- 1Second Clinical Medical College, Yunnan University of Traditional Chinese Medicine, Kunming, China
- 2Department of Orthopedic, Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
- 3Department of Emergency, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China
- 4School of Medicine, Shanghai Jiao Tong University, Shanghai, China
- 5Department of Orthopedic, Affiliated Renhe Hospital of Shanghai University (Renhe Hospital, Baoshan District), Shanghai, China
The unique hypoxic microenvironment of the intervertebral disc, characterized by its avascularity and restricted nutrient exchange, drives a shift in cellular metabolism towards anaerobic glycolysis. This metabolic adaptation results in the accumulation of significant lactate levels. Increasing evidence indicates that lactate plays a pivotal role in regulating cell differentiation and fate in both physiological and pathological contexts, particularly in complex conditions such as degenerative diseases and cancer. Lactate is not merely a metabolic byproduct; it also modulates cellular signaling pathways and promotes lactylation. In the lactate-enriched microenvironment of the intervertebral disc, understanding the regulatory mechanisms of lactate and lactylation is essential for mitigating intervertebral disc degeneration and improving therapeutic outcomes. Targeting lactate production and transport—particularly through lactate dehydrogenases (LDHs) and monocarboxylate transporters (MCTs)—holds significant therapeutic promise. This review highlights the critical role of lactate in intervertebral disc degeneration progression and discusses potential therapeutic strategies aimed at modulating lactate metabolism to enhance treatment efficacy.
1 Introduction
Intervertebral disc degeneration (IDD) is characterized by reduced disc height, diminished hydration, and impaired ability to absorb pressure loads (Kang et al., 2023). Low back pain (LBP), the primary clinical manifestation of IDD, severely impacts patients’ quality of life and generates substantial socioeconomic burdens, contributing to increased healthcare costs and productivity losses (Wu et al., 2020). With the aging global population, the prevalence of IDD is steadily increasing, with projections indicating that by 2025, more than 2.1 billion people will be over the age of 60 (Madhu et al., 2021; Rustenburg et al., 2018; Tonomura et al., 2020). Current management strategies for IDD include both conservative and surgical approaches. Conservative therapies, including physical modalities, pharmacotherapy, and behavioral interventions, offer symptomatic relief but do not halt the progression of degeneration (Xin et al., 2022). Surgical procedures, such as discectomy and spinal fusion, may provide immediate benefits; however, they carry the risk of adjacent segment degeneration, chronic pain, and neurological complications (Mirza and Deyo, 2007). Currently, no clinically validated pharmacological interventions effectively halt or reverse the progression of IDD (Liu et al., 2023). Emerging regenerative strategies, such as stem cell/exosome therapy, gene editing, and molecular targeting, show promising therapeutic potential (Sheyn et al., 2019; Liao et al., 2019; Kamali et al., 2021; Krut et al., 2021). However, significant gaps remain in their clinical translation. Therefore, it is urgent to find effective strategies for treating IDD.
Recent studies have found that lactate plays a significant role in the onset and progression of discogenic degeneration, revealing its multifaceted impact beyond being merely a metabolic byproduct (Grunhagen et al., 2006; Bibby et al., 2005; Wang D. et al., 2021). A key breakthrough in lactate-related research of IDD was the discovery of lactylation, a post-translational modification that bridges metabolism and epigenetics (Zhang et al., 2019). Lactylation is not confined to histones but extends to non-histone proteins, influencing various cellular processes. This modification strengthens the relationship between metabolic states and epigenetic regulation, accelerating the progression of IDD (Sun et al., 2025; Sheng et al., 2024). Lactate-induced lactylation operates through multiple molecular mechanisms and signaling pathways, often interacting with other epigenetic modifications to promote disease progression (Certo et al., 2022; Hu XT. et al., 2024). Notably, lactate and lactylation represent an underexplored therapeutic frontier in IDD. More recently, emerging classical post-translational modifications (PTMs), such as lactylation (Zhang et al., 2019), palmitoylation (Qu et al., 2021), and succinylation (Zhao et al., 2023), have gained attention due to their biological relevance. Therefore, further promotion and consolidation of lactate-related research are urgently needed. This review rationalizes the pathological and physiological effects of lactate, elucidates the molecular mechanisms underlying lactate-driven IDD, and discusses lactate-targeted therapeutic strategies for IDD.
2 Intervertebral disc degeneration
IDD is a highly prevalent spinal disorder that commonly presents as pain and restricted mobility (Dowdell et al., 2017). Anatomically, the disc comprises three distinct components: the outer annulus fibrosus (AF), the inner nucleus pulposus (NP), and the cartilaginous endplates (CEP) (Pan et al., 2018). Current evidence has identified several key pathomechanistic drivers of IDD, including extracellular matrix (ECM) degradation (Roughley et al., 2006; Liang et al., 2022; Wei et al., 2019), oxidative stress (Rahal et al., 2014; Chen X. et al., 2024; Ma et al., 2019; Cheng et al., 2022), cellular senescence (Silwal et al., 2023; Cheng et al., 2025; Shi et al., 2023), and dysregulated autophagy/apoptosis (Madhu et al., 2021; Cheng et al., 2025; Hu et al., 2021; Kang et al., 2020), however, the pathogenesis of IDD is complex and not limited to these factors (Figure 1). The intervertebral disc (IVD) is characterized by an avascular structure and a hypoxic microenvironment, which predominantly relies on glycolysis for energy production. This metabolic reliance leads to the accumulation of substantial amounts of lactate within the IVD (Shen et al., 2022). Only a small portion of this lactate is recycled by nucleus pulposus cells (NPCs) or diffused to surrounding tissues, while efflux mediated by monocarboxylate transporters remains minimal (Bibby et al., 2005; Soukane et al., 2007). The unique structural and metabolic characteristics of the IVD have led to increased focus on lactate and lactylation as important factors in the pathophysiology of IDD.
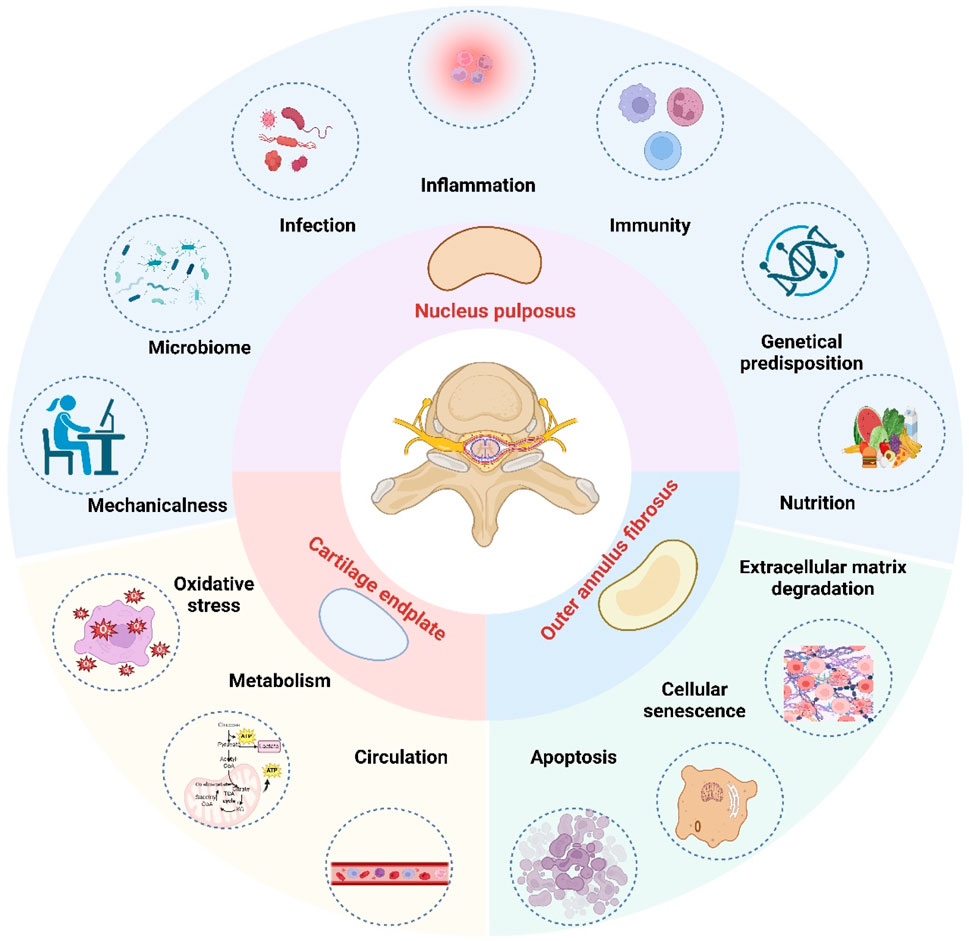
Figure 1. The etiology of intervertebral disc degeneration. In the hypoxic and lactic acidifying environment of the intervertebral disc, the most affected area is the nucleus pulposus, followed by the cartilage endplate and the outer annulus fibrosus. The etiology of intervertebral disc degeneration is multifactorial. Key pathological drivers include oxidative stress, inflammation, apoptosis, cellular senescence, extracellular matrix degradation, nutritional deficiency, metabolic imbalance, genetic predispositions, mechanical stress, autoimmune response, microbial involvement, infection, and circulatory insufficiency. (Figure created with http://www.BioRender.com, accessed on 5 October 2025).
3 Lactate metabolism
3.1 History of research on lactate metabolism
The discovery and study of lactic acid (C3H6O3) dates back to the 18th century, when Swedish chemist Carl Wilhelm Scheele first isolated this organic acid from sour milk in 1780 (Kompanje et al., 2007; Mülle et al., 2023). Later, in 1808, renowned chemist Jöns Jacob Berzelius further revealed that athletic muscle tissue also produces lactic acid, establishing its connection to energy metabolism (Dawson et al., 1978). Lactate is the ionized form of lactic acid. Chemically, lactic acid is a three-carbon hydroxycarboxylic acid, which dissociates in aqueous solution to produce lactate ions (C3H5O3-) and hydrogen ions. For a long time, lactate was merely regarded as a byproduct of glycolysis and metabolic waste (Mäki-Arvela et al., 2014). However, the discovery of the Warburg effect and extensive modern research suggest that lactate is not only a crucial energy substrate for skeletal, cardiac, and brain tissues but also acts as a key metabolic signaling molecule in regulating various cellular processes involved in cell fate determination (Grunhagen et al., 2006; Wang and Dang, 2024; Rabinowitz and Enerbäck, 2020; Fendt, 2024); thus, the biological role of lactate has expanded beyond its metabolic functions (Vander Heiden et al., 2009). As research into lactate and lactylation in IDD continues to grow, strategies targeting lactate and lactylation have gained considerable interest as potential therapeutic interventions for IDD.
3.2 Lactate production
During high energy demand, glucose is catabolized through the glycolytic pathway, ultimately generating pyruvate. Lactate dehydrogenase (LDH) plays a key catalytic role in converting most of the pyruvate to lactate (Sharma et al., 2022). The physiological significance of lactate production lies primarily in maintaining the NAD+ pool required for glycolysis through the coupled oxidation of NADH to NAD+ (Cluntun et al., 2021; Arce-Molina et al., 2020), and in sustaining the reaction catalyzed by 3-phosphoglycerol aldehyde dehydrogenase, thereby maintaining glycolytic flux and ATP production (Henderson et al., 2007; Mitchell, 1961). To regulate lactate metabolism, the body has established intricate metabolic regulatory mechanisms. The pyruvate dehydrogenase (PDH) complex catalyzes the irreversible conversion of pyruvate to acetyl-CoA, which then enters the tricarboxylic acid (TCA) cycle for complete oxidation (Breda et al., 2019). Alternatively, excess lactate can be converted back into glucose via gluconeogenesis (Andreucci et al., 2020; H et al., 2012). This dynamic equilibrium mechanism satisfies energy demands while preventing metabolic acidosis due to excessive lactate accumulation.
The avascular, hypoxic microenvironment of the IVD forces reliance on anaerobic glycolysis, particularly in NPCs, as the primary energy-producing pathway. This metabolic adaptation leads to significant lactate production within the IVD (Bibby et al., 2005; Soukane et al., 2007) (Figure 2). Physiological homeostasis is maintained through the dynamic regulation of lactate flux, including its synthesis, transmembrane transport, and clearance mechanisms (Silagi et al., 2020; Silagi et al., 2021). However, in degenerative conditions, lactate concentrations increase significantly, with the highest accumulation observed in the central NP regions of degenerated discs (Shi et al., 2019; Malandrino et al., 2011). This metabolic disruption arises from compromised nutrient and metabolite diffusion through adjacent capillary networks (Shen et al., 2022; Yang et al., 2020), resulting in NP lactate levels that are 8–10 times higher than those in circulating plasma (Shen et al., 2022; Silagi et al., 2018). Pathologically elevated lactate levels directly inhibit ECM biosynthesis and promote NPCs apoptosis (Shen et al., 2022; Shi et al., 2019), accelerating IVD structural degradation and exacerbating discogenic pain pathways (Shen et al., 2022; Keshari et al., 2008; Byvaltsev et al., 2018). In addition, this metabolic disturbance creates an acidic microenvironment that promotes ECM acidification, inhibits proteoglycan biosynthesis, and activates pro-inflammatory cascades, all of which accelerate the progression of IDD (Shi et al., 2019; Ma et al., 2024; Zhao et al., 2021). Emerging evidence suggests that lactate accumulation and its derivative lactylation play central roles in driving IDD pathogenesis (Shen et al., 2022). Therefore, understanding the mechanistic role of lactylation in IVD degeneration is critical for advancing the knowledge of NP cell pathophysiology and for developing targeted therapeutic strategies against IDD.
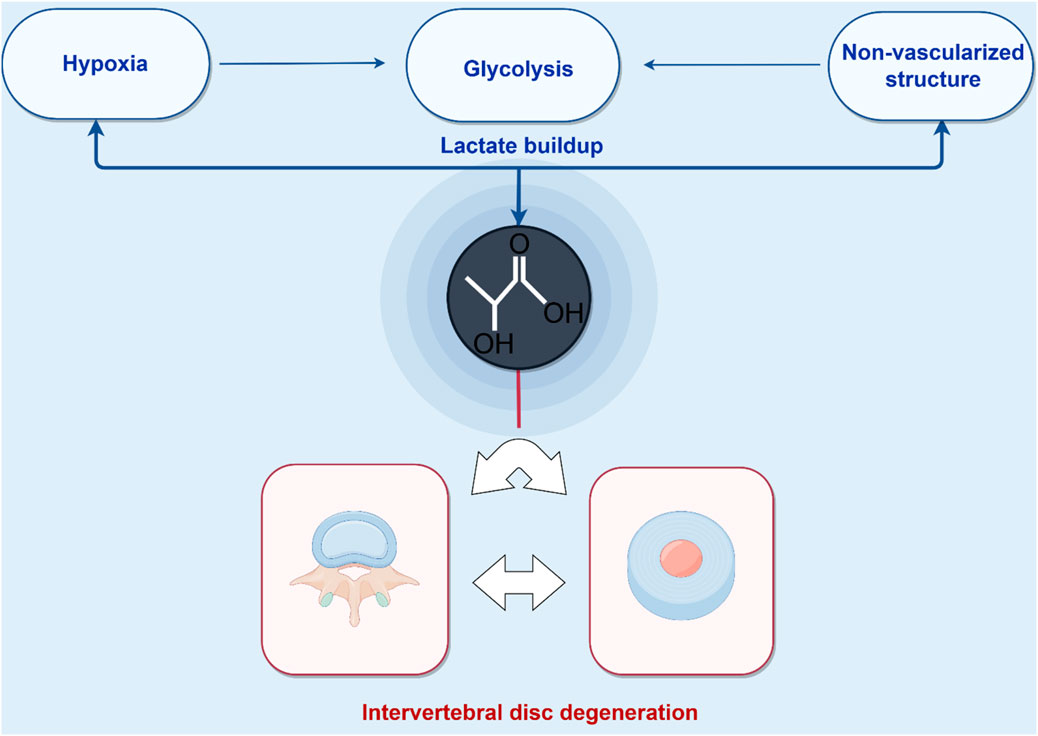
Figure 2. Pathological lactate accumulation in the intervertebral disc microenvironment. Energy homeostasis in the avascular nucleus pulposus (NP) critically depends on glycolytic flux. Lactate, a key byproduct of glycolysis, accumulates and drives lysine lactylation on histone and non-histone proteins, establishing a feed-forward loop that accelerates intervertebral disc degeneration (IDD). (Figure created with http://www.home-for-researchers.com, accessed on 5 October 2025).
3.3 Lactate transport and shuttle
Lactate transport within cells is primarily mediated by the monocarboxylate transporter (MCT) family, including isoforms such as MCT1 and MCT4. These transporters facilitate transmembrane lactate transport through the H+/lactate cotransport mechanism, with transport direction determined by the transmembrane proton and lactate concentration gradients (Slomiany et al., 2009; Fang et al., 2023; Fan M. et al., 2023). MCT-mediated lactate efflux also removes protons, maintaining intracellular pH homeostasis while acidifying the extracellular microenvironment (Fan M. et al., 2023; Chen et al., 2025). Among the MCT family, MCT1 is a basally expressed transporter regulated by c-Myc, widely distributed across various cell types, and responsible for the transport of lactate and pyruvate (Doherty et al., 2014). In contrast, MCT4, a hypoxia-inducible and efficient lactate transporter, is particularly expressed in glycolytically active tissues, such as white muscle fibers and tumor cells (Fang et al., 2023; Singh et al., 2023). Notably, the NP of the IVD, a glycolysis-dependent tissue, upregulates MCT expression during degeneration, promoting the formation of an acidic microenvironment (Wang CY. et al., 2022). Aberrant expression of monocarboxylate transporters (MCT1/4) exacerbates lactate retention, creating a self-perpetuating pathological loop (Wang D. et al., 2021). This acidic microenvironment, in turn, maintains high glycolytic activity through a positive feedback mechanism, significantly influencing the survival and function of NPCs (Wang CY. et al., 2022). These findings provide new insights into the molecular mechanisms of IDD.
Since its introduction in 1985, the lactate shuttle theory has become a fundamental concept in metabolic research, continuously validated and expanded. The theory systematically describes the dynamic transport of lactate, both as a glycolytic end product and as a substrate for oxidative respiration, across cells, tissues, and organs. Specifically, the lactate shuttle not only elucidates the molecular mechanisms of lactate transmembrane transport but also highlights its critical role as a metabolic bridge connecting anaerobic glycolysis with aerobic respiration (Brooks, 2018; Li et al., 2022; Wang Y. et al., 2022). Notably, this metabolic connection is maintained even under normoxic conditions (Brooks, 2009; Halestra and p, 2013). From a physiological perspective, the lactate shuttle serves three key functions: first, lactate acts as an important energy carrier for the organism; second, it is a key precursor for gluconeogenesis; and third, lactate functions as an autocrine, paracrine, and endocrine-like signaling molecule.
A notable example is the muscle-hepatic Cori cycle, which accomplishes three major physiological functions: it effectively prevents muscle lactic acidosis during exercise, sustains muscle ATP supply, and provides a more significant gluconeogenic substrate than dietary sources by converting muscle-generated lactate into glucose via hepatic gluconeogenesis, which is then resupplied to the muscle (Manoj et al., 2022; Ku et al., 2024; Soeters et al., 2021). Additionally, lactate can be transported between cells in a non-channel-dependent manner, expanding our understanding of lactate’s metabolic networks beyond the classical MCT transporter protein-mediated pathway (Chen et al., 2025; Py et al., 2005). Finally, research has demonstrated lactate-dependent metabolic symbiosis within the disc, where lactate produced by hypoxic, glycolytic NP cells is oxidatively phosphorylated by oxygenated AF cells for energy and substrate production. This paradigm shift challenges the traditional view of disc lactate as a waste product, recognizing it instead as a valuable biofuel (Wang D. et al., 2021).
3.4 Biological functions of lactate
3.4.1 Redox steady-state-buffer
Lactate, as a key metabolic intermediate, plays a pivotal role in cellular redox signaling. Its metabolic processes maintain electron homeostasis through specific pathways, including the conversion of NADH to NAD+ and H+ and the lactate-pyruvate interconversion mediated by LDH (Titov et al., 2016). These reactions involve the oxidation of reduced coenzymes (NAD+/NADP+) to generate electrons via the mitochondrial respiratory chain or the lactic acid fermentation pathway, thereby regulating redox balance. Notably, lactate also serves as a regulator of oxidative phosphorylation (OXPHOS) and is involved in maintaining intracellular redox homeostasis (Ying, 2008).
3.4.2 Circulating carbohydrate fuel of the TCA cycle
Recent studies show that lactate can also serve as a major carbon source for the TCA cycle (Hui et al., 2020). Using the 13C isotope tracer technique, studies revealed that (1) the circulating turnover flux of lactate in mammals is significantly higher than that of glucose, reaching 1.1 and 2.5 times the turnover flux of glucose in fed and fasted states, respectively; (2) while glucose is the primary precursor of lactate, other metabolic pathways also contribute to lactate production; (3) 13C labeling experiments confirmed that lactate is widely involved in TCA cycle metabolism in various tissues and organs (Hui et al., 2017). Additionally, lactate has been shown to function as an important energy carrier for the central nervous system, both by maintaining the energy homeostasis of preopiomelanocortin (POMC) neurons and directly supporting the energy demands of excitatory neural activities in the brain. These findings have fundamentally altered our understanding of lactate’s metabolic role, positioning it as a central hub in energy metabolism (Dienel, 2019; Gómez-Valadés et al., 2021).
4 The multifaceted functions of lactate in intervertebral disc degeneration
4.1 Decreased proteoglycan content
An acidic environment significantly inhibits proteoglycan biosynthesis in cartilage tissue. To explore this mechanism, researchers systematically evaluated the effects of lactate concentration and pH changes on IVD matrix synthesis in experimental samples, including bovine caudal IVDs and human IVD tissues obtained from percutaneous nucleotomy. The rate of proteoglycan synthesis was found to be highly sensitive to extracellular pH, with optimal synthesis efficiency occurring within a range close to physiological pH, as measured by 35S-sulfate and 3H-proline isotope labeling assays (Ohshima and Urban, 1992). Notably, factors that promote lactate accumulation (such as hypoxia, smoking, or mechanical vibration) can inhibit proteoglycan synthesis by lowering local pH (Ohshima and Urban, 1992). This ongoing impairment in synthesis can ultimately lead to a progressive reduction in proteoglycan content within the disc matrix, accelerating the process of IDD. These findings provide essential experimental evidence for understanding the pathological links between mechanical stress, metabolic disorders, and IDD.
Under physiological conditions, the IVD environment typically maintains a relatively neutral pH (pH 7.2-7) (Urban, 2002). However, when this homeostasis shifts toward an acidic environment, the normal metabolic functions of the disc are significantly impaired. Urban and his team have demonstrated through a series of experiments that a decrease in pH triggers a series of pathological changes: on one hand, it promotes the upregulation of ECM-degrading enzymes, such as matrix metalloproteinases (MMPs), and on the other hand, it inhibits the synthesis of key matrix components, such as proteoglycans (Bibby et al., 2005; Bibby et al., 2002; Urban and Mcmullin, 1988). This metabolic disturbance not only disrupts the structural integrity of the IVD but also negatively impacts its biomechanical properties, which may ultimately lead to abnormal disc function and contribute to chronic LBP. These findings provide important experimental support for understanding the causal relationship between the acidic microenvironment and IDD.
4.2 Inflammation and apoptosis
Lactate plays a dual role in tissue repair and regeneration (Rabinowitz and Enerbäck, 2020). At physiological concentrations, lactate acts as an important metabolic signaling molecule, promoting wound healing by regulating cellular signaling, stimulating neovascularization, and enhancing fibroplasia, among other mechanisms (Zhang et al., 2019; Brooks, 2018). However, pathological lactate accumulation triggers a range of harmful effects, including excessive recruitment of inflammatory cells, mitochondrial dysfunction, and increased production of reactive oxygen species (ROS), which ultimately lead to the death of tissue-resident cells and hinder regenerative processes (Ivashkiv, 2020; Pucino et al., 2017). In the pathological context of IDD, lactate levels in the NP tissue can be 8 to 10 times higher than in the surrounding plasma due to impaired diffusion of metabolites and nutrients through adjacent capillaries (Yang et al., 2020; Silagi et al., 2018). This abnormal lactate buildup not only inhibits ECM synthesis but also promotes NP cell apoptosis (Shi et al., 2019), leading to structural damage in the IVD and discogenic pain (Shen et al., 2022; Keshari et al., 2008). Additionally, 2 mM lactate promotes NP cell proliferation, while 6 mM lactate slightly inhibits proliferation and induces autophagy and apoptosis in NP cells (Wu et al., 2014).
Notably, lactate gradually accumulates in the degenerating region, from the periphery of the AF toward the center of the NP, forming a gradient distribution along with a decreasing pH. This acid-base gradient change activates multiple inflammatory pathways, further intensifying the local inflammatory response (Ma et al., 2024; Horner et al., 2002; Zhang et al., 2024a). In this hyper-inflammatory environment, the regenerative capacity of NP cells is significantly impaired, leading to a severe imbalance between ECM synthesis and degradation. This imbalance ultimately triggers disc herniation and painful symptoms (Ma et al., 2024; Liu et al., 2017). Moreover, the inflammatory microenvironment in the degenerated disc accelerates disease progression through a dual mechanism: it directly disrupts the metabolic function of NP cells, worsening ECM metabolic disorders (Gaudet and Popovich, 2014; Li et al., 2023), and it triggers a cascade effect that recruits more inflammatory cells, further amplifying the inflammatory response (Ma et al., 2024; Gao et al., 2023). This vicious cycle leads to irreversible pathological changes.
4.3 Pyroptosis and signaling
Unlike conventional cell death, pyroptosis is a form of programmed necrosis with pro-inflammatory properties. It is characterized by cellular swelling until membrane rupture, resulting in the leakage of intracellular contents and triggering a more intense inflammatory response (Broz, 2025). In recent years, inflammation-mediated cellular pyroptosis has been recognized as a key player in IDD (Wang et al., 2024). Extracellular lactate not only promotes the activation of NLRP3 inflammasomes, leading to degeneration of the ECM in the NP, but also significantly enhances the inflammatory response and increases the level of cellular pyroptosis in NP tissues (Okajima, 2013; Chen et al., 2022; Song et al., 2025). At the molecular level, the NLRP3 inflammasome serves as the central molecular machinery mediating typical cellular juxtaposition (Coll et al., 2022; Hsu et al., 2021). Atypical inflammasomes, composed primarily of caspase-4/5/11, specifically recognize lipopolysaccharides in the cytoplasm and induce cellular pyroptosis by cleaving the downstream effector protein GSDMD (Song et al., 2025; Miao et al., 2023). In addition, inflammatory cytokines accelerate the IDD process by activating multiple signaling pathways, including NF-κB, MAPK, and PI3K/Akt, triggering intracellular inflammatory cascades and pyroptosis (Song et al., 2025; Liu et al., 2018; Zhou et al., 2024; Guo et al., 2023). These findings provide a new theoretical basis for understanding the lactate-NLRP3-pyroptosis axis in IDD.
Acid-sensing ion channels (ASICs) are important members of the proton-gated ion channel family, consisting of six isoforms (ASIC1a, 1b, 2a, 2b, 3, and 4) that can be activated by ligands such as extracellular acidosis, lactate, and arachidonic acid (Omerbašić et al., 2015; Zhou et al., 2016). These channels are widely expressed in various mammalian tissues, including the nervous system, articular chondrocytes, and cells of the musculoskeletal system and IVD (Song et al., 2025; Ruan et al., 2021; Gilbert et al., 2016). The expression levels of ASIC1, ASIC2, and ASIC3 are significantly upregulated in degenerative NP cells (Song et al., 2025). Extracellular lactate not only promotes the activation of NLRP3 inflammasomes through ASIC activation, leading to degeneration of the NP ECM, but also significantly enhances the inflammatory response and the level of cellular pyroptosis in NP tissues (Song et al., 2025). This discovery provides a novel molecular perspective for understanding the pathological mechanisms of the acidic microenvironment in IDD.
4.4 Metabolic regulation
In severely degenerated human NP and senescent SD rat NP tissues, decreased glutamine levels were accompanied by lactate accumulation and enhanced protein lactylation (Zhang et al., 2024b). Mechanistic studies showed that exogenous glutamine supplementation could rectify this imbalance by: (1) reducing lactate production by inhibiting the glycolytic pathway; (2) downregulating the lactylation of the AMPKα subunit; (3) promoting the activation of AMPKα phosphorylation. Further experiments confirmed that glutamine supplementation reduces the senescence phenotype of NP cells, enhances cellular autophagy activity, and promotes ECM synthesis through these dual regulatory mechanisms (Zhang et al., 2024b). This study reveals the central role of glutamine metabolic regulation in maintaining NP cells homeostasis through dual mechanisms: inhibiting glycolysis and lactate production, and activating AMPK phosphorylation signaling, thereby providing a novel theoretical basis for metabolic-targeted strategies against IDD.
4.5 Oxidative stress and cellular senescence
High lactate concentrations can accelerate tissue degeneration through various molecular mechanisms. Lactate interacts with Akt proteins, inducing cellular senescence and oxidative stress by modulating the Akt/p21/p27/cyclin D1 and Akt/Nrf2/HO-1 signaling pathways (Zhang et al., 2024a). This effect has been clearly demonstrated in NPCs. Notably, the association between lactate accumulation and senescence has been observed across different species, with the Drosophila model showing a correlation between lactate levels and the senescence process (Iatsenko et al., 2018). In the nervous system, elevated lactate levels serve as a key biomarker of brain aging (Ross et al., 2010), and their accumulation promotes the progression of neurodegenerative diseases such as Alzheimer’s disease (Wang Q. et al., 2022; Mullins et al., 2018). Moreover, lactate induces tissue damage by triggering oxidative stress during peripheral nerve regeneration disorders and arterial calcification (Zhang et al., 2024a; Zhu et al., 2019; Jia et al., 2021). Collectively, these findings underscore the critical role of lactate metabolism disorders in various degenerative diseases.
In addition to the roles of lactate mentioned above, recent study has revealed that in myelofibrosis (MF), the abnormal accumulation of lactate can remodel the bone marrow microenvironment, driving the fibrotic progression of the disease by inducing an immunosuppressive state and promoting collagen deposition (Spampinato et al., 2025). This study clearly identifies lactate as a core regulator of immune escape and fibrotic transformation in MF, and proposes targeting the lactate transporter MCT1 as an innovative anti-fibrotic strategy (Spampinato et al., 2025). This provides a highly valuable parallel perspective for understanding the potential function of lactate in IDD: NPCs during degeneration also exhibit significantly activated glycolysis and substantial lactate accumulation (Xu et al., 2025; Peng et al., 2024). Could the large amount of lactate produced by NPCs under high mechanical load and hypoxic conditions in degenerated discs similarly drive the imbalance between destruction and repair of the annulus fibrosus through analogous mechanisms? Furthermore, the MCT1 blockade strategy proposed in MF research also offers important insights for exploring new therapeutic targets to delay or reverse disc fibrosis.
5 Lactylation: a bridge between metabolism and epigenetics
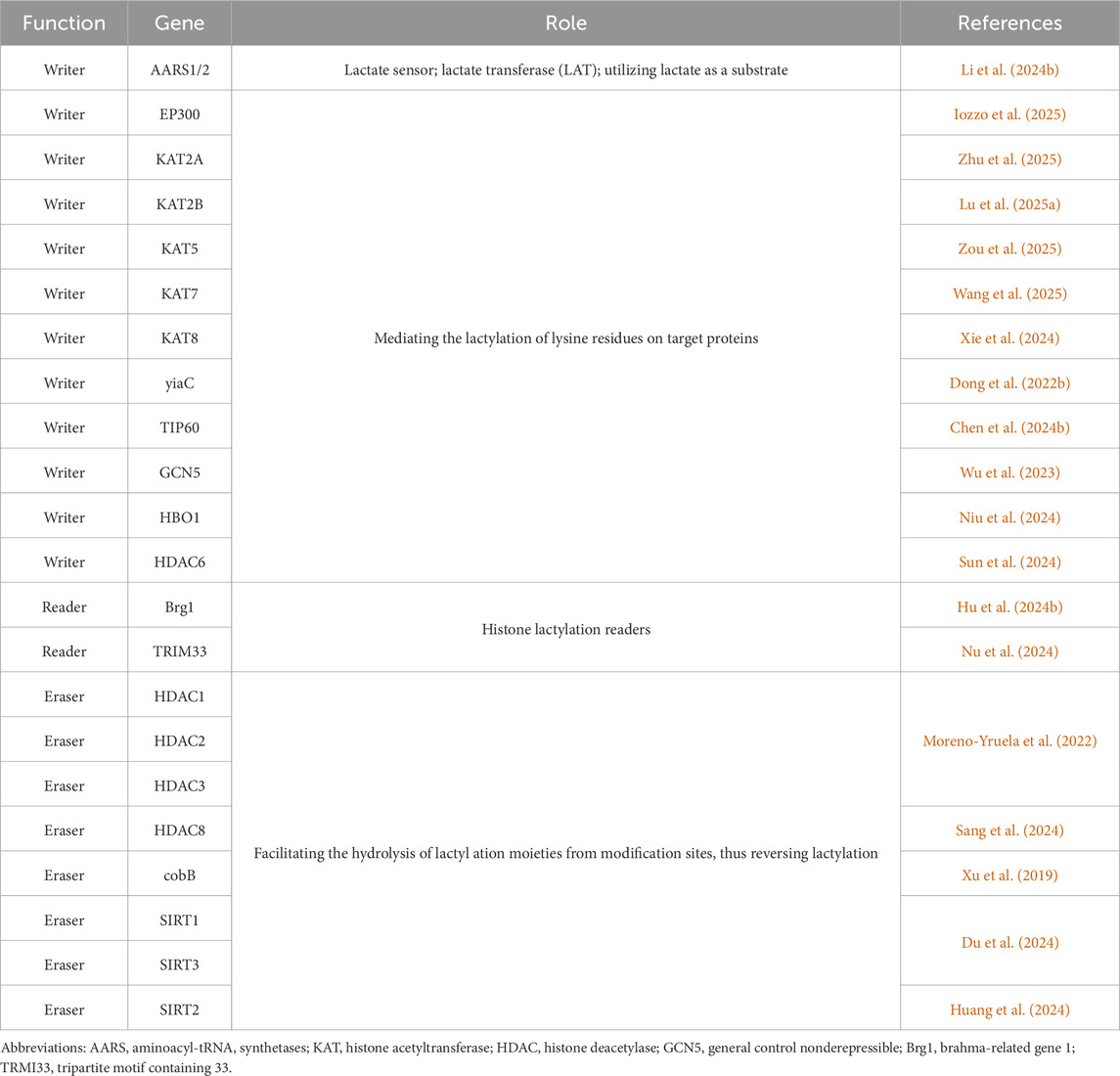
Histone lactylation was first documented in 2019 by Zhang et al. (Zhang et al., 2019) (Figure 3). This PTM involves the covalent attachment of lactate to lysine residues via amide bonds, forming lactylated lysine (Kla). Notably, lactylation redefines lactate—previously considered a metabolic byproduct—as an epigenetic regulator, positioning it as an emerging research frontier (Zhang et al., 2019). Biochemically, lactylation is dependent on lactate’s α-hydroxycarboxylic acid structure (Chen H. et al., 2024; Jing et al., 2025), and it is catalyzed by lactyltransferase enzymes (such as p300) through lactyl-CoA intermediates (Zhang et al., 2019). We summarize inhibitors of lactylation and enzymes associated with lactylation (Tables 1, 2). The levels of lactylation increase under conditions of elevated intracellular lactate concentrations, particularly during hypoxia or hyperglycemia, thus linking metabolic flux directly to gene regulation (Zhang et al., 2019). Notably, site-specific modifications, such as histone H3K18la, drive pro-inflammatory gene transcription, highlighting lactylation’s critical role in immunometabolic reprogramming (Piao et al., 2025). This paradigm establishes a novel metabolic-epigenetic axis, challenging the classical view of metabolites as merely energy substrates or signaling molecules.
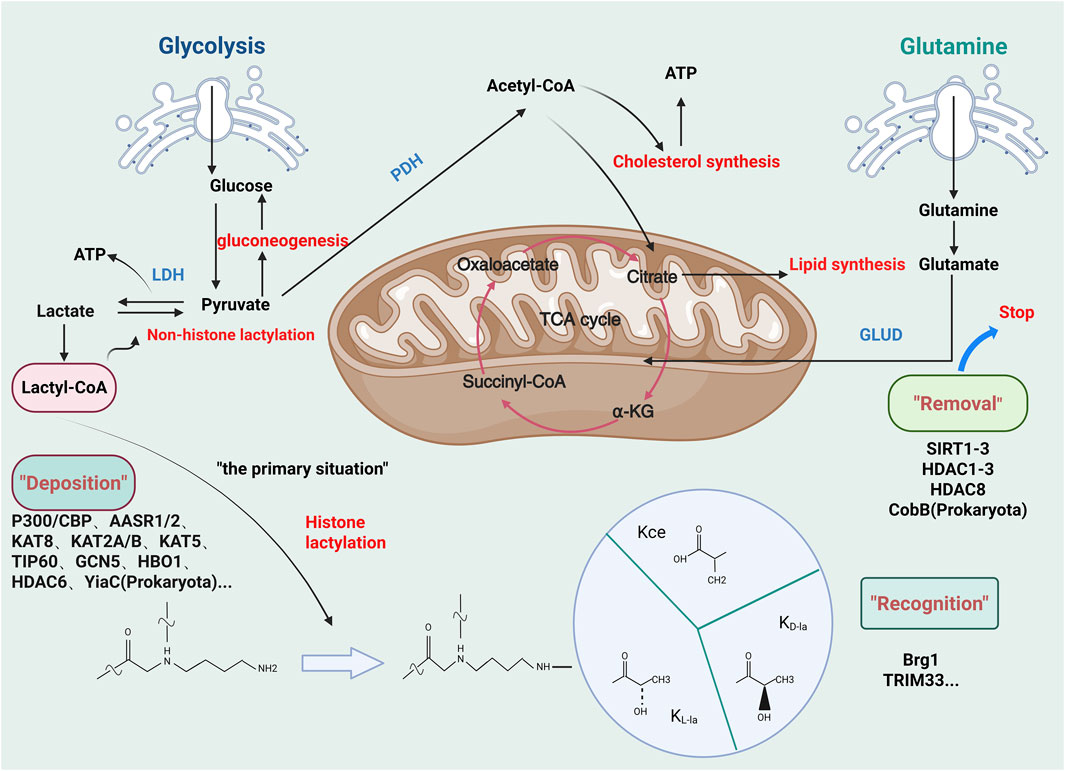
Figure 3. Regulatory mechanisms involved in lactylation. Glycolysis generates pyruvate, which leads to lactate accumulation in intervertebral disc degeneration (IDD). This lactate pool promotes lysine lactylation (KL-la), a novel post-translational modification mediated by L-lactate. Related isomers, including N-ε-(carboxyethyl)-lysine (Kce) and D-lactate-lysine (KD-la), share similar molecular structures but differ in stereochemistry. Lactylation mechanisms can be categorized into enzymatic and non-enzymatic pathways based on precursor specificity. The enzymatic KL-la cascade, the most studied pathway, operates through a tripartite enzymatic machinery: “Writer” (deposition), “Reader” (recognition), and “Eraser” (removal) proteins. (Figure created at http://www.BioRender.com on 7 October 2025).
Lactylation facilitates the crosstalk between metabolism and epigenetics (Zhang et al., 2019; Fan H. et al., 2023; Wang et al., 2023; Izzo and Wellen, 2019). Recent single-cell RNA sequencing analyses demonstrate that during the progression of IDD, NPCs exhibit enhanced glycolytic activity, with the resulting lactate excess leading to NPC dysfunction through the activation of the ferroptosis pathway (Sun et al., 2025). At the molecular level, lactate regulates acyl-CoA synthetase long-chain family member 4 (ACSL4) through a dual mechanism: it promotes lactylation at the histone H3K18 site, which upregulates ACSL4 transcription, and it directly enhances ACSL4 protein lactylation at the K412 site (Sun et al., 2025). Furthermore, lactate exacerbates ACSL4 lactylation by inhibiting Sirtuin-3 (SIRT3) expression (Sun et al., 2025). These findings establish a new theoretical framework for understanding lactate-mediated epigenetic regulation in IDD.
6 Clinical applications and intervention strategies
Lactate homeostasis imbalance plays a pivotal role in the pathogenesis of IDD and could serve as a potential therapeutic target. In terms of molecular mechanisms, lactate metabolism is regulated by two key proteins: LDH, which catalyzes the conversion of pyruvate to lactate, and inhibition of LDH activity effectively reduces lactate production (Sharma et al., 2022; Chen et al., 2025; An et al., 2023). MCTs are responsible for lactate transmembrane transport, and targeted interventions can block lactate release (Py et al., 2005). Importantly, modulating lactate levels could serve as an independent therapeutic strategy, potentially synergizing with conventional adjunctive therapies such as physical therapy and rehabilitation exercises, providing new avenues for the comprehensive treatment of IDD.
6.1 Detection of lactate
Advanced molecular and imaging techniques offer valuable insights into the mechanisms and functional importance of fluctuating tissue lactate levels during the progression of IDD. Magnetic resonance spectroscopy (MRS) and T2 relaxation time (T2r) measurement techniques can act as sensitive imaging biomarkers for early IDD, enabling early diagnosis through the detection of subtle changes in metabolic activity within the disc matrix (Bhari Thippeswamy et al., 2025). In a study involving 236 normal volunteer discs and 215 disc samples from patients with LBP, the molecular profiles of several metabolites, including lactate, were obtained using MRS technology, while disc hydration and collagen content were assessed via T2r measurements (Bhari Thippeswamy et al., 2025). Notably, results indicated that even within discs of the same Pfirrmann classification (PF1), metabolite concentrations such as lactate differed significantly between normal volunteers and patients with LBP (Bhari Thippeswamy et al., 2025). Additionally, the combination of a clinical 3T magnetic resonance (MR) scanner and short-echo water suppression point-resolved spectroscopy (PRESS) technology enables noninvasive, reproducible quantitative detection of metabolic changes associated with IDD (Zuo et al., 2009). The study systematically analyzed bovine IVDs with papain-induced degeneration (N = 17) and human cadaveric IVDs (N = 27) based on Pfirrmann grading of T2-weighted images. Using the 1H PRESS technique, the researchers successfully quantified metabolite concentrations in the carbohydrate zone (Carb), choline head group (Cho), N-acetyl zone (N-acetyl), and lipid and lactate complex (Lac + Lip), and compared metabolic differences between samples with varying levels of degeneration (Zuo et al., 2009). These innovative methods provide a reliable clinical tool for the early diagnosis and metabolic assessment of IDD.
As an emerging area of research in epigenetic modifications, the study of protein lactylation (especially non-histone lactylation) is still in its early stages, underscoring the urgent need for the development of highly sensitive and specific detection techniques. Wan’s research team made a pioneering discovery that during tandem mass spectrometry, lactoyl-lysine (K-lac) forms characteristic cyclic immonium (CycIm) ions, providing a breakthrough for the precise identification of protein lactylation (Wan et al., 2022). The team systematically validated the sensitivity and specificity of this method using affinity-enriched lactylproteome proteomics combined with in-depth informatics evaluation of non-lactylated spectral databases (Wan et al., 2022). Meanwhile, Sun’s group developed an innovative chemical probe, sodium (S)-2-hydroxypent-4-ynoate (YnLac), which can specifically integrate fluorescent or affinity tags into lactated proteins via metabolic labeling strategies. This enables fluorescence visualization of lactylation for detection and proteomics analysis. These technological advances have established a crucial methodological foundation for the in-depth analysis of the biological functions of lactylation (Sun et al., 2022). In conclusion, there is an urgent need to develop high-throughput, high-resolution detection systems to systematically uncover the dynamic regulation of lactate/lactylation in different disc cell populations and their molecular mechanisms in the degeneration process. This will form the theoretical foundation for the development of precise therapeutic strategies targeting lactate metabolism.
Future studies could further integrate single-cell multi-omics technologies to provide finer resolution for studies on disc lactate/lactylation. By combining single-cell transcriptome sequencing (scRNA-seq) with lactate/lactylation proteomic analysis, it will be possible to precisely distinguish the lactate/lactylation characteristics of different cell subgroups, such as NP and AF cells. Additionally, cell-type-specific lactate/lactylation maps can be established, allowing the identification of key gene networks significantly correlated with lactate/lactylation levels in specific cell populations. This multimodal analysis will not only elucidate the metabolic heterogeneity in IDD but also provide novel molecular markers for disease classification and prognostic assessment.
6.2 Targeting LDHs
Studies have shown that LDHA inhibitors can effectively reduce lactate levels by blocking the final step of glycolysis, thereby inhibiting tumor progression (Le et al., 2010). Furthermore, in vitro and in vivo studies have demonstrated that the inhibition of glycolytic activity resulting from LDHA knockdown confers antitumor effects in pancreatic ductal adenocarcinoma (PDAC) (Li F. et al., 2024). However, another study found that high expression of SIRT1 correlates with elevated LDHA expression, suggesting that increased glycolysis generates more ATP to support physiological activities, thereby preventing the negative effects of incomplete OXPHOS on NP cells (Wu et al., 2021). These findings prompt us to consider: Could targeting LDH to inhibit the glycolytic process be a viable approach for treating IDD? Although research on targeting LDH for IDD treatment remains scarce, LDH is undoubtedly a highly promising therapeutic target, providing a crucial therapeutic strategy for delaying the degeneration of nucleus pulposus cells in intervertebral discs.
6.3 Targeting MCTs
Recent study has found that inhibition of lactate influx by the monocarboxylate transporter (MCT)-1 inhibitor, AZD3965, reversed the effect of lactate on GAG accumulation and MMP3 expression and further improved NP cell degeneration in the NPD model (Wang CY. et al., 2022). Currently, MCT inhibitors are primarily used in tumor therapy, with first-generation broad-spectrum inhibitors like α-cyano-4-hydroxycinnamic acid (CHC) (Miranda-Gonçalves et al., 2013; Amorim et al., 2015) and photothialdehydes benzenesulfonates (Poole and Halestrap, 1990), as well as second-generation inhibitors such as AR-C155858 (Andersen et al., 2016; Lopez et al., 2023; Richiardone et al., 2024), BAY-8002 (Wang N. et al., 2021; Vera et al., 2024), and SR13800 (Chatterjee et al., 2023; Khan et al., 2020; Padilla et al., 2022). These drugs have shown promising effects in regulating tumor metabolism, but their potential for treating IDD needs systematic evaluation. Despite the rapid progress in developing MCT inhibitors, some issues also need attention. These inhibitors may interfere with normal cellular energy metabolism, potentially causing severe nonspecific toxic responses. Notably, a recent innovative strategy in oncology therapy—blocking the pathway linking metabolic reprogramming and proteomic remodeling in tumor cells by inhibiting aminoacyl-tRNA synthetase 1 (AARS1) (Li H. et al., 2024; Zong et al., 2024; Li et al., 2025)—offers a new approach for treating IDD. However, there is a gap in research on AARS1 and its cognate protein AARS2 inhibitors in the context of IDD.
This opens a new research direction for developing therapeutic strategies targeting lactate metabolism in IDD. Future studies could build on oncology research to explore the potential application of these targeted drugs in IDD treatment, with special attention to their selectivity and safety.
6.4 Other intervention strategies
Recent advances in nanomaterials science have introduced new possibilities for treating metabolic diseases. A recent study reported a microfluidics-based nanoenzyme functionalized delivery system (MS@MCL), which involves grafting manganese dioxide (MnO2)-lactate oxidase (LOX) composite nanoenzymes onto the surface of hyaluronate methacrylate (HAMA) microspheres via chemical bonding (Shen et al., 2022). This system offers several significant advantages: (1) the uniform porous structure created through microfluidics significantly enhances encapsulation efficiency and injection performance; (2) chemical grafting via amide reaction ensures localized enzyme enrichment and activity enhancement; and (3) sustained oxygen-promoted lactate consumption capacity, along with an extended in vivo half-life. The study demonstrated for the first time that nanoenzyme-functionalized injections can significantly promote the regenerative repair of ischemic tissues by modulating the local lactate microenvironment, offering a novel approach to the treatment of IDD (Shen et al., 2022). However, to achieve a major breakthrough in lactate-targeted therapeutics, several key scientific challenges must still be addressed: first, the molecular regulatory mechanisms of lactate/lactylation and their dynamic changes need to be thoroughly elucidated; second, the interaction network of lactate metabolism-related signaling pathways, along with their spatial and temporal specificity, must be analyzed; and finally, the metabolic characteristics of the heterogeneity of different cell types and their impact on therapeutic responses should be deeply investigated. These breakthroughs in basic research will provide the theoretical foundation and technical guidance needed for developing a new generation of precision nanotherapy strategies.
Additionally, the natural small-molecule compound cryptotanshinone (Cry) could effectively alleviate lactate-induced oxidative stress by modulating the STAT3/SIRT3 signaling pathway (Lu JJ. et al., 2025). Experimental data showed that Cry intervention significantly inhibited the senescence of IVD cells, reduced apoptosis rates, and effectively delayed ECM degradation (Lu JJ. et al., 2025). The highly specific binding of Cry to the STAT3 protein was confirmed through molecular docking and surface plasmon resonance (SPR) techniques. In a rat model of IDD, the Cry-treated group exhibited significant improvement in disease progression (Lu JJ. et al., 2025). These findings provide an experimental basis for treating IDD with natural small-molecule compounds. However, research on small-molecule compounds targeting lactate metabolism and lactylation for IDD remains limited, highlighting an urgent need for the development of small-molecule chemosynthesis strategies.
7 Conclusion and future directions
This review synthesizes evidence that lactate and lactylation contribute to the progression of IDD. It classifies inhibitors of lactylation regulation and summarizes the enzymes involved in this metabolic-epigenetic modification. Targeting lactate metabolism has emerged as a promising therapeutic strategy for IDD due to its abundance in degenerating discs, its broad role in cellular processes, and its impact on disc cells. However, despite its potential, most lactate-targeted therapies remain in the preclinical stage, with only a few progressing to clinical trials. Significant challenges remain in the development of effective lactate/lactylation-targeted therapies. One major hurdle is determining the quantitative contribution of lactate modulation to therapeutic efficacy. Additionally, the specificity of the disc structure complicates the achievement of effective drug concentrations, further hindering therapeutic success. Moreover, the true pharmacological mechanisms and therapeutic benefits of current lactate inhibitors versus lactate-targeted therapies remain unclear, particularly in relation to the high off-target effects and low specificity to lactate metabolism.
To overcome these challenges, future research should focus on several promising areas to improve therapeutic outcomes. One key area is the design and optimization of novel small-molecule inhibitors that exhibit enhanced specificity and efficacy for NP cells. These inhibitors should selectively disrupt lactate-associated pathways, reducing the metabolic fitness of NP cells. Furthermore, advancements in materials science are needed to develop efficient drug delivery systems that improve bioavailability and precise targeting of therapeutic agents. Such innovations are crucial to ensure that drugs reach their intended site of action in sufficient concentrations while minimizing side effects on healthy tissues. Additionally, greater attention should be given to developing rational and synergistic drug combination strategies. These combination therapies can target multiple pathways simultaneously, maximizing the overall therapeutic effect. On the other hand, future studies on IDD should integrate artificial intelligence (AI) and utilize spatial multi-omics triangulation strategies. For instance, spatial metabolomics can be used to identify and map hotspots of lactate accumulation in pathological regions. Mechanistic decoding can be achieved through joint epigenome-transcriptome profiling to unravel lactate-metabolite regulatory networks. Additionally, spatial transcriptomics and single-cell multi-omics can be applied to map cell types and determine lactate heterogeneity across different cells and microdomains. This synergistic approach will correlate spatiotemporal lactate gradients with lactylation dynamics, reveal cell type-specific metabolic-epigenetic crosstalk, and identify RNA-lactylation interactomes. Ultimately, these efforts will generate spatially resolved maps of IDD pathogenesis, facilitating the development of precision therapies targeting lactate/lactylation pathways.
In conclusion, while lactate-targeted therapies show considerable promise for treating IDD, they remain in the early stages of development. Advancing these therapies will require multidisciplinary collaboration and substantial efforts from researchers across various fields to unlock their full potential. A comprehensive understanding of how lactate interacts with the metabolic and epigenetic processes involved in IDD is crucial for developing more effective therapeutic strategies.
Author contributions
YL: Writing – original draft. JY: Writing – original draft. ZW: Writing – original draft. JF: Investigation, Writing – review and editing. HL: Conceptualization, Writing – review and editing. ZeL: Writing – review and editing. JD: Methodology, Writing – review and editing. ZhL: Writing – review and editing, Data curation. QM: Investigation, Writing – review and editing. CL: Formal Analysis, Writing – review and editing. HM: Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was performed with the support of the Project of Shanghai Science and Technology Commission (23ZR1447400), Supported by the Scientific Research Fund Project of Yunnan Provincial Department of Education (2025Y0641).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Amorim, R., Pinheiro, C., Miranda-Gonçalves, V., Pereira, H., Moyer, M. P., Preto, A., et al. (2015). Monocarboxylate transport inhibition potentiates the cytotoxic effect of 5-fluorouracil in colorectal cancer cells. Cancer Lett. 365, 68–78. doi:10.1016/j.canlet.2015.05.015
An, Y. J., Jo, S., Kim, J. M., Kim, H. S., Kim, H. Y., Jeon, S. M., et al. (2023). Lactate as a major epigenetic carbon source for histone acetylation via nuclear LDH metabolism. Exp. Mol. Med. 55, 2238–2247. doi:10.1038/s12276-023-01095-w
Andersen, A. P., Flinck, M., Oernbo, E. K., Pedersen, N. B., Viuff, B. M., and Pedersen, S. F. (2016). Roles of acid-extruding ion transporters in regulation of breast cancer cell growth in a 3-dimensional microenvironment. Mol. Cancer 15, 45. doi:10.1186/s12943-016-0528-0
Andreucci, E., Peppicelli, S., Ruzzolini, J., Bianchini, F., Biagioni, A., Papucci, L., et al. (2020). The acidic tumor microenvironment drives a stem-like phenotype in melanoma cells. J. Mol. Med. Berl. 98, 1431–1446. doi:10.1007/s00109-020-01959-y
Arce-Molina, R., Cortés-Molina, F., Sandoval, P. Y., Galaz, A., Alegría, K., Schirmeier, S., et al. (2020). A highly responsive pyruvate sensor reveals pathway-regulatory role of the mitochondrial pyruvate carrier MPC. Elife, 9. doi:10.7554/eLife.53917
Bhari Thippeswamy, P., Rajasekaran, S., Ramachandran, K., Easwaran, M., Ramadevi, S. S., Sri Vijay Anand, K. S., et al. (2025). Role of magnetic resonance spectroscopy and T2 relaxometry as imaging biomarker of early lumbar intervertebral disc degeneration. Glob. Spine J. 15, 2736–2743. doi:10.1177/21925682241311515
Bibby, S. R., Fairbank, J. C., Urban, M. R., and Urban, J. P. (2002). Cell viability in scoliotic discs in relation to disc deformity and nutrient levels. Spine (Phila Pa 1976) 27, 2220–2228. ; discussion 27-8. doi:10.1097/00007632-200210150-00007
Bibby, S. R., Jones, D. A., Ripley, R. M., and Urban, J. P. (2005). Metabolism of the intervertebral disc: effects of low levels of oxygen, glucose, and pH on rates of energy metabolism of Bovine nucleus pulposus cells. Spine (Phila Pa 1976) 30, 487–496. doi:10.1097/01.brs.0000154619.38122.47
Boudreau, A., Purkey, H. E., Hitz, A., Robarge, K., Peterson, D., Labadie, S., et al. (2016). Metabolic plasticity underpins innate and acquired resistance to LDHA inhibition. Nat. Chem. Biol. 12, 779–786. doi:10.1038/nchembio.2143
Breda, C. N. S., Davanzo, G. G., Basso, P. J., Saraiva Câmara, N. O., and Moraes-Vieira, P. M. M. (2019). Mitochondria as central hub of the immune system. Redox Biol. 26, 101255. doi:10.1016/j.redox.2019.101255
Brooks, G. A. (2009). Cell-cell and intracellular lactate shuttles. J. Physiol. 587, 5591–5600. doi:10.1113/jphysiol.2009.178350
Brooks, G. A. (2018). The science and translation of lactate shuttle theory. Cell Metab. 27, 757–785. doi:10.1016/j.cmet.2018.03.008
Broz, P. (2025). Pyroptosis: molecular mechanisms and roles in disease. Cell Res. 35, 334–344. doi:10.1038/s41422-025-01107-6
Byvaltsev, V. A., Kolesnikov, S. I., Bardonova, L. A., Belykh, E. G., Korytov, L. I., Giers, M. B., et al. (2018). Assessment of lactate production and Proteoglycans synthesis by the intact and degenerated intervertebral disc cells under the influence of activated macrophages: an in vitro study. Bull. Exp. Biol. Med. 166, 170–173. doi:10.1007/s10517-018-4307-3
Certo, M., Llibre, A., Lee, W., and Mauro, C. (2022). Understanding lactate sensing and signalling. Trends Endocrinol. Metab. 33, 722–735. doi:10.1016/j.tem.2022.07.004
Chatterjee, P., Bhowmik, D., and Roy, S. S. (2023). A systemic analysis of monocarboxylate transporters in ovarian cancer and possible therapeutic interventions. Channels (Austin) 17, 2273008. doi:10.1080/19336950.2023.2273008
Chen, Q., Xin, M., Wang, L., Li, L., Shen, Y., Geng, Y., et al. (2022). Inhibition of LDHA to induce eEF2 release enhances thrombocytopoiesis. Blood 139, 2958–2971. doi:10.1182/blood.2022015620
Chen, X., Jaiswal, A., Costliow, Z., Herbst, P., Creasey, E. A., Oshiro-Rapley, N., et al. (2022). pH sensing controls tissue inflammation by modulating cellular metabolism and endo-lysosomal function of immune cells. Nat. Immunol. 23, 1063–1075. doi:10.1038/s41590-022-01231-0
Chen, X., Zhang, A., Zhao, K., Gao, H., Shi, P., Chen, Y., et al. (2024a). The role of oxidative stress in intervertebral disc degeneration: mechanisms and therapeutic implications. Ageing Res. Rev. 98, 102323. doi:10.1016/j.arr.2024.102323
Chen, H., Li, Y., Li, H., Chen, X., Fu, H., Mao, D., et al. (2024b). NBS1 lactylation is required for efficient DNA repair and chemotherapy resistance. Nature 631, 663–669. doi:10.1038/s41586-024-07620-9
Chen, J., Huang, Z., Chen, Y., Tian, H., Chai, P., Shen, Y., et al. (2025). Lactate and lactylation in cancer. Signal Transduct. Target Ther. 10, 38. doi:10.1038/s41392-024-02082-x
Cheng, J., Zhang, R., Xu, Z., Ke, Y., Sun, R., Yang, H., et al. (2021). Early glycolytic reprogramming controls microglial inflammatory activation. J. Neuroinflammation 18, 129. doi:10.1186/s12974-021-02187-y
Cheng, F., Yang, H., Cheng, Y., Liu, Y., Hai, Y., and Zhang, Y. (2022). The role of oxidative stress in intervertebral disc cellular senescence. Front. Endocrinol. (Lausanne) 13, 1038171. doi:10.3389/fendo.2022.1038171
Cheng, Z., Gan, W., Xiang, Q., Zhao, K., Gao, H., Chen, Y., et al. (2025). Impaired degradation of PLCG1 by chaperone-mediated autophagy promotes cellular senescence and intervertebral disc degeneration. Autophagy 21, 352–373. doi:10.1080/15548627.2024.2395797
Cluntun, A. A., Badolia, R., Lettlova, S., Parnell, K. M., Shankar, T. S., Diakos, N. A., et al. (2021). The pyruvate-lactate axis modulates cardiac hypertrophy and heart failure. Cell Metab. 33, 629–48.e10. doi:10.1016/j.cmet.2020.12.003
Coll, R. C., Schroder, K., and Pelegrín, P. (2022). NLRP3 and pyroptosis blockers for treating inflammatory diseases. Trends Pharmacol. Sci. 43, 653–668. doi:10.1016/j.tips.2022.04.003
Dawson, M. J., Gadian, D. G., and Wilkie, D. R. (1978). Muscular fatigue investigated by phosphorus nuclear magnetic resonance. Nature 274, 861–866. doi:10.1038/274861a0
Dienel, G. A. (2019). Brain glucose metabolism: integration of energetics with function. Physiol. Rev. 99, 949–1045. doi:10.1152/physrev.00062.2017
Doherty, J. R., Yang, C., Scott, K. E., Cameron, M. D., Fallahi, M., Li, W., et al. (2014). Blocking lactate export by inhibiting the myc target MCT1 disables glycolysis and glutathione synthesis. Cancer Res. 74, 908–920. doi:10.1158/0008-5472.Can-13-2034
Dong, X., Zhang, Q., Yu, X., Wang, D., Ma, J., Ma, J., et al. (2022a). Metabolic lactate production coordinates vasculature development and progenitor behavior in the developing mouse neocortex. Nat. Neurosci. 25, 865–875. doi:10.1038/s41593-022-01093-7
Dong, H., Zhang, J., Zhang, H., Han, Y., Lu, C., Chen, C., et al. (2022b). YiaC and CobB regulate lysine lactylation in Escherichia coli. Nat. Commun. 13, 6628. doi:10.1038/s41467-022-34399-y
Dowdell, J., Erwin, M., Choma, T., Vaccaro, A., Iatridis, J., and Cho, S. K. (2017). Intervertebral disk degeneration and repair. Neurosurgery 80, S46–s54. doi:10.1093/neuros/nyw078
Du, R., Gao, Y., Yan, C., Ren, X., Qi, S., Liu, G., et al. (2024). Sirtuin 1/sirtuin 3 are robust lysine delactylases and sirtuin 1-mediated delactylation regulates glycolysis. iScience 27, 110911. doi:10.1016/j.isci.2024.110911
Fan, M., Yang, K., Wang, X., Chen, L., Gill, P. S., Ha, T., et al. (2023a). Lactate promotes endothelial-to-mesenchymal transition via Snail1 lactylation after myocardial infarction. Sci. Adv. 9, eadc9465. doi:10.1126/sciadv.adc9465
Fan, H., Yang, F., Xiao, Z., Luo, H., Chen, H., Chen, Z., et al. (2023b). Lactylation: novel epigenetic regulatory and therapeutic opportunities. Am. J. Physiol. Endocrinol. Metab. 324, E330–E338. doi:10.1152/ajpendo.00159.2022
Fang, Y., Liu, W., Tang, Z., Ji, X., Zhou, Y., Song, S., et al. (2023). Monocarboxylate transporter 4 inhibition potentiates hepatocellular carcinoma immunotherapy through enhancing T cell infiltration and immune attack. Hepatology 77, 109–123. doi:10.1002/hep.32348
Fendt, S. M. (2024). 100 years of the warburg effect: a cancer metabolism endeavor. Cell 187, 3824–3828. doi:10.1016/j.cell.2024.06.026
Gao, Y., Chen, X., Zheng, G., Lin, M., Zhou, H., and Zhang, X. (2023). Current status and development direction of immunomodulatory therapy for intervertebral disk degeneration. Front. Med. (Lausanne) 10, 1289642. doi:10.3389/fmed.2023.1289642
Gaudet, A. D., and Popovich, P. G. (2014). Extracellular matrix regulation of inflammation in the healthy and injured spinal cord. Exp. Neurol. 258, 24–34. doi:10.1016/j.expneurol.2013.11.020
Gilbert, H. T. J., Hodson, N., Baird, P., Richardson, S. M., and Hoyland, J. A. (2016). Acidic pH promotes intervertebral disc degeneration: acid-Sensing ion channel -3 as a potential therapeutic target. Sci. Rep. 6, 37360. doi:10.1038/srep37360
Gómez-Valadés, A. G., Pozo, M., Varela, L., Boudjadja, M. B., Ramírez, S., Chivite, I., et al. (2021). Mitochondrial cristae-remodeling protein OPA1 in POMC neurons couples Ca(2+) homeostasis with adipose tissue lipolysis. Cell Metab. 33, 1820–35.e9. doi:10.1016/j.cmet.2021.07.008
Grunhagen, T., Wilde, G., Soukane, D. M., Shirazi-Adl, S. A., and Urban, J. P. (2006). Nutrient supply and intervertebral disc metabolism. J. Bone Jt. Surg. Am. 88 (Suppl. 2), 30–35. doi:10.2106/jbjs.E.01290
Guo, D., Cheng, K., Song, C., Liu, F., Cai, W., Chen, J., et al. (2023). Mechanisms of inhibition of nucleus pulposus cells pyroptosis through SDF1/CXCR4-NFkB-NLRP3 axis in the treatment of intervertebral disc degeneration by duhuo jisheng decoction. Int. Immunopharmacol. 124, 110844. doi:10.1016/j.intimp.2023.110844
Herzig, S., Raemy, E., Montessuit, S., Veuthey, J. L., Zamboni, N., Westermann, B., et al. (2012). Identification and functional expression of the mitochondrial pyruvate carrier. Science 337, 93–96. doi:10.1126/science.1218530
Halestrap, A. P. (2013). Monocarboxylic acid transport. Compr. Physiol. 3, 1611–1643. doi:10.1002/cphy.c130008
Halford, S., Veal, G. J., Wedge, S. R., Payne, G. S., Bacon, C. M., Sloan, P., et al. (2023). A phase I dose-escalation study of AZD3965, an oral monocarboxylate transporter 1 inhibitor, in patients with advanced cancer. Clin. Cancer Res. 29, 1429–1439. doi:10.1158/1078-0432.Ccr-22-2263
Henderson, G. C., Horning, M. A., Wallis, G. A., and Brooks, G. A. (2007). Pyruvate metabolism in working human skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 292, E366. doi:10.1152/ajpendo.00363.2006
Horner, H. A., Roberts, S., Bielby, R. C., Menage, J., Evans, H., and Urban, J. P. (2002). Cells from different regions of the intervertebral disc: effect of culture system on matrix expression and cell phenotype. Spine (Phila Pa 1976) 27, 1018–1028. doi:10.1097/00007632-200205150-00004
Hsu, S. K., Li, C. Y., Lin, I. L., Syue, W. J., Chen, Y. F., Cheng, K. C., et al. (2021). Inflammation-related pyroptosis, a novel programmed cell death pathway, and its crosstalk with immune therapy in cancer treatment. Theranostics 11, 8813–8835. doi:10.7150/thno.62521
Hu, S., Chen, L., Al Mamun, A., Ni, L., Gao, W., Lin, Y., et al. (2021). The therapeutic effect of TBK1 in intervertebral disc degeneration via coordinating selective autophagy and autophagic functions. J. Adv. Res. 30, 1–13. doi:10.1016/j.jare.2020.08.011
Hu, X. T., Wu, X. F., Xu, J. Y., and Xu, X. (2024a). Lactate-mediated lactylation in human health and diseases: progress and remaining challenges. J. Adv. Res. 75, 229–248. doi:10.1016/j.jare.2024.11.010
Hu, X., Huang, X., Yang, Y., Sun, Y., Zhao, Y., Zhang, Z., et al. (2024b). Dux activates metabolism-lactylation-MET network during early iPSC reprogramming with Brg1 as the histone lactylation reader. Nucleic Acids Res. 52, 5529–5548. doi:10.1093/nar/gkae183
Huang, H., Chen, K., Zhu, Y., Hu, Z., Wang, Y., Chen, J., et al. (2024). A multi-dimensional approach to unravel the intricacies of lactylation related signature for prognostic and therapeutic insight in colorectal cancer. J. Transl. Med. 22, 211. doi:10.1186/s12967-024-04955-9
Hui, S., Ghergurovich, J. M., Morscher, R. J., Jang, C., Teng, X., Lu, W., et al. (2017). Glucose feeds the TCA cycle via circulating lactate. Nature 551, 115–118. doi:10.1038/nature24057
Hui, S., Cowan, A. J., Zeng, X., Yang, L., Teslaa, T., Li, X., et al. (2020). Quantitative fluxomics of circulating metabolites. Cell Metab. 32, 676–88.e4. doi:10.1016/j.cmet.2020.07.013
Iatsenko, I., Boquete, J. P., and Lemaitre, B. (2018). Microbiota-derived lactate activates production of reactive oxygen species by the intestinal NADPH oxidase nox and shortens drosophila lifespan. Immunity 49, 929–42.e5. doi:10.1016/j.immuni.2018.09.017
Iozzo, M., Pardella, E., Giannoni, E., and Chiarugi, P. (2025). The role of protein lactylation: a kaleidoscopic post-translational modification in cancer. Mol. Cell 85, 1263–1279. doi:10.1016/j.molcel.2025.02.011
Ivashkiv, L. B. (2020). The hypoxia-lactate axis tempers inflammation. Nat. Rev. Immunol. 20, 85–86. doi:10.1038/s41577-019-0259-8
Izzo, L. T., and Wellen, K. E. (2019). Histone lactylation links metabolism and gene regulation. Nature 574, 492–493. doi:10.1038/d41586-019-03122-1
Jaschke, N. P., Breining, D., Hofmann, M., Pählig, S., Baschant, U., Oertel, R., et al. (2024). Small-molecule CBP/p300 histone acetyltransferase inhibition mobilizes leukocytes from the bone marrow via the endocrine stress response. Immunity 57, 364–78.e9. doi:10.1016/j.immuni.2024.01.005
Jia, L., Liao, M., Mou, A., Zheng, Q., Yang, W., Yu, Z., et al. (2021). Rheb-regulated mitochondrial pyruvate metabolism of Schwann cells linked to axon stability. Dev. Cell 56, 2980–94.e6. doi:10.1016/j.devcel.2021.09.013
Jing, F., Zhang, J., Zhang, H., and Li, T. (2025). Unlocking the multifaceted molecular functions and diverse disease implications of lactylation. Biol. Rev. Camb Philos. Soc. 100, 172–189. doi:10.1111/brv.13135
Kamali, A., Ziadlou, R., Lang, G., Pfannkuche, J., Cui, S., Li, Z., et al. (2021). Small molecule-based treatment approaches for intervertebral disc degeneration: current options and future directions. Theranostics 11, 27–47. doi:10.7150/thno.48987
Kang, L., Liu, S., Li, J., Tian, Y., Xue, Y., and Liu, X. (2020). The mitochondria-targeted anti-oxidant MitoQ protects against intervertebral disc degeneration by ameliorating mitochondrial dysfunction and redox imbalance. Cell Prolif. 53, e12779. doi:10.1111/cpr.12779
Kang, L., Zhang, H., Jia, C., Zhang, R., and Shen, C. (2023). Epigenetic modifications of inflammation in intervertebral disc degeneration. Ageing Res. Rev. 87, 101902. doi:10.1016/j.arr.2023.101902
Keshari, K. R., Lotz, J. C., Link, T. M., Hu, S., Majumdar, S., and Kurhanewicz, J. (2008). Lactic acid and Proteoglycans as metabolic markers for discogenic back pain. Spine (Phila Pa 1976) 33, 312–317. doi:10.1097/BRS.0b013e31816201c3
Khan, A., Valli, E., Lam, H., Scott, D. A., Murray, J., Hanssen, K. M., et al. (2020). Targeting metabolic activity in high-risk neuroblastoma through monocarboxylate transporter 1 (MCT1) inhibition. Oncogene 39, 3555–3570. doi:10.1038/s41388-020-1235-2
Kompanje, E. J., Jansen, T. C., Van Der Hoven, B., and Bakker, J. (2007). The first demonstration of lactic acid in human blood in shock by johann joseph scherer (1814-1869) in January 1843. Intensive Care Med. 33, 1967–1971. doi:10.1007/s00134-007-0788-7
Krut, Z., Pelled, G., Gazit, D., and Gazit, Z. (2021). Stem cells and exosomes: new therapies for intervertebral disc degeneration. Cells 10, 2241. doi:10.3390/cells10092241
Kulik, U., Moesta, C., Spanel, R., and Borlak, J. (2024). Dysfunctional cori and krebs cycle and inhibition of lactate transporters constitute a mechanism of primary nonfunction of fatty liver allografts. Transl. Res. 264, 33–65. doi:10.1016/j.trsl.2023.09.006
Lasko, L. M., Jakob, C. G., Edalji, R. P., Qiu, W., Montgomery, D., Digiammarino, E. L., et al. (2017). Discovery of a selective catalytic p300/CBP inhibitor that targets lineage-specific tumours. Nature 550, 128–132. doi:10.1038/nature24028
Le, A., Cooper, C. R., Gouw, A. M., Dinavahi, R., Maitra, A., Deck, L. M., et al. (2010). Inhibition of lactate dehydrogenase A induces oxidative stress and inhibits tumor progression. Proc. Natl. Acad. Sci. U. S. A. 107, 2037–2042. doi:10.1073/pnas.0914433107
Li, X., Yang, Y., Zhang, B., Lin, X., Fu, X., An, Y., et al. (2022). Lactate metabolism in human health and disease. Signal Transduct. Target Ther. 7, 305. doi:10.1038/s41392-022-01151-3
Li, Y., Tian, X., He, W., Jin, C., Yang, C., Pan, Z., et al. (2023). Fucoidan-functionalized gelatin methacryloyl microspheres ameliorate intervertebral disc degeneration by restoring redox and matrix homeostasis of nucleus pulposus. Int. J. Biol. Macromol. 250, 126166. doi:10.1016/j.ijbiomac.2023.126166
Li, F., Si, W., Xia, L., Yin, D., Wei, T., Tao, M., et al. (2024a). Positive feedback regulation between glycolysis and histone lactylation drives oncogenesis in pancreatic ductal adenocarcinoma. Mol. Cancer 23, 90. doi:10.1186/s12943-024-02008-9
Li, H., Liu, C., Li, R., Zhou, L., Ran, Y., Yang, Q., et al. (2024b). AARS1 and AARS2 sense L-lactate to regulate cGAS as global lysine lactyltransferases. Nature 634, 1229–1237. doi:10.1038/s41586-024-07992-y
Li, X., Zhang, C., Mei, Y., Zhong, W., Fan, W., Liu, L., et al. (2025). Irinotecan alleviates chemoresistance to anthracyclines through the inhibition of AARS1-mediated BLM lactylation and homologous recombination repair. Signal Transduct. Target Ther. 10, 214. doi:10.1038/s41392-025-02302-y
Liang, H., Luo, R., Li, G., Zhang, W., Song, Y., and Yang, C. (2022). The proteolysis of ECM in intervertebral disc degeneration. Int. J. Mol. Sci. 23, 1715. doi:10.3390/ijms23031715
Liao, Z., Luo, R., Li, G., Song, Y., Zhan, S., Zhao, K., et al. (2019). Exosomes from mesenchymal stem cells modulate endoplasmic reticulum stress to protect against nucleus pulposus cell death and ameliorate intervertebral disc degeneration in vivo. Theranostics 9, 4084–4100. doi:10.7150/thno.33638
Liu, J., Tao, H., Wang, H., Dong, F., Zhang, R., Li, J., et al. (2017). Biological behavior of human nucleus pulposus mesenchymal stem cells in response to changes in the acidic environment during intervertebral disc degeneration. Stem Cells Dev. 26, 901–911. doi:10.1089/scd.2016.0314
Liu, Z. C., Wang, Z. L., Huang, C. Y., Fu, Z. J., Liu, Y., Wei, Z. C., et al. (2018). Duhuo jisheng decoction inhibits SDF-1-induced inflammation and matrix degradation in human degenerative nucleus pulposus cells in vitro through the CXCR4/NF-κB pathway. Acta Pharmacol. Sin. 39, 912–922. doi:10.1038/aps.2018.36
Liu, W., Ma, Z., Wang, Y., and Yang, J. (2023). Multiple nano-drug delivery systems for intervertebral disc degeneration: current status and future perspectives. Bioact. Mater 23, 274–299. doi:10.1016/j.bioactmat.2022.11.006
Lopez, E., Karattil, R., Nannini, F., Weng-Kit Cheung, G., Denzler, L., Galvez-Cancino, F., et al. (2023). Inhibition of lactate transport by MCT-1 blockade improves chimeric antigen receptor T-cell therapy against B-cell malignancies. J. Immunother. Cancer 11, e006287. doi:10.1136/jitc-2022-006287
Lu, Z., Fang, P., Li, S., Xia, D., Zhang, J., Wu, X., et al. (2025a). Lactylation of histone H3k18 and Egr1 promotes endothelial glycocalyx degradation in sepsis-induced acute lung injury. Adv. Sci. (Weinh) 12, e2407064. doi:10.1002/advs.202407064
Lu, J. J., Zhang, Q. C., Chen, Y. T., Yuan, G. C., Huang, Y. K., Wu, T., et al. (2025b). Cryptotanshinone attenuates lactate-induced nucleus pulposus cells injury by modulating the STAT3/SIRT3 signaling axis. Phytomedicine 145, 157021. doi:10.1016/j.phymed.2025.157021
Ma, K., Chen, S., Li, Z., Deng, X., Huang, D., Xiong, L., et al. (2019). Mechanisms of endogenous repair failure during intervertebral disc degeneration. Osteoarthr. Cartil. 27, 41–48. doi:10.1016/j.joca.2018.08.021
Ma, T., Wu, J., Chen, S., Bian, J., Gao, G., and Nong, L. (2024). pH-Responsive modified HAMA microspheres regulate the inflammatory microenvironment of intervertebral discs. ACS Appl. Mater Interfaces 16, 63295–63305. doi:10.1021/acsami.4c14475
Madhu, V., Guntur, A. R., and Risbud, M. V. (2021). Role of autophagy in intervertebral disc and cartilage function: implications in health and disease. Matrix Biol. 100-101, 207–220. doi:10.1016/j.matbio.2020.12.002
Mäki-Arvela, P., Simakova, I. L., Salmi, T., and Murzin, D. Y. (2014). Production of lactic acid/lactates from biomass and their catalytic transformations to commodities. Chem. Rev. 114, 1909–1971. doi:10.1021/cr400203v
Malandrino, A., Noailly, J., and Lacroix, D. (2011). The effect of sustained compression on oxygen metabolic transport in the intervertebral disc decreases with degenerative changes. PLoS Comput. Biol. 7, e1002112. doi:10.1371/journal.pcbi.1002112
Manoj, K. M., Nirusimhan, V., Parashar, A., Edward, J., and Gideon, D. A. (2022). Murburn precepts for lactic-acidosis, cori cycle, and warburg effect: interactive dynamics of dehydrogenases, protons, and oxygen. J. Cell Physiol. 237, 1902–1922. doi:10.1002/jcp.30661
Miao, R., Jiang, C., Chang, W. Y., Zhang, H., An, J., Ho, F., et al. (2023). Gasdermin D permeabilization of mitochondrial inner and outer membranes accelerates and enhances pyroptosis. Immunity 56, 2523–41.e8. doi:10.1016/j.immuni.2023.10.004
Miranda-Gonçalves, V., Honavar, M., Pinheiro, C., Martinho, O., Pires, M. M., Pinheiro, C., et al. (2013). Monocarboxylate transporters (MCTs) in gliomas: expression and exploitation as therapeutic targets. Neuro Oncol. 15, 172–188. doi:10.1093/neuonc/nos298
Mirza, S. K., and Deyo, R. A. (2007). Systematic review of randomized trials comparing lumbar fusion surgery to nonoperative care for treatment of chronic back pain. Spine (Phila Pa 1976) 32, 816–823. doi:10.1097/01.brs.0000259225.37454.38
Mitchell, P. (1961). Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature 191, 144–148. doi:10.1038/191144a0
Moreno-Yruela, C., Zhang, D., Wei, W., Bæk, M., Liu, W., Gao, J., et al. (2022). Class I histone deacetylases (HDAC1-3) are histone lysine delactylases. Sci. Adv. 8, eabi6696. doi:10.1126/sciadv.abi6696
Moretti, L., Li, B., Kim, K. W., Chen, H., and Lu, B. (2010). AT-101, a pan-Bcl-2 inhibitor, leads to radiosensitization of non-small cell lung cancer. J. Thorac. Oncol. 5, 680–687. doi:10.1097/JTO.0b013e3181d6e08e
Müller, J., Radej, J., Horak, J., Karvunidis, T., Valesova, L., Kriz, M., et al. (2023). Lactate: the fallacy of oversimplification. Biomedicines 11, 3192. doi:10.3390/biomedicines11123192
Mullins, R., Reiter, D., and Kapogiannis, D. (2018). Magnetic resonance spectroscopy reveals abnormalities of glucose metabolism in the Alzheimer's brain. Ann. Clin. Transl. Neurol. 5, 262–272. doi:10.1002/acn3.530
Nguyen, N. T. B., Gevers, S., Kok, R. N. U., Burgering, L. M., Neikes, H., Akkerman, N., et al. (2025). Lactate controls cancer stemness and plasticity through epigenetic regulation. Cell Metab. 37, 903–919.e10. doi:10.1016/j.cmet.2025.01.002
Ni, C., Zhou, L., Yang, S., Ran, M., Luo, J., Cheng, K., et al. (2025). Oxymatrine, a novel TLR2 agonist, promotes megakaryopoiesis and thrombopoiesis through the STING/NF-κB pathway. J. Pharm. Anal. 15, 101054. doi:10.1016/j.jpha.2024.101054
Niu, Z., Chen, C., Wang, S., Lu, C., Wu, Z., Wang, A., et al. (2024). HBO1 catalyzes lysine lactylation and mediates histone H3K9la to regulate gene transcription. Nat. Commun. 15, 3561. doi:10.1038/s41467-024-47900-6
Nuñez, R., Sidlowski, P. F. W., Steen, E. A., Wynia-Smith, S. L., Sprague, D. J., Keyes, R. F., et al. (2024). The TRIM33 bromodomain recognizes histone lysine lactylation. ACS Chem. Biol. 19, 2418–2428. doi:10.1021/acschembio.4c00248
Ohshima, H., and Urban, J. P. (1992). The effect of lactate and pH on proteoglycan and protein synthesis rates in the intervertebral disc. Spine (Phila Pa 1976) 17, 1079–1082. doi:10.1097/00007632-199209000-00012
Okajima, F. (2013). Regulation of inflammation by extracellular acidification and proton-sensing GPCRs. Cell Signal 25, 2263–2271. doi:10.1016/j.cellsig.2013.07.022
Omerbašić, D., Schuhmacher, L. N., Bernal Sierra, Y. A., Smith, E. S., and Lewin, G. R. (2015). ASICs and Mammalian mechanoreceptor function. Neuropharmacology 94, 80–86. doi:10.1016/j.neuropharm.2014.12.007
Ouyang, F., Li, Y., Wang, H., Liu, X., Tan, X., Xie, G., et al. (2024). Aloe emodin alleviates radiation-induced heart disease via blocking P4HB lactylation and mitigating kynurenine metabolic disruption. Adv. Sci. (Weinh) 11, e2406026. doi:10.1002/advs.202406026
Padilla, J., Lee, B. S., Zhai, K., Rentz, B., Bobo, T., Dowling, N. M., et al. (2022). A heme-binding transcription factor BACH1 regulates lactate catabolism suggesting a combined therapy for triple-negative breast cancer. Cells 11, 1177. doi:10.3390/cells11071177
Pan, Z., Sun, H., Xie, B., Xia, D., Zhang, X., Yu, D., et al. (2018). Therapeutic effects of gefitinib-encapsulated thermosensitive injectable hydrogel in intervertebral disc degeneration. Biomaterials 160, 56–68. doi:10.1016/j.biomaterials.2018.01.016
Peng, Y., Chen, X., Zhang, Q., Liu, S., Wu, W., Li, K., et al. (2024). Enzymatically bioactive nucleus pulposus matrix hydrogel microspheres for exogenous stem cells therapy and endogenous repair strategy to achieve disc regeneration. Adv. Sci. (Weinh) 11, e2304761. doi:10.1002/advs.202304761
Piao, Y., Zhai, N., Zhang, X., Zhao, W., and Li, M. (2025). Post-translational modifications in hepatocellular carcinoma: unlocking new frontiers in immunotherapy. Front. Immunol. 16, 1554372. doi:10.3389/fimmu.2025.1554372
Poole, R. C., and Halestrap, A. P. (1990). Inhibition and labelling of the erythrocyte lactate transporter by stilbene disulphonates. Biochem. Soc. Trans. 18, 1245–1246. doi:10.1042/bst0181245
Pucino, V., Bombardieri, M., Pitzalis, C., and Mauro, C. (2017). Lactate at the crossroads of metabolism, inflammation, and autoimmunity. Eur. J. Immunol. 47, 14–21. doi:10.1002/eji.201646477
Py, G., Eydoux, N., Lambert, K., Chapot, R., Koulmann, N., Sanchez, H., et al. (2005). Role of hypoxia-induced anorexia and right ventricular hypertrophy on lactate transport and MCT expression in rat muscle. Metabolism 54, 634–644. doi:10.1016/j.metabol.2004.12.007
Qu, M., Zhou, X., Wang, X., and Li, H. (2021). Lipid-induced S-palmitoylation as a vital regulator of cell signaling and disease development. Int. J. Biol. Sci. 17, 4223–4237. doi:10.7150/ijbs.64046
Rabinowitz, J. D., and Enerbäck, S. (2020). Lactate: the ugly duckling of energy metabolism. Nat. Metab. 2, 566–571. doi:10.1038/s42255-020-0243-4
Rahal, A., Kumar, A., Singh, V., Yadav, B., Tiwari, R., Chakraborty, S., et al. (2014). Oxidative stress, prooxidants, and antioxidants: the interplay. Biomed. Res. Int. 2014, 761264. doi:10.1155/2014/761264
Richiardone, E., Al Roumi, R., Lardinois, F., Giolito, M. V., Ambroise, J., Boidot, R., et al. (2024). MCT1-dependent lactate recycling is a metabolic vulnerability in colorectal cancer cells upon acquired resistance to anti-EGFR targeted therapy. Cancer Lett. 598, 217091. doi:10.1016/j.canlet.2024.217091
Ross, J. M., Öberg, J., Brené, S., Coppotelli, G., Terzioglu, M., Pernold, K., et al. (2010). High brain lactate is a hallmark of aging and caused by a shift in the lactate dehydrogenase A/B ratio. Proc. Natl. Acad. Sci. U. S. A. 107, 20087–20092. doi:10.1073/pnas.1008189107
Roughley, P. J., Melching, L. I., Heathfield, T. F., Pearce, R. H., and Mort, J. S. (2006). The structure and degradation of aggrecan in human intervertebral disc. Eur. Spine J. 15 (Suppl. 3), S326–S332. doi:10.1007/s00586-006-0127-7
Ruan, N., Tribble, J., Peterson, A. M., Jiang, Q., Wang, J. Q., and Chu, X. P. (2021). Acid-sensing ion channels and mechanosensation. Int. J. Mol. Sci. 22, 4810. doi:10.3390/ijms22094810
Rustenburg, C. M. E., Emanuel, K. S., Peeters, M., Lems, W. F., Vergroesen, P. A., and Smit, T. H. (2018). Osteoarthritis and intervertebral disc degeneration: quite different, quite similar, JOR Spine, 1 (03), 12–15. doi:10.1002/jsp2.1033
Sang, C., Li, X., Liu, J., Chen, Z., Xia, M., Yu, M., et al. (2024). Reversible acetylation of HDAC8 regulates cell cycle. EMBO Rep. 25, 3925–3943. doi:10.1038/s44319-024-00210-w
Sharma, D., Singh, M., and Rani, R. (2022). Role of LDH in tumor glycolysis: regulation of LDHA by small molecules for cancer therapeutics. Semin. Cancer Biol. 87, 184–195. doi:10.1016/j.semcancer.2022.11.007
Shen, J., Chen, A., Cai, Z., Chen, Z., Cao, R., Liu, Z., et al. (2022). Exhausted local lactate accumulation via injectable nanozyme-functionalized hydrogel microsphere for inflammation relief and tissue regeneration. Bioact. Mater 12, 153–168. doi:10.1016/j.bioactmat.2021.10.013
Sheng, L., Xu, H., Wang, Y., Ni, J., Xiang, T., Xu, H., et al. (2024). Systematic analysis of lysine lactylation in nucleus pulposus cells. iScience 27, 111157. doi:10.1016/j.isci.2024.111157
Sheyn, D., Ben-David, S., Tawackoli, W., Zhou, Z., Salehi, K., Bez, M., et al. (2019). Human iPSCs can be differentiated into notochordal cells that reduce intervertebral disc degeneration in a porcine model. Theranostics 9, 7506–7524. doi:10.7150/thno.34898
Shi, J., Zhou, X., Wang, Z., Kurra, S., Niu, J., and Yang, H. (2019). Increased lactic acid content associated with extracellular matrix depletion in a porcine disc degeneration induced by superficial annular lesion. BMC Musculoskelet. Disord. 20, 551. doi:10.1186/s12891-019-2937-x
Shi, Y., Li, H., Chu, D., Lin, W., Wang, X., Wu, Y., et al. (2023). Rescuing nucleus pulposus cells from senescence via dual-functional greigite nanozyme to alleviate intervertebral disc degeneration. Adv. Sci. (Weinh) 10, e2300988. doi:10.1002/advs.202300988
Silagi, E. S., Schoepflin, Z. R., Seifert, E. L., Merceron, C., Schipani, E., Shapiro, I. M., et al. (2018). Bicarbonate recycling by HIF-1-Dependent carbonic anhydrase isoforms 9 and 12 is critical in maintaining intracellular pH and viability of nucleus pulposus cells. J. Bone Min. Res. 33, 338–355. doi:10.1002/jbmr.3293
Silagi, E. S., Novais, E. J., Bisetto, S., Telonis, A. G., Snuggs, J., Le Maitre, C. L., et al. (2020). Lactate efflux from intervertebral disc cells is required for maintenance of spine health. J. Bone Min. Res. 35, 550–570. doi:10.1002/jbmr.3908
Silagi, E. S., Schipani, E., Shapiro, I. M., and Risbud, M. V. (2021). The role of HIF proteins in maintaining the metabolic health of the intervertebral disc. Nat. Rev. Rheumatol. 17, 426–439. doi:10.1038/s41584-021-00621-2
Silwal, P., Nguyen-Thai, A. M., Mohammad, H. A., Wang, Y., Robbins, P. D., Lee, J. Y., et al. (2023). Cellular senescence in intervertebral disc aging and degeneration: molecular mechanisms and potential therapeutic opportunities. Biomolecules 13, 686. doi:10.3390/biom13040686
Singh, M., Afonso, J., Sharma, D., Gupta, R., Kumar, V., Rani, R., et al. (2023). Targeting monocarboxylate transporters (MCTs) in cancer: how close are we to the clinics? Semin. Cancer Biol. 90, 1–14. doi:10.1016/j.semcancer.2023.01.007
Slomiany, M. G., Grass, G. D., Robertson, A. D., Yang, X. Y., Maria, B. L., Beeson, C., et al. (2009). Hyaluronan, CD44, and emmprin regulate lactate efflux and membrane localization of monocarboxylate transporters in human breast carcinoma cells. Cancer Res. 69, 1293–1301. doi:10.1158/0008-5472.Can-08-2491
Soeters, P. B., Shenkin, A., Sobotka, L., Soeters, M. R., De Leeuw, P. W., and Wolfe, R. R. (2021). The anabolic role of the warburg, Cori-cycle and crabtree effects in health and disease. Clin. Nutr. 40, 2988–2998. doi:10.1016/j.clnu.2021.02.012
Song, C., Liu, F., Wu, X., Zhou, D., Mei, Y., Wei, Z., et al. (2025). ASIC1a mediated nucleus pulposus cells pyroptosis and glycolytic crosstalk as a molecular basis for intervertebral disc degeneration. Inflamm. Res. 74, 29. doi:10.1007/s00011-025-02003-w
Soukane, D. M., Shirazi-Adl, A., and Urban, J. P. (2007). Computation of coupled diffusion of oxygen, glucose and lactic acid in an intervertebral disc. J. Biomech. 40, 2645–2654. doi:10.1016/j.jbiomech.2007.01.003
Spampinato, M., Giallongo, C., Giallongo, S., Spina, E., Duminuco, A., Longhitano, L., et al. (2025). Lactate accumulation promotes immunosuppression and fibrotic transformation of bone marrow microenvironment in myelofibrosis. J. Transl. Med. 23, 69. doi:10.1186/s12967-025-06083-4
Sun, Y., Chen, Y., and Peng, T. (2022). A bioorthogonal chemical reporter for the detection and identification of protein lactylation. Chem. Sci. 13, 6019–6027. doi:10.1039/d2sc00918h
Sun, S., Xu, Z., He, L., Shen, Y., Yan, Y., Lv, X., et al. (2024). Metabolic regulation of cytoskeleton functions by HDAC6-catalyzed α-tubulin lactylation. Nat. Commun. 15, 8377. doi:10.1038/s41467-024-52729-0
Sun, K., Shi, Y., Yan, C., Wang, S., Han, L., Li, F., et al. (2025). Glycolysis-derived lactate induces ACSL4 expression and lactylation to activate ferroptosis during intervertebral disc degeneration. Adv. Sci. (Weinh) 12, e2416149. doi:10.1002/advs.202416149
Titov, D. V., Cracan, V., Goodman, R. P., Peng, J., Grabarek, Z., and Mootha, V. K. (2016). Complementation of mitochondrial electron transport chain by manipulation of the NAD+/NADH ratio. Science 352, 231–235. doi:10.1126/science.aad4017
Tong, H., Jiang, Z., Song, L., Tan, K., Yin, X., He, C., et al. (2024). Dual impacts of serine/glycine-free diet in enhancing antitumor immunity and promoting evasion via PD-L1 lactylation. Cell Metab. 36, 2493–510.e9. doi:10.1016/j.cmet.2024.10.019
Tonomura, H., Nagae, M., Takatori, R., Ishibashi, H., Itsuji, T., and Takahashi, K. (2020). The potential role of hepatocyte growth factor in degenerative disorders of the synovial joint and spine. Int. J. Mol. Sci. 21, 8717. doi:10.3390/ijms21228717
Urban, J. P. (2002). The role of the physicochemical environment in determining disc cell behaviour. Biochem. Soc. Trans. 30, 858–864. doi:10.1042/bst0300858
Urban, J. P., and Mcmullin, J. F. (1988). Swelling pressure of the lumbar intervertebral discs: influence of age, spinal level, composition, and degeneration. Spine (Phila Pa 1976) 13, 179–187. doi:10.1097/00007632-198802000-00009
Vander Heiden, M. G., Cantley, L. C., and Thompson, C. B. (2009). Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324, 1029–1033. doi:10.1126/science.1160809
Vera, M. J., Ponce, I., Almarza, C., Ramirez, G., Guajardo, F., Dubois-Camacho, K., et al. (2024). CCL2 and lactate from chemotherapeutics-treated fibroblasts drive malignant traits by metabolic rewiring in low-migrating breast cancer cell lines. Antioxidants (Basel) 13, 801. doi:10.3390/antiox13070801
Wan, M., Liu, Z., Li, T., Chen, H., Wang, Q., Chen, T., et al. (2021). Zwitterion-Based hydrogen sulfide nanomotors induce multiple acidosis in tumor cells by destroying tumor metabolic symbiosis. Angew. Chem. Int. Ed. Engl. 60, 16139–16148. doi:10.1002/anie.202104304
Wan, N., Wang, N., Yu, S., Zhang, H., Tang, S., Wang, D., et al. (2022). Cyclic immonium ion of lactyllysine reveals widespread lactylation in the human proteome. Nat. Methods 19, 854–864. doi:10.1038/s41592-022-01523-1
Wang, Y., and Dang, C. V. (2024). The warburg effect revisited through blood and electron flow. Cancer Res. 84, 2046–2048. doi:10.1158/0008-5472.Can-24-0474
Wang, D., Hartman, R., Han, C., Zhou, C. M., Couch, B., Malkamaki, M., et al. (2021a). Lactate oxidative phosphorylation by annulus fibrosus cells: evidence for lactate-dependent metabolic symbiosis in intervertebral discs. Arthritis Res. Ther. 23, 145. doi:10.1186/s13075-021-02501-2
Wang, N., Jiang, X., Zhang, S., Zhu, A., Yuan, Y., Xu, H., et al. (2021b). Structural basis of human monocarboxylate transporter 1 inhibition by anti-cancer drug candidates. Cell 184, 370–83.e13. doi:10.1016/j.cell.2020.11.043
Wang, C. Y., Hsieh, M. K., Hu, Y. J., Bit, A., and Lai, P. L. (2022a). Monocarboxylate transporter 1-mediated lactate accumulation promotes nucleus pulposus degeneration under hypoxia in a 3D multilayered nucleus pulposus degeneration model. Eur. Cell Mater 43, 53–65. doi:10.22203/eCM.v043a06
Wang, Y., Stancliffe, E., Fowle-Grider, R., Wang, R., Wang, C., Schwaiger-Haber, M., et al. (2022b). Saturation of the mitochondrial NADH shuttles drives aerobic glycolysis in proliferating cells. Mol. Cell 82, 3270–83.e9. doi:10.1016/j.molcel.2022.07.007
Wang, Q., Duan, L., Li, X., Wang, Y., Guo, W., Guan, F., et al. (2022c). Glucose metabolism, neural cell senescence and alzheimer's disease. Int. J. Mol. Sci. 23, 4351. doi:10.3390/ijms23084351
Wang, B., Chen, D., Jiang, R., Ntim, M., Lu, J., Xia, M., et al. (2022d). TIP60 buffers acute stress response and depressive behaviour by controlling PPARγ-mediated transcription. Brain Behav. Immun. 101, 410–422. doi:10.1016/j.bbi.2022.01.022
Wang, T., Ye, Z., Li, Z., Jing, D. S., Fan, G. X., Liu, M. Q., et al. (2023). Lactate-induced protein lactylation: a bridge between epigenetics and metabolic reprogramming in cancer. Cell Prolif. 56, e13478. doi:10.1111/cpr.13478
Wang, N., Rong, W., Xie, Y., Chen, S., Xi, Z., and Deng, R. (2024). Visualizing the bibliometrics of the inflammatory mechanisms in intervertebral disc degeneration. Exp. Gerontol. 188, 112380. doi:10.1016/j.exger.2024.112380
Wang, M., Mu, G., Qiu, B., Wang, S., Tao, C., Mao, Y., et al. (2025). Competitive antagonism of KAT7 crotonylation against acetylation affects procentriole formation and colorectal tumorigenesis. Nat. Commun. 16, 2379. doi:10.1038/s41467-025-57546-7
Wei, Q., Zhang, X., Zhou, C., Ren, Q., and Zhang, Y. (2019). Roles of large aggregating proteoglycans in human intervertebral disc degeneration. Connect. Tissue Res. 60, 209–218. doi:10.1080/03008207.2018.1499731
Wu, W., Zhang, X., Hu, X., Wang, X., Sun, L., Zheng, X., et al. (2014). Lactate down-regulates matrix systhesis and promotes apoptosis and autophagy in rat nucleus pulposus cells. J. Orthop. Res. 32, 253–261. doi:10.1002/jor.22503
Wu, A., March, L., Zheng, X., Huang, J., Wang, X., Zhao, J., et al. (2020). Global low back pain prevalence and years lived with disability from 1990 to 2017: estimates from the global burden of disease study 2017. Ann. Transl. Med. 8, 299. doi:10.21037/atm.2020.02.175
Wu, L., Shen, J., Zhang, X., and Hu, Z. (2021). LDHA-mediated glycolytic metabolism in Nucleus pulposus cells is a potential therapeutic target for intervertebral disc degeneration. Biomed. Res. Int. 2021, 9914417. doi:10.1155/2021/9914417
Wu, C. J., Yuan, D. Y., Liu, Z. Z., Xu, X., Wei, L., Cai, X. W., et al. (2023). Conserved and plant-specific histone acetyltransferase complexes cooperate to regulate gene transcription and plant development. Nat. Plants 9, 442–459. doi:10.1038/s41477-023-01359-3
Xie, B., Zhang, M., Li, J., Cui, J., Zhang, P., Liu, F., et al. (2024). KAT8-catalyzed lactylation promotes eEF1A2-mediated protein synthesis and colorectal carcinogenesis. Proc. Natl. Acad. Sci. U. S. A. 121, e2314128121. doi:10.1073/pnas.2314128121
Xin, J., Wang, Y., Zheng, Z., Wang, S., Na, S., and Zhang, S. (2022). Treatment of intervertebral disc degeneration. Orthop. Surg. 14, 1271–1280. doi:10.1111/os.13254
Xu, Z., Zhang, H., Zhang, X., Jiang, H., Liu, C., Wu, F., et al. (2019). Interplay between the bacterial protein deacetylase CobB and the second messenger c-di-GMP. Embo J. 38, e100948. doi:10.15252/embj.2018100948
Xu, H., Wei, K., Ni, J., Deng, X., Wang, Y., Xiang, T., et al. (2025). Matrix stiffness regulates nucleus pulposus cell glycolysis by MRTF-A-dependent mechanotransduction. Bone Res. 13, 23. doi:10.1038/s41413-025-00402-7
Yang, S., Zhang, F., Ma, J., and Ding, W. (2020). Intervertebral disc ageing and degeneration: the antiapoptotic effect of oestrogen. Ageing Res. Rev. 57, 100978. doi:10.1016/j.arr.2019.100978
Yin, J., Forn-Cuní, G., Surendran, A. M., Lopes-Bastos, B., Pouliopoulou, N., Jager, M. J., et al. (2024). Lactate secreted by glycolytic conjunctival melanoma cells attracts and polarizes macrophages to drive angiogenesis in zebrafish xenografts. Angiogenesis 27, 703–717. doi:10.1007/s10456-024-09930-y
Ying, W. (2008). NAD+/NADH and NADP+/NADPH in cellular functions and cell death: regulation and biological consequences. Antioxid. Redox Signal 10, 179–206. doi:10.1089/ars.2007.1672
Zhang, D., Tang, Z., Huang, H., Zhou, G., Cui, C., Weng, Y., et al. (2019). Metabolic regulation of gene expression by histone lactylation. Nature 574, 575–580. doi:10.1038/s41586-019-1678-1
Zhang, Y., Liu, L., Qi, Y., Lou, J., Chen, Y., Liu, C., et al. (2024a). Lactic acid promotes nucleus pulposus cell senescence and corresponding intervertebral disc degeneration via interacting with Akt. Cell Mol. Life Sci. 81, 24. doi:10.1007/s00018-023-05094-y
Zhang, Y., Huang, Z., Han, W., Wu, J., Li, S., Qin, T., et al. (2024b). Glutamine suppresses senescence and promotes autophagy through glycolysis inhibition-mediated AMPKα lactylation in intervertebral disc degeneration. Commun. Biol. 7, 325. doi:10.1038/s42003-024-06000-3
Zhao, K., An, R., Xiang, Q., Li, G., Wang, K., Song, Y., et al. (2021). Acid-sensing ion channels regulate nucleus pulposus cell inflammation and pyroptosis via the NLRP3 inflammasome in intervertebral disc degeneration. Cell Prolif. 54, e12941. doi:10.1111/cpr.12941
Zhao, G., Zhen, J., Liu, X., Guo, J., Li, D., Xie, J., et al. (2023). Protein post-translational modification by lysine succinylation: biochemistry, biological implications, and therapeutic opportunities. Genes Dis. 10, 1242–1262. doi:10.1016/j.gendis.2022.03.009
Zhou, R. P., Wu, X. S., Wang, Z. S., Xie, Y. Y., Ge, J. F., and Chen, F. H. (2016). Novel insights into acid-sensing ion channels: implications for degenerative diseases. Aging Dis. 7, 491–501. doi:10.14336/ad.2015.1213
Zhou, D., Mei, Y., Song, C., Cheng, K., Cai, W., Guo, D., et al. (2024). Exploration of the mode of death and potential death mechanisms of nucleus pulposus cells. Eur. J. Clin. Invest 54, e14226. doi:10.1111/eci.14226
Zhu, Y., Ji, J. J., Yang, R., Han, X. Q., Sun, X. J., Ma, W. Q., et al. (2019). Lactate accelerates calcification in VSMCs through suppression of BNIP3-mediated mitophagy. Cell Signal 58, 53–64. doi:10.1016/j.cellsig.2019.03.006
Zhu, J., Wang, R., Yang, C., Shao, X., Zhang, Y., Hou, J., et al. (2024). Blocking tumor-platelet crosstalk to prevent tumor metastasis via reprograming glycolysis using biomimetic membrane-hybridized liposomes. J. Control Release 366, 328–341. doi:10.1016/j.jconrel.2023.12.052
Zhu, R., Ye, X., Lu, X., Xiao, L., Yuan, M., Zhao, H., et al. (2025). ACSS2 acts as a lactyl-CoA synthetase and couples KAT2A to function as a lactyltransferase for histone lactylation and tumor immune evasion. Cell Metab. 37, 361–76.e7. doi:10.1016/j.cmet.2024.10.015
Zong, Z., Xie, F., Wang, S., Wu, X., Zhang, Z., Yang, B., et al. (2024). Alanyl-tRNA synthetase, AARS1, is a lactate sensor and lactyltransferase that lactylates p53 and contributes to tumorigenesis. Cell 187, 2375–92.e33. doi:10.1016/j.cell.2024.04.002
Zou, Y., Cao, M., Tai, M., Zhou, H., Tao, L., Wu, S., et al. (2025). A feedback loop driven by H4K12 lactylation and HDAC3 in macrophages regulates lactate-induced collagen synthesis in fibroblasts via the TGF-β signaling. Adv. Sci. (Weinh) 12, e2411408. doi:10.1002/advs.202411408
Keywords: intervertebral disc degeneration, lactate, lactylation, metabolism, functions, intervention strategies
Citation: Liu Y, Yang J, Wang Z, Fu J, Liu H, Lu Z, Dong J, Li Z, Mao Q, Li C and Ma H (2025) Lactate and lactylation in intervertebral disc degeneration. Front. Mol. Biosci. 12:1685975. doi: 10.3389/fmolb.2025.1685975
Received: 16 August 2025; Accepted: 20 October 2025;
Published: 18 November 2025.
Edited by:
Francois-Pierre Martin, H&H Group, SwitzerlandReviewed by:
Sun Jung Kim, Northwell Health, United StatesSerena Castelli, San Raffaele Telematic University, Italy
Copyright © 2025 Liu, Yang, Wang, Fu, Liu, Lu, Dong, Li, Mao, Li and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hui Ma, c2g5X3NwaW5lQDE2My5jb20=
†These authors have contributed equally to this work
 Yadong Liu1,2†
Yadong Liu1,2† Jing Fu
Jing Fu Zhong Li
Zhong Li Hui Ma
Hui Ma