- 1Department of Neurology, Tokyo Women’s Medical University School of Medicine, Tokyo, Japan
- 2Department of Neurology, Suita Municipal Hospital, Suita, Japan
Background: Patients with Parkinson’s disease (PD) have a high risk of sarcopenia. Herein, we evaluated the prevalence of sarcopenia and factors associated with it in ambulatory patients with PD.
Methods: Ambulatory patients with PD up to Hoehn and Yahr stage III were included and evaluated based on age, sex, disease duration, levodopa equivalent daily dose, cognitive impairment, swallowing disturbance, history of falling, the Japanese version of the movement disorder society-sponsored revision of the unified PD rating scale (MDS-UPDRS) parts I–IV, quality of life (QoL), and blood test (total protein, albumin, and anemia). Cognitive impairment was assessed using the Japanese version of Mini-Mental State Examination and the Japanese version of the Montreal Cognitive Assessment, whereas swallowing disturbance was assessed using the Japanese version of the Swallowing Disturbance Questionnaire. QoL was assessed using the Parkinson’s Disease Questionnaire (PDQ-8). Sarcopenia was diagnosed based on handgrip strength, five-time chair stand test, and skeletal muscle mass.
Results: Overall, 97 patients with PD (55 males), with a mean age of 69.8 years and a mean disease duration of 7.3 years, were included. The prevalence of sarcopenia was 33.0%. There were significant differences between the sarcopenia and the non-sarcopenia groups in age, sex, swallowing disturbance, MDS-UPDRS part III total score, and the sub-items arising from a chair, postural stability, and global spontaneity of movement (p < 0.05). There was no association between the presence of sarcopenia and the PDQ-8 total and sub-item scores. The factors that contributed most to sarcopenia were being female, cognitive impairment, and swallowing disturbance.
Conclusion: In clinical management, it is important to assess muscle strength and evaluate sarcopenia, particularly in patients with PD who have being female, cognitive impairment, or swallowing disturbance.
1 Introduction
Parkinson’s disease (PD) causes weight loss, fatigue, decreased activity, and slowed gait (Marinus et al., 2018), as well as sarcopenia, a loss of muscle mass that can lead to decreased muscle strength and physical function (Yazar et al., 2018; Peball et al., 2019). The general prevalence of sarcopenia is 7.5%–13.8% (Akune et al., 2014; Yoshida et al., 2014; Yoshimura et al., 2017), whereas that of sarcopenia in patients with PD is 17%–50%, depending on the subject and assessment method (Vetrano et al., 2018; Cai et al., 2021).
In 1989, Rosenberg posited the importance of age-related loss of skeletal muscle mass and promoted the term sarcopenia (Rosenberg, 1989). Sarcopenia, defined as the age-related loss of muscle mass and function, is increasingly recognized as a serious medical and economic problem in an aging society (Cruz-Jentoft et al., 2010) and has become a significant global public health focus (Shafiee et al., 2017). The causes of sarcopenia remain undetermined, and the prevailing theories consider it multifactorial, with the normal aging process increasing the risk of sarcopenia (Cruz-Jentoft et al., 2010).
Although previous reports have investigated the prevalence of sarcopenia in patients with PD (Ponsoni et al., 2023), the association between PD severity and sarcopenia (Vetrano et al., 2018), and changes in body composition and sarcopenia in patients with PD (Tan et al., 2018), there have been no reports of an association between sarcopenia and cognitive impairment, swallowing disturbance, or quality of life (QoL) in patients with PD. The Parkinson’s Disease Questionnaire-8 (PDQ-8), a shortened version of PDQ-39, was developed to reduce respondent burden and increase the convenience of use for patients with PD in clinical settings (Peto et al., 1998; Hagell et al., 2003; Chen et al., 2017).
PDQ-8 was constructed by taking one question from each domain of PDQ-39 (Peto et al., 1998; Chen et al., 2017). It is an established tool for assessing patients with PD that is frequently used in clinical studies (Martinez-Martin et al., 2011). Accordingly, in this study, we assessed QoL using PDQ-8 for convenience and examined the prevalence of sarcopenia in patients with PD and the factors affecting sarcopenia, including motor and non-motor symptoms and QoL in patients with PD, using the AWGS (2019 ASIAN working group for sarcopenia) diagnostic criteria.
2 Materials and methods
2.1 Participants
Ambulatory patients with PD up to Hoehn and Yahr (H-Y) stages I-III enrolled from December 1, 2021, to June 1, 2024, were included. As an inclusion criterion and for dementia screening, patients with a score ≤23 on the Japanese version of the Mini-Mental State Examination (MMSE-J) were excluded. PD was clinically diagnosed using the MDS clinical diagnostic criteria (Postuma et al., 2015).
2.2 Evaluation of clinical symptoms
Sarcopenia was diagnosed using the diagnostic criteria of AWGS (Chen et al., 2014). The AWGS diagnostic criteria are shown in Supplementary File 1.
In this study, physical performance was assessed using a five-time chair stand test, and appendicular skeletal muscle mass was assessed using a body component analyzer (InBody720®; InBody, Seoul, S. Korea). The InBody device is based on bioelectrical impedance analysis. Patients were evaluated based on age, sex, disease duration, H-Y stage, and history of falls. Cognitive impairment was assessed using the Japanese version of the Montreal Cognitive Assessment (MoCA-J), with scores of ≤25 on the MoCA-J considered abnormal. Swallowing disturbances were assessed using the Japanese version of the Swallowing Disturbance Questionnaire (SDQ-J) (Supplementary File 2): the SDQ-J total score of ≥11 points was classified as the swallowing disturbance group, and <11 points as the no swallowing disturbance group (Yamamoto et al., 2012).
The severity of PD was assessed using the H-Y stage, and clinical symptoms were evaluated using the Japanese version of the movement disorder society-sponsored revision of the unified Parkinson’s disease rating scale (MDS-UPDRS) parts I–IV (Kashihara et al., 2014). H-Y stage and MDS-UPDRS part III were assessed in the on-state. MDS-UPDRS part I focuses on non-motor symptoms in daily living, in which the patient or caregiver completes a 13-item questionnaire; part II focuses on motor symptoms in daily living, in which the patient or caregiver completes a 13-item questionnaire; part III covers objective motor symptoms, in which the assessor rates 18 motor symptoms; part IV covers motor complications, in which the assessor rates six questions regarding symptom variability and dyskinesia. The scores were evaluated as follows: 0, normal; 1, very mild; 2, mild; 3, moderate; 4, severe. QoL was assessed using the PDQ-8 (Peto et al., 1998; Supplementary File 3).
Each medication was evaluated using the levodopa equivalent daily dose (LEDD) (Jost et al., 2023). Biochemical factors (total protein [TP], albumin [Alb], and anemia) were evaluated using blood tests. An abnormal TP value was defined as ≤6.4 g/dl, and an abnormal Alb value was defined as ≤3.5 g/dl. The anemia group was defined as a group with a hemoglobin (Hb) value of <14 g/dL for male patients and <12 g/dL for female patients, and a hematocrit (Ht) value of <40% for male patients and <35% for female patients. All patients underwent magnetic resonance imaging to rule out other diseases. This study was approved by the Institutional Review Board of Tokyo Women’s Medical University (Approval No. 20210148). It was conducted in accordance with the Ethical Guidelines for Clinical Research in Japan and the Declaration of Helsinki. Written informed consent was obtained from all patients before the study commenced.
2.3 Statistical analysis
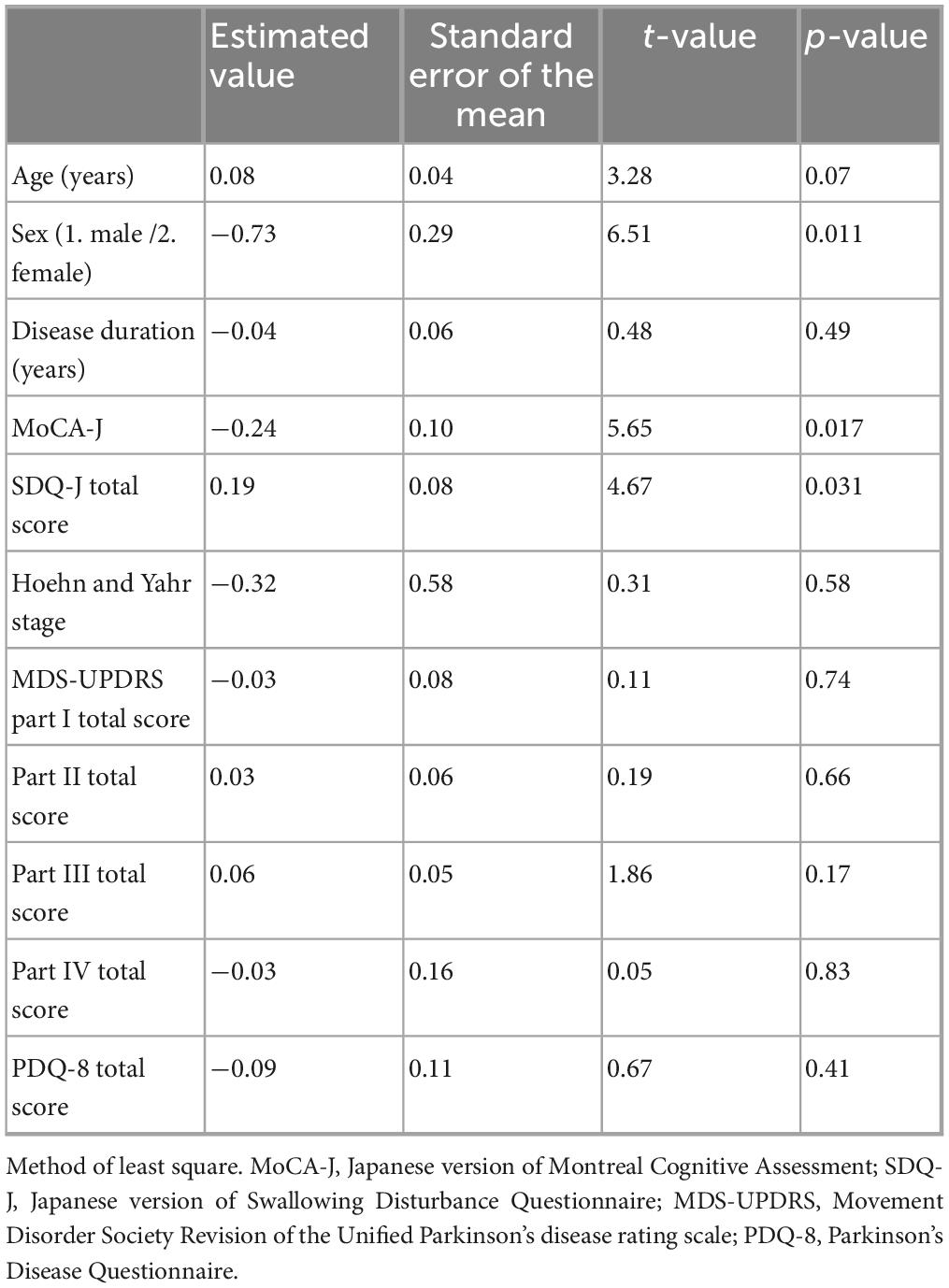
Data are expressed as the mean ± SD. JMP Pro statistical software (version 16; SAS Institute, Tokyo, Japan) was used for statistical analysis. The Wilcoxon test was used to compare age, disease duration, LEDD, MMSE-J, MoCA-J, H-Y stage, MDS-UPDRS parts I-V, PDQ-8, and biochemical factors (TP, Alb) between the non-sarcopenia and sarcopenia groups. The chi-squared test was used to compare sex, the presence or absence of swallowing disturbance, the presence or absence of falls, and anemia between the non-sarcopenia and sarcopenia groups. Factors related to the non-sarcopenia and sarcopenia groups (age, sex, disease duration, MoCA-J, presence or absence of swallowing disturbance, H-Y stage, MDS-UPDRS parts I-IV total score, and PDQ-8 total score) were analyzed using logistic regression analysis. Statistical significance was set at p < 0.05.
3 Results
3.1 Patient background
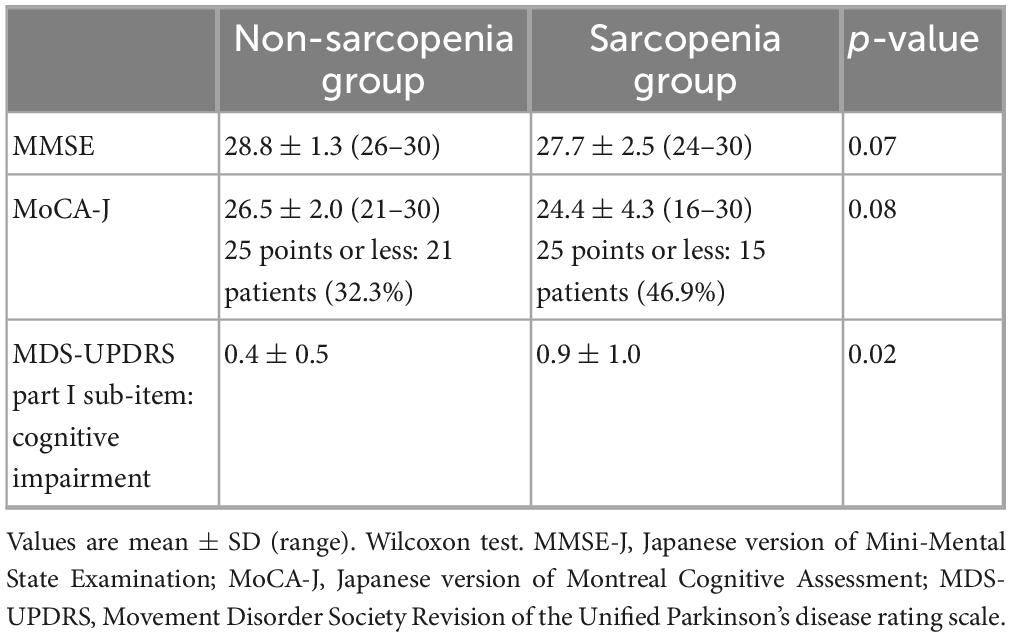
We included 97 patients with PD (55 males and 42 females), with a mean age of 69.8 ± 9.6 years (44–89 years) and disease duration of 7.3 ± 6.0 years (0.5–26 years). The mean LEDD was 477.0 ± 306.2 mg/day, the mean MMSE-J was 28.5 points, the mean MoCA-J was 25.8 points (25 points or less: 36 patients), and the mean SDQ-J total score was 4.8 ± 4.7 (swallowing disturbance group: 13 patients). Furthermore, 12, 66, and 19 patients had H-Y stage I, II, and III, respectively. The mean MDS-UPDRS parts I–IV total scores were 10.2 ± 5.6, 11.3 ± 7.1, 15.0 ± 7.9, and 1.4 ± 2.1 for parts I, II, III, and IV, respectively. The mean PDQ-8 total score was 5.4 ± 4.4 (Table 1).
3.2 Patient backgrounds and clinical characteristics of the non-sarcopenia and sarcopenia groups
Patient backgrounds and clinical characteristics are listed in Table 2. The prevalence of sarcopenia was 33%, with 65 patients in the non-sarcopenia group (43 males and 22 females) and 32 patients in the sarcopenia group (12 males and 20 females). Patients with PD in the non-sarcopenia group had a mean age of 67.3 ± 10.1 years (44–89 years), disease duration of 7.4 ± 6.0 years (0.5–22 years), and H-Y stage of 7 patients I, 51 patients II, and 7 patients III. Patients with PD in the sarcopenia group had an age of 74.8 ± 5.7 years (60–86 years), disease duration of 7.1 ± 5.9 years (0.5–26 years), and H-Y stage of 5 patients I, 15 patients II, and 12 patients III. There were significant differences between the non-sarcopenia and sarcopenia groups in age and sex (female) (p < 0.05).
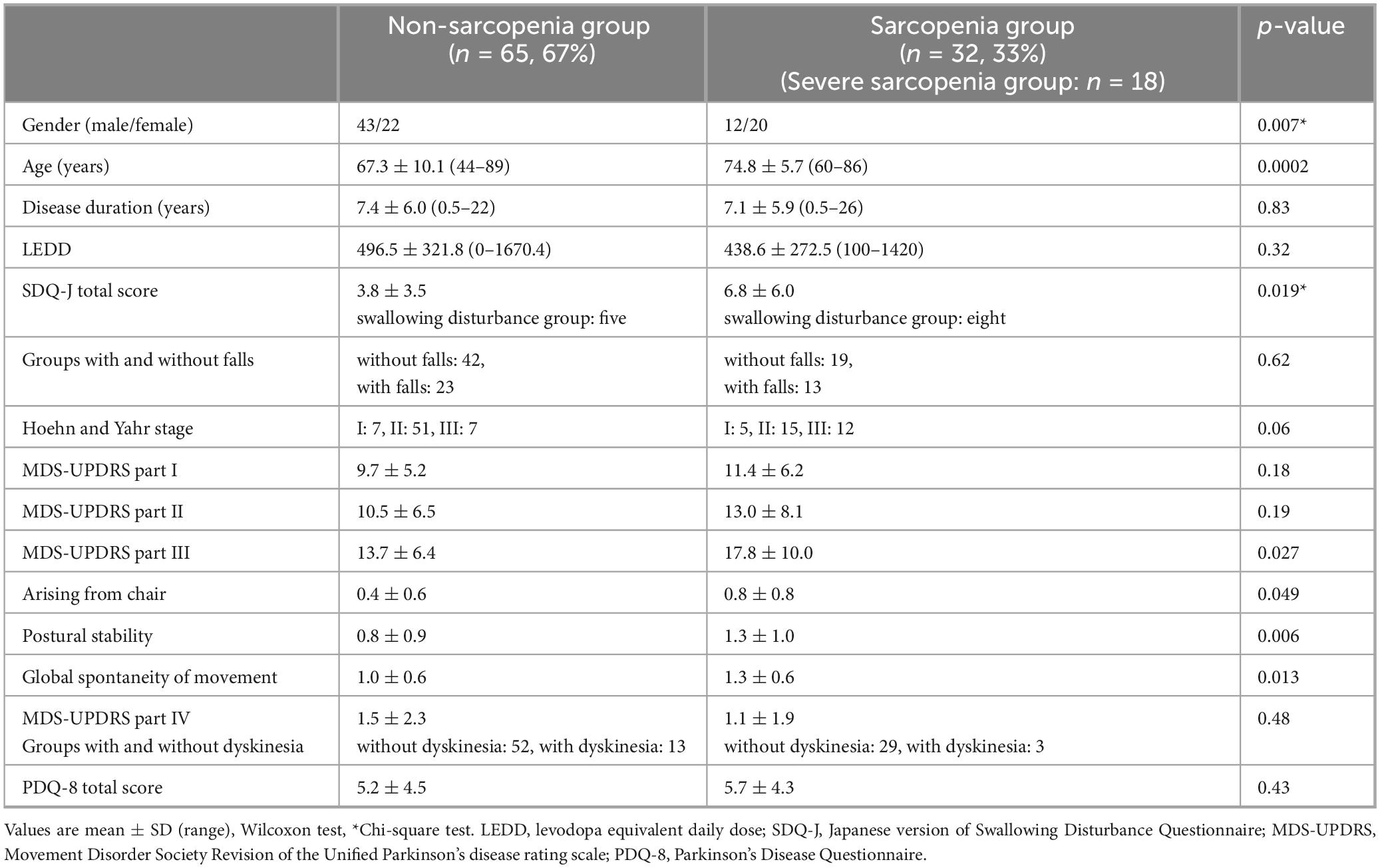
Table 2. Background and clinical characteristics of patients with Parkinson’s disease in the non-sarcopenia and sarcopenia groups.
3.3 Comparison of SDQ, fallings, MDS-UPDRS, PDQ-8, blood test between non-sarcopenia and sarcopenia group
The results in Table 2 show a significant difference between the non-sarcopenia and sarcopenia groups in the SDQ-J total score. A comparison of falls and the presence of sarcopenia showed no significant difference in falls between the two groups.
The MDS-UPDRS part III total score was significantly higher in the sarcopenia group than the non-sarcopenia group. The mean part III total score was 13.7 ± 6.4 in the non-sarcopenia group and 17.8 ± 10.0 in the sarcopenia group. There were significant differences in the part III sub-items, “arising from a chair,” “postural stability,” and “global spontaneity of movement.” In addition, among the sarcopenia assessment items (grip strength, five-time chair stand test, and skeletal muscle mass), a significant difference was observed between the part III total score and grip strength (p = 0.0051) (Figure 1). Grip strength also showed significant differences in several part III subitems, including hand movements, toe tapping, arising from a chair, postural stability, global spontaneity of movement, postural tremor, and kinetic tremor. Furthermore, there was no significant difference in the MDS-UPDRS part I, II, and IV total scores between the two groups; however, the sub-items of part I “cognitive impairment” and the part II sub-item “personal hygiene” were significantly different (p < 0.05).
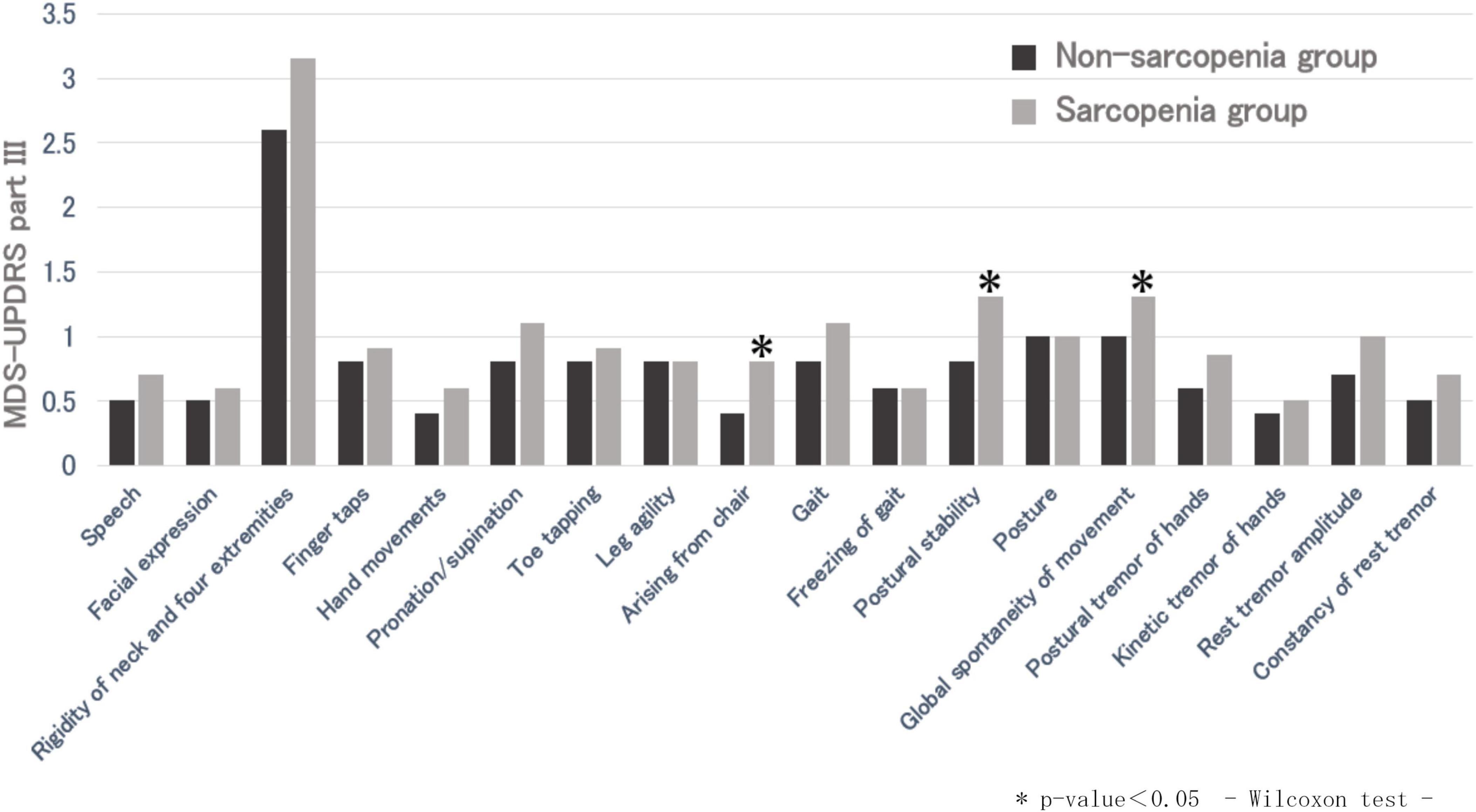
Figure 1. Comparison of MDS-UPDRS part III between non-sarcopenic and sarcopenic groups. *p-value < 0.05, Wilcoxon test.
There was no significant difference between the non-sarcopenia and sarcopenia groups in the PDQ-8 total score and the PDQ-8 sub-items. There was no significant difference between the non-sarcopenia and sarcopenia groups in TP (p = 0.98), Alb (p = 0.85), and Anemia (p = 0.70) by blood test.
3.4 Differences in cognitive function between non-sarcopenic and sarcopenic groups
In the comparison of cognitive function between the non-sarcopenia and sarcopenia groups, a significant difference was observed in the cognitive impairment of the MDS-UPDRS part I sub-items (Table 3). In contrast, there were no significant differences between the two groups in the MMSE-J or MoCA-J scores (Table 3).
3.5 Factors associated with sarcopenia
Factors contributing to sarcopenia were examined, including patient background (age, sex, disease duration, MoCA-J, SDQ-J total score), H-Y stage, MDS-UPDRS parts I–IV total scores, and PDQ-8 total score were examined, and the presence of sarcopenia was associated with gender (female), cognitive impairment, and swallowing disturbance (Table 4).
4 Discussion
The general prevalence of sarcopenia is reportedly 7.5%–13.8%, depending on the definition and data used (Akune et al., 2014; Yoshida et al., 2014; Yoshimura et al., 2017). In this study, the prevalence of sarcopenia in patients with PD was 33.0%, indicating a significantly higher prevalence than that in healthy persons. In this study, it was difficult to classify sarcopenia as primary or secondary; however, disuse muscle atrophy and weakness, which occur as secondary disorders in patients with PD, could be considered secondary sarcopenia due to “reduced activity,” according to the European Working Group on Sarcopenia in Older People (EWGSOP) (Cruz-Jentoft et al., 2010). In addition, some participants may have shown decreased performance on the five-time chair stand test and reduced grip strength due to insufficient dopaminergic treatment. In general, adequate dopaminergic therapy is defined as LEDD of approximately 600–800 mg/day or higher, according to a previous report (Tomlinson et al., 2010). In this study, the mean LEDD was 476.6 ± 306.4 mg/day, which may indicate that some patients did not receive an adequate dopaminergic dose.
In the comparison of the non-sarcopenic and sarcopenic group, MDS-UPDRS part I, which assesses subjective non-motor symptoms, and part II, which assesses subjective motor symptoms, were not significantly different between the two groups, suggesting that the presence of sarcopenia and subjective symptoms in patients with PD are poorly related. In this study, patients with sarcopenia showed higher the part III total scores than those without sarcopenia, particularly in subitems related to lower-limb function such as “arising from a chair” and “postural stability.” In addition, grip strength showed significant differences with the part III total score and several subitems, suggesting that overall motor severity is closely linked to reduced muscle strength. Based on these findings, sarcopenia in patients with PD is primarily associated with motor impairment rather than non-motor symptoms, underscoring the importance of assessing and maintaining muscle strength in clinical management. Part IV was not significantly different between the non-sarcopenia and sarcopenia group, suggesting that sarcopenia is not associated with motor complications.
Both the PDQ-8 total score and sub-items were not significantly different between the non-sarcopenic and sarcopenic groups of patients with PD in this study, suggesting that the presence of sarcopenia is poorly related to QoL in patients with PD. The EWGSOP defines sarcopenia as “a syndrome characterized by a progressive and generalized loss of muscle mass and strength, with risk of physical dysfunction, reduced QoL, and death” (Cruz-Jentoft et al., 2010). Although sarcopenia is associated with QoL in older Japanese patients (Tanimoto et al., 2012), there have been no previous reports on sarcopenia and QoL in patients with PD. Previous studies examining the PDQ-8 and MDS-UPDRS found that the PDQ-8 was correlated with parts I and II of the MDS-UPDRS, suggesting an association between QoL and subjective symptoms (Skorvanek et al., 2018). It was presumed that subjective motor and non-motor symptoms of PD may be more related to QoL in patients with PD than to the loss of muscle mass or strength.
The comparison of cognitive impairment between the non-sarcopenia and sarcopenia groups showed significant differences in MDS-UPDRS part I cognitive impairment. Cognitive impairment is one of the common non-motor symptoms in patients with PD and can affect attention, executive function, and visuospatial abilities (Aarsland et al., 2010). These cognitive deficits may lead to reduced physical activity and poor nutritional intake, thereby contributing to the development and progression of sarcopenia. An association between sarcopenia and cognitive impairment has been reported (Cabett Cipolli et al., 2019), and sarcopenia has been associated with mild cognitive impairment and depression in older women (Lee et al., 2018), and some reports have shown the effect of malnutrition on cognitive impairment in older adults (Petersson and Philippou, 2016). Sequential lifestyle changes, including impaired exercise and inactivity, may be associated with sarcopenia and cognitive impairment (Cabett Cipolli et al., 2019), a finding that also supports the previous reports of sarcopenia in patients with PD in this study.
In this study, sarcopenia in patients with PD was most strongly associated with being female, cognitive impairment, and swallowing disturbance. Some studies have reported no sex differences in sarcopenia in patients with PD (Cai et al., 2021; Ponsoni et al., 2023), whereas others have reported that sex differences in women are independently associated with sarcopenia (Lima et al., 2020). Androgens are important in the maintenance of muscle mass, and low plasma testosterone levels may cause or accelerate muscle- and age-related diseases, such as sarcopenia (Basualto-Alarcón et al., 2014; Wu et al., 2020). The association between sex, female hormones and PD has long been indicated; (Kumagai et al., 2014; Cerri et al., 2019) however, the relationship between the presence or absence of sarcopenia and sex in patients with PD is controversial and has not been established in the literature. These results also suggest that cognitive impairment and swallowing disturbances are associated with sarcopenia in patients with PD, even in the absence of undernutrition. In older adults, an association between swallowing and chewing functions has been reported, along with generalized sarcopenia (Shiozu et al., 2015; Murakami et al., 2015). The mechanism underlying swallowing disturbance caused by sarcopenia is considered to be secondary sarcopenia of the whole body and swallowing-related muscles due to inactivity, malnutrition, and disease (Wakabayashi and Sakuma, 2014). Although data on the association between sarcopenia and swallowing disturbance in patients with PD are lacking, our results suggest a link between the swallowing disturbances caused by the disease and sarcopenia. The results of this study also suggest the importance of assessing muscle strength and evaluating sarcopenia, particularly in patients with PD who have being female, cognitive impairment, or swallowing disturbance.
This study has some limitations. The presence of sarcopenia prior to the diagnosis of PD could not be evaluated, and the medical background of the patients with PD could not be considered; therefore, it is undeniable that there may have been a bias in the cases. Moreover, although an association between PD severity and sarcopenia has been reported previously (Vetrano et al., 2018), this study did not include H-Y stage IV and thus could not evaluate patients with severe PD. In addition, the motor score (MDS-UPDRS part III) was evaluated in the “on” state and was not compared with that in the “off” state, warranting consideration in future studies.
5 Conclusion
The prevalence of sarcopenia in patients with mild to moderate PD was higher than in the general population, and the most contributing factors were being female, cognitive impairment, and swallowing disturbance. In clinical management, it is important to assess muscle strength and evaluate sarcopenia, particularly in patients with such risk factors.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary material.
Ethics statement
The studies involving humans were approved by Institutional Review Board of Tokyo Women’s Medical University (Approval No. 20210148). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
RM: Data curation, Formal analysis, Investigation, Writing – original draft. KK: Project administration, Supervision, Writing – review & editing. KT: Supervision, Writing – review & editing. MI: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This research was supported by YOSHIOKA Yayoi Memorial Research Fellowship Grant.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2025.1718723/full#supplementary-material
References
Aarsland, D., Brønnick, K., Larsen, J. P., Tysnes, O. B., Alves, G., and Norwegian, et al. (2010). Cognitive impairment in incident, untreated Parkinson disease: The Norwegian ParkWest study. Neurology 72, 1121–1126. doi: 10.1212/01.wnl.0000340981.37492.96
Akune, T., Muraki, S., Oka, H., Tanaka, S., Kawaguchi, H., Nakamura, K., et al. (2014). Exercise habits during middle age are associated with lower prevalence of Sarcopenia: The ROAD study. Osteoporos. Int. 25, 1081–1088. doi: 10.1007/s00198-013-2550-z
Basualto-Alarcón, C., Varela, D., Duran, J., Maass, R., and Estrada, M. (2014). Sarcopenia and androgens: A link between pathology and treatment. Front. Endocrinol. 5:217. doi: 10.3389/fendo.2014.00217
Cabett Cipolli, G., Sanches Yassuda, M., and Aprahamian, I. (2019). Sarcopenia is associated with cognitive impairment in older adults: a systematic review and meta-analysis. J. Nutr. Health Aging 23, 525–531. doi: 10.1007/s12603-019-1188-8
Cai, Y., Feng, F., Wei, Q., Jiang, Z., Ou, R., and Shang, H. (2021). Sarcopenia in patients with Parkinson’s disease: A systematic review and meta-analysis. Front. Neurol. 12:598035. doi: 10.3389/fneur.2021.598035
Cerri, S., Mus, L., and Blandini, F. (2019). Parkinson’s disease in women and men: what’s the difference? J. Parkinsons Dis. 9, 501–515. doi: 10.3233/JPD-191683
Chen, K., Yang, Y. J., Liu, F. T., Li, D. K., Bu, L. L., Yang, K., et al. (2017). Evaluation of PDQ-8 and its relationship with PDQ-39 in China: A three-year longitudinal study. Health Qual. Life Outcomes 15:170. doi: 10.1186/s12955-017-0742-5
Chen, L. K., Liu, L. K., Woo, J., Assantachai, P., Auyeung, T. W., Bahyah, K. S., et al. (2014). Sarcopenia in Asia: Consensus report of the Asian Working Group for Sarcopenia. J. Am. Med. Dir. Assoc. 15, 95–101. doi: 10.1016/j.jamda.2013.11.025
Cruz-Jentoft, A. J., Baeyens, J. P., Bauer, J. M., Boirie, Y., Cederholm, T., Landi, F., et al. (2010). Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing 39, 412–423. doi: 10.1093/ageing/afq034
Hagell, P., Whalley, D., McKenna, S. P., and Lindvall, O. (2003). Health status measurement in Parkinson’s disease: validity of the PDQ-39 and Nottingham health profile. Mov. Disord. 18, 773–783. doi: 10.1002/mds.10438
Jost, S. T., Kaldenbach, M.-A., Antonini, A., Martinez-Martin, P., Timmermann, L., Odin, P., et al. (2023). Levodopa dose equivalency in Parkinson’s disease: Updated systematic review and proposals. Mov. Disord. 38, 1236–1252. doi: 10.1002/mds.29410
Kashihara, K., Kondo, T., Mizuno, Y., Kikuchi, S., Kuno, S., Hasegawa, K., et al. (2014). Official Japanese version of the movement disorder society-unified Parkinson’s disease rating scale: Validation against the original English version. Mov. Disord. Clin. Pract. 1, 200–212. doi: 10.1002/mdc3.12058
Kumagai, T., Nagayama, H., Ota, T., Nishiyama, Y., Mishina, M., and Ueda, M. (2014). Sex differences in the pharmacokinetics of levodopa in elderly patients with Parkinson disease. Clin. Neuropharmacol. 37, 173–176. doi: 10.1097/WNF.0000000000000051
Lee, I., Cho, J., Hong, H., Jin, Y., Kim, D., and Kang, H. (2018). Sarcopenia is associated with cognitive impairment and depression in elderly Korean women. Iran. J. Public Health 47, 327–334.
Lima, D. P., de Almeida, S. B., Bonfadini, J. C., de Luna, J. R. G., de Alencar, M. S., Pinheiro-Neto, E. B., et al. (2020). Clinical correlates of Sarcopenia and falls in Parkinson’s disease. PLoS One 15:e0227238. doi: 10.1371/journal.pone.0227238
Marinus, J., Zhu, K., Marras, C., Aarsland, D., and van Hilten, J. J. (2018). Risk factors for non-motor symptoms in Parkinson’s disease. Lancet Neurol. 17, 559–568. doi: 10.1016/S1474-4422(18)30127-3
Martinez-Martin, P., Jeukens-Visser, M., Lyons, K. E., Rodriguez-Blazquez, C., Selai, C., Siderowf, A., et al. (2011). Health-related quality-of-life scales in Parkinson’s disease: Critique and recommendations. Mov. Disord. 26, 2371–2380. doi: 10.1002/mds.23834
Murakami, M., Hirano, H., Watanabe, Y., Sakai, K., Kim, H., and Katakura, A. (2015). Relationship between chewing ability and Sarcopenia in Japanese community-dwelling older adults. Geriatr. Gerontol. Int. 15, 1007–1012. doi: 10.1111/ggi.12399
Peball, M., Mahlknecht, P., Werkmann, M., Marini, K., Murr, F., Herzmann, H., et al. (2019). Prevalence and associated factors of Sarcopenia and frailty in Parkinson’s disease: A cross-sectional study. Gerontology 65, 216–228. doi: 10.1159/000492572
Petersson, S. D., and Philippou, E. (2016). Mediterranean diet, cognitive function, and dementia: A systematic review of the evidence. Adv. Nutr. 7, 889–904. doi: 10.3945/an.116.012138
Peto, V., Jenkinson, C., and Fitzpatrick, R. (1998). PDQ-39: A review of the development, validation and application of a Parkinson’s disease quality of life questionnaire and its associated measures. J. Neurol. 245(Suppl. 1), S10–S14. doi: 10.1007/pl00007730
Ponsoni, A., Sardeli, A. V., Costa, F. P., and Mourão, L. F. (2023). Prevalence of Sarcopenia in Parkinson’s disease: A systematic review and meta-analysis. Geriatr. Nurs. 49, 44–49. doi: 10.1016/j.gerinurse.2022.11.006
Postuma, R. B., Berg, D., Stern, M., Poewe, W., Olanow, C. W., Oertel, W., et al. (2015). MDS clinical diagnostic criteria for Parkinson’s disease. Mov. Disord. 30, 1591–1601. doi: 10.1002/mds.26424
Rosenberg, I. H. (1989). Summary comments. Am. J. Clin. Nutr. 50, 1231–1233. doi: 10.1093/ajcn/50.5.1231
Shafiee, G., Keshtkar, A., Soltani, A., Ahadi, Z., Larijani, B., and Heshmat, R. (2017). Prevalence of Sarcopenia in the world: A systematic review and meta- analysis of general population studies. J. Diab. Metab. Disord. 16:21. doi: 10.1186/s40200-017-0302-x
Shiozu, H., Higashijima, M., and Koga, T. (2015). Association of Sarcopenia with swallowing problems, related to nutrition and activities of daily living of elderly individuals. J. Phys. Ther. Sci. 27, 393–396. doi: 10.1589/jpts.27.393
Skorvanek, M., Martinez-Martin, P., Kovacs, N., Zezula, I., Rodriguez-Violante, M., Corvol, J. C., et al. (2018). Relationship between the MDS-UPDRS and quality of life: A large multicenter study of 3206 patients. Parkinsonism Relat. Disord. 52, 83–89. doi: 10.1016/j.parkreldis.2018.03.027
Tan, A. H., Hew, Y. C., Lim, S. Y., Ramli, N. M., Kamaruzzaman, S. B., Tan, M. P., et al. (2018). Altered body composition, sarcopenia, frailty, and their clinico-biological correlates in Parkinson’s disease. Parkinsonism Relat. Disord. 56, 58–64. doi: 10.1016/j.parkreldis.2018.06.020
Tanimoto, Y., Watanabe, M., Sun, W., Sugiura, Y., Tsuda, Y., Kimura, M., et al. (2012). Association between Sarcopenia and higher-level functional capacity in daily living in community-dwelling elderly subjects in Japan. Arch. Gerontol. Geriatr. 55, e9–e13. doi: 10.1016/j.archger.2012.06.015
Tomlinson, C. L., Stowe, R., Patel, S., Rick, C., Gray, R., and Clarke, C. E. (2010). Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov. Disord. 25, 2649–2653. doi: 10.1002/mds.23429
Vetrano, D. L., Pisciotta, M. S., Laudisio, A., Lo Monaco, M. R., Onder, G., Brandi, V., et al. (2018). Sarcopenia in Parkinson disease: Comparison of different criteria and association with disease severity. J. Am. Med. Dir. Assoc. 19, 523–527. doi: 10.1016/j.jamda.2017.12.005
Wakabayashi, H., and Sakuma, K. (2014). Rehabilitation nutrition for Sarcopenia with disability: A combination of both rehabilitation and nutrition care management. J. Cachexia Sarcopenia Muscle 5, 269–277. doi: 10.1007/s13539-014-0162-x
Wu, Y. N., Chen, M. H., Chiang, P. L., Lu, C. H., Chen, H. L., Yu, C. C., et al. (2020). Associations between brain structural damage and core muscle loss in patients with Parkinson’s disease. J. Clin. Med. 9:239. doi: 10.3390/jcm9010239
Yamamoto, T., Ikeda, K., Usui, H., Miyamoto, M., and Murata, M. (2012). Validation of the Japanese translation of the swallowing disturbance questionnaire in Parkinson’s disease patients. Qual. Life Res. 21, 1299–1303. doi: 10.1007/s11136-011-0041-2
Yazar, T., Yazar, H. O., Zayimoğlu, E., and Çankaya, S. (2018). Incidence of Sarcopenia and dynapenia according to stage in patients with idiopathic Parkinson’s disease. Neurol. Sci. 39, 1415–1421. doi: 10.1007/s10072-018-3439-6
Yoshida, D., Suzuki, T., Shimada, H., Park, H., Makizako, H., Doi, T., et al. (2014). Using two different algorithms to determine the prevalence of Sarcopenia. Geriatr. Gerontol. Int. 14(Suppl. 1), 46–51. doi: 10.1111/ggi.12210
Keywords: Parkinson’s disease, sarcopenia, gender, cognitive impairment, swallowing disturbance
Citation: Morimoto R, Kitagawa K, Todo K and Iijima M (2025) Clinical analysis of sarcopenia prevalence and its influencing factors in patients with Parkinson’s disease. Front. Aging Neurosci. 17:1718723. doi: 10.3389/fnagi.2025.1718723
Received: 04 October 2025; Revised: 18 November 2025; Accepted: 19 November 2025;
Published: 08 December 2025.
Edited by:
Atsushi Takeda, National Hospital Organization Sendai Nishitaga Hospital, JapanReviewed by:
Tadashi Ichikawa, Saitama Prefectural Rehabilitation Center, JapanHidetomo Murakami, Showa University, Japan
Copyright © 2025 Morimoto, Kitagawa, Todo and Iijima. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mutsumi Iijima, aWlqaW1hLm11dHN1bWlAdHdtdS5hYy5qcA==
 Remi Morimoto
Remi Morimoto Kazuo Kitagawa
Kazuo Kitagawa Kenichi Todo1
Kenichi Todo1 Mutsumi Iijima
Mutsumi Iijima