- 1Center for Clinical Research on Neurological Diseases, The First Affiliated Hospital, Dalian Medical University, Dalian, China
- 2Liaoning Provincial Key Laboratory for Research on the Pathogenic Mechanisms of Neurological Diseases, The First Affiliated Hospital, Dalian Medical University, Dalian, China
- 3Institute for Systems Genetics, West China Hospital, Sichuan University, Chengdu, China
Nuclear receptor related 1 protein (NURR1), a transcription factor as key player for maintaining dopamine neuron functions and regulating neuroinflammation in the central nerves system, is a potential susceptibility gene for Parkinson’s disease (PD). To ascertain whether the expression levels of NURR1 gene and inflammatory cytokines are altered in patients with PD, we measured their mRNA levels in the peripheral blood mononuclear cells (PBMCs) in 312 PD patients, 318 healthy controls (HC), and 332 non-PD neurological disease controls (NDCs) by quantitative real-time PCR. Our data showed that NURR1 gene expression was significantly decreased in the PBMCs of PD as compared with that of HC and NDC (p < 0.01). Since NURR1 was reported to have regulating effects on neuroinflammation, we assessed the expression levels of cytokines (TNF-α, IL-1β, IL-4, IL-6, and IL-10) in the PBMCs of PD and controls (HC and NDC). Our results showed that the expression levels of those cytokines were significantly higher than those of controls. Statistical analysis revealed that NURR1 expression presented a negative correlation with the expression of TNF-α, IL-1β, IL-6, and IL-10, and collectively the measurements of NURR1 plus those cytokines significantly improve the diagnostic accuracy. All these findings suggested that NURR1 is likely to be involved in the process of PD by mediating the neuroinflammation, and the combination of NURR1 and cytokines assessment in the PBMCs can be potential biomarkers for PD diagnosis.
Introduction
Parkinson’s disease (PD) is a chronic and progressive neurodegenerative disorder occurring as a result of the loss of dopamine (DA) neurons within the substantia nigra (SN) (Dickson, 2012). As the second most common neurodegenerative disorder, PD affects 1% of people older than age 60, and 3% at the age 80 years or older (Erkkinen et al., 2018). Although many important discoveries have been made during the 200 years of PD research history (Li and Le, 2017), PD diagnosis is still mainly based on the identification of motor symptoms whereas pathologicalchanges and non-motor manifestations emerge years prior to motor symptoms, indicating that earlier diagnosis and treatments are necessary (Van Laar and Jain, 2004). Currently, an increasing number of studies focus on the peripheral biomarkers utilizing biofluids, which may reflect the disease related molecular changes in the brain. Commonly peripheral blood mononuclear cells (PBMCs) have been used to measure specific alteration in DA components, enzyme activities, DA receptors, and transporters in PD (Caronti et al., 2001; Pellicano et al., 2007; Buttarelli et al., 2011), gene expression profile in PBMCs was widely investigated to identify potential biomarkers of PD.
Causal genes for Mendelian-inherited PD have been reported, including SNCA, PRKN, UCH-L1, PINK1, DJ-1, LRRK2, GBA, and VPS35 (Mizuta et al., 2006; Martin et al., 2011; Williams-Gray and Worth, 2016). Among the potential susceptibility genes which may act as molecular biomarkers of PD, we were particular interested in NURR1, a transcription factor belonging to the nuclear receptor 4 family (Wang et al., 2003; Huang et al., 2004). NURR1 is not only highly expressed in midbrain DA neurons (Castillo et al., 1998; Sakurada et al., 1999), but also in other tissues, such as PBMCs (Mages et al., 1994; Jankovic et al., 2005). NURR1 is known to play a crucial role in the development and differentiation of midbrain DA neurons (Jankovic et al., 2005; Decressac et al., 2013; Dong et al., 2016). Our previously study has shown that Nurr1-null mice have selective agenesis of DA neurons in the SN and ventral tegmental area (Le et al., 1999). Several studies also suggested that dysfunction in NURR1 gene may play a role in PD (Le et al., 2003; Chu et al., 2006). Our earlier study has documented that NURR1 gene expression is significantly decreased in the PBMCs of PD as compared with healthy control (HC) and neurological disease control (NDC) (Le et al., 2008; Liu et al., 2012). Based on those reports, it is believed that alteration in NURR1 could be a potential molecular biomarker of PD.
NURR1 has also been considered to be a part of anti-inflammatory pathway in microglia, which protects DA neurons against inflammation-induced death (Saijo et al., 2009; Decressac et al., 2013). Mounting evidence supports the role of inflammation as a measurable driving force of PD pathology (Deleidi and Gasser, 2013). Neuroinflammation is associated with activated microglia and altered levels of inflammatory mediators in the brain of PD (Heneka et al., 2014). Many researches have revealed that significantly higher levels of inflammatory cytokines, such as tumor necrosis factor (TNF)-α, interleukin (IL)-1β, IL-4, IL-6 are found in the brain and cerebrospinal fluid (CSF) of PD (Mogi et al., 1996; Nagatsu and Sawada, 2005; Sawada et al., 2006). The pathological effects in the brain may have an implication in the peripheral blood, therefore, detecting the levels of inflammatory cytokines in the peripheral blood may be able to evaluate the inflammatory status of PD (Chen et al., 2008; Reale et al., 2009).
In the present study, we recruited 312 patients with diagnosed PD, 318 HC and 332 non-PD NDC, and measured the levels of NURR1 and inflammatory cytokines (TNF-α, IL-1β, IL-4, IL-6, and IL-10) mRNA in their PBMCs. The purpose of this study was to determine whether the expression levels of NURR1 gene and inflammatory cytokines in their PBMCs were specifically altered in PD as compared with HC and NDC in a relatively larger number of Chinese population, and to evaluate the relationship between NURR1 and cytokines expression levels in the PBMCs, which may provide further evidence that NURR1 is involved in the process of PD by mediating the neuroinflammation pathway.
Materials and Methods
Participants and Blood Sampling
In this study, we collected a total of 962 PBMCs samples: 312 from patients with sporadic PD, 318 from HC, and 332 from patients with various NDC (Table 1). Among 312 PD patients, 82 were of recent-onset without any anti-PD treatment, the other 230 patients were treated with anti-PD medications. NDC consisted of 48 cerebrovascular diseases, 42 Alzheimer disease (AD), 40 epilepsy, 36 peripheral neuropathy, 31 migraine, 24 myasthenia gravis, 22 essential tremor, 21 parkinsonism (including 11 vascular parkinsonism and 10 multiple system atrophy), 21 anxiety/sleep disorders, 17 restless legs syndrome, 12 motor neuron disease, 11 multiple sclerosis, 4 myelopathy, and 3 chorea minor. PD patients were examined and diagnosed by at least two experienced neurologists from the First Affiliated Hospital of Dalian Medical University according to the Movement Disorder Society Clinical Diagnostic Criteria for Parkinson’s disease (Postuma et al., 2015). PD disease severity was assessed by Modified Hoehn – Yahr (H-Y) staging (Goetz et al., 2004). PD patients were excluded if they had any other major neurological, ongoing infectious/autoimmune, or serious metabolic disorders. HC subjects were recruited from the Health Examination Center of the First Affiliated Hospital of Dalian Medical University, showing they did not have any obvious neurological disorders or non-neurological disorders. All subjects (or their caregivers) recruited to our studies provided a written informed consent agreeing to participate the project. This study has been granted ethical approval by the Ethics Committee of the First Affiliated Hospital of Dalian Medical University (approval number: LCKY2014-29).
Peripheral blood samples were collected by direct venipuncture at the First Affiliated Hospital of Dalian Medical University. Peripheral blood (2 ml) was drawn from cubital vein into ethylene diamine tetra-acetic acid (EDTA) containing blood collection tubes. PBMCs were separated from Human Peripheral Lymphocyte Separation Medium (Haoyang, Tianjin, China) by centrifugation at 450g for 20 min at room temperature (20 ± 2°C) no later than 4 h and were stored at −80°C immediately until RNA preparation.
PBMCs mRNA Extraction and Quantification
Total RNAs from PBMCs were extracted using the mirVana miRNA Isolation Kit (Ambion, Carlsbad, CA, United States). The mRNA levels of NURR1, TNF-α, IL-1β, IL-4, IL-6, and IL-10 in PBMCs were also measured by quantitative real-time PCR. PCR was carried out using ABI 7500 fast real-time PCR system (Applied Biosystems, Foster City, CA, United States) in a total volume of 20 μl for each reaction. GAPDH gene was used as an internal control. The specific primers targeting PBMCs NURR1, TNF-α, IL-1β, IL-4, IL-6, and IL-10 were presented in Table 2. After 94°C for 30 s, the experimental reaction consisted of 40 cycles of 94°C for 5 s and 60°C for 34 s, the target genes as well as GAPDH gene was detected by the fluorescent dye SYBR Green I (TransGen, Beijing, China). The value of threshold cycle (Ct) was generated at every cycle during a run. Fluorescent reading from real-time PCR reaction was quantitatively analyzed by determining the difference of Ct (delta Ct) between Ct of the target genes and GAPDH, and the target genes expression were determined using the 2−dCt method.
Statistical Analysis
Quantitative data were expressed as mean ± standard error of mean (SEM). The dichotomous variables were compared by using chi-square test and continuous variables were compared with independent t-test. A one-way ANOVA followed by a Tukey-Kramer test as a post hoc analysis was performed using the GraphPad Prism software version 7 (GraphPad Inc., San Diego, CA, United States) to evaluate the differences in the mean value of the relative NURR1 and cytokines expression. Correlations were evaluated using Spearman’s correlation coefficient (R). The correlations were reported at an α level of 0.05. Receiver operating characteristic (ROC) curves and areas under the curves (AUC) were used to evaluate the prediction performance of the potential biomarkers. The other statistical analysis in this research was performed with the SPSS software version 13.0 (SPSS Inc., Chicago, IL, United States). All statistical checks were carried out two-sided and a p-value <0.05 was considered as statistical significance.
Results
Characteristics of Study Population
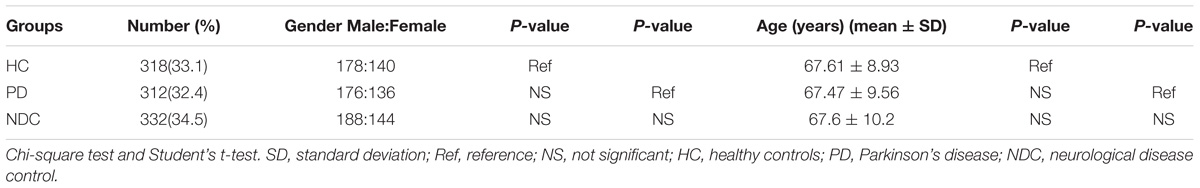
All subjects we collected were ethnic Chinese. The demographic characteristics of PD patients and control subjects were summarized in Table 1. No significant difference in both gender and age was found among patients with PD, HC, and NDC.
NURR1 Gene Expression in PBMCs of All Three Groups
We determined the NURR1 mRNA level in the PBMCs of all three groups by quantitative real-time PCR technique. We found that the NURR1 mRNA level in the PBMCs of patients with PD was significantly lower than that of HC (decreased by 61%, p < 0.01), and NDC (decreased by 54%, p < 0.01). There was no difference of NURR1 expression between HC and NDC groups (Figure 1). In the individual groups of NDC, some changes were found in the expression levels of NURR1 as compared with PD, but no statistical differences were reached (Supplementary Table S1).
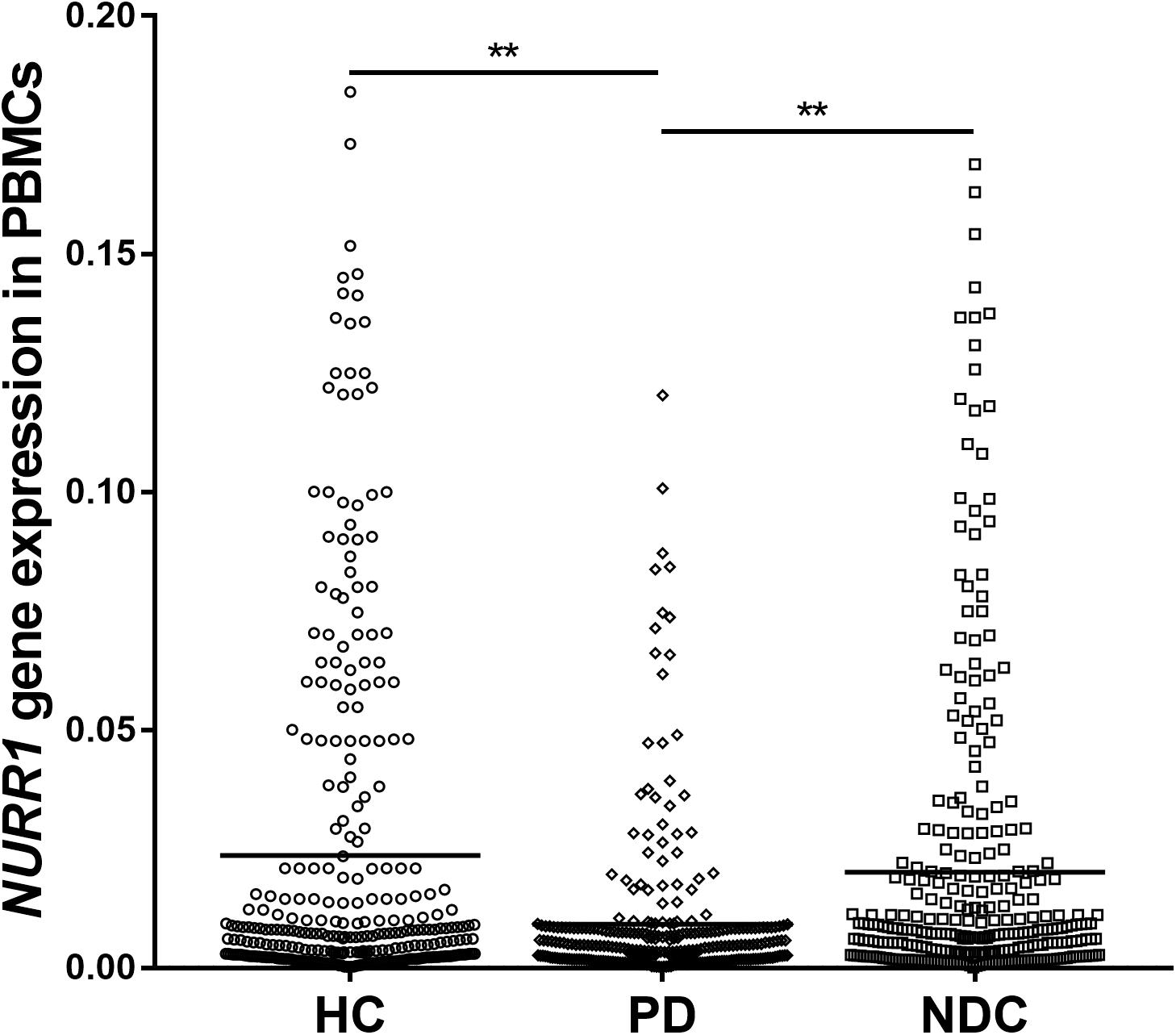
FIGURE 1. Scatter plots of NURR1 gene relative mRNA expression level in the PBMCs of HC (n = 318), PD (n = 312), and NDC (n = 332), which was determined using real-time PCR assays. Horizontal bars represent mean value. The NURR1 gene mRNA level in the PBMCs was markedly low in patients with PD (mean ± SEM, 0.009 ± 0.0009) as compared with HC (mean ± SEM, 0.023 ± 0.0021) and various NDC (mean ± SEM, 0.019 ± 0.002). ∗∗p < 0.01.
The Impacts of Disease Duration, Severity, and Medications on NURR1 Expression in PD
We analyzed disease duration (years after onset of disease symptoms) and severity (H-Y scores) in 312 patients with PD. We divided disease duration into four stages: 1–2 years (n = 82), 3–5 years (n = 83), 6–10 years (n = 105), and 10–20 years (n = 42) and demonstrated that the level of NURR1 expression was slightly down-regulated during the disease progression, but no significant statistical difference (Figure 2A). The disease severity of PD was divided into five stages: H-Y 1–1.5 (n = 59), H-Y 2 (n = 89), H-Y 2.5 (n = 93), H-Y 3 (n = 55) and H-Y 4–5 (n = 16). Again, a slightly down-regulation of NURR1 expression with higher H-Y scores was found, there was no significant statistical difference (Figure 2B).
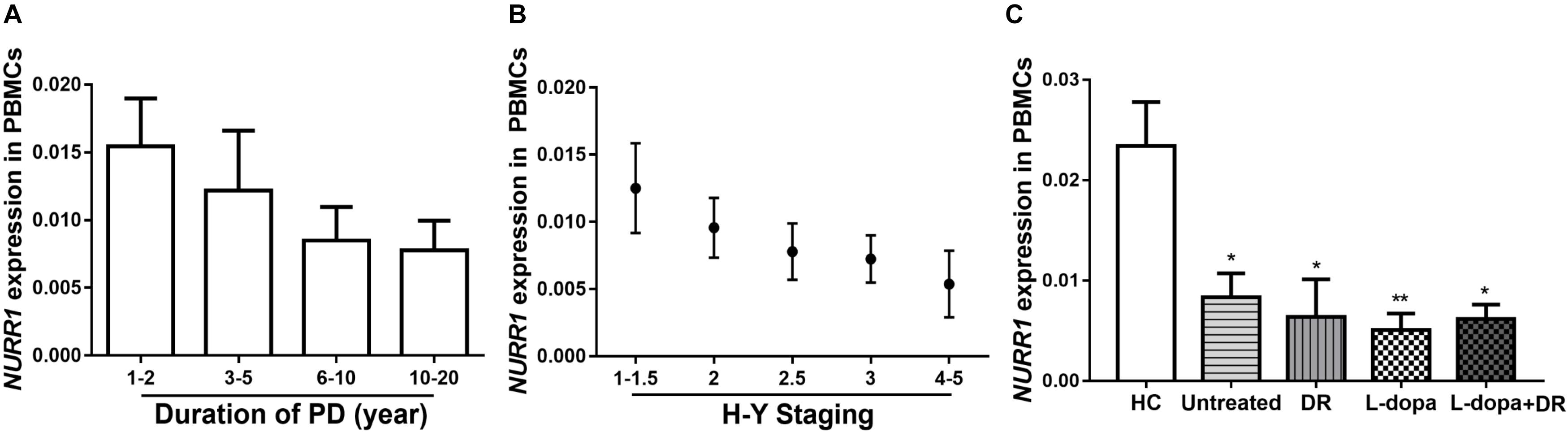
FIGURE 2. The expression level of NURR1 in PD and HC. The effects of (A) disease course, (B) disease severity, and (C) medication on the expression level of NURR1 in PBMCs. The results are the mean ± SEM values. ∗p < 0.05 and ∗∗p < 0.01 relative to HC.
We also divided the PD patients into four groups according to the use of medications. Among the 312 PD patients, 82 were untreated with any anti-PD medications, the remaining patients were treated with anti-PD medications, including 89 were treated with l-dopa monotherapy, 28 treated with DA receptor agonists (DR) monotherapy, and other 113 patients were treated with the combination of DA agonists and l-dopa. All these four groups were significantly lower expression level of NURR1 than HC, but there was no significant difference among the four groups (Figure 2C).
TNF-α, IL-1β, IL-4, IL-6, and IL-10 Expressions in the PBMCs of All Recruited Subjects
Since NURR1 plays important role in regulating neuroinflammation, we then measured mRNA levels of several cytokines in their PBMCs. Our data showed significantly higher levels of TNF-α (p < 0.001), IL-1β (p < 0.001), IL-4 (p < 0.01), IL-6 (p < 0.05) and IL-10 (p < 0.05) in PD than those in HC. Moreover, the levels of TNF-α (p < 0.05), IL-1β (p < 0.01), IL-4 (p < 0.05), IL-6 (p < 0.05), and IL-10 (p < 0.05) expression were also markedly higher in PD than NDC (Figure 3). In the NDC group, there were slight to moderate differences among different diseases regarding the five cytokines expression levels, but no statistical differences were reached (Supplementary Table S2).
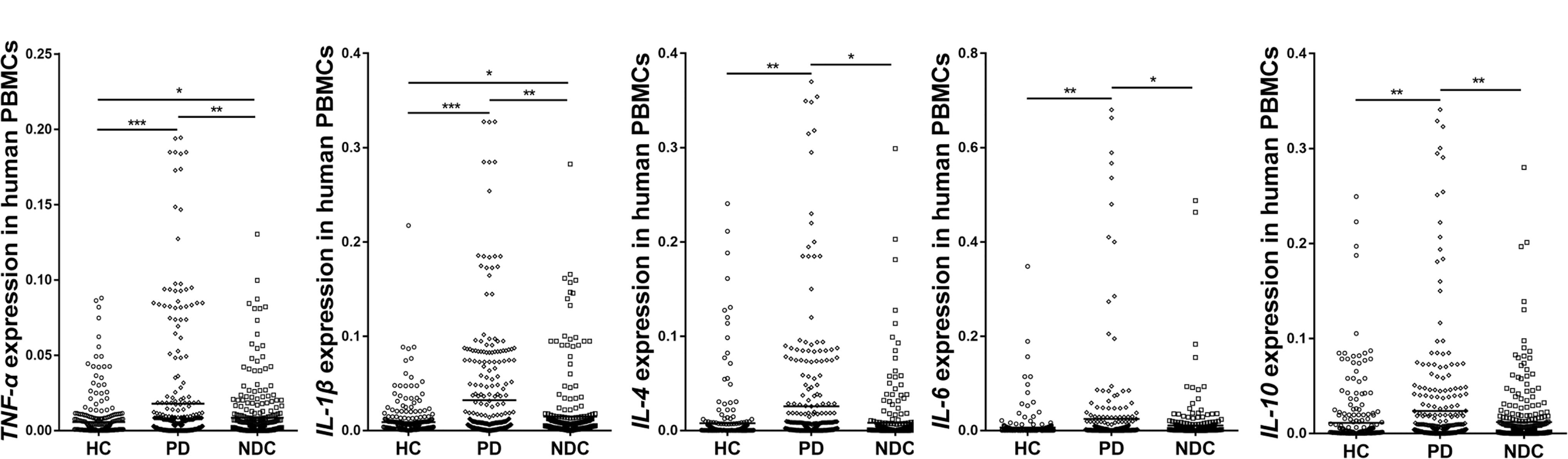
FIGURE 3. Scatter plots of TNF-α, IL-1β, IL-4, IL-6, and IL-10 relative mRNA expression levels in the PBMCs of HC (n = 318), PD (n = 312), and NDC (n = 332). Significantly higher levels of TNF-α (p < 0.001), IL-1β (p < 0.001), IL-4 (p < 0.01), IL-6 (p < 0.05), and IL-10 (p < 0.01) were seen in PD patients than those in HC. The levels of TNF-α (p < 0.01), IL-1β (p < 0.01), IL-4 (p < 0.05), IL-6 (p < 0.05), and IL-10 (p < 0.01) expression were also markedly higher in PD than NDC. Horizontal bars represent mean value. ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001.
We further analyzed the expression levels of cytokines in different disease duration and severity of PD, no significant differences were found among different groups (Supplementary Figures S1, S2).
The Influence of Medications on Cytokines Expression
Generally, the influence of anti-PD medications on PBMCs cytokines expression was minimal. Our results showed that the levels of TNF-α, IL-1β, and IL-6 expression in PD patients with anti-PD medications were slightly lower than that of untreated patients, while the levels of IL-4 and IL-10 expression in PD with all types of anti-PD medications were slightly higher than that of untreated PD. However, there was no statistical difference between them (Figure 4).
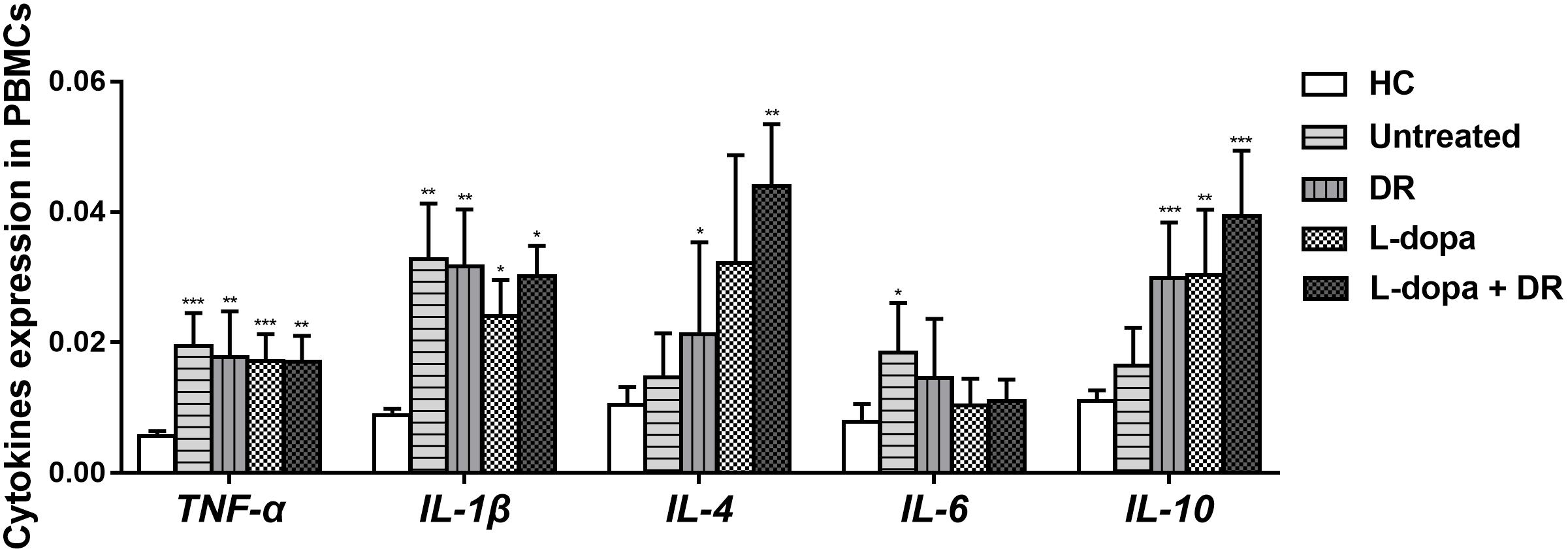
FIGURE 4. The levels of TNF-α, IL-1β, IL-4, IL-6, and IL-10 expression in PD patients with different anti-PD medications. The results are the mean ± SEM values. ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001 relative to HC. DR: DA receptor agonists monotherapy; L-dopa: l-dopa monotherapy; L-dopa+DR: combination of DA agonists and l-dopa.
Correlations Between the Expression Levels of NURR1, TNF-α, IL-1β, IL-4, IL-6, and IL-10
We performed a correlation analysis between expressions of NURR1 and TNF-α, IL-1β, IL-4, IL-6, and IL-10. Our results showed that the level of NURR1 presented a negative correlation with TNF-α (r = −0.232, p < 0.01), IL-1β (r = −0.101, p < 0.05), IL-6 (r = −0.123, p < 0.05) and IL-10 (r = −0.129, p < 0.05). Moreover, positive correlations were also found among the expression levels of TNF-α, IL-1β, IL-4, IL-6, and IL-10 (Table 3).
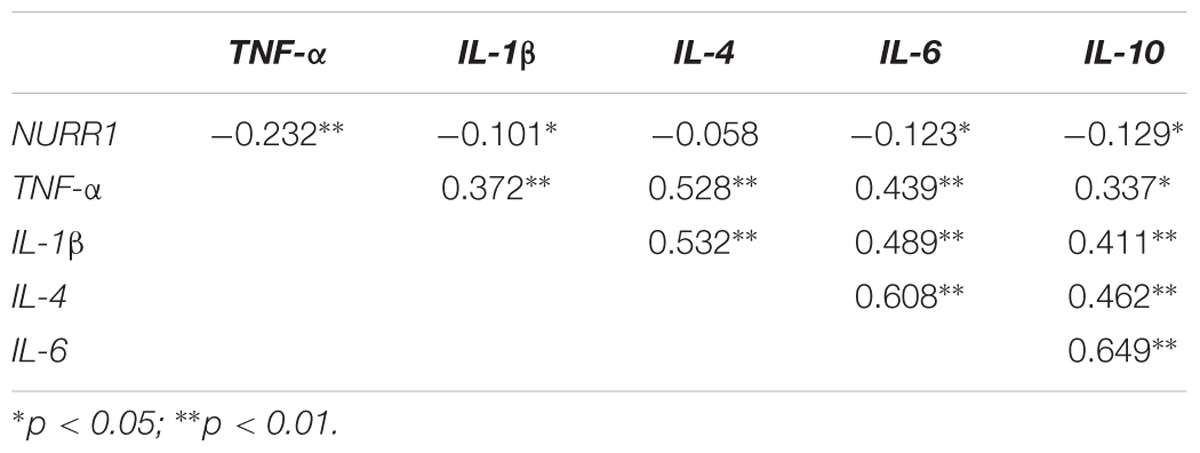
TABLE 3. Spearman correlation coefficient (R) between the expression levels of NURR1, TNF-α, IL-1β, IL-4, IL-6, and IL-10 in the PBMCs of PD.
Performance of Combined Expression Levels of NURR1, TNF-α, IL-1β, IL-4, IL-6, and IL-10 for PD Diagnosis
We evaluated the performance of combined expression of PBMCs NURR1, TNF-α, IL-1β, IL-4, IL-6, and IL-10 for the PD diagnosis by the AUC values based on the ROC curve analysis. The AUCs of NURR1, TNF-α, IL-1β, IL-4, IL-6, and IL-10 were 0.64 (95% CI, 0.6–0.69), 0.62 (95% CI, 0.58–0.67), 0.65 (95% CI, 0.61–0.7), 0.65 (95% CI, 0.61–0.7), 0.6 (95% CI, 0.55–0.65), 0.64 (95% CI, 0.59–0.68), respectively. The combination of PBMCs NURR1 with these cytokines significantly enhanced the discriminatory accuracy between PD and HC, with an increased AUC as 0.73 (95% CI, 0.69–0.77) (p < 0.05, Figure 5).
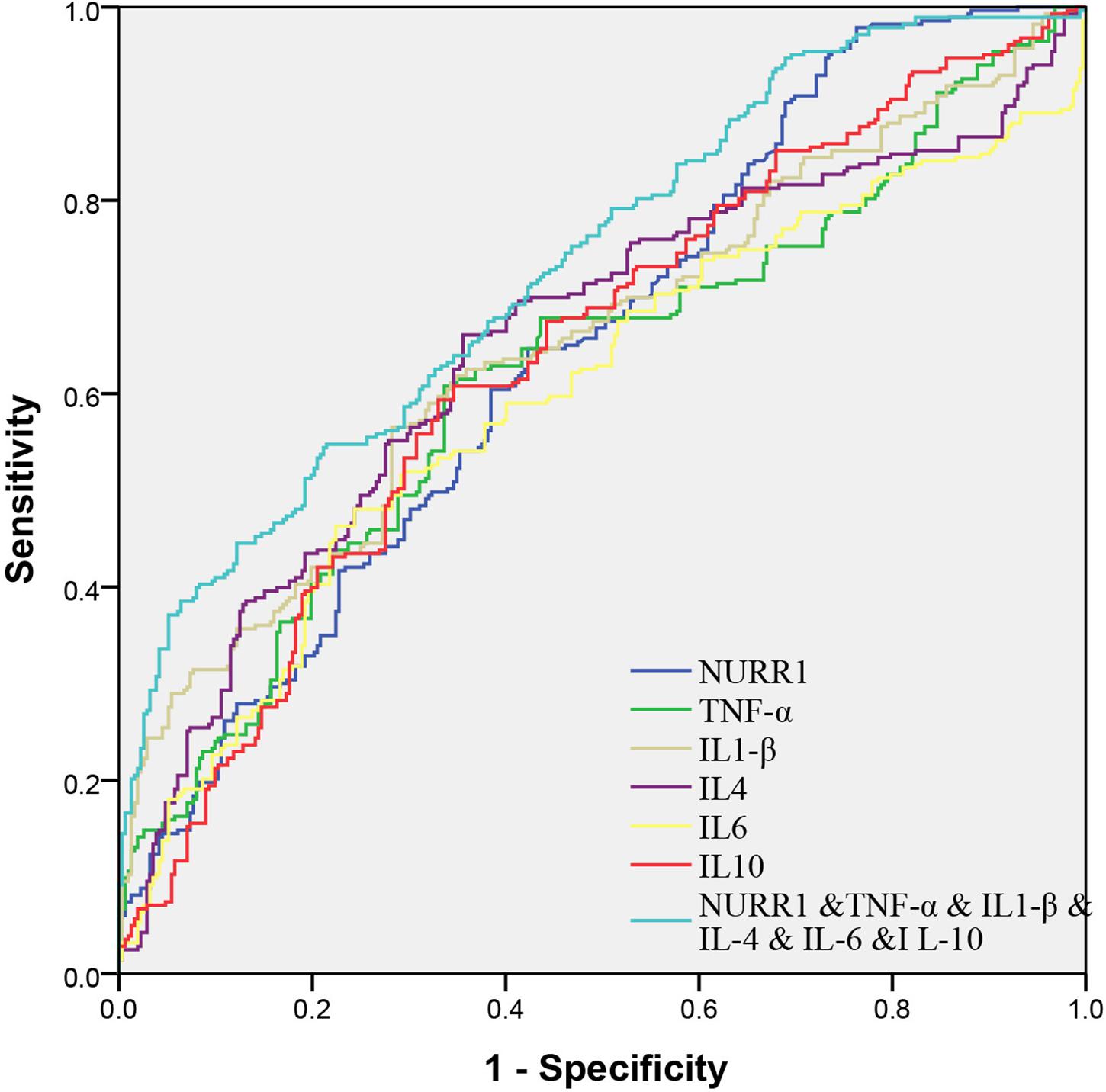
FIGURE 5. Receiver operating characteristic curves for NURR1, TNF-α, IL-1β, IL-4, IL-6, and IL-10 of PD versus HC. The AUCs value of the combination of NURR1 with cytokines was 0.73 (95% CI, 0.69–0.77; p < 0.05), which was out performed those of NURR1, TNF-α, IL-1β, IL-4, IL-6, and IL-10 alone.
Discussion
In this study, we measured the levels of NURR1 and inflammatory cytokines (TNF-α, IL-1β, IL-4, IL-6, and IL-10) mRNA in the PBMCs of a relatively larger number of Chinese population. In order to determine the alterations of PBMCs NURR1 and cytokines in PD are specific, we particularly recruited 332 various NDC and compared the expression levels of NURR1 and inflammatory cytokines in PD with not only HC but also NDC. We carried out the assays of five key inflammatory cytokines simultaneously in the PBMCs to assess the complex inflammatory changes in PD. Although there were numerous reports of cytokine changes in PD, a comprehensive analysis of NURR1 and cytokines expression changes has, to the best of our knowledge, not been performed. We demonstrated for the first time that the level of NURR1 presented a negative correlation with cytokines in the PBMCs of PD, and the combination of PBMCs NURR1 and cytokines assessment may improve the diagnostic performance of PD.
Our study confirmed that the expression level of NURR1 in the PBMCs of PD was significantly lower than that of HC and NDC, which is consistent with our previous reports (Le et al., 2008; Liu et al., 2012). The genetic variant resulting in reduced expression of NURR1 was reported to be associated with PD (Le et al., 2003; Hering et al., 2004; Jacobsen et al., 2008). NURR1 has not only been found to be down-regulated in the brains of PD patients (Chu et al., 2006), but also in the peripheral blood of PD patients (Montarolo et al., 2016). These results indicating that NURR1 dysfunction may contribute to the PD pathogenesis and the disease progression, acting in the brain and in peripheral inflammatory cells.
Based on the evidence that NURR1 is able to prevent DA neurons from inflammation-induced death through the anti-inflammatory pathway (Bensinger and Tontonoz, 2009; Saijo et al., 2009), we suspected that the decreased level of NURR1 gene in the pathogenesis of PD may give rise to the expression of inflammatory cytokines. Our study documented that the levels of all measured cytokines were significantly higher in the PBMCs of PD in comparison to the controls. Recently a numerous of studies have reported similar findings in protein levels from samples of serum or plasma (Chen et al., 2008; Hofmann et al., 2009; Reale et al., 2009; Koziorowski et al., 2012). Cytokines are considered key players in the neuroinflammatory cascades associated with the degenerative process in PD (Litteljohn and Hayley, 2012). However, the exact mechanisms by which cytokine levels are elevated in the peripheral blood of PD patients remain controversial. It is believed that neuroinflammation in the central nervous system of PD may induces a systemic inflammatory response to activate mononuclear cells in the peripheral blood to express and produces more cytokines during the disease development and progression of PD (Neumann and Wekerle, 1998; Reale et al., 2009). In the various diseases of NDC, some changes were found in the expression levels of cytokines as compared with PD, but no statistical differences were reached. This could be because of the small number of patients enrolled in the individual groups of NDC. As to the influence of medications on cytokines expression in PD, no statistical difference was found between the anti-PD medications groups and the untreated group, indicating that anti-PD medications may have a minimal effect on the immunological processes of PD.
In this study, we documented that NURR1 presented a negative correlation with those of TNF-α, IL-1β, IL-6, and IL-10 in the PBMCs of PD patients. Consistent with our findings, Saijo et al. (2009) showed that NURR1 acted in microglia and astrocytes to suppress the production of inflammatory mediators and protect against DA neuron degeneration. Another study suggested that the levels of pro-inflammatory cytokines produced by primary microglia was significantly decreased in the presence of NURR1 overexpression (Chen et al., 2018). These reports suggest that NURR1 may play a significant role in regulating inflammation in PD. Although statistical analysis showed a negative correlation between NURR1 and cytokines in our study, it is still unclear how NURR1 mediates the neuroinflammation in peripheral circulation, and more experiments are needed in the future to clarify that. Furthermore, we also found that the combination of PBMCs NURR1 and cytokines can enhance the discriminatory accuracy between PD and HC, indicating that combination of NURR1 and cytokines expression in PBMCs could be utilized as collective biomarkers for PD diagnosis.
Conclusion
This study may draw the following conclusions: (1) NURR1 gene expression is significantly decreased in the PBMCs PD patients as compared with HC and NDC. (2) The levels of inflammatory cytokines (TNF-α, IL-1β, IL-4, IL-6, and IL-10) were significantly higher in the PBMCs of PD patients in comparison to the controls (HC and NDC). (3) NURR1 presented a negative correlation with TNF-α, IL-1β, IL-6, and IL-10. (4) The combination of PBMCs NURR1 and cytokines assessment could be used as biomarkers and improve the performance of PD diagnosis.
Author Contributions
TL, ZY, SL, CC, BS, and WL designed the project of this manuscript and revised the paper. TL and ZY carried out all the experiments. TL, ZY, CC, and BS contributed to statistical analyses and results interpretation. TL, ZY, SL, CC, and BS contributed to drafting of the manuscript. WL contributed to research concept and research administration. All authors edited and approved the final version of the manuscript.
Funding
This work was supported by the National Basic Research Program of China (81430021 and 81771521).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2018.00392/full#supplementary-material
References
Bensinger, S., and Tontonoz, P. (2009). A Nurr1 pathway for neuroprotection. Cell 137, 26–28. doi: 10.1016/j.cell.2009.03.024
Buttarelli, F., Fanciulli, A., Pellicano, C., and Pontieri, F. (2011). The dopaminergic system in peripheral blood lymphocytes: from physiology to pharmacology and potential applications to neuropsychiatric disorders. Curr. Neuropharmacol. 9, 278–288. doi: 10.2174/157015911795596612
Caronti, B., Antonini, G., Calderaro, C., Ruggieri, S., Palladini, G., Pontieri, F., et al. (2001). Dopamine transporter immunoreactivity in peripheral blood lymphocytes in Parkinson’s disease. J. Neural. Transm. 108, 803–807. doi: 10.1007/s007020170030
Castillo, S., Baffi, J., Palkovits, M., Goldstein, D., Kopin, I., Witta, J., et al. (1998). Dopamine biosynthesis is selectively abolished in substantia nigra/ventral tegmental area but not in hypothalamic neurons in mice with targeted disruption of the Nurr1 gene. Mol. Cell. Neurosci. 11, 36–46. doi: 10.1006/mcne.1998.0673
Chen, H., O’Reilly, E., Schwarzschild, M., and Ascherio, A. (2008). Peripheral inflammatory biomarkers and risk of Parkinson’s disease. Am. J. Epidemiol. 167, 90–95. doi: 10.1093/aje/kwm260
Chen, X., Qian, Y., Wang, X., Tang, Z., Xu, J., Lin, H., et al. (2018). Nurr1 promotes neurogenesis of dopaminergic neuron and represses inflammatory factors in the transwell coculture system of neural stem cells and microglia. CNS Neurosci. Ther. (Suppl. 1), doi: 10.1111/cns.12825 [Epub ahead of print].
Chu, Y., Le, W., Kompoliti, K., Jankovic, J., Mufson, E., and Kordower, J. (2006). Nurr1 in Parkinson’s disease and related disorders. J. Comp. Neurol. 494, 495–514. doi: 10.1002/cne.20828
Decressac, M., Volakakis, N., Bjorklund, A., and Perlmann, T. (2013). NURR1 in Parkinson disease–from pathogenesis to therapeutic potential. Nat. Rev. Neurol. 9, 629–636. doi: 10.1038/nrneurol.2013.209
Deleidi, M., and Gasser, T. (2013). The role of inflammation in sporadic and familial Parkinson’s disease. Cell. Mol. Life Sci. 70, 4259–4273. doi: 10.1007/s00018-013-1352-y
Dickson, D. (2012). Parkinson’s disease and parkinsonism: neuropathology. Cold Spring Harb. Perspect. Med. 2:a009258. doi: 10.1101/cshperspect.a009258
Dong, J., Li, S., Mo, J., Cai, H., and Le, W. (2016). Nurr1-based therapies for Parkinson’s disease. CNS Neurosci. Ther. 22, 351–359. doi: 10.1111/cns.12536
Erkkinen, M., Kim, M., and Geschwind, M. (2018). Clinical neurology and epidemiology of the major neurodegenerative diseases. Cold Spring Harb. Perspect. Biol. 10:a033118. doi: 10.1101/cshperspect.a033118
Goetz, C., Poewe, W., Rascol, O., Sampaio, C., Stebbins, G., Counsell, C., et al. (2004). Movement disorder society task force report on the Hoehn and Yahr staging scale: status and recommendations. Mov. Disord. 19, 1020–1028. doi: 10.1002/mds.20213
Heneka, M., Kummer, M., and Latz, E. (2014). Innate immune activation in neurodegenerative disease. Nat. Rev. Immunol. 14, 463–477. doi: 10.1038/nri3705
Hering, R., Petrovic, S., Mietz, E., Holzmann, C., Berg, D., Bauer, P., et al. (2004). Extended mutation analysis and association studies of Nurr1 (NR4A2) in Parkinson disease. Neurology 62, 1231–1232. doi: 10.1212/01.wnl.0000118285.18383.90
Hofmann, K., Schuh, A., Saute, J., Townsend, R., Fricke, D., Leke, R., et al. (2009). Interleukin-6 serum levels in patients with Parkinson’s disease. Neurochem. Res. 34, 1401–1404. doi: 10.1007/s11064-009-9921-z
Huang, Y., Cheung, L., Rowe, D., and Halliday, G. (2004). Genetic contributions to Parkinson’s disease. Brain Res. Rev. 46, 44–70. doi: 10.1016/j.brainresrev.2004.04.007
Jacobsen, K., MacDonald, H., Lemonde, S., Daigle, M., Grimes, D., Bulman, D., et al. (2008). A Nurr1 point mutant, implicated in Parkinson’s disease, uncouples ERK1/2-dependent regulation of tyrosine hydroxylase transcription. Neurobiol. Dis. 29, 117–122. doi: 10.1016/j.nbd.2007.08.003
Jankovic, J., Chen, S., and Le, W. (2005). The role of Nurr1 in the development of dopaminergic neurons and Parkinson’s disease. Prog. Neurobiol. 77, 128–138. doi: 10.1016/j.pneurobio.2005.09.001
Koziorowski, D., Tomasiuk, R., Szlufik, S., and Friedman, A. (2012). Inflammatory cytokines and NT-proCNP in Parkinson’s disease patients. Cytokine 60,762–766. doi: 10.1016/j.cyto.2012.07.030
Le, W., Conneely, O., Zou, L., He, Y., Saucedo-Cardenas, O., Jankovic, J., et al. (1999). Selective agenesis of mesencephalic dopaminergic neurons in Nurr1-deficient mice. Exp. Neurol. 159, 451–458. doi: 10.1006/exnr.1999.7191
Le, W., Pan, T., Huang, M., Xu, P., Xie, W., Zhu, W., et al. (2008). Decreased NURR1 gene expression in patients with Parkinson’s disease. J. Neurol. Sci. 273, 29–33. doi: 10.1016/j.jns.2008.06.007
Le, W., Xu, P., Jankovic, J., Jiang, H., Appel, S., Smith, R., et al. (2003). Mutations in NR4A2 associated with familial Parkinson disease. Nat. Genet. 33, 85–89. doi: 10.1038/ng1066
Li, S., and Le, W. (2017). Milestones of Parkinson’s disease research: 200 years of history and beyond. Neurosci. Bull. 33, 598–602. doi: 10.1007/s12264-017-0178-2
Litteljohn, D., and Hayley, S. (2012). Cytokines as potential biomarkers for Parkinson’s disease: a multiplex approach. Methods Mol. Biol. 934, 121–144. doi: 10.1007/978-1-62703-071-7_7
Liu, H., Wei, L., Tao, Q., Deng, H., Ming, M., Xu, P., et al. (2012). Decreased NURR1 and PITX3 gene expression in Chinese patients with Parkinson’s disease. Eur. J. Neurol. 19, 870–875. doi: 10.1111/j.1468-1331.2011.03644.x
Mages, H., Rilke, O., Bravo, R., Senger, G., and Kroczek, R. (1994). NOT, a human immediate-early response gene closely related to the steroid/thyroid hormone receptor NAK1/TR3. Mol. Endocrinol. 8, 1583–1591. doi: 10.1210/mend.8.11.7877627
Martin, I., Dawson, V., and Dawson, T. (2011). Recent advances in the genetics of Parkinson’s disease. Annu. Rev. Genomics Hum. Genet. 12, 301–325. doi: 10.1146/annurev-genom-082410-101440
Mizuta, I., Satake, W., Nakabayashi, Y., Ito, C., Suzuki, S., Momose, Y., et al. (2006). Multiple candidate gene analysis identifies α-synuclein as a susceptibility gene for sporadic Parkinson’s disease. Hum. Mol. Genet. 15, 1151–1158. doi: 10.1093/hmg/ddl030
Mogi, M., Harada, M., Narabayashi, H., Inagaki, H., Minami, M., and Nagatsu, T. (1996). Interleukin (IL)-1β, IL-2, IL-4, IL-6 and transforming growth factor-α levels are elevated in ventricular cerebrospinal fluid in juvenile parkinsonism and Parkinson’s disease. Neurosci. Lett. 211, 13–16. doi: 10.1016/0304-3940(96)12706-3
Montarolo, F., Perga, S., Martire, S., Navone, D., Marchet, A., Leotta, D., et al. (2016). Altered NR4A subfamily gene expression level in peripheral blood of Parkinson’s and Alzheimer’s disease patients. Neurotox. Res. 30, 338–344. doi: 10.1007/s12640-016-9626-4
Nagatsu, T., and Sawada, M. (2005). Inflammatory process in Parkinson’s disease: role for cytokines. Curr. Pharm. Des. 11, 999–1016. doi: 10.2174/1381612053381620
Neumann, H., and Wekerle, H. (1998). Neuronal control of the immune response in the central nervous system: linking brain immunity to neurodegeneration. J. Neuropathol. Exp. Neurol. 57, 1–9. doi: 10.1097/00005072-199801000-00001
Pellicano, C., Buttarelli, F., Circella, A., Tiple, D., Giovannelli, M., Benincasa, D., et al. (2007). Dopamine transporter immunoreactivity in peripheral blood lymphocytes discriminates Parkinson’s disease from essential tremor. J. Neural Transm. 114, 935–938. doi: 10.1007/s00702-006-0623-2
Postuma, R., Berg, D., Stern, M., Poewe, W., Olanow, C., Oertel, W., et al. (2015). MDS clinical diagnostic criteria for Parkinson’s disease. Mov. Disord. 30, 1591–1601. doi: 10.1002/mds.26424
Reale, M., Iarlori, C., Thomas, A., Gambi, D., Perfetti, B., Nicola, M., et al. (2009). Peripheral cytokines profile in Parkinson’s disease. Brain Behav. Immun. 23, 55–63. doi: 10.1016/j.bbi.2008.07.003
Saijo, K., Winner, B., Carson, C., Collier, J., Boyer, L., Rosenfeld, M., et al. (2009). A Nurr1/CoREST pathway in microglia and astrocytes protects dopaminergic neurons from inflammation-induced death. Cell 137, 47–59. doi: 10.1016/j.cell.2009.01.038
Sakurada, K., Ohshima-Sakurada, M., Palmer, T., and Gage, F. (1999). Nurr1, an orphan nuclear receptor, is a transcriptional activator of endogenous tyrosine hydroxylase in neural progenitor cells derived from the adult brain. Development 126, 4017–4026.
Sawada, M., Imamura, K., and Nagatsu, T. (2006). Role of cytokines in inflammatory process in Parkinson’s disease. J. Neural Transm. Suppl. 70, 373–381. doi: 10.1007/978-3-211-45295-0
Van Laar, A., and Jain, S. (2004). Non-motor symptoms of Parkinson disease: update on the diagnosis and treatment. Neurologist 10,185–194.
Wang, Z., Benoit, G., Liu, J., Prasad, S., Aarnisalo, P., Liu, X., et al. (2003). Structure and function of Nurr1 identifies a class of ligand-independent nuclear receptors. Nature 423, 555–560. doi: 10.1038/nature01645
Keywords: NURR1, Parkinson’s disease, biomarkers, cytokines, neuroinflammation
Citation: Li T, Yang Z, Li S, Cheng C, Shen B and Le W (2018) Alterations of NURR1 and Cytokines in the Peripheral Blood Mononuclear Cells: Combined Biomarkers for Parkinson’s Disease. Front. Aging Neurosci. 10:392. doi: 10.3389/fnagi.2018.00392
Received: 27 June 2018; Accepted: 12 November 2018;
Published: 29 November 2018.
Edited by:
Hi-Joon Park, Kyung Hee University, South KoreaReviewed by:
Franc Llorens, Centro de Investigación Biomédica en Red sobre Enfermedades Neurodegenerativas (CIBERNED), SpainLiu Jun, Shanghai Jiao Tong University, China
Copyright © 2018 Li, Yang, Li, Cheng, Shen and Le. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weidong Le, d2RsZUBzaWJzLmFjLmNu
 Tianbai Li
Tianbai Li Zhaofei Yang
Zhaofei Yang Song Li
Song Li Cheng Cheng
Cheng Cheng Bairong Shen
Bairong Shen Weidong Le
Weidong Le