- Department of Psychology, Centre for Applied Behavioural Sciences, Heriot-Watt University, Edinburgh, United Kingdom
Introduction: Possession of one or two e4 alleles of the apolipoprotein E (APOE) gene is associated with cognitive decline and dementia risk. Some evidence suggests that physical activity may benefit carriers of the e4 allele differently.
Method: We conducted a systematic review and meta-analysis of studies which assessed APOE differences in the association between physical activity and: lipid profile, Alzheimer's disease pathology, brain structure and brain function in healthy adults. Searches were carried out in PubMed, SCOPUS, Web of Science and PsycInfo.
Results: Thirty studies were included from 4,896 papers screened. Carriers of the e4 allele gained the same benefit from physical activity as non-carriers on most outcomes. For brain activation, e4 carriers appeared to gain a greater benefit from physical activity on task-related and resting-state activation and resting-state functional connectivity compared to non-carriers. Post-hoc analysis identified possible compensatory mechanisms allowing e4 carriers to maintain cognitive function.
Discussion: Though there is evidence suggesting physical activity may benefit e4 carriers differently compared to non-carriers, this may vary by the specific brain health outcome, perhaps limited to brain activation. Further research is required to confirm these findings and elucidate the mechanisms.
Introduction
While research has supported the potential benefit of physical activity across a range of cognitive and brain health outcomes, there are indications that not all individuals experience this to the same extent. Possession of the e4 allele of the apolipoprotein E (APOE) gene, a risk factor for cognitive decline and dementia, may moderate the association between physical activity and brain health. Research has suggested that individuals possessing the e4 allele may actually benefit more from physical activity, compared to non-carriers. However, findings are variable, both across individual studies and the brain health outcomes considered. The current systematic review explored whether APOE moderated the association between physical activity and brain health, including specific cardiovascular or cerebrovascular markers implicated in the mechanisms.
Physical Activity and Brain Health
Understanding how lifestyle affects the brain is crucial for maintaining our cognitive abilities as we get older. Even in the absence of any diagnosed cognitive impairment, cognitive abilities follow different trajectories through the lifespan. The typical progression involves relative stability or slight increases from our mid-twenties through to our fifties, followed by a gradual decline from our sixties (Schaie et al., 2004). A similar pattern can be seen for brain structure and health (Vinke et al., 2018).
Physical activity is a modifiable lifestyle factor associated with preserved cognitive ability (Erickson et al., 2019). Encouragingly, randomised controlled trials suggest a causative role, with physical activity interventions resulting in improved cognitive performance. For example, executive function (Stern et al., 2019) and spatial memory (Erickson et al., 2011) improved in those undertaking an aerobic exercise intervention compared to a control group engaging only in stretching exercises. Physical activity may also predict future cognitive change. In a longitudinal study which assessed cognitive ability four times between the ages of 79 and 90, greater physical activity undertaken between the ages of 60 and 75 was associated with less cognitive decline over the 11-year period (Gow et al., 2017).
The mechanisms through which physical activity benefits cognition may involve a range of physiological and brain health outcomes. One part of this mechanism is cholesterol, which is transported in the blood by lipoproteins. Higher low density lipoprotein cholesterol (LDL), often referred to as “bad cholesterol,” indicates surplus cholesterol in the blood. In contrast, “good” high density lipoprotein cholesterol (HDL) transports cholesterol back to the liver for disposal. Due to the different effects of LDL and HDL, combined measurements of total cholesterol (TC) can be misleading (Mann et al., 2014). However, assessments of LDL and HDL separately demonstrate a clear association between physical activity and lipid profile, with physically active individuals having reduced LDL (Sarzynski et al., 2015) and increased HDL (Thompson et al., 1997; Kodama et al., 2007).
Physical activity may also be associated with Alzheimer's disease (AD) pathology. The neuropathological hallmarks of AD are senile plaques that contain amyloid beta (Aβ) and intracellular neurofibrillary tangles which consist of tau proteins. Higher levels of brain Aβ are associated with poorer cognitive ability and increased risk of dementia. The most reliable measurement of Aβ is with a tracer such as Pittsburgh compound B (PiB) during positron emission tomography (PET). In a cross-sectional study, physically active individuals had a lower association between PiB-PET Aβ burden with age compared to inactive individuals (Okonkwo et al., 2014). Aβ can also be measured within cerebrospinal fluid (CSF), with lower CSF Aβ associated with higher PiB-PET Aβ measures (Fagan et al., 2006). This negative association was supported by a meta-analysis of 131 studies (Olsson et al., 2016), and is thought to be due to higher levels of Aβ aggregated in plaques in the brain leaving less Aβ available to be secreted to the CSF. Cross-sectional evidence suggests that physical activity is positively associated with CSF Aβ (Law et al., 2018), consistent with physical activity being associated with reduced brain Aβ. Blood plasma Aβ mirrors the profile seen in CSF (Blennow and Zetterberg, 2018), with plasma Aβ being lower in individuals with high PiB-PET Aβ (Ovod et al., 2017). Finally, erythrocytes (red blood cells) can be used to measure Aβ (Lan et al., 2015). While less research has been conducted in this area, erythrocyte Aβ accumulation increases with age, and the profile does not follow the reversed pattern seen in CSF and blood plasma (Kiko et al., 2012). Less is known about the association between physical activity and tau. A recent review concluded that evidence for an association between physical activity and reduced tau (and brain Aβ) is robust in mice, with longitudinal studies potentially supporting a causative effect, but that more research is needed to confirm the association in humans (Brown et al., 2019).
Physical activity also appears to have a positive effect on brain structure. Higher levels of physical activity have been associated with larger grey matter (GM) volumes, particularly in frontal and temporal regions (Bugg and Head, 2011). A randomised controlled trial revealed increased frontal cortical thickness in participants who engaged in aerobic exercise, supporting a causal relationship (Stern et al., 2019). White matter (WM) structure, another key factor in maintaining brain health, has also been positively associated with being physically active (Marks et al., 2007). Evidence again suggests a causal relationship, with a 6-month randomised aerobic exercise intervention resulting in increased WM volume (Colcombe et al., 2006).
An aspect of brain structure which is less easy to interpret is WM integrity, which is inferred from measures of water diffusion in brain tissue. When diffusion is constrained along an axis, it is said to be anisotropic, and is thought to reflect the structure of axons. While lower mean diffusivity (MD) and higher fractional anisotropy (FA) suggest more constrained diffusion of water and therefore better WM integrity, crossing neural fibres mean that this conclusion must be made with caution. Diffusion can appear more isotropic as axons intersect in complex architectural regions despite high structural integrity (Pierpaoli and Basser, 1996; Madden et al., 2009). It is therefore suggested that these measures are not automatically interpreted as indicating WM integrity (Jones et al., 2013). Cerebrovascular health is another important factor for maintaining cognitive ability. The presence of white matter hyperintensities (WMH) observed by MRI are thought to indicate poorer cerebrovascular health (Wardlaw et al., 2015), and physical fitness has been associated with reduced WMH (Ritchie et al., 2017).
In terms of brain activation, physical activity is associated with the strength of task-related neural activation. A meta-analysis of 20 studies which investigated a range of cognitive tasks reported that physical activity was associated with parietal lobe activation, specifically in the precuneus (Yu et al., 2021), which is often affected in the early stages of AD (Jacobs et al., 2012). Communication between brain regions may also benefit from physical activity. During an executive control task, physically active individuals showed greater functional connectivity compared to physically inactive participants (Kamijo et al., 2011). Though functional connectivity is generally considered beneficial, strong synchronicity between two regions could be indicative of a deficit, given evidence of oscillatory hypersynchrony in AD mice (Vico Varela et al., 2019). This must therefore be considered when interpreting the association between physical activity and functional connectivity.
Apolipoprotein E and Brain Health
Though physical activity is a promising target for promoting brain health, it is important to know whether it benefits everyone equally. Research has focused on a number of potential moderators of physical activity-brain health associations, including genetic factors such as APOE genotype. Apolipoprotein E is a protein involved in cholesterol transportation (Mahley, 1988; Bennet et al., 2007). The gene (APOE) which codes for this protein comes in three different versions—or alleles—known as e2, e3, and e4. The e4 allele is estimated to have a frequency of 14.4% in the UK (Corbo and Scacchi, 1999) and is associated with increased risk of AD (Corder et al., 1993), vascular dementia (VD) (Chuang et al., 2010), and stroke (Khan et al., 2013). Around 95% of AD cases are sporadic late onset, and e4 possession confers the strongest known genetic risk for late onset AD (Rocchi et al., 2003). Estimates of the variance in late onset AD diagnosis explained by APOE range from 6 to 13% (Ridge et al., 2013, 2016).
Possession of the e4 allele is also associated with cognitive decline within what might be considered “typical” age-related changes, though some of those “typical” changes may actually result from prodromal stages of dementia, with decline identified up to 6 years prior to diagnosis (Wilson et al., 2011). Whatever the mechanism, a meta-analysis demonstrated impaired cognitive ability in middle-aged e4 carriers compared to non-carriers, suggesting a cognitive phenotype prior to clinical diagnosis (Wisdom et al., 2011). APOE e4 possession has been associated with poorer outcomes in lipid profile (Leoni et al., 2010; Ferguson et al., 2020), Aβ burden (Liu et al., 2015), GM volume (Wishart et al., 2006), WM integrity (Persson et al., 2006; Operto et al., 2018), cerebrovascular health (Rojas et al., 2018; Lyall et al., 2019), task-related neural activation (Bondi et al., 2005) and functional connectivity (Canuet et al., 2012), i.e., the factors that appear to benefit from engagement in physical activity described earlier.
APOE Moderation of the Association Between Physical Activity and Brain Health
Evidence suggests that the benefit of physical activity for brain health may differ by APOE status, however, findings have been inconsistent. For example, studies have shown cognitive ability to be associated with physical activity in either e4 carriers (Pizzie et al., 2014) or e4 non-carriers only (Obisesan et al., 2012). Other studies have shown an association between physical activity and cognitive ability in both e4 carriers and non-carriers (Sabia et al., 2010; Rodriguez et al., 2018). A recent systematic review investigating the association between physical activity, dementia risk and brain health suggested that e4 carriers might show a stronger association between physical activity and amyloid burden, and that in some cases only e4 carriers, and in others both carriers and non-carriers, showed an association between physical activity and functional neuroimaging outcomes (de Frutos-Lucas et al., 2020c). The authors concluded that while there was some evidence of moderation by APOE, the overall picture was inconclusive.
In the present review, we considered the moderating effect of APOE on the association between physical activity and a broader range of outcomes including lipid profile (LDL, HDL, TC), AD pathology (Aβ and tau), brain structure (GM volume, WM volume, WM integrity and cerebrovascular health) and brain activation (task-related activation, resting-state activation, resting-state functional connectivity). In addition to narrative syntheses, we conducted additional meta-analyses where possible to empirically investigate the nature and extent of any APOE moderation.
Methods
This systematic review and meta-analysis is reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). A protocol (CRD42020164913) for this review was registered with PROSPERO and the record can be accessed online: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=164913.
Search Strategy
Initial searches were conducted in February 2020 for peer reviewed studies written in English in PubMed, PsycINFO, Web of Science and SCOPUS. Search strings included terms relating to physical activity (e.g., “physical activity” or “exercise”), APOE (e.g., “apolipoprotein E” or “e4”), and the outcomes (e.g., “amyloid” or “grey matter”) (see Supplementary Table 1 for full search terms). A second search was carried out to include all studies published up to 31st December 2020. To yield additional studies, reference lists of review papers returned from the searches were examined along with searches of the lead author's records.
Inclusion Criteria
Cross-sectional, longitudinal and intervention studies with adults aged 18 or over were eligible for inclusion. Studies including healthy participants or those with mild cognitive impairment were included, but studies which only assessed participants diagnosed with dementia were excluded. Studies were required to examine the association between total physical activity or physical fitness and one of the outcomes with a comparison of the association by APOE status. This could be through a statistical assessment of a physical activity by APOE interaction, or by stratified analyses for e4 carriers and non-carriers. Carriers included participants carrying either one e4 allele (heterozygotes) or two e4 alleles (homozygotes).
Selection Process
Search results were combined in EndNote and duplicates removed. Titles and abstracts were screened by one reviewer (AP). Full text screening was carried out independently by two reviewers (AP and CM) with any discrepancies discussed until consensus was achieved.
Data Extraction
Study characteristics extracted included study design, population, outcome(s), physical activity measure and APOE genotype. If cross-sectional data and longitudinal change were reported in the same paper, longitudinal outcomes were extracted. Data extracted included main effects of physical activity and APOE, and the interaction term if applicable. Associations between physical activity and the outcome were extracted for e4 carriers and non-carriers separately. Where relevant data were not reported, an email request was sent to the authors. One reminder email was sent after 3 weeks if there had been no response.
Analysis
Narrative syntheses consisted of a discussion of the association between physical activity and each outcome, and whether the association differed depending on APOE genotype. For meta-analyses to be possible, at least 5 studies were required. As TC levels can be misleading, they were not deemed suitable for meta-analysis, and as high LDL represents a negative outcome and HDL represents a positive outcome, they were assessed in two separate meta-analyses. Similarly, interpretation of WM integrity is ambiguous where there are crossing neural fibres, so only a narrative synthesis was deemed possible.
When meta-analysis was possible, effect sizes of associations between physical activity and the outcome from each study were included separately for e4 carriers and non-carriers. A subgroup analysis was used to determine whether any association between physical activity and the outcomes differed by APOE status.
Where an outcome was analysed with different measurements or techniques, all effect sizes were included in the meta-analysis. To account for the resulting dependency from multiple effect sizes being obtained from the same sample, a multilevel model was used. Simulations suggest that multilevel models provide appropriate estimates of mean effects and confidence intervals (Van den Noortgate et al., 2014), and are considered superior to alternatives such as computing an average or selecting one effect size from each study as these do not utilise the available data (Cheung, 2019). Analyses were conducted in R Core Team (2020) using the metafor v2.4-0 package (Viechtbauer, 2010) with effect sizes nested within their respective study. Comparisons were made between the full multilevel model and a model with the study level held constant at zero to determine whether the multilevel model provided a better fit. Where the Bayesian Information Criterion (BIC) and the Akaike Information Criterion (AIC) were significantly lower in the multilevel model, the multilevel meta-analysis was used (Assink and Wibbelink, 2016), but where the full model did not provide a better fit, the standard meta-analysis was retained.
Due to the expected heterogeneity among study designs and outcomes, random effects models were used. In contrast to a fixed effect model which assumes one true effect size, a random effects model assumes a distribution of true effect sizes. Heterogeneity was assessed with the I2 statistic, which indicates the extent to which studies differ over and above random sampling error. Where heterogeneity was high, study characteristics and forest plots were examined to identify differences which could explain this heterogeneity. Where appropriate, post-hoc sensitivity analyses were carried out with potential sources of heterogeneity removed from meta-analyses to identify where studies differed.
The metric used to estimate summary effects was Pearson's r. If this was not reported, the Campbell Collaboration effect size calculator (https://campbellcollaboration.org/research-resources/effect-size-calculator.html) was used to convert r from either (1) standardised or unstandardised regression coefficient and sample size; (2) means, standard deviations and sample sizes (where there were more than two physical activity groups, the most active and the least active were used); (3) t-test t-value and sample sizes; or (4) t-test p-value and sample sizes. Where rho was reported, this was used instead of Pearson's r as this was preferable to omitting the data.
Where necessary, the sign of a correlation was reversed to ensure that associations between physical activity and outcomes were consistent. For example, effect sizes for the associations between physical activity and CSF Aβ and blood plasma Aβ were reversed so that positive values represented greater brain Aβ burden. One study reversed the PiB PET Aβ sign so that larger positive values corresponded to lower Aβ burden (Vemuri et al., 2016), reported as a positive correlation though interpreted as a higher level of physical activity being associated with less Aβ. In the current review, that correlation was reported consistent with effect sizes from other studies considering PiB PET and erythrocytes, where a negative correlation indicated that brain Aβ burden was lower in those reporting higher physical activity. For functional brain outcomes, shorter latencies resulted in a negative correlation with physical activity, and these were reversed so that a positive correlation indicated a better outcome associated with physical activity.
Some studies which reported a non-significant physical activity by APOE interaction did not present the stratified data. Where these data could not be obtained after email request, the missing data were imputed. A technique common in meta-analyses where non-significant odds ratios are unavailable is to set the odds ratio to 1. As the aim of the analysis was to use a subgroup analysis to assess whether the association between physical activity and the outcome differed by APOE status, where the stratified effects for e4 carriers and non-carriers were not available separately, the Pearson's r main effect of physical activity for e4 carriers and non-carriers combined was used for both e4 carriers and non-carriers individually, effectively setting the difference across APOE to 0. If the physical activity main effect was also not reported, this was set to 0 for both e4 carriers and non-carriers. Where there was a significant physical activity by APOE interaction but one of the stratified analyses was non-significant and not reported, this was set to 0. The alpha level for significance tests for all analyses was p = 0.05 or a 95% confidence interval.
Publication Bias
Contour enhanced funnel plots were generated using the metafor v2.4-0 package (Viechtbauer, 2010) in R Studio and used to visually investigate publication bias. When multiple outcomes from one study were included in the analysis, all effect sizes were included in the funnel plot grouped by symbol to aid judgement. Subgroups of effect sizes for e4 carriers and non-carriers were colour coded so that a judgement of any bias across APOE genotype could be made.
Study Quality
Study quality was assessed using the National Heart, Lung and Blood Institute's Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies. The tool includes 14 items designed to assess study quality, assessing, for example, how participants were selected and compared; whether exposures and outcomes were valid and reliable; and whether potential confounds had been accounted for. An overall judgement determined whether each study was good, fair or poor. The assessment tool does not specify a scoring system for determining overall quality but is designed to help the user focus on key aspects of study quality from which an overall judgement can be made. Though all items were used to form an overall judgement, items 6, 7, 8, and 14 were critical in judging a study as good or bad. These items focused on the possible variance in the physical activity measures, whether those were taken prior to the outcome measure with sufficient time for an effect to be seen, and whether key confounding variables were accounted for. Assessment was carried out independently by two reviewers (AP and CM) with any discrepancies discussed until consensus was achieved.
Results
Study Selection
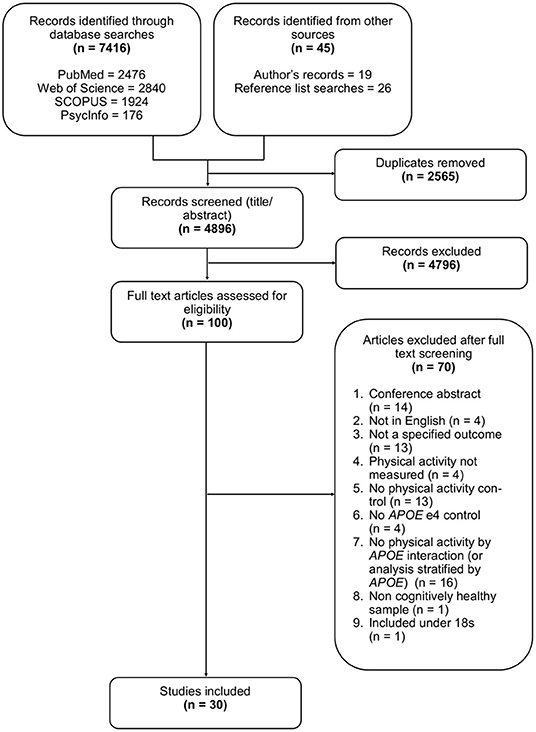
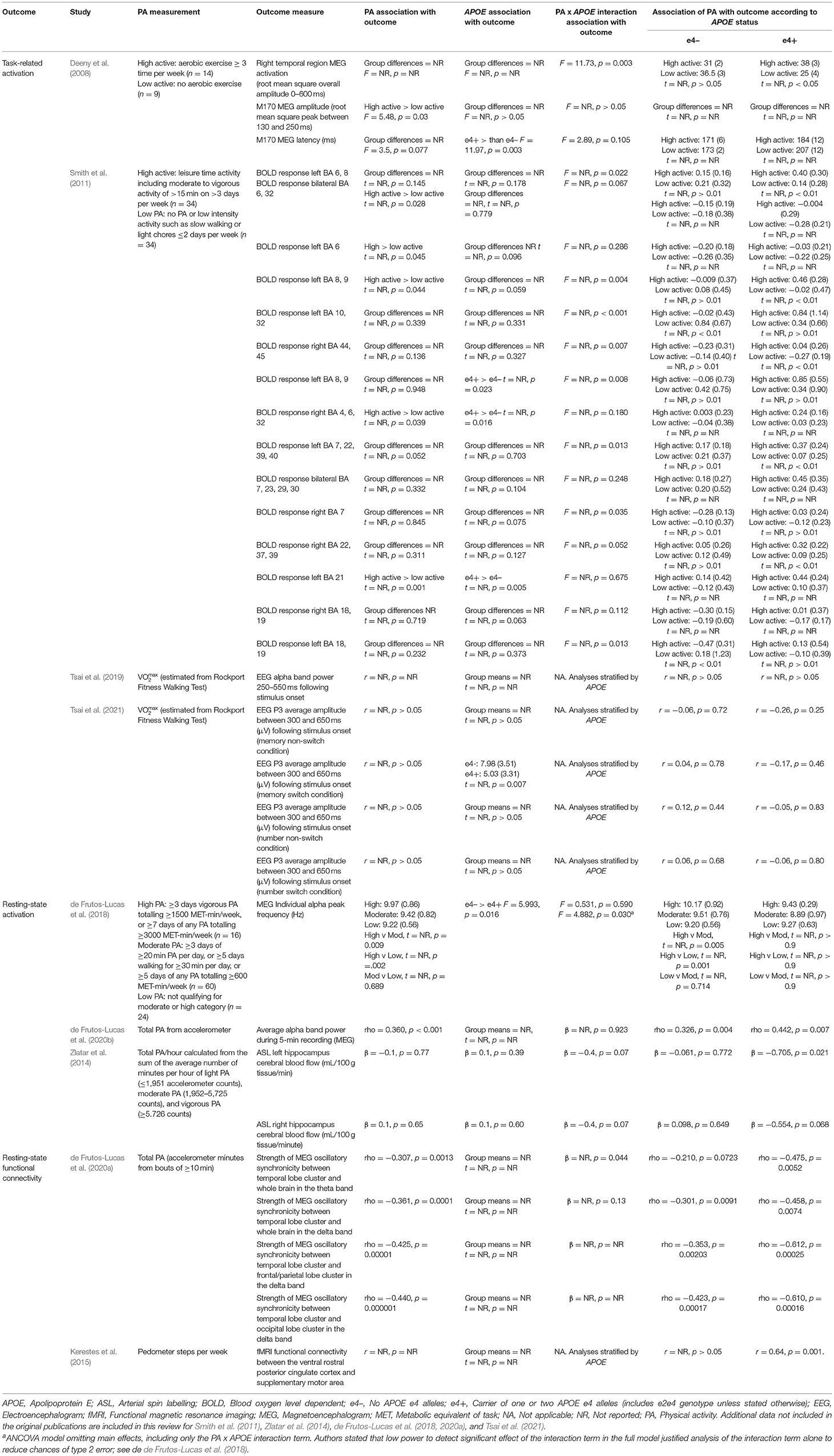
After reviewing the titles and abstracts of 4,896 studies, 100 underwent full text review, with 30 selected for inclusion, some of which contributed to multiple outcomes. Of the 30 studies, eight assessed lipid profile, eight assessed AD pathology, six assessed brain structure, and nine assessed brain activation. Full details of the search results and selection process are illustrated in Figure 1, and study characteristics are given in Table 1.
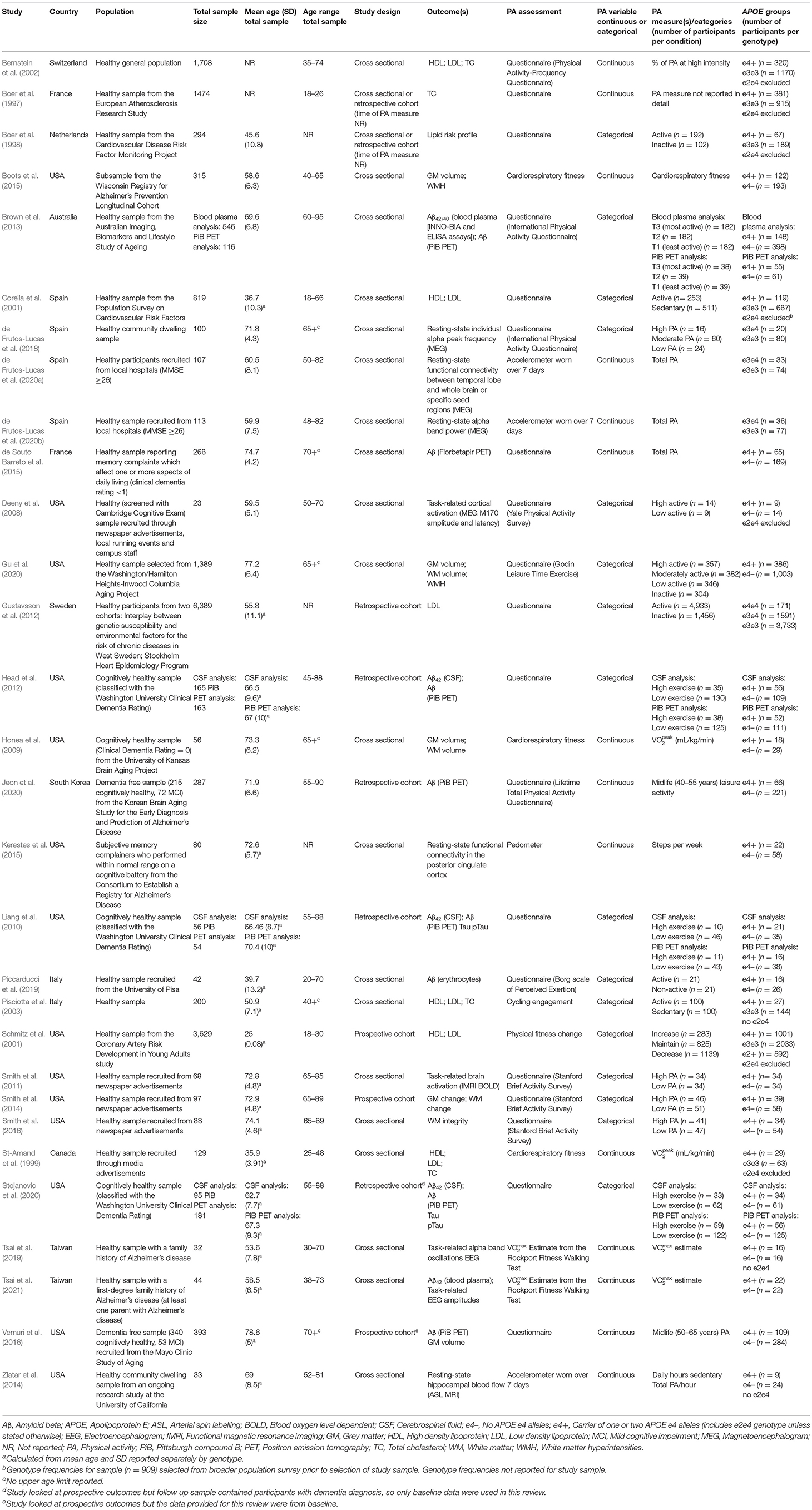
Table 1. Study characteristics for all included studies assessing APOE differences in the association between physical activity and all outcomes.
Lipid Profile
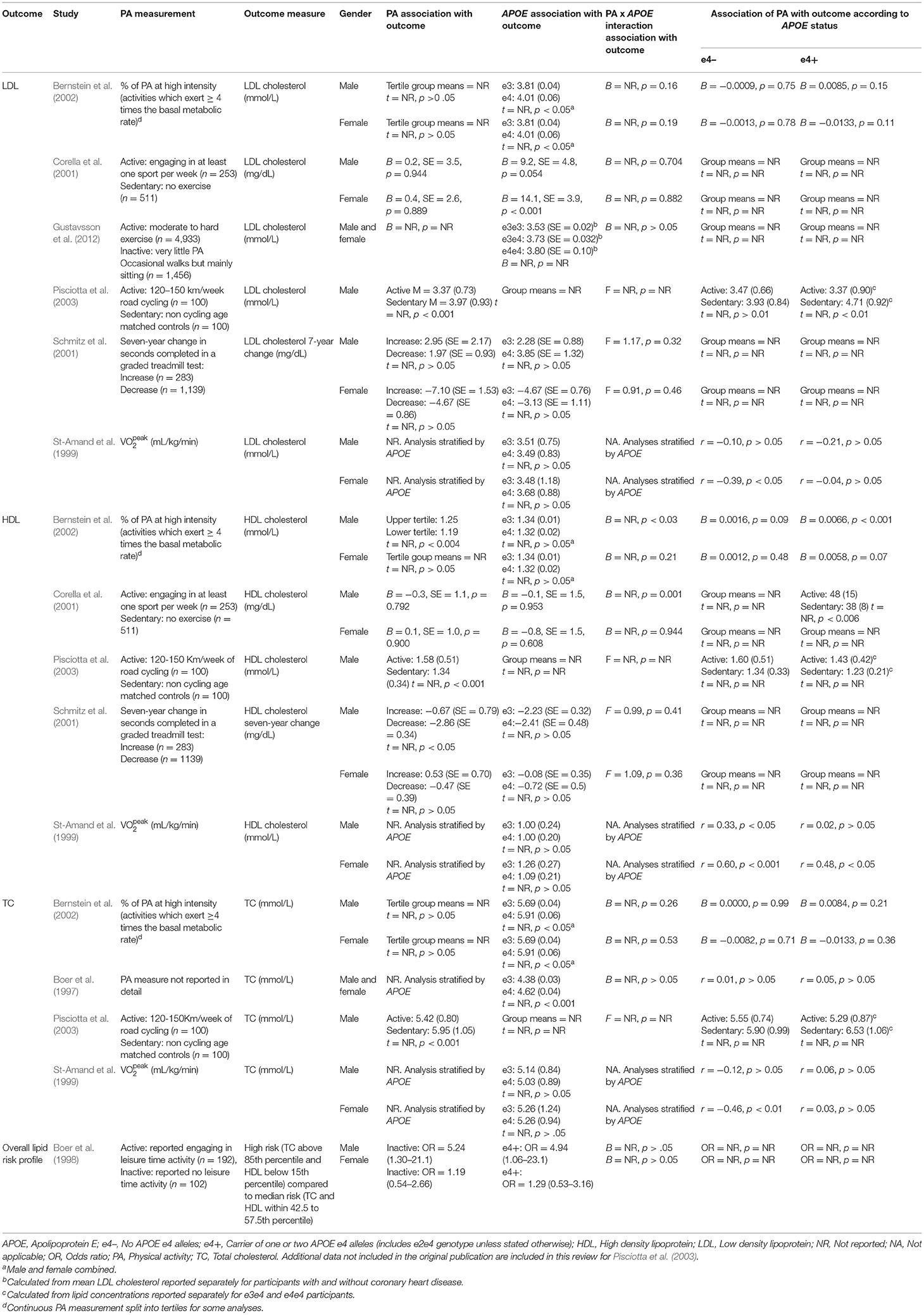
Of the eight studies assessing lipid profile, six assessed LDL, five assessed HDL, and four assessed TC. One study did not assess lipoprotein levels individually, instead calculating an overall lipid risk score as a dichotomous outcome (see Table 2 for lipid data).
Low Density Lipoproteins
Of the six studies which assessed LDL, none showed moderation of the physical activity-LDL association by APOE. A meta-analysis was conducted with 10 effect sizes each for e4 carriers and non-carriers, five of which were substituted with the physical activity main effect from e4 carriers and non-carriers combined. Analysis of the AICs and BICs indicated that the multilevel model was a significantly better fit than the standard model (p = 0.014; see Supplementary Table 2 for model fit statistics). Physical activity was not significantly associated with LDL (r = −0.08, p = 0.17), and this was also the case for e4 carriers (r = 0.08, p = 0.18) and non-carriers (r = −0.07, p = 0.18) separately. The moderation test indicated that there was no significant difference between APOE subgroups [F(1, 18) = 0.04, p = 0.84] (see Figure 2).
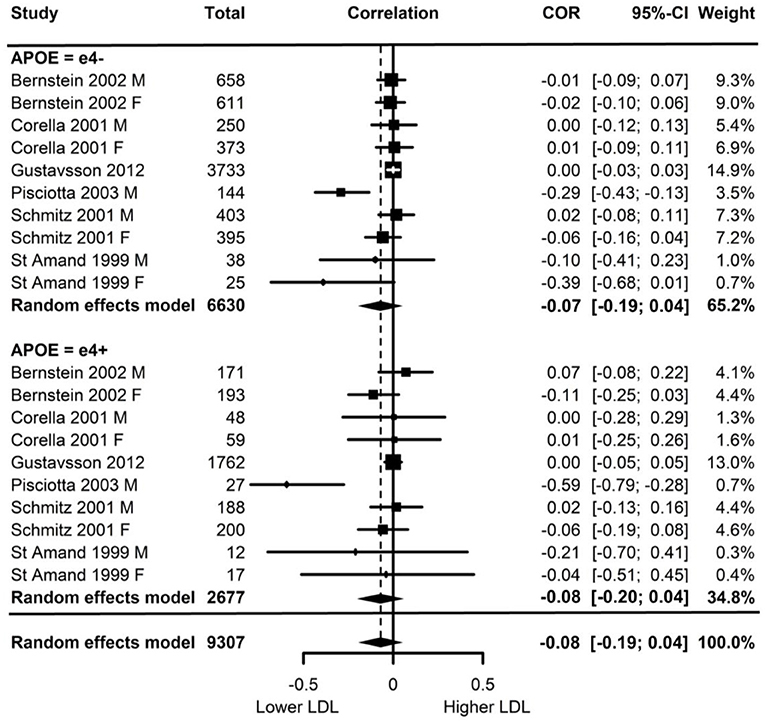
Figure 2. Forest plot indicating the association between physical activity and LDL with carrier (e4+) and non-carrier (e4–) subgroups. Subgroup moderation test indicated no significant difference between APOE groups (p = 0.84).
Visual inspection of the funnel plot (Supplementary Figure 1) indicated possible publication bias with smaller studies more likely to be published if demonstrating an association between physical activity and reduced LDL, however, this bias did not differ by APOE status.
Heterogeneity was high (I2 = 84.5%) and all of this variance was at the between cluster level (that is, effect sizes differed between studies but not within a study). Post-hoc investigation identified physical activity measurement, LDL measurement, and study design as possible sources of the between cluster heterogeneity. As the metrics used to quantify LDL can be directly converted, this was unlikely to be a source of heterogeneity. For study design, one study (Schmitz et al., 2001) assessed the association between physical activity and 7-year longitudinal change in LDL in contrast to the cross-sectional nature of the other studies. A sensitivity analysis with this longitudinal study removed again indicated high heterogeneity (I2 = 88.4%) with all of this variance was at the between cluster level.
High Density Lipoproteins
Of the five studies which assessed HDL, two provided evidence of APOE moderation of the physical activity-HDL association. A meta-analysis was conducted with nine effect sizes each for e4 carriers and non-carriers, three of which were substituted with the physical activity main effect from e4 carriers and non-carriers combined. AICs and BICs indicated that the multilevel model was a significantly better fit than the standard model (p = 0.03; see Supplementary Table 2 for model fit statistics). Physical activity was significantly associated with HDL (r = 0.16, p = 0.02), and this was also the case in the e4 carriers (r = 0.20, p = 0.01) and non-carriers (r = 0.15, p = 0.03) separately. The moderation test indicated that there was no significant difference between APOE subgroups [F(1, 16) = 1.86, 0.19] (Figure 3).
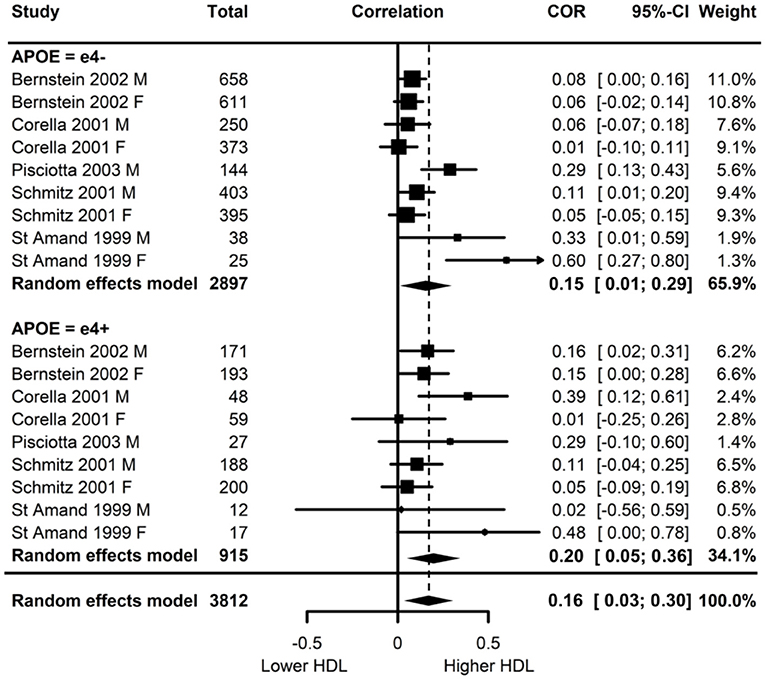
Figure 3. Forest plot indicating the association between physical activity and HDL with carrier (e4+) and non-carrier (e4–) subgroups. Subgroup moderation test indicated no significant difference between APOE groups (p = 0.10).
Visual inspection of the funnel plot (Supplementary Figure 1) indicated possible publication bias with smaller studies more likely to be published if demonstrating an association between physical activity and increased HDL, however, this bias did not differ by APOE status.
Heterogeneity was high (I2 = 77.5%) and all at the between cluster level. Post-hoc investigation identified a similar pattern to the LDL analyses, with physical activity measurement, HDL measurement, and study design as possible sources of the between cluster heterogeneity. A sensitivity analysis with the longitudinal study removed made minimal difference, with high heterogeneity (I2 = 79.6%) again all at the between cluster level.
Total Cholesterol
Four studies assessed TC. One study did not report the physical activity by APOE interaction result or the stratified data (Pisciotta et al., 2003). Two studies reported the interaction result, both of which were not significant (Boer et al., 1997; Bernstein et al., 2002). The remaining study carried out stratified analyses across APOE and gender and only female non-carriers demonstrated a significant association between physical activity and TC (r = −0.46, p < 0.01; St-Amand et al., 1999).
Overall Lipid Risk Profile
One study assessed whether physical activity predicted a high-risk lipid profile (Boer et al., 1997). Participants with TC levels above the 85th percentile and HDL below the 15th percentile were compared to a medium risk profile consisting of participants with TC and HDL levels in the middle 15th percentile. There was no physical activity by APOE interaction.
Alzheimer's Disease Pathology
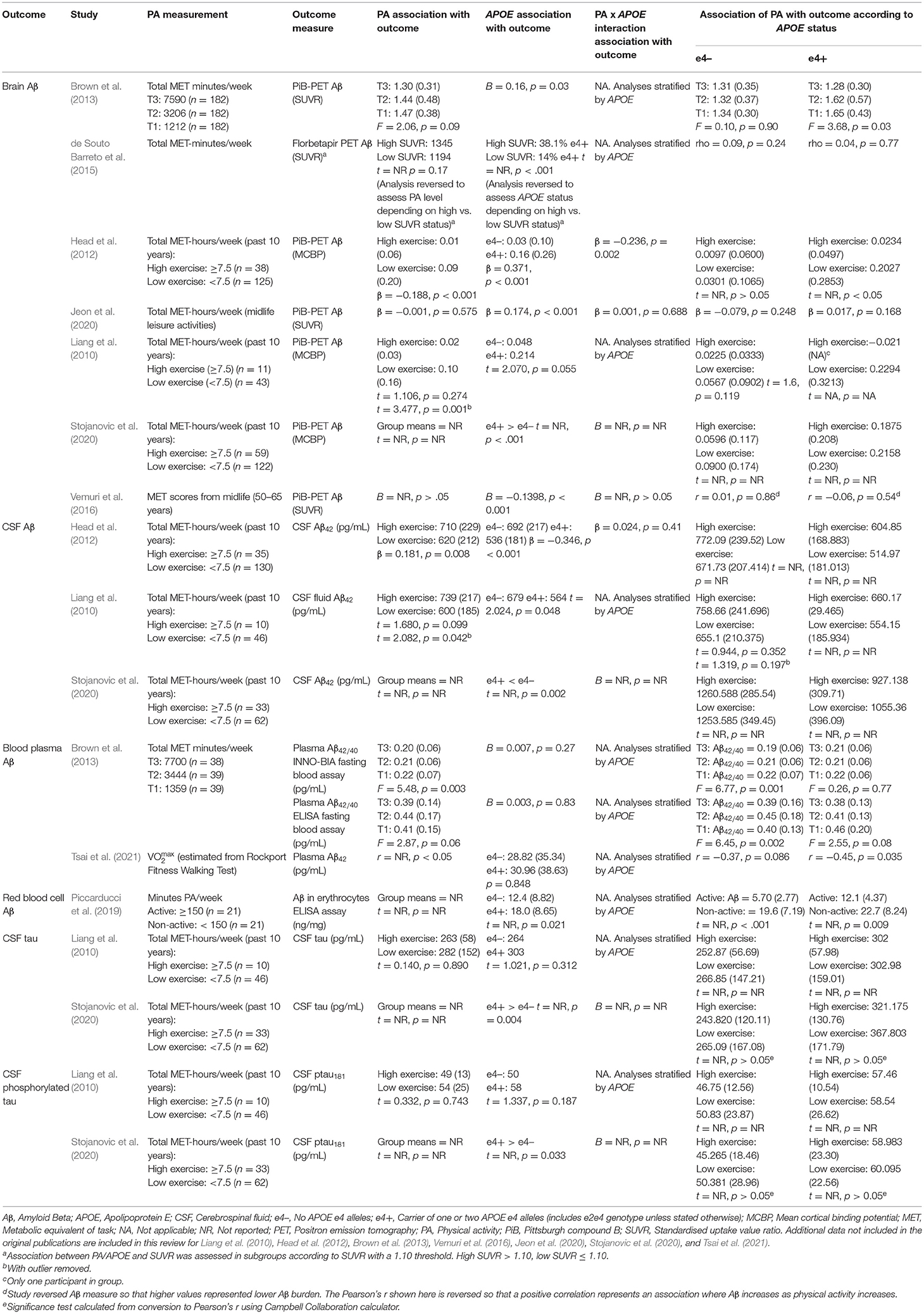
Eight of the studies investigated AD pathology, with all eight assessing Aβ and two also assessing tau (see Table 3 for AD pathology data).
Amyloid Beta
Of the eight studies assessing Aβ, two provided evidence of moderation of the physical activity-Aβ association by APOE. All effect sizes were available, resulting in a full meta-analysis on the eight studies. AICs and BICs indicated that the multilevel model was a significantly better fit than the standard model (p = 0.01, see Supplementary Table 2 for model fit statistics). Physical activity was not significantly associated with Aβ (r = −0.13, p = 0.19), and this was also the case in e4 carriers (r = −0.15, p = 0.15) and non-carriers (r = −0.12, p = 0.24) separately (Figure 4). The moderation test indicated that there was no significant difference between APOE subgroups [F(1, 24) = 0.38, p = 0.54].
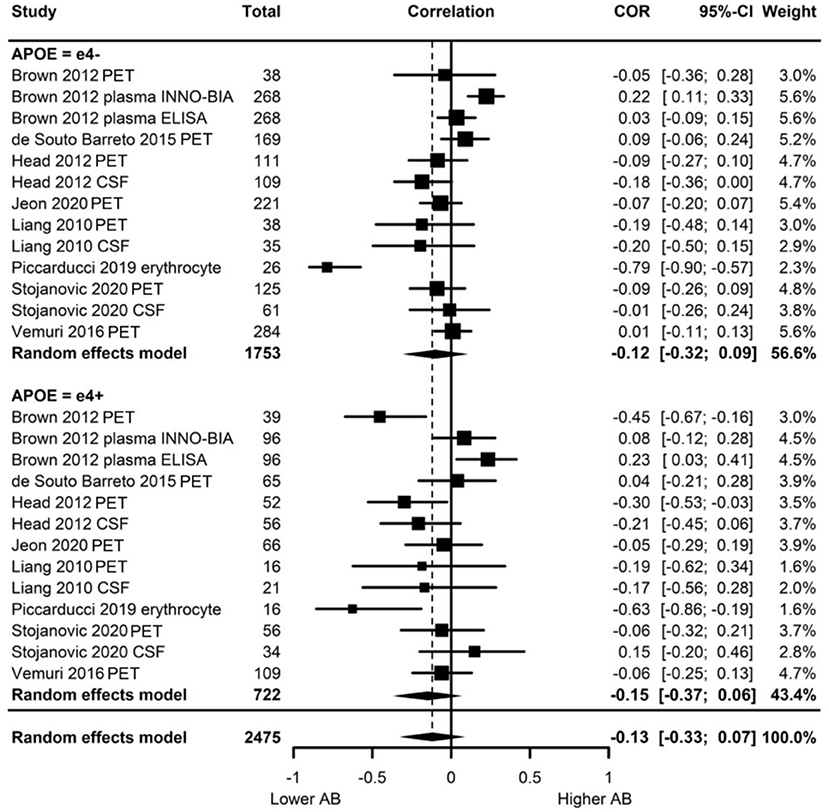
Figure 4. Forest plot indicating the association between physical activity and Aβ with carrier (e4+) and non-carrier (e4–) subgroups. Subgroup moderation test indicated no significant difference between APOE groups (p = 0.54).
Visual inspection of the funnel plot (Supplementary Figure 1) indicated possible publication bias with smaller studies more likely to be published if demonstrating an association between physical activity and reduced Aβ, however, this bias did not differ by APOE status. Heterogeneity was high (I2 = 86.5%), with 80.0% of the heterogeneity at the between cluster level. No sensitivity analyses to explain the heterogeneity were identified.
Tau
Of the two studies assessing tau, one (Liang et al., 2010) found no main effects of physical activity and APOE on either tau or phosphorylated tau, and it did not investigate outcomes stratified by APOE. The other (Stojanovic et al., 2020) found a main effect of APOE, with e4 carriers having higher levels of both tau and phosphorylated tau. However, physical activity was not associated with tau or phosphorylated tau in e4 carriers or non-carriers.
Brain Structure
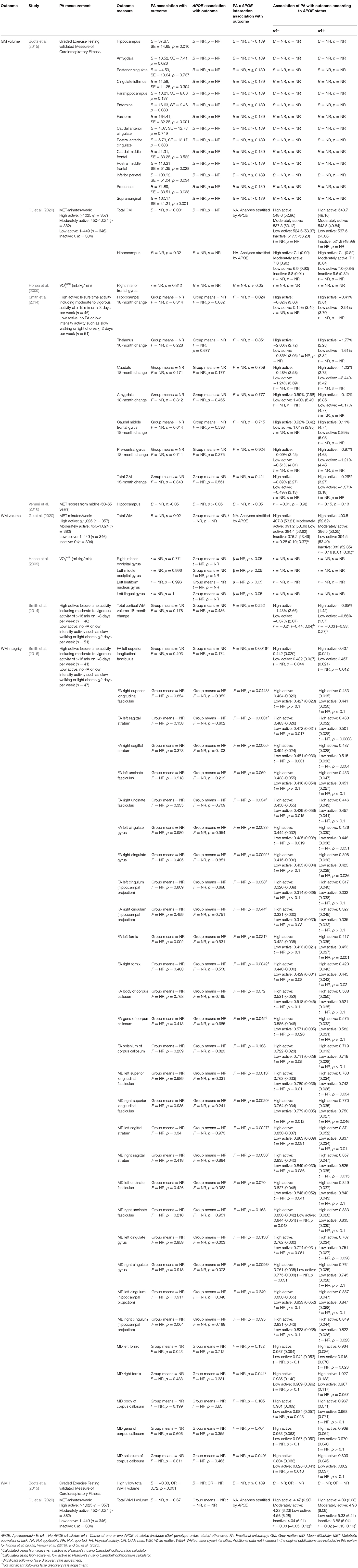
Of the six studies which assessed brain structure, five assessed GM volume, three assessed WM volume, one assessed WM integrity and two assessed cerebrovascular health (see Table 4 for brain structure data).
Grey Matter Volume
Of the five studies which assessed grey matter volume, one provided evidence of APOE moderation of the physical activity-GM association. A meta-analysis was carried out with 25 effect sizes each for e4 carriers and non-carriers, 15 of which were substituted with the physical activity main effect from e4 carriers and non-carriers combined. AICs and BICs indicated that the full multilevel model was a significantly better fit than the standard model (p = 0.002; see Supplementary Table 2 for full model fit statistics). Physical activity was significantly associated with GM (r = 0.10, p = 0.03). A subgroup analysis revealed that physical activity was significantly associated with GM volume in e4 carriers (r = 0.12, p = 0.02) but not in e4 non-carriers (r = 0.09, p = 0.06) (Figure 5). However, the moderation test did not indicate a significant difference between e4 carriers and non-carriers [F(1, 48) = 1.30, p = 0.26].
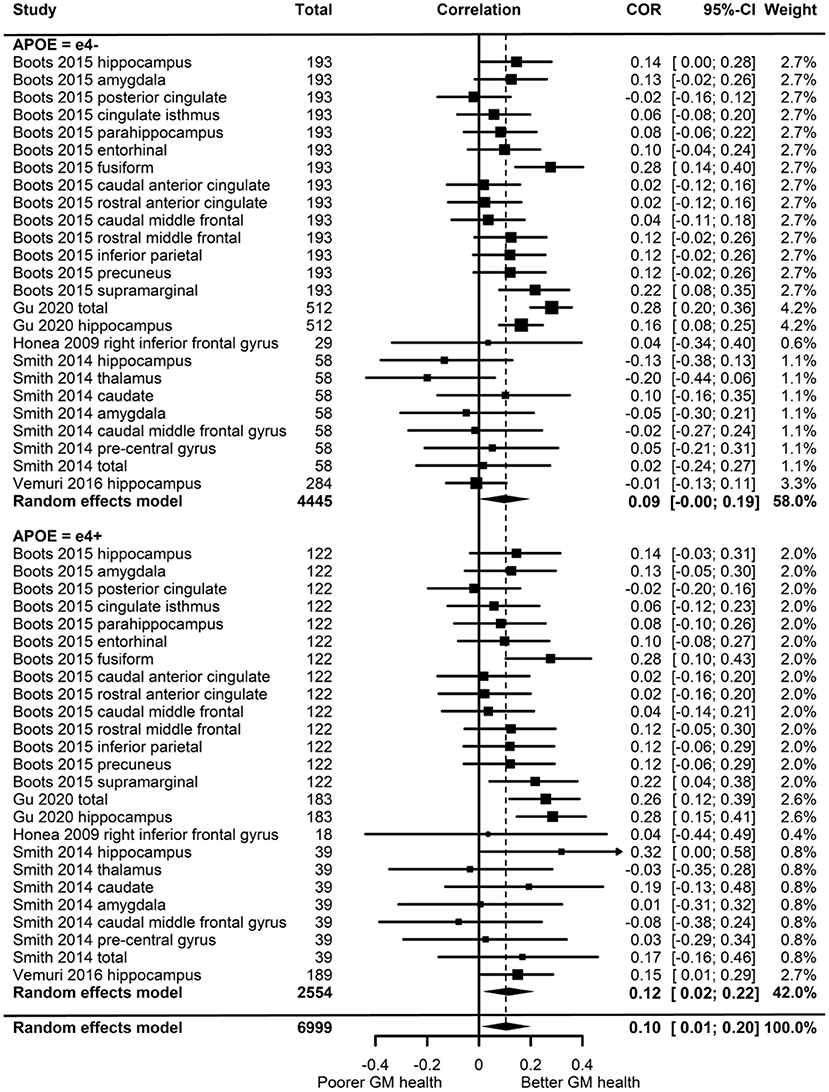
Figure 5. Forest plot indicating the association between physical activity and GM with carrier (e4+) and non-carrier (e4–) subgroups. Subgroup moderation test indicated no significant difference between APOE groups (p = 0.26).
Visual inspection of the funnel plot (Supplementary Figure 1) did not suggest publication bias. Heterogeneity was moderate (I2 = 54.7%), with 49.1% of the heterogeneity at the between cluster level. Post-hoc investigation identified physical activity measurement and study design as possible sources of between cluster heterogeneity. One study (Smith et al., 2014) assessed the association between physical activity and eighteen-month change in GM volume, while the others assessed cross-sectional associations. A sensitivity analysis with the longitudinal study removed made minimal difference, with moderate overall heterogeneity (I2 = 58.3%) which was mostly at the between cluster level (I2 = 50.3%).
White Matter Volume
From the three studies which assessed WM volume, four of the six effect sizes for e4 carriers and non-carriers were not reported, and neither were their physical activity main effects. One study (Gu et al., 2020) only reported stratified APOE data and showed significant positive associations between physical activity and WM volume for both e4 carriers and non-carriers. Highly active e4 carriers had 17.5 cm3 higher WM volume compared to inactive e4 carriers, whereas active non-carriers had 31.6 cm3 higher WM volume compared to inactive non-carriers. Without an interaction test, it was not possible to confirm whether this difference was significant. The other two studies did investigate physical activity by APOE interactions. Honea et al. (2009) investigated WM in four regions, and Smith et al. (2014) investigated cortical WM change over 18 months. Both studies reported no difference in the association between physical activity and WM volume by APOE status.
White Matter Integrity
One study (Smith et al., 2016) assessed the association between physical activity and WM integrity. Of the 15 association and commissural fibre tracts assessed, there were seven significant interactions between physical activity and APOE on FA, and six significant interactions on MD. For e4 carriers, active participants unexpectedly demonstrated lower FA and higher MD. For non-carriers, active participants demonstrated the expected pattern of higher FA and lower MD. Post-hoc analysis by the author suggested that WM integrity measures were complicated due to crossing neural fibres, and the findings potentially indicated that e4 carriers benefit from physical activity as well as non-carriers (for more detail, see Smith et al., 2016).
Cerebrovascular Health
Two studies assessed cerebrovascular health indicated by WMH. One (Boots et al., 2015) demonstrated a main effect of physical activity on WMH, with more active participants having lower WMH (better cerebrovascular health). There was no significant physical activity by APOE interaction, suggesting that both e4 carriers and non-carriers benefited from being physically active. The other study (Gu et al., 2020) assessed the association between physical activity and WMH stratified by APOE, but neither e4 carriers nor non-carriers demonstrated an association between physical activity and cerebrovascular health.
Brain Activation
Of the nine studies which assessed brain activation, four assessed activation during cognitive tasks, three assessed resting-state activation, and two assessed resting-state functional connectivity. These studies consisted of a mixture of fMRI, EEG and MEG, and studies which assessed activation were considered separately from studies which assessed connectivity (see Table 5 for brain activation data).
Task-Related and Resting-State Activity
Of the seven studies which assessed brain activation, four provided evidence of APOE moderation of the physical activity-brain activation association. A meta-analysis was carried out with 27 effect sizes each for e4 carriers and non-carriers, one of which was substituted with the main effect for e4 carriers and non-carriers combined, and one substituted with 0 due to the main effect not being reported. The multilevel model was not a significantly (p = 0.19) better fit than the standard model (see Supplementary Table 2 for model fit statistics). Overall, physical activity was significantly associated with brain activation (r = 0.13, p = 0.01). A moderation test indicated that the association between physical activity and brain activation was significantly different across APOE subgroups [F(1, 52) = 18.03, p < 0.01]; subgroup analyses indicated that the association was significant for e4 carriers (r = 0.31, p < 0.01), but not non-carriers (r = −0.03, p = 0.58) (Figure 6). Heterogeneity was 52.8%. Visual inspection of the funnel plot did not suggest publication bias (Supplementary Figure 1).
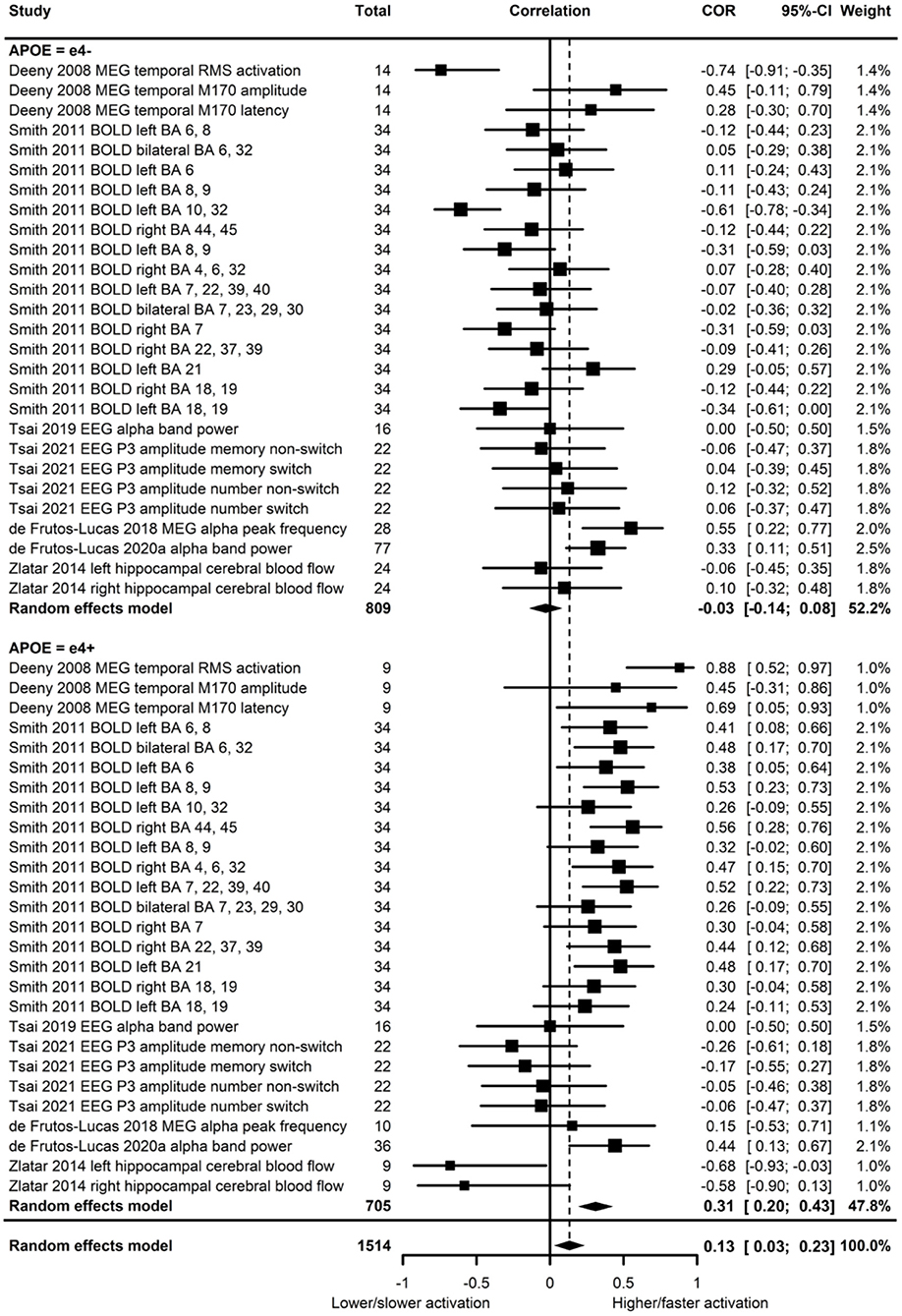
Figure 6. Forest plot indicating the association between physical activity and functional brain activation with carrier (e4+) and non-carrier (e4–) subgroups. Subgroup moderation test indicated a significant difference between APOE groups (p < 0.01).
For the studies which demonstrated significant physical activity by APOE interactions on brain activation, we considered whether there were differences in cognitive ability across APOE to assess if there was evidence of compensatory mechanisms in e4 carriers. In the study by Deeny et al. (2008), there was no difference between e4 carriers and non-carriers on the Cambridge Cognition Examination, nor on the working memory task used for MEG analysis. However, physical activity was associated with greater and faster neural activation in e4 carriers. In Smith et al. (2011), memory performance did not differ between e4 carriers and non-carriers, but physical activity was associated with increased BOLD activation more consistently in e4 carriers. In addition, spatial extent analysis indicated greater volume of activation in physically active e4 carriers only, and greater fMRI BOLD response in some regions indicated higher activation in e4 carriers. Zlatar et al. (2014) did not report cognitive differences across APOE, though the significant interaction between APOE and physical activity indicated the association between physical activity and resting-state cerebral blood flow was in e4 carriers only. However, the direction was reversed, with higher physical activity associated with lower cerebral blood flow.
Resting-State Functional Connectivity
Two studies assessed functional connectivity. de Frutos-Lucas et al. (2020a) assessed oscillatory synchronicity, which has been associated with dysfunction in AD. Physical activity was negatively associated with synchronicity, and while the association was consistently stronger in e4 carriers, only one of the four analyses demonstrated a significant physical activity by APOE interaction. Specifically, synchronicity between a temporal lobe cluster and the whole brain indicated that e4 carriers had an association between physical activity and reduced synchronicity (rho = −0.475, p < 0.01), but not non-carriers (rho = −0.210, p = 0.07). There were no differences in cognitive ability across APOE. Kerestes et al. (2015) investigated functional connectivity in the default mode network. Stratified analysis revealed a moderate association in e4 carriers (r = 0.64, p = 0.001), but no association in non-carriers. There were no differences in cognitive ability across APOE.
Study Quality
Study quality judgements are shown in Supplementary Figure 2. None of the studies met/failed all four criteria (items 6, 7, 8, and 14) deemed essential for an overall judgement of good or bad, respectively, thus all studies were judged as fair overall. Figure 7 shows how many studies met each of the 14 criteria, demonstrating key areas for improvement. Only nine studies assessed physical activity levels prior to the outcome measurement, and only six of these allowed sufficient time for the effects of physical activity to be seen. Furthermore, only two studies measured physical activity over time. While three studies reported that participation rates were 50% or more, it was not possible to rate this for 21 studies. Finally, only 11 studies sufficiently controlled for potential confounds.
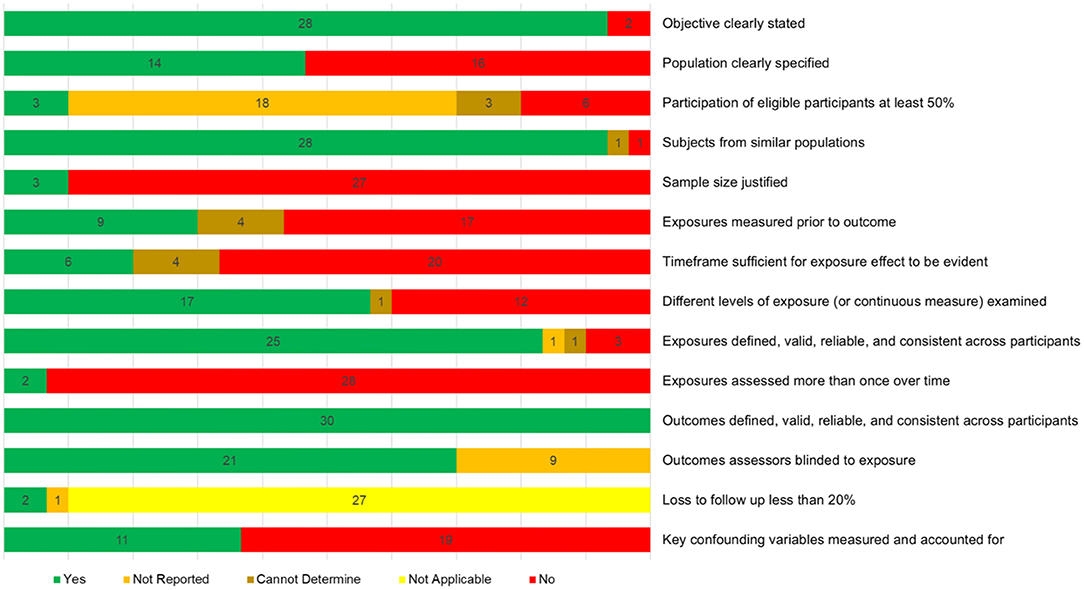
Figure 7. Quality assessment summary showing how many studies were given each of the five possible judgements for each of the assessment criteria.
Discussion
Meta-analyses indicated that physical activity was associated with better outcomes for HDL, GM and brain activation, but not for LDL and Aβ. Narrative syntheses revealed that one of three studies demonstrated an association between physical activity and WM volume (Gu et al., 2020); one study reported an association between physical activity and WM integrity (Smith et al., 2016); one of two studies demonstrated an association between physical activity and cerebrovascular health (Boots et al., 2015); and two of two studies reported an association between physical activity and functional connectivity (Kerestes et al., 2015; de Frutos-Lucas et al., 2020a).
In terms of APOE moderation, meta-analyses only indicated significant APOE differences in the association between physical activity and brain activation, with an association in e4 carriers but not non-carriers. Narrative syntheses provided some support for a difference in the association between physical activity and functional connectivity by APOE status. One study reported an association between physical activity and functional connectivity in both e4 carriers and non-carriers in three of the four analyses, and an association only in e4 carriers in the other analysis (de Frutos-Lucas et al., 2020a). The other study investigating functional connectivity found an association with physical activity only in e4 carriers (Kerestes et al., 2015).
Lipid Profile
The meta-analyses indicated that physical activity was associated with HDL but not LDL, and no moderation by APOE for either. Publication bias was more likely for studies demonstrating a significant association with physical activity, but this pattern did not differ by APOE status. Assessment of TC was carried out narratively due to the complexity of interpreting TC levels. While one study (St-Amand et al., 1999) suggested APOE might moderate the association between physical activity and lipid profile, the other three studies (Boer et al., 1997; Bernstein et al., 2002; Pisciotta et al., 2003) did not. The one study (Boer et al., 1998) which looked at lipid risk profile as the outcome also suggested no moderating effect of APOE.
Overall, the results partially support the suggestion that physical activity benefits lipid profile. For HDL, the results indicate a beneficial association between physical activity and HDL, though this did not differ by APOE status. That is, those carrying the e4 allele are able to gain the same benefit from physical activity in terms of HDL levels as those without.
Alzheimer's Disease Pathology
Based on the meta-analysis, physical activity was not associated with Aβ measured from PiB PET, CSF, blood plasma and erythrocytes, and the association did not differ by APOE status. Publication bias was as likely for e4 carriers and non-carriers, suggesting missing studies did not affect our ability to detect APOE differences. Although the results were consistent with higher levels of physical activity being associated with lower levels of Aβ, the overall association was not significant. This is supported by a recent review (Brown et al., 2019) which suggested that evidence for the association between physical activity and lower Aβ is more convincing in mice than in humans, and more work is needed to confirm whether physical activity is an effective means of reducing Aβ accumulation in humans.
Brain Structure
The meta-analysis indicated that physical activity was significantly associated with GM volume. Interestingly, the subgroup analysis indicated that this association was only significant in e4 carriers, but the test of moderation was not significant (p = 0.06). There did not appear to be any publication bias, thus the overall association could be a reasonably accurate representation of the true effect. Indeed, there is evidence that physical activity and fitness is related to GM volume (Erickson et al., 2014), though further work is needed to confirm whether physical activity similarly benefits e4 carriers and non-carriers.
Only one study assessed WM integrity, demonstrating evidence of APOE differences in the association with physical activity (Smith et al., 2016). To accurately determine whether physical activity benefits WM integrity, it is important to note the limitations of WM integrity measurement. MD and FA measure the dispersion of water, which is used to infer the structural integrity of axons. However, in regions where axons cross, dispersion can appear high even when structural integrity is good (Pierpaoli and Basser, 1996; Madden et al., 2009). A greater understanding of the effect that crossing WM tracts have on measures of WM integrity would aid the interpretation of APOE differences in the association between physical activity and WM integrity, in addition to further studies simultaneously considering physical activity WM, and APOE status. With only one study, no firm conclusions can be made.
For cerebrovascular health, one of the two studies found evidence of an association between physical activity and WMH, but neither study provided evidence of a difference across APOE. Though there is evidence that physical activity does benefit cerebrovascular health (Wardlaw et al., 2015; Ritchie et al., 2017), there is no suggestion those benefits would differ by APOE status, albeit based on a limited number of studies.
Brain Activation
For task-related and resting-state brain activation, the meta-analyses suggested that physical activity was associated with greater or faster brain activation in e4 carriers only. This effect appears to have been driven by two studies (Deeny et al., 2008; Smith et al., 2011), which contributed two-thirds of the effect sizes. Given that the model used for this analysis was not a multilevel model, the use of multiple effect sizes on the same participants might have spuriously indicated a significant effect in e4 carriers. However, when a multilevel random effects model was fitted to account for multiple effect sizes from each study, the result was unchanged (the multilevel model did not improve the model fit). The better fit of the standard model suggested that the multiple outcomes within a study were adding independent variance to the model.
Post-hoc investigation of the studies with APOE moderation revealed evidence that the association between physical activity and brain activation could be related to compensatory mechanisms in e4 carriers. Higher brain activation may be a mechanism through which the negative effect of e4 possession is masked. In a memory encoding task, a comparison of the blood oxygen level dependent (BOLD) response during the presentation of new pictures compared to a repeated picture facilitated an assessment of the “effort” needed to encode new memories. A greater BOLD response during memory encoding was seen in e4 carriers across occipital, parietal and frontal regions. However, with no difference in memory performance across APOE groups, it seems that e4 carriers “worked harder” to achieve comparable cognitive performance (Bondi et al., 2005). Brain activation when not engaged in a task also appears to show compensation for e4 possession. Resting-state cerebral blood flow was higher in e4 carriers, but there was no difference in brain activation during a memory task (Fleisher et al., 2009; Bangen et al., 2012). This upregulation of resting-state blood flow could enable sufficient cerebral blood flow during tasks in those with underlying neurological deficits, thus representing another potential compensatory mechanism.
Our meta-analysis indicated that physical activity was only associated with brain activation in e4 carriers, however, cognitive ability did not differ across APOE in the two studies which appeared to drive the effect (Deeny et al., 2008; Smith et al., 2011). Deeny et al. (2008) and Smith et al. (2011) both found physical activity to be associated with greater brain activation. Smith et al. (2011) also found evidence of greater brain activation in e4 carriers compared to non-carriers and suggested that physical activity could facilitate the neural upregulation necessary for e4 carriers to maintain cognitive ability during early neurodegeneration. If this suggestion is correct, it might be expected that active e4 carriers would show greater cognitive ability than inactive e4 carriers. This was the case for participants in the Deeny et al. (2008) study, but not in the Smith et al. (2011) study. Further studies are therefore required to determine whether and how physical activity might facilitate neural upregulation in e4 carriers, and the resultant effect on cognitive ability.
In contrast to the physical activity-related upregulation reported by Deeny et al. (2008) and Smith et al. (2011), Zlatar et al. (2014) demonstrated the opposite effect. In e4 carriers only, physical activity was associated with lower cerebral blood flow. As cognitive ability did not differ by APOE, Zlatar et al. (2014) interpreted these findings as demonstrating a compensatory mechanism in physically inactive e4 carriers, whereby resting-state cerebral blood flow was upregulated. This interpretation contradicts the suggestion that physical activity facilitates upregulation, instead implicating a lack of physical activity as a reason for upregulation becoming necessary. The association between physical activity and cognitive ability in e4 carriers was not reported, so it is not clear whether physical activity-related differences in cerebral blood flow influenced cognitive ability. Overall, our meta-analysis provides some support for the beneficial effect of physical activity in facilitating compensation in e4 carriers, but further studies are needed to confirm this given the limited number of studies available.
Functional connectivity was investigated in two studies, with both providing evidence for the association between physical activity and functional connectivity differing by APOE. One study found reduced oscillatory hypersynchrony to be associated with physical activity in both e4 carriers and non-carriers, though potentially stronger in carriers (de Frutos-Lucas et al., 2020a). The other found better functional connectivity to be associated with physical activity in e4 carriers only (Kerestes et al., 2015). No differences in cognitive ability across APOE in these studies again indicates a possibility of physical activity aiding e4 carriers to compensate for deficits. Compensation may differ from upregulation and involve structural differences which facilitate communication between different brain regions.
Though these two methods of compensation share similarities in facilitating brain activation which maintains cognitive ability during early neurodegeneration, they may differ in other ways. Upregulation of brain activation is achieved by increased blood flow during a task (Buckner et al., 1996), whereas enhanced functional connectivity may also require structural differences in the form of connexions between distinct brain regions (van den Heuvel and Hulshoff Pol, 2010). The evidence in this review does not provide support for a beneficial effect of physical activity on general brain health in e4 carriers but does provide some support for a beneficial effect of physical activity in promoting the required neural architecture (Kamijo et al., 2011) and task-related neural upregulation (Yu et al., 2021) to facilitate compensation which allow e4 carriers to maintain cognitive ability during the early stages of neurodegeneration. As this is based on a small number of studies, further research is needed to confirm and further elucidate these mechanisms.
Study Quality
Heterogeneity
As expected, there was evidence of heterogeneity across the meta-analyses. For LDL, heterogeneity was high and all of the I2 variance was between clusters. As each cluster contained effect sizes which used the same measure of physical activity and the same measurement of LDL, the only possible source of heterogeneity within a cluster was gender, and four of the six studies reported effect sizes separately for male and female participants. The within cluster homogeneity suggested that gender was not a source of heterogeneity. One potential difference between clusters was the LDL measurement, but as the two LDL metrics used (mmol/L and mg/dL) can be directly converted, this was unlikely to have caused heterogeneity. As a sensitivity analysis with the only longitudinal study removed made little difference to the heterogeneity, physical activity appears to be the most likely source. The pattern of heterogeneity was similar for HDL, with high heterogeneity all at the between study level cluster again demonstrating that the physical activity measurement was the most likely cause.
Heterogeneity among studies assessing Aβ was high with most of this variance at the between cluster level. In contrast to the models for LDL and HDL, where the outcomes were unlikely to represent a potential source of heterogeneity, the Aβ model included different methods of measuring the outcome. As some studies used multiple outcome measures, these differences could be evident even within a cluster. However, the amount of within cluster variance was low with the majority between clusters, suggesting that the Aβ measurement method was not a substantial source of heterogeneity. While some of the between cluster heterogeneity could have been due to differences in the Aβ measurement, as there were different combinations of measurements in each cluster, it seems likely again that the biggest source of heterogeneity among the studies was the measurement of physical activity.
Though heterogeneity in the GM volume model was lower than for the lipid and Aβ analyses, there was still moderate heterogeneity, with most of this at the between study cluster level. Post-hoc analyses indicated the measurement of physical activity and study design as potential sources of heterogeneity. Removing the one longitudinal study made little difference, with moderate heterogeneity mostly at the between cluster level, again suggesting physical activity measurement as the main source of heterogeneity.
Study Quality Assessment
All studies were judged as fair following assessment with the National Heart, Lung and Blood Institute's Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies. While a clear metric would have been desirable, this tool is only designed to be used as a guide to aid authors in making an overall quality judgement. One criterion which all studies met was using outcomes that were defined, valid and reliable. Given the objective nature of the measures used for outcomes, it is perhaps unsurprising that they did not appear to contribute heterogeneity to the analyses. Measures of the exposure, i.e., physical activity, were also generally good, with 25 of the 30 studies deemed to have used defined, valid and reliable measures. However, given that the measures of physical activity appeared to introduce substantial heterogeneity into the analyses, the use of a consistent tool for measuring physical activity would improve the literature.
It would also be desirable for future studies to assess physical activity multiple times prior to the outcome being measured and with sufficient time for any potential benefits to become evident. In addition, more detailed reporting of participation rates would allow stronger conclusions to be drawn on the representativeness of the results (albeit within the context of the specific samples). Finally, robust controlling for potential confounds would facilitate stronger conclusions that physical activity itself is beneficial after ruling out factors such as blood pressure and BMI.
APOE allele frequencies were generally poorly reported, with only three studies explicitly stating that frequencies did not deviate from the Hardy-Weinberg equilibrium (Corella et al., 2001; Pisciotta et al., 2003; Gustavsson et al., 2012). Given that some studies selected participants for analysis based on APOE status, it was not possible to determine whether the samples reflected a representative selection of participants in terms of e4 possession.
Limitations
One limitation of this review is that all studies were observational, being either cross-sectional, retrospective cohort or prospective cohort studies. Randomised controlled trials would provide stronger evidence for a causal association between physical activity and brain health. A second limitation is that not all data were available for meta-analyses. While attempts were made to acquire the missing data and no eligible studies were omitted due to this, only the meta-analysis for Aβ did not contain any estimated data points. The conclusions drawn from the meta-analyses on LDL, HDL, GM volume and functional brain activation therefore include a degree of uncertainty.
It is also worth noting that most studies did not investigate allele dose. In smaller studies, this is not possible due to the low number of people carrying two e4 alleles. While combining heterozygotes and homozygotes is not problematic, it meant that it was not possible to consider whether physical activity differentially benefits homozygotes, who are at the highest genetic risk. In addition, many studies did not demonstrate a significant main effect of APOE, which might be expected. If any increased benefit from physical activity in e4 carriers is only seen in those who are experiencing the negative effects of e4 possession, then analysis on those who are yet to experience the negative effects may fail to identify an increased benefit of physical activity. The lower participation of individuals with poorer health, including Alzheimer's (Tyrrell et al., 2021) could potentially explain why no APOE effect was observed.
Finally, a common approach among studies in this review was to assess the association between physical activity and the outcome separately for e4 carriers and non-carriers. While this stratified approach helps to identify whether the association differs by APOE, it does not determine whether any observed difference is statistically significant.
Future Directions
While there is some evidence for a greater benefit of physical activity in e4 carriers, this appears to be dependent upon the outcome being assessed. Our findings suggest a nuanced pattern where physical activity does not benefit e4 carriers differently for lipids, Alzheimer's disease pathology, GM volume, WM volume or cerebrovascular health, but might for functional brain outcomes. Future studies could focus on brain activation and brain structure which facilitates functional connectivity to consider whether physical activity allows e4 carriers to maintain cognitive ability during the early stages of neurodegeneration. If physical activity facilitates improved neural processing, it might be expected that e4 carriers would benefit more from physical activity on cerebrovascular health, which was not supported by the current analyses. With only two studies on this outcome, more are needed to consider this possibility. If physical activity benefits cerebrovascular health to a greater extent in e4 carriers, it would provide support for compensation by neural upregulation in e4 carriers. If e4 carriers do not benefit more, this could indicate that any apparent compensation is through structural changes which facilitate efficient communication between distinct brain regions.
Detecting subtle associations would be aided if future studies could reduce heterogeneity within the literature, for example by using objective measurements of physical activity such as accelerometer data. Considering how best to measure physical activity would facilitate an exploration of whether findings differ based on self-report compared to objective measures, ultimately determining whether future studies should focus exclusively on objective measures. Furthermore, measures of physical fitness and fitness-related health measures could elucidate specific biological outcomes related to being physically active that are involved in any mechanism through which e4 carriers benefit from physical activity.
Future studies could also compare analyses in those already showing evidence of age-related decline to those who are not to see if any greater benefit from physical activity in e4 carriers is only seen in those who need to compensate. Analysis of the interaction between physical activity and the outcome would allow a judgement on whether the association is significantly different in e4 carriers compared to non-carriers. Finally, analysis in large scale datasets where there are enough e4 homozygotes could uncover whether there is a difference in the benefit gained from physical activity in those at the highest genetic risk.
Conclusion
The current review indicates that those carrying the APOE e4 allele gain at least the same benefit from physical activity as those without. There is tentative support that the benefit of physical activity might be greater for e4 carriers specifically in relation to brain activation. However, the evidence is limited and further research is required to confirm this.
Data Availability Statement
The datasets presented in this article are not readily available because the results were drawn from published studies for inclusion in the systematic review, and where relevant, meta-analyses. All data relevant to the analyses are presented within the manuscript so no additional data posting is necessary. Requests to access the datasets should be directed to Alan J. Gow, YS5qLmdvd0Body5hYy51aw==.
Author Contributions
AP, MD, and AG contributed to conception and design of the study. AP developed the systematic review protocol, with input from MD and AG. AP conducted the systematic review with support from CM on study screening and reviewing. AP performed the statistical analysis and wrote the first draft of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Funding
The work was supported by a PhD Scholarship from the Centre for Applied Behavioural Sciences at Heriot-Watt University.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We wish to thank Dr. Daniel R. Hale for providing advice in relation to aspects of the meta-analyses. We also wish to thank Professor Stefano Bertolini (Pisciotta et al., 2003), Dr. Belinda Brown (Brown et al., 2013), Jaisalmer de Frutos-Lucas (de Frutos-Lucas et al., 2018, 2020a), Dr. Yian Gu (Gu et al., 2020), Professor Denise Head (Liang et al., 2010; Head et al., 2012), Dr. Robyn Honea (Honea et al., 2009), Dr. So Yeon Jeon (Jeon et al., 2020), Dr. J. Carson Smith (Smith et al., 2011), Marta Stojanovic (Stojanovic et al., 2020), Dr. Andy Tsai (Tsai et al., 2021), Dr. Prashanthi Vemuri (Vemuri et al., 2016), and Dr. Zvinka Zlatar (Zlatar et al., 2014) for providing data for this review.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2021.815439/full#supplementary-material
References
Assink, M., and Wibbelink, C. J. M. (2016). Fitting three-level meta-analytic models in R: a step-by-step tutorial. Quant. Methods Psychol. 12, 154–174. doi: 10.20982/tqmp.12.3.p154
Bangen, K. J., Restom, K., Liu, T. T., Wierenga, C. E., Jak, A. J., Salmon, D. P., et al. (2012). Assessment of Alzheimer's disease risk with functional magnetic resonance imaging: an arterial spin labeling study. J. Alzheimer's Dis. 3, S59–S74. doi: 10.3233/JAD-2012-120292
Bennet, A. M., di Angelantonio, E., Ye, Z., Wensley, F., Dahlin, A., Ahlbom, A., et al. (2007). Association of apolipoprotein e genotypes with lipid levels and coronary risk. J. Am. Med. Assoc. 298, 1300–1311. doi: 10.1001/jama.298.11.1300
Bernstein, M. S., Costanza, M. C., James, R. W., Morris, M. A., Cambien, F., Raoux, S., et al. (2002). Physical activity may modulate effects of ApoE genotype on lipid profile. Arterioscler. Thromb. Vasc. Biol. 22, 133–140. doi: 10.1161/hq0102.101819
Blennow, K., and Zetterberg, H. (2018). Biomarkers for Alzheimer's disease: current status and prospects for the future. J. Intern. Med. 284, 643–663. doi: 10.1111/joim.12816
Boer, J. M. A., Ehnholm, C., Menzel, H. J., Havekes, L. M., Rosseneu, M., O'Reilly, D. S. J., et al. (1997). Interactions between lifestyle-related factors and the ApoE polymorphism on plasma lipids and apolipoproteins: the ears study. Arterioscler. Thromb. Vasc. Biol. 17, 1675–1681. doi: 10.1161/01.ATV.17.9.1675
Boer, J. M. A., Feskens, E. J. M., Schouten, E. G., Havekes, L. M., Seidell, J. C., and Kromhout, D. (1998). Lipid profiles reflecting high and low risk for coronary heart disease: contribution of apolipoprotein E polymorphism and lifestyle. Atherosclerosis 136, 395–402. doi: 10.1016/S0021-9150(97)00231-1
Bondi, M. W., Houston, W. S., Eyler, L. T., and Brown, G. G. (2005). fMRI evidence of compensatory mechanisms in older adults at genetic risk for Alzheimer disease. Neurology 64, 501–508. doi: 10.1212/01.WNL.0000150885.00929.7E
Boots, E. A., Schultz, S. A., Oh, J. M., Larson, J., Edwards, D., Cook, D., et al. (2015). Cardiorespiratory fitness is associated with brain structure, cognition, and mood in a middle-aged cohort at risk for Alzheimer's disease. Brain Imaging Behav. 9, 639–649. doi: 10.1007/s11682-014-9325-9
Brown, B. M., Peiffer, J., and Rainey-Smith, S. R. (2019). Exploring the relationship between physical activity, beta-amyloid and tau: a narrative review. Ageing Res. Rev. 50, 9–18. doi: 10.1016/j.arr.2019.01.003
Brown, B. M., Peiffer, J. J., Taddei, K., Lui, J. K., Laws, S. M., Gupta, V. B., et al. (2013). Physical activity and amyloid-β plasma and brain levels: results from the Australian imaging, biomarkers and lifestyle study of ageing. Mol. Psychiatry 18, 875–881. doi: 10.1038/mp.2012.107
Buckner, R. L., Bandettini, P. A., O'Craven, K. M., Savoy, R. L., Petersen, S. E., Raichle, M. E., et al. (1996). Detection of cortical activation during averaged single trials of a cognitive task using functional magnetic resonance imaging. Proc. Nat. Acad. Sci. 93, 14878–14883. doi: 10.1073/pnas.93.25.14878
Bugg, J. M., and Head, D. (2011). Exercise moderates age-related atrophy of the medial temporal lobe. Neurobiol. Aging 32, 506–514. doi: 10.1016/j.neurobiolaging.2009.03.008
Canuet, L., Tellado, I., Couceiro, V., Fraile, C., Fernandez-Novoa, L., Ishii, R., et al. (2012). Resting-state network disruption and APOE genotype in Alzheimer's disease: a lagged functional connectivity study. PLoS ONE 7:e46289. doi: 10.1371/journal.pone.0046289
Cheung, M. W.. (2019). A guide to conducting a meta-analysis with non-independent effect sizes. Neuropsychol. Rev. 29, 387–396. doi: 10.1007/s11065-019-09415-6
Chuang, Y. F., Hayden, K. M., Norton, M. C., Tschanz, J., Breitner, J. C. S., Welsh-Bohmer, K. A., et al. (2010). Association between APOE ε4 allele and vascular dementia: the cache county study. Dement. Geriatr. Cogn. Disord. 29, 248–253. doi: 10.1159/000285166
Colcombe, S. J., Erickson, K. I., Scalf, P. E., Kim, J. S., Prakash, R., McAuley, E., et al. (2006). Aerobic exercise training increases brain volume in aging humans. J. Gerontol. Series A Biol. Sci. Med. Sci. 61, 1166–1170. doi: 10.1093/gerona/61.11.1166
Corbo, R. M., and Scacchi, R. (1999). Apolipoprotein E (APOE) allele distribution in the world. Is APOE * 4 a ‘thrifty' allele? Ann. Hum. Genet. 63, 301–310. doi: 10.1046/j.1469-1809.1999.6340301.x
Corder, E. H., Saunders, A. M., Strittmatter, W. J., Schmechel, D. E., Gaskell, P. C., Small, G. W., et al. (1993). Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science 261, 921–923. doi: 10.1126/science.8346443
Corella, D., Guillén, M., Sáiz, C., Portolés, O., Sabater, A., Cortina, S., et al. (2001). Environmental factors modulate the effect of the APOE genetic polymorphism on plasma lipid concentrations: ecogenetic studies in a Mediterranean Spanish population. Metab. Clin. Exp. 50, 936–944. doi: 10.1053/meta.2001.24867
de Frutos-Lucas, J., Cuesta, P., López-Sanz, D., Peral-Suárez, Á., Cuadrado-Soto, E., Ramírez-Toranõ, F., et al. (2020a). The relationship between physical activity, apolipoprotein e ϵ4 carriage, and brain health. Alzheimers Res. Ther. 12:48. doi: 10.1186/s13195-020-00608-3
de Frutos-Lucas, J., Cuesta, P., Ramírez-Toraño, F., Nebreda, A., Cuadrado-Soto, E., Peral-Suárez, Á., et al. (2020b). Age and APOE genotype affect the relationship between objectively measured physical activity and power in the alpha band, a marker of brain disease. Alzheimers Res. Ther. 12:113. doi: 10.1186/s13195-020-00681-8
de Frutos-Lucas, J., Frost, N., Erickson, K. I., Serrano, J. M., Maestu, F., Laws, S. M., et al. (2020c). Does APOE genotype moderate the relationship between physical activity, brain health and dementia risk? A systematic review. Ageing Res. Rev. 64:101173. doi: 10.1016/j.arr.2020.101173
de Frutos-Lucas, J., López-Sanz, D., Zuluaga, P., Rodríguez-Rojo, I. C., Luna, R., López, M. E., et al. (2018). Physical activity effects on the individual alpha peak frequency of older adults with and without genetic risk factors for Alzheimer's disease: a MEG study. Clin. Neurophysiol. 129, 1981–1989. doi: 10.1016/j.clinph.2018.06.026
de Souto Barreto, P., Andrieu, S., Payoux, P., Demougeot, L., Rolland, Y., and Vellas, B. (2015). Physical activity and amyloid-β brain levels in elderly adults with intact cognition and mild cognitive impairment. J. Am. Geriatr. Soc. 63, 1634–1639. doi: 10.1111/jgs.13530
Deeny, S. P., Poeppel, D., Zimmerman, J. B., Roth, S. M., Brandauer, J., Witkowski, S., et al. (2008). Exercise, APOE, and working memory: MEG and behavioral evidence for benefit of exercise in epsilon4 carriers. Biol. Psychol. 78, 179–187. doi: 10.1016/j.biopsycho.2008.02.007
Erickson, K. I., Hillman, C., Stillman, C. M., Ballard, R. M., Bloodgood, B., Conroy, D. E., et al. (2019). Physical activity, cognition, and brain outcomes: a review of the 2018 physical activity guidelines. Med. Sci. Sports Exerc. 51, 1242–1251. doi: 10.1249/MSS.0000000000001936
Erickson, K. I., Leckie, R. L., and Weinstein, A. M. (2014). Physical activity, fitness, and gray matter volume. Neurobiol. Aging 35 (Suppl. 2):S20. doi: 10.1016/j.neurobiolaging.2014.03.034
Erickson, K. I., Voss, M. W., Prakash, R. S., Basak, C., Szabo, A., Chaddock, L., et al. (2011). Exercise training increases size of hippocampus and improves memory. Proc. Natl. Acad. Sci. U. S. A. 108, 3017–3022. doi: 10.1073/pnas.1015950108
Fagan, A. M., Mintun, M. A., Mach, R. H., Lee, S. Y., Dence, C. S., Shah, A. R., et al. (2006). Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid Abeta;42 in humans. Ann. Neurol. 59, 512–519. doi: 10.1002/ana.20730
Ferguson, A., Tank, R., Lyall, L., Ward, J., Celis-Morales, C., Strawbridge, R., et al. (2020). Alzheimer's disease susceptibility gene apolipoprotein e (APOE) and blood biomarkers in UK Biobank (N=395,769). J. Alzheimers Dis. 76, 1541–1551. doi: 10.3233/JAD-200338
Fleisher, A. S., Podraza, K. M., Bangen, K. J., Taylor, C., Sherzai, A., Sidhar, K., et al. (2009). Cerebral perfusion and oxygenation differences in Alzheimer's disease risk. Neurobiol. Aging 30, 1737–1748. doi: 10.1016/j.neurobiolaging.2008.01.012
Gow, A. J., Pattie, A., and Deary, I. J. (2017). Lifecourse activity participation from early, mid, and later adulthood as determinants of cognitive aging: the lothian birth cohort 1921. J. Gerontol. Series B Psychol. Sci. Soc. Sci. 72, 25–37. doi: 10.1093/geronb/gbw124
Gu, Y., Beato, J. M., Amarante, E., Chesebro, A. G., Manly, J. J., Schupf, N., et al. (2020). Assessment of leisure time physical activity and brain health in a multiethnic cohort of older adults. JAMA Network Open 3:2026506. doi: 10.1001/jamanetworkopen.2020.26506
Gustavsson, J., Mehlig, K., Leander, K., Strandhagen, E., Björck, L., Thelle, D. S., et al. (2012). Interaction of apolipoprotein E genotype with smoking and physical inactivity on coronary heart disease risk in men and women. Atherosclerosis 220, 486–492. doi: 10.1016/j.atherosclerosis.2011.10.011
Head, D., Bugg, J. M., Goate, A. M., Fagan, A. M., Mintun, M. A., Benzinger, T., et al. (2012). Exercise engagement as a moderator of the effects of APOE genotype on amyloid deposition. Arch. Neurol. 69, 636–643. doi: 10.1001/archneurol.2011.845
Honea, R. A., Thomas, G. P., Harsha, A., Anderson, H. S., Donnelly, J. E., Brooks, W. M., et al. (2009). Cardiorespiratory fitness and preserved medial temporal lobe volume in alzheimer disease. Alzheimer Dis. Assoc. Disord. 23, 188–197. doi: 10.1097/WAD.0b013e31819cb8a2
Jacobs, H. I. L., van Boxtel, M. P. J., Jolles, J., Verhey, F. R. J., and Uylings, H. B. M. (2012). Parietal cortex matters in Alzheimer's disease: an overview of structural, functional and metabolic findings. Neurosci. Biobehav. Rev. 36, 297–309. doi: 10.1016/j.neubiorev.2011.06.009
Jeon, S. Y., Byun, M. S., Yi, D., Lee, J. H., Ko, K., Sohn, B. K., et al. (2020). Midlife lifestyle activities moderate APOE ε4 effect on in vivo Alzheimer's disease pathologies. Front. Aging Neurosci. 12:42. doi: 10.3389/fnagi.2020.00042
Jones, D. K., Knösche, T. R., and Turner, R. (2013). White matter integrity, fiber count, and other fallacies: the do's and don'ts of diffusion MRI. Neuroimage 73, 239–254. doi: 10.1016/j.neuroimage.2012.06.081
Kamijo, K., Takeda, Y., and Hillman, C. H. (2011). The relation of physical activity to functional connectivity between brain regions. Clin. Neurophysiol. 122, 81–89. doi: 10.1016/j.clinph.2010.06.007
Kerestes, R., Phal, P. M., Steward, C., Moffat, B. A., Salinas, S., Cox, K. L., et al. (2015). Alterations in dorsal and ventral posterior cingulate connectivity in APOE ε 4 carriers at risk of Alzheimer's disease. BJPsych Open 1, 139–148. doi: 10.1192/bjpo.bp.115.001339
Khan, T. A., Shah, T., Prieto, D., Zhang, W., Price, J., Fowkes, G. R., et al. (2013). Apolipoprotein E genotype, cardiovascular biomarkers and risk of stroke: systematic review and meta-analysis of 14,015 stroke cases and pooled analysis of primary biomarker data from up to 60,883 individuals. Int. J. Epidemiol. 42, 475–492. doi: 10.1093/ije/dyt034
Kiko, T., Nakagawa, K., Satoh, A., Tsuduki, T., Furukawa, K., Arai, H., et al. (2012). Amyloid β Levels in human red blood cells. PLoS ONE 7:e49620. doi: 10.1371/journal.pone.0049620
Kodama, S., Tanaka, S., Saito, K., Shu, M., Sone, Y., Onitake, F., et al. (2007). Effect of aerobic exercise training on serum levels of high-density lipoprotein cholesterol: a meta-analysis. Arch. Intern. Med. 167, 999–1008. doi: 10.1001/archinte.167.10.999
Lan, J., Liu, J., Zhao, Z., Xue, R., Zhang, N., Zhang, P., et al. (2015). The peripheral blood of Ab binding RBC as a biomarker for diagnosis of Alzheimer's disease. Age Ageing 44, 458–464. doi: 10.1093/ageing/afv009
Law, L. L., Rol, R. N., Schultz, S. A., Dougherty, R. J., Edwards, D. F., Koscik, R. L., et al. (2018). Moderate intensity physical activity associates with CSF biomarkers in a cohort at risk for Alzheimer's disease. Alzheimers Dement. Diagn. Assess. Dis. Monit. 10, 188–195. doi: 10.1016/j.dadm.2018.01.001
Leoni, V., Solomon, A., and Kivipelto, M. (2010). The biology of tau and its role in tauopathies links between ApoE, brain cholesterol metabolism, tau and amyloid β-peptide in patients with cognitive impairment. Biochem. Soc. Trans. 38, 1021–1025. doi: 10.1042/BST0381021
Liang, K. Y., Mintun, M. A., Fagan, A. M., Goate, A. M., Bugg, J. M., Holtzman, D. M., et al. (2010). Exercise and Alzheimer's disease biomarkers in cognitively normal older adults. Ann. Neurol. 68, 311–318. doi: 10.1002/ana.22096
Liu, Y., Yu, J.-T., Wang, H. F., Han, P.-R., Tan, C.-C., Wang, C., et al. (2015). APOE genotype and neuroimaging markers of Alzheimer's disease: systematic review and meta-analysis. J. Neurol. Neurosurg. Psychiatry 86, 127–134. doi: 10.1136/jnnp-2014-307719
Lyall, D. M., Cox, S. R., Lyall, L. M., Celis-Morales, C., Cullen, B., Mackay, D. F., et al. (2019). Association between APOE e4 and white matter hyperintensity volume, but not total brain volume or white matter integrity. Brain Imaging Behav. doi: 10.1007/s11682-019-00069-9
Madden, D. J., Bennett, I. J., and Song, A. W. (2009). Cerebral white matter integrity and cognitive aging: contributions from diffusion tensor imaging. Neuropsychol. Rev. 19, 415–435. doi: 10.1007/s11065-009-9113-2
Mahley, R. W.. (1988). Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science 240, 622–630. doi: 10.1126/science.3283935
Mann, S., Beedie, C., and Jimenez, A. (2014). Differential effects of aerobic exercise, resistance training and combined exercise modalities on cholesterol and the lipid profile: review, synthesis and recommendations. Sports Med. 44, 211–221. doi: 10.1007/s40279-013-0110-5
Marks, B. L., Madden, D. J., Bucur, B., Provenzale, J. M., White, L. E., Cabeza, R., et al. (2007). Role of aerobic fitness and aging on cerebral white matter integrity. Ann. N. Y. Acad. Sci. 1097, 171–174. doi: 10.1196/annals.1379.022
Obisesan, T. O., Umar, N., Paluvoi, N., and Gillum, R. F. (2012). Association of leisure-time physical activity with cognition by apolipoprotein-e genotype in persons aged 60 years and over: the National Health and Nutrition Examination Survey (NHANES-III). Clin. Interv. Aging 7, 35–45. doi: 10.2147/CIA.S26794
Okonkwo, O. C., Schultz, S. A., Oh, J. M., Larson, J., Edwards, D., Cook, D., et al. (2014). Physical activity attenuates age-related biomarker alterations in preclinical AD. Neurology 83, 1753–1760. doi: 10.1212/WNL.0000000000000964
Olsson, B., Lautner, R., Andreasson, U., Öhrfelt, A., Portelius, E., Bjerke, M., et al. (2016). CSF and blood biomarkers for the diagnosis of Alzheimer's disease: a systematic review and meta-analysis. Lancet Neurol. 15, 673–684. doi: 10.1016/S1474-4422(16)00070-3
Operto, G., Cacciaglia, R., Grau-Rivera, O., Falcon, C., Brugulat-Serrat, A., Ródenas, P., et al. (2018). White matter microstructure is altered in cognitively normal middle-aged APOE-ϵ4 homozygotes. Alzheimers Res. Ther. 10:48. doi: 10.1186/s13195-018-0375-x
Ovod, V., Ramsey, K. N., Mawuenyega, K. G., Bollinger, J. G., Hicks, T., Schneider, T., et al. (2017). Amyloid β concentrations and stable isotope labeling kinetics of human plasma specific to central nervous system amyloidosis. Alzheimers Dement. 13, 841–849. doi: 10.1016/j.jalz.2017.06.2266
Persson, J., Lind, J., Larsson, A., Ingvar, M., Cruts, M., van Broeckhoven, C., et al. (2006). Altered brain white matter integrity in healthy carriers of the APOE ε4 allele: a risk for AD? Neurology 66, 1029–1033. doi: 10.1212/01.wnl.0000204180.25361.48
Piccarducci, R., Daniele, S., Fusi, J., Chico, L., Baldacci, F., Siciliano, G., et al. (2019). Impact of ApoE polymorphism and physical activity on plasma antioxidant capability and erythrocyte membranes. Antioxidants 8:538. doi: 10.3390/antiox8110538
Pierpaoli, C., and Basser, P. J. (1996). Toward a quantitative assessment of diffusion anisotropy. Magnet. Resonan. Med. 36, 893–906. doi: 10.1002/mrm.1910360612
Pisciotta, L., Cantafora, A., Piana, A., Masturzo, P., Cerone, R., Minniti, G., et al. (2003). Physical activity modulates effects of some genetic polymorphisms affecting cardiovascular risk in men aged over 40 years. Nutr. Metab. Cardiovasc. Dis. 13, 202–210. doi: 10.1016/S0939-4753(03)80012-1
Pizzie, R., Hindman, H., Roe, C. M., Head, D., Grant, E., Morris, J. C., et al. (2014). Physical activity and cognitive trajectories in cognitively normal adults: the adult children study. Alzheimer Dis. Assoc. Disord. 28, 50–57. doi: 10.1097/WAD.0b013e31829628d4
R Core Team (2020). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available online at: https://www.R-project.org/
Ridge, P. G., Hoyt, K. B., Boehme, K., Mukherjee, S., Crane, P. K., Haines, J. L., et al. (2016). Assessment of the genetic variance of late-onset Alzheimer's disease. Neurobiol. Aging 41, 200.e13–200.e20. doi: 10.1016/j.neurobiolaging.2016.02.024
Ridge, P. G., Mukherjee, S., Crane, P. K., and Kauwe, J. S. K. (2013). Alzheimer's disease: analyzing the missing heritability. PLoS ONE 8:e79771. doi: 10.1371/journal.pone.0079771
Ritchie, S. J., Tucker-Drob, E. M., Cox, S. R., Dickie, D. A., del, C., Valdés Hernández, M., et al. (2017). Risk and protective factors for structural brain ageing in the eighth decade of life. Brain Struct. Funct. 222, 3477–3490. doi: 10.1007/s00429-017-1414-2
Rocchi, A., Pellegrini, S., Siciliano, G., and Murri, L. (2003). Causative and susceptibility genes for Alzheimer's disease: a review. Brain Res. Bull. 61, 1–24. doi: 10.1016/S0361-9230(03)00067-4
Rodriguez, F. S., Schroeter, M. L., Arélin, K., Veronica Witte, A., Baber, R., Burkhardt, R., et al. (2018). APOE e4–genotype and lifestyle interaction on cognitive performance: results of the LIFE-adult-study. Health Psychol. 37, 194–205. doi: 10.1037/hea0000515
Rojas, S., Brugulat-Serrat, A., Bargall,ó, N., Minguillón, C., Tucholka, A., Falcon, C., et al. (2018). Higher prevalence of cerebral white matter hyperintensities in homozygous APOE-ε4 allele carriers aged 45–75: results from the ALFA study. J. Cereb. Blood Flow Metab. 38, 250–261. doi: 10.1177/0271678X17707397
Sabia, S., Kivimaki, M., Kumari, M., Shipley, M. J., and Singh-Manoux, A. (2010). Effect of apolipoprotein e 4 on the association between health behaviors and cognitive function in late midlife. Mol. Neurodegener. 5:23. doi: 10.1186/1750-1326-5-23
Sarzynski, M. A., Burton, J., Rankinen, T., Blair, S. N., Church, T. S., Després, J. P., et al. (2015). The effects of exercise on the lipoprotein subclass profile: a meta-analysis of 10 interventions. Atherosclerosis 243, 364–372. doi: 10.1016/j.atherosclerosis.2015.10.018
Schaie, K. W., Willis, S. L., and Caskie, G. I. L. (2004). The seattle longitudinal study: relationship between personality and cognition. Aging Neuropsychol. Cogn. 11, 304–324. doi: 10.1080/13825580490511134
Schmitz, K. H., Schreiner, P. J., Jacobs, D. R., Leon, A. S., Liu, K., Howard, B., et al. (2001). Independent and interactive effects of Apolipoprotein E phenotype and cardiorespiratory fitness on plasma lipids. Ann. Epidemiol. 11, 94–103. doi: 10.1016/S1047-2797(00)00174-5
Smith, J. C., Lancaster, M. A., Nielson, K. A., Woodard, J. L., Seidenberg, M., Durgerian, S., et al. (2016). Interactive effects of physical activity and APOE-ε4 on white matter tract diffusivity in healthy elders. Neuroimage 131, 102–112. doi: 10.1016/j.neuroimage.2015.08.007
Smith, J. C., Nielson, K. A., Woodard, J. L., Seidenberg, M., Durgerian, S., Antuono, P., et al. (2011). Interactive effects of physical activity and APOE-ε4 on BOLD semantic memory activation in healthy elders. Neuroimage 54, 635–644. doi: 10.1016/j.neuroimage.2010.07.070
Smith, J. C., Nielson, K. A., Woodard, J. L., Seidenberg, M., Durgerian, S., Hazlett, K. E., et al. (2014). Physical activity reduces hippocampal atrophy in elders at genetic risk for Alzheimer's disease. Front. Aging Neurosci. 6:61. doi: 10.3389/fnagi.2014.00061
St-Amand, J., Prud'homme, D., Moorjani, S., Nadeau, A., Tremblay, A., Bouchard, C., et al. (1999). Apolipoprotein E polymorphism and the relationships of physical fitness to plasma lipoprotein-lipid levels in men and women. Med. Sci. Sports Exerc. 31, 692–697. doi: 10.1097/00005768-199905000-00011
Stern, Y., Mackay-Brandt, A., Lee, S., McKinley, P., McIntyre, K., Razlighi, Q., et al. (2019). Effect of aerobic exercise on cognition in younger adults: a randomized clinical trial. Neurology 92, E905–E916. doi: 10.1212/WNL.0000000000007003
Stojanovic, M., Jin, Y., Fagan, A. M., Benzinger, T. L., Hassenstab, J., Cruchaga, C., et al. (2020). Physical exercise and longitudinal trajectories in Alzheimer disease biomarkers and cognitive functioning. Alzheimer Dis. Assoc. Disord. 34, 212–219. doi: 10.1097/WAD.0000000000000385
Thompson, P. D., Yurgalevitch, S. M., Flynn, M. M., Zmuda, J. M., Spannaus-Martin, D., Saritelli, A., et al. (1997). Effect of prolonged exercise training without weight loss on high- density lipoprotein metabolism in overweight men. Metab. Clin. Exp. 46, 217–223. doi: 10.1016/S0026-0495(97)90305-X
Tsai, C. L., Erickson, K. I., Sun, H. S., Kuo, Y. M., and Pai, M. C. (2021). A cross-sectional examination of a family history of Alzheimer's disease and ApoE epsilon 4 on physical fitness, molecular biomarkers, and neurocognitive performance. Physiol. Behav. 230, 113268. doi: 10.1016/j.physbeh.2020.113268
Tsai, C. L., Sun, H., Kuo, Y. M., and Pai, M. C. (2019). The role of physical fitness in cognitive-related biomarkers in persons at genetic risk of familial Alzheimer's disease. J. Clin. Med. 8:1639. doi: 10.3390/jcm8101639
Tyrrell, J., Zheng, J., Beaumont, R., Hinton, K., Richardson, T. G., Wood, A. R., et al. (2021). Genetic predictors of participation in optional components of UK Biobank. Nat. Commun. 12, 1–13. doi: 10.1038/s41467-021-21073-y
van den Heuvel, M. P., and Hulshoff Pol, H. E. (2010). Exploring the brain network: a review on resting-state fMRI functional connectivity. Eur. Neuropsychopharmacol. 20, 519–534. doi: 10.1016/j.euroneuro.2010.03.008
Van den Noortgate, W., López-López, J. A., Marín-Martínez, F., and Sánchez-Meca, J. (2014). Meta-analysis of multiple outcomes: a multilevel approach. Behav. Res. Methods 47, 1274–1294. doi: 10.3758/s13428-014-0527-2
Vemuri, P., Lesnick, T. G., Przybelski, S. A., Knopman, D. S., Machulda, M., Lowe, V. J., et al. (2016). Effect of intellectual enrichment on AD biomarker trajectories. Neurology 86, 1128–1135. doi: 10.1212/WNL.0000000000002490
Vico Varela, E., Etter, G., and Williams, S. (2019). Excitatory-inhibitory imbalance in Alzheimer's disease and therapeutic significance. Neurobiol. Dis. 127, 605–615. doi: 10.1016/j.nbd.2019.04.010
Viechtbauer, W.. (2010). Conducting meta-analyses in R with the metafor. J. Stat. Softw. 36, 1–48. doi: 10.18637/jss.v036.i03
Vinke, E. J., de Groot, M., Venkatraghavan, V., Klein, S., Niessen, W. J., Ikram, M. A., et al. (2018). Trajectories of imaging markers in brain aging: the Rotterdam Study. Neurobiol. Aging 71, 32–40. doi: 10.1016/j.neurobiolaging.2018.07.001
Wardlaw, J. M., Valdés Hernández, M. C., and Muñoz-Maniega, S. (2015). What are white matter hyperintensities made of? Relevance to vascular cognitive impairment. J. Am. Heart Assoc. 4, 1–19. doi: 10.1161/JAHA.114.001140
Wilson, R. S., Leurgans, S. E., Boyle, P. A., and Bennett, D. A. (2011). Cognitive decline in prodromal Alzheimer's disease and mild cognitive impairment. Arch. Neurol. 68:351. doi: 10.1001/archneurol.2011.31
Wisdom, N. M., Callahan, J. L., and Hawkins, K. A. (2011). The effects of apolipoprotein E on non-impaired cognitive functioning: a meta-analysis. Neurobiol. Aging 32, 63–74. doi: 10.1016/j.neurobiolaging.2009.02.003
Wishart, H. A., Saykin, A. J., McAllister, T. W., Rabin, L. A., McDonald, B. C., Flashman, L. A., et al. (2006). Regional brain atrophy in cognitively intact adults with a single APOE ε4 allele. Neurology 67, 1221–1224. doi: 10.1212/01.wnl.0000238079.00472.3a
Yu, Q., Herold, F., Becker, B., Klugah-Brown, B., Zhang, Y., Perrey, S., et al. (2021). Cognitive benefits of exercise interventions: an fMRI activation likelihood estimation meta-analysis. Brain Struct. Funct. 226, 601–619. doi: 10.1007/s00429-021-02247-2
Keywords: Alzheimer's disease, apolipoprotein E, brain function, brain structure, lipid profile, meta-analysis, physical activity
Citation: Pearce AM, Marr C, Dewar M and Gow AJ (2022) Apolipoprotein E Genotype Moderation of the Association Between Physical Activity and Brain Health. A Systematic Review and Meta-Analysis. Front. Aging Neurosci. 13:815439. doi: 10.3389/fnagi.2021.815439
Received: 15 November 2021; Accepted: 17 December 2021;
Published: 28 January 2022.
Edited by:
Anja Soldan, Johns Hopkins University, United StatesReviewed by:
Chia-Liang Tsai, National Cheng Kung University, TaiwanDonald Lyall, University of Glasgow, United Kingdom
Copyright © 2022 Pearce, Marr, Dewar and Gow. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alan J. Gow, YS5qLmdvd0Body5hYy51aw==
 Andrew M. Pearce
Andrew M. Pearce Calum Marr
Calum Marr Michaela Dewar
Michaela Dewar Alan J. Gow
Alan J. Gow