- 1Biochemistry Ph.D. Program
- 2Department of Chemistry and Biochemistry
- 3Biomolecular Sciences Institute, Florida International University, Miami, FL, United States
N6-methyladenosine (m6A) modification in mRNAs and non-coding RNAs is a newly identified epitranscriptomic mark. It provides a fine-tuning of gene expression to serve as a cellular response to endogenous and exogenous stimuli. m6A is involved in regulating genes in multiple cellular pathways and functions, including circadian rhythm, cell renewal, differentiation, neurogenesis, immunity, among others. Disruption of m6A regulation is associated with cancer, obesity, and immune diseases. Recent studies have shown that m6A can be induced by oxidative stress and DNA damage to regulate DNA repair. Also, deficiency of the m6A eraser, fat mass obesity-associated protein (FTO) can increase cellular sensitivity to genotoxicants. These findings shed light on the novel roles of m6A in modulating DNA repair and genome integrity and stability through responding to DNA damage. In this mini-review, we discuss recent progress in the understanding of a unique role of m6As in mRNAs, lncRNAs, and microRNAs in DNA damage response and regulation of DNA repair and genome integrity and instability.
Introduction
N6-methyladenosine (m6A) is one of the most abundant epitranscriptomic marks in mRNA of eukaryotic cells that occurs at the consensus motif, RRACH (R is G or A or U, and H is U, A, or C) (Dominissini et al., 2012; Meyer et al., 2012; Zhao et al., 2017). It is regulated by its writer, adenosine methyltransferase, METTL3/METTL14 and erasers, RNA demethylases, fat mass obesity-associated protein (FTO), and Alk B homolog 5 (ALKBH5). m6A is usually present at the 5′- and 3′-untranslated regions (5′- and 3′-UTRs) and protein-encoding sequences of mRNAs (Svobodova Kovarikova et al., 2020; Wang et al., 2020). It can also occur in long-noncoding RNAs (lncRNAs) (Meyer et al., 2012), primary microRNAs (pri-miRNA) among others (Iwanami and Brown, 1968; Saneyoshi et al., 1969; Alarcon et al., 2015a; Alarcon et al., 2015b; Yang et al., 2017).
M6A in mRNAs was initially discovered by several groups in the 1970s (Desrosiers et al., 1974; Perry and Kelley, 1974). However, its cellular functions have not been extensively explored until 2012, when the methylated RNA immunoprecipitation-sequencing (MeRIP-seq) became available for its genome mapping, and its writers, erasers, and readers were discovered (Meyer and Jaffrey, 2017). In this mini-review, we focus on recent progress in understanding the function of m6A in mRNA, lncRNAs, and microRNAs and its unique role in regulating DNA repair and genome stability.
Mapping of m6A in mRNAs
M6A can be mapped in a transcriptome-wide manner using MeRIP-seq (Meyer et al., 2012). It can be detected at nucleotide-specific resolution using crosslinking immunoprecipitation (miCLIP)-seq (Linder et al., 2015) during which an anti-m6A antibody is UV-crosslinked to the site containing an m6A at mRNAs. Subsequently, reverse transcription of mRNAs results in C to T transition or truncation at +1 position relative to m6A in cDNAs leading to the mapping of m6A located at specific nucleotides. The detection of m6A has been further advanced by the newly-developed DART-seq (deamination adjacent to RNA modification targets) from the Meyer group (Meyer, 2019). This approach uses the APOBEC1 fused with the m6A-binding YTH domain to induce C-to-U deamination at the sites next to m6A. The Us are then detected by RNA-seq. DART-seq is sensitive and specific in mapping m6A with a detection limit as low as 10 ng total RNA. It can also quantitatively estimate m6A abundance in individual RNA transcripts and determine the dynamic changes of m6A induced by genome stressors in cells. The long-read DART-seq is particularly advantageous in mapping the m6A profiles in different RNA isoforms. Thus, DART-seq has demonstrated its versatile advantages in mapping transcriptome-wide m6A over other approaches.
m6A and Cellular Function and Diseases
m6A Mediates Multiple Cellular Functions
m6A can regulate various cellular functions by affecting pre-mRNA splicing (Alarcon et al., 2015a), mRNA degradation (Wang et al., 2014), mRNA and circRNA translation (Wang et al., 2015; Yang et al., 2017), primary microRNA processing (Alarcon et al., 2015a), and protein-RNA interactions (Liu et al., 2015). m6A can directly modulate RNA splicing by altering the structures of pre-mRNAs. This increases the accessibility of the heterogeneous nuclear ribonucleoprotein C (hnRNP C) RNA binding motif, facilitating the hnRNP C-RNA interaction and the splicing of pre-mRNA (Liu et al., 2015). m6A can also regulate RNA alternative splicing (Alarcon et al., 2015a). Depletion of METTL3 can affect the alternative splicing to alter global gene expression (Alarcon et al., 2015a). Depletion of hnRNPA2B1 with a similar function to METTL3 leads to the same alternation on the global gene expression as METTL3 depletion (Alarcon et al., 2015a). m6A can also modulate mRNA stability by reducing the binding of the human antigen R protein (HuR) to the 3′-UTR region of mRNAs and promoting the mRNAs-microRNAs interaction (Wang et al., 2014). m6A can regulate mRNA translation through YTHDF1 that binds to m6A and help load m6A-coded mRNAs to ribosomes, thereby stimulating translation initiation (Wang et al., 2015). On the other hand, m6A in circRNA is involved in the initiation of the cap-independent translation. The effect can be further enhanced by METTLE3/14 or diminished by FTO. m6A-mediated circRNA translation is also YTHDF3-dependent (Yang et al., 2017). m6A in pri-miRNA can promote its conversion to pre-miRNA (Alarcon et al., 2015a). This is accomplished by the recognition of m6A by the hnRNPA2B1, promoting its interaction with the DiGeorge Syndrome Critical Region 8 (DGCR8). Subsequently, Drosha, the ribonuclease III enzyme, is recruited to form a microprocessor complex. The hnRNPA2B1-DGCR8 interaction then facilitates DGCR8 binding at the junction between the flanking single-stranded RNA and the stem region in the hairpin structure of pri-miRNA, leading to its cleavage by Drosha (Alarcon et al., 2015a).
m6A is Associated With Diseases
An association between m6A and different types of cancer has been implicated. The mutations of m6A regulatory genes, METTL3, METTL14, YTHDF1, YTHDF2, FTO, and ALKBH5, have been identified in acute lymphoblastic leukemia, multiple myeloma, and acute myeloid leukemia (AML). Also, copy number variations, predominantly loss of ALKBH5, are found in about 10% of AML patients (Kwok et al., 2017; He et al., 2018). Possible mechanisms by which m6A mediates cancer progression are implicated in recent studies. An altered m6A level can result in abnormal cellular differentiation in cancer (He et al., 2018). Increased m6A in glioma stem-like cells (GSCs) can lead to cellular resistance to radiation through the SOX2-dependent DNA repair (Visvanathan et al., 2018). In addition, m6A can mediate the interplay between METTL3 and the oncoprotein HBXIP to stimulate its expression and mediate breast cancer development (Cai et al., 2018). On the other hand, decreased m6A promotes endometrial carcinogenesis through an AKT-dependent mechanism by downregulating PHLPP2 and upregulating mTORC2 (Liu et al., 2018). Increased m6A reduces AKT activity in the endometrium by enhancing PHLPP2 translation and mTORC2 degradation (Liu et al., 2018). m6A can also regulate the stemness of ovarian cancer stem cells by stabilizing the mRNA of the phosphodiesterase, PDE1C, and PDE4B and stimulating the stemness of ovarian cancer cells through the cyclic adenosine monophosphate (cAMP) signaling pathway (Huang et al., 2020). Thus, m6A can promote or prevent tumorigenesis upon specific genes and cell types.
m6A Regulates R-Loop formation and Genome Stability
A recent study shows that m6A also occurs at the RNA strand of R-loops with a wide distribution at the genome in human pluripotent stem cells (iPSCs) (Abakir et al., 2020). The accumulation of m6A on R-loops can exist throughout all the cell cycle phases. Depletion of METTL3 and m6A reader, YTH-domain family member 2 (YTHDF2) promotes the accumulation of R-loops, leading to double-strand breaks in iPSCs suggesting that m6A prevents the formation of R-loops and DNA damage in iPSCs. Furthermore, m6A can lead to the resolution of R-loops induced by DNA damage through the tonicity-responsive enhancer-binding protein (TonEBP). TonEBP binds to R-loops and recruits METTL3 that generates m6A on the RNA strand of the R-loop. TonEBP then recruits RNaseH1 to cleave the RNA strand resolving the R-loops (Kang et al., 2021). In contrast, m6A in R-loop in some somatic cells promotes the formation of R-loops (Yang et al., 2019). The results suggest that m6A plays a distinct role in regulating the formation of R-loops, DNA damage, and genome stability in different types of cells.
m6A may also coordinate with ten-eleven translocation 1 (TET1) that can be recruited to R-loops by the growth arrest and DNA damage protein 45A (GADD45A) (Arab et al., 2019) to modulate active DNA demethylation and gene expression. GADD45A can promote active DNA demethylation and TCF21 expression on the R-loop formed at the antisense lncRNA, TARID at the TCF21 promoter (Arab et al., 2019). It is possible that m6A on the lncRNA R-loop can facilitate TET1-mediated DNA demethylation leading to the accumulation of DNA demethylation products such as 5hmC. Thus, cells may also coordinate m6A with DNA methylation and demethylation to regulate R-loop formation, genomic stability, and gene expression.
m6A Regulates DNA Repair Affecting Genome Stability
m6A Regulates DNA Repair to Modulate Genome Stability
m6A and UV Damage Repair
m6A can be induced by UV irradiation and recruited to UV-damaged sites to promote DNA repair and cellular resistance to UV damage (Xiang et al., 2017). It has been shown that m6A generated by METTL3 facilitates UV damage response and repair. This may be mediated by the translesion DNA polymerase κ (Xiang et al., 2017). Moreover, it has been found that upon the UV-induced damage, m6A-coded RNAs in the cytoplasm can be translocated back to the nucleus and accumulate at the UV-induced DNA damage sites (Svobodova Kovarikova et al., 2020). It is proposed that m6A may guide NER to remove the damage through noncoding RNAs (Svobodova Kovarikova et al., 2020).
m6A Mediates Double-Strand Break Repair to Prevent Genome Instability
m6A can also mediate double-strand DNA (dsDNA) break repair (Zhang et al., 2020). It can be induced by dsDNA breaks through the activation of METTL3, i.e., the phosphorylation at serine 43 (S43) of METTL3 by ATM. m6A then recruits YTHDC1 to m6A-coded RNAs resulting in the formation of RNA-DNA hybrid at dsDNA breakage sites. Subsequently, RAD51 and BRCA1 are recruited to the dsDNA breaks accomplishing the homologous recombination-mediated dsDNA break repair and preventing genome instability (Zhang et al., 2020).
m6A and Direct DNA Repair
The role of m6A in regulating direct DNA repair is supported by the fact that in mice, m6A recognition by YTHDF1 can facilitate the cap-dependent translation (CIT) to stimulate the translation of the direct DNA repair enzyme, O6-methylguanine-DNA methyltransferase (MGMT), extending mouse life span (Ozkurede et al., 2019). Moreover, increased METTL3/14 and decreased ALKBH5 and FTO promote the extension of mouse life span (Ozkurede et al., 2019), further suggesting that m6A promotes direct DNA repair preventing DNA damage-induced genome instability.
m6A Processing Proteins and DNA Damage and Repair
m6A and DNA Damage induced by Anti-cancer Drugs
An important research area related to m6A is to understand the roles of m6A in modulating anti-cancer drug resistance through DNA repair, as this is essentially important for the development of m6A regulatory proteins as new anti-cancer drug targets. It has been found that m6A increases the stability of the transcription factor-activating enhancer-binding protein 2C (TFAP2C) mRNA, promoting seminoma cell survival under cisplatin treatment. This suggests that DNA repair genes are upregulated by TFAP2C through m6A, conferring cellular resistance to the drug-induced DNA damage (Wei et al., 2020). In contrast, decreased m6A in β-catenin mRNA promotes the resistance of cervical squamous cell carcinoma to chemo-radiotherapy by upregulating the excision repair cross-complementation group 1 (ERCC1) (Zhou et al., 2018). The results suggest that m6A plays a dual role in modulating the resistance of anti-cancer therapies through DNA damage repair upon the types of cancer and their treatments. The cellular pathways that can mediate anti-cancer drug resistance through m6A have been discussed in detail in a recent review (Li, B. et al., 2020).
m6A, FTO, AlkB, and DNA Damage
The roles of m6A in regulating the cellular accumulation of DNA damage induced by genotoxicants and their repair can be studied by examining the effects of its writers, readers, and erasers on the cellular sensitivity to DNA damaging agents. It has been shown that FTO knockout in mouse osteoblasts confers the cellular sensitivity to UV and H2O2, promoting cell death (Zhang et al., 2019). Further analysis demonstrates that FTO erases m6A to enhance the stability of mRNAs of Hspa1a and DNA repair genes, thereby protecting cells from DNA damage (Zhang et al., 2019). ALKBH1, on the other hand, has both m6A demethylation activity and AP lyase activity that can cleave abasic (AP) sites in DNA through the β-elimination. Since the two types of enzymatic activities share the overlapped active sites (Müller et al., 2017; Müller et al., 2018), m6A demethylation in RNA by ALKBH1 may compete with its AP lyase activity resulting in the accumulation of AP sites in the genome. The coordination between the m6A demethylation and DNA damage repair of ALKBH1 needs to be further elucidated.
m6A, DNA Damage and Repair, and Bone Development in Mice
m6A can also regulate bone development through the modulation of DNA repair. Zhang et al. have shown that mice with FTO global knockout (FTOKO) or selective knockout in osteoblasts (FTOOcKO) exhibit decreased bone volume in an age-dependent manner. They further demonstrate that FTO KO promotes the accumulation of DNA double-strand breaks in mouse osteoblasts induced by DNA damaging agents and the metabolic stress, high-fat diet while it decreases the level of Hspa1a and Kdm2a protein that is associated with DNA repair (Zhang et al., 2019). These effects promote apoptosis and the death of osteoblasts. The results suggest that the removal of m6A by FTO is essential for mammalian osteoblast survival and differentiation, and bone development.
m6A and Its Crosstalk With Histone Modifications
m6A can also crosstalk with histone modifications. m6A generated in the coding sequence and 3′-UTR can be guided by histone H3 trimethylation at lysine-36 (H3K36me3) and deposited to mRNAs at specific gene loci. METTL14 can bind to H3K36me3 to facilitate the assembly of the m6A methyltransferase complex (MTC) that further interacts with RNA polymerase II, thereby stimulating gene locus-specific deposition of m6A in nascent mRNA transcripts (Huang et al., 2019). On the other hand, m6A also regulates the demethylation of H3K9me2 by recruiting the lysine demethylase 3B (KDM3B) to its target chromatin regions. m6A in the chromatin-related mRNAs is bound by YTHDC1, which then recruits KDM3B to its substrate leading to the demethylation of H3K9me2 (Li, Y. et al., 2020). Moreover, m6A can interplay with histone modifications by regulating the expression of histone modification enzymes. m6A on the mRNA of lysine demethylase 6B (KDM6B) is bound by YTHDF2, causing the degradation of KDM6B mRNAs, reducing the KDM6B protein level, and resulting in a high level of H3K27me3 in proinflammatory cytokine gene like IL6 (Wu et al., 2020). Also, m6A can specifically increase the protein level rather than the mRNA level of the histone methyltransferase to stimulate the trimethylation of H3K27 (Chen et al., 2019).
m6A and Telomerase RNA
m6A also occurs on the consensus motif, GGACU, located in the duplex region of the secondary structure of human telomerase RNA (hTR) (Han et al., 2020). ALKBH5 can be recruited to hTR to remove m6A, and this prevents the assembly of the telomerase complex inhibiting telomerase activity. Thus, m6A in hTR can maintain telomere stability by sustaining telomerase activity.
m6A and Regulation of lncRNA and Pri-miRNA
m6A in lncRNAs varies upon cell lines, tissue types, and growth stage (Meyer et al., 2012; Han et al., 2019; Xiao et al., 2019). It preferentially occurs in the lncRNAs that undergo alternative splicing (Xiao et al., 2019). m6A can mediate the interaction between the large intergenic coding RNA 1281 (linc1281) and the pluripotency-related let-7 family miRNAs to sequester the miRNAs leading to maintenance of mouse embryonic stem cell identity (Yang et al., 2018). m6A at specific sites of the X-inactive specific transcript (XIST) mediates the transcriptional silencing of the genes on the X chromosome by recruiting YTHDC1, promoting XIST-regulated gene repression (Patil et al., 2016).
Also, m6A can modulate the interaction between lncRNAs and their binding proteins. m6A in the metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) promotes the binding of hnRNPG, hnRNPC, and METTL16 to the MALAT1 transcripts, thereby altering the level of the lncRNA (Zhou et al., 2016). m6A in the Olfr29-ps1 present in myeloid-derived suppressor cells (MDSCs) plays a significant role in MDSC immunosuppression and differentiation by stimulating the production and stability of Olfr29-ps1 (Shang et al., 2019).
m6A on the promoter-associated non-coding RNA-D (pncRNA-D) plays a critical role in regulating cell cycle progression by modulating the interaction between the RNA and the fused in sarcoma/translocated (FUS/TLS) protein in liposarcoma (Yoneda et al., 2020). m6A recruits YTHDC1 to prevent the FUS/TLS protein from binding to pncRNA-D, thereby increasing the cyclin D1 expression.
m6A can be deposited to the pri-miRNA of microRNA, which is processed by the microprocessor complex of the pri-miRNAs that contains the RNA binding protein DGCR8 and Drosha type III ribonuclease (Denli et al., 2004; Gregory et al., 2004; Han et al., 2004; Landthaler et al., 2004). m6A on pri-miRNA is bound by hnRNPA2B1 protein that recruits DGCR8 stimulating nuclear miRNA processing (Alarcon et al., 2015a). METTL3 depletion reduces the binding of DGCR8 to pri-miRNAs resulting in the decrease of matured miRNAs and accumulation of unprocessed pri-miRNAs (Alarcon et al., 2015b). METTL14 can directly recruit DCGR8 on the m6A-coded pri-miRNA to stimulate the expression of the oncosuppressor, miR-126a, in hepatocellular carcinoma (HCC) (Ma et al., 2017). This suggests that m6A governs HCC metastasis by regulating miRNA levels. m6A can also modulate microRNA expression by regulating the stability of the AGO2 mRNA and increasing the AGO2 protein level (Min et al., 2018). In addition, m6A is involved in regulating the oncogenic miR-25-3p in pancreatic duct epithelial cells induced by cigarette smoke condensate (CSC) (Zhang et al., 2019). CSC can cause the hypomethylation at the promoter region of METTL3 to upregulate the methyltransferase, increasing m6A. Subsequently, this upregulates miR-25-3p, thereby promoting malignant phenotypes of pancreatic cancer cells (Zhang et al., 2019).
Discussion
Current studies support that m6A is involved in DNA repair and genome maintenance in an R-loop-dependent manner (Figure 1). However, Our understanding of the multidimensional roles of m6A in regulating DNA repair and genome stability is just in its infancy. An important question that needs to be addressed is the differential roles of m6A and its regulators in mediating diversified DNA damage response and repair pathways that maintain genome stability. Another outstanding question that needs to be elucidated in the future is the interplay among m6A, noncoding RNAs, and DNA and histone epigenetics in modulating DNA repair and genome stability. In addition, a fast and straightforward high throughput platform and technology need to be developed for determining the stoichiometry of m6A and other RNA modifications that coexist in RNA transcripts. These future studies should significantly advance our understanding of the molecular mechanisms underlying m6A-mediated DNA repair, genome stability, and cellular function.
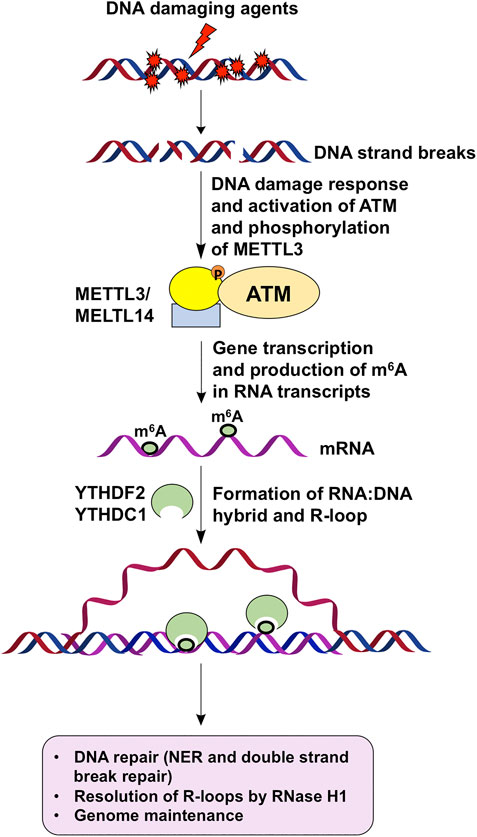
FIGURE 1. m6A regulates DNA repair to maintain genome stability via R-loops. DNA damage agents induce strand breaks to activate DNA damage response and ATM, which phosphorylates and activates METTL3. DNA damage response then leads to gene transcription and production of m6A by activated METTL3 on mRNA transcripts. Subsequently, m6A-coated mRNAs hybrid with their DNA template to form R-loops. m6A readers such as YTHDF2 and YTHDC1 then bind to m6A on R-loops to facilitate DNA repair and resolution of R-loops through RNase H1, thereby leading to genome maintenance.
Author Contributions
YL developed the concept. FQ, PST, and YL wrote the manuscript. YL edited the manuscript. All authors approved the submitted version of the manuscript.
Funding
This work was supported by NIH grant ES023569 to YL.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
PST is supported by Florida International University Dissertation Year Fellowship.
References
Abakir, A., Giles, T. C., Cristini, A., Foster, J. M., Dai, N., Starczak, M., et al. (2020). N(6)-methyladenosine regulates the stability of RNA:DNA hybrids in human cellsN6-methyladenosine regulates the stability of RNA:DNA hybrids in human cells. Nat. Genet. 52, 48–55. doi:10.1038/s41588-019-0549-x
Alarcon, C. R., Goodarzi, H., Lee, H., Liu, X., Tavazoie, S., and Tavazoie, S. F. (2015a). HNRNPA2B1 is a mediator of m(6)A-dependent nuclear RNA processing events. Cell. 162, 1299–1308. doi:10.1016/j.cell.2015.08.011
Alarcon, C. R., Lee, H., Goodarzi, H., Halberg, N., and Tavazoie, S. F. (2015b). N6-methyladenosine marks primary microRNAs for processing. Nature 519, 482–485. doi:10.1038/nature14281
Arab, K., Karaulanov, E., Musheev, M., Trnka, P., SchaferSchäfer, A., Grummt, I., et al. (2019). GADD45A binds R-loops and recruits TET1 to CpG island promoters. Nat. Genet. 51, 217–223. doi:10.1038/s41588-018-0306-6
Cai, X., Wang, X., Cao, C., Gao, Y., Zhang, S., Yang, Z., et al. (2018). HBXIP-elevated methyltransferase METTL3 promotes the progression of breast cancer via inhibiting tumor suppressor let-7g. Cancer Lett. 415, 11–19. doi:10.1016/j.canlet.2017.11.018
Chen, J., Zhang, Y. C., Huang, C., Shen, H., Sun, B., Cheng, X., et al. (2019). m(6)A regulates neurogenesis and neuronal development by modulating histone methyltransferase Ezh2m6A regulates neurogenesis and neuronal development by modulating histone methyltransferase Ezh2. Genomics Proteomics Bioinf. 17, 154–168. doi:10.1016/j.gpb.2018.12.007
Denli, A. M., Tops, B. B., Plasterk, R. H., Ketting, R. F., and Hannon, G. J. (2004). Processing of primary microRNAs by the Microprocessor complex. Nature 432, 231–235. doi:10.1038/nature03049
Desrosiers, R., Friderici, K., and Rottman, F. (1974). Identification of methylated nucleosides in messenger RNA from Novikoff hepatoma cells. Proc. Natl. Acad. Sci. USA 71, 3971–3975. doi:10.1073/pnas.71.10.3971
Dominissini, D., Moshitch-Moshkovitz, S., Schwartz, S., Salmon-Divon, M., Ungar, L., Osenberg, S., et al. (2012). Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature 485, 201–206. doi:10.1038/nature11112
Gregory, R. I., Yan, K. P., Amuthan, G., Chendrimada, T., Doratotaj, B., Cooch, N., et al. (2004). The Microprocessor complex mediates the genesis of microRNAs. Nature 432, 235–240. doi:10.1038/nature03120
Han, J., Lee, Y., Yeom, K. H., Kim, Y. K., Jin, H., and Kim, V. N. (2004). The Drosha-DGCR8 complex in primary microRNA processing. Genes Dev. 18, 3016–3027. doi:10.1101/gad.1262504
Han, S., Zhao, B. S., Myers, S. A., Carr, S. A., He, C., and Ting, A. Y. (2020). RNA-protein interaction mapping via MS2- or Cas13-based APEX targeting. Proc. Natl. Acad. Sci. USA 117, 22068–22079. doi:10.1073/pnas.2006617117
Han, Y., Feng, J., Xia, L., Dong, X., Zhang, X., Zhang, S., et al. (2019). CVm6A: a visualization and exploration database for m(6)As in cell LinesCVm6A: a visualization and exploration database for m6As in cell lines. Cells 8, 168. doi:10.3390/cells8020168
He, L., Li, J., Wang, X., Ying, Y., Xie, H., Yan, H., et al. (2018). The dual role of N6-methyladenosine modification of RNAs is involved in human cancers. J. Cell Mol. Med. 22, 4630–4639. doi:10.1111/jcmm.13804
Huang, H., Wang, Y., Kandpal, M., Zhao, G., Cardenas, H., Ji, Y., et al. (2020). FTO-dependent N (6)-methyladenosine modifications inhibit ovarian cancer stem cell self-renewal by blocking cAMP SignalingFTO-dependent N6-methyladenosine modifications inhibit ovarian cancer stem cell self-renewal by blocking cAMP signaling. Cancer Res. 80, 3200–3214. doi:10.1158/0008-5472.CAN-19-4044
Huang, H., Weng, H., Zhou, K., Wu, T., Zhao, B. S., Sun, M., et al. (2019). Histone H3 trimethylation at lysine 36 guides m(6)A RNA modification co-transcriptionallyHistone H3 trimethylation at lysine 36 guides m6A RNA modification co-transcriptionally. Nature 567, 414–419. doi:10.1038/s41586-019-1016-7
Iwanami, Y., and Brown, G. M. (1968). Methylated bases of ribosomal ribonucleic acid from HeLa cells. Arch. Biochem. Biophys. 126, 8–15. doi:10.1016/0003-9861(68)90553-5
Kang, H. J., Cheon, N. Y., Park, H., Jeong, G. W., Ye, B. J., Yoo, E. J., et al. (2021). TonEBP recognizes R-loops and initiates m6A RNA methylation for R-loop resolution. Nucleic Acids Res. 49, 269–284. doi:10.1093/nar/gkaa1162
Kwok, C. T., Marshall, A. D., Rasko, J. E., and Wong, J. J. (2017). Genetic alterations of m(6)A regulators predict poorer survival in acute myeloid leukemiaGenetic alterations of m6A regulators predict poorer survival in acute myeloid leukemia. J. Hematol. Oncol. 10, 39. doi:10.1186/s13045-017-0410-6
Landthaler, M., Yalcin, A., and Tuschl, T. (2004). The human DiGeorge syndrome critical region gene 8 and its D. melanogaster homolog are required for miRNA biogenesis. Curr. Biol. 14, 2162–2167. doi:10.1016/j.cub.2004.11.001
Li, B., Jiang, J., Assaraf, Y. G., Xiao, H., Chen, Z. S., and Huang, C. (2020). Surmounting cancer drug resistance: new insights from the perspective of N(6)-methyladenosine RNA modificationSurmounting cancer drug resistance: new insights from the perspective of N6-methyladenosine RNA modification. Drug Resist. Updat. 53, 100720. doi:10.1016/j.drup.2020.100720
Li Y., Y., Xia, L., Tan, K., Ye, X., Zuo, Z., Li, M., et al. (2020). N(6)-Methyladenosine co-transcriptionally directs the demethylation of histone H3K9me2N6-Methyladenosine co-transcriptionally directs the demethylation of histone H3K9me2. Nat. Genet. 52, 870–877. doi:10.1038/s41588-020-0677-3
Linder, B., Grozhik, A. V., Olarerin-George, A. O., Meydan, C., Mason, C. E., and Jaffrey, S. R. (2015). Single-nucleotide-resolution mapping of m6A and m6Am throughout the transcriptome. Nat. Methods 12, 767–772. doi:10.1038/nmeth.3453
Liu, J., Eckert, M. A., Harada, B. T., Liu, S. M., Lu, Z., Yu, K., et al. (2018). m(6)A mRNA methylation regulates AKT activity to promote the proliferation and tumorigenicity of endometrial cancerm6A mRNA methylation regulates AKT activity to promote the proliferation and tumorigenicity of endometrial cancer. Nat. Cell Biol. 20, 1074–1083. doi:10.1038/s41556-018-0174-4
Liu, N., Dai, Q., Zheng, G., He, C., Parisien, M., and Pan, T. (2015). N(6)-methyladenosine-dependent RNA structural switches regulate RNA-protein interactions. Nature 518, 560–564. doi:10.1038/nature14234
Ma, J. Z., Yang, F., Zhou, C. C., Liu, F., Yuan, J. H., Wang, F., et al. (2017). METTL14 suppresses the metastatic potential of hepatocellular carcinoma by modulating N(6) -methyladenosine-dependent primary MicroRNA processingMETTL14 suppresses the metastatic potential of hepatocellular carcinoma by modulating N6 -methyladenosine-dependent primary MicroRNA processing. Hepatology. 65, 529–543. doi:10.1002/hep.28885
Meyer, K. D. (2019). DART-seq: an antibody-free method for global m(6)A detectionDART-seq: an antibody-free method for global m6A detection. Nat. Methods 16, 1275–1280. doi:10.1038/s41592-019-0570-0
Meyer, K. D., and Jaffrey, S. R. (2017). Rethinking m(6)A readers, writers, and ErasersRethinking m6A readers, writers, and erasers. Annu. Rev. Cell Dev. Biol. 33, 319–342. doi:10.1146/annurev-cellbio-100616-060758
Meyer, K. D., Saletore, Y., Zumbo, P., Elemento, O., Mason, C. E., and Jaffrey, S. R. (2012). Comprehensive analysis of mRNA methylation reveals enrichment in 3' UTRs and near stop codons. Cell 149, 1635–1646. doi:10.1016/j.cell.2012.05.003
Min, K. W., Zealy, R. W., Davila, S., Fomin, M., Cummings, J. C., Makowsky, D., et al. (2018). Profiling of m6A RNA modifications identified an age-associated regulation of AGO2 mRNA stability. Aging Cell. 17, e12753. doi:10.1111/acel.12753
Müller, T. A., Struble, S. L., Meek, K., and Hausinger, R. P. (2018). Characterization of human AlkB homolog 1 produced in mammalian cells and demonstration of mitochondrial dysfunction in ALKBH1-deficient cells. Biochem. Biophys. Res. Commun. 495, 98–103. doi:10.1016/j.bbrc.2017.10.158
Müller, T. A., Tobar, M. A., Perian, M. N., and Hausinger, R. P. (2017). Biochemical characterization of AP lyase and mA demethylase activities of human AlkB homologue 1 (ALKBH1)Biochemical characterization of AP lyase and m6A demethylase activities of human AlkB homologue 1 (ALKBH1). Biochemistry 56, 1899–1910. doi:10.1021/acs.biochem.7b00060
Ozkurede, U., Kala, R., Johnson, C., Shen, Z., Miller, R. A., and Garcia, G. (2019). Cap-independent mRNA translation is upregulated in long-lived endocrine mutant mice. J. Mol. Endocrinol. 63, 123–138. doi:10.1530/JME-19-0021
Patil, D. P., Chen, C. K., Pickering, B. F., Chow, A., Jackson, C., Guttman, M., et al. (2016). m(6)A RNA methylation promotes XIST-mediated transcriptional repression. Nature 537, 369–373. doi:10.1038/nature19342
Perry, R. P., and Kelley, D. E. (1974). Existence of methylated messenger RNA in mouse L cells. Cell 1, 37–42. doi:10.1016/0092-8674(74)90153-6
Saneyoshi, M., Harada, F., and Nishimura, S. (1969). Isolation and characterization of N6-methyladenosine from Escherichia coli valine transfer RNA. Biochim. Biophys. Acta 190, 264–273. doi:10.1016/0005-2787(69)90078-1
Shang, W., Gao, Y., Tang, Z., Zhang, Y., and Yang, R. (2019). The pseudogene Olfr29-ps1 promotes the suppressive function and differentiation of monocytic MDSCs. Cancer Immunol. Res. 7, 813–827. doi:10.1158/2326-6066.CIR-18-0443
Svobodova Kovarikova, A., Stixova, L., Kovarik, A., Komurkova, D., Legartova, S., Fagherazzi, P., et al. (2020). N(6)-Adenosine methylation in RNA and a reduced m3G/TMG level in non-coding RNAs appear at microirradiation-induced DNA lesions. Cells 9, 360. doi:10.3390/cells9020360
Visvanathan, A., Patil, V., Arora, A., Hegde, A. S., Arivazhagan, A., Santosh, V., et al. (2018). Essential role of METTL3-mediated m(6)A modification in glioma stem-like cells maintenance and radioresistanceEssential role of METTL3-mediated m6A modification in glioma stem-like cells maintenance and radioresistance. Oncogene 37, 522–533. doi:10.1038/onc.2017.351
Wang, W., Sun, B., Xia, Y., Sun, S., and He, C. (2020). RNA N6-methyladenosine-related gene contribute to clinical prognostic impact on patients with liver cancer. Front. Genet. 11, 306. doi:10.3389/fgene.2020.00306
Wang, X., Zhao, B. S., Roundtree, I. A., Lu, Z., Han, D., Ma, H., et al. (2015). N(6)-methyladenosine modulates messenger RNA translation efficiency. Cell 161, 1388–1399. doi:10.1016/j.cell.2015.05.014
Wang, Y., Li, Y., Toth, J. I., Petroski, M. D., Zhang, Z., and Zhao, J. C. (2014). N6-methyladenosine modification destabilizes developmental regulators in embryonic stem cells. Nat. Cell Biol. 16, 191–198. doi:10.1038/ncb2902
Wei, J., Yin, Y., Zhou, J., Chen, H., Peng, J., Yang, J., et al. (2020). METTL3 potentiates resistance to cisplatin through m A modification of TFAP2C in seminomaMETTL3 potentiates resistance to cisplatin through m6 A modification of TFAP2C in seminoma. J. Cell Mol. Med. 24, 11366–11380. doi:10.1111/jcmm.15738
Wu, C., Chen, W., He, J., Jin, S., Liu, Y., Yi, Y., et al. (2020). Interplay of m(6)A and H3K27 trimethylation restrains inflammation during bacterial infection. Sci. Adv. 6, eaba0647. doi:10.1126/sciadv.aba0647
Xiang, Y., Laurent, B., Hsu, C. H., Nachtergaele, S., Lu, Z., Sheng, W., et al. (2017). RNA m(6)A methylation regulates the ultraviolet-induced DNA damage responseRNA m6A methylation regulates the ultraviolet-induced DNA damage response. Nature 543, 573–576. doi:10.1038/nature21671
Xiao, S., Cao, S., Huang, Q., Xia, L., Deng, M., Yang, M., et al. (2019). The RNA N(6)-methyladenosine modification landscape of human fetal tissues The RNA N6-methyladenosine modification landscape of human fetal tissues. Nat. Cell Biol. 21, 651–661. doi:10.1038/s41556-019-0315-4
Yang, D., Qiao, J., Wang, G., Lan, Y., Li, G., Guo, X., et al. (2018). N6-Methyladenosine modification of lincRNA 1281 is critically required for mESC differentiation potential. Nucleic Acids Res. 46, 3906–3920. doi:10.1093/nar/gky130
Yang, X., Liu, Q. L., Xu, W., Zhang, Y. C., Yang, Y., Ju, L. F., et al. (2019). m(6)A promotes R-loop formation to facilitate transcription terminationm6A promotes R-loop formation to facilitate transcription termination. Cell Res. 29, 1035–1038. doi:10.1038/s41422-019-0235-7
Yang, Y., Fan, X., Mao, M., Song, X., Wu, P., Zhang, Y., et al. (2017). Extensive translation of circular RNAs driven by N-methyladenosineExtensive translation of circular RNAs driven by N6-methyladenosine. Cell Res. 27, 626–641. doi:10.1038/cr.2017.31
Yoneda, R., Ueda, N., Uranishi, K., Hirasaki, M., and Kurokawa, R. (2020). Long noncoding RNA pncRNA-D reduces cyclin D1 gene expression and arrests cell cycle through RNA m(6)A modificationLong noncoding RNA pncRNA-D reduces cyclin D1 gene expression and arrests cell cycle through RNA m6A modification. J. Biol. Chem. 295, 5626–5639. doi:10.1074/jbc.RA119.011556
Zhang, C., Chen, L., Peng, D., Jiang, A., He, Y., Zeng, Y., et al. (2020). METTL3 and N6-methyladenosine promote homologous recombination-mediated repair of DSBs by modulating DNA-RNA hybrid accumulation. Mol. Cell. 79, 425–442.e7. doi:10.1016/j.molcel.2020.06.017
Zhang J., J., Bai, R., Li, M., Ye, H., Wu, C., Wang, C., et al. (2019). Excessive miR-25-3p maturation via N(6)-methyladenosine stimulated by cigarette smoke promotes pancreatic cancer progression Excessive miR-25-3p maturation via N6-methyladenosine stimulated by cigarette smoke promotes pancreatic cancer progression. Nat. Commun. 10, 1858. doi:10.1038/s41467-019-09712-x
Zhang, Q., Riddle, R. C., Yang, Q., Rosen, C. R., Guttridge, D. C., Dirckx, N., et al. (2019). The RNA demethylase FTO is required for maintenance of bone mass and functions to protect osteoblasts from genotoxic damage. Proc. Natl. Acad. Sci. USA 116, 17980–17989. doi:10.1073/pnas.1905489116
Zhao, B. S., Roundtree, I. A., and He, C. (2017). Post-transcriptional gene regulation by mRNA modifications. Nat. Rev. Mol. Cell Biol. 18, 31–42. doi:10.1038/nrm.2016.132
Zhou, K. I., Parisien, M., Dai, Q., Liu, N., Diatchenko, L., Sachleben, J. R., et al. (2016). N(6)-Methyladenosine modification in a long noncoding RNA hairpin predisposes its conformation to protein binding. J. Mol. Biol. 428, 822–833. doi:10.1016/j.jmb.2015.08.021
Zhou, S., Bai, Z. L., Xia, D., Zhao, Z. J., Zhao, R., Wang, Y. Y., et al. (2018). FTO regulates the chemo-radiotherapy resistance of cervical squamous cell carcinoma (CSCC) by targeting β-catenin through mRNA demethylationFTO regulates the chemo-radiotherapy resistance of cervical squamous cell carcinoma (CSCC) by targeting β-catenin through mRNA demethylation. Mol. Carcinog. 57, 590–597. doi:10.1002/mc.22782
Keywords: N6-methyladenosine, DNA damage, DNA repair, genome stability, histone modifications
Citation: Qu F, Tsegay PS and Liu Y (2021) N6-Methyladenosine, DNA Repair, and Genome Stability. Front. Mol. Biosci. 8:645823. doi: 10.3389/fmolb.2021.645823
Received: 24 December 2020; Accepted: 09 February 2021;
Published: 09 April 2021.
Edited by:
Arijit Dutta, The University of Texas Health Science Center at San Antonio, United StatesReviewed by:
Alexey Ruzov, University of Nottingham, United KingdomIvan V. Rosado, Institute of Biomedicine of Seville (IBIS), Spain
Copyright © 2021 Qu, Tsegay and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuan Liu, eXVhbGl1QGZpdS5lZHU=
 Fei Qu1
Fei Qu1 Yuan Liu
Yuan Liu