl et al., 2014). In S. pneumoniae, which possess the ComCDE competence regulatory system, the exit from competence is regulated by multiple ComX dependent- and independent mechanisms (Chastanet et al., 2001; Bergé et al., 2003; Mortier-Barrière et al., 2007; Piotrowski et al., 2009; Martin et al., 2013; Mirouze et al., 2013; Weng et al., 2013) In S. mutans and S. thermophilus which both utilize the ComRS system to regulate competence development, MecA negatively regulates competence development by targeting the ClpC-ClpP protease activity to ComX (Boutry et al., 2012; Tian et al., 2013). Moreover, in vitro degradation of ComX by ClpC-ClpP was shown to be strictly dependent on MecA (Wahl et al., 2014).
In some streptococci including S. pneumoniae ComX regulates secondary processes including expression of stress response pathways and fratricin, a cell wall hydrolase which provides a predatory mechanism to lyse non-competent pneumococci and acquire DNA (Kausmally et al., 2005; Håvarstein et al., 2006; Claverys et al., 2007). Conservation of this predatory mechanism has been proposed in other streptococci based on gene homologies and the presence of CIN-boxes in promoter regions (Berg et al., 2012). Recently, in S. mutans a bacteriocin-like molecule was identified that is induced by its competence-inducing peptide, causing autolysis in part of the population (Perry et al., 2009; Lemme et al., 2011).
We recently identified a pheromone-induced mechanism of competence in Streptococcus suis, an important pig pathogen and zoonotic agent of human meningitis (Zaccaria et al., 2014). The competence system of S. suis appears to be similar to the ComRS-driven mechanism that has been discovered in S. mutans, S. thermophilus, and S. pyogenes, although S. suis belongs to a different phylogenetic group (Zaccaria et al., 2014).
A time-series transcriptome study of competence development has been previously reported for streptococcal species using a two-component system to regulate peptide-induced competence development (Dagkessamanskaia et al., 2004; Vickerman et al., 2007) but as far as we are aware similar studies have not been performed for a streptococcal species harboring a ComRS system as the proximal switch. The aims of this study were to identify the ComR-regulated genes and in S. suis using genome-wide transcriptomics and identify their function based on orthology and the construction of specific knockout mutants. At three biologically relevant times after pheromone induction of competence (Zaccaria et al., 2014), S. suis RNA was extracted and hybridized to commercially available whole-genome microarrays. We found that induction and repression of major DNA repair and RNA metabolic genes occurred within 5 and 15 min, indicating that uptake, processing and incorporation of exogenous DNA into the S. suis genome occurs effectively within 15–30 min. Our data were used to predict the S. suis transformasome by orthology and pinpoint processes that are both crucial to genomic integrity and gene transfer. These processes are therefore not only relevant from a fundamental biological viewpoint but could also be targets of future antimicrobials.
Materials and Methods
Bacterial Strains and Culture Conditions
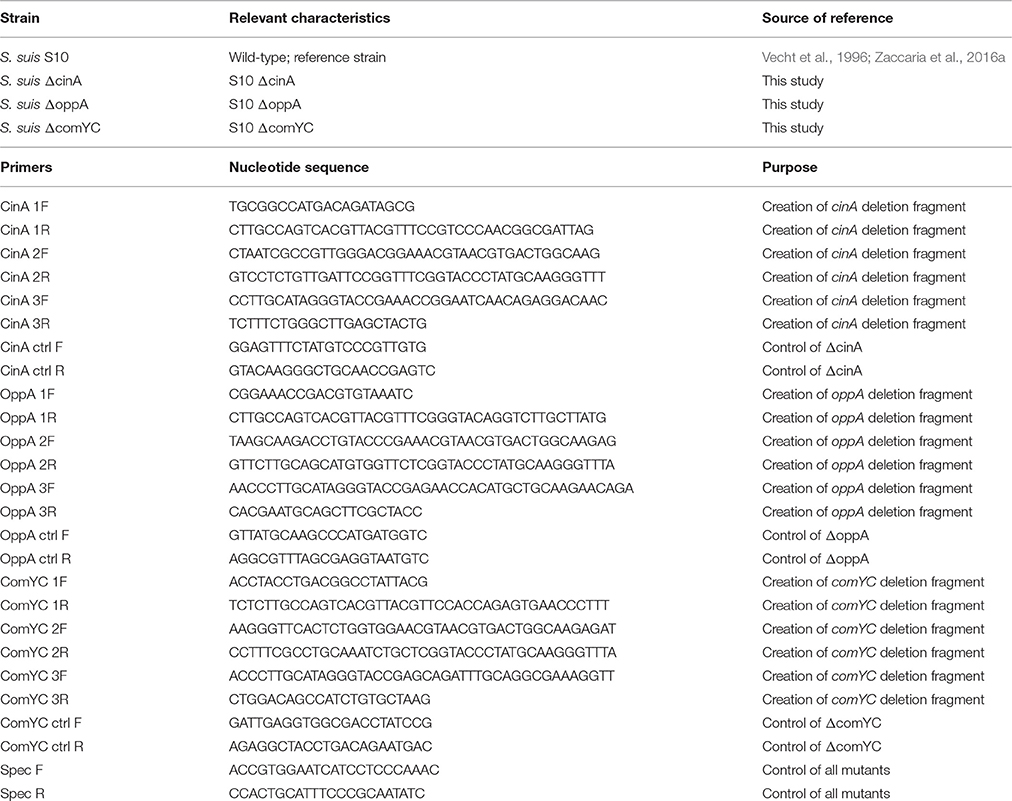
The S. suis strains used in the present study are listed in Table 1. S. suis strain S10 is a virulent isolate from an infected pig, and its genome is 99% identical to the genome of S. suis 2 strain P1/7 (de Greeff et al., 2011), a sequenced reference strain of which the genome had been annotated previously (Holden et al., 2009). S. suis was grown at 37°C at 5% atmospheric CO2 in Todd Hewitt Broth (THB, Thermo Scientific, Oxoid) or on THB plates containing 1.2% of agar (BD). When required the medium was supplemented with spectinomycin (Invitrogen) and/or chloramphenicol (Sigma) at a concentration of 100 and 5 μg/ml, respectively. Insertional deletion mutants of the genes cinA, oppA, and comYC were constructed in S. suis strain S10 by Gene Splicing Overlap Extension PCR (SOE-PCR) and allelic replacement as previously described (Zaccaria et al., 2014). The primers used for SOE-PCR are shown in Table 1. Successful deletion of the genes was verified by colony PCR using primer combinations based on DNA sequences of the inserted DNA and proximal chromosomal DNA (Table 1) and verified by sequencing of the amplicons. Growth phase was determined by measuring optical density at 600 nm (OD600nm) using a SpectraMax M5 reader (Molecular Devices LLC).
RNA Extraction
RNA was isolated and purified from S. suis cultures at different times after induction of competence. Briefly S. suis S10 was grown to OD600nm 0.04. Thirty-five mL of culture was collected and transforming DNA (pNZ8048, 350 μg) in EB buffer (10 mM Tris-Cl, pH 8.5) was added to the bacteria together with synthetic XIP (GNWGTWVEE) at a final concentration of 250 μM. At 5, 15, and 45 min after the addition of XIP, 10 mL aliquots of the cultures were centrifuged for 2 min at 8000 g at RT and the bacterial pellets resuspended in 2.5 mL PBS plus 5 mL RNAprotect buffer (Qiagen). After 5 min incubation the bacterial suspension was centrifuged, the supernatant aspirated, and bacterial pellet immediately frozen in liquid nitrogen. The frozen pellet was dissolved in 110 μL of TE containing proteinase K and lysozyme (1.25 and 15 μg/ml, respectively) and incubated for 10 min at room temperature with vortex mixing every 2 min. Then 700 μL of RLT buffer (Promega) containing 7 μL of freshly added β-mercaptoethanol was added and the bacteria disrupted using a FastPrep-24 (MP Biomedicals, Solon, OH) for 20 s at 6.0 m/s. Total RNA was purified using the RNeasy Mini Kit (Qiagen). The quality and the concentration of RNA were determined using the Experion System (Bio-Rad) and measurement of the A260/A280 ratio (NanoDrop 8000 UV-Vis Spectrophotometer). Complementary DNA (cDNA) was synthesized using the SuperScript III Reverse Transcriptase kit (Invitrogen) using aminoallyl-dUTPs in place of UTP and purified with the Illustra CyScribe GFX Purification Kit (GE Healthcare). The cDNA was labeled with CyDye Post-Labeling Reactive Dye Pack (GE Healthcare) using the manufacturer's recommended protocol. RNA was also purified from control cultures to which no XIP or no transforming DNA was added using the above method.
Microarray Transcriptome Analysis
An S. suis oligoarray (8 × 15 K) containing in situ synthesized 60-mers was produced by Agilent Technologies (Santa Clara, USA), based on the genome sequence of S. suis P1/7 (Holden et al., 2009). A total of 7651 unique 60-mers having a theoretical melting temperature of ~81°C and representing 1960 ORFs were selected as described (Saulnier et al., 2011). Genes were represented by 4 (91%), 3 (4%), 2 (2%), or 1 probe(s) (3%). Twenty-five putative genes were not represented on the array because no unique probe satisfying the selection criteria could be selected. Co-hybridization with labeled cDNA probes was performed on these oligonucleotide arrays at 42°C for 16 h in hybridization chambers (Slidehyb#1, Ambion, Austin, USA). The data were normalized using Lowess normalization (Yang et al., 2002) as available in MicroPrep (van Hijum et al., 2003) and corrected for inter-slide differences on the basis of total signal intensity per slide using Postprep (van Hijum et al., 2003). Significance of differential gene expression was based on false discovery rate (FDR) values lower than 0.05. The data discussed in this publication have been deposited in NCBI's Gene Expression Omnibus and are accessible through GEO Series accession number GSE74507.
Microarray Data Analysis
Within the dataset gene expression data with high standard deviation (>250) or very low expression values (i.e., not having at least four observations with absolute value higher than 20) or that were not altered by the induction of competence (maximal value minus minimal value of at least 200) were filtered out using Cluster 3.0. Further details on the software and settings can be found in the online handbook (http://bonsai.hgc.jp/~mdehoon/software/cluster/cluster3.pdf). Heatmaps were generated by the MultiExperimental Viewer (MeV) program (http://www.tm4.org/mev.html) (Saeed et al., 2006).
Transformation Experiments
S. suis strains were grown overnight in THB broth in an incubator 37°C with 5% CO2. The overnight culture was then diluted 1:40 into pre-warmed THB broth, and grown at 37°C without shaking. When the culture reached an OD600nm of ~0.04, aliquots of 100 μL were transferred to 1.5 mL Eppendorf Safe Lock Tubes™ and combined with transforming DNA (1.2 μg of pNZ8048) in EB buffer (10 mM Tris-Cl, pH 8.5) and 5 μl of XIP at a final concentration of 250 μM. After 2 h of incubation at 37°C in the presence of 5% CO2, the samples were diluted and plated onto THB agar plates containing antibiotic.
Results and Discussion
The Competence Pheromone Induces Distinct Clusters of Differentially Regulated Genes at Specific Time-Points
Competence pheromone-induced transcriptional changes were identified by microarray analysis of RNA isolated from bacteria at 5, 15, and 45 min in the presence and absence of the competence pheromone and in the presence of exogenous DNA as previously described (Zaccaria et al., 2014). Additionally, the peptide pheromone was added without adding DNA, to identify possible effects of DNA addition. Five minutes after addition of the competence pheromone, 556 differentially expressed genes were up-regulated more than two-fold and 215 genes were down-regulated more than two-fold. At 15 min 148 and 185 differentially expressed genes were respectively up-regulated or down-regulated. At 45 min 140 and 48 were up- and downregulated, respectively. Genes that were not expressed or did not change expression at the multiple time points and controls were removed by filtering the data as described in Methods. Genes with altered expression were clustered according to their relative expression values at the different time points (Figure 1). Four major clusters were observed (Figure 1), the first of which (cluster 1) contains genes that were down-regulated upon induction of competence until 45 min post-addition of peptide (Figure 1). Cluster 1 contains 13 of the 14 fatty acid biosynthetic pathway genes and three genes involved in cell envelope metabolism. This reflects the finding that cell division and basal metabolic processes are halted during competence development (Zaccaria et al., 2016b) to avoid recombination of transforming DNA during DNA replication which is potentially dangerous to genome integrity. Cluster 2 contains genes of diverse functions including a cation-transporting ATPase, a putative peptidase and a predicted transcriptional regulator that were up-regulated only at 15 min. Cluster 3 genes were all highly (>4 fold) up-regulated at 5 min, after which their expression decreased until 45 min, when expression reached the same level as measured for the uninduced control samples. Cluster 3 contained 37 genes including comX, the sigma factor controlling the competence regulon. Of these 37 genes, 8 genes were annotated to be involved in DNA repair and recombination, and four genes were annotated as homologs of the multi-protein Type 4 pilus-like DNA uptake and transport apparatus recently described as the “transformasome” in S. pneumoniae (Laurenceau et al., 2013). Cluster 4 contains 28 genes that were highly expressed at 5 and 15 min and downregulated at 45 min including the comYA-YH operon encoding homologs of pneumococcal proteins forming the transformasome DNA uptake apparatus, genes encoding chaperones groES and groEL, a putative fratricin gene, three genes in the mevalonate pathway and an operon (SSU0038-SSU0045) of unknown function.
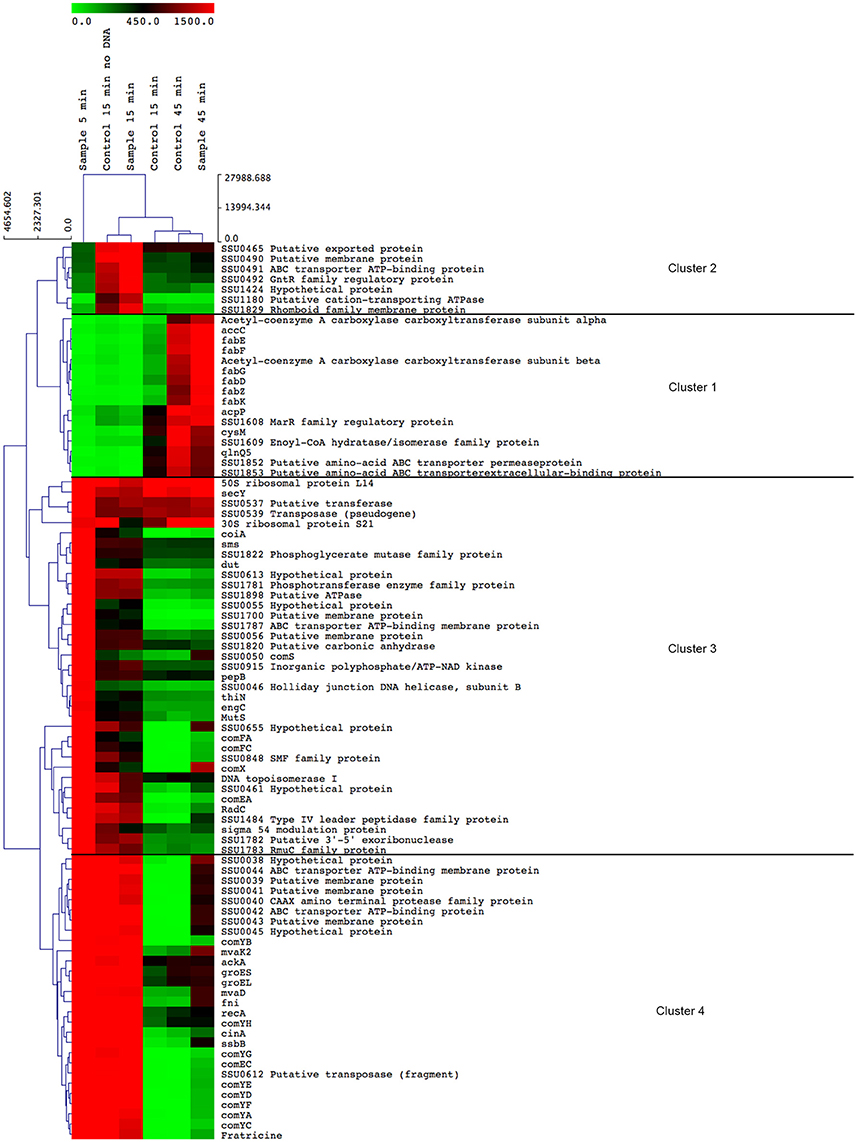
Figure 1. Heatmap displaying the most differentially expressed genes in S. suis during competence. Genes were filtered using Cluster 3.0 (See Methods) and were clustered using average linkage and Euclidian distance using MultiExperiment Viewer (MeV, see Methods). The color scale at the top depicts the normalized, unlogged expression values of the genes indicated on the right. The heatmap colors represent gene expression levels from the lowest value (zero, light green) to the highest level (~3000, bright red). To not lose resolution of intermediary expression levels, a highest cut-off value of 1500 was applied. The time at which bacterial samples were rapidly centrifuged and suspended in RNAprotect buffer is indicated above each column. “Control” indicates bacterial cultures to which no inducing peptide was added. “No DNA” indicates that no transforming DNA was added together with the competence inducing peptide.
The S. suis Transformasome Is Regulated by ComX via a Conserved CIN-box
To identify the consensus motifs interacting with ComX in S. suis (i.e., CIN-box), promoters of genes or operons that were highly upregulated at 5 and 15 min in the presence of the competence peptide but in not the control samples at the same points were searched for consensus motifs using MEME (http://meme-suite.org/doc/overview.html). The conserved consensus 9 nt motif (YTACGAAYW) identified in S. suis is similar to the CIN-box of S. pneumoniae (Figure 2). In S. suis the CIN-box genes were present in nine operons, four of which were identified using the FIMO module of the MEME software suite (See Methods). The S. suis CIN-box genes encode homologs of all the known transformasome proteins in S. pneumoniae, S. mutans, and S. thermophilus (Table 2), showing its conservation across streptococcal species (Peterson et al., 2000, 2004; Vickerman et al., 2007).
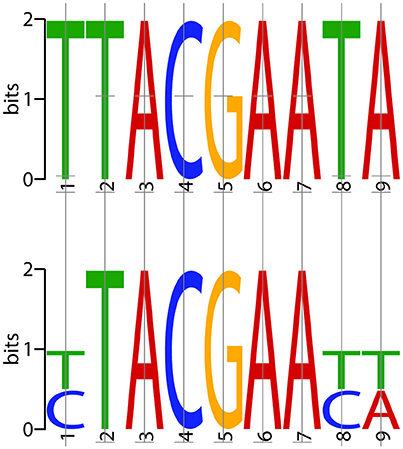
Figure 2. Consensus CIN-box of S. pneumoniae and S. suis. The consensus CIN-box identified in S. pneumoniae (top) is compared to that identified for S. suis (bottom) using MEME with the FIMO plug in (http://meme-suite.org/doc/overview.html). The MEME motifs represent the probability of each possible DNA nucleotide appearing at each possible position in an occurrence of the motif. The possible letters are A, C, G, and T, adenine, cytosine, guanine, or thymine, covalently linked to a phosphodiester backbone. The height of the individual letters in a stack represents the probability of the letter at that position.
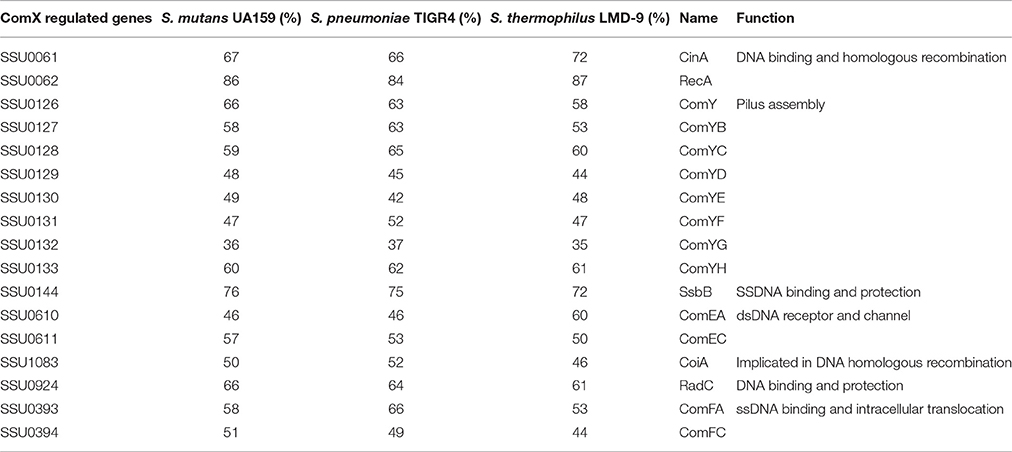
Table 2. Transformasome genes under ComX regulation with their homologs in S. mutans, S. pneumonia, and S. thermophilus and their (putative) function.
The S. suis CIN-box regulon contains a dedicated set of genes, the comYA-YH operon (comGA-GH in S. pneumoniae), with homology to the Type 4 pili (T4P) of Gram-positive bacteria, predicted to encode a putative ATPase (ComYA), a membrane protein (ComYB) and five other proteins corresponding to the major pilin (ComYC) and to the minor pilins (ComYD, ComYE, ComYF, ComYG, and ComYH; Laurenceau et al., 2013). With the exception of recA, that has its own promoter and is expressed constitutively, the S. suis CIN-box regulated genes were all highly expressed at 5 min and thereafter showed decreased expression, eventually returning to basal levels after 45 min. This pattern of temporal expression has also been described in S. pneumoniae with the difference that the ComX regulated genes of S. pneumoniae peak at 15 min after induction of competence rather than 5 min as we observed in S. suis (see clusters 3 and 4, Figure 1).
Deletion of the Major Pilin Gene comYC Prevents DNA Transformation
To verify if the conserved ComY operon was necessary for competence in S. suis we generated a S. suis deletion mutant of the pilin comYC and verified the mutation by PCR and sequencing (Table 1). The comYC deletion in S. suis prevented subsequent attempts to obtain DNA transformation after competence induction with the SigX-inducing-peptide (XIP) providing further evidence for the conserved role of ComYC in competence for DNA transformation.
Regulation and Function of DNA Processing and Recombination Enzymes in Competence
In S. pneumoniae it is thought that once the pilus is polymerized, a channel is formed that passes through the cell wall and the capsule, allowing the exogenous DNA to be internalized into the cytoplasm (Petersen et al., 2005; Laurenceau et al., 2015). Before or concomitant with DNA translocation, the activity of EndA generates a single stranded DNA (ssDNA) molecule. Additionally, CoiA, DprA, and RecA, a DNA-dependent ATPase, promote formation of the recombination synapse, heteroduplex formation and strand exchange between homologous DNAs (Desai and Morrison, 2006; Morrison et al., 2007).
In S. suis genes coiA, radC, recA, and cinA which are involved in the formation of the recombination synapse, heteroduplex formation and strand exchange were regulated by ComX (Table 2). In contrast to S. pneumoniae and S. mutans, EndA, a DNA specific nuclease that converts the dsDNA bound by ComEA and ComEC (Lacks et al., 1974; Mirouze et al., 2013) into ssDNA before or concomitant with delivery into the cytoplasm through ComEC (Seitz et al., 2014), and SsbA, single stranded binding protein A, which protects the transforming ssDNA from degradation were not regulated by ComX (Attaiech et al., 2011).
Pneumococcus endA deletion mutants were found to accumulate DNA at the cell surface (Lacks et al., 1974). There is probably a similar role for EndA in competence development in S. suis but we were unable to verify this as we were not able to obtain endA gene deletion mutants suggesting EndA has an additional essential role in S. suis. This is consistent with an absence of a CIN-box in the endA promoter and the constitutive expression of endA in S. suis. Expression of cinA and the downstream gene recA, is strongly enhanced at 5 and 15 min after XIP exposure. Unlike cinA, recA was constitutively expressed at a lower basal level in the absence of XIP, reflecting its “housekeeping” role in DNA recombination. To determine whether the predicted S. suis ortholog of CinA may have a role in competence, a cinA deletion mutant (ΔcinA) was generated using previously described methods (Zaccaria et al., 2014) and verified by PCR and sequencing. The ΔcinA mutant resulted in substantially reduced DNA transformation efficiency compared to the parent wild-type (WT) strain S10 (about 8% of WT efficiency) suggesting that CinA has an important but not essential role in DNA transformation (Figure 3) as shown for other bacteria (Masure et al., 1998; Mair et al., 2012).
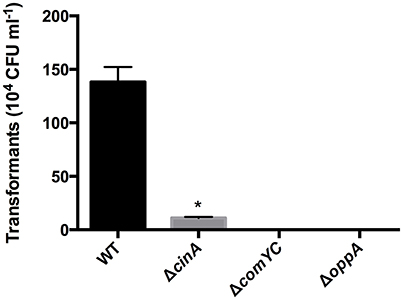
Figure 3. Number of transformants obtained with ΔcinA, ΔcomYC, and ΔoppA deletion mutants in S. suis strain S10. The mutants described above and in Table 1 were made using Gene Splicing Overlap Extension PCR (SOE-PCR) and allelic replacement as previously described (Zaccaria et al., 2014). The efficiency of transformation was determined by plating and enumeration of chloramphenicol resistant colonies after competence induction and transformation with plasmid pNZ8084. *Indicates a significant reduction in transformation efficiency.
Expression Profiles of the Transcriptional Regulators of Competence, comR and comX
In all the streptococcal species in which natural competence has been demonstrated, its activation leads to expression of the alternative sigma factor X (comX or sigX). We have previously shown that comX is essential for natural transformation in S. suis S10 (Zaccaria et al., 2014). In our transcriptome data comX expression was strongly up-regulated at 5 min (1300-fold compared with the control), relatively mildly up-regulated at 15 min (105-fold) and up-regulated again at 45 min (327-fold). This fluctuation in comX expression could be due to an oscillation of the positive and negative feedback loops controlling its transcription (Haustenne et al., 2015).
Despite the high amount of comX expression at the 45 min time point, the CIN-box genes under its direct regulation were not increased in expression at 45 min compared to 15 min in the XIP-induced samples with DNA provided exogenously, although they were expressed significantly higher compared to uninduced control samples.
We have shown that ComR is required for competence induction in S. suis (Zaccaria et al., 2014) and postulated that ComR interacts with the mature ComS pheromone to induce comX and comS, in a similar way as described for other streptococci possessing the ComRS regulatory system (Figure 5) (Mashburn-Warren et al., 2010; Gardan et al., 2013). In support of this hypothesis, the expression of comS is similar to comX expression, characterized by a strong induction at 5 min, a decline at 15 min and an induction at 45 min. The comR regulator is constitutively expressed in broth cultures and slightly increased 5 min after peptide-induced competence (2.3-fold compared to the control) but then rapidly decreases to its prior expression level at 15 min. Microarray expression values for comX in the uninduced state are close to zero, preventing S. suis from entering the competence state, whereas in the uninduced state comS the competence inducing peptide precursor was expressed at low levels (Figure 1).
Where Is the Exit?—Regulation of Competence Shut down
In S. suis expression of MecA, the adapter protein regulating ClpCP-mediated degradation of ComX in S. thermophilus, was not substantially different over the time course of competence induction. mecA expression was down-regulated at 5 min (0.42-fold) and 45 min (0.88-fold) and up-regulated at 15 min (1.28-fold). Similar fold changes in the expression were measured for ClpC at the same respective time points. These findings make it unlikely that only these two proteins are responsible for degrading ComX and exiting the competence state. One other candidate gene that may regulate competence exit is the dprA gene that is regulated by ComX and has a dual role in the natural transformation system of S. pneumoniae. In the later species DprA promotes the homologous recombination facilitating RecA binding to the ssDNA but can also bind to the phosphorylated form of the ComE response regulator (ComE~P), preventing its interaction with the ComX promoter (Mirouze et al., 2013; Weng et al., 2013), thereby shutting down competence. The S. suis dprA possesses a CIN-box in its promoter and was highly induced 5 min after competence induction. However, it seems unlikely to have a role in competence shutdown because S. suis utilizes ComRS rather than the two-component system ComCDE as a regulatory switch for comX expression. S. thermophilus, which also uses ComRS as the proximal switch for comX expression does not appear to utilize DprA for shutting down comX expression.
Conserved Function of the Oligopeptide Permease Gene Cluster of S. suis in Competence Induction
In all investigated ComRS systems efficient binding of ComR to its operator motif is strictly dependent on the presence of the XIP pheromone (Fleuchot et al., 2011, 2013; Fontaine et al., 2013; Aggarwal et al., 2015). In other streptococci, XIP mediates the quorum sensing mechanism of competence induction by its transport back into the cell via the Opp oligopeptide ABC type transporter (Gardan et al., 2009; Mashburn-Warren et al., 2010). In S. suis the five genes encoding the Opp transporter are organized in two transcriptional units, an operon of four genes and OppA which encodes the subunit A of the Opp transporter complex. The expression of the full transporter system did not significantly change during competence induction. We generated a knockout of oppA, the component of the transporter that recognizes XIP, and were unable to transform this mutant. Although this is consistent with oppA deletion in other streptococcal species, we cannot completely rule out that the slower growth of the OppA mutant impacted on competence development.
S. suis Contains a CIN-Box-Regulated Homolog of Fratricin and a Putative Bacteriocin-Producing Operon
Downstream of a CIN-box promoter we identified a fratricin-like gene in S. suis (SSU1911) that contains an N-terminal CHAP (Cysteine, Histidine-dependent Amidohydrolases/Peptidases) domain and two SH3b (central Src homology 3b) domains, which are also present in pneumococcal fratricin (Berg et al., 2012). After induction of S. suis competence by XIP, a gene encoding a homolog of fratricin was up-regulated at 5, 15, and 45 min by 722-, 208-, and 22-fold, respectively. The expression profile of this gene was similar to the expression profile of the ComX-regulated genes of S. suis (Figure 4) suggesting that S. suis also produces a fratricin-like protein during competence development. S. pneumoniae fratricin, a cell wall hydrolase, was shown to lyse non-competent pneumococci and closely related bacterial species, thereby ensuring that the transforming DNA has overall a high level of homology to the competent recipients, favoring beneficial DNA recombination over detrimental genetic events (Kausmally et al., 2005; Håvarstein et al., 2006; Claverys et al., 2007; Berg et al., 2012). We speculate that the S. suis fratricin-like protein may have a similar role to that of pneumococcal fratricin, although we could not identify a homolog of the candidate fratricin immunity gene ComM, described in S. pneumoniae (Håvarstein et al., 2006; Eldholm et al., 2010).
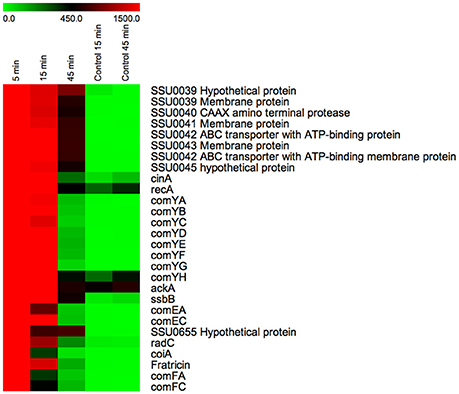
Figure 4. Temporal expression of the genes under direct regulation of ComX. Heatmap displaying the temporal expression of predicted ComX-regulated genes during competence development. The color scale at the top depicts the normalized, unlogged expression values of the genes indicated on the right. The heatmap colors represent gene expression levels from the lowest value (zero, light green) to the highest level (~3000, bright red). To not lose resolution of intermediary expression levels, a highest cut-off value of 1500 was applied to the heatmap display. The time at which bacterial samples were rapidly centrifuged and suspended in RNAprotect buffer is indicated above each column. “Control” indicates bacterial cultures to which no inducing peptide was added.
Interestingly, we also identified a CIN-box regulated operon consisting of eight genes that do not show significant homology with other competence genes. The operon SSU0038-45, comprises three putative membrane proteins, one CAAX amino terminal protease and two ABC transporters with ATPase activity. In addition, we measured high expression of two relatively small putative ORFs (SSU0038 and SSU0045) represented on the microarray but not annotated in S. suis genome. These ORFs are predicted to encode two small (42 and 57 amino acids) proteins with unknown function but their size and association with a CAAX peptidase and two ABC transporters suggests a possible role as bacteriocins. This is also supported by the peptide leader sequence of SSU0045 that features a double-glycine motif which is a characteristic of bacteriocins that are secreted by ABC transporters (van Belkum et al., 1997). In S. mutans, competence induction directly controls bacteriocin production (Reck et al., 2015). Also in S. gordonii, a locus with a CIN-box in the promoter region that encodes a bacteriocin has been reported. The competence-related bacteriocin peptide in S. gordonii also contains a double-glycine motif for export via an ABC-type transport system (Håvarstein et al., 1995) and is active against S. gordonii and S. mitis (Heng et al., 2007).
Concluding Remarks
As far as we are aware this is the first transcriptomics study of the complete time course of competence development in a streptococcal species harboring a ComRS system. This time-resolved overview of the genetic regulation of competence revealed the ComX regulon, comprising all genes encoding homologs of all the known transformasome proteins in S. pneumoniae, S. mutans, and S. thermophilus (Figure 5, Table 2). Additionally, we showed that deletion mutants of the major pilin gene comYC, which is required for formation of the DNA binding pilus is necessary for peptide-induced DNA transformation. A mutant of cinA, encoding a protein involved in DNA binding and recombination was strongly attenuated for DNA transformation. OppA encoding the binding subunit of the general oligopeptide transporter was required for competence development suggesting it transports XIP into the bacteria where it binds to ComR. In contrast to previous studies with S. pneumoniae, endA, encoding a DNA specific nuclease that converts the dsDNA bound by ComEA and ComEC into ssDNA during uptake by the transformasome (Lacks et al., 1974; Mirouze et al., 2013) could not be deleted in S. suis suggesting it might have an additional essential role in this species. S. suis expresses a fratricin-like gene and a putative bacteriocin and associated transport system during competence development, which we speculate may play roles in acquiring DNA as described for other species. The induction of competence was transient with expression of the ComX-regulated genes peaking at around 5 min after addition of the peptide and declining substantially at 15 min, despite continued presence of comX transcripts to 45 min. The transient nature of competence development is assumed to avoid potentially adverse effects of genetic recombination on genome integrity during cell division and is associated with a suppression of basal metabolism (Zaccaria et al., 2016b). From the transcriptomics data alone it was not possible to identify genes regulating exit from competence and further studies are needed to elucidate the mechanisms involved.
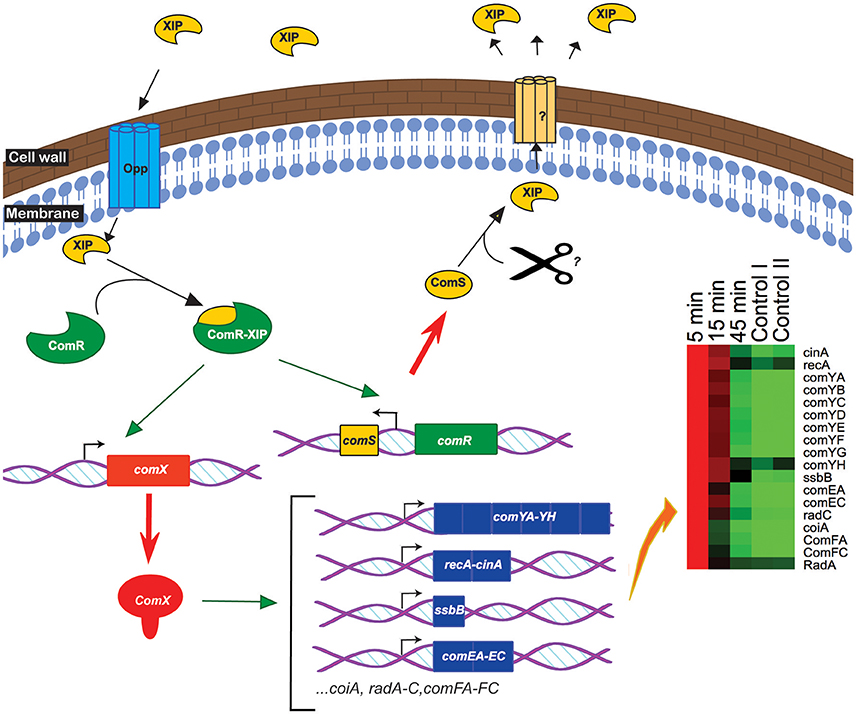
Figure 5. Simplified model of competence induction in S. suis. Extracellular SigX-inducing-peptide (XIP) enters the bacteria via the Opp transporter system. Intracellularly, the transcriptional regulator ComR binds to XIP and the ComR-XIP complex promotes the expression of comS, encoding the full-length form of the XIP pheromone, and of comX (green arrow). ComX activates the expression (green arrow) of the late-competence genes involved in the transformasome having a CIN-box in their promoter (heatmap). ComS is processed and secreted by an unknown mechanism inducing a positive feedback loop.
Author Contributions
JW, EZ, Pv, and MW contributed substantially to aspects of the design of the experimental work and the interpretation of data. EZ, JW, and Pv contributed to the conception of the whole study. EZ performed the experimental work. MW, Pv, and EZ were involved in the acquisition and analysis of data. JW and EZ wrote the draft manuscript. Pv, MW, EZ, and JW revised the intellectual content of the manuscript, and approved the final version for submission. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding
This work was supported by the European Commission, as part of the Framework Programme 7, Marie Curie Initial Training Network - STARS (Contract No. PITN-GA-2009-238490).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank Iris van Swam, and Roger Bongers NIZO Food Research Ede, for assistance with transcriptomics.
References
Aggarwal, C., Jimenez, J. C., Lee, H., Chlipala, G. E., Ratia, K., and Federle, M. J. (2015). Identification of quorum-sensing inhibitors disrupting signaling between rgg and short hydrophobic peptides in streptococci. mBio 6, e00393–315. doi: 10.1128/mBio.00393-15
Attaiech, L., Olivier, A., Mortier-Barrière, I., Soulet, A. L., Granadel, C., Martin, B., et al. (2011). Role of the single-stranded DNA-binding protein SsbB in pneumococcal transformation: maintenance of a reservoir for genetic plasticity. PLoS Genet. 7:e1002156. doi: 10.1371/journal.pgen.1002156
Berg, K. H., Biørnstad, T. J., Johnsborg, O., and Håvarstein, L. S. (2012). Properties and biological role of streptococcal fratricins. Appl. Environ. Microbiol. 78, 3515–3522. doi: 10.1128/AEM.00098-12
Bergé, M., Mortier-Barrière, I., Martin, B., and Claverys, J. P. (2003). Transformation of Streptococcus pneumoniae relies on DprA- and RecA-dependent protection of incoming DNA single strands. Mol. Microbiol. 50, 527–536. doi: 10.1046/j.1365-2958.2003.03702.x
Boutry, C., Wahl, A., Delplace, B., Clippe, A., Fontaine, L., and Hols, P. (2012). Adaptor protein MecA is a negative regulator of the expression of late competence genes in Streptococcus thermophilus. J. Bacteriol. 194, 1777–1788. doi: 10.1128/JB.06800-11
Campbell, E. A., Choi, S. Y., and Masure, H. R. (1998). A competence regulon in Streptococcus pneumoniae revealed by genomic analysis. Mol. Microbiol. 27, 929–939. doi: 10.1046/j.1365-2958.1998.00737.x
Chastanet, A., Prudhomme, M., Claverys, J. P., and Msadek, T. (2001). Regulation of Streptococcus pneumoniae clp genes and their role in competence development and stress survival. J. Bacteriol. 183, 7295–7307. doi: 10.1128/JB.183.24.7295-7307.2001
Claverys, J. P., Martin, B., and Håvarstein, L. S. (2007). Competence-induced fratricide in streptococci. Mol. Microbiol. 64, 1423–1433. doi: 10.1111/j.1365-2958.2007.05757.x
Dagkessamanskaia, A., Moscoso, M., Hénard, V., Guiral, S., Overweg, K., Reuter, M., et al. (2004). Interconnection of competence, stress and CiaR regulons in Streptococcus pneumoniae: competence triggers stationary phase autolysis of ciaR mutant cells. Mol. Microbiol. 51, 1071–1086. doi: 10.1111/j.1365-2958.2003.03892.x
de Greeff, A., Wisselink, H. J., de Bree, F. M., Schultsz, C., Baums, C. G., Thi, H. N., et al. (2011). Genetic diversity of Streptococcus suis isolates as determined by comparative genome hybridization. BMC Microbiol. 11:161. doi: 10.1186/1471-2180-11-161
Desai, B. V., and Morrison, D. A. (2006). An unstable competence-induced protein, CoiA, promotes processing of donor DNA after uptake during genetic transformation in Streptococcus pneumoniae. J. Bacteriol. 188, 5177–5186. doi: 10.1128/JB.00103-06
Desai, K., Mashburn-Warren, L., Federle, M. J., and Morrison, D. A. (2012). Development of competence for genetic transformation of Streptococcus mutans in a chemically defined medium. J. Bacteriol. 194, 3774–3780. doi: 10.1128/JB.00337-12
Dong, G., Tian, X. L., Gomez, Z. A., and Li, Y. H. (2014). Regulated proteolysis of the alternative sigma factor SigX in Streptococcus mutans: implication in the escape from competence. BMC Microbiol. 14:183. doi: 10.1186/1471-2180-14-183
Eldholm, V., Johnsborg, O., Straume, D., Ohnstad, H. S., Berg, K. H., Hermoso, J. A., et al. (2010). Pneumococcal CbpD is a murein hydrolase that requires a dual cell envelope binding specificity to kill target cells during fratricide. Mol. Microbiol. 76, 905–917. doi: 10.1111/j.1365-2958.2010.07143.x
Federle, M. J., and Morrison, D. A. (2012). One if by land, two if by sea: signalling to the ranks with CSP and XIP. Mol. Microbiol. 86, 241–245. doi: 10.1111/mmi.12029
Fleuchot, B., Gitton, C., Guillot, A., Vidic, J., Nicolas, P., Besset, C., et al. (2011). Rgg proteins associated with internalized small hydrophobic peptides: a new quorum-sensing mechanism in streptococci. Mol. Microbiol. 80, 1102–1119. doi: 10.1111/j.1365-2958.2011.07633.x
Fleuchot, B., Guillot, A., Mézange, C., Besset, C., Chambellon, E., Monnet, V., et al. (2013). Rgg-associated SHP signaling peptides mediate cross-talk in streptococci. PLoS ONE 8:e66042. doi: 10.1371/journal.pone.0066042
Fontaine, L., Dandoy, D., Boutry, C., Delplace, B., de Frahan, M. H., Fremaux, C., et al. (2010). Development of a versatile procedure based on natural transformation for marker-free targeted genetic modification in Streptococcus thermophilus. Appl. Environ. Microbiol. 76, 7870–7877. doi: 10.1128/AEM.01671-10
Fontaine, L., Goffin, P., Dubout, H., Delplace, B., Baulard, A., Lecat-Guillet, N., et al. (2013). Mechanism of competence activation by the ComRS signalling system in Streptococci. Mol. Microbiol. 87, 1113–1132. doi: 10.1111/mmi.12157
Gardan, R., Besset, C., Gitton, C., Guillot, A., Fontaine, L., Hols, P., et al. (2013). Extracellular life cycle of ComS, the competence-stimulating peptide of Streptococcus thermophilus. J. Bacteriol. 195, 1845–1855. doi: 10.1128/JB.02196-12
Gardan, R., Besset, C., Guillot, A., Gitton, C., and Monnet, V. (2009). The oligopeptide transport system is essential for the development of natural competence in Streptococcus thermophilus strain LMD-9. J. Bacteriol. 191, 4647–4655. doi: 10.1128/JB.00257-09
Guo, Q., Ahn, S. J., Kaspar, J., Zhou, X., and Burne, R. A. (2014). Growth phase and pH influence peptide signaling for competence development in Streptococcus mutans. J. Bacteriol. 196, 227–236. doi: 10.1128/JB.00995-13
Haustenne, L., Bastin, G., Hols, P., and Fontaine, L. (2015). Modeling of the ComRS signaling pathway reveals the limiting factors controlling competence in Streptococcus thermophilus. Front. Microbiol. 6:1413. doi: 10.3389/fmicb.2015.01413
Håvarstein, L. S., Coomaraswamy, G., and Morrison, D. A. (1995). An unmodified heptadecapeptide pheromone induces competence for genetic transformation in Streptococcus pneumoniae. Proc. Natl. Acad. Sci. U.S.A. 92, 11140–11144. doi: 10.1073/pnas.92.24.11140
Håvarstein, L. S., Martin, B., Johnsborg, O., Granadel, C., and Claverys, J. P. (2006). New insights into the pneumococcal fratricide: relationship to clumping and identification of a novel immunity factor. Mol. Microbiol. 59, 1297–1307. doi: 10.1111/j.1365-2958.2005.05021.x
Heng, N. C., Tagg, J. R., and Tompkins, G. R. (2007). Competence-dependent bacteriocin production by Streptococcus gordonii DL1 (Challis). J. Bacteriol. 189, 1468–1472. doi: 10.1128/JB.01174-06
Higgins, C. F. (2001). ABC transporters: physiology, structure and mechanism–an overview. Res. Microbiol. 152, 205–210. doi: 10.1016/S0923-2508(01)01193-7
Holden, M. T., Hauser, H., Sanders, M., Ngo, T. H., Cherevach, I., Cronin, A., et al. (2009). Rapid evolution of virulence and drug resistance in the emerging zoonotic pathogen Streptococcus suis. PLoS ONE 4:e6072. doi: 10.1371/journal.pone.0006072
Johnston, C., Martin, B., Fichant, G., Polard, P., and Claverys, J. P. (2014). Bacterial transformation: distribution, shared mechanisms and divergent control. Nat. Rev. Microbiol. 12, 181–196. doi: 10.1038/nrmicro3199
Kausmally, L., Johnsborg, O., Lunde, M., Knutsen, E., and Håvarstein, L. S. (2005). Choline-binding protein D (CbpD) in Streptococcus pneumoniae is essential for competence-induced cell lysis. J. Bacteriol. 187, 4338–4345. doi: 10.1128/JB.187.13.4338-4345.2005
Lacks, S., Greenberg, B., and Neuberger, M. (1974). Role of a deoxyribonuclease in the genetic transformation of Diplococcus pneumoniae. Proc. Natl. Acad. Sci. U.S.A. 71, 2305–2309. doi: 10.1073/pnas.71.6.2305
Laurenceau, R., Krasteva, P. V., Diallo, A., Ouarti, S., Duchateau, M., Malosse, C., et al. (2015). Conserved Streptococcus pneumoniae spirosomes suggest a single type of transformation pilus in competence. PLoS Pathog. 11:e1004835. doi: 10.1371/journal.ppat.1004835
Laurenceau, R., Péhau-Arnaudet, G., Baconnais, S., Gault, J., Malosse, C., Dujeancourt, A., et al. (2013). A type IV pilus mediates DNA binding during natural transformation in Streptococcus pneumoniae. PLoS Pathog. 9:e1003473. doi: 10.1371/journal.ppat.1003473
Lemme, A., Gröbe, L., Reck, M., Tomasch, J., and Wagner-Döbler, I. (2011). Subpopulation-specific transcriptome analysis of competence-stimulating-peptide-induced Streptococcus mutans. J. Bacteriol. 193, 1863–1877. doi: 10.1128/JB.01363-10
Luo, P., and Morrison, D. A. (2003). Transient association of an alternative sigma factor, ComX, with RNA polymerase during the period of competence for genetic transformation in Streptococcus pneumoniae. J. Bacteriol. 185, 349–358. doi: 10.1128/JB.185.1.349-358.2003
Mair, R. W., Senadheera, D. B., and Cvitkovitch, D. G. (2012). CinA is regulated via ComX to modulate genetic transformation and cell viability in Streptococcus mutans. FEMS Microbiol. Lett. 331, 44–52. doi: 10.1111/j.1574-6968.2012.02550.x
Martin, B., Soulet, A. L., Mirouze, N., Prudhomme, M., Mortier-Barrière, I., Granadel, C., et al. (2013). ComE/ComE~P interplay dictates activation or extinction status of pneumococcal X-state (competence). Mol. Microbiol. 87, 394–411. doi: 10.1111/mmi.12104
Mashburn-Warren, L., Morrison, D. A., and Federle, M. J. (2010). A novel double-tryptophan peptide pheromone controls competence in Streptococcus spp. via an Rgg regulator. Mol. Microbiol. 78, 589–606. doi: 10.1111/j.1365-2958.2010.07361.x
Mashburn-Warren, L., Morrison, D. A., and Federle, M. J. (2012). The cryptic competence pathway in Streptococcus pyogenes is controlled by a peptide pheromone. J. Bacteriol. 194, 4589–4600. doi: 10.1128/JB.00830-12
Masure, H. R., Pearce, B. J., Shio, H., and Spellerberg, B. (1998). Membrane targeting of RecA during genetic transformation. Mol. Microbiol. 27, 845–852. doi: 10.1046/j.1365-2958.1998.00732.x
Mirouze, N., Bergé, M. A., Soulet, A. L., Mortier-Barrière, I., Quentin, Y., Fichant, G., et al. (2013). Direct involvement of DprA, the transformation-dedicated RecA loader, in the shut-off of pneumococcal competence. Proc. Natl. Acad. Sci. U.S.A. 110, E1035–E1044. doi: 10.1073/pnas.1219868110
Morrison, D. A., Guedon, E., and Renault, P. (2013). Competence for natural genetic transformation in the Streptococcus bovis group streptococci S. infantarius and S. macedonicus. J. Bacteriol. 195, 2612–2620. doi: 10.1128/JB.00230-13
Morrison, D. A., Mortier-Barrière, I., Attaiech, L., and Claverys, J. P. (2007). Identification of the major protein component of the pneumococcal eclipse complex. J. Bacteriol. 189, 6497–6500. doi: 10.1128/JB.00687-07
Mortier-Barrière, I., Velten, M., Dupaigne, P., Mirouze, N., Piétrement, O., McGovern, S., et al. (2007). A key presynaptic role in transformation for a widespread bacterial protein: DprA conveys incoming ssDNA to RecA. Cell 130, 824–836. doi: 10.1016/j.cell.2007.07.038
Perry, J. A., Jones, M. B., Peterson, S. N., Cvitkovitch, D. G., and Lévesque, C. M. (2009). Peptide alarmone signalling triggers an auto-active bacteriocin necessary for genetic competence. Mol. Microbiol. 72, 905–917. doi: 10.1111/j.1365-2958.2009.06693.x
Petersen, F. C., Tao, L., and Scheie, A. A. (2005). DNA binding-uptake system: a link between cell-to-cell communication and biofilm formation. J. Bacteriol. 187, 4392–4400. doi: 10.1128/JB.187.13.4392-4400.2005
Peterson, S., Cline, R. T., Tettelin, H., Sharov, V., and Morrison, D. A. (2000). Gene expression analysis of the Streptococcus pneumoniae competence regulons by use of DNA microarrays. J. Bacteriol. 182, 6192–6202. doi: 10.1128/JB.182.21.6192-6202.2000
Peterson, S. N., Sung, C. K., Cline, R., Desai, B. V., Snesrud, E. C., Luo, P., et al. (2004). Identification of competence pheromone responsive genes in Streptococcus pneumoniae by use of DNA microarrays. Mol. Microbiol. 51, 1051–1070. doi: 10.1046/j.1365-2958.2003.03907.x
Piotrowski, A., Burghout, P., and Morrison, D. A. (2009). spr1630 is responsible for the lethality of clpX mutations in Streptococcus pneumoniae. J. Bacteriol. 191, 4888–4895. doi: 10.1128/JB.00285-09
Reck, M., Tomasch, J., and Wagner-Döbler, I. (2015). The Alternative sigma factor SigX controls bacteriocin synthesis and competence, the two quorum sensing regulated traits in Streptococcus mutans. PLoS Genet. 11:e1005353. doi: 10.1371/journal.pgen.1005353
Saeed, A. I., Bhagabati, N. K., Braisted, J. C., Liang, W., Sharov, V., Howe, E. A., et al. (2006). TM4 microarray software suite. Methods Enzymol. 411, 134–193. doi: 10.1016/S0076-6879(06)11009-5
Saulnier, D. M., Santos, F., Roos, S., Mistretta, T. A., Spinler, J. K., Molenaar, D., et al. (2011). Exploring metabolic pathway reconstruction and genome-wide expression profiling in Lactobacillus reuteri to define functional probiotic features. PLoS ONE 6:e18783. doi: 10.1371/journal.pone.0018783
Seaton, K., Ahn, S. J., and Burne, R. A. (2015). Regulation of competence and gene expression in Streptococcus mutans by the RcrR transcriptional regulator. Mol. Oral Microbiol. 30, 147–159. doi: 10.1111/omi.12079
Seaton, K., Ahn, S. J., Sagstetter, A. M., and Burne, R. A. (2011). A transcriptional regulator and ABC transporters link stress tolerance, (p)ppGpp, and genetic competence in Streptococcus mutans. J. Bacteriol. 193, 862–874. doi: 10.1128/JB.01257-10
Seitz, P., Pezeshgi Modarres, H., Borgeaud, S., Bulushev, R. D., Steinbock, L. J., Radenovic, A., et al. (2014). ComEA is essential for the transfer of external DNA into the periplasm in naturally transformable Vibrio cholerae cells. PLoS Genet. 10:e1004066. doi: 10.1371/journal.pgen.1004066
Tian, X. L., Dong, G., Liu, T., Gomez, Z. A., Wahl, A., Hols, P., et al. (2013). MecA protein acts as a negative regulator of genetic competence in Streptococcus mutans. J. Bacteriol. 195, 5196–5206. doi: 10.1128/JB.00821-13
van Belkum, M. J., Worobo, R. W., and Stiles, M. E. (1997). Double-glycine-type leader peptides direct secretion of bacteriocins by ABC transporters: colicin V secretion in Lactococcus lactis. Mol. Microbiol. 23, 1293–1301. doi: 10.1046/j.1365-2958.1997.3111677.x
van Hijum, S. A., García de la Nava, J., Trelles, O., Kok, J., and Kuipers, O. P. (2003). MicroPreP: a cDNA microarray data pre-processing framework. Appl. Bioinformatics 2, 241–244.
Vecht, U., Wisselink, H. J., Stockhofe-Zurwieden, N., and Smith, H. E. (1996). Characterization of virulence of the Streptococcus suis serotype 2 reference strain Henrichsen S 735 in newborn gnotobiotic pigs. Vet. Microbiol. 51, 125–136. doi: 10.1016/0378-1135(96)00028-4
Vickerman, M. M., Iobst, S., Jesionowski, A. M., and Gill, S. R. (2007). Genome-wide transcriptional changes in Streptococcus gordonii in response to competence signaling peptide. J. Bacteriol. 189, 7799–7807. doi: 10.1128/JB.01023-07
Wahl, A., Servais, F., Drucbert, A. S., Foulon, C., Fontaine, L., and Hols, P. (2014). Control of natural transformation in salivarius Streptococci through specific degradation of sigmaX by the MecA-ClpCP protease complex. J. Bacteriol. 196, 2807–2816. doi: 10.1128/JB.01758-14
Weng, L., Piotrowski, A., and Morrison, D. A. (2013). Exit from competence for genetic transformation in Streptococcus pneumoniae is regulated at multiple levels. PLoS ONE 8:e64197. doi: 10.1371/journal.pone.0064197
Yang, Y. H., Dudoit, S., Luu, P., Lin, D. M., Peng, V., Ngai, J., et al. (2002). Normalization for cDNA microarray data: a robust composite method addressing single and multiple slide systematic variation. Nucleic Acids Res. 30:e15. doi: 10.1093/nar/30.4.e15
Zaccaria, E., Cao, R., Wells, J. M., and van Baarlen, P. (2016a). A zebrafish larval model to assess virulence of porcine Streptococcus suis strains. PLoS ONE 11:e0151623. doi: 10.1371/journal.pone.0151623
Zaccaria, E., van Baarlen, P., de Greeff, A., Morrison, D. A., Smith, H., and Wells, J. M. (2014). Control of competence for DNA transformation in Streptococcus suis by genetically transferable pherotypes. PLoS ONE 9:e99394. doi: 10.1371/journal.pone.0099394
Keywords: Streptococcus suis, competence, DNA transformation, sigma factor X, fratricin, pillus, bacteriocins
Citation: Zaccaria E, Wels M, van Baarlen P and Wells JM (2016) Temporal Regulation of the Transformasome and Competence Development in Streptococcus suis. Front. Microbiol. 7:1922. doi: 10.3389/fmicb.2016.01922
Received: 02 May 2016; Accepted: 16 November 2016;
Published: 20 December 2016.
Edited by:
Thomas Dandekar, University of Würzburg, GermanyReviewed by:
Sascha Brunke, Hans Knöll Institute (LG), GermanyShivangi Agarwal, Northwestern University, USA
Copyright © 2016 Zaccaria, Wels, van Baarlen and Wells. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jerry M. Wells, amVycnkud2VsbHNAd3VyLm5s
 Edoardo Zaccaria
Edoardo Zaccaria Michiel Wels2
Michiel Wels2 Peter van Baarlen
Peter van Baarlen Jerry M. Wells
Jerry M. Wells