- 1Institute of Molecular Biology and Biotechnology of Prokaryotes, University of Ulm, Ulm, Germany
- 2Institute of Bio- and Geosciences, IBG-1: Biotechnology, Forschungszentrum Jülich GmbH, Jülich, Germany
- 3Institute of Biotechnology, RWTH Aachen University, Aachen, Germany
Bacteriocins are antimicrobial peptides applied in food preservation and are interesting candidates as alternatives to conventional antibiotics or as microbiome modulators. Recently, we established Corynebacterium glutamicum as a suitable production host for various bacteriocins including garvicin Q (GarQ). Here, we establish secretion of GarQ by C. glutamicum via the Sec translocon achieving GarQ titers of about 7 mg L–1 in initial fermentations. At neutral pH, the cationic peptide is efficiently adsorbed to the negatively charged envelope of producer bacteria limiting availability of the bacteriocin in culture supernatants. A combination of CaCl2 and Tween 80 efficiently reduces GarQ adsorption to C. glutamicum. Moreover, cultivation in minimal medium supplemented with CaCl2 and Tween 80 improves GarQ production by C. glutamicum to about 15 mg L–1 but Tween 80 resulted in reduced GarQ activity at later timepoints. Using a reporter strain and proteomic analyses, we identified HtrA, a protease associated with secretion stress, as another potential factor limiting GarQ production. Transferring production to HtrA-deficient C. glutamicum K9 improves GarQ titers to close to 40 mg L–1. Applying conditions of low aeration prevented loss in activity at later timepoints and improved GarQ titers to about 100 mg L–1. This is about 50-fold higher than previously shown with a C. glutamicum strain employing the native GarQ transporter GarCD for secretion and in the range of levels observed with the native producer Lactococcus petauri B1726. Additionally, we tested several synthetic variants of GarQ and were able to show that exchange of the methionine in position 5 to a phenylalanine (GarQM5F) results in markedly increased activity against Lactococcus lactis and Listeria monocytogenes. In summary, our findings shed light on several aspects of recombinant GarQ production that may also be of relevance for production with natural producers and other bacteriocins.
1 Introduction
Bacteriocins are ribosomally synthesized antimicrobial peptides or proteins that kill sensitive bacteria or inhibit their growth (Darbandi et al., 2022). Bacteriocins of lactic acid bacteria (LAB), especially model peptides like nisin and pediocin, have been widely studied (Chikindas et al., 2018; Silva et al., 2018) and are classified into modified (class I), non-modified (class II) peptides and thermo-labile proteins (class III) (Alvarez-Sieiro et al., 2016; Acedo et al., 2018). A more recent review adds a class IV with lipid or carbohydrate moieties to the classification (Darbandi et al., 2022). While the majority of the known LAB bacteriocins are secreted by dedicated ABC-transporters (Nes et al., 1996; Zheng and Sonomoto, 2018), there are examples of class II bacteriocins (e.g., divergicin A or enterocin P) that are secreted by the general secretory (Sec) pathway by their native producers (Worobo et al., 1995; Nes et al., 1996; Herranz and Driessen, 2005). Depending on the secretion pathway, different N-terminal signal peptides (SPs) are required guiding the prepeptide to the transporter. After or during translocation, the SP is cleaved by a signal peptidase and the active peptide is released (Nes et al., 1996; Zheng and Sonomoto, 2018). In case of dedicated ABC-transporters, cleavage occurs directly after a double-glycine-motif in the SP (Nes et al., 1996; Zheng and Sonomoto, 2018). In contrast, Sec-dependent SPs have a tripartite structure with a positively charged N-terminal part, a hydrophobic central region and a polar C-terminal region containing the conserved AXA recognition site for signal peptidase cleavage (Freudl, 2018). Interestingly, double-glycine-type and Sec-dependent SPs can be exchanged, and it was demonstrated that the Sec pathway can be employed for heterologous bacteriocin production (McCormick et al., 1996; Van Belkum et al., 1997; Martín et al., 2007; Borrero et al., 2011; Feito et al., 2023).
Due to their antimicrobial activity against important human and animal pathogens, bacteriocins are interesting alternatives for conventional antimicrobials in a number of applications (Cotter et al., 2013; Meade et al., 2020; Soltani et al., 2021). However, their therapeutic application and market entry of new bacteriocins are hampered by a lack in easy and cost-effective production and purification at industrially relevant titers (Abbasiliasi et al., 2017; Johnson et al., 2017). Currently processes for industrial scale production of bacteriocins rely on natural producer strains grown on complex media often supplemented with milk or whey (de Arauz et al., 2009; Abbasiliasi et al., 2017), which requires expensive downstream processing and/or results in low product purity. Also, some bacteriocins are naturally produced by pathogenic bacteria that are not suitable for biotechnological production (EFSA Panel on Biological Hazards et al., 2022). One possibility that addresses these obstacles is transfer of production of bacteriocins to recombinant strains of industrial workhorse organisms.
In recent years, we explored the potential of the Gram-positive diderm bacterium Corynebacterium glutamicum as a host for recombinant bacteriocin production (Goldbeck et al., 2021; Desiderato et al., 2022; Weixler et al., 2022; Christmann et al., 2023). Due to its long history as a biotechnological platform organism, many genetic tools, cheap and defined minimal media and industrial-scale processes are established (Becker et al., 2018; Wolf et al., 2021; Lee and Jeong, 2022). C. glutamicum has several advantages for recombinant bacteriocin production, e.g., the GRAS (generally regarded as safe) status, low extracellular protease activity (Vertès, 2013) and functional protein secretion pathways (Freudl, 2018). Secretion of various recombinant proteins via the Sec pathway of C. glutamicum was successfully demonstrated in the mg L–1 to g L–1 scale (Lee and Jeong, 2022).
The linear and non-modified bacteriocin garvicin Q (GarQ) is a class IId peptide naturally produced by Lactococcus garvieae and Lactococcus petauri strains (Tosukhowong et al., 2012; Desiderato et al., 2022). The peptide has activity against a wide range of Gram-positive bacteria including Listeria spp., Lactococcus spp. and Enterococcus spp. (Tymoszewska et al., 2017). Similar to the class IIa bacteriocin pediocin PA-1, GarQ uses the transmembrane subunits IIC and IID of the mannose phosphotransferase system (PTSMan) as receptor to exert its activity (Tymoszewska et al., 2017). Sensitive bacteria are then killed by disruption of membrane integrity and intracellular pH homeostasis (Desiderato et al., 2022). The ABC-transporter GarC and likely the accessory protein GarD are responsible for processing and secretion of GarQ via an N-terminal SP containing the conserved double-glycine-motif (Tosukhowong et al., 2012; Desiderato et al., 2022). Recently established recombinant production of GarQ in C. glutamicum using GarCD (Desiderato et al., 2022) but this recombinant strain produced about 4-fold lower levels of GarQ compared to the natural producer L. petauri B1726 (Desiderato et al., 2022).
The cell envelope of L. petauri B1726 has the typical topology of Gram-positive LAB with a cytoplasmic membrane and a thick peptidoglycan sacculus (Chapot-Chartier and Kulakauskas, 2014). Successful expression of membrane proteins such as GarCD depends on several factors such as membrane lipid composition and can be challenging or even impossible if the intended heterologous host has a cell envelope architecture that differs from the native host (Opekarová and Tanner, 2003). Corynebacteria are diderm bacteria that also stain Gram-positive but have an unusual, complex cell envelope consisting of a cytoplasmic membrane, a peptidoglycan layer that is linked to a arabinogalactan polymer and an outer membrane of mycolic acids similar to outer membrane of Gram-negative bacteria (Burkovski, 2013). Thus, wrong insertion into the cell envelope or compromised functionality of GarCD in C. glutamicum may constitute a bottleneck for recombinant production of GarQ by C. glutamicum.
In the present study, we established and improve production of GarQ by C. glutamicum via the Sec-pathway because we consider GarQ and interesting and suitable bacteriocin for heterologous production using this biotechnological workhorse organism for several reasons. First of all, natural GarQ producers L. garvieae and L. petauri are biosafety level 2 pathogens hampering large-scale production of GarQ with these organisms. Second, C. glutamicum lacks a PTSMan. Thus, C. glutamicum is not sensitive to GarQ, which is a prerequisite for the production at high titers. Furthermore, GarQ is a linear peptide without any posttranslational modifications and therefore an ideal candidate for secretion via the Sec-pathway. Release of GarQ into culture supernatants was improved by minimizing adsorption of the peptide to the producer cells. GarQ titers in supernatants were further improved by using a HtrA-deficient strain and conditions of low oxygenation. The optimized production strategy resulted in stable production of active GarQ at titers above 100 μg ml–1.
2 Materials and methods
2.1 Strains and growth conditions
Corynebacterium glutamicum was routinely cultivated at 30°C and under shaking conditions (130 rpm). For growth experiments, C. glutamicum strains were cultivated overnight (O/N) in 2xTY medium. Bacteria of these O/N cultures were washed with sterile 0.9% (w/v) NaCl and used to inoculate a modified CGXII minimal medium without urea containing glucose at 2% (w/v) as carbon source (CGXII-U) (Desiderato et al., 2022) to an optical density (OD600) of ∼2. For optimization of recombinant GarQ production, 0.5 g L–1 CaCl2 and/or 0.5% (v/v) Tween 80 were supplemented as indicated. These media will be referred to as CGXII-U+C (CGXII-U with CaCl2), CGXII-U+T (CGXII-U with Tween 80), and CGXII-U+CT (CGXII-U with CaCl2 and Tween 80). Lactococcus lactis IL1403/pNZ-pHin2Lm was cultivated in M17 medium with 0.5% (w/v) glucose at 30°C with aeration on an orbital shaker. Listeria monocytogenes EGDe/pNZ-pHin2Lm was cultivated in BHI medium at 37°C with aeration on an orbital shaker. For GarQ production by the native producer, L. petauri B1726 was cultivated statically in M17 medium supplemented with 2% (w/v) glucose at 30°C overnight in Schott glass bottles. Media were supplemented with 25 μg kanamycin ml–1 (C. glutamicum), 10 or 7.5 μg ml–1 chloramphenicol (L. lactis or C. glutamicum) and/or 0.2 mM IPTG (C. glutamicum) as appropriate. All strains are listed in Table 1.
2.2 Molecular biology procedures
Cloning procedures were performed using standard reagents following instructions of the manufacturers. Primers were purchased from a commercial service provider (Eurofins Genomics, Germany) and sequences are listed in Supplementary Table 1. For construction of the Sec-SP library (plasmids pCMEx8-SPXYZ-garQ), an automated protocol for plasmid construction using an OT-2 robot (Opentrons, USA) was applied as previously described (Müller et al., 2022). Briefly, the garQ gene lacking the nucleotide sequence for its native SP was amplified from pPBEx2-garQICDCgl and inserted into pCMEx8-[SPXYZ] by Golden Gate Assembly using the type II restriction enzyme BsaI (New England Biolabs). Restriction digestion and capillary electrophoresis were used to check for positive clones. For construction of pXMJ19-SPywaD-garQ-v1, the gene coding for a fusion peptide consisting of the Sec-dependent SP of YwaD of B. subtilis and GarQ was amplified together with its ribosomal binding site from pCMEx8-SPywa-garQ and new restriction sites were introduced by primers with appropriate adapters containing PstI and BamHI restriction sites. The PCR fragment was digested with these enzymes and ligated into linearized pXMJ19. For construction of pXMJ19-SPywaD-garQ-v2, SPywaD and garQ were separately amplified from pXMJ19-SPywaD-garQ-v1 lacking the codons for the two amino acid linker between SP and GarQ. The two fragments were fused by overlapping extension PCR and sub-cloned into pJET1.2 (ThermoFisher, Germany) by blunt-end ligation. Then SPywaD-garQ-v2 was excised from the sub-cloning vector using restriction by XbaI and PstI and ligated into linearized pXMJ19. For construction of pXMJ19-SPywaD-garQM5F, a SPywaD-garQM5F gene with the mutations for the M5F amino acid exchange was synthesized by a commercial service provider (Eurofins Genomics) and inserted into XbaI/PstI-linearized pXMJ19. All constructs were verified by Sanger sequencing by a commercial service provider (Microsynth Seqlab, Germany).
2.3 pHluorin2 assay
The pHluorin2 assay was performed according to a previously published protocol using reporter strains L. lactis IL1403/pNZ-pHin2Lm or L. monocytogenes EGDe/pNZ-pHin2Lm (Desiderato et al., 2022; Reich et al., 2022) and Listeria minimal buffer (LMB) with slight modifications. Instead of 100 mM 3-(N-morpholino)propanesulfonic acid (MOPS) as a buffering agent, 200 mM 2-(N-morpholino)ethanesulfonic acid were used. The assay is based on the two distinct excitation peaks of pHluorin2 at 400 and 480 nm. Fluorescence intensity (FI) at these two excitation peaks shifts in response to changes in pH in a ratiometric manner: with increasing pH, FI after excitation at 400 nm increases and FI after excitation at 480 nm decreases. Under steady-state conditions, biosensor bacteria expressing pHluorin2 are able to maintain an intracellular pH (pHi) of 7.6–8.0 even in LMB with a pH of 6.2. However, when exposed to compounds that disrupt membrane integrity, pHi will rapidly change to the pH of LMB, and this shift can be detected by the changes in FIs after excitation at 400 and 480 nm.
For quantification of GarQ activities, standards with defined concentration (range 0.03–2.0 μg ml–1) of chemically synthesized peptides were used to establish a calibration curve by plotting ratios of pHluorin2 fluorescence intensities at 520 nm after excitation at 400 and 480 nm (ratio RFU 400/480) against peptide concentration. The equation of the calibration curve was calculated as a nonlinear regression using a semilogarithmic fit with least square fitting. The coefficient of determination R2 was > 0.96 in all cases. These calibration curves were used to calculate mass concentration of GarQ in unknown samples based on measurements of ratio RFU 400/480. Statistical analysis was performed by ANOVA with the Bonferroni post-test to calculate p-values adjusted for multiple comparisons. GraphPad Prism 6 software was sued to compute nonlinear regression and ANOVA. Chemically synthesized GarQ was obtained from the Functional Peptidomics core facility of Ulm University. Dried, synthetic GarQ had a purity of > 95% and was dissolved in HPLC water for further use.
2.4 Purification and identification of GarQ
GarQ was purified from culture supernatants of C. glutamicum by sequential hydrophobic interaction chromatography (HIC) and reverse-phase chromatography (RPC) as previously described (Desiderato et al., 2022). After HIC, GarQ was eluted with HPLC-grade water. Elution buffer in RPC was 80% acetonitrile and 0.05% TFA in water and GarQ eluted at 30% elution buffer.
Mass spectrometry analysis of purified GarQ peptide fractions was performed based on a previously described method (Desiderato et al., 2022). Lyophilized peptide fractions were concentrated to 1 mg ml–1. Aliquots of peptide solutions were subjected to tryptic digestion, with 1 μg trypsin in a total volume of 100 μl for 5 h at 42°C as recommended by the supplier. Solutions of intact and digested peptides were diluted 1:2 with LC-MS-grade H2O prior to Liquid chromatography mass spectrometry (LC-MS).
Liquid chromatography mass spectrometry was conducted with an Agilent 1260 Infinity system (Agilent Technologies, Waldbronn, Germany) coupled to a quadrupole time-of-flight mass spectrometer (TripleTOF6600, AB Sciex, Darmstadt, Germany). LC was performed with an Ascentis® Express Peptide ES-C18, 2.7 μm HPLC column (53307-U, Merck, Darmstadt, Germany) with a flow rate of 200 μL min–1 and the mobile phases (A) 0.1% formic acid in water and (B) acetonitrile. The elution gradient was as follows: 0 min, 3% B; 70 min, 40% B; 78 min 40% B, 79 min 60% B, 89 min 60% B, 90 min 3% B followed by a 12 min equilibration time between injections. Column temperature was set to 21 °C and injection volume to 10 μL. MS was conducted with a TurboV ion source operated in positive ionization mode. Ion spray voltage was set to 5.5 kV, source temperature to 450 °C, curtain gas to 35 psi, and the support gases GS1/GS2 to 50 psi/50 psi. All gases were nitrogen.
For analysis of undigested peptide, the quadrupole time-of-flight (QToF) mass spectrometer was operated in ToF mode with a dwell time of 250 ms and for digested peptide, the QToF mass spectrometer was operated in data-dependent acquisition (DDA) mode. Based on a ToF survey scan with a dwell time of 250 ms, product ion scans with a dwell-time of 100 ms were automatically performed for a maximum of 40 ions with a mass-to-charge ratio > 300 m/z and a charge of 2 to 4 passing an intensity threshold of 150 cps. After acquisition, mass-to-charge ratios were excluded from the potential candidate-ion list for 12 s. Declustering potential was set to 120 V, collision energy spread to 5 V and mass tolerance to 25 ppm.
Acquired mass spectra were analyzed with PeakView 2.1 (AB Sciex, Darmstadt, Germany). Protein identification was performed with the ProteinPilot 5.1 software (AB Sciex, Darmstadt, Germany).
2.5 Adsorption assay
The adsorption assays were performed as previously described (Desiderato et al., 2022) with slight modifications. Overnight cultures of C. glutamicum were washed three times with phosphate-buffered saline. The universal buffer UB3 (20 mM HEPES, 20 mM Bis-Tris and 20 mM sodium acetate) was used to allow adsorption studies at different pH values without changing the buffer composition (Brooke et al., 2015). Bacteria were resuspended at an OD600 of 3 in UB3 buffer set to different pH containing HIC purified GarQ. After incubation of the peptide with bacteria for 1 h at 30°C, bacteria were pelleted by centrifugation (11,000 × g, 5 min, room temperature) and the adsorption supernatant (ASN) was collected. In parallel, GarQ was incubated at the same conditions and buffer composition without bacteria as a control. Antimicrobial activity of two-fold serial dilution of ASN and control samples was then determined in growth inhibition assay using L. lactis IL1403 as indicator strain as described previously (Desiderato et al., 2022). To calculate bacteriocin activity, a nonlinear fit based on the Gompertz function was calculated using GraphPad Prism 6 software. The coefficient of determination R2 of the Gompertz function was above 0.97 in all cases, indicating that the function is suitable. Arbitrary units (AU) of bacteriocin activity of sample was defined as the X-value of the inflection point (1/K) of the Gompertz function. The percentage of adsorbed GarQ was calculated according to following formula:
2.6 Flow cytometry analysis
Corynebacterium glutamicum strains were cultivated in CGXII-U or normal CGXII minimal medium (with urea). Samples were taken at the end of exponential phase and in stationary phase. Cells were diluted 1:100 in PBS and 50 μl of cell suspension was analyzed for fluorescence intensity of the eYFP reporter (FI eYFP) of C. glutamicum K9 strains in an Amnis® CellStream® device (Luminex) equipped with a 488 nm laser (emission wavelength 528 nm). The flow speed was set to “slow.” The laser powers were: 15% for forward scatter (FSC) and side scatter (SSC) and 40% for the 488 nm laser (excitation of eYFP). Bacteria were gated based on FSC and SSC and FI eYFP was recorded for at least 5,000 bacteria per sample. Flow cytometry data was analyzed using the FlowJo analysis software (v10).
2.7 Proteomic analysis
For proteomic analysis, C. glutamicum ATCC13032 was cultivated in a four-fold parallel bioreactor setup (DASGIP® Parallel Bioreactor System, Eppendorf SE, Germany) with a filling volume of 1 L each. Bacteria were grown in CGXII-U medium without MOPS and pH was maintained at defined levels (pH = 5 or 8) using 4 M KOH or 6 M H3PO4. Ample oxygen supply was guaranteed by using an oxygen controller set to 30% dissolved oxygen and using a cascade of stirrer speed, gas flow and oxygen concentration in inlet air. The bioreactors were inoculated to an OD600 of 1.5. Samples for proteome analysis were taken in exponential and stationary growth phase in technical triplicates. For each replicate, 1.6 ml culture broth were samples to a proteome vial and centrifuged for 5 min at 4°C and 21,500 × g. The supernatant was discarded, and pellets were frozen in liquid nitrogen and stored at −80°C. Proteomic analysis was carried out as described elsewhere (Unrean et al., 2018). The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE (Perez-Riverol et al., 2022) partner repository with the dataset identifier PXD046499.
3 Results
3.1 Identification of a suitable Sec-dependent secretion signal
To establish Sec-dependent secretion of GarQ, an existing library of known Sec-dependent SPs of Bacillus subtilis was used to replace the native double-glycine-type signal sequence by automized plasmid construction (Müller et al., 2022). This yielded constructs of 23 different N-terminal SPs fused to the GarQ-coding sequence. Following transformation into C. glutamicum, all strains were cultivated in CGXII-U medium and GarQ activity was determined in culture supernatants after 24 h of cultivation by pHluorin2 assay (Figure 1A). A significant response of the sensor L. lactis IL1403/pNZ-pHin2Lm was detected with supernatants of a total of 10 strains of the library. Highest activity and thus most efficient secretion was observed with the SP of the ywaD gene (SPywaD) and the corresponding SPywaD-garQ fusion was selected for further experiments.
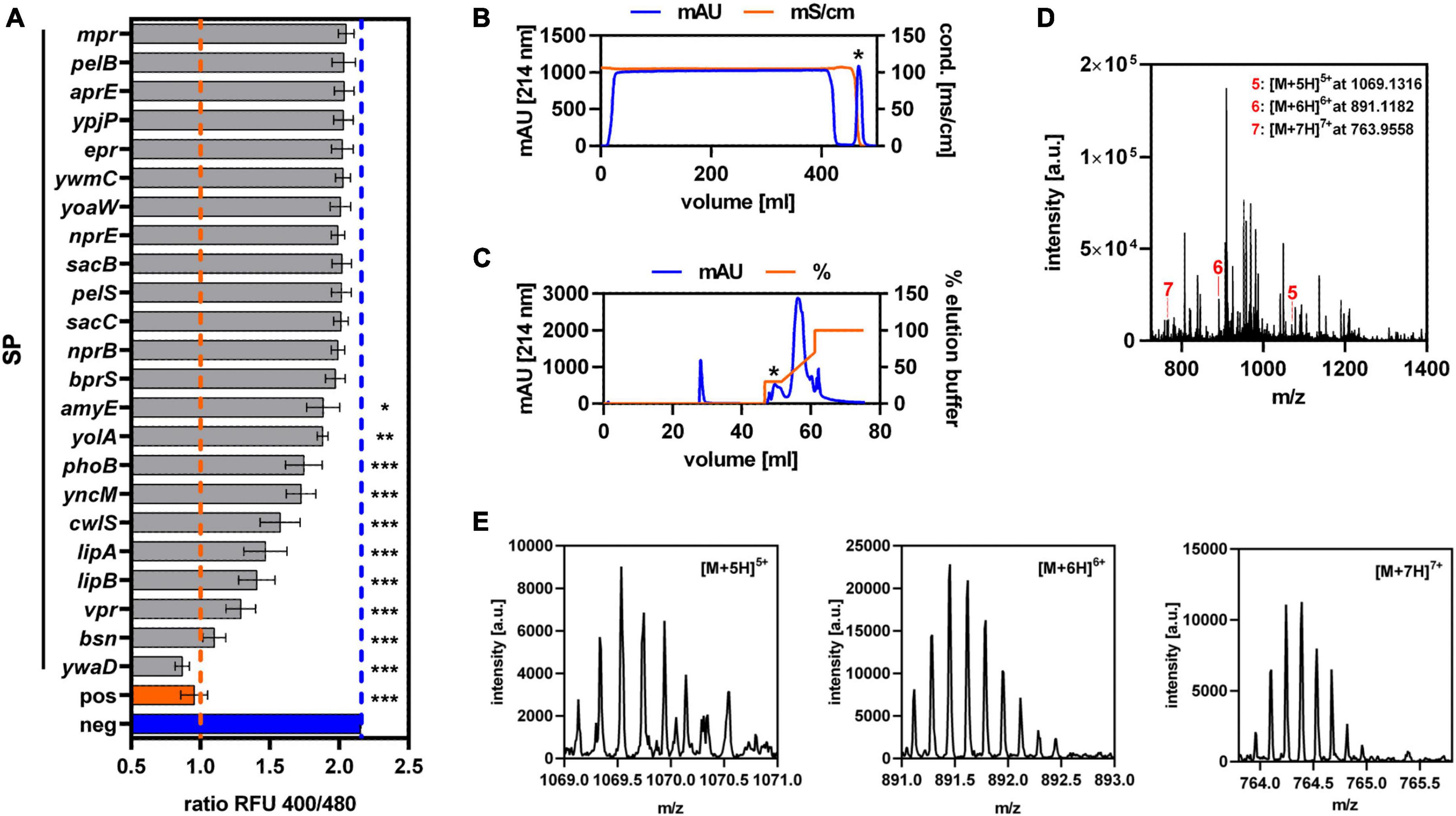
Figure 1. Establishment of Sec-dependent secretion of GarQ by C. glutamicum. (A) Screening of 23 different Sec-dependent SPs of Bacillus subtilis for secretion of GarQ by pHluorin2 assay using L. lactis IL1403/pNZ-pHin2Lm as biosensor. Values are ratios of fluorescence intensity (emission at 520 nm) of the biosensor after excitation at 400 and 480 nm (ratio RFU 400/480) and are mean ± standard deviation (SD) of three independent experiments (i.e., supernatants of independent cultivations of the strains of the SP library). As controls, biosensors treated with purified GarQ (pos) or buffer only (neg) were used. Statistical analysis was performed by ANOVA with the Bonferroni post-test to calculate p-values adjusted for multiple comparisons and untreated biosensors (i.e., neg) set as the control condition (*p < 0.05; **p < 0.01; ***p < 0.001). (B,C) Purification of GarQ from supernatants of C. glutamicum CR099/pXMJ19-SPywaD-garQ-v2 by hydrophobic interaction chromatography (B) and reverse-phase chromatography (C). Peaks containing antimicrobial activity as indicated by pHluorin2 assays (marked by *) were selected for further purification and mass spectrometry. (D) LC–MS analysis of combined RPC elution fraction harboring antimicrobial activity. The indicated peaks (red lines and numbers) with m/z ratios corresponding to the calculated monoisotopic mass of GarQ (5340.63 Da) carrying five (5: [M+5H]5+, m/z = 1069.1316), six (6: [M+6H]6+, m/z = 891.1182), or seven (7: [M+7H]7+, m/z = 763.9558) positive charges. (E) Zoom on the peaks of the [M+5H]5+ (m/z spacing: 0.200), [M+6H]6+ (m/z spacing: 0.167), and [M+7H]7+ (m/z spacing: 0.143) ions indicated in panel (D). In (B–E) results of one representative of at least three independent experiments are shown.
As a remnant of the cloning procedure, GarQ derivatives expressed using the constructs of the SP library contain a linker of two amino acid residues (glutamate and phenylalanine) at the N-terminus of GarQ following cleavage by the signal peptidase. To obtain GarQ by Sec-dependent secretion without these two amino acids, we generated a second version of our construct. Both strains, C. glutamicum CR099/pXMJ19-SPywaD-garQ-v1 (with linker) and C. glutamicum CR099/pXMJ19-SPywaD-garQ-v2 (without linker), showed comparable growth characteristics and had comparable levels of GarQ activity in their supernatants (Supplementary Figure 1). This indicates that presence/absence of the linker had no effect on activity and/or secretion. To ensure that GarQ is secreted and processed properly by the Sec translocon, the active compound was purified from culture supernatants of C. glutamicum CR099/pXMJ19-SPywaD-garQ-v2 by sequential HIC and RPC (Figures 1B, C). Three distinct peaks with mass/charge ratios (m/z) of 1069.1316, 891.1182, or 763.9558, respectively, were identified by LC-MS of pooled RPC fractions containing antimicrobial activity (Figures 1D, E). These peaks correspond to m/z of the five-, six and seven-fold protonated ions of GarQ and are in good agreement with the calculated monoisotopic mass of natural GarQ (5340.63 Da). Trypsin digestion and subsequent mass spectrometry analysis further confirmed the presence and correct processing of GarQ in culture supernatants of the recombinant C. glutamicum strain (data not shown).
For better estimation of mass concentrations of GarQ, we established calibration curves for quantification of GarQ by the pHluorin2 assay using standards of chemically synthesized GarQ of known concentration serially diluted in CGXII-U medium (Supplementary Figure 2). Using this approach, we determined that C. glutamicum CR099/pXMJ19-SPywaD-garQ-v2 produces 7.2 ± 1.5 mg L–1 GarQ after cultivation in CGXII-U medium for 24 h. This is about 3.5-fold higher than levels observed with the previously published GarQ production strain C. glutamicum CR099/pPBEx2-garQICDCgl (Desiderato et al., 2022) under the same conditions (2.0 ± 0.3 mg L–1). However, it is still by far lower than GarQ levels in supernatants of the native producer L. petauri B1726 grown in GM17 for 24 h (198.6 ± 5.6 mg L–1).
3.2 Extracellular pH affects adsorption of GarQ to C. glutamicum
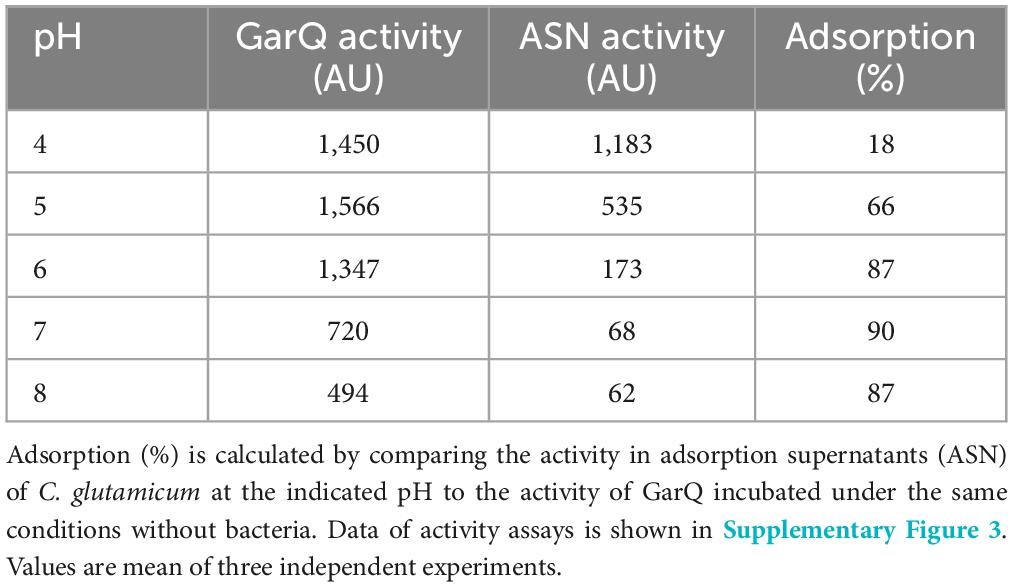
Production of bacteriocins by their native producers is affected by pH and generally LAB produce higher titers in acidic conditions (Abbasiliasi et al., 2017). Likewise, we previously reported that acidic pH enhances recombinant production of pediocin PA-1 and GarQ by C. glutamicum (Desiderato et al., 2022; Christmann et al., 2023). Also, GarQ is strongly adsorbed by C. glutamicum and other bacterial cells (Desiderato et al., 2022) at neutral pH and we have made similar observations for other bacteriocins including nisin Z and pediocin PA-1 (data not shown). To better understand the adsorption of GarQ by C. glutamicum cells, we tested the impact of different pH values (4, 5, 6, 7, and 8) on adsorption (Table 2; Supplementary Figure 3).
In general, we observed a decrease in adsorption at lower pH. At pH = 6–8, about 90% of the peptide adsorbed to the cells. At pH = 5 adsorption was reduced to 66% and at pH = 4 only 18% of the peptide were adsorbed by bacteria. Concomitantly, we also observed that activity of GarQ was reduced at pH = 7–8.
3.3 CaCl2 and Tween 80 reduce adsorption of GarQ and increases GarQ levels in supernatants
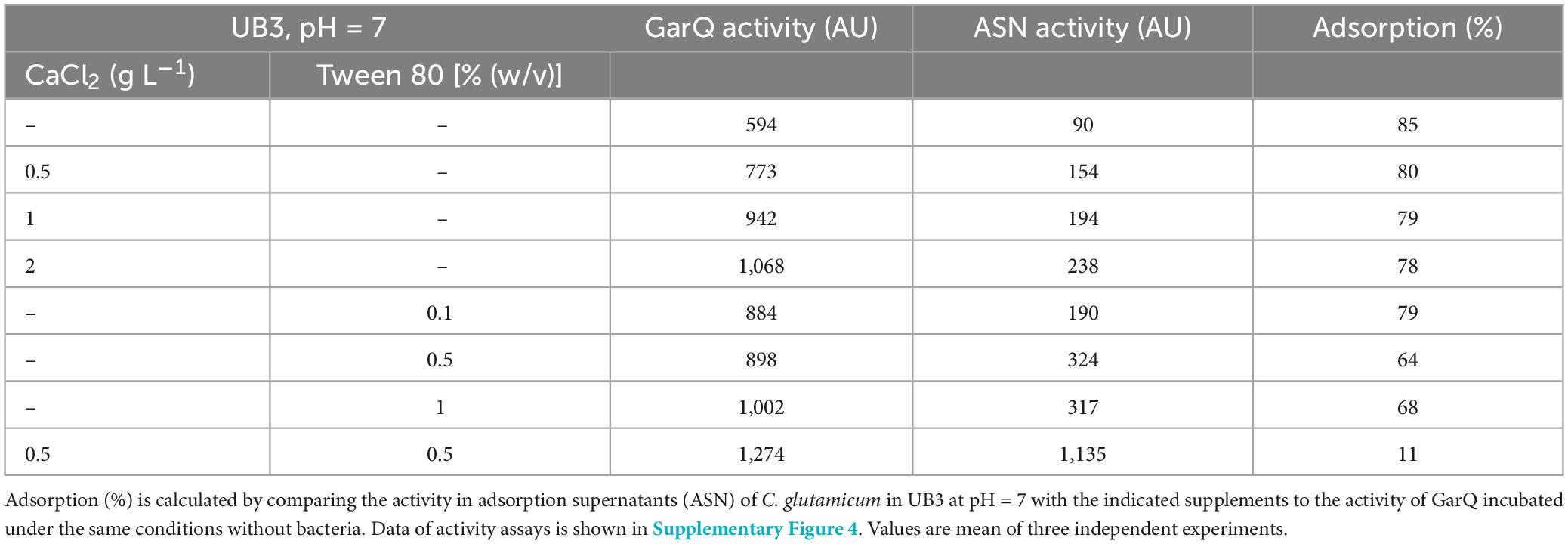
To reduce adsorption of GarQ to biomass of C. glutamicum and improve release into the supernatant, we sought to modify cultivation conditions. In previous studies, CaCl2 was shown to improve the heterologous production of secreted proteins with C. glutamicum (Teramoto et al., 2011; Freier et al., 2016), to improve resistance of C. glutamicum to nisin, and to increase recombinant pediocin PA-1 production (Weixler et al., 2022; Christmann et al., 2023). Tween 80 is a non-ionic detergent that is used as a solubilizer for proteins and as emulsifying agent in food (Linke, 2009; Duquesne and Sturgis, 2010). Thus, we tested the effects of CaCl2 and Tween 80 on adsorption of GarQ to C. glutamicum (Table 3; Supplementary Figure 4).
The effects of Tween 80 and CaCl2 individually were rather moderate. Supplementation with 0.5–2 g L–1 CaCl2 reduced adsorption to ∼80% and the addition of 0.5% (w/v) Tween 80 reduced adsorption to 64%. Interestingly, a combination of 0.5 g L–1 CaCl2 and 0.5% (w/v) Tween 80 strongly reduced adsorption of GarQ to 11%, suggesting a synergistic effect.
Based on these results, we cultivated C. glutamicum CR099/pXMJ19-ywaDSPgarQ-v2 in CGXII-U, CGXII-U+C (containing 0.5 g L–1 CaCl2), CGXII-U+T [with 0.5% (w/v) Tween 80], or CGXII-U+CT (containing both supplements) and compared GarQ levels in supernatants (Figure 2). Supplementation with CaCl2 alone increased GarQ titers at all timepoints analyzed and a maximum of 15.1 ± 0.4 mg L–1 GarQ was observed after 24 h. When only Tween 80 was supplemented, the GarQ production in exponential phase was comparable to that CGXII-U without supplements (2.6 ± 0.4 mg L–1) at t = 4 h but decreased in stationary phase. In CGXII-U+CT, we observed increased GarQ levels after 4 h of cultivation (13.3 ± 0.7 mg L–1) but the activity decreased drastically to 2.0 ± 0.5 mg L–1 after 24 h.
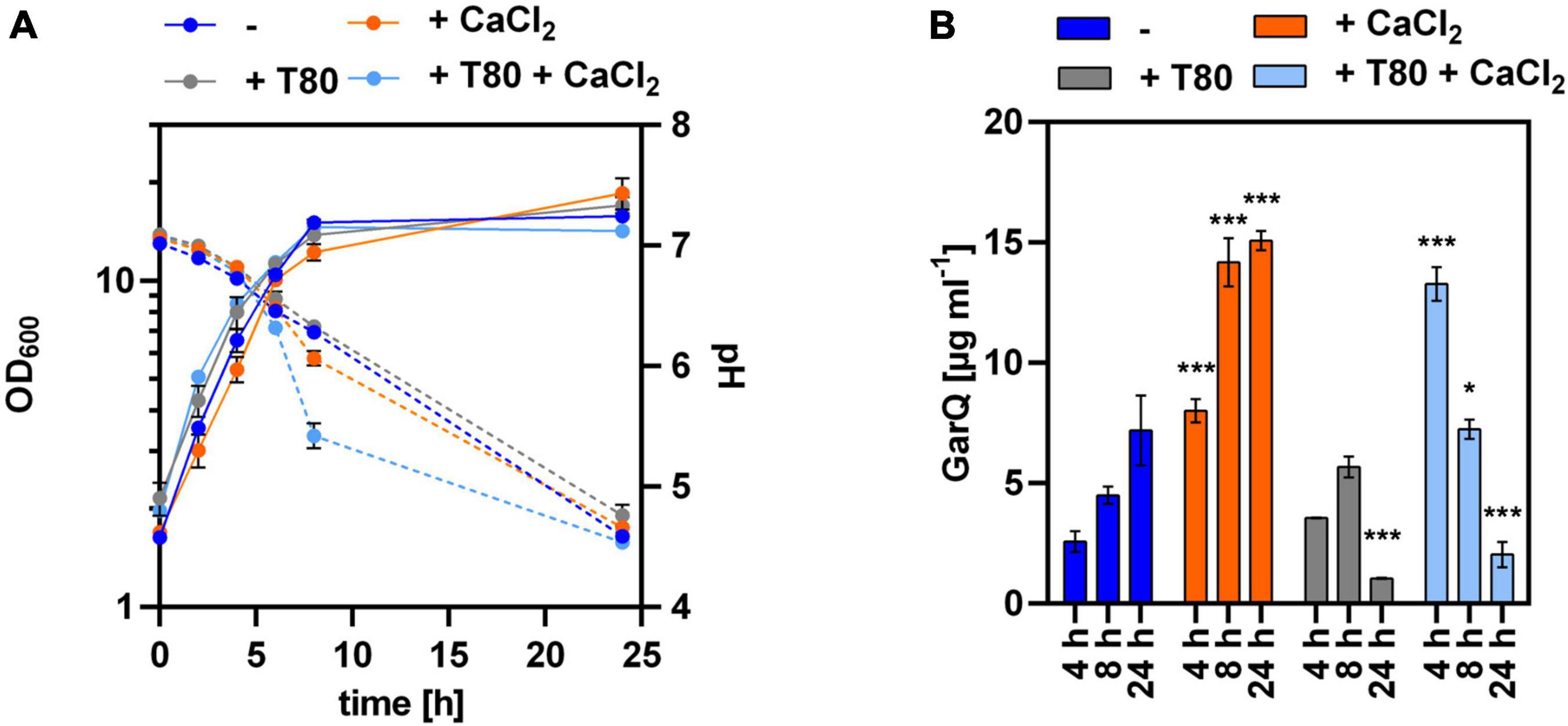
Figure 2. Impact of Tween 80 and CaCl2 supplementation on recombinant GarQ production by C. glutamicum. (A) Biomass (OD600; solid lines) and pH (broken lines) in supernatants of C. glutamicum CR099/pXMJ19-SPywaD-garQ-v2 during cultivation in CGXII-U (dark blue; -), CGXII-U+C (orange; +CaCl2), CGXII-U+T (gray, T80), or CGXII-U+CT (light blue, T80+CaCl2). (B) Concentrations of GarQ in supernatants of these cultures at the indicated timepoints were determined by pHluorin2 assays using L. lactis IL1403/pNZ-pHin2Lm as biosensor. Mass concentrations were calculated using a calibration curve established with standards of synthetic GarQ. All values are mean ± SD of n = 3 independent cultivations per condition. Statistical analysis was performed comparing levels of GarQ between conditions at each timepoint by ANOVA and Bonferroni’s post-test to calculate p-values adjusted for multiple comparisons with cultures in CGXII-U (–) set as control condition (*p < 0.05; ***p < 0.001).
3.4 A C. glutamicum strain lacking HtrA protease shows increased GarQ production
Corynebacterium glutamicum is generally believed to have little to no extracellular protease activity (Vertès, 2013). However, the dramatic decrease in GarQ activity in supernatants of C. glutamicum CR099/pXMJ19-ywaDSPgarQ-v2 grown in CGXII-U+CT at later timepoints of the cultivation prompted us to reconsider proteolytic degradation as a potential problem under these conditions. For other bacteria, it is described that high level heterologous expression of secreted proteins and presence of bacteriocins induces cell envelop/secretion stress and expression of proteases for cleavage of unfolded or misfolded proteins (Jordan et al., 2008; Neef et al., 2020). A protease previously associated with Sec-dependent secretion stress in C. glutamicum is HtrA (Jurischka et al., 2020). This was reported for C. glutamicum K9, a biosensor that reports secretion stress by expression of the yellow fluorescent protein eYFP from a gene cassette replacing the chromosomal htrA gene. To investigate whether HtrA may play a role in reducing GarQ levels, we performed similar experiments under the conditions of GarQ production. C. glutamicum K9 was transformed with pXMJ19-SPywaD–garQ-v2 and the obtained strain as well as its two parental strains K9 and ATCC13032 were grown in standard CGXII or CGXII-U, and eYFP fluorescence was analyzed by flow cytometry (Figure 3).
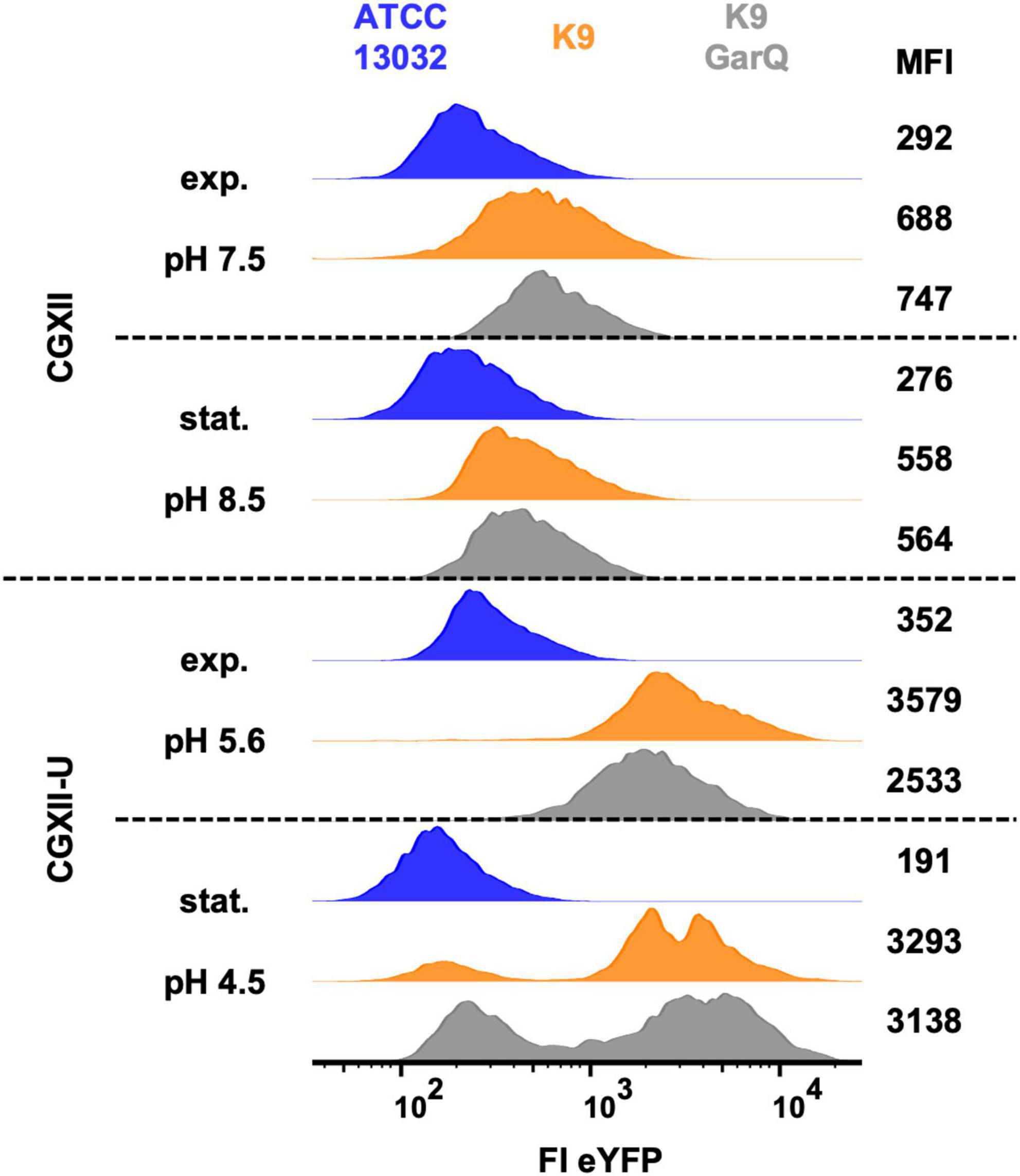
Figure 3. Analysis of C. glutamicum ATCC13032 (blue), K9 (orange), and K9/pXMJ19-SPywaD-garQ-v2 (K9-GarQ, gray) for htrA-reporter activity. All strains were cultivated in CGXII (upper panels) or CGXII-U (lower panels). Samples for flow cytometry were taken in exponential (exp.) or stationary (stat.) growth phase, pH in supernatants was measured and single bacteria were analyzed for eYFP fluorescence intensity (FI-eYFP) by flow cytometry. Shown are histogram plots of FI eYFP of at least n = 5,000 bacterial cells per condition with mean fluorescence intensity (MFI). Results of one representative of at least two independent experiments per condition are shown.
In normal CGXII medium, pH was slightly increased to 8.5 in stationary growth phase and the two K9 strains showed only marginally increased eYFP fluorescence (approx. 2-fold higher mean fluorescence intensity; MFI) compared to the negative control, i.e., wildtype strain ATCC13032. As observed previously, pH in cultures grown in CGXII-U dropped markedly to 5.6 in exponential and to 4.5 in stationary growth phase. At the same time, eYFP expression of the two K9 strains was markedly increased. Compared to the wildtype strain ATCC13032, MFI of C. glutamicum K9 was increased approx. 10-fold and for C. glutamicum K9/pXMJ19-SPywaD-garQ-v2 a 7-fold increase in eYFP fluorescence was observed. Although for a subpopulation of these strains eYFP fluorescence returned to baseline in stationary growth phase, still a considerable fraction remains eYFP positive. No clear difference was observed between C. glutamicum K9 and K9/pXMJ19-SPywaD–garQ-v2. These results indicate that HtrA expression is increased in acidic environments but is not affected by expression/secretion of GarQ.
Additionally, LC-MS proteome analysis of C. glutamicum ATCC13032 cultivated in bioreactors in CGXII-U without MOPS at defined pH values confirmed that HtrA levels are increased at acidic extracellular pH. At pH = 5, signals for HtrA protein were increased 7.6-fold (p = 2.09E-08) and 2.4-fold (p = 0.01) in exponential and stationary growth phase, respectively, compared to pH = 8.
Based on these results, we hypothesized that increased expression of htrA under the conditions of GarQ production may cause proteolytic degradation of the product and result in decreased activity in supernatants at later timepoints of the fermentation. To test this hypothesis, we analyzed GarQ production by C. glutamicum K9/pXMJ19-SPywaD–garQ-v2 lacking a functional htrA gene (Figure 4). When grown in CGXII-U+CT, GarQ titers of 38.6 ± 3.3 mg L–1 were observed after 4 h of cultivation. This is a ∼3-fold increase compared to the highest GarQ levels observed with C. glutamicum CR099/pXMJ19-SPywaD–garQ-v2, i.e., the strain harboring an intact htrA gene (Figure 2). Also, GarQ concentrations remained high at t = 8 h of fermentation. Of note, lack of a functional htrA gene had no effect on GarQ levels in CGXII-U, CGXII-U+C or CGXII-U+T. This suggests that HtrA causes proteolytic degradation of GarQ when expressed and deletion of htrA improves production only under the optimized conditions, i.e., growth in CGXII-U+CT, i.e., when both CaCl2 and Tween 80 are supplemented. Nevertheless, we still observed a decrease in GarQ activities at later timepoints of the fermentation (14.6 ± 2.8 mg L–1 GarQ at t = 24 h) indicating that there are also other factors that reduce levels of the active product.
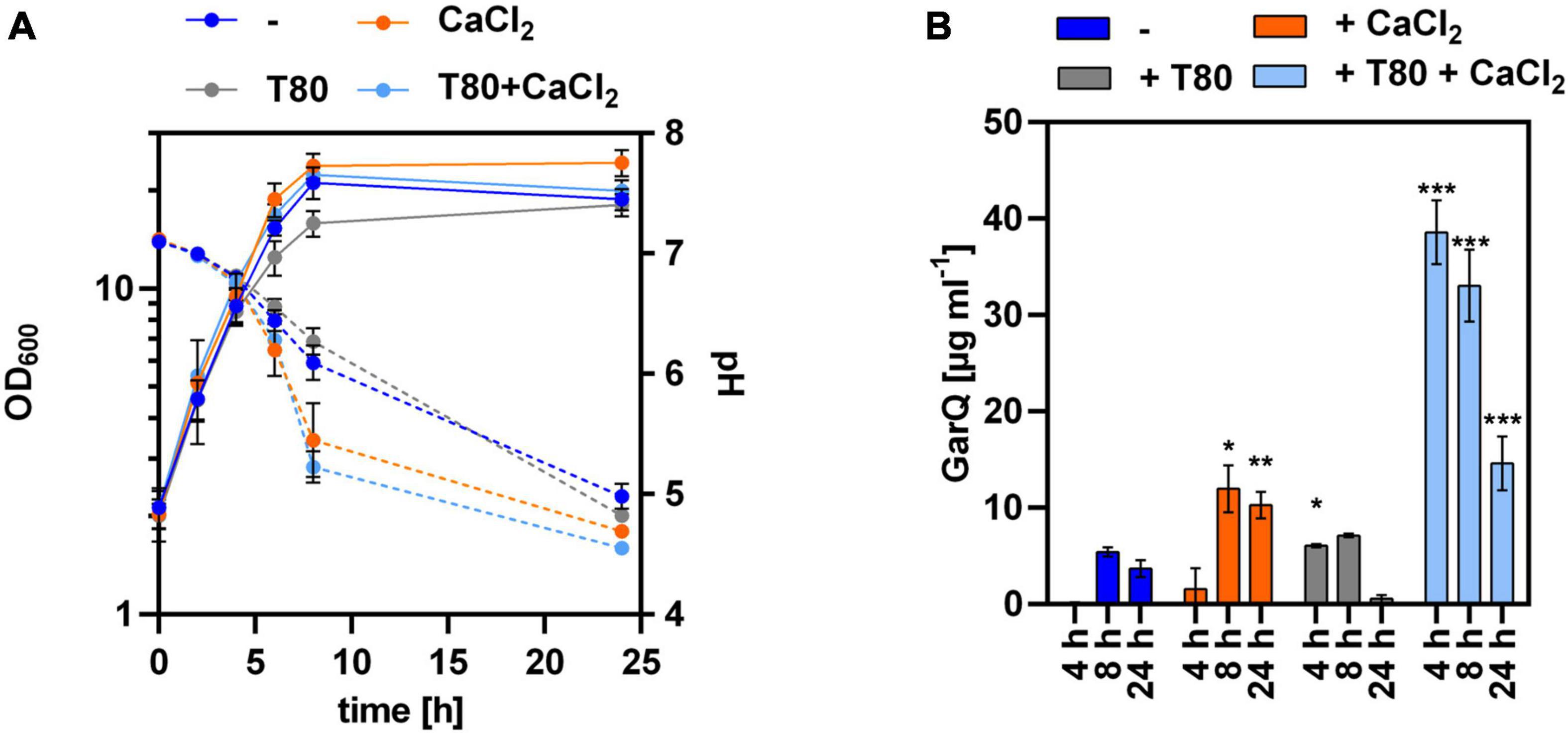
Figure 4. Lack of HtrA protease increases GarQ levels produced by C. glutamicum. (A) Biomass (OD600; solid lines) and pH (broken lines) in supernatants of C. glutamicum K9/pXMJ19-SPywaD-garQ during cultivation in CGXII-U (dark blue; -), CGXII-U+C (orange; +CaCl2), CGXII-U+T (gray, T80), or CGXII-U+CT (light blue, T80+CaCl2). (B) Concentrations of GarQ in supernatants of these cultures at the indicated timepoints were determined by pHluorin2 assays using L. lactis IL1403/pNZ-pHin2Lm as biosensor. Mass concentrations were calculated using a calibration curve established with standards of synthetic GarQ. All values are mean ± SD of n = 3 independent cultivations per condition. Statistical analysis was performed comparing levels of GarQ between conditions at each timepoint by ANOVA and Bonferroni’s post-test to calculate p-values adjusted for multiple comparisons with cultures in CGXII-U (–) set as control condition (*p < 0.05; **p < 0.01; ***p < 0.001).
3.5 Cultivation under low oxygen conditions improves recombinant GarQ production
Recently, we were able to show that fermentation under low oxygen conditions in bioreactor or non-baffled flasks improves production of the class IIa bacteriocin pediocin PA-1 (Goldbeck et al., 2021; Christmann et al., 2023). A possible explanation for this effect is that pediocin PA-1 is (partially) inactivated by oxidation of the methionine residue in position 31 (Bédard et al., 2018). Similar to pediocin PA-1, GarQ contains a methionine residue at position 5 of the active peptide. Hence, inactivation of the peptide by oxidation of this methionine may occur. Indeed, re-evaluation of the LC-MS data of the purified GarQ fraction (Figure 1) revealed that mass spectra also contained signals with of 1072.326 m/z (spacing: 0.200) and 893.772 m/z (spacing: 0.167) corresponding to the five- and six-fold protonated ions of oxidized GarQ (Supplementary Figure 5A). We also observed a loss of GarQ activity by about 50% after overnight incubation at 30°C, 130 rpm compared to samples stored at −20°C (Supplementary Figure 5B).
To test if reduced aeration might positively impact on GarQ production, we cultivated C. glutamicum K9/pXMJ19-SPywaD–garQ-v2 in non-baffled shake flasks (Figure 5) and observed a slight increase in GarQ titers to 47.6 ± 1.3 mg L–1 in cultures grown in CGXII-U+CT at 4 h of fermentation. However, in contrast to all previous experiments GarQ concentrations further increased toward the end of the fermentation and reached a maximum of 102.8 ± 1.1 mg L–1 after 24 h of cultivation. Similarly, increased levels of GarQ were observed in CGXII-U+T at 4 h (18.6 ± 3.2 mg L–1) and 8 h (56.0 ± 12.1 mg L–1) of cultivation. Of note, reduced aeration had no impact on GarQ levels in CGXII-U or CGXII-U+C.
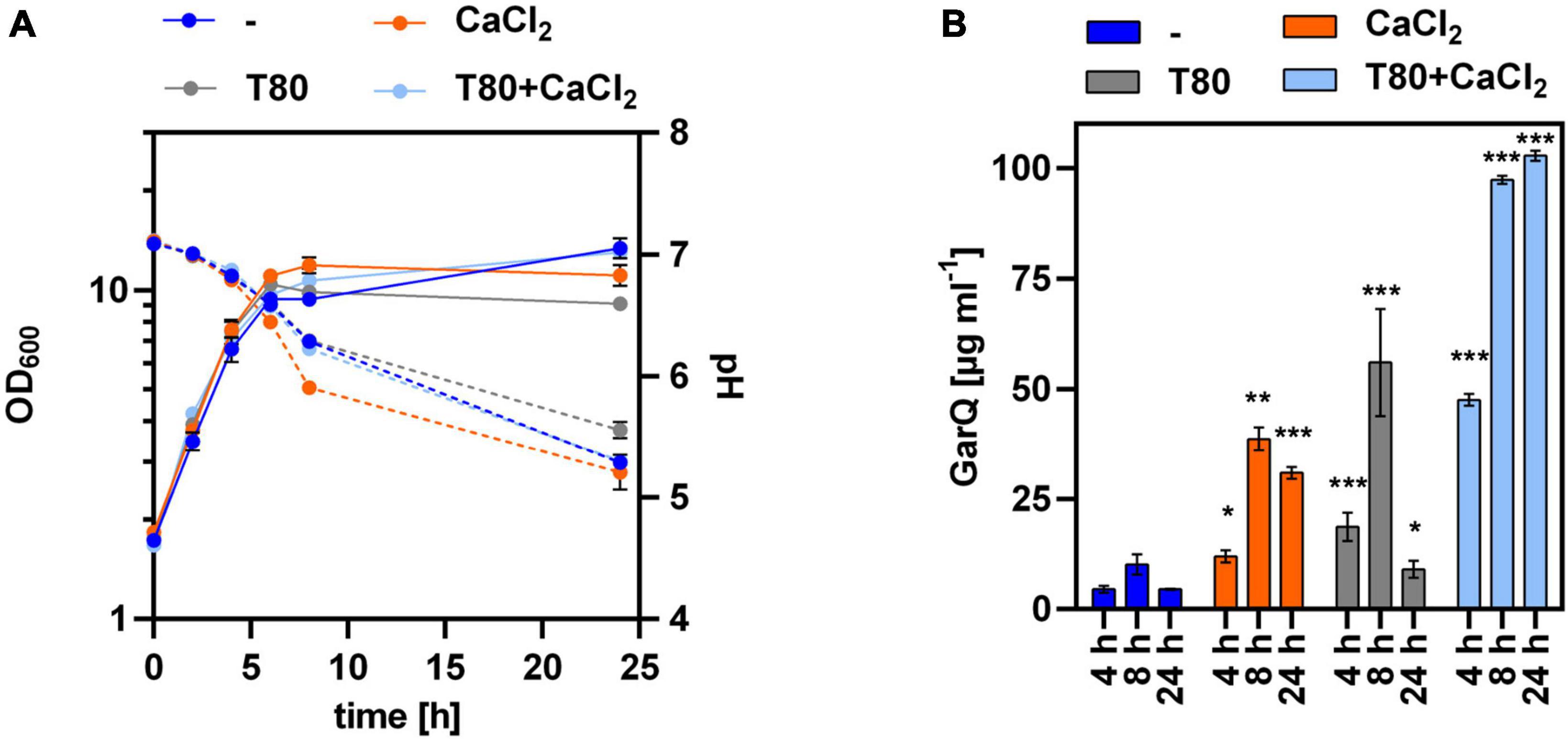
Figure 5. GarQ production by C. glutamicum is increased in conditions of reduced aeration. (A) Biomass (OD600; solid lines) and pH (broken lines) in supernatants of C. glutamicum K9/pXMJ19-SPywaD-garQ during cultivation in CGXII-U (dark blue; -), CGXII-U+C (orange; +CaCl2), CGXII-U+T (gray, T80), or CGXII-U+CT (light blue, T80+CaCl2) in non-baffled flasks. (B) Concentrations of GarQ in supernatants of these cultures at the indicated timepoints determined by pHluorin2 assays using L. lactis IL1403/pNZ-pHin2Lm as biosensor. Mass concentrations were calculated using a calibration curve established with standards of synthetic GarQ. All values are mean ± SD of n = 3 independent cultivations per condition. Statistical analysis was performed comparing levels of GarQ between conditions at each timepoint by ANOVA and Bonferroni’s post-test to calculate p-values adjusted for multiple comparisons with cultures in CGXII-U (–) set as control condition (*p < 0.05; **p < 0.01; ***p < 0.001).
3.6 Activity of a natural GarQ variants
Activity of the class II bacteriocin pediocin PA-1 was shown to be sensitive to oxidation of a methionine residue. As GarQ contains a methionine in position 5 of the active peptide, we hypothesized that GarQ may be inactivated by oxidation of this methionine and cultivation in low oxygen conditions may reduce oxidative inactivation Garvicin AG2 is a natural variant of GarQ, in which the methionine in position 5 is changed to a phenylalanine (Maldonado-Barragán et al., 2022). To test if this peptide may be protected against oxidative inactivation, we purchased a synthetic GarQ variant with the M5F amino acid exchange (GarQM5F) from a commercial supplier and compared its activity to native GarQ in pHluorin2 assays using L. lactis LMG2785/pNZ-pHinLm or L. monocytogenes EGDe/pNZ-pHinLm (Figure 6A).
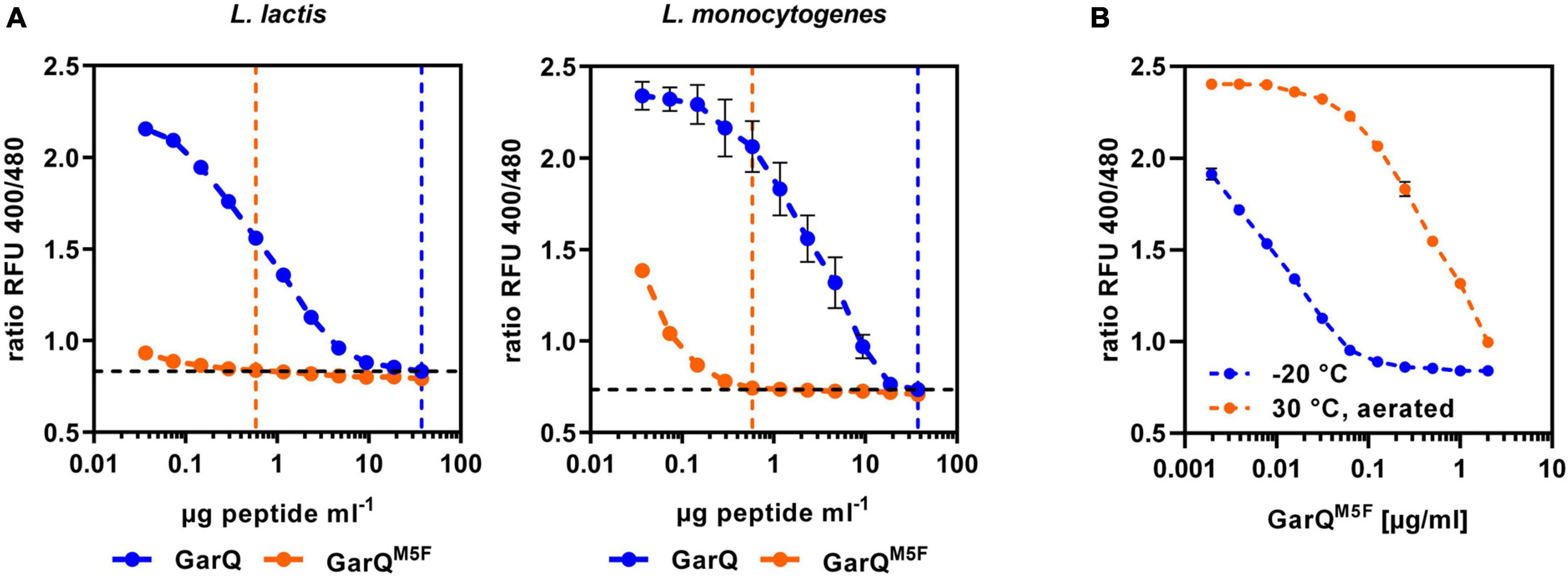
Figure 6. A natural variant of GarQ shows altered properties. (A) Activity of GarQ (dark blue) and GarQM5F (orange) or (B) GarQM5F incubated O/N at 30°C with aeration on a rotary shaker (orange) or stored at –20°C (blue) was determined by pHluorin2 assays. L. lactis IL1403/pNZ-pHin2Lm [left panel in (A,B)] or L. monocytogenes EGDe/pNZ-pHin2Lm [right panel in (A)] were used as sensors. Values are ratios of fluorescence intensity of the biosensor (emission at 520 nm) after excitation at 400 and 480 nm (ratio RFU 400/480) and are mean ± SD of n = 3 independent experiments.
Surprisingly, GarQM5F has markedly higher activity against both biosensors than the native peptide as concentration of GarQM5F to achieve a complete shift in fluorescence ratios was 64-fold lower than for GarQ. To test if GarQM5F is also protected against oxidative inactivation, we performed activity assays of GarQM5F after incubation O/N at 30°C with aeration or stored at −20°C. In contrast to our predictions, activity of GarQM5F was reduced by aeration (Figure 6B) and this effect was even more pronounced than oxidative inactivation of the wildtype GarQ (Supplementary Figure 5). This indicates that GarQM5F has markedly improved activity, but oxidation of the methionine seems not to be the reason for reduced activity of GarQ after prolonged cultivation or incubation under oxygenated conditions.
Based on the interesting properties of GarQM5F, we generated a C. glutamicum production strain again employing Sec-dependent secretion using the YwaD SP. The strain C. glutamicum K9/pXMJ19-SPywaD-garQM5F was cultivated in CGXII-U+CT in baffled flasks and activities were determined in supernatants at different timepoints during cultivation (Figure 7). Surprisingly, activities were approx. 40-fold lower than with the isogenic strain producing wildtype GarQ cultivated under the same conditions (Figure 4). As observed in all other experiments conducted in baffled flasks, GarQM5F activity was decreased by 10-fold at 24 h of incubation again supporting that methionine oxidation is not the reason for loss in activity of GarQ and GarQM5F during recombinant production under high oxygen conditions.
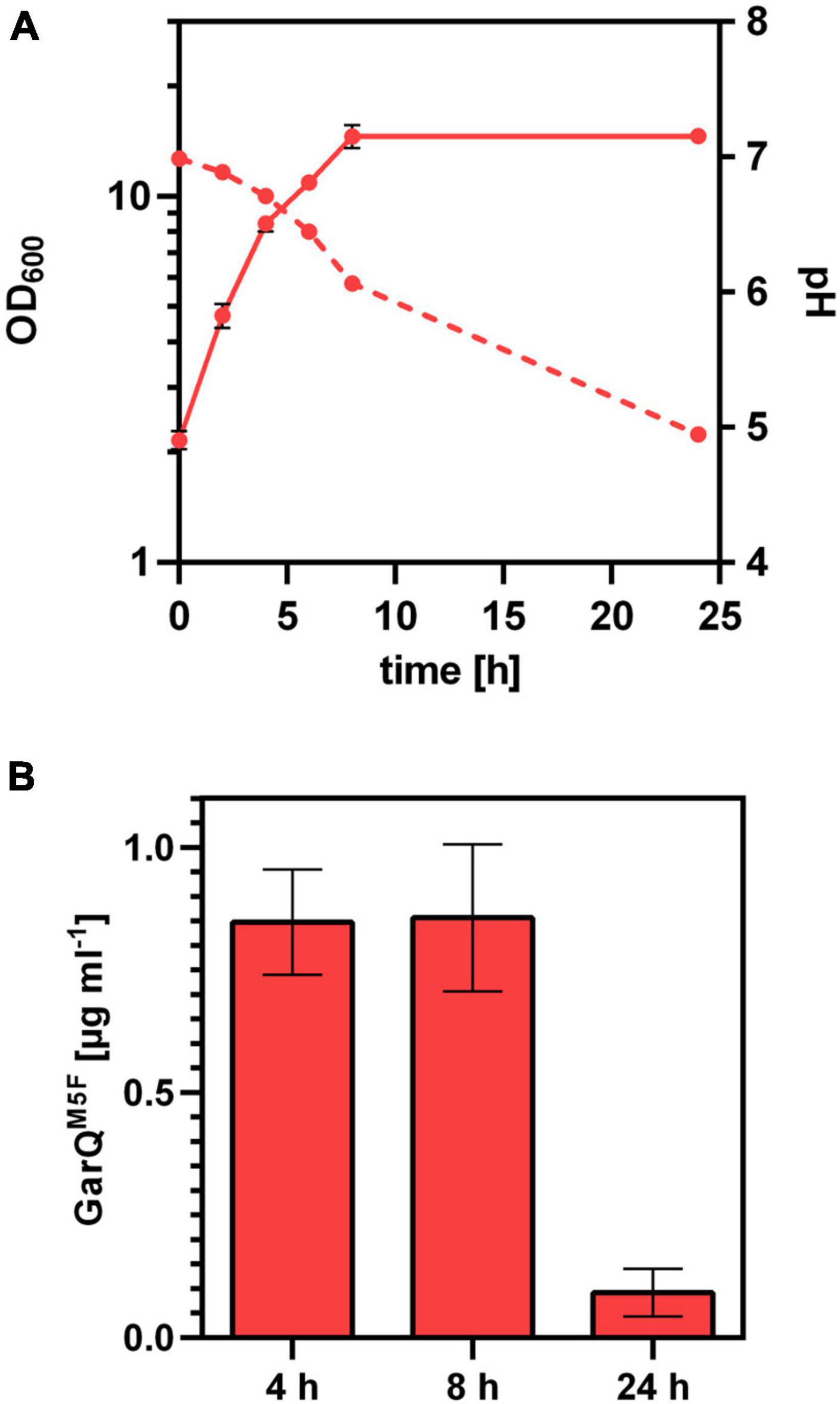
Figure 7. Recombinant production of GarQM5F. (A) Biomass (OD600; solid lines) und pH (broken lines) in supernatants of C. glutamicum K9/pXMJ19-SPywaD-garQM5F during cultivation in CGXII-U+CT. (B) Concentration of GarQM5F in supernatants of these cultures at the indicated timepoints were determined by pHluorin2 assays using L. lactis IL1403/pNZ-pHin2Lm as biosensor. Mass concentrations were calculated using a calibration curve established with standards of synthetic GarQM5F. All values are mean ± SD of n = 3 independent cultivations per condition.
4 Discussion
Bacteriocins are a heterogeneous group of antimicrobial peptides and proteins produced by bacteria and a few of these peptides are approved and marketed as preservatives in food and beverages and in animal and pet feed (Mills et al., 2011; Chikindas et al., 2018; Silva et al., 2018). As antibiotic resistant variants of a wide range of infectious agents are an increasing concern of human health on a global scale and some bacteriocins are active against these antibiotic resistant pathogens (Ovchinnikov et al., 2016, 2017, 2020), bacteriocins also offer opportunities to combat these infections. However, commercial available bacteriocins are produced exclusively with their natural producers organisms on complex media and are sold as semi-purified preparations or crude fermentates, which limits their use in clinical applications (Abbasiliasi et al., 2017; Johnson et al., 2017; Juturu and Wu, 2018).
To allow easy and cost-effective production and purification at industrially relevant titers, we recently established C. glutamicum as a host for recombinant bacteriocin production (Goldbeck et al., 2021; Desiderato et al., 2022; Weixler et al., 2022; Christmann et al., 2023). The approach applied so far is based on expression systems for the prepeptide of a bacteriocin together with the modification and secretion machineries of the native producer. This strategy is essential for bacteriocins containing post-translational modifications such as nisin but might not be required for linear and non-modified peptides. In fact, it was shown for several bacteriocins that the double glycine SPs can be successfully replaced by Sec-dependent SPs (McCormick et al., 1996; Martín et al., 2007; Borrero et al., 2011; Back et al., 2016) and recently a fusion of the SP of lactococcal protein Usp45 and GarQ allowed recombinant GarQ production by L. lactis (Feito et al., 2023). To identify a suitable Sec-dependent SP for GarQ secretion by C. glutamicum we screened an available library of B. subtilis SPs. This library was previously used to screen for secretion of cutinase alone or fused to split-GFP in both B. subtilis and C. glutamicum (Hemmerich et al., 2016; Müller et al., 2022) and it was shown that efficient expression is affected by differences in G+C content of the coding sequences for SP (from low G+C B. subtilis) and the target protein itself (which when derived from C. glutamicum has high G+C content). For recombinant expression, the same signal peptide was used for GarQ and the GarQM5F variant yet a 40-fold difference in active peptide were observed. Further studies are needed to investigate, why a single amino acid exchange at the N-terminus of the mature bacteriocin close to the SP cleavage site has such a profound impact on secretion efficiency.
Bacteriocins are generally hydrophobic and positively charged peptides and a number of studies show adsorption of bacteriocins to the negatively charged cell envelope of bacteria (Parente et al., 1994; De Vuyst et al., 1996; Callewaert and De Vuyst, 2000). This may constitute a major bottleneck for recombinant bacteriocin production. Indeed, we previously observed that GarQ efficiently adsorbs to various sensitive and insensitive bacteria including C. glutamicum (Desiderato et al., 2022). As C. glutamicum does not encode the genes for a PTSMan and is resistant to GarQ (Desiderato et al., 2022), absorption of GarQ is independent of the described receptor and is probably caused by electrostatic interactions of the cationic peptide with the negatively charged cell envelope. In the present study, we confirm and extend these results showing that adsorption of GarQ to C. glutamicum is pH-dependent and decreases at acidic pH as observed for several other bacteriocins (Yang et al., 1992). This may explain why acidification of the supernatant during cultivation is needed to obtain measurable GarQ titers with recombinant C. glutamicum strains (Desiderato et al., 2022). Similarly, fermentation at acidic pH boosted recombinant production of pediocin PA-1 with C. glutamicum (Christmann et al., 2023).
To address the problem of high adsorption of GarQ to biomass of the envelope of producer bacteria, we optimized medium composition by supplementation with CaCl2 and Tween 80. For nisin Z it was shown that addition of CaCl2 to the cultivation broth stimulates bacteriocin production in L. lactis (Matsusaki et al., 1996). The authors hypothesized that Ca2+ displaces cell adsorbed nisin. For C. glutamicum, it was shown that supplementation with CaCl2 results in increased production of secreted GFP (Teramoto et al., 2011; Freier et al., 2016) and Freier et al. (2016) hypothesized that CaCl2 might reduce electrostatic interaction of GFP and C. glutamicum cells by neutralizing the negative charges of the cell envelope. Further, supplementation with CaCl2 decreased nisin susceptibility and increased recombinant pediocin PA-1 production of C. glutamicum (Weixler et al., 2022; Christmann et al., 2023). In line with these findings, supplementation with CaCl2 led to a small decrease in adsorption of GarQ and resulted in a 2-fold increase in GarQ production by C. glutamicum.
Detergents are commonly used for solubilization and purification of amphiphilic membrane proteins (Linke, 2009; Duquesne and Sturgis, 2010) and the non-ionic detergent Tween 80 is described to reduce adsorption and increase production of bacteriocins (Garver and Muriana, 1994; Huot et al., 1996; Aymerich et al., 2000; Keren et al., 2004). We found that Tween 80 reduces adsorption of GarQ to C. glutamicum and, in combination, with CaCl2 increases titers of GarQ in supernatants of our C. glutamicum producer strains. Tween 80 also boosted GarQ activity and stabilized activity at neutral to alkaline pH in in vitro assays. An increase of bacteriocin activity in the presence of Tween 80 was also observed for lacticin RM (Keren et al., 2004). The stabilizing effect of Tween 80 on bacteriocin activity at neutral pH might be attributed to increased solubility of the peptide.
It is possible that CaCl2 and/or Tween 80 positively impact on expression of GarQ leading to increased levels of active GarQ in supernatants. However, our expression platform in C. glutamicum consists of an IPTG-inducible promoter (Ptac) on a widely used plasmid (pXMJ19). Teramoto et al. have used a similar plasmid and the same IPTG-inducible promoter to show that CaCl2 at 2 g/L positively affects secretion of GFP (and amylase) by C. glutamicum but had no effect on gfp transcription or levels of cytosolic GFP (Teramoto et al., 2011). Thus, an effect of CaCl2 on garQ transcription is rather unlikely. Similar data on Tween 80 is, to our knowledge not available. To conclusively rule out effects of Tween 80 alone or in combination with CaCl2 on expression of GarQ, more comprehensive analyses by transcriptomics and proteomics are required.
Although C. glutamicum is considered to have low extracellular protease activity (Vertès, 2013; Freudl, 2018), several studies have shown that recombinant protein production by C. glutamicum can be improved by deleting proteases (Peng et al., 2019; Zhang et al., 2019; Liu et al., 2022). In a recent study, we investigated a possible contribution of extracellular protease activity to reduced GarQ levels during production with C. glutamicum (Desiderato et al., 2022). For these experiments, the C. glutamicum CR099 was grown over night in standard 2xTY medium. The conditions used for recombinant production GarQ production in the present study are, however, completely different, i.e., CGXII-U medium and acidic pH of the culture. A protease previously associated with Sec-dependent secretion stress in C. glutamicum is HtrA (Jurischka et al., 2020). HtrA proteases are widely distributed in pro- and eukaryotes and are quality control chaperones that play important roles during heat stress, pH stress and virulence (Hansen and Hilgenfeld, 2013; Backert et al., 2018). In L. monocytogenes and Helicobacter pylori, expression of HtrA is increased at low pH (Merrell et al., 2003; Stack et al., 2005). In line with these observations, we found increased levels of HtrA in C. glutamicum during cultivation under conditions of GarQ production in CGXII-U at acidic pH. More importantly, employing htrA-deficient C. glutamicum K9 for production instead of C. glutamicum CR099 resulted in increased GarQ titers (Figure 4). Similar observation were reported in L. lactis and E. coli showing that deletion of HtrA proteases improved recombinant protein production and stability (Baneyx and Georgiou, 1991; Meerman and Georgiou, 1994; Miyoshi et al., 2002; Cortes-Perez et al., 2006). It is quite possible that HtrA of C. glutamicum is involved in maintaining protein quality at low pH.
Another obstacle for production or storage described for pediocin PA-1 is the loss of activity by oxidation of a methionine residue (Johnsen et al., 2000). We previously observed that titers of active pediocin PA-1 are increased during recombinant production at low levels of dissolved oxygen in bioreactors or reduced aeration in non-baffled flasks (Goldbeck et al., 2021; Christmann et al., 2023). Similarly, cultivation of C. glutamicum K9/pXMJ19-SPywaD-garQ in non-baffled flasks was beneficial for GarQ production. Of note, the natural producer L. petauri B1726 shows high GarQ production during cultivation at static conditions (Desiderato et al., 2022). Wildtype GarQ contains a methionine in position 5 of the active peptide. Loss in activity of GarQ incubated O/N with aeration (Supplementary Figure 5) and increased activity of the GarQM5F compared to GarQ (Figure 6A) support the notion that methionine oxidation may be involved in reduced GarQ in supernatants of C. glutamicum producers at high oxygen levels. However, loss of activity of GarQM5F after O/N incubation with aeration was even more pronounced than for wildtype GarQ (Figure 6B), and reduced activity at the end of fermentations in baffled flasks was also observed for GarQM5F (Figure 7B). This points toward other reasons for loss in activity or product under conditions with high aeration. In general, shaking of protein solutions generates air/liquid interfaces and hydrophobic interactions at these interfaces can lead to protein aggregation (Wang et al., 2010). It is possible that GarQ/GarQM5F are adsorbed to the surface of the glassware used for experiments by hydrophobic interactions. Also, vigorous shaking in baffled flasks may cause aggregation of the hydrophobic peptides GarQ and GarQM5F. This would explain why activity remains on a high level in fermentation in non-baffled flasks. Similar observations were made for production of a secreted hydrophobic protein with Pichia pastoris (Woo et al., 2006). Here, aggregation of the protein was minimized by reducing stirrer speed and area of air/liquid interface.
Previously, we have shown successful production of other bacteriocins with C. glutamicum including pediocin PA-1, another bacteriocin that uses the PTSMan as a receptor. We have also tried to the class I bacteriocin nisin. However, C. glutamicum is highly sensitive to nisin and a number of approaches to increase nisin resistance have failed (Weixler et al., 2021). Thus, we established a two-step process with production of the inactive precursor of nisin, which is then activated during downstream processing (Weixler et al., 2022). Further research is aimed at extending the range of bacteriocins (and other bioactive peptides) that can be produced wit C. glutamicum. However, the strategy for production has to be developed for each new product according to the level of resistance of C. glutamicum.
In summary, we show for the first time recombinant production of a bacteriocin by C. glutamicum using its native Sec translocon. By optimizing media and cultivation conditions and deletion of a quality control protease induced by pH stress, production and secretion of GarQ by C. glutamicum were improved to over 100 mg L–1. This is a 50-fold increase to GarQ levels obtained with a previously published first generation C. glutamicum producer strain employing the GarCD transporter. Although this is still 2-fold lower than GarQ levels obtained with the native producer Lactococcus garvieae production with C. glutamicum offers several advantages. On the one hand, natural producers are BSL2 pathogens while C. glutamicum is a biotechnological workhorse organism and generally recognized as safe. On the other hand, natural producers require rich, complex media to support growth whereas production processes with C. glutamicum on defined minimal media that allow downstream purification in pharmaceutical grade are well established. We also elucidate a number of factors that limit production of GarQ by C. glutamicum. This includes extracellular pH, HtrA protease activity, oxygenation, and, possibly, peptide aggregation. As these factors are not specific for GarQ but are rather related to general features of bacteriocins and/or associated with the production host, our findings may be of relevance for production of other bacteriocins using natural or recombinant producers.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: ProteomeXchange Consortium–PXD046499.
Author contributions
CD: Data curation, Investigation, Visualization, Writing—original draft, Writing—review and editing, Formal analysis. CM: Formal analysis, Investigation, Writing—review and editing, Methodology. AS: Formal analysis, Investigation, Writing—review and editing. BG: Formal analysis, Investigation, Writing—review and editing. KH: Formal analysis, Investigation, Writing—review and editing. BS: Formal analysis, Investigation, Writing—review and editing. VS: Formal analysis, Investigation, Methodology, Writing—review and editing. AR: Formal analysis, Investigation, Methodology, Writing—review and editing. MO: Formal analysis, Methodology, Writing—review and editing, Conceptualization, Funding acquisition, Project administration, Resources, Supervision. BE: Conceptualization, Formal analysis, Funding acquisition, Methodology, Project administration, Resources, Supervision, Writing—review and editing. CR: Conceptualization, Funding acquisition, Methodology, Project administration, Resources, Supervision, Writing—review and editing, Data curation, Investigation, Visualization, Writing—original draft.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was funded by grants of the BMBF consortium AMPLIFY to CR, BE (Grant No. 031B0826A), and MO (Grant No. 031B0826C). The funding bodies had no role in the design of the study, analysis of the data, or writing of the manuscript.
Acknowledgments
We gratefully acknowledge Dr. Oliver Goldbeck for valuable advice and helpful discussions during preparation of the manuscript and Bianca Klein for help with proteomic analyses.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2023.1254882/full#supplementary-material
References
Abbasiliasi, S., Tan, J. S. S., Tengku Ibrahim, T. A. A., Bashokouh, F., Ramakrishnan, N. R. R., Mustafa, S., et al. (2017). Fermentation factors influencing the production of bacteriocins by lactic acid bacteria: A review. RSC Adv. 7, 29395–29420. doi: 10.1039/C6RA24579J
Acedo, J. Z., Chiorean, S., Vederas, J. C., and van Belkum, M. J. (2018). The expanding structural variety among bacteriocins from Gram-positive bacteria. FEMS Microbiol. Rev. 42, 805–828. doi: 10.1093/femsre/fuy033
Alvarez-Sieiro, P., Montalbán-López, M., Mu, D., and Kuipers, O. P. (2016). Bacteriocins of lactic acid bacteria: Extending the family. Appl. Microbiol. Biotechnol. 100, 2939–2951. doi: 10.1007/s00253-016-7343-9
Aymerich, T., Artigas, M. G., Garriga, M., Monfort, J. M., and Hugas, M. (2000). Effect of sausage ingredients and additives on the production of enterocin A and B by Enterococcus faecium CTC492. Optimization of in vitro production and anti-listerial effect in dry fermented sausages. J. Appl. Microbiol. 88, 686–694. doi: 10.1046/j.1365-2672.2000.01012.x
Back, A., Borges, F., Mangavel, C., Paris, C., Rondags, E., Kapel, R., et al. (2016). Recombinant pediocin in Lactococcus lactis: Increased production by propeptide fusion and improved potency by co-production with PedC. Microbial Biotechnol. 9, 466–477. doi: 10.1111/1751-7915.12285
Backert, S., Bernegger, S., Skórko-Glonek, J., and Wessler, S. (2018). Extracellular HtrA serine proteases: An emerging new strategy in bacterial pathogenesis. Cell Microbiol. 20:e12845. doi: 10.1111/cmi.12845
Baneyx, F., and Georgiou, G. (1991). Construction and characterization of Escherichia coli strains deficient in multiple secreted proteases: Protease III degrades high-molecular-weight substrates in vivo. J. Bacteriol. 173, 2696–2703. doi: 10.1128/jb.173.8.2696-2703.1991
Baumgart, M., Unthan, S., Rückert, C., Sivalingam, J., Grünberger, A., Kalinowski, J., et al. (2013). Construction of a prophage-free variant of Corynebacterium glutamicum ATCC 13032 for use as a platform strain for basic research and industrial biotechnology. Appl. Environ. Microbiol. 79, 6006–6015. doi: 10.1128/AEM.01634-13
Becker, J., Rohles, C. M., and Wittmann, C. (2018). Metabolically engineered Corynebacterium glutamicum for bio-based production of chemicals, fuels, materials, and healthcare products. Metab. Eng. 50, 122–141. doi: 10.1016/j.ymben.2018.07.008
Bédard, F., Hammami, R., Zirah, S., Rebuffat, S., Fliss, I., and Biron, E. (2018). Synthesis, antimicrobial activity and conformational analysis of the class IIa bacteriocin pediocin PA-1 and analogs thereof. Sci. Rep. 8:9029. doi: 10.1038/s41598-018-27225-3
Borrero, J., Jiménez, J. J., Gútiez, L., Herranz, C., Cintas, L. M., and Hernández, P. E. (2011). Protein expression vector and secretion signal peptide optimization to drive the production, secretion, and functional expression of the bacteriocin enterocin A in lactic acid bacteria. J. Biotechnol. 156, 76–86. doi: 10.1016/j.jbiotec.2011.07.038
Brooke, D., Movahed, N., and Bothner, B. (2015). Universal buffers for use in biochemistry and biophysical experiments. AIMS Biophys. 2, 336–342. doi: 10.3934/biophy.2015.3.336
Burkovski, A. (2013). Cell envelope of corynebacteria: Structure and influence on pathogenicity. ISRN Microbiol. 2013, 1–11. doi: 10.1155/2013/935736
Callewaert, R., and De Vuyst, L. (2000). Bacteriocin production with Lactobacillus amylovorus DCE 471 is improved and stabilized by fed-batch fermentation. Appl. Environ. Microbiol. 66, 606–613. doi: 10.1128/aem.66.2.606-613.2000
Chapot-Chartier, M. P., and Kulakauskas, S. (2014). Cell wall structure and function in lactic acid bacteria. Microb. Cell Fact. 13, (Suppl. 1):S9. doi: 10.1186/1475-2859-13-s1-s9
Chikindas, M. L., Weeks, R., Drider, D., Chistyakov, V. A., and Dicks, L. M. (2018). Functions and emerging applications of bacteriocins. Curr. Opin. Biotechnol. 49, 23–28. doi: 10.1016/j.copbio.2017.07.011
Christmann, J., Cao, P., Becker, J., Desiderato, C. K., Goldbeck, O., Riedel, C. U., et al. (2023). High-efficiency production of the antimicrobial peptide pediocin PA-1 in metabolically engineered Corynebacterium glutamicum using a microaerobic process at acidic pH and elevated levels of bivalent calcium ions. Microb. Cell Fact. 22:41. doi: 10.1186/s12934-023-02044-y
Cortes-Perez, N. G., Poquet, I., Oliveira, M., Gratadoux, J. J., Madsen, S. M., Miyoshi, A., et al. (2006). Construction and characterization of a Lactococcus lactis strain deficient in intracellular ClpP and extracellular HtrA proteases. Microbiology 152(Pt 9), 2611–2618. doi: 10.1099/mic.0.28698-0
Cotter, P. D., Ross, R. P., and Hill, C. (2013). Bacteriocins — a viable alternative to antibiotics? Nat. Rev. Microbiol. 11, 95–105. doi: 10.1038/nrmicro2937
Darbandi, A., Asadi, A., Mahdizade Ari, M., Ohadi, E., Talebi, M., Halaj Zadeh, M., et al. (2022). Bacteriocins: Properties and potential use as antimicrobials. J. Clin. Lab. Anal. 36:e24093. doi: 10.1002/jcla.24093
de Arauz, L. J., Jozala, A. F., Mazzola, P. G., and Vessoni Penna, T. C. (2009). Nisin biotechnological production and application: A review. Trends Food Sci. Technol. 20, 146–154. doi: 10.1016/j.tifs.2009.01.056
De Vuyst, L., Callewaert, R., and Crabbé, K. (1996). Primary metabolite kinetics of bacteriocin biosynthesis by Lactobacillus amylovorus and evidence for stimulation of bacteriocin production under unfavourable growth conditions. Microbiology 142, 817–827. doi: 10.1099/00221287-142-4-817
Desiderato, C. K., Hasenauer, K. M., Reich, S. J., Goldbeck, O., Holivololona, L., Ovchinnikov, K. V., et al. (2022). Garvicin Q: Characterization of biosynthesis and mode of action. Microb Cell Fact. 21:236. doi: 10.1186/s12934-022-01952-9
Duquesne, K., and Sturgis, J. N. (2010). Membrane protein solubilization. Methods Mol. Biol. 601, 205–217. doi: 10.1007/978-1-60761-344-2_13
EFSA Panel on Biological Hazards, Koutsoumanis, K., Allende, A., Alvarez-Ordóñez, A., Bolton, D., Bover-Cid, S., et al. (2022). Update of the list of QPS-recommended biological agents intentionally added to food or feed as notified to EFSA 15: Suitability of taxonomic units notified to EFSA until September 2021. EFSA J. 20:e07045. doi: 10.2903/j.efsa.2022.7045
Feito, J., Araújo, C., Arbulu, S., Contente, D., Gómez-Sala, B., Díaz-Formoso, L., et al. (2023). Design of Lactococcus lactis strains producing garvicin A and/or Garvicin Q, either alone or together with nisin A or Nisin Z and high antimicrobial activity against Lactococcus garvieae. Foods 12:1063. doi: 10.3390/foods12051063
Freier, L., Hemmerich, J., Schöler, K., Wiechert, W., Oldiges, M., and von Lieres, E. (2016). Framework for Kriging-based iterative experimental analysis and design: Optimization of secretory protein production in Corynebacterium glutamicum. Eng. Life Sci. 16, 538–549. doi: 10.1002/elsc.201500171
Freudl, R. (2018). Signal peptides for recombinant protein secretion in bacterial expression systems. Microb Cell Fact. 17:52. doi: 10.1186/s12934-018-0901-3
Garver, K. I., and Muriana, P. M. (1994). Purification and partial amino acid sequence of curvaticin FS47, a heat-stable bacteriocin produced by Lactobacillus curvatus FS47. Appl. Environ. Microbiol. 60, 2191–2195. doi: 10.1128/aem.60.6.2191-2195.1994
Goldbeck, O., Desef, D. N., Ovchinnikov, K. V., Perez-Garcia, F., Christmann, J., Sinner, P., et al. (2021). Establishing recombinant production of pediocin PA-1 in Corynebacterium glutamicum. Metab. Eng. 68, 34–45. doi: 10.1016/J.YMBEN.2021.09.002
Hanahan, D. (1983). Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 166, 557–580. doi: 10.1016/S0022-2836(83)80284-8
Hansen, G., and Hilgenfeld, R. (2013). Architecture and regulation of HtrA-family proteins involved in protein quality control and stress response. Cell Mol. Life Sci. 70, 761–775. doi: 10.1007/s00018-012-1076-4
Hemmerich, J., Rohe, P., Kleine, B., Jurischka, S., Wiechert, W., Freudl, R., et al. (2016). Use of a Sec signal peptide library from Bacillus subtilis for the optimization of cutinase secretion in Corynebacterium glutamicum. Microb Cell Fact. 15:208. doi: 10.1186/s12934-016-0604-6
Herranz, C., and Driessen, A. J. (2005). Sec-mediated secretion of bacteriocin enterocin P by Lactococcus lactis. Appl. Environ. Microbiol. 71, 1959–1963. doi: 10.1128/aem.71.4.1959-1963.2005
Huot, E., Barrena-Gonzalez, C., and Petitdemange, H. (1996). Tween 80 effect on bacteriocin synthesis by Lactococcus lactis subsp. cremoris J46. Lett. Appl. Microbiol. 22, 307–310. doi: 10.1111/j.1472-765x.1996.tb01167.x
Johnsen, L., Fimland, G., Eijsink, V., and Nissen-Meyer, J. (2000). Engineering increased stability in the antimicrobial peptide pediocin PA-1. Appl. Environ. Microbiol. 66, 4798–4802. doi: 10.1128/AEM.66.11.4798-4802.2000
Johnson, E. M., Jung, D. Y.-G., Jin, D. Y.-Y., Jayabalan, D. R., Yang, D. S. H., and Suh, J. W. (2017). Bacteriocins as food preservatives: Challenges and emerging horizons. Crit. Rev. Food Sci. Nutr. 58, 2743–2767. doi: 10.1080/10408398.2017.1340870
Jordan, S., Hutchings, M. I., and Mascher, T. (2008). Cell envelope stress response in Gram-positive bacteria. FEMS Microbiol. Rev. 32, 107–146. doi: 10.1111/j.1574-6976.2007.00091.x
Jurischka, S., Bida, A., Dohmen-Olma, D., Kleine, B., Potzkei, J., Binder, S., et al. (2020). A secretion biosensor for monitoring Sec-dependent protein export in Corynebacterium glutamicum. Microb Cell Fact. 19:11. doi: 10.1186/s12934-019-1273-z
Juturu, V., and Wu, J. C. (2018). Microbial production of bacteriocins: Latest research development and applications. Biotechnol. Adv. 36, 2187–2200. doi: 10.1016/j.biotechadv.2018.10.007
Keren, T., Yarmus, M., Halevy, G., and Shapira, R. (2004). Immunodetection of the bacteriocin lacticin RM: Analysis of the influence of temperature and Tween 80 on its expression and activity. Appl. Environ. Microbiol. 70, 2098–2104. doi: 10.1128/aem.70.4.2098-2104.2004
Lee, S. M., and Jeong, K. J. (2022). Advances in synthetic biology tools and engineering of Corynebacterium glutamicum as a platform host for recombinant protein production. Biotechnol. Bioprocess Eng. 5, 1–15. doi: 10.1007/s12257-022-0219-1
Linke, D. (2009). Detergents: An overview. Methods Enzymol. 463, 603–617. doi: 10.1016/s0076-6879(09)63034-2
Liu, X., Meng, L., Wang, X., Yang, Y., and Bai, Z. (2022). Effect of Clp protease from Corynebacterium glutamicum on heterologous protein expression. Protein Expr. Purif. 189:105928. doi: 10.1016/j.pep.2021.105928
Maldonado-Barragán, A., Alegría-Carrasco, E., Blanco, M. D. M., Vela, A. I., Fernández-Garayzábal, J. F., Rodríguez, J. M., et al. (2022). Garvicins AG1 and AG2: Two Novel Class IId Bacteriocins of Lactococcus garvieae Lg-Granada. Int. J. Mol. Sci. 23:4685. doi: 10.3390/ijms23094685
Martín, M., Gutiérrez, J., Criado, R., Herranz, C., Cintas, L. M., and Hernández, P. E. (2007). Chimeras of mature pediocin PA-1 fused to the signal peptide of enterocin P permits the cloning, production, and expression of pediocin PA-1 in Lactococcus lactis. J. Food Prot. 70, 2792–2798. doi: 10.4315/0362-028x-70.12.2792
Matsusaki, H., Endo, N., Sonomoto, K., and Ishizaki, A. (1996). Lantibiotic nisin Z fermentative production by Lactococcus lactis 10-1: Relationship between production of the lantibiotic and lactate and cell growth. Appl. Microbiol. Biotechnol. 45, 36–40. doi: 10.1007/s002530050645
McCormick, J. K., Worobo, R. W., and Stiles, M. E. (1996). Expression of the antimicrobial peptide carnobacteriocin B2 by a signal peptide-dependent general secretory pathway. Appl. Environ. Microbiol. 62, 4095–4099. doi: 10.1128/aem.62.11.4095-4099.1996
Meade, E., Slattery, M. A., and Garvey, M. (2020). Bacteriocins, potent antimicrobial peptides and the fight against multi drug resistant species: Resistance is futile? Antibiotics 9:32. doi: 10.3390/antibiotics9010032
Meerman, H. J., and Georgiou, G. (1994). Construction and characterization of a set of E. coli strains deficient in all known loci affecting the proteolytic stability of secreted recombinant proteins. Biotechnology 12, 1107–1110. doi: 10.1038/nbt1194-1107
Merrell, D. S., Goodrich, M. L., Otto, G., Tompkins, L. S., and Falkow, S. (2003). pH-regulated gene expression of the gastric pathogen Helicobacter pylori. Infect. Immun. 71, 3529–3539. doi: 10.1128/iai.71.6.3529-3539.2003
Mills, S., Stanton, C., Hill, C., and Ross, R. P. (2011). New developments and applications of bacteriocins and peptides in foods. Annu. Rev. Food Sci. Technol. 2, 299–329. doi: 10.1146/annurev-food-022510-133721
Miyoshi, A., Poquet, I., Azevedo, V., Commissaire, J., Bermudez-Humaran, L., Domakova, E., et al. (2002). Controlled production of stable heterologous proteins in Lactococcus lactis. Appl. Environ. Microbiol. 68, 3141–3146. doi: 10.1128/aem.68.6.3141-3146.2002
Müller, C., Bakkes, P. J., Lenz, P., Waffenschmidt, V., Helleckes, L. M., Jaeger, K.-E., et al. (2022). Accelerated strain construction and characterization of C. glutamicum protein secretion by laboratory automation. Appl. Microbiol. Biotechnol. 106, 4481–4497. doi: 10.1007/s00253-022-12017-7
Neef, J., Bongiorni, C., Schmidt, B., Goosens, V. J., and van Dijl, J. M. (2020). Relative contributions of non-essential Sec pathway components and cell envelope-associated proteases to high-level enzyme secretion by Bacillus subtilis. Microb Cell Fact. 19:52. doi: 10.1186/s12934-020-01315-2
Nes, I. F., Diep, D. B., Håvarstein, L. S., Brurberg, M. B., Eijsink, V., and Holo, H. (1996). Biosynthesis of bacteriocins in lactic acid bacteria. Antonie Van Leeuwenhoek 70, 113–128. doi: 10.1007/bf00395929
Opekarová, M., and Tanner, W. (2003). Specific lipid requirements of membrane proteins–a putative bottleneck in heterologous expression. Biochim. Biophys. Acta 1610, 11–22. doi: 10.1016/s0005-2736(02)00708-3
Ovchinnikov, K. V., Chi, H., Mehmeti, I., Holo, H., Nes, I. F., and Diep, D. B. (2016). Novel group of leaderless multipeptide bacteriocins from gram-positive bacteria. Appl. Environ. Microbiol. 82, 5216–5224. doi: 10.1128/AEM.01094-16
Ovchinnikov, K. V., Kranjec, C., Thorstensen, T., Carlsen, H., and Diep, D. B. (2020). Successful development of bacteriocins into therapeutic formulation for treatment of MRSA-skin infection in a murine model. Antimicrobial Agents Chemother. 64:e00829-20. doi: 10.1128/aac.00829-20.
Ovchinnikov, K. V., Kristiansen, P. E., Straume, D., Jensen, M. S., Aleksandrzak-Piekarczyk, T., Nes, I. F., et al. (2017). The leaderless bacteriocin enterocin K1 is highly potent against Enterococcus faecium: A study on structure, target spectrum and receptor. Front. Microbiol. 8:774. doi: 10.3389/fmicb.2017.00774
Parente, E., Ricciardi, A., and Addario, G. (1994). Influence of pH on growth and bacteriocin production by Lactococcus lactis subsp.lactis 14ONWC during batch fermentation. Appl. Microbiol. Biotechnol. 41, 388–394. doi: 10.1007/BF01982525
Peng, F., Liu, X., Wang, X., Chen, J., Liu, M., Yang, Y., et al. (2019). Triple deletion of clpC, porB, and mepA enhances production of small ubiquitin-like modifier-N-terminal pro-brain natriuretic peptide in Corynebacterium glutamicum. J. Ind. Microbiol. Biotechnol. 46, 67–79. doi: 10.1007/s10295-018-2091-8
Perez-Riverol, Y., Bai, J., Bandla, C., García-Seisdedos, D., Hewapathirana, S., Kamatchinathan, S., et al. (2022). The PRIDE database resources in 2022: A hub for mass spectrometry-based proteomics evidences. Nucleic Acids Res. 50, D543–D552. doi: 10.1093/nar/gkab1038
Reich, S. J., Stohr, J., Goldbeck, O., Fendrich, B., Crauwels, P., and Riedel, C. U. (2022). Improved fluorescent Listeria spp. biosensors for analysis of antimicrobials by flow cytometry. Microbiology Open 11:e1304. doi: 10.1002/mbo3.1304
Silva, C. C. G., Silva, S. P. M., and Ribeiro, S. C. (2018). Application of bacteriocins and protective cultures in dairy food preservation. Front. Microbiol. 9:594. doi: 10.3389/fmicb.2018.00594
Soltani, S., Hammami, R., Cotter, P. D., Rebuffat, S., Said, L. B., Gaudreau, H., et al. (2021). Bacteriocins as a new generation of antimicrobials: Toxicity aspects and regulations. FEMS Microbiol. Rev. 45:fuaa039. doi: 10.1093/femsre/fuaa039
Stack, H. M., Sleator, R. D., Bowers, M., Hill, C., and Gahan, C. G. (2005). Role for HtrA in stress induction and virulence potential in Listeria monocytogenes. Appl. Environ. Microbiol. 71, 4241–4247. doi: 10.1128/aem.71.8.4241-4247.2005
Teramoto, H., Watanabe, K., Suzuki, N., Inui, M., and Yukawa, H. (2011). High yield secretion of heterologous proteins in Corynebacterium glutamicum using its own Tat-type signal sequence. Appl. Microbiol. Biotechnol. 91, 677–687. doi: 10.1007/s00253-011-3281-8
Tosukhowong, A., Zendo, T., Visessanguan, W., Roytrakul, S., Pumpuang, L., Jaresitthikunchai, J., et al. (2012). Garvieacin Q, a Novel Class II Bacteriocin from Lactococcus garvieae BCC 43578. Appl. Environ. Microbiol. 78, 1619–1623. doi: 10.1128/AEM.06891-11
Tymoszewska, A., Diep, D. B., Wirtek, P., and Aleksandrzak-Piekarczyk, T. (2017). The non-lantibiotic bacteriocin garvicin Q Targets Man-PTS in a broad spectrum of sensitive bacterial genera. Sci. Rep. 7:8359. doi: 10.1038/s41598-017-09102-7
Unrean, P., Gätgens, J., Klein, B., Noack, S., and Champreda, V. (2018). Elucidating cellular mechanisms of Saccharomyces cerevisiae tolerant to combined lignocellulosic-derived inhibitors using high-throughput phenotyping and multiomics analyses. FEMS Yeast Res. 18:foy106. doi: 10.1093/femsyr/foy106
Van Belkum, M. J., Worobo, R. W., and Stiles, M. E. (1997). Double-glycine-type leader peptides direct secretion of bacteriocins by ABC transporters: Colicin V secretion in Lactococcus lactis. Mol. Microbiol. 23, 1293–1301. doi: 10.1046/j.1365-2958.1997.3111677.x
Vertès, A. A. (2013). “Protein secretion systems of Corynebacterium glutamicum,” in Corynebacterium glutamicum: Biology and Biotechnology, eds H. Yukawa and M. Inui (Berlin: Springer), 351–389.
Wang, W., Nema, S., and Teagarden, D. (2010). Protein aggregation–pathways and influencing factors. Int. J. Pharm. 390, 89–99. doi: 10.1016/j.ijpharm.2010.02.025
Weixler, D., Berghoff, M., Ovchinnikov, K. V., Reich, S., Goldbeck, O., Seibold, G. M., et al. (2022). Recombinant production of the lantibiotic nisin using Corynebacterium glutamicum in a two-step process. Microb Cell Fact. 21:11. doi: 10.1186/s12934-022-01739-y
Weixler, D., Goldbeck, O., Seibold, G. M., Eikmanns, B. J., and Riedel, C. U. (2021). Towards improved resistance of Corynebacterium glutamicum against nisin. bioRxiv[Preprint]. Available online at: https://www.biorxiv.org/content/10.1101/2021.08.09.454123v1 (accessed August 9, 2021).
Wolf, S., Becker, J., Tsuge, Y., Kawaguchi, H., Kondo, A., Marienhagen, J., et al. (2021). Advances in metabolic engineering of Corynebacterium glutamicum to produce high-value active ingredients for food, feed, human health, and well-being. Essays Biochem. 65, 197–212. doi: 10.1042/ebc20200134
Woo, J. H., Liu, Y. Y., and Neville, D. M. Jr. (2006). Minimization of aggregation of secreted bivalent anti-human T cell immunotoxin in Pichia pastoris bioreactor culture by optimizing culture conditions for protein secretion. J. Biotechnol. 121, 75–85. doi: 10.1016/j.jbiotec.2005.07.004
Worobo, R. W., Van Belkum, M. J., Sailer, M., Roy, K. L., Vederas, J. C., and Stiles, M. E. (1995). A signal peptide secretion-dependent bacteriocin from Carnobacterium divergens. J. Bacteriol. 177, 3143–3149. doi: 10.1128/jb.177.11.3143-3149.1995
Yang, R., Johnson, M. C., and Ray, B. (1992). Novel method to extract large amounts of bacteriocins from lactic acid bacteria. Appl. Environ. Microbiol. 58, 3355–3359. doi: 10.1128/aem.58.10.3355-3359.1992
Zhang, W., Yang, Y., Liu, X., Liu, C., and Bai, Z. (2019). Development of a secretory expression system with high compatibility between expression elements and an optimized host for endoxylanase production in Corynebacterium glutamicum. Microb Cell Fact. 18:72. doi: 10.1186/s12934-019-1116-y
Keywords: Corynebacterium glutamicum, bacteriocin, recombinant production, Sec-dependent secretion, HtrA protease
Citation: Desiderato CK, Müller C, Schretzmeier A, Hasenauer KM, Gnannt B, Süpple B, Reiter A, Steier V, Oldiges M, Eikmanns BJ and Riedel CU (2024) Optimized recombinant production of the bacteriocin garvicin Q by Corynebacterium glutamicum. Front. Microbiol. 14:1254882. doi: 10.3389/fmicb.2023.1254882
Received: 07 July 2023; Accepted: 12 December 2023;
Published: 08 January 2024.
Edited by:
Paul David Cotter, Seqbiome Ltd., IrelandReviewed by:
Duolong Zhu, Baylor College of Medicine, United StatesTamara Aleksandrzak-Piekarczyk, The Polish Academy of Sciences, Poland
Copyright © 2024 Desiderato, Müller, Schretzmeier, Hasenauer, Gnannt, Süpple, Reiter, Steier, Oldiges, Eikmanns and Riedel. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Christian U. Riedel, christian.riedel@uni-ulm.de
 Christian K. Desiderato
Christian K. Desiderato Carolin Müller2,3
Carolin Müller2,3 Marco Oldiges
Marco Oldiges Bernhard J. Eikmanns
Bernhard J. Eikmanns Christian U. Riedel
Christian U. Riedel