- Department of Neurology, The First Affiliated Hospital of China Medical University, Shenyang, China
Background: Recent studies have reported that homocysteine (Hcy) may play a vital role in the pathogenesis of vascular dementia (VaD) and Alzheimer's disease (AD). Our study explored the relationship between the plasma Hcy and folate levels and the risk of dementia.
Methods: We searched Embase, PubMed, and Web of Science for published literature, including case-control studies and prospective cohort studies, and performed a systematic analysis.
Results: The results of our meta-analysis, consisting of case-control studies, showed higher levels of Hcy and lower levels of folate in dementia, AD, and VaD patients than those in non-demented controls (for dementia: SMD = 0.812, 95% CI [0.689, 0.936], p = 0.000 for Hcy; SMD = −0.677, 95% CI [−0.828, −0.525], p = 0.000 for folate). AD patients showed significantly lower plasma Hcy levels compared to VaD patients (SMD = −0.278, 95% CI [−0.466, −0.09], p = 0.000). Subgroup analysis revealed that ethnicity, average age, and dementia type had no significant effect on this association. Furthermore, from the analysis of prospective cohort studies, we identified that elevated plasma Hcy levels were associated with an increased risk of dementia, AD, and VaD (RRdementia = 1.22, 95% CI [1.08, 1.36]; RRAD = 1.07, 95% CI [1.04, 1.11]; RRVaD = 1.13, 95% CI [1.04, 1.23]). In addition, every 5 μmol/L increase in the plasma Hcy level was associated with a 9% increased risk of dementia and a 12% increased risk of AD.
Conclusion: Hcy and folic acid are potential predictors of the occurrence and development of AD. A better understanding of their function in dementia could provide evidence for clinicians to rationalize clinical intervention strategies.
Introduction
Dementia is a severe threat to the health of older people and places a heavy burden on their families and society (Brookmeyer et al., 2007). Alzheimer's disease (AD) is the most common form of dementia and is characterized by β-amyloid (Aβ)-containing plaques and neurofibrillary tangles (NFT) (Lane et al., 2018). Vascular dementia (VaD) is the second most common form of dementia and has similar clinical manifestations that are caused by specific ischemic lesions, such as thalamus and white matter. There has not yet been an effective therapy for dementia, and it is urgent to identify considerable methods to delay cognitive decline. It has been reported that subjects with vascular risk factors are more susceptible to VaD and AD. Plasma total homocysteine (Hcy), as a common risk factor for vascular diseases, could be modified by supplementation with folic acid and B vitamin as well as relevant with dementia (Beydoun et al., 2014).
Several recent studies have focused on the role of Hcy, vitamin B12, and folate as possible participants in the neurodegenerative processes. Hcy is a sulfur-containing amino acid produced during methionine metabolism. Hcy remethylation requires folate as the methyl donor and vitamin B12 as the co-factor; deficiencies of vitamin B12 or folate would lead to hyperhomocysteinemia. Additionally, Hcy can also be converted to cystathionine via transsulfuration (Joosten, 2001). Thus, Hcy and folate play an important role in the transfer of methyl groups in cellular metabolism, which contributes to the pathogenesis of AD (Smith and Refsum, 2016).
The association between Hcy levels and dementia risk has been studied for many years. However, the results remain controversial (Clarke et al., 1998; Leblhuber et al., 2000; Miller et al., 2002; Quadri et al., 2004). A previous meta-analysis by Van Dam et al. demonstrated higher Hcy levels and lower vitamin B12 and folic acid levels in AD patients (Van Dam and Van Gool, 2009). They also indicated that folate, vitamin B6, and vitamin B12 levels were related to the risk of developing AD. Subsequently, a meta-analysis of relevant epidemiological studies by Beydoun et al. indicated that a high Hcy level represented a modifiable risk factor for cognitive dysfunction and was a strong predictor of the incidence of AD (Beydoun et al., 2014). A study of 1,249 elderly patients performed by Ariogul et al. failed to find any correlation between Hcy levels, folate levels, and cognitive function (Ariogul et al., 2005). Another study by Faux et al. showed that Hcy levels were higher in female AD patients compared to those in female healthy control subjects, however, that was not observed in male subjects (Faux et al., 2011). Moreover, current researches also found that factors of ethnicity and age might contribute to the inheritance and development of dementia. Therefore, we performed an updated comprehensive meta-analysis to further evaluate the association of plasma Hcy and folate levels with dementia risk covering all currently available data in order to provide evidence for clinical practice. To achieve this, we first performed a systematic analysis of case–control studies on Hcy and folate levels in dementia patients and divided these into subgroups according to dementia type, geographical ethnicity, average age, and gender distribution. We then focused on prospective studies to explore the association between Hcy levels and the risk of developing AD and VaD.
Materials and Methods
Search Strategy
We searched the electronic databases PubMed, Web of Science, and EMBASE to identify suitable studies published prior to December 2020 using specific keywords, using the following search strategies: (1) Homocysteine OR “Hcy” OR Hyperhomocysteinemia OR Folate OR Folic acid and (2) Dementia OR Cognitive OR Cognition OR Alzheimer OR Vascular dementia. Some articles were identified via manual screening of relevant references from other studies on the subject.
Inclusion and Exclusion Criteria
The inclusion criteria for the meta-analysis of case-control studies were that the studies must provide the number of dementia patients and controls and clearly present means and standard deviations (SD) of Hcy and/or folic acid levels for both patients and controls. For the meta-analysis of prospective cohort studies, studies must present blood Hcy concentration levels, adjusted relative risk (RR), and 95% confidence intervals (CI) for dementia or AD occurrence.
The exclusion criteria were as follows: (1) we selected population-based case-control studies and cohort studies to extract raw data, case report, review, letters were all excluded; (2) we designated two independent reviewers to scrutinize the manuscripts of retrieval, irrelevant titles and abstracts were excluded; and (3) studies with incomplete data were excluded such as undefined included cohorts and imprecise level of Hcy and folic acid.
Study Quality and Data Extraction
Two reviewers independently assessed the included studies. The extracted data included the name of the first author, publication year, the ethnicity of samples, the number of subjects, gender distribution, mean age, mean follow-up duration, mean and SD of Hcy and folate levels for patients and controls, source and detection method used for Hcy and folate analyses, categories of plasma Hcy, and multivariable-adjusted effects (RR and 95% CI for each exposure category). Different Hcy and folate concentration measurement units were converted to a standard format (μmol/L for Hcy and nmol/L for folate).
The Newcastle-Ottawa Scale (NOS) (Stang, 2010) was used to assess the quality of the included studies. The NOS contains three domains (study selection, group comparability, and cohort exposure) covering four, two, and three points. The total NOS score ranged from zero to nine stars, and six to nine points were considered high quality. All the selected articles scored six or more stars in this system (Supplementary Table 1).
All the included studies were approved by ethics committees and conducted according to the Code of Ethics of the World Medical Association (Declaration of Helsinki). All the participants of the included studies provided written informed consent.
Statistical Analysis
The standard mean differences (SMD) with a 95% confidence interval (CI) were calculated to explore the differences in Hcy and folate levels between the dementia and control groups. The pooled RR with 95% CI was used to evaluate the relationship between the Hcy and folate levels and the risk of dementia. Heterogeneity was evaluated using the Q-test and the I2-test. I2 > 50% represented high heterogeneity. Thus, the random-effects model was applicable. Subgroup analyses—stratified by dementia type (AD and VaD), geographical ethnicity (Caucasian or Asian), average age (60 ≤ age < 70, 70 ≤ age < 80, or age ≥ 80), and gender distribution (male/total ≥ 50%, male/total < 50%)—were also conducted. A dose-response meta-analysis was used to assess the potential linear and non-linear dose-response relationships between blood Hcy levels and the risk of dementia. Sensitivity analysis was used to assess the results after removing any of the studies one at a time. The risk of publication bias was assessed using Egger's test and Begg's test. A value of p ≥ 0.05 indicated no publication bias. If publication bias was observed, we applied the trim and fill method to recalculate the results. All meta-analyses were performed using Stata version 15.1.
Results
Study Selection and Characteristics
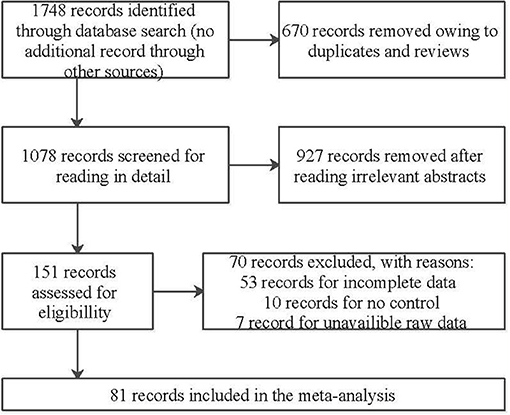
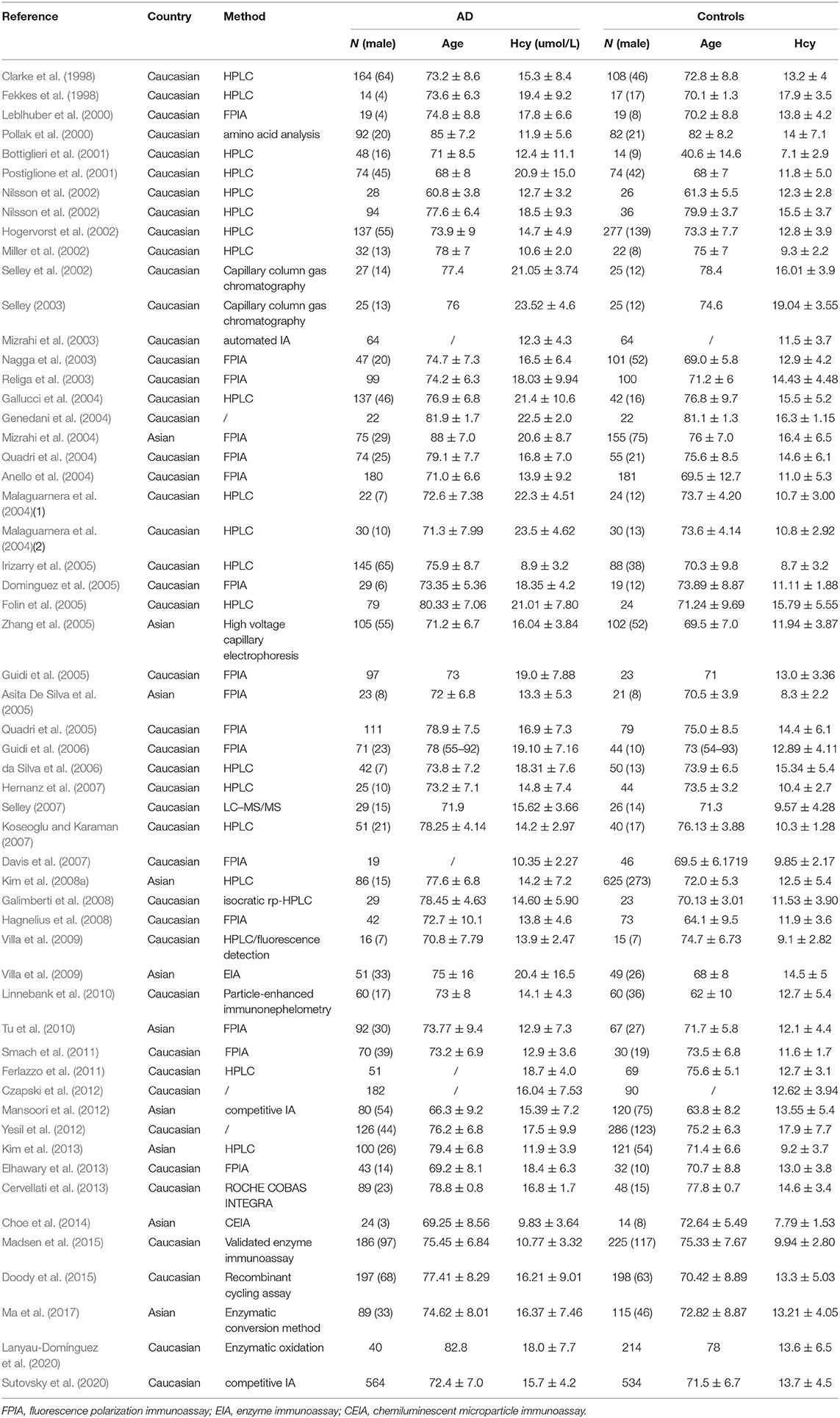
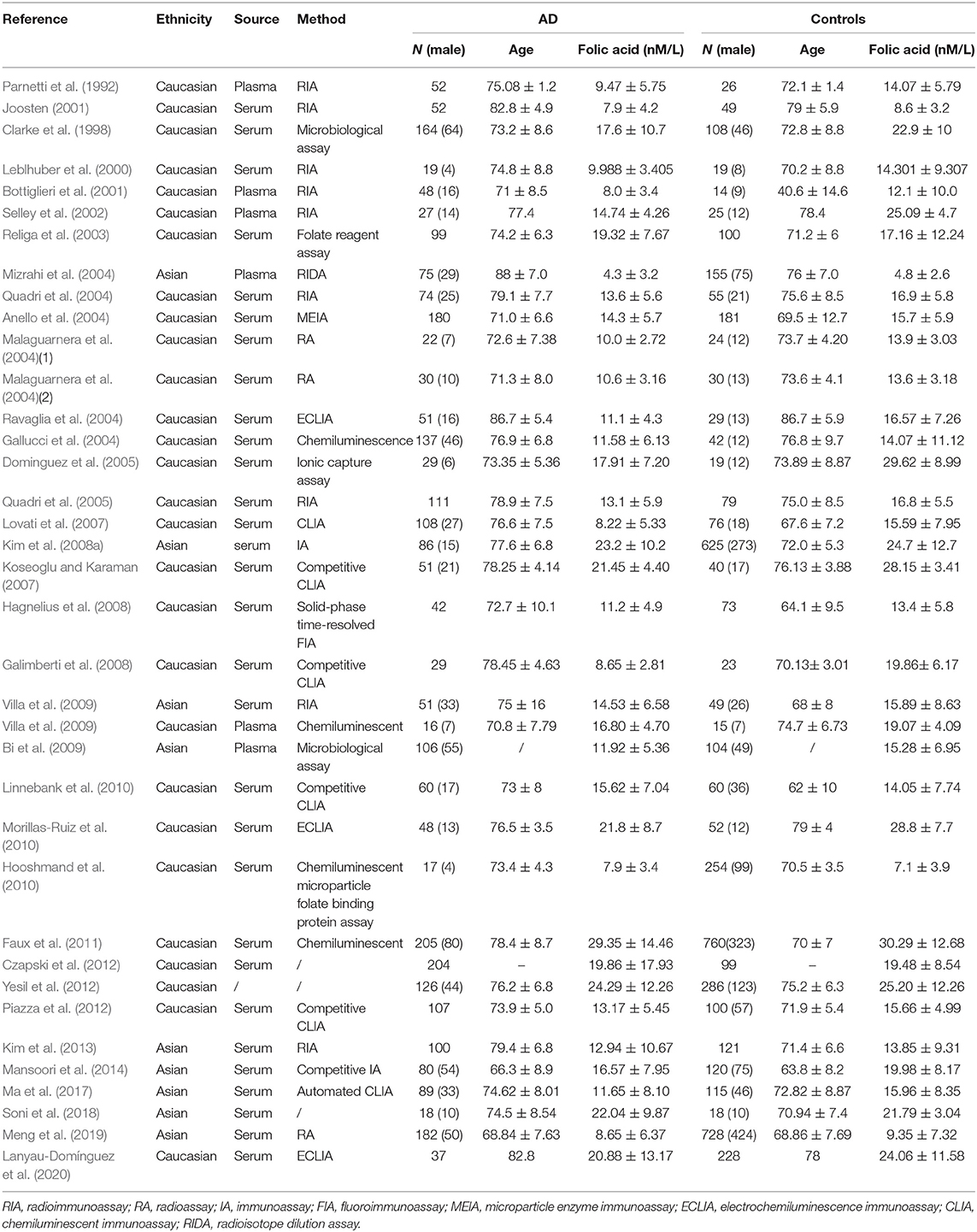
We initially identified 1,746 relevant articles from available databases, including Embase, Pubmed, and Web of Science, and 1,078 of the articles were left after removing duplications and reviews. We excluded 927 articles after reading titles or abstracts and selected 151 full-text articles for eligibility. Subsequently, 70 articles were removed on account of failing to data extraction after careful perusal, thus 81 articles were included in this meta-analysis eventually. Thereinto, 55 and 37 articles were retrospective researches relevant to Hcy and folic acid, and 13 articles and 5 articles were prospective aiming at Hcy and folic acid, respectively. A flow diagram of the retrieval process is illustrated in Figure 1. The baseline characteristics of the included studies are presented in Tables 1, 2, Supplementary Tables 3, 4. In our study, serum or plasma folate and plasma Hcy levels were studied. A total of 3,240 dementia patients (2,932 for AD and 308 for VaD) and 4,901 non-dementia controls were enrolled for the analysis of folate. Five thousand one hundred and fifty-one cases (4,547 for AD and 604 for VaD) and 5,113 controls were examined for Hcy. In addition, 15,134 samples and 1,771 cases were enrolled in our meta-analysis, based on information obtained from prospective studies (Supplementary Table 2).
Meta-analysis
Plasma Hcy and Folate Levels in Dementia Patients
We discovered that heterogeneity in the SMD of Hcy and folate existed in all sets of the analyzed groups among various studies (P < 0.05) (Supplementary Figure 1). Further, a random-effects model was used to take additional account of study variation. Our study found that plasma Hcy levels were higher in dementia patients than in controls, with an SMD of 0.812 (95% CI [0.689–0.936], p = 0.000) (Figure 2). Our study also found that folate levels were lower in dementia patients than in controls, with an SMD of −0.568 (95% CI [−0.705, −0.431], p = 0.000) (Figure 3). Begg's and Egger's tests indicated publication bias (p = 0.000 and p = 0.000, respectively). Therefore, we used the trim and fill method to recalculate the pooled effect size, and the results were slightly altered but still similar to our original risk estimate (SMD = 0.812, 95% CI [0.689, 0.936], p = 0.000 for Hcy; SMD = −0.677, 95% CI [−0.828, −0.525], p = 0.000 for folate) (Supplementary Figure 2). Sensitivity analysis revealed that the results were identical to the primary results after removing any one of the studies one at a time. This indicated that no single study exerted a substantial influence on the pooled effect size (ES) (Supplementary Figures 3, 4).
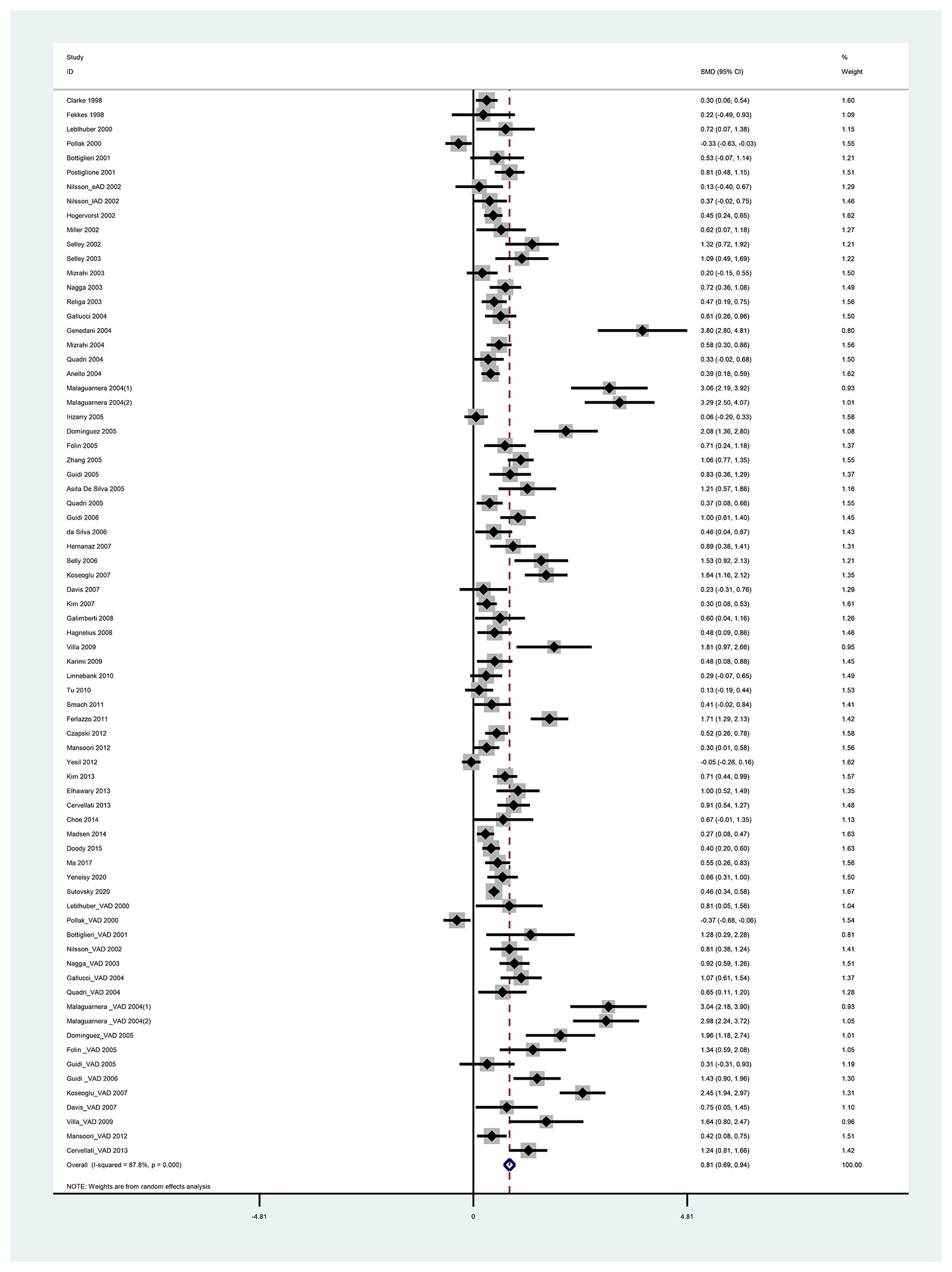
Figure 2. Forest plot of standard mean difference (SMD) and 95% confidence interval (95%CI) in dementia and control group for plasma homocysteine.
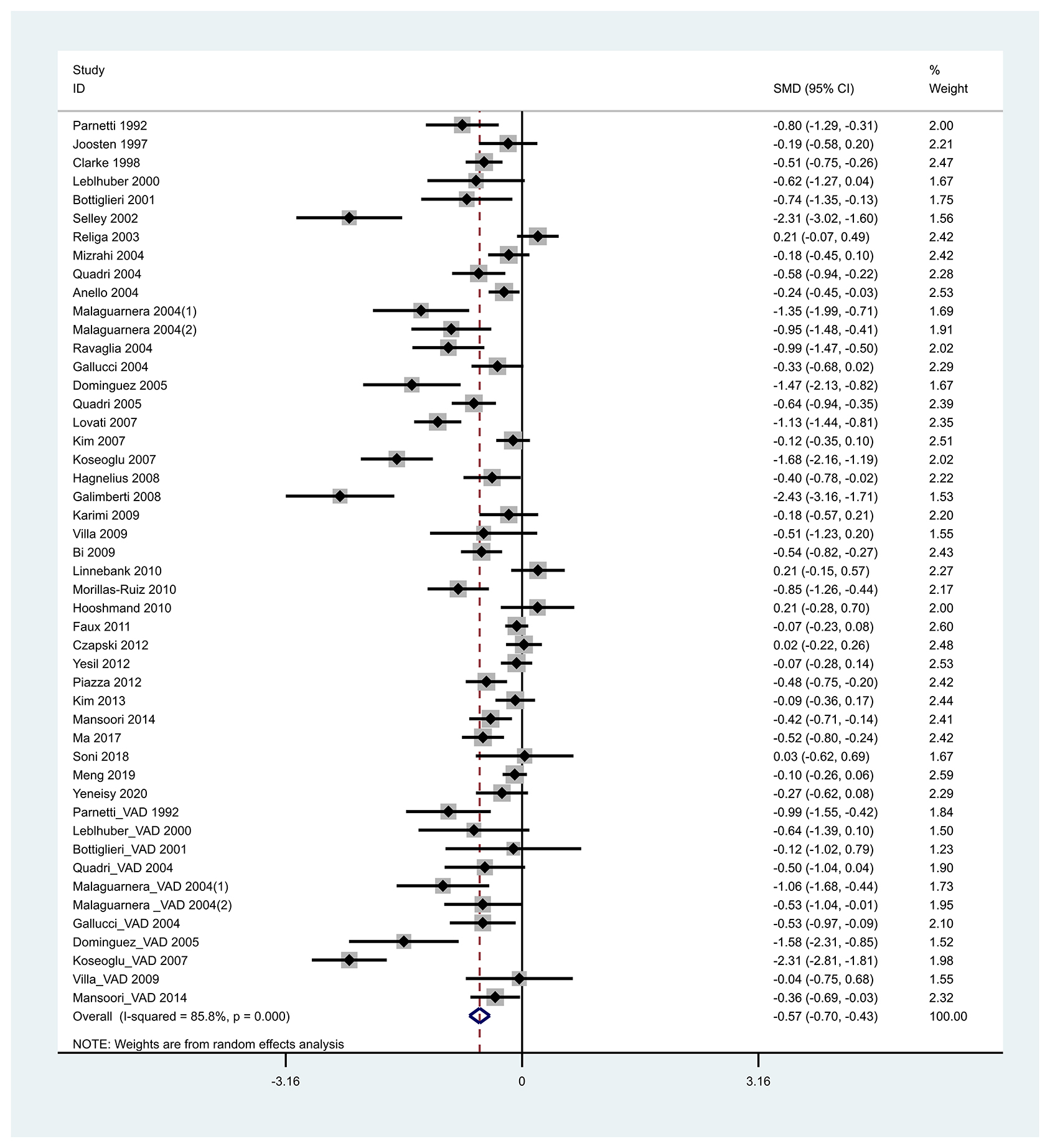
Figure 3. Forest plot of standard mean difference (SMD) and 95% confidence interval (95%CI) in dementia and control group for folic acid.
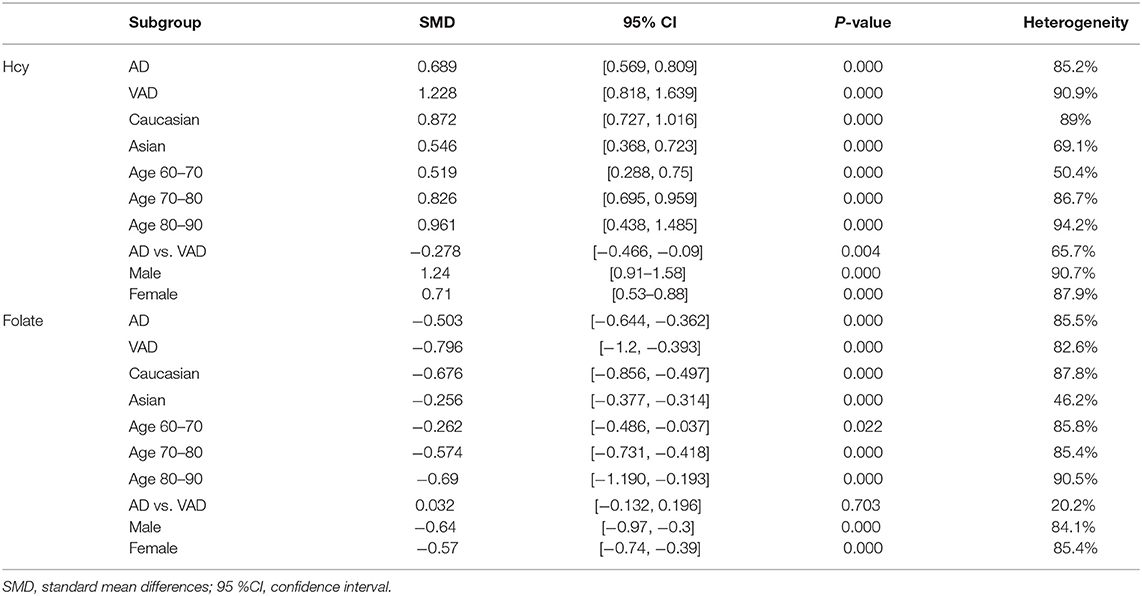
Subgroup Analysis
To further explore the effect of geographical ethnicity, average age, gender, and dementia type on plasma Hcy and folate levels in dementia patients and controls, subgroup analyses were performed. The 55 studies for Hcy and 37 studies for folate levels were divided into two subgroups based on the dementia type. Subgroup analysis showed lower folic acid levels in AD patients, with an SMD of −0.503 (95% CI [−0.644, −0.362], p < 0.05), and higher Hcy levels in AD patients, with an SMD of 0.689 (95% CI [0.569, 0.809], p < 0.05), than in the controls. This indicated significant differences in plasma Hcy and folate levels between AD patients and controls. Further, we also found that Hcy levels were higher and folate levels were lower in VaD patients than in the controls. Additionally, high Hcy and low folic acid levels in dementia patients were found in the Caucasian and Asian cohorts. Ethnicity also exhibited no significant effect on this association. Further subgroup analysis based on age (age 60–70, SMD = 0.519 [0.288, 0.75], p = 0.000; age 70–80, SMD = 0.826 [0.695, 0.959], p = 0.000; age 80–90, SMD = 0.961 [0.438, 1.485], p = 0.000) showed higher levels of Hcy in dementia patients than in healthy controls. In both male and female subgroups, higher Hcy and lower folic acid levels were observed in dementia patients than in controls. Plasma folate levels were significantly lower in dementia patients with a mean age of 70–80 and 80–90 years than in controls. However, dementia patients with an average age of 60–70 years had marginally lower folic acid levels (SMD = −0.262, [−0.486, −0.037], p = 0.022). The above results are shown in Supplementary Figures 5–12, Table 3.
Comparison of Hcy and Folic Acid Levels in AD and VaD Patients
A significant reduction in the plasma Hcy level was observed in AD patients compared to VaD patients (SMD = −0.278, 95% CI [−0.466, −0.09], p = 0.000) (Figure 4). Additionally, there were no significant differences in folate levels between AD and VaD patients (SMD = 0.032, 95% CI [−0.132, 0.196], p = 0.703).
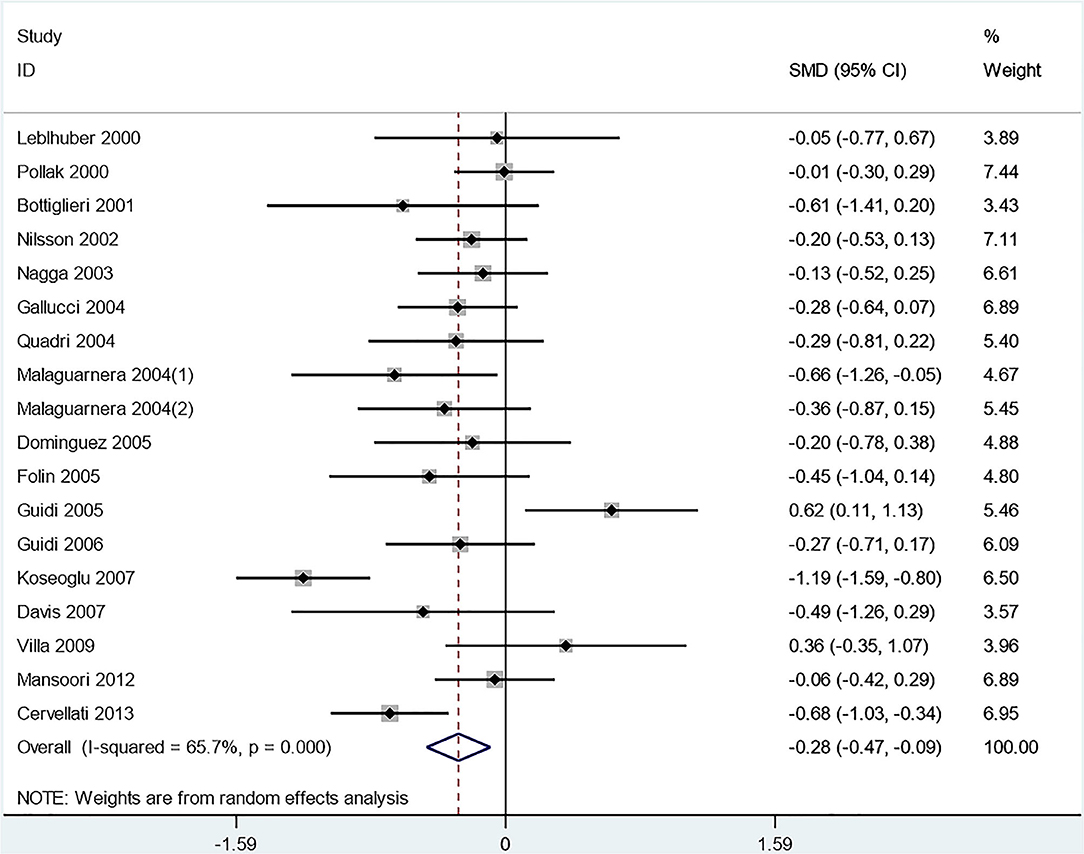
Figure 4. Forest plot of standard mean difference (SMD) and 95% confidence interval (95%CI) in AD and VaD for plasma homocysteine.
Hcy Level and Risk of Dementia
In total, 13 prospective studies on Hcy levels and risk of dementia were included in our study. A summary of these studies is shown in Supplementary Table 1. The pooled overall RR was 1.22, 95% CI (1.08, 1.36), which suggests that an elevated Hcy level may be correlated with a significantly increased risk of all-cause dementia. High serum Hcy levels were also associated with an increased risk of AD and VaD occurrence (RR 1.07, 95% CI [1.04, 1.11]; RR 1.13, 95% CI [1.04, 1.23]) (Figure 5). Five studies were included in our meta-analysis to explore the association between folic acid level and the risk of AD. We found that a low folic acid level was associated with an increased risk of AD (RR 1.731, 95% CI [1.122, 2.34]) (Figure 6).
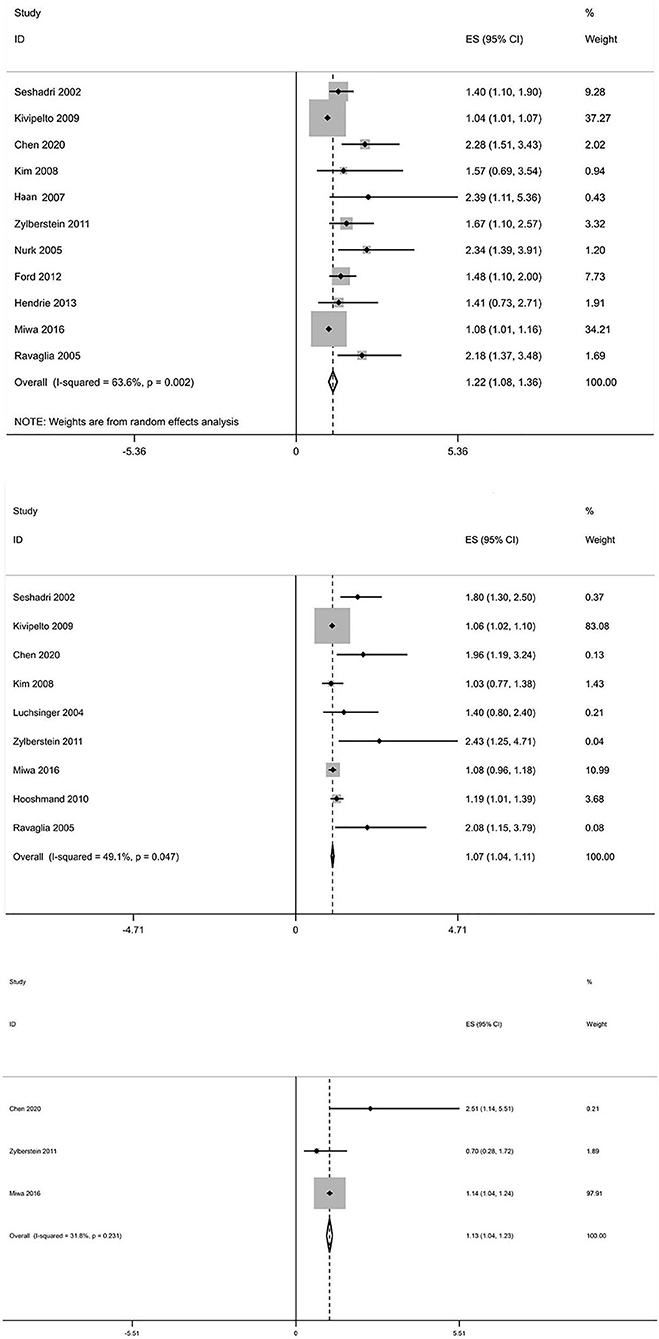
Figure 5. Forest plot of relative risk (RR) and 95% confidence interval (95%CI) in dementia (all-caused dementia, AD, and VaD) and control group for homocysteine.
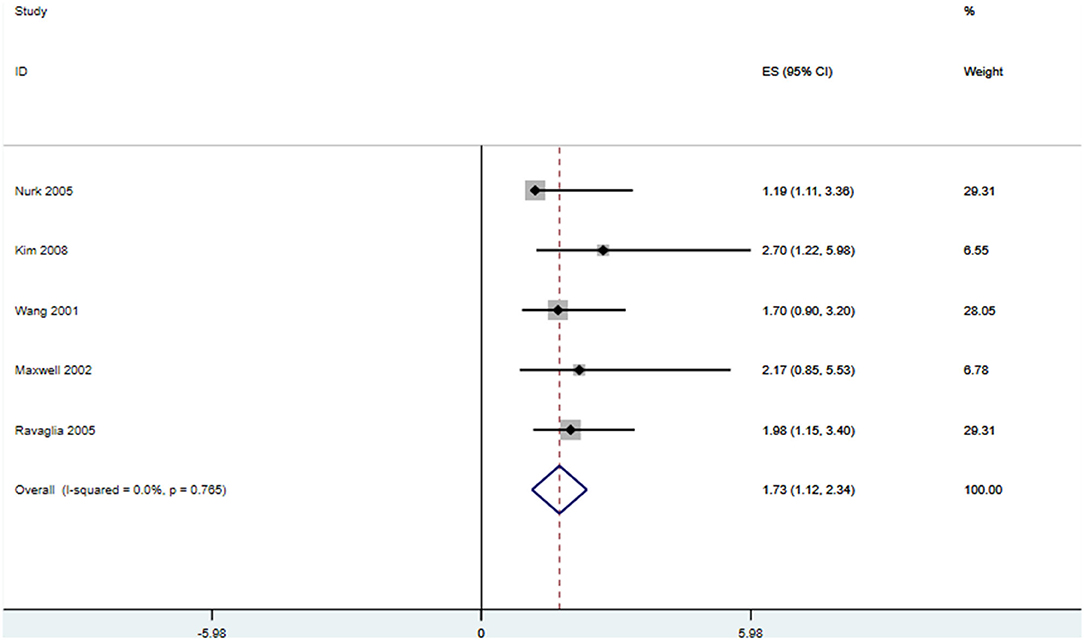
Figure 6. Forest plot of relative risk (RR) and 95% confidence interval (95%CI) in AD and control group for folic acid.
Begg's and Egger's tests indicated no publication bias. For further linear dose–response analysis, eight studies about Hcy with more than two categories were included, with a total of 15,134 participants and 1,627 all-cause dementia cases. There was no significance for non-linear dose-response analysis (p-non-linear = 0.801). The summary RR of dementia was 1.09 (95% CI [1.057–1.131]) per 5 μmol/L of increased Hcy (Figure 7). In other words, it was estimated that every 5 μmol/L increase in the plasma Hcy level was associated with a 9% increase in the risk of dementia. The summary RR of AD per 5 μmol/L increment in Hcy was 1.12 (95% CI [1.067–1.178]). However, the summary RR was 1.046 (95% CI [0.951–1.14]) for every 5-unit increase with no significance for VaD. In addition, the results of the sensitivity analysis were not substantially different. This indicates that our results are reliable.
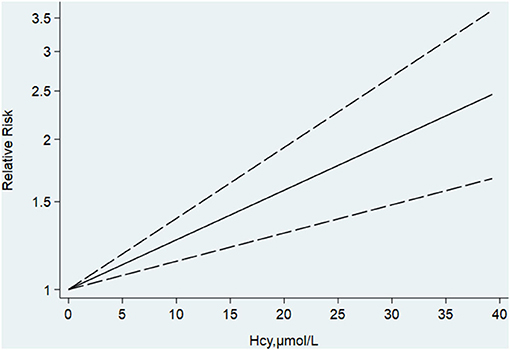
Figure 7. Dose-response analysis of homocysteine and risk of AD. The solid line represents the best fitting cubic spline model.
Discussion
Our meta-analysis included 81 articles, and 15 of these were prospective cohort studies. Drawing on this, we systematically analyzed the association between the Hcy and folate levels and the risk of dementia. The results of the meta-analysis not only confirmed that dementia patients had higher Hcy and lower folic acid levels than the controls but also suggested that subjects with high Hcy levels and low folic acid levels might be susceptible to AD. We found a linear trend that for every 5 μmol/L increase in Hcy levels, the risk of dementia and AD increased by 9 and 12%, respectively. To our knowledge, the present meta-analysis is the largest and most comprehensive evaluation of the correlation between Hcy and folate levels and the risk of dementia.
A recent meta-analysis of retrospective studies by Shen et al. demonstrated that AD was significantly associated with high Hcy levels and low serum folate levels. They also suggested that high Hcy and low folate levels may be associated with an increased risk of occurrence of AD (Shen and Ji, 2015). Another meta-analysis by Zhang et al. indicated that all the subtypes of the trial of org 10,172 in acute stroke treatment (TOAST) of ischemic stroke in Chinese patients showed significantly higher Hcy levels compared to the controls (Zhang et al., 1998). Our meta-analysis confirmed that higher levels of Hcy and lower levels of folic acid were observed in patients with dementia than in non-demented controls, which was consistent with the previous meta-analysis results. Further, we found that plasma Hcy levels were higher in VaD patients than in AD patients. In our meta-analysis, we included 26 studies (Fekkes et al., 1998; Pollak et al., 2000; Wang et al., 2001; Maxwell et al., 2002; Malaguarnera et al., 2004; Irizarry et al., 2005; Zhang et al., 2005; Guidi et al., 2006; Davis et al., 2007; Galimberti et al., 2008; Kim et al., 2008a; Bi et al., 2009; Hooshmand et al., 2010; Tu et al., 2010; Ferlazzo et al., 2011; Mansoori et al., 2012, 2014; Piazza et al., 2012; Yesil et al., 2012; Elhawary et al., 2013; Choe et al., 2014; Doody et al., 2015; Madsen et al., 2015; Ma et al., 2017; Soni et al., 2018; Meng et al., 2019; Lanyau-Domínguez et al., 2020; Sutovsky et al., 2020) (21 for Hcy and 13 for folate) that were not included in previous studies. Subgroup analysis by ethnicity revealed that dementia patients in Caucasian and Asian cohorts both had high Hcy levels and low folic acid levels. It was also determined that ethnicity exhibited no significant effect on this association. Further subgroup analysis by average age showed that plasma Hcy levels were significantly higher in dementia patients of every age group than in healthy controls. However, no significant difference was observed in folate levels between dementia patients with an average age of 60–70 years and healthy controls. Recent studies (Hainsworth et al., 2016) showed that older patients need vitamin B supplementation to reduce cognitive impairment, which is in accordance with our results. It seems that folate and vitamin B exhibit a significant role in the dementia of elder people. A previous study indicated that there is a gender difference in the relationship between Hcy levels and the risk of AD. However, our subgroup analysis concluded that both women and men with dementia had higher Hcy levels and lower folate levels than the controls, and the gender exerted no effect on this relationship. Sensitivity analyses suggested that the primary pooled effect size of our analysis remained stable after the exclusion of any single study.
Individual studies on the association between plasma Hcy level and the risk of dementia occurrence did not reach a consensus (Luchsinger et al., 2004; Ford and Almeida, 2012; Miwa et al., 2016). Recently, Zhou et al. performed a meta-analysis of prospective cohort studies and found that an increased level of blood Hcy was associated with the risk of developing AD but not VaD or cognitive impairment (Zhou and Chen, 2019). Our meta-analysis supplemented four studies (Nurk et al., 2005; Haan et al., 2007; Kim et al., 2008b; Chen et al., 2020) and demonstrated a positive association between high levels of Hcy and the risk of dementia, AD, and VaD, which is partly different from the results of the earlier researches. We also identified that higher Hcy levels and lower folate levels were associated with an increased risk of developing dementia and AD. More importantly, a linear dose-dependent relationship between Hcy levels (per 5 μmol/L increase) and the risk of developing AD (12% increase) was found in our study. This finding suggests that every 5 μmol/L increment in plasma Hcy is linearly associated with a 12% increase in AD risk. It has been noted that an increased level of plasma Hcy contributes to the occurrence and development of AD but not VaD.
To date, three trials (FACIT, WAFACS, and VITACOG) have reported the beneficial effects of Hcy-lowering interventions (Hainsworth et al., 2016). The FACIT and WAFACS trials indicated significant effects of B6, B12, and folate on slowing cognitive impairment in patients with high plasma Hcy levels (Douaud et al., 2013). Nieraad et al. suggested that the B-vitamin intervention showed promise to become a preventive therapeutic for AD by the evidence of immediate normalization of Hcy after B-vitamins supplement in the mouse model (Nieraad et al., 2021). Nevertheless, vitamin B supplementation slowed brain atrophy in AD-related regions in subjects with high plasma Hcy levels. It should also be noted that the timing of the Hcy-lowering intervention is critical and that vitamin supplementation exerts a protective effect on AD after 15 months. Hcy as a key mediator in AD development, and Hcy-lowering intervention may be an effective treatment for cognitive impairment.
The following may be the potential Hcy-related mechanisms leading to dementia. First, high Hcy levels can produce superoxide anions, hydrogen peroxide, and hydroxyl anions, which act as promoters of neurodegenerative events and cause endothelial damage and neuronal death (Faraci and Lentz, 2004; Sharma et al., 2015). Moreover, hyperhomocysteinemia is associated with increased hippocampal and cortical atrophy through increasing hippocampal neuron vulnerability to excitotoxic insults and amyloid-peptide toxicity. Hcy may also have a neurotoxic effect on neurons by stimulating the activation of N-methyl-D-aspartate glutamate receptors, resulting in cell death. In addition, hyperhomocysteinemia also affects the synthesis of methionine and S-adenosylmethionine, which could inhibit the methylation reaction, neurotransmitter metabolism, and membrane phospholipids (Lipton et al., 1997; Sachdev et al., 2002). Finally, high Hcy levels can generate β-amyloid peptides through hydrolysis of the amyloid precursor protein. This can lead to the formation of amyloid plaques in neurons (Pacheco-Quinto et al., 2006; Prince et al., 2014). Thus, hyperhomocysteinemia might have versatile roles in cognitive impairment, and complex mechanisms are worthy to be explored. Furthermore, independent of high Hcy levels, folic acid also plays an important role in cognitive function. Folate deficiency causes oxidative stress and generates reactive oxygen species that are responsible for neuronal deterioration and cellular death in areas of the brain involved in AD (Robinson et al., 2018). It has been reported that a folic-acid-deficient diet can promote hippocampal neurodegeneration in amyloid precursor protein mutant transgenic mice (Pacheco-Quinto et al., 2006). Specific mechanisms of Hcy and folate related to the occurrence and development of AD are still not completely lighted, and more exploration is needed to find effective therapy to delay the progression of cognitive decline and dementia.
The strengths of the current study include comprehensive analyses. First, our investigation included prospective studies and retrospective case-control studies providing a comprehensive perspective on the association between Hcy and folate levels and dementia. Second, our current meta-analysis examined the relationship between blood Hcy levels and the risk of cognitive disorders including AD and VaD. Third, we performed subgroup, sensitivity, and dose-response analyses, which provided reliable and precise estimates. Lastly, multiple studies including several cases and participants were included in our study. However, some limitations of our meta-analysis should be considered. First, the heterogeneity among studies was significant owing to differences in geographical location, analytical methods, cut-off values, and patient selection. Second, most studies included in our analysis do not belong to the randomized controlled trial (RCT) category in the strict sense. Randomized placebo-controlled trials of Hcy-lowering treatment are needed to determine the effect of such treatment on the progression of dementia. In addition, although the RR with fully adjusted models was used in our study, different adjustments for confound risk among the different studies could potentially influence the results. Not all the included studies took account of the adjustment for cardiovascular risk factors, MMSE score, vitamin B, duration of follow-up, and other factors. Lastly, only studies with full texts and published papers were included in our meta-analysis; therefore, publication bias may have affected our findings.
In conclusion, our meta-analysis is the first to report that patients with dementia, including AD and VaD, show higher levels of Hcy and lower levels of folic acid compared to non-demented controls. We also revealed a possible influence of elevated plasma Hcy levels and reduced folate levels on the risk of dementia. Moreover, every 5 μmol/L increase in the plasma Hcy level was associated with a 12% increase in the risk of AD. The above findings indicate that Hcy and folic acid may contribute to the incidence of dementia. Our study provides vital evidence for clinicians to rationalize clinical intervention strategies. Moreover, longitudinal studies are needed to further elucidate the relationship between Hcy, folic acid, and dementia.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
RZ and XL: designed the experiments. RZ, XL, and JZ: performed the experiments. XL, QW, and HC: analyzed the data. RZ and QW: wrote the paper. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the Natural Science Foundation of Liaoning province of China (2019-MS-364) and the National Natural Science Foundation of China (81501006).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are deeply grateful to all participants of this study.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2021.665114/full#supplementary-material
References
Anello, G., Guéant-Rodríguez, R. M., Bosco, P., Guéant, J. L., Romano, A., Namour, B., et al. (2004). Homocysteine and methylenetetrahydrofolate reductase polymorphism in Alzheimer's disease. Neuroreport 15, 859–861. doi: 10.1097/00001756-200404090-00025
Ariogul, S., Cankurtaran, M., Dagli, N., Khalil, M., and Yavuz, B. (2005). Vitamin B12, folate, homocysteine and dementia: are they really related? Arch. Gerontol. Geriatr. 40, 139–146. doi: 10.1016/j.archger.2004.07.005
Asita De Silva, H., Gunatilake, S. B., Johnston, C., Warden, D., and Smith, A. D. (2005). Medial temporal lobe atrophy, apolipoprotein genotype, and plasma homocysteine in Sri Lankan patients with Alzheimer's disease. Exp. Aging Res. 31, 345–354. doi: 10.1080/03610730590948221
Beydoun, M. A., Beydoun, H. A., Gamaldo, A. A., Teel, A., Zonderman, A. B., and Wang, Y. (2014). Epidemiologic studies of modifiable factors associated with cognition and dementia: systematic review and meta-analysis. BMC Public Health 14:643. doi: 10.1186/1471-2458-14-643
Bi, X. H., Zhao, H. L., Zhang, Z. X., and Zhang, J. W. (2009). Association of RFC1 A80G and MTHFR C677T polymorphisms with Alzheimer's disease. Neurobiol. Aging 30, 1601–1607. doi: 10.1016/j.neurobiolaging.2007.12.010
Bottiglieri, T., Parnetti, L., Arning, E., Ortiz, T., Amici, S., Lanari, A., et al. (2001). Plasma total homocysteine levels and the C677T mutation in the methylenetetrahydrofolate reduc-tase (MTHFR) gene: a study in an Italian population with dementia. Mech. Ageing Dev. 122, 2013–2023. doi: 10.1016/s0047-6374(01)00307-4
Brookmeyer, R., Johnson, E., Ziegler-Graham, K., and Arrighi, H. M. (2007). Forecasting the global burden of Alzheimer's disease. Alzheimer's Dement. 3, 186–191. doi: 10.1016/j.jalz.2007.04.381
Cervellati, C., Romani, A., Seripa, D., Cremonini, E., Bosi, C., Magon, S., et al. (2013). Oxidative balance, homocysteine, and uric acid levels in older patients with late onset Alzheimer's disease or vascular dementia. J. Neurol. Sci. 337, 156–161. doi: 10.1016/j.jns.2013.11.041
Chen, S., Honda, T., Ohara, T., Hata, J., Hirakawa, Y., Yoshida, D., et al. (2020). Serum homocysteine and risk of dementia in Japan[J]. J. Neurol. Neurosurg. Psychiatry 91, 540–546. doi: 10.1136/jnnp-2019-322366
Choe, Y. M., Sohn, B. K., Choi, H. J., Byun, M. S., Seo, E. H., Han, J. Y., et al. (2014). Association of homocysteine with hippocampal volume independent of cerebral amyloid and vascular burden. Neurobiol. Aging 35, 1519–1525. doi: 10.1016/j.neurobiolaging.2014.01.013
Clarke, R., Smith, A. D., Jobst, K. A., Refsum, H., Sutton, L., and Ueland, P. M. (1998). Folate, vitamin B12, and serum total homocysteine levels in confirmed Alzheimer disease. Arch. Neurol. 55, 1449–1455. doi: 10.1001/archneur.55.11.1449
Czapski, G. A., Maruszak, A., Styczyńska, M., Ż ekanowski, C., Safranow, K., and Strosznajder, J. B. (2012). Association between plasma biomarkers, CDK5 polymorphism and the risk of Alzheimer's disease. Acta Neurobiol. Exp. 72, 397–411.
da Silva, V. C., Ramos, F. J., Fretitas, E. M., de Brito-Margues, P. R., Cavalcanti, M. N., Almeida, D., et al. (2006). Alzheimer's disease in Brazilian elderly has a relation with homocysteine but not with MTHFR polymorphisms. Arq. Neuropsiquiatr. 64, 941–945. doi: 10.1590/s0004-282x2006000600010
Davis, G. K., Baboolal, N. S., Seales, D., Ramchandani, J., McKell, S., and McRae, A. (2007). Potential biomarkers for dementia in Trinidad and Tobago. Neurosci. Lett. 424, 27–30. doi: 10.1016/j.neulet.2007.07.011
Dominguez, R. O., Marschoff, E. R., Guareschi, E. M., Famulari, A. L., Pagano, M. A., and Serra, J. A. (2005). Homocysteine, vitamin B 12 and folate in Alzheimer's and vascular dementias: the paradoxical effect of the superimposed type II diabetes mellitus condition. Clin. Chim Acta. 359, 163–170. doi: 10.1016/j.cccn.2005.03.049
Doody, R. S., Demirovic, J., Ballantyne, C. M., Chan, W., Barber, R., Powell, S., et al. (2015). Lipoprotein-associated phospholipase A2, homocysteine, and Alzheimer's disease. Alzheimer's Dement. 1, 464–471. doi: 10.1016/j.dadm.2015.08.001
Douaud, G., Refsum, H., De Jager, C. A., Jacoby, R., Nichols, T. E., Smith, S. M., et al. (2013). Preventing Alzheimer's diseaserelated gray matter atrophy by B-vitamin treatment. Proc. Natl. Acad. Sci. U.S.A. 110, 9523–9528. doi: 10.1073/pnas.1301816110
Elhawary, N. A., Hewedi, D., Arab, A., Teama, S., Shaibah, H., Tayeb, M. T., et al. (2013). The MTHFR 677T allele may influence the severity and biochemical risk factors of Alzheimer's disease in an Egyptian population. Dis. Markers 35, 439–446. doi: 10.1155/2013/524106
Faraci, F. M., and Lentz, S. R. (2004). Hyperhomocysteinemia, oxidative stress, and cerebral vascular dysfunction. Stroke 35, 345–347. doi: 10.1161/01.STR.0000115161.10646.67
Faux, N. G., Ellis, K. A., Porter, L., Fowler, C. J., Laws, S. M., Martins, R. N., et al. (2011). Homocysteine, vitamin B12, and folic acid levels in Alzheimer's disease, mild cognitive impairment, and healthy elderly: baseline characteristics in subjects of the Australian Imaging Biomarker Lifestyle study. J. Alzheimer's Dis. 27, 909–922. doi: 10.3233/JAD-2011-110752
Fekkes, D., Van Der Cammen, T. J. M., Van Loon, C. P. M., Verschoor, C., Van Harskamp, F., De Koning, I., et al. (1998). Abnormal amino acid metabolism in patients with early stage Alzheimer dementia. J. Neural Trans. 105, 287–294. doi: 10.1007/s007020050058
Ferlazzo, N., Gorgone, G., Caccamo, D., Currò, M., Condello, S., Pisani, F., et al. (2011). The 894G> T (Glu298Asp) variant in the endothelial NOS gene and MTHFR polymorphisms influence homocysteine levels in patients with cognitive decline. Neuromol. Med. 13, 167–174. doi: 10.1007/s12017-011-8148-8
Folin, M., Baiguera, S., Gallucci, M., Conconi, M. T., Di Liddo, R., Zanardo, A., et al. (2005). A cross-sectional study of homocysteine-, NO-levels, and CT-findings in Alzheimer dementia, vascular dementia and controls. Biogerontology. 6, 255–260. doi: 10.1007/s10522-005-2622-3
Ford, A. H., and Almeida, O. P. (2012). Effect of homocysteine lowering treatment on cognitive function: a systematic review and meta-analysis of randomized controlled trials. J. Alzheimer's Dis. 29, 133–149. doi: 10.3233/JAD-2012-111739
Galimberti, G., Conti, E., Zini, M., Piazza, F., Fenaroli, F., Isella, V., et al. (2008). Post-methionine load test: a more sensitive tool to reveal hyperhomocysteinemia in Alzheimer patients? Clin. Biochem. 41, 914–916. doi: 10.1016/j.clinbiochem.2008.03.015
Gallucci, M., Zanardo, A., De Valentin, L., and Vianello, A. (2004). Homocysteine in Alzheimer disease and vascular dementia. Arch. Gerontol. Geriatr. Suppl. 2004, 195–200. doi: 10.1016/j.archger.2004.04.027
Genedani, S., Rasio, G., Cortelli, P., Antonelli, F., Guidolin, D., Galantucci, M., et al. (2004). Studies on homocysteine and dehydroepiandrosterone sulphate plasma levels in Alzheimer's disease patients and in Parkinson's disease patients. Neurotox. Res. 6, 327–332. doi: 10.1007/BF03033443
Guidi, I., Galimberti, D., Lonati, S., Novembrino, C., Bamonti, F., Tiriticco, M., et al. (2006). Oxidative imbalance in patients with mild cognitive impairment and Alzheimer's disease. Neurobiol. Aging. 27, 262–269. doi: 10.1016/j.neurobiolaging.2005.01.001
Guidi, I., Galimberti, D., Venturelli, E., Lovati, C., Del Bo, R., Fenoglio, C., et al. (2005). Influence of the Glu298Asp polymorphism of NOS3 on age at onset and homocysteine levels in AD patients. Neurobiol. Aging 26, 789–794. doi: 10.1016/j.neurobiolaging.2004.07.003
Haan, M. N., Miller, J. W., Aiello, A. E., Whitmer, R. A., Jagust, W. J., Mungas, D. M., et al. (2007). Homocysteine, B vitamins, and the incidence of dementia and cognitive impairment: results from the Sacramento Area Latino Study on Aging. Am. J. Clin. Nutr. 85, 511–517. doi: 10.1093/ajcn/85.2.511
Hagnelius, N. O., Wahlund, L. O., and Nilsson, T. K. (2008). CSF/serum folate gradient: Physiology and determinants with special reference to dementia. Dement. Geriatr. Cogn. Disord. 25, 516–523. doi: 10.1159/000129696
Hainsworth, A. H., Yeo, N. E., Weekman, E. M., and Wilcock, D. M. (2016). Homocysteine, hyperhomocysteinemia and vascular contributions to cognitive impairment and dementia (VCID). Biochim. Biophys. Acta 1862, 1008–1017. doi: 10.1016/j.bbadis.2015.11.015
Hernanz, A., De la Fuente, M., Navarro, M., and Frank, A. (2007). Plasma aminothiol compounds, but not serum tumor necrosis factor receptor II and soluble receptor for advanced glycation end products, are related to the cognitive impairment in Alzheimer's disease and mild cognitive impairment patients. Neuroimmunomodulation 14, 163–167. doi: 10.1159/000110641
Hogervorst, E., Ribeiro, H. M., Molyneux, A., Budge, M., and Smith, A. D. (2002). Plasma homocysteine levels, cerebrovascular risk factors, and cerebral white matter changes (leukoaraiosis) in patients with Alzheimer disease. Arch. Neurol. 59, 787–793. doi: 10.1001/archneur.59.5.787
Hooshmand, B., Solomon, A., Kåreholt, I., Leivisk,ä, J., Rusanen, M., Ahtiluoto, S., et al. (2010). Homocysteine and holotranscobalamin and the risk of Alzheimer disease: a longitudinal study. Neurology 75, 1408–1414. doi: 10.1212/WNL.0b013e3181f88162
Irizarry, M. C., Gurol, M. E., Raju, S., Diaz-Arrastia, R., Locascio, J. J., Tennis, M., et al. (2005). Association of homocysteine with plasma amyloid β protein in aging and neurodegenerative disease. Neurology 65, 1402–1408. doi: 10.1212/01.wnl.0000183063.99107.5c
Joosten, E. (2001). Homocysteine, vascular dementia and Alzheimer's disease. Clin. Chem. Lab. Med. 39, 717–720. doi: 10.1515/CCLM.2001.119
Kim, G., Kim, H., Kim, K. N., Son, J. I., Kim, S. Y., Tamura, T., et al. (2013). Relationship of cognitive function with B vita-min status, homocysteine, and tissue factor pathway inhibitor in cognitively impaired elderly: a cross-sectional survey. J. Alzheimers Dis. 33, 853–862. doi: 10.3233/JAD-2012-121345
Kim, J. M., Stewart, R., Kim, S. W., Shin, I. S., Yang, S. J., Shin, H. Y., et al. (2008b). Changes in folate, vitamin B12 and homocysteine associated with incident dementia. J. Neurol. Neurosurg. Psychiatry 79, 864–868. doi: 10.1136/jnnp.2007.131482
Kim, J. M., Stewart, R., Kim, S. W., Yang, S. J., Shin, I. S., Shin, H. Y., et al. (2008a). Methylenetetrahydrofolate reductase gene and risk of Alzheimer's disease in Koreans. Int. J. Geriatr Psychiatry 23, 454–459. doi: 10.1002/gps.1903
Koseoglu, E., and Karaman, Y. (2007). Relations between homocysteine, folate and vitamin B12 in vascular dementia and in Alzheimer disease. Clin. Biochem. 40, 859–863. doi: 10.1016/j.clinbiochem.2007.04.007
Lane, C. A., Hardy, J., and Schott, J. M. (2018). Alzheimer's disease. Eur. J. Neurol. 25, 59–70. doi: 10.1111/ene.13439
Lanyau-Domínguez, Y., Macías-Matos, C., Llibre-Rodriguez, J. J., María, G., Suárez-Medina, R., Eugenia, M., et al. (2020). Levels of vitamins and homocysteine in older adults with Alzheimer disease or mild cognitive impairment in Cuba. MEDICC Rev. 22, 40–47. doi: 10.37757/MR2020.V22.N4.14
Leblhuber, F., Walli, J., Artner-Dworzak, E., Vrecko, K., Widner, B., Reibnegger, G., et al. (2000). Hyperhomocysteinemia in dementia. J. Neural Trans. 107, 1469–1474. doi: 10.1007/s007020070010
Linnebank, M., Popp, J., Smulders, Y., Smith, D., Semm-ler, A., Farkas, M., et al. (2010). S-adenosylmethionine is decreased in the cerebrospinal fluid of patients with Alzheimer's disease. Neurodegener. Dis. 7, 373–378. doi: 10.1159/000309657
Lipton, S. A., Kim, W. K., Choi, Y. B., Kumar, S., D'Emilia, D. M., Rayudu, P. V., et al. (1997). Neurotoxicity associated with dual actions of homocysteine at the N-methyl-D-aspartate receptor. Proc. Natl. Acad. Sci. U.S.A. 94, 5923–5928. doi: 10.1073/pnas.94.11.5923
Lovati, C., Galimberti, D., Pomati, S., Capiluppi, E., Dolci, A., Scapellato, L., et al. (2007). Serum folate concentrations in patients with cortical and subcortical dementias. Neurosci. Lett. 420, 213–216. doi: 10.1016/j.neulet.2007.04.060
Luchsinger, J. A., Tang, M. X., Shea, S., Miller, J., Green, R., and Mayeux, R. (2004). Plasma homocysteine levels and risk of Alzheimer disease. Neurology 62, 1972–1976. doi: 10.1212/01.WNL.0000129504.60409.88
Ma, F., Wu, T., Zhao, J., Ji, L., Song, A., Zhang, M., et al. (2017). Plasma homocysteine and serum folate and vitamin B12 levels in mild cognitive impairment and Alzheimer's disease: a case-control study. Nutrients 9:725. doi: 10.3390/nu9070725
Madsen, S. K., Rajagopalan, P., Joshi, S. H., Toga, A. W., Thompson, P. M., and Alzheimer's Disease Neuroimaging Initiative (ADNI). (2015). Higher homocysteine associated with thinner cortical gray matter in 803 participants from the Alzheimer's Disease Neuroimaging Initiative. Neurobiol. Aging 36, S203–S210. doi: 10.1016/j.neurobiolaging.2014.01.154
Malaguarnera, M., Ferri, R., Bella, R., Alagona, G., Carnemolla, A., and Pennisi, G. (2004). Homocysteine, vitamin B12 and folate in vascular dementia and in Alzheimer disease. Clin. Chem. Lab. Med. 42, 1032–1035. doi: 10.1515/CCLM.2004.208
Mansoori, N., Tripathi, M., Alam, R., Luthra, K., Sharma, S., Lakshmy, R., et al. (2014). Serum folic acid and RFC A80G polymorphism in Alzheimer's disease and vascular dementia. Am. J. Alzheimers Dis. Other Demen. 29, 38–44. doi: 10.1177/1533317513505131
Mansoori, N., Tripathi, M., Luthra, K., Alam, R., Lakshmy, R., Sharma, S., et al. (2012). MTHFR (677 and 1298) and IL-6-174 G/C genes in pathogenesis of Alzheimer's and vascular dementia and their epistatic interaction. Neurobiol. Aging 33:1003. e1–8. doi: 10.1016/j.neurobiolaging.2011.09.018
Maxwell, C. J., Hogan, D. B., and Ebly, E. M. (2002). Serum folate levels and subsequent adverse cerebrovascular outcomes in elderly persons. Dement. Geriatr. Cogn. Disord. 13, 225–234. doi: 10.1159/000057701
Meng, H. X., Li, Y., Zhang, W., Zhao, Y. R., Niu, X. Y., and Guo, J. H. (2019). The relationship between cognitive impairment and homocysteine in a B12 and folate deficient population in China: a cross-sectional study. Medicine 98:e17970. doi: 10.1097/MD.0000000000017970
Miller, J. W., Green, R., Mungas, D. M., Reed, B. R., and Jagust, W. J. (2002). Homocysteine, vitamin B6, and vascular disease in AD patients. Neurology 58, 1471–1475. doi: 10.1212/WNL.58.10.1471
Miwa, K., Tanaka, M., Okazaki, S., Yagita, Y., Sakaguchi, M., Mochizuki, H., et al. (2016). Increased total homocysteine levels predict the risk of incident dementia independent of cerebral small-vessel diseases and vascular risk factors. J. Alzheimer's Dis. 49, 503–513. doi: 10.3233/JAD-150458
Mizrahi, E. H., Bowirrat, A., Jacobsen, D. W., Korczyn, A. D., Traore, F., Petot, G. J., et al. (2004). Plasma homocysteine, vitamin B12 and folate in Alzheimer's patients and healthy Arabs in Israel. J. Neurol. Sci. 227, 109–113. doi: 10.1016/j.jns.2004.08.011
Mizrahi, E. H., Jacobsen, D. W., Debanne, S. M., Traore, F., Lerner, A. J., Friedland, R. P., et al. (2003). Plasma total homocysteine levels, dietary vitamin B6 and folate intake in AD and healthy aging. J. Nutr. Health Aging 7, 160–165.
Morillas-Ruiz, J. M., Rubio-Perez, J. M., Albaladejo, M. D., Zafrilla, P., Parra, S., and Vidal-Guevara, M. L. (2010). Effect of an antioxidant drink on homocysteine levels in Alzheimer's patients. J. Neurol. Sci. 299, 175–178. doi: 10.1016/j.jns.2010.08.050
Nagga, K., Rajani, R., Mardh, E., Borch, K., Mardh, S., and Marcusson, J. (2003). Cobalamin, folate, methylmalonic acid, homocys-teine, and gastritis markers in dementia. Dement. Geriatr. Cogn. Disord. 16, 269–275. doi: 10.1159/000072812
Nieraad, H., De Bruin, N., Arne, O., Hofmann, M. C. J., Gurke, R., Schmidt, D., et al. (2021). Effects of Alzheimer-like pathology on homocysteine and homocysteic acid levels—an exploratory in vivo kinetic study. Int. J. Mol. Sci. 22:927. doi: 10.3390/ijms22020927
Nilsson, K., Gustafson, L., and Hultberg, B. (2002). Relation between plasma homocysteine and Alzheimer's disease. Dement. Geriatr. Cogn. Disord. 14, 7–12. doi: 10.1159/000058327
Nurk, E., Refsum, H., Tell, G. S., Engedal, K., Vollset, S. E., Ueland, P. M., et al. (2005). Plasma total homocysteine and memory in the elderly: the Hordaland Homocysteine Study. Ann. Neurol. 58, 847–857. doi: 10.1002/ana.20645
Pacheco-Quinto, J., De Turco, E. B. R., DeRosa, S., Howard, A., Cruz-Sanchez, F., Sambamurti, K., et al. (2006). Hyperhomocysteinemic Alzheimer's mouse model of amyloidosis shows increased brain amyloid β peptide levels. Neurobiol. Dis. 22, 651–656. doi: 10.1016/j.nbd.2006.01.005
Parnetti, L., Mecocci, P., Reboldi, G. P., Santucci, C., Brunetti, M., Gaiti, A., et al. (1992). Platelet MAO-B activity and vitamin B12 in old age dementias. Mol. Chem. Neuropathol. 16, 23–32. doi: 10.1007/BF03159958
Piazza, F., Galimberti, G., Conti, E., Isella, V., Perlangeli, M. V., Speranza, T., et al. (2012). Increased tissue factor pathway inhibitor and homocysteine in Alzheimer's disease. Neurobiol. Aging 33, 226–233. doi: 10.1016/j.neurobiolaging.2010.02.016
Pollak, R. D., Pollak, A., Idelson, M., Bejarano-Achache, I., Doron, D., and Blumenfeld, A. (2000). The C677T mutation in the methylenetetrahydrofolate reductase (MTHFR) gene and vascular dementia. J. Am. Geriatr. Soc. 48, 664–668. doi: 10.1111/j.1532-5415.2000.tb04725.x
Postiglione, A., Milan, G., Ruocco, A., Gallotta, G., Guiotto, G., and Di Minno, G. (2001). Plasma folate, vitamin B(12), and total homocysteine and homozygosity for the C677T mutation of the 5,10-methylene tetrahydrofolate reductase gene in patients with Alzheimer's dementia. A case-control study. Gerontology. 47, 324–329. doi: 10.1159/000052822
Prince, M., Albanese, E., Guerchet, M., and Prina, M. (2014). Nutrition and dementia: a review of available research. Alzheimer's Disease International (ADI).
Quadri, P., Fragiacomo, C., Pezzati, R., Zanda, E., Forloni, G., Tettamanti, M., et al. (2004). Homocysteine, folate, and vitamin B-12 in mild cognitive impairment, Alzheimer disease, and vascular dementia. Am. J. Clin. Nutr. 80, 114–122. doi: 10.1093/ajcn/80.1.114
Quadri, P., Fragiacomo, C., Pezzati, R., Zanda, E., Tettamanti, M., and Lucca, U. (2005). Homocysteine and B vitamins in mild cognitive impairment and dementia. Clin. Chem. Lab Med. 43, 1096–1100. doi: 10.1515/CCLM.2005.191
Ravaglia, G., Forti, P., Maioli, F., Bianchi, G., Martelli, M., Talerico, T., et al. (2004). Plasma amino acid concentrations in patients with amnestic mild cognitive impairment or Alzheimer disease. Am. J. Clin. Nutr. 80, 483–438. doi: 10.1093/ajcn/80.2.483
Religa, D., Styczynska, M., Peplonska, B., Gabryelewicz, T., Pfef-fer, A, Chodakowska, M., et al. (2003). Homocysteine, apolipoproteine E and methylenetetrahydrofolate reductase in Alzheimer's disease and mild cognitive impairment. Dement. Geriatr. Cogn. Disord. 16, 64–70. doi: 10.1159/000070677
Robinson, N., Grabowski, P., and Rehman, I. (2018). Alzheimer's disease pathogenesis: is there a role for folate? Mechan. Ageing Dev. 174, 86–94. doi: 10.1016/j.mad.2017.10.001
Sachdev, P. S., Valenzuela, M., Wang, X. L., Looi, J. C., and Brodaty, H. (2002). Relationship between plasma homocysteine levels and brain atrophy in healthy elderly individuals. Neurology 58, 1539–1541. doi: 10.1212/WNL.58.10.1539
Selley, M. L. (2003). Increased concentrations of homocysteine and asymmetric dimethylarginine and decreased concentra-tions of nitric oxide in the plasma of patients with Alzheimer's disease. Neurobiol. Aging. 24, 903–907. doi: 10.1016/s0197-4580(03)00007-1
Selley, M. L. (2007). A metabolic link between S adenosyl-homocysteine and polyunsaturatcd fatty acid metabolism in Alzheimer's disease. Neurobiol. Aging. 28, 1834–1839. doi: 10.1016/j.neurobiolaging.2006.08.003
Selley, M. L., Close, D. R., and Stern, S. E. (2002). The effect of increased concentrations of homocysteine on the concentration of (E)-4-hydroxy-2-nonenal in the plasma and cerebrospinal fluid of patients with Alzheimer's disease. Neurobiol. Aging. 23, 383–388. doi: 10.1016/s0197-4580(01)00327-x
Sharma, M., Tiwari, M., and Tiwari, R. K. (2015). Hyperhomocysteinemia: impact on neurodegenerative diseases. Basic Clin. Pharmacol. Toxicol. 117, 287–296. doi: 10.1111/bcpt.12424
Shen, L., and Ji, H. F. (2015). Associations between homocysteine, folic acid, vitamin B12 and Alzheimer's disease: insights from meta-analyses. J. Alzheimer's Dis. 46, 777–790. doi: 10.3233/JAD-150140
Smach, M. A., Jacob, N., Golmard, J. L., Charfeddine, B., Lammouchi, T., Ben Othman, L., et al. (2011). Folate and homocysteine in the cerebrospinal fluid of patients with Alzheimer's disease or dementia: a case control study. Eur. Neurol. 65, 270–278. doi: 10.1159/000326301
Smith, A. D., and Refsum, H. (2016). Homocysteine, B vitamins, and cognitive impairment. Annu. Rev. Nutr. 36, 211–239. doi: 10.1146/annurev-nutr-071715-050947
Soni, R. M., Tiwari, S. C., Mahdi, A. A., and Kohli, N. (2018). Serum homocysteine and behavioral and psychological symptoms of dementia: is there any correlation in Alzheimer's Disease? Ann. Neurosci. 25, 152–159. doi: 10.1159/000487068
Stang, A. (2010). Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Europ. J. Epidemiol. 25, 603–605. doi: 10.1007/s10654-010-9491-z
Sutovsky, S., Petrovic, R., Fischerova, M., Haverlikova, V., Ukropcova, B., Ukropec, J., et al. (2020). Allelic distribution of genes for apolipoprotein E and MTHFR in patients with Alzheimer's disease and their epistatic interaction. J. Alzheimer's Dis. 77, 1095–1105. doi: 10.3233/JAD-200321
Tu, M. C., Huang, C. W., Chen, N. C., Chang, W. N., Lui, C. C., Chen, C. F., et al. (2010). Hyperhomocysteinemia in Alzheimer dementia patients and cognitive decline after 6 months follow-up period. Acta Neurol. Taiwan 19, 168–177.
Van Dam, F., and Van Gool, W. A. (2009). Hyperhomocysteinemia and Alzheimer's disease: a systematic review. Arch. Gerontol. Geriatr. 48, 425–430. doi: 10.1016/j.archger.2008.03.009
Villa, P., Bosco, P., Ferri, R., Perri, C., Suriano, R., Costantini, B., et al. (2009). Fasting and post-methionine homocysteine levels in Alzheimer's disease and vascular dementia. Int. J. Vitam. Nutr. Res. 79, 166–172. doi: 10.1024/0300-9831.79.3.166
Wang, H. X., Wahlin, A., Basun, H., Fastbom, J., Winblad, B., and Fratiglioni, L. (2001). Vitamin B(12) and folate in relation to the development of Alzheimer's disease. Neurology 56, 1188–1194. doi: 10.1212/WNL.56.9.1188
Yesil, Y., Kuyumcu, M. E., Cankurtaran, M., Uz, B., Kara, A., Kilic, M. K., et al. (2012). Increased mean platelet volume (MPV) indicating the vascular risk in Alzheimer's disease (AD). Arch. Gerontol. Geriatr. 55, 257–260. doi: 10.1016/j.archger.2011.09.016
Zhang, T., Jiang, Y., Zhang, S., Tie, T., Cheng, Y., Su, X., et al. (1998). The association between homocysteine and ischemic stroke subtypes in Chinese: a meta-analysis. Medicine (Baltimore) 99:e19467. doi: 10.1097/MD.0000000000019467
Zhang, Y. D., Ke, X. Y., Shen, W., and Liu, Y. (2005). Relationship of homocysteine and gene polymorphisms of its related metabolic enzymes with Alzheimer's disease. Chin. Med. Sci. J. 20, 247–251.
Keywords: Alzheimer's disease, vascular dementia, folic acid, homocysteine, meta-analysis
Citation: Wang Q, Zhao J, Chang H, Liu X and Zhu R (2021) Homocysteine and Folic Acid: Risk Factors for Alzheimer's Disease—An Updated Meta-Analysis. Front. Aging Neurosci. 13:665114. doi: 10.3389/fnagi.2021.665114
Received: 07 February 2021; Accepted: 09 April 2021;
Published: 26 May 2021.
Edited by:
Sergey Bachurin, Institute of Physiologically Active Compounds (RAS), RussiaReviewed by:
Aleksey Ustyugov, Institute of Physiologically Active Compounds (RAS), RussiaYong Ji, Capital Medical University, China
Copyright © 2021 Wang, Zhao, Chang, Liu and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ruixia Zhu, enJ4XzIwMDYyNjMxM0AxNjMuY29t
 Qianwen Wang
Qianwen Wang Ruixia Zhu
Ruixia Zhu