- 1Department of Neurology, Xiangya Hospital, Central South University, Changsha, China
- 2Department of Geriatrics, Xiangya Hospital, Central South University, Changsha, China
- 3National Clinical Research Center for Geriatric Disorders, Changsha, China
- 4Key Laboratory of Hunan Province in Neurodegenerative Disorders, Central South University, Changsha, China
- 5Parkinson's Disease Center of Beijing Institute for Brain Disorders, Beijing, China
Background: LRRK2 variants have been demonstrated to have distinct distributions in different populations. However, researchers have thus far chosen to focus on relatively few variants, such as R1628P, G2019S, and G2385R. We therefore investigated the relationship between common LRRK2 variants and PD risk in various populations.
Methods: Using a set of strict inclusion criteria, six databases were searched, resulting in the selection of 94 articles covering 49,299 cases and 47,319 controls for final pooled analysis and frequency analysis. Subgroup analysis were done for Africans, European/West Asians, Hispanics, East Asians, and mixed populations. Statistical analysis was carried out using the Mantel-Haenszel approach to determine the relationship between common LRRK2 variants and PD risk, with the significance level set at p < 0.05.
Results: In the absence of obvious heterogeneities and publication biases among the included studies, we concluded that A419V, R1441C/G/H, R1628P, G2019S, and G2385R were associated with increased PD risk (p: 0.001, 0.0004, < 0.00001, < 0.00001, and < 0.00001, respectively), while R1398H was associated with decreased risk (p: < 0.00001). In East Asian populations, A419V, R1628P, and G2385R increased risk (p: 0.001, < 0.00001, < 0.00001), while R1398H had the opposite effect (p: 0.0005). G2019S increased PD risk in both European/West Asian and mixed populations (p: < 0.00001, < 0.00001), while R1441C/G/H increased risk in European/West Asian populations only (p: 0.0004).
Conclusions: We demonstrated that LRRK2 variant distribution is different among various populations, which should inform decisions regarding the development of future genetic screening strategies.
Introduction
Parkinson's disease (PD) is one of the most common neurodegenerative diseases, affecting ~2% of people over the age of 60 years, and the most common cause of movement disorders, including bradykinesia, resting tremor, rigidity, and postural instability or gait difficulty. Non-motor symptoms, such as depression, olfactory dysfunction, and constipation are also common in PD (Schapira, 2006). The pathological hallmark of PD is Lewy Body aggregation in neurons and the loss of dopamine neurons in the substantia nigra compacta and corpus striatum.
The pathogenesis of PD is as yet unclear, but genetic and environmental factors, as well as aging are thought to contribute to PD risk. Since the discovery of the PARK1 locus, further autosomal-dominant or -recessive disease genes have been identified (Paisan-Ruiz, 2009). The most common among the former is the leucine-rich repeat kinase 2 (LRRK2) gene, which encodes for a protein containing armadillo (ARM), Ras of complex proteins (ROC), C-terminal of ROC (COR), mitogen-activated protein kinase kinase kinase (MAPKKK), and WD40 domains, in addition to others (Kruger, 2008).
To date, nearly a hundred LRRK2 variants have been identified. Of these, G2019S, R1628P, and G2385R have traditionally received much of the attention, and there have already been a number of meta-analyses on the role of these variants in PD risk in people of different ethnicities (Xie et al., 2014; Liu et al., 2016; Zhang et al., 2016; Zhao and Kong, 2016). These variants each possess distinct geographical distributions. G2019S, which is the most frequently-occurring variant, accounts for 3–6% of familial PD cases among patients in European populations (Guo et al., 2006) and nearly 14% of Ashkenazi Jews (AJ) (Luzon-Toro et al., 2007), whereas G2385R and R1628P were found to be more common among East Asian PD patients (Fu et al., 2013).
If strict selection criteria and quality control methods are employed, meta-analysis can be an immensely powerful tool, allowing researchers to pool data from original studies and effectively expanding the sample size (Haines et al., 2008) to provide unbiased evidence with far-reaching clinical implications (Wolf, 2015). In the case of genetic analyses in particular, where original studies are inevitably limited by the diversity of their subject pool, and by their limited sample sizes a meta-analytical approach can be invaluable in providing convincing evidence for the effects of a specific gene on disease risks. In the recent crop of articles, researchers have begun to shift their focus to the other less well-known LRRK2 variants. Given that others have not yet done so, we decided in this study to perform a complete analysis of all relevant original association studies relating to the LRRK2 variants that have been identified thus far.
Methods
Literature Search
The English-language Medline and Embase databases, as well as the Chinese-language Wanfang, CNKI, and VIP databases were searched manually on Nov 29, 2018, using the keywords “parkinson*,” “PD,” “LRRK2,” “PARK8,” “polymorphisms,” “SNP,” “gene,” “variant,” and “mutation.” Overlapping articles among databases were deleted in EndNote.
Selection Criteria
The PICOS (participants, interventions, controls, outcomes, and study types) approach was used to define inclusion criteria. For this study, “participants” were patients diagnosed with PD according to accepted standards, such as the UK PD Society Brain Bank Clinical Diagnostic Criteria (Hughes et al., 1992) and other widely accepted criteria (Calne et al., 1992; Bower et al., 1999; Gelb et al., 1999); “Interventions” consisted of gDNA analysis performed using accepted methods based on PCR; “Controls” were people without PD or related diseases (movement disorders, neurodegenerative diseases etc.); We accepted the definition of PD patients or controls by the authors of the original articles if there were no description of the criteria; “Outcomes” were complete data (complete number of patients or controls carrying either homozygous or heterozygous polymorphisms of LRRK2) and at least four original articles reported on the same variant, and “study types” consisted of original case-control studies, cohort studies (Supplementary Table 1).
Data Extraction
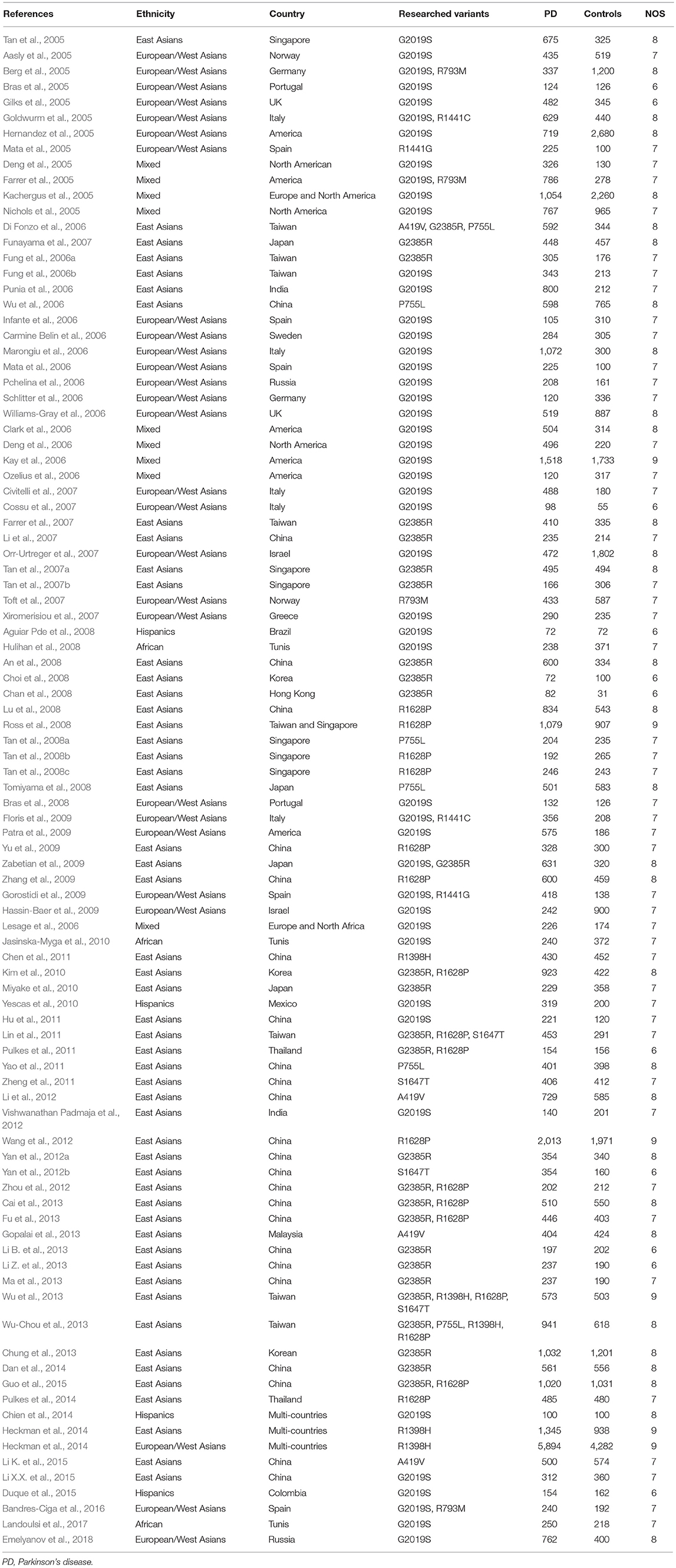
Complete data, including containing the name of the first author, the publication year of the study, subject ethnicity, the country in which the study was performed, the gene and gene variants analyzed, the number of cases and controls and subject genotypes (both homozygotes and heterozygotes) were extracted from all selected original studies, and are detailed in the Table 1, Supplementary Table 2. Pooled analysis was performed in cases where sufficient data was provided to allow for the calculation of odds ratios (OR) and 95% confidence intervals (CI). If studies had enough data to calculated frequency of variants, we included the articles in the frequency analysis. Newcastle-Ottawa Scale (NOS) was used to perform quality control on all included studies. Data extraction was performed by Li S and Yuan Z, in consultation with Qiying S. Results relating to R1628P were provided by ZY, a co-author on this study, and were based on (Zhang et al., 2017).
Statistical Analysis
Statistical analysis was performed using Revman 5.3 software. The pooled analyses were conducted if there were at least four original studies. Meta-analyses were conducted on total populations and subgroup analyses by ethnicity (Africans, European/West Asians, Hispanics, East Asians, mixed:composed of at least two different groups) (Risch et al., 2002; Zhang et al., 2018). In cases where the Q statistic P > 0.1 and I2 statistic ≤ 50%, a fixed-effect model was used, otherwise, a random-effect model was applied for pooled analysis instead. All pooled results were graphed using forest plots and publication biases were showed using funnel plots. Subgroup analysis using the Mantel-Haenszel statistical method was performed to determine how the common LRRK2 variants affect PD risk and the level of significance was set at p < 0.05. Sensitivity analysis was performed by sequentially deleting each included article, and observing how the pooled OR and 95%CI was affected by their removal. Genotype frequency (GF) and minor allele frequency (MAF) were calculated of each LRRK2 variants in our analyses.
Results
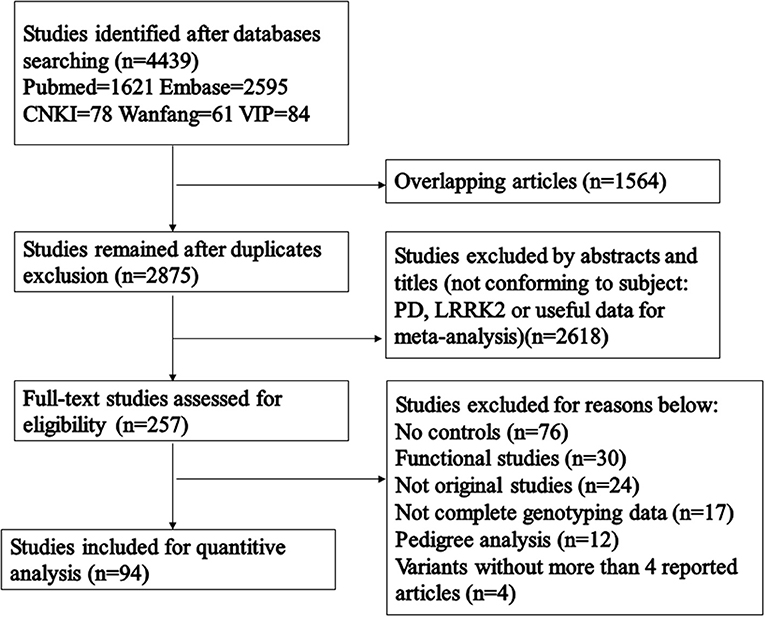
A total of 4,439 articles were included following our initial database search (Figure 1). 2,875 articles remained after repeated articles had been eliminated, and a further 2,618 articles were excluded after we had manually reviewed their titles and abstracts. Among the 257 articles that received a full-text review, 163 were excluded due to no controls, functional studies, not original studies, not complete genotyping data, pedigree analysis and articles studying variants with no more than 4 reported articles. Eventually, 94 relevant articles were included in the final analysis, covering 49,299 cases and 47,319 control subjects (Table 1). As shown in Table 1, all included articles were of high quality. Subgroup analysis was performed for each of the major ethnic groups (Africans, European/West Asians, Hispanics, East Asians, mixed: composed of at least two different groups). Results of the pooled analysis were graphed using forest plots (Supplementary Figure 1), and variant frequencies in PD patients of different ethnicities were further calculated (Figure 2). All analysis was performed using a fixed-effect model due to there being relatively little heterogeneity among the included studies.
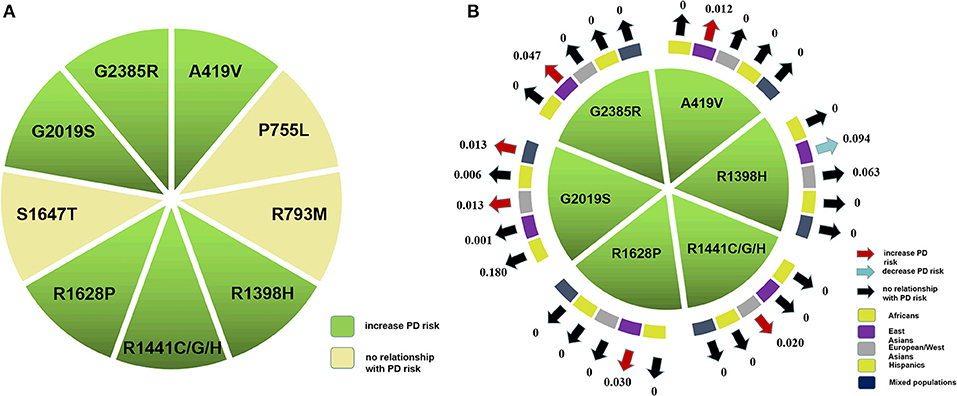
Figure 2. Nine LRRK2 variants were included in the meta-analysis. (A) Six variants which had statistical differences in our pooled analysis. (B) Red, blue, and black arrows indicate that the associated variant increases, decreases, or has no bearing on PD risk, respectively. The arrow pointed to MAF of a specific variant.
Comprehensive Analysis of LRRK2 Variants in Different Ethnicities
Based on our comprehensive analysis of the common LRRK2 variants, we concluded that A419V, R1398H, R1441C/G/H, R1628P, G2019S, and G2385R were associated with greater PD risk (p: 0.001, 0.0004, < 0.00001, < 0.00001, and < 0.00001, respectively), while R1398H was associated with decreased PD risk (< 0.00001; Figure 2). Of the high-risk variants, G2019S posed the greatest degree of risk, followed by R1441C/G/H, A419V, G2385R, and R1628P in descending order, as demonstrated by their OR values, which ranged from 13.16 to 1.83 (Table 2).
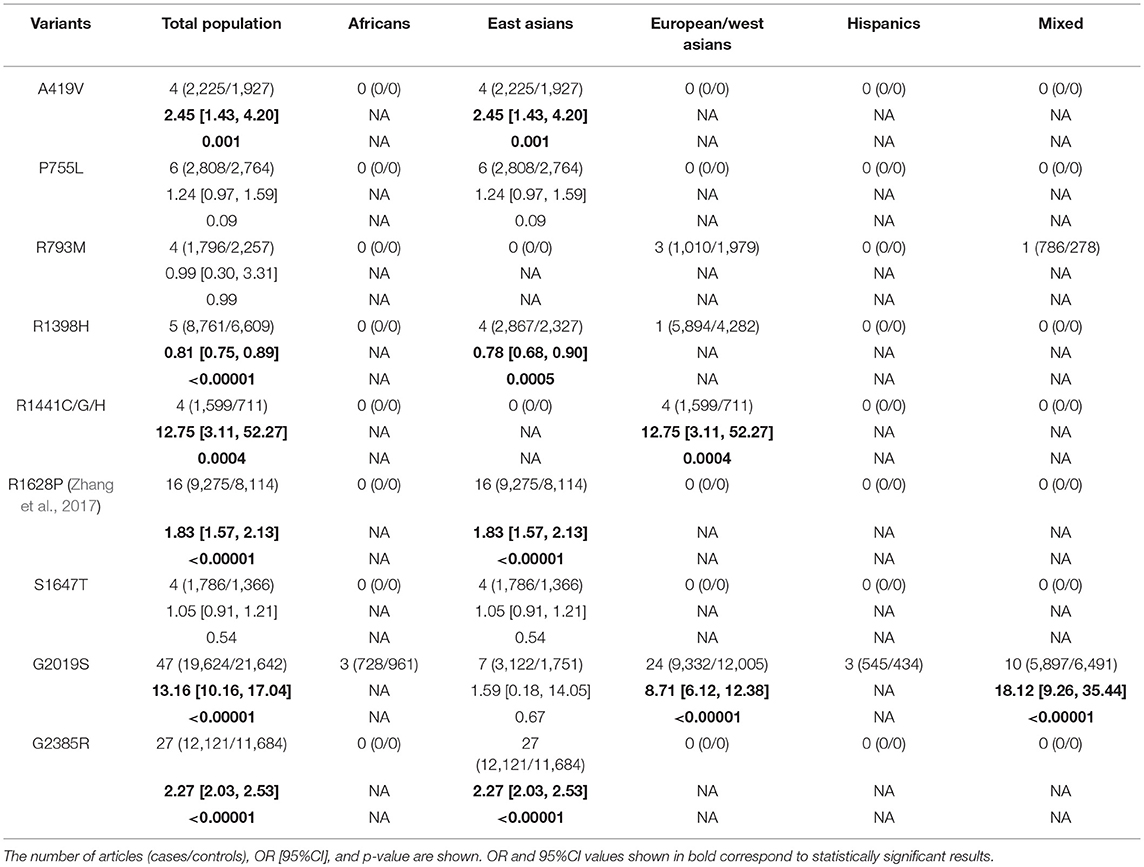
Table 2. Relationship between common LRRK2 and overall PD risk and for populations of specific ethnicities.
By ethnicity, our analysis indicated that A419V, R1628P, and G2385R were associated with increased PD risk in East Asian populations (p: 0.001, < 0.00001, and < 0.00001), while R1398H had the opposite effect (p: 0.0005). The G2019S variant was found to increase PD risk in European/West Asian and mixed populations (p: < 0.00001 and < 0.00001), while R1441C/G/H increased risk for European/West Asians only (p: 0.0004; Table 2).
LRRK2 Variant Frequency in PD Patients and Control Individuals of Different Ethnicities
Among the LRRK2 variants which were of statistical significance in previous meta-analyses, the most frequently-occurring LRRK2 variants in PD patients were, in descending order, R1398H, G2385R, R1628P, and A419V, which had MAF ranging from 0.094 to 0.012 in East Asian populations (Figure 2; Supplementary Table 3). In European/West Asians, the MAF of the high-risk variants G2019S and R1441C/G/H were 0.013 and 0.020, respectively (Figure 2; Supplementary Table 3), and G2019S occurred within mixed populations at a total frequency of 0.013. Further, we found that A419V, R1628P, and G2385R appeared to be specific for Asian populations, while R1441C/G/H were European/West Asians-specific. Even though the total genotype frequency for other variants, such as S1647T and P755L were higher compared to those of G2385R or G2019S, a significant difference in their distribution between cases and control subjects was not apparent.
Sensitivity Analysis and Publication Bias
After sequentially deleting each included article, the pooled OR and 95% CI of each variant was not changed significantly, and the pooled results of each grouped and subgroup analysis remained stable. Publication biases were not obvious from the funnel plots of all responsive variants (Supplementary Figure 2).
Discussion
The current meta-analysis and systematic review is, as far as we are aware, the most comprehensive analysis of common LRRK2 variants in PD to date, and revealed population heterogeneity to be a prominent factor in LRRK2 allelic distribution.
Previous studies of ethnicity-specific LRRK2 variation have focused primarily on the common variants G2019S, G2385R, and R1628P (Xie et al., 2014; Liu et al., 2016; Zhang et al., 2016; Zhao and Kong, 2016), showing that G2019S was more common in European and North American populations while G2385R and R1628P existed only in Asian populations (Guo et al., 2006). Our study replicated these results, and further demonstrated the importance of other variants, such as P755L, A419V, and R1398H. We demonstrated that G2019S, R1441C/G/H, A419V, G2385R, and R1628P, in descending order, carried the highest overall degrees of PD risk, as indicated by their ORs, which ranged from 13.16 to 1.83.
In East Asian populations, G2385R, R1628P, and A419V (arranged in descending order according to the frequency of their occurrence) were found to increase PD risk. Although previous studies have been quite successful at identifying high-frequency risk variants, our findings serve to highlight the fact that even those that occur at lower frequencies, such as A419V, should not be neglected, particularly in East Asian populations, even if clinically significant data may only be accessed in these cases through the use of larger sample sizes that are possible only with collaborative multi-center projects. We also determined that G2019S can increase PD risk in European/West Asian and mixed populations, while R1441C/G/H increases PD risk in European/West Asians only. The pooled analysis and frequency analysis of variants in LRRK2 supported the differences in geographic distributions of LRRK2 variants.
The 51-exon LRRK2 gene has always posed a challenge for researchers interested in screening for the gene due to its large size. For the sake of improving the efficiency and economy of genetic diagnosis, it is thus of great importance to prioritize the identification of specific variations instead of sequencing the entire gene (Foroud, 2005). Based on calculations of the ORs associated with each of the LRRK2 variants, we suggest that A419V, G2385R, and R1628P, and G2019S and R1441C/G/H should be screened for first in East Asians and European/West Asians, respectively. Variants with high OR and that occur at higher rates, such as G2385R in East Asian populations and G2019S in European/West Asian populations, should be, in particular, prioritized above all others.
Our meta-analysis also revealed additional details of how mutations affect LRRK2 function, which could have potential ramifications with regards to our understanding of the mechanisms that underlie PD and the development of future treatment strategies. We determined, for instance, that all of the high-risk variants carried mutations in the exon regions of the LRRK2 gene, and in the functional domains of the LRRK2 protein (Figure 3). While the specific pathological mechanisms of these mutations are as yet unclear, it is possible that they can lead to an increase in the kinase activity of the protein, thereby increasing disease risk as well (West et al., 2005), as in the case of the G2019S “gain of function” feature (Luzon-Toro et al., 2007). If this is demonstrated to be true on a more general basis, it may be promising to target the variations in these vital regions for therapeutic purposes.
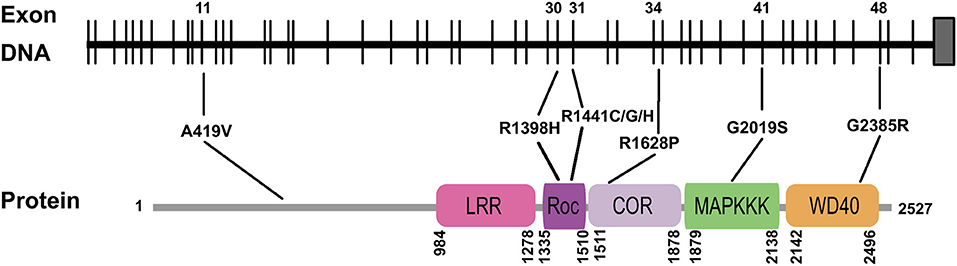
Figure 3. Schematic figure illustrating the distribution of mutations associated with various LRRK2 variants demonstrated by meta-analysis to have a significant effect on PD risk. Only exons containing these mutations are shown. Note particular variants linked to mutations in functional domains. LRR, leucine rich repeat; ROC, Ras of complex proteins; COR, C-terminal of Roc; MAPKKK, kinase domain of MAPK; WD40, β-propeller.
Certain LRRK2 variants appear to be linked with clinical phenotypes. For instance, motor fluctuations were more frequent in the G2385R carriers than in non-carriers in PD patients. G2019S had better olfactory function and less likely to have depression than G2385R carriers (West et al., 2005). In a large meta-analysis of LRRK2 related clinical features by our group, we found that LRRK2-G2019S-related PD patients were likely to be female, had higher rates of early-onset PD and family history. Moreover, they tended to have high scores of Schwab & England, low Geriatric Depression Scale (GDS) scores, high University of Pennsylvania Smell Identification Test (UPSIT) scores and responded well to Levodopa. G2385R carriers tended to have family history, lower Hoehn and Yahr rating (H-Y) and higher Mini Mental State Examination (MMSE) scores. However, both G2019S and G2385R carriers were more likely to develop motor complications than non-carriers (Shu et al., 2018). Therefore, for the purposes of clinical genetic counseling and testing, the symptoms exhibited by the patient could be useful in guiding the decision of which LRRK2 variant to screen for. Additionally, identifying the specific clinical features associated with carrier of particular LRRK2 variants could also be useful for neurologists in prescribing the correct symptomatic treatments.
There are, of course, limitations to this study. Firstly, although we have endeavored to include every published case-control study in the meta-analysis, certain pieces of unpublished data, or articles that were written in languages other than English or Chinese may have been omitted unintentionally. Secondly, due to the lack of sufficient data, variants in populations other than East Asians and European/West Asians cannot be analyzed, and it is possible that biases may exist in our pooled analysis of different ethnic groups and in their demographic information, such as age and gender. Thirdly, stratified analysis can inadvertently increase the possibility of there being false positives, especially as the sample size is limited.
Conclusion
In conclusion, we found that LRRK2 variants A419V, G2019S, R1441C/G/H, G2385R, and R1628P were associated with increased PD risk while R1398H was associated with decreased risk. In East Asian populations, A419V, G2385R, and R1628P increased risk, while R1398H had the opposite effect. G2019S increased the risk in European/West Asian and mixed populations while R1441C/G/H increased the risk of PD in European/West Asians. Combined with frequency analysis, we suggest that A419V, G2385R, and R1628P should receive top priority for screening in East Asian populations and that a greater focus be placed on G2019S and R1441C/G/H in European/West Asian populations.
Author Contributions
LS, YZ, and BT: chose the topic and designed the experiments; LS, YZ, and QS: performed the analysis; LS, YZ, QS, and BT: analyzed the data; LS, YZ, and BT: wrote the manuscript; HP: data management and figure modification.
Funding
This work was supported by grants from the National Natural Science Foundation of China (No. 81430023, No. 81401059), the National Key Plan for Scientific Research and Development of China (NO. 2016YFC1306000, 2017YFC0909100) and Hunan Provincial Innovation Foundation for Postgraduate (NO. CX2017B066).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2019.00013/full#supplementary-material
References
Aasly, J. O., Toft, M., Fernandez-Mata, I., Kachergus, J., Hulihan, M., White, L. R., et al. (2005). Clinical features of LRRK2-associated Parkinson's disease in central Norway. Ann. Neurol. 57, 762–765. doi: 10.1002/ana.20456
Aguiar Pde, C., Lessa, P. S., Godeiro, C. Jr., Barsottini, O., Felicio, A. C., Borges, V., et al. (2008). Genetic and environmental findings in early-onset Parkinson's disease Brazilian patients. Mov. Disord. 23, 1228–1233. doi: 10.1002/mds.22032
An, X. K., Peng, R., Li, T., Burgunder, J. M., Wu, Y., Chen, W. J., et al. (2008). LRRK2 Gly2385Arg variant is a risk factor of Parkinson's disease among Han-Chinese from mainland China. Eur. J. Neurol. 15, 301–305. doi: 10.1111/j.1468-1331.2007.02052.x
Bandres-Ciga, S., Price, T. R., Barrero, F. J., Escamilla-Sevilla, F., Pelegrina, J., Arepalli, S., et al. (2016). Genome-wide assessment of Parkinson's disease in a Southern Spanish population. Neurobiol. Aging 45, 213e213–213e219. doi: 10.1016/j.neurobiolaging.2016.06.001
Berg, D., Schweitzer, K. J., Leitner, P., Zimprich, A., Lichtner, P., Belcredi, P., et al. (2005). Type and frequency of mutations in the LRRK2 gene in familial and sporadic Parkinson's disease. Brain 128(Pt 12), 3000–3011. doi: 10.1093/brain/awh666
Bower, J. H., Maraganore, D. M., McDonnell, S. K., and Rocca, W. A. (1999). Incidence and distribution of parkinsonism in Olmsted County, Minnesota, 1976–1990. Neurology 52, 1214–1220.
Bras, J., Guerreiro, R., Ribeiro, M., Morgadinho, A., Januario, C., Dias, M., et al. (2008). Analysis of Parkinson disease patients from Portugal for mutations in SNCA, PRKN, PINK1 and LRRK2. BMC Neurol. 8:1. doi: 10.1186/1471-2377-8-1
Bras, J. M., Guerreiro, R. J., Ribeiro, M. H., Januario, C., Morgadinho, A., Oliveira, C. R., et al. (2005). G2019S dardarin substitution is a common cause of Parkinson's disease in a Portuguese cohort. Mov. Disord. 20, 1653–1655. doi: 10.1002/mds.20682
Cai, J., Lin, Y., Chen, W., Lin, Q., Cai, B., Wang, N., et al. (2013). Association between G2385R and R1628P polymorphism of LRRK2 gene and sporadic Parkinson's disease in a Han-Chinese population in south-eastern China. Neurol. Sci. 34, 2001–2006. doi: 10.1007/s10072-013-1436-3
Calne, D. B., Snow, B. J., and Lee, C. (1992). Criteria for diagnosing Parkinson's disease. Ann. Neurol. 32(Suppl.), S125–S127.
Carmine Belin, A., Westerlund, M., Sydow, O., Lundstromer, K., Hakansson, A., Nissbrandt, H., et al. (2006). Leucine-rich repeat kinase 2 (LRRK2) mutations in a Swedish Parkinson cohort and a healthy nonagenarian. Mov. Disord. 21, 1731–1734. doi: 10.1002/mds.21016
Chan, D. K., Ng, P. W., Mok, V., Yeung, J., Fang, Z. M., Clarke, R., et al. (2008). LRRK2 Gly2385Arg mutation and clinical features in a Chinese population with early-onset Parkinson's disease compared to late-onset patients. J Neural Transm (Vienna) 115, 1275–1277. doi: 10.1007/s00702-008-0065-0
Chen, L., Zhang, S., Liu, Y., Hong, H., Wang, H., Zheng, Y., et al. (2011). LRRK2 R1398H polymorphism is associated with decreased risk of Parkinson's disease in a Han Chinese population. Parkinsonism Relat. Disord. 17, 291–292. doi: 10.1016/j.parkreldis.2010.11.012
Chien, H. F., Figueiredo, T. R., Hollaender, M. A., Tofoli, F., Takada, L. T., Pereira Lda, V., et al. (2014). Frequency of the LRRK2 G2019S mutation in late-onset sporadic patients with Parkinson's disease. Arq. Neuropsiquiatr. 72, 356–359. doi: 10.1590/0004-282X20140019
Choi, J. M., Woo, M. S., Ma, H. I., Kang, S. Y., Sung, Y. H., Yong, S. W., et al. (2008). Analysis of PARK genes in a Korean cohort of early-onset Parkinson disease. Neurogenetics 9, 263–269. doi: 10.1007/s10048-008-0138-0
Chung, S. J., Jung, Y., Hong, M., Kim, M. J., You, S., Kim, Y. J., et al. (2013). Alzheimer's disease and Parkinson's disease genome-wide association study top hits and risk of Parkinson's disease in Korean population. Neurobiol. Aging 34, 2695 e2691–2697. doi: 10.1016/j.neurobiolaging.2013.05.022
Civitelli, D., Tarantino, P., Nicoletti, G., Ciro Candiano, I. C., Annesi, F., De Marco, E. V., et al. (2007). LRRK2 G6055A mutation in Italian patients with familial or sporadic Parkinson's disease. Clin. Genet. 71, 367–370. doi: 10.1111/j.1399-0004.2007.00771.x
Clark, L. N., Wang, Y., Karlins, E., Saito, L., Mejia-Santana, H., Harris, J., et al. (2006). Frequency of LRRK2 mutations in early- and late-onset Parkinson disease. Neurology 67, 1786–1791. doi: 10.1212/01.wnl.0000244345.49809.36
Cossu, G., van Doeselaar, M., Deriu, M., Melis, M., Molari, A., Di Fonzo, A., et al. (2007). LRRK2 mutations and Parkinson's disease in Sardinia–a Mediterranean genetic isolate. Parkinsonism Relat. Disord. 13, 17–21. doi: 10.1016/j.parkreldis.2006.06.010
Dan, X., Wang, C., Ma, J., Feng, X., Wang, T., Zheng, Z., et al. (2014). MAPT IVS1 + 124 C > G modifies risk of LRRK2 G2385R for Parkinson's disease in Chinese individuals. Neurobiol. Aging 35, 1780 e1787–1780 e1710. doi: 10.1016/j.neurobiolaging.2014.01.025
Deng, H., Le, W., Guo, Y., Hunter, C. B., Xie, W., Huang, M., et al. (2006). Genetic analysis of LRRK2 mutations in patients with Parkinson disease. J. Neurol. Sci. 251, 102–106. doi: 10.1016/j.jns.2006.09.017
Deng, H., Le, W., Guo, Y., Hunter, C. B., Xie, W., and Jankovic, J. (2005). Genetic and clinical identification of Parkinson's disease patients with LRRK2 G2019S mutation. Ann. Neurol. 57, 933–934. doi: 10.1002/ana.20510
Di Fonzo, A., Wu-Chou, Y. H., Lu, C. S., van Doeselaar, M., Simons, E. J., Rohe, C. F., et al. (2006). A common missense variant in the LRRK2 gene, Gly2385Arg, associated with Parkinson's disease risk in Taiwan. Neurogenetics 7, 133–138. doi: 10.1007/s10048-006-0041-5
Duque, A. F., Lopez, J. C., Benitez, B., Hernandez, H., Yunis, J. J., Fernandez, W., et al. (2015). Analysis of the LRRK2 p.G2019S mutation in Colombian Parkinson's disease patients. Colomb Med (Cali). 46, 117–121.
Emelyanov, A. K., Usenko, T. S., Tesson, C., Senkevich, K. A., Nikolaev, M. A., Miliukhina, I. V., et al. (2018). Mutation analysis of Parkinson's disease genes in a Russian data set. Neurobiol. Aging 71, 267 e267–267 e210. doi: 10.1016/j.neurobiolaging.2018.06.027
Farrer, M., Stone, J., Mata, I. F., Lincoln, S., Kachergus, J., Hulihan, M., et al. (2005). LRRK2 mutations in Parkinson disease. Neurology 65, 738–740. doi: 10.1212/01.wnl.0000169023.51764.b0
Farrer, M. J., Stone, J. T., Lin, C. H., Dachsel, J. C., Hulihan, M. M., Haugarvoll, K., et al. (2007). Lrrk2 G2385R is an ancestral risk factor for Parkinson's disease in Asia. Parkinsonism Relat. Disord. 13, 89–92. doi: 10.1016/j.parkreldis.2006.12.001
Floris, G., Cannas, A., Solla, P., Murru, M. R., Tranquilli, S., Corongiu, D., et al. (2009). Genetic analysis for five LRRK2 mutations in a Sardinian parkinsonian population: importance of G2019S and R1441C mutations in sporadic Parkinson's disease patients. Parkinsonism Relat. Disord. 15, 277–280. doi: 10.1016/j.parkreldis.2008.06.009
Foroud, T. (2005). LRRK2: both a cause and a risk factor for Parkinson disease? Neurology 65, 664–665. doi: 10.1212/01.wnl.0000179342.58181.c9
Fu, X., Zheng, Y., Hong, H., He, Y., Zhou, S., Guo, C., et al. (2013). LRRK2 G2385R and LRRK2 R1628P increase risk of Parkinson's disease in a Han Chinese population from Southern Mainland China. Parkinsonism Relat. Disord. 19, 397–398. doi: 10.1016/j.parkreldis.2012.08.007
Funayama, M., Li, Y., Tomiyama, H., Yoshino, H., Imamichi, Y., Yamamoto, M., et al. (2007). Leucine-rich repeat kinase 2 G2385R variant is a risk factor for Parkinson disease in Asian population. Neuroreport 18, 273–275. doi: 10.1097/WNR.0b013e32801254b6
Fung, H. C., Chen, C. M., Hardy, J., Hernandez, D., Singleton, A., and Wu, Y. R. (2006a). Lack of G2019S LRRK2 mutation in a cohort of Taiwanese with sporadic Parkinson's disease. Mov. Disord. 21, 880–881. doi: 10.1002/mds.20814
Fung, H. C., Chen, C. M., Hardy, J., Singleton, A. B., and Wu, Y. R. (2006b). A common genetic factor for Parkinson disease in ethnic Chinese population in Taiwan. BMC Neurol. 6:47. doi: 10.1186/1471-2377-6-47
Gelb, D. J., Oliver, E., and Gilman, S. (1999). Diagnostic criteria for Parkinson disease. Arch. Neurol. 56, 33–39.
Gilks, W. P., Abou-Sleiman, P. M., Gandhi, S., Jain, S., Singleton, A., Lees, A. J., et al. (2005). A common LRRK2 mutation in idiopathic Parkinson's disease. Lancet 365, 415–416. doi: 10.1016/S0140-6736(05)17830-1
Goldwurm, S., Di Fonzo, A., Simons, E. J., Rohe, C. F., Zini, M., Canesi, M., et al. (2005). The G6055A (G2019S) mutation in LRRK2 is frequent in both early and late onset Parkinson's disease and originates from a common ancestor. J. Med. Genet. 42:e65. doi: 10.1136/jmg.2005.035568
Gopalai, A. A., Lim, S. Y., Aziz, Z. A., Lim, S. K., Tan, L. P., Chong, Y. B., et al. (2013). Lack of association between the LRRK2 A419V variant and Asian Parkinson's disease. Ann. Acad. Med. Singap. 42, 237–240.
Gorostidi, A., Ruiz-Martinez, J., Lopez de Munain, A., Alzualde, A., and Marti Masso, J. F. (2009). LRRK2 G2019S and R1441G mutations associated with Parkinson's disease are common in the Basque Country, but relative prevalence is determined by ethnicity. Neurogenetics 10, 157–159. doi: 10.1007/s10048-008-0162-0
Guo, J. F., Li, K., Yu, R. L., Sun, Q. Y., Wang, L., Yao, L. Y., et al. (2015). Polygenic determinants of Parkinson's disease in a Chinese population. Neurobiol. Aging 36, 1765 e1761–1765 e1766. doi: 10.1016/j.neurobiolaging.2014.12.030
Guo, L., Wang, W., and Chen, S. G. (2006). Leucine-rich repeat kinase 2: relevance to Parkinson's disease. Int. J. Biochem. Cell Biol. 38, 1469–1475. doi: 10.1016/j.biocel.2006.02.009
Haines, T., McKnight, L., Duku, E., Perry, L., and Thoma, A. (2008). The role of systematic reviews in clinical research and practice. Clin. Plast. Surg. 35, 207–214. doi: 10.1016/j.cps.2007.10.003
Hassin-Baer, S., Laitman, Y., Azizi, E., Molchadski, I., Galore-Haskel, G., Barak, F., et al. (2009). The leucine rich repeat kinase 2 (LRRK2) G2019S substitution mutation. Association with Parkinson disease, malignant melanoma and prevalence in ethnic groups in Israel. J. Neurol. 256, 483–487. doi: 10.1007/s00415-009-0117-x
Heckman, M. G., Elbaz, A., Soto-Ortolaza, A. I., Serie, D. J., Aasly, J. O., Annesi, G., et al. (2014). Protective effect of LRRK2 p.R1398H on risk of Parkinson's disease is independent of MAPT and SNCA variants. Neurobiol. Aging 35, 266 e265–e214. doi: 10.1016/j.neurobiolaging.2013.07.013
Hernandez, D., Paisan Ruiz, C., Crawley, A., Malkani, R., Werner, J., Gwinn-Hardy, K., et al. (2005). The dardarin G 2019 S mutation is a common cause of Parkinson's disease but not other neurodegenerative diseases. Neurosci. Lett. 389, 137–139. doi: 10.1016/j.neulet.2005.07.044
Hu, Z. X., Peng, D. T., Cai, M., Pu, J. L., Lei, X. G., Yin, X. Z., et al. (2011). A study of six point mutation analysis of LRRK2 gene in Chinese mainland patients with Parkinson's disease. Neurol. Sci. 32, 741–742. doi: 10.1007/s10072-010-0453-8
Hughes, A. J., Daniel, S. E., Kilford, L., and Lees, A. J. (1992). Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J. Neurol. Neurosurg. Psychiatr. 55, 181–184.
Hulihan, M. M., Ishihara-Paul, L., Kachergus, J., Warren, L., Amouri, R., Elango, R., et al. (2008). LRRK2 Gly2019Ser penetrance in Arab-Berber patients from Tunisia: a case-control genetic study. Lancet Neurol. 7, 591–594. doi: 10.1016/S1474-4422(08)70116-9
Infante, J., Rodriguez, E., Combarros, O., Mateo, I., Fontalba, A., Pascual, J., et al. (2006). LRRK2 G2019S is a common mutation in Spanish patients with late-onset Parkinson's disease. Neurosci. Lett. 395, 224–226. doi: 10.1016/j.neulet.2005.10.083
Jasinska-Myga, B., Kachergus, J., Vilarino-Guell, C., Wider, C., Soto-Ortolaza, A. I., Kefi, M., et al. (2010). Comprehensive sequencing of the LRRK2 gene in patients with familial Parkinson's disease from North Africa. Mov. Disord. 25, 2052–2058. doi: 10.1002/mds.23283
Kachergus, J., Mata, I. F., Hulihan, M., Taylor, J. P., Lincoln, S., Aasly, J., et al. (2005). Identification of a novel LRRK2 mutation linked to autosomal dominant parkinsonism: evidence of a common founder across European populations. Am. J. Hum. Genet. 76, 672–680. doi: 10.1086/429256
Kay, D. M., Bird, T. D., Zabetian, C. P., Factor, S. A., Samii, A., Higgins, D. S., et al. (2006). Validity and utility of a LRRK2 G2019S mutation test for the diagnosis of Parkinson's disease. Genet. Test. 10, 221–227. doi: 10.1089/gte.2006.10.221
Kim, J. M., Lee, J. Y., Kim, H. J., Kim, J. S., Shin, E. S., Cho, J. H., et al. (2010). The LRRK2 G2385R variant is a risk factor for sporadic Parkinson's disease in the Korean population. Parkinsonism Relat. Disord. 16, 85–88. doi: 10.1016/j.parkreldis.2009.10.004
Kruger, R. (2008). LRRK2 in Parkinson's disease–drawing the curtain of penetrance: a commentary. BMC Med. 6:33. doi: 10.1186/1741-7015-6-33
Landoulsi, Z., Benromdhan, S., Ben Djebara, M., Damak, M., Dallali, H., Kefi, R., et al. (2017). Using KASP technique to screen LRRK2 G2019S mutation in a large Tunisian cohort. BMC Med. Genet. 18:70. doi: 10.1186/s12881-017-0432-5
Lesage, S., Durr, A., Tazir, M., Lohmann, E., Leutenegger, A. L., Janin, S., et al. (2006). LRRK2 G2019S as a cause of Parkinson's disease in North African Arabs. N. Engl. J. Med. 354, 422–423. doi: 10.1056/NEJMc055540
Li, B., Zhen, C., Wang, X., Chen, X., and Zhang, X. (2013). Association between G2385R polymorphisms of LRRK2 gene and Parkinson's disease. Zhejiang Med. J. 881–884.
Li, C., Ting, Z., Qin, X., Ying, W., Li, B., Guo Qiang, L., et al. (2007). The prevalence of LRRK2 Gly2385Arg variant in Chinese Han population with Parkinson's disease. Mov. Disord. 22, 2439–2443. doi: 10.1002/mds.21763
Li, K., Tang, B. S., Liu, Z. H., Kang, J. F., Zhang, Y., Shen, L., et al. (2015). LRRK2 A419V variant is a risk factor for Parkinson's disease in Asian population. Neurobiol. Aging 36, 2908 e2911–e2905. doi: 10.1016/j.neurobiolaging.2015.07.012
Li, N. N., Tan, E. K., Chang, X. L., Mao, X. Y., Zhang, J. H., Zhao, D. M., et al. (2012). Genetic analysis of LRRK2 A419V variant in ethnic Chinese. Neurobiol. Aging 33, 1849 e1841–1843. doi: 10.1016/j.neurobiolaging.2012.02.013
Li, X. X., Liao, Q., Xia, H., and Yang, X. L. (2015). Association between Parkinson's disease and G2019S and R1441C mutations of the LRRK2 gene. Exp. Ther. Med. 10, 1450–1454. doi: 10.3892/etm.2015.2659
Li, Z., An, X., Lin, Q., Ma, Q., and Zeng, J. (2013). Single nucleotide polymorphism analysis for LRRK2 rs34778348: G > A of Parkinson's disease. J. Xiamen Univ. (Nat. Sci.) 52, 860–865. doi: 10.6043/j.issn.0438-0479.2013.06.022
Lin, C. H., Wu, R. M., Tai, C. H., Chen, M. L., and Hu, F. C. (2011). Lrrk2 S1647T and BDNF V66M interact with environmental factors to increase risk of Parkinson's disease. Parkinsonism Relat. Disord. 17, 84–88. doi: 10.1016/j.parkreldis.2010.11.011
Liu, Z., Guo, J., Wang, Y., Li, K., Kang, J., Wei, Y., et al. (2016). Lack of association between IL-10 and IL-18 gene promoter polymorphisms and Parkinson's disease with cognitive impairment in a Chinese population. Sci. Rep. 6:19021. doi: 10.1038/srep19021
Lu, C. S., Wu-Chou, Y. H., van Doeselaar, M., Simons, E. J., Chang, H. C., Breedveld, G. J., et al. (2008). The LRRK2 Arg1628Pro variant is a risk factor for Parkinson's disease in the Chinese population. Neurogenetics 9, 271–276. doi: 10.1007/s10048-008-0140-6
Luzon-Toro, B., Rubio de la Torre, E., Delgado, A., Perez-Tur, J., and Hilfiker, S. (2007). Mechanistic insight into the dominant mode of the Parkinson's disease-associated G2019S LRRK2 mutation. Hum. Mol. Genet. 16, 2031–2039. doi: 10.1093/hmg/ddm151
Ma, Q., An, X., Li, Z., Zhang, H., Huang, W., Cai, L., et al. (2013). P268S in NOD2 associates with susceptibility to Parkinson's disease in Chinese population. Behav. Brain Funct. 9:19. doi: 10.1186/1744-9081-9-19
Marongiu, R., Ghezzi, D., Ialongo, T., Soleti, F., Elia, A., Cavone, S., et al. (2006). Frequency and phenotypes of LRRK2 G2019S mutation in Italian patients with Parkinson's disease. Mov. Disord. 21, 1232–1235. doi: 10.1002/mds.20890
Mata, I. F., Ross, O. A., Kachergus, J., Huerta, C., Ribacoba, R., Moris, G., et al. (2006). LRRK2 mutations are a common cause of Parkinson's disease in Spain. Eur. J. Neurol. 13, 391–394. doi: 10.1111/j.1468-1331.2006.01256.x
Mata, I. F., Taylor, J. P., Kachergus, J., Hulihan, M., Huerta, C., Lahoz, C., et al. (2005). LRRK2 R1441G in Spanish patients with Parkinson's disease. Neurosci. Lett. 382, 309–311. doi: 10.1016/j.neulet.2005.03.033
Miyake, Y., Tsuboi, Y., Koyanagi, M., Fujimoto, T., Shirasawa, S., Kiyohara, C., et al. (2010). LRRK2 Gly2385Arg polymorphism, cigarette smoking, and risk of sporadic Parkinson's disease: a case-control study in Japan. J. Neurol. Sci. 297, 15–18. doi: 10.1016/j.jns.2010.07.002
Nichols, W. C., Pankratz, N., Hernandez, D., Paisan-Ruiz, C., Jain, S., Halter, C. A., et al. (2005). Genetic screening for a single common LRRK2 mutation in familial Parkinson's disease. Lancet 365, 410–412. doi: 10.1016/S0140-6736(05)17828-3
Orr-Urtreger, A., Shifrin, C., Rozovski, U., Rosner, S., Bercovich, D., Gurevich, T., et al. (2007). The LRRK2 G2019S mutation in Ashkenazi Jews with Parkinson disease: is there a gender effect? Neurology 69, 1595–1602. doi: 10.1212/01.wnl.0000277637.33328.d8
Ozelius, L. J., Senthil, G., Saunders-Pullman, R., Ohmann, E., Deligtisch, A., Tagliati, M., et al. (2006). LRRK2 G2019S as a cause of Parkinson's disease in Ashkenazi Jews. N. Engl. J. Med. 354, 424–425. doi: 10.1056/NEJMc055509
Paisan-Ruiz, C. (2009). LRRK2 gene variation and its contribution to Parkinson disease. Hum. Mutat. 30, 1153–1160. doi: 10.1002/humu.21038
Patra, B., Parsian, A. J., Racette, B. A., Zhao, J. H., Perlmutter, J. S., and Parsian, A. (2009). LRRK2 gene G2019S mutation and SNPs [haplotypes] in subtypes of Parkinson's disease. Parkinsonism Relat. Disord. 15, 175–180. doi: 10.1016/j.parkreldis.2008.05.004
Pchelina, S. N., Yakimovskii, A. F., Ivanova, O. N., Emelianov, A. K., Zakharchuk, A. H., and Schwarzman, A. L. (2006). G2019S LRRK2 mutation in familial and sporadic Parkinson's disease in Russia. Mov. Disord. 21, 2234–2236. doi: 10.1002/mds.21134
Pulkes, T., Papsing, C., Mahasirimongkol, S., Busabaratana, M., Kulkantrakorn, K., and Tiamkao, S. (2011). Frequencies of LRRK2 variants in Thai patients with Parkinson's disease: evidence for an R1628P founder. J. Neurol. Neurosurg. Psychiatr. 82, 1179–1180. doi: 10.1136/jnnp.2009.194597
Pulkes, T., Papsing, C., Thakkinstian, A., Pongpakdee, S., Kulkantrakorn, K., Hanchaiphiboolkul, S., et al. (2014). Confirmation of the association between LRRK2 R1628P variant and susceptibility to Parkinson's disease in the Thai population. Parkinsonism Relat. Disord. 20, 1018–1021. doi: 10.1016/j.parkreldis.2014.06.013
Punia, S., Behari, M., Govindappa, S. T., Swaminath, P. V., Jayaram, S., Goyal, V., et al. (2006). Absence/rarity of commonly reported LRRK2 mutations in Indian Parkinson's disease patients. Neurosci. Lett. 409, 83–88. doi: 10.1016/j.neulet.2006.04.052
Risch, N., Burchard, E., Ziv, E., and Tang, H. (2002). Categorization of humans in biomedical research: genes, race and disease. Genome Biol 3:comment2007.1–comment2007.12.
Ross, O. A., Wu, Y. R., Lee, M. C., Funayama, M., Chen, M. L., Soto, A. I., et al. (2008). Analysis of Lrrk2 R1628P as a risk factor for Parkinson's disease. Ann. Neurol. 64, 88–92. doi: 10.1002/ana.21405
Schapira, A. H. (2006). The importance of LRRK2 mutations in Parkinson disease. Arch. Neurol. 63, 1225–1228. doi: 10.1001/archneur.63.9.1225
Schlitter, A. M., Woitalla, D., Mueller, T., Epplen, J. T., and Dekomien, G. (2006). The LRRK2 gene in Parkinson's disease: mutation screening in patients from Germany. J. Neurol. Neurosurg. Psychiatr. 77, 891–892. doi: 10.1136/jnnp.2005.083022
Shu, L., Zhang, Y., Pan, H., Xu, Q., Guo, J., Tang, B., et al. (2018). Clinical heterogeneity among LRRK2 variants in Parkinson's disease: a meta-analysis. Front. Aging Neurosci. 10:283. doi: 10.3389/fnagi.2018.00283
Tan, E. K., Lim, H. Q., Yuen, Y., and Zhao, Y. (2008a). Pathogenicity of LRRK2 P755L variant in Parkinson's disease. Mov. Disord. 23, 734–736. doi: 10.1002/mds.21852
Tan, E. K., Shen, H., Tan, L. C., Farrer, M., Yew, K., Chua, E., et al. (2005). The G2019S LRRK2 mutation is uncommon in an Asian cohort of Parkinson's disease patients. Neurosci. Lett. 384, 327–329. doi: 10.1016/j.neulet.2005.04.103
Tan, E. K., Tan, L. C., Lim, H. Q., Li, R., Tang, M., Yih, Y., et al. (2008b). LRRK2 R1628P increases risk of Parkinson's disease: replication evidence. Hum. Genet. 124, 287–288. doi: 10.1007/s00439-008-0544-2
Tan, E. K., Tang, M., Tan, L. C., Wu, Y. R., Wu, R. M., Ross, O. A., et al. (2008c). Lrrk2 R1628P in non-Chinese Asian races. Ann. Neurol. 64, 472–473. doi: 10.1002/ana.21467
Tan, E. K., Zhao, Y., Skipper, L., Tan, M. G., Di Fonzo, A., Sun, L., et al. (2007a). The LRRK2 Gly2385Arg variant is associated with Parkinson's disease: genetic and functional evidence. Hum. Genet. 120, 857–863. doi: 10.1007/s00439-006-0268-0
Tan, E. K., Zhao, Y., Tan, L., Lim, H. Q., Lee, J., Yuen, Y., et al. (2007b). Analysis of LRRK2 Gly2385Arg genetic variant in non-Chinese Asians. Mov. Disord. 22, 1816–1818. doi: 10.1002/mds.21658
Toft, M., Haugarvoll, K., Ross, O. A., Farrer, M. J., and Aasly, J. O. (2007). LRRK2 and Parkinson's disease in Norway. Acta Neurol. Scand. Suppl. 187, 72–75. doi: 10.1111/j.1600-0404.2007.00852.x
Tomiyama, H., Mizuta, I., Li, Y., Funayama, M., Yoshino, H., Li, L., et al. (2008). LRRK2 P755L variant in sporadic Parkinson's disease. J. Hum. Genet. 53, 1012–1015. doi: 10.1007/s10038-008-0336-5
Vishwanathan Padmaja, M., Jayaraman, M., Srinivasan, A. V., Srikumari Srisailapathy, C. R., and Ramesh, A. (2012). The SNCA (A53T, A30P, E46K) and LRRK2 (G2019S) mutations are rare cause of Parkinson's disease in South Indian patients. Parkinsonism Relat. Disord. 18, 801–802. doi: 10.1016/j.parkreldis.2012.02.012
Wang, C., Cai, Y., Zheng, Z., Tang, B. S., Xu, Y., Wang, T., et al. (2012). Penetrance of LRRK2 G2385R and R1628P is modified by common PD-associated genetic variants. Parkinsonism Relat. Disord. 18, 958–963. doi: 10.1016/j.parkreldis.2012.05.003
West, A. B., Moore, D. J., Biskup, S., Bugayenko, A., Smith, W. W., Ross, C. A., et al. (2005). Parkinson's disease-associated mutations in leucine-rich repeat kinase 2 augment kinase activity. Proc. Natl. Acad. Sci. U.S.A. 102, 16842–16847. doi: 10.1073/pnas.0507360102
Williams-Gray, C. H., Goris, A., Foltynie, T., Brown, J., Maranian, M., Walton, A., et al. (2006). Prevalence of the LRRK2 G2019S mutation in a UK community based idiopathic Parkinson's disease cohort. J. Neurol. Neurosurg. Psychiatr. 77, 665–667. doi: 10.1136/jnnp.2005.085019
Wolf, L. A. (2015). Clinical research: the importance of meta-analysis and systematic reviews in determining appropriate practice changes. J. Emerg. Nurs. 41, 360–361. doi: 10.1016/j.jen.2015.04.015
Wu, T., Zeng, Y., Ding, X., Li, X., Li, W., Dong, H., et al. (2006). A novel P755L mutation in LRRK2 gene associated with Parkinson's disease. Neuroreport 17, 1859–1862. doi: 10.1097/WNR.0b013e328010521c
Wu, Y. R., Chang, K. H., Chang, W. T., Hsiao, Y. C., Hsu, H. C., Jiang, P. R., et al. (2013). Genetic variants ofLRRK2 in Taiwanese Parkinson's disease. PLoS ONE 8:e82001. doi: 10.1371/journal.pone.0082001
Wu-Chou, Y. H., Chen, Y. T., Yeh, T. H., Chang, H. C., Weng, Y. H., Lai, S. C., et al. (2013). Genetic variants of SNCA and LRRK2 genes are associated with sporadic PD susceptibility: a replication study in a Taiwanese cohort. Parkinsonism Relat. Disord. 19, 251–255. doi: 10.1016/j.parkreldis.2012.10.019
Xie, C. L., Pan, J. L., Wang, W. W., Zhang, Y., Zhang, S. F., Gan, J., et al. (2014). The association between the LRRK2 G2385R variant and the risk of Parkinson's disease: a meta-analysis based on 23 case-control studies. Neurol. Sci. 35, 1495–1504. doi: 10.1007/s10072-014-1878-2
Xiromerisiou, G., Hadjigeorgiou, G. M., Gourbali, V., Johnson, J., Papakonstantinou, I., Papadimitriou, A., et al. (2007). Screening for SNCA and LRRK2 mutations in Greek sporadic and autosomal dominant Parkinson's disease: identification of two novel LRRK2 variants. Eur. J. Neurol. 14, 7–11. doi: 10.1111/j.1468-1331.2006.01551.x
Yan, H., Li, H., and Yang, X. (2012a). Correlation between LRRK2 gene S1647T polymorphism and Parkinson′s disease in Xinjiang Uygur and Han Nationalities. J. Med. Postgrad. 25, 729–733. doi: 10.3969/j.issn.1008-8199.2012.07.011
Yan, H., Ma, Q., Yang, X., Wang, Y., Yao, Y., and Li, H. (2012b). Correlation between LRRK2 gene G2385R polymorphisms and Parkinson's disease. Mol. Med. Rep. 6, 879–883. doi: 10.3892/mmr.2012.1008
Yao, L. Y., Guo, J. F., Wang, L., Yu, R. H., Sun, Q. Y., Pan, Q., et al. (2011). LRRK2 Pro755Leu variant in ethnic Chinese population with Parkinson's disease. Neurosci. Lett. 495, 35–38. doi: 10.1016/j.neulet.2011.03.030
Yescas, P., Lopez, M., Monroy, N., Boll, M. C., Rodriguez-Violante, M., Rodriguez, U., et al. (2010). Low frequency of common LRRK2 mutations in Mexican patients with Parkinson's disease. Neurosci. Lett. 485, 79–82. doi: 10.1016/j.neulet.2010.08.029
Yu, L., Hu, F., Zou, X., Jiang, Y., Liu, Y., He, X., et al. (2009). LRRK2 R1628P contributes to Parkinson's disease susceptibility in Chinese Han populations from mainland China. Brain Res. 1296, 113–116. doi: 10.1016/j.brainres.2009.08.047
Zabetian, C. P., Yamamoto, M., Lopez, A. N., Ujike, H., Mata, I. F., Izumi, Y., et al. (2009). LRRK2 mutations and risk variants in Japanese patients with Parkinson's disease. Mov. Disord. 24, 1034–1041. doi: 10.1002/mds.22514
Zhang, P., Wang, Q., Jiao, F., Yan, J., Chen, L., He, F., et al. (2016). Association of LRRK2 R1628P variant with Parkinson's disease in Ethnic Han-Chinese and subgroup population. Sci. Rep. 6:35171. doi: 10.1038/srep35171
Zhang, Y., Shu, L., Sun, Q., Zhou, X., Pan, H., Guo, J., et al. (2018). Integrated genetic analysis of racial differences of common GBA variants in Parkinson's disease: a meta-analysis. Front. Mol. Neurosci. 11:43. doi: 10.3389/fnmol.2018.00043
Zhang, Y., Sun, Q., Yi, M., Zhou, X., Guo, J., Xu, Q., et al. (2017). Genetic analysis of LRRK2 R1628P in Parkinson's disease in Asian populations. Parkinsons Dis. 2017:8093124. doi: 10.1155/2017/8093124
Zhang, Z., Burgunder, J. M., An, X., Wu, Y., Chen, W., Zhang, J., et al. (2009). LRRK2 R1628P variant is a risk factor of Parkinson's disease among Han-Chinese from mainland China. Mov. Disord. 24, 1902–1905. doi: 10.1002/mds.22371
Zhao, H., and Kong, Z. (2016). Relationship between LRRK2 R1628P polymorphism and Parkinson's disease in Asian populations. Oncotarget 7, 46890–46898. doi: 10.18632/oncotarget.10378
Zheng, Y., Liu, Y., Wu, Q., Hong, H., Zhou, H., Chen, J., et al. (2011). Confirmation of LRRK2 S1647T variant as a risk factor for Parkinson's disease in southern China. Eur. J. Neurol. 18, 538–540. doi: 10.1111/j.1468-1331.2010.03164.x
Keywords: Parkinson's disease, LRRK2, Asian, European, variant
Citation: Shu L, Zhang Y, Sun Q, Pan H and Tang B (2019) A Comprehensive Analysis of Population Differences in LRRK2 Variant Distribution in Parkinson's Disease. Front. Aging Neurosci. 11:13. doi: 10.3389/fnagi.2019.00013
Received: 27 September 2018; Accepted: 14 January 2019;
Published: 30 January 2019.
Edited by:
Hanting Zhang, West Virginia University, United StatesReviewed by:
Guoyuan Yang, Shanghai Jiao Tong University, ChinaPeng Jin, Emory University School of Medicine, United States
Copyright © 2019 Shu, Zhang, Sun, Pan and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Beisha Tang, YnN0YW5nNzM5OEAxNjMuY29t
†These authors have contributed equally to this work and are co-first authors
 Li Shu
Li Shu Yuan Zhang
Yuan Zhang Qiying Sun
Qiying Sun Hongxu Pan1
Hongxu Pan1 Beisha Tang
Beisha Tang