- 1Department of Neurology, Ewha Womans University School of Medicine, Seoul, Republic of Korea
- 2Department of Neurology, Ewha Womans University Seoul Hospital, Seoul, Republic of Korea
- 3Department of Neurology, Ewha Womans University Mokdong Hospital, Seoul, Republic of Korea
- 4Division of Psychiatry, Department of Mental Health Care of Older People, University College London, London, United Kingdom
- 5Department of Medical Nutrition (AgeTech-Service Convergence Major), Kyung Hee University, Yongin, Republic of Korea
- 6Clinical Trial Center, Ewha Womans University Mokdong Hospital, Seoul, Republic of Korea
- 7Department of Psychiatry, Ajou University School of Medicine, Suwon, Republic of Korea
- 8Department of Neurology, Ajou University School of Medicine, Suwon, Republic of Korea
- 9CJ Bioscience Inc., Seoul, Republic of Korea
- 10Department of Nuclear Medicine, Ewha Womans University School of Medicine, Seoul, Republic of Korea
- 11Department of Neurology, Inha University College of Medicine, Incheon, Republic of Korea
Background: The SoUth Korean study to PrEvent cognitive impaiRment and protect BRAIN health through lifestyle intervention in at-risk elderly people (SUPERBRAIN) is a part of the World-Wide Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (WW-FINGERS) network. This study aimed to demonstrate the effects of the SUPERBRAIN-based multidomain intervention with nutritional supplements in amyloid positive emission tomography (PET) proven early symptomatic Alzheimer’s disease patients.
Methods: Forty-six participants who were diagnosed with mild cognitive impairment or mild dementia and were positive in the amyloid PET study randomized into three groups: group A, the multidomain intervention with nutritional supplements; group B, nutritional supplements only; and a control group. The primary outcome was a change in the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) total scale index score after an 8-week intervention. Secondary outcomes, including gut microbiome data, were also analyzed.
Results: The RBANS total scale index score improved significantly in group A compared with group B (p < 0.032) and compared with the control group (p < 0.001). After intervention, beta diversity of the gut microbiome between group A and the control group increased, and patients in group A were more enriched with Bifidobacterium.
Conclusion: SUPERBRAIN-based multidomain intervention with nutritional supplements improves cognition and gut microbiota in patients with early symptomatic Alzheimer’s disease who were amyloid-positive by PET.
1. Introduction
Alzheimer’s disease (AD) and related dementias are the most common neurodegenerative diseases (World Health Organization, 2012, 2017). However, as curative pharmacological treatments for AD dementia are still lacking (Hung and Fu, 2017; Alzheimer’s Association, 2022), nonpharmacological treatments to prevent and modify cognitive impairment have been employed. Although single-domain interventions have shown modest outcomes and inconsistent results (Hill et al., 2017; de Souto et al., 2018; Radd-Vagenas et al., 2018; Ding et al., 2020), multidomain interventions have emerged as an alternative approach that considers the multifactorial causes of AD dementia (Coley et al., 2008; Scarmeas et al., 2009; Andrieu et al., 2015).
The Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER) trial reported that participants who received 24 months of multidomain intervention had better cognitive outcomes than control participants (Ngandu et al., 2015). To better adapt this intervention for use in other countries (World Health Organization, 2019), the World-Wide FINGERS (WW-FINGERS) network was launched. As a part of this worldwide network, the SoUth Korean study to PrEvent cognitive impaiRment and protect BRAIN health through lifestyle intervention in at-risk elderly people (SUPERBRAIN) was established as a multidomain intervention adjusted to the Korean context (Park et al., 2020). The feasibility study of the SUPERBRAIN presented significant improvement in cognition in intervention groups compared with a control group (Moon et al., 2021), and its magnetic resonance imaging results showed interval cortical thickening in facility-based multidomain intervention groups compared with the other groups (Moon et al., 2022).
In contrast, other clinical trials, such as the Multidomain Alzheimer Preventive Trial (MAPT) and Prevention of Dementia by Intensive Vascular Care (PreDIVA) trials, did not reveal significant effects from the interventions (van Charante et al., 2016; Andrieu et al., 2017). These inconsistent results require explanation, considering the positive results from the FINGER trial because of its well-selected target population (Solomon et al., 2021). The ancillary study of the MAPT revealed that a positive amyloid status could indicate a target population for multidomain intervention (Delrieu et al., 2019). Furthermore, a subgroup analysis of the original FINGER study reported that APOE ε4 carriers, who may have higher levels of brain amyloid pathology, showed a beneficial effect of the multidomain intervention on general cognition and memory within the group compared to non-carriers (Solomon et al., 2018). These findings called for further studies of the effects of multidomain interventions in amyloid-positive participants.
Recent trials have focused on a population at risk of dementia; that is, those not yet diagnosed with dementia. However, multidomain intervention might also be effective for patients with dementia because components of multidomain interventions are still available as treatment options for patients with dementia (Livingston et al., 2017, 2020). Consequently, this study targeted individuals with amyloid PET-proven early symptomatic AD, encompassing those with mild cognitive impairment (MCI) as well as individuals with dementia with Clinical Dementia Rating Scale Sum of Boxes (CDR-SB) score of less than 5.
We also enhanced the SUPERBRAIN multidomain intervention protocol for nutrition. Despite receiving nutritional guidance, patients with cognitive impairment find it challenging to eat balanced meals every day. To compensate for this, a nutritional supplement in the form of a multinutrient drink was added to the nutrition protocol of the SUPERBRAIN.
Therefore, this study investigated whether the effects of the multidomain intervention with nutritional supplements are superior to nutritional supplements alone or to no intervention (the control group) and whether the effects of nutritional supplements are superior to those of the control group.
2. Materials and methods
2.1. Study design
This study was a single-center, outcome assessor–blinded randomized controlled trial with a three-arm parallel design, and the intervention period was 8 weeks. The participants in group A received multidomain intervention with nutritional supplements, whereas those in group B received only nutritional supplements. A waitlist control group was also established in which participants were informed that the multidomain intervention program would be provided to them after the study. The baseline study was executed within 8 weeks before the start of the intervention, and the final study was completed within 4 weeks after the end of the last intervention. This study was registered with the Clinical Research Information Service (KCT0007253).
The study was conducted in accordance with the International Conference on Harmonization Good Clinical Practices Guidelines (Dixon, 1998). Written informed consent was obtained from all participants before enrollment. The Ewha Womans University, Seoul Hospital Institutional Review Board (IRB) approved this trial (SEUMC 2020-08-008-001).
2.2. Participants
The minimum number of participants required for adequate statistical power was calculated based on the effect size of changes in the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) total scale index score from a previous study (Calapai et al., 2017). The calculation was performed using G*Power version 3.1.9.7 (Heinrich Heine University, Düsseldorf, Germany). For 80% of the statistical power, at least 13 participants were required in each group. Considering a drop-out rate of approximately 20%, the final sample size was determined to be 16 participants per group.
Participants were recruited from individuals who visited the outpatient clinic of Ewha Womans University Seoul Hospital because of cognitive decline from March 2, 2021, to August 13, 2022. The inclusion criteria were: older adults aged 60–85 years; clinically diagnosed with MCI (Albert et al., 2011) or probable AD dementia (McKhann et al., 2011); Korean Mini-Mental State Examination Second Edition (K-MMSE-2) score of 17–27 (Han et al., 2008; Baek et al., 2016); Clinical Dementia Rating (CDR) (Morris, 1991) score of 0.5–1; CDR-SB (Choi et al., 2003) score of 0.5–5; having a caregiver with whom they could attend interviews and programs; and the subject agrees to participate in the study with written consent.
Participants were excluded if they had organic brain diseases or degenerative diseases known to be major causes of cognitive decline, including brain tumor, stroke, normal pressure hydrocephalus, Parkinson’s disease, Lewy body dementia, vascular dementia, and autoimmune encephalitis. In addition, individuals were excluded if they had infectious or metabolic diseases that may cause cognitive impairment, such as neurosyphilis, AIDS dementia, vitamin B12 deficiency, folate deficiency, and hypothyroidism. Other exclusion criteria were major psychiatric illness, epilepsy with intractable seizures, acute illness or acute infectious disease, probable encephalopathy caused by chronic liver or kidney disease, chronic pulmonary or cardiovascular disease under treatment, and any medical condition that would prevent cooperation with the interventions. Participants were also excluded if they had significant vision or hearing impairment, were illiterate, were unable to cooperate until the end of the study, were unable to safely complete the exercise program, had concurrent participation in another intervention trial, or refused to participate in the study.
Amyloid PET scans were conducted on the recruited participants. Participants who were determined as having amyloid pathology were enrolled in the study. Ultimately, the enrolled participants were amyloid PET-proven early symptomatic AD with a CDR-SB score below 5.
2.3. Amyloid PET
We used 18F-florbetaben manufactured by DuChemBio Co., Ltd. (Seoul, Korea) following the approval process of the Korean Ministry of Food and Drug Safety. Delayed-phase 3D list-mode dynamic PET images were acquired over a 20-min period 90–110 min after the bolus injection of 308.12 ± 10.93 MBq 18F-florbetaben. A spiral computed tomography scan of the brain was performed with the following parameters: 120 kV, 30 mA, and a slice thickness of 1.0 mm to correct for attenuation in the PET emission data. The participants’ heads were fixed with a head holder and a vacuum fixation cushion to reduce motion artifacts. The standard PET data were reconstructed into a 128 × 128 matrix (voxel size: 3.18 × 3.18 × 2.02 mm3) using the built-in 3D ordered subset expectation maximization algorithm (iteration: 4; subset: 12).
A visual reading by a nuclear medicine specialist was used to determine amyloid PET positivity.
2.4. Randomization
Participants were randomized to each group in a 1:1:1 ratio. The permuted block randomization method was applied using a macro in SAS software (SAS Institute Inc., Cary, NC, USA) with a block size of six. Only the independent statistical specialists knew the whole allocation sequence. Outcome assessors were not involved in the interventions, and participants were prohibited from discussing their assigned group when they met the outcome assessors.
2.5. Nutritional supplements
Memory Pack Plus (Daesang Life Science Corporation, Korea) was used as the nutritional supplement for this study. It is a multinutrient drink designed for brain health that contains eicosapentaenoic acid, docosahexaenoic acid, and phosphatidylserine (Supplementary material 1). It is aseptically packed as a 150 mL carton, and each carton contains 150 kcal.
2.6. Interventions
The protocol and contents of the multidomain intervention were based on the facility-based multidomain intervention of SUPERBRAIN (Park et al., 2020), which includes the following five components: the monitoring and managing of metabolic and vascular risk factors, cognitive training, physical exercise, nutritional guidance, and motivational enhancement. The number and frequency of each session were adjusted for a shorter study period than the previous SUPERBRAIN. Education on vascular risk factors was provided at the first visit with a booklet for the participants. The blood pressure, alcohol drinking, smoking, body weight, and abdominal circumference of each participant were monitored, and the results were discussed with each participant every 4 weeks. Each week, exercise and cognitive training sessions were done twice on the same visit day. Cognitive training sessions and nutritional guidance sessions were conducted with individual participants, whereas exercise sessions were conducted in a group of two participants. The participants attended nutritional guidance sessions once every 3 weeks. The details of the activities are described in Supplementary material 2. The motivation program was given as an in-person session at the beginning of the intervention and was followed by weekly text messages sent by the study coordinator.
Memory Pack Plus cartons were provided to group A and B participants. Both groups were told to drink two cartons daily. Participants in the control group received dementia-prevention education from a guideline booklet at the beginning of the study, and general medical care was provided to them.
2.7. Adherence and adverse events
Adherence to cognitive training, exercise, vascular-risk-factor monitoring, and nutritional guidance was assessed using the cumulative attendance rate for the 8-week intervention. The adherence to intake of nutritional supplements was assessed by calculating the number of remaining supplement cartons.
Adherence (%) to nutrient supplements The study coordinator monitored the occurrence of adverse events.
2.8. Primary outcome
The primary outcome was a change in the total scale index score of the RBANS from baseline to after intervention and using a reference population of Korean adults to normalize the data (Randolph et al., 1998). We also evaluated five subdomain index scores: immediate memory, delayed memory, visuoconstruction, language and attention. Higher scores indicate better performance for all index scores.
2.9. Secondary outcomes
The secondary outcomes included global cognition, evaluated using K-MMSE-2 and CDR-SB. We also evaluated activities of daily living by the Korean Instrumental Activities of Daily Living scale (K-IADL) (Chin et al., 2018), depression by the 15-item Geriatric Depression Scale (GDS-15), and caregiver burden by the Zarit Burden Interview (ZBI). Physical performance was evaluated using the Short Physical Performance Battery (SPPB), grip power, and 30-s sit-to-stand test (endurance evaluation). Body composition was assessed using the body mass index and measurements of body fat, skeletal muscle mass, and visceral fat. The Nutrition Quotient for Elderly (NQ-E) was used to assess consumption of vegetables, fruits, beans, fish, milk, dairy products, eggs, water, fast food, pastries, and sweet food (Chung et al., 2018). Higher scores indicate better performance for K-MMSE-2, SPPB, and NQ-E. Lower scores indicate better performance for CDR-SB, K-IADL, GDS-15, and ZBI.
The secondary outcomes were modified from the first clinical trial enrollment. According to the results of the previous feasibility study of SUPERBRAIN (Moon et al., 2021), outcomes related to cognition, caregiver burden and physical performance were added. To assess nutrition, we replaced the mini-nutrition assessment that was a registered item in the initial trial registration with the NQ-E, which had shown a significant effect in the feasibility study. These changes were reported to the IRB, and further approval was obtained.
2.10. Exploratory outcomes
Fasting blood samples were collected from all participants in serum separation tubes and K2EDTA tubes. Total plasma cortisol and serum brain-derived neurotrophic factor (BDNF) were measured at baseline and after the interventions. BDNF was measured by Human Free BDNF enzyme-linked immunosorbent assay kit (R&D Systems, Minneapolis, MN, USA), and cortisol was measured using an ADVIA Centaur chemiluminescence immunoassay kit (Siemens Healthcare GmbH, Munich, Germany).
Stool collection kits containing buffer (CJ Bioscience Inc., Seoul, Korea) were provided to participants, and they were instructed on how to collect stools. Stool was collected 0–2 days before the baseline and final study visits. The kits were stored at room temperature and were shipped to CJ Bioscience Inc. for analysis within 1 week of the collection date.
We conducted 16S ribosomal RNA (rRNA) gene sequencing, taxonomic profiling, and functional profiling. The V3–V4 hypervariable region of the 16S rRNA gene was amplified with primers 341F and 805R using the direct polymerase chain reaction method. NEBNext Ultra II FS DNA Library Prep Kit for Illumina (New England Biolabs Inc., Ipswich, MA, USA) was used to construct DNA libraries. Sequencing of prepared DNA libraries by CJ Bioscience Inc. was conducted using the Illumina MiSeq platform (Illumina, San Diego, CA, USA) with 2 × 300 base pair kits.
The paired-end raw 16S rRNA sequence data were uploaded to the EzBioCloud and processed using a web-based EzBioCloud microbiome taxonomic profile tool.1 High-quality sequence reads were assigned to the “species group” at 97% sequence similarity using the PKSSU4.0 database.
Sex is a potential confounder of the microbiome outcome as it may influence both behavior that related to adherence to intervention components and the gut microbiome (Shobeiri et al., 2022). We confirmed that there were no significant differences in demographic characteristics, including sex, between the three experimental groups before conducting the microbiome analysis, thus controlling for the effect of sex.
Electroencephalogram data were obtained, but the data could not be read because of a technical problem. Therefore, analysis of electroencephalogram data was omitted from this study. This change was reported to the IRB, and further approval was obtained.
2.11. Statistical analyses
Statistical analyses were performed using a modified intention-to-treat approach. Baseline characteristics of each group were analyzed using analysis of variance (ANOVA) for continuous variables and chi-squared tests for categorical variables. To assess the effect of each intervention, outcomes were analyzed using a linear mixed-effect model. Comparisons between two groups were conducted using Bonferroni-adjusted post hoc analyses. Subgroup analyses for RBANS index scores were performed by disease stages (MCI and mild dementia) and sex. The analyses were performed using SPSS version 25.0 (IBM Corp., Armonk, NY, USA).
For gut microbiome analyses, the EzBioCloud Media Transfer Protocol server was used. The alpha diversity was calculated using the Chao1 and Shannon matrices, and its significance was assessed by Mann–Whitney U test. The generalized UniFrac metric was used for calculating the beta diversity, and the significance was assessed with permutational multivariate analysis of variance (PERMANOVA). Linear discriminant analysis effect size was performed to investigate the taxonomic differences among the groups. We also compared three experimental groups with an external healthy population (healthy control), and this microbiome analyses were performed using the Ez-Mx platform (CJ Bioscience Inc., Seoul, Korea) (Oh et al., 2022).
Statistical significance for each analysis was set at p < 0.05.
3. Results
3.1. Baseline characteristics of participants
Between October 12, 2020, and November 23, 2021, 76 patients were assessed for eligibility: 3 withdrew consent, and 24 failed screening due to negative amyloid PET results. We randomly assigned 49 participants with positive amyloid PET results and who were diagnosed with MCI or mild dementia to three groups: group A (n = 16), group B (n = 16), and the control group (n = 17). After randomization, three participants, one from each group, withdrew consent before the start of the interventions (Figure 1).
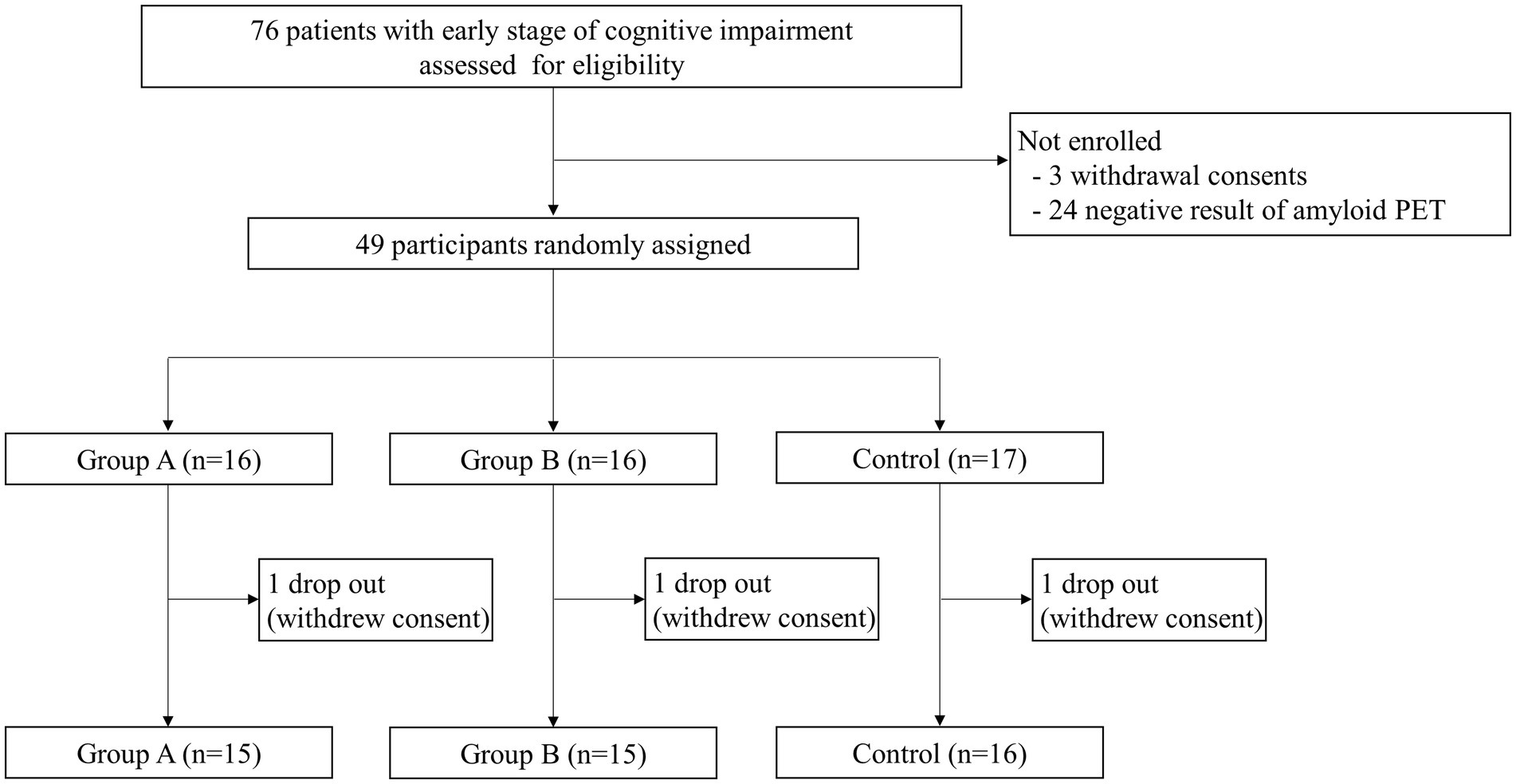
Figure 1. Study design. A total of 76 patients were screened for eligibility. Of those, 27 patients were not enrolled due to consent withdrawal or negative amyloid PET results. Thus, 49 participants with early-stage Alzheimer’s disease were randomly divided into three groups. Of these, three participants (one from each group) dropped out due to withdrawal of consent, and 46 participants completed the study.
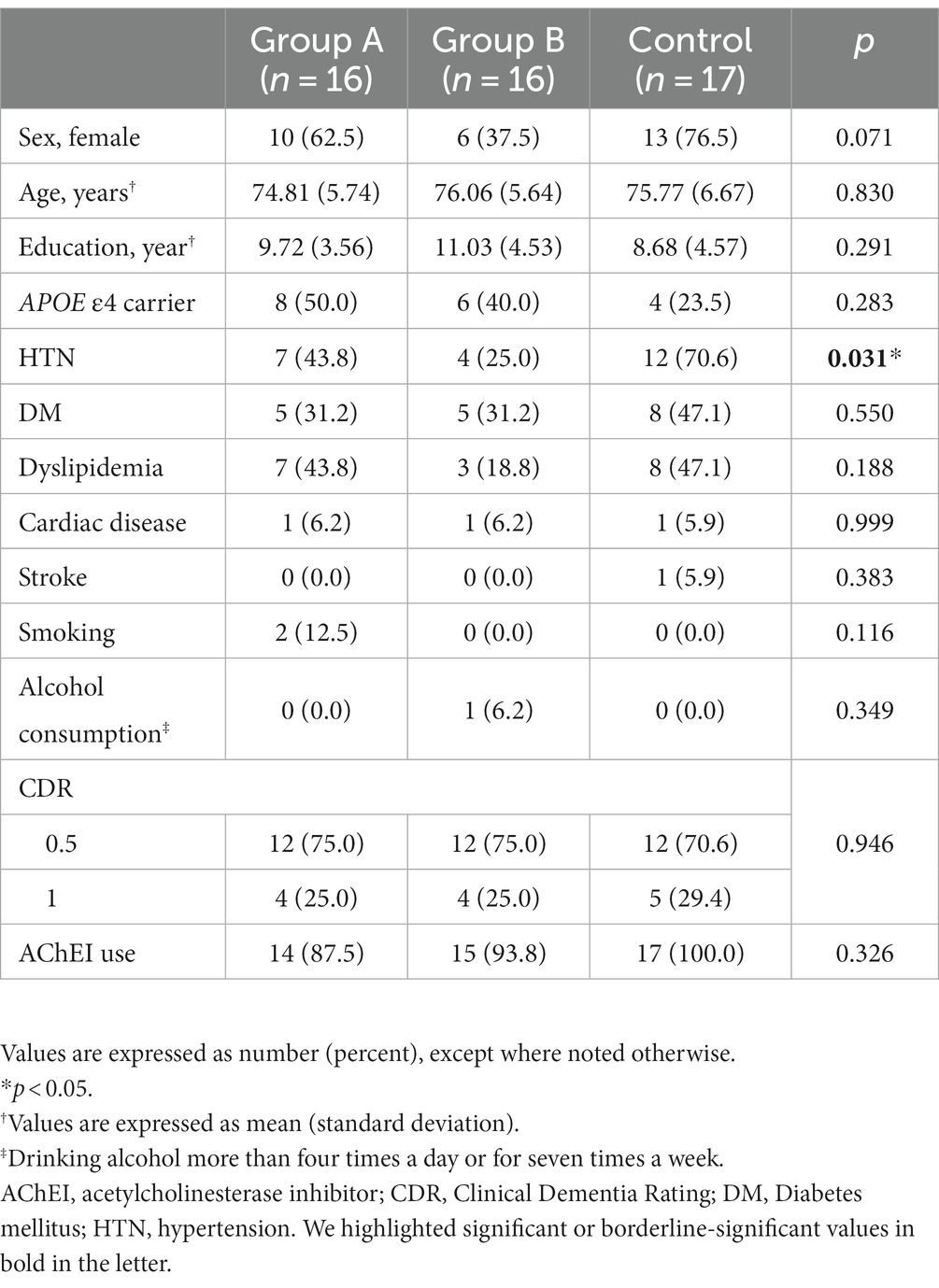
There were no significant differences in sex, age, education, and frequency of APOE ε4 carriers among the three groups. There were no significant differences among the three groups regarding the number of participants with diabetes mellitus, hyperlipidemia, or history of cardiac disease or stroke, but the number of participants with hypertension was significantly different among the three groups. The presence of hypertension was adjusted as a covariate in the analyses of primary and secondary outcomes. Cognitive function, physical performance, and nutritional status were similar among the three groups at baseline (Tables 1–3 and Supplementary material 3).
3.2. Adherence and adverse events
In group A, the adherence rates were 96.1% for the cognitive program, 94.0% for the exercise program, 100.0% for the vascular risk-factor monitoring, and 100.0% for the nutritional guidance. The adherence rate for the intake of nutritional supplements was 99.1% in group A and 83.7% in group B.
In group A, male participants showed adherence rates of 93.8% for the cognitive program, 87.5% for the exercise program, and 100.0% for supplement intake, while female participants demonstrated adherence rates of 99.5, 99.5, and 98.9%, respectively. In Group B, adherence rates for nutritional supplement intake were 83.3% for males and 84.8% for females.
No adverse event was reported during the entire intervention period.
3.3. Primary outcome
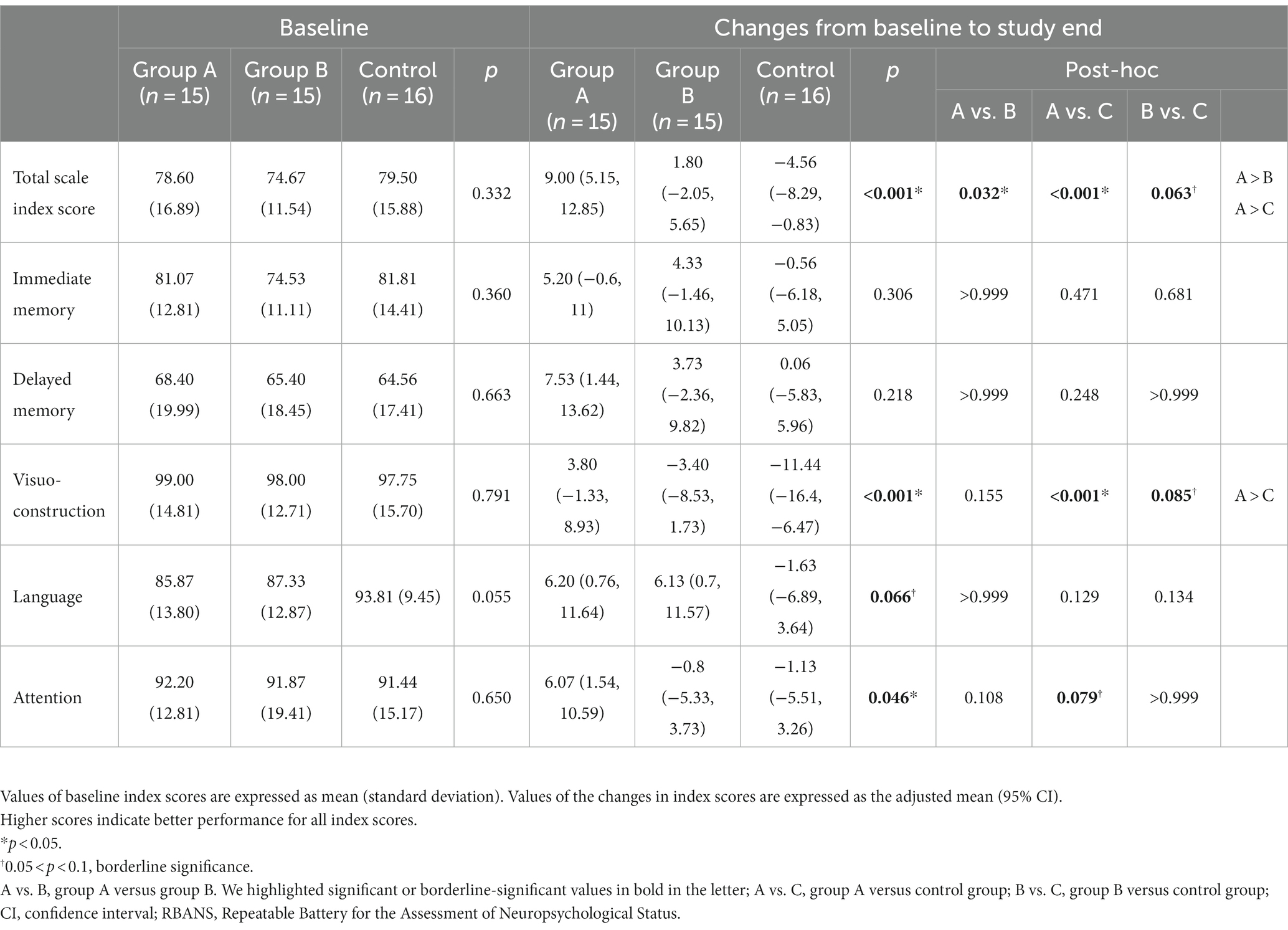
At baseline, there were no differences in the RBANS total scale index score and subdomain index scores among the three groups (Table 2). After intervention, the linear mixed-effect model revealed a significant difference in changes in RBANS total scale index score among the three groups. The adjusted means (95% confidence intervals) were as follows: group A, 9.00 (5.15, 12.85); group B, 1.80 (−2.05, 5.65); and control group, −4.56 (−8.29, 0.83); p < 0.001, effect size f2 = 0.530. Post hoc analysis revealed that the total scale index scores improved more for group A than for group B (p = 0.032) and more for group A than for the control group (p < 0.001). The visuoconstruction domain index score improved significantly different among the groups (p < 0.001), and post hoc analysis showed that the index score of group A improved more than that of the control group (p < 0.001, effect size f2 = 0.362). In the attention domain, the difference among the three groups was significant (p = 0.046), but a post hoc analysis found only a borderline significance in that group A improved more than the control group (p = 0.079) (Table 2).
3.4. Secondary outcomes
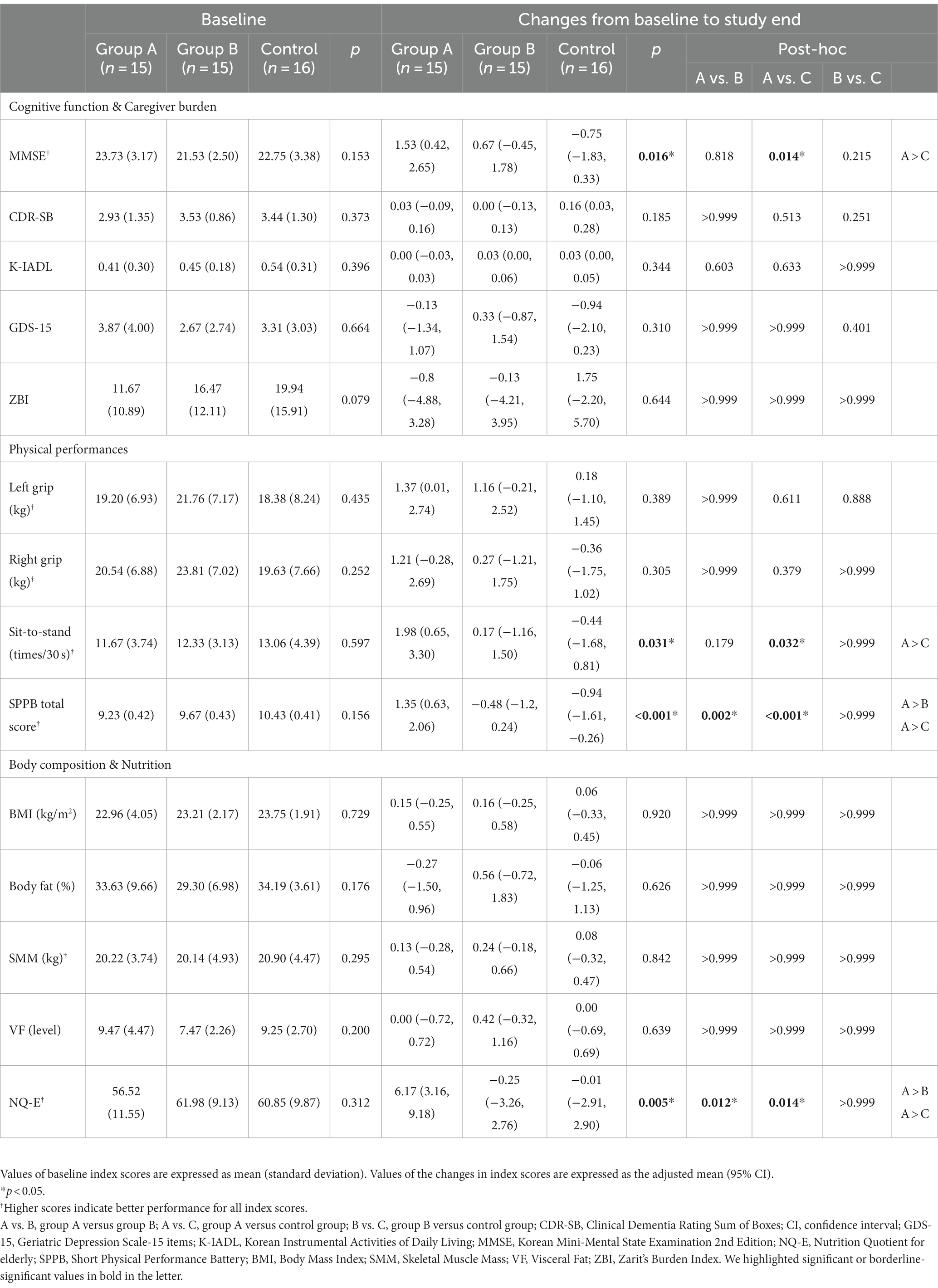
At baseline, there were no differences among the three groups for the K-MMSE-2, K-IADL, GDS-15, ZBI, physical performance, body composition, and nutrition score (Table 3).
After intervention, changes in K-MMSE-2 scores differed significantly among the three groups (p = 0.016) and the changes were the highest in treatment group A (p = 0.014) (Table 3). The change in the sit-to-stand time was significantly improved in group A compared with the control group (p = 0.032). The SPPB score improved significantly in group A compared with group B (p = 0.002) and in group A compared with the control group (p < 0.001) (Table 3).
3.5. Exploratory outcomes
At baseline and after intervention, there were no differences in plasma cortisol and serum BDNF concentrations among the three groups (Figures 2A,B). However, after intervention, serum BDNF concentrations increased in groups A and B but decreased in the control group; these differences were not statistically significant (p = 0.288) (Figure 2A). There were no differences in plasma cortisol concentration among the three groups after intervention (p = 0.787) (Figure 2B).
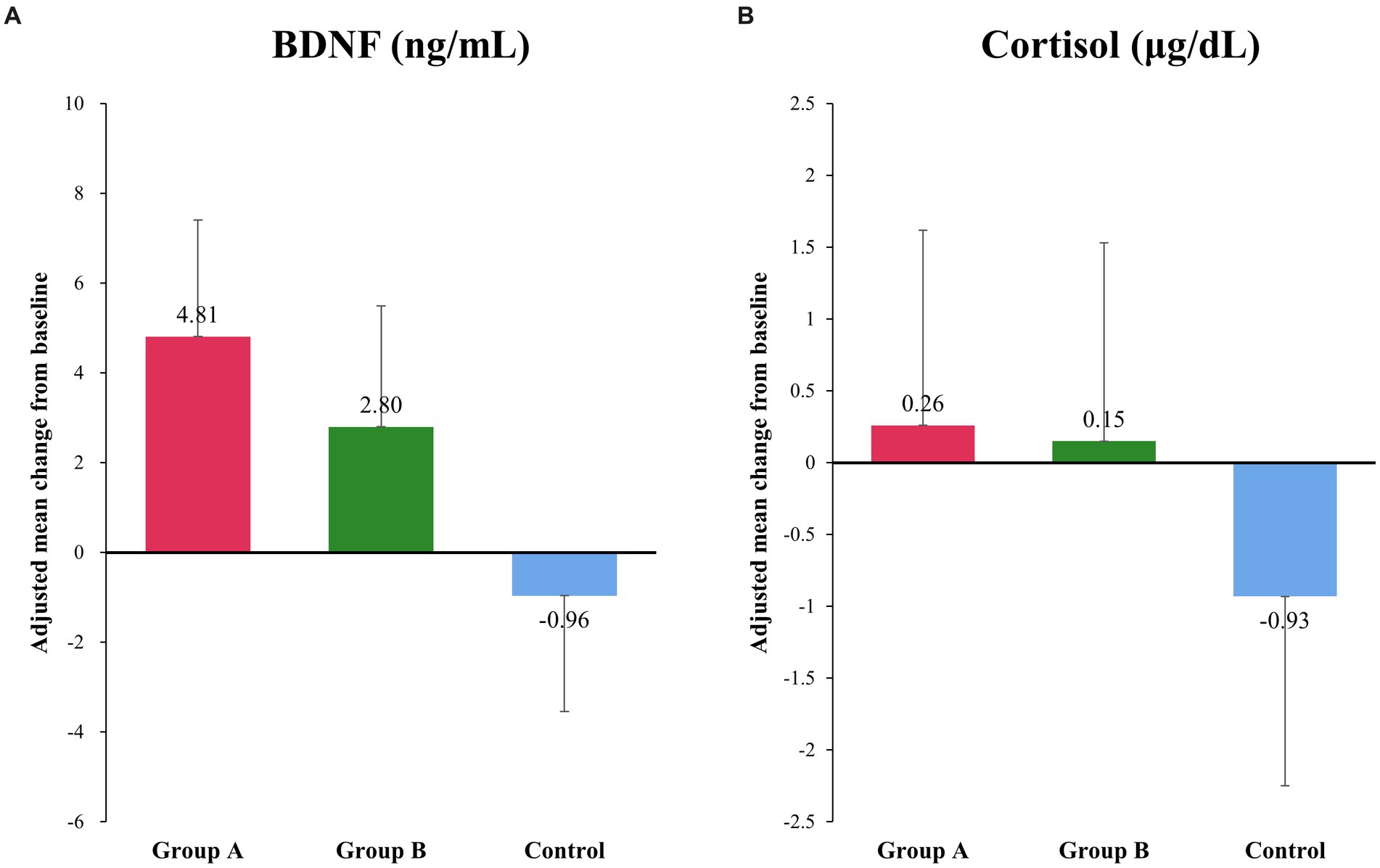
Figure 2. Changes in serum BDNF and plasma cortisol concentration. (A) There was an increase in the serum concentration of BDNF after interventions, whereas a decrease was observed in the control group, although the difference was not statistically significant. Adjusted mean (95% confidence interval) in μg/dL: group A, 4.81 (−0.44, 10.06); group B, 2.80 (−2.64, 8.23); and control group, −0.96 (−6.16, 4.24); p = 0.288. (B) There was no significant difference in the plasma cortisol level among different groups. Adjusted mean (95% confidence interval) in ng/mL: group A, 0.26 (−2.49, 3.02); group B, 0.15 (−2.67, 2.97); and control group, −0.93 (−3.6, 1.74); p = 0.787.
The gut microbiome data analysis showed that the changes in alpha diversity were not statistically different in the three groups (Figures 3A–C). PERMANOVA for beta diversity showed no differences among the three groups at baseline (p = 0.453) (Figure 3D). However, beta diversity after intervention was significantly different among the three groups (p = 0.033) and between group A and the control group (p = 0.013) (Figure 3E). Comparison of the linear discriminant analysis effect size between group A and the control group after intervention showed that group A was more enriched with Faecalibacterium and Bifidobacterium than was the control group (Figure 3F). After intervention, group B presented more enriched with Eubacterium and Clostridium than the control group (Figure 3G).

Figure 3. Gut microbiome analysis. There were no significant changes in alpha diversity in group A (A), group B (B), and the control group (C) before and after intervention. (D) The beta diversity at baseline showed no significant difference among the groups. (E) However, the beta diversity after intervention was significantly different between group A and the control group. (F) Linear discriminant analysis effect size analysis between group A and the control group showed that the genera Faecalibacterium and Bifidobacterium were more abundant in group A than in the control group after the intervention. (G) Group B were more enriched with Eubacterium and Clostridium than the control group, but the control group presented no characteristic genera. LDA, Linear discriminant analysis.
The gut microbiome data before and after the intervention in each experimental group were compared to that of healthy control (Supplementary material 4). After an 8-week intervention period, group A’s microbiota profile shifted toward that of the healthy control, while there were no significant changes observed in the microbiota profiles of group B and the control group (Supplementary materials 4A–E). UniFrac distances calculated between the healthy control group and each of the experimental groups also revealed that the distance was notably shorter in group A compared to the other groups (Supplementary material 4F). Moreover, Faecalibacterium was a genus that displayed a similar abundance between group A and the healthy control group but exhibited a significant difference in abundance when compared to the control group (Supplementary material 4G).
3.6. Subgroup analyses
Within MCI subgroup analyses, the results for RBANS total scale index scores and five subdomain index scores showed the similar pattern of significance as in the main analysis (Supplementary material 4). The linear mixed effects model showed a significant difference in changes in RBANS total scale index score among the three groups. The post hoc analysis showed that the total scale index scores improved more for group A than for group B (p = 0.003) and more for group A than for the control group (p < 0.001). The index score for the visual construction domain improved significantly in group A compared to the control group (p = 0.001). In the attention domain, the difference between group A and the control group was significant (p = 0.038). There was no significant group difference among mild dementia participants (Supplementary material 5).
Subgroup analysis for sex, showed a slightly different pattern in RBANS total scale and subdomain index scores. In the male group, changes in RBANS total scale index scores improved more for group A than for group B (p = 0.003) and more for group A than for the control group (p < 0.001), similar to the main analysis (Supplementary material 6). However, there was no significant group difference in subdomain index scores. In the female group, group A showed significant improvement in RBANS total scale index scores only compared to the control group, but not to group B (Supplementary material 7). Changes in visuoconstruction index scores of the female group were presented that group A improved significantly compared to the control group (p = 0.003). In addition, the attention index score showed a significant result that group A improved better than the control group (p = 0.023).
4. Discussion
To our knowledge, this is the first study to reveal the efficacy of a multidomain intervention with nutritional supplements on cognition and the gut microbiome in amyloid PET-proven early symptomatic AD patients. The RBANS total scale index scores improved significantly in patients with early symptomatic AD who received an 8-week multidomain intervention with nutritional supplements compared with patients who received nutritional supplements only or who received no intervention. Additionally, physical fitness was also significantly improved in the participants who received multidomain intervention with nutritional supplements.
Studies of multidomain interventions in patients with MCI have shown inconsistent results. The effect of multidomain interventions is difficult to decipher because of heterogeneity among the studies. Most trials that did not show the effectiveness of multidomain interventions combined only two main domains of interventions: exercise and cognitive training (Fiatarone Singh et al., 2014; Fogarty et al., 2016; Anderson-Hanley et al., 2018; Combourieu Donnezan et al., 2018; Shimada et al., 2018). In comparison, the multidomain intervention protocol of our study encompassed exercise, cognition, nutrition, and vascular risk-factor management, simultaneously providing a more comprehensive lifestyle modification to improve patient outcomes. The MAPT trial is similar to our study, as it also studied the effect of ready-to-eat nutritional supplements (omega-3 capsules) in addition to exercise and cognitive training. The subgroup analysis of patients with MCI presented positive effects; however, there was no cognitive improvement after their interventions (Andrieu et al., 2017). Thus, our study is the first trial that verifies the effectiveness of multidomain intervention with nutritional supplements on MCI and mild dementia, specifically in patients with proven Alzheimer’s pathology.
According to a previous study, among participants with mild to moderate dementia and MMSE scores ranging from 9 to 28, the RBANS total scale index score correlated well with the variety of activities of daily living (Freilich and Hyer, 2007). Therefore, significant changes in RBANS scores, particularly in group A, may signify improvements in practical functionality as perceived by patients and caregivers. However, there were no definite group differences in changes of CDR-SB or K-IADL in our study. As such, future investigations should aim to target deeper into these practical aspects to provide a more comprehensive understanding of the impact of the multidomain intervention on patients’ daily lives.
Group A showed favorable changes in attention subdomain index scores when compared to the control group. These findings align with the FINGER study, which reported enhancements in processing speed—a cognitive function closely associated with attention (Ngandu et al., 2015). This relationship is further supported by studies highlighting the effectiveness of computerized cognitive training in improving attention (Hill et al., 2017; Sherman et al., 2017), even in short-term interventions (Finn and McDonald, 2011; Hagovska and Olekszyova, 2016). Furthermore, our study underscores the positive impact of physical exercise on the attention domain (Yaguez et al., 2011). On the other hand, the best improvement of group A on visuoconstruction index score is consistent with the result of the SUPERBRAIN feasibility study that the facility-based multidomain intervention group improved on the visuoconstruction index score compared to the control group (Moon et al., 2021). However, this study showed an insignificant result in the delayed memory subdomain while the previous SUPERBRAIN study did. This difference could be explained by the different study design between both studies, and small sample size. The required sample size to evaluate the subdomains of the RBANS was found to be 13–64 participants based on the ANOVA model. Therefore, the interpretation of the subdomain index scores is limited, and a larger number of participants than in this study is required to assess significance with adequate power.
The difference between group B and the control group showed only borderline significance in our primary outcome. This is consistent with previous studies that revealed insignificant effect of providing nutritional supplements alone instead of combining with multidomain interventions (Shah et al., 2013; Soininen et al., 2017). Consequently, it is emphasized that significant effects can occur only when multidomain intervention is accompanied with nutritional supplements. However, since there was no experimental arm with the multidomain intervention alone in this study, it is not investigated whether the multidomain intervention with nutritional supplements is superior to that with the multidomain intervention alone. Therefore, further study is required to investigate the difference between the multidomain intervention with nutritional supplements and the multidomain intervention alone. Furthermore, because our studies were conducted in participants with early symptomatic AD, similar to participants in randomized controlled trials of newer disease-modifying therapies (Sims et al., 2023; van Dyck et al., 2023), future studies investigating the effects of combining multidomain interventions with disease-modifying therapy may further our understanding of AD treatment.
With cautious interpretation due to the small sample size for the RBANS subdomain index scores, this study showed borderline significance of superiority of group B over the control group for the visuoconstruction domain index score and total scale index score, while there was no difference in memory-function change between group B and the control group. In contrast, previous studies have shown that memory function improved more in patients who received nutritional supplements compared with those in control groups (Scheltens et al., 2010, 2012). This discrepancy with previous studies might be caused by the differences in the ingredients and proportions of different nutritional supplement products and the length of the intervention period.
The previous SUPERBRAIN trial revealed significant changes in biomarkers in the treatment group compared with the control group (Moon et al., 2021). However, in our study, the change in BDNF concentration among the three groups after intervention was not statistically significant. The reason might be that the number of exercise programs per week and the total number of sessions decreased from the previous study. Despite statistical insignificance, changes in BDNF concentration tended to increase in the treatment groups and decrease in the control group, which suggests a possible multidomain intervention mechanism for cognitive improvement.
Meanwhile, cortisol levels revealed no such tendency among the groups, because awakening and blood-sampling time were not strictly controlled on the examination days. Changes in the exercise protocol are also one of the reasons for the insignificant result for cortisol levels. Furthermore, some studies have suggested that physical activity, a nonpharmacological intervention, alters the dynamics of cortisol secretion rather than the cortisol concentration at a specific time point (Tortosa-Martinez et al., 2015); therefore, more sophisticated study designs and considerations are required to draw convincing conclusions from cortisol data.
The 8-week-long intervention did not change the richness and diversity scales of the gut microbiome within the groups, but it significantly changed beta diversity between group A and the control group. Moreover, microbiota of group A shifted toward that of the external healthy population. Although there is interspecies variability within a genus, Faecalibacterium were more abundant in group A than in the control group, and that was also the genera that showed statistically similar abundance to the external healthy population. Bifidobacterium was also more abundant in group A than in the control group. These are genera that are generally known to produce aminobutyric acid. The production of aminobutyric acid by some bacteria is a possible mechanism for brain-protective effects (Strandwitz, 2018). In studies in mice, exercise increased butyrate-producing bacteria (Abraham et al., 2019), and Bifidobacterium was depleted in mice fed high-fat diets (Nam et al., 2017; Sah et al., 2017; Sanguinetti et al., 2018). In a human study, Bifidobacterium was depleted in older adults (Gavini et al., 2001) and in patients with AD dementia compared with a control group (Vogt et al., 2017). These studies possibly explain why patients diagnosed with early symptomatic AD in group A had more butyrate-producing bacteria than the control group who received no intervention. Moreover, no difference in beta diversity between group B and the control group suggests that the microbiota change does not occur after simple nutrition supplementation alone. As group A showed differences in the microbiota compared with the control group, it is clear that comprehensive lifestyle modifications, including nutritional guidance, exercise, and cognitive training, are more critical for microbiota change than a simple nutritional supplement.
A low adherence rate is a common limitation of multidomain lifestyle modification studies. A recent study revealed a positive correlation between the adherence rate to the intervention and improvement in cognition (Lam et al., 2015). The improvement in outcomes might be caused by the high adherence rates in our study (>90% in all groups). There are three possible reasons for these high adherence rates. First, there was a strategy to enroll participants on the waiting list while they were motivated. All participants were educated together about dementia prevention without knowing which group they would be assigned to so that control group participants were equally motivated to participate in the program. Subsequently, the participants were informed of their assigned group, and the control group participants were enrolled on the waitlist for the same program. This maintained the motivation of the control group and gave them the expectation of receiving the multidomain intervention program at the end of the study. Second, because the program was delivered in a two-person group or as individual sessions, it was possible for trained therapists to provide a more appropriate level of training content for a participant than in large group sessions. In addition, caregivers attended the sessions and assisted the participants. Finally, a short intervention period was crucial for achieving a high adherence rate.
One limitation of our study is the relatively short intervention period compared to some other multidomain intervention trials, which have ranged from 8 weeks to 6 years (Fiatarone Singh et al., 2014; Ngandu et al., 2015; van Charante et al., 2016; Andrieu et al., 2017; McMaster et al., 2020). This shorter duration was influenced by ethical considerations, as we were mindful of the delay in treatment for participants in the control group who were waitlisted. Additionally, the ongoing COVID-19 pandemic posed challenges in terms of resource utilization, healthcare facility availability, and time constraints, which are common factors limiting the feasibility of conducting long-term studies. To address this limitation, it is essential to establish a long-term cohort study to evaluate the conversion rate from MCI to probable dementia compared with the general population.
The original SUPERBRAIN feasibility study demonstrated the effectiveness of a home-based multidomain intervention (Moon et al., 2021). However, implementing a home-based intervention in our study presented certain challenges. Notably, the cognitive stages of our study participants differed from those in the original SUPERBRAIN study, as our participants were diagnosed with MCI or mild dementia, whereas the original study included individuals with better cognitive scores. Training participants to use tablet applications for home-based interventions, as done in the original SUPERBRAIN study, was relatively straightforward. However, even in that study, it took 8 weeks to train participants to use the application independently, alongside weekly group sessions held at the facilities. Given the cognitive challenges faced by our specific population, implementing a home-based intervention within an 8-week timeframe was deemed unsuitable for achieving effective cognitive outcomes. Therefore, we chose a facility-based intervention to ensure a more structured and supervised cognitive training approach for both participants and caregivers. Consequently, while our multidomain intervention is fundamentally based on the FINGER and SUPERBRAIN protocols, we designed a new protocol tailored to our distinct target population.
Another limitation of our study is the small sample size. Several previous studies conducted in Asia have used the RBANS as the primary outcome measure, with sample sizes ranging from 48 to 98 participants per experimental arm (Cheng et al., 2012; Kita et al., 2019; Ng et al., 2018, 2021). Notably, only one study from China reported a significant intervention effect on RBANS (Cheng et al., 2012), while the majority did not. It is important to highlight that the intervention employed in the Chinese study significantly differed from ours, as it did not encompass a multidomain approach and targeted healthy elderly individuals, which is distinct from the early symptomatic AD participants in our study.
Due to the scarcity of multidomain intervention studies specifically targeting early symptomatic AD, we faced challenges in determining the required sample size a priori. We computed our sample size based on a study that demonstrated a significant intervention effect on RBANS, the primary outcome measure (Calapai et al., 2017). The Cohen’s f2 effect size for our primary outcome, the RBANS total scale index score, was calculated to be 0.53 (Selya et al., 2012), and its statistical power of 99.3%. Furthermore, our study adhered to the proposed criterion from a study assessing the RBANS anchor-based minimum clinically important difference (MCID) in Chinese subjects, where a difference of 8 points was considered meaningful (Phillips et al., 2015). The adjusted mean of group A met this criterion, and the proportion of the participants meeting this criterion within the group was 60.0%. This confirms the effect of the multidomain intervention with nutritional supplements. Therefore, the main results of our study are statistically reliable, despite the limitation of small sample size.
In the subgroup analysis of the MCI group, the results for the RBANS index scores showed a pattern of significance similar to that observed in the main analysis. This is because the number of MCI participants in each experimental arm is equal to or greater than eleven, which is an adequate sample size to detect an effect on the RBANS. Subgroup analysis within the mild dementia group revealed that there was no significant difference in changes in RBANS total scale index scores among the three groups. The interpretation of this result must be constrained by the critical influence of the very small sample size within the early stage of dementia groups (group A: n = 4, group B: n = 3, control: n = 5). In another subgroup analysis exploring the impact of sex, it was observed that Group A yielded superior results compared to group B within the male subgroup while no such distinction was evident within the female group. Nevertheless, the interpretation of this result remains constrained by the small size of the subgroups.
In this study, our multidomain intervention with nutritional supplements demonstrated notable improvements in cognition, physical performance, and the gut microbiome when compared to patients who received nutritional supplements alone or those who received no intervention. While patients who received nutritional supplements alone showed a trend toward enhanced cognition compared to those who received no intervention, the multidomain approach emerged as the most effective.
These findings offer compelling support for the use of multidomain interventions with nutritional supplements in patients with early symptomatic AD. However, to gain a more profound understanding of the intervention’s impact at specific disease stages and among different sexes, further research with larger sample sizes is warranted. Such endeavors will provide more definitive insights and contribute to advancing our knowledge of effective therapeutic strategies for this population.
Data availability statement
The 16S rRNA gene sequencing data presented in the study are deposited in the National Center for Biotechnology Information Sequence Read Archive, accession number PRJNA1025333.
Ethics statement
The studies involving humans were approved by Ewha Womans University, Seoul Hospital Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants, with the involvement of their legal guardians/next of kin.
Author contributions
EL: Data curation, Formal analysis, Writing – review & editing, Writing – original draft. GK: Data curation, Writing – review & editing. HP: Writing – review & editing, Conceptualization, Methodology. HK: Data curation, Writing – review & editing. YKP: Data curation, Methodology, Supervision, Writing – review & editing. HL: Formal analysis, Methodology, Writing – review & editing. CH: Methodology, Supervision, Writing – review & editing. SM: Methodology, Supervision, Writing – review & editing. SC: Conceptualization, Data curation, Formal analysis, Methodology, Supervision, Writing – review & editing. JJ: Conceptualization, Data curation, Formal analysis, Methodology, Supervision, Writing – review & editing. WK: Formal analysis, Visualization, Writing - review & editing. H-SO: Formal analysis, Visualization, Writing - review & editing. H-JY: Data curation, Formal analysis, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by grants from Daesang Life Science Corporation, the National Research Council of Science & Technology (NST) Aging Convergence Research Center (CRC22011-600), the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI) and Korea Dementia Research Center (KDRC), funded by the Ministry of Health & Welfare and Ministry of Science and ICT, Republic of Korea (HU20C0271 and HU21C0016), and the Original Technology Research Program for Brain Science through the National Research Foundation of Korea (NRF) (NRF-2018M3C7A1057137 and NRF-2020M3E5D2A01084721) and the Institute of Information & communications Technology Planning & Evaluation (IITP) (No. 2022-0-00448) funded by the Ministry of Science and ICT, Republic of Korea. These funding sources were not involved in the study design; in the collection, analysis, and interpretation of data; in the writing of the manuscript; and in the decision to submit the article for publication.
Acknowledgments
The authors thank all participants and their family for their dedicated participation during the COVID-19 pandemic.
Conflict of interest
SM receives a research grant from Hyundai Pharmaceutical Co., Ltd. CH receives research support from Eisai Korea Inc. JJ receives research grants from Chong Kun Dang Pharmaceutical Corp., and consults for PeopleBio Co., Ltd. SM, CH, JJ, YKP, and SC are shareholders of Rowan Inc. SC consults for Hyundai Pharmaceutical Co., Ltd., and PeopleBio Co. Ltd. WK and H-SO were employed by CJ Bioscience Inc.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2023.1266955/full#supplementary-material
Footnotes
References
Abraham, D., Feher, J., Scuderi, G. L., Szabo, D., Dobolyi, A., Cservenak, M., et al. (2019). Exercise and probiotics attenuate the development of Alzheimer’s disease in transgenic mice: role of microbiome. Exp. Gerontol. 115, 122–131. doi: 10.1016/j.exger.2018.12.005
Albert, M. S., DeKosky, S. T., Dickson, D., Dubois, B., Feldman, H. H., Fox, N. C., et al. (2011). The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 7, 270–279. doi: 10.1016/j.jalz.2011.03.008
Alzheimer’s Association (2022, 2022). 2022 Alzheimer’s disease facts and figures. Alzheimers Dement. 18, 700–789. doi: 10.1002/alz.12638
Anderson-Hanley, C., Barcelos, N. M., Zimmerman, E. A., Gillen, R. W., Dunnam, M., Cohen, B. D., et al. (2018). The aerobic and cognitive exercise study (ACES) for community-dwelling older adults with or at-risk for mild cognitive impairment (MCI): neuropsychological, neurobiological and neuroimaging outcomes of a randomized clinical trial. Front. Aging Neurosci. 10:76. doi: 10.3389/fnagi.2018.00076
Andrieu, S., Coley, N., Lovestone, S., Aisen, P. S., and Vellas, B. (2015). Prevention of sporadic Alzheimer’s disease: lessons learned from clinical trials and future directions. Lancet Neurol. 14, 926–944. doi: 10.1016/S1474-4422(15)00153-2
Andrieu, S., Guyonnet, S., Coley, N., Cantet, C., Bonnefoy, M., Bordes, S., et al. (2017). Effect of long-term omega 3 polyunsaturated fatty acid supplementation with or without multidomain intervention on cognitive function in elderly adults with memory complaints (MAPT): a randomised, placebo-controlled trial. Lancet Neurol. 16, 377–389. doi: 10.1016/S1474-4422(17)30040-6
Baek, M. J., Kim, K., Park, Y. H., and Kim, S. (2016). The validity and reliability of the mini-mental state examination-2 for detecting mild cognitive impairment and Alzheimer’s disease in a Korean population. PLoS One 11:e0163792. doi: 10.1371/journal.pone.0163792
Calapai, G., Bonina, F., Bonina, A., Rizza, L., Mannucci, C., Arcoraci, V., et al. (2017). A randomized, double-blinded, clinical trial on effects of a Vitis vinifera extract on cognitive function in healthy older adults. Front. Pharmacol. 8:776. doi: 10.3389/fphar.2017.00776
Cheng, Y., Wu, W., Feng, W., Wang, J., Chen, Y., Shen, Y., et al. (2012). The effects of multi-domain versus single-domain cognitive training in non-demented older people: a randomized controlled trial. BMC Med, 10, 30. doi: 10.1186/1741-7015-10-30
Chin, J., Park, J., Yang, S. J., Yeom, J., Ahn, Y., Baek, M. J., et al. (2018). Re-standardization of the Korean-instrumental activities of daily living (K-IADL): clinical usefulness for various neurodegenerative diseases. Dement. Neurocogn. Disord. 17, 11–22. doi: 10.12779/dnd.2018.17.1.11
Choi, S. H., Lee, B. H., Kim, S., Hahm, D. S., Jeong, J. H., Yoon, S. J., et al. (2003). Interchanging scores between clinical dementia rating scale and global deterioration scale. Alzheimer Dis. Assoc. Disord. 17, 98–105. doi: 10.1097/00002093-200304000-00008
Chung, M.-J., Kwak, T.-K., Kim, H.-Y., Kang, M.-H., Lee, J.-S., Chung, H. R., et al. (2018). Development of NQ-E, nutrition quotient for Korean elderly: item selection and validation of factor structure. J. Nutr. Health 51:87. doi: 10.4163/jnh.2018.51.1.87
Coley, N., Andrieu, S., Gardette, V., Gillette-Guyonnet, S., Sanz, C., Vellas, B., et al. (2008). Dementia prevention: methodological explanations for inconsistent results. Epidemiol. Rev. 30, 35–66. doi: 10.1093/epirev/mxn010
Combourieu Donnezan, L., Perrot, A., Belleville, S., Bloch, F., and Kemoun, G. (2018). Effects of simultaneous aerobic and cognitive training on executive functions, cardiovascular fitness and functional abilities in older adults with mild cognitive impairment. Ment. Health Phys. Act. 15, 78–87. doi: 10.1016/j.mhpa.2018.06.001
Delrieu, J., Payoux, P., Carrie, I., Cantet, C., Weiner, M., Vellas, B., et al. (2019). Multidomain intervention and/or omega-3 in nondemented elderly subjects according to amyloid status. Alzheimers Dement. 15, 1392–1401. doi: 10.1016/j.jalz.2019.07.008
de Souto, B. P., Demougeot, L., Vellas, B., and Rolland, Y. (2018). Exercise training for preventing dementia, mild cognitive impairment, and clinically meaningful cognitive decline: a systematic review and meta-analysis. J. Gerontol. A Biol. Sci. Med. Sci. 73, 1504–1511. doi: 10.1093/gerona/glx234
Ding, J., Davis-Plourde, K. L., Sedaghat, S., Tully, P. J., Wang, W., Phillips, C., et al. (2020). Antihypertensive medications and risk for incident dementia and Alzheimer's disease: a meta-analysis of individual participant data from prospective cohort studies. Lancet Neurol. 19, 61–70. doi: 10.1016/S1474-4422(19)30393-X
Dixon, J. R. Jr. (1998). The international conference on harmonization good clinical practice guideline. Qual. Assur. 6, 65–74. doi: 10.1080/105294199277860
Fiatarone Singh, M. A., Gates, N., Saigal, N., Wilson, G. C., Meiklejohn, J., Brodaty, H., et al. (2014). The study of mental and resistance training (SMART) study-resistance training and/or cognitive training in mild cognitive impairment: a randomized, double-blind, double-sham controlled trial. J. Am. Med. Dir. Assoc. 15, 873–880. doi: 10.1016/j.jamda.2014.09.010
Finn, M., and McDonald, S. (2011). Computerised cognitive training for older persons with mild cognitive impairment: a pilot study using a randomised controlled trial design. Brain Impairment. 12, 187–199. doi: 10.1375/brim.12.3.187
Fogarty, J. N., Murphy, K. J., McFarlane, B., Montero-Odasso, M., Wells, J., Troyer, A. K., et al. (2016). Taoist tai chi® and memory intervention for individuals with mild cognitive impairment. J. Aging Phys. Act. 24, 169–180. doi: 10.1123/japa.2014-0062
Freilich, B. M., and Hyer, L. A. (2007). Relation of the repeatable battery for assessment of neuropsychological status to measures of daily functioning in dementia. Psychol. Rep. 101, 119–129. doi: 10.2466/pr0.101.1.119-129
Gavini, F., Cayuela, C., Antoine, J.-M., Lecoq, C., Lefebvre, B., Membré, J.-M., et al. (2001). Differences in the distribution of Bifidobacterial and Enterobacterial species in human faecal microflora of three different (children, adults, elderly) age groups. Microb. Ecol. Health Dis. 13, 40–45. doi: 10.1080/089106001750071690
Hagovska, M., and Olekszyova, Z. (2016). Impact of the combination of cognitive and balance training on gait, fear and risk of falling and quality of life in seniors with mild cognitive impairment. Geriatr. Gerontol. Int. 16, 1043–1050. doi: 10.1111/ggi.12593
Han, C., Jo, S. A., Jo, I., Kim, E., Park, M. H., and Kang, Y. (2008). An adaptation of the Korean mini-mental state examination (K-MMSE) in elderly Koreans: demographic influence and population-based norms (the AGE study). Arch. Gerontol. Geriatr. 47, 302–310. doi: 10.1016/j.archger.2007.08.012
Hill, N. T., Mowszowski, L., Naismith, S. L., Chadwick, V. L., Valenzuela, M., and Lampit, A. (2017). Computerized cognitive training in older adults with mild cognitive impairment or dementia: a systematic review and meta-analysis. Am. J. Psychiatry 174, 329–340. doi: 10.1176/appi.ajp.2016.16030360
Hung, S. Y., and Fu, W. M. (2017). Drug candidates in clinical trials for Alzheimer’s disease. J. Biomed. Sci. 24:47. doi: 10.1186/s12929-017-0355-7
Kita, M., Kobayashi, K., Obara, K., Koikeda, T., Umeda, S., and Ano, Y. (2019). Supplementation With Whey Peptide Rich in beta-Lactolin Improves Cognitive Performance in Healthy Older Adults: A Randomized, Double-Blind, Placebo-Controlled Study. Front Neurosci, 13, 399. doi: 10.3389/fnins.2019.00399
Lam, L. C., Chan, W. C., Leung, T., Fung, A. W., and Leung, E. M. (2015). Would older adults with mild cognitive impairment adhere to and benefit from a structured lifestyle activity intervention to enhance cognition?: a cluster randomized controlled trial. PLoS One 10:e0118173. doi: 10.1371/journal.pone.0118173
Livingston, G., Huntley, J., Sommerlad, A., Ames, D., Ballard, C., Banerjee, S., et al. (2020). Dementia prevention, intervention, and care: 2020 report of the lancet commission. Lancet 396, 413–446. doi: 10.1016/S0140-6736(20)30367-6
Livingston, G., Sommerlad, A., Orgeta, V., Costafreda, S. G., Huntley, J., Ames, D., et al. (2017). Dementia prevention, intervention, and care. Lancet 390, 2673–2734. doi: 10.1016/S0140-6736(17)31363-6
McKhann, G. M., Knopman, D. S., Chertkow, H., Hyman, B. T., Jack, C. R. Jr., Kawas, C. H., et al. (2011). The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 7, 263–269. doi: 10.1016/j.jalz.2011.03.005
McMaster, M., Kim, S., Clare, L., Torres, S. J., Cherbuin, N., D'Este, C., et al. (2020). Lifestyle risk factors and cognitive outcomes from the multidomain dementia risk reduction randomized controlled trial, body brain life for cognitive decline (BBL-CD). J. Am. Geriatr. Soc. 68, 2629–2637. doi: 10.1111/jgs.16762
Moon, S. Y., Hong, C. H., Jeong, J. H., Park, Y. K., Na, H. R., Song, H. S., et al. (2021). Facility-based and home-based multidomain interventions including cognitive training, exercise, diet, vascular risk management, and motivation for older adults: a randomized controlled feasibility trial. Aging 13, 15898–15916. doi: 10.18632/aging.203213
Moon, S. Y., Kim, S., Choi, S. H., Hong, C. H., Park, Y. K., Na, H. R., et al. (2022). Impact of multidomain lifestyle intervention on cerebral cortical thickness and serum brain-derived neurotrophic factor: the SUPERBRAIN exploratory sub-study. Neurotherapeutics 19, 1514–1525. doi: 10.1007/s13311-022-01276-x
Nam, K. N., Mounier, A., Wolfe, C. M., Fitz, N. F., Carter, A. Y., Castranio, E. L., et al. (2017). Effect of high fat diet on phenotype, brain transcriptome and lipidome in Alzheimer’s model mice. Sci. Rep. 7:4307. doi: 10.1038/s41598-017-04412-2
Ngandu, T., Lehtisalo, J., Solomon, A., Levälahti, E., Ahtiluoto, S., Antikainen, R., et al. (2015). A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet 385, 2255–2263. doi: 10.1016/S0140-6736(15)60461-5
Ng, T. P., Ling, L. H. A., Feng, L., Nyunt, M. S. Z., Feng, L., Niti, M., et al. (2018). Cognitive effects of multi-domain interventions among pre-frail and frail community-living older persons: randomized controlled trial. J Gerontol A Biol Sci Med Sci, 73, 806–812. doi: 10.1093/gerona/glx207
Ng, P. E. M., Nicholas, S. O., Wee, S. L., Yau, T. Y., Chan, A., Chng, I., et al. (2021). Implementation and effectiveness of a multi-domain program for older adults at risk of cognitive impairment at neighborhood senior centres. Sci Rep, 11, 3787. doi: 10.1038/s41598-021-83408-5
Oh, H.-S., Min, U., Jang, H., Kim, N., Lim, J., Chalita, M., et al. (2022). Proposal of a health gut microbiome index based on a meta-analysis of Korean and global population datasets. J. Microbiol. 60, 533–549. doi: 10.1007/s12275-022-1526-0
Park, H. K., Jeong, J. H., Moon, S. Y., Park, Y. K., Hong, C. H., Na, H. R., et al. (2020). South Korean study to prevent cognitive impairment and protect brain health through lifestyle intervention in at-risk elderly people: protocol of a multicenter, randomized controlled feasibility trial. J. Clin. Neurol. 16, 292–303. doi: 10.3988/jcn.2020.16.2.292
Phillips, R., Qi, G., Collinson, S. L., Ling, A., Feng, L., Cheung, Y. B., et al. (2015). The minimum clinically important difference in the repeatable battery for the assessment of neuropsychological status. Clin. Neuropsychol. 29, 905–923. doi: 10.1080/13854046.2015.1107137
Radd-Vagenas, S., Duffy, S. L., Naismith, S. L., Brew, B. J., Flood, V. M., and Fiatarone Singh, M. A. (2018). Effect of the Mediterranean diet on cognition and brain morphology and function: a systematic review of randomized controlled trials. Am. J. Clin. Nutr. 107, 389–404. doi: 10.1093/ajcn/nqx070
Randolph, C., Tierney, M. C., Mohr, E., and Chase, T. N. (1998). The repeatable battery for the assessment of neuropsychological status (RBANS): preliminary clinical validity. J. Clin. Exp. Neuropsychol. 20, 310–319. doi: 10.1076/jcen.20.3.310.823
Sah, S. K., Lee, C., Jang, J. H., and Park, G. H. (2017). Effect of high-fat diet on cognitive impairment in triple-transgenic mice model of Alzheimer’s disease. Biochem. Biophys. Res. Commun. 493, 731–736. doi: 10.1016/j.bbrc.2017.08.122
Sanguinetti, E., Collado, M. C., Marrachelli, V. G., Monleon, D., Selma-Royo, M., Pardo-Tendero, M. M., et al. (2018). Microbiome-metabolome signatures in mice genetically prone to develop dementia, fed a normal or fatty diet. Sci. Rep. 8:4907. doi: 10.1038/s41598-018-23261-1
Scarmeas, N., Luchsinger, J. A., Schupf, N., Brickman, A. M., Cosentino, S., Tang, M. X., et al. (2009). Physical activity, diet, and risk of Alzheimer disease. JAMA 302, 627–637. doi: 10.1001/jama.2009.1144
Scheltens, P., Kamphuis, P. J., Verhey, F. R., Olde Rikkert, M. G., Wurtman, R. J., Wilkinson, D., et al. (2010). Efficacy of a medical food in mild Alzheimer’s disease: a randomized, controlled trial. Alzheimers Dement. 6, 1–10.e1. doi: 10.1016/j.jalz.2009.10.003
Scheltens, P., Twisk, J. W., Blesa, R., Scarpini, E., von Arnim, C. A., Bongers, A., et al. (2012). Efficacy of Souvenaid in mild Alzheimer’s disease: results from a randomized, controlled trial. J. Alzheimers Dis. 31, 225–236. doi: 10.3233/JAD-2012-121189
Selya, A. S., Rose, J. S., Dierker, L. C., Hedeker, D., and Mermelstein, R. J. (2012). A practical guide to calculating Cohen’s f(2), a measure of local effect size, from PROC MIXED. Front. Psychol. 3:111. doi: 10.3389/fpsyg.2012.00111
Shah, R. C., Kamphuis, P. J., Leurgans, S., Swinkels, S. H., Sadowsky, C. H., Bongers, A., et al. (2013). The S-connect study: results from a randomized, controlled trial of Souvenaid in mild-to-moderate Alzheimer’s disease. Alzheimers Res. Ther. 5:59. doi: 10.1186/alzrt224
Sherman, D. S., Mauser, J., Nuno, M., and Sherzai, D. (2017). The efficacy of cognitive intervention in mild cognitive impairment (MCI): a meta-analysis of outcomes on neuropsychological measures. Neuropsychol. Rev. 27, 440–484. doi: 10.1007/s11065-017-9363-3
Shimada, H., Makizako, H., Doi, T., Park, H., Tsutsumimoto, K., Verghese, J., et al. (2018). Effects of combined physical and cognitive exercises on cognition and mobility in patients with mild cognitive impairment: a randomized clinical trial. J. Am. Med. Dir. Assoc. 19, 584–591. doi: 10.1016/j.jamda.2017.09.019
Shobeiri, P., Kalantari, A., Teixeira, A. L., and Rezaei, N. (2022). Shedding light on biological sex differences and microbiota-gut-brain axis: a comprehensive review of its roles in neuropsychiatric disorders. Biol. Sex Differ. 13:12. doi: 10.1186/s13293-022-00422-6
Sims, J. R., Zimmer, J. A., Evans, C. D., Lu, M., Ardayfio, P., Sparks, J., et al. (2023). Donanemab in early symptomatic Alzheimer disease: the TRAILBLAZER-ALZ 2 randomized clinical trial. JAMA 330, 512–527. doi: 10.1001/jama.2023.13239
Soininen, H., Solomon, A., Visser, P. J., Hendrix, S. B., Blennow, K., Kivipelto, M., et al. (2017). 24-month intervention with a specific multinutrient in people with prodromal Alzheimer’s disease (LipiDiDiet): a randomised, double-blind, controlled trial. Lancet Neurol. 16, 965–975. doi: 10.1016/S1474-4422(17)30332-0
Solomon, A., Stephen, R., Altomare, D., Carrera, E., Frisoni, G. B., Kulmala, J., et al. (2021). Multidomain interventions: state-of-the-art and future directions for protocols to implement precision dementia risk reduction. A user manual for brain health services-part 4 of 6. Alzheimers Res. Ther. 13:171. doi: 10.1186/s13195-021-00875-8
Solomon, A., Turunen, H., Ngandu, T., Peltonen, M., Levälahti, E., Helisalmi, S., et al. (2018). Effect of the apolipoprotein E genotype on cognitive change during a multidomain lifestyle intervention: a subgroup analysis of a randomized clinical trial. JAMA Neurol. 75, 462–470. doi: 10.1001/jamaneurol.2017.4365
Strandwitz, P. (2018). Neurotransmitter modulation by the gut microbiota. Brain Res. 2018, 128–133. doi: 10.1016/j.brainres.2018.03.015
Tortosa-Martinez, J., Clow, A., Caus-Pertegaz, N., Gonzalez-Caballero, G., Abellan-Miralles, I., and Saenz, M. J. (2015). Exercise increases the dynamics of diurnal cortisol secretion and executive function in people with amnestic mild cognitive impairment. J. Aging Phys. Act. 23, 550–558. doi: 10.1123/japa.2014-0006
van Charante, E. P. M., Richard, E., Eurelings, L. S., van Dalen, J.-W., Ligthart, S. A., van Bussel, E. F., et al. (2016). Effectiveness of a 6-year multidomain vascular care intervention to prevent dementia (preDIVA): a cluster-randomised controlled trial. Lancet 388, 797–805. doi: 10.1016/S0140-6736(16)30950-3
van Dyck, C. H., Swanson, C. J., Aisen, P., Bateman, R. J., Chen, C., Gee, M., et al. (2023). Lecanemab in early Alzheimer’s disease. N. Engl. J. Med. 388, 9–21. doi: 10.1056/NEJMoa2212948
Vogt, N. M., Kerby, R. L., Dill-McFarland, K. A., Harding, S. J., Merluzzi, A. P., Johnson, S. C., et al. (2017). Gut microbiome alterations in Alzheimer’s disease. Sci. Rep. 7:13537. doi: 10.1038/s41598-017-13601-y
World Health Organization. Dementia: a public health priority. Geneva: World Health Organization; (2012).
World Health Organization. Global action plan on the public health response to dementia 2017–2025. Geneva: World Health Organization; (2017).
World Health Organization. Risk reduction of cognitive decline and dementia: WHO guidelines. Geneva: World Health Organization; (2019).
Keywords: Alzheimer’s disease, dementia, mild cognitive impairment, prevention, multidomain intervention, nutritional supplements, gut microbiome
Citation: Lee EH, Kim GH, Park HK, Kang HJ, Park YK, Lee HA, Hong CH, Moon SY, Kang W, Oh H-S, Yoon H-J, Choi SH and Jeong JH (2023) Effects of the multidomain intervention with nutritional supplements on cognition and gut microbiome in early symptomatic Alzheimer’s disease: a randomized controlled trial. Front. Aging Neurosci. 15:1266955. doi: 10.3389/fnagi.2023.1266955
Edited by:
Guillermo Felipe López Sánchez, University of Murcia, SpainReviewed by:
Chinedu Udeh-Momoh, Imperial College London, United KingdomMin Chen, Icahn School of Medicine at Mount Sinai, United States
Copyright © 2023 Lee, Kim, Park, Kang, Park, Lee, Hong, Moon, Kang, Oh, Yoon, Choi and Jeong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Seong Hye Choi, c2VvbmdoeWVAaW5oYS5hYy5rcg==; Jee Hyang Jeong, amplb25nQGV3aGEuYWMua3I=
 Eun Hye Lee
Eun Hye Lee Geon Ha Kim
Geon Ha Kim Hee Kyung Park
Hee Kyung Park Hae Jin Kang
Hae Jin Kang Yoo Kyoung Park
Yoo Kyoung Park Hye Ah Lee
Hye Ah Lee Chang Hyung Hong7
Chang Hyung Hong7 So Young Moon
So Young Moon Seong Hye Choi
Seong Hye Choi Jee Hyang Jeong
Jee Hyang Jeong