- 1Frontiers Science Center for Deep Ocean Multispheres and Earth System, College of Marine Life Sciences, Ocean University of China, Qingdao, China
- 2State Key Laboratory of Microbial Technology, Marine Biotechnology Research Center, Shandong University, Qingdao, China
- 3Laboratory for Marine Biology and Biotechnology, Pilot National Laboratory for Marine Science and Technology, Qingdao, China
- 4School of Bioengineering, Qilu University of Technology, Jinan, China
- 5School of Life Sciences, University of Warwick, Coventry, United Kingdom
Dimethylsulfide (DMS) and dimethylsulfoxide (DMSO) are widespread in marine environment, and are important participants in the global sulfur cycle. Microbiol oxidation of DMS to DMSO represents a major sink of DMS in marine surface waters. The SAR11 clade and the marine Roseobacter clade (MRC) are the most abundant heterotrophic bacteria in the ocean surface seawater. It has been reported that trimethylamine monooxygenase (Tmm, EC 1.14.13.148) from both MRC and SAR11 bacteria likely oxidizes DMS to generate DMSO. However, the structural basis of DMS oxidation has not been explained. Here, we characterized a Tmm homolog from the SAR11 bacterium Pelagibacter sp. HTCC7211 (Tmm7211). Tmm7211 exhibits DMS oxidation activity in vitro. We further solved the crystal structures of Tmm7211 and Tmm7211 soaked with DMS, and proposed the catalytic mechanism of Tmm7211, which comprises a reductive half-reaction and an oxidative half-reaction. FAD and NADPH molecules are essential for the catalysis of Tmm7211. In the reductive half-reaction, FAD is reduced by NADPH. In the oxidative half-reaction, the reduced FAD reacts with O2 to form the C4a-(hydro)peroxyflavin. The binding of DMS may repel the nicotinamide ring of NADP+, and make NADP+ generate a conformational change, shutting off the substrate entrance and exposing the active C4a-(hydro)peroxyflavin to DMS to complete the oxidation of DMS. The proposed catalytic mechanism of Tmm7211 may be widely adopted by MRC and SAR11 bacteria. This study provides important insight into the conversion of DMS into DMSO in marine bacteria, leading to a better understanding of the global sulfur cycle.
Introduction
Dimethylsulfide (DMS), one of the major biogenic sulfur species emitted into the atmosphere from oceans, is an important participant in the global sulfur cycle (Andreae, 1990; Simo, 2001; Zhang et al., 2019). Approximately 300 Tg of DMS is produced annually mainly by dimethylsulfoniopropionate (DMSP) cleavage through various DMSP lyases (Curson et al., 2011; Johnston et al., 2016), among which 13–37 Tg is transferred into the atmosphere through ocean-atmosphere sulfur flux (Ksionzek et al., 2016). In the air, DMS may contribute to the formation of the cloud condensation nuclei and thus act as a global coolant (Charlson et al., 1987; Lidbury et al., 2016). DMS loss in marine surface waters is mediated by different processes, including photochemical oxidation and biological consumption, with the latter being a major component of the global sink for DMS (Brimblecombe and Shooter, 1986; Kiene and Bates, 1990; Lidbury et al., 2016). Microorganisms can transform DMS into different compounds, such as dimethylsulfoxide (DMSO), methanethiol, sulfate, thiosulfate and tetrathionate (deZwart et al., 1996; Vila-Costa et al., 2006; del Valle et al., 2007; Boden et al., 2010, 2011; Lidbury et al., 2016). In surface seawater, microbial oxidation to DMSO is a major fate of DMS (Lidbury et al., 2016), which accounts for approximately 70% of the total oxidized DMS in the Sargasso Sea (del Valle et al., 2007). DMSO is ubiquitous in aquatic environments, and is likely to function as cryoprotectant, free-radical scavenger or intracellular electrolyte modifier in marine organisms (Lee and De Mora, 1999; Asher et al., 2017; Speeckaert et al., 2018).
The SAR11 clade and the marine Roseobacter clade (MRC) are the most abundant heterotrophic bacteria in the ocean surface seawater, and are active participants in marine carbon, nitrogen, sulfur, and phosphorus cycles (Morris et al., 2002; Buchan et al., 2005; Rusch et al., 2007; Chen, 2012; Carini et al., 2015; Sebastián et al., 2016; Tsementzi et al., 2016). Previous studies have shown that trimethylamine monooxygenase (Tmm, EC 1.14.13.148) from both MRC and SAR11 bacteria likely oxidizes DMS to generate DMSO (Chen et al., 2011; Lidbury et al., 2016). It is estimated that ∼20% of the bacteria in the surface ocean contain tmm homologs (Chen et al., 2011). Physiological experiments demonstrated that MRC can oxidize DMS to DMSO using Tmm (Lidbury et al., 2016), and it is deduced that SAR11 bacteria may also play a vital role in the conversion of DMS to DMSO in marine environment (Chen et al., 2011; Lidbury et al., 2016). However, the catalytic mechanism underpinning DMS oxidation to DMSO by Tmm remains understudied.
Tmm is a bacterial flavin-containing monooxygenase (FMO), which belongs to the class B flavoprotein monooxygenases (Chen et al., 2011; Paul et al., 2021). FMOs are a widespread class of enzymes that are involved in the metabolism of xenobiotics (Cho et al., 2011). FMOs oxygenate a wide range of substrates, such as nitrogen-containing and sulfur-containing compounds (van Berkel et al., 2006). Tmm is also reported to act on various substrates, including trimethylamine (TMA), dimethylamine (DMA), DMS, indole, and methimazole (Chen et al., 2011). The catalytic process of Tmm to oxidize TMA, indole or methimazole can be divided into two half-reactions: a reductive half-reaction followed by an oxidative half-reaction (Beaty and Ballou, 1981a,b; Cho et al., 2011; Li et al., 2017). In the reductive half-reaction, the cofactor flavin adenine dinucleotide (FAD) is reduced by NADPH. In the oxidative half-reaction, the reduced FAD reacts with an oxygen molecule, generating the C4a-(hydro)peroxyflavin, which is relatively stable in vitro (Alfieri et al., 2008). An oxygen atom from the C4a-(hydro)peroxyflavin is transferred to the substrate to complete the oxidation cycle (Alfieri et al., 2008; Orru et al., 2010). However, the detailed structural basis for DMS oxidation is still lacking. Considering the important roles of DMS and DMSO in the global sulfur cycle, the structural basis of DMS oxidation to DMSO by Tmm warrants further investigation.
The SAR11 bacterium Pelagibacter sp. HTCC7211 was isolated from the oligotrophic Sargasso Sea (Sun et al., 2011). It has been reported that the recombinant Tmm from strain HTCC7211 (Tmm7211) could catalyze the oxidation of TMA to trimethylamine N-oxide (TMAO) (Chen et al., 2011). In this study, the Tmm7211 gene was synthesized and over-expressed in Escherichia coli. The recombinant Tmm7211 also exhibits DMS oxidation activity in vitro. The crystal structures of Tmm7211 and Tmm7211 soaked with DMS were solved. The catalytic mechanism of DMSO production through DMS oxidation was proposed by structural analyses and mutational assays.
Materials and Methods
Gene Cloning, Point Mutations, and Protein Expression and Purification
The 1335-bp full-length tmm gene from Pelagibacter sp. HTCC7211 was synthesized by the Beijing Genomics Institute (China). The gene was then subcloned into the pET28a (Novagen, United States) vector with an N-terminal His tag. The point mutations in Tmm7211 were introduced using PCR-based method and verified by DNA sequencing. The Tmm7211 protein and its mutants were expressed in E. coli BL21 (DE3). The cells were cultured at 37°C in Lysogeny Broth medium to an OD600 of 0.8–1.0 and then induced at 20°C for 14 h with 0.5 mM isopropyl β-D-1-thiogalactopyranoside (IPTG). The proteins were purified first with Ni2+-NTA resin (Qiagen, Germany) and then fractionated on a Superdex-200 column (GE Healthcare, United States). The protein concentration was determined with the Pierce BCA Protein Assay Kit (Thermo Fisher Scientific, United States), and a nine-point calibration curve of bovine serum albumin (BSA) standards was used according to the user guide.
Gel Filtration Analysis
A Superose 6 column was used for gel filtration analysis, because it possesses a wider fractionation range than the Superdex-200 column. The Superose 6 column was calibrated in the buffer containing 10 mM Tris-HCl (pH 8.0) and 100 mM NaCl using the following standards from GE Healthcare: thyroglobulin (669 kDa), ferritin (440 kDa), aldolase (158 kDa), conalbumin (75 kDa), carbonic anhydrase (29 kDa), ribonuclease A (13.7 kDa), and aprotinin (6.5 kDa). The void volume of Superose 6 column was determined with Blue Dextran 2000 (2,000 kDa).
Spectrophotometric Analysis
The UV spectra of Tmm7211 (0.1 mM protein in the buffer containing 10 mM Tris-HCl (pH 8.0) and 100 mM NaCl) were measured by a V550 UV/VIS spectrophotometer (Jasco, Japan) in a cell with 1.0 cm path length (Response: Medium; Band width: 1.0 nm). The spectra of the mixture of Tmm7211 and NADPH were measured immediately after NADPH (0.1 mM) was added into the protein solution.
High Performance Liquid Chromatography Analysis
The DMSO produced by the enzymatic activity of Tmm7211 toward DMS was measured by high performance liquid chromatography (HPLC) (Dionex, America) on a SunFire C18 column (Waters, America). The detection wavelength was 210 nm because DMSO exhibited an absorbance maximum at ∼210 nm. The samples were eluted in HPLC buffer (2.5% (v/v) acetonitrile, 0.2% (v/v) phosphoric acid in double-distilled H2O) over 20 min at a flow rate of 1 ml/min. The reaction system contained 6 mM DMS (Sigma-Aldrich, America), 1.5 mM NADPH (Sigma-Aldrich, America), 0.15 mM Tmm7211, 10 mM Tris-HCl (pH 7.0) and 100 mM NaCl. The reaction was performed at 25°C, pH 7.0 for 3 h, and terminated by adding 10% phosphoric acid. The reaction system was centrifuged at 15,000 g for 15 min, and then the supernatant (20 μl) was injected for HPLC analysis. The control group had the same reaction system except that Tmm7211 was not added.
Enzyme Assays
In the absence of DMS, the consumption of NADPH was less than 3% of that in the presence of DMS, indicating the NADPH-oxidase activity (also known as uncoupling) of Tmm7211 is rather weak under the experimental conditions. Because monitoring NADPH oxidation is more sensitive than monitoring DMSO formation, the enzymatic activity of Tmm7211 was measured by following the decrease of absorbance at 340 nm (ε340 = 6.22 mM–1 cm–1 for NADPH) (Alfieri et al., 2008). The reaction mixture for detecting the enzymatic activity of Tmm7211 contains 1 μM Tmm7211, 0.25 mM NADPH, 1 mM DMS, 10 mM Tris-HCl (pH 7.0) and 100 mM NaCl. The reaction mixture without Tmm7211 was set as the control. For the measurements of the apparent KM values of Tmm7211, substrate (DMS, TMA, DMA or methimazole) of different concentrations was added into the reaction system containing 1 μM Tmm7211 and 0.25 mM NADPH. For the measurements of the apparent KM values of Tmm7211 and its mutants toward NADPH, different concentrations of NADPH were added into the reaction system containing 1.5 μM purified enzyme and 1 mM DMS. The optimal pH and the optimal temperature of Tmm7211 were determined using DMS as the substrate. For measurement of the optimal temperature of Tmm7211, a buffer containing 10 mM Tri-HCl (pH 8.0) and 100 mM NaCl was pre-incubated at different temperatures for 30 min, and then 1 μM Tmm7211, 0.25 mM NADPH and 1 mM DMS were added into the buffer. The mixture was incubated at different temperatures for 6 min before detection of NADPH oxidation at 340 nm using a V550 UV/VIS spectrophotometer (Jasco, Japan). The optimum of pH was examined at 25°C (the optimal temperature for Tmm7211 enzymatic activity) using Bis-Tris buffer for pH 6–7, Tris buffer for pH 7–9 and glycine buffer for pH 9–10.
Crystallization and Data Collection
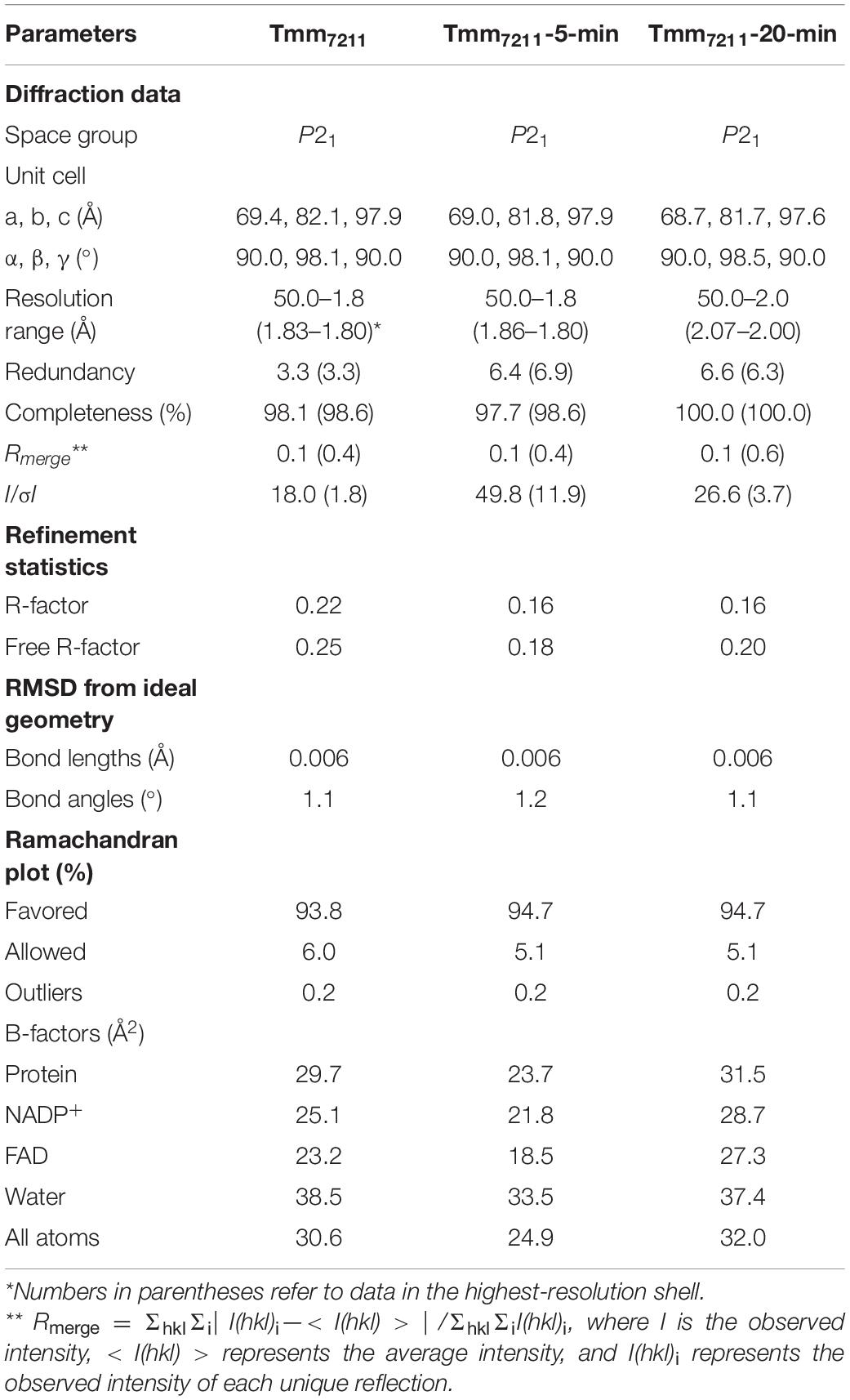
The purified Tmm7211 protein was concentrated to ∼8 mg ml–1 in 10 mM Tris-HCl (pH 8.0) and 100 mM NaCl. To obtain crystals of Tmm7211, NADPH with a final concentration of 5 mM was added into the protein solution before crystallization. Initial crystallization trials for Tmm7211 were performed at 18°C using the sitting drop vapor diffusion method. Diffraction-quality crystals of Tmm7211 were obtained in hanging drops containing 0.2 M ammonium citrate tribasic, 0.1 M imidazole (pH 7.0) and 20% (w/v) polyethylene glycol monomethyl ether 2,000 at 18°C after a 3-week incubation. To obtain the crystals of Tmm7211 soaked with DMS, Tmm7211 crystals were soaked in 20 mM DMS for 5 and 20 min, respectively. X-ray diffraction data were collected on the BL17U1 (Wang et al., 2018) and BL18U1 beamlines at the Shanghai Synchrotron Radiation Facility. The initial diffraction data sets were processed using the HKL3000 program with its default settings (Minor et al., 2006).
Structure Determination and Refinement
The crystals of Tmm7211 and Tmm7211 soaked with DMS belong to the P21 space group. The crystal structures of Tmm7211 and Tmm7211 soaked with DMS were determined by molecular replacement using the CCP4 program phaser (Winn et al., 2011) with the crystal structure of a bacterial Tmm (PDB code: 5IPY) as the search model. The refinement of these structures were performed using WinCoot (Emsley et al., 2010) and Phenix (Adams et al., 2010). Default parameters in CCP4, WinCoot and Phenix were used. All the structure figures were processed using the program PyMOL.1
Circular-Dichroism Spectroscopic Assays
CD spectroscopic assays for Tmm7211 and all its mutants were carried out on a J-1,500 Spectrometer (Jasco, Japan) in a 1 mm pathlength cuvette at 25°C. The concentration of the proteins was 8.0 μM in the buffer of 10 mM Tris-HCl (pH 8.0) containing 100 mM NaCl. The buffer without proteins was used for baseline and blank measurements. The spectra were collected from 250 to 200 nm at a scan speed of 500 nm min–1 with a band width of 1 nm. Each sample was scanned for three times. The noise level is < 0.05 mdeg.
Coexistence Analysis of Enzymes Involved in Dimethylsulfide Metabolism
Related protein sequences DddD (Pseudomonas putida, WP_062573753.1), DddK (Candidatus Pelagibacter ubique HTCC1062, WP_011281678.1), DddP (Mesorhizobium, WP_109668646.1), DddQ (Mesorhizobium loti, WP_10966 8666.1), DddW (Ruegeria pomeroyi, WP_011046214.1), DddL (Puniceibacterium antarcticum SM1211, WP_099909581.1), DddY (Alcaligenes faecalis, WP_123051132.1), DMSOR (Rhodobacter capsulatus, Q52675.2), Tmm (Pelagibaca abyssi, APZ51459.1), DdhA (Sagittula stellata E-37, EBA07058.1), MddA (Pseudomonas deceptionensis, WP_048359798.1), DsoB (Acinetobacter sp. 20B, BAA23331.1) and DmoA (Hyphomicrobium sulfonivorans, E9JFX9.1) were obtained from National Center for Biotechnology Information (NCBI) database2 as seed sequences. For multifunctional strains screening, the seed sequences were used to search against the genomes of isolated strains on the IMG/M metagenomics database (Chen et al., 2019) with parameters of similarity > 40%, E-value of < 10–50 and coverage > 70% to elevate the accuracy and precision of blast hits. Data processing was performed via scripts compiled in Python code.3 The biological networks of related proteins were built via software Cytoscape 3.8.0 (Kohl et al., 2011).
Accession Numbers
The structures of Tmm7211, Tmm7211-5-min and Tmm7211-20-min have been deposited in the Protein Data Bank (PDB) under the accession codes 7D4K, 7D4M, and 7D4N, respectively.
Results
Expression and Characterization of Tmm7211
The tmm gene of Pelagibacter sp. HTCC7211 contains 1335 nucleotides and encodes a protein of 444 amino acid residues, with a calculated molecular mass of 52 kDa. Tmm7211 shares ∼53% amino acid sequence identity with RnTmm, a previously reported Tmm homolog from an MRC strain Roseovarius nubinhibens ISM (Li et al., 2017). Full-length tmm of strain HTCC7211 was synthesized and was expressed in E. coli BL21 (DE3) cells, and the recombinant Tmm7211 was purified (Figure 1A) and characterized. The purified Tmm7211 is yellow, suggesting that FAD has been already bound in the recombinant Tmm7211 during protein expression in E. coli, which is further supported by spectroscopic analysis. The purified Tmm7211 exhibited the typical absorbance maxima (around 372 and 442 nm) of fully oxidized FMOs (Figure 1B; Alfieri et al., 2008; Orru et al., 2010). Addition of equimolar amount of NADPH should lead to the formation of the enzyme-(hydro)peroxyflavin-NADP+ complex exhibiting a typical absorbance maximum at around 360 nm (Alfieri et al., 2008; Orru et al., 2010). However, the absorption spectrum showed that the absorbance maximum of Tmm7211 with the addition of equimolar amount of NADPH was around 350 nm (Figure 1B). Because NADPH absorbs at 340 nm (Alfieri et al., 2008), this spectrum probably reflected a mixture of the enzyme-(hydro)peroxyflavin-NADP+ complex and some residual NADPH, which may due to some inactive enzymes in the purified Tmm7211 solution. Incubation of recombinant Tmm7211 with DMS and NADPH yielded DMSO and NADP+ (Figure 1C), demonstrating that Tmm7211 has DMS oxidation activity in vitro. The optimal temperature for Tmm7211 enzymatic activity toward DMS was ∼25°C (Figure 1D), and the optimal pH was 7.0 (Figure 1E). The optimal temperature of Tmm7211 is lower than that of RnTmm toward TMA (30°C) (Li et al., 2017). Furthermore, Tmm7211 only retained ∼40% of its highest enzymatic activity at 30°C, whereas RnTmm still retained ∼70% of its highest enzymatic activity at 40°C (Li et al., 2017), suggesting that Tmm7211 is more sensitive to high temperature than RnTmm.
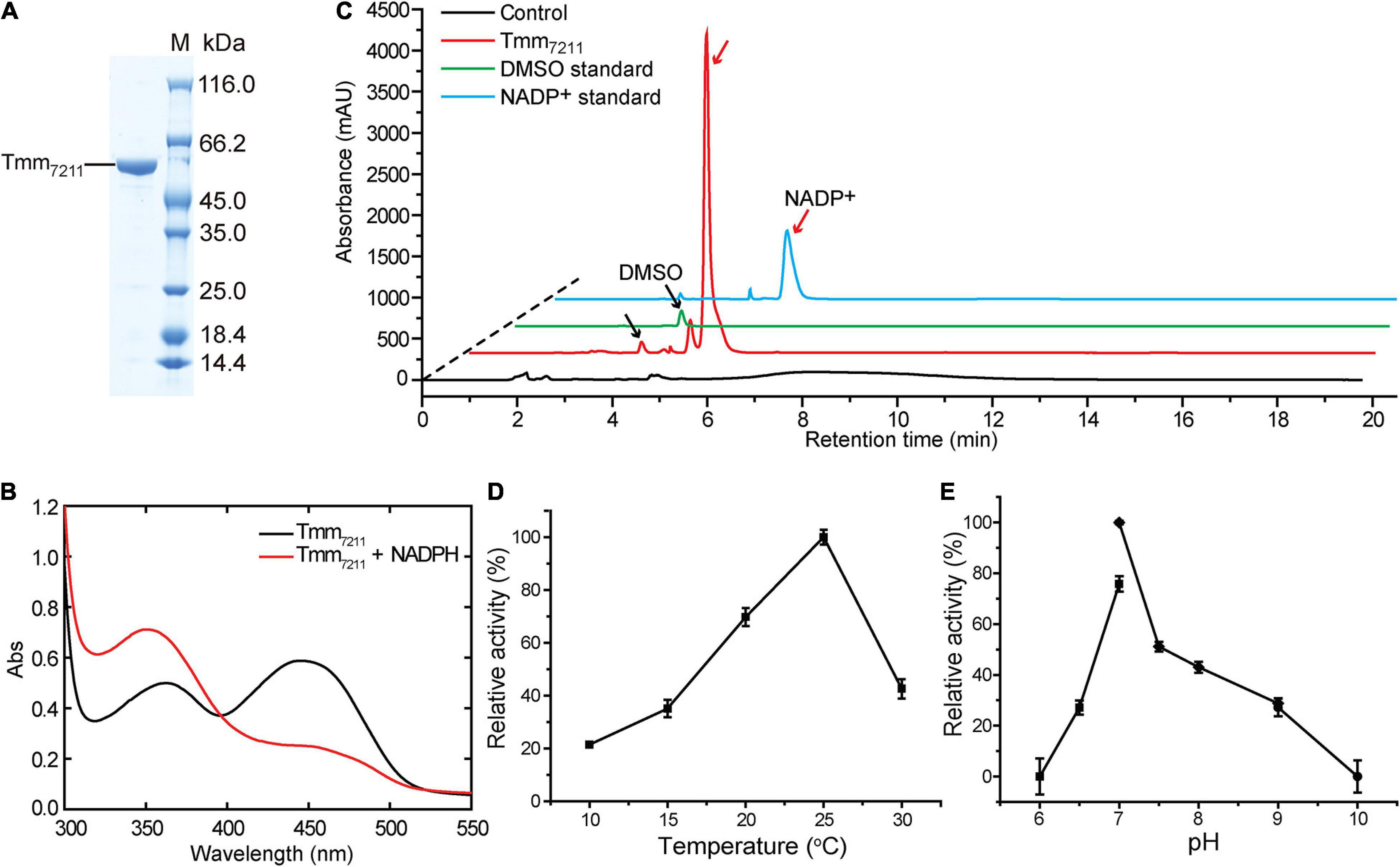
Figure 1. Characterization of Tmm7211. (A) SDS-PAGE analysis of the purified Tmm7211 protein. (B) Absorbance spectra of Tmm7211. Black line, the absorbance spectrum of the purified Tmm7211. Red line, the absorbance spectrum of the purified Tmm7211 with the addition of equimolar amount of NADPH (0.1 mM) under aerobic conditions. (C) HPLC assay of the enzymatic activity of the recombinant Tmm7211 on DMS. The peaks of DMSO and NADP+ monitored at 210 nm were indicated with black and red arrows, respectively. The reaction system without Tmm7211 was used as the control. The DMSO (1.25 mM) and NADP+ (0.4 mM) standards were used as positive controls. (D) Effect of temperature on the enzymatic activity of Tmm7211. (E) Effect of pH on the enzymatic activity of Tmm7211. The optimal pH was examined at 25°C using Bis-Tris buffer for pH 6–7, Tris buffer for pH 7–9 and glycine buffer for pH 9–10. The standard errors are from three independent experiments.
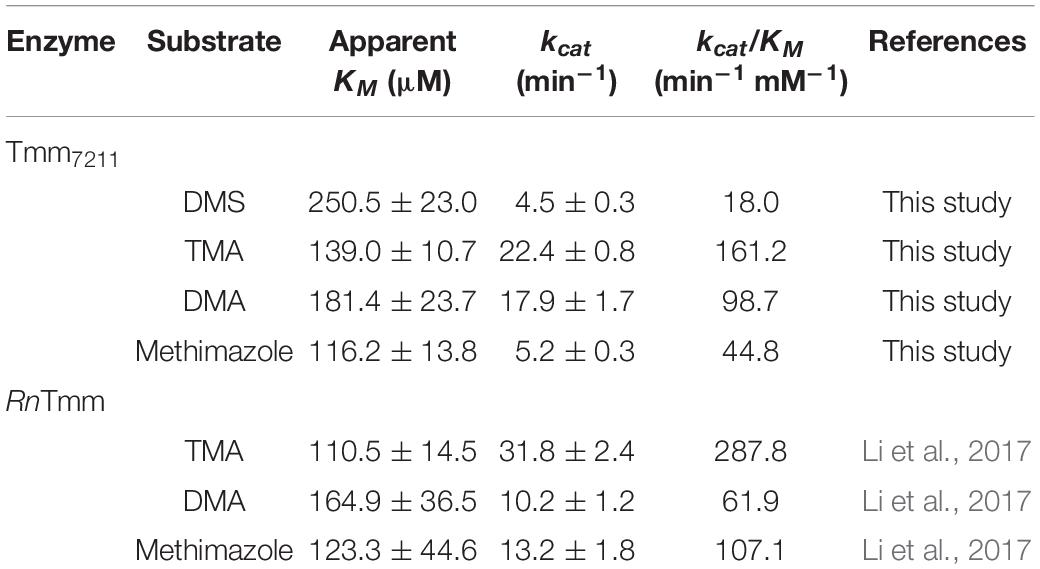
The substrate specificity of Tmm7211 was also analyzed. Tmm7211 can oxidize DMS, TMA, DMA, and methimazole, with TMA showing the highest affinity (Table 1). In general, the apparent KM values of Tmm7211 to different substrates are slightly higher than those of RnTmm, and the kcat values of Tmm7211 are lower (Table 1; Li et al., 2017), indicating that the enzymatic activity of Tmm7211 is lower than that of RnTmm in vitro.
Overall Structure of Tmm7211
To gain insight into the putative active site of Tmm7211, we solved the crystal structure of Tmm7211 to 1.8 Å (Table 2). The crystals of Tmm7211 belong to the P21 space group, with two molecules arranged as a dimer in an asymmetric unit (Figures 2A,B). Gel filtration analysis (Figure 2C) indicated that Tmm7211 functions as a dimer in solution, which is supported by the result of the PISA server prediction.4 After structural refinement, the NADP+ and FAD molecules can be clearly observed in the structure (Figure 2A). The overall structure of Tmm7211 is similar to those of other reported bacterial FMOs (Alfieri et al., 2008; Cho et al., 2011; Li et al., 2017), with the root mean square deviations (RMSDs) between Tmm7211 and other bacterial FMOs of no more than 0.6 Å. Tmm7211 also comprises an NADPH binding domain and an FAD binding domain (Figure 2B). These two domains are connected through two hinge regions (Ser163–Pro168 and Cys268–Leu272) (Figure 2B).
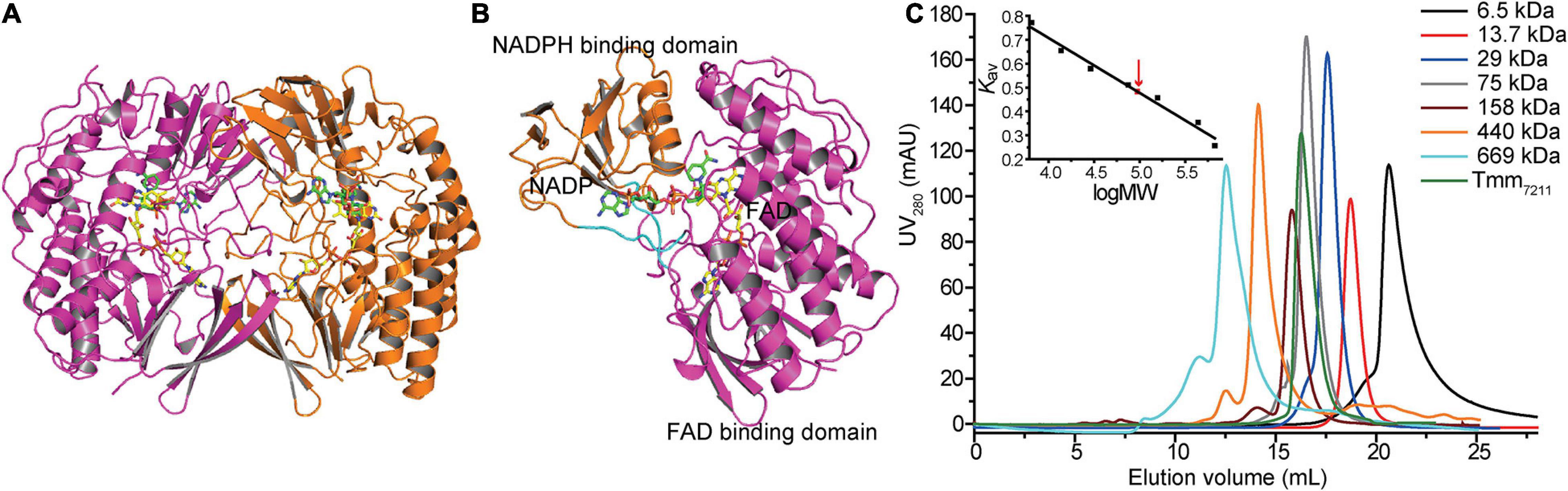
Figure 2. Overall structure of Tmm7211. (A) Two monomers of Tmm7211 arranged as a dimer in an asymmetric unit. The monomers are colored in magenta and orange, respectively. (B) The overall structure of Tmm7211 monomer. Tmm7211 contains an NADPH binding domain (colored in orange) and a FAD binding domain (colored in magenta) connected through two hinge regions (colored in cyan). The NADP+ molecule and the FAD molecule are shown as sticks colored in green and yellow, respectively. (C) Gel filtration analysis of Tmm7211. Inset, semilog plot of the molecular mass of all standards used vs. their Kav values (black squares). The red arrow indicates the position of the Kav value of Tmm7211 (0.48) interpolated in the regression line. Tmm7211 monomer has a calculated molecular mass of 52 kDa. The apparent molecular mass of Tmm7211 is 95 kDa, indicating that Tmm7211 is a dimer in solution.
To obtain the crystal structure of Tmm7211 in complex with DMS, we first tried to co-crystalize Tmm7211 with DMS. However, this failed, probably due to the volatile nature of DMS that has a low boiling point (∼37°C) in the crystallization buffer. Next, we tried the soaking method and solved two crystal structures of Tmm7211 soaked with DMS for different soaking time (Table 2). For briefness, the crystal structures of Tmm7211 soaked with DMS for 5 min and for 20 min were termed as Tmm7211-5-min and Tmm7211-20-min, respectively. The overall structures of Tmm7211 soaked with DMS are similar to that of Tmm7211, with the RMSD between Tmm7211 and Tmm7211-5-min of 0.1 Å, and the RMSD between Tmm7211 and Tmm7211-20-min of 0.2 Å.
Residues Involved in Binding NADP+ and Flavin Adenine Dinucleotide
From the surface view of Tmm7211, we can only observe part of the NADP+ molecule and the FAD molecule was not visible (Figure 3A). The nicotinamide ring of NADP+ is located inside Tmm7211, and the entire FAD molecule is deeply bound in the protein (Figures 3A,B). The binding of NADP+ and FAD mainly depends on hydrogen bonds formed between Tmm7211 residues and them (Figures 3C,D). For NADP+ binding, residues Trp70 and Arg409 form hydrogen bonds with the nicotinamide ring, Asn72 and Gln315 interact with the ribose ring via water-mediated hydrogen-bonds, and Tyr170, Ser202, Ser203, Ser205, Arg226, His227, and Asn288 interact with the other parts of NADP+ (Figure 3C). For FAD binding, residues Asn72 and Thr318 interact with the isoalloxazine ring, Glu37 forms hydrogen bonds with the ribose ring, Val125 forms a hydrogen bond with the adenine moiety, and Gly10, Leu45, Trp46, Gly160, Ser163, and Gln315 interact with the other parts of FAD (Figure 3D).
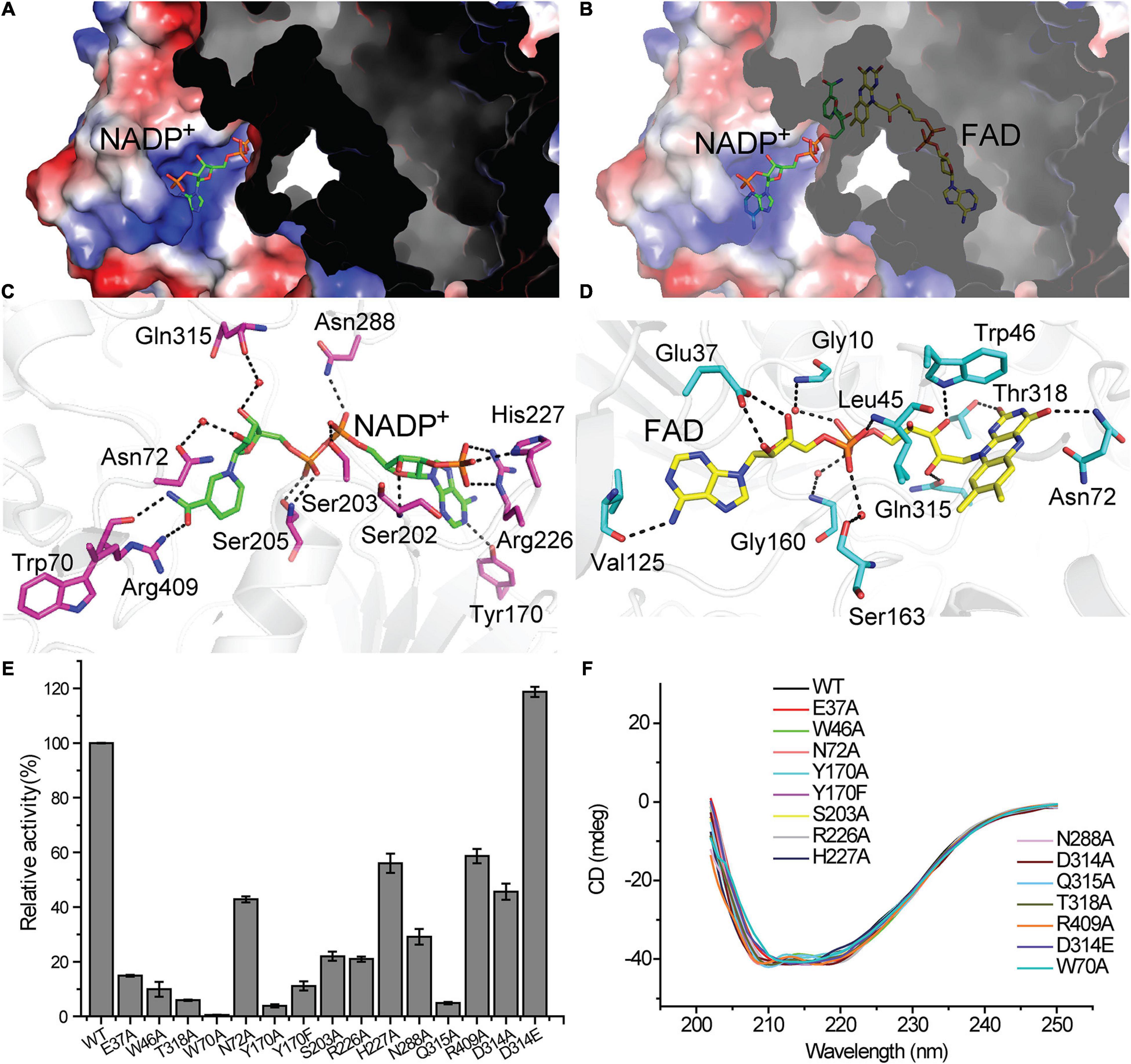
Figure 3. The binding of the NADP+ molecule and the FAD molecule in Tmm7211. The NADP+ molecule and the FAD molecule are shown as sticks colored in green and yellow, respectively. (A) Electrostatic surface of Tmm7211. The NADP+ molecule is partially visible through the surface. (B) Electrostatic surface of Tmm7211 after the transparency of surface was set to 40%. (C) Interactions between NADP+ and Tmm7211 residues. (D) Interactions between FAD and Tmm7211 residues. Water molecules are shown in red dots. The possible hydrogen bonds are represented by dashed lines. (E) The enzymatic activities of WT Tmm7211 and its mutants. The activity of WT Tmm7211 is taken as 100%. The standard errors are from three independent experiments. (F) CD spectra of WT Tmm7211 and its mutants.
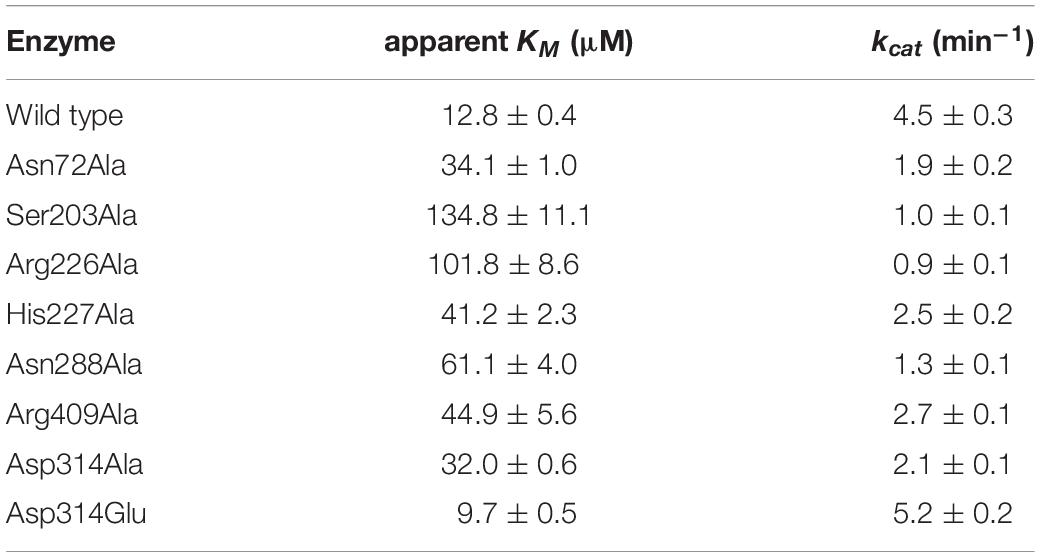
To confirm the importance of Tmm7211 residues involved in binding NADP+ and FAD, we generated site-directed mutations to the related residues and quantified the enzymatic activities of the mutants. All mutants had significantly decreased activity (Figure 3E), suggesting that these residues play important roles for the correct binding of NADP+ or FAD. Moreover, mutants Asn72Ala, Ser203Ala, Arg226Ala, His227Ala, Asn288Ala, and Arg409Ala all exhibited higher apparent KM values toward NADPH than wild type (WT) Tmm7211 (Table 3), further supporting their roles in binding NADP+/NADPH. CD spectroscopy analysis showed that the secondary structures of the mutants exhibited little deviation from that of WT Tmm7211 (Figure 3F), indicating that the decreases in the enzymatic activities of the mutants are caused by residue replacement rather than structural changes.
Conformational Change of NADP+ During Soaking Dimethylsulfide
To elucidate the catalytic mechanism of Tmm7211 for DMS oxidation, it is important to ascertain the location of DMS. Despite the two structures of Tmm7211 soaked with DMS were solved, the explicit electron density of DMS in the structures could not be identified. Previous structural analyses demonstrated that the substrate of bacterial FMOs with a ring structure, such as indole and methimazole, is located in the position of the nicotinamide ring of NADP+, forming stacking interactions with the isoalloxazine ring of FAD (Eswaramoorthy et al., 2006; Cho et al., 2011; Li et al., 2017). There is no direct interaction between the residues of bacterial FMOs and the substrates (Eswaramoorthy et al., 2006; Cho et al., 2011; Li et al., 2017). For substrates with no ring structure, such as DMS and TMA, there may be no effective interactions to stabilize their conformations, which may be the reason why we could not find the DMS molecule in the structures of Tmm7211 soaked with DMS.
By comparing the structures of Tmm7211, Tmm7211-5-min and Tmm7211-20-min, we noticed that with the extension of soaking time, the electron densities of the nicotinamide ring and the ribose ring of NADP+ become increasingly weaker (Figures 4A–C), indicating that the nicotinamide ring and the ribose ring become flexible during soaking. Combined with previous studies that the indole and methimazole molecules located in the binding site of NADP+ (Eswaramoorthy et al., 2006; Cho et al., 2011; Li et al., 2017), this result suggests that DMS may also be bound in the position of the nicotinamide ring of NADP+, and that the entry of DMS may repel NADP+, leading to a conformational change of the nicotinamide ring and the ribose ring.
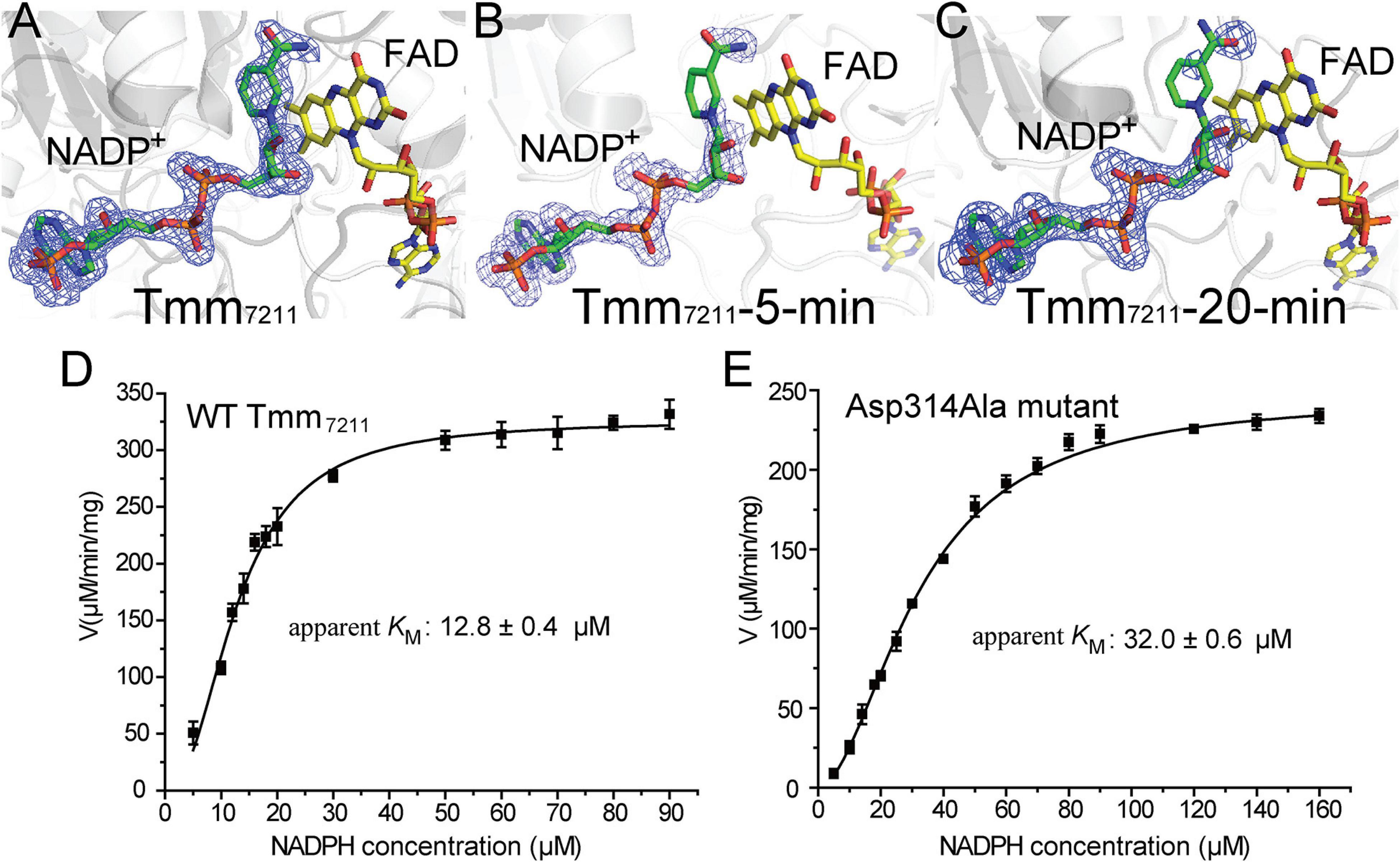
Figure 4. Analysis of the conformational change of NADP+. (A) Structure of Tmm7211. (B) Structure of Tmm7211-5-min. (C) Structure of Tmm7211-20-min. The NADP+ molecule and the FAD molecule are shown as sticks colored in green and yellow, respectively. The Fo-Fc densities for NADP+ are contoured in blue meshes at 3.0σ. (D) Kinetic analysis of WT Tmm7211 toward NADPH. (E) Kinetic analysis of the mutant Asp314Ala toward NADPH.
The conformational change of NADP+ was also observed in RnTmm when soaked with TMA, and this conformational change was shown to be important for TMA oxidation (Li et al., 2017). After conformational change, the ribose ring of NADP+ in RnTmm forms a hydrogen bond with Asp317, shutting off the substrate entrance to promote a protected micro-environment for catalysis (Li et al., 2017). Because the electron densities of the ribose ring in Tmm7211-5-min and Tmm7211-20-min are rather poor (Figures 4B,C), we could not ascertain whether the ribose ring can form a hydrogen bond with Asp314 of Tmm7211, the equivalent residue to Asp317 of RnTmm. To further probe this, we generated mutants Asp314Ala and Asp314Glu, and measured their enzymatic activities. The enzymatic activity and the apparent KM of Tmm7211 toward NADPH are only slightly affected by Asp314Glu mutation (Figure 3E and Table 3), probably due to the similar properties of aspartic acid and glutamic acid. However, although the residue Asp314 is far away from the catalytic center of Tmm7211, the mutation of Asp314 to alanine decreased the activity of Tmm7211 significantly (Figure 3E), suggesting that Asp314 is involved in the catalytic reaction of Tmm7211. The CD spectrum of the mutant Asp314Ala was indistinguishable from that of WT Tmm7211, suggesting that the enzymatic activity loss in the mutant is caused by residue replacement rather than structural alteration of the enzyme (Figure 3F). In addition, the Asp314Ala mutation increased the apparent KM of Tmm7211 toward NADPH (Figures 4D,E), suggesting that this residue participates in binding NADP+/NADPH. Because structural analysis shows that the residue Asp314 is too far away to participate in NADP+ binding before DMS enters the catalytic center (Figure 3C), this result suggests that, after DMS enters the catalytic center, NADP+ in Tmm7211 likely undergoes a conformational change and forms a new hydrogen bond with Asp314, which is important for the catalysis of DMS oxidation.
Discussion
DMS and DMSO are widespread in marine environment, and the oxidation of DMS to DMSO is an important biogeochemical reaction. Tmm7211 is a bacterial FMO which is able to catalyze the conversion of DMS to DMSO. Based on our results and previous studies of bacterial FMOs (Alfieri et al., 2008; Cho et al., 2011; Li et al., 2017), the structural basis of Tmm7211 for DMS oxidation to DMSO is proposed (Figure 5). The catalytic cycle of Tmm7211 consists of a reductive half-reaction and an oxidative half-reaction, similar to other bacterial FMOs (Alfieri et al., 2008; Cho et al., 2011; Li et al., 2017). In the reductive half-reaction, FAD is reduced by NADPH. In the oxidative half-reaction, the reduced FAD reacts with an oxygen molecule and the (hydro)peroxyflavin intermediate forms (Figures 1B, 5), which is a common trait for FMOs (Krueger and Williams, 2005; Eswaramoorthy et al., 2006; Alfieri et al., 2008). The nicotinamide ring of NADP+ buried in Tmm7211 protects the C4a-(hydro)peroxyflavin from solvent attack (Beaty and Ballou, 1981b; Li et al., 2017). The (hydro)peroxyflavin intermediate is stable in cells and behaves like a “cocked gun,” awaiting a suitable substrate (Alfieri et al., 2008; Lidbury et al., 2016). After entering the catalytic pocket, DMS may occupy the position of the nicotinamide ring of NADP+ and likely makes NADP+ generate a conformational change, leading to two consequences: (1), NADP+ forms a hydrogen bond with Asp314, shutting off the substrate entrance and creating a protected micro-environment for catalysis; (2), the nicotinamide ring of NADP+ no longer protects the C4a-(hydro)peroxyflavin, exposing the active C4a-(hydro)peroxyflavin to DMS to complete the oxidation cycle (Figure 5). After the reaction, DMSO, NADP+ and a water molecule are released and the oxidized FAD is regenerated, enabling Tmm7211 to get ready for the next catalytic cycle.
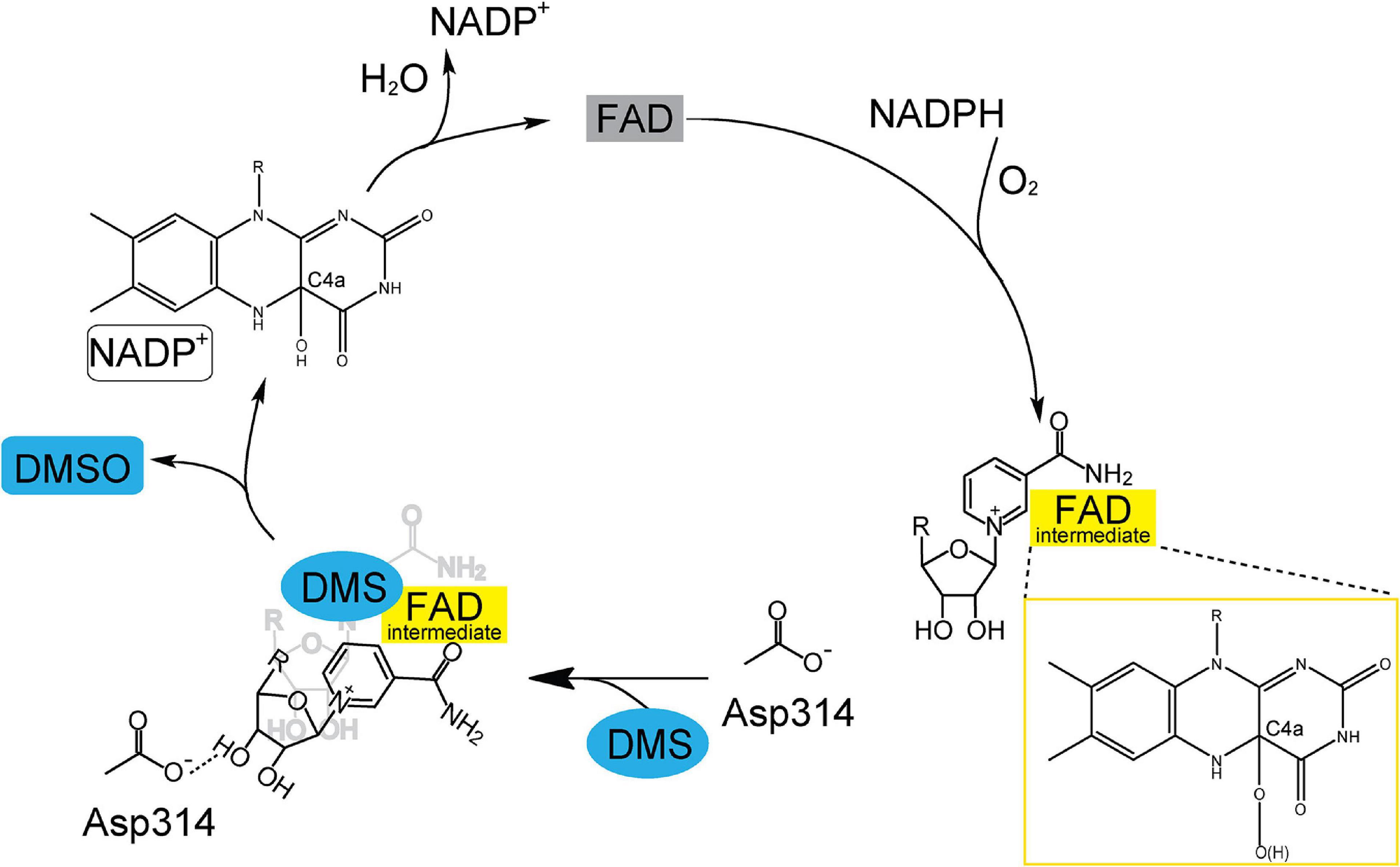
Figure 5. A proposed catalytic cycle of Tmm7211 oxidizing DMS to generate DMSO. In the reductive half-reaction, FAD is reduced by NADPH. In the oxidative half-reaction, the reduced FAD reacts with O2, and a C4a-(hydro)peroxyflavin (FAD intermediate) is formed. The nicotinamide ring of NADP+ protects the FAD intermediate from solvent attack. When DMS enters the catalytic pocket, NADP+ generates a conformational change to form a hydrogen bond with Asp314, shutting off the substrate entrance and exposing the FAD intermediate to DMS. After the reaction, DMSO, NADP+ and a water molecule are released and the oxidized FAD is regenerated.
To elucidate the catalytic mechanism of Tmm7211 to oxidize DMS, it is important to obtain structures of Tmm7211 and Tmm7211 in complex with DMS. Here, although we solved Tmm7211 structures in three states, all our attempts to obtain the structure of Tmm7211 in complex with DMS failed. As such, we propose the structural basis for DMS oxidation to DMSO by Tmm7211 based on our structural and mutational analyses, and the previous studies of bacterial FMOs (Alfieri et al., 2008; Cho et al., 2011; Li et al., 2017). Tmm7211 shares ∼53% sequence identity with three other reported bacterial FMOs from Nitrincola lacisaponensis (NiFMO) (Loncar et al., 2019), Methylophaga sp. strain SK1 (mFMO) (Alfieri et al., 2008) and R. nubinhibens ISM (RnTmm) (Li et al., 2017). The overall structure as well as the locations of NADP+ and FAD of Tmm7211 are similar to those of mFMO (PDB code: 2VQ7), RnTmm (PDB code: 5IPY) and NiFMO (PDB code: 6HNS) (Figure 6), with the RMSDs between Tmm7211 and mFMO, RnTmm and NiFMO of 0.5 Å, 0.6 Å and 0.5 Å, respectively. This suggests that Tmm7211 may adopt a similar catalytic mechanism to oxidize DMS as these bacterial FMOs. Indeed, the catalytic mechanism of Tmm7211 oxidizing DMS we proposed is similar to that of RnTmm oxidizing TMA (Li et al., 2017), which includes a reductive half-reaction and an oxidative half-reaction. Asp317 of RnTmm was reported to form a hydrogen bond with the ribose ring of NADP+ after its conformational change (Li et al., 2017). Here, our structural and biochemical results indicate that Asp314 of Tmm7211 also likely forms a hydrogen bond with NADP+ after its conformational change, which is important for the catalysis of DMS oxidation. Moreover, sequence analysis showed that the residue Asp314 and most residues involved in binding NADP+ and FAD in Tmm7211 are highly conserved in the Tmm sequences in both MRC and SAR11 bacteria (Li et al., 2017), suggesting that these residues are important residues in bacterial Tmms and that MRC and SAR11 bacteria containing Tmm may adopt a similar mechanism in oxidizing both DMS and TMA. Despite these analyses, further efforts, especially attempts to obtain the Tmm-DMS complex structure, are needed to provide more details of the catalytic mechanism of Tmm to oxidize DMS.
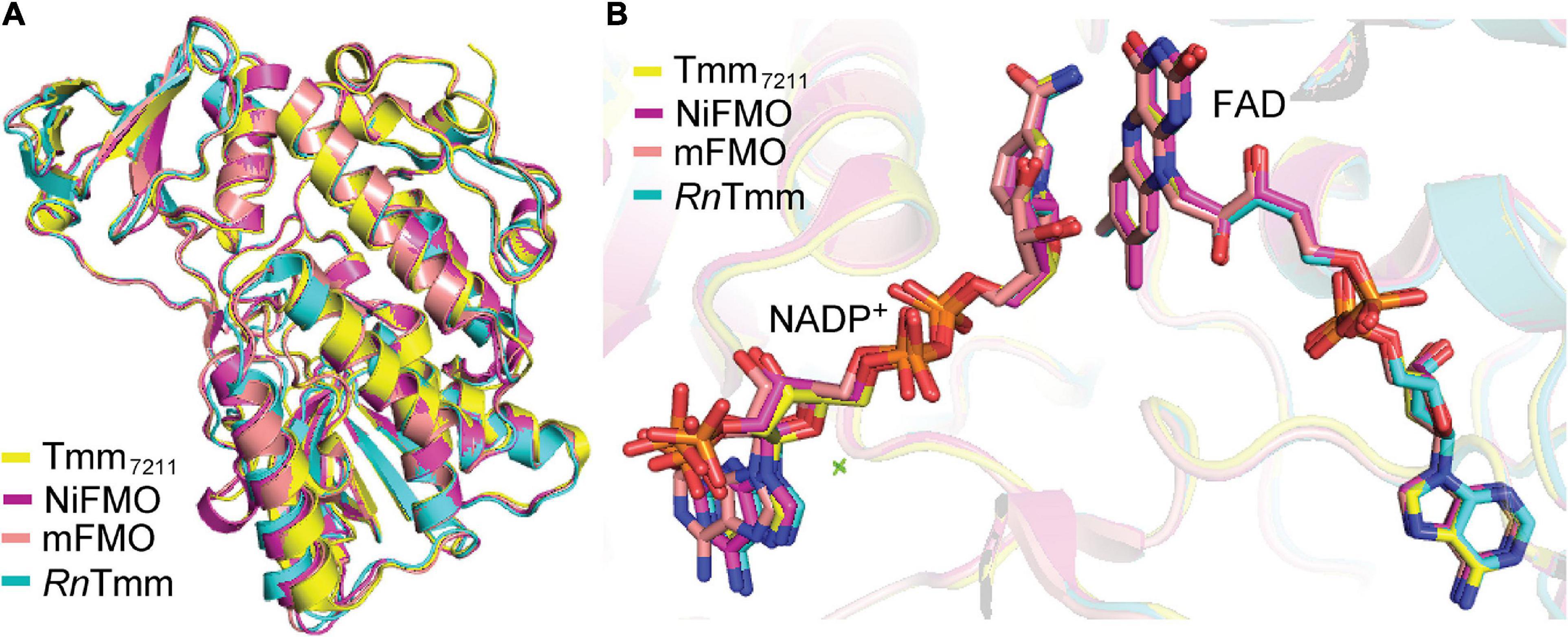
Figure 6. Structural comparisons between Tmm7211 and three other reported bacterial FMOs. (A) Superimposition of the structures of Tmm7211, NiFMO (PDB code: 6HNS), mFMO (PDB code: 2VQ7) and RnTmm (PDB code: 5IPY). Structures of Tmm7211, NiFMO, mFMO and RnTmm are colored in yellow, purple, salmon and cyan, respectively. (B) The locations of the NADP+ and FAD in the four structures. The NADP+ and FAD molecules are shown as sticks.
The volatile DMS is predominately produced from microbial degradation of DMSP through various DMSP dethiomethylases (colloquial “DMSP lyases,” EC 4.4.1.3) and the DMSP CoA-transferase/lyase DddD (EC 2.3.1.x) (Curson et al., 2011; Acolombri et al., 2014; Johnston et al., 2016). Despite this, DMS can also be generated from DMSO reduction catalyzed by the DMSO reductase DMSOR (EC 1.8.5.3) (Bray et al., 2001), and from methanethiol (MeSH) via the methyltransferase MddA (EC 2.1.1.334, methanethiol S-methyltransferase) (Carrion et al., 2017; Boden and Hutt, 2019). In addition to Tmm, three other enzymes, namely DMS dehydrogenase DdhABC (EC 1.8.2.4, DMS:cytochrome c reductase) (McDevitt et al., 2002; Boden and Hutt, 2019), assimilatory DMS S-monooxygenase DsoABCDEF (EC 1.14.13.245) (Horinouchi et al., 1997; Boden and Hutt, 2019) and dissimilatory DMS monooxygenase DmoAB (EC 1.14.13.131) (Boden et al., 2011; Boden and Hutt, 2019), also participate in the bacterial consumption of DMS. DdhABC and DsoABCDEF convert DMS to DMSO, while DmoAB converts DMS to MeSH (Horinouchi et al., 1997; McDevitt et al., 2002; Boden et al., 2011). To investigate the prevalence and the coexistence of the enzymes involved in DMS metabolism, we searched homologs of DMS metabolism related enzymes using the IMG/M database (Chen et al., 2019). In total, 3,182 non-redundant bacterial genomes were filtered out to possess at least one type of enzymes related to DMS metabolism, among which 415 contain more than one types of DMS related genes. All these enzyme combinations yielded 22 different one-to-one enzyme configuration modes (Supplementary Figure 1). The relatively frequent co-occurrence between Tmm and DMSP dethiomethylases suggests that some bacteria may channel DMS generated from DMSP cleavage to DMS oxidation to DMSO.
Both previous metagenomic analysis (Chen et al., 2011) and the coexistence analysis presented here suggest a high potential of the oxidation of DMS to DMSO via Tmm catalysis. It was also reported that the oxidation of DMS in MRC is methylated amine-dependent (Lidbury et al., 2016). However, the wide distribution of methylated amines in marine environments (Wang and Lee, 1990; Chen et al., 2011) suggests that Tmm may be functional in DMS oxidation under physiological conditions. Considering that the oxidation to DMSO is a major fate of DMS in surface seawater and the ubiquity of DMSO in marine environments (Lidbury et al., 2016), there should exist active microbiol processes to consume DMSO. The microorganisms and the metabolic pathways involved in DMSO metabolisms warrant further investigation.
Conclusion
The oxidation of oceanic DMS to DMSO is an important step in the global sulfur cycle, which can be catalyzed by Tmm (Lidbury et al., 2016). Tmm is present in ∼20% of the bacteria in the surface ocean, and is particularly common in the cosmopolitan marine heterotrophs such as MRC and SAR11 bacteria (Chen et al., 2011). In this study, the recombinant Tmm7211 protein from the SAR11 bacterium Pelagibacter sp. HTCC7211 was purified and characterized. The crystal structures of Tmm7211 and Tmm7211 soaked with DMS were also solved. Based on structural analysis and mutational assays, the catalytic mechanism for Tmm7211 oxidizing DMS was proposed, which may be widely adopted by MRC and SAR11 bacteria. This study offers a better understanding of how marine bacteria oxidize DMS to generate DMSO.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Author Contributions
C-YL and Y-ZZ designed the research. X-LC and H-HF directed the research. C-YL, X-JW, NZ, and Z-JT performed the experiments. PW, W-PZ, and YC helped in data analysis. C-YL and X-LC wrote the manuscript. YC edited the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Key Research and Development Program of China (Grants Nos. 2016YFA0601303 and 2018YFC1406700), the National Science Foundation of China (Grants Nos. 91851205, 42076229, 31630012, U1706207, 31870052, 31800107, 91751101, 41706152, and 41676180), the Fundamental Research Funds for the Central Universities (Grant No. 202041011), the Major Scientific and Technological Innovation Project (MSTIP) of Shandong Province (Grant No. 2019JZZY010817), the Program of Shandong for Taishan Scholars (Grant No. tspd20181203), AoShan Talents Cultivation Program supported by Qingdao National Laboratory for Marine Science and Technology (Grant Nos. 2017ASTCP-OS14 and QNLM2016ORP0310), the grant of Laboratory for Marine Biology and Biotechnology (Grant No. OF2019NO02), the Pilot National Laboratory for Marine Science and Technology (Qingdao), and the Natural Science Foundation of Shandong Province (Grant No. ZR2017LD013).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank the staffs from the BL17U1 and BL18U1 beamline of National Facility for Protein Sciences Shanghai (NFPS) and Shanghai Synchrotron Radiation Facility, for assistance during data collection. We thank Caiyun Sun from State Key laboratory of Microbial Technology of Shandong University for her help in enzyme assays.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2021.735793/full#supplementary-material
Footnotes
- ^ http://www.pymol.org/
- ^ https://www.ncbi.nlm.nih.gov/
- ^ https://www.python.org/
- ^ http://www.ebi.ac.uk/pdbe/prot_int/pistart.html
References
Acolombri, U., Laurino, P., Lara-Astiaso, P., Vardi, A., and Tawfik, D. S. (2014). DddD is a CoA-transferase/lyase producing dimethyl sulfide in the marine environment. Biochemistry 53, 5473–5475. doi: 10.1021/bi500853s
Adams, P. D., Afonine, P. V., Bunkoczi, G., Chen, V. B., Davis, I. W., Echols, N., et al. (2010). PHENIX: a comprehensive python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221. doi: 10.1107/S0907444909052925
Alfieri, A., Malito, E., Orru, R., Fraaije, M. W., and Mattevi, A. (2008). Revealing the moonlighting role of NADP in the structure of a flavin-containing monooxygenase. Proc. Natl. Acad. Sci. U. S. A. 105, 6572–6577. doi: 10.1073/pnas.0800859105
Andreae, M. O. (1990). Ocean-atmosphere interactions in the global biogeochemical sulfur cycle. Mar. Chem. 30, 1–29. doi: 10.1016/0304-4203(90)90059-l
Asher, E. C., Dacey, J. W. H., Stukel, M., Long, M. C., and Tortell, P. D. (2017). Processes driving seasonal variability in DMS, DMSP, and DMSO concentrations and turnover in coastal Antarctic waters. Limnol. Oceanogr. 62, 104–124. doi: 10.1002/lno.10379
Beaty, N. B., and Ballou, D. P. (1981a). The oxidative half-reaction of liver microsomal FAD-containing monooxygenase. J. Biol. Chem. 256, 4619– 4625.
Beaty, N. B., and Ballou, D. P. (1981b). The reductive half-reaction of liver microsomal FAD-containing monooxygenase. J. Biol. Chem. 256, 4611–4618. doi: 10.1016/0165-022X(81)90075-0
Boden, R., Borodina, E., Wood, A. P., Kelly, D. P., Murrell, J. C., and Schafer, H. (2011). Purification and characterization of dimethylsulfide monooxygenase from Hyphomicrobium sulfonivorans. J. Bacteriol. 193, 1250–1258. doi: 10.1128/JB.00977-10
Boden, R., and Hutt, L. P. (2019). “Bacterial metabolism of C1 sulfur compounds,” in Aerobic Utilization of Hydrocarbons, Oils, and Lipids, ed. F. Rojo (Cham: Springer International Publishing), 421–463.
Boden, R., Kelly, D. P., Murrell, J. C., and Schafer, H. (2010). Oxidation of dimethylsulfide to tetrathionate by Methylophaga thiooxidans sp. nov.: a new link in the sulfur cycle. Environ. Microbiol. 12, 2688–2699. doi: 10.1111/j.1462-2920.2010.02238.x
Bray, R. C., Adams, B., Smith, A. T., Richards, R. L., Lowe, D. J., and Bailey, S. (2001). Reactions of dimethylsulfoxide reductase in the presence of dimethyl sulfide and the structure of the dimethyl sulfide-modified enzyme. Biochemistry 40, 9810–9820. doi: 10.1021/bi010559r
Brimblecombe, P., and Shooter, D. (1986). Photo-oxidation of dimethylsulphide in aqueous solution. Mar. Chem. 19, 343–353. doi: 10.1016/0304-4203(86)90055-1
Buchan, A., Gonzalez, J. M., and Moran, M. A. (2005). Overview of the marine Roseobacter lineage. Appl. Environ. Microbiol. 71, 5665–5677. doi: 10.1128/AEM.71.10.5665-5677.2005
Carini, P., Van Mooy, B. A., Thrash, J. C., White, A., Zhao, Y., Campbell, E. O., et al. (2015). SAR11 lipid renovation in response to phosphate starvation. Proc. Natl. Acad. Sci. U. S. A. 112, 7767–7772. doi: 10.1073/pnas.1505034112
Carrion, O., Pratscher, J., Curson, A. R. J., Williams, B. T., Rostant, W. G., Murrell, J. C., et al. (2017). Methanethiol-dependent dimethylsulfide production in soil environments. ISME J. 11, 2379–2390. doi: 10.1038/ismej.2017.105
Charlson, R. J., Lovelock, J. E., Andreae, M. O., and Warren, S. G. (1987). Oceanic phytoplankton, atmospheric sulphur, cloud albedo and climate. Nature 326, 655–661. doi: 10.1038/326655a0
Chen, I. M. A., Chu, K., Palaniappan, K., Pillay, M., Ratner, A., Huang, J. H., et al. (2019). IMG/M v.5.0: an integrated data management and comparative analysis system for microbial genomes and microbiomes. Nucleic Acids Res. 47, D666–D677. doi: 10.1093/nar/gky901
Chen, Y. (2012). Comparative genomics of methylated amine utilization by marine Roseobacter clade bacteria and development of functional gene markers (tmm, gmaS). Environ. Microbiol. 14, 2308–2322. doi: 10.1111/j.1462-2920.2012.02765.x
Chen, Y., Patel, N. A., Crombie, A., Scrivens, J. H., and Murrell, J. C. (2011). Bacterial flavin-containing monooxygenase is trimethylamine monooxygenase. Proc. Natl. Acad. Sci. U. S. A. 108, 17791–17796. doi: 10.1073/pnas.1112928108
Cho, H. J., Cho, H. Y., Kim, K. J., Kim, M. H., Kim, S. W., and Kang, B. S. (2011). Structural and functional analysis of bacterial flavin-containing monooxygenase reveals its ping-pong-type reaction mechanism. J. Struct. Biol. 175, 39–48. doi: 10.1016/j.jsb.2011.04.007
Curson, A. R. J., Todd, J. D., Sullivan, M. J., and Johnston, A. W. B. (2011). Catabolism of dimethylsulphoniopropionate: microorganisms, enzymes and genes. Nat. Rev. Microbiol. 9, 849–859. doi: 10.1038/nrmicro2653
del Valle, D. A., Kieber, D. J., and Kiene, R. P. (2007). Depth-dependent fate of biologically-consumed dimethylsulfide in the Sargasso Sea. Mar. Chem. 103, 197–208. doi: 10.1016/j.marchem.2006.07.005
deZwart, J. M. M., Nelisse, P. N., and Kuenen, J. G. (1996). Isolation and characterization of Methylophaga sulfidovorans sp nov: an obligately methylotrophic, aerobic, dimethylsulfide oxidizing bacterium from a microbial mat. FEMS Microbiol. Ecol. 20, 261–270. doi: 10.1111/j.1574-6941.1996.tb00324.x
Emsley, P., Lohkamp, B., Scott, W. G., and Cowtan, K. (2010). Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501. doi: 10.1107/S0907444910007493
Eswaramoorthy, S., Bonanno, J. B., Burley, S. K., and Swaminathan, S. (2006). Mechanism of action of a flavin-containing monooxygenase. Proc. Natl. Acad. Sci. U. S. A. 103, 9832–9837. doi: 10.1073/pnas.0602398103
Horinouchi, M., Kasuga, K., Nojiri, H., Yamane, H., and Omori, T. (1997). Cloning and characterization of genes encoding an enzyme which oxidizes dimethyl sulfide in Acinetobacter sp. strain 20B. FEMS Microbiol. Lett. 155, 99–105. doi: 10.1111/j.1574-6968.1997.tb12692.x
Johnston, A. W. B., Green, R. T., and Todd, J. D. (2016). Enzymatic breakage of dimethylsulfoniopropionate - a signature molecule for life at sea. Curr. Opin. Chem. Biol. 31, 58–65. doi: 10.1016/j.cbpa.2016.01.011
Kiene, R. P., and Bates, T. S. (1990). Biological removal of dimethyl sulphide from sea water. Nature 345, 702–705. doi: 10.1038/345702a0
Kohl, M., Wiese, S., and Warscheid, B. (2011). “Cytoscape: software for visualization and analysis of biological networks,” in Data Mining in Proteomics: From Standards to Applications, eds M. Hamacher, M. Eisenacher, and C. Stephan (Totowa, NJ: Humana Press), 291–303.
Krueger, S. K., and Williams, D. E. (2005). Mammalian flavin-containing monooxygenases: structure/function, genetic polymorphisms and role in drug metabolism. Pharmacol. Ther. 106, 357–387. doi: 10.1016/j.pharmthera.2005.01.001
Ksionzek, K. B., Lechtenfeld, O. J., McCallister, S. L., Schmitt-Kopplin, P., Geuer, J. K., Geibert, W., et al. (2016). Dissolved organic sulfur in the ocean: biogeochemistry of a petagram inventory. Science 354, 456–459. doi: 10.1126/science.aaf7796
Lee, P. A., and De Mora, S. J. (1999). Intracellular dimethylsulfoxide (DMSO) in unicellular marine algae: speculations on its origin and possible biological role. J. Phycol. 35, 8–18. doi: 10.1046/j.1529-8817.1999.3510008.x
Li, C. Y., Chen, X. L., Zhang, D., Wang, P., Sheng, Q., Peng, M., et al. (2017). Structural mechanism for bacterial oxidation of oceanic trimethylamine into trimethylamine N-oxide. Mol. Microbiol. 103, 992–1003. doi: 10.1111/mmi.13605
Lidbury, I., Krober, E., Zhang, Z., Zhu, Y., Murrell, J. C., Chen, Y., et al. (2016). A mechanism for bacterial transformation of dimethylsulfide to dimethylsulfoxide: a missing link in the marine organic sulfur cycle. Environ. Microbiol. 18, 2754–2766. doi: 10.1111/1462-2920.13354
Loncar, N., Fiorentini, F., Bailleul, G., Savino, S., Romero, E., Mattevi, A., et al. (2019). Characterization of a thermostable flavin-containing monooxygenase from Nitrincola lacisaponensis (NiFMO). Appl. Microbiol. Biotechnol. 103, 1755–1764. doi: 10.1007/s00253-018-09579-w
McDevitt, C. A., Hanson, G. R., Noble, C. J., Cheesman, M. R., and McEwan, A. G. (2002). Characterization of the redox centers in dimethyl sulfide dehydrogenase from Rhodovulum sulfidophilum. Biochemistry 41, 15234–15244. doi: 10.1021/bi026221u
Minor, W., Cymborowski, M., Otwinowski, Z., and Chruszcz, M. (2006). HKL-3000: the integration of data reduction and structure solution–from diffraction images to an initial model in minutes. Acta Crystallogr. D Biol. Crystallogr. 62, 859–866. doi: 10.1107/S0907444906019949
Morris, R. M., Rappe, M. S., Connon, S. A., Vergin, K. L., Siebold, W. A., Carlson, C. A., et al. (2002). SAR11 clade dominates ocean surface bacterioplankton communities. Nature 420, 806–810. doi: 10.1038/nature01240
Orru, R., Pazmiño, D. E., Fraaije, M. W., and Mattevi, A. (2010). Joint functions of protein residues and NADP(H) in oxygen activation by flavin-containing monooxygenase. J. Biol. Chem. 285, 35021–35028. doi: 10.1074/jbc.M110.161372
Paul, C. E., Eggerichs, D., Westphal, A. H., Tischler, D., and van Berkel, W. J. H. (2021). Flavoprotein monooxygenases: versatile biocatalysts. Biotechnol. Adv. 51, 107712. doi: 10.1016/j.biotechadv.2021.107712
Rusch, D. B., Halpern, A. L., Sutton, G., Heidelberg, K. B., Williamson, S., Yooseph, S., et al. (2007). The Sorcerer II Global Ocean Sampling expedition: northwest Atlantic through eastern tropical Pacific. PLoS Biol. 5:e77. doi: 10.1371/journal.pbio.0050077
Sebastián, M., Smith, A. F., González, J. M., Fredricks, H. F., Van Mooy, B., Koblížek, M., et al. (2016). Lipid remodelling is a widespread strategy in marine heterotrophic bacteria upon phosphorus deficiency. ISME J. 10, 968–978. doi: 10.1038/ismej.2015.172
Simo, R. (2001). Production of atmospheric sulfur by oceanic plankton: biogeochemical, ecological and evolutionary links. Trends Ecol. Evol. 16, 287–294. doi: 10.1016/s0169-5347(01)02152-8
Speeckaert, G., Borges, A. V., Champenois, W., Royer, C., and Gypens, N. (2018). Annual cycle of dimethylsulfoniopropionate (DMSP) and dimethylsulfoxide (DMSO) related to phytoplankton succession in the Southern North Sea. Sci. Total Environ. 622-623, 362–372. doi: 10.1016/j.scitotenv.2017.11.359
Sun, J., Steindler, L., Thrash, J. C., Halsey, K. H., Smith, D. P., Carter, A. E., et al. (2011). One carbon metabolism in SAR11 pelagic marine bacteria. PLoS One 6:e23973. doi: 10.1371/journal.pone.0023973
Tsementzi, D., Wu, J., Deutsch, S., Nath, S., Rodriguez, R. L., Burns, A. S., et al. (2016). SAR11 bacteria linked to ocean anoxia and nitrogen loss. Nature 536, 179–183. doi: 10.1038/nature19068
van Berkel, W. J., Kamerbeek, N. M., and Fraaije, M. W. (2006). Flavoprotein monooxygenases, a diverse class of oxidative biocatalysts. J. Biotechnol. 124, 670–689. doi: 10.1016/j.jbiotec.2006.03.044
Vila-Costa, M., Del Valle, D. A., Gonzalez, J. M., Slezak, D., Kiene, R. P., Sanchez, O., et al. (2006). Phylogenetic identification and metabolism of marine dimethylsulfide-consuming bacteria. Environ. Microbiol. 8, 2189–2200. doi: 10.1111/j.1462-2920.2006.01102.x
Wang, Q. S., Zhang, K. H., Cui, Y., Wang, Z. J., Pan, Q. Y., Liu, K., et al. (2018). Upgrade of macromolecular crystallography beamline BL17U1 at SSRF. Nucl. Sci. Tech. 29:68.
Wang, X. C., and Lee, C. (1990). The distribution and adsorption behavior of aliphatic-amines in marine and lacustrine sediments. Geochim. Cosmochim. Acta 54, 2759–2774. doi: 10.1016/0016-7037(90)90010-I
Winn, M. D., Ballard, C. C., Cowtan, K. D., Dodson, E. J., Emsley, P., Evans, P. R., et al. (2011). Overview of the CCP4 suite and current developments. Acta Crystallogr. D Biol. Crystallogr. 67, 235–242. doi: 10.1107/S0907444910045749
Keywords: DMS, DMSO, flavin-containing monooxygenase, SAR11, catalytic mechanism
Citation: Wang X-J, Zhang N, Teng Z-J, Wang P, Zhang W-P, Chen X-L, Zhang Y-Z, Chen Y, Fu H-H and Li C-Y (2021) Structural and Mechanistic Insights Into Dimethylsulfoxide Formation Through Dimethylsulfide Oxidation. Front. Microbiol. 12:735793. doi: 10.3389/fmicb.2021.735793
Received: 05 July 2021; Accepted: 02 September 2021;
Published: 24 September 2021.
Edited by:
Rich Boden, University of Plymouth, United KingdomReviewed by:
Willem J. H. Van Berkel, Wageningen University and Research, NetherlandsDenyce Wicht, Suffolk University, United States
Copyright © 2021 Wang, Zhang, Teng, Wang, Zhang, Chen, Zhang, Chen, Fu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hui-Hui Fu, fuhuihui@ouc.edu.cn; Chun-Yang Li, lcy@ouc.edu.cn
†These authors have contributed equally to this work
 Xiu-Juan Wang1,2,3†
Xiu-Juan Wang1,2,3† Zhao-Jie Teng
Zhao-Jie Teng Peng Wang
Peng Wang Wei-Peng Zhang
Wei-Peng Zhang Xiu-Lan Chen
Xiu-Lan Chen Yu-Zhong Zhang
Yu-Zhong Zhang Yin Chen
Yin Chen Hui-Hui Fu
Hui-Hui Fu Chun-Yang Li
Chun-Yang Li