- 1Civin Neuropathology Laboratory, Banner Sun Health Research Institute, Sun City, AZ, United States
- 2Division of Behavioral Neurology, Department of Neurology, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan
- 3Department of Neurology, National Taiwan University Hospital, College of Medicine, National Taiwan University, Taipei, Taiwan
- 4Department of Neurology, Taipei Medical University, Taipei, Taiwan
- 5Department of Neurology, Shuang Ho Hospital, Taipei Medical University, New Taipei City, Taiwan
- 6Department of Neurology, Kaohsiung Chang Gung Memorial Hospital, Kaohsiung, Taiwan
- 7Department of Internal Medicine, National Taiwan University Hospital, College of Medicine, National Taiwan University, Taipei, Taiwan
- 8Clinical Neurochemistry Laboratory, Sahlgrenska University Hospital, Mölndal, Sweden
- 9Department of Psychiatry and Neurochemistry, Institute of Neuroscience and Physiology, University of Gothenburg, Mölndal, Sweden
- 10Lou Ruvo Center for Brain Health, Cleveland Clinic Nevada, Las Vegas, NV, United States
- 11Department of Neurology, Renai Branch, Taipei City Hospital, Taipei, Taiwan
- 12Department of Neurology, National Yang-Ming University, Taipei, Taiwan
- 13Department of Neurology, Taipei Veterans General Hospital, Taipei, Taiwan
- 14MagQu Company Limited, New Taipei City, Taiwan
- 15MagQu LLC, Surprise, AZ, United States
- 16Hatsuta Neurology Clinic, Osaka, Japan
- 17Department of Neurology, Osaka City University Graduate School of Medicine, Osaka, Japan
- 18Department of Physiology, School of Medicine, Keio University, Tokyo, Japan
- 19Departemnt of Neurology, Sun Yat-Sen Memorial Hospital, Sun Yat-Sen University, Guangzhou, China
- 20Department of Neurology, Foshan Hospital of Sun Yat-Sen University, Foshan, China
A Corrigendum on
Age-Dependent Relationship Between Plasma Aβ40 and Aβ42 and Total Tau Levels in Cognitively Normal Subjects
by Lue, L.-F., Pai, M.-C., Chen, T.-F., Hu, C.-J., Huang, L.-K., Lin, W.-C., et al. (2019). Front. Aging Neurosci. 11:222. doi: 10.3389/fnagi.2019.00222
In the original article, there was an error. The name of a participating site was incorrectly written as “Keio University Hospital.” The correct site is “Hatsuta Neurology Clinic.”
A correction has therefore been made to the table and legend for Table 1:
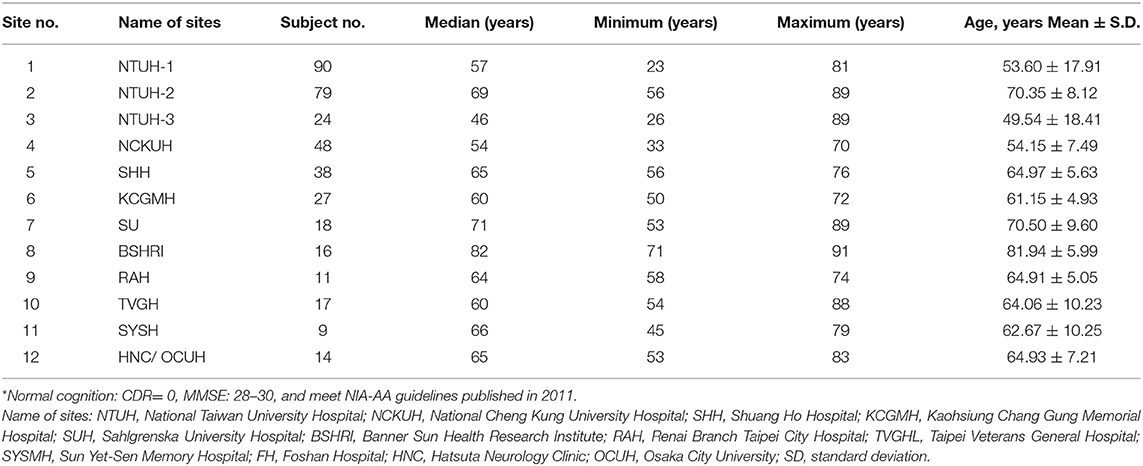
Table 1. The means and standard deviations (SD) of age (years) of the normal-cognition subjects in each participating site*.
Additionally, a correction has also been made to the Materials and Methods section, subsection Participating Sites:
“A total of 391 cognitively normal subjects aged 23–91 were enrolled from 2010 to 2018 from the following six hospitals in Taiwan: National Taiwan University Hospital (NTUH), Taipei Medical University Shuang-Ho Hospital (SHH), Renai Branch of Taipei City Hospital (RAH), Taipei Veterans General Hospital (TVGH), National Cheng Kung University Hospital (NCKUH), and Kaohsiung Chang Gung Memorial Hospital (KCGMH); Sahlgrenska University Hospital (SUH) in Guttenberg, Sweden; Banner Sun Health Research Institute (BSHRI) in Sun City, AZ, USA; two hospitals in the cities of Foshan, Foshan Hospital (FH) and Guangzhou, Sun Yat-Sen Memorial Hospital (SYSMH), Guangdong, China; and finally two hospitals in Japan: Hatsuta Neurology Clinic (HNC) in Osaka, and Osaka City University Hospital (OCUH) in Osaka. All participants were older than 21 years of age and gave their own written informed consent. The study was approved by the Institutional Review Board (IRB) or Research Ethics Committee (REC) of each participating hospital in the respective countries, namely, NTUH REC, Taipei Medical University-Joint IRB for SHH, Taipei City Hospital REC for RAH, TVGH IRB, NCKUH IRB, KCGMH IRB, Central Ethical Review Board-University of Gothenburg for SUH, Banner Health IRB for BSHRI, Sun Yat-Sen University Hospital (SYSUH) Cancer Center IRB, Asai Dermatology Clinic IRB and Osaka City University IRB.”
And the subsection Cognition Assessment and Criteria for Recruitment:
“The purpose of the recruitment criteria was to exclude subjects with diagnoses of MCI and dementia. All study sites followed the NIA-AA criteria for the diagnosis of dementia and MCI due to AD (Albert et al., 2011; McKhann et al., 2011). In addition to clinical criteria, basic cognitive assessment tools [Mini-Mental State Examination (MMSE) and Clinical Dementia Rating (CDR)] were also used. The criteria for normal cognition were MMSE ≥ 28 and CDR = 0. Brain imaging and CSF biomarkers were used as supplementary tools. Brain (FDG)-PET were used by HNC/OCUH, Japan, and Subjects from SUH, Sweden had CSF Ab > 530 pg/ml and t-Tau <350 pg/ml (Sutphen et al., 2015; Teunissen et al., 2018). Subjects who had acute or chronic systemic diseases or neuropsychiatric disorders, visual or auditory dysfunction severe enough to interfere with cognitive assessments were all excluded.”
The authors apologize for these errors and state that this does not change the scientific conclusions of the article in any way. The original article has been updated.
References
Albert, M. S., DeKosky, S. T., Dickson, D., Dubois, B., Feldman, H. H., Fox, N. C., et al. (2011). The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 7, 270–279. doi: 10.1016/j.jalz.2011.03.008
McKhann, G. M., Knopman, D. S., Chertkow, H., Hyman, B. T., Jack, C. R. Jr., Kawas, C. H., et al. (2011). The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 7, 263–269. doi: 10.1016/j.jalz.2011.03.005
Sutphen, C. L., Jasielec, M. S., Shah, A. R., Macy, E. M., Xiong, C., Vlassenko, A. G., et al. (2015). Longitudinal cerebrospinal fluid biomarker changes inpreclinical Alzheimer disease during middle age. JAMA Neurol. 72, 1029–1042. doi: 10.1001/jamaneurol.2015.1285
Keywords: Alzheimer, plasma, amyloid, tau, immunomagnetic reduction, cognitively normal subjects
Citation: Lue L-F, Pai M-C, Chen T-F, Hu C-J, Huang L-K, Lin W-C, Wu C-C, Jeng J-S, Blennow K, Sabbagh MN, Yan S-H, Wang P-N, Yang S-Y, Hatsuta H, Morimoto S, Takeda A, Itoh Y, Liu J, Xie H and Chiu M-J (2019) Corrigendum: Age-Dependent Relationship Between Plasma Aβ40 and Aβ42 and Total Tau Levels in Cognitively Normal Subjects. Front. Aging Neurosci. 11:292. doi: 10.3389/fnagi.2019.00292
Received: 01 October 2019; Accepted: 09 October 2019;
Published: 08 November 2019.
Approved by:
Frontiers Editorial Office, Frontiers Media SA, SwitzerlandCopyright © 2019 Lue, Pai, Chen, Hu, Huang, Lin, Wu, Jeng, Blennow, Sabbagh, Yan, Wang, Yang, Hatsuta, Morimoto, Takeda, Itoh, Liu, Xie and Chiu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ming-Jang Chiu, bWpjaGl1QG50dS5lZHUudHc=
 Lih-Fen Lue
Lih-Fen Lue Ming-Chyi Pai2
Ming-Chyi Pai2 Ta-Fu Chen
Ta-Fu Chen Chaur-Jong Hu
Chaur-Jong Hu Li-Kai Huang
Li-Kai Huang Wei-Che Lin
Wei-Che Lin Chau-Chung Wu
Chau-Chung Wu Jian-Shing Jeng
Jian-Shing Jeng Kaj Blennow
Kaj Blennow Marwan N. Sabbagh
Marwan N. Sabbagh Pei-Ning Wang
Pei-Ning Wang Shieh-Yueh Yang
Shieh-Yueh Yang Satoru Morimoto
Satoru Morimoto Akitoshi Takeda
Akitoshi Takeda Haiqun Xie
Haiqun Xie Ming-Jang Chiu
Ming-Jang Chiu