- 1Department of Neurology, The First Affiliated Hospital of Guangzhou Medical University, Guangzhou, China
- 2Department of Physiology, School of Basic Medical Sciences, Guangzhou Medical University, Guangzhou, China
Background: Triggering receptor expressed on myeloid cells 2 (TREM2) is a microglial receptor exclusively expressed in the central nervous system (CNS). It contributes to abnormal protein aggregation in neurodegenerative disorders, but its role in Parkinson’s disease (PD) is still unclear.
Methods: In this case-control study, we measured the concentration of the soluble fragment of TREM2 (sTREM2) in PD patients, evaluated their sleep conditions by the PD sleep scale (PDSS), and analyzed the relationship between sTREM2 and PD symptoms.
Results: We recruited 80 sporadic PD patients and 65 healthy controls without disease-related variants in TREM2. The concentration of sTREM2 in the CSF was significantly higher in PD patients than in healthy controls (p < 0.01). In the PD group, the concentration of sTREM2 had a positive correlation with α-syn in the CSF (Pearson r = 0.248, p = 0.027). Receiver operating characteristic curve (ROC) analyses showed that sTREM2 in the CSF had a significant diagnostic value for PD (AUC, 0.791; 95% CI, 0.711–0.871, p < 0.05). The subgroup analysis showed that PD patients with sleep disorders had a significantly higher concentration of sTREM2 in their CSF (p < 0.01). The concentration of sTREM2 in the CSF had a negative correlation with the PDSS score in PD patients (Pearson r = −0.555, p < 0.01). The ROC analyses showed that sTREM2 in the CSF had a significant diagnostic value for sleep disorders in PD (AUC, 0.733; 95% CI, 0.619–0.846, p < 0.05).
Conclusion: Our findings suggest that CSF sTREM2 may be a potential biomarker for PD and it could help predict sleep disorders in PD patients, but multicenter prospective studies with more participants are still needed to confirm its diagnostic value in future.
Introduction
Parkinson’s disease (PD) is a common neurodegenerative disease that is characterized by an impaired dopaminergic (DA) neurotransmitter system, aggregation of α-synuclein (α-syn) and microglial neuroinflammation-mediated neuroinflammation in the midbrain (Block and Hong, 2005; Wang et al., 2015; Rai and Singh, 2020; Rai et al., 2021). In addition to motor symptoms, PD patients suffer from non-motor disorders such as sleep disorders (Stefani and Högl, 2020). Sleep disorders affect 60–98% of PD patients as one of the most common non-motor symptoms and it often appears as the first symptom (Chaudhuri et al., 2006; Stefani and Högl, 2020). The regulation of sleep depends on the comprehensive functions of multiple brain regions and a series of neurotransmitters, including dopamine, 5-hydroxytryptamine, norepinephrine, and other PD-related molecules (Alexandre et al., 2006; Meles et al., 2017; Oh et al., 2018; Hayat et al., 2020). The mechanism of sleep disorders in PD patients may involve the loss of DA neurons and the deposition of α-syn in the brain stem, basal ganglia and hypothalamus (Dolores et al., 2016; Doppler et al., 2017; Garrido et al., 2020; Stefani et al., 2021). It has been suggested that sleep disorders have a certain value for the prediction and diagnosis of PD (Barasa et al., 2021).
Microglia perform functions including monitoring changes in neuronal activity, regulating learning and memory, and acting as local phagocytes or damage sensors in the brain (Colonna and Butovsky, 2017). Microglia engulf extracellular aggregated α-syn mediated by special receptors such as lymphocyte activation gene 3 (Lag-3) (Guo Y. et al., 2019; Gu et al., 2021). The dysfunction of microglia impairs the clearance of extracellular α-syn, which may lead to neurodegeneration of DA neurons in PD (Filippini et al., 2019; Li et al., 2019). Triggering receptor expressed by myeloid cells 2 (TREM2) is expressed exclusively by microglia in the central nervous system (CNS) (Dempsey, 2015). Microglia may recognize abnormal extracellular signals through the TREM2 receptor, a process that participates in neuron degeneration (Udeochu et al., 2018; Lee et al., 2021). In PD animal models, TREM2 deficiency can aggravate α-syn-induced neurodegeneration and neuroinflammation in the CNS, and its overexpression remarkably reduces toxicity-induced apoptosis of DA neurons (Ren et al., 2018; Guo Y. et al., 2019). This result suggests that TREM2 may enhance microglial phagocytosis function and inhibit neuroinflammation and neurodegeneration in the brain (Leyns et al., 2017; Udeochu et al., 2018; Guo Y. et al., 2019). In clinical studies, single nucleotide polymorphisms (SNPs) of exon 2 of the TREM2 gene, including rs75932628, rs143332484 and rs2234253, which are closely related to the genetic susceptibility to Alzheimer’s disease (AD) (Sims et al., 2017; Zhou et al., 2019), have not been demonstrated to show a relationship with PD (Li et al., 2016; Mengel et al., 2016). The extracellular segment of TREM2, recoded by exon 2, can be proteolytically cleaved and released into the extracellular space as soluble fragments (sTREM2) in the cerebrospinal fluid (CSF) (Kleinberger et al., 2014; Piccio et al., 2016). sTREM2 is thought to be an ideal biomarker for microglia-involved neurological disorders (Bekris et al., 2018).
Recent studies have shown that the concentration of sTREM2 in the CSF is increased in individuals with neuroinflammation in the CNS (Bekris et al., 2018; Ren et al., 2018). The concentration of sTREM2 in the CSF is closely correlated with aging (Henjum et al., 2016; Mecca et al., 2018). A higher concentration of sTREM2 was found to be associated with poor sleep characteristics in amyloid-positive individuals (Boespflug and Iliff, 2018; Wilckens et al., 2018; Belsare et al., 2020; Hu et al., 2021). sTREM2 in the CSF has a close relationship with tau-mediated neurodegenerative diseases such as AD (Piccio et al., 2016; Henjum et al., 2018; Sayed et al., 2018). The concentration of sTREM2 in the CSF was found to be elevated in PD patients with a positive-tau biomarker signature (Wilson et al., 2020). The α-syn protein shares similar pathobiology to tau and amyloid and is prone to pathological misfolding and aggregation in the CNS (Veys et al., 2021). The relationship between sTREM2 and α-syn is still not clear. Thus, in this study, we measured the concentrations of sTREM2 in the CSF, evaluated its correlation with PD and sleep disorders, and speculated its role in PD (Figure 1).
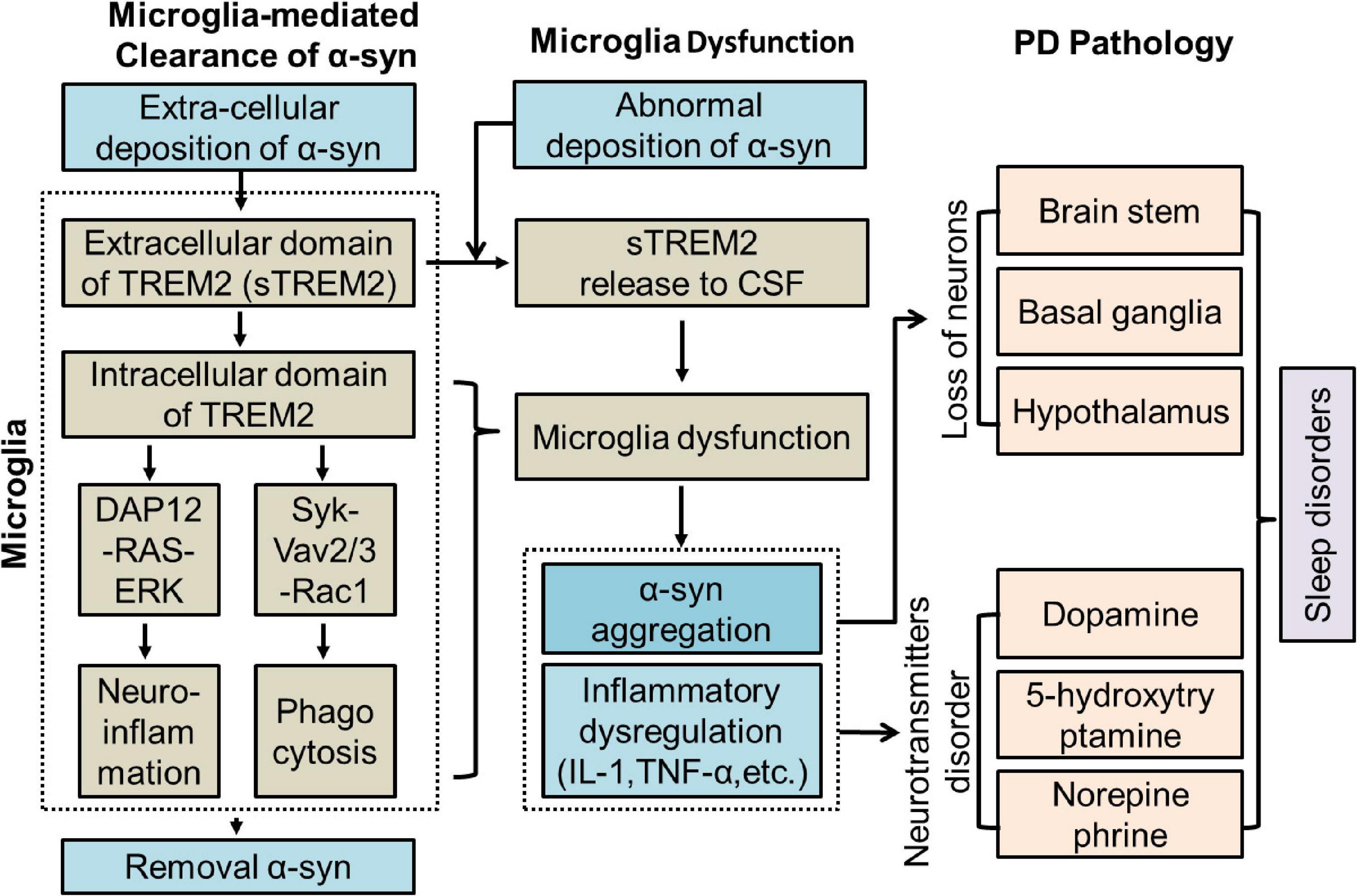
Figure 1. Potential role of TREM2 in PD. CSF, Cerebrospinal fluid; DAP12, DNAX-activating protein of 12 kDa; ERK, extracellular signal-regulated kinase 3; IL-1, interleukin-1; PD, Parkinson’s Disease; sTREM2, soluble TREM2; TNF-α, tumor necrosis factor-α.
Materials and Methods
Subjects
A total of 145 Han Chinese subjects, including 80 sporadic PD patients and 65 healthy controls matched by age, sex, and ethnicity, were recruited from the First Affiliated Hospital of Guangzhou Medical University and Guangdong 999 Brain Hospital from January 2017 to August 2021. PD was diagnosed according to the United Kingdom Parkinson’s Disease Society Brain Bank clinical diagnostic criteria for Parkinson’s disease. According to the results of the PD sleep scale (PDSS) (Tse et al., 2005), the PD patients were divided into a PDSS ≥ 90 group with no or mild sleep disorder and a PDSS < 90 group with moderate or severe sleep disorder. The global cognitive functions of all participants were assessed by the Montreal Cognitive Assessment test (MoCA) (Nasreddine et al., 2005). The conditions of the PD patients were evaluated by the International Parkinson and Movement Disorder Society (MDS)-sponsored revision of the Unified Parkinson’s Disease Rating Scale (UPRDS) and the Hoehn and Yahr stage (H&Y). Healthy control participants without a history of PD, other neurodegenerative diseases or chronic neuropsychiatric disorders were recruited from the surrounding community. The study protocols were approved by our institutional review boards. All samples were analyzed after obtaining informed consent from the participants.
Plasma and Cerebrospinal Fluid Collection
Peripheral blood samples were collected from all participants within 2 weeks after CSF collection, and genomic DNA was extracted by a Blood DNA Kit (QIAGEN, Dutch). Exon 2 of TREM2 was amplified by polymerase chain reaction (PCR) in an ABI 7900HT Fast Real-Time PCR system (Applied Biosystems, United States). The primers were designed by Primer Software Version 5.0 (Premier, Canada) as follows: 5′-3′ AAGTGCCTCCAGAATAGACC, 3′-5′ CCTTGGTAGCCCTGAGTAG. Sanger sequencing was used to investigate polymorphisms in exon 2 of the TREM2 gene using an ABI 3730XL system at Beijing Genomics Institute. Variant frequencies of TREM2 were compared by Fisher’s exact test. The CSF samples without hemolysis or discoloration were collected in polypropylene tubes, stored at −80°C, and subjected to a maximum of two freeze-thaw cycles prior to the detection of sTREM2 or α-syn.
Cerebrospinal Fluid Soluble Triggering Receptor Expressed on Myeloid Cells 2 and α-Syn Measurement
The concentrations of sTREM2 and α-syn were detected by enzyme-linked immunosorbent assay (ELISA) as previously described (Peng et al., 2020). After the freeze-thaw cycles, the Human TREM2 DuoSet ELISA (R&D Systems: #DY1828–05, Minneapolis, MN, United States) and Human alpha-Synuclein DuoSet ELISA (R&D Systems: #DY1338–05, Minneapolis, MN, United States) were used to measure the concentration of sTREM2 and α-syn in the CSF according to the manufacturer’s instructions (Peng et al., 2020). Briefly, 96-well streptavidin plates were blocked with 3% bovine serum albumin (BSA) and coated with the capture antibodies. The capture antibodies were anti-human TREM2 (#844598, R&D) or anti-α-syn (#844224, R&D). The samples and recombinant human TREM2 (#844600, R&D) or the α-syn (#844226, R&D) standard were incubated overnight with the capture antibodies. After three washes with phosphate-buffered saline (PBS)/0.05% Tween-20 (PBS-T) buffer, a biotinylated mouse anti-human TREM2 (#844599, R&D) or anti-α-syn (#844225, R&D) mAb was added and incubated for 2 h. Then, the detection was performed with a SpectraMax M2 plate reader (Molecular Devices, United States). Recombinant soluble human TREM2 or α-syn was used to construct the ELISA standard curve. The sample was discarded if its intraplate coefficient of variation (CV) > 15% or intraplate CV > 5%. Samples were distributed in a randomized manner and detected by an operator blinded to the clinical information. Samples were re-measured with the same reagents on the same day.
Statistical Analyses
Differences in the demographic data between the PD and control groups were assessed using the Chi-square test for categorical variables and the t-test for the age variable. The α-syn and sTREM2 concentration data had skewed distributions and were analyzed by Mann-Whitney U tests. Differences in SNPs of TREM2 with rare frequencies were analyzed using Fisher’s exact test. Receiver operating characteristic (ROC) curve analysis was used to evaluate the diagnostic performance of the CSF sTREM2 concentration by the area under the ROC curve (AUC). The Pearson correlation coefficients were used for correlational analyses. The p-values were two-tailed since all hypotheses tested were two-sided, and a significance level of α = 0.01 was selected. GraphPad Prism (GraphPad Software Inc., Version 6, United States) and SPSS version 21.0 (SPSS Inc., United States) were used for the analyses.
Results
Characteristics of the Participants
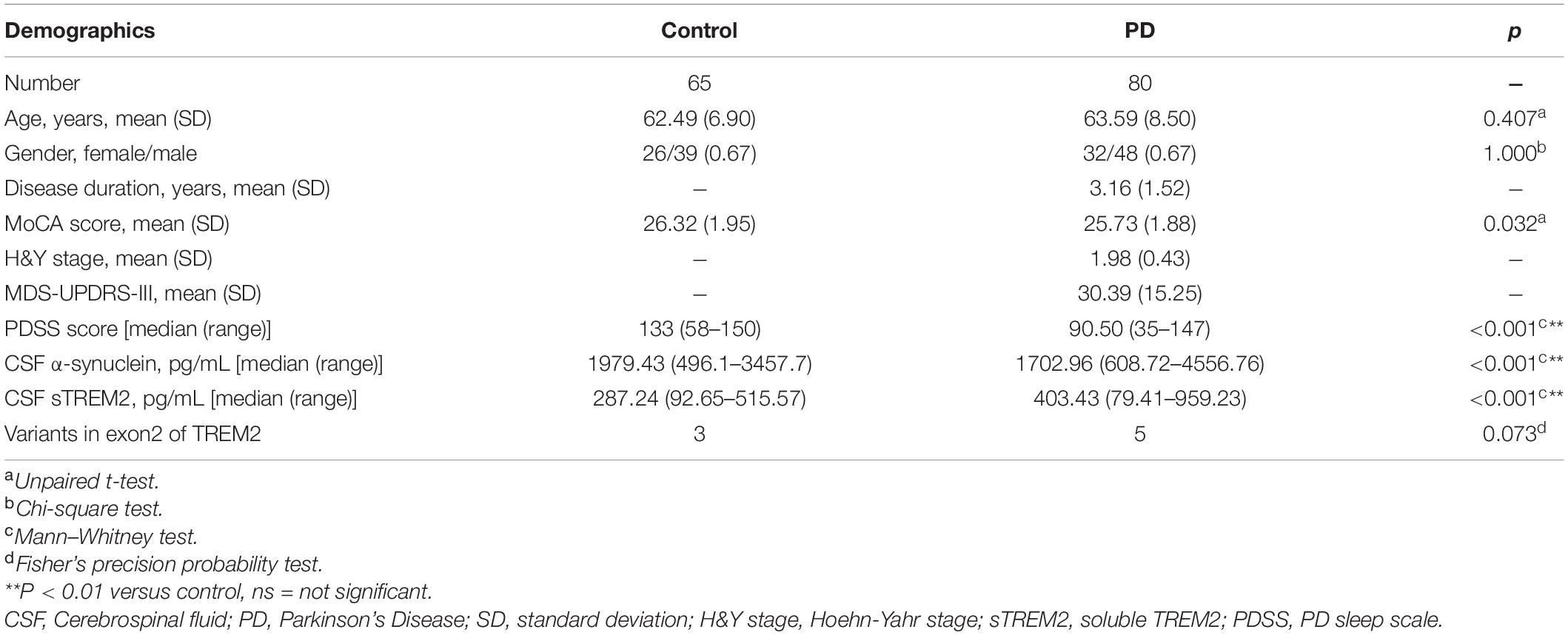
The demographic characterization of the PD cases and controls is presented in Table 1. A total of 80 sporadic PD patients and 65 age- and sex-matched controls were recruited as two groups. The PD group recruited patients diagnosed at an early H&Y stage of disease (Unpaired t-test, 1.98 ± 0.43 years) with a short disease duration (3.1 ± 1.5 years) and a moderate MDS-UPDRS-III score (30.39 ± 15.25). Compared with the controls, the PD group had no significant difference in MoCA scores (Unpaired t-test, p = 0.032), which is used to evaluate global cognition and executive function. As expected, performance on the PDSS, a test sensitive to assessing sleep disorders in subjects with PD, was significantly lower in the PD group than in the healthy control group (p < 0.001). The PD groups had a significantly lower concentration of α-syn in the CSF (Mann–Whitney test, p < 0.001) and a higher concentration of sTREM2 in the CSF (Mann–Whitney test, p < 0.001). Exon 2 encodes the main body of the extracellular segment in TREM2 that can be proteolytically cleaved and released into CSF as a soluble peptide named sTREM2. To avoid the side effects of gene mutations, targeted sequencing of exon 2 of TREM2 was performed (Piccio et al., 2016). Three variants (p. S81N, p. R47H, p. G58D) were shared by the control and PD groups, while the other two variants (p. A105 V, p. A62H) were only detected in PD patients. The percentage of variants on exon 2 of TREM2 was not significantly different between the two groups (Fisher’s test, p = 0.073).
Cerebrospinal Fluid Soluble Triggering Receptor Expressed on Myeloid Cells 2 Has Potential Value as a Biomarker of Parkinson’s Disease
The TREM2 receptor may regulate the microglial response to engulf aggregated α-syn and it could be used as a sensor in PD. CSF sTREM2, reflecting microglial function in the CNS, was evaluated as a characteristic hallmark of AD (Wood, 2017). Compared with the healthy control group, the PD group had a significantly decreased concentration of α-syn (Mann–Whitney U test, p < 0.001) and an increased concentration of sTREM2 (Mann–Whitney U test, p < 0.001) in the CSF (Figures 2A,B). The increasing trend of CSF sTREM2 was positively correlated with the trend of CSF α-syn in PD patients (Pearson r = 0.248, p = 0.027, Figure 2C). CSF α-syn has been proven to be a useful biomarker for the diagnosis of PD, suggesting that CSF sTREM2, closely related to CSF α-syn, may also have a similar function as a biomarker. The ROC curve analysis revealed that CSF sTREM2 could differentiate PD patients from age-matched healthy controls, with an area under the curve (AUC) of 0.791 (95% CI = 0.711–0.871, p < 0.001, Figure 2D).
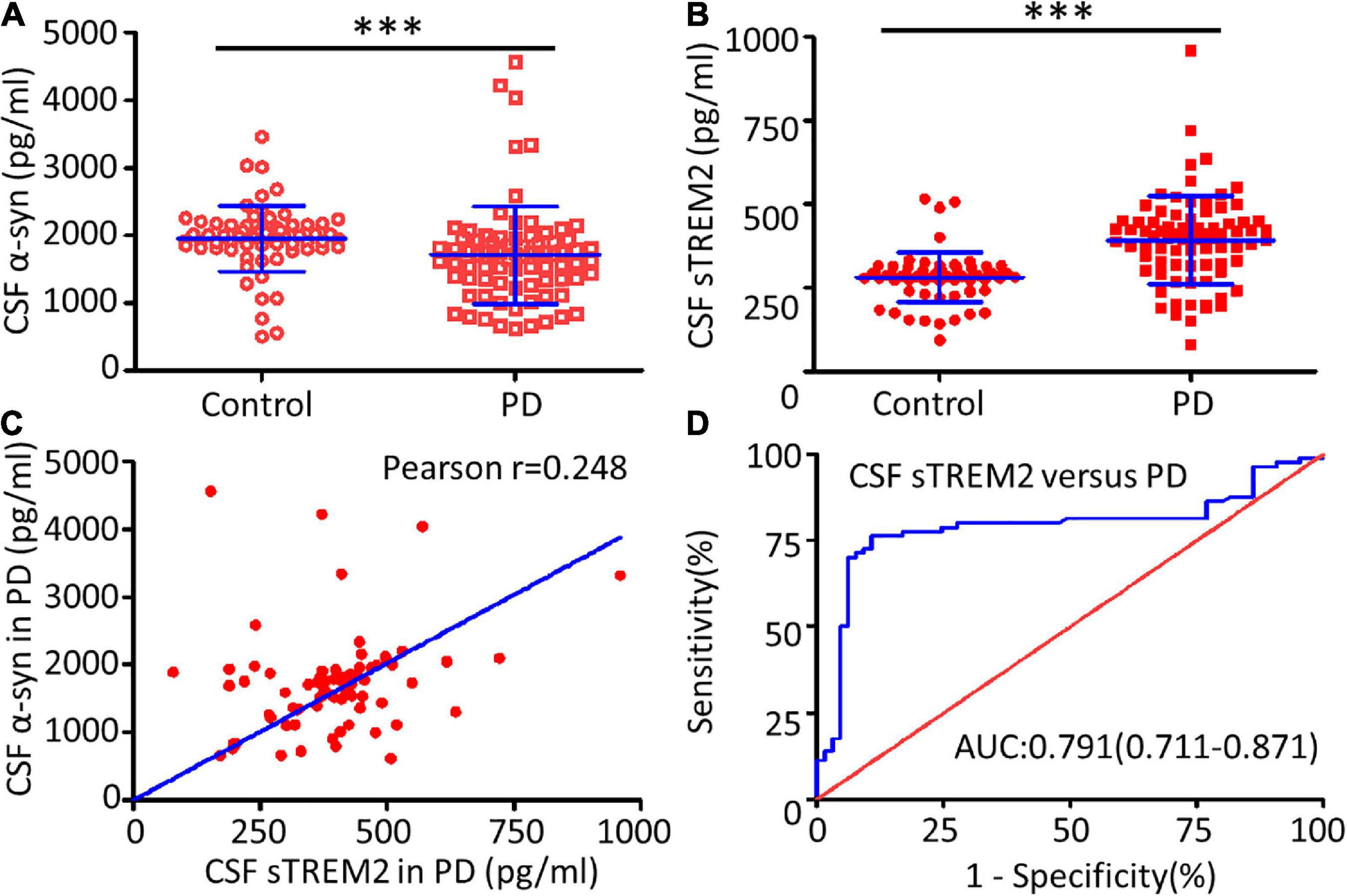
Figure 2. Correlation between CSF sTREM2 and PD. The concentrations of α-syn (A) and sTREM2 (B) in the CSF from PD patients and controls were determined by ELISA. The samples consisted of 80 PD patients and 65 controls. The sTREM2 values are shown as medians ± interquartile ranges with blue bars. The Mann–Whitney test was used for comparisons. (C) A significant association was observed between sTREM2 and α-syn in the CSF from PD patients (Pearson r = 0.248, p = 0.027) by Pearson correlation coefficient analysis. (D) ROC curve analysis of CSF sTREM2 in discriminating PD patients from healthy controls. The area under the curve was 0.791 (95% CI = 0.711–0.871). ∗∗∗p < 0.001 versus control.
Cerebrospinal Fluid Soluble Triggering Receptor Expressed on Myeloid Cells 2 Concentration Is Positively Associated With Sleep Disorders in Parkinson’s Disease
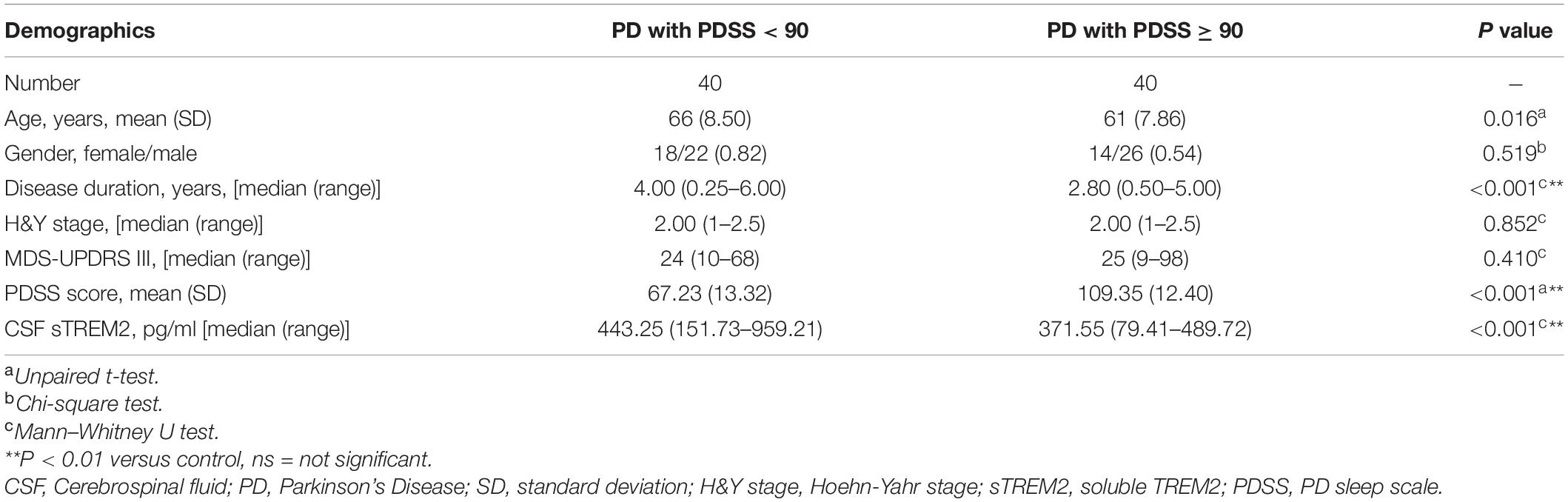
Sleep disorders, as one of the earliest non-motor symptoms of PD, may show an increased prevalence or a worsening of symptoms with disease progression (Stefani and Högl, 2020). According to the PDSS score used for the assessment of sleep disorders, the PD patients were further subdivided into two subgroups: the PDSS ≥ 90 group with no or mild sleep disorders and the PDSS < 90 group with moderate or severe sleep disorders. The two groups differed in disease duration (Mann–Whitney U test, p < 0.001), with a higher concentration of CSF sTREM2 in the PDSS ≥ 90 group (Mann–Whitney test, p < 0.001), but there was no difference in age (Unpaired t-test, p = 0.016), sex (Chi-square test, p = 0.491), H&Y stage (Mann–Whitney U test, p = 0.852) or MDS-UPDRS III score (Mann–Whitney U test, p = 0.410, Table 2).
Compared with the no or mild sleep disorder (PDSS ≥ 90) group, the PD patients with a moderate or severe sleep disorder (PDSS < 90) had a significantly increased concentration of sTREM2 in their CSF (Mann–Whitney test, p < 0.001, Figure 3A). The increasing trend of the CSF sTREM2 was negatively correlated with a closely decreasing trend of the PDSS score in PD patients (Pearson correlation analysis, Pearson r = -0.555, p < 0.001) but had no significant relationship with the UPDRS III score, which was used for the assessment of motor symptoms in PD (Pearson correlation analysis, Pearson r = 0.058, p = 0.607, Figures 3B,C). The ROC curve analysis revealed that CSF sTREM2 could differentiate PD patients with a moderate or severe sleep disorder from those with no or mild sleep disorder, with an AUC of 0.733 (95% CI = 0.619–0.846, p < 0.001, Figure 3D). This result suggested that CSF sTREM2 may have potential as a biomarker for disease progression in PD.
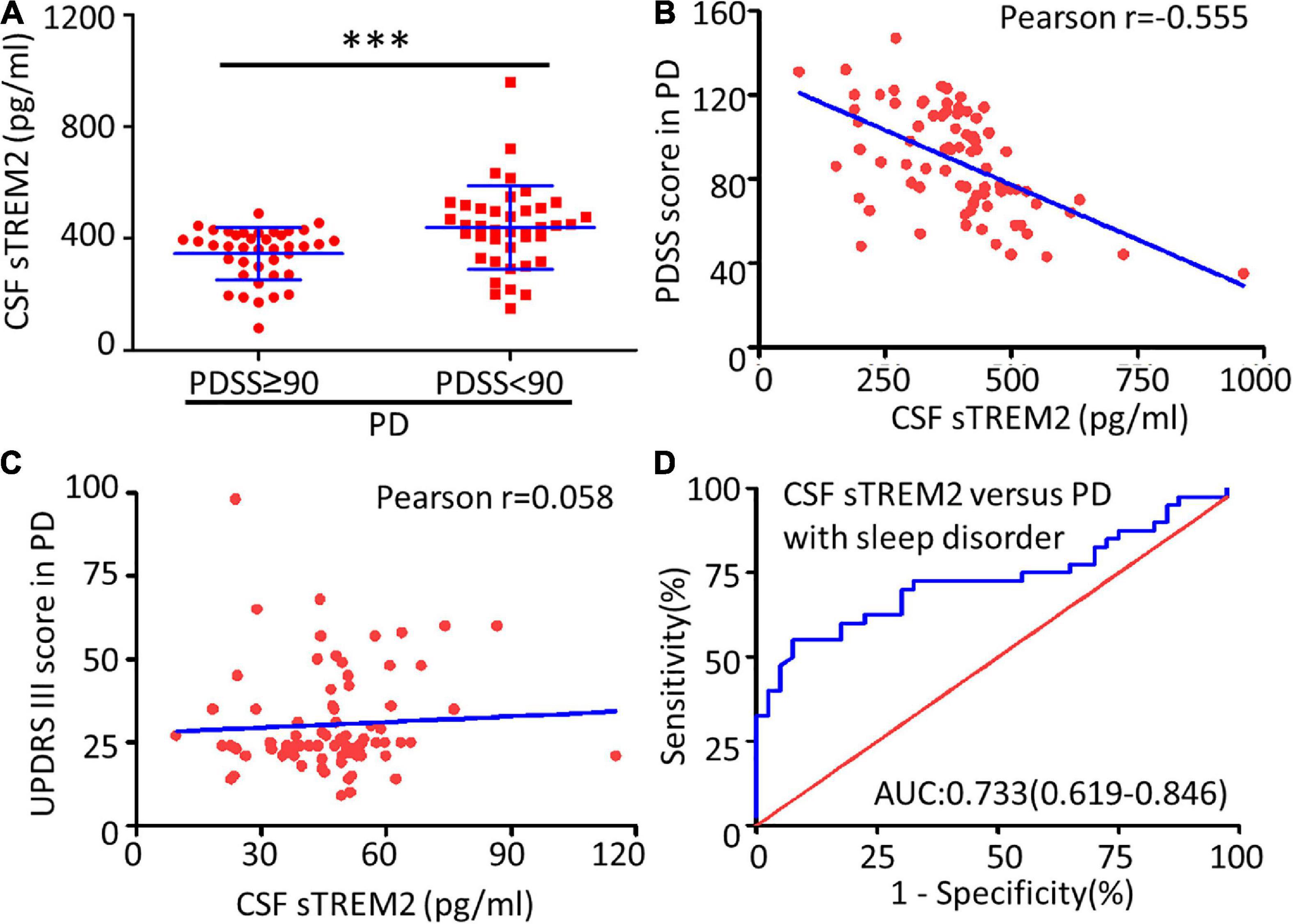
Figure 3. Correlation between CSF sTREM2 and PD with a sleep disorder. (A) The concentration of sTREM2 in CSF from PD patients with PDSS ≥ 90 (n = 40) or PDSS < 90 (n = 40) was determined by ELISA. The sTREM2 values are shown as medians ± interquartile ranges with blue bars. The Mann–Whitney test was used for comparisons. Pearson correlation coefficients were used. The concentration of sTREM2 in the CSF had a significant association with the PDSS score in PD patients (Pearson r = -0.555, p < 0.001) in (B) but not with the UPDRS III score (Pearson r = 0.058, p = 0.607) in (C). (D) ROC curve analysis of CSF sTREM2 in discriminating moderate- or severe-sleep disorder (PDSS < 90) from no- or mild-sleep disorder with PDSS ≥ 90 in PD. The area under the curve was 0.733 (95% CI = 0.619–0.846). ∗∗∗p < 0.001 versus control.
Discussion
The clinical presentation and progression of neurodegenerative disease shows substantial biological heterogeneity (Chen-Plotkin et al., 2018). Biological function-based biomarkers may help to differentiate subgroups of PD by their rate of progression accompanied by motor or non-motor symptoms (Chen-Plotkin et al., 2018). In the present study, we measured the levels of sTREM2 in CSF, evaluated its association with PD onset, and found that CSF sTREM2 is a promising candidate biomarker for PD, with higher sTREM2 levels corresponding to lower α-syn levels in CSF at disease onset. In the subgroup analysis, we found that higher sTREM2 levels were correlated with worse sleep disorders, as measured by the PDSS scale. Taken together, our data suggest that sTREM2 levels in CSF may reflect the progression of PD, indicating it is a valuable biomarker.
TREM2 receptors are exclusively expressed on microglia in the CNS (Dempsey, 2015). sTREM2 in the CSF is a released extracellular segment of the single-pass transmembrane protein of TREM2 after proteolytic cleavage (Kleinberger et al., 2014; Piccio et al., 2016). The physiological- and disease-associated functions of sTREM2 are not clear. sTREM2 has been suggested to have protective functions on microglial activity and viability (Zhong et al., 2017). Before release from the membrane, sTREM2 acts as a receptor and recognizes and binds to special ligands, such as apoptotic neurons, misfolded proteins, and cellular debris (Yeh et al., 2017). After release into the CSF, sTREM2 can directly bind monomeric Aβ42 (mAβ42) and potently inhibit mAβ42 polymerization (Henjum et al., 2018). The concentration of sTREM2 in the CSF is a sensitive marker of microglial activation and was reported to correlate with the prevalence of tau-mediated AD (Henjum et al., 2018; Wilson et al., 2020). In addition, the CSF sTREM2 level reflects TREM2 expression, and TREM2-triggered microglial activity in the CNS is an ideal biomarker for microglia-involved disease (Brendel et al., 2017; Hu et al., 2021). High sTREM2 concentrations are found mainly in the preclinical stage of AD, frontotemporal dementia, vascular dementia, and aging adults (Henjum et al., 2016; Suárez-Calvet et al., 2016; Wang et al., 2020; van der Ende et al., 2021). α-syn in PD has similar mechanisms of aggregation and degradation as Aβ in AD (Rai and Singh, 2020; Rai et al., 2020; Cong et al., 2021; Rai et al., 2021). They share a GAV motif (VGGAVVAGV) in peptides which may be involved with initiating protein aggregation or fibrillization, and similar degradation mechanisms including intra- and extra- cellular aggregation, microglial activation and endocytosis (Lin et al., 2010; Schechter et al., 2020; Lashuel, 2021). It suggests that TREM2 may contribute to microglial recognition and endocytosis of α-syn. In our study, we found that the levels of sTREM2 in the CSF of PD patients were higher than those of controls and positively correlated with the levels of α-syn in the CSF, an association previously observed in PD patients (Peng et al., 2020). We found that an elevated concentration of sTREM2 in CSF may help to differentiate PD patients from healthy controls with an AUC of 0.791 (CI = 0.711–0.871). To avoid the effects of gene mutation, we sequenced exon 2 of the TREM2 gene encoding the sTREM2 peptides and found that the distribution of SNPs was not significantly different between the PD and control groups. Our study suggested that sTREM2 may be an ideal biomarker for PD diagnosis, but more supporting data from multicenter studies are still needed.
Sleep disorders include insomnia, daytime somnolence, rapid eye movement (REM) sleep behavior disorder, periodic limb movement, etc. (Tse et al., 2005). Sleep disorder, present in 64.1% of PD patients, was listed as the second most frequent non-motor symptom (Pont-Sunyer et al., 2015; Lysen et al., 2019). Shorter sleep duration and worse sleep quality are associated with a higher risk of parkinsonism in the first 2 years (Lysen et al., 2019). Sleep disorders appear at any stage of PD and become aggravated with disease progression (Videnovic, 2018). The pathogenesis of sleep disorders in PD is still not clear. The role of microglia in sleep disorders should not be underestimated. Microglial inflammatory responses, which are involved in neurodegenerative diseases such as PD and AD, can induce sleep disorders and are regulated by the circadian clock (Fonken et al., 2015). Peripheral blood samples from narcolepsy patients show increased levels of interleukin (IL)-6, tumor necrosis factor (TNF)-α, and major histocompatibility complex (MHC) class II, and reduced levels of chemokine receptor (CCR) 1 and CCR3, which are derived from microglia and macrophages, and IL-1 and TNF-α, which are well known to promote sleep in humans (Agnes et al., 2017). Another key factor related to sleep disorders is α-syn (Dolores et al., 2016). Abnormal α-syn, normally localized to presynaptic terminals of neurons, forms pathological amyloid aggregates deposited in multiple brain regions and induces abnormal neurotransmitter release, such as sleep-related interleukins (Dolores et al., 2016; Doppler et al., 2017; Garrido et al., 2020; Stefani et al., 2021). Phosphorylated α-syn deposits in the colon, saliva, glands, and skin can be used to differentiate subjects with isolated rapid eye movement sleep behavior disorder (RBD) from controls with 24–89% sensitivity and 78–100% specificity (Doppler et al., 2017). The TREM2 receptor is involved in the regulation of microglial function and α-syn cleaning (Guo Y. et al., 2019), but its relationship with sleep disorders in PD is not clear. In our study, we found that the levels of sTREM2 in the CSF among patients with worse sleep disorders were lower in PD and negatively correlated with the PDSS score, as was its association with α-syn in CSF. The elevated concentration of sTREM2 in the CSF can be used to differentiate PD patients with sleep disorders with an AUC of 0.733 (CI = 0.619–0.846). These results further support the relationship between sTREM2 and PD and suggest that sTREM2 may be useful to evaluate sleep disorders in PD progression.
Conclusion
Taken together, we demonstrated that the concentration of sTREM2 in the CSF may represent a biomarker for monitoring the progression of PD, and an increased concentration of sTREM2 in the CSF was positively correlated with α-syn levels and sleep disorders. These findings may provide novel insights into a pivotal role for sTREM2, which serves as a biomarker of TREM2-mediated microglial function and can help to evaluate the development of PD.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Medical Ethics Committee of Guangzhou Medical University. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
MM and PX conceived and designed the study and interpreted the experiments. YT, LW, and WG performed the study and prepared the initial draft of the manuscript. MM, PX, XZ, ZW, YL, and YT supervised the project. WD, YW, JQ, GP, YL, MZ, WG, ZZ, LD, HL, PY, and XC provided the clinical samples and data. All authors read and approved the final submission.
Funding
This work was supported by research grants from National Key R&D Program of China (2016YFC1306601 and 2017YFC1306002), the National Natural Science Foundation of China (81701254, 82171240, 81870856, 81870992, and 82071416), General Project of Basic and Applied Basic Research of Guangzhou Bureau of Science and Technology (2060206), Yang-cheng Scholar Project of Guangzhou Municipal Bureau of Education (202032790), and General Project of Natural Science Foundation of Guangdong Province (2021A1515011043). Thanks for the support from Guangdong 999 Brain Hospital over the past years.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2021.753210/full#supplementary-material
References
Agnes, N., Wigren, H. M., and Marie-Eve, T. (2017). Roles of Microglial Phagocytosis and Inflammatory Mediators in the Pathophysiology of Sleep Disorders. Front. Cell Neurosci. 11:250. doi: 10.3389/fncel.2017.00250
Alexandre, C., Popa, D., Fabre, V., Bouali, S., Venault, P., Lesh, K. P., et al. (2006). Early life blockade of 5-hydroxytryptamine 1A receptors normalizes sleep and depression-like behavior in adult knock-out mice lacking the serotonin transporter. J. Neurosci. 26, 5554–5564. doi: 10.1523/JNEUROSCI.5156-05.2006
Barasa, A., Wang, J., and Dewey, R. B. (2021). Probable REM Sleep Behavior Disorder Is a Risk Factor for Symptom Progression in Parkinson Disease. Front. Neurol. 12:651157. doi: 10.3389/fneur.2021.651157
Bekris, L. M., Khrestian, M., Dyne, E., Shao, Y., Pillai, J., Rao, S., et al. (2018). Soluble TREM2 and biomarkers of central and peripheral inflammation in neurodegenerative disease. J. Neuroimmunol. 319, 19–27. doi: 10.1016/j.jneuroim.2018.03.003
Belsare, K., Wu, H., and Degrado, W. (2020). Interaction of sTREM2 with Amyloid Beta: implication on the Protective Role of sTREM2 in Alzheimer’s Disease. FASEB J. 34, 1–1. doi: 10.1096/fasebj.2020.34.s1.01844
Block, M. L., and Hong, J. S. (2005). Microglia and inflammation-mediated neurodegeneration: multiple triggers with a common mechanism. Prog. Neurobiol. 76, 77–98. doi: 10.1016/j.pneurobio.2005.06.004
Boespflug, E. L., and Iliff, J. J. (2018). The Emerging Relationship Between Interstitial Fluid-Cerebrospinal Fluid Exchange, Amyloid-β, and Sleep. Biol. Psychiatry 83:328. doi: 10.1016/j.biopsych.2017.11.031
Brendel, M., Kleinberger, G., Probst, F. J., Jaworska, A., Overhoff, F., Blume, T., et al. (2017). Increase of TREM2 during Aging of an Alzheimer’s Disease Mouse Model Is Paralleled by Microglial Activation and Amyloidosis. Front. Aging Neurosci. 9:8. doi: 10.3389/fnagi.2017.00008
Chaudhuri, K. R., Healy, D. G., and Schapira, A. H. (2006). Non-motor symptoms of Parkinson’s disease: diagnosis and management. Lancet Neurol. 5, 235–245. doi: 10.1016/S1474-4422(06)70373-8
Chen-Plotkin, A. S., Albin, R., Alcalay, R., Babcock, D., and Zhang, J. (2018). Finding useful biomarkers for Parkinson’s disease. Sci. Transl. Med. 10:eaam6003. doi: 10.1126/scitranslmed.aam6003
Colonna, M., and Butovsky, O. (2017). Microglia Function in the Central Nervous System During Health and Neurodegeneration. Annu. Rev. Immunol. 35:441. doi: 10.1146/annurev-immunol-051116-052358
Cong, C., Zhang, W., Qian, X., Qiu, W., and Ma, C. (2021). Significant overlap of α-synuclein, amyloid-β, and phospho-tau pathologies in neuropathological diagnosis of lewy-related pathology. J. Alzheimers Dis. 80, 447–458. doi: 10.3233/JAD-201548
Dolores, V., Alex, I., Eduardo, T., Iban, A., Joan, B., Isabel, V., et al. (2016). Assessment of α-synuclein in submandibular glands of patients with idiopathic rapid-eye-movement sleep behaviour disorder: a case-control study. Lancet Neurol. 15, 708–718. doi: 10.1016/S1474-4422(16)00080-6
Doppler, K., Jentschke, H. M., Schulmeyer, L., Vadasz, D., Janzen, A., Luster, M., et al. (2017). Dermal phospho-alpha-synuclein deposits confirm REM sleep behaviour disorder as prodromal Parkinson’s disease. Acta Neuropathol. 133, 535–545. doi: 10.1007/s00401-017-1684-z
Filippini, A., Gennarelli, M., and Russo, I. (2019). α-Synuclein and Glia in Parkinson’s Disease: a Beneficial or a Detrimental Duet for the Endo-Lysosomal System? Cell Mol. Neurobiol. 39, 161–168. doi: 10.1007/s10571-019-00649-9
Fonken, L. K., Frank, M. G., Kitt, M. M., Barrientos, R. M., Watkins, L. R., and Maier, S. F. (2015). Microglia inflammatory responses are controlled by an intrinsic circadian clock. Brain Behav. Immun. 45, 171–179. doi: 10.1016/j.bbi.2014.11.009
Garrido, A., Iranzo, A., Stefani, A., Serradell, M., Muñoz-Lopetegi, A., and Marrero, P. (2020). Lack of Asymmetry of Nigrostriatal Dopaminergic Function in Healthy Subjects. Mov. Disord. 35, 1072–1076. doi: 10.1002/mds.28019
Gu, H., Yang, X., Mao, X., Xu, E., and Dawson, T. M. (2021). Lymphocyte Activation Gene 3 (Lag3) Contributes to α-Synucleinopathy in α-Synuclein Transgenic Mice. Front. Cell Neurosci. 15:656426. doi: 10.3389/fncel.2021.656426
Guo, W., Zhou, M., Qiu, J., Lin, Y., Chen, X., Huang, S., et al. (2019). Association of LAG3 genetic variation with an increased risk of PD in Chinese female population. J. Neuroinflamm. 16:270. doi: 10.1186/s12974-019-1654-6
Guo, Y., Wei, X., Yan, H., Qin, Y., Yan, S., Liu, J., et al. (2019). TREM2 deficiency aggravates α-synuclein-induced neurodegeneration and neuroinflammation in Parkinson’s disease models. FASEB J. 33, 12164–12174. doi: 10.1096/fj.201900992R
Hayat, H., Regev, N., Matosevich, N., Sales, A., Krom, A. J., Berg, L., et al. (2020). Locus coeruleus norepinephrine activity mediates sensory-evoked awakenings from sleep. Sci. Adv. 6:eaaz4232. doi: 10.1126/sciadv.aaz4232
Henjum, K. I Almdahl, S., Årskog, V., Minthon, L., Hansson, O., Fladby, T., et al. (2016). Cerebrospinal fluid soluble TREM2 in aging and Alzheimer’s disease. Alzheimers Res. Ther. 8:17. doi: 10.1186/s13195-016-0182-1
Henjum, K., Quist-Paulsen, E., Zetterberg, H., Blennow, K., Nilsson, L., and Watne, L. O. (2018). CSF sTREM2 in delirium-relation to Alzheimer’s disease CSF biomarkers Aβ42, t-tau and p-tau. J. Neuroinflamm. 15:304. doi: 10.1186/s12974-018-1331-1
Hu, H. Y., Ma, L. Z., Hu, H., Bi, Y. L., Ma, Y. H., and Shen, X. N. (2021). Associations of Sleep Characteristics with Cerebrospinal Fluid sTREM2 in Cognitively Normal Older Adults: the CABLE Study. Neurotox Res. 39, 1372–1380. doi: 10.1007/s12640-021-00383-5
Kleinberger, G., Yamanishi, Y., Suárez-Calvet, M., Czirr, E., Lohmann, E., Cuyvers, E., et al. (2014). TREM2 mutations implicated in neurodegeneration impair cell surface transport and phagocytosis. Sci. Transl. Med. 6:243ra86. doi: 10.1126/scitranslmed.3009093
Lashuel, H. A. (2021). Alpha-synuclein oligomerization and aggregation: all models are useful but only if we know what they model. J. Neurochem. 157, 891–898. doi: 10.1111/jnc.15275
Lee, S. H., Meilandt, W. J., Xie, L., Gandham, V. D., Ngu, H., Barck, K. H., et al. (2021). Trem2 restrains the enhancement of tau accumulation and neurodegeneration by β-amyloid pathology. Neuron 109, 1283–1301.e6. doi: 10.1016/j.neuron.2021.02.010
Leyns, C., Ulrich, J. D., Finn, M. B., Stewart, F. R., and Holtzman, D. M. (2017). TREM2 deficiency attenuates neuroinflammation and protects against neurodegeneration in a mouse model of tauopathy. Proc. Natl. Acad. Sci. U. S. A. 114, 11524–11529. doi: 10.1073/pnas.1710311114
Li, N., Wu, Y., Zhu, L., Huang, Y., Liu, Z., and Shi, M. (2019). Extracellular microvesicles-derived from microglia treated with unaggregated α-synuclein attenuate mitochondrial fission and toxicity-induced by Parkinsonian toxin MPP+. Biochem. Biophys. Res. Commun. 517, 642–647. doi: 10.1016/j.bbrc.2019.07.084
Li, Z., Zhong, L., Gu, L., Huang, W., Shi, X., Zhang, X., et al. (2016). Association study of TREM2 polymorphism rs75932628 with leucoaraiosis or Parkinson’s disease in the Han Chinese population. BMJ Open 6:e009499. doi: 10.1136/bmjopen-2015-009499
Lin, X., Zhang, F., Xie, Y., Bao, W., He, J. H., and Hu, H. (2010). Secondary structural formation of alpha-synuclein amyloids as revealed by g-factor of solid-state circular dichroism. Biopolymers 83, 226–232. doi: 10.1002/bip.20550
Lysen, T. S., Darweesh, S. K. L., Ikram, M. K., Luik, A. I., and Ikram, M. A. (2019). Sleep and the risk of parkinsonism and Parkinson’s disease: a population-based study. Brain 42, 2013–2022. doi: 10.1093/brain/awz113
Mecca, C., Giambanco, I., Donato, R., and Arcuri, C. (2018). Microglia and aging: the role of the TREM2-DAP12 and CX3CL1-CX3CR1 Axes. Int. J. Mol. Sci. 19:318. doi: 10.3390/ijms19010318
Meles, S. K., Vadasz, D., Renken, R. J., Sittig-Wiegand, E., Mayer, G., Depboylu, C., et al. (2017). FDG PET, dopamine transporter SPECT, and olfaction: combining biomarkers in REM sleep behavior disorder. Mov. Disord. 32, 1482–1486. doi: 10.1002/mds.27094
Mengel, D., Thelen, M., Balzer-Geldsetzer, M., Ling, C. S., Bach, J. P., Schaeffer, E., et al. (2016). TREM2 rare variant p.R47H is not associated with Parkinson’s disease. Parkinsonism Relat. Disord. 23, 109–111. doi: 10.1016/j.parkreldis.2015.11.026
Nasreddine, Z. S., Phillips, N. A., Bédirian, V., Charbonneau, S., and Chertkow, H. (2005). The Montreal Cognitive Assessment, MoCA: a Brief Screening Tool For Mild Cognitive Impairment. J. Am. Geriatr. Soc. 53, 695–699. doi: 10.1111/j.1532-5415.2005.53221.x
Oh, J., Petersen, C., Walsh, C. M., Bittencourt, J. C., Neylan, T. C., and Grinberg, L. T. (2018). The role of co-neurotransmitters in sleep and wake regulation. Mol. Psychiatry 24, 1284–1295. doi: 10.1038/s41380-018-0291-2
Peng, G., Qiu, J., Liu, H., Zhou, M., and Xu, P. (2020). Analysis of Cerebrospinal Fluid Soluble TREM2 and Polymorphisms in Sporadic Parkinson’s Disease in a Chinese Population. J. Mol. Neurosci. 70, 294–301. doi: 10.1007/s12031-019-01424-7
Piccio, L., Deming, Y., Del-Guila, J. L., Ghezzi, L., Holtzman, D. M., Fagan, A. M., et al. (2016). Cerebrospinal fluid soluble TREM2 is higher in Alzheimer disease and associated with mutation status. Acta Neuropathol. 131, 925–933. doi: 10.1007/s00401-016-1533-5
Pont-Sunyer, C., Hotter, A., Gaig, C., Seppi, K., Compta, Y., Katzenschlager, R., et al. (2015). The Onset of Nonmotor Symptoms in Parkinson’s disease (The ONSET PD Study). Mov. Disord. 30, 229–237. doi: 10.1002/mds.26077
Rai, S. N., Chaturvedi, V. K., Singh, P., Singh, B. K., and Singh, M. P. (2020). Mucuna pruriens in Parkinson’s and in some other diseases: recent advancement and future prospective. 3 Biotech 10:522. doi: 10.1007/s13205-020-02532-7
Rai, S. N., and Singh, P. (2020). Advancement in the modelling and therapeutics of Parkinson’s disease. J. Chem. Neuroanat. 104:101752. doi: 10.1016/j.jchemneu.2020.101752
Rai, S. N., Singh, P., Varshney, R., Chaturvedi, V. K., Vamanu, E., Singh, M. P., et al. (2021). Promising drug targets and associated therapeutic interventions in Parkinson’s disease. Neural Regen. Res. 16, 1730--1739. doi: 10.4103/1673-5374.306066
Ren, M., Guo, Y., Wei, X., Yan, S., Qin, Y., Zhang, X., et al. (2018). TREM2 overexpression attenuates neuroinflammation and protects dopaminergic neurons in experimental models of Parkinson’s disease. Exp. Neurol. 302, 205–213. doi: 10.1016/j.expneurol.2018.01.016
Sayed, F. A., Telpoukhovskaia, M., Kodama, L., Li, Y., Zhou, Y., Le, D., et al. (2018). Differential effects of partial and complete loss of TREM2 on microglial injury response and tauopathy. Proc. Natl. Acad. Sci. U. S. A. 115:201811411. doi: 10.1073/pnas.1811411115
Schechter, M., Atias, M., Elhadi, S. A., Davidi, D., and Sharon, R. (2020). α-synuclein facilitates endocytosis by elevating the steady-state levels of phosphatidylinositol 4,5-bisphosphate. J. Biol. Chem. 295, 18076–18090. doi: 10.1074/jbc.RA120.015319
Sims, R., van der Lee, S. J., Naj, A. C., Bellenguez, C., Badarinarayan, N., Jakobsdottir, J., et al. (2017). Rare coding variants in PLCG2, ABI3, and TREM2implicate microglial-mediated innate immunity in Alzheimer’s disease. Nat. Genet. 49, 1373–1384. doi: 10.1038/ng.3916
Stefani, A., and Högl, B. (2020). Sleep in Parkinson’s disease. Neuropsychopharmacology 45, 121–128. doi: 10.1038/s41386-019-0448-y
Stefani, A., Iranzo, A., Holzknecht, E., Perra, D., Bongianni, M., and Gaig, C. (2021). Alpha-synuclein seeds in olfactory mucosa of patients with isolated rapid-eye-movement sleep behaviour disorder. Brain 144, 1118–1126. doi: 10.1093/brain/awab005
Suárez-Calvet, M., Kleinberger, G., Caballero, M. A., Brendel, M., Rominger, A., Alcolea, D., et al. (2016). sTREM2 cerebrospinal fluid levels are a potential biomarker for microglia activity in early-stage Alzheimer’s disease and associate with neuronal injury markers. EMBO Mol. Med. 8, 466–476. doi: 10.15252/emmm.201506123
Tse, W., Liu, Y., Barthlen, G. M., HäLbig, T. D., Tolgyesi, S. V., Gracies, J. M., et al. (2005). Clinical usefulness of the Parkinson’s disease sleep scale. Parkinsonism Relat. Disord. 11, 317–321. doi: 10.1016/j.parkreldis.2005.02.006
Udeochu, J., Sayed, F. A., and Gan, L. (2018). TREM2 and Amyloid Beta: a Love-Hate Relationship. Neuron 97:991. doi: 10.1016/j.neuron.2018.02.018
van der Ende, E. L., Morenas-Rodriguez, E., Mcmillan, C., Grossman, M., and Seelaar, H. (2021). CSF sTREM2 is elevated in a subset in grn-related frontotemporal dementia. Neurobiol. Aging 103, 158.e1–158.e5. doi: 10.1016/j.neurobiolaging.2021.02.024
Veys, L., Van Houcke, J., Aerts, J., Van Pottelberge, S., Mahieu, M., Coens, A., et al. (2021). Absence of Uptake and Prion-Like Spreading of Alpha-Synuclein and Tau After Intravitreal Injection of Preformed Fibrils. Front. Aging Neurosci. 12:614587. doi: 10.3389/fnagi.2020.614587
Videnovic, A. (2018). Disturbances of Sleep and Alertness in Parkinson’s Disease. Curr. Neurol. Neurosci. 18:29. doi: 10.1007/s11910-018-0838-2
Wang, Q., Liu, Y., and Zhou, J. (2015). Neuroinflammation in Parkinson’s disease and its potential as therapeutic target. Transl. Neurodegener. 4:19. doi: 10.1186/s40035-015-0042-0
Wang, Q., Yang, W., Zhang, J., Zhao, Y., and Xu, Y. (2020). TREM2 Overexpression Attenuates Cognitive Deficits in Experimental Models of Vascular Dementia. Neural Plast. 2, 1–10. doi: 10.1155/2020/8834275
Wilckens, K. A., Dana, T., Snitz, B. E., Julie, P., Aizenstein, H. J., Oscar, L., et al. (2018). Sleep moderates the relationship between amyloid beta and memory recall. Neurobiol. Aging 71, 142–148. doi: 10.1016/j.neurobiolaging.2018.07.011
Wilson, E. N., Swarovski, M. S., Patricia, L., Marian, S., Zuckerman, A. J., Wang, Q., et al. (2020). Soluble TREM2 is elevated in Parkinson’s disease subgroups with increased CSF tau. Brain 143, 932–943. doi: 10.1093/brain/awaa021
Wood, H. (2017). Alzheimer disease: soluble TREM2 in CSF sheds light on microglial activation in AD. Nat. Rev. Neurol. 13:65. doi: 10.1038/nrneurol.2016.203
Yeh, F. L., Hansen, D. V., and Sheng, M. (2017). TREM2, Microglia, and Neurodegenerative Diseases. Trends Mol. Med. 23, 512–533. doi: 10.1016/j.molmed.2017.03.008
Zhong, L., Chen, X. F., Wang, T., Wang, Z., Liao, C., Wang, Z., et al. (2017). Soluble TREM2 induces inflammatory responses and enhances microglial survival. J. Exp. Med. 214, 597–607. doi: 10.1084/jem.20160844
Keywords: Parkinson’s disease, TREM2 (triggering receptor expressed on myeloid cells), biomarker, sleep disorder (SD), cerebrospinal fluid (CSF)
Citation: Mo M, Tang Y, Wei L, Qiu J, Peng G, Lin Y, Zhou M, Dai W, Zhang Z, Chen X, Liu H, Ding L, Ye P, Wu Y, Zhu X, Wu Z, Guo W and Xu P (2021) Soluble Triggering Receptor Expressed on Myeloid Cells 2 From Cerebrospinal Fluid in Sleep Disorders Related to Parkinson’s Disease. Front. Aging Neurosci. 13:753210. doi: 10.3389/fnagi.2021.753210
Received: 04 August 2021; Accepted: 07 September 2021;
Published: 29 September 2021.
Edited by:
Yi Tang, Xuanwu Hospital, Capital Medical University, ChinaReviewed by:
Haigang Ren, Soochow University, ChinaSachchida Nand Rai, Centre of Biotechnology, University of Allahabad, India
Copyright © 2021 Mo, Tang, Wei, Qiu, Peng, Lin, Zhou, Dai, Zhang, Chen, Liu, Ding, Ye, Wu, Zhu, Wu, Guo and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mingshu Mo, bW9taW5nc2h1MTIzQDE2My5jb20=; Pingyi Xu, cHl4MTIzQDE2My5jb20=
†These authors have contributed equally to this work
 Mingshu Mo
Mingshu Mo Yuting Tang1†
Yuting Tang1† Miaomiao Zhou
Miaomiao Zhou