- 1Department of Neurology, Qingdao Municipal Hospital, Qingdao University, Qingdao, China
- 2Department of Neurology, Qingdao Municipal Hospital, Nanjing Medical University, Nanjing, China
Background: The relationship between the low-density lipoprotein/high-density lipoprotein (LDL/HDL) ratio, an indicator of lipid metabolism and assessment of cardiovascular disease (CVD) risk, and Alzheimer’s disease (AD) is unclear.
Methods: Multivariable Cox regression analyses were used to examine the association of LDL/HDL ratio and the risk of AD. Multiple linear regression and mixed effects models used to assess associations between LDL/HDL and cognitive function, AD pathology, and brain structure. Mediation analyses examined AD pathology’s potential mediating role between the LDL/HDL ratio and cognition.
Results: Higher LDL/HDL ratio correlated with lower AD risk (HR 0.644 [0.431, 0.962]). In the linear regression analyses, the LDL/HDL ratio were positively associated with cognition. Longitudinally, the LDL/HDL ratio also positively with cognitive measures. Besides, higher LDL/HDL ratio were associated Aβ42 and decreased Tau, pTau, Tau/Aβ42, pTau/Aβ42, and pTau/Tau. The LDL/HDL ratio was positively associated with brain structures such as hippocampal volume. Mediation analyses revealed AD pathology mediated the association between LDL/HDL ratio and cognition.
Conclusion: The LDL/HDL ratio is associated with AD risk, cognition, AD biomarkers and brain structure and can affect cognition by AD biomarkers.
Introduction
Alzheimer’s disease (AD) is a neurodegenerative condition marked by gradual cognitive deterioration and hindered daily living capabilities, imposing a tremendous burden on individuals and society (Xu et al., 2015; Yang et al., 2013). Recent studies have shown that abnormalities in lipid metabolism are closely associated with the onset and progression of AD (de Bruijn and Ikram, 2014). Reduced levels of high-density lipoprotein (HDL) and increased levels of low-density lipoprotein (LDL) are frequently observed abnormalities in lipid metabolism, and they are closely associated with vascular dysfunction, inflammatory responses, and oxidative stress, which may contribute to the development of AD.
The National Cholesterol Education Program outlines target LDL and HDL cholesterol levels for assessing heart disease risk (Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults, 2001) The LDL/HDL ratio, a key indicator of lipid balance, reflects both atherogenic and cardioprotective lipid fractions, making it an effective metric for evaluating overall cardiovascular health and predicting cardiovascular events (Arsenault et al., 2011; Fernandez and Webb, 2008). Elevated LDL/HDL ratios have been strongly associated with coronary heart disease risk, atherosclerosis, and other cardiovascular conditions (Schnabel et al., 2010). This underscores the ratio’s utility in assessing not only heart disease risk but also potential implications for cerebral health.
Moreover, increasing evidence suggests that cardiovascular disease (CVD) is an important risk factor for AD (Saeed et al., 2023). Vascular pathology may accelerate the progression of AD by reducing cerebral perfusion, disrupting the integrity of the blood–brain barrier (BBB), and promoting the deposition of amyloid-β and tau proteins (Stakos et al., 2020). Therefore, evaluating lipid-related cardiovascular risk factors may play a significant role in the prediction and prevention of AD.
Given the robust predictive value of the LDL/HDL ratio for cardiovascular conditions and the established link between cardiovascular health and cognitive function, it is imperative to examine the potential association between the LDL/HDL ratio and Alzheimer’s disease. Despite known correlations, little research has specifically addressed how the LDL/HDL ratio relates to cognitive function, AD biomarkers, and brain structure. This study aims to fill this gap by exploring the relationships between the LDL/HDL ratio, AD risk, biomarkers, cognitive measures, and brain structure, and to elucidate the mechanisms by which lipid metabolism might influence cognitive functions, thereby providing insights into the interconnected pathways of lipid metabolism, cardiovascular health, and neurodegeneration.
Methods
Participants
A total of 616 non-dementia adults were gathered from the Alzheimer’s Disease Neuroimaging Initiative (ADNI). These participants, ranging in age from 55 to 90 years, provided us with comprehensive data from the ADNI study, including essential clinical characteristics, biochemical biomarkers of AD, imaging data, and cognitive assessment data. Participants with missing LDL, HDL, ethnicity, marriage, education, APOEε4 carrier status, cognitive diagnosis, systolic, diastolic, total cholesterol (TC), diabetes and CVD data and dementia were excluded. More information is available at http://adni.loni.usc.edu (Petersen et al., 2010).
In addition, we examined data from the CABLE study from 1,105 adults without dementia. The CABLE experiment is detailed in the Supplementary file. Patients with missing LDL, HDL, cerebrospinal fluid (CSF) biomarkers, cognitive measures, and covariate data were excluded from the study, in addition to patients with dementia. In the CABLE database, in addition to the previous exclusion criteria, those with >15% variability in CSF batch were also excluded from the study.
Laboratory parameters
The ADNI database provided all laboratory and anthropometric parameters and medical history data. LDL and HDL levels were measured. APOEε4 genotyping was conducted at the ADNI Biomarker Core Laboratory, University of Pennsylvania, to identify participants with at least one APOEε4 allele, designating them the APOEε4 positive status (Shaw et al., 2009). Since the majority of the included population lacked a diagnosis of diabetes, we used fasting blood glucose (FDG) levels instead of missing components, and an FDG ≥ 7.0 mmol/L was considered to have diabetes (American Diabetes Association, 2021).
CSF AD biomarkers
Cerebrospinal fluid samples were collected and placed in 10-mL polypropylene tubes using a lumbar puncture. Within 2 h, these samples were transported to the laboratory. Subsequently, the CSF samples underwent centrifugation at 2,000 g for 10 min. Samples not detected in time for thawing and freezing procedures did not exceed two cycles prior to analysis (Shaw et al., 2009; Sheng et al., 2025). The INNO-BIA AlzBio3 immunoassay (Innogenetics-Fujirebio, Ghent, Belgium) was used to measure CSF Aβ42, Tau, and phosphorylated Tau (pTau181) concentrations (pg/mL).
The procedures for collecting CSF have previously been documented in CABLE. To quantify CSF levels of Aβ40, Aβ42, Tau, and pTau, an enzyme-linked immunosorbent assay kit (Innotest; Fujirebio, Ghent, Belgium) was used on a microplate reader (Multiskan MK3; Thermo Fisher Scientific, Waltham, MA) (Zhang et al., 2024).
Incorporating Tau/Aβ42 and pTau/Aβ42 ratios is crucial, given their enhanced predictive value for brain Aβ deposition and cognitive decline compared to their individual expression (Racine et al., 2016; Snider et al., 2009).
Definition of incident AD and cognitive measures
Alzheimer’s disease was diagnosed in patients who met the criteria for probable AD established by the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association (McKhann et al., 1984). The ADNI used a series of scales to assess cognitive functions, encompassing assessments of global cognition using the Mini-Mental State Examination (MMSE) and the cognitive domain of the Alzheimer’s Disease Assessment Scale (ADAS), in addition to specific cognitive domains such as episodic memory (MEM), executive function (EF), language (LAN), and visual–spatial functions (VS) (Fletcher et al., 2021). Higher ADAS scores and lower MMSE, MEM, EF, LAN, and VS scores indicate poorer cognitive functioning.
Neuroimaging
Brain structural magnetic resonance imaging (MRI) data were acquired using either a Siemens Trio 3.0-T or a Vision 1.5-T imaging system. The image analysis software Freesurfer (versions 4.3 and 5.1) was utilized for processing to estimate regional brain volumes from the MRI images (Jack et al., 2008; Zhang et al., 2024). Our analyses included measurements of whole brain volume hippocampus, middle temporal, and entorhinal cortex.
Statistical analyses
For continuous variables, mean (standard deviation) or median (interquartile range) were used to represent normal or non-normal distributions, respectively. Analysis of Variance or the Kruskal–Wallis test was employed for analysis accordingly. Categorical variables were depicted as numbers (n) and percentages (%), with chi-square tests employed for assessment. Outliers exceeding three standard deviations were eliminated from the statistical analysis involving the LDL/HDL ratio. Relationships between the LDL/HDL ratio and AD biomarkers, cognition, and brain structure were explored via multiple linear regression. Subgroup analyses were conducted based on age, sex, ethnicity, APOEε4 carrier status, cognitive diagnosis, diabetes, CVD and hypercholesterolemia. Multicollinearity was assessed, and all variance inflation factors (VIFs) were below 2, indicating no significant collinearity among covariates (Supplementary Table 1). In addition, we used data from the CABLE cohort (currently limited to cross-sectional data, with longitudinal follow-up ongoing) to confirm the associations between the LDL/HDL ratio and AD biomarkers and cognitive function observed in the ADNI dataset.
To explore the relationship between the LDL/HDL ratio and the risk of AD, we used the Cox proportional risk model to estimate the hazard ratio (HR) and 95% confidence interval (CI) for AD. Restricted cubic spline (RCS) regression analyses were employed to examine the relationships between LDL/HDL ratio and AD risk. Linear mixed-effects models were then employed to delineate the longitudinal relationships among the LDL/HDL ratio, AD pathology, cognition, and brain structure.
Mediation analyses, following Baron and Kenny’s approach, were conducted to ascertain whether AD pathology mediated the association of the LDL/HDL ratio with cognition (Baron and Kenny, 1986). In addition, the magnitude of attenuation or indirect effects was estimated and significance was ascertained via 10,000 self-directed iterations.
In all analyses, adjustments were made for age, sex, ethnicity, marriage, education, APOEε4 carrier status, cognitive diagnosis, systolic, diastolic, total cholesterol, diabetes and cardiovascular disease as covariates. Given that all outcome variables were standardized to z-scores in the model, the coefficient represents the standardized effect. Statistical analyses were performed using R version 4.2.0, with statistical significance set at p < 0.05 for all analyses.
Results
Participants’ characteristics
In the ADNI database, 616 participants were included, with a mean age of 73.20 ± 7.10 years. Among them, 43.3% were female, and 66.9% had a history of CVD. Our analysis revealed that the mean LDL/HDL ratio was 1.27 ± 0.35 in the group with normal cognition, which was lower compared to those with MCI (1.36 ± 0.38) (Table 1).
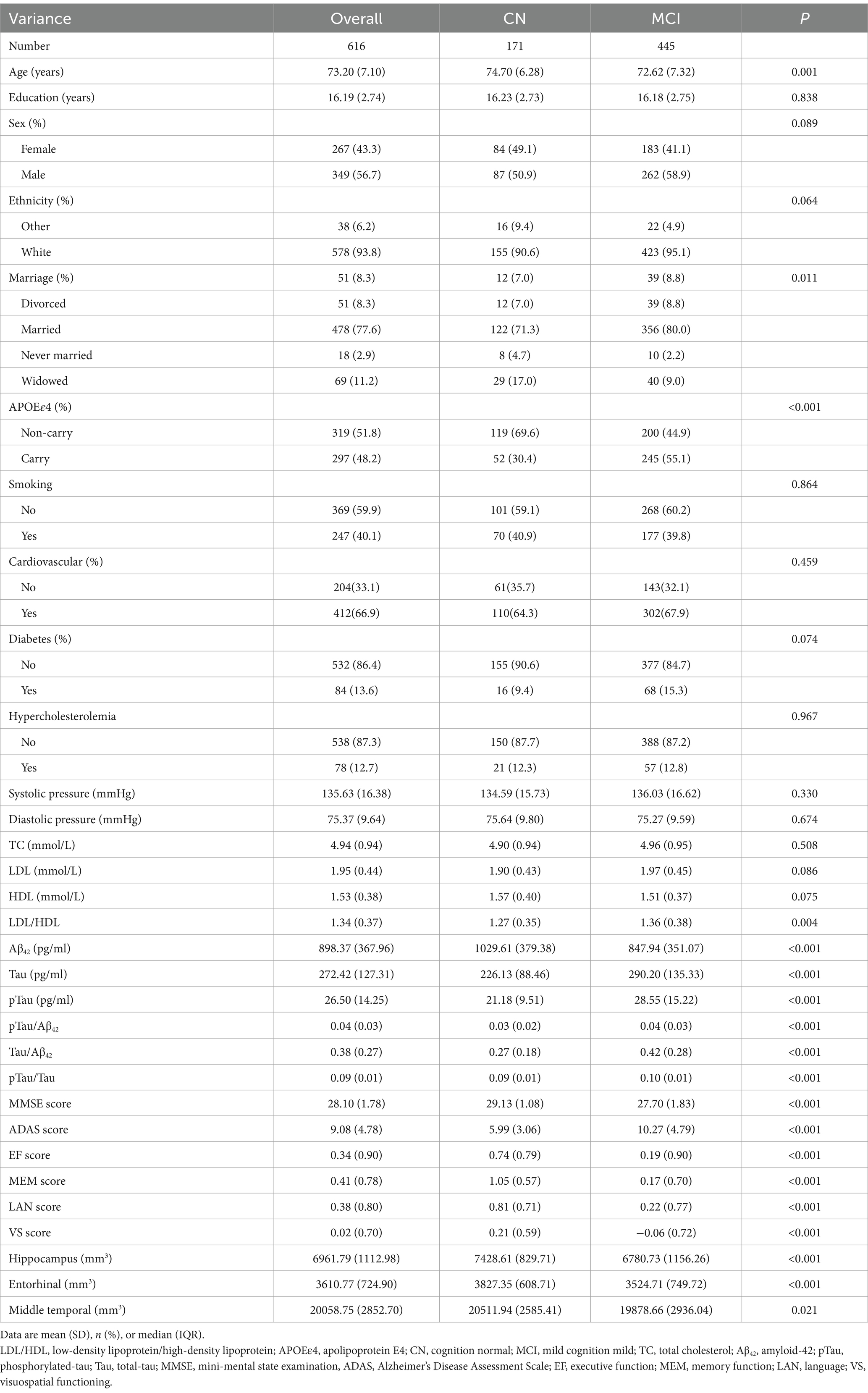
Table 1. Characteristics based on the LDL/HDL ratio of 616 participants obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI).
In the CABLE database, 1,105 individuals were included, with a mean age of 62.96 ± 10.06 years. Among them, 42.7% were female, and 44.3% had a history of CVD (Supplementary Table 2).
LDL/HDL ratio and AD risk
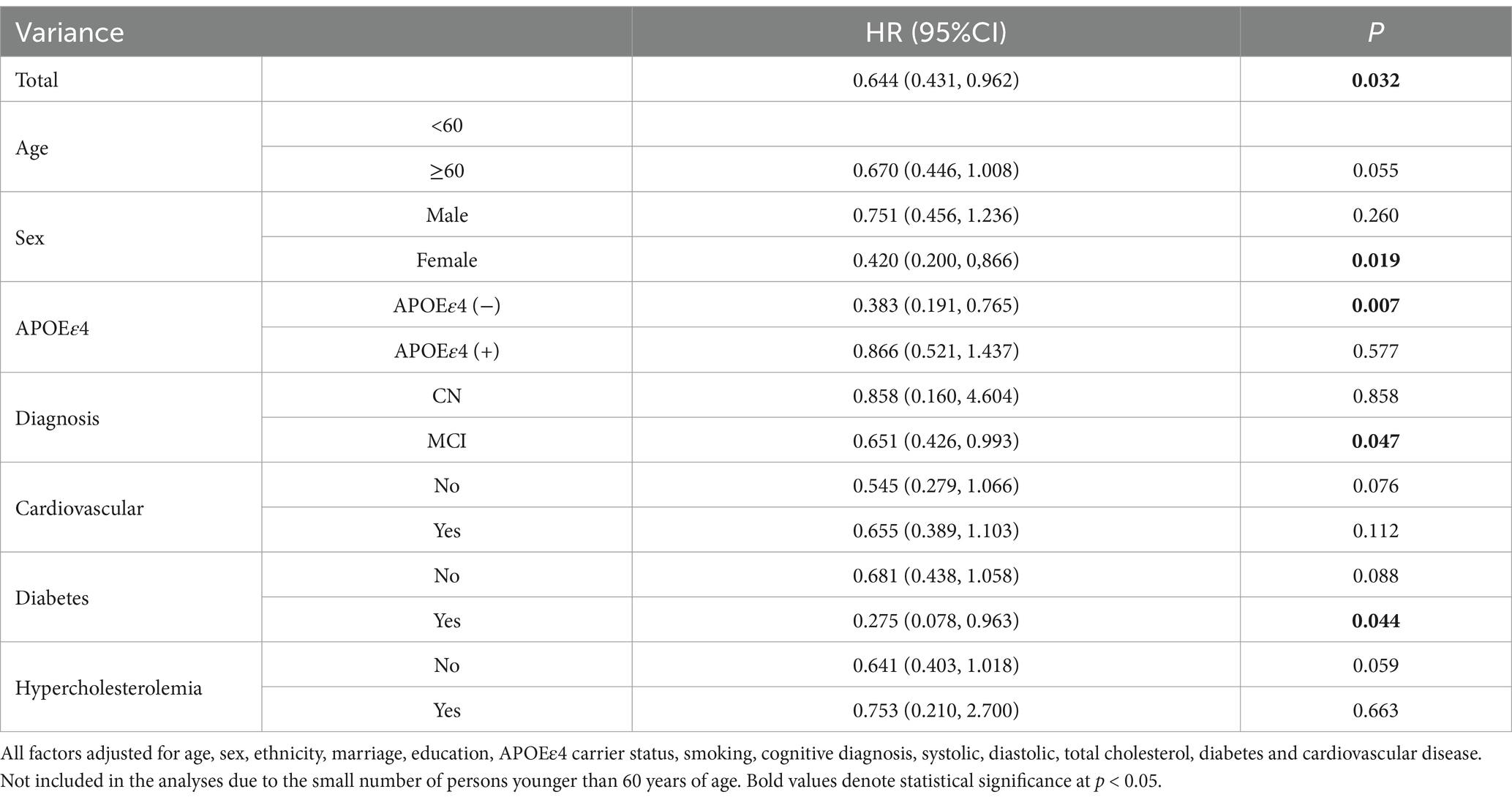
During a follow-up period of up to 16 years, we analyzed patient groups with different LDL/HDL ratio using Cox proportional risk model. In the adjusted risk model, we observed that for each unit increase in the LDL/HDL ratio, the risk of developing AD decreased by 35.6% (HR 0.644, 95% CI [0.431, 0.962]). Furthermore, in women, individuals who did not carry the APOEε4 gene, MCI participate and diabetes, we observed a decreased risk of AD by 58.0% (HR 0.420, 95% CI [0.200, 0.866]), 61.7% (HR 0.383, 95% CI [0.191, 0.765]), 34.9% (HR 0.651, 95% CI [0.426, 0.993]) and 72.5% (HR 0.275, 95% CI [0.078, 0.963]) respectively, with increasing LDL/HDL ratio (Table 2). RCS results did not find a non-linear relationship between LDL/HDL ratio and risk of AD (Supplementary Figure 1).
Association of LDL/HDL ratio with cognitive measures
In the ADNI database, the LDL/HDL ratio showed positive correlations with MMSE (β = 0.080, p = 0.048), EF (β = 0.118, p = 0.004), MEM (β = 0.101, p = 0.006), and LAN (β = 0.086, p = 0.034), while exhibiting negative correlations with ADAS (β = −0.120, p = 0.002) (Figure 1 and Supplementary Table 3). Subgroup analyses revealed significant associations between LDL/HDL ratio and cognitive function, in older adults aged over 60, females, participants carrying the APOEε4 gene, individuals with MCI, and participants without diabetes. In additional, we investigated longitudinal associations between LDL/HDL ratio and cognitive function, we found that the LDL/HDL ratio was positively associated with MMSE scores (β = 0.026, p = 0.047), MEM scores (β = 0.015, p = 0.033), LAN scores (β = 0.017, p = 0.048) and negatively associated with ADAS scores (β = −0.020, p = 0.050). These findings suggest potential links between LDL/HDL ratio and changes in cognitive function overtime (Supplementary Table 4).
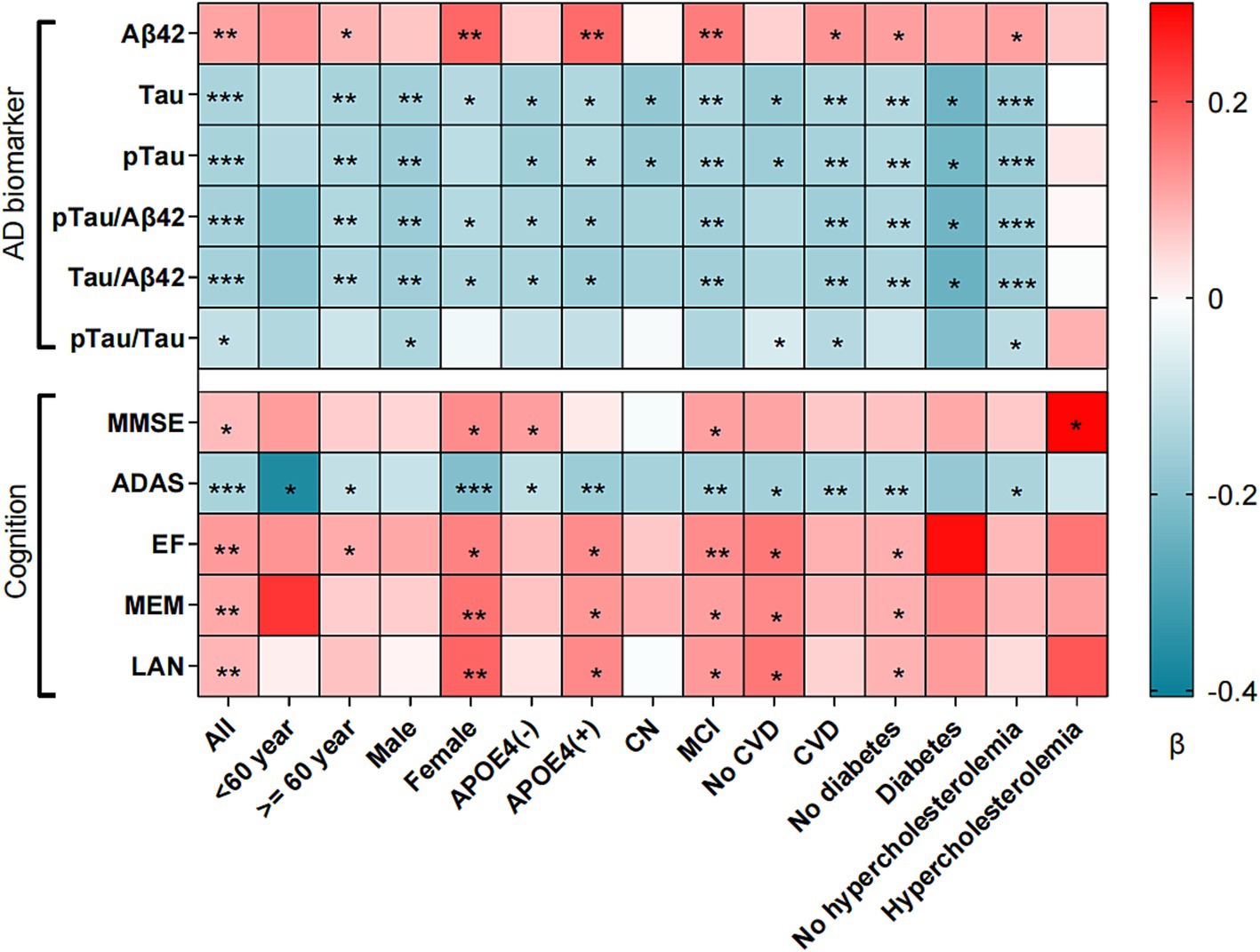
Figure 1. Association between LDL/HDL ratio and cognition, AD biomarker and brain structure. LDL/HDL, low-density lipoprotein/high-density lipoprotein; AD, Alzheimer’s disease; Aβ42, Amyloid-42; pTau, phosphorylated-tau; Tau, total-tau; MMSE, mini-mental state examination, ADAS, Alzheimer’s Disease Assessment Scale; EF, executive function; MEM, memory function; LAN, language; VS, visuospatial functioning; APOEε4, apolipoprotein E4; CN, cognition normal; MCI, mild cognition mild; CVD, cardiovascular disease. *p < 0.05, **p < 0.01, ***p < 0.001. All factors adjusted for age, sex, ethnicity, marriage, education, APOEε4 carrier status, smoking, cognitive diagnosis, systolic, diastolic, total cholesterol, diabetes and cardiovascular disease.
Association of LDL/HDL ratio with CSF AD biomarkers
In the ADNI database, higher LDL/HDL ratios are associated with higher levels of Aβ42 (β = 0.108, p = 0.009). Conversely, with increasing LDL/HDL ratio, levels of Tau (β = −0.140, p < 0.001), pTau (β = −0.141, p < 0.001), Tau/Aβ42 (β = −0.147, p < 0.001), pTau/Aβ42 (β = −0.145, p < 0.001), and pTau/Tau (β = −0.101, p = 0.018) gradually decreased (Figure 2 and Supplementary Table 5).
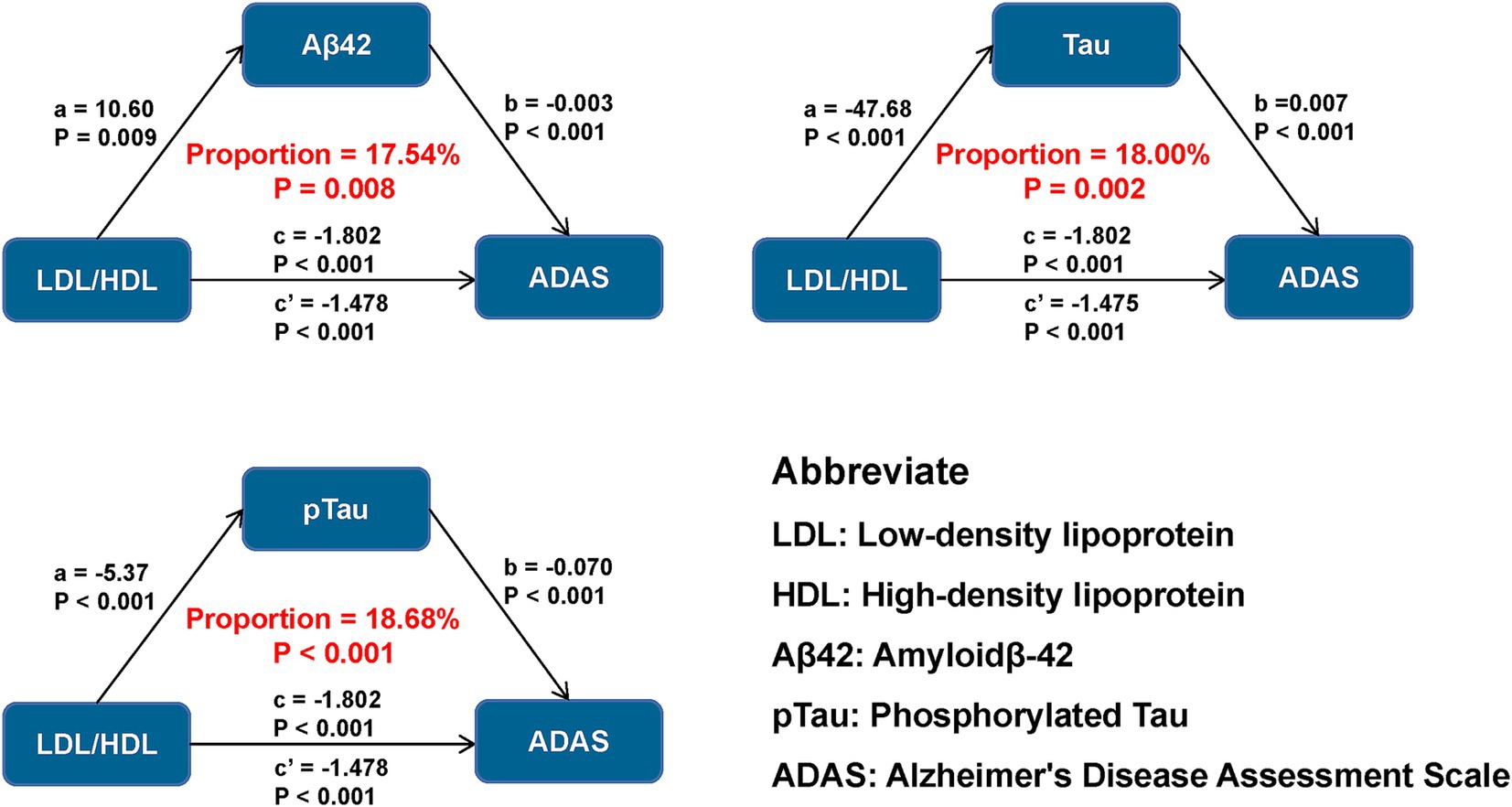
Figure 2. Mediation analyses of LDL/HDL ratio and baseline cognition with AD biomarkers as mediators. LDL/HDL, low-density lipoprotein/high-density lipoprotein; AD, Alzheimer’s disease; Aβ42, amyloid-42; pTau, phosphorylated-tau; Tau, total-tau; MMSE, mini-mental state examination, ADAS, Alzheimer’s Disease Assessment Scale; EF, executive function; MEM, memory function; LAN, language; VS, visuospatial functioning.
In the CABLE database, the LDL/HDL ratio was also negatively correlated with the levels of Tau (β = −0.064, p = 0.046) protein and pTau (β = −0.085, p = 0.008) protein (Supplementary Table 6). Similarly, in the CABLE database, we observed a negative association between the LDL/HDL ratio and the levels of Tau protein and pTau protein in older adults aged over 60, women, participants carrying the APOEε4 gene, participants without diabetes, and participants without hypercholesterolemia, which is consistent with the findings from the ADNI (Supplementary Table 6).
Association of LDL/HDL ratio with brain structure
Analysis of brain images showed that LDL/HDL was positively correlated with hippocampus (β = 0.102, p = 0.027), entorhinal cortex (β = 0.093, p = 0.041), and middle temporal volume (β = 0.104, p = 0.021). Subgroup analyses showed that LDL/HDL was also positively correlated with whole brain volume in those with MCI and diabetes mellitus (Supplementary Table 7).
Causal mediation analyses
We examined whether the association between LDL/HDL ratio and cognitive measures was mediated by AD pathology. Our results suggest that the LDL/HDL ratio may exert direct effects on cognitive measures, as well as influence global cognition and specific cognitive domains by modulating Aβ42 levels, explaining between 17.54 and 27.19% of the total effect (Figure 2). Furthermore, the LDL/HDL ratio was associated with influence in cognitive function by decreasing Tau and pTau levels, as well as the ratios of pTau/Aβ42, Tau/Aβ42, and pTau/Tau, which accounted for 18.00–35.50%, 18.68–36.45%, 25.40–43.92%, 26.80–46.09%, and 11.54–19.05% of the total effect, respectively. Notably, the impact of LDL/HDL ratio on ADAS11 through AD pathology was relatively small compared to other cognitive measures analyzed, but had the greatest effect on MEM (Supplementary Table 8).
Discussion
This study used found that LDL/HDL ratio was positively associated with CSF Aβ42 levels and inversely associated with Tau and pTau levels, and found the same trend in another database. Besides, LDL/HDL ratio was associated with cognitive performance. As for brain imaging, the LDL/HDL ratio was positively correlated with hippocampal, entorhinal cortex and middle temporal volume. There was a positive association between the LDL/HDL ratio and cognitive level over time, and the LDL/HDL ratio could also influence changes in cognitive level by affecting the three AD pathologic biomarkers and their ratios.
Research indicates that the relationship between CVD risk and cognitive function may differ across racial groups, potentially due to physiological, genetic, lifestyle, and environmental factors (Bailey et al., 2021). Notably, while the ADNI cohort predominantly consists of White participants and the CABLE cohort primarily includes Asians, our findings show similar trends in the association between LDL/HDL ratio and AD biomarkers (such as Tau and pTau) across both cohorts. This suggests that despite the ethnic differences, the underlying mechanisms linking lipid levels to AD pathology may be consistent.
Previous studies have been more inclined to consider the relationship between lipids and risk of AD development, cognitive measures, and brain imaging, and have paid less attention to direct associations between lipids and CSF biomarkers (Turri et al., 1867; Yu et al., 2023; Andrews et al., 2021). Our study bridges this gap. A study of a cognitively normal population reported no association between lipids and AD biomarkers (Jansson et al., 2022), which is inconsistent with our findings, and in addition to differences in the populations selected, the small sample size of this study may have been important in failing to find an association between lipids and AD (Peters et al., 2021).
Some studies suggest that higher LDL levels are associated with better memory function in older populations. For example, a study from Japan found that elevated LDL levels were linked to improved memory performance in elderly individuals (Katsumata et al., 2013). Similarly, a cohort study from China reported an inverse association between high LDL levels and dementia risk (Zhou et al., 2018). These findings align with the results of our study, which demonstrated a positive correlation between the LDL/HDL ratio and cognitive function. We hypothesize that the selective permeability of the BBB to different types of cholesterol may play a crucial role in this relationship (Poliakova and Wellington, 2023). HDL can cross the BBB through specific transport mechanisms, such as the SR-BI receptor, where it reduces Aβ deposition and suppresses inflammation, exerting neuroprotective effects. In contrast, LDL has limited ability to cross the BBB under normal physiological conditions, restricting its direct impact on the central nervous system (Rhea and Banks, 2021). This mechanism may explain why elevated peripheral LDL levels do not necessarily result in cognitive decline and may even be associated with better cognitive performance in certain elderly populations. Additionally, some studies suggest that higher LDL levels in older adults may reflect better nutritional status or overall health, whereas lower cholesterol levels could be linked to brain atrophy and poorer outcomes (Zhou et al., 2018; Pfrieger, 2003). Age-related differences may also contribute to these findings. A meta-analysis indicated that while high LDL levels may be a potential risk factor for AD, this association diminishes or disappears in populations over 70 years of age (Zhou et al., 2020). Similar to cognition, HDL is a protective factor for brain structure. HDL is positively correlated with volumes of the bilateral temporal pole, middle temporal gyrus and para-hippocampal regions (Ward et al., 2010); lower levels of HDL are associated with smaller hippocampal volumes (Wolf et al., 2004) LDL, on the other hand, is significantly correlated with decreased volume of total white matter and a decrease in the hippocampus (Wang et al., 2021).
Our mediation analysis suggests that the LDL/HDL ratio indirectly impacts cognitive function through its effects on CSF biomarkers such as Aβ42, Tau, and pTau. This modulation affects global cognition and specific cognitive domains, including executive function, memory, and language. Specifically, higher LDL/HDL ratios were associated with better performance in these cognitive domains by reducing Tau and pTau levels and increasing Aβ42 levels. Compared to other studies, our findings are consistent with those indicating that lipid levels influence AD progression through biochemical pathways involving these biomarkers. Previous research shows that elevated LDL increases AD risk, especially with APOEε4, while higher HDL levels are protective. These pathways likely involve the BBB and inflammatory processes, impacting biomarker levels and cognitive functions (Liu et al., 2021).
The LDL/HDL ratio, as an indicator for assessing CVD risk, an elevated ratio also reduces the risk of AD. It is worth noting that most participants in the ADNI database used in this study had LDL/HDL ratios below 2.5, which corresponds to a population with low CVD risk. This could explain the inverse association observed between LDL/HDL and AD risk in this study. Furthermore, a large proportion of participants were diagnosed with mild cognitive impairment (MCI). Early interventions, particularly the use of statins and dietary improvements, may have contributed to reducing AD risk. Statins not only lower peripheral cholesterol levels but also reduce neuroinflammation by inhibiting microglial activation and pro-inflammatory cytokine release. Additionally, they enhance BBB tegrity by stabilizing endothelial function, which may limit the entry of peripheral inflammatory mediators into the brain. These effects can reduce Aβ accumulation and tau hyperphosphorylation, thereby mitigating neurodegeneration (Zingel et al., 2021). Dietary improvements, such as adherence to heart-healthy patterns like the Mediterranean diet, have been associated with improved lipid profiles and reduced CVD risk (Middleton and Riboli, 2023).
Experimental and clinical evidence suggests that Aβ may play a role in the link between CVD and AD (Stakos et al., 2020), and Aβ40 levels were significantly and independently associated with subclinical atherosclerosis and coronary artery disease (Stamatelopoulos et al., 2015), suggesting a link between CVD and AD pathology. The higher the risk and severity of CVD, the more pronounced the impact on cognition. Studies have shown that there is an association between total CVD burden and cognitive deterioration (de Galan et al., 2009); the degree of cognitive impairment in patients with heart failure correlates with the severity of left ventricular systolic dysfunction (Zuccalà et al., 1997); and common CVDs such as stroke, hypertension, and coronary heart disease are associated with cognitive impairment (Abete et al., 2014). Our results show slow cognitive decline in people at low CVD risk. Song et al. (2020) found that a higher Framingham General Cardiovascular Risk Score was associated with smaller hippocampal volume and whole-brain volume, which is similar to our findings of low CVD risk with greater hippocampal volume.
The LDL/HDL ratio can influence the onset and progression of AD for the following reasons. Firstly, LDL and HDL can affect the BBB through a number of pathways. High levels of LDL may promote the formation of atherosclerosis, leading to damage to the arterial endothelium and the development of inflammatory responses (Pentikäinen et al., 2000). The inflammatory response leads to an increase in the permeability of vascular endothelial cells, which in turn affects the BBB (Galea, 2021). Second, an increase in the LDL/HDL ratio may directly affect the permeability of the BBB by influencing the composition and structure of the cell membrane (Muralidharan et al., 2022). LDL and HDL can affect the fluidity and stability of cell membranes, which in turn affects the permeability of the BBB (Rhea and Banks, 2021).
Our study has some strengths. First, we validated the association between the LDL/HDL ratio and biomarkers of AD in human CSF using two databases. Second, we illustrated the association between LDL/HDL ratio and AD from three aspects: pathology, cognitive measures, and brain imaging. Finally, the LDL/HDL ratio can affect global cognition and cognitive domains through three pathologic markers. Our study also has some limitations. First, our finding needs to be tested in a larger longitudinal cohort. Second, although we validated our findings in the CABLE cohort, differences in age distribution and cardiovascular characteristics between CABLE and ADNI mean that the results can only partially support the conclusions drawn from the ADNI cohort. In addition, although the LDL/HDL ratio could eliminate some of the effects of statins on LDL levels, some factors that can affect LDL, HDL levels such as the use of cholesterol-lowering and glucose-lowering medications, hormones, and diet, were not taken into account. Finally, the generalization of the findings is limited by the fact that participants in the ADNI database had LDL/HDL < 2.5.
Conclusion
We found that the LDL/HDL ratio is associated with AD pathology, cognition, and brain structure and can influence cognitive changes by affecting AD pathology. Although our findings indicate that a higher LDL/HDL ratio is associated with a reduced risk of AD and slower cognitive decline, elevated LDL/HDL levels are generally linked to an increased risk of CVD, which may ultimately heighten the risk of AD. Therefore, improving metabolic and cardiovascular health is essential for effective AD prevention.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the institutional review board of Qingdao Municipal Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
ZZ: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. LM: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft. LH: Conceptualization, Writing – original draft. YZ: Investigation, Writing – original draft. HG: Software, Writing – original draft. LW: Data curation, Writing – original draft. WZ: Writing – review & editing. LT: Funding acquisition, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by grants from the National Natural Science Foundation of China (82271475 and 82201587).
Acknowledgments
Thanks to my colleagues for the construction of the CABLE database. Data collection and sharing for this project was funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2025.1457160/full#supplementary-material
Reference
Abete, P., Della-Morte, D., Gargiulo, G., Basile, C., Langellotto, A., Galizia, G., et al. (2014). Cognitive impairment and cardiovascular diseases in the elderly. A heart-brain continuum hypothesis. Ageing Res. Rev. 18, 41–52. doi: 10.1016/j.arr.2014.07.003
American Diabetes Association (2021). 2. Classification and diagnosis of diabetes: standards of medical Care in Diabetes-2021. Diabetes Care 44, S15–s33. doi: 10.2337/dc21-S002
Andrews, S. J., Fulton-Howard, B., O'Reilly, P., Marcora, E., and Goate, A. M.collaborators of the Alzheimer's Disease Genetics Consortium (2021). Causal associations between modifiable risk factors and the Alzheimer's phenome. Ann. Neurol. 89, 54–65. doi: 10.1002/ana.25918
Arsenault, B. J., Boekholdt, S. M., and Kastelein, J. J. (2011). Lipid parameters for measuring risk of cardiovascular disease. Nat. Rev. Cardiol. 8, 197–206. doi: 10.1038/nrcardio.2010.223
Bailey, M. J., Soliman, E. Z., McClure, L. A., Howard, G., Howard, V. J., Judd, S. E., et al. (2021). Relation of atrial fibrillation to cognitive decline (from the REasons for geographic and racial differences in stroke [REGARDS] study). Am. J. Cardiol. 148, 60–68. doi: 10.1016/j.amjcard.2021.02.036
Baron, R. M., and Kenny, D. A. (1986). The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J. Pers. Soc. Psychol. 51, 1173–1182. doi: 10.1037/0022-3514.51.6.1173
de Bruijn, R. F., and Ikram, M. A. (2014). Cardiovascular risk factors and future risk of Alzheimer's disease. BMC Med. 12:130. doi: 10.1186/s12916-014-0130-5
de Galan, B. E., Zoungas, S., Chalmers, J., Anderson, C., Dufouil, C., Pillai, A., et al. (2009). Cognitive function and risks of cardiovascular disease and hypoglycaemia in patients with type 2 diabetes: the action in diabetes and vascular disease: Preterax and Diamicron modified release controlled evaluation (ADVANCE) trial. Diabetologia 52, 2328–2336. doi: 10.1007/s00125-009-1484-7
Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (2001). Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III). JAMA 285, 2486–2497. doi: 10.1001/jama.285.19.2486
Fernandez, M. L., and Webb, D. (2008). The LDL to HDL cholesterol ratio as a valuable tool to evaluate coronary heart disease risk. J. Am. Coll. Nutr. 27, 1–5. doi: 10.1080/07315724.2008.10719668
Fletcher, E., Gavett, B., Crane, P., Soldan, A., Hohman, T., Farias, S., et al. (2021). A robust brain signature region approach for episodic memory performance in older adults. Brain 144, 1089–1102. doi: 10.1093/brain/awab007
Galea, I. (2021). The blood-brain barrier in systemic infection and inflammation. Cell. Mol. Immunol. 18, 2489–2501. doi: 10.1038/s41423-021-00757-x
Jack, C. R., Bernstein, M. A., Fox, N. C., Thompson, P., Alexander, G., Harvey, D., et al. (2008). The Alzheimer's disease neuroimaging initiative (ADNI): MRI methods. J. Magn. Reson. Imaging 27, 685–691. doi: 10.1002/jmri.21049
Jansson, D., Wang, M., Thomas, R. G., Erickson, M. A., Peskind, E. R., Li, G., et al. (2022). Markers of cerebrovascular injury, inflammation, and plasma lipids are associated with Alzheimer's disease cerebrospinal fluid biomarkers in cognitively Normal persons. J. Alzheimers Dis. 86, 813–826. doi: 10.3233/JAD-215400
Katsumata, Y., Todoriki, H., Higashiuesato, Y., Yasura, S., Ohya, Y., Willcox, D. C., et al. (2013). Very old adults with better memory function have higher low-density lipoprotein cholesterol levels and lower triglyceride to high-density lipoprotein cholesterol ratios: KOCOA project. J. Alzheimers Dis. 34, 273–279. doi: 10.3233/JAD-121138
Liu, L., Li, H., Iyer, H., Liu, A. J., Zeng, Y., and Ji, J. S. (2021). Apolipoprotein E induced cognitive dysfunction: mediation analysis of lipids and glucose biomarkers in an elderly cohort study. Front. Aging Neurosci. 13:727289. doi: 10.3389/fnagi.2021.727289
McKhann, G., Drachman, D., Folstein, M., Katzman, R., Price, D., and Stadlan, E. M. (1984). Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA work group under the auspices of Department of Health and Human Services Task Force on Alzheimer's disease. Neurology 34, 939–944. doi: 10.1212/WNL.34.7.939
Middleton, L. T., and Riboli, E. (2023). Dietary cholesterol and dementia risk. J. Prev Alzheimers Dis. 10, 746–747. doi: 10.14283/jpad.2023.66
Muralidharan, J., Papandreou, C., Soria-Florido, M. T., Sala-Vila, A., Blanchart, G., Estruch, R., et al. (2022). Cross-sectional associations between HDL structure or function, cell membrane fatty acid composition, and inflammation in elderly adults. J. Nutr. 152, 789–795. doi: 10.1093/jn/nxab362
Pentikäinen, M. O., Oörni, K., Ala-Korpela, M., and Kovanen, P. T. (2000). Modified LDL – trigger of atherosclerosis and inflammation in the arterial intima. J. Intern. Med. 247, 359–370. doi: 10.1046/j.1365-2796.2000.00655.x
Peters, R., Xu, Y., Antikainen, R., Beckett, N., Gussekloo, J., Jagger, C., et al. (2021). Evaluation of high cholesterol and risk of dementia and cognitive decline in older adults using individual patient Meta-analysis. Dement. Geriatr. Cogn. Disord. 50, 318–325. doi: 10.1159/000519452
Petersen, R. C., Aisen, P. S., Beckett, L. A., Donohue, M. C., Gamst, A. C., Harvey, D. J., et al. (2010). Alzheimer's disease neuroimaging initiative (ADNI): clinical characterization. Neurology 74, 201–209. doi: 10.1212/WNL.0b013e3181cb3e25
Pfrieger, F. W. (2003). Cholesterol homeostasis and function in neurons of the central nervous system. Cell. Mol. Life Sci. 60, 1158–1171. doi: 10.1007/s00018-003-3018-7
Poliakova, T., and Wellington, C. L. (2023). Roles of peripheral lipoproteins and cholesteryl ester transfer protein in the vascular contributions to cognitive impairment and dementia. Mol. Neurodegener. 18:86. doi: 10.1186/s13024-023-00671-y
Racine, A. M., Koscik, R. L., Nicholas, C. R., Clark, L. R., Okonkwo, O. C., Oh, J. M., et al. (2016). Cerebrospinal fluid ratios with Aβ42 predict preclinical brain β-amyloid accumulation. Alzheimers Demen. 2, 27–38. doi: 10.1016/j.dadm.2015.11.006
Rhea, E. M., and Banks, W. A. (2021). Interactions of lipids, lipoproteins, and apolipoproteins with the blood-brain barrier. Pharm. Res. 38, 1469–1475. doi: 10.1007/s11095-021-03098-6
Saeed, A., Lopez, O., Cohen, A., and Reis, S. E. (2023). Cardiovascular disease and Alzheimer's disease: the heart-brain Axis. J. Am. Heart Assoc. 12:e030780. doi: 10.1161/JAHA.123.030780
Schnabel, R. B., Larson, M. G., Yamamoto, J. F., Sullivan, L. M., Pencina, M. J., Meigs, J. B., et al. (2010). Relations of biomarkers of distinct pathophysiological pathways and atrial fibrillation incidence in the community. Circulation 121, 200–207. doi: 10.1161/CIRCULATIONAHA.109.882241
Shaw, L. M., Vanderstichele, H., Knapik-Czajka, M., Clark, C. M., Aisen, P. S., Petersen, R. C., et al. (2009). Cerebrospinal fluid biomarker signature in Alzheimer's disease neuroimaging initiative subjects. Ann. Neurol. 65, 403–413. doi: 10.1002/ana.21610
Sheng, Z., Wang, L., Chen, M., Zhong, F., Wu, S., Liang, S., et al. (2025). Cerebrospinal fluid β2-microglobulin promotes the tau pathology through microglia-astrocyte communication in Alzheimer's disease. Alzheimers Res. Ther. 17:2. doi: 10.1186/s13195-024-01665-8
Snider, B. J., Fagan, A. M., Roe, C., Shah, A. R., Grant, E. A., Xiong, C., et al. (2009). Cerebrospinal fluid biomarkers and rate of cognitive decline in very mild dementia of the Alzheimer type. Arch. Neurol. 66, 638–645. doi: 10.1001/archneurol.2009.55
Song, R., Xu, H., Dintica, C. S., Pan, K. Y., Qi, X., Buchman, A. S., et al. (2020). Associations between cardiovascular risk, structural brain changes, and cognitive decline. J. Am. Coll. Cardiol. 75, 2525–2534. doi: 10.1016/j.jacc.2020.03.053
Stakos, D. A., Stamatelopoulos, K., Bampatsias, D., Sachse, M., Zormpas, E., Vlachogiannis, N. I., et al. (2020). The Alzheimer's disease amyloid-Beta hypothesis in cardiovascular aging and disease: JACC focus seminar. J. Am. Coll. Cardiol. 75, 952–967. doi: 10.1016/j.jacc.2019.12.033
Stamatelopoulos, K., Sibbing, D., Rallidis, L. S., Georgiopoulos, G., Stakos, D., Braun, S., et al. (2015). Amyloid-beta (1-40) and the risk of death from cardiovascular causes in patients with coronary heart disease. J. Am. Coll. Cardiol. 65, 904–916. doi: 10.1016/j.jacc.2014.12.035
Turri, M., Marchi, C., Adorni, M. P., Calabresi, L., and Zimetti, F. (1867). Emerging role of HDL in brain cholesterol metabolism and neurodegenerative disorders. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 1867:159123. doi: 10.1016/j.bbalip.2022.159123
Wang, M., Li, Y., Cong, L., Hou, T., Luo, Y., Shi, L., et al. (2021). High-density lipoprotein cholesterol and brain aging amongst rural-dwelling older adults: a population-based magnetic resonance imaging study. Eur. J. Neurol. 28, 2882–2892. doi: 10.1111/ene.14939
Ward, M. A., Bendlin, B. B., McLaren, D. G., Hess, T. M., Gallagher, C. L., Kastman, E. K., et al. (2010). Low HDL cholesterol is associated with lower gray matter volume in cognitively healthy adults. Front. Aging Neurosci. 2:2. doi: 10.3389/fnagi.2010.00029
Wolf, H., Hensel, A., Arendt, T., Kivipelto, M., Winblad, B., and Gertz, H. J. (2004). Serum lipids and hippocampal volume: the link to Alzheimer's disease? Ann. Neurol. 56, 745–749. doi: 10.1002/ana.20289
Xu, W., Tan, L., Wang, H. F., Jiang, T., Tan, M. S., Tan, L., et al. (2015). Meta-analysis of modifiable risk factors for Alzheimer's disease. J. Neurol. Neurosurg. Psychiatry 86, jnnp-2015-310548–jnnp-2015-310306. doi: 10.1136/jnnp-2015-310548
Yang, Z., Lin, P. J., and Levey, A. (2013). Monetary costs of dementia in the United States. N. Engl. J. Med. 369:489. doi: 10.1056/NEJMc1305541
Yu, Y., Yan, P., Cheng, G., Liu, D., Xu, L., Yang, M., et al. (2023). Correlation between serum lipid profiles and cognitive impairment in old age: a cross-sectional study. Gen Psychiatr. 36:e101009. doi: 10.1136/gpsych-2023-101009
Zhang, Z., Chen, X., and Sheng, Z. (2024). Association of triglyceride glucose-body mass index with Alzheimer's disease pathology, cognition and brain structure in non-demented people. Sci. Rep. 14:16097. doi: 10.1038/s41598-024-67052-3
Zhang, Z., Sheng, Z., Liu, J., Zhang, D., Wang, H., Wang, L., et al. (2024). The association of the triglyceride-glucose index with Alzheimer's disease and its potential mechanisms. J. Alzheimers Dis. 102, 77–88. doi: 10.1177/13872877241284216
Zhou, F., Deng, W., Ding, D., Zhao, Q., Liang, X., Wang, F., et al. (2018). High low-density lipoprotein cholesterol inversely relates to dementia in community-dwelling older adults: the Shanghai aging study. Front. Neurol. 9:952. doi: 10.3389/fneur.2018.00952
Zhou, Z., Liang, Y., Zhang, X., Xu, J., Lin, J., Zhang, R., et al. (2020). Low-density lipoprotein cholesterol and Alzheimer's disease: a systematic review and Meta-analysis. Front. Aging Neurosci. 12:5. doi: 10.3389/fnagi.2020.00005
Zingel, R., Bohlken, J., Riedel-Heller, S., Barth, S., and Kostev, K. (2021). Association between low-density lipoprotein cholesterol levels, statin use, and dementia in patients followed in German general practices. J. Alzheimers Dis. 79, 37–46. doi: 10.3233/JAD-201176
Keywords: Alzheimer’s disease, low-density lipoprotein/high-density lipoprotein, pathology, cognition, brain structure
Citation: Zhang Z, Ma L, Huang L, Zhu Y, Guo H, Wang L, Zhang W and Tan L (2025) Association of low-density lipoprotein/high-density lipoprotein ratio with cognition, Alzheimer’s disease biomarkers and brain structure. Front. Aging Neurosci. 17:1457160. doi: 10.3389/fnagi.2025.1457160
Edited by:
Vijay Karkal Hegde, Texas Tech University, United StatesReviewed by:
Wenhui Qu, Cornell University, United StatesMd Abu Bakkar Siddik, Texas Tech University, United States
Copyright © 2025 Zhang, Ma, Huang, Zhu, Guo, Wang, Zhang and Tan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lan Tan, ZHIudGFubGFuQDE2My5jb20=; Wei Zhang, am56aGFuZ3dlaUAxMjYuY29t
 Zihao Zhang
Zihao Zhang Lingzhi Ma1
Lingzhi Ma1 Lan Tan
Lan Tan