- 1School of Kinesiology and Health, Capital University of Physical Education and Sports, Beijing, China
- 2College of Physical Education and Sport, Langfang Normal University, Langfang, Hebei, China
- 3School of Physical Education and Sport Science, Fujian Normal University, Fuzhou, Fujian, China
Background: Recent studies indicate that older adults (aged 55 years and above) represent a critical period for changes in circulating brain derived neurotrophic factor (BDNF) levels and cognitive function. Regular aerobic exercise (AE) has been recognized as a promising non-pharmacological strategy to influence neuroplasticity and cognitive function, primarily through the regulation of BDNF. However, inconsistent findings have been reported regarding the specific effects of three AE modalities—walking, running, and cycling—on circulating BDNF levels in older adults. This study aimed to systematically evaluate the effects of these AE modalities on BDNF levels in the elderly through a meta-analysis, and to further compare the relative effectiveness of various exercise protocols using network meta-analysis.
Methods: A systematic search was conducted from database inception to June 10, 2025, in PubMed, Web of Science, Embase, the Cochrane Library, and Scopus. The methodological quality and risk of bias of the included studies were assessed using the Cochrane Risk of Bias 2 tool. Meta-analysis and network meta-analysis were conducted using Stata software (version 18, StataCorp LLC, United States).
Results: A total of 17 studies involving 900 older participants were included. Meta-analysis indicated that three AE modalities significantly increased circulating BDNF levels (SMD = 0.62, 95% CI: 0.06 to 1.18, p = 0.03). Subgroup analysis revealed that the intervention effect was significantly influenced by participants’ health status (p < 0.01). Specifically, the interventions had positive effects in healthy individuals and those with mild cognitive impairment. Exercise-related variables such as modality, intensity, and the interval were identified as potential moderators. Network meta-analysis demonstrated that protocols involving low-intensity short-duration walking (WLS) were superior to other exercise protocols (p < 0.05), and protocols involving moderate-intensity short-duration walking (WMS) were more effective in increasing BDNF levels than high-intensity long-duration walking (p < 0.05). Surface under the cumulative ranking curve results further supported the superiority of WLS (99.9%) and WMS (83.7%) over other exercise protocols.
Conclusion: Walking, running, and cycling are effective for improving circulating BDNF levels in older adults; however, the magnitude of improvement depends on participants’ health status and specific exercise prescription. Interventions involving walking at low to moderate intensity demonstrated favorable efficacy. This effect may be more favorable in healthy individuals and those with mild cognitive impairment. Future studies should further investigate the influence of total exercise volume on outcomes and adopt more rigorous and standardized protocols to facilitate the development of standardized exercise strategies, thereby improving comparability and reducing heterogeneity in future analyses.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/, Identifier CRD420251068909.
1 Introduction
With advancing age, the progressive accumulation of deleterious cellular components and molecular markers significantly impairs the body’s ability to cope with internal and external stressors, rendering the brain increasingly susceptible to a state of chronic, low-grade neuroinflammation (Rezaee et al., 2022). As one of the core systems involved in responding to and resisting stress, the nervous system exhibits high sensitivity to such exposure. Consequently, any risk factors associated with the nervous system, including psychological stress and emotional trauma, may induce neuronal apoptosis and synaptic plasticity impairments (Barde, 2025). Notably, aging further intensifies these processes, leading to brain atrophy, microstructural white matter damage, and alterations in neural activity patterns (Raz et al., 2010), thereby elevating the risk of psychiatric and neurodegenerative disorders in the elderly, including mild cognitive impairment (MCI), Alzheimer’s disease (AD), and Parkinson’s disease (PD) (Mattson and Magnus, 2006). Although cognitive decline often occurs with aging (manifested as a high incidence of cognitive disorders among older adults) (Morrison and Baxter, 2012), existing evidence has demonstrated that certain health-promoting behaviors may enhance cognitive performance and delay its deterioration. Among these, physical exercise has been identified as one of the most promising interventions due to its cost-effectiveness, accessibility, and efficiency (Erickson et al., 2022; Gates et al., 2013).
While general exercise guidelines are available for all ages, whether these guidelines are optimal or sufficient for improving cognitive function in older adults remains debated, and the underlying mechanisms are not fully understood. Therefore, further investigation into the cognitive benefits of physical exercise in aging populations remains critically important. A systematic review revealed that supervised exercise interventions significantly improved cognitive function in adults aged 50 years and older, regardless of whether participants were cognitively intact or exhibited cognitive impairments (Northey et al., 2018). Similarly, recent findings have indicated that moderate-intensity aerobic exercise (AE) not only contributes to improved glycemic control in older individuals with type 2 diabetes (T2D), but also enhances cerebral vascular hemodynamics, and significantly increases circulating levels of brain-derived neurotrophic factor (BDNF) (Ploydang et al., 2023). These findings suggest that the cognitive benefits of exercise may be mediated through multiple neurobiological mechanisms, among which BDNF has been identified as a critical regulatory factor underlying exercise-induced improvements in cognitive function.
BDNF, a frequently studied dimeric polypeptide and member of the neurotrophin family, plays a crucial role in neuronal differentiation, axonal growth, survival, and synaptic plasticity. However, its levels naturally decline with age (von Bohlen und Halbach, 2010). BDNF is widely expressed in the brain, particularly in the hippocampus, amygdala, cerebral cortex, and cerebellum, and it can potentially cross the blood–brain barrier to play a key role in enhancing learning, memory, and cognitive function (Jemni et al., 2023). Notably, the transport of BDNF across the blood–brain barrier may be influenced by its form. For instance, platelet-bound BDNF may be released more slowly than freely circulating BDNF, potentially altering its bioavailability and affecting peripheral BDNF levels (Dell'Oste et al., 2024). BDNF is initially synthesized in its precursor form (proBDNF), which is then cleaved by proteases into its biologically active mature form (mBDNF) (Antonijevic et al., 2024). mBDNF is released during synaptic activity or neuronal excitation (including physical exercise), where it binds to its high-affinity receptor TrkB (tropomyosin receptor kinase B), activating a series of downstream signaling pathways which may be associated with potential benefits for cognitive function and synaptic plasticity (Li et al., 2022).
In addition to its effects on neuroplasticity, BDNF has been associated with increased hippocampal volume in older adults. Studies have demonstrated that AE can significantly improve circulating BDNF levels, increases hippocampal volume, and enhances the gray matter volume in several cortical regions (Firth et al., 2018; Prakash et al., 2010; Sharma et al., 2024). For instance, Erickson et al. (2011) conducted a one-year moderate-intensity walking training program (50 to 75% HRR) in healthy older adults and found significant increases in hippocampal volume, which were closely associated with elevated serum BDNF levels. However, inconsistent findings were reported by Enette et al. (2020), who conducted a 9-week cycling training program for elderly individuals with AD. Despite significant improvements in participants’ 6-min walking test performance and quality of life, no significant changes in plasma BDNF levels or cognitive function after the intervention. Similarly, Maass et al. (2016) reported that a 3-month AE intervention did not significantly alter circulating BDNF levels or cognitive performance in the elderly.
Despite some inconsistencies, clinical trial evidence suggests that appropriately designed AE protocols can significantly modulate BDNF levels, enhance brain structure, and contribute to the prevention of age-related psychiatric and neurodegenerative disorders (Rezaee et al., 2022). Notably, different exercise types exert differential effects on BDNF regulation. In particular, compared to resistance exercise, AE appears to confer greater benefits in promoting neuroplasticity and enhancing cognitive function (Hvid et al., 2017; Tsai et al., 2019). However, the current literature remains inconclusive regarding the specific effects of three AE modalities—walking, running, and cycling—on circulating BDNF levels in older adults. This study aims to systematically evaluate the impact of these AE modalities on circulating BDNF in the elderly. Furthermore, a network meta-analysis will be conducted to identify the most effective AE protocols. Restricting the analysis to these modalities enables a more coherent comparison of their effects and enhances the applicability of findings to practical exercise prescriptions. This study is expected to clarify the comparative efficacy of commonly practiced AE protocols in modulating BDNF and provide a theoretical basis for developing individualized exercise prescriptions that may support cognitive health in aging populations, while also offering methodological references for the prevention of cognitive decline–related psychiatric and neurodegenerative conditions.
2 Methods
This meta-analysis has been registered on the International Prospective Register of Systematic Reviews (PROSPERO), with registration number CRD420251068909, and was conducted in accordance with systematic reviews and meta-analyses, as outlined by the PRISMA statement (Page et al., 2021).
2.1 Search strategy
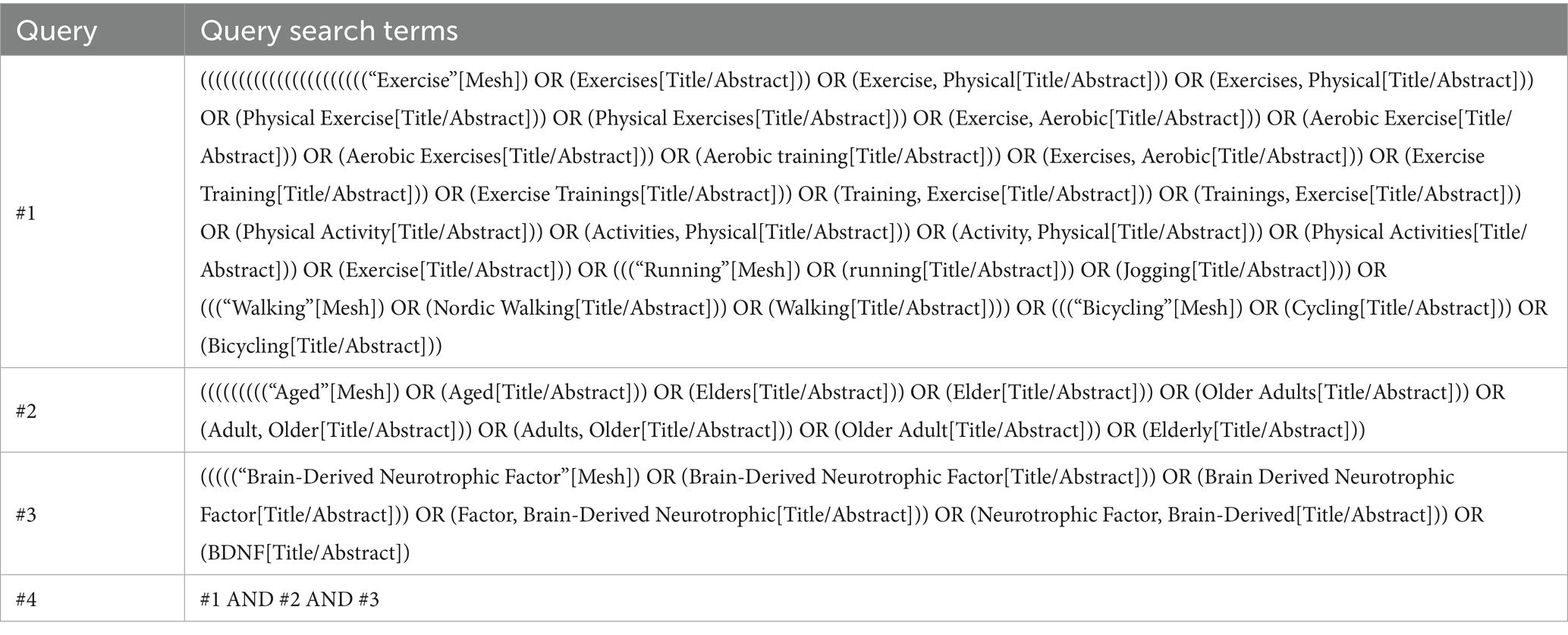
A systematic search was conducted from database inception to June 10, 2025, in PubMed, Web of Science, Embase, the Cochrane Library, and Scopus. Boolean operators (AND, OR) were used to search randomized controlled trials (RCTs) related to the effects of walking, running, and cycling on BDNF levels in older adults. The search strategy incorporated the following terms: “Exercise,” “Physical Exercise,” “Aerobic Training,” “Aerobic Exercise,” “Walking,” “Jogging,” “Running,” “Cycling,” “Bicycling,” “Aged,” “Elder,” “Older Adults,” “Older Adult,” “Elderly,” “Brain-Derived Neurotrophic Factor,” and “BDNF.” A detailed search strategy for PubMed is provided in Table 1.
2.2 Eligibility criteria
2.2.1 Inclusion criteria
1. Study Population: Older adults aged ≥55 years, regardless of sex or health status; this age cutoff was chosen since recent studies indicate it is a critical period for changes in BDNF levels and cognitive function (Kim et al., 2025; Wang et al., 2024).
2. Intervention: Participants perform AE in the modality of walking, running, or cycling, with exercise interventions lasting a minimum of 4 weeks (Cardoso et al., 2024; Gholami et al., 2025; Ribeiro et al., 2021), if an intervention simultaneously involved two of these modalities, it was also eligible to comprehensively capture the effects of exercise on BDNF.
3. Control Group: The control group consisted of participants engaging in non-AE (e.g., stretching) or maintaining their usual lifestyle.
4. Outcome Measures: Studies have reported plasma or serum BDNF levels before and after the intervention, with no restrictions on detection methods.
5. Study Design: RCTs.
2.2.2 Exclusion criteria
Studies were excluded if they were non-randomized controlled trials, self-controlled trials, animal studies, duplicate publications, review articles, or conference abstracts. Studies with unclearly described interventions, or those involving exercise modalities other than walking, running, or cycling (e.g., combined aerobic and resistance training, Tai Chi, dance), were also excluded. These three modalities were selected to reduce heterogeneity across exercise types and to facilitate the comparison of specific intervention protocols in the network meta-analysis. In addition, studies that did not report changes in BDNF levels, lacked extractable statistical data, or included control groups involving resistance training or other interventions that could confound comparisons were excluded.
2.3 Study selection and data extraction
After removing duplicate references using EndNote (version 20.6, Clarivate, United States), two authors (Y.C. and Y.L.) independently screened article titles and abstracts according to the pre-established inclusion and exclusion criteria. Subsequently, potentially eligible studies were selected for full-text review to determine final eligibility. The two authors extracted study characteristics using Microsoft Excel, including first author, publication year, participant demographics, specific parameters of the exercise intervention (e.g., type, frequency, intensity, duration), and outcome measures. Any disagreements were resolved through discussion, and if necessary, consultation with a third author (Z.L.) to ensure accuracy and consistency.
2.4 Bias risk and methodological quality assessment of included studies
The Cochrane Risk of Bias 2 (RoB 2) tool was used to assess the risk of bias and methodological quality of the included studies (Sterne et al., 2019). This tool consists of five domains: (1) randomization process; (2) deviations from intended interventions; (3) missing outcome data; (4) measurement of the outcome; and (5) selection of the reported result. Based on a comprehensive assessment of these five domains, the two authors (Y.C. and Y.L.) independently evaluated the studies using three categories: “low risk,” “some concerns,” or “high risk.” Any discrepancies in the assessment were resolved through discussion.
2.5 Statistical methods
Meta-analysis was performed using Stata software (version 18, StataCorp LLC, United States) (Antoniou et al., 2019). Given the inconsistency in measurement units across studies, the standardized mean difference (SMD) and its 95% confidence interval (95% CI) were used for the pooled effect analysis, with Hedges’ g applied to correct for biases arising from small sample sizes (Taylor and Alanazi, 2023). Heterogeneity was assessed using the I2 statistic, where values of I2 at 25, 50, and 75% indicate low, moderate, and high levels of heterogeneity, respectively. If I2 exceeded 50%, indicating significant heterogeneity, a random-effects model was used to pool the effect sizes; otherwise, a fixed-effects model was applied (Higgins et al., 2003). The interpretation of effect size was as follows: SMD < 0.4 indicates a small effect, 0.4–0.8 indicates a medium effect, and SMD > 0.8 indicates a large effect (Kons et al., 2023). To explore the potential sources of heterogeneity, subgroup analysis was performed for categorical variables, while meta-regression was used for continuous variables. Funnel plots were generated, and publication bias was quantitatively assessed using Egger’s test. If significant bias was detected, results were further corrected using the trim-and-fill method. Finally, sensitivity analysis was conducted using the leave-one-out method to assess the robustness of the pooled results.
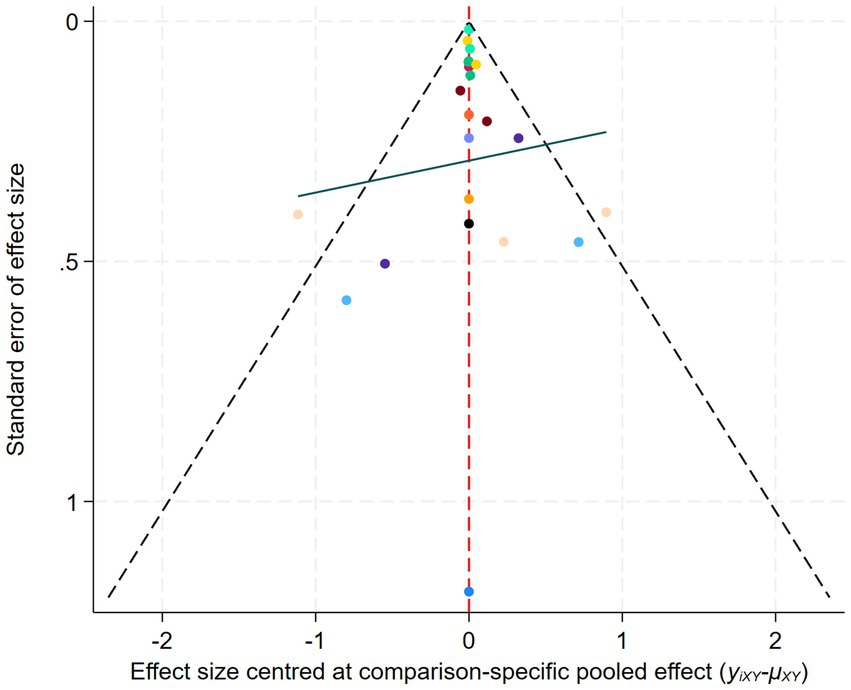
In the network meta-analysis, a network geometry plot was constructed to visualize the comparative relationships among different AE protocols (Chaimani et al., 2013). In the presence of open loops in the network, a consistency model was applied. If closed loops were present, further testing for loop inconsistency was conducted to examine the consistency between direct and indirect comparison results. A p-value greater than 0.05 indicated good consistency between direct and indirect evidence, and a consistency model was used; if p < 0.05, the sources of inconsistency were further investigated. Subsequently, surface under the cumulative ranking curve (SUCRA) was employed to rank the exercise protocols in order to identify the optimal intervention. Lastly, a comparison-adjusted funnel plot was used to assess potential publication bias and small sample effects (Higgins et al., 2012).
3 Results
3.1 Literature search and screening
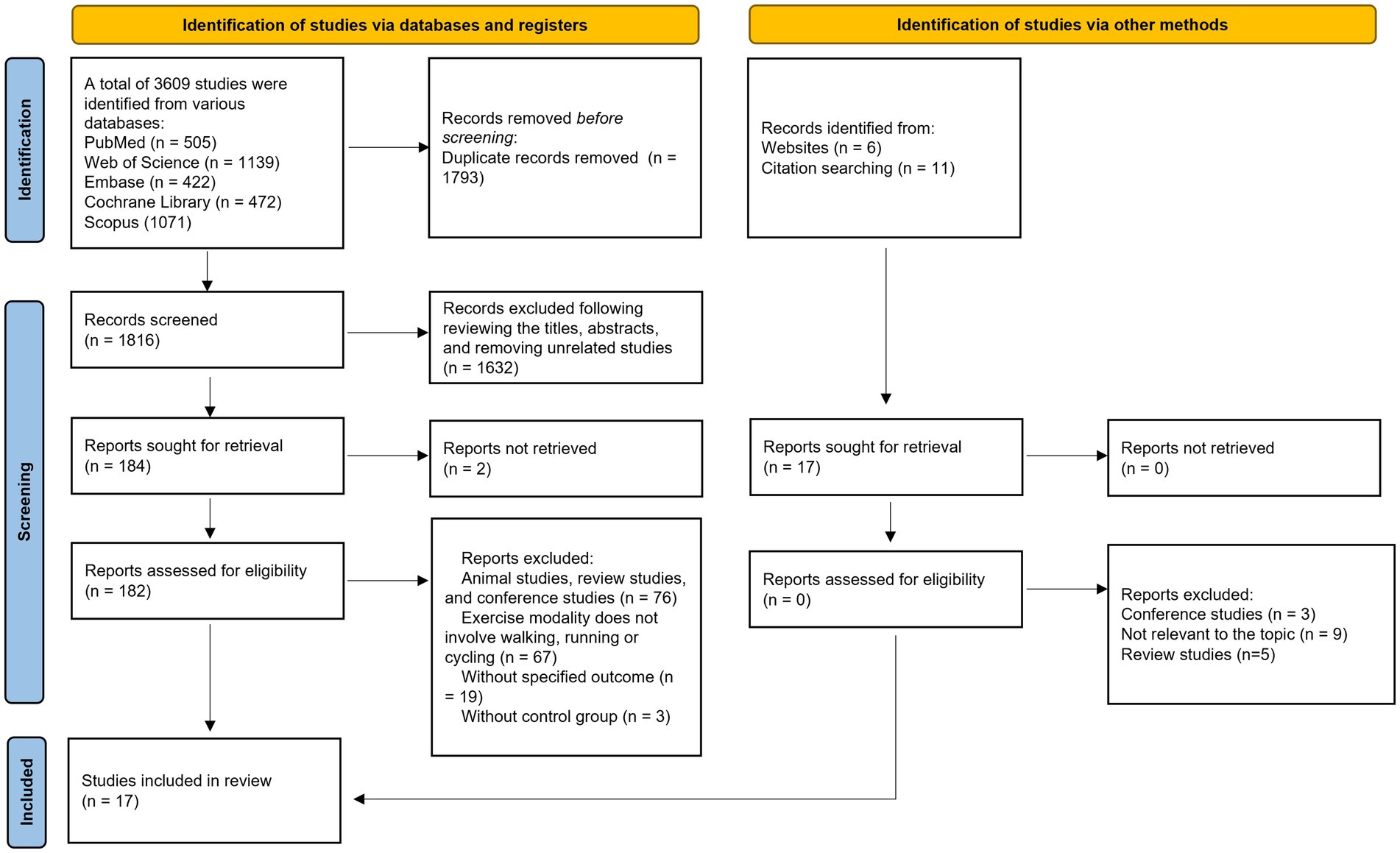
The primary flow of literature screening is illustrated in Figure 1. After initial retrieval, removal of duplicates, title and abstract screening, and full-text assessment, a total of 17 studies met the inclusion criteria (Chou et al., 2022; Enette et al., 2020; Erickson et al., 2011; Fungwe et al., 2019; Hsu et al., 2021; Kohanpour et al., 2017; Kovacevic et al., 2020; Li et al., 2021; Lin et al., 2025; Maass et al., 2016; Matura et al., 2017; Osali, 2020; Ploydang et al., 2023; Rackoll et al., 2024; Reed et al., 2022; Segura et al., 2020; Stein et al., 2023).
3.2 Characteristics and quality assessment of included studies
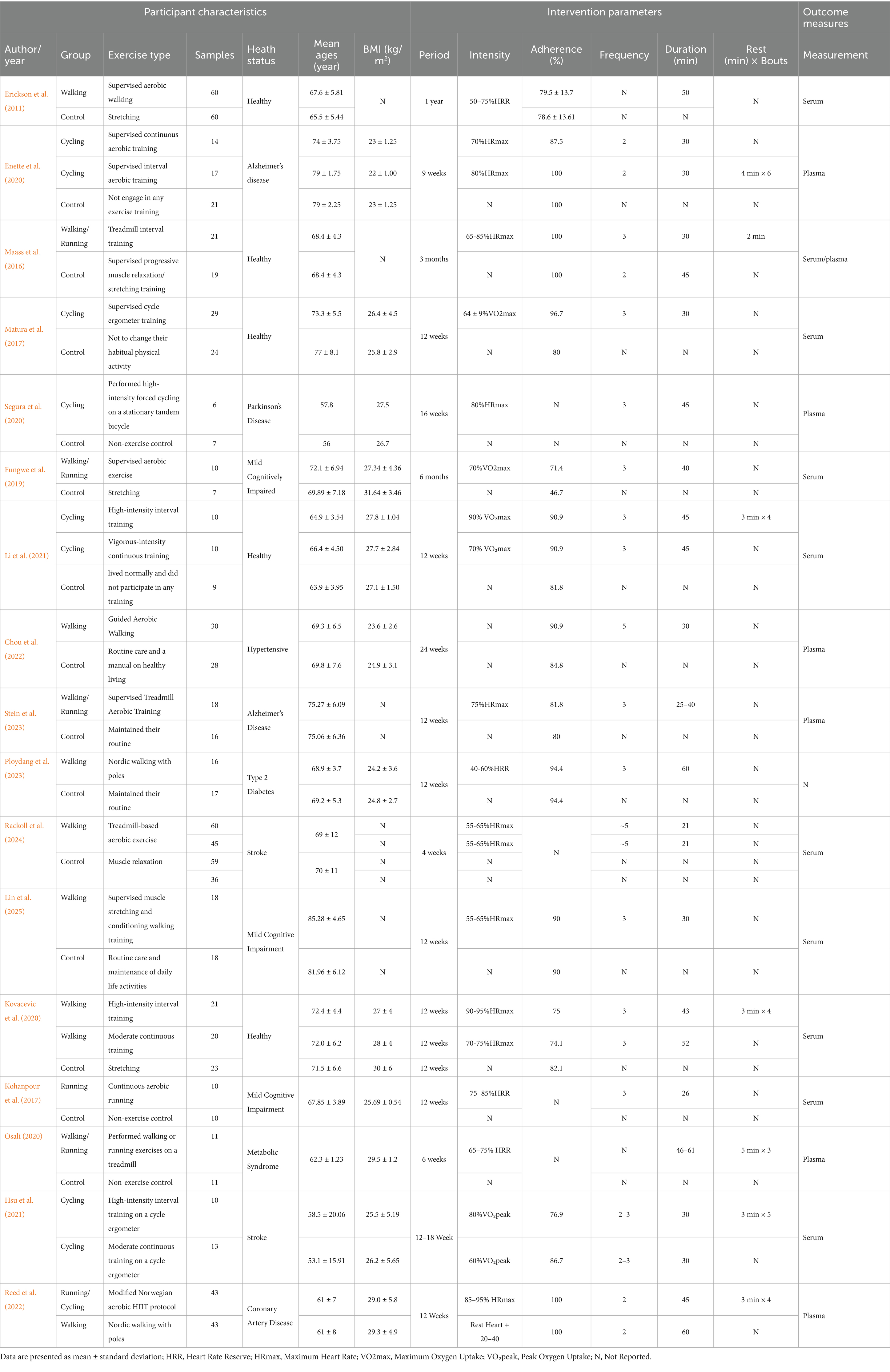
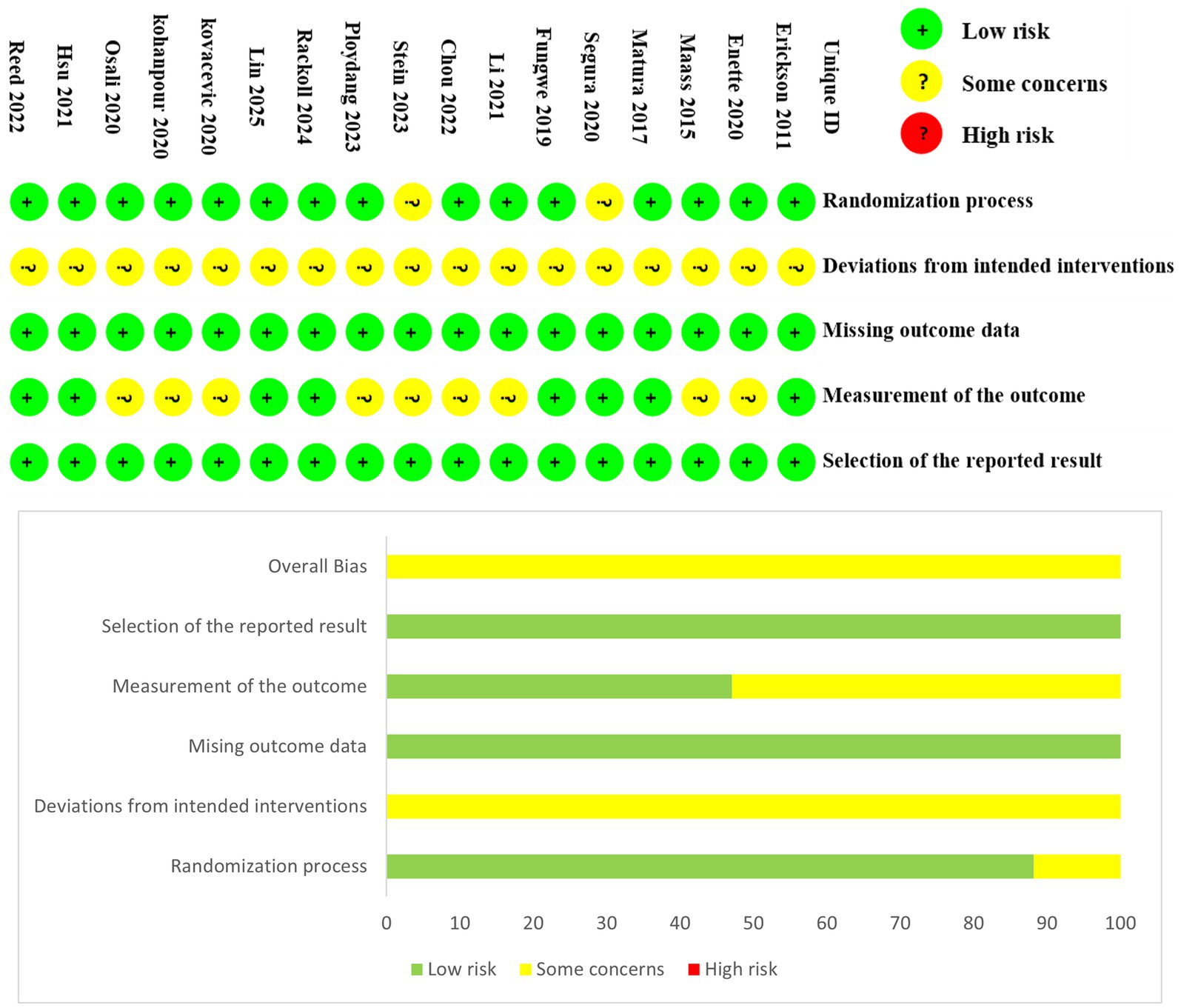
The 17 included studies involved a total of 900 participants. Among these, 15 studies were incorporated into the traditional meta-analysis, while 2 studies, which involved specific intervention comparisons, were included in the network meta-analysis (Hsu et al., 2021; Reed et al., 2022). Overall, in the included studies, the mean age of participants was 69 years. The exercise interventions involved walking, running, and cycling, with mean session lasting approximately 38 min, performed 2–5 times per week. Mean adherence to the exercise protocols was reported to be 86.4%. Exercise intensity was primarily measured using heart rate or oxygen consumption, ranging from 40 to 95% of heart rate and 60–90% of oxygen uptake. The control groups received interventions such as stretching or relaxation, routine care or health education, or were instructed to maintain their routine. Detailed characteristics of the studies are presented in Table 2. The risk of bias and methodological quality assessments are shown in Figure 2. Notably, due to the inherent nature of exercise interventions, the implementation of double-blind RCTs poses considerable challenges; consequently, most studies were rated as “some concerns” in the second domain of the RoB 2 (Cheng et al., 2025).
3.3 Meta-analysis
3.3.1 Pooled effect size results
Interventions were coded according to exercise modality (walking [W], running [R], cycling [C] and Mix [H]), exercise intensity (low [L], moderate [M], high [V]) (Gholami et al., 2025), and intervention duration (greater than 30 min [L] and less than or equal to 30 min [S]) to facilitate network comparison. The heterogeneity test revealed I2 = 93.70%, τ2 = 1.48, Q(19) = 132.96, p < 0.01, indicating significant heterogeneity among the studies. Therefore, a random-effects model was employed to pool the effect sizes. The results showed that the three AE modalities significantly increased circulating BDNF levels compared with the control group (SMD = 0.62, 95% CI: 0.06 to 1.18, p = 0.03) (Figure 3).
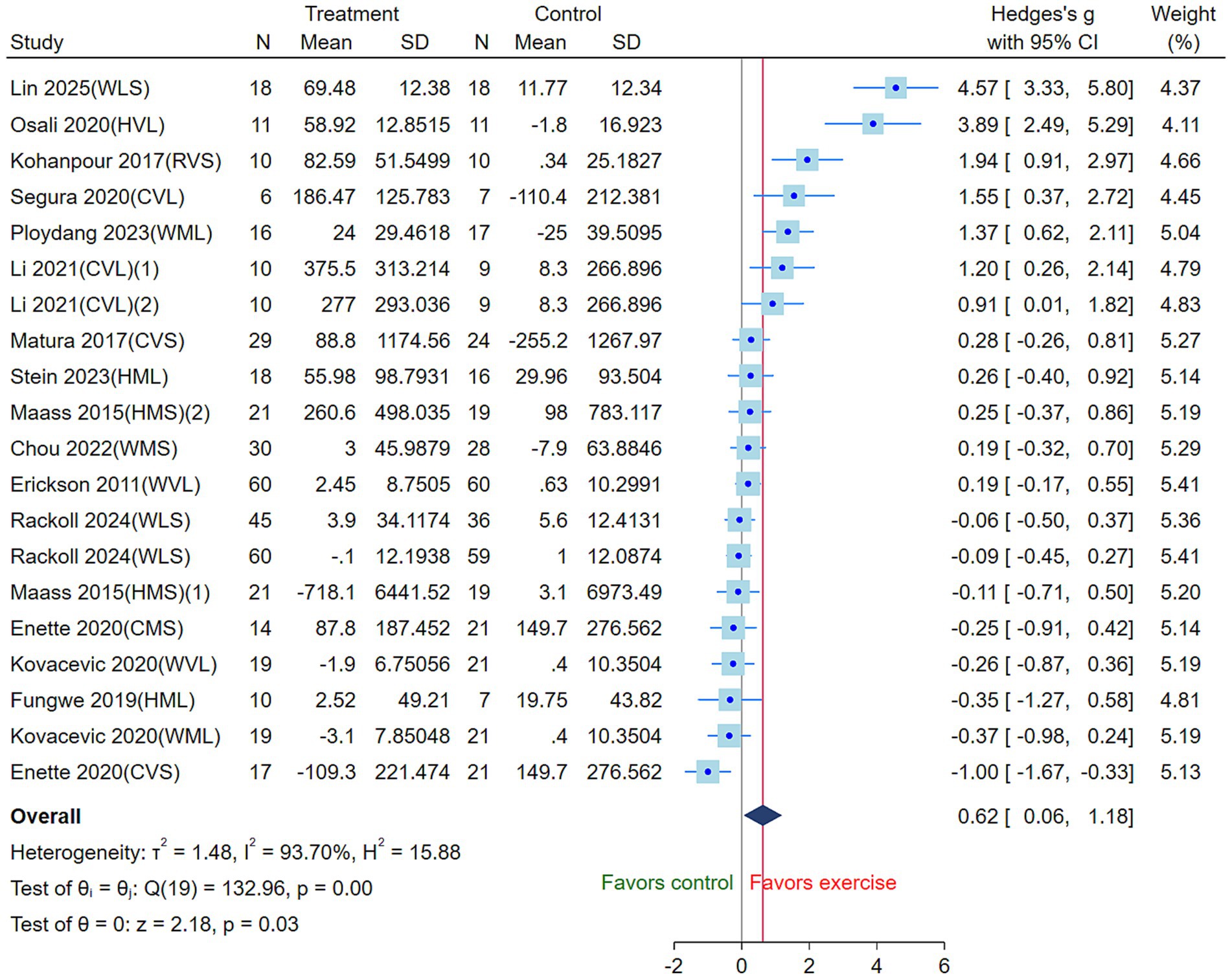
Figure 3. Effects of three aerobic exercise modalities on circulating BDNF levels in the elderly (CVS, high-intensity short-duration cycling; CVL, high-intensity long-duration cycling; CMS, moderate-intensity short-duration cycling; WVL, high-intensity long-duration walking; WMS, moderate-intensity short-duration walking; WML, moderate-intensity long-duration walking; WLS, low-intensity short-duration walking; RVS, high-intensity short-duration running; HVL, high-intensity long-duration mixed exercise; HMS, moderate-intensity short-duration mixed exercise; HML, moderate-intensity long-duration mixed exercise) (Square, the Hedges’s g for study; CI, Confidence Interval; τ2, tau-squared; I2, I-squared; H2, H-squared; Q, Q statistics; z, Z-score).
3.3.2 Subgroup analysis and meta-regression
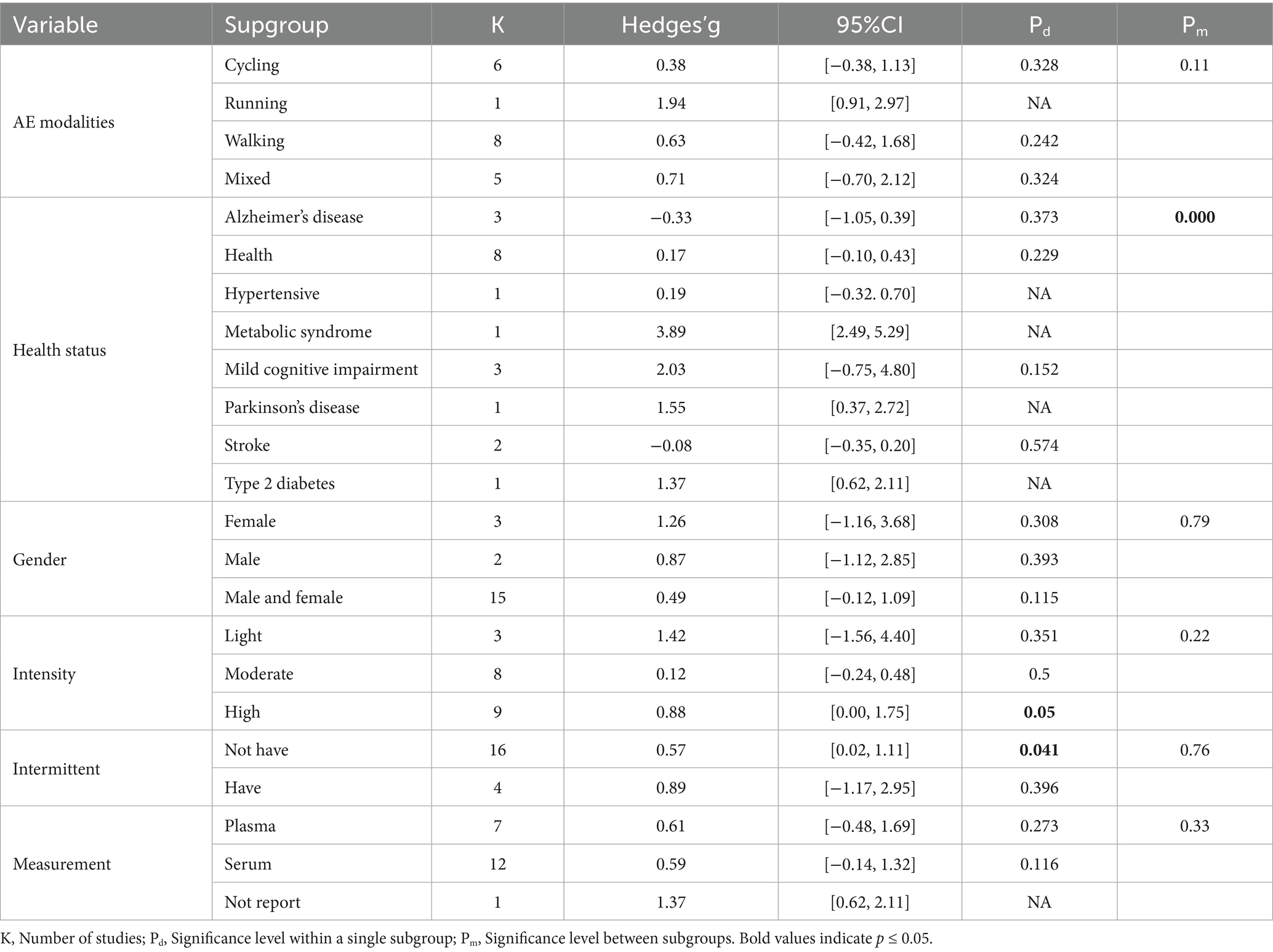
To further investigate potential sources of heterogeneity and the effects of different variables on BDNF intervention outcomes, subgroup analyses were conducted for categorical variables (Table 3), while meta-regression was performed for continuous variables (Table 4). Regarding intervention modality, running demonstrated a positive effect on BDNF levels in the elderly (Kohanpour et al., 2017), with its 95% CI crossing the null effect line. However, this result was based on a single study and should be interpreted with caution. Other exercise modalities showed positive but non-significant effects (p > 0.05), with no significant differences between groups (p > 0.05). Subgroup analysis by health status indicated that exercise interventions yielded some beneficial effects on BDNF levels in healthy individuals (Erickson et al., 2011; Kovacevic et al., 2020; Li et al., 2021; Maass et al., 2016; Matura et al., 2017) and those with MCI (SMD > 0) (Fungwe et al., 2019; Kohanpour et al., 2017; Lin et al., 2025), whereas no significant effects were observed in individuals with AD (Enette et al., 2020; Stein et al., 2023) or stroke (Rackoll et al., 2024). Notably, significant differences were found across health status subgroups (p < 0.01), suggesting that health status is an important moderator of BDNF intervention effects.
Subgroup analysis by sex revealed no significant differences between males and females (p > 0.05). In terms of exercise intensity, the high-intensity exercise group showed a borderline positive effect (p = 0.05), while the low and moderate-intensity groups did not reach statistical significance (p > 0.05), and the differences between these groups were not significant (p > 0.05). Additionally, the non-intermittent exercise group exhibited a significant intervention effect (p < 0.05), while the intermittent exercise group showed no significant difference (p > 0.05), with no significant differences between the groups (p > 0.05). Finally, no statistical significance was found regardless of whether the measurement site was plasma or serum, and the differences between groups were not significant (p > 0.05).
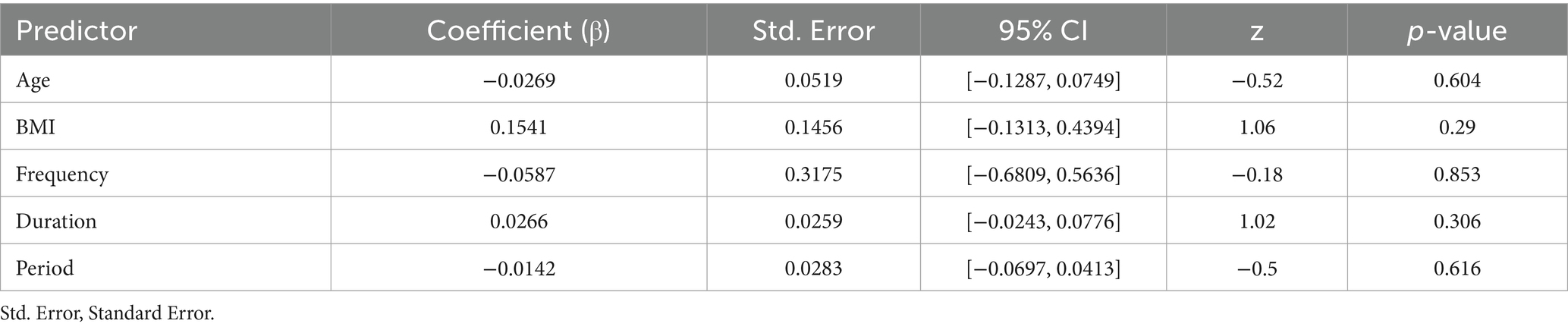
The results of the meta-regression indicate that variables including age, BMI, duration (min), frequency, and period (weeks) did not have a significant effect on the intervention outcomes (p > 0.05), indicating that these variables are unlikely to be primary moderators of the intervention effects (Figure 4) (Table 4).
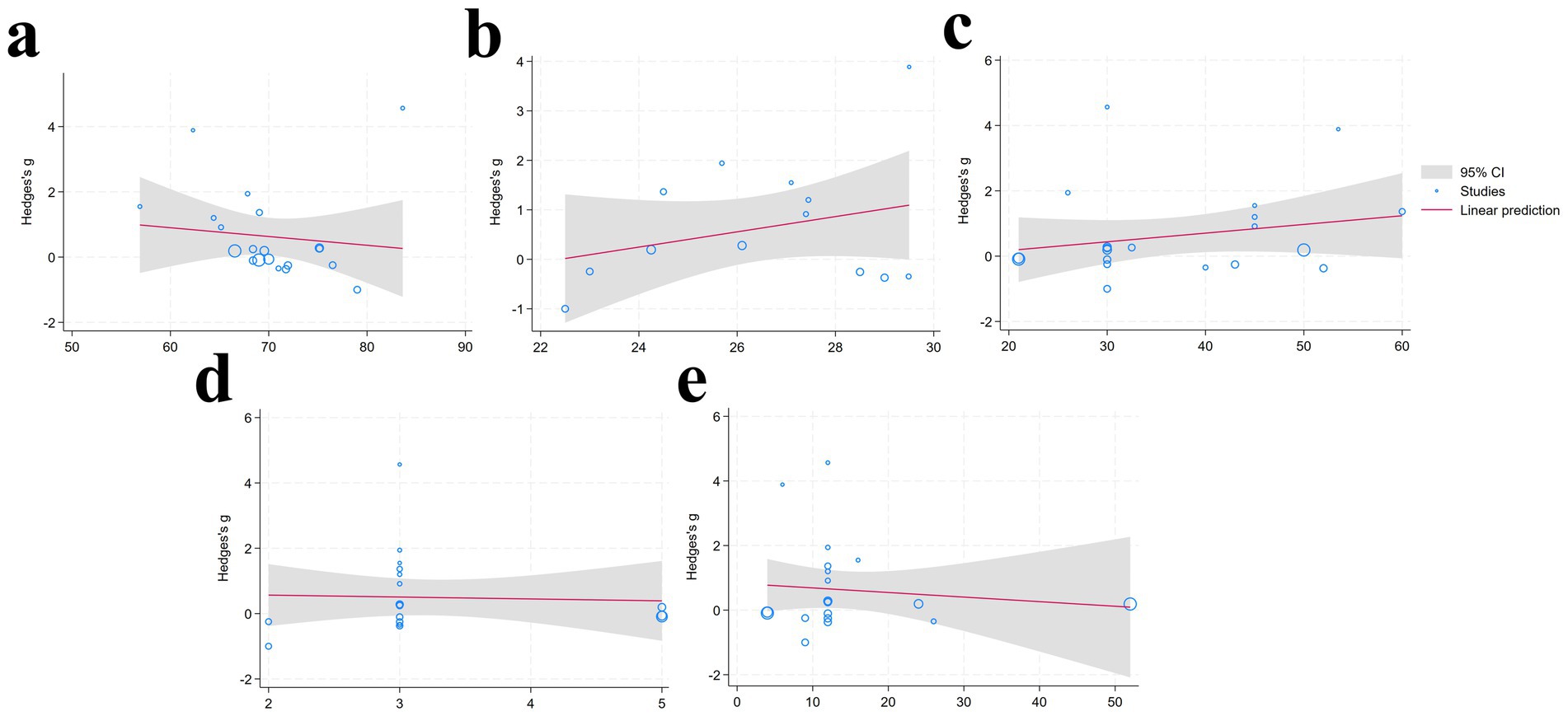
Figure 4. Meta-regression analysis results [(a): Age; (b) BMI; (c) duration; (d) frequency; (e) period] (95% CI, Gray shaded region around the regression line; Studies, Blue circular; Linear prediction, Red solid line depicting the fitted linear regression model).
3.3.3 Publication bias and sensitivity analysis
Visual inspection of the funnel plot suggested the presence of potential publication bias, which was supported by the Egger test (p < 0.05) (Supplementary material). To address this, we used the trim-and-fill method to impute missing studies. After adding three missing studies, the Hedges’ g and its 95% CI increased from 0.624 (0.063 to 1.184) to 0.917 (0.373 to 1.460), and the results remained significant, indicating that the potentially missing studies did not significantly affect the overall effect (Figure 5). Subsequently, a leave-one-out meta-analysis was performed by removing each study (Supplementary material). The results showed that regardless of which study was excluded, the pooled effect size remained within the 95% CI, confirming the robustness and reliability of the findings.
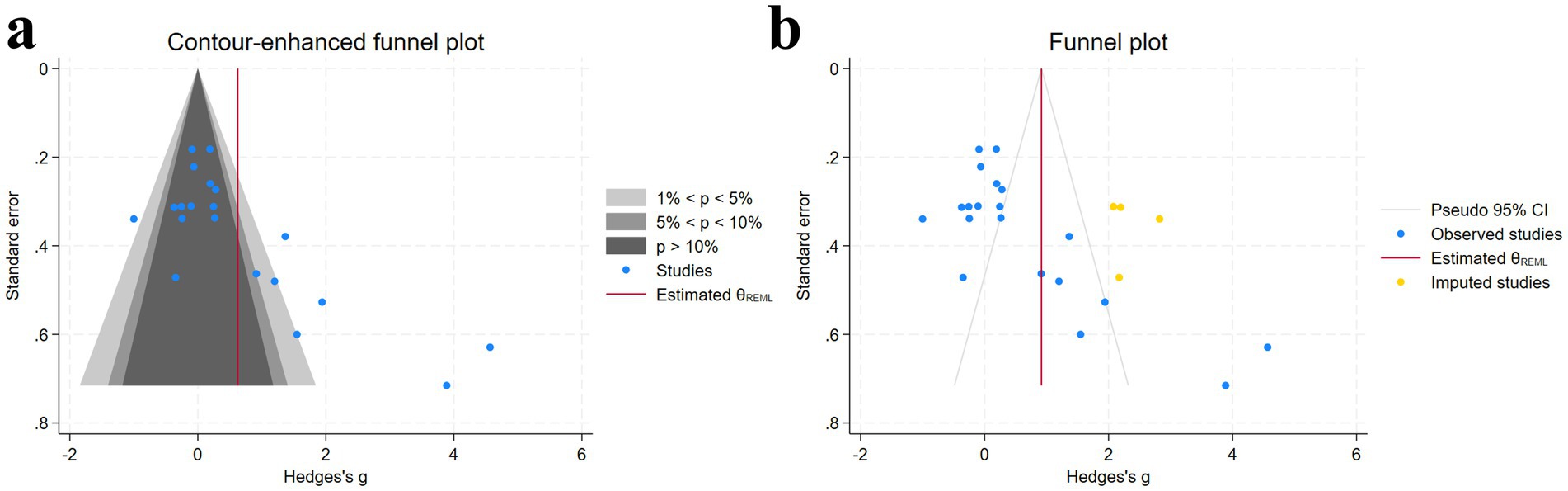
Figure 5. Funnel plot and trim-and-fill method [(a) funnel plot; (b) trim-and-fill method] [Gray regions, Statistical significance contours; Blue dots, Individual studies included in the meta-analysis; Red line, Estimated overall effect size (θREML); Yellow dots, Imputed studies (data imputed to address missing studies or publication bias)].
3.4 Network meta-analysis
3.4.1 Network evidence diagram
The network evidence diagram is presented in Figure 6. Each node in the diagram represents an intervention, with the node size proportional to the sample size involved in the corresponding intervention. The lines connecting nodes indicate the existence of direct comparisons between two interventions, and the thickness of each line corresponds to the number of such direct comparisons. The network evidence diagram reveals direct comparisons between CVS and CMS, as well as between HVL and WML, whereas other intervention pairs lack direct comparisons and therefore require indirect comparison through network meta-analysis.
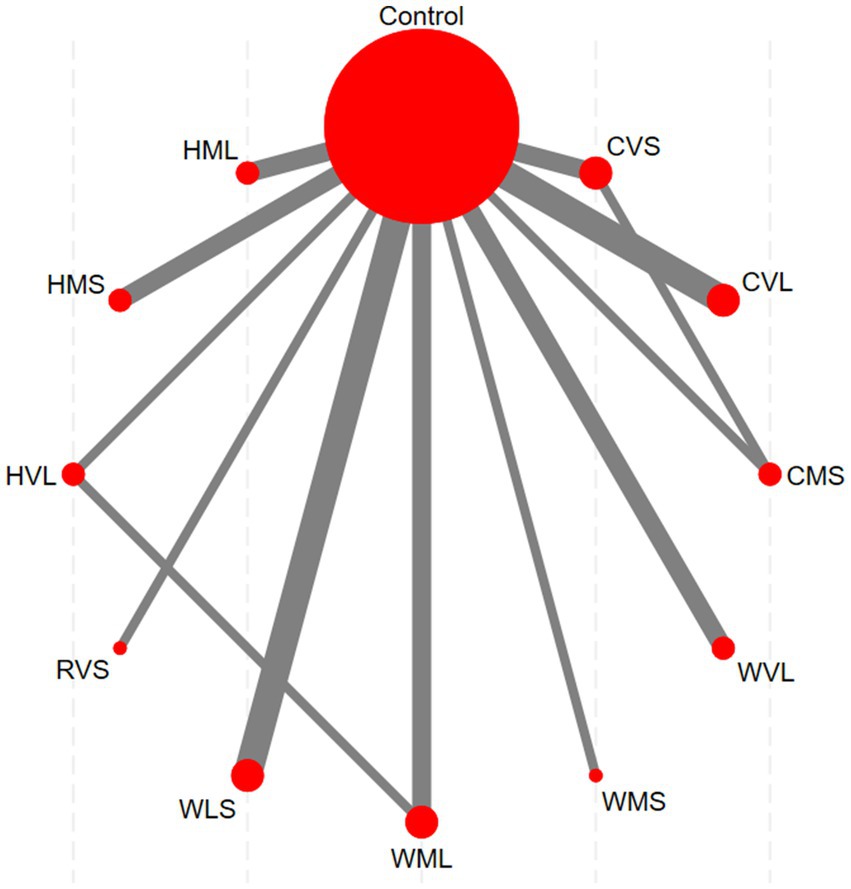
Figure 6. Network evidence diagram (Red nodes: represent individual interventions; Gray lines: indicate direct comparisons between two interventions).
3.4.2 Pairwise comparisons between protocols
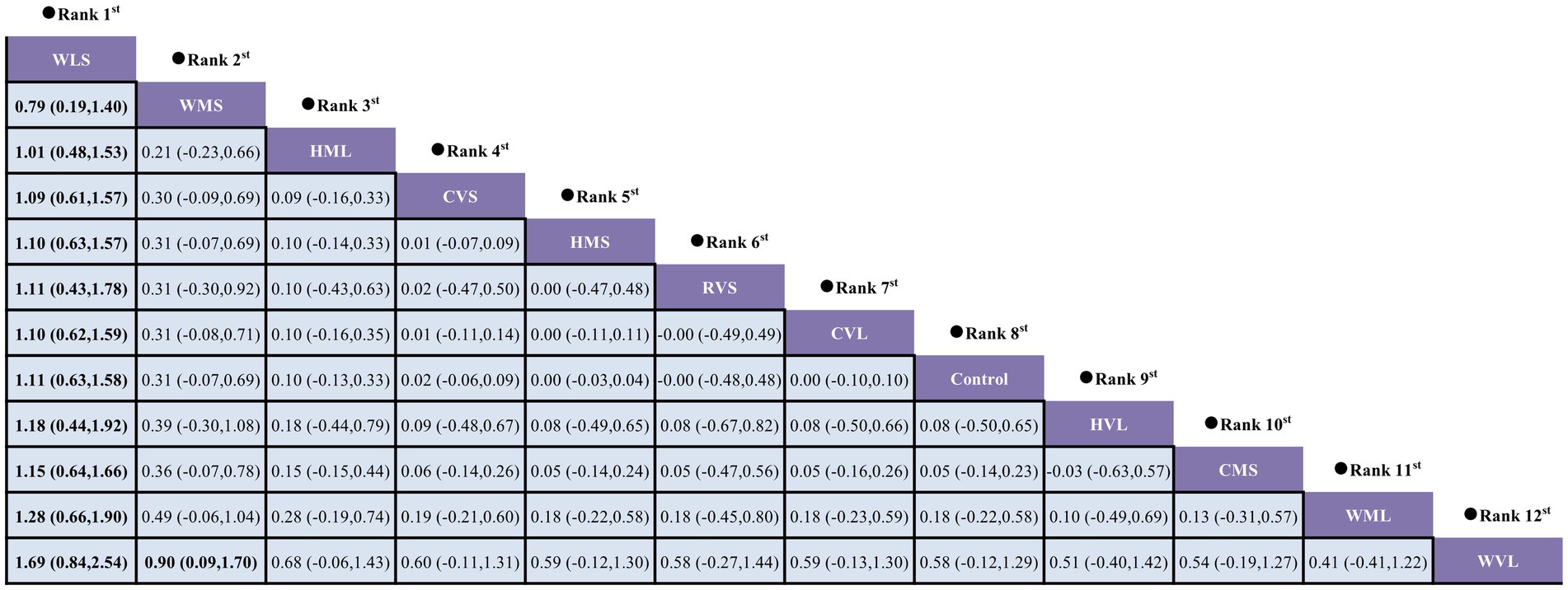
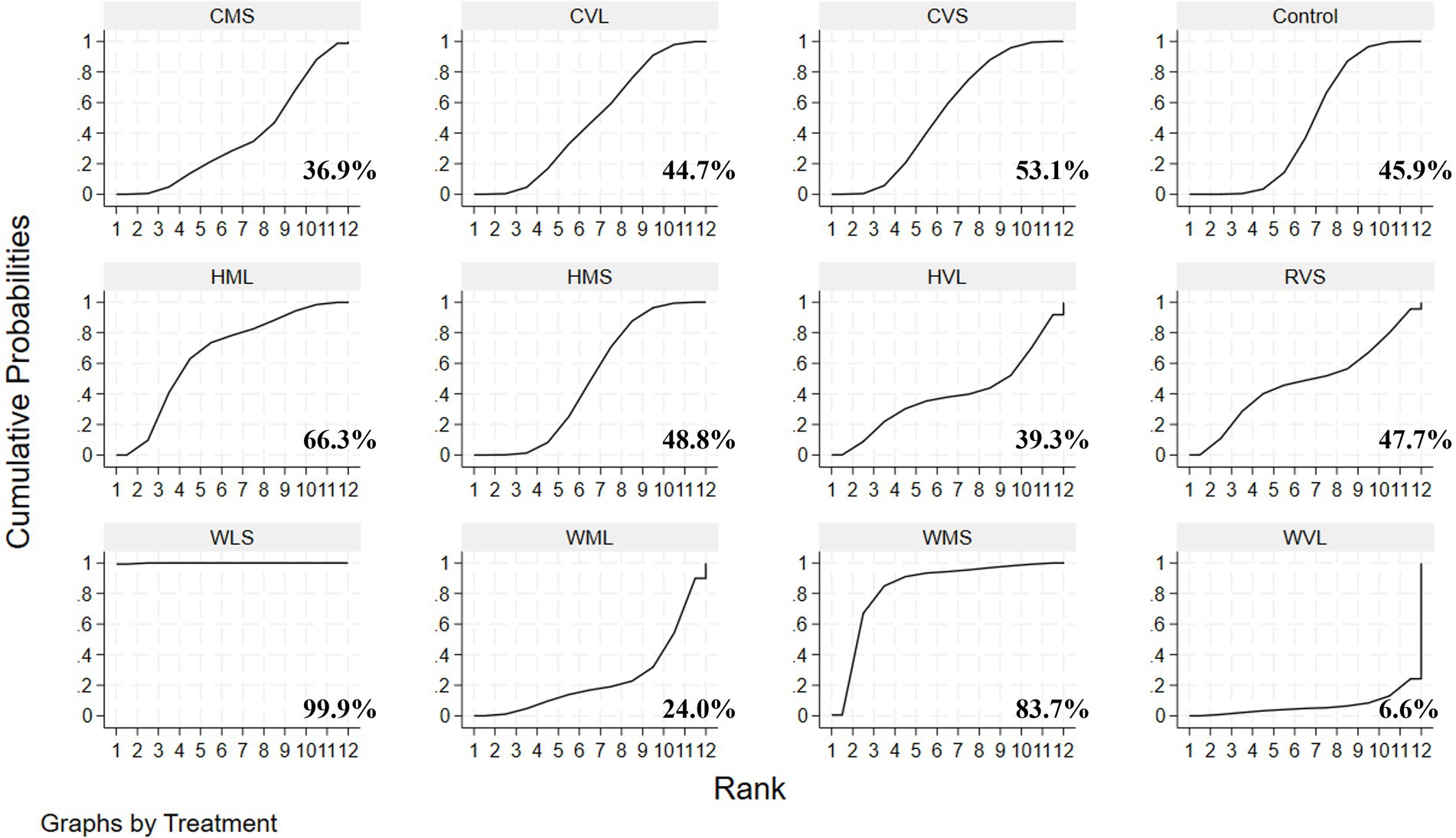
A loop inconsistency test was conducted to examine the consistency between direct and indirect evidence. The results showed good consistency among comparisons (p > 0.05); therefore, a consistency model was used for the network meta-analysis. Results indicated that protocols involving WLS was superior to other AE protocols; additionally, protocols involving WMS also outperformed WVL (SMD = 0.90, 95% CI: 0.09 to 1.70). These comparisons reached statistical significance (p < 0.05) (Figure 7). Based on SUCRA rankings, protocols involving WLS (99.9%) and WMS (83.7%) were the top-ranked interventions (Figure 8). Finally, visual inspection of the funnel plot showed no obvious publication bias (Figure 9).
4 Discussion
This study systematically evaluated the effects of walking, running, and cycling on circulating BDNF levels in elderly. The meta-analysis results indicated that three AE modalities significantly improved circulating BDNF levels in elderly. Although our review did not directly assess cognition, neuroplasticity, or neurodegeneration, existing evidence suggests that AE may have potential benefits for these functions through BDNF and could help slow age-related decline in brain function (Hayes et al., 2014). It is noteworthy that the degree of improvement in BDNF levels was significantly influenced by the participants’ health status, suggesting that health status may be an important modulating factor in the impact of AE on circulating BDNF. Specifically, the interventions had positive effects in healthy individuals and those with MCI, whereas the effects were less evident in individuals with AD or stroke. Furthermore, training variables such as the modality of intervention, exercise intensity, and interval may also serve as potential influencing factors. To compare the relative advantages of different AE protocols in improving BDNF levels, a network meta-analysis was further conducted. Pairwise comparisons and SUCRA results revealed that exercise protocols involving low- to moderate-intensity walking (e.g., WLS vs. WMS) may exert the most favorable effect on circulating BDNF. These findings have important practical implications, suggesting that when developing individualized exercise prescriptions to improve BDNF in the elderly, priority should be given to sustainable walking interventions of appropriate intensity.
A large body of research has demonstrated that AE is an effective non-pharmacological intervention for improving cognitive impairment in older adults, and it is widely used in the prevention and treatment of neurological diseases such as MCI, AD, and PD (Baker et al., 2010; Segura et al., 2020; Yang et al., 2025). In fact, these benefits are largely attributed to the ability of AE to promote neuroplasticity. Neuroplasticity refers to the brain’s capacity to change and adapt based on experiences and environmental factors. Specifically, it can modify its structure and form new neural networks, with BDNF expression being a key regulatory mechanism (Ben Ezzdine et al., 2025; Khalil, 2025). Research has shown that AE can enhance the release of BDNF, thereby improving dendritic spine integrity and activating various signaling pathways associated with neuroplasticity. Specifically, BDNF promotes dendritic spine formation by activating TrkB receptors, which trigger a series of signaling pathways such as PI3K/Akt, RAS/ERK, and PLCγ1/PKC (Alonso et al., 2004).
The present study further demonstrates that the three AE modalities elicit varying degrees of improvement in circulating BDNF levels across different health statuses. Specifically, positive intervention effects (SMD > 0) are observed in healthy individuals and those with MCI, hypertension, metabolic syndrome (MetS), PD, and T2D, whereas in patients with AD and stroke, AE does not have a positive effect on BDNF levels (SMD < 0). Previous studies have demonstrated that AD patients exhibit extracellular deposition of amyloid-β plaques and intracellular hyperphosphorylation of tau protein. These pathological features disrupt axonal transport of BDNF, reduce its synaptic availability, and impair TrkB receptor-mediated signaling pathways, potentially negatively impacting circulating BDNF levels (Lima Giacobbo et al., 2019). Additionally, platelets serve as a major reservoir of BDNF (Boukhatem et al., 2024), and skeletal muscle can secrete BDNF during physical activity (Schnyder and Handschin, 2015). In AD patients, reduced physical activity and muscle atrophy may lead to decreased release of BDNF from both platelets and skeletal muscle into the circulation, further contributing to lower peripheral BDNF levels (Knaepen et al., 2010). Similar to AD, patients with MCI also exhibit significantly lower circulating BDNF levels compared to healthy individuals, suggesting that early stages of neurodegenerative disease may negatively affect BDNF expression and function (Miranda et al., 2019). In PD, reduced mRNA and protein expression of BDNF in the substantia nigra pars compacta has also been observed (Howells et al., 2000), further supporting the notion that neurodegenerative disorders adversely impact neural structure and function by impairing BDNF signaling.
Beyond these conditions, evidence has shown that individuals with coronary heart disease, T2D, MetS, or sedentary lifestyles also exhibit a generally reduced peripheral BDNF levels (Huang et al., 2014), which may be closely linked to their lower cardiorespiratory fitness and adverse metabolic status. These conditions are frequently characterized by chronic low-grade inflammation, insulin resistance, endothelial dysfunction, and insufficient physical activity (Booth et al., 2012). Collectively, these pathophysiological alterations are likely to impair the synthesis and release of BDNF, thereby contributing to diminished peripheral BDNF concentrations (Arentoft et al., 2009; Júdice et al., 2021; Motamedi et al., 2017; Shobeiri et al., 2023). Lower circulating BDNF concentrations are not only associated with an elevated risk of cardiovascular and cerebrovascular diseases but may also be linked to impaired cognitive recovery (Pikula et al., 2013). Notably, our subgroup analysis revealed that the effects of exercise interventions on BDNF recovery were limited in individuals with AD and stroke, possibly due to irreversible pathological damage in the brain (Ma et al., 2025; Mohd Khairi et al., 2024). It should be noted that the number of studies included in the subgroup analyses for hypertension, AD, MetS, PD, and T2D was relatively small. Therefore, these findings should be interpreted with caution.
Subsequently, a network meta-analysis was conducted to perform pairwise comparisons and SUCRA ranking, revealing that protocols involving walking at low to moderate intensity, with a duration not exceeding 30 min per session (e.g., WLS and WMS), was the most effective for enhancing circulating BDNF levels in older adults. Notably, an intriguing observation emerged during the analysis: the overall ranking results from the network meta-analysis were inconsistent with those obtained from traditional subgroup analyses. Specifically, in the subgroup analysis, higher exercise intensity and continuous (non-intermittent) exercise protocols appeared to positively influence circulating BDNF levels in older adults. However, when exercise modality, intensity, and duration were considered collectively in the network meta-analysis, walking at low to moderate intensity with short-duration interventions emerged as the optimal combination. Moreover, as only two additional studies were included in the network meta-analysis, this may have contributed to the discrepancy between its results and those of the subgroup analysis. It is noteworthy that, as the included studies did not report total exercise volume, the results for sessions lasting less than 30 min should be interpreted with caution.
Another important factor to consider is exercise intensity. According to the classification of Gholami et al. (2025) exercise intensity, Erickson et al. (2011) reported that although 1 year of high-intensity aerobic walking produced some improvements in hippocampal volume, changes in serum BDNF levels did not differ significantly. Furthermore, Kovacevic et al. (2020) investigated the effects of exercise intensity on BDNF and found that after 12 weeks of AE intervention, there were no significant differences in BDNF levels between the HIIT, MICT, and Control groups. In contrast, Ploydang et al. (2023) found that 12 weeks of moderate-intensity aquatic Nordic walking significantly increased serum BDNF. These findings are consistent with our results, suggesting that protocols involving moderate-intensity AE (e.g., aquatic Nordic walking) may be more favorable for enhancing BDNF. The underlying mechanisms may include the following. First, high-intensity AE substantially elevates stress hormones, such as cortisol, which may suppress BDNF expression and offset some positive effects of exercise (Clos et al., 2021; Jeon and Ha, 2015). Second, lactate can stimulate BDNF production via the PGC-1α/FNDC5 pathway; however, excessive lactate accumulation may lead to blood acidification and metabolic stress, thereby limiting circulating BDNF levels (Brooks et al., 2023; Müller et al., 2020).
Exercise duration is another critical factor. Current evidence indicates that even 30-min AE sessions can effectively increase circulating BDNF levels in older adults. For example, Lin et al. (2025) reported that 12 weeks of walking training (55–65% HRmax) significantly elevated serum BDNF. Therefore, we speculate that exercise sessions limited to 30 min may improve BDNF levels through moderate neural stimulation, limited stress hormone release, and optimized lactate signaling, thereby promoting neuroplasticity. However, this interpretation should be made with caution given the high heterogeneity and variability across study samples. Regarding whether AE should be performed with intervals, we did not consider this a primary dimension for categorizing interventions for two main reasons. First, although continuous exercise showed a slight advantage over intermittent exercise in subgroup analyses of BDNF enhancement, inter-group differences were not statistically significant. Second, given that older adults are more susceptible to exercise-induced fatigue and intolerance due to physiological decline, the use of intervals should be flexibly adjusted based on individual responses (Chmelo et al., 2015). These findings indicate that, for older adults, enhancing BDNF levels requires a multifactorial approach, rather than focusing on any single exercise parameter. Future research should further explore the combined effects of exercise intensity, duration, and intervention period to optimize exercise prescriptions and maximize improvements in BDNF levels and cognitive function in older adults.
This study has several limitations that warrant attention. First, although strictly RCTs were included, the inherent characteristics of exercise interventions make it difficult to conduct truly double-blind RCTs in practice. Nevertheless, awareness of group allocation is unlikely to directly affect objective biological outcomes such as blood BDNF levels. Second, the lack of significant subgroup differences for variables such as age, BMI, duration, frequency, or intervention period does not necessarily indicate that these factors have no effect. Rather, this may be due to the narrow ranges represented in the included studies or methodological inconsistencies. Future studies are needed to further clarify the influence of these factors. Moreover, as only two additional studies were included in the network meta-analysis, this may have contributed to the discrepancy between its results and those of the subgroup analysis, representing an additional limitation of the study. Additionally, considerable heterogeneity was observed across studies, which may be related to baseline activity levels, BDNF assay methodology, timing of blood sampling, and adherence rates. For certain outcomes, only a limited number of trials were included in the subgroups, potentially restricting the robustness and generalizability of our findings. Moreover, this study focused on walking, running, and cycling to more clearly assess their effects on BDNF. To reduce heterogeneity and improve comparability, we excluded activities like dancing and Tai Chi, which are more variable and harder to standardize. While this strengthened the internal validity of our analysis, it may limit the generalizability of the findings. Finally, most studies currently use plasma or serum BDNF levels as assessment indicators. While previous studies have indicated that peripheral BDNF can partially reflect brain BDNF levels, this indirect measurement method still has limitations and may be influenced by external factors such as sample processing methods. Future research should explore more sensitive and specific measurement techniques for directly assessing brain BDNF levels to enhance the reliability and interpretability of this biomarker.
5 Conclusion
Walking, running, and cycling are effective for improving circulating BDNF levels in older adults; however, the magnitude of improvement depends on participants’ health status and specific exercise prescription. Interventions involving walking at low to moderate intensity demonstrated favorable efficacy. This effect may be more favorable in healthy individuals and those with mild cognitive impairment. Future studies should further investigate the influence of total exercise volume on outcomes and adopt more rigorous and standardized protocols to facilitate the development of standardized exercise strategies, thereby improving comparability and reducing heterogeneity in future analyses.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
YC: Investigation, Writing – original draft, Formal analysis, Visualization, Conceptualization, Data curation, Project administration, Methodology. YL: Formal analysis, Data curation, Investigation, Conceptualization, Methodology, Writing – original draft. JM: Writing – review & editing, Investigation. ZL: Investigation, Writing – review & editing. EH: Resources, Writing – review & editing, Funding acquisition, Conceptualization, Supervision. SB: Conceptualization, Supervision, Funding acquisition, Writing – review & editing, Resources.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by Emerging Interdisciplinary Platform for Medicine and Engineering in Sports (EIPMES).
Acknowledgments
The authors thank all participants for their honest effort and commitment to the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2025.1673786/full#supplementary-material
References
Alonso, M., Medina, J. H., and Pozzo-Miller, L. (2004). ERK1/2 activation is necessary for BDNF to increase dendritic spine density in hippocampal CA1 pyramidal neurons. Learn. Mem. 11, 172–178. doi: 10.1101/lm.67804
Antonijevic, M., Dallemagne, P., and Rochais, C. (2024). Inducing neuronal regeneration and differentiation via the BDNF/TrkB signaling pathway: a key target against neurodegenerative diseases? Neural Regen. Res. 19, 495–496. doi: 10.4103/1673-5374.380896
Antoniou, S. A., Koelemay, M., Antoniou, G. A., and Mavridis, D. (2019). A practical guide for application of network Meta-analysis in evidence synthesis. Eur. J. Vasc. Endovasc. Surg. 58, 141–144. doi: 10.1016/j.ejvs.2018.10.023
Arentoft, A., Sweat, V., Starr, V., Oliver, S., Hassenstab, J., Bruehl, H., et al. (2009). Plasma BDNF is reduced among middle-aged and elderly women with impaired insulin function: evidence of a compensatory mechanism. Brain Cogn. 71, 147–152. doi: 10.1016/j.bandc.2009.04.009
Baker, L. D., Frank, L. L., Foster-Schubert, K., Green, P. S., Wilkinson, C. W., McTiernan, A., et al. (2010). Effects of aerobic exercise on mild cognitive impairment: a controlled trial. Arch. Neurol. 67, 71–79. doi: 10.1001/archneurol.2009.307
Barde, Y. A. (2025). The physiopathology of brain-derived neurotrophic factor. Physiol. Rev. 105, 2073–2140. doi: 10.1152/physrev.00038.2024
Ben Ezzdine, L., Dhahbi, W., Dergaa, I., Ceylan, H., Guelmami, N., Ben Saad, H., et al. (2025). Physical activity and neuroplasticity in neurodegenerative disorders: a comprehensive review of exercise interventions, cognitive training, and AI applications. Front. Neurosci. 19:1502417. doi: 10.3389/fnins.2025.1502417
Booth, F. W., Roberts, C. K., and Laye, M. J. (2012). Lack of exercise is a major cause of chronic diseases. Compr Physiol 2, 1143–1211. doi: 10.1002/cphy.c110025
Boukhatem, I., Fleury, S., Jourdi, G., and Lordkipanidzé, M. (2024). The intriguing role of platelets as custodians of brain-derived neurotrophic factor. Res Pract Thromb Haemost 8:102398. doi: 10.1016/j.rpth.2024.102398
Brooks, G. A., Osmond, A. D., Arevalo, J. A., Duong, J. J., Curl, C. C., Moreno-Santillan, D. D., et al. (2023). Lactate as a myokine and exerkine: drivers and signals of physiology and metabolism. J. Appl. Physiol. (1985) 134, 529–548. doi: 10.1152/japplphysiol.00497.2022
Cardoso, S. V., Fernandes, S. R., and Tomás, M. T. (2024). Therapeutic importance of exercise in neuroplasticity in adults with neurological pathology: systematic review. Int. J. Exerc. Sci. 17, 1105–1119. doi: 10.70252/vzwf7949
Chaimani, A., Higgins, J. P., Mavridis, D., Spyridonos, P., and Salanti, G. (2013). Graphical tools for network meta-analysis in STATA. PLoS One 8:e76654. doi: 10.1371/journal.pone.0076654
Cheng, Y., Yi, Y., Bo, S., Mao, J., and Ma, J. (2025). Optimization of high-intensity resistance exercise protocols for improving bone mineral density in the elderly without chronic diseases: a systematic review and network meta-analysis. Front. Physiol. 16:200. doi: 10.3389/fphys.2025.1589200
Chmelo, E. A., Crotts, C. I., Newman, J. C., Brinkley, T. E., Lyles, M. F., Leng, X., et al. (2015). Heterogeneity of physical function responses to exercise training in older adults. J. Am. Geriatr. Soc. 63, 462–469. doi: 10.1111/jgs.13322
Chou, C. C., Chien, L. Y., Lin, M. F., Wang, C. J., and Liu, P. Y. (2022). Effects of aerobic walking on memory, subjective cognitive complaints, and brain-derived neurotrophic factor among older hypertensive women. Biol. Res. Nurs. 24, 484–492. doi: 10.1177/10998004221098974
Clos, P., Lepers, R., and Garnier, Y. M. (2021). Locomotor activities as a way of inducing neuroplasticity: insights from conventional approaches and perspectives on eccentric exercises. Eur. J. Appl. Physiol. 121, 697–706. doi: 10.1007/s00421-020-04575-3
Dell'Oste, V., Palego, L., Betti, L., Fantasia, S., Gravina, D., Bordacchini, A., et al. (2024). Plasma and platelet brain-derived neurotrophic factor (BDNF) levels in bipolar disorder patients with post-traumatic stress disorder (PTSD) or in a major depressive episode compared to healthy controls. Int. J. Mol. Sci. 25:529. doi: 10.3390/ijms25063529
Enette, L., Vogel, T., Merle, S., Valard-Guiguet, A. G., Ozier-Lafontaine, N., Neviere, R., et al. (2020). Effect of 9 weeks continuous vs. interval aerobic training on plasma BDNF levels, aerobic fitness, cognitive capacity and quality of life among seniors with mild to moderate Alzheimer's disease: a randomized controlled trial. Eur. Rev. Aging Phys. Act. 17:2. doi: 10.1186/s11556-019-0234-1
Erickson, K. I., Donofry, S. D., Sewell, K. R., Brown, B. M., and Stillman, C. M. (2022). Cognitive aging and the promise of physical activity. Annu. Rev. Clin. Psychol. 18, 417–442. doi: 10.1146/annurev-clinpsy-072720-014213
Erickson, K. I., Voss, M. W., Prakash, R. S., Basak, C., Szabo, A., Chaddock, L., et al. (2011). Exercise training increases size of hippocampus and improves memory. Proc. Natl. Acad. Sci. USA 108, 3017–3022. doi: 10.1073/pnas.1015950108
Firth, J., Stubbs, B., Vancampfort, D., Schuch, F., Lagopoulos, J., Rosenbaum, S., et al. (2018). Effect of aerobic exercise on hippocampal volume in humans: A systematic review and meta-analysis. NeuroImage 166, 230–238. doi: 10.1016/j.neuroimage.2017.11.007
Fungwe, T. V., Ngwa, J. S., Ntekim, O. E., Allard, J. S., Nadarajah, S., Wolday, S., et al. (2019). Exercise training induced changes in nuclear magnetic resonance-measured lipid particles in mild cognitively impaired elderly African American volunteers: A pilot study. Clin. Interv. Aging 14, 2115–2123. doi: 10.2147/cia.S195878
Gates, N., Fiatarone Singh, M. A., Sachdev, P. S., and Valenzuela, M. (2013). The effect of exercise training on cognitive function in older adults with mild cognitive impairment: a meta-analysis of randomized controlled trials. Am. J. Geriatr. Psychiatry 21, 1086–1097. doi: 10.1016/j.jagp.2013.02.018
Gholami, F., Mesrabadi, J., Iranpour, M., and Donyaei, A. (2025). Exercise training alters resting brain-derived neurotrophic factor concentration in older adults: A systematic review with meta-analysis of randomized-controlled trials. Exp. Gerontol. 199:112658. doi: 10.1016/j.exger.2024.112658
Hayes, S. M., Alosco, M. L., and Forman, D. E. (2014). The effects of aerobic exercise on cognitive and neural decline in aging and cardiovascular disease. Curr. Geriatr. Rep. 3, 282–290. doi: 10.1007/s13670-014-0101-x
Higgins, J. P., Jackson, D., Barrett, J. K., Lu, G., Ades, A. E., and White, I. R. (2012). Consistency and inconsistency in network meta-analysis: concepts and models for multi-arm studies. Res. Synth. Methods 3, 98–110. doi: 10.1002/jrsm.1044
Higgins, J. P., Thompson, S. G., Deeks, J. J., and Altman, D. G. (2003). Measuring inconsistency in meta-analyses. BMJ 327, 557–560. doi: 10.1136/bmj.327.7414.557
Howells, D. W., Porritt, M. J., Wong, J. Y., Batchelor, P. E., Kalnins, R., Hughes, A. J., et al. (2000). Reduced BDNF mRNA expression in the Parkinson's disease substantia nigra. Exp. Neurol. 166, 127–135. doi: 10.1006/exnr.2000.7483
Hsu, C. C., Fu, T. C., Huang, S. C., Chen, C. P., and Wang, J. S. (2021). Increased serum brain-derived neurotrophic factor with high-intensity interval training in stroke patients: A randomized controlled trial. Ann. Phys. Rehabil. Med. 64:101385. doi: 10.1016/j.rehab.2020.03.010
Huang, T., Larsen, K. T., Ried-Larsen, M., Møller, N. C., and Andersen, L. B. (2014). The effects of physical activity and exercise on brain-derived neurotrophic factor in healthy humans: A review. Scand. J. Med. Sci. Sports 24, 1–10. doi: 10.1111/sms.12069
Hvid, L. G., Nielsen, M. K. F., Simonsen, C., Andersen, M., and Caserotti, P. (2017). Brain-derived neurotrophic factor (BDNF) serum basal levels is not affected by power training in mobility-limited older adults—a randomized controlled trial. Exp. Gerontol. 93, 29–35. doi: 10.1016/j.exger.2017.03.019
Jemni, M., Zaman, R., Carrick, F. R., Clarke, N. D., Marina, M., Bottoms, L., et al. (2023). Exercise improves depression through positive modulation of brain-derived neurotrophic factor (BDNF). A review based on 100 manuscripts over 20 years. Front. Physiol. 14:1102526. doi: 10.3389/fphys.2023.1102526
Jeon, Y. K., and Ha, C. H. (2015). Expression of brain-derived neurotrophic factor, IGF-1 and cortisol elicited by regular aerobic exercise in adolescents. J. Phys. Ther. Sci. 27, 737–741. doi: 10.1589/jpts.27.737
Júdice, P. B., Magalhães, J. P., Hetherington-Rauth, M., Correia, I. R., and Sardinha, L. B. (2021). Sedentary patterns are associated with BDNF in patients with type 2 diabetes mellitus. Eur. J. Appl. Physiol. 121, 871–879. doi: 10.1007/s00421-020-04568-2
Khalil, M. H. (2025). The impact of walking on BDNF as a biomarker of neuroplasticity: A systematic review. Brain Sci. 15:254. doi: 10.3390/brainsci15030254
Kim, K., Byun, M. S., Yi, D., Jung, J. H., Sohn, B. K., Jung, G., et al. (2025). Serum BDNF and progression to MCI in cognitively normal older adults: a prospective cohort study. J. Prev Alzheimers Dis. 12:100210. doi: 10.1016/j.tjpad.2025.100210
Knaepen, K., Goekint, M., Heyman, E. M., and Meeusen, R. (2010). Neuroplasticity—exercise-induced response of peripheral brain-derived neurotrophic factor: a systematic review of experimental studies in human subjects. Sports Med. 40, 765–801. doi: 10.2165/11534530-000000000-00000
Kohanpour, M.-A., Peeri, M., and Azarbayjani, M.-A. (2017). The effects of aerobic exercise with lavender essence use on cognitive state and serum brain-derived neurotrophic factor levels in elderly with mild cognitive impairment. J. Herbmed. Pharmacol. 6, 80–84.
Kons, R. L., Orssatto, L. B. R., Ache-Dias, J., De Pauw, K., Meeusen, R., Trajano, G. S., et al. (2023). Effects of plyometric training on physical performance: an umbrella review. Sports Med Open 9:4. doi: 10.1186/s40798-022-00550-8
Kovacevic, A., Fenesi, B., Paolucci, E., and Heisz, J. J. (2020). The effects of aerobic exercise intensity on memory in older adults. Appl. Physiol. Nutr. Metab. 45, 591–600. doi: 10.1139/apnm-2019-0495
Li, X., Han, T., Zou, X., Zhang, H., Feng, W., Wang, H., et al. (2021). Long-term high-intensity interval training increases serum neurotrophic factors in elderly overweight and obese Chinese adults. Eur. J. Appl. Physiol. 121, 2773–2785. doi: 10.1007/s00421-021-04746-w
Li, Y., Li, F., Qin, D., Chen, H., Wang, J., Wang, J., et al. (2022). The role of brain derived neurotrophic factor in central nervous system. Front. Aging Neurosci. 14:986443. doi: 10.3389/fnagi.2022.986443
Lima Giacobbo, B., Doorduin, J., Klein, H. C., Dierckx, R., Bromberg, E., and de Vries, E. F. J. (2019). Brain-derived neurotrophic factor in brain disorders: focus on neuroinflammation. Mol. Neurobiol. 56, 3295–3312. doi: 10.1007/s12035-018-1283-6
Lin, L., He, Y. X., Wen, Q., Liu, J. Y., Dai, Y., Fei, Y. Z., et al. (2025). Evaluation of the efficacy of tai chi on the cognitive function of patients with mild cognitive dysfunction and research on its mechanism. Front. Aging Neurosci. 17:1435996. doi: 10.3389/fnagi.2025.1435996
Ma, L., Zhang, M., Chen, T., Wang, L., and Deng, Q. (2025). Electroacupuncture inhibits neuronal pyroptosis in ischemic brain injury through modulating SIRT5-mediated NEK7 succinylation. Brain Res. Bull. 220:111173. doi: 10.1016/j.brainresbull.2024.111173
Maass, A., Düzel, S., Brigadski, T., Goerke, M., Becke, A., Sobieray, U., et al. (2016). Relationships of peripheral IGF-1, VEGF and BDNF levels to exercise-related changes in memory, hippocampal perfusion and volumes in older adults. NeuroImage 131, 142–154. doi: 10.1016/j.neuroimage.2015.10.084
Mattson, M. P., and Magnus, T. (2006). Ageing and neuronal vulnerability. Nat. Rev. Neurosci. 7, 278–294. doi: 10.1038/nrn1886
Matura, S., Fleckenstein, J., Deichmann, R., Engeroff, T., Füzéki, E., Hattingen, E., et al. (2017). Effects of aerobic exercise on brain metabolism and grey matter volume in older adults: results of the randomised controlled SMART trial. Transl. Psychiatry 7:e1172. doi: 10.1038/tp.2017.135
Miranda, M., Morici, J. F., Zanoni, M. B., and Bekinschtein, P. (2019). Brain-derived neurotrophic factor: A key molecule for memory in the healthy and the pathological brain. Front. Cell. Neurosci. 13:363. doi: 10.3389/fncel.2019.00363
Mohd Khairi, N. A. A., Hanafi, M. H., Kassim, N. K., and Ibrahim, A. H. (2024). The levels of biomarkers interleukin 1 (IL-1) and brain-derived neurotrophic factor (BDNF) in non-invasive conventional rehabilitation and robotic rehabilitation among brain injury patients: A narrative review. Cureus 16:e68332. doi: 10.7759/cureus.68332
Morrison, J. H., and Baxter, M. G. (2012). The ageing cortical synapse: hallmarks and implications for cognitive decline. Nat. Rev. Neurosci. 13, 240–250. doi: 10.1038/nrn3200
Motamedi, S., Karimi, I., and Jafari, F. (2017). The interrelationship of metabolic syndrome and neurodegenerative diseases with focus on brain-derived neurotrophic factor (BDNF): kill two birds with one stone. Metab. Brain Dis. 32, 651–665. doi: 10.1007/s11011-017-9997-0
Müller, P., Duderstadt, Y., Lessmann, V., and Müller, N. G. (2020). Lactate and BDNF: key mediators of exercise induced neuroplasticity? J. Clin. Med. 9:1136. doi: 10.3390/jcm9041136
Northey, J. M., Cherbuin, N., Pumpa, K. L., Smee, D. J., and Rattray, B. (2018). Exercise interventions for cognitive function in adults older than 50: a systematic review with meta-analysis. Br. J. Sports Med. 52, 154–160. doi: 10.1136/bjsports-2016-096587
Osali, A. (2020). Aerobic exercise and nano-curcumin supplementation improve inflammation in elderly females with metabolic syndrome. Diabetol. Metab. Syndr. 12:26. doi: 10.1186/s13098-020-00532-4
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71. doi: 10.1136/bmj.n71
Pikula, A., Beiser, A. S., Chen, T. C., Preis, S. R., Vorgias, D., DeCarli, C., et al. (2013). Serum brain-derived neurotrophic factor and vascular endothelial growth factor levels are associated with risk of stroke and vascular brain injury: Framingham study. Stroke 44, 2768–2775. doi: 10.1161/strokeaha.113.001447
Ploydang, T., Khovidhunkit, W., Tanaka, H., and Suksom, D. (2023). Nordic walking in water on cerebrovascular reactivity and cognitive function in elderly patients with type 2 diabetes. Med. Sci. Sports Exerc. 55, 1803–1811. doi: 10.1249/mss.0000000000003216
Prakash, R. S., Snook, E. M., Motl, R. W., and Kramer, A. F. (2010). Aerobic fitness is associated with gray matter volume and white matter integrity in multiple sclerosis. Brain Res. 1341, 41–51. doi: 10.1016/j.brainres.2009.06.063
Rackoll, T., Doppelbauer, L., Neumann, K., and Heinrich Nave, A., (2024). Dynamics of serum BDNF after aerobic exercise in subacute stroke patients: a sub-analysis of the randomized controlled Phys-stroke trial. medRxiv [Preprint]. medRxiv: 2024.09.24.24314322.
Raz, N., Ghisletta, P., Rodrigue, K. M., Kennedy, K. M., and Lindenberger, U. (2010). Trajectories of brain aging in middle-aged and older adults: regional and individual differences. NeuroImage 51, 501–511. doi: 10.1016/j.neuroimage.2010.03.020
Reed, J. L., Terada, T., Cotie, L. M., Tulloch, H. E., Leenen, F. H., Mistura, M., et al. (2022). The effects of high-intensity interval training, Nordic walking and moderate-to-vigorous intensity continuous training on functional capacity, depression and quality of life in patients with coronary artery disease enrolled in cardiac rehabilitation: A randomized controlled trial (CRX study). Prog. Cardiovasc. Dis. 70, 73–83. doi: 10.1016/j.pcad.2021.07.002
Rezaee, Z., Marandi, S. M., and Alaei, H. (2022). Molecular mechanisms of exercise in brain disorders: a focus on the function of brain-derived neurotrophic factor-a narrative review. Neurotox. Res. 40, 1115–1124. doi: 10.1007/s12640-022-00527-1
Ribeiro, D., Petrigna, L., Pereira, F. C., Muscella, A., Bianco, A., and Tavares, P. (2021). The impact of physical exercise on the circulating levels of BDNF and NT 4/5: A review. Int. J. Mol. Sci. 22:814. doi: 10.3390/ijms22168814
Schnyder, S., and Handschin, C. (2015). Skeletal muscle as an endocrine organ: PGC-1α, myokines and exercise. Bone 80, 115–125. doi: 10.1016/j.bone.2015.02.008
Segura, C., Eraso, M., Bonilla, J., Mendivil, C. O., Santiago, G., Useche, N., et al. (2020). Effect of a high-intensity tandem bicycle exercise program on clinical severity, functional magnetic resonance imaging, and plasma biomarkers in Parkinson's disease. Front. Neurol. 11:656. doi: 10.3389/fneur.2020.00656
Sharma, A., Sharma, N., Singh, R. K., and Chahal, A. (2024). Effects of aerobic training on brain architecture, hippocampal volume, cardiorespiratory parameters, and health-related quality of life among patients with schizophrenia: A systematic review. Indian J. Psychiatry 66, 997–1013. doi: 10.4103/indianjpsychiatry.indianjpsychiatry_735_23
Shobeiri, P., Behnoush, A. H., Khalaji, A., Teixeira, A. L., and Rezaei, N. (2023). Peripheral levels of the brain-derived neurotrophic factor in coronary artery disease: a systematic review and meta-analysis. J. Tehran Heart Cent. 18, 244–255. doi: 10.18502/jthc.v18i4.14823
Stein, A. M., Coelho, F. G. M., Vital-Silva, T. M., Rueda, A. V., Pereira, J. R., Deslandes, A. C., et al. (2023). Aerobic training and circulating neurotrophins in Alzheimer's disease patients: a controlled trial. Exp. Aging Res. 49, 1–17. doi: 10.1080/0361073x.2022.2048586
Sterne, J. A. C., Savović, J., Page, M. J., Elbers, R. G., Blencowe, N. S., Boutron, I., et al. (2019). RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 366:l4898. doi: 10.1136/bmj.l4898
Taylor, J. M., and Alanazi, S. (2023). Cohen's and hedges' g. J. Nurs. Educ. 62, 316–317. doi: 10.3928/01484834-20230415-02
Tsai, C. L., Pai, M. C., Ukropec, J., and Ukropcová, B. (2019). Distinctive effects of aerobic and resistance exercise modes on neurocognitive and biochemical changes in individuals with mild cognitive impairment. Curr. Alzheimer Res. 16, 316–332. doi: 10.2174/1567205016666190228125429
von Bohlen und Halbach, O. (2010). Involvement of BDNF in age-dependent alterations in the hippocampus. Front. Aging Neurosci. 2:36. doi: 10.3389/fnagi.2010.00036
Wang, X., Hu, P., Ai, Y., Zhou, S., Li, Y., Zhou, P., et al. (2024). Dual group-based trajectories of physical activity and cognitive function in aged over 55: a nationally representative cohort study. Front. Public Health 12:1450167. doi: 10.3389/fpubh.2024.1450167
Keywords: aerobic exercise, brain-derived neurotrophic factor, older adults, meta-analysis, walking, running, cycling
Citation: Cheng Y, Liu Y, Ma J, Li Z, Han E and Bo S (2025) Effects of three aerobic exercise modalities (walking, running, and cycling) on circulating brain-derived neurotrophic factor in older adults: a systematic review and meta-analysis. Front. Aging Neurosci. 17:1673786. doi: 10.3389/fnagi.2025.1673786
Edited by:
Vicki A. Nejtek, University of North Texas Health Science Center, United StatesReviewed by:
Richard J. Elsworthy, University of Birmingham, United KingdomChristopher Mackay, The University of Queensland, Australia
Kirby Doshier, University of North Texas, United States
Copyright © 2025 Cheng, Liu, Ma, Li, Han and Bo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Enli Han, aGFuZW5saTE5NzZAMTI2LmNvbQ==; Shumin Bo, Ym9zaHVtaW5AMTYzLmNvbQ==
†These authors have contributed equally to this work
 Yang Cheng
Yang Cheng Yuxiang Liu2†
Yuxiang Liu2† Jing Ma
Jing Ma Zhen Li
Zhen Li