- 1Department of Neurology, Affiliated Hospital of North Sichuan Medical College, Nanchong, Sichuan, China
- 2Sichuan Key Laboratory of Medical Imaging, Department of Radiology, Affiliated Hospital of North Sichuan Medical College, Nanchong, Sichuan, China
- 3Department of Geriatrics, Nanchong Central Hospital, Nanchong, Sichuan, China
Background: Drug-induced Parkinsonism (DIP) is a secondary Parkinsonism with limited research on its hippocampal structural changes. This study explores hippocampal subfield volumes in DIP compared to Parkinson’s disease (PD) and healthy controls (HCs), investigating correlations with cognitive (Montreal Cognitive Assessment, MoCA), emotional (Hamilton Depression Rating Scale, HAMD; Hamilton Anxiety Rating Scale, HAMA), and motor (Unified Parkinson’s Disease Rating Scale, UPDRS) symptoms.
Methods: A total of 19 DIP patients, 20 PD patients, and 20 HCs were enrolled. MRI-based hippocampal subfield volumes were assessed using FreeSurfer, and clinical scores were evaluated for cognitive, emotional, and motor functions. Statistical analyses compared group differences and examined correlations.
Results: Significant atrophy was observed in the DIP group in multiple hippocampal subfields compared to HCs, including the presubiculum, subiculum, Granule cell and molecular layer of the dentate gyrus (GC-ML-DG), molecular_layer_HP, Cornu ammonis (CA) 1, CA4, hippocampal tail, and fimbria. MoCA scores positively correlated with volumes in bilateral hippocampus and subfields such as subiculum and CA4, while HAMD scores mainly showed negative correlations in both DIP and PD group. UPDRS scores revealed group-specific patterns, with DIP showing stronger associations between non-motor symptoms and hippocampal volume.
Conclusion: This study first reported significant hippocampal subfield atrophy in DIP, distinct from PD, and links structural changes to cognitive, emotional, and motor impairments. These findings advance understanding of DIP pathophysiology and underscore the hippocampus’s role in non-motor symptoms.
Introduction
Drug-induced parkinsonism (DIP) is one of the most common forms of secondary parkinsonism (Bondon-Guitton et al., 2011; Savica et al., 2017; Shiraiwa et al., 2018), resulting from the use of medications that block dopamine receptors or deplete dopamine levels (Feldman et al., 2022; Margolesky, 2019), and its prevalence and incidence of DIP increased in the recent years (Han et al., 2019). Although DIP and Parkinson’s disease (PD) are both subtypes of parkinsonism (Shin and Chung, 2012; Wenning et al., 2011), and DIP shares several clinical features with PD, such as bradykinesia and rigidity, the underlying neurobiological mechanisms of DIP remain poorly understood, with limited research focusing on its structural and functional brain changes. Unlike PD, which has been extensively studied, DIP has received far less attention, leaving significant gaps in our understanding of its neuropathological basis.
Neuroimaging offers valuable insights for diagnosing DIP, particularly in cases with clinical presentations that closely resemble PD (Pitton Rissardo and Caprara, 2023). Current MRI studies on DIP remain limited, with existing research primarily focusing on alterations in the substantia nigra (Sung et al., 2016) and white matter (Lee et al., 2017). Our previous study found structural (volume) alterations in the subcortical nuclei of DIP patients (Zhou et al., 2024). These studies underscore the scarcity of neuroimaging investigations into DIP, highlighting a need for further exploration to better understand its pathophysiology and distinguish it from PD.
The hippocampus serves as a critical brain region involved in cognitive processes and emotional regulation (Li et al., 2020; Zhang et al., 2016). Moreover, the hippocampus is divided into several substructures, each with distinct functions and vulnerabilities in neurodegenerative diseases. Its substructures, such as the dentate gyrus and CA regions, have distinct roles in memory, learning, and mood regulation and are known to be affected in neurodegenerative and psychiatric disorders. Previous studies have reported that hippocampal alteration is associated with cognitive function in PD patients (Yildiz et al., 2015), indicating its potential as a biomarker for disease progression and treatment response. Beyer et al. (2013) found that memory deficits in recall and recognition have been linked to hippocampal atrophy in newly diagnosed PD patients, particularly in verbal memory tasks. Furthermore, Low et al. (2019) have reported atrophy in specific hippocampal subfields, such as CA1, in individuals who progressed to PD dementia. These findings underscore the strong association between hippocampal dysfunction and cognitive impairment in PD. Cognitive impairment and emotional disturbances can also occur in patients with DIP. However, it remains unclear whether these changes are associated with hippocampal structural alterations in DIP patients, and to date, no studies have specifically investigated the relationship between hippocampal subfield volumes and clinical symptoms, such as cognitive deficits, depressive symptoms, and motor dysfunction, in this population.
Hence, our study is the first to explore the relationship between hippocampal subfield volumes and cognitive and emotional functioning in DIP patients, potentially offering new insights into the pathophysiology of DIP and its management.
This research not only provides new perspectives on the hippocampal structural alterations associated with DIP, but also helps us understand the potential relationship between these changes and cognitive, emotional, and motor symptoms.
Methods
Participants
The research protocol received approval from the Local Ethical Committee (Approval no. 2021ER0105-1), and all participants signed written informed consent forms.
The study involved participants who were part of a previous cohort study (Zhou et al., 2024). A total of 19 patients with DIP, 20 patients diagnosed with PD, and 20 healthy control participants (HCs) were enrolled. The diagnosis of PD was made according to the 2016 Chinese diagnostic guidelines. DIP cases were confirmed based on the following criteria: (1) exhibiting parkinsonism symptoms; (2) absence of prior parkinsonism before exposure to causative drugs; (3) symptom manifestation following drug usage; and (4) being right-handed. Exclusion criteria for DIP participants included: (1) a diagnosis of primary Parkinson’s disease or other identifiable causes of parkinsonism; (2) MRI contraindications (e.g., claustrophobia or presence of metallic implants); (3) structural brain damage or motion artifacts on MRI; (4) history of neurological disorders (e.g., stroke, head injury); or (5) unwillingness to participate.
For PD participants, inclusion criteria were: (1) diagnosis per the 2016 Chinese PD criteria; (2) voluntary consent; and (3) right-handedness. Exclusion criteria included: (1) secondary or atypical parkinsonism; (2) inability to cooperate with symptom evaluations; (3) MRI contraindications; (4) significant structural abnormalities or motion artifacts on MRI; and (5) unwillingness to participate.
HCs were age-and sex-matched, with all participants being right-handed. The exclusion criteria for HCs included: (1) psychiatric or neurological disorders; (2) MRI contraindications; (3) significant structural abnormalities or motion artifacts on MRI; and (4) unwillingness to participate.
The evaluation of clinical symptoms was conducted using the Unified Parkinson’s Disease Rating Scale (UPDRS) (Goetz et al., 2008) and the Hoehn-Yahr (H-Y) staging scale (Goetz et al., 2004). The assessment of motor symptoms was carried out with the UPDRS-III and H-Y staging scale, whereas the evaluation of non-motor symptoms and daily living experiences related to motor functions was performed using the UPDRS-I and UPDRS-II, respectively. Cognitive assessment was conducted using the Montreal Cognitive Assessment (MoCA). Additionally, the patients’ emotional state was evaluated through the Hamilton Depression Rating Scale (HAMD) and the Hamilton Anxiety Rating Scale (HAMA).
MRI scan
MRI data were collected using a 3.0 T scanner (GE Discovery MR750, USA) equipped with a 32-channel head coil. High-resolution 3D-T1-weighted imaging was performed with the following parameters: repetition time (TR) of 8.3 ms, echo time (TE) of 3.3 ms, flip angle of 15°, field of view (FOV) of 240 × 240 mm, image matrix of 240 × 240, and slice thickness of 1.0 mm with no interslice gap.
Imaging analysis
The hippocampal subfields segmentation was performed using FreeSurfer version 7.1.1. This automated process included several steps: correcting motion artifacts in T1-weighted images, aligning images to the Talairach coordinate system, adjusting for B1 field inhomogeneities, and applying a hybrid watershed algorithm for skull stripping. Subsequent stages involved labeling volumes, segmenting subcortical regions, refining subcortical structures, and constructing cortical models. The analysis specifically extracted volumes of 12 hippocampal subfields per hemisphere, including the Cornu ammonis (CA) 1, CA3, CA4, etc. (Figure 1). Additionally, the intracranial volume (ICV) was calculated. This hippocampal subfield segmentation approach has been widely used in neuroimaging research (Iglesias et al., 2015; Sämann et al., 2022).
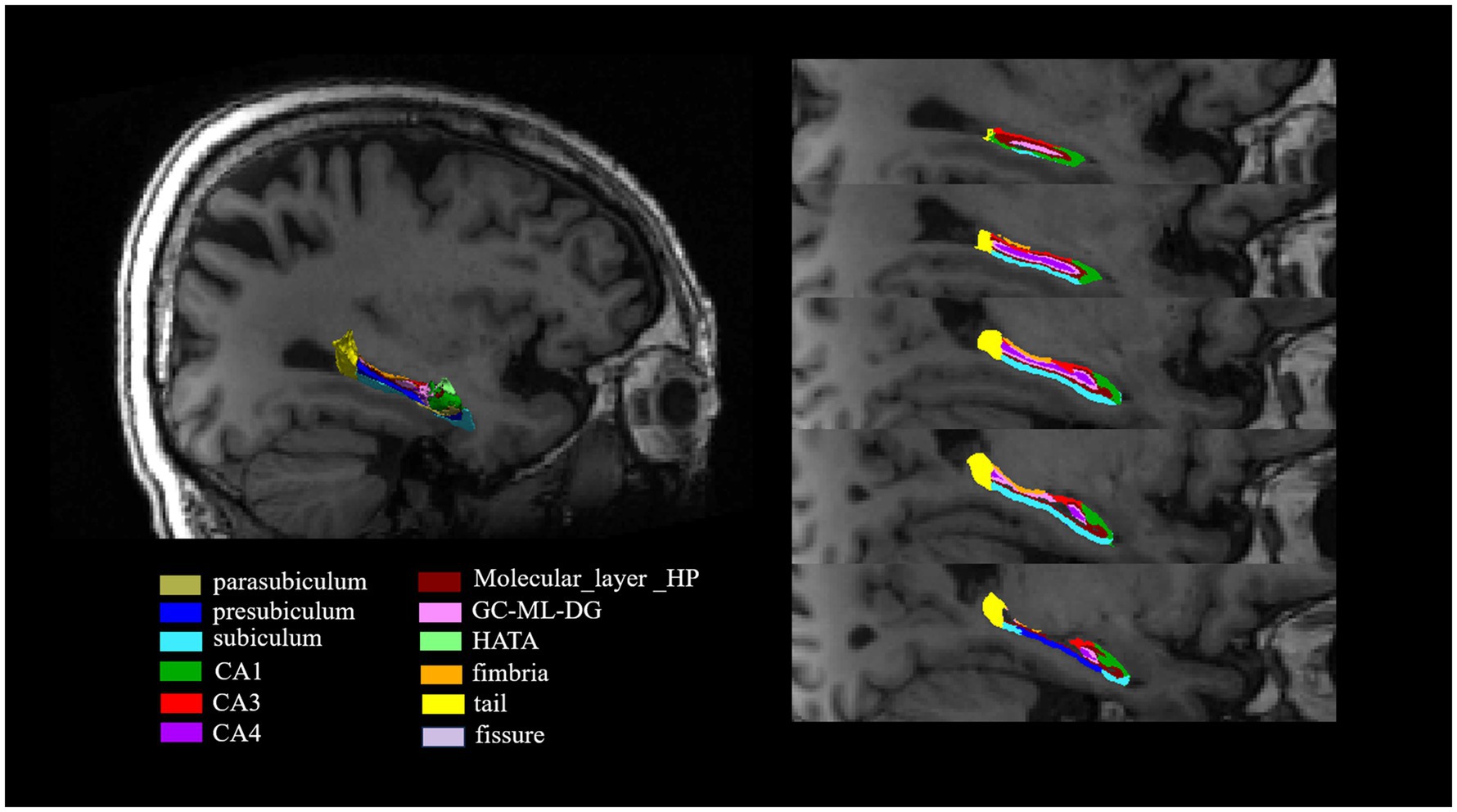
Figure 1. The segmentation of hippocampal subfields on T1-weighted MRI images. The hippocampus was segmented into the following subfields: parasubiculum, presubiculum, subiculum, CA1, CA3, CA4, molecular_layer_HP, GC-ML-DG, HATA, fimbria, tail and fissure. CA, Cornu ammonis; GC-ML-DG, Granule cell and molecular layer of the dentate gyrus; HATA, Hippocampus-amygdala transition area.
Statistical analysis
Continuous variables were expressed as either the mean or median. For H-Y staging scale, stages 2 and above were combined into a single group due to the limited number of patients in the higher stages (Ziegler et al., 2013). Comparisons of demographic and clinical features were conducted using analysis of variance (ANOVA), Mann–Whitney U-tests, or Chi-Squared tests. To examine hippocampal volume parameters among three groups, analysis of covariance (ANCOVA) was applied, followed by post-hoc analyses. p-values were adjusted for multiple comparisons using the false discovery rate (FDR). The relationship between clinical parameters and subcortical volume was explored through partial correlation analysis, controlling for sex, age, and ICV.
All statistical analyses were carried out with SPSS software (Version 23.0), with p < 0.05 considered statistically significant.
Results
Demographics
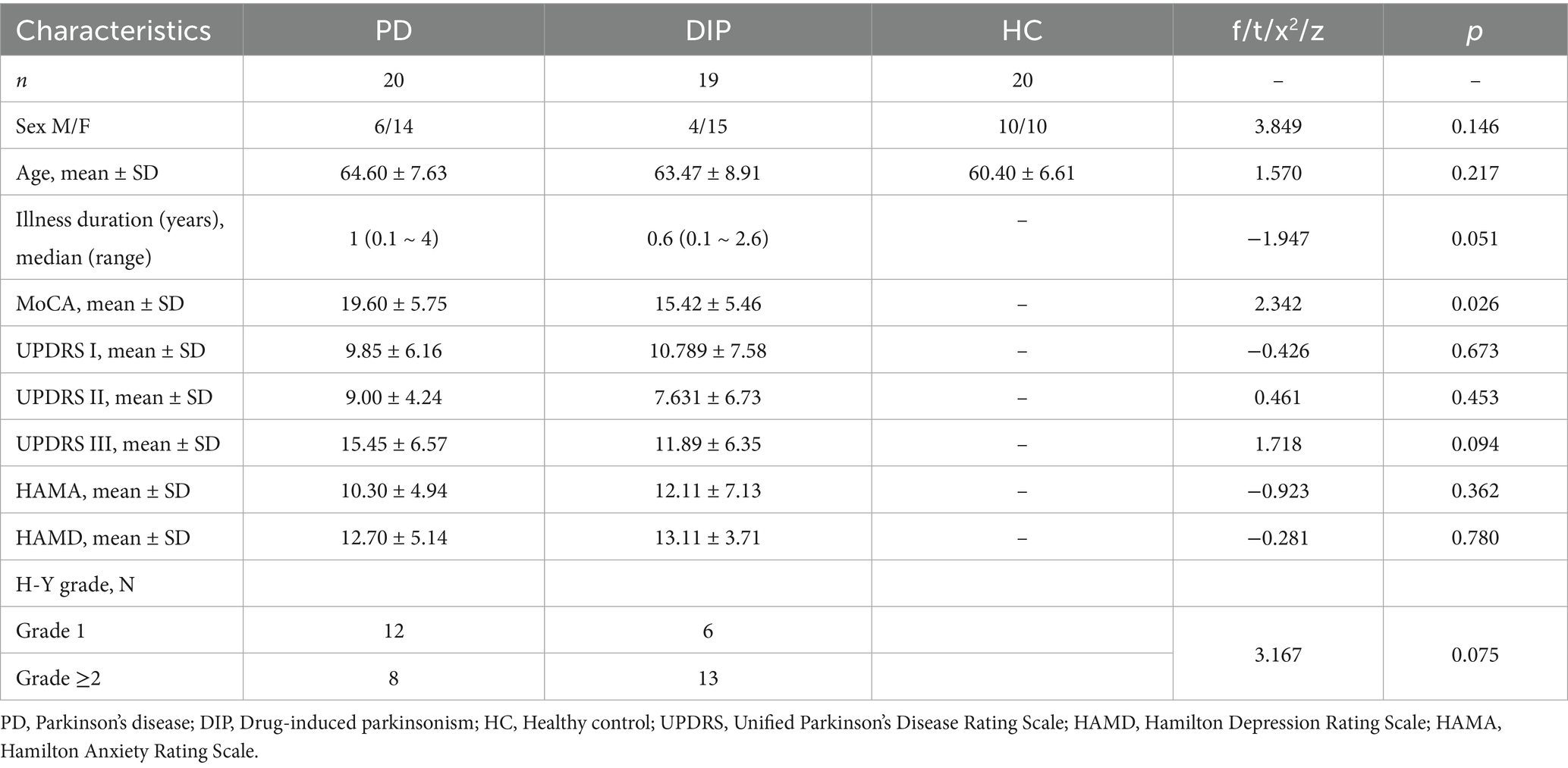
The demographic and clinical characteristics of these subject groups are showed in the Table 1. There were no significant differences in age, sex distribution among the three groups. The duration of illness was marginally longer in the PD group than in the DIP group, though this difference was not statistically significant. Cognitive performance, assessed using the MoCA score, showed a significant group difference, with the DIP group scoring lower than the PD group. Scores on the UPDRS subscales (I, II, and III) showed no significant differences among groups. Emotional states, evaluated using the HAMA and HAMD score, were similar across groups, with no statistically significant differences (p > 0.05).
Comparison of hippocampal and subfield volumes
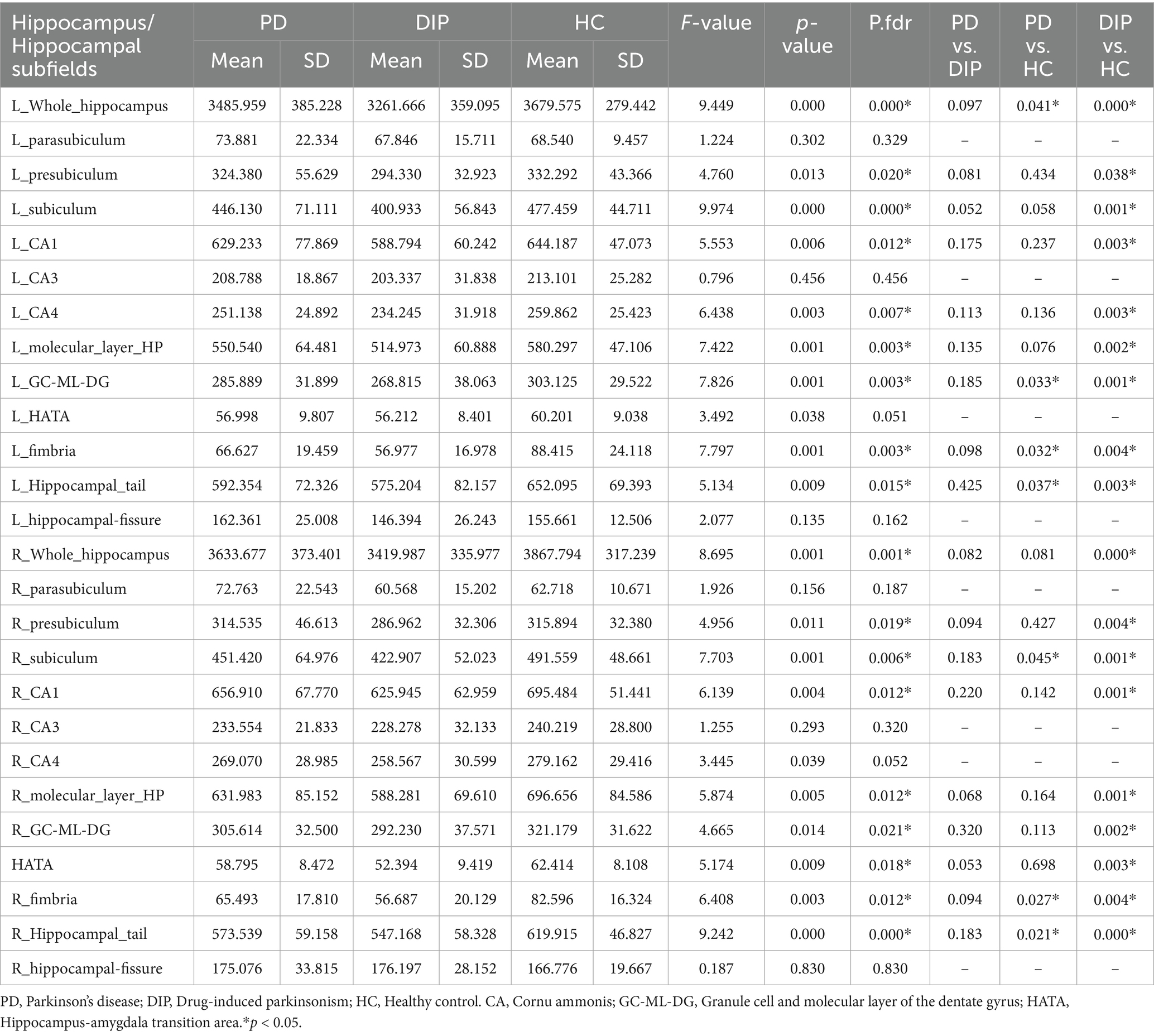
The results revealed significant differences in hippocampal subfield volumes across the three groups, with the DIP group showing widespread reductions compared to HCs. Both the bilateral whole hippocampal volumes were significantly smaller in the DIP group, alongside reductions in several subfields, including the presubiculum, subiculum, Granule cell and molecular layer of the dentate gyrus (GC-ML-DG), Molecular_layer_HP, CA1, CA4, hippocampal tail, and fimbria. These reductions were bilateral and were more pronounced in DIP compared to HC, with some subfields also differing from the PD group. Overall, the DIP group exhibited the more severe hippocampal atrophy among the groups studied (Table 2 and Figure 2).
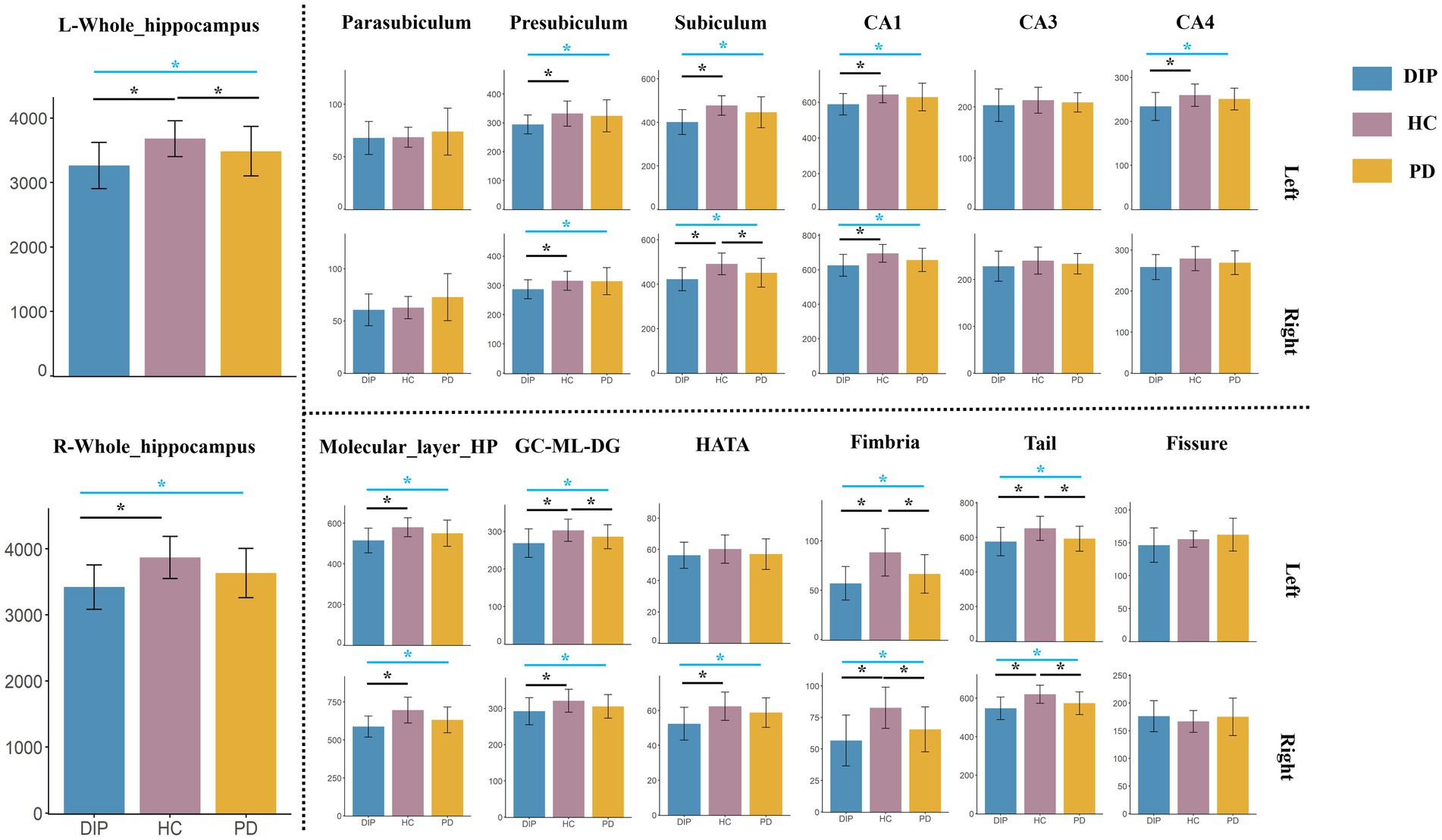
Figure 2. Comparison of Hippocampal Subfield Volumes among DIP, HC, and PD. The blue lines indicate the results of ANCOVA among these three groups, while the black lines represent post-hoc analysis. *Represents statistically significant differences (p < 0.05) in either the ANOVA or post-hoc comparisons.
Correlation analysis
The results of the correlation analysis are presented in the following heatmap (Figure 3 and Supplementary Tables 1, 2).
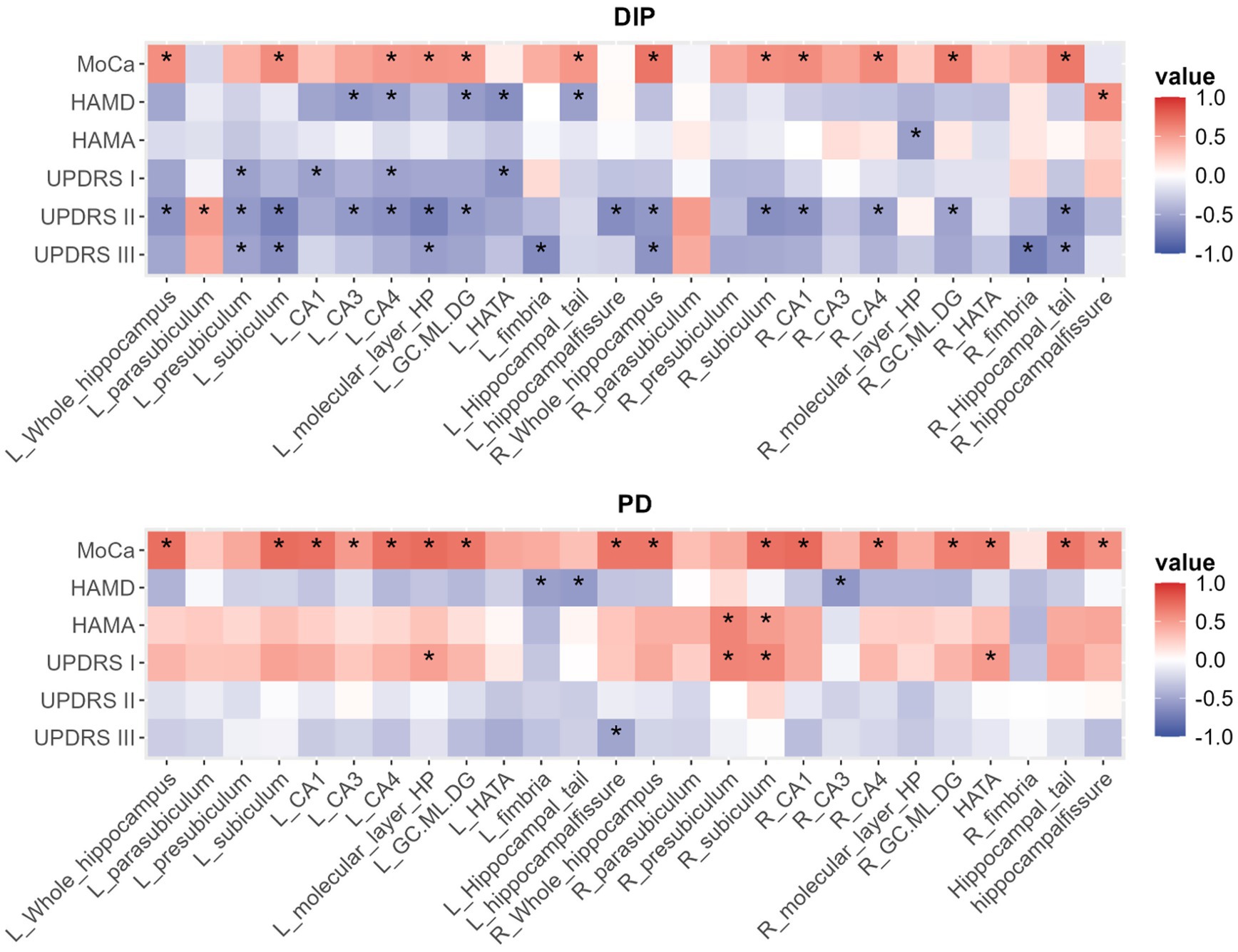
Figure 3. The heatmap of correlations between hippocampal volume parameters and related various clinical measures in DIP and PD group, with *indicating p < 0.05.
In both DIP and PD groups, MoCA scores were positively correlated with bilateral total hippocampal volumes and several subfield volumes, while HAMD scores predominantly exhibited negative correlations with hippocampal volume metrics. According to the heatmap results, the DIP group displayed a greater number of statistically significant correlations between hippocampal volume metrics and UPDRS scores compared to the PD group, with most of these correlations being negative.
Subgroup analysis based on H-Y staging scale
Patients were grouped using an H-Y stage threshold of grade 2, with those at grade 2 and above combined into a single group. The volumes of the hippocampus and its subfields were compared in both the DIP and PD groups at different H-Y stages, respectively. The results showed no statistically significant differences in the whole hippocampal volume or its subfield volumes between the two groups (Supplementary Tables 3, 4).
Discussion
This study is the first to investigate hippocampal subfield volume alterations in patients with DIP and their associations with clinical parameters, including cognitive performance (MoCA), emotional states (HAMD), and motor/non-motor symptoms (UPDRS). Our findings revealed significant reductions in hippocampal subfields in the DIP group compared to HCs, revealing a distinct pattern of hippocampal atrophy in DIP. Moreover, the DIP group exhibited more severe hippocampal volume loss than the PD group, which may due to specific mechanistic differences between primary and secondary parkinsonism. These structural changes were significantly correlated with clinical outcomes, particularly cognitive and emotional dysfunction, suggesting that hippocampal atrophy may play a critical role in the course of DIP.
We observed widespread reductions in hippocampal subfields, including the presubiculum, subiculum, GC-ML-DG, molecular_layer_HP, CA1, CA4, hippocampal tail, and fimbria, reflect severe structural damage in DIP, with pronounced atrophy in DIP compared to PD. On one hand, hippocampal alterations are possibly caused by neuronal damage (Fan et al., 2018; Zhu et al., 2018). Our results may suggest that hippocampal atrophy occurs in both DIP and PD, potentially reflecting a common structural vulnerability. Previous researches reported that cognitive impairments in PD have been linked to reductions in hippocampal volume (Xu et al., 2020; Carlesimo et al., 2012). Recent study (Low et al., 2019) found atrophy in the CA1 subfield of the hippocampus in developed PD dementia. Hippocampal volume decrease might serve as a common biomarker of cognitive vulnerability in both degenerative and non-degenerative parkinsonian syndromes.
On the other hand, the more extensive and severe hippocampal atrophy observed in DIP compared to PD when compared to HCs, might reflect unique pathological processes influenced by differences in clinical characteristics. The disease course in DIP is typically shorter than in PD (López-Sendón et al., 2012), and its progression was more quickly (Shiraiwa et al., 2018). This may suggest that hippocampal volume reductions in DIP may be attributed to the acute effects of drug exposure, contrasting with the gradual, progressive neurodegenerative changes observed in PD. Additionally, DIP patients exhibited significantly lower MoCA scores compared to PD patients, indicating more pronounced cognitive impairment, consistent with earlier studies indicating that neurological deficits in DIP are more pronounced than those in PD (Shin and Chung, 2012). We speculated that cognitive vulnerability in DIP may exacerbate hippocampal susceptibility. So, these factors may collectively contribute to the more severe hippocampal atrophy observed in DIP patients.
Cognitive impairment, as reflected by lower MoCA scores in the DIP group, was strongly associated with reduced hippocampal subfield volumes. Specifically, subfields such as the subiculum, CA4, and CA1 showed significant positive correlations with MoCA scores. Firstly, we found the DIP patients exhibited more pronounced cognitive impairment, in addition to the previously mentioned relationship between cognitive impairment and hippocampal changes, cognitive impairment itself has been reported as a significant risk factor in the onset and progression of DIP (López-Sendón et al., 2012). Secondly, a positive correlation was observed between MoCA scores and hippocampal subfield volumes, suggesting that smaller hippocampal volumes were linked to more severe cognitive deficits. Although the causal relationship between these two factors remains unclear, it is possible that the reduction in hippocampal volume could be a result of prolonged cognitive decline, or conversely, that hippocampal atrophy may contribute to the worsening of cognitive function. This underscores the complex interplay between structural brain changes and cognitive performance in DIP, warranting further investigation to elucidate the direction of causality.
The negative correlations between HAMD scores and hippocampal subfield volumes, particularly in the HATA and CA3, suggest that smaller hippocampal volumes are linked to greater depressive symptoms in DIP patients. These regions are crucial for emotional regulation, for example, CA3 is an important subfield involved in depression (Nolan et al., 2020; Roddy et al., 2019). All of them have been reported decreased volume in depression in the majority researches (Nolan et al., 2020; Sun et al., 2023). Moreover, these correlations were stronger in DIP than in PD, indicating that hippocampal atrophy may have a more significant impact on mood disturbances in DIP patients. In addition to the interplay with hippocampal structural changes, gender differences in the occurrence of DIP may also be another contributing factor. Since DIP has a higher prevalence in female (Shin and Chung, 2012), who also have a higher incidence of depression (Eid et al., 2019; Marx et al., 2023), and our DIP sample also included a higher proportion of female participants. So, gender-specific factors may contribute to the more pronounced mood disturbances observed in the DIP group.
While the hippocampus is primarily associated with cognitive and emotional functions, exploring its relationship with motor symptoms may be significant in DIP and PD. Molina et al. (2016) reported that motor impairments in PD patients can predict cognitive deficits in schizophrenia patients, suggesting a potential mediating role of the hippocampus. Ledoux et al. (2014) found that hippocampal dysfunctions can affect motor-related tasks. These results suggested that the hippocampus may play a role in integrating cognitive and motor functions, which could have implications for understanding the pathophysiology of related disorders. In our study, the heatmap analysis revealed distinct patterns of correlation between UPDRS I/II/III and hippocampal subfield volumes in both DIP and PD groups. These findings highlight group-specific differences in how hippocampal subfields relate to motor and non-motor symptoms in DIP and PD. Previous studies have suggested the hippocampus, interacting with the basal ganglia and prefrontal cortex, likely contributed to motor and cognitive integration within the broader cortico-basal ganglia-thalamic circuits (Maurice et al., 2015; Ursino et al., 2020). Notably, the more extensive correlations in the DIP group, particularly with UPDRS-I and III, may suggest a heightened hippocampal involvement in this condition, potentially reflecting its unique pathophysiology. Further research is needed to clarify these associations and to determine whether hippocampal structural changes could serve as biomarkers for symptom severity or progression in DIP and PD.
Additionally, subgroup analysis based on Hoehn and Yahr (H-Y) staging showed no significant differences in hippocampal volumes or subfield volumes between the DIP and PD groups. This lack of significant structural differences may be attributed to the relatively short disease duration in our sample, suggesting that hippocampal structural changes might not yet be detectable.
Although subgroup analysis based on H-Y staging scale did not show significant differences in hippocampal volumes between the DIP and PD groups, we observed significant correlations between hippocampal volumes parameters and UPDRS-III scores. Compared with UPDRS, the H-Y scale can provide a limited evaluation of the severity of motor symptoms (Movement Disorder Society Task Force on Rating Scales for Parkinson's Disease, 2003; Yamada et al., 2022). Another possible explanation is that UPDRS-III, as a continuous measure, offers greater sensitivity in quantifying the severity of motor symptoms. In contrast, the H-Y scale is categorical and provides a general information of disease stage. Furthermore, the relatively small sample sizes within H-Y subgroups may limit the statistical power to detect subtle group differences. Further researches with larger samples and longitudinal follow-up are needed to confirm our findings.
This study has several limitations. Firstly, the relatively small sample size may have reduced the statistical power, potentially affecting the ability to detect minor differences, especially in subgroup analyses. Future studies with larger cohorts are needed to validate these findings. Second, the cross-sectional design precludes causal inferences about the relationship between hippocampal atrophy and clinical outcomes. Longitudinal studies would be valuable to explore how hippocampal changes progress over time and their potential reversibility upon cessation of causative drugs. Third, while the study focused on volumetric changes in hippocampal subfields, functional alterations or connectivity changes were not assessed. Combining structural MRI with functional imaging techniques, such as resting-state fMRI, could provide a more comprehensive understanding of hippocampal dysfunction in DIP. Finally, the heterogeneity of the drugs causing DIP in the recruited population may introduce variability in the observed effects. Stratifying patients by the specific causative agents in future research could clarify drug-specific impacts on hippocampal subfields.
In conclusion, our research provides new perspectives on the structural changes in hippocampal subfields associated with DIP, focusing on significant atrophy in specific regions and their correlations with cognitive, emotional, and motor symptoms. Our findings highlight the need for greater clinical attention to hippocampal alterations in DIP, particularly given its significant impact on cognition, mood, and even motor-related symptoms. Moreover, understanding the unique patterns of hippocampal atrophy in DIP may provide insights into the broader mechanisms underlying drug-induced neurotoxicity and secondary parkinsonism.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Research Ethics Committee of the Affiliated Hospital of North Sichuan Medical College. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
WZ: Conceptualization, Data curation, Writing – original draft, Writing – review & editing, Formal analysis, Methodology. MT: Conceptualization, Data curation, Formal analysis, Methodology, Software, Writing – original draft, Writing – review & editing. BC: Conceptualization, Data curation, Formal analysis, Methodology, Writing – original draft, Writing – review & editing. LS: Data curation, Formal analysis, Methodology, Writing – original draft. HL: Data curation, Methodology, Writing – original draft. YF: Data curation, Methodology, Writing – original draft. NL: Data curation, Funding acquisition, Software, Writing – original draft. SZ: Conceptualization, Data curation, Funding acquisition, Project administration, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by grants from the Key Project of the Primary Health 13 Development Research Center of Sichuan Province Program (SWFZ17-Z-13); the Natural Science Foundation of Sichuan Province (24NFSC0490) and Sichuan Science and Technology Program (2024ZYD0272).
Acknowledgments
We thank all the participants for participating in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2025.1566785/full#supplementary-material
References
Beyer, M. K., Bronnick, K. S., Hwang, K. S., Bergsland, N., Tysnes, O. B., Larsen, J. P., et al. (2013). Verbal memory is associated with structural hippocampal changes in newly diagnosed Parkinson's disease. J. Neurol. Neurosurg. Psychiatry 84, 23–28. doi: 10.1136/jnnp-2012-303054
Bondon-Guitton, E., Perez-Lloret, S., Bagheri, H., Brefel, C., Rascol, O., and Montastruc, J. L. (2011). Drug-induced parkinsonism: a review of 17 years' experience in a regional pharmacovigilance center in France. Mov. Disorders Off. J. Movement Disorder Soc. 26, 2226–2231. doi: 10.1002/mds.23828
Carlesimo, G. A., Piras, F., Assogna, F., Pontieri, F. E., Caltagirone, C., and Spalletta, G. (2012). Hippocampal abnormalities and memory deficits in Parkinson disease: a multimodal imaging study. Neurology 78, 1939–1945. doi: 10.1212/WNL.0b013e318259e1c5
Eid, R. S., Gobinath, A. R., and Galea, L. A. M. (2019). Sex differences in depression: insights from clinical and preclinical studies. Prog. Neurobiol. 176, 86–102. doi: 10.1016/j.pneurobio.2019.01.006
Fan, C., Song, Q., Wang, P., Li, Y., Yang, M., Liu, B., et al. (2018). Curcumin protects against chronic stress-induced dysregulation of neuroplasticity and depression-like behaviors via suppressing IL-1β pathway in rats. Neuroscience 392, 92–106. doi: 10.1016/j.neuroscience.2018.09.028
Feldman, M., Marmol, S., and Margolesky, J. (2022). Updated perspectives on the Management of Drug-Induced Parkinsonism (DIP): insights from the clinic. Ther. Clin. Risk Manag. 18, 1129–1142. doi: 10.2147/TCRM.S360268
Goetz, C. G., Poewe, W., Rascol, O., Sampaio, C., Stebbins, G. T., Counsell, C., et al. (2004). Movement disorder society task force report on the Hoehn and Yahr staging scale: status and recommendations. Mov. Disord. 19, 1020–1028. doi: 10.1002/mds.20213
Goetz, C. G., Tilley, B. C., Shaftman, S. R., Stebbins, G. T., Fahn, S., Martinez-Martin, P., et al. (2008). Movement disorder society-sponsored revision of the unified Parkinson's disease rating scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov. Disord. 23, 2129–2170. doi: 10.1002/mds.22340
Han, S., Kim, S., Kim, H., Shin, H. W., Na, K. S., and Suh, H. S. (2019). Prevalence and incidence of Parkinson's disease and drug-induced parkinsonism in Korea. BMC Public Health 19:1328. doi: 10.1186/s12889-019-7664-6
Iglesias, J. E., Augustinack, J. C., Nguyen, K., Player, C. M., Player, A., Wright, M., et al. (2015). A computational atlas of the hippocampal formation using ex vivo, ultra-high resolution MRI: application to adaptive segmentation of in vivo MRI. NeuroImage 115, 117–137. doi: 10.1016/j.neuroimage.2015.04.042
Ledoux, A. A., Boyer, P., Phillips, J. L., Labelle, A., Smith, A., and Bohbot, V. D. (2014). Structural hippocampal anomalies in a schizophrenia population correlate with navigation performance on a wayfinding task. Front. Behav. Neurosci. 8:88. doi: 10.3389/fnbeh.2014.00088
Lee, Y., Ho Choi, Y., Lee, J. J., Lee, H. S., Sohn, Y. H., Lee, J. M., et al. (2017). Microstructural white matter alterations in patients with drug induced parkinsonism. Hum. Brain Mapp. 38, 6043–6052. doi: 10.1002/hbm.23809
Li, J., Shang, Y., Wang, L., Zhao, B., Sun, C., Li, J., et al. (2020). Genome integrity and neurogenesis of postnatal hippocampal neural stem/progenitor cells require a unique regulator Filia. Sci. Adv. 6:aba0682. doi: 10.1126/sciadv.aba0682
López-Sendón, J. L., Mena, M. A., and de Yébenes, J. G. (2012). Drug-induced parkinsonism in the elderly: incidence, management and prevention. Drugs Aging 29, 105–118. doi: 10.2165/11598540-000000000-00000
Low, A., Foo, H., Yong, T. T., Tan, L. C. S., and Kandiah, N. (2019). Hippocampal subfield atrophy of CA1 and subicular structures predict progression to dementia in idiopathic Parkinson's disease. J. Neurol. Neurosurg. Psychiatry 90, 681–687. doi: 10.1136/jnnp-2018-319592
Margolesky, J. (2019). Approaching drug-induced parkinsonism from a neurohospitalist perspective. Expert. Rev. Neurother. 19, 93–95. doi: 10.1080/14737175.2019.1569515
Marx, W., Penninx, B., Solmi, M., Furukawa, T. A., Firth, J., Carvalho, A. F., et al. (2023). Major depressive disorder. Nat. Rev. Dis. Primers 9:44. doi: 10.1038/s41572-023-00454-1
Maurice, N., Deltheil, T., Melon, C., Degos, B., Mourre, C., Amalric, M., et al. (2015). Bee venom alleviates motor deficits and modulates the transfer of cortical information through the basal ganglia in rat models of Parkinson's disease. PLoS One 10:e0142838. doi: 10.1371/journal.pone.0142838
Molina, J. L., González Alemán, G., Florenzano, N., Padilla, E., Calvó, M., Guerrero, G., et al. (2016). Prediction of neurocognitive deficits by parkinsonian motor impairment in schizophrenia: a study in neuroleptic-naïve subjects, unaffected first-degree relatives and healthy controls from an indigenous population. Schizophr. Bull. 42, 1486–1495. doi: 10.1093/schbul/sbw023
Movement Disorder Society Task Force on Rating Scales for Parkinson's Disease (2003). The unified Parkinson's disease rating scale (UPDRS): status and recommendations. Mov. Disord. 18, 738–750. doi: 10.1002/mds.10473
Nolan, M., Roman, E., Nasa, A., Levins, K. J., O'Hanlon, E., O'Keane, V., et al. (2020). Hippocampal and amygdalar volume changes in major depressive disorder: a targeted review and focus on stress. Chronic Stress 4:2470547020944553. doi: 10.1177/2470547020944553
Pitton Rissardo, J., and Caprara, A. L. F. (2023). Neuroimaging techniques in differentiating Parkinson's disease from drug-induced parkinsonism: a comprehensive review. Clinics Prac 13, 1427–1448. doi: 10.3390/clinpract13060128
Roddy, D. W., Farrell, C., Doolin, K., Roman, E., Tozzi, L., Frodl, T., et al. (2019). The hippocampus in depression: more than the sum of its parts? Advanced hippocampal substructure segmentation in depression. Biol. Psychiatry 85, 487–497. doi: 10.1016/j.biopsych.2018.08.021
Sämann, P. G., Iglesias, J. E., Gutman, B., Grotegerd, D., Leenings, R., Flint, C., et al. (2022). FreeSurfer-based segmentation of hippocampal subfields: a review of methods and applications, with a novel quality control procedure for ENIGMA studies and other collaborative efforts. Hum. Brain Mapp. 43, 207–233. doi: 10.1002/hbm.25326
Savica, R., Grossardt, B. R., Bower, J. H., Ahlskog, J. E., Mielke, M. M., and Rocca, W. A. (2017). Incidence and time trends of drug-induced parkinsonism: a 30-year population-based study. Mov. Disorders Off. J. Soc. 32, 227–234. doi: 10.1002/mds.26839
Shin, H. W., and Chung, S. J. (2012). Drug-induced parkinsonism. J. Clin. Neurol. 8, 15–21. doi: 10.3988/jcn.2012.8.1.15
Shiraiwa, N., Tamaoka, A., and Ohkoshi, N. (2018). Clinical features of drug-induced parkinsonism. Neurol. Int. 10:7877. doi: 10.4081/ni.2018.7877
Sun, Y., Hu, N., Wang, M., Lu, L., Luo, C., Tang, B., et al. (2023). Hippocampal subfield alterations in schizophrenia and major depressive disorder: a systematic review and network meta-analysis of anatomic MRI studies. JPN 48, E34–e49. doi: 10.1503/jpn.220086
Sung, Y. H., Noh, Y., Lee, J., and Kim, E. Y. (2016). Drug-induced parkinsonism versus idiopathic Parkinson disease: utility of Nigrosome 1 with 3-T imaging. Radiology 279, 849–858. doi: 10.1148/radiol.2015151466
Ursino, M., Véronneau-Veilleux, F., and Nekka, F. (2020). A non-linear deterministic model of action selection in the basal ganglia to simulate motor fluctuations in Parkinson's disease. Chaos 30:083139. doi: 10.1063/5.0013666
Wenning, G. K., Litvan, I., and Tolosa, E. (2011). Milestones in atypical and secondary Parkinsonisms. Mov. Disord. 26, 1083–1095. doi: 10.1002/mds.23713
Xu, R., Hu, X., Jiang, X., Zhang, Y., Wang, J., and Zeng, X. (2020). Longitudinal volume changes of hippocampal subfields and cognitive decline in Parkinson's disease. Quant. Imaging Med. Surg. 10, 220–232. doi: 10.21037/qims.2019.10.17
Yamada, H., Nakamori, M., Nezu, T., Kotozaki, T., Kitamura, J., Ohshita, T., et al. (2022). Clinical factors predicting voluntary driving cessation among patients with Parkinson's disease. Behav. Neurol. 2022, 1–6. doi: 10.1155/2022/4047710
Yildiz, D., Erer, S., Zarifoğlu, M., Hakyemez, B., Bakar, M., Karli, N., et al. (2015). Impaired cognitive performance and hippocampal atrophy in Parkinson disease. Turk. J. Med. Sci. 45, 1173–1177. doi: 10.3906/sag-1408-68
Zhang, Y., Cao, S. X., Sun, P., He, H. Y., Yang, C. H., Chen, X. J., et al. (2016). Loss of MeCP2 in cholinergic neurons causes part of RTT-like phenotypes via α7 receptor in hippocampus. Cell Res. 26, 728–742. doi: 10.1038/cr.2016.48
Zhou, W., Tang, M., Sun, L., Lin, H., Tan, Y., Fan, Y., et al. (2024). Subcortical structure alteration in patients with drug-induced Parkinsonism: evidence from neuroimaging. IBRO Neurosci. Rep. 16, 436–442. doi: 10.1016/j.ibneur.2024.03.001
Zhu, X. L., Chen, J. J., Han, F., Pan, C., Zhuang, T. T., Cai, Y. F., et al. (2018). Novel antidepressant effects of Paeonol alleviate neuronal injury with concomitant alterations in BDNF, Rac1 and RhoA levels in chronic unpredictable mild stress rats. Psychopharmacology 235, 2177–2191. doi: 10.1007/s00213-018-4915-7
Keywords: Drug-induced Parkinsonism, Parkinson’s disease, hippocampus, subfields, cognitive impairment
Citation: Zhou W, Tang M, Cheng B, Sun L, Lin H, Fan Y, Liu N and Zhang S (2025) Exploring cognitive and emotional symptoms associated with hippocampal subfield atrophy in drug-induced Parkinsonism. Front. Aging Neurosci. 17:1566785. doi: 10.3389/fnagi.2025.1566785
Edited by:
Alberto Cacciola, University of Messina, ItalyReviewed by:
Anupa A. Vijayakumari, Cleveland Clinic, United StatesCharles Okanda Nyatega, Mbeya University of Science and Technology, Tanzania
Sebastiano Vacca, Northwell Health, United States
Copyright © 2025 Zhou, Tang, Cheng, Sun, Lin, Fan, Liu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shushan Zhang, c3VzYW40NDhAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Wei Zhou1†
Wei Zhou1† MengYue Tang
MengYue Tang Ling Sun
Ling Sun Yang Fan
Yang Fan Nian Liu
Nian Liu Shushan Zhang
Shushan Zhang