- 1Department of Physical Education Teaching, Shanghai Sanda University, Shanghai, China
- 2School of Physical Education, Shanghai University of Sport, Shanghai, China
- 3College of Physical Education, Yangzhou University, Yangzhou, China
- 4Faculty of Sports Science Ningbo University, Ningbo, China
Background: Cognitive decline in older adults is a pressing public health concern, with emerging evidence suggesting that both muscle strength and neural function may influence cognitive outcomes. However, the integrative mechanisms linking these domains remain insufficiently understood.
Objective: This study aimed to explore whether resting-state EEG characteristics and working memory mediate the relationship between muscle strength and global cognitive function in older adults with cognitive impairment. Methods: A cross-sectional study was conducted among 137 older adults (mean age = 72.65 ± 7.75) with cognitive impairment. Muscle strength was assessed using grip strength and 30 s chair stand tests. Resting-state EEG power across six frequency bands was recorded from 16 electrodes. Working memory was evaluated using a two-back task, and cognitive function was assessed via the MoCA. Mediation analyses were performed using the PROCESS macro (Model 4), controlling for age, sex, education, and BMI.
Results: Grip strength showed significant direct effects on cognitive function (β = 0.399, p < 0.001), with partial mediation by both working memory (β = 0.070, p < 0.05) and resting-state EEG (β = 0.150, p < 0.01). In contrast, lower limb strength was mediated only by working memory (β = 0.078, p < 0.05), while EEG-based mediation was not significant. The overall model explained 50.7% of the variance in cognitive outcomes.
Conclusion: This study highlights the distinct mediating roles of working memory and EEG features in the muscle strength–cognition relationship. Grip strength, as a potential biomarker, may reflect central nervous system integrity and serve as a target for cognitive health interventions in aging populations.
1 Introduction
According to projections by the World Health Organization (WHO), the global population aged 60 years and above will reach 2.1 billion by 2050, accounting for 22% of the total population (Bajwa et al., 2019). Population aging is closely associated with increased prevalence of age-related neurodegenerative diseases (Vassilaki et al., 2025). Identifying modifiable risk factors and developing targeted interventions to mitigate cognitive decline in older adults has therefore become an urgent public health priority (He et al., 2023). Cognitive impairment in aging individuals typically manifests as deficits in memory, attention, executive function, language, and visuospatial skills (Xin et al., 2025). As cognitive capacity declines, so does autonomy—placing growing demands on caregivers and generating substantial burdens on healthcare systems and families (Connors et al., 2019).
Among the key predictors of cognitive health, working memory plays a central role, as it is particularly sensitive to age-related neural deterioration. Cognitive decline is often rooted in structural and functional degeneration of the prefrontal, temporal, and parietal cortices (Kamio et al., 2025), with working memory impairment being a common early symptom (D’esposito and Postle, 2015). From a neurobiological perspective, age-related disruptions in cortical connectivity are thought to drive this deterioration (Wang et al., 2011). Meanwhile, muscle strength—including both upper- and lower-limb performance—has emerged as a potentially modifiable factor linked to working memory and general cognition (Cai et al., 2023; Veronese et al., 2016; Chou et al., 2019). Longitudinal studies have demonstrated that individuals with reduced muscular strength in mid-to-late life are at elevated risk for cognitive impairment and dementia.
Muscle strength declines progressively with age, beginning as early as the third decade of life. By age 40, strength decreases by approximately 16%, with reductions reaching over 40% after the age of 60 (Keller and Engelhardt, 2013). Similarly, working memory peaks around age 30 and undergoes accelerated decline after 60 (Wang et al., 2011). This temporal overlap suggests a possible synchrony between neuromuscular and cognitive aging trajectories (Liao et al., 2024).
Resting-state electroencephalography (EEG)—a non-invasive technique with excellent temporal resolution—has emerged as a valuable tool for characterizing neural mechanisms of age-related cognitive decline (Puri et al., 2025). EEG captures spontaneous neural oscillations under task-free conditions, reflecting the brain’s intrinsic capacity for information processing (Si et al., 2019; Li et al., 2015). Age-associated EEG alterations include global slowing of peak frequencies, reductions in alpha-band power (8–12 Hz), and increases in delta (1–4 Hz) and theta (4–8 Hz) power across cortical regions (Klimesch, 2012; Dujardin et al., 1995; Klass and Brenner, 1995). These spectral changes are closely linked to declines in attention, memory, and executive functioning.
Taken together, the literature supports a multifactorial model of cognitive aging—one that integrates physical frailty, neural degradation, and disrupted cognitive resource allocation. Specifically, emerging evidence suggests that resting-state EEG biomarkers (as proxies for neural efficiency), muscle strength (as an index of physiological reserve), and working memory (as a core cognitive domain) may interactively influence cognitive outcomes in older adults. This framework prompts important mechanistic questions: What are the pathways by which muscular strength affects cognition? Do EEG features and working memory mediate these effects? Are the pathways consistent across different limb strengths (e.g., grip vs. lower-body performance)?
To address these knowledge gaps, the present study aimed to clarify the neurocognitive pathways linking muscle strength and cognitive function in older adults with cognitive impairment. Specifically, we posed the following research questions:1. Do working memory and resting-state EEG characteristics mediate the association between muscle strength and global cognitive performance? 2. Which specific EEG features (e.g., frontal theta power, parietal alpha frequency) are most predictive of cognitive function? 3. Do upper-limb (e.g., grip strength) and lower-limb (e.g., chair stand performance) strength indicators exert differential effects through distinct neural and cognitive pathways? Based on these questions, we proposed three hypotheses: H1: Muscle strength, working memory, and resting-state EEG parameters form an integrated predictive model for cognitive function. H2: Muscle strength is significantly correlated with both resting-state EEG features and working memory, which in turn are associated with cognitive performance. H3: Working memory and EEG spectral power jointly mediate the relationship between muscle strength and cognition.
To test these hypotheses, we conducted a cross-sectional analysis among older adults with cognitive impairment, incorporating standardized assessments of muscle strength (upper and lower limbs), working memory, and resting-state EEG. Path analysis was used to identify direct and indirect effects among these variables. Our goal is to clarify the neurophysiological mechanisms by which muscular fitness influences cognitive function, thereby informing strategies for early detection and intervention in age-related cognitive decline.
2 Research objects and methods
2.1 Sample size and power analysis
A priori power analysis was conducted using G*Power 3.1 to determine the minimum sample size required for multiple regression with three main predictors (muscle strength, EEG features, and working memory). Assuming a medium effect size (f2 = 0.15), an alpha level of 0.05, and desired statistical power of 0.80, the required sample size was 89 participants. The final analytic sample (N = 137) exceeds this threshold, ensuring adequate statistical power for detecting medium-sized effects in the mediation models.
2.2 Recruitment of subjects
Using convenience sampling method from March to May 2024, recruitment posters and leaflets were distributed in Chenfu Jiayuan and Qiangwei Jiuli communities of Songjiang District, and Luyan community of Jinshan District in Shanghai. A total of 145 eligible older adults were recruited based on voluntary participation. During testing, 1 participant was excluded due to exercise contraindications, 2 due to poor hearing/vision, and 5 failed to complete EEG acquisition, resulting in 137 final participants. This study complied with the latest version of the Helsinki Declaration and was approved by Shanghai University of Sport Ethics Committee (102772020RT060). This study was reported in accordance with the STROBE statement for cross-sectional studies. All participants voluntarily participated in the experiment and signed informed consent forms.
2.2.1 Inclusion criteria
① Aged 60 years or older; ② Right-handedness; ③ Good physical condition; ④ Absence of severe cardiovascular diseases; ⑤ Normal vision and hearing; ⑥ Normal mental status with verbal communication ability and willingness to cooperate with investigation; ⑦ Willingness to sign informed consent. All participants were included in the analysis based on cognitive screening (MoCA score <26).
2.2.2 Exclusion criteria
① Severe cardiovascular diseases or major organic diseases; ② Severe musculoskeletal disorders preventing prolonged standing; ③ Contraindications to physical exercise; ④ Long-term or recent use of medications affecting physical activity, psychotropic drugs, cholinergic inhibitors, or related substances.
2.3 Test process
All tests were conducted between 13:30 and 16:30. Participants were required to visit the laboratory twice. During the first visit, experimental procedures were explained, and participants completed a basic information form and the Montreal Cognitive Assessment (MoCA) scale. The second visit involved EEG signal acquisition and working memory testing. Additional assessments included height, weight, handgrip strength tests, and a 30 s chair stand test. The testing procedure is shown in Figure 1. Participants were instructed to refrain from vigorous exercise and avoid consuming caffeinated or alcoholic beverages 24 h prior to testing. All participants voluntarily participated in the experiment, signed informed consent forms, and the study adhered to the latest version of the Helsinki Declaration.
2.3.1 Test tool
2.3.1.1 Self-designed basic information questionnaire
Collected participants’ demographic data including name, age, gender, BMI, marital status, educational level, occupation, place of residence, presence/absence of exercise contraindications, smoking and alcohol consumption history, decline in hearing or vision, social activities, medical history, and medication usage.
2.3.1.2 Montreal cognitive assessment (MoCA)
Global cognitive function was assessed using the Beijing version of the Montreal Cognitive Assessment (MoCA), a widely validated screening tool for mild cognitive impairment (MCI). The MoCA evaluates eight cognitive domains including visuospatial/executive function, naming, memory, attention, language fluency, abstract reasoning, delayed recall, and orientation, with a maximum score of 30 (higher scores indicate better cognition). The cutoff score of <26 was used to identify cognitive impairment. To control for educational level, 1 or 2 points were added as recommended. The MoCA was selected over other tools (e.g., MMSE) due to its higher sensitivity in detecting early-stage MCI, especially in community-dwelling older adults. The Beijing version has been validated in Chinese populations, with a reported test–retest reliability of 0.857 (Yang et al., 2018).
2.3.1.3 Muscle strength test
This study assessed muscle strength using handgrip strength and the 30 s chair stand test. Handgrip strength reflects whole-body strength and physical function with high practicality and sensitivity. Participants stood upright with arms extended by their sides, gripping a dynamometer with maximum force for 3–5 s. Each hand was tested three times (with 30 s rests between trials), and the mean value was calculated. The 30 s chair stand test demonstrates strong validity and reliability for evaluating lower limb muscle strength in older adults, and a simple assessment model using this test as reference values has been preliminarily established. Participants stood in front of a 43 cm-high chair with arms crossed over the chest. Upon starting the timer, they repeatedly stood up and sat down as quickly as possible within 30 s. Strict protocols were enforced: maintaining upright posture without touching the backrest, and requiring full knee extension during standing.
2.3.1.4 Working memory test
The N-back task paradigm, a classic psychological experiment, was used to assess working memory. Stimuli (digits) were programmed using E-Prime 2.0 software. Participants responded to stimuli via keyboard according to task rules, with reaction time and accuracy recorded. Three cognitive load levels (0-back, 1-back, 2-back) were implemented. 0-back: Compare current digit with “0” – press “1” for match, “2” for mismatch. 1-back (starting from 2nd digit): Press “1” if identical to previous digit, else “2.” 2-back (starting from 3rd digit): Press “1” if matching the digit two steps back, else “2.” Instructions and practice trials preceded formal testing, with each load level repeated 5 times. Each trial began with a 500 ms fixation cross “+,” followed by random presentation of 10 digits (0–9) for 500 ms each, then a 2000 ms blank screen. Unresponded trials automatically advanced. A “Rest 30s” prompt appeared after each task block. Total testing duration was approximately 15 min.
2.3.1.5 EEG signal acquisition
Resting-state EEG was recorded using a 16-channel amplifier-equipped system (NCERP-190012, Shanghai Nuocheng Electric Co., Ltd.), with electrodes placed according to the international 10–20 system at Fp1, Fp2, F3, F4, F7, F8, C3, C4, P3, P4, O1, O2, T3, T4, T5, and T6. The ground electrode was placed on GND, and signals were re-referenced offline to linked mastoids (A1, A2). Impedances were maintained below 5 kΩ. Participants were tested in a soundproof, dimly lit, and ventilated room free of sensory-stimulating devices (e.g., mobile phones). After an adaptation period, participants were seated comfortably with hands resting, eyes closed, and instructed to remain awake, relaxed, and motionless during the 5 min eyes-closed (EC) resting-state recording. EEG signals were recorded at a sampling rate of 500 Hz and filtered in real-time using a 0.3 Hz high-pass, 30 Hz low-pass, and 50 Hz notch filter. Raw EEG data were further preprocessed in MATLAB using the EEGLAB toolbox. Preprocessing steps included: Offline re-referencing to bilateral mastoids (A1, A2). Band-pass filtering between 0.3–30 Hz and a 50 Hz notch filter to suppress powerline noise. Visual inspection for gross artifacts. Independent Component Analysis (ICA) to identify and remove components related to eye movements (EOG) and muscle activity. No regression-based removal of physiological signals (e.g., ECG, EMG) was performed, and this limitation is acknowledged in the Discussion section. EEG spectral power was calculated using Fast Fourier Transform (FFT) across the following frequency bands: delta (1–4 Hz), theta (4–8 Hz), alpha-1 (8–10.5 Hz), alpha-2 (10.5–13 Hz), beta-1 (13–20 Hz), and beta-2 (20–30 Hz), using left-closed-right-open interval definitions. Frequency boundary values were assigned to the higher band. The entire 5 min EC segment was retained without epoching. Regions of interest included frontal (Fp1, F7) and central (C4) electrodes, consistent with prior studies on cognitive aging and EEG biomarkers.
2.4 Statistical analysis
Pearson correlation and stepwise regression analyses were used to examine the associations between resting-state EEG features, muscle strength, working memory, and cognitive function in older adults. Based on these associations, path models were constructed with muscle strength as the independent variable and cognitive function as the outcome, while controlling for age, BMI, and years of education. Two separate mediation models were specified, treating EEG spectral indices and working memory metrics as mediator variables, respectively. The PROCESS macro (v4.1) Model 4 was used to estimate direct and indirect effects with 5,000 bootstrap resamples to calculate 95% confidence intervals (CIs). All variables were standardized (z-scores) prior to modeling. Statistical significance was assessed using two-tailed tests with α = 0.05, where p-values < 0.05, < 0.01, and < 0.001 indicated increasing levels of significance. To quantify the strength of the mediation, the proportion mediated was calculated as follows:
Where a represents the effect of the independent variable on the mediator, b the effect of the mediator on the dependent variable, and c′ the direct effect of the independent variable on the dependent variable after adjusting for the mediator. The ratio was multiplied by 100 to express the mediated effect as a percentage of the total effect. In addition, hierarchical multiple regression analysis was conducted to evaluate the incremental predictive contribution of muscle strength, working memory, and EEG features to cognitive performance. Predictors were entered in sequential blocks, beginning with demographic covariates, followed by physical, cognitive, and neural variables. Multicollinearity among predictors was assessed using the Variance Inflation Factor (VIF). A VIF close to 1 suggests no multicollinearity; values between 1 and 5 were considered acceptable; values ≥5 indicated moderate multicollinearity; and VIF ≥ 10 signaled severe multicollinearity, warranting further model diagnostics. The independence of residuals was evaluated using the Durbin–Watson statistic (DW), with acceptable values ranging from 1.5 to 2.5, indicating no substantial autocorrelation.
3 Results
3.1 Basic information of the subjects
The study enrolled 137 participants (age: 72.65 ± 7.754 years; BMI: 24.044 ± 3.258 kg/m2), with 56.20% male and 43.80% female. Comparative analysis of demographic characteristics between mild and moderate–severe cognitive impairment groups (Table 1) revealed statistically significant differences in age and education years (all p < 0.05), while other variables showed no significant group differences (all p > 0.05). These findings suggest that advanced age is associated with greater cognitive impairment severity in older adults, whereas longer educational attainment correlates with reduced impairment levels.
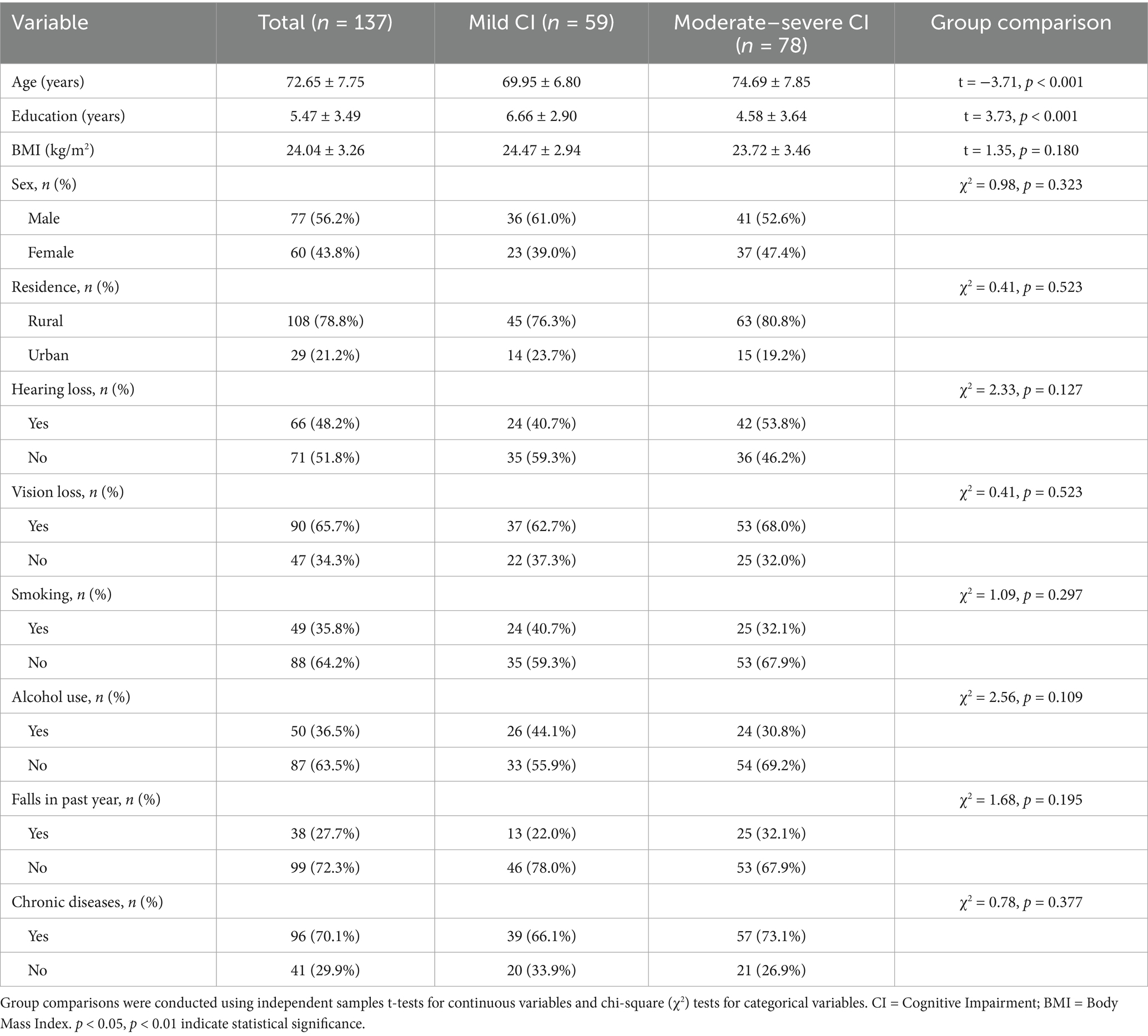
Table 1. Group differences in demographic variables between older adults with mild and moderate-to-severe cognitive impairment.
3.2 Model construction of resting electroencephalogram characteristics, muscle strength, working memory and cognitive function
Hierarchical stepwise regression analysis (Table 2) demonstrated a model fit of R2 = 0.507 (F(1,127) = 18.564, p < 0.001). In the final step of the hierarchical regression analysis, the combined predictors—muscle strength, resting-state EEG features, and working memory—explained 50.7% of the total variance in MoCA scores (R2 = 0.507), indicating strong overall model fit. Variance inflation factors (VIF) ranged between 1 and 5, and the Durbin-Watson statistic (DW = 1.974) fell within 1.5–2.5, both indicating acceptable multicollinearity and independence of residuals. Bootstrap validation results are presented in Table 3. The findings reveal multivariate relationships among resting-state EEG features, muscle strength, working memory, and cognitive function. These combined characteristics serve as significant predictors, providing empirical support for developing preventive strategies and predictive models of cognitive decline in older adults.
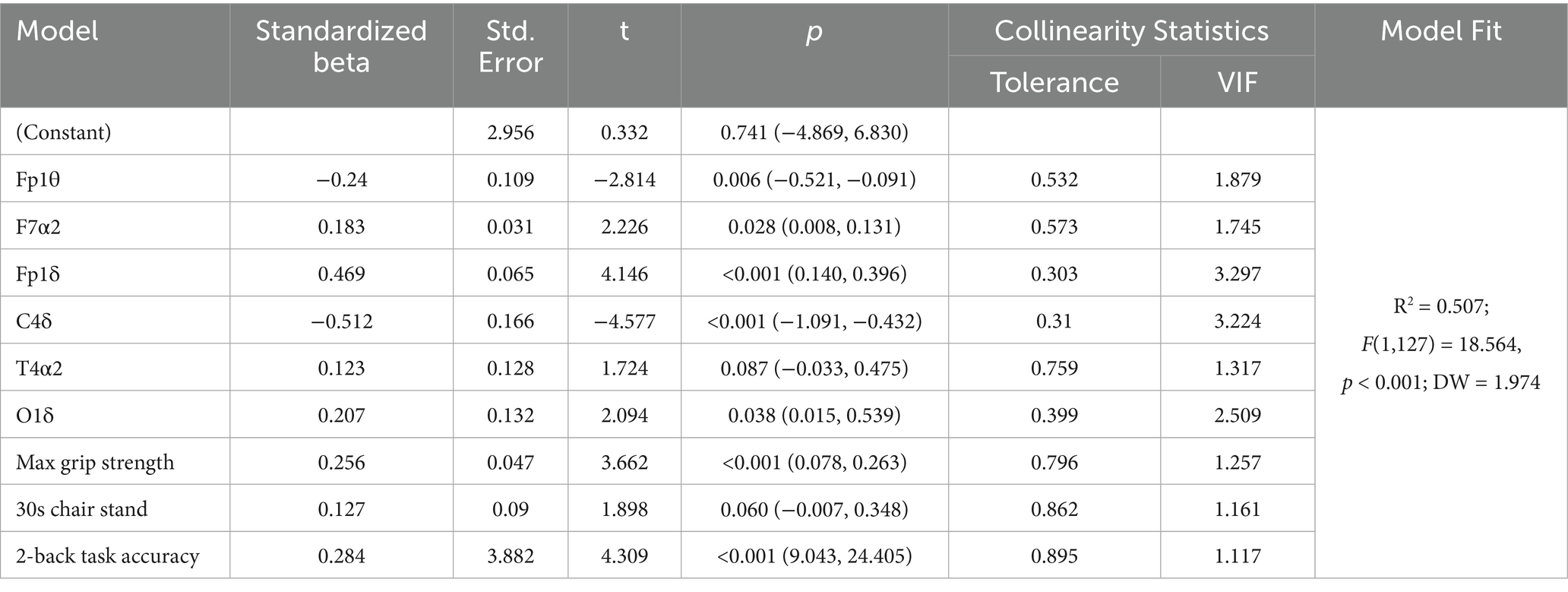
Table 2. Multivariate analysis of resting electroencephalogram characteristics, muscle strength, working memory and cognitive function.
3.3 The realization path of cognitive function improvement in the elderly with cognitive impairment
3.3.1 The relationship between resting electroencephalogram characteristics, muscle strength, working memory and cognitive function in the elderly with cognitive impairment
Correlation analyses across variables in the study cohort (Table 4) revealed no significant correlation between working memory and resting-state EEG features, while other primary variables demonstrated varying degrees of association (absolute r-values ranging from 0.201 to 0.726). The results confirm that resting-state EEG features, muscle strength, working memory, and cognitive function exhibit interconnected relationships.
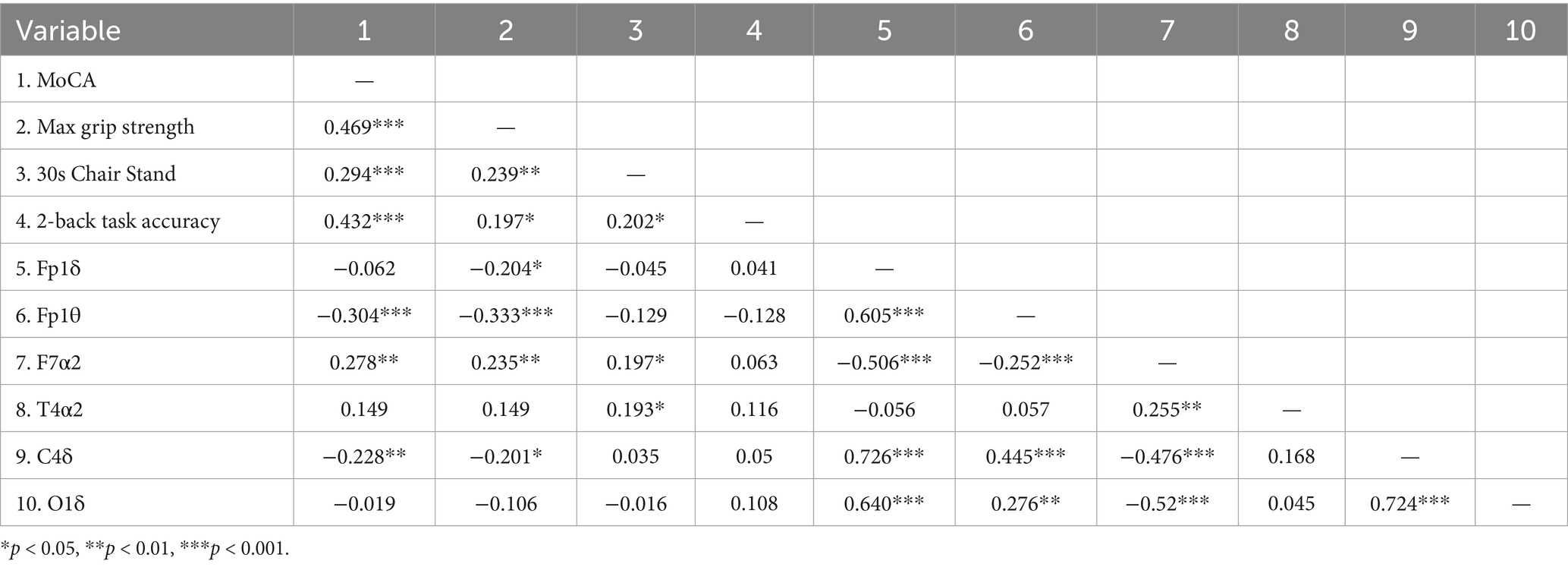
Table 4. Matrix of correlation coefficients between resting EEG characteristics, muscle strength, working memory, and cognitive function.
3.3.2 Potential associated pathways for muscle strength, resting EEG characteristics, working memory, and cognitive function
Building on the hierarchical regression analyses, we further explored potential pathways linking muscle strength, resting-state EEG features, working memory, and cognitive function. Mediation analyses were performed using Model 4 of the PROCESS macro, with covariates including age, BMI, and education controlled, to examine the mediating roles of resting-state EEG activity and working memory (see Figure 2).
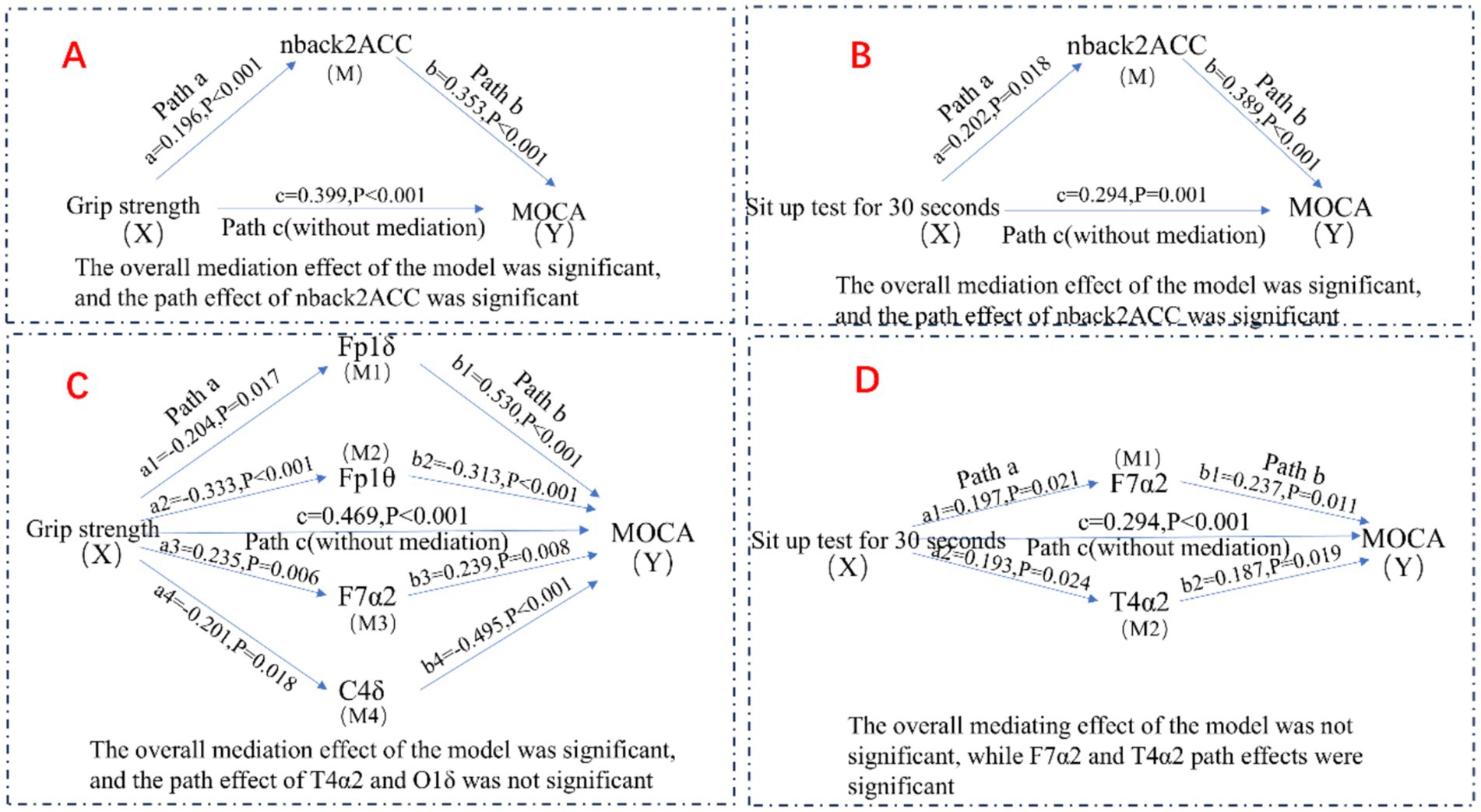
Figure 2. Potential association pathways for muscle strength, resting EEG characteristics, working memory, and cognitive function.
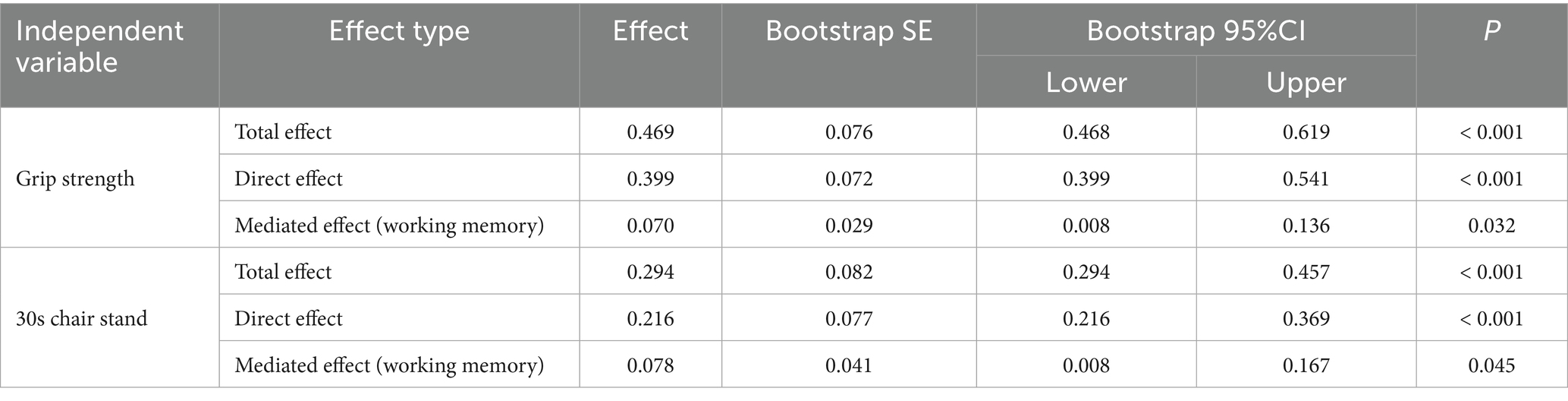
As shown in Tables 5, 6, grip strength was significantly associated with cognitive performance, with a total effect of β = 0.469 (95% CI [0.469, 0.619]). The direct effect, controlling for the working memory mediator, remained significant (β = 0.399, 95% CI [0.399, 0.541], p < 0.001), while the indirect effect via working memory was also significant (β = 0.070, 95% CI [0.008, 0.136]), accounting for 14.93% of the total effect. Similarly, lower-limb strength (30 s chair stand test) showed a total effect of β = 0.294 (95% CI [0.294, 0.467]) on cognitive function. The direct effect remained significant (β = 0.216, 95% CI [0.216, 0.369]), and the indirect effect via working memory was β = 0.078 (95% CI [0.008, 0.167]), explaining 26.53% of the total effect.
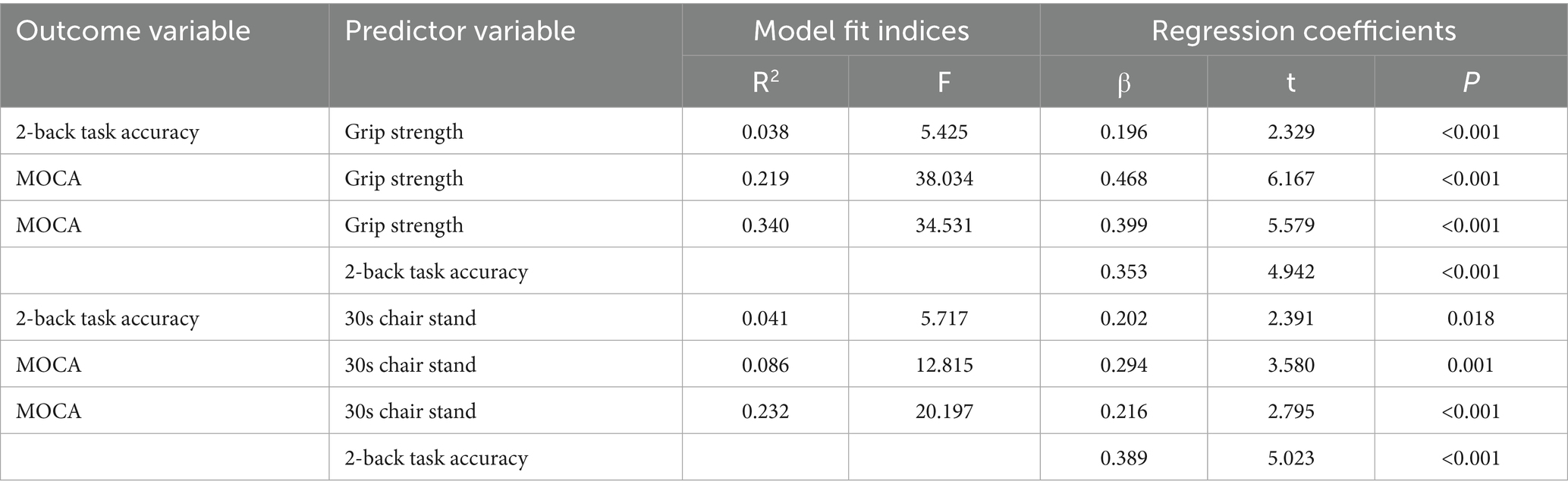
Table 5. Regression analysis of the mediating model of working memory between muscle strength and cognitive function.
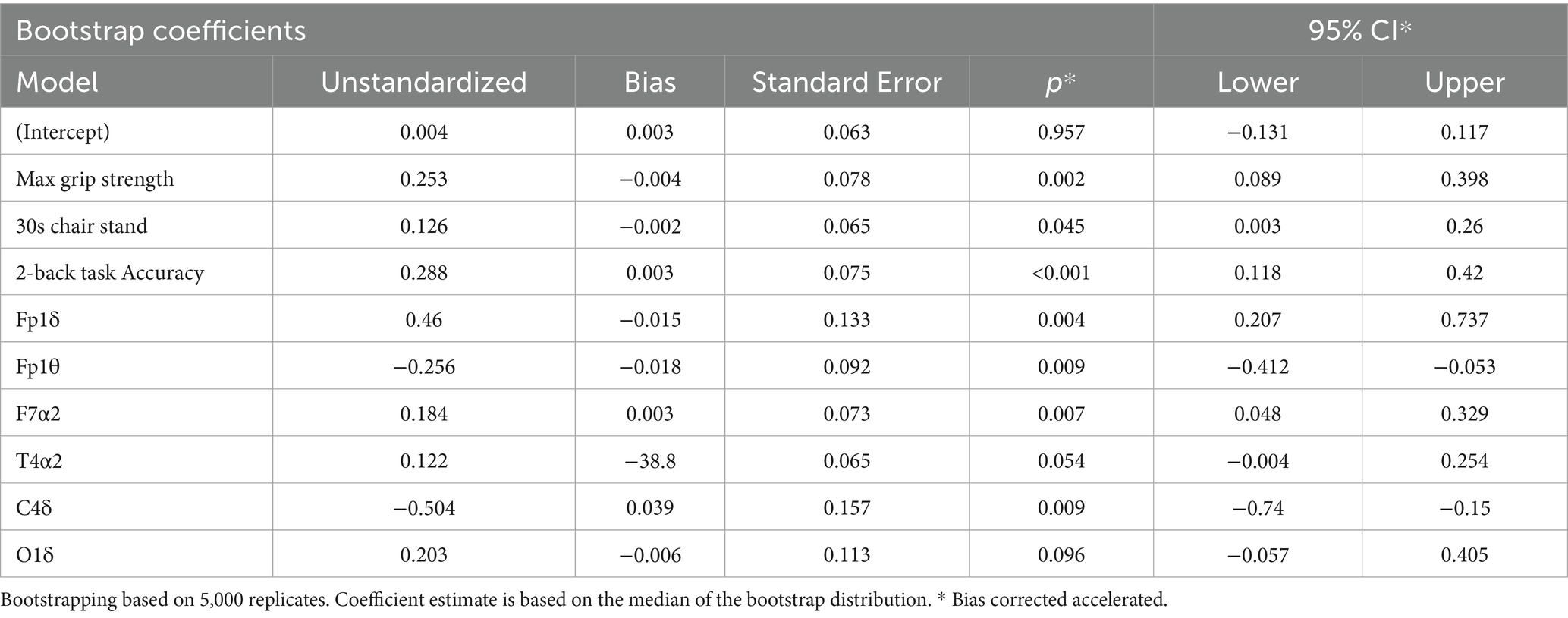
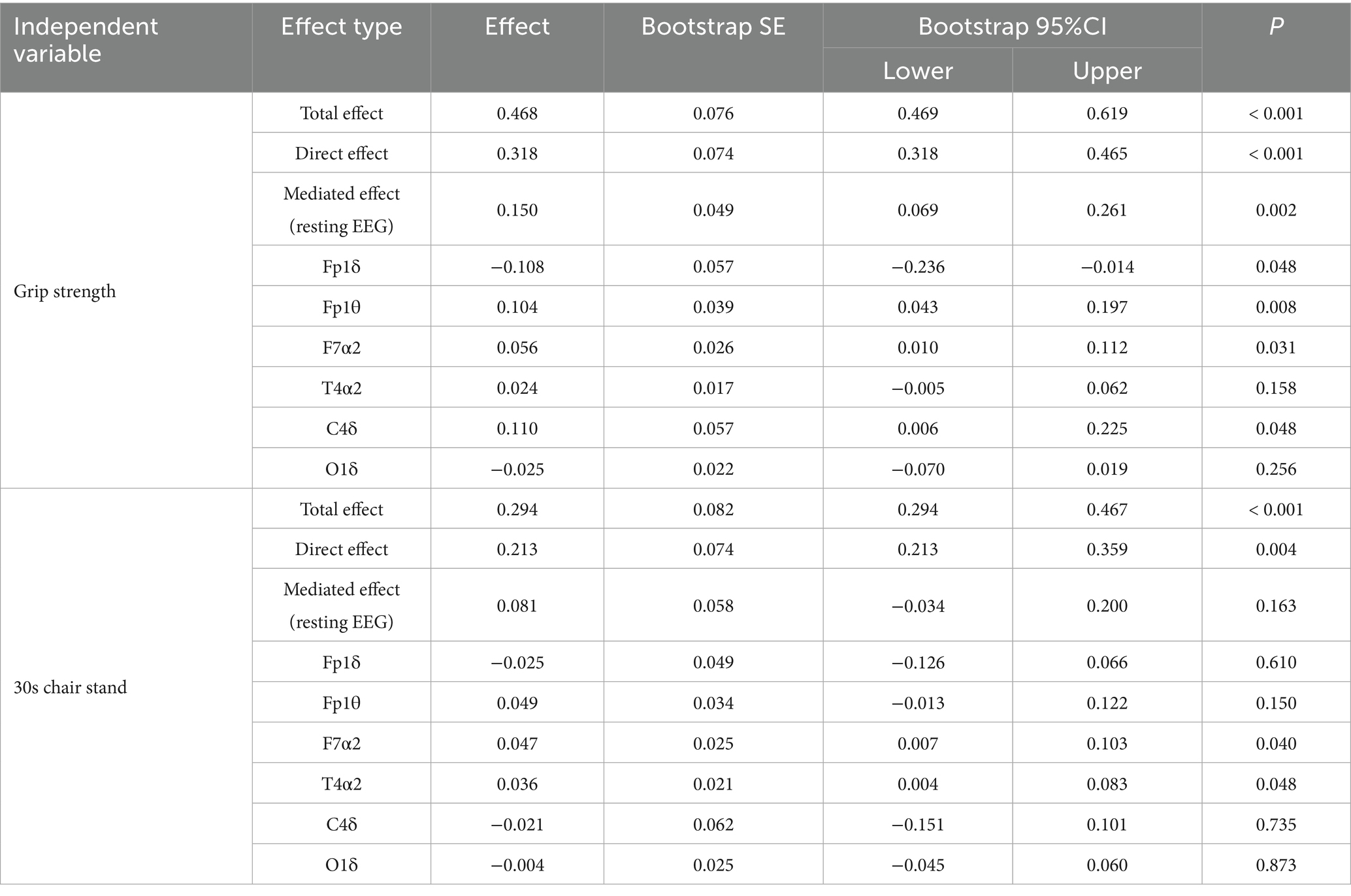
For the EEG-based model (Tables 7, 8), grip strength showed a total effect of β = 0.468 (95% CI [0.469, 0.619]), with a direct effect of β = 0.318 (95% CI [0.318, 0.465], p < 0.001), and a significant indirect effect via resting-state EEG features (β = 0.150, 95% CI [0.069, 0.261]), accounting for 32.05% of the total effect. Specific EEG markers—Fp1θ, F7α2, and C4δ—showed statistically significant mediation paths (see Table 8). In contrast, lower-limb strength also had a significant total effect on cognition (β = 0.294), with a direct effect of β = 0.213 (95% CI [0.213, 0.359], p < 0.001), but the indirect effect via EEG was non-significant (β = 0.081, 95% CI [−0.034, 0.200]), as the confidence interval included zero.
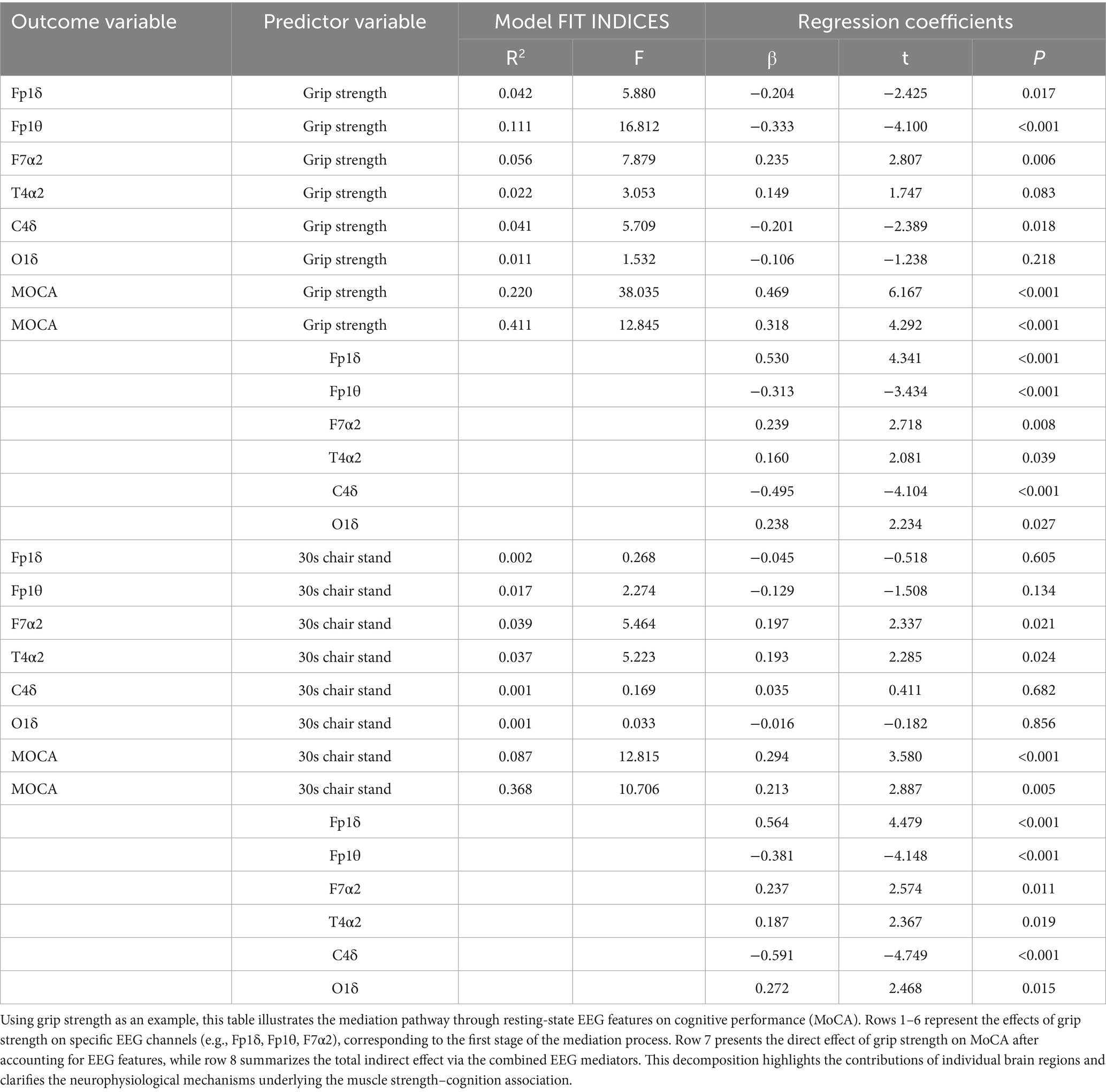
Table 7. Regression analysis of the mediating model of resting EEG characteristics between muscle strength and cognitive function.
In summary, these findings indicate that working memory significantly mediates the association between both upper and lower limb muscle strength and cognitive function, whereas resting-state EEG features mediate only the grip strength–cognition relationship. These results suggest that grip strength provides a unique neurophysiological pathway to cognition, while working memory serves as a broader cognitive mediator across different muscular systems.
4 Discussion
The study demonstrates significant associations between age, educational attainment, and cognitive impairment in older adults. Furthermore, multifactorial relationships involving resting-state EEG features; muscle strength; working memory; and cognitive functioning were identified, with their combination constituting key determinants of cognitive performance.
The study identified both advanced age and educational attainment as key determinants of cognitive decline. Aging emerged as a robust risk factor for cognitive deterioration in older adults, consistent with previous literature (Xin et al., 2025). Age-related changes—such as reductions in brain volume (particularly in the hippocampus and prefrontal cortex), diminished synaptic plasticity, neurotransmitter depletion, and cerebrovascular degeneration—have been well documented as contributors to cognitive vulnerability (Missonnier et al., 2004). These neurobiological alterations manifest as progressive deficits across multiple cognitive domains, including memory, processing speed, and executive function (He et al., 2023). In contrast, higher educational attainment exhibited protective effects, with greater years of education associated with reduced severity of cognitive impairment (Nie and Hu, 2025). Education may exert neuroprotective influences by enhancing neural network complexity and efficiency, thereby facilitating cognitive reserve mechanisms that buffer against neuropathological damage (Elkana and Beheshti, 2025). Epidemiological evidence indicates that each additional year of education is associated with a 7–11% reduction in dementia risk (Xu et al., 2016), with particularly pronounced benefits observed in verbal memory and executive functioning (Archer et al., 2018; Grande et al., 2016). Importantly, although age and education both significantly shape cognitive trajectories, they likely do so through distinct neurobiological mechanisms and interact differentially with other modifiers of cognitive aging. These findings underscore the need to consider both vulnerability and resilience factors in understanding individual variability in age-related cognitive outcomes.
This study demonstrated that resting-state EEG metrics—specifically Fp1δ, Fp1θ, F7α2, and C4δ in frontoparietal regions—along with muscular strength (upper and lower extremities) and working memory capacity, jointly predicted cognitive performance. These findings support our original hypothesis and provide novel electrophysiological evidence for the utility of EEG spectral features as biomarkers of cognitive impairment. The robust association between muscular strength, particularly handgrip, and cognitive function is consistent with prior studies (Xin et al., 2025; Cai et al., 2023; Nie and Hu, 2025; Sternäng et al., 2016; Burtscher and Burtscher, 2024). However, inconsistent results reported by Ritchie et al. (2016) may reflect methodological differences in cognitive assessment protocols and participant characteristics. Notably, oscillatory activity in resting-state frontal, parietal, and occipital cortices has been shown to couple with motor function (Ho et al., 2025), and specific EEG features—such as alpha-1 power at F7/F8 and T5/T6—are sensitive to interindividual differences in muscular strength (Xin et al., 2025). Working memory also emerged as a key cognitive predictor, with its decline reflecting neurodegenerative changes in the structural and functional integrity of prefrontal, temporal, and parietal regions. These findings reinforce the multifactorial etiology of cognitive impairment, wherein neuromuscular decline and cognitive dysfunction may be linked via shared pathophysiological pathways, including low-grade inflammation, insulin resistance, oxidative stress, and dysregulation of neurotrophic factors (Dercon et al., 2021; Gregorio and Battaglia, 2024; Tanaka et al., 2024; Nazzi et al., 2024; Zabetian-Targhi et al., 2021).
This study found that both resting-state EEG features (in the frontoparietal regions) and working memory independently mediated the relationship between muscle strength and cognitive decline; however, no significant joint mediation effect was observed between EEG and working memory. In the EEG mediation model, frontoparietal oscillatory activity (Fp1δ, Fp1θ, F7α2, C4δ) emerged as a key neurophysiological factor underlying the muscle–cognition association. Previous research suggests that muscle strength may enhance cognitive function by upregulating activation in specific prefrontal regions, and that older adults with higher levels of physical activity and greater muscle strength exhibit a 30–46% reduced risk of cognitive decline (Herold et al., 2019; Lubitz et al., 2017). In the working memory model, working memory significantly mediated the association between muscle strength and cognitive performance. As a core dimension of cognition (Elliott et al., 2011; Bopp and Verhaeghen, 2020), the capacity to retain information in working memory is essential for maintaining cognitive resilience in older age (Seo and Lee, 2022). Notably, no significant correlation was found between working memory and resting-state EEG features. We speculate that this discrepancy may stem from excessive cognitive load during the working memory task. The participants in this study had a mean age of 72.65 ± 7.754 years and exhibited substantial cognitive impairment, which may have rendered the 2-back task too demanding for their cognitive capacity, potentially introducing measurement bias. Therefore, future studies should incorporate larger samples and varied working memory load levels to validate and expand upon these findings.
Our findings revealed that handgrip strength exerted a more pronounced effect on resting-state EEG features and working memory performance, while lower-limb muscle strength did not significantly mediate EEG-cognition associations. Specifically, grip strength demonstrated robust positive correlations with working memory performance (reaction speed and accuracy) (Mavros et al., 2017), as well as synergistic associations with higher-order cognitive domains such as verbal fluency and episodic memory (Nie and Hu, 2025; Ruan et al., 2020). Its decline may serve as an early warning marker for working memory deterioration (Liu et al., 2022). This phenomenon likely stems from the unique physiological specialization of the upper-limb neuromuscular system (Gabriel et al., 2006; Zhang et al., 2023), where the hand acts as a primary effector of fine motor control and somatosensory feedback. Its function is tightly coordinated by prefrontal and parietal cortical regions. Grip training may therefore enhance cognitive performance through mechanisms such as improved synaptic plasticity and increased secretion of brain-derived neurotrophic factor (BDNF), facilitating more efficient engagement of cognition-related neural networks (Veronese et al., 2016; Chou et al., 2019).
In contrast, lower-limb muscle strength exhibited a weaker mediating role in EEG activity. This may be attributable to distinct corticospinal control pathways, reduced relevance of lower-limb precision in standard cognitive assessments, and the relatively limited cognitive demands imposed by gross motor tasks (Xin et al., 2025; Duchowny et al., 2022). Additionally, the lack of significance may partially reflect insufficient statistical power due to modest sample size. Future studies should expand participant cohorts to further elucidate the neurophysiological underpinnings linking lower-limb strength, brain oscillatory dynamics, and cognitive function.
5 Limitations and future prospects
Several limitations merit acknowledgment. First, the cross-sectional design precludes causal inference; thus, mediation pathways should be interpreted as associative rather than mechanistic. Second, although adjustments were made for key covariates (e.g., age, BMI, education), residual confounding from unmeasured factors—such as diet quality, sleep architecture, vascular integrity, and habitual physical activity—cannot be excluded. Prospective studies incorporating ecological momentary assessment and multimodal biomarkers (e.g., actigraphy, omega-3 index) are warranted. Third, while ICA and visual inspection attenuated major EEG artifacts, formal regression of physiological signals (e.g., ECG, EMG) was not performed, limiting signal specificity. Fourth, absolute spectral power was favored over ratio metrics (e.g., theta/alpha) to enhance robustness in resting-state elderly EEG, though the latter may offer complementary insights and should be considered in future analyses. Finally, functional status was not directly assessed via standardized ADL instruments (e.g., Barthel Index, Lawton IADL), limiting our ability to contextualize neuromuscular-cognitive interplay in daily living domains.
6 Conclusion
Upper-limb muscle strength predicts cognitive function by enhancing prefrontal-parietal network efficiency and optimizing working memory, whereas lower-limb strength shows limited association. These findings suggest that grip strength may serve as a modifiable biomarker and potential target for interventions aimed at preserving cognitive health in aging populations. However, causal relationships cannot be inferred due to the cross-sectional design. Future longitudinal studies are warranted to validate these mediation pathways and assess the efficacy of targeted neuromuscular interventions.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Shanghai University of Sport (102772020RT059). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
SG: Writing – review & editing, Resources, Visualization, Writing – original draft, Formal analysis, Project administration, Funding acquisition, Methodology, Software, Supervision, Investigation, Validation, Conceptualization, Data curation. SJ: Funding acquisition, Visualization, Conceptualization, Software, Investigation, Writing – review & editing, Resources, Project administration, Writing – original draft, Validation, Supervision, Formal analysis, Data curation, Methodology. XiW: Methodology, Writing – review & editing, Writing – original draft, Supervision. AW: Writing – review & editing, Writing – original draft, Formal analysis, Data curation. SL: Writing – original draft, Writing – review & editing, Validation, Supervision. FD: Methodology, Funding acquisition, Resources, Writing – review & editing, Writing – original draft. TM: Conceptualization, Writing – original draft, Investigation, Writing – review & editing. XuW: Data curation, Methodology, Writing – review & editing, Conceptualization, Software, Validation, Supervision, Investigation, Resources, Formal analysis, Writing – original draft, Visualization, Funding acquisition, Project administration.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by Shanghai University of Sport, the Shanghai Key Laboratory of Human Performance (11DZ2261100) and the National Social Science Foundation of China (22BTY076).
Acknowledgments
We thank all the participating patients and their schools for their trust, commitment, and cooperation, which made this study possible. We would like to thank Shanghai Nuocheng Electric Co., Ltd. for providing equipment support and technical assistance in data collection.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2025.1641209/full#supplementary-material
References
Archer, J. A., Lee, A., Qiu, A., and Chen, S. H. A. (2018). Working memory, age and education: a lifespan fmri study. PLoS One 13:e0194878. doi: 10.1371/journal.pone.0194878
Bajwa, R. K., Goldberg, S. E., Van Der Wardt, V., Burgon, C., Di Lorito, C., Godfrey, M., et al. (2019). A randomised controlled trial of an exercise intervention promoting activity, independence and stability in older adults with mild cognitive impairment and early dementia (praised)—a protocol. Trials 20:815. doi: 10.1186/s13063-019-3871-9
Bopp, K. L., and Verhaeghen, P. (2020). Aging and n-Back performance: a Meta-analysis. J. Gerontol. B Psychol. Sci. Soc. Sci. 75, 229–240. doi: 10.1093/geronb/gby024
Burtscher, J., and Burtscher, M. (2024). Training muscles to keep the aging brain fit. J. Sport Health Sci. 13, 761–763. doi: 10.1016/j.jshs.2024.04.006
Cai, Z., Wang, X., and Wang, Q. (2023). Does muscle strength predict working memory? A cross-sectional fnirs study in older adults. Front. Aging Neurosci. 15:1243283. doi: 10.3389/fnagi.2023.1243283
Chou, M. Y., Nishita, Y., Nakagawa, T., Tange, C., Tomida, M., Shimokata, H., et al. (2019). Role of gait speed and grip strength in predicting 10-year cognitive decline among community-dwelling older people. BMC Geriatr. 19:186. doi: 10.1186/s12877-019-1199-7
Connors, M. H., Seeher, K., Teixeira-Pinto, A., Woodward, M., Ames, D., and Brodaty, H. (2019). Mild cognitive impairment and caregiver burden: a 3-year-longitudinal study. Am. J. Geriatr. Psychiatry 27, 1206–1215. doi: 10.1016/j.jagp.2019.05.012
Dercon, Q., Nicholas, J. M., James, S. N., Schott, J. M., and Richards, M. (2021). Correction to: grip strength from midlife as an indicator of later-life brain health and cognition: evidence from a British birth cohort. BMC Geriatr. 21:518. doi: 10.1186/s12877-021-02445-x
D’esposito, M., and Postle, B. R. (2015). The cognitive neuroscience of working memory. Annu. Rev. Psychol. 66, 115–142. doi: 10.1146/annurev-psych-010814-015031
Duchowny, K. A., Ackley, S. F., Brenowitz, W. D., Wang, J., Zimmerman, S. C., Caunca, M. R., et al. (2022). Associations between handgrip strength and dementia risk, cognition, and neuroimaging outcomes in the UK biobank cohort study. JAMA Netw. Open 5:e2218314. doi: 10.1001/jamanetworkopen.2022.18314
Dujardin, K., Bourriez, J. L., and Guieu, J. D. (1995). Event-related desynchronization (Erd) patterns during memory processes: effects of aging and task difficulty. Electroencephalogr. Clin. Neurophysiol. 96, 169–182. doi: 10.1016/0168-5597(94)00284-L
Elkana, O., and Beheshti, I. (2025). The protective role of education in white matter lesions and cognitive decline. Brain Cogn. 187:106304. doi: 10.1016/j.bandc.2025.106304
Elliott, E. M., Cherry, K. E., Brown, J. S., Smitherman, E. A., Jazwinski, S. M., Yu, Q., et al. (2011). Working memory in the oldest-old: evidence from output serial position curves. Mem. Cogn. 39, 1423–1434. doi: 10.3758/s13421-011-0119-7
Gabriel, D. A., Kamen, G., and Frost, G. (2006). Neural adaptations to resistive exercise: mechanisms and recommendations for training practices. Sports Med. 36, 133–149. doi: 10.2165/00007256-200636020-00004
Grande, G., Cucumo, V., Cova, I., Ghiretti, R., Maggiore, L., Lacorte, E., et al. (2016). Reversible mild cognitive impairment: the role of comorbidities at baseline evaluation. J Alzheimer’s Dis 51, 57–67. doi: 10.3233/JAD-150786
Gregorio, F. D., and Battaglia, S. (2024). The intricate brain-body interaction in psychiatric and neurological diseases. Adv. Clin. Exp. Med. 33, 321–326. doi: 10.17219/acem/185689
He, S., Zhang, J., Yang, R., and Yuan, P. (2023). Spatial distribution of cognitive dysfunction and its risk factors in Chinese population aged 45 years and above. Nan Fang Yi Ke Da Xue Xue Bao 43, 611–619. doi: 10.12122/j.issn.1673-4254.2023.04.15
Herold, F., Törpel, A., Schega, L., and Müller, N. G. (2019). Functional and/or structural brain changes in response to resistance exercises and resistance training lead to cognitive improvements—a systematic review. Eur. Rev. Aging Phys. Act. 16:10. doi: 10.1186/s11556-019-0217-2
Ho, M. C., Fu, H. L., Kao, S. C., Moreau, D., Liang, W. K., Kuo, H. Y., et al. (2025). Quadriceps strength and temporal preparation in elderly adults: the mediating role of Beta oscillation. Eur. J. Neurosci. 61:e70101. doi: 10.1111/ejn.70101
Kamio, S., Hagiwara, A., Kamagata, K., Uchida, W., Nakaya, M., Sekine, T., et al. (2025). Association between cognitive function and relaxation rates of the cerebral cortex. J. Neurol. Sci. 472:123466. doi: 10.1016/j.jns.2025.123466
Keller, K., and Engelhardt, M. (2013). Strength and muscle mass loss with aging process. Age and strength loss. Muscles Ligam Tendons J 3, 346–350. doi: 10.32098/mltj.04.2013.17
Klass, D. W., and Brenner, R. P. (1995). Electroencephalography of the elderly. J. Clin. Neurophysiol. 12, 116–131. doi: 10.1097/00004691-199503000-00002
Klimesch, W. (2012). Α-Band oscillations, attention, and controlled access to stored information. Trends Cogn. Sci. 16, 606–617. doi: 10.1016/j.tics.2012.10.007
Li, F., Liu, T., Wang, F., Li, H., Gong, D., Zhang, R., et al. (2015). Relationships between the resting-state network and the P3: evidence from a scalp Eeg study. Sci. Rep. 5:15129. doi: 10.1038/srep15129
Liao, J., Wang, J., Jia, S., Cai, Z., and Liu, H. (2024). Correlation of muscle strength, working memory, and activities of daily living in older adults. Front. Aging Neurosci. 16:1453527. doi: 10.3389/fnagi.2024.1453527
Liu, S. W., Li, M., Zhu, J. T., Zhang, Y. C., Wu, Y. H., Liu, C. F., et al. (2022). Correlation of muscle strength with cognitive function and medial temporal lobe atrophy in patients with mild to moderate Alzheimer’s disease. Zhonghua Yi Xue Za Zhi 102, 2786–2792. doi: 10.3760/cma.j.cn112137-20220406-00715
Lubitz, A. F., Niedeggen, M., and Feser, M. (2017). Aging and working memory performance: electrophysiological correlates of high and low performing elderly. Neuropsychologia 106, 42–51. doi: 10.1016/j.neuropsychologia.2017.09.002
Mavros, Y., Gates, N., Wilson, G. C., Jain, N., Meiklejohn, J., Brodaty, H., et al. (2017). Mediation of cognitive function improvements by strength gains after resistance training in older adults with mild cognitive impairment: outcomes of the study of mental and resistance training. J. Am. Geriatr. Soc. 65, 550–559. doi: 10.1111/jgs.14542
Missonnier, P., Gold, G., Leonards, U., Costa-Fazio, L., Michel, J. P., Ibez, V., et al. (2004). Aging and working memory: early deficits in Eeg activation of posterior cortical areas. J. Neural Transm. (Vienna) 111, 1141–1154. doi: 10.1007/s00702-004-0159-2
Nazzi, C., Avenanti, A., and Battaglia, S. (2024). The involvement of antioxidants in cognitive decline and neurodegeneration: Mens Sana in Corpore Sano. Antioxidants (Basel) 13:701. doi: 10.3390/antiox13060701
Nie, W., and Hu, J. (2025). The relationship between grip strength and cognitive impairment: evidence from Nhanes 2011-2014. Brain Behav. 15:e70381. doi: 10.1002/brb3.70381
Puri, D. V., Gawande, J. P., Kachare, P. H., and al-Shourbaji, I. (2025). Optimal time-frequency localized wavelet filters for identification of Alzheimer’s disease from Eeg signals. Cogn. Neurodyn. 19:12. doi: 10.1007/s11571-024-10198-7
Ritchie, S. J., Tucker-Drob, E. M., Starr, J. M., and Deary, I. J. (2016). Do cognitive and physical functions age in concert from age 70 to 76? Evidence from the Lothian birth cohort 1936. Span. J. Psychol. 19:E90. doi: 10.1017/sjp.2016.85
Ruan, Y., Shi, Y., Guo, Y. F., Sun, S. Y., Huang, Z. Z., Wang, Y. Z., et al. (2020). Association between grip strength, rapid gait speed and cognition in people aged 50 and above in Shanghai during 2009-2010. Zhonghua Yu Fang Yi Xue Za Zhi 54, 1414–1420. doi: 10.3760/cma.j.cn112150-20200714-01003
Seo, J. H., and Lee, Y. (2022). Association of physical activity with sarcopenia evaluated based on muscle mass and strength in older adults: 2008-2011 and 2014 - 2018 Korea National Health and nutrition examination surveys. BMC Geriatr. 22:217. doi: 10.1186/s12877-022-02900-3
Si, Y., Jiang, L., Tao, Q., Chen, C., Li, F., Jiang, Y., et al. (2019). Predicting individual decision-making responses based on the functional connectivity of resting-state Eeg. J. Neural Eng. 16:066025. doi: 10.1088/1741-2552/ab39ce
Sternäng, O., Reynolds, C. A., Finkel, D., Ernsth-Bravell, M., Pedersen, N. L., and Dahl Aslan, A. K. (2016). Grip strength and cognitive abilities: associations in old age. J. Gerontol. B Psychol. Sci. Soc. Sci. 71, 841–848. doi: 10.1093/geronb/gbv017
Tanaka, M., Battaglia, S., Giménez-Llort, L., Chen, C., Hepsomali, P., Avenanti, A., et al. (2024). Innovation at the intersection: emerging translational research in neurology and psychiatry. Cells 13:790. doi: 10.3390/cells13100790
Vassilaki, M., Aakre, J. A., Lesnick, T. G., Kremers, W. K., Graff-Radford, J., Knopman, D. S., et al. (2025). Patterns of factors in the National Institute on Aging health disparities research framework domains and mild cognitive impairment risk. AJPM Focus 4:100324. doi: 10.1016/j.focus.2025.100324
Veronese, N., Stubbs, B., Trevisan, C., Bolzetta, F., de Rui, M., Solmi, M., et al. (2016). What physical performance measures predict incident cognitive decline among intact older adults? A 4.4year follow up study. Exp. Gerontol. 81, 110–118. doi: 10.1016/j.exger.2016.05.008
Wang, M., Gamo, N. J., Yang, Y., Jin, L. E., Wang, X. J., Laubach, M., et al. (2011). Neuronal basis of age-related working memory decline. Nature 476, 210–213. doi: 10.1038/nature10243
Xin, X., Liu, Q., Jia, S., Li, S., Wang, P., and Wang, X. (2025). Correlation of muscle strength, information processing speed and cognitive function in the elderly with cognitive impairment--evidence from EEG. Front. Aging Neurosci. 17:1496725. doi: 10.3389/fnagi.2025.1496725
Xu, W., Tan, L., Wang, H. F., Tan, M. S., Tan, L., Li, J. Q., et al. (2016). Education and risk of dementia: dose-response Meta-analysis of prospective cohort studies. Mol. Neurobiol. 53, 3113–3123. doi: 10.1007/s12035-015-9211-5
Yang, L., Tang, X., Zhou, N., Zhang, M., and Yan, J. (2018). Application and reliability verification of the Beijing version of the Montreal Cognitive Assessment in the assessment of cognitive function in adults with Osahs. J. Clin. Otorhinol. Head Neck Surg. 32, 58–64. doi: 10.13201/j.issn.1001-1781.2018.01.012
Zabetian-Targhi, F., Srikanth, V. K., Smith, K. J., Oddy PhD, W. H., Beare, R., Moran, C., et al. (2021). Associations between the dietary inflammatory index, brain volume, small vessel disease, and global cognitive function. J. Acad. Nutr. Diet. 121, 915–24.e3. doi: 10.1016/j.jand.2020.11.004
Keywords: cognitive impairment, EEG characteristics, muscle strength, working memory, cognitive function
Citation: Song Y, Jia S, Wang X, Wang A, Li S, Ding F, Ma T and Wu X (2025) Muscle strength, EEG biomarkers, and working memory as interacting predictors of cognitive function in cognitively impaired older adults. Front. Aging Neurosci. 17:1641209. doi: 10.3389/fnagi.2025.1641209
Edited by:
Adérito Ricardo Duarte Seixas, Escola Superior de Saúde Fernando Pessoa, PortugalReviewed by:
Benedictor Alexander Nguchu, University of Science and Technology of China, ChinaMohammed Jajere Adamu, Tianjin University, China
Ricardo Iván Bravo-Chávez, Autonomous University of Hidalgo State, Mexico
Copyright © 2025 Song, Jia, Wang, Wang, Li, Ding, Ma and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xueping Wu, d3V4dWVwaW5nQHN1cy5lZHUuY24=
 Yagang Song
Yagang Song Shuqi Jia
Shuqi Jia Xing Wang
Xing Wang Aiwei Wang
Aiwei Wang Shufan Li
Shufan Li Feng Ding2
Feng Ding2