- 1Department of Cardiology, the Fourth Affiliated Hospital of School of Medicine, International School of Medicine, International Institutes of Medicine, Zhejiang University, Yiwu, China
- 2Shanghai Weiyu High School, Shanghai, China
- 3Xuhui District Central Hospital, Shanghai, China
Background: Cognitive impairment is closely linked to immune system dysfunction, with increasing research interest in the underlying mechanisms and potential therapeutic targets. Bibliometric analysis provides a comprehensive approach to understanding research trends, influential contributions, and emerging topics in this interdisciplinary field.
Methods: This study conducted a bibliometric analysis of publications related to the immune system and cognitive impairment from 1985 to 2024, retrieved from the Web of Science Core Collection. CiteSpace (6.4. R1), VOSviewer (1.6.20), and R-bibliometrix (R 4.3.0) were employed to analyze publication trends, co-authorship networks, keyword clustering, and co-citation patterns. Key metrics, including the H-index, G-index, and M-index, were computed to assess academic influence.
Results: A total of 3,737 publications were analyzed, revealing a significant increase in research output since 2021. The United States and China emerged as leading contributors, with a robust presence of collaborative networks. Keyword and co-citation analysis identified core research themes, including neuroinflammation, microglia activation, gut microbiota, TREM2-mediated immune responses, and inflammasomes. Emerging topics such as the gut–brain axis, metabolic syndromes, and immune regulation in neurodegenerative diseases have gained prominence in recent years. Highly cited papers highlighted the role of immune dysregulation in Alzheimer’s disease, multiple sclerosis, and HIV-associated neurocognitive disorders.
Conclusion: This bibliometric analysis provides a comprehensive overview of research trends in immune-related cognitive impairment. The findings indicate an increasing focus on neuroinflammatory mechanisms, immune cell interactions, and novel immunotherapeutic strategies. Future research is expected to further explore the gut–immune–brain axis and precision medicine approaches in managing cognitive disorders. These findings may facilitate early detection strategies and novel interventions targeting immune–cognitive interactions, such as gut–brain axis modulation.
1 Introduction
The immune system in vertebrates consists of two primary subsystems: the innate and adaptive immune systems (Mohmmad-Rezaei et al., 2021). The innate immune system responds to pathogens through germline-encoded receptors, which are expressed particularly in cell types that are not clonally distributed. Pattern recognition receptors (PRRs), such as Toll-like receptors (TLRs), recognize microbial pathogens and damage-associated molecular patterns (DAMPs)—including misfolded proteins, denatured DNA, and lipopolysaccharides (LPS)—playing a crucial role in initiating NF-κB signaling and inflammation (Barton et al., 2014). The cellular components of the innate immune system consist of dendritic cells (DCs), monocytes, macrophages (as well as microglia in the brain), and natural killer T cells. The innate immune system responds rapidly to triggers, but its response is relatively nonspecific. In contrast, the adaptive immune response can be very specific. This specificity depends on the large repertoires of antigen receptors on T and B cells (TCRs and BCRs), the cellular elements of the adaptive immune system (Clark, 2006).
Both the innate and adaptive immune responses are present in the central nervous system (CNS), although the immune niche is safeguarded as “immune privileged” in the homeostatic brain parenchyma. Various studies on neurodegenerative diseases, including Alzheimer’s disease (AD), have demonstrated the involvement of both innate and adaptive immune responses in the initiation and progression of the disease, indicating the remodeling of the immune state and the cooperation of both arms of immunity within the brain parenchyma (Amor and Woodroofe, 2014).
Recent studies have shown that high levels of circulating C-reactive protein, IL-1, IL-6, tumor necrosis factor-α (TNF-α), and CD4 + T-cell count might increase the risk of dementias (Gate et al., 2021; Contreras et al., 2022; Zhao et al., 2024). This new focus has been supported by epidemiological studies that link chronic inflammatory diseases (for example, diabetes, autoimmune diseases, and severe infections) to an increased risk of dementia (Gudala et al., 2013; Sipilä et al., 2021; Cooper et al., 2023). It is widely believed that in a healthy state, the blood–brain barrier (BBB) protects the CNS from peripheral neurotoxic molecules and pathogens, keeping the CNS immune privileged (Wei et al., 2024). However, aging and peripheral inflammation that arises from low-grade systemic inflammation and infections can disrupt this function (Geloso et al., 2024; Quiros-Roldan et al., 2024; Tamatta et al., 2025). A dysfunctional BBB might promote the expression of endothelial adhesion molecules and chemokines, lead to the migration of peripheral leukocytes into the CNS (Geloso et al., 2024), and then result in the activation of the central immune system and exposure of the CNS to prolonged neuroinflammation and subsequent neurodegeneration.
Cognitive impairment is a common complication of various diseases that can affect the neurological system, such as HIV infection and multiple sclerosis. An increasing number of studies have explored the potential risk factors and underlying mechanisms of cognitive impairment, including the role of the immune system. However, the pathogenesis of cognitive impairment varies significantly across conditions and remains poorly understood. This complexity underscores the need for more integrated, cross-disease investigations that incorporate both clinical and immunological perspectives. While prior bibliometric studies have mapped general trends, gaps remain in cross-disease immune mechanisms (e.g., comparing Alzheimer’s disease, HIV, and multiple sclerosis) and the longitudinal evolution of research themes. This study aims to address these gaps by analyzing 40-year trends, emerging molecular topics (e.g., TREM2 and NLRP3), and global collaboration networks to identify underexplored therapeutic targets. In this study, bibliometric methods were adopted to summarize the previous studies on the immune system and cognitive impairment. Based on this study, we aim to summarize the hotspots in this area and indicate potential directions for future research.
2 Materials and methods
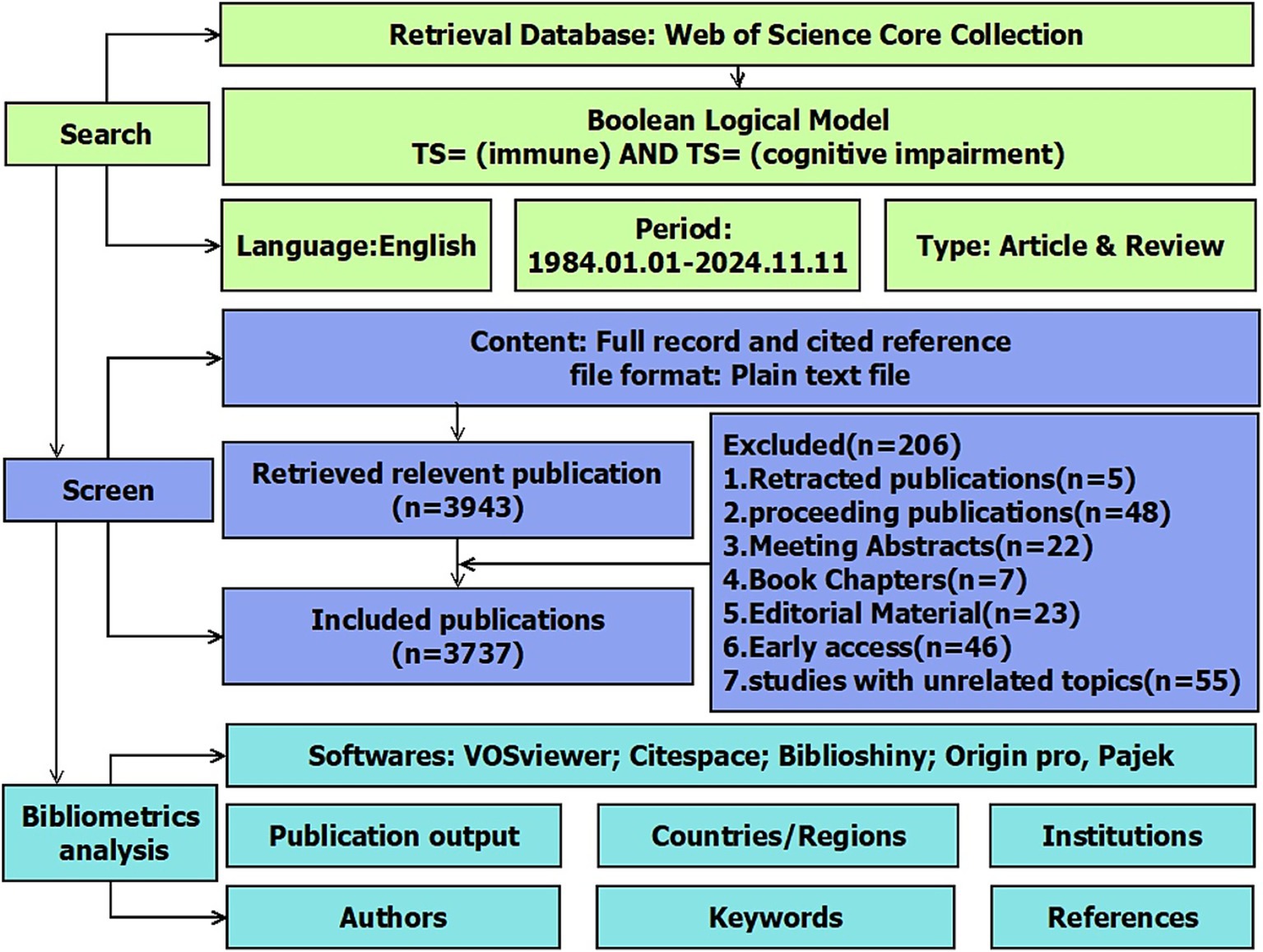
2.1 Data sources and collection
This study utilized the Web of Science (WOS) as its primary database. To mitigate potential errors from database updates, we promptly acquired all source data from the WOS Core Collection within a single day (November 11, 2024). We included early access and in-press articles available at that time, provided they had confirmed metadata and DOI numbers. Given concerns about redundancy and peripheral literature with “topic” retrieval, data were retrieved from the Science Citation Index Expanded web database in the WOS Core Collection. We considered the comprehensiveness, timeliness, and relevance of the sources. We used the following Boolean search string in the Web of Science Core Collection: TS = (“cognitive impairment”) AND TS = (“immune”). Synonyms and truncated terms (e.g., “neurodegeneration*”) were not included to ensure specificity. Excluded records included 5 retracted articles, 48 conference proceedings, and other non-peer-reviewed document types (Figure 1). From 1985 to 2024, a total of 3,737 publications were obtained, all of which were reviews and articles in English, downloaded under the name “XXX_download.txt” for further bibliometric visualization and analysis. The exact operational procedure is shown in Figure 1.
2.2 Data analysis
In the study, CiteSpace (version 6.4. R1) software, developed by Prof. Chaomei Chen, was employed for burst analysis and clustering of keywords and references. VOSviewer (version 1.6.20) was used to visually represent co-occurrence networks of countries, institutions, journals, authors, and keywords, as well as co-citation networks of authors and journals. R-bibliometrix (version R 4.3.0) and R-Studio were used to analyze detailed publication trends for countries, institutions, authors, and journals. Moreover, metrics such as the H-index, G-index, and M-index were computed for countries, journals, and authors. However, we acknowledge that these indicators may underrepresent newly emerging scholars due to citation lag. OriginPro 2024 (version 10.1.0.178) was used for visualizing publication and citation numbers. Pajek offers various layout algorithms and beautification options for VOSviewer, enhancing the clarity and presentation of network structures. In this study, it was mainly used for optimizing the layout of keyword co-occurrence networks. The document selection and analysis process is illustrated in Figure 1.
3 Results
3.1 Trend and annual count
Plotting the distribution of literature over time can effectively evaluate the state of research in this discipline and further predict its dynamics and trends. Figure 2 displays the annual distribution of literature related to cognitive impairment and immune research on the WoS for the past 40 years. From the trends in publication and citation, it is evident that the number of citations for these articles has increased steadily year by year. We divided the research into three phases, reflecting annual publication trends. From 1985 to 2000, the field saw limited development, with only ten articles published per year, marking the “preparation period.” The second phase (2001 to 2020) exhibited a gradual upward trend with a moderate growth curve. The third phase (2021 to 2024) experienced a surge, surpassing 400 annual publications and reaching 1,602 articles (Figure 2), indicating that cognitive impairment and immune research has entered a rapid development stage. The increase in publications from 2021 to 2024 may be attributed to growing attention to neuroimmune mechanisms during the COVID-19 pandemic. This trend is likely to continue in the coming years as researchers continue to uncover the complex relationships between cognitive health and immune function, which may lead to new breakthroughs and treatments for cognitive impairment.
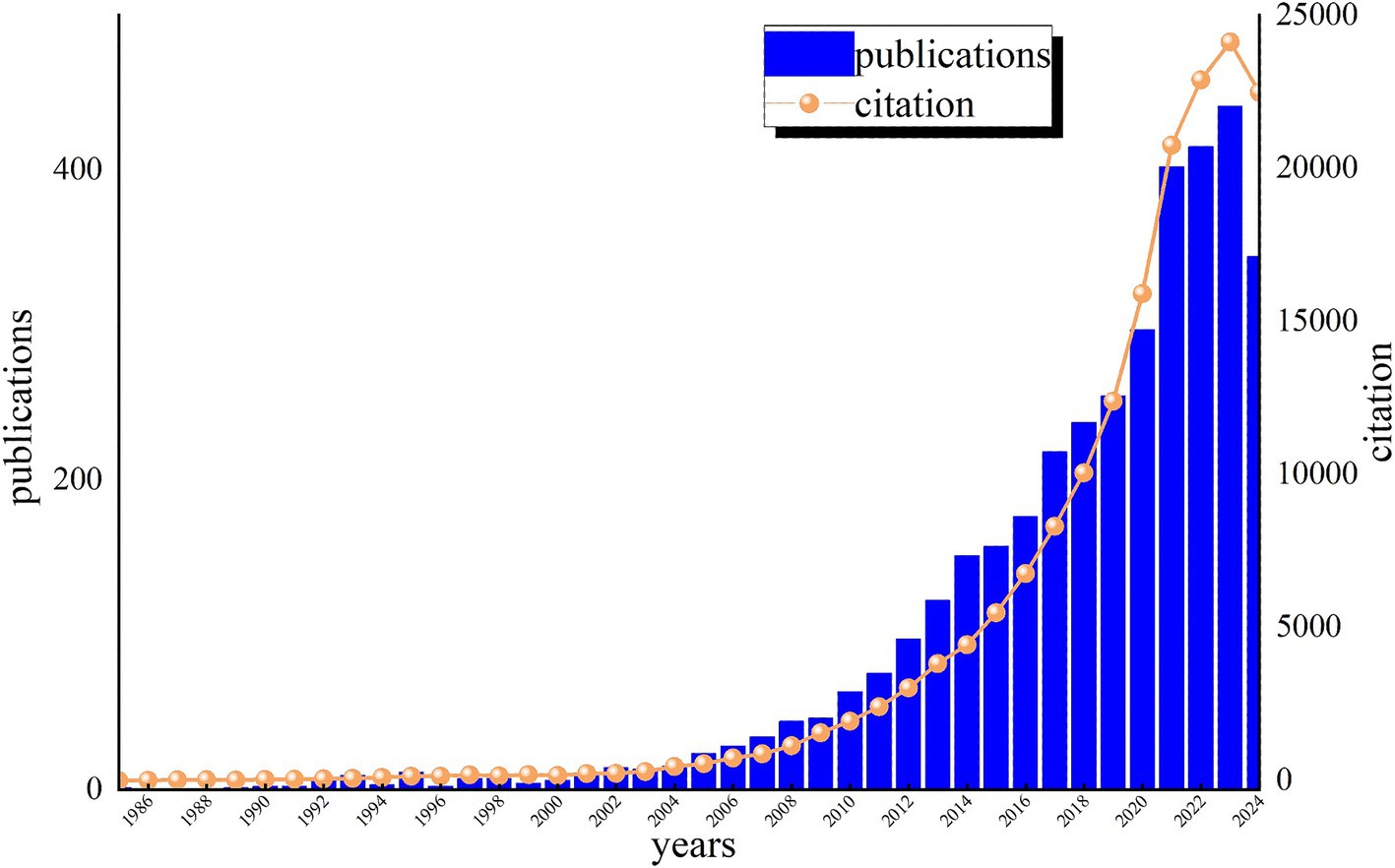
Figure 2. Annual publications and citations of cognitive impairment and immune research on the Web of Science (WOS).
3.2 Analysis of article output characteristics
3.2.1 Co-countries analysis
Analysis of co-authorship involving countries, institutions, and authors facilitates the identification of potential partners, providing valuable insights for acquiring academic resources, fostering scholarly collaborations, and evaluating academic outcomes. A total of 58 countries actively contribute to cognitive impairment and immune research. Key contributors include the USA (1,419) and China (726) (Figure 3A). Publications from other countries or regions numbered fewer than 350, including Italy (314), the United Kingdom (290), Germany (236), and Canada (208). The USA dominates with the highest citations (88,373) and total link strength (TLS, 821). International collaboration shows relatively fewer joint publications (26.11%) (Figure 3B), with USA-centric collaborations occupying nine of the top 10 partnership positions, mainly with China.
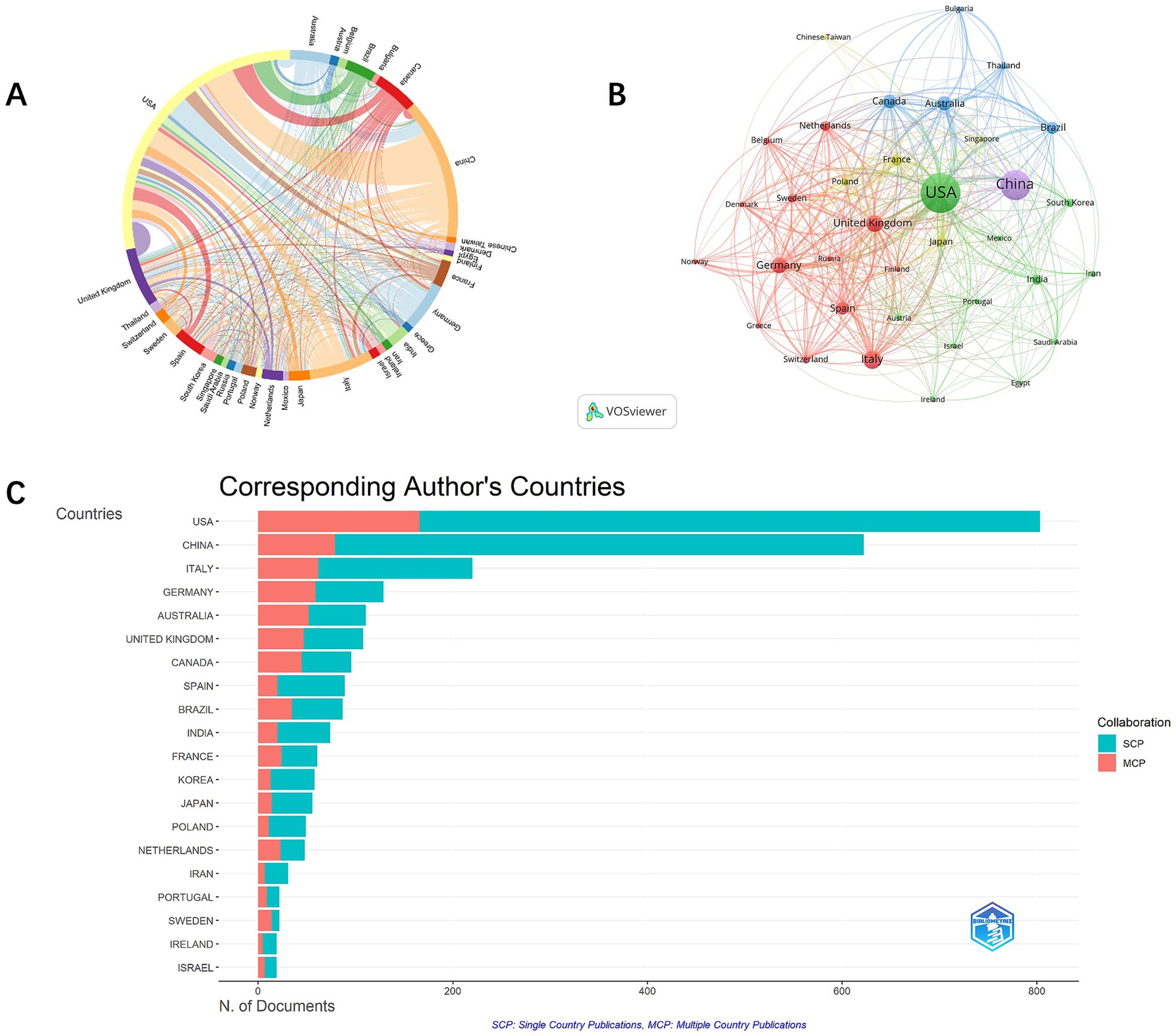
Figure 3. Chord diagram of countries co-authorship analysis. (A). Network visualization map of countries co-authorship analysis. (B). The number of citations and publications in the top 20 cited countries (C).
3.2.2 Co-institutions analysis
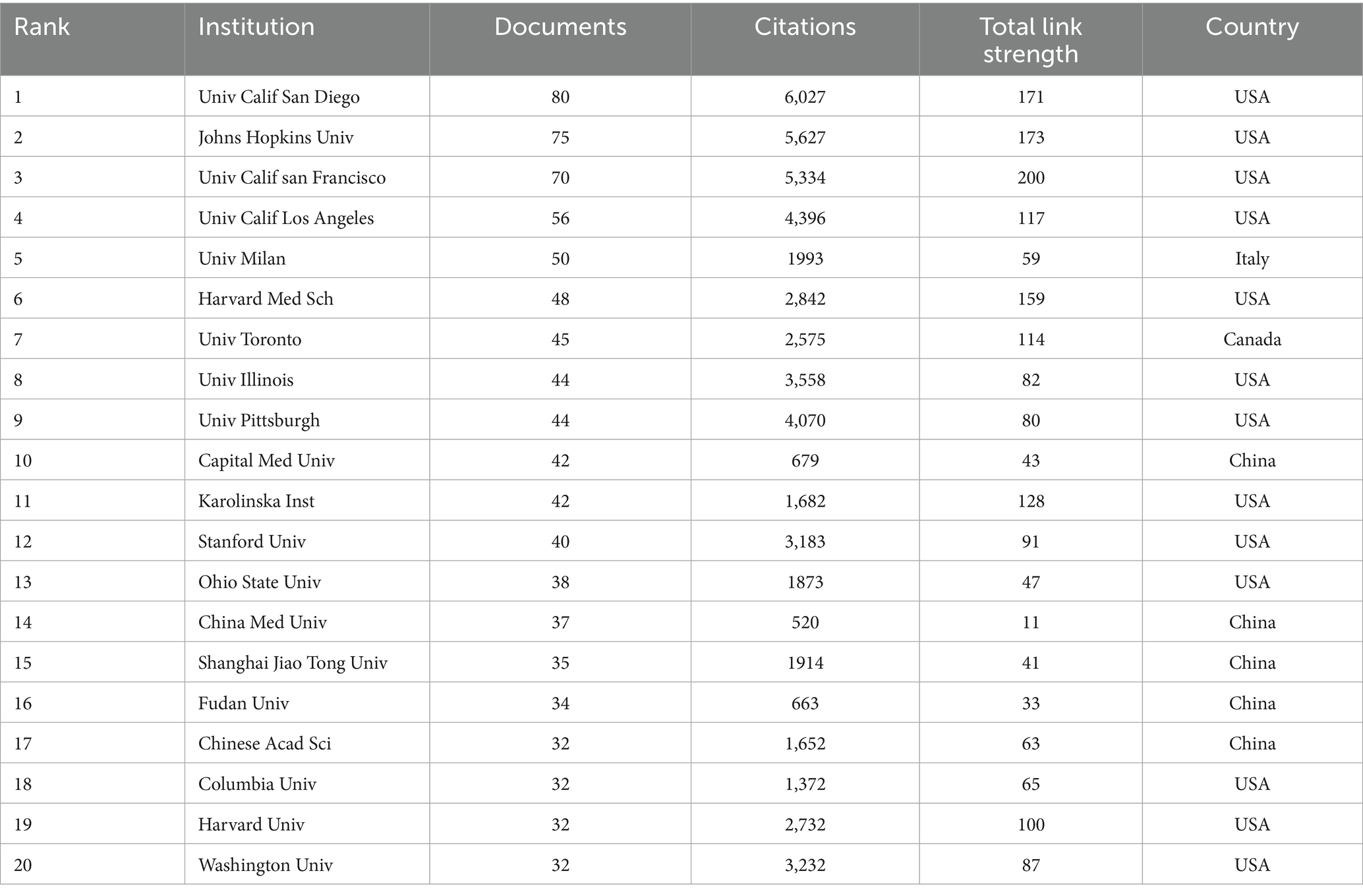
A total of 4,418 institutions were included in the analysis, with 465 institutions having a minimum publication output of 5. The top ten contributors are predominantly from the USA. The University of California, San Diego leads with 80 articles and the highest citations (6,027). Despite ranking third in output, the University of California, San Francisco excels in TLS (200) (Table 1; Figures 4A,B. Five Chinese institutions are among the top 20 institutions in terms of contributions. However, most of them have relatively lower citation rates, with the exception of Shanghai Jiao Tong University (35 articles, 1,914 citations) and the Chinese Academy of Sciences (32 articles, 1,652 citations). Despite high publication output, Chinese institutions exhibited lower citation rates, possibly due to more recent publication dates, language barriers, and limited international collaborations. As shown in Figures 4C,D, from 2021 to 2024, a total of 2,715 institutions published articles, with 195 institutions having a minimum publication count of more than 5 articles. The analysis shows that institutions in the United States still have the highest publication numbers, followed by those in China and Europe (Table 2). Among them, the publications of Chinese institutions are relatively recent.
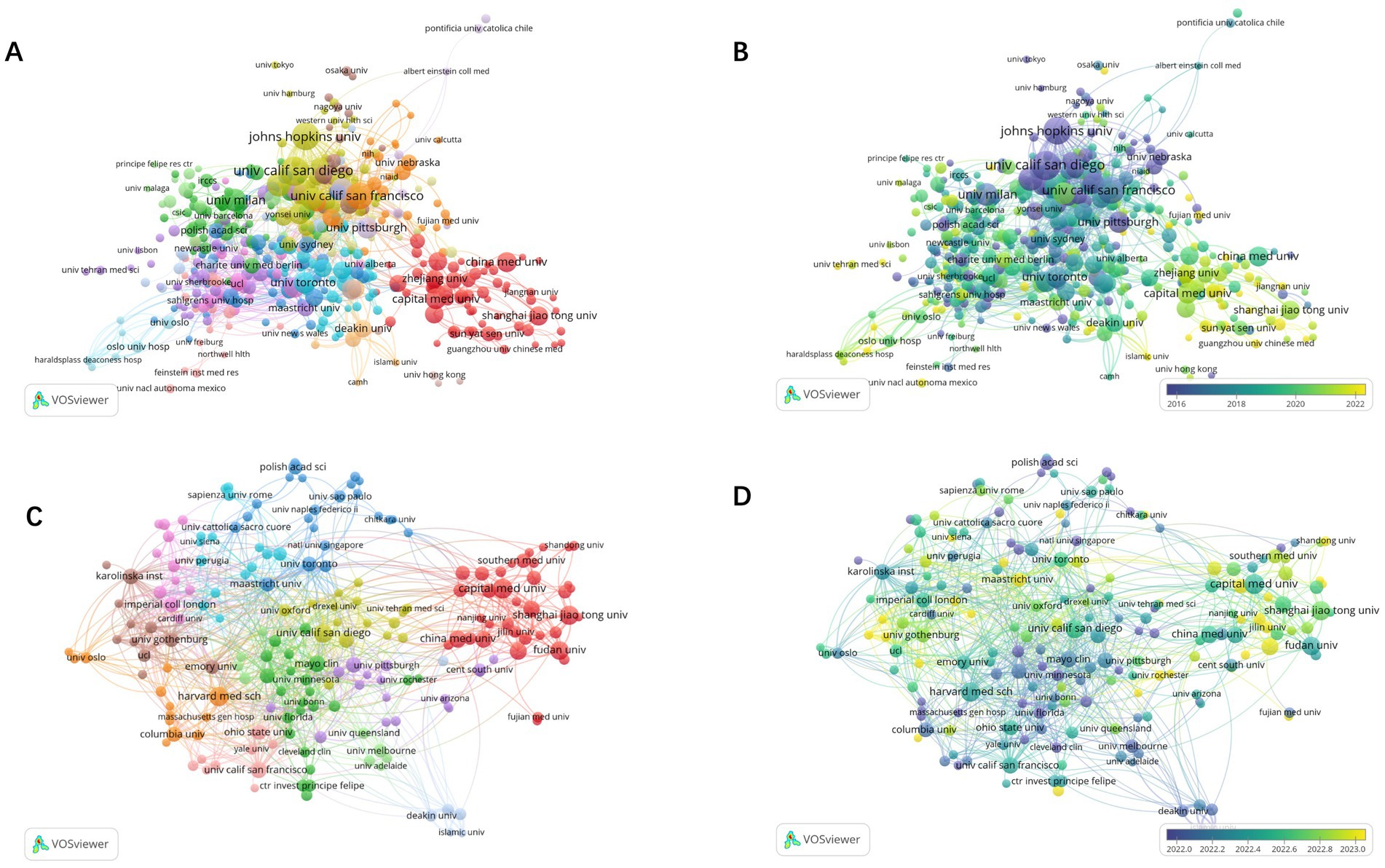
Figure 4. Network visualization map of institutions co-authorship analysis from 1985 to 2024 (A). Overlay visualization map of institutions co-authorship analysis from 1985 to 2024 (B). Network visualization map of institutions co-authorship analysis from 2021 to 2024 (C). Overlay visualization map of institutions co-authorship analysis from 2021 to 2024 (D).
3.2.3 Co-author analysis
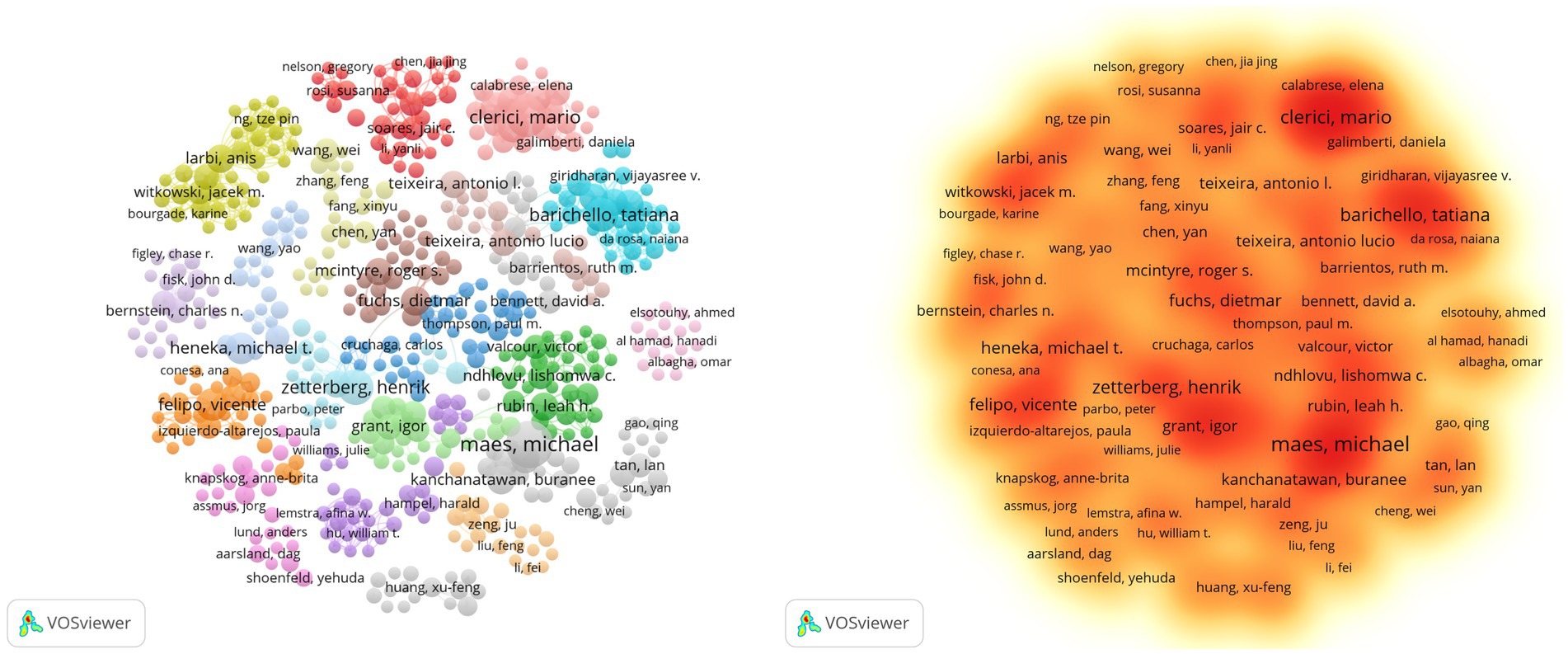
This analysis, as shown in Supplementary Table S1 and Figure 5, reveals 21,430 contributors, with 193 authors having over five articles. Notable authors include Michael Maes (the highest articles: 29; citations: 1,465; TLS: 38), Mario Clerici (articles: 21; citations: 989; TLS: 109), and Michael T. Heneka (articles: 12; citations: 1,552; TLS: 5). Additionally, Tony Wyss-Coray, Michael T. Heneka, and Michal Schwartz, despite a publication count of only 6–12 articles, rank among the top three in citation frequency, showcasing their high-quality contributions. Michael Maes focuses his research on inflammation-related psychiatric disorders such as major depressive disorder, bipolar disorder, and schizophrenia, as well as cognitive impairments associated with these conditions (Al-Hakeim et al., 2018; Maes and Kanchanatawan, 2021). Mario Clerici specializes in investigating the impact of inflammatory mediators, such as cytokines and chemokines, on cognitive function in both normal and neurodegenerative disease states, with his research spanning the fields of molecular biology and genetics (Krasemann et al., 2017; Mancuso et al., 2019, 2020). Heneka emphasizes that neuroinflammation induced by the immune system may be a significant contributor to the onset of neurodegenerative cognitive impairments, in which microglia play a dual role (Heneka et al., 2015). He particularly focuses on microglia and the NLRP3 inflammasome as potential therapeutic targets, exploring their protective effects in this context. Based on Figure 6C, it is evident that while some high-producing authors have experienced a decline in their output in recent years, Heneka has consistently maintained a high level of quality output since 2014. This demonstrates his continued dedication and credibility in his field.
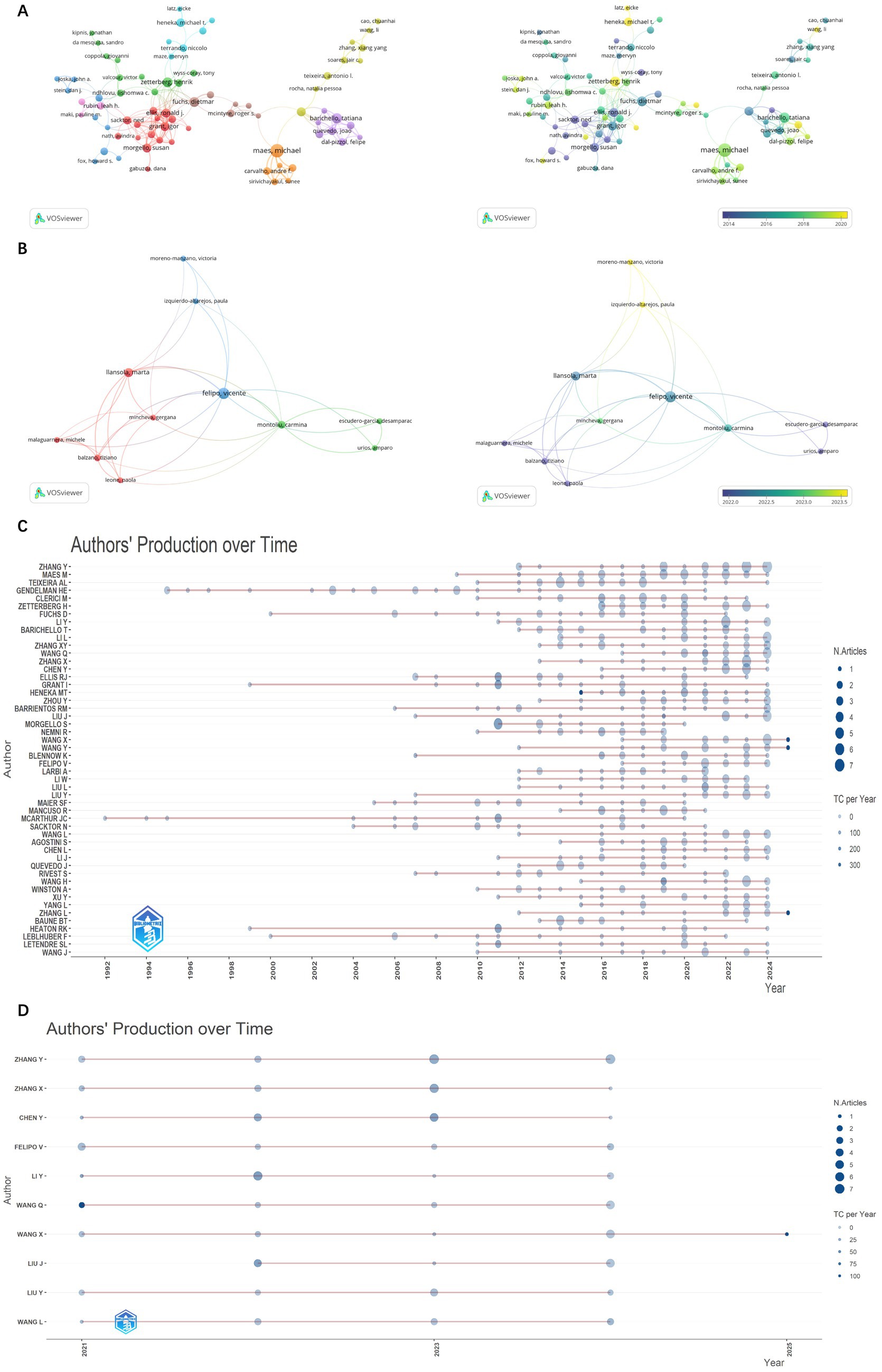
Figure 6. Network visualization map and overlay visualization map of authors citation analysis from 1985 to 2024 (A). Network visualization map and overlay visualization map of authors citation analysis from 2021 to 2024 (B). The number of citations and publications in the top 50 cited authors from 1992 to 2024 (C). The number of citations and publications in the top 10 cited authors from 2021 to 2024 (D).
During 2021–2024, there are 10,988 contributors, with 174 authors having over three articles. Based on the analysis of publication number, there are some differences compared to the data from 1985 to 2024 (as shown in Figures 6A,B and Supplementary Table S2). Vicente Felipo occupies a central position in author collaboration, based on Figure 6D, he has maintained a stable publication output since 2021, through whom many authors are able to co-publish papers. Meanwhile, we can observe that although the number of publications is not high, Victoria Moreno-Manzano, Paula Izquierdo-Altarejos, and Mincheva, Gergana, who are from the same institution and collaborate with Vicente Felipo have published very recent work. They all mainly studied the reversal effect of extracellular vesicles on neuroinflammation in metabolic and neurodegenerative diseases, as well as their improvement on cognition (Wang et al., 2024). They also proposed that neuroinflammation not only leads to neuronal damage and death but also affects the connectivity and function of neural networks.
Co-citation analysis, a methodology in which a third author or paper concurrently cites two authors or papers, elucidates these intricately interwoven associations. A total of 194 authors who published more than 5 papers have been co-cited, illustrating the clustering information of co-cited authors analyzed using CiteSpace. By analyzing co-cited author clusters, researchers can discern highly co-cited scholars and their impact within a specific field. We used CiteSpace to analyze the author co-citation clustering for the two time periods of 1985–2024 and 2021–2024, as shown in Supplementary Tables S3, S4. Across these timeframes, it is evident that HIV-associated neurocognitive disorders garnered brief attention. Over a longer timespan (such as the entire 1985–2024 period), AD, microglia, T cells, and the NLRP3 inflammasome have consistently been research hotspots. Research in these areas may have continued to deepen, forming stable research communities and knowledge systems.
Emerging research areas: In the more recent timeframe (2021–2024), areas such as gut microbiota and trem2 have begun to receive increasing attention. These new areas may have introduced new research directions and academic controversies, potentially driving the development of related fields.
3.3 Analysis of the journals
The criteria for assessing a journal’s influence include publication volume, citation frequency, and Impact Factor (IF). Among the top ten journals, only three have an IF over 10: NATURE (IF 50.5), SCIENCE (IF 44.8), and NEURON (IF 14.7). J neurosci leads in citations (6531).
The purpose of co-citation analysis is to identify the papers frequently cited and the journals publishing these papers within a research field. By utilizing VOSviewer to create a co-citation map of journals, we set a threshold of a minimum of 500 co-citations for each journal, resulting in the selection of 119 journals for the co-citation analysis of cited journals. The final co-citation relationship diagram is presented in Table 3.
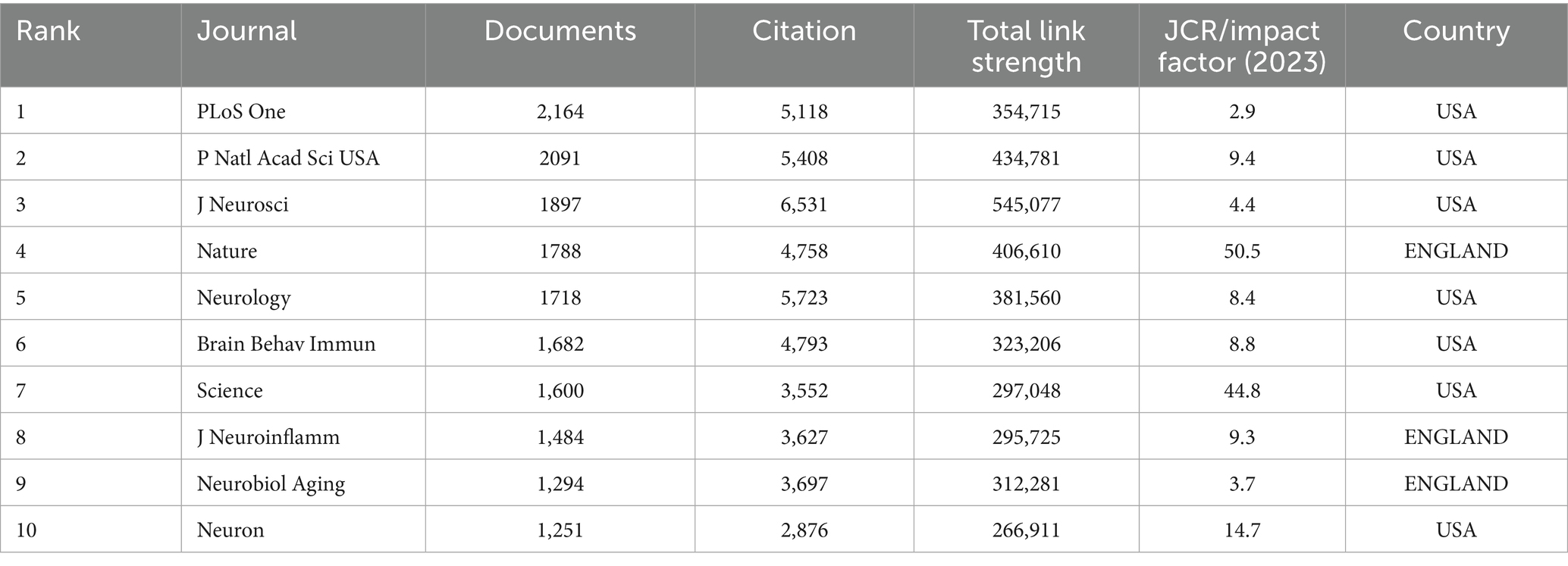
Table 3. The top 10 journals contributed to publications on immune and cognitive impairment research.
The co-citation network mainly consists of three clusters, corresponding to the three colors in Figure 7A. The top three journals in terms of citation count are J Neurosci (6,531 citations), Neurology (5,723 citations), and P Natl Acad Sci USA (5,408 citations). Among the three clusters, the journals in the red cluster primarily focus on aging, dementia, and biochemistry, while the journals in the green cluster mainly concentrate on the pathophysiology and pharmacology related to psycho-behavioral impairments. The journals heatmap indicates that the co-cited journals from 2021 to 2024 are more concentrated than before and appear in cluster form. In 2021, published articles emphasized the diversification of research methods and the application of new technologies. The magazines in 2022 covered more cross-disciplinary research across multiple professional fields, highlighting the integration of basic research and clinical applications. In 2023, while continuing the trend of multidisciplinary intersection, the published articles focused on the relationship between immunity and cognition in disease states, with an emphasis on exploring mechanisms. In 2024, the magazines were more focused on aging-related topics and continued to explore the relationship between neuro-immunity in disease models (Figures 7A,B).
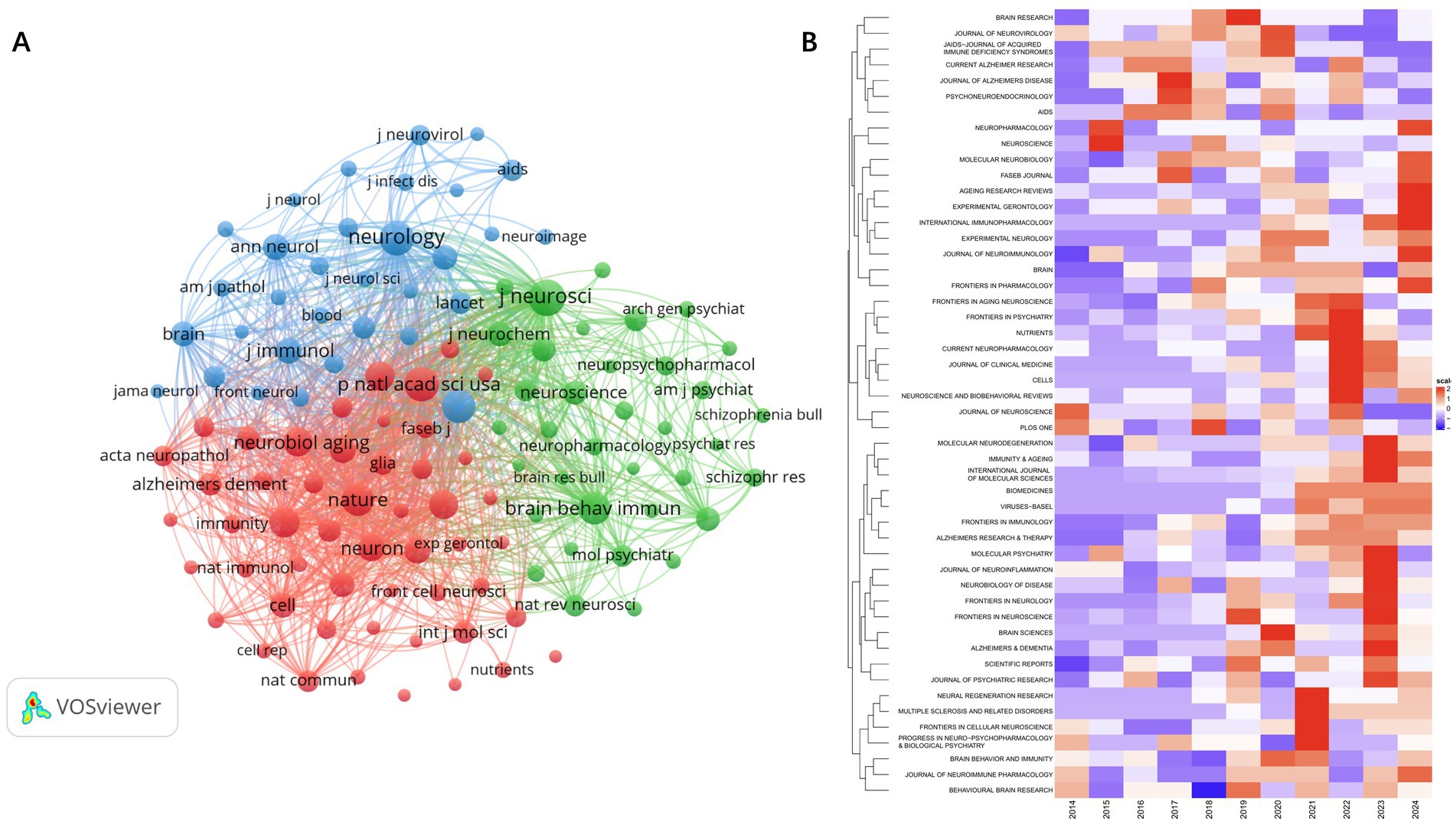
Figure 7. Network visualization map of source citation analysis (A). Heatmap of source citation analysis (B).
3.4 Analysis of keywords
Keywords play a pivotal role in summarizing the themes and content of articles. Based on bibliometric keyword trend analysis it can be seen that the keywords have become prominent and concentrated during the period from 2014 to 2024 (Figure 8A).
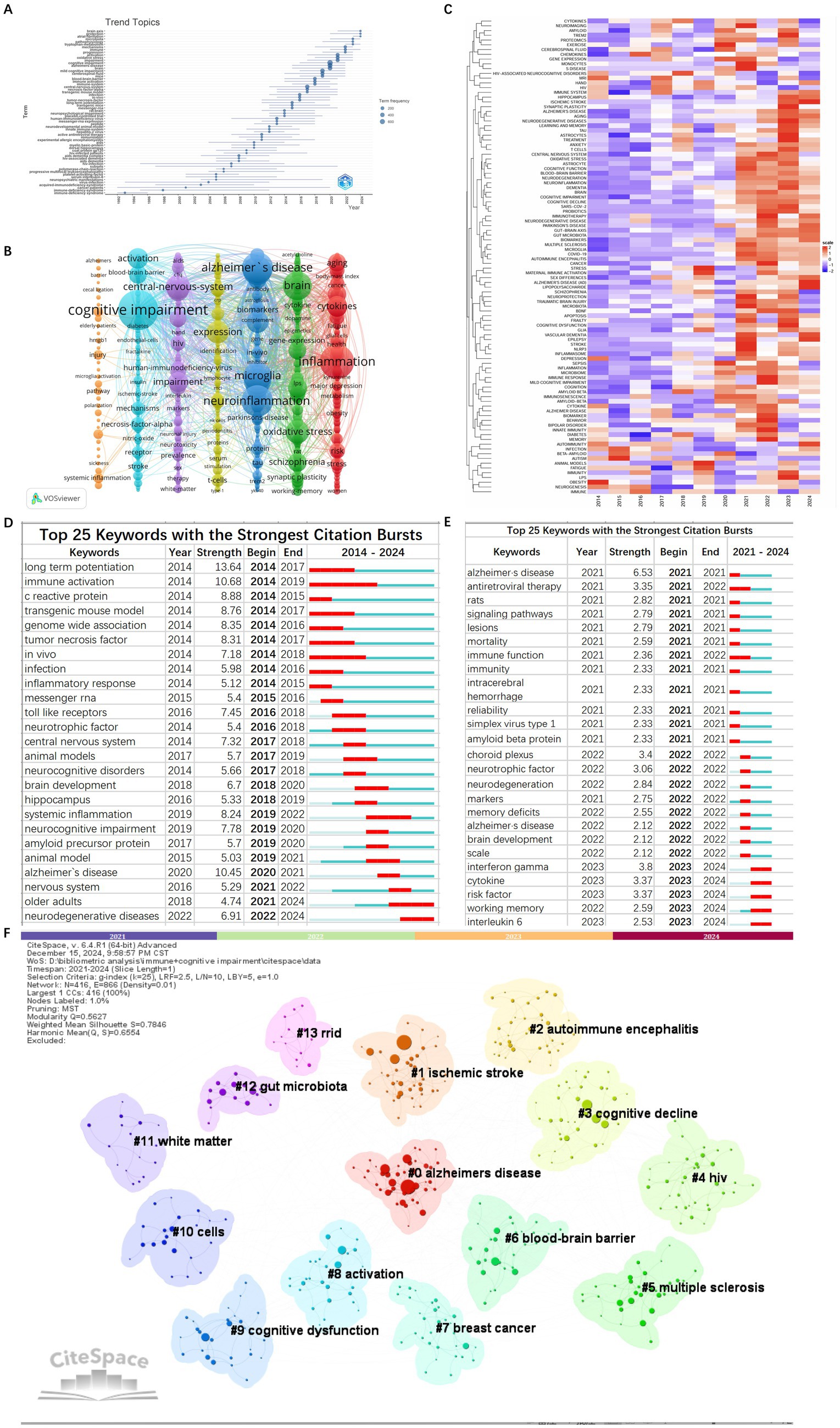
Figure 8. Bibliometrix keyword trend analysis in the field of immune and cognitive impairment studies (A). Keywords co-occurrence network visualization map in the field of immune and cognitive impairment studies (B). Heatmap of keywords analysis (C). The top 25 keywords with the strongest citation bursts from 2014 to 2024 (D). The top 25 keywords with the strongest citation bursts from 2021 to 2024 (E). The cluster visualization map of keywords with the strongest citation bursts (F).
The extraction of 13,988 keywords unveiled the top three: cognitive impairment (971), inflammation (716), and neuroinflammation (604) (Figure 8B).
We have analyzed the keywords over the past decade. The keyword heatmap reveals that, in addition to the persistent focus on cognitive impairment, neuroinflammation, and AD, new keywords such as ischemic stroke, astrocytes, neuroprotection, immunotherapy, and vascular dementia have emerged from 2021 to 2024 (Figure 8C).
Keyword burst detection identifies terms with markedly increased frequencies signaling emerging research directions. Burst analysis identifies 25 keywords withrobust citation bursts including long-term potentiation (13.642014–2017) immune activation (10.682014–2019) and alzheimer’s diseases (10.452020–2021). In the recent 4 years (2021–2024) keywords with significant bursts include alzheimers diseases (6.532021) choroid plexus (3.42022) antiretroviral (3.352021–2022) cytokine (3.372023–2024) risk factor (3.372023–2024) and working memory (2.592023–2024) (Figures 8D–F) indicating that future research may increase the focus on vascular factors. The transition from early mechanistic terms such as ‘long-term potentiation’ to immune-related keywords like ‘cytokines’ further reflects a thematic shift in the field—from classical neurophysiological mechanisms to immune-mediated models of cognitive impairment.
Cluster analysis of keywords enables a comprehensive and precise examination of relationships among keywords. Cluster analysis generated reveals 10 keyword clusters using CiteSpace: #0 microglia, #1 Alzheimer’s disease, #2 autoimmune encephalitis, #3 hiv, #4 schizophrenia, #5 multiple sclerosis, #6 hippocampus, #7 gut microbiota, #8 kynurenine pathway, #9 immunne response, #10 neurodegenerative disease. #0, #1, and #2 emerged as the top 3 clusters. Figures 9A,B illustrate the developmental trajectory of keyword clusters, highlighting ongoing advancements in clusters #5, #7, and #8. These continuous advancements underscore the current dynamism of these research domains in immune and cognitive impairment studies.
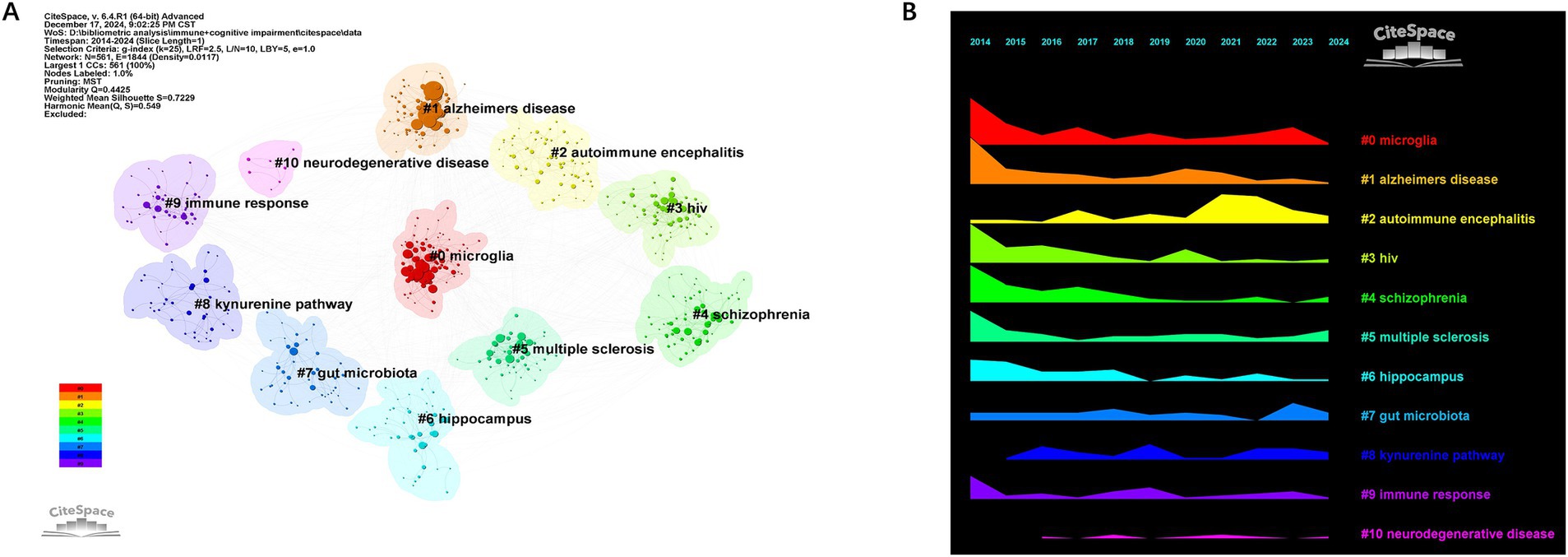
Figure 9. The cluster visualization map of keywords analysis (A). The timeline chart of keywords analysis (B).
3.5 Seminal literature: landmark research
Highly cited literature often signifies impactful and innovative research in its field. Analyzing these studies provides insights into advancements, trends, and future directions in immune and cognitive impairment studies.
Scrutinizing the countries, institutions, and authors associated with this literature identifies key contributors in the immune and cognitive impairment domain, aiding global research understanding and fostering future collaborations.
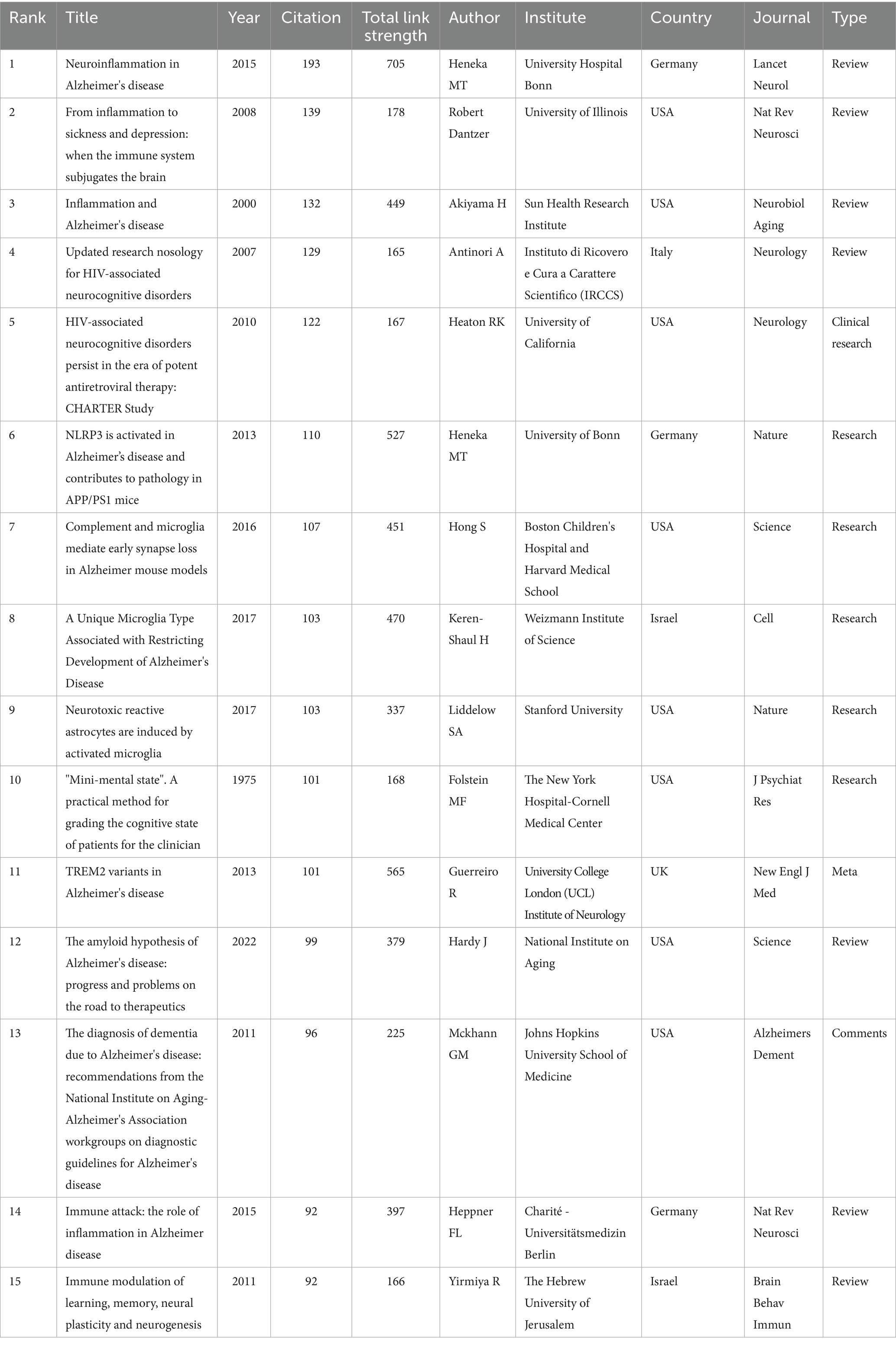
Among the top 15 cited works, there are 6 reviews, 6 articles, 1 meta-analysis, and 2 comments. The USA is the primary contributor (8), followed by Germany (3) and Israel (2). Table 4 highlights the top 15 articles, including Heneka MT’s 2015 review in LANCET NEUROL, which garnered the highest number of citations (193). It reviewed that the pathogenesis of Alzheimer’s disease is not restricted to the neuronal compartment but includes strong interactions with immunological mechanisms in the brain. Modulation of risk factors and targeting these immune mechanisms could lead to future therapeutic or preventive strategies for Alzheimer’s disease. Other highly cited articles predominantly discuss the role of neuroinflammation in various neurocognitive disorders, including Alzheimer’s disease, addressing immune activation, neuroinflammatory mechanisms, TREM2, NLRP3, cognitive impairment, and their relationship with disease progression.
They also explore the impact of various factors, such as age, activation of the peripheral immune system, and immune cell function, on neuroinflammation and cognitive function.
3.6 Analysis of co-cited references
3.6.1 Clusters and timeline of research
Co-citation reflects the referencing of two or more papers by other scholarly works, showcasing their interconnectedness within the research domain (27). Co-citation analysis elucidates foundational knowledge and the frontiers of academic research, enhancing our understanding of the field’s structure and evolution. CiteSpace was employed for a 2014–2024 co-citation analysis, resulting in 18 principal clusters. The clustering results (Q = 0.79, S = 0.93) highlight reliability (Figure 10A). The largest cluster is #0 ykl40. There are 13 principal clusters for the 2021–2024 co-citation analysis (Q = 0.77, S = 0.93) (Figure 10B). When comparing the two sets of data, the gut microbiota, T cells, metabolic syndrome, MCP-1, and NLPR3 have all garnered significant attention. The emergence of terms such as “microglia” and “inflammasome” during this period indicates an increased interest in studying the role of microglia in immunity and cognitive impairments from 2021 to 2024. As immune cells in the brain, microglia’s role in neuroinflammation, neurodegenerative diseases, and other pathological processes related to cognitive impairments may become a research hotspot. Figure 10C shows the temporal evolution of the main clusters. It is evident that clusters #8 and #11 have received sustained attention. Outlining their critical research focuses, most of the literature in Cluster 8 on metabolic syndrome consists of reviews. These discuss the involvement of neuroinflammation and microglial activation in the pathogenesis of Alzheimer’s disease and other neurodegenerative disorders. They emphasize the role of microglia, the resident immune cells of the brain, in responding to neuronal damage and contributing to inflammation. Several articles mention the role of astrocytes, another type of glial cell, in CNS inflammation and their crosstalk with microglia and other cells. The interaction between astrocytes and microglia is highlighted as a crucial aspect of neuroinflammatory processes. In addition, the role of inflammation and gut microbiota in mediating inflammatory pathways in AD is also explored.
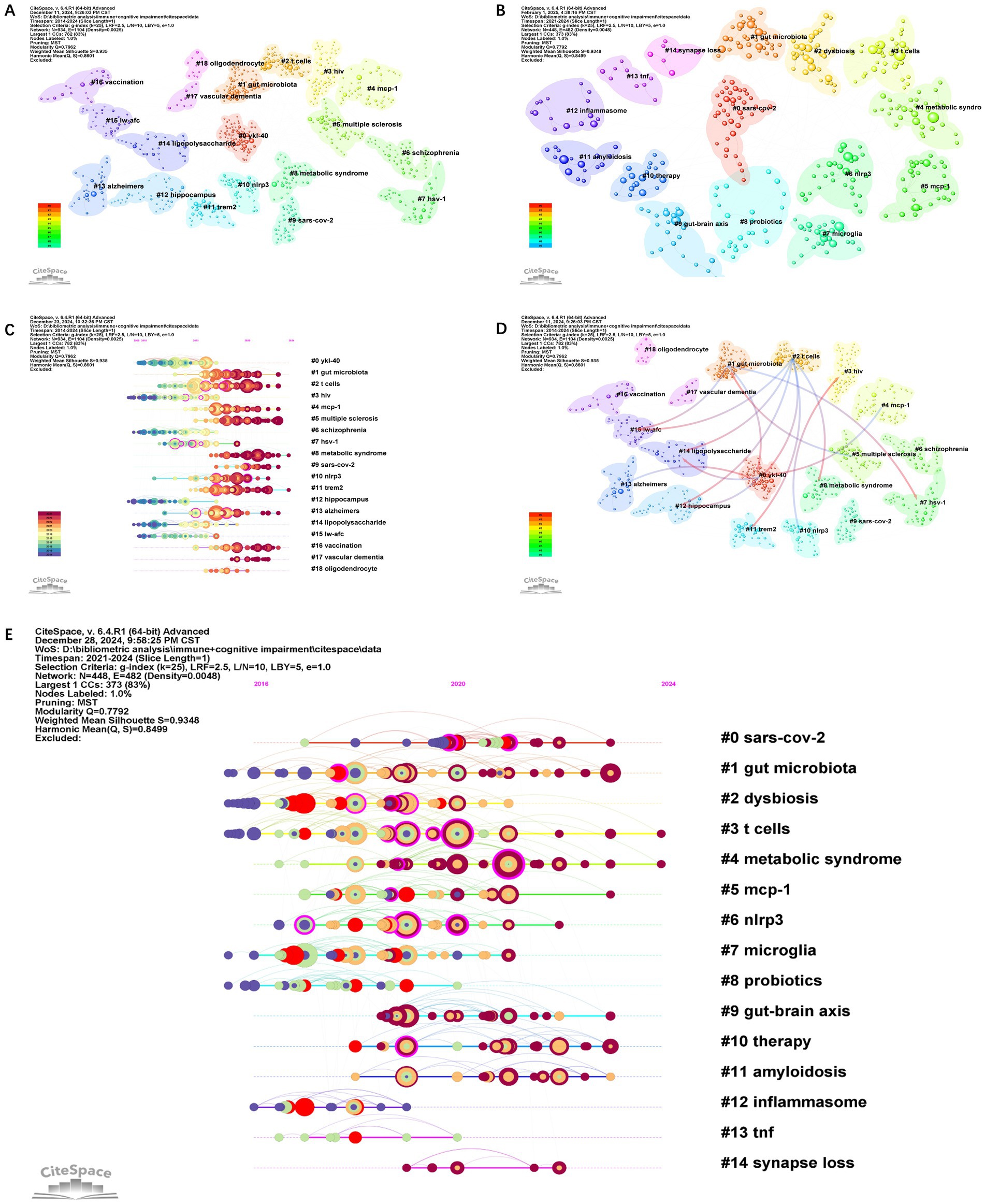
Figure 10. The co-citation cluster visualization map of references from 2014 to 2024 (A). The co-citation cluster visualization map of references from 2021 to 2024 (B). The co-citation network visualizations map of keywords from 2014 to 2024 (C). The co-citation cluster visualization map of references from 2014 to 2024 (D). The co-citation network visualizations map of keywords from 2021 to 2024 (E).
The cluster#11 trem2 is a recent cluster, with the average publication year of the literature being 2018. Nine out of the top ten articles in the #11 cluster have exhibited citation bursts; three of these articles have continued to receive attention until 2024, indicating that this cluster will become an emerging trend in the field. These articles are predominantly published in high-impact journals such as Nature and Cell. Most of the literature in Cluster 11 primarily focused on the genetic and molecular aspects of Alzheimer’s disease (AD), with a strong emphasis on microglia, immunity, and genetic risk loci. Mathys H presents a single-cell transcriptomic analysis of Alzheimer’s disease, providing insights into the gene expression profiles of different cell types, including microglia, in AD brains (Mathys et al., 2019). Jonathan Kipnis and others highlight the role of the TREM2-APOE pathway in driving microglial dysfunction in AD and other neurodegenerative diseases (Gratuze et al., 2023). David H. Holtzman and others present the complexity and dynamic nature of microglial function in health and disease (Chen et al., 2023; Parhizkar et al., 2023). Some articles revisit or reevaluate the amyloid hypothesis (the idea that the accumulation of amyloid-beta plaques in the brain is a central cause of AD) (Pimplikar, 2009; Armstrong, 2011), while others explore alternative or additional pathways, such as tau pathology, lipid processing, and immune dysfunction (Cao et al., 2018; Yamazaki et al., 2019). They implicate microglial-mediated innate immunity in the disease process and highlight the importance of microglial function and dysfunction in neurodegeneration. Genetic meta-analyses analyze large datasets to uncover genetic variations that increase the risk of developing AD and have identified new risk loci for Alzheimer’s disease (Lambert et al., 2013a,b; Sims et al., 2017; Lancaster et al., 2019).
3.6.2 Most co-cited papers
Table 5 highlights the top 10 articles, including those of cluster #2 T cells dominate, with other clusters such as #4 MCP1, #8 metabolic syndrome, #10 NLRP3, #11 Trem 2, and #13 Alzheimer’s. Cluster dependencies show that Cluster #2 T cells is a relatively new group that cites the most literature from other clusters, such as clusters #0, #8, #11, #12, #14, and #15. The most highly cited literature, Heneka MT’s 2015 review, also originates from this cluster. Figure 10D shows that cluster #1 gut microbiota cites articles from clusters #0, #7, and #15. The cluster with the most citations is cluster #0 YKL-40, whose articles are cited by clusters #1, #2, #4, and #13. #0 YKL-40 and #15 lw-afc are cited by clusters #1 gut microbiota and #2 T cells. Heneka MT’s 2015 review in LANCET NEUROL and Keren-Shaul H’s 2017 article in CELL garnered the highest number of citations (8,468) (Table 5). Heneka MT reviewed that Alzheimer’s disease pathogenesis is not restricted to the neuronal compartment but includes strong interactions with immunological mechanisms in the brain. Modulating risk factors and targeting these immune mechanisms could lead to future therapeutic or preventive strategies for Alzheimer’s disease. Keren-Shaul et al. (2017) affirmed that immunohistochemical staining of mouse and human brain slices reveals a microglial type associated with neurodegenerative diseases (disease-associated microglia, DAM) containing Aβ particles. Single-cell analysis shows that DAM activation occurs in a Trem2-independent and then Trem2-dependent two-step process, potentially restricting neurodegeneration (Zhou et al., 2020). Other highly cited articles broadly cover several research aspects and questions related to dementia and neuroinflammation. Inflammation is a recurring theme throughout, highlighting its pivotal role in AD and other neurodegenerative diseases. Specifically, they delve into five points:
Immune Cells in AD: Studies examine the role of clonally expanded CD8 T cells and microglia in patrolling the cerebrospinal fluid and neuroinflammatory processes. The involvement of TREM2 variants and neutrophils in AD is also explored, highlighting the complexity of the immune system in the disease.
Neuroinflammation and Microglial Activation: Multiple articles focus on microglial activation and neuroinflammation, including their roles in synapse loss, tau pathology, and the transcriptional phenotype of dysfunctional microglia. These studies aim to understand how neuroinflammatory processes contribute to dementia pathogenesis.
Biological Mechanisms and Genetic Risk Factors: Research frameworks and genetic meta-analyses are presented to define AD biologically and identify new risk loci. Topics such as Aβ, tau, immunity, and lipid processing are implicated in AD risk, providing insights into the disease’s underlying mechanisms.
Gut Microbiome and Brain Health: Alterations in the gut microbiome are associated with dementia, suggesting a gut–brain axis in the disease. Pro-inflammatory gut bacterial taxa and peripheral inflammation markers are linked to brain amyloidosis in cognitively impaired elderly, further supporting this connection.
Blood–Brain Barrier and Inflammation: The breakdown of the blood–brain barrier in AD and other neurodegenerative disorders is discussed, emphasizing inflammation as a central mechanism in AD pathogenesis. Many articles aim to bridge the gap between basic research and clinical applications, discussing potential therapeutic strategies and implications for future treatments.
3.6.3 Burst analysis
The eruption of citations has provided a valuable means of observing the evolution of research focal points. A total of 567 references underwent citation bursts. Figure 11A displays the 21 articles with the highest citation burst strength during 2014–2024, underscoring the flux of comparable thematic trends. The article with the highest citation burst strength was Heneka MT’s 2015 review in LANCET NEUROL. In CiteSpace, from 2014 to 2024, 567 documents experienced citation bursts (Figure 11A displays only the top 21), with 8 in #2 T cells. From 2021 to 2024, 308 documents still experienced citation bursts (Figure 11B displays only the top 16), with 3 in #1 gut microbiota, indicating these areas as key current and future focuses of immune and cognitive impairment research.
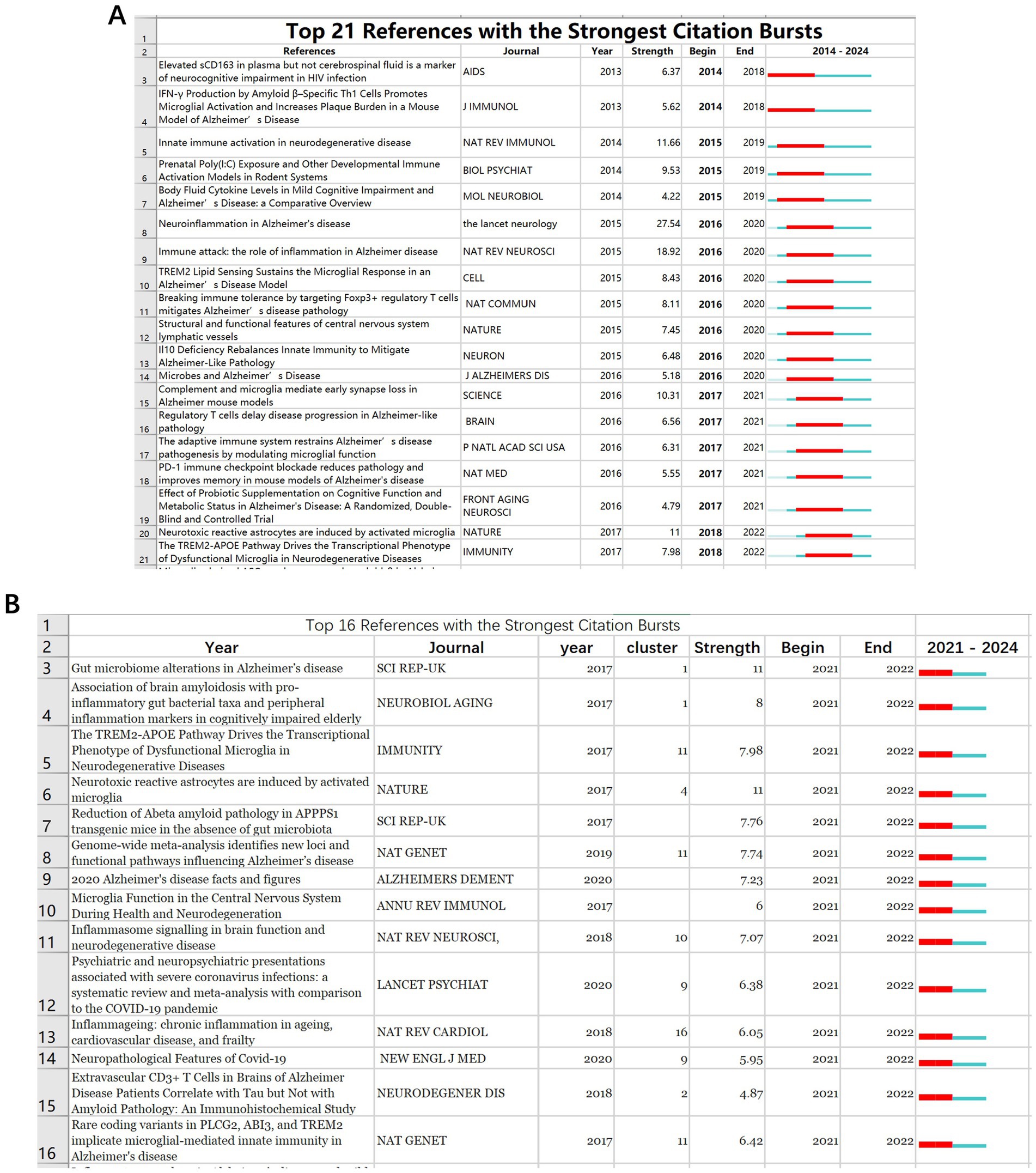
Figure 11. The top 25 references with the strongest citation bursts from 2014 to 2024 (A). The top 16 references with the strongest citation bursts from 2021 to 2024 (B).
4 Discussion
4.1 General information
This study conducted a systematic bibliometric analysis of the literature related to cognitive impairment and immunity from 1985 to 2024 by retrieving 3,737 documents (2,562 articles; 1,175 reviews) and 216,933 references.
Results of this study showed that researchers from 96 countries and regions engaged in immune system and cognitive impairment studies, highlighting the global prominence of this topic. The top ten countries in terms of publication output account for 70% of the total literature. The United States and China significantly outnumber other nations. The United States had the highest number of publications in the early stages, and its papers also had the highest citation counts. However, in recent years, Chinese authors have emerged with a significant number of publications, particularly in the field related to gut microbiota and cognition.
Universities played a pivotal role in this context. Among the top 10 institutions, a majority are situated in the USA. Among the top 20 publishing countries, five research institutions in China have distinguished themselves. An analysis of relevant publications from 2021 to 2024 shows that six out of the top 10 publishing institutions are from China. This indicates that China has shown tremendous potential in research on immunity and cognitive impairments in recent years.
In the long course of time, some authors may achieve remarkable accomplishments early in their careers but gradually lose momentum. However, Professor Heneka MT has consistently produced high-quality papers and maintained a focus on his research areas of neuroinflammation, microglia, and the NLRP3 inflammasome. This demonstrates his dedicated effort and expertise in this field and showcases his academic authority. Maes, Michael, and Zetterberg, Henrik, have consistently sustained the momentum of their research endeavors, boasting a considerable number of publications. Professor Michael Maes has dedicated himself to exploring psychiatry and molecular neuroscience, as well as long-COVID-associated mental ailments after 2021. Professor Henrik Zetterberg’s primary research interests encompass the role of neuroinflammation in the etiology of neurodegenerative diseases and the study of associated cerebrospinal fluid and blood biomarkers (Heneka et al., 2015; Zetterberg and Blennow, 2021). Post-2021, he has intensified his efforts on the early diagnosis of diseases and the clinical utilization of biomarkers, as well as the interplay between neurodegenerative diseases and other systemic conditions (Kelly et al., 2023; Hong et al., 2025; Pedersen et al., 2025). Professor Vicente Felipo has long concentrated his research on the association between metabolic diseases, neuroinflammation, and cognitive impairments (Mekhora et al., 2024; Sørensen et al., 2024). Nevertheless, in recent years, his attention has shifted to the function of cellular vesicles in this process, especially emphasizing the treatment of neuroinflammation and cognitive impairment-related diseases using extracellular vesicles (Upadhya and Shetty, 2019; Tang et al., 2020; Ruan et al., 2021; Yuan et al., 2021). The number of studies in the immune and cognitive impairment field has shown a significant growing trend, especially from 2021 to 2024, entering a rapid development stage, with 1,602 publications in 2024, and the number of citations of the literature has increased steadily year by year. This indicates that research on the relationship between cognitive impairment and immunity has attracted extensive attention in the academic community, and research enthusiasm continues to rise.
4.2 Critical research topics in the domain of immune and cognitive impairment, hotspots and frontiers
The analysis of keywords and references yields valuable insights into the primary research focus. In recent years, interferon gamma, cytokines, risk factors, working memory, interleukin-6, older adults, and neurodegenerative diseases have emerged as the strongest citation burst keywords. This suggests that immune regulators such as interferon gamma and interleukin-6, among other cytokines, may serve as risk factors for cognitive function in the elderly population and are closely related to the onset and progression of neurodegenerative diseases. Keyword clustering analysis indicated that each cluster had a clear theme and a certain depth. For example, the #7 gut microbiota cluster not only involves the association between changes in gut microbiota composition and cognitive impairment but also deeply explores the mechanisms by which gut microbiota affects neuroinflammation, neurotransmitter metabolism, and synaptic plasticity through the “gut–brain axis” (Gareau et al., 2011; Sampson et al., 2016; Vogt et al., 2017; Strandwitz, 2018; Cryan et al., 2019). Evidence suggests that early-life interactions between the microbiota and the immune system play a critical role in immune programming, which may have long-term consequences for brain development and cognitive function (Donald and Finlay, 2023). The #9 immune response cluster focuses on the immune response process in cognitive impairment, including research on “inflammatory factors,” “immune cell activation,” and “autoantibodies,” and deeply analyzes the dual role of the immune response in the occurrence and development of cognitive impairment, specifically how the imbalance of the normal immune response leads to neuropathological damage and cognitive decline (Heneka et al., 2015; Heppner et al., 2015; Hong et al., 2016; Schwartz and Deczkowska, 2016; Prinz and Priller, 2017; Dalmau and Graus, 2018). Additionally, there are close associations and synergies between different clusters. For example, the #0 microglia cluster is closely related to the #1 Alzheimer’s disease cluster. Microglia play an important role in the pathological process of Alzheimer’s disease. Their abnormal activation can lead to neuroinflammation, which in turn promotes the deposition of β-amyloid and the hyperphosphorylation of tau protein, aggravating the condition of Alzheimer’s disease (Heppner et al., 2015; Maphis et al., 2015; Hong et al., 2016; Keren-Shaul et al., 2017; Sarlus and Heneka, 2017; Li Y. et al., 2023). The #7 gut microbiota cluster and the #9 immune response cluster interact through the “gut–brain axis.” The imbalance of gut microbiota can trigger a systemic inflammatory response, affect the balance of the immune system, and then affect the immune status of the central nervous system, which is closely related to the occurrence and development of cognitive impairment (Sampson et al., 2016; Harach et al., 2017; Li N. et al., 2023; Zha et al., 2025). The associations between these clusters highlight the importance of cross-topic research. Future research should focus on integrating the research content of different clusters and deeply exploring the complex network relationships between cognitive impairment and immunity.
The keyword timeline diagram indicates that microglia, neurodegeneration, gut microbiota, and the kynurenine pathway are current research hotspots. The Alzheimer’s disease cluster has been a research hotspot for decades. Over time, the research has deepened, gradually developing from a simple description of pathological features to the research on immune mechanisms and the exploration of immunotherapy strategies. It is expected that future research will continue to focus on precision medicine and personalized treatment. The emerging cluster, such as the #7 gut microbiota cluster, has rapidly gained attention in recent years, reflecting the importance attached to the interaction between gut microbiota–brain–immune system in the research field. The identification of TREM2 and gut microbiota as emerging research foci suggests promising directions for therapeutic intervention. Modulating the gut–immune–brain axis, for instance, has demonstrated potential in improving cognitive outcomes in both preclinical and early clinical studies. Future research is expected to make breakthroughs in revealing new pathogenesis and developing innovative treatment methods.
Analyzing highly cited literature facilitates the identification of research outcomes with significant impact and offers crucial insights for future research directions. The top 15 highly cited publications underscore the importance of foundational research and theoretical reviews in the field of immune and cognitive impairment. However, they also highlight the clinical translation of findings in immune and cognitive impairment is still in its early stages. Basic research has clearly indicated that neuroinflammation plays a pivotal role in the pathogenesis of Alzheimer’s disease (AD). Microglia, the resident immune cells in the central nervous system, become activated and undergo functional changes in AD, serving as primary contributors to neuroinflammation. Microglia exhibit diverse phenotypes under different conditions, exerting multifaceted influences on the progression of AD. The M1 pro-inflammatory phenotype, triggered by pathogen-associated molecular patterns (PAMPs) or damage-associated molecular patterns (DAMPs), releases neurotoxic substances, leading to neuronal damage and synaptic dysfunction, thereby accelerating the progression of AD. In contrast, the M2 anti-inflammatory/repair phenotype, triggered by anti-inflammatory factors (such as IL-4, IL-10, and TGF-β), promotes the clearance of Aβ, alleviates neuroinflammation, and protects neurons, thereby slowing the pathological progression of AD. Disease-associated microglia (DAM) is a unique phenotype of microglia that expresses specific genetic markers (such as TREM2 and APOE). DAM may play a protective role in the early stages of AD, but in the late stages of the disease, it may shift to a pro-inflammatory phenotype, exacerbating pathological damage. TREM2 mutations may increase the risk of AD by affecting the Aβ clearance capacity and inflammatory responses of microglia. Furthermore, other cells such as astrocytes and T cells interact with microglia through the release of inflammatory cytokines like IFN-γ, IL-1β, and TNF-α, collectively impacting the pathological process of AD (Heneka et al., 2015; Heppner et al., 2015; Liddelow et al., 2017; Gate et al., 2020). Variants of immune-related genes, such as TREM2, as well as the TREM2-APOE pathway, can drive transcriptional phenotypic changes in microglia, affecting their function and the pathogenesis of AD (Guerreiro et al., 2013; Krasemann et al., 2017; Ulrich et al., 2018; Parhizkar et al., 2019).
Neuroinflammation, gut microbiota, and genetic factors are currently prominent topics in cognition-related research. The analysis of keywords and co-citation clusters reveals evolving focal points and developmental trends in immune and cognitive impairment research. Cluster analysis of co-cited literature from 1985 to 2024 shows that the neuroinflammatory marker YKL-40, gut microbiota, and T cells form major clusters. For the co-cited literature cluster analysis from 2021 to 2024, dysbiosis of the gut ecosystem and probiotics, the gut–brain axis and therapy, inflammasomes and amyloidosis, and the emergence of microglia indicate that there has been increased focus on the specific states of gut dysbiosis and the use of probiotics to regulate gut microbiota, which may subsequently influence immunity and cognitive impairments. At the same time, these changes may play a significant role in immunity and cognitive impairments through the gut–brain axis. The co-occurrence of “inflammasome, ““amyloidosis,” and “microglia” indicates that the bidirectional role of microglia in cognition and inflammation is increasingly recognized. Inflammasomes, amyloidosis, and microglia interact and influence each other during the onset and progression of immune and cognitive disorders. The activation of inflammasomes can promote microglial activation and inflammatory responses, accelerating the process of amyloidosis; conversely, amyloidosis can activate inflammasomes and microglia, forming a vicious cycle that jointly drives the development of immune abnormalities and cognitive impairments. An in-depth study of their relationships helps to reveal the pathogenesis of immune and cognitive disorder-related diseases, providing a theoretical basis for the development of new therapeutic strategies. The relevance of metabolic syndrome, T cells, and TREM2 remains strong. TREM2, metabolic syndrome, YKL-40, LW-AFC, and lipopolysaccharide are all related to T cells, while YKL-40 and LW-AFC are also associated with gut microbiota. #2cluster T cells is also a major cluster generating a high number of citations. In view of current research trends, researchers should pay more attention to emerging hotspots. For example, in TREM2-related research, more studies should deeply analyze its signaling pathway and mechanism of action in neurodegenerative diseases such as Alzheimer’s disease, and explore the development of drugs targeting TREM2, including small molecule inhibitors or agonists, and TREM2-based immunomodulatory therapies, which are expected to provide new breakthroughs for the treatment of neurodegenerative diseases.
4.3 Strengths and limitations
Compared with the literature focusing on the immune mechanisms of Alzheimer’s disease, this study covered a wider range of cognitive impairment-related diseases, such as cognitive impairment caused by HIV infection and multiple sclerosis. The research perspective was more comprehensive and could analyze the immune commonalities and differences in cognitive impairment caused by different etiologies. However, in terms of the depth of research on the immune-related aspects of Alzheimer’s disease, it may not be as detailed as the research specifically targeting this disease. Additionally, regional disparities in citation impact should be acknowledged, which may stem from differences in language use, journal visibility, and international collaboration networks—particularly affecting institutions from non-English-speaking countries.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
BZ: Conceptualization, Methodology, Visualization, Writing – original draft, Writing – review & editing. JX: Methodology, Investigation, Formal analysis, Writing – original draft. MZ: Validation, Writing – original draft. JH: Investigation, Project administration, Supervision, Writing – review & editing. CF: Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Correction note
A correction has been made to this article. Details can be found at: 10.3389/fnagi.2025.1675716.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2025.1587575/full#supplementary-material
References
Al-Hakeim, H. K., Al-Kufi, S. N., Al-Dujaili, A. H., and Maes, M. (2018). Serum interleukin levels and insulin resistance in major depressive disorder. CNS Neurol. Disord. Drug Targets 17, 618–625. doi: 10.2174/1871527317666180720155300
Amor, S., and Woodroofe, M. N. (2014). Innate and adaptive immune responses in neurodegeneration and repair. Immunology 141, 287–291. doi: 10.1111/imm.12134
Armstrong, R. A. (2011). The pathogenesis of Alzheimer's disease: a reevaluation of the "amyloid cascade hypothesis". Int. J. Alzheimers Dis. 2011:630865. doi: 10.4061/2011/630865
Barton, G. M., Pasare, C., and Medzhitov, R. (2014). “Toll-like receptors and control of adaptive immunity” in The innate immune response to infection. eds. S. H. E. Kaufmann, R. Medzhitov, and S. Gordon (Washington, DC, USA: ASM Press), 271–285.
Cao, J., Hou, J., Ping, J., and Cai, D. (2018). Advances in developing novel therapeutic strategies for Alzheimer’s disease. Mol. Neurodegener. 13:64. doi: 10.1186/s13024-018-0299-8
Chen, X., Firulyova, M., Manis, M., Herz, J., Smirnov, I., Aladyeva, E., et al. (2023). Microglia-mediated T cell infiltration drives neurodegeneration in tauopathy. Nature 615, 668–677. doi: 10.1038/s41586-023-05788-0
Clark, R. A. (2006). Misbehaving macrophages in the pathogenesis of psoriasis. J. Clin. Invest. 116, 2084–2087. doi: 10.1172/JCI29441
Contreras, J. A., Aslanyan, V., Albrecht, D. S., Mack, W. J., and Alzheimer’s Disease Neuroimaging Initiative (ADNI)Pa, J. (2022). Higher baseline levels of CSF inflammation increase risk of incident mild cognitive impairment and Alzheimer’s disease dementia. Alzheimers Dement. Diagn. Assess. Dis. Monit. 14:e12346. doi: 10.1002/dad2.12346
Cooper, J., Pastorello, Y., and Slevin, M. (2023). A meta-analysis investigating the relationship between inflammation in autoimmune disease, elevated CRP, and the risk of dementia. Front. Immunol. 14:1087571. doi: 10.3389/fimmu.2023.1087571
Cryan, J. F., O’Riordan, K. J., Cowan, C. S. M., Sandhu, K. V., Bastiaanssen, T. F. S., Boehme, M., et al. (2019). The microbiota-gut-brain axis. Physiol. Rev. 99, 1877–2013. doi: 10.1152/physrev.00018.2018
Dalmau, J., and Graus, F. (2018). Antibody-mediated encephalitis. N. Engl. J. Med. 378, 840–851. doi: 10.1056/NEJMra1708712
Donald, K., and Finlay, B. B. (2023). Early-life interactions between the microbiota and immune system: impact on immune system development and atopic disease. Nat. Rev. Immunol. 23, 735–748. doi: 10.1038/s41577-023-00874-w
Gareau, M. G., Wine, E., Rodrigues, D. M., Cho, J. H., Whary, M. T., Philpott, D. J., et al. (2011). Bacterial infection causes stress-induced memory dysfunction in mice. Gut 60, 307–317. doi: 10.1136/gut.2009.202515
Gate, D., Saligrama, N., Leventhal, O., Yang, A. C., Unger, M. S., Middeldorp, J., et al. (2020). Clonally expanded CD8 T cells patrol the cerebrospinal fluid in Alzheimer’s disease. Nature 577, 399–404. doi: 10.1038/s41586-019-1895-7
Gate, D., Tapp, E., Leventhal, O., Shahid, M., Nonninger, T. J., Yang, A. C., et al. (2021). CD4+ T cells contribute to neurodegeneration in Lewy body dementia. Science 374, 868–874. doi: 10.1126/science.abf7266
Geloso, M. C., Zupo, L., and Corvino, V. (2024). Crosstalk between peripheral inflammation and brain: focus on the responses of microglia and astrocytes to peripheral challenge. Neurochem. Int. 180:105872. doi: 10.1016/j.neuint.2024.105872
Gratuze, M., Schlachetzki, J. C. M., D’Oliveira Albanus, R., Jain, N., Novotny, B., Brase, L., et al. (2023). TREM2-independent microgliosis promotes tau-mediated neurodegeneration in the presence of ApoE4. Neuron 111, 202–219.e7. doi: 10.1016/j.neuron.2022.10.022
Gudala, K., Bansal, D., Schifano, F., and Bhansali, A. (2013). Diabetes mellitus and risk of dementia: a meta-analysis of prospective observational studies. J. Diabetes Investig. 4, 640–650. doi: 10.1111/jdi.12087
Guerreiro, R., Wojtas, A., Bras, J., Carrasquillo, M., Rogaeva, E., Majounie, E., et al. (2013). TREM2 variants in Alzheimer’s Disease. N. Engl. J. Med. 368, 117–127. doi: 10.1056/NEJMoa1211851
Harach, T., Marungruang, N., Duthilleul, N., Cheatham, V., Mc Coy, K. D., Frisoni, G., et al. (2017). Reduction of Abeta amyloid pathology in APPPS1 transgenic mice in the absence of gut microbiota. Sci. Rep. 7:41802. doi: 10.1038/srep41802
Heneka, M. T., Carson, M. J., Khoury, J. E., Landreth, G. E., Brosseron, F., Feinstein, D. L., et al. (2015). Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 14, 388–405. doi: 10.1016/S1474-4422(15)70016-5
Heppner, F. L., Ransohoff, R. M., and Becher, B. (2015). Immune attack: the role of inflammation in Alzheimer disease. Nat. Rev. Neurosci. 16, 358–372. doi: 10.1038/nrn3880
Hong, S., Beja-Glasser, V. F., Nfonoyim, B. M., Frouin, A., Li, S., Ramakrishnan, S., et al. (2016). Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science 352, 712–716. doi: 10.1126/science.aad8373
Hong, H., Fu, Q., Gu, P., Zhao, J., Dai, J., Xu, K., et al. (2025). Investigating the common genetic architecture and causality of metabolic disorders with neurodegenerative diseases. Diabetes Obes. Metab. 27, 1337–1349. doi: 10.1111/dom.16130
Kelly, J., Moyeed, R., Carroll, C., Luo, S., and Li, X. (2023). Blood biomarker-based classification study for neurodegenerative diseases. Sci. Rep. 13:17191. doi: 10.1038/s41598-023-43956-4
Keren-Shaul, H., Spinrad, A., Weiner, A., Matcovitch-Natan, O., Dvir-Szternfeld, R., Ulland, T. K., et al. (2017). A unique microglia type associated with restricting development of Alzheimer’s Disease. Cell 169, 1276–1290.e17. doi: 10.1016/j.cell.2017.05.018
Krasemann, S., Madore, C., Cialic, R., Baufeld, C., Calcagno, N., El Fatimy, R., et al. (2017). The TREM2-APOE pathway drives the transcriptional phenotype of dysfunctional microglia in neurodegenerative diseases. Immunity 47, 566–581.e9. doi: 10.1016/j.immuni.2017.08.008
Lambert, J.-C., Grenier-Boley, B., Harold, D., Zelenika, D., Chouraki, V., Kamatani, Y., et al. (2013a). Genome-wide haplotype association study identifies the FRMD4A gene as a risk locus for Alzheimer’s disease. Mol. Psychiatry 18, 461–470. doi: 10.1038/mp.2012.14
Lambert, J.-C., Ibrahim-Verbaas, C. A., Harold, D., Naj, A. C., Sims, R., Bellenguez, C., et al. (2013b). Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer’s disease. Nat. Genet. 45, 1452–1458. doi: 10.1038/ng.2802
Lancaster, T. M., Hill, M. J., Sims, R., and Williams, J. (2019). Microglia – mediated immunity partly contributes to the genetic association between Alzheimer’s disease and hippocampal volume. Brain Behav. Immun. 79, 267–273. doi: 10.1016/j.bbi.2019.02.011
Li, N., Tan, S., Wang, Y., Deng, J., Wang, N., Zhu, S., et al. (2023). Akkermansia muciniphila supplementation prevents cognitive impairment in sleep-deprived mice by modulating microglial engulfment of synapses. Gut Microbes 15:2252764. doi: 10.1080/19490976.2023.2252764
Li, Y., Xu, H., Wang, H., Yang, K., Luan, J., and Wang, S. (2023). TREM2: potential therapeutic targeting of microglia for Alzheimer’s disease. Biomed. Pharmacother. 165:115218. doi: 10.1016/j.biopha.2023.115218
Liddelow, S. A., Guttenplan, K. A., Clarke, L. E., Bennett, F. C., Bohlen, C. J., Schirmer, L., et al. (2017). Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541, 481–487. doi: 10.1038/nature21029
Maes, M., and Kanchanatawan, B. (2021). In (deficit) schizophrenia, a general cognitive decline partly mediates the effects of neuro-immune and neuro-oxidative toxicity on the symptomatome and quality of life. CNS Spectr. 1–10, 1–10. doi: 10.1017/S1092852921000419
Mancuso, R., Agostini, S., Hernis, A., Zanzottera, M., Bianchi, A., and Clerici, M. (2019). Circulatory miR-223-3p discriminates between Parkinson’s and Alzheimer’s patients. Sci. Rep. 9:9393. doi: 10.1038/s41598-019-45687-x
Mancuso, R., Cabinio, M., Agostini, S., Baglio, F., and Clerici, M. (2020). HSV-1-specific IgG3 titers correlate with brain cortical thinning in individuals with mild cognitive impairment and Alzheimer’s Disease. Vaccine 8:255. doi: 10.3390/vaccines8020255
Maphis, N., Xu, G., Kokiko-Cochran, O. N., Jiang, S., Cardona, A., Ransohoff, R. M., et al. (2015). Reactive microglia drive tau pathology and contribute to the spreading of pathological tau in the brain. Brain 138, 1738–1755. doi: 10.1093/brain/awv081
Mathys, H., Davila-Velderrain, J., Peng, Z., Gao, F., Mohammadi, S., Young, J. Z., et al. (2019). Single-cell transcriptomic analysis of Alzheimer’s disease. Nature 570, 332–337. doi: 10.1038/s41586-019-1195-2
Mekhora, C., Lamport, D. J., and Spencer, J. P. E. (2024). An overview of the relationship between inflammation and cognitive function in humans, molecular pathways and the impact of nutraceuticals. Neurochem. Int. 181:105900. doi: 10.1016/j.neuint.2024.105900
Mohmmad-Rezaei, M., Arefnezhad, R., Ahmadi, R., Abdollahpour-Alitappeh, M., Mirzaei, Y., Arjmand, M., et al. (2021). An overview of the innate and adaptive immune system in atherosclerosis. IUBMB Life 73, 64–91. doi: 10.1002/iub.2425
Parhizkar, S., Arzberger, T., Brendel, M., Kleinberger, G., Deussing, M., Focke, C., et al. (2019). Loss of TREM2 function increases amyloid seeding but reduces plaque-associated ApoE. Nat. Neurosci. 22, 191–204. doi: 10.1038/s41593-018-0296-9
Parhizkar, S., Gent, G., Chen, Y., Rensing, N., Gratuze, M., Strout, G., et al. (2023). Sleep deprivation exacerbates microglial reactivity and aβ deposition in a TREM2 -dependent manner in mice. Sci. Transl. Med. 15:eade6285. doi: 10.1126/scitranslmed.ade6285
Pedersen, C. C., Maple-Grødem, J., and Lange, J. (2025). A systematic review of biofluid phosphorylated α-synuclein in Parkinson’s disease. Parkinsonism Relat. Disord. 132:107240. doi: 10.1016/j.parkreldis.2024.107240
Pimplikar, S. W. (2009). Reassessing the amyloid cascade hypothesis of Alzheimer’s disease. Int. J. Biochem. Cell Biol. 41, 1261–1268. doi: 10.1016/j.biocel.2008.12.015
Prinz, M., and Priller, J. (2017). The role of peripheral immune cells in the CNS in steady state and disease. Nat. Neurosci. 20, 136–144. doi: 10.1038/nn.4475
Quiros-Roldan, E., Sottini, A., Natali, P. G., and Imberti, L. (2024). The impact of immune system aging on infectious diseases. Microorganisms 12:775. doi: 10.3390/microorganisms12040775
Ruan, J., Miao, X., Schlüter, D., Lin, L., and Wang, X. (2021). Extracellular vesicles in neuroinflammation: pathogenesis, diagnosis, and therapy. Mol. Ther. 29, 1946–1957. doi: 10.1016/j.ymthe.2021.04.020
Sampson, T. R., Debelius, J. W., Thron, T., Janssen, S., Shastri, G. G., Ilhan, Z. E., et al. (2016). Gut microbiota regulate motor deficits and Neuroinflammation in a model of Parkinson’s Disease. Cell 167, 1469–1480.e12. doi: 10.1016/j.cell.2016.11.018
Sarlus, H., and Heneka, M. T. (2017). Microglia in Alzheimer’s disease. J. Clin. Invest. 127, 3240–3249. doi: 10.1172/JCI90606
Schwartz, M., and Deczkowska, A. (2016). Neurological disease as a failure of brain–immune crosstalk: the multiple faces of neuroinflammation. Trends Immunol. 37, 668–679. doi: 10.1016/j.it.2016.08.001
Sims, R., van der Lee, S. J., Naj, A. C., Bellenguez, C., Boland, A., Jakobsdottir, J., et al. (2017). Rare coding variants in PLCG2, ABI3, and TREM2 implicate microglial-mediated innate immunity in Alzheimer’s disease. Nat. Genet. 49, 1373–1384. doi: 10.1038/ng.3916
Sipilä, P. N., Heikkilä, N., Lindbohm, J. V., Hakulinen, C., Vahtera, J., Elovainio, M., et al. (2021). Hospital-treated infectious diseases and the risk of dementia: a large, multicohort, observational study with a replication cohort. Lancet Infect. Dis. 21, 1557–1567. doi: 10.1016/S1473-3099(21)00144-4
Sørensen, M., Andersen, J. V., Bjerring, P. N., and Vilstrup, H. (2024). Hepatic encephalopathy as a result of ammonia-induced increase in GABAergic tone with secondary reduced brain energy metabolism. Metab. Brain Dis. 40:19. doi: 10.1007/s11011-024-01473-x
Strandwitz, P. (2018). Neurotransmitter modulation by the gut microbiota. Brain Res. 1693, 128–133. doi: 10.1016/j.brainres.2018.03.015
Tamatta, R., Pai, V., Jaiswal, C., Singh, I., and Singh, A. K. (2025). Neuroinflammaging and the immune landscape: the role of autophagy and senescence in aging brain. Biogerontology 26:52. doi: 10.1007/s10522-025-10199-x
Tang, T.-T., Wang, B., Lv, L.-L., and Liu, B.-C. (2020). Extracellular vesicle-based Nanotherapeutics: emerging frontiers in anti-inflammatory therapy. Theranostics 10, 8111–8129. doi: 10.7150/thno.47865
Ulrich, J. D., Ulland, T. K., Mahan, T. E., Nyström, S., Nilsson, K. P., Song, W. M., et al. (2018). ApoE facilitates the microglial response to amyloid plaque pathology. J. Exp. Med. 215, 1047–1058. doi: 10.1084/jem.20171265
Upadhya, D., and Shetty, A. K. (2019). Extracellular vesicles as therapeutics for brain injury and Disease. Curr. Pharm. Des. 25, 3500–3505. doi: 10.2174/1381612825666191014164950
Vogt, N. M., Kerby, R. L., Dill-McFarland, K. A., Harding, S. J., Merluzzi, A. P., Johnson, S. C., et al. (2017). Gut microbiome alterations in Alzheimer’s disease. Sci. Rep. 7:13537. doi: 10.1038/s41598-017-13601-y
Wang, L., Zhang, X., Yang, Z., Wang, B., Gong, H., Zhang, K., et al. (2024). Extracellular vesicles: biological mechanisms and emerging therapeutic opportunities in neurodegenerative diseases. Transl. Neurodegener. 13:60. doi: 10.1186/s40035-024-00453-6
Wei, C., Jiang, W., Wang, R., Zhong, H., He, H., Gao, X., et al. (2024). Brain endothelial GSDMD activation mediates inflammatory BBB breakdown. Nature 629, 893–900. doi: 10.1038/s41586-024-07314-2
Yamazaki, Y., Zhao, N., Caulfield, T. R., Liu, C.-C., and Bu, G. (2019). Apolipoprotein E and Alzheimer disease: pathobiology and targeting strategies. Nat. Rev. Neurol. 15, 501–518. doi: 10.1038/s41582-019-0228-7
Yuan, Q., Li, X., Zhang, S., Wang, H., and Wang, Y. (2021). Extracellular vesicles in neurodegenerative diseases: insights and new perspectives. Genes Dis. 8, 124–132. doi: 10.1016/j.gendis.2019.12.001
Zetterberg, H., and Blennow, K. (2021). Moving fluid biomarkers for Alzheimer’s disease from research tools to routine clinical diagnostics. Mol. Neurodegener. 16:10. doi: 10.1186/s13024-021-00430-x
Zha, X., Liu, X., Wei, M., Huang, H., Cao, J., Liu, S., et al. (2025). Microbiota-derived lysophosphatidylcholine alleviates Alzheimer’s disease pathology via suppressing ferroptosis. Cell Metab. 37, 169–186.e9. doi: 10.1016/j.cmet.2024.10.006
Zhao, Z., Zhang, J., Wu, Y., Xie, M., Tao, S., Lv, Q., et al. (2024). Plasma IL-6 levels and their association with brain health and dementia risk: a population-based cohort study. Brain Behav. Immun. 120, 430–438. doi: 10.1016/j.bbi.2024.06.014
Keywords: cognitive impairment, immune system, bibliometric, neuroinflammation, microglia, gut microbiota, TREM2, co-citation
Citation: Zou B, Xiang J, Zhang M, Huang J and Feng C (2025) A bibliometric analysis of the immune system and cognitive impairment: trends from 1985 to 2024. Front. Aging Neurosci. 17:1587575. doi: 10.3389/fnagi.2025.1587575
Edited by:
Zhi-bei Qu, Fudan University, ChinaCopyright © 2025 Zou, Xiang, Zhang, Huang and Feng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chao Feng, ODAxMzAxMEB6anUuZWR1LmNu; Jing Huang, aHVhbmdqaW5nLmRvY0Bob3RtYWlsLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Beibei Zou
Beibei Zou Jinxi Xiang2†
Jinxi Xiang2† Jing Huang
Jing Huang Chao Feng
Chao Feng