- 1Department of Otorhinolaryngology Head and Neck Surgery, College of Medicine, Kyung Hee University Medical Center, Seoul, Republic of Korea
- 2Medical Research Center for Bioreaction to Reactive Oxygen Species and Biomedical Science Institute, Core Research Institute, Kyung Hee University, Seoul, Republic of Korea
- 3Department of Laboratory Medicine, College of Medicine, Kyung Hee University, Seoul, Republic of Korea
- 4Department of Occupational and Environmental Medicine, School of Medicine, Kyung Hee University, Seoul, Republic of Korea
- 5Department of Precision Medicine, Graduate School, Kyung Hee University, Seoul, Republic of Korea
- 6Department of Integrative Medicine, College of Medicine, Kyung Hee University, Seoul, Republic of Korea
Oxidative stress contributes to the pathogenesis of facial nerve injury (FNI), yet the role of antioxidants in driving regeneration and functional recovery remains incompletely defined. This narrative review synthesizes studies published between 2008 and 2025 that evaluated antioxidant interventions in FNI across animal and limited human contexts. We systematically searched five databases and included 19 studies assessing oxidative stress markers and neural outcomes following antioxidant administration. To avoid overgeneralization, we stratified findings by injury model—crush/compression, transection with anastomosis, and ischemic/viral—and by primary endpoints (electrophysiology/behavior vs. histology/biochemistry), and, where reported, by intervention timing and dose. Antioxidants commonly reduced reactive oxygen species and modulated survival and inflammatory pathways, supporting neuroprotection and, in some models, improved electrophysiological or behavioral readouts. However, benefits varied by model and regimen: crush injuries showed earlier functional gains, whereas transection models more often demonstrated histological improvement without consistent short-term functional recovery; ischemic/viral studies frequently lacked standardized electrophysiological confirmation. Outcomes were also contingent on timing and dose (with earlier initiation and moderate dosing generally more favorable), and select combinations showed additive effects in preclinical settings. Overall, the evidence is predominantly preclinical, heterogeneous in dosing/timing/formulations, and limited by small sample sizes and inconsistent functional outcomes. Antioxidant strategies should therefore be considered hypothesis-generating rather than clinically recommendable at this time. Future research should use model-appropriate, standardized functional endpoints, prespecify timing/dose exploration, evaluate rational combinations, and conduct well-powered clinical trials to establish efficacy, optimal use, and safety.
1 Introduction
1.1 Facial nerve
The facial nerve, also known as the seventh cranial nerve, is a critical component of the cranial nervous system responsible for controlling the muscles involved in facial expression. The motor nerve fibers of the facial nerve are myelinated and include sensory, gustatory, and parasympathetic components. Anatomically, the facial nerve originates at the pontomedullary junction of the brainstem, passes through the internal auditory canal, and converges at the geniculate ganglion. This sensory ganglion is located within the facial canal and contains sensory nerve cell bodies that transmit taste from the anterior two-thirds of the tongue and sensation from the palate, auricle, and external auditory canal. After passing through the temporal bone and exiting the skull via the stylomastoid foramen, the facial nerve branches into small motor fibers extending to the posterior auricular nerve, auricular muscles, and stylohyoid muscle. It then enters the parotid gland and divides into the upper temporal and lower cervicofacial branches, further branching into five terminal branches: the temporal, zygomatic, buccal, marginal mandibular, and cervical branches. These branches control various facial-expression muscles, enabling movements such as raising the eyebrows, closing the eyes, smiling, and frowning. The facial nerve contains parasympathetic fibers that regulate salivary and lacrimal gland secretion, as well as gustatory sensory fibers for the anterior two-thirds of the tongue. This multifunctionality is essential for maintaining facial symmetry and various autonomic functions. Due to its complex anatomical pathways and diverse functions, the facial nerve is susceptible to injury from trauma, inflammation, and surgery, potentially leading to aesthetic and physiological issues such as facial asymmetry, dry eyes, and loss of taste. Understanding the anatomical and physiological complexity of the facial nerve is crucial for diagnosing and treating disorders related to facial nerve damage (Ho et al., 2015; Huang et al., 2023; Pauna et al., 2024; Seneviratne and Patel, 2025).
1.2 Facial nerve injury (FNI)
Facial nerve injury (FNI) significantly impacts a patient’s quality of life, leading to facial asymmetry that can complicate social interactions and cause psychological stress and social isolation. Additional physiological issues such as dry eyes, dry mouth, and speech difficulties can further diminish overall health and quality of life. FNI can result from various pathological conditions, including congenital malformation, trauma, inflammatory disease, tumors, and surgical interventions. Bell’s palsy, the most common cause of unilateral facial paralysis, occurs acutely and is often associated with viral infections. In most cases, partial or complete recovery from Bell’s palsy occurs within several months without treatment. FNI is prevalent in adults and closely associated with surgical procedures such as parotidectomy, mastoidectomy, and facial cosmetic surgeries. In adults, the prevalence of facial nerve paralysis is approximately 30 per 100,000 individuals annually. Post-injury, symptoms such as facial muscle weakness or paralysis can lead to facial asymmetry, difficulty closing the eyes, loss of nasolabial folds, and drooping of the mouth corner. The distal axons and myelin of the injured nerve undergo Wallerian degeneration (WD), characterized by the degeneration and disintegration of axons and myelin. Schwann cell proliferation and macrophage-mediated debris clearance create an environment conducive to nerve regeneration. The peripheral nervous system (PNS) has a faster myelin clearance rate and thus a higher regenerative capacity than the central nervous system (CNS). The regeneration capability of an axon may vary depending on the site and severity of the injury and the patient’s age. Cellular changes involved in axonal regeneration include Schwann cell activation, blood-nerve barrier disruption, and the recruitment of immune cells such as macrophages and cytokine-producing cells. Relevant molecular changes include metalloproteinase-mediated extracellular matrix modulation, neurotrophic factor upregulation, and cytokine production. These cellular and molecular changes create conditions that induce axon regeneration from the proximal nerve stump. Nerve injury also increases oxidative stress, exacerbating cellular damage and dysfunction. Oxidative stress results from the excessive production of free radicals (FR) and reactive oxygen species (ROS), which can damage cell membranes, proteins, and DNA. While the initial inflammatory response facilitates debris clearance and growth factor release, excessive or chronic inflammation can inhibit nerve regeneration. Therefore, therapeutic strategies after nerve injury should focus on precisely regulating the initial inflammatory response and oxidative stress to promote nerve regeneration. Given the complexity and prevalence of FNI and the limited availability of large-scale human studies, preclinical models, particularly small animal studies, play a crucial role in advancing our understanding of the mechanisms and potential therapeutic approaches. (Stoll and Müller, 1999; Cha et al., 2008; Gaudet et al., 2011; Nellis et al., 2017; Reich, 2017; Nocera and Jacob, 2020).
1.3 Oxidative stress
Oxidative stress occurs when there is an imbalance between the production of ROS and FR and the body’s ability to counteract their harmful effects through its antioxidant defense systems. ROS are chemically reactive molecules containing oxygen, including superoxide radicals (O2•−), hydrogen peroxide (H2O2), hydroxyl radicals (•OH), and singlet oxygen (1O2), naturally generated as byproducts of intracellular metabolic processes. Under normal physiological conditions, ROS play essential roles in cell signaling, immune response, and homeostasis. However, excessive ROS production can damage critical cellular structures, including cell membranes, proteins, and DNA, leading to cellular damage and dysfunction. This oxidative stress outcome can significantly exacerbate cellular damage and hinder nerve regeneration following FNI. The imbalance caused by excessive accumulation of ROS and FR leads to the severe degradation of essential biomolecules such as proteins, lipids, and DNA, ultimately impairing cellular function and recovery processes. Oxidative damage is particularly harmful to neurons, as it leads to mitochondrial dysfunction, decreased ATP production, and depletion of the energy necessary for cell survival. Mitochondrial dysfunction can further increase ROS production, creating a vicious cycle of oxidative stress. ROS can also induce lipid peroxidation of cell membranes, compromising their structural integrity and function. This damage can trigger inflammatory signaling pathways. While the initial inflammatory response is crucial for clearing debris and initiating healing, chronic inflammation can lead to further damage and delayed recovery. Excessive ROS generated during neuroinflammatory processes can induce apoptosis or necrosis of nerve cells, reducing the number of viable cells necessary for regeneration. ROS can also alter the signaling pathways of growth factors essential for nerve regeneration, further hindering the regenerative process. Although ROS are primarily generated in mitochondria, they can also arise from enzymatic activities in pathways such as cellular respiration and arachidonic acid metabolism. Cells possess intrinsic antioxidant systems involving enzymes such as superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx), which neutralize ROS. However, this system often fails to fully manage high ROS levels, leading to oxidative stress. The use of antioxidant therapies aimed at mitigating oxidative stress may be a promising strategy to promote peripheral nerve recovery. Preclinical studies provide essential insights into the oxidative stress mechanisms and the potential efficacy of antioxidant interventions, which are crucial for developing future clinical applications (Pizzino et al., 2017; Juan et al., 2021; Adamu et al., 2024).
1.4 Facial nerve injury and oxidative stress
FNI can arise from various causes, including viral and bacterial infection, cerebral infarction, trauma, tumors, and surgery. When the facial nerve is damaged, an active anterograde degeneration process called Wallerian degeneration (WD) occurs at the distal ends of the terminal branches, leading to increased ROS production and oxidative stress. After FNI, ROS and free radicals (FR) significantly affect various intracellular signaling pathways that complexly regulate physiological processes such as apoptosis, inflammatory responses, and cell survival. Facial nerve transection inhibits tubulin polymerization and disrupts microtubule assembly, hindering nerve regeneration. Reactive species such as nitric oxide (NO) and peroxynitrite oxidize polyunsaturated fatty acids, accumulate in neuronal membranes, and produce toxic aldehydes that compromise membrane integrity and function. Inhibition of NO and peroxynitrite can promote axonal regeneration and recovery after peripheral nerve injury (PNI). Increased ROS levels activate glial cells, such as microglia and astrocytes, which play a dual role in the injury response. These cells secrete pro-inflammatory cytokines [e.g., interleukin-1 beta (IL-1β) and tumor necrosis factor-alpha (TNF-α)] that can exacerbate inflammation and accelerate neurodegeneration. Overactivated glial cells release neurotoxic mediators that worsen neuroinflammation and cause further neuronal damage. However, glial cells also contribute to the antioxidant defense by producing enzymes that neutralize ROS and by secreting neurotrophic factors that support axonal regeneration and remyelination. Therefore, reducing excessive glial cell activation and inflammation while enhancing their neuroprotective functions is crucial for promoting nerve recovery. Human herpesvirus 7 (HHV7) infection increases ROS production, leading to facial nerve damage and oxidative stress through increased malondialdehyde (MDA) levels and decreased superoxide dismutase (SOD) activity. These findings underscore the need for integrated therapeutic strategies that regulate FR and ROS, enhance mitochondrial health, and control inflammation to improve nerve injury recovery. Efforts to comprehensively address these oxidative processes will be essential for developing effective treatments aimed at addressing FNI and improving patient outcomes. This review emphasizes preclinical models, specifically small animal studies, due to their prevalence in the literature and their ability to provide detailed insights into the oxidative processes and therapeutic potential of antioxidants in FNI. These models are instrumental in elucidating the underlying mechanisms and guiding the development of effective treatment strategies for FNI (Chang et al., 2021; Yeh and Liu, 2021; Oh et al., 2024; Lee et al., 2025).
2 Research methods
Although extensive research has examined the role of antioxidants in facial nerve injury (FNI) and various diseases, the field has lacked comprehensive reviews specifically addressing the impact of antioxidant therapy on nerve regeneration post-injury. To fill this gap, one of the authors (S.W.B.) conducted a thorough literature search across five electronic databases—Cochrane Libraries, EMBASE, Google Scholar, PubMed, and SCOPUS—covering publications from January 2008 to May 2025. Keywords such as “facial nerve injury” and “antioxidants” were used in the search, which focused on studies published in English and included both human and animal studies investigating the association between FNI and antioxidants. This search strategy retrieved a total of 154 studies. Exclusions included studies not related to the topic (87), studies not involving the facial nerve (17), non-English publications (14), review articles (9), and duplicate studies (8). Ultimately, 19 studies were selected (Figure 1) and analyzed for information on changes in oxidative stress and nerve regeneration following post-FNI antioxidant administration. This review aims to provide insights into the potential therapeutic efficacy of antioxidants on FNI by synthesizing their impact on the pathogenesis of facial nerve degeneration and regeneration (Figure 1). The literature search was designed to focus on preclinical studies, with an emphasis on small animal models, to investigate the effects of antioxidant treatments on facial nerve injury. This focus was chosen due to the limited availability of large animal and human studies in this area and the critical insights these models provide into the efficacy and mechanisms of antioxidant therapies.
3 Results
3.1 Oxidative stress factors associated with facial nerve injury
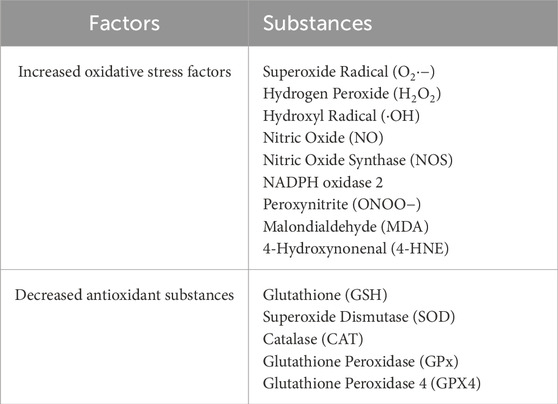
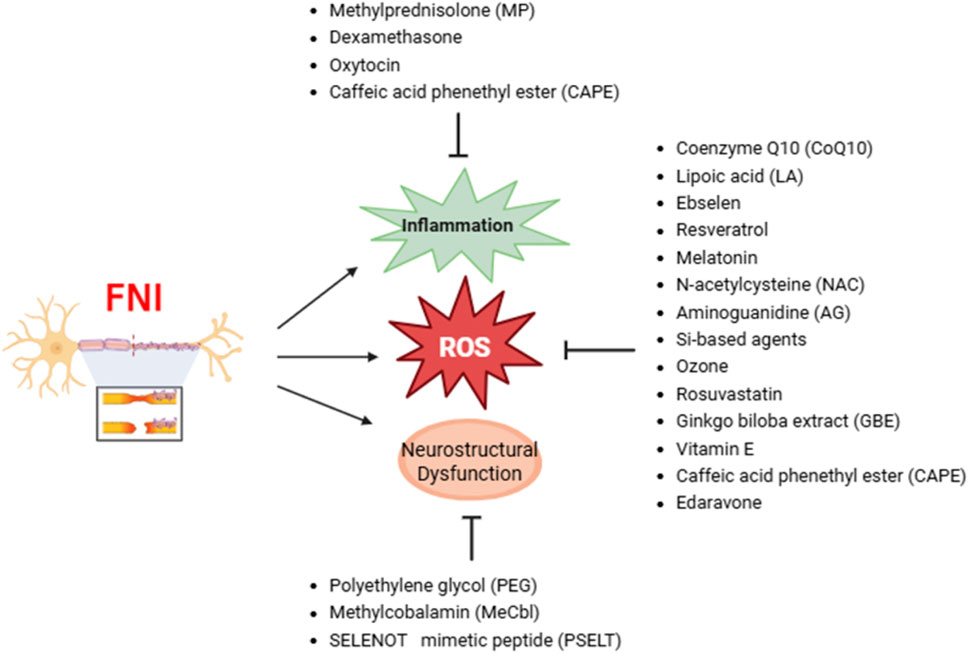
Facial nerve injury (FNI) is associated with an increase in oxidative stress, which plays a critical role in nerve degeneration. Experimental models of FNI, induced through various methods such as nerve transection, compression, and viral infection, show a significant increase in oxidative stress markers and a decrease in antioxidant substances (Table 1). This oxidative stress is characterized by elevated levels of superoxide radicals (O2·−), hydrogen peroxide (H2O2), hydroxyl radicals (·OH), nitric oxide (NO), nitric oxide synthase (NOS), NADPH oxidase 2, peroxynitrite (ONOO−), malondialdehyde (MDA), and 4-hydroxynonenal (4-HNE). Concurrently, there are decreases in key antioxidants such as glutathione (GSH), superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), and glutathione peroxidase 4 (GPX4).
3.2 Antioxidants effective against facial nerve injury
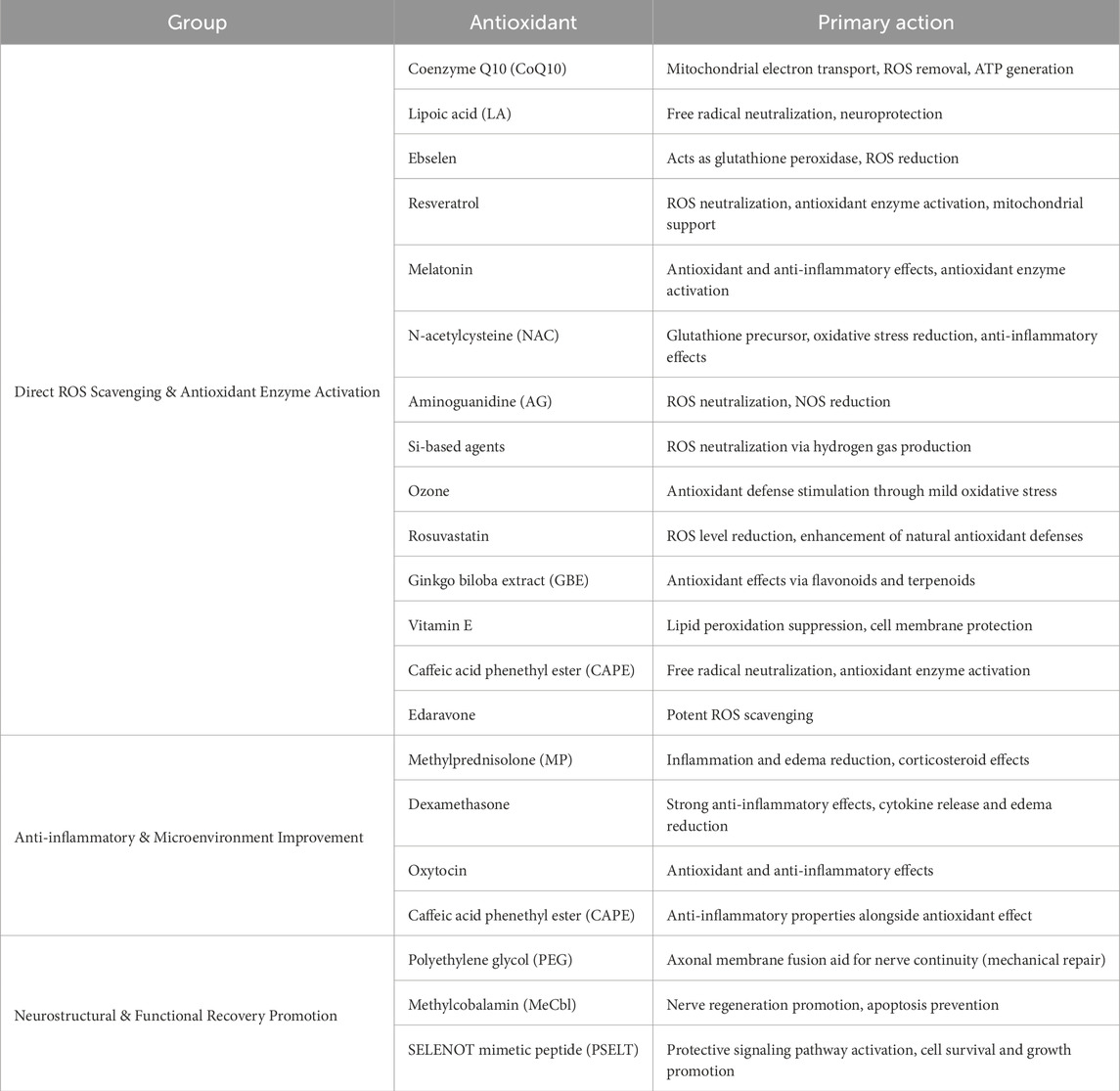
The present review of 19 studies indicates that the administration of antioxidants is associated with functional recovery, improved nerve conduction, and increased levels of histological markers for nerve recovery. Facial nerve regeneration is a complex process influenced by oxidative stress, inflammation, apoptosis, and cellular metabolic disturbances following nerve injury. Antioxidants intervene at various points in these pathological cascades to create an environment conducive to regeneration. Their mechanisms of action include direct scavenging of ROS, modulation of survival and recovery-related signaling pathways, stabilization of mitochondria, and support of neurotrophic activity. These actions contribute to maintaining nerve integrity, promoting axonal regrowth, and enhancing Schwann cell remyelination (Table 2).
3.2.1 Coenzyme Q10 (CoQ10)
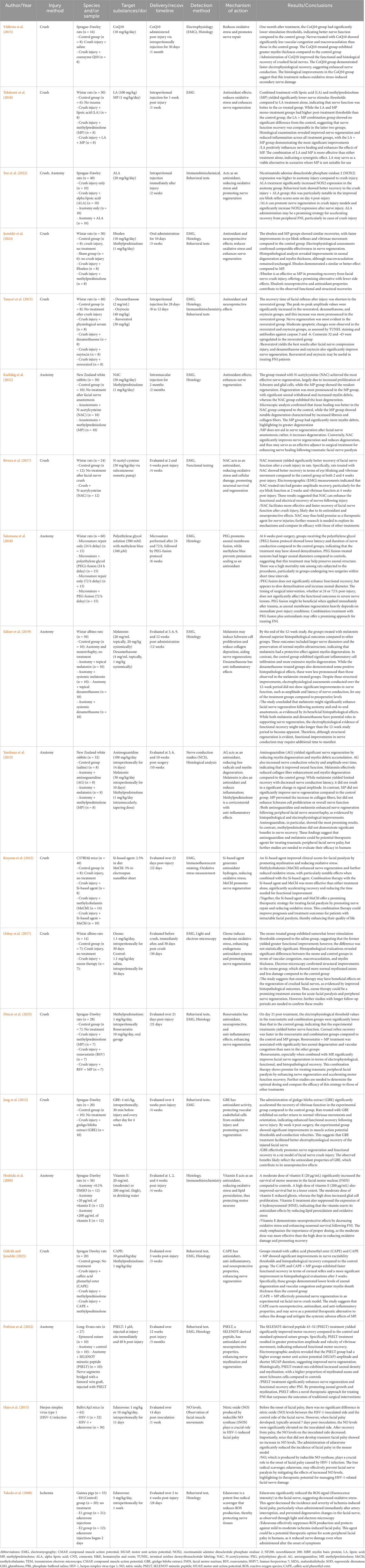
Coenzyme Q10 (CoQ10) plays a crucial role in generating ATP through the electron transport chain within mitochondria, and ATP is essential for maintaining cellular functions. CoQ10 acts as a potent antioxidant, helping to scavenge ROS within cells. This reduces cellular damage and oxidative stress, prevents lipid peroxidation and DNA damage, and protects cell and mitochondrial membranes from damage. CoQ10 also possesses anti-inflammatory properties that can reduce inflammatory responses and prevent additional inflammation-induced damage following nerve injury, thereby promoting nerve regeneration and accelerating nerve function recovery. After PNI, CoQ10 was shown to promote nerve regeneration and reduce oxidative stress and inflammation (Vachirarojpisan et al., 2024). Wistar rats administered CoQ10 following sciatic nerve injury exhibited faster nerve regeneration, with increased numbers and diameters of regenerated nerve fibers (Moradi et al., 2014). Following compression-induced FNI, CoQ10 administration increased myelin thickness, reduced secondary nerve damage due to oxidative stress, and improved both functional and histological recovery of the injured facial nerve (Yildirim et al., 2015). These results suggest that CoQ10 administration can improve peripheral facial nerve damage by suppressing oxidative stress and reducing inflammation.
3.2.2 Lipoic acid (LA) and alpha-lipoic acid (ALA)
Lipoic acid (LA) and alpha-lipoic acid (ALA) are potent antioxidants that help neutralize free radicals and reduce oxidative stress. These properties are crucial in managing nerve injuries, as oxidative stress can exacerbate nerve damage and impede recovery. LA has been shown to have neuroprotective effects, contributing to maintaining nerve function and promoting nerve regeneration. In diabetic neuropathy, LA administration improved neuropathic symptoms by reducing oxidative stress (Nagamatsu et al., 1995), LA and methylprednisolone (MP) play complementary roles in treating facial nerve injury (FNI), with combination treatment yielding better outcomes in terms of recovering nerve function and reducing inflammation (Tekdemir et al., 2018). Additionally, ALA prevents further damage following peripheral facial nerve transection injury by reducing oxidative stress through the removal of excessive ROS (Yoo et al., 2022). However, conflicting evidence exists regarding the efficacy of ALA. A Cochrane review concluded that ALA treatment in diabetic peripheral neuropathy (DPN) patients has little or no effect on neuropathy symptoms and functional impairment compared to placebo after 6 months of treatment (Baicus et al., 2024). This finding suggests that while ALA has shown potential benefits in some studies, its overall effectiveness in DPN may be limited. The conflicting results highlight the complexity of ALA’s action and suggest that its efficacy may vary depending on factors such as dosage, duration of treatment, and patient population. These inconsistencies indicate the need for further research to determine the optimal use of ALA and LA in nerve injury management and to clarify their roles as therapeutic agents in conditions associated with oxidative stress.
3.2.3 Methylprednisolone (MP)
Methylprednisolone (MP) is a potent corticosteroid known for its ability to reduce inflammation and edema around damaged nerves, thereby promoting nerve healing. By decreasing inflammation, MP can reduce axonal degeneration and promote axonal regeneration, facilitating the overall recovery of nerve function. It is particularly effective in treating various nerve injuries, such as facial nerve palsy, and is widely used in clinical settings (Tekdemir et al., 2018). However, the use of MP is not without controversy. Some studies suggest that MP might have negative effects on nerve regeneration, potentially due to its interference with other physiological processes necessary for nerve recovery (Karlidag et al., 2012). For instance, in a traumatic peripheral facial nerve injury model, MP inhibited the enhancement of collagen fiber without enhancing Schwann cell proliferation or overall nerve function (Yanilmaz et al., 2015). Despite these concerns, MP administration significantly reduces edema and inflammation at the nerve injury site, relieving pressure on the nerve and promoting recovery. MP has shown effectiveness in promoting recovery from facial nerve compression injuries, contributing to functional and structural recovery through its neuroprotective and antioxidant effects (Şeneldir et al., 2024). It significantly improves axonal degeneration, vascular congestion caused by facial nerve palsy, and enhances electrophysiological recovery (Dincer et al., 2025). MP can thus be a useful therapeutic agent in managing nerve injuries by effectively promoting nerve recovery and contributing to functional and structural recovery through its neuroprotective and antioxidant effects (Gölcük and Şeneldir, 2025). MP can thus be a useful therapeutic agent in managing nerve injuries by effectively promoting nerve recovery and contributing to functional and structural recovery through its neuroprotective and antioxidant effects. The Second National Spinal Cord Injury Study (NASCIS-II) study on methylprednisolone for acute spinal cord injury was that methylprednisolone therapy, when administered within 8 h of injury, did not improve motor score recovery in patients with acute cervical or thoracic traumatic spinal cord injury and was associated with a higher rate of complications (Evaniew et al., 2015). Thus, while MP can be a useful therapeutic agent in managing nerve injuries by promoting nerve recovery and contributing to functional and structural recovery, its application should be carefully considered, taking into account the potential for complications and the mixed evidence regarding its efficacy.
3.2.4 Dexamethasone
Dexamethasone is a glucocorticoid that reduces inflammation and suppresses the immune response by inhibiting the release of pro-inflammatory cytokines and mediators. This reduction in inflammation minimizes secondary damage to nerve tissue post-injury, thereby promoting healing and regeneration. By reducing inflammation, dexamethasone also decreases edema around the damaged nerve to improve the microenvironment and facilitate the supply of essential nutrients and oxygen, which are crucial for the nerve recovery process. Although dexamethasone is not primarily recognized as an antioxidant, its ability to control inflammation and stabilize the cellular environment provides effective neuroprotection following nerve injury. For instance, following sciatic nerve injury, dexamethasone has been shown to reduce tissue edema and demyelination, promote remyelination, and help improve gastrocnemius muscle atrophy (Feng and Yuan, 2015). Another study demonstrated that dexamethasone administration after facial nerve compression injury improved functional recovery, as evidenced by increased amplitude values in EMG. In FNI caused by transection, dexamethasone exhibited anti-inflammatory effects, reducing edema and inflammation (Edizer et al., 2019). The safety of low-dose, preservative-free perineural dexamethasone suggests potential neuroprotective effects when used as an adjuvant to local anesthetics. However, concerns are raised about potential neurotoxicity, especially when it is combined with local anesthetics at higher concentrations or when formulations contain sulfites. Additionally, the review highlights the need for caution in patients with preexisting neuropathies due to their increased susceptibility to ischemic damage (De Cassai et al., 2025). These findings collectively indicate that dexamethasone can promote regeneration and recovery after nerve injury through its anti-inflammatory and neuroprotective effects. Nonetheless, clinical use should be carefully considered, especially regarding dosage and patient population, to mitigate potential risks associated with its use.
3.2.5 Ebselen
Ebselen is a neuroprotective agent that protects cellular components from oxidative damage by modulating enzyme cofactors, metalloproteins, and gene expression, exhibiting antioxidant and anti-inflammatory effects, and regulating the immune system. Ebselen acts as an analogue of glutathione peroxidase, an enzyme that neutralizes ROS and reduces oxidative stress. These properties enable ebselen to protect nerve cells from oxidative damage, which is a common result of nerve injury. In animal models of spinal cord injury, ebselen administration reduced oxidative stress due to spinal cord injury and increased the levels of antioxidant enzymes, thereby protecting neurons (Kalayci et al., 2005). After ischemia/reperfusion injury, ebselen inhibited excessive glutamate release and enhanced the inhibitory effects of GABA to alleviate nerve damage (Seo et al., 2009). Additionally, following peripheral facial nerve compression injury, ebselen administration yielded recovery patterns (Şeneldir et al., 2024). These findings indicate that ebselen exhibits neuroprotective effects in various nerve injury models, aiding in the protection and regeneration of nerve cells by reducing oxidative stress and exerting anti-inflammatory and antioxidant actions.
3.2.6 Oxytocin
Oxytocin is a hormone essential for social bonding, childbirth, and lactation; it is produced in the hypothalamus and secreted by the pituitary gland. Beyond its well-known role in enhancing social interactions and maternal behaviors, oxytocin also reduces oxidative stress through powerful antioxidant actions by increasing the activity of antioxidant enzymes such as SOD and glutathione peroxidase, which neutralize harmful ROS. Oxytocin also reduces lipid peroxidation to help maintain cell membrane integrity, lowers inflammation, and reduces oxidative stress to protect cells from oxidative damage. In a sciatic nerve transection injury model, oxytocin administration resulted in higher amplitudes and shorter latency times in EMG, with increased vascular diameter and thickness, suggesting that it promotes revascularization and improves functional recovery (Gümüs et al., 2015). Oxytocin administration can also promote nerve recovery by upregulating nerve growth factor (NGF), Nrf2, and irisin (Tosyalı et al., 2023). Following facial nerve compression injury, oxytocin administration effectively modulated apoptosis and promoted facial nerve regeneration to yield significant improvements in functional recovery (Tanyeri et al., 2015). These findings suggest that oxytocin has potential as a therapeutic agent that may promote nerve regeneration and recovery through its neuroprotective and anti-inflammatory actions following PNI.
3.2.7 Resveratrol
Resveratrol is a well-known polyphenolic compound; it is found in various plants, especially grapes, red wine, berries, and peanuts, and has antioxidant, anti-inflammatory, and cardioprotective effects. In PNI, in particular, resveratrol contributes to reducing oxidative stress by neutralizing ROS. Resveratrol increases the activity of antioxidant enzymes in the body, reduces inflammation, and promotes mitochondrial function. In a sciatic nerve compression injury model, resveratrol administration increased the protein expression of vascular endothelial growth factor (VEGF) and the number of axons in the damaged nerve, and thus enhanced nerve regeneration (Ding et al., 2018). Resveratrol also promoted nerve recovery by accelerating myelin clearance through the autophagy of Schwann cells (Zhang et al., 2020). Following facial nerve compression injury, resveratrol administration significantly improved blink reflex recovery and functional recovery, as evidenced by increased amplitudes in EMG In particular, resveratrol modulated apoptosis and increased intercellular levels of connexins (connexin 32 and 43), which are important for cell communication and tissue integrity during the healing process, and thus aided nerve recovery (Tanyeri et al., 2015). These findings suggest that resveratrol can play an important role in promoting recovery after nerve injury.
3.2.8 Melatonin
Melatonin is a hormone that is primarily produced by the pineal gland in the brain. It is well known to regulate the sleep-wake cycle, but it also possesses powerful antioxidant, anti-inflammatory, and neuroprotective effects. Based on these properties, extensive research has examined melatonin’s potential therapeutic effects in various conditions, including nerve injury. Melatonin reduces oxidative stress by stimulating antioxidant enzymes, such as SOD, catalase (Ct), and peroxidase (Uyanikgil et al., 2017). Schwann cells express melatonin receptors to enable melatonin signaling. Melatonin promotes the reorganization, migration, dedifferentiation, and regeneration of Schwann cells (Klymenko and Lutz, 2022). In promoting peripheral nerve recovery, melatonin inhibits ROS generation through mitophagy, maintains autophagic flow, and suppresses mitochondrial apoptosis [48]. After FNI, intraperitoneal injection of melatonin yielded more extensive systemic effects and better preservation of nerve diameter and myelin thickness compared to controls or local administration. Melatonin also increased the activity of antioxidant enzymes, such as SOD and glutathione peroxidase, to further protect cells from oxidative damage (Edizer et al., 2019). Following traumatic peripheral FNI, melatonin administration reduced the collagen fiber increase and myelin degeneration seen in controls, and thus enhanced nerve regeneration (Yanilmaz et al., 2015). Melatonin shows potential as a neuroprotective agent that can promote recovery following PNI. However, structure–function translation has been inconsistent in severe injury settings. In an axotomy/anastomosis model, melatonin yielded superior histopathology but did not produce significant short-term electrophysiological gains (e.g., EMG amplitude/latency) over a 12-week window, underscoring model- and dose-contingent effects on function. Comparative data in transection contexts further suggest that while melatonin can outperform steroids on select structural or electrophysiological parameters, near-term functional improvements are not uniformly observed and may require longer follow-up to emerge. Taken together, melatonin shows promise as an experimental antioxidant/neuroprotective agent in FNI, with benefits influenced by injury severity, timing, and dose. Translation will require standardized functional endpoints (EMG, reflexes, conduction), formal dose–response evaluation, and extended follow-up to determine whether structural gains consistently convert into clinically meaningful functional recovery.
3.2.9 N-acetylcysteine (NAC)
NAC is a medication and dietary supplement derived from L-cysteine, known for its powerful antioxidant properties and its use as a mucolytic agent in respiratory diseases. NAC acts as a precursor for glutathione (GSH) in the body, playing a crucial role in regulating intracellular oxidative stress by increasing intracellular concentrations of GSH. Additionally, NAC inhibits the activation of nuclear factor kappa B (NF-κB), thereby reducing the levels of inflammatory cytokines such as TNF-α and interleukins (IL-6, IL-1β) (Tenório et al., 2021). Following sciatic nerve compression injury, NAC administration reduced perineurial thickness, axonal degeneration, axonal lysis, edema, inflammation, muscle atrophy, and myopathy through its antioxidant effects (Sarıtaş et al., 2024). In the context of facial nerve injuries, NAC has been shown to improve nerve regeneration and reduce nerve degeneration by increasing the proliferation of Schwann cells and modulating glial cell activity (Karlidag et al., 2012). NAC likely influences glial activation by reducing oxidative stress and inflammation, which can help prevent the overactivation of glial cells such as microglia and astrocytes. This modulation can decrease the release of neurotoxic mediators and promote a more supportive environment for nerve repair. In rats with compression-induced facial nerve injury (FNI), NAC administration resulted in better recovery in terms of eye blinking and vibrissae movement compared to controls at both 2 and 4 weeks post-injury. Electromyography (EMG) measurements indicated greater amplitude recovery, with eye-blink function recovering more rapidly by week 2 and vibrissae function by week 4 (Rivera et al., 2017). These results suggest that NAC can enhance the functional and electrical recovery of nerves following injury, in part by modulating glial cell responses to create a more favorable environment for nerve regeneration.
3.2.10 Polyethylene glycol (PEG)
PEG is a highly biocompatible and soluble compound that is widely used in the medical field. In FNI, it functions by fusing damaged axonal membranes to restore axonal continuity, enhance nerve conduction and functional recovery, and restore action potentials at the injury site. PEG also helps maintain nerve function by preventing Wallerian degeneration, which is the degeneration of the axonal portion of a neuron upon its separation from the cell body (Smith et al., 2025). The application of PEG solution to the sciatic nerve at the cut axonal end was found to preserve neuromuscular junctions and prevent muscle atrophy, leading to the recovery of sensory and motor functions. This so-called “PEG fusion” provides a rapid and effective method for PNI repair, yielding significantly better functional outcomes compared to traditional nerve suturing techniques (Ghergherehchi et al., 2019). After sciatic nerve transection, PEG administration yielded functional recovery in EMG and inclined plane tests, increased levels of nerve growth factor (NGF), reduced fibrosis, and increased axonal density, indicating that it promotes neuroprotection and regeneration (Tunç et al., 2025). In a study involving transection of the mandibular branch of the facial nerve, the PEG fusion group exhibited larger axonal diameters compared to the control group, indicating that PEG fusion had a positive impact on preserving axonal structure (Salomone et al., 2018). These results suggest that PEG can promote functional and structural recovery following peripheral nerve injury.
3.2.11 Aminoguanidine (AG)
Aminoguanidine (AG) is a compound known for its potent antioxidant effects and its ability to inhibit the formation of advanced glycation end-products (AGEs) and inducible nitric oxide synthase (iNOS). AG also demonstrated antioxidant activity against brain damage induced by doxorubicin (DOX), reducing levels of malondialdehyde (MDA) and glutathione peroxidase (GPx) while increasing the activity of glutathione S-transferase (GST) [54]. The increase in oxidative stress due to ischemia was mitigated by AG treatment, which increased the oxidized glutathione ratio (GSH/GSSG), activated GST, and reduced lipid peroxidation (Pasten et al., 2021). After ischemia was induced by occlusion of the right common iliac artery and femoral artery, AG administration was associated with functional recovery in hindlimb function tests, reduced endoneurial edema, and decreases in ROS and apoptosis (Alipour et al., 2011). Following traumatic peripheral FNI, AG administration reduced myelin degeneration and myelin debris accumulation, and EMG showed increases in nerve conduction velocity and amplitude over time, indicating improved nerve function (Yanilmaz et al., 2015). In PNI, AG decreases ROS and reduces the production of NO, which can form harmful peroxynitrite when combined with superoxide; thus, AG reduces oxidative stress in this context. Together, these results indicate that AG promotes nerve recovery and functional improvement by protecting nerve cells and maintaining structural integrity after injury.
3.2.12 Methylcobalamin (MeCbl)
Methylcobalamin (MeCbl), the active form of vitamin B12, is essential for nerve health and effective in promoting nerve regeneration after PNI. MeCbl enhances the body’s antioxidant defenses to neutralize harmful ROS and contribute to reducing oxidative stress. Following sciatic nerve compression, MeCbl administration resulted in recovery of motor and sensory functions, as evidenced by improvements in the sciatic function index (SFI) and von Frey filament tests. MeCbl enhanced nerve conduction velocity, promoted nerve regeneration, and increased myelination to aid in axonal repair (Suzuki et al., 2017). In a rat model of sciatic nerve injury, continuous MeCbl administration via an osmotic mini-pump increased the activities of Erk1/2 and Akt, which are key signaling proteins involved in axon growth and neuron survival, and thereby promoted recovery of motor and sensory functions (Okada et al., 2010). After facial nerve compression injury, MeCbl promoted nerve regeneration and further reduced oxidative stress; its effects were particularly pronounced when combined with silicone-based drugs (Koyama et al., 2022). These findings demonstrate that MeCbl is effective in managing oxidative stress and facilitating nerve repair.
3.2.13 Si-based agents
Silicon-based agents react with water to produce hydrogen gas, which acts as a selective antioxidant by targeting and neutralizing nerve cell-damaging ROS, hydroxyl radicals, and peroxynitrite. These agents exert anti-inflammatory effects by reducing pro-inflammatory cytokines and factors, show anti-apoptotic effects by inhibiting pathways leading to cell death, and yield anti-fibrotic effects by decreasing fibrosis-related markers (Koyama et al., 2023). In the case of PNI, oxidative stress due to excessive ROS can hinder nerve regeneration. Silicon-based agents protect nerve cells and create a more favorable environment for nerve regeneration by reducing oxidative stress, making them effective in promoting recovery from nerve damage-related conditions such as facial paralysis. After compression-induced FNI, silicon-based agents improved nerve regeneration by promoting myelination and reducing oxidative stress (Koyama et al., 2022). Due to these properties, silicon-based agents are reported to contribute to the treatment of nerve injuries.
3.2.14 Ozone therapy
Ozone therapy involves the use of ozone gas to stimulate the body’s natural antioxidant defense system, inducing appropriate oxidative stress to exert anti-inflammatory effects, eradicate pathogenic microorganisms, and promote tissue regeneration. Due to its small molecular structure, ozone quickly penetrates cells and tissues to eliminate pathogens and generate oxygen, promoting cellular and tissue recovery. Ozone therapy is effective in alleviating neuropathic pain by promoting efferocytosis of macrophages and activating AMPK/Gas6-MerTK/SOCS3 signaling to reduce neuroinflammation. In a chronic constriction injury (CCI) model, ozone therapy alleviated pain through this mechanism (Ruan et al., 2024). Following sciatic nerve transection, ozone therapy improved function in the SFI and withdrawal reflex (WDR) by enhancing antioxidant enzyme activity, promoting nerve fiber myelination, and increasing functional recovery (Ogut et al., 2020). After compression-induced FNI, ozone therapy improved damage to myelinated axons and demonstrated beneficial effects on regeneration (Ozbay et al., 2017). These results suggest that ozone therapy is effective in reducing oxidative stress associated with PNI and promoting nerve regeneration.
3.2.15 Rosuvastatin
Rosuvastatin is primarily used to lower cholesterol levels, but it also possesses antioxidant properties that may be beneficial in treating PNI. Additionally, rosuvastatin enhances the body’s natural antioxidant defenses, improves endothelial function, and reduces inflammation, thereby creating a favorable environment for nerve healing and regeneration. Following sciatic nerve compression injury, rosuvastatin administration increased motor function recovery in the SFI test, increased the amplitude of compound muscle action potentials (CMAP), and improved nerve conduction. It also increased the number and thickness of myelinated fibers and enhanced the expression levels of neurotrophic factors, such as nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF), to promote nerve cell function and recovery (Abdolmaleki et al., 2020). In a peripheral facial paralysis animal model, rosuvastatin administration contributed to electrophysiological recovery and significantly improved axonal degeneration and vascular congestion, and thus had beneficial effects on facial nerve regeneration (Dincer et al., 2025). In sum, rosuvastatin has shown potential as a therapeutic agent in the treatment of PNI.
3.2.16 Ginkgo biloba extract (GBE)
Ginkgo biloba extract (GBE) is derived from the leaves of the ginkgo biloba tree and is known for its medicinal properties. It contains flavonoids and terpenoids, which have potent antioxidant and anti-inflammatory effects and help protect vascular endothelial cells from oxidative damage by scavenging free radicals (Singh et al., 2019). GBE also promotes microcirculation, which is crucial for nerve health and recovery. The extensive antioxidant properties of GBE may be beneficial in the prevention and treatment of diseases associated with free radical-induced damage (Rong et al., 1996). In an animal model of compression-induced facial nerve injury, recovery of vibrissae function was faster in the GBE-treated group compared to controls, and there were significant improvements in muscle action potential thresholds and conduction velocity in the GBE group (Jang et al., 2012). This suggests that GBE treatment facilitates electrophysiological recovery of the injured facial nerve.
3.2.17 Vitamin E
Vitamin E is a fat-soluble vitamin primarily known for the antioxidant activity of its α-tocopherol form. It is particularly effective in inhibiting lipid peroxidation, thereby protecting cell membranes from oxidative stress. Vitamin E also reduces oxidative stress by scavenging ROS, preventing nerve cell degeneration. These protective actions lead to reduced inflammation and enhanced recovery, making this vitamin a potentially useful therapeutic agent for diseases related to nerve damage. Vitamin E has demonstrated beneficial effects on motor function, pain hypersensitivity, and histopathological changes after nerve injury, promoting neuroprotection and regeneration by inhibiting oxidative stress pathways (Tamaddonfard et al., 2014). In a model of transection-induced FNI, vitamin E significantly increased the survival rate of motor neurons in the facial nucleus of the CNS and demonstrated neuroprotective effects by reducing lipid peroxidation and oxidative stress (Hoshida et al., 2009). However, the effects of vitamin E are not universally positive. A study on the nematode Caenorhabditis elegans revealed that high concentrations of vitamin E (400 μg/mL) can adversely affect thermosensation and thermotaxis learning. The study showed that such high doses impaired presynaptic function and decreased the fluorescent intensities of key neurons involved in thermosensation and learning. These findings suggest that while vitamin E has significant neuroprotective properties, its administration at high concentrations can lead to adverse effects, particularly on neuronal development and function (Li et al., 2013). These protective actions of vitamin E, when administered at appropriate doses, may notably promote neuroprotection and regeneration by inhibiting pathways related to oxidative stress following nerve injury. Benefits are dose- and context-dependent. Moderate dosing has been associated with functional and histological improvements, whereas high exposures have produced paradoxical or adverse effects (e.g., increased gliosis or neuronal dysfunction in select models), underscoring the need for formal dose–response evaluation and safety monitoring. Accordingly, clinical translation will require standardized functional endpoints and careful dose-finding before recommendations can be made.
3.2.18 Caffeic acid phenethyl ester (CAPE)
CAPE is a bioactive compound that is found in bee propolis and known for its anti-inflammatory, antioxidant, and neuroprotective properties. As an antioxidant, CAPE neutralizes free radicals, inhibits lipid peroxidation, enhances antioxidant enzyme activity, and reduces oxidative stress and inflammation by inhibiting the NF-κB pathway. After spinal cord injury, CAPE downregulated DRP1 while activating SIRT1 and PGC1α to alleviate mitochondrial dysfunction and reduce inflammation and oxidative stress (Zhang et al., 2024). CAPE activates the Nrf2 pathway to increase antioxidant enzyme expression, stabilize ROS, and protect nerve cells. CAPE also inhibits the NF-κB pathway, thereby reducing pro-inflammatory cytokine expression and preventing neuroinflammation (Pérez et al., 2023). In a facial nerve crush model, CAPE administration promoted nerve regeneration; when it was combined with MP functional recovery was faster, as measured in terms of corneal reflex. Histopathological evaluation of the CAPE + MP group showed lower levels of axonal degeneration and vascular congestion, with thicker myelin sheaths (Gölcük and Şeneldir, 2025). These findings suggest that CAPE, with its neuroprotective, antioxidant, and anti-inflammatory effects, could serve as a potential therapeutic alternative that could be used to reduce the dosage and mitigate the systemic side effects of MP.
3.2.19 SELENOT mimetic peptide (PSELT)
SELENOT mimetic peptide (PSELT), which is a synthetic peptide derived from the selenoprotein, SELENOT, is known for its antioxidant and neuroprotective properties. PSELT effectively reduces oxidative stress in PNI by neutralizing ROS, enhancing endogenous antioxidant defenses, and preventing lipid peroxidation. Additionally, PSELT modulates protective signaling pathways and reduces inflammation to create a favorable environment for nerve regeneration and functional recovery. PSELT administration improved motor coordination and spontaneous motor activity in mice treated with MPTP by targeting oxidative stress and regulating gene expression to promote cell survival (Alsharif et al., 2021). Following facial nerve transection, PSELT administration enhanced motor recovery, with higher mean motor unit action potential amplitude and shorter duration, with increased axonal density and myelination, and thus improved nerve regeneration and functional recovery (Pothion et al., 2022). Through its antioxidant and neuroprotective actions, PSELT can regulate key signaling pathways and reduce oxidative stress and inflammation, thereby enhancing nerve regeneration and functional recovery.
3.2.20 Edaravone
Edaravone is a potent antioxidant that acts as a FR scavenger, effectively neutralizing unstable molecules that cause oxidative stress and cellular damage. By removing ROS and other FR, edaravone protects nerve cells from oxidative damage, reduces inflammation, and promotes nerve recovery. Due to its antioxidant and anti-inflammatory effects, edaravone has been clinically used in treating acute cerebral ischemia and amyotrophic lateral sclerosis (ALS). In a placental ischemia model, it reduced brain inflammation and oxidative stress, and improved fetal survival, growth, and maternal organ function (Yamashita and Abe, 2024). Edaravone decreased oxidative stress markers such as malondialdehyde (MDA), increased the levels of antioxidants such as SOD and GSH/GSSG, and reduced neuroinflammation through decreased activation of hippocampal microglia (Iba1+ cells) (Ma et al., 2024). However, not all studies report positive outcomes with edaravone. Some research suggests that supraphysiologic doses may lead to delayed recovery or adverse effects. It is crucial to consider the dose–response relationship, as varying doses can significantly impact the therapeutic outcomes. Additionally, while edaravone’s ability to reduce oxidative stress is effective in treating nerve damage-related conditions, such as Bell’s palsy caused by HSV-1 infection, potential off-target effects, particularly at high doses, should be addressed (Hato et al., 2013). Given its strong antioxidant and anti-inflammatory properties, edaravone shows potential as an effective therapeutic agent for preventing damage to cell membranes and tissues in conditions such as ischemic nerve injury (Takeda et al., 2008). Nonetheless, further research is needed to fully understand the optimal dosing and potential risks associated with its use in different clinical scenarios (Table 3) (Figure 2).
3.3 Antioxidants effective against FNI
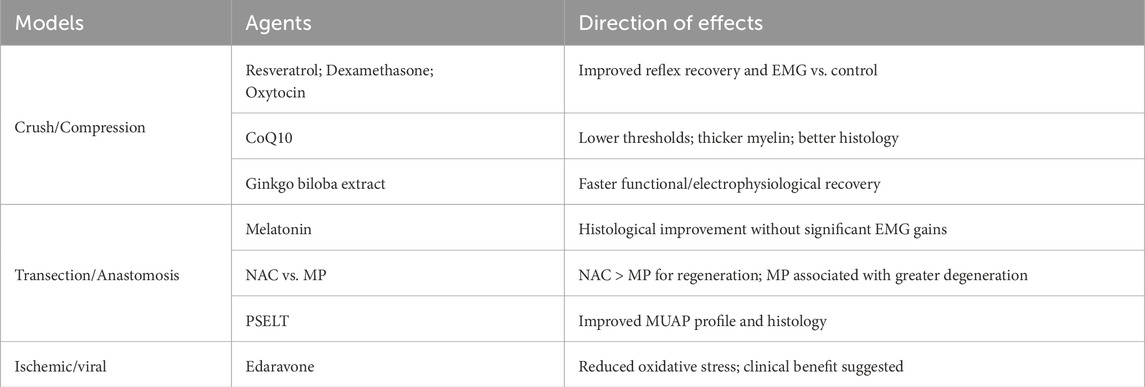
3.3.1 Crush/compression models
In crush/compression models, several antioxidants improved functional readouts. Resveratrol, dexamethasone, and oxytocin shortened reflex recovery times and increased EMG amplitudes versus controls; ebselen achieved functional and histological recovery comparable to methylprednisolone, suggesting a potential steroid-sparing alternative. CoQ10 lowered stimulation thresholds and increased myelin thickness with favorable histology, and Ginkgo biloba extract accelerated vibrissae function recovery with improved conduction metrics. Given the intrinsically higher regenerative potential and substantial spontaneous recovery in crush injuries, these gains are interpreted with caution and not extrapolated to more severe models.
3.3.2 Transection/anastomosis models
In transection/anastomosis settings, structural improvements often outpaced short-term functional gains. For example, melatonin yielded superior histopathology without significant EMG improvement within 12 weeks. N-acetylcysteine outperformed methylprednisolone after anastomosis, with better EMG and histology and less degeneration, underscoring the risk of extrapolating steroid benefits across models. SELENOT-mimetic peptides (PSELT) improved MUAP characteristics and axonal metrics, suggesting mechanistic promise that warrants longer functional follow-up. PEG-fusion mitigated demyelination and increased axonal diameter but provided limited functional enhancement over the study window. Comparator data further indicate advantages over steroids in select parameters: aminoguanidine and melatonin showed superior electrophysiological and structural profiles to methylprednisolone in transection settings, although short-term functional gains were not always consistent.
3.3.3 Ischemic/viral models
In ischemic/viral contexts, edaravone reduced oxidative stress markers and suggested clinical benefit; however, assessments often relied on clinical observation without standardized electrophysiology. Standardized head-to-head confirmation against both no-treatment and steroid comparators is needed to define its incremental effect size and dose window (Table 4).
3.4 Clinical and translational implications
3.4.1 Crush/compression
Several antioxidants (e.g., resveratrol, dexamethasone, oxytocin, ebselen, coenzyme Q10, and Ginkgo biloba extract) were associated with earlier improvements in electrophysiological or behavioral readouts versus controls in crush/compression models. Given the intrinsically high spontaneous recovery in these injuries, such gains should be interpreted as supportive signals rather than definitive efficacy and should not be extrapolated to more severe injuries without confirmatory data. Coenzyme Q10 and Ginkgo biloba extract, for instance, improved conduction metrics and myelination in preclinical studies, suggesting potential as adjuncts pending standardized endpoints and human validation.
3.4.2 Transection/anastomosis
In transection/anastomosis settings, structural improvements (e.g., myelination, axonal caliber) frequently outpaced near-term functional recovery. In some comparisons, N-acetylcysteine showed advantages over steroids on EMG and histology; SELENOT-mimetic peptides (PSELT) and PEG-fusion improved axonal metrics and reduced demyelination, yet provided limited short-window functional enhancement—indicating the need for longer follow-up and standardized electrophysiological endpoints before clinical recommendations can be made. Melatonin yielded histopathologic benefits with inconsistent short-term EMG gains, underscoring model- and dose-contingent translation challenges.
3.4.3 Infectious/ischemic FNI
In infectious/ischemic contexts, edaravone reduced oxidative stress markers with suggested clinical benefit; however, assessments often relied on clinical observation without standardized electrophysiology. Head-to-head confirmation against both no-treatment and steroid comparators is required to define incremental effect size and dosing windows.
3.4.4 Degenerative/metabolic contexts (aging, diabetes, vascular disease)
Most included studies used young, otherwise healthy animals. Because nerve regenerative capacity declines with age and comorbidities elevate oxidative stress, treatment effects may attenuate in older or metabolically compromised patients; dose windows and safety margins may also differ. Translational studies should therefore prespecify age/comorbidity strata and oxidative-stress baselines when designing trials.
3.5 Clinician-facing translation and practical use
The model-stratified signals synthesized above can be translated into a pragmatic, clinician-facing framework that outlines how antioxidants and related agents might be used as adjuncts in real-world scenarios, while avoiding overgeneralization from preclinical data. We summarize potential use by injury mechanism, therapeutic timing, route of administration, monitoring strategy, safety considerations, and evidence level. These statements are intended to guide research and shared decision-making rather than to recommend off-label treatment.
3.5.1 Traumatic/compressive injuries (acute crush/compression)
In acute crush or compressive injuries-particularly within the first 72 h when some residual conduction is expected and spontaneous recovery is common-adjunctive antioxidants may be considered alongside standard care to potentially accelerate electrophysiological recovery. Preclinical studies report earlier reflex recovery and higher EMG amplitudes with resveratrol, dexamethasone, and oxytocin; ebselen achieved functional and histological recovery comparable to methylprednisolone, suggesting a possible steroid-sparing role. Coenzyme Q10 lowered stimulation thresholds and increased myelin thickness with favorable histology, and Ginkgo biloba extract accelerated recovery of vibrissae function with improved conduction metrics. Because crush models have intrinsically high spontaneous recovery, any apparent gains should be confirmed with standardized ENoG/EMG, blink reflex, and clinical grading before being interpreted as drug effects. Safety requires attention to bleeding risk with Ginkgo in patients on antithrombotics, potential CYP-mediated interactions with resveratrol, and the hyperglycemia/infection risks of dexamethasone. These signals are supportive but should not be extrapolated to more severe injuries without confirmatory data.
3.5.2 Transection/anastomosis (post-repair)
Following transection and primary repair, structural improvements (myelination, axonal caliber) frequently precede measurable functional gains. In this context, N-acetylcysteine has shown advantages over methylprednisolone on EMG and histology with less degeneration, supporting consideration as an early adjunct after anastomosis rather than defaulting to steroids. Melatonin has yielded histopathologic benefits without consistent short-term EMG improvements, indicating that function may lag behind structure in the early window. SELENOT-mimetic peptides (PSELT) and PEG-fusion have improved axonal metrics and reduced demyelination, respectively, yet have provided limited functional enhancement over short follow-up intervals and remain investigational. Clinically, we suggest planning extended follow-up of at least 6–12 months with MUAP and CMAP analyses and, where available, high-resolution imaging to capture delayed functional convergence. For safety, clinicians should be aware of gastrointestinal intolerance to NAC and rare bronchospasm with intravenous formulations. Comparative signals favoring NAC and, in select parameters, aminoguanidine or melatonin over steroids underscore the need to avoid uncritical extrapolation of steroid benefits across models.
3.5.3 Infectious/ischemic presentations
In ischemic or viral facial palsy, edaravone has reduced oxidative stress markers and, in animal models, decreased the incidence and severity of palsy; however, many assessments have relied on clinical observation rather than standardized electrophysiology. Accordingly, any adjunctive use should be embedded in protocols that include head-to-head comparisons against both no-treatment and steroid controls, with predefined electrophysiological endpoints to quantify incremental benefit, and with renal function monitoring for safety. Until such data are available, edaravone should be regarded as a hypothesis-generating adjunct rather than a routine therapy in these indications.
3.5.4 A pragmatic algorithm for use
A practical approach begins by triaging patients by mechanism (traumatic/compressive, transection/anastomosis, infectious/ischemic) and timing (acute 0–72 h, subacute 3–14 d, chronic >3 mo). Standard care is applied per guidelines, followed by a screen for contraindications and drug–drug interactions. An antioxidant adjunct is considered when preclinical signals match the mechanism of injury and safety is acceptable, recognizing the high spontaneous recovery seen in crush models and the structural–functional lag typical of transection settings. Patients are monitored with ENoG/EMG, blink reflex, and standardized facial scoring over 2–12 weeks, and continuation or de-escalation is determined by predefined thresholds and longitudinal trends. This algorithm prioritizes standardized electrophysiology and longer follow-up in models where short-term functional gains are unlikely to capture the full effect size.
4 Conclusion
FNI profoundly affects quality of life. Oxidative stress and consequent mitochondrial dysfunction contribute to impaired nerve recovery. Based on mechanistic and predominantly preclinical evidence (with limited clinical data), our synthesis suggests that antioxidants-including CoQ10, ALA, melatonin, NAC, MeCbl, resveratrol, and others-may mitigate reactive oxygen species and inflammation and support neuroprotection in experimental models. To avoid overgeneralization, we emphasize model-specific differences: crush/compression models more often show early electrophysiological or behavioral gains, whereas transection/anastomosis models frequently exhibit histological improvement without consistent short-term functional recovery; ischemic/viral contexts remain under-evaluated with standardized electrophysiological endpoints. Outcomes also depend on regimen: earlier initiation and appropriate dosing tend to be more favorable in preclinical settings, and select combinations may show additive effects; however, these remain hypothesis-generating. Taken together-with heterogeneity in dosing, timing, formulations, small sample sizes, and inconsistent functional gains-current data do not support clinical recommendations. Direct clinical evidence for specific supplement-drug combinations is scarce, and safety/interaction profiles require systematic evaluation. We therefore refrain from prescriptive guidance and present combination approaches as testable hypotheses. Future work should prioritize well-powered randomized trials in FNI that optimize formulation, dosing, and timing; incorporate biomarker-based stratification and standardized functional and patient-reported outcomes with adequate follow-up; prespecify timing/dose exploration and combination arms; and rigorously monitor adverse events and pharmacologic interactions. Such studies are essential to determine whether antioxidant strategies can be translated into effective, evidence-based care that improves neurological recovery and patients’ quality of life.
Author contributions
JL: Data curation, Methodology, Software, Writing – original draft. SB: Conceptualization, Methodology, Project administration, Validation, Writing – original draft. SK: Investigation, Methodology, Writing – review and editing. KP: Investigation, Methodology, Writing – review and editing. J-HR: Methodology, Software, Writing – review and editing. SY: Conceptualization, Funding acquisition, Supervision, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (NRF 2018R1A6A1A03025124) (NRF 2022R1A2C1091779). The funders had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Correction note
This article has been corrected with minor changes. These changes do not impact the scientific content of the article.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdolmaleki, A., Zahri, S., and Bayrami, A. (2020). Rosuvastatin enhanced functional recovery after sciatic nerve injury in the rat. Eur. J. Pharmacol. 882, 173260. doi:10.1016/j.ejphar.2020.173260
Adamu, A., Li, S., Gao, F., and Xue, G. (2024). The role of neuroinflammation in neurodegenerative diseases: current understanding and future therapeutic targets. Front. Aging Neurosci. 16, 1347987. doi:10.3389/fnagi.2024.1347987
Alipour, M., Gholami, M. R., Jafari Anarkooli, I., Sohrabi, D., Tajki, J., and Pourheidar, M. (2011). Intraperitoneal aminoguanidine improves sciatic nerve ischemia-reperfusion injury in male sprague-dawley rats. Cell Mol. Neurobiol. 31, 765–773. doi:10.1007/s10571-011-9682-5
Alsharif, I., Boukhzar, L., Lefranc, B., Godefroy, D., Aury-Landas, J., Rego, J.-L. do, et al. (2021). Cell-penetrating, antioxidant SELENOT mimetic protects dopaminergic neurons and ameliorates motor dysfunction in Parkinson’s disease animal models. Redox Biol. 40, 101839. doi:10.1016/j.redox.2020.101839
Baicus, C., Purcarea, A., von Elm, E., Delcea, C., and Furtunescu, F. L. (2024). Alpha-lipoic acid for diabetic peripheral neuropathy. Cochrane Database Syst. Rev. 1, CD012967. doi:10.1002/14651858.CD012967.pub2
Cha, C.Il, Hong, C. K., Park, M. S., and Yeo, S. G. (2008). Comparison of facial nerve paralysis in adults and children. Yonsei Med. J. 49, 725–734. doi:10.3349/ymj.2008.49.5.725
Chang, B., Guan, H., Wang, X., Chen, Z., Zhu, W., Wei, X., et al. (2021). Cox4i2 triggers an increase in reactive oxygen species, leading to ferroptosis and apoptosis in HHV7 infected schwann cells. Front. Mol. Biosci. 8, 660072. doi:10.3389/fmolb.2021.660072
De Cassai, A., Santonastaso, D. P., Coppolino, F., D’Errico, C., Melegari, G., Dost, B., et al. (2025). Perineural dexamethasone: neurotoxicity or neuroprotection? A systematic review of preclinical evidence. J. Anesth. analgesia Crit. care 5, 50. doi:10.1186/s44158-025-00271-w
Dincer, U., Verim, A., Becerik, Ç., Gürsan, N., Tepe Karaca, Ç., and Toros, S. Z. (2025). The effect of Rosuvastatin on facial nerve regeneration after facial nerve injury: an experimental animal study. Ann. Otology, Rhinology and Laryngology 134, 134–141. doi:10.1177/00034894241291814
Ding, Z., Cao, J., Shen, Y., Zou, Y., Yang, X., Zhou, W., et al. (2018). Resveratrol promotes nerve regeneration via activation of p300 acetyltransferase-mediated VEGF signaling in a rat model of sciatic nerve crush injury. Front. Neurosci. 12, 341. doi:10.3389/fnins.2018.00341
Edizer, D. T., Donmez, Z., Gul, M., Yigit, O., Yigitcan, B., Adatepe, T., et al. (2019). Effects of melatonin and dexamethasone on facial nerve neurorrhaphy. J. Int. Adv. Otol. 15, 43–50. doi:10.5152/iao.2018.3273
Evaniew, N., Noonan, V. K., Fallah, N., Kwon, B. K., Rivers, C. S., Ahn, H., et al. (2015). Methylprednisolone for the treatment of patients with acute spinal cord injuries: a propensity score-matched cohort study from a Canadian multi-center spinal cord injury registry. J. Neurotrauma 32, 1674–1683. doi:10.1089/neu.2015.3963
Feng, X., and Yuan, W. (2015). Dexamethasone enhanced functional recovery after sciatic nerve crush injury in rats. Biomed. Res. Int. 2015, 627923–627929. doi:10.1155/2015/627923
Gaudet, A. D., Popovich, P. G., and Ramer, M. S. (2011). Wallerian degeneration: gaining perspective on inflammatory events after peripheral nerve injury. J. Neuroinflammation 8, 110. doi:10.1186/1742-2094-8-110
Ghergherehchi, C. L., Mikesh, M., Sengelaub, D. R., Jackson, D. M., Smith, T., Nguyen, J., et al. (2019). Polyethylene glycol (PEG) and other bioactive solutions with neurorrhaphy for rapid and dramatic repair of peripheral nerve lesions by PEG-fusion. J. Neurosci. Methods 314, 1–12. doi:10.1016/j.jneumeth.2018.12.015
Gölcük, V. A., and Şeneldir, L. (2025). The effect of caffeic acid phenethyl ester on facial nerve regeneration. Acta Otolaryngol. 145, 270–276. doi:10.1080/00016489.2024.2433704
Gümüs, B., Kuyucu, E., Erbas, O., Kazimoglu, C., Oltulu, F., and Bora, O. A. (2015). Effect of oxytocin administration on nerve recovery in the rat sciatic nerve damage model. J. Orthop. Surg. Res. 10, 161. doi:10.1186/s13018-015-0301-x
Hato, N., Kohno, H., Yamada, H., Takahashi, H., and Gyo, K. (2013). Role of nitric oxide in the onset of facial nerve palsy by HSV-1 infection. JAMA Otolaryngology–Head and Neck Surg. 139, 1339–1342. doi:10.1001/jamaoto.2013.5542
Ho, M.-L., Juliano, A., Eisenberg, R. L., and Moonis, G. (2015). Anatomy and pathology of the facial nerve. Am. J. Roentgenol. 204, W612–W619. doi:10.2214/AJR.14.13444
Hoshida, S., Hatano, M., Furukawa, M., and Ito, M. (2009). Neuroprotective effects of vitamin E on adult rat motor neurones following facial nerve avulsion. Acta Otolaryngol. 129, 330–336. doi:10.1080/00016480802210431
Huang, H., Lin, Q., Rui, X., Huang, Y., Wu, X., Yang, W., et al. (2023). Research status of facial nerve repair. Regen. Ther. 24, 507–514. doi:10.1016/j.reth.2023.09.012
Jang, C. H., Cho, Y. B., and Choi, C. H. (2012). Effect of ginkgo biloba extract on recovery after facial nerve crush injury in the rat. Int. J. Pediatr. Otorhinolaryngol. 76, 1823–1826. doi:10.1016/j.ijporl.2012.09.009
Juan, C. A., Pérez de la Lastra, J. M., Plou, F. J., and Pérez-Lebeña, E. (2021). The chemistry of reactive oxygen species (ROS) revisited: outlining their role in biological macromolecules (DNA, lipids and proteins) and induced pathologies. Int. J. Mol. Sci. 22, 4642. doi:10.3390/ijms22094642
Kalayci, M., Coskun, O., Cagavi, F., Kanter, M., Armutcu, F., Gul, S., et al. (2005). Neuroprotective effects of ebselen on experimental spinal cord injury in rats. Neurochem. Res. 30, 403–410. doi:10.1007/s11064-005-2615-2
Karlidag, T., Yildiz, M., Yalcin, S., Colakoglu, N., Kaygusuz, I., and Sapmaz, E. (2012). Evaluation of the effect of methylprednisolone and N-acetylcystein on anastomotic degeneration and regeneraton of the facial nerve. Auris Nasus Larynx 39, 145–150. doi:10.1016/j.anl.2011.03.004
Klymenko, A., and Lutz, D. (2022). Melatonin signalling in Schwann cells during neuroregeneration. Front. Cell Dev. Biol. 10, 999322. doi:10.3389/fcell.2022.999322
Koyama, Y., Harada, S., Sato, T., Kobayashi, Y., Yanagawa, H., Iwahashi, T., et al. (2022). Therapeutic strategy for facial paralysis based on the combined application of Si-based agent and methylcobalamin. Biochem. Biophys. Rep. 32, 101388. doi:10.1016/j.bbrep.2022.101388
Koyama, Y., Kobayashi, Y., Kobayashi, H., and Shimada, S. (2023). Diverse possibilities of Si-Based agent, a unique new antioxidant. Antioxidants (Basel) 12, 1061. doi:10.3390/antiox12051061
Lee, J., Yeo, J. H., Kim, S. S., Lee, J. M., and Yeo, S. G. (2025). Production and role of free radicals and reactive oxygen species after facial nerve injury. Antioxidants 14, 436. doi:10.3390/antiox14040436
Li, Y., Li, Y., Wu, Q., Ye, H., Sun, L., Ye, B., et al. (2013). High concentration of vitamin E decreases thermosensation and thermotaxis learning and the underlying mechanisms in the nematode Caenorhabditis elegans. PLoS One 8, e71180. doi:10.1371/journal.pone.0071180
Ma, Y.-Y., Li, X., Yu, Z.-Y., Luo, T., Tan, C.-R., Bai, Y.-D., et al. (2024). Oral antioxidant edaravone protects against cognitive deficits induced by chronic hypobaric hypoxia at high altitudes. Transl. Psychiatry 14, 415. doi:10.1038/s41398-024-03133-1
Moradi, Z., Azizi, S., and Hobbenaghi, R. (2014). The effect of ubiquinone on functional recovery and morphometric indices of sciatic nerve regeneration. Iran. J. Vet. Res. 15, 392–396.
Nagamatsu, M., Nickander, K. K., Schmelzer, J. D., Raya, A., Wittrock, D. A., Tritschler, H., et al. (1995). Lipoic acid improves nerve blood flow, reduces oxidative stress, and improves distal nerve conduction in experimental diabetic neuropathy. Diabetes Care 18, 1160–1167. doi:10.2337/diacare.18.8.1160
Nellis, J. C., Ishii, M., Byrne, P. J., Boahene, K. D. O., Dey, J. K., and Ishii, L. E. (2017). Association among facial paralysis, depression, and quality of life in facial plastic surgery patients. JAMA Facial Plast. Surg. 19, 190–196. doi:10.1001/jamafacial.2016.1462
Nocera, G., and Jacob, C. (2020). Mechanisms of Schwann cell plasticity involved in peripheral nerve repair after injury. Cell. Mol. Life Sci. 77, 3977–3989. doi:10.1007/s00018-020-03516-9
Ogut, E., Yildirim, F. B., Sarikcioglu, L., Aydin, M. A., and Demir, N. (2020). Neuroprotective effects of ozone therapy after sciatic nerve cut injury. Kurume Med. J. 65, 137–144. doi:10.2739/kurumemedj.MS654002
Oh, Y. J., Yon, D. K., Choi, Y. S., Lee, J., Yeo, J. H., Kim, S. S., et al. (2024). Induction of nitric oxide and its role in facial nerve regeneration according to the method of facial nerve injury. Antioxidants 13, 741. doi:10.3390/antiox13060741
Okada, K., Tanaka, H., Temporin, K., Okamoto, M., Kuroda, Y., Moritomo, H., et al. (2010). Methylcobalamin increases Erk1/2 and Akt activities through the methylation cycle and promotes nerve regeneration in a rat sciatic nerve injury model. Exp. Neurol. 222, 191–203. doi:10.1016/j.expneurol.2009.12.017
Ozbay, I., Ital, I., Kucur, C., Akcılar, R., Deger, A., Aktas, S., et al. (2017). Effects of ozone therapy on facial nerve regeneration. Braz J. Otorhinolaryngol. 83, 168–175. doi:10.1016/j.bjorl.2016.02.009
Pasten, C., Lozano, M., Rocco, J., Carrión, F., Alvarado, C., Liberona, J., et al. (2021). Aminoguanidine prevents the oxidative stress, inhibiting elements of inflammation, endothelial activation, mesenchymal markers, and confers a renoprotective effect in renal ischemia and reperfusion injury. Antioxidants 10, 1724. doi:10.3390/antiox10111724
Pauna, H. F., Silva, V. A. R., Lavinsky, J., Hyppolito, M. A., Vianna, M. F., Gouveia, M. de C. L., et al. (2024). Task force of the Brazilian Society of otology — evaluation and management of peripheral facial palsy. Braz J. Otorhinolaryngol. 90, 101374. doi:10.1016/j.bjorl.2023.101374
Pérez, R., Burgos, V., Marín, V., Camins, A., Olloquequi, J., González-Chavarría, I., et al. (2023). Caffeic acid phenethyl ester (CAPE): biosynthesis, derivatives and formulations with neuroprotective activities. Antioxidants 12, 1500. doi:10.3390/antiox12081500
Pizzino, G., Irrera, N., Cucinotta, M., Pallio, G., Mannino, F., Arcoraci, V., et al. (2017). Oxidative stress: harms and benefits for human health. Oxid. Med. Cell Longev. 2017, 8416763. doi:10.1155/2017/8416763
Pothion, H., Lihrmann, I., Duclos, C., Riou, G., Cartier, D., Boukhzar, L., et al. (2022). The SELENOT mimetic PSELT promotes nerve regeneration by increasing axonal myelination in a facial nerve injury model in female rats. J. Neurosci. Res. 100, 1721–1731. doi:10.1002/jnr.25098
Reich, S. G. (2017). Bell’s palsy. Contin. (Minneap Minn) 23, 447–466. doi:10.1212/CON.0000000000000447
Rivera, A., Raymond, M., Grobman, A., Abouyared, M., and Angeli, S. I. (2017). The effect of n-acetyl-cysteine on recovery of the facial nerve after crush injury. Laryngoscope Investig. Otolaryngol. 2, 109–112. doi:10.1002/lio2.68
Rong, Y., Geng, Z., and Lau, B. H. (1996). Ginkgo biloba attenuates oxidative stress in macrophages and endothelial cells. Free Radic. Biol. Med. 20, 121–127. doi:10.1016/0891-5849(95)02016-0
Ruan, S., Jia, R., Hu, L., Liu, Y., Tian, Q., Jiang, K., et al. (2024). Ozone promotes macrophage efferocytosis and alleviates neuropathic pain by activating the AMPK/Gas6-MerTK/SOCS3 signaling pathway. Front. Immunol. 15, 1455771. doi:10.3389/fimmu.2024.1455771
Salomone, R., Jácomo, A. L., Nascimento, S. B. do, Lezirovitz, K., Hojaij, F. C., Costa, H. J. Z. R., et al. (2018). Polyethylene glycol fusion associated with antioxidants: a new promise in the treatment of traumatic facial paralysis. Head. Neck 40, 1489–1497. doi:10.1002/hed.25122
Sarıtaş, T. B., Ertürk, C., Büyükdoğan, H., Yıldırım, B., Gündüz, N., and Selek, Ş. (2024). Effects of N-acetylcysteine on sciatic nerve healing: a histopathological, functional, and biochemical study of the rat sciatic nerve. Jt. Dis. Relat. Surg. 35, 618–627. doi:10.52312/jdrs.2024.1784
Şeneldir, L., Gölcük, V. A., Tanyeri Toker, G., Verim, A., and Güneş, P. (2024). The effectiveness of ebselen in facial nerve crush injury: an experimental study. J. Int. Adv. Otol. 20, 507–516. doi:10.5152/iao.2024.231360
Seo, J. Y., Lee, C. H., Cho, J. H., Choi, J. H., Yoo, K.-Y., Kim, D. W., et al. (2009). Neuroprotection of ebselen against ischemia/reperfusion injury involves GABA shunt enzymes. J. Neurol. Sci. 285, 88–94. doi:10.1016/j.jns.2009.05.029
Singh, S. K., Srivastav, S., Castellani, R. J., Plascencia-Villa, G., and Perry, G. (2019). Neuroprotective and antioxidant effect of Ginkgo biloba extract against AD and other neurological disorders. Neurotherapeutics 16, 666–674. doi:10.1007/s13311-019-00767-8
Smith, T. A., Zhou, L., Ghergherehchi, C. L., Mikesh, M., Yang, C. Z., Tucker, H. O., et al. (2025). Polyethylene glycol has immunoprotective effects on sciatic allografts, but behavioral recovery and graft tolerance require neurorrhaphy and axonal fusion. Neural Regen. Res. 20, 1192–1206. doi:10.4103/NRR.NRR-D-23-01220
Stoll, G., and Müller, H. W. (1999). Nerve injury, axonal degeneration and neural regeneration: basic insights. Brain Pathol. 9, 313–325. doi:10.1111/j.1750-3639.1999.tb00229.x
Suzuki, K., Tanaka, H., Ebara, M., Uto, K., Matsuoka, H., Nishimoto, S., et al. (2017). Electrospun nanofiber sheets incorporating methylcobalamin promote nerve regeneration and functional recovery in a rat sciatic nerve crush injury model. Acta Biomater. 53, 250–259. doi:10.1016/j.actbio.2017.02.004
Takeda, T., Takeda, S., Takumida, M., Okada, T., Kakigi, A., Nakatani, H., et al. (2008). Protective effects of edaravone against ischemia-induced facial palsy. Auris Nasus Larynx 35, 321–327. doi:10.1016/j.anl.2007.08.009
Tamaddonfard, E., Farshid, A. A., Maroufi, S., Kazemi-Shojaei, S., Erfanparast, A., Asri-Rezaei, S., et al. (2014). Effects of safranal, a constituent of saffron, and vitamin E on nerve functions and histopathology following crush injury of sciatic nerve in rats. Phytomedicine 21, 717–723. doi:10.1016/j.phymed.2013.10.031
Tanyeri, G., Celik, O., Erbas, O., Oltulu, F., and Yilmaz Dilsiz, O. (2015). The effectiveness of different neuroprotective agents in facial nerve injury: an experimental study. Laryngoscope 125, E356–E364. doi:10.1002/lary.25554
Tekdemir, E., Tatlipinar, A., Özbeyli, D., Tekdemir, Ö., and Kınal, E. (2018). The effects of lipoic acid and methylprednisolone on nerve healing in rats with facial paralysis. Acta Otolaryngol. 138, 537–541. doi:10.1080/00016489.2017.1420914
Tenório, M. C., dos, S., Graciliano, N. G., Moura, F. A., Oliveira, A. C. M. de, and Goulart, M. O. F. (2021). N-Acetylcysteine (NAC): impacts on human health. Antioxidants 10, 967. doi:10.3390/antiox10060967
Tosyalı, H. K., Bora, E. S., Çınaroğlu, O. S., and Erbaş, O. (2023). Oxytocin mitigates peripheral nerve damage via Nrf2 and irisin pathway. Eur. Rev. Med. Pharmacol. Sci. 27, 11340–11350. doi:10.26355/eurrev_202312_34573
Tunç, E., Bora, E. S., and Erbaş, O. (2025). Harnessing polyethylene glycol 3350 for enhanced peripheral nerve repair: a path to accelerated recovery. Med. (B Aires) 61, 624. doi:10.3390/medicina61040624
Uyanikgil, Y., Cavusoglu, T., Kılıc, K. D., Yigitturk, G., Celik, S., Tubbs, R. S., et al. (2017). Useful effects of melatonin in peripheral nerve injury and development of the nervous system. J. Brachial Plex. Peripher Nerve Inj. 12, e1–e6. doi:10.1055/s-0036-1597838
Vachirarojpisan, T., Srivichit, B., Vaseenon, S., Powcharoen, W., and Imerb, N. (2024). Therapeutic roles of coenzyme Q10 in peripheral nerve injury-induced neurosensory disturbances: mechanistic insights from injury to recovery. Nutr. Res. 129, 55–67. doi:10.1016/j.nutres.2024.07.011
Yamashita, T., and Abe, K. (2024). Update on antioxidant therapy with edaravone: expanding applications in neurodegenerative diseases. Int. J. Mol. Sci. 25, 2945. doi:10.3390/ijms25052945
Yanilmaz, M., Akduman, D., Sagun, Ö. F., Haksever, M., Yazicilar, O., Orhan, I., et al. (2015). The effects of aminoguanidine, methylprednisolone, and melatonin on nerve recovery in peripheral facial nerve neurorrhaphy. J. Craniofacial Surg. 26, 667–672. doi:10.1097/SCS.0000000000001503
Yeh, T.-Y., and Liu, P.-H. (2021). Inhibition of nitric oxide production enhances the activity of facial nerve tubulin polymerization and the ability of tau to promote microtubule assembly after neurorrhaphy. Neurochem. Int. 150, 105183. doi:10.1016/j.neuint.2021.105183
Yildirim, G., Kumral, T. L., Berkiten, G., Saltürk, Z., Sünnetçi, G., Öztürkçü, Y., et al. (2015). The effect of coenzyme Q10 on the regeneration of crushed facial nerve. J. Craniofac Surg. 26, 277–280. doi:10.1097/SCS.0000000000001201
Yoo, M. C., Ryu, I. Y., Choi, J. W., Lee, J. M., Byun, J. Y., and Yeo, S. G. (2022). Nicotinamide adenine dinucleotide phosphate oxidase 2 expression and effects of alpha lipoic acid on recovery in a rat model of facial nerve injury. Biomedicines 10, 291. doi:10.3390/biomedicines10020291
Zhang, J., Ren, J., Liu, Y., Huang, D., and Lu, L. (2020). Resveratrol regulates the recovery of rat sciatic nerve crush injury by promoting the autophagy of Schwann cells. Life Sci. 256, 117959. doi:10.1016/j.lfs.2020.117959
Zhang, Y., Deng, Q., Hong, H., Qian, Z., Wan, B., and Xia, M. (2024). Caffeic acid phenethyl ester inhibits neuro-inflammation and oxidative stress following spinal cord injury by mitigating mitochondrial dysfunction via the SIRT1/PGC1α/DRP1 signaling pathway. J. Transl. Med. 22, 304. doi:10.1186/s12967-024-05089-8
Keywords: facial nerve injury, oxidative stress, antioxidants, reactive oxygen species, nerveregeneration
Citation: Lee JM, Byun SW, Kim SS, Park KS, Ryoo J-H and Yeo SG (2025) The role of antioxidants in facial nerve injury. Front. Mol. Biosci. 12:1663998. doi: 10.3389/fmolb.2025.1663998
Received: 11 July 2025; Accepted: 19 September 2025;
Published: 01 October 2025; Correcteed: 06 October 2025.
Edited by:
Palash Mandal, Charotar University of Science and Technology, IndiaReviewed by:
Sitthisak Thongrong, University of Phayao, ThailandVitor Hugo Panhóca, University of São Paulo, São Carlos, Brazil
Copyright © 2025 Lee, Byun, Kim, Park, Ryoo and Yeo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Seung Geun Yeo, eWVvMnBhcmtAZ21haWwuY29t, eWVvMnBhcmtAa2h1LmFjLmty
†These authors have contributed equally to this work
 Jae Min Lee
Jae Min Lee Seong Wook Byun1†
Seong Wook Byun1† Sung Soo Kim
Sung Soo Kim Kyung Sun Park
Kyung Sun Park