- 1Department of Immunology, School of Medical Sciences, Universiti Sains Malaysia, Kubang Kerian, Malaysia
- 2Department of Chemical Pathology, School of Medical Sciences, Universiti Sains Malaysia, Kubang Kerian, Malaysia
- 3Universite De Lorraine, CNRS, LCPM, Nancy, France
- 4School of Chemical and Energy Engineering, Universiti Teknologi Malaysia, Skudai, Malaysia
- 5School of Health and Biomedical Sciences, RMIT University, Bundoora, VIC, Australia
- 6Advanced Materials and Nanobiotechnology Laboratory, TardigradeNano LLC, Irvine, CA, United States
Recent years have witnessed an unprecedented growth in the research area of nanomedicine. There is an increasing optimism that nanotechnology applied to medicine will bring significant advances in the diagnosis and treatment of various diseases, including colorectal cancer (CRC), a type of neoplasm affecting cells in the colon or the rectum. Recent findings suggest that the role of microbiota is crucial in the development of CRC and its progression. Dysbiosis is a condition that disturbs the normal microbial environment in the gut and is often observed in CRC patients. In order to detect and treat precancerous lesions, new tools such as nanotechnology-based theranostics, provide a promising option for targeted marker detection or therapy for CRC. Because the presence of gut microbiota influences the route of biomarker detection and the route of the interaction of nanoparticle/drug complexes with target cells, the development of nanoparticles with appropriate sizes, morphologies, chemical compositions and concentrations might overcome this fundamental barrier. Metallic particles are good candidates for nanoparticle-induced intestinal dysbiosis, but this aspect has been poorly explored to date. Herein, we focus on reviewing and discussing nanotechnologies with potential applications in CRC through the involvement of gut microbiota and highlight the clinical areas that would benefit from these new medical technologies.
Introduction
Colorectal cancer (CRC) is the second most common cancer in females and third most common cancer in males worldwide. Over time, it has become a leading cause of morbidity and mortality. There is a broad geographical variation in the incidence of CRC globally, and there has been a rapid rise in its incidence in Asian countries for the past few years (Siegel et al., 2020). In Malaysia, for example, the National Cancer Patient Registry has reported that CRC is the second most common cancer in both males and females, with a total number of 4,501 cases diagnosed from 2008 to 2013 (Abu Hassan et al., 2016). In general, CRC incidence is higher in the developed countries as compared to the developing ones; however, the burden of this disease is rising globally, including that in the low-to-middle-income nations (Siegel et al., 2020).
Currently, ample research is being conducted in order to find the definite cause of CRC. Based on the actual findings, it is theorized that the bacteria present in the human colon are linked to the development of carcinogenesis (Saus et al., 2019). Large numbers of bacterial cells live in commensal relationships with the host. However, once the gastric ecosystem is altered, various bacterial species become prone to develop pathogenic phenotypes (Sekirov et al., 2010). In recent years, there has been a surge of interest in assessing the relationship between the gut microbiota and the gut modifications that eventually lead to CRC. From here on, modification in the composition of the gut microbe is suggested as the cause underlying the development of colorectal malignancies (Saus et al., 2019).
Nanomedicine can be broadly defined as comprehensive monitoring, control, construction, repair, defense and/or improvement of all human biological systems, working from the molecular level to more complex wholes, with the use of nanomaterials. Nanoparticles (NPs) have been increasingly applied in the disease diagnosis and treatment during the last few decades. This use of NPs for medical purposes has led to encouraging prospects of their use for the betterment of human health (Riaz Rajoka et al., 2021). Diagnostic and therapeutic research on nanomedicine formulations has brought about a number of effective platforms, including those for combined diagnosis, targeted drug delivery and therapy (Saini et al., 2010). Still, despite the immense prospect of nanomedicine for improving human health, the prognosis for advanced stages of CRC is still relatively poor. The current treatment method for CRC includes surgery and/or chemotherapy. Although CRC is curable, the survival rates are still low. With the current advancements in the world of nanomedicine, it is expected that utilization of NPs will be a vital future approach in CRC theragnostics (Werner and Heinemann, 2016). Correspondingly, this review primarily elaborates on the dysbiosis condition that leads to the development of CRC, and the clinical aspect of NPs and their limitations based on published research.
Microbiota
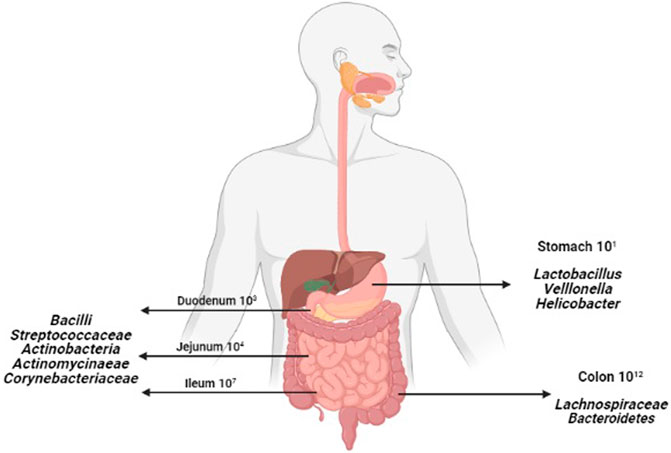
Human body is inhabited by a vast number of bacteria, archaea, viruses and unicellular eukaryotes. The collection of microorganisms that live in peaceful coexistence with their hosts has been referred to as the microbiota, microflora or normal flora (Dieterich et al., 2018). By far the most heavily colonized organ is the gastrointestinal tract (GIT); the colon alone is estimated to host over 70% of all the microbes in the human body (Jandhyala et al., 2015). The concentration of microbiota increases steadily along the GIT. Hence, lesser concentrations of microbiota are found in our stomach and significantly higher in the colon (Anselmo and Mitragotri, 2019), as shown in Figure 1.
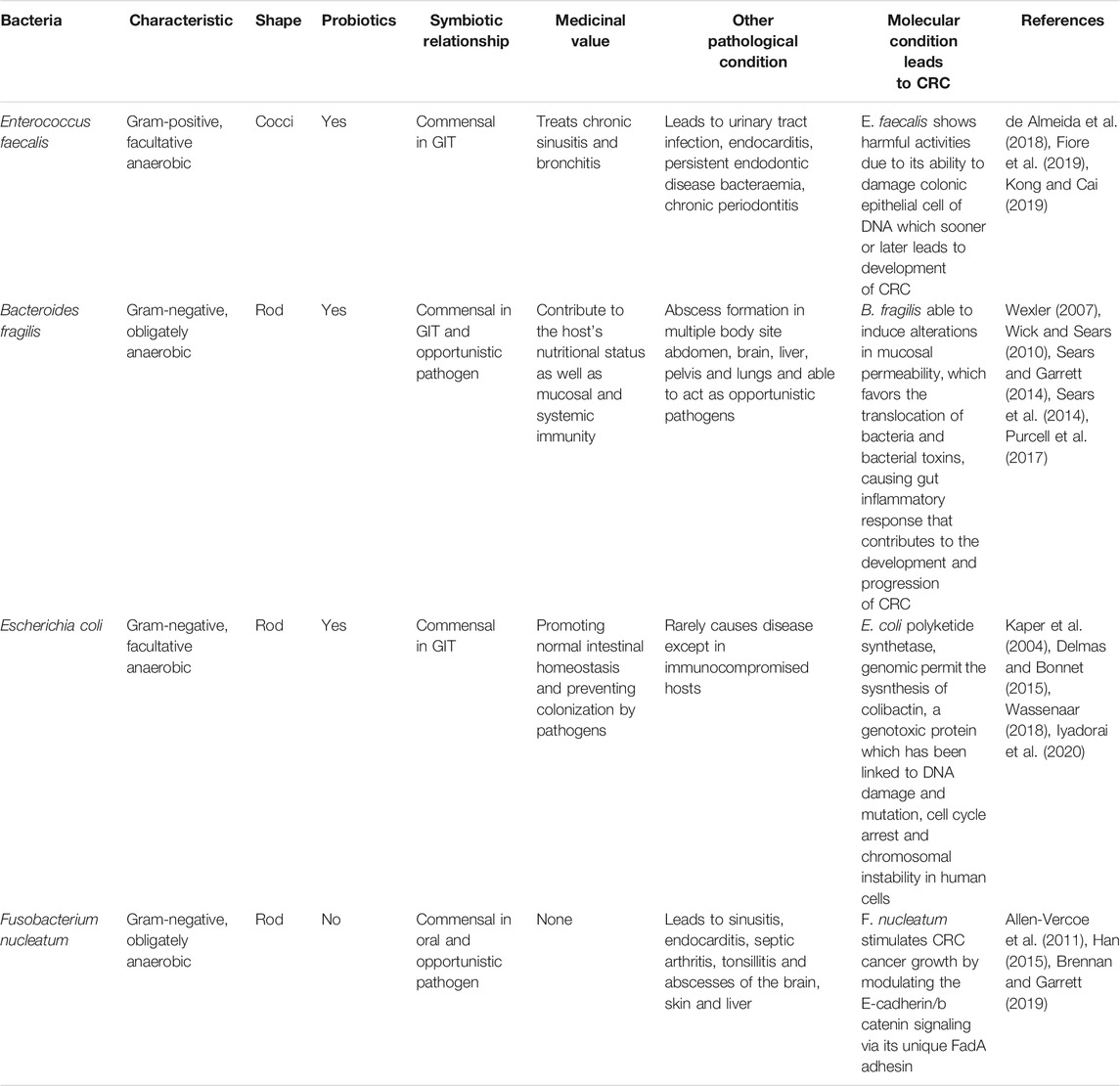
The GIT shelters trillions of microbes which mostly live in a harmonized relationship with the host. In spite of the fact that most of the microbiome shows a favorable symbiotic relationship with the host, when the microbiome composition and function get disturbed, preconditions for various diseases arise, including cancers, obesity, metabolic diseases, diabetes, allergies, depression and disorders of the immune system (Quigley, 2013). Here, a selective group of bacteria such as Enterococcus faecalis, Bacteroides fragilis, Escherichia coli and Fusobacterium nucleatum are seen as fundamental causes of the pathogenesis of CRC (Kong and Cai, 2019). These bacteria are listed in Table 1.
Immunomodulation Through Host-Microbiota Interactions
Host-microbiota interactions are fundamental for the development of immune system. The early life colonization of the human gut mucosal surfaces plays a pivotal role in the maturation of the human immune system (Zheng et al., 2020). Gut microbial community composition is modified by many environmental factors, such as geographical location, host diet, and administration of antibiotics and other medicines. Local immune responses are triggered by microbes via interactions with immune cells that express pattern recognition receptors (PRRs). Local dendritic cells (DCs) get activated by microbes or microbe-derived elements such as constituents, products, metabolites via interactions with PRRs. Activated DCs travel from the gastrointestinal tract to mesenteric lymph nodes (mLNs), where they present microbe-derived antigens and subsequently induce the differentiation of immature T cells into effector T cells, particularly regulatory T cells (Tregs) and T helper 17 (Th17) cells (Inamura, 2021). A subset of these effector T cells migrates back to the gastrointestinal tract and influences local immune responses. The remaining population enters the systemic circulation and influences systemic immunity. The conversion of the immune system from a pro-inflammatory to an anti-inflammatory state occurs through the release of anti-inflammatory cytokines such as IL-10, TGF-β or the engagement of DCs mediated by Tregs. Conversely, Th17 cells mediate the conversion of the immune system to a pro-inflammatory state by secreting immunostimulatory cytokines (e.g., IL-17) or by activating and recruiting neutrophils. This intriguing relationship strongly suggests the vital role of microbes in Th17 cell activation (Inamura 2021).
Impact of Nanoparticles and Gut Microbiota
NPs could be present in food in the form as additives, supplements and packaging and directly impact the composition and/or metabolic activities of the gut microbiota (Lamas et al., 2020). Furthermore, inorganic NPs, particularly, such as silver, titanium dioxide, silicon dioxide and zinc oxide have been shown to affect gut microbiota through their interaction with immune system. This alteration in gut microbiota-immune axis is associated with many chronic diseases such as inflammatory bowel disease (IBD), diabetes and even colorectal cancer (Ni et al., 2017; Meijnikman et al., 2018; Richard et al., 2018). Silver NPs are used in hundreds of commercial products due to their anti-microbial properties and intentional and accidental uptake of silver NPs, which might affect the gut microbiome, are underestimated. Dahiya et al. (2018) have reviewed evidence from both animal and human studies and concluded that silver NPs altered the gut microbiota, thus making the host susceptible to certain diseases. They also illustrated that the mucus present in the gut lining prevents absorption of silver NPs into intestinal cells, hence the interaction with gut microbiota. Meanwhile, titanium dioxide NPs, which are commonly available in daily products, also cause changes in both gut microbiota morphology and metabolism (Juan et al., 2018; Mao et al., 2019; Zhangjian et al., 2019). Specifically, a mix of commensal and pathogenic bacteria was modulated upon administration of titanium dioxide NPs and manifested as oxidative stress and inflammatory responses in the intestine (Zhangjian et al., 2019). However, another study indicates that titanium dioxide NPs have limited effect on gut microbiota compared to silver NPs that drastically changes the commensal density. In this study, titanium dioxide NPs formed large agglomerates that appeared to loosely interact with microbial cells, whereas Silver NPs were found both within and outside of microbial cells. This explains the significantly lower effect of titanium dioxide NPs on community growth (Agans et al., 2019). There is also optimistic opportunity of NPs in their interaction with gut microbiota. Selenium NPs are shown to be a promising tool in livestock industry due to their efficacy in intestinal pathogen control (Gangadoo et al., 2019). These NPs are observed to reduce emerging poultry pathogen, Enterococcus cecorum, without any significant disturbance to the gut total commensal community. Another type of NPs, fullerenol NPs with good biocompatibility properties, markedly increased the production of short-chain fatty acids (SCFAs)-producing bacteria in vivo (Li et al., 2018). Fullerenol NPs contain furan- and pyran-like structure which is similar to those of polysaccharides in dietary fiber such as inulin, thus the capacity to promote this kind of gut microbes. The increase of this gut main metabolites-producing bacteria is accompanied with anti-hyperlipidemic effect of fullerenol NPs when both triglycerides and total cholesterol levels in liver and blood were decreased. Interestingly, fabrication of fullerenol NPs to form carbonyls in low pH would induce a peroxidase-like activity and eradicate Helicobacter pylori both in vivo and in vitro (Zhang C. et al., 2020). In addition, approach to design NPs with natural sources and line up into a systematic drug delivery tool is demonstrated to be beneficial to gut microbiota. Curcumin- and ginger-derived NPs are shown to improve absorption by gut microbiota, so that they can produce their respective effects (Ohno et al., 2017; Teng et al., 2018). Curcumin NPs are shown to suppress development of mouse colitis through the increase of butyrate-producing bacteria and regulatory T cells (Tregs) while ginger NPs is design to contain microRNA that could ameliorate mouse colitis. Another natural sources such as milk is developed as extracellular vesicles and these NPs modulated intestinal SCFAs metabolites and increased intestinal immunity (Tong et al., 2020).
Nanomedicines and Colorectal Cancer
The unique optical, magnetic, and electrical properties of metallic NPs such as gold and silver have rendered these systems a great tool for the detection of several markers of cancer. For example, fluorescent gold NPs have been used to detect mechano-growth factor (MGF), a unique marker of colon cancer (Kasprzak and Szaflarski, 2020). Another study demonstrated that the incorporation of theragnostic gold nanorods as cores inside tumour-specific antibody shells as allowed to make the distinction between the different types of tumors and destroy the surrounding tumor cells (Lee et al., 2015). A non-invasive NP-based breath sensor was also shown to distinguish between the healthy and cancerous patients, including those with CRC (Peng et al., 2010). This nanomaterial-based sensor array would analyze volatile organic compounds associated with and differently regulated in different diseases using cross-reactive absorption sites on nanomaterials such as gold NPs, thus producing a unique breath fingerprint (Xu et al., 2013). Local staging of colon cancer is routinely done with computerized tomography (CT) scans and magnetic resonance imaging (MRI), which both benefit from the use of NPs, which can improve the sensitivity for the detection of tumor invasion (Nerad et al., 2017). In MRI, superparamagnetic iron oxide (γ-Fe2O3 or Fe3O4) NPs coated with a polymer are demonstrated as promising contrast agent for MRI, whereas conjugation with doxorubicin further displayed the multimodality of these NPs in both diagnostic and therapeutic assays.
Conjugation of these NPs to chemotherapeutic drugs did not only effectively kill tumor cells via active targeting, but also inhibited tumor growth using near-infrared (NIR) irradiation due to the photothermal activity of the NPs (Fan et al., 2019). In another study, Fe2O3-gold NPs were observed to accumulate within the tumor mass instead of other organs in a colon cancer model, while also enhancing the tumor detection selectivity as MRI contrast agents in a pancreatic cancer model (Kumagai et al., 2010). Conventional chemotherapy has been reported to be associated with severe side effects, mainly due to the nonspecific biodistribution, whereby both the cancer cells and the healthy cells are affected by the therapy. In CRC, common side effects over the course of chemotherapy include fatigue, diarrhea, constipation and dyspnoea, as reported in routine healthcare settings (Pearce et al., 2017). However, over the past three decades, the concept of enhanced permeability retention (EPR) effect conditioned by the hypervasculature in solid tumors, as proposed by Matsumura and Maeda (1986), greatly improved the logistics of cancer treatments using NPs (Matsumura and Maeda, 1986). Nowadays, the application of NPs in cancer therapeutics enhances the efficacy of the conventional cancer management, particularly relative to that of chemotherapy. This enhanced efficacy is largely owing to the passive targeting of tumors by the NPs, which are able to pass through the tumor vascular pores and accumulate the nanomedicine in the tumor zone. Furthermore, the poor response to anticancer drugs due to the development of multidrug resistance (MDR) phenotypes has shown improvement following the incorporation of NPs in various chemo-photothermal therapies (Wang et al., 2017; Jiang et al., 2020).
Various preclinical and clinical studies have explored the potential of nanomedicines, including metallic NPs, in the CRC treatment. Drugs such as 5-fluorouracil (5-FU) and doxorubicin (DOX) have been incorporated into NP delivery systems to enhance the efficacy of the treatment of CRC. One example of a recent in vitro study using NPs was that of enhancing the antitumour efficacy in a CRC model, as reported by Jiang et al. (2020). In this study, DOX-resistant SW620/Ad300 cells treated with a nanocomposite based on mesoporous silica-coated gold nanorods (GNRs/mSiO2) loaded with DOX showed a higher toxicity compared to the free DOX treatment, the reason being the increased intracellular DOX accumulation achieved by the NP drug carrier. Apart from biocompatibility, the GNRs exhibit a highly efficient photothermal conversion, which enabled the chemo-photothermal therapy on CRC cells through functionalization with anticancer drugs (Zhou et al., 2017). In another study, manganese-based (Mn) NPs stabilized by l-arginine were developed as a nanocarrier system to deliver 5-FU to colon cancer cell lines (Jain et al., 2020). In that case, cell viability was significantly reduced after the treatment with 5-FU-loaded Mn-based NPs as compared to the treatment with free 5-FU, being twice lower in the former specimen group than in the latter.
Previously, CRLX101, a 30–40 nm NP consisting of camptothecin (CPT), a topoisomerase 1 (topo-1) and hypoxia-inducible factor 1-alpha (HIF-1α) inhibitor, conjugated to cyclodextrin and polyethylene glycol (PEG) was designed to enhance the CPT efficacy against CRC (Weiss et al., 2013). Promising findings of CRLX101 in a human-phase trial with advanced solid tumors has led to further studies in other in vitro and in vivo systems to support or complement the ongoing clinical trials. Nonetheless, the local recurrence of tumors has been a major concern in these tumor treatments. Thus, CRLX101 with 5-FU incorporation was investigated in a subcutaneous mouse xenograft model of CRC following radiotherapy (Tian et al., 2017). It was reported that the nano-formulation had the highest therapeutic effect by eradicating tumor repopulation after chemoradiotherapy. Meanwhile, a comparative study involving the use of gold-based NPs in a radio-iodide cancer treatment reported that iodine-131 alone reduced the tumor growth by 15%, while the incorporation of iodine-131 inside the NPs reduced the tumors by 50%, suggesting that functionalized NPs may open up a new venue for radiopharmaceutical therapies in cancer management (Le Goas et al., 2019).
The branch of nanomedicine is emerging into the biomimetic NPs, particularly cell membrane-based NPs to avoid the immune system clearance and, thereby, increase their therapeutic effects. This biomimetic approach mimics the native cells to mediate the interactions at the nano-bio interface as the fate of NPs in vivo is governed by their physicochemical surface (Sushnitha et al., 2020). The presence of natural cell membrane coatings to camouflage NPs offers the ability to remain the NPs in circulation and eventually to achieve targeted delivery. Red blood cell (RBC) membrane-coated NPs have been one of the most studied cell membrane-based nanocarriers in drug targeting delivery to enhance tumor cell death. Fabricating NPs with RBC membrane suited in vivo circulation due to the expression of CD47 protein on their surface to ensure self-recognition by the RES to allow longer circulation (Liu et al., 2019). In addition, RBCs have limited nuclei and are easily isolated from the blood donor (Zhang et al., 2017). Poly(lactic-co-glycolic acid) (PLGA) NPs loaded with gambogic acid (GA) possessed antitumour potential when covered with RBC membrane obtained by BALB/c mice. They found that RBC membrane-GA/PLGA NPs showed significant inhibition of tumor growth in CRC in vivo model via necrosis. Comparing GA toxicity with RBC membrane-coated NPs to the free GA without NPs, they observed the higher median survival rate recorded at 30 days compared to the free-GA group that lasted for 4 days only. It should be noted that, RBC membrane-coated NPs also improved the poor aqueous solubility of GA, just as with the poor water solubility of the available chemotherapy drug in the market such as paclitaxel.
Still, to date, limited numbers of clinical trials have been conducted on CRC treatments using nanomedicine. The most prominent NPs used in these studies have been polymer-based. For instance, a phase I clinical study using PEGylated liposomal mitomycin C (NCT01705002) and PEG conjugate of SN38 (NCT00931840) showed that both nano-formulations were well-tolerated at higher doses compared to the free antitumour drugs (Norris et al., 2014; Golan et al., 2015). Nevertheless, further studies are warranted to evaluate therapeutic efficacies of nanomedicine formulations in CRC management. However, to our knowledge, no clinical trials using metallic NPs in CRC treatments have been reported yet. Still, considering the number of promising preclinical findings, the metallic NPs are expected to be utilized in human-phase trials with CRC patients in due time.
Advantages and Challenges in Nanomedicine
Nanomedicine aims to provide more efficient tools for prevention and treatment of various diseases as it possess potential advantages (Desai, 2012), and might someday provide answers to long lasting problems in medical research, such as poor drug solubility and lack of target specificity for therapeutic compounds (Bawa, 2011). This field give new perspectives for the biomedical area as well as clinical for prevention, diagnosis, and treatment of severe diseases such as cancer (Chow and Ho, 2013). Making remarkable enhancements in drug delivery systems (Butcher, Mortimer et al., 2016), medical imaging and diagnosis platforms have been accounted as nanotechnology effects in health and medicine (Bayford et al., 2017).
Biological Barrier
NPs impart a range of properties to drugs that they carry into a living organism. Due to specific characteristics such as efficient transport through capillary blood vessels, longer circulation duration, higher binding capacity to biomolecules, higher accumulation in target tissues, and reduced inflammatory and oxidative stress in tissue, various nanomedicines have been developed and commercially applied in clinical and non-clinical areas as their characteristics are differ from those of conventional medicine (Liu et al., 2011). This range of improved properties often translates to a greater therapeutic efficacy of nanomedicines than that of conventional medicines. These features also endow nanomedicines with lower toxicity, improved bioavailability, and enhanced pharmacokinetics compared to the conventional drug therapies. Thus, nanomedicines overcome obstacles that limit conventional drugs, and have the potential to meet future market demands for disease treatments (Wang et al., 2018).
Specific Surface Area
Namomedicines often exhibit greater therapeutic efficacy than conventional small molecular drugs due to their size, large specific surface area, and flexibility of surface functionalization. These features endow nanomedicines with low toxicity, improved bioavailability, and enhanced pharmacokinetics and therapeutics effect. Thus, nanomedicines overcome obstacles that limit conventional drugs, and have the potential to meet future market demands for disease treatments (Wang et al., 2018). Nanomedicine is used in versatile aspects of the disease management process, including diagnosis, treatment and monitoring (Sajja et al., 2009). In view of the ability of NP carriers to slow down the release of drugs and prolong the pharmacokinetic half-life for many of them, nanomedicine has a key role in the controlled delivery of drugs or other biological products to improve the human health.
Solubility and Permeability
NPs are also capable of ensuring the solubility and permeability of insoluble drugs across biological barriers. Drugs with low bioavailability can thus be delivered directly to the target site (Galvin et al., 2012). Moreover, the large surface area and a higher reactivity of nanomedicines may allow for the dose reduction of a drug, which can improve toxicity profiles and patient compliance (Bawa, 2011; Galvin et al., 2012). The large surface area of nanomedicines can also increase the dissolution rate, the saturation solubility, and the intracellular uptake of the drugs, thereby improving in vivo performance (Bawa, 2011). In addition, combining encapsulation, release modalities, and surface modifications to improve targeting or bioavailability could improve the therapeutic efficacy (Bharali and Mousa, 2010). Currently, 65% of nanomedicines undergoing clinical trials focus on cancer applications, as nanomedicine can offer great contributions for a better treatment and early diagnosis of this disease (Germain et al., 2020). In addition to the cancer treatments, nanomedicine has also been used to combat infectious, ophthalmic, neurological and other disease, as well as to facilitate gene editing, immunotherapies, and so on.
Challenges
The development of nanomedicines is growing rapidly; however, each of the nanomedicines has its own challenges such as safety, biological challenges, scale-up, cost, and regulation (Grenha, 2011). In addition, the development of nanomedicines requires massive preclinical research, carefully conducted clinical trials, and appropriate clinical indications (Etheridge et al., 2013). The drug development process takes years, and any mistakes or negative results may delay these process (Etheridge et al., 2013). Thus, to understand the current situation of nanomedicine, including common challenges and future demands for nanomedicines development (Moghimi et al., 2005) is important.
Specific and Precise Targeting
One of the highest priorities for the next generation of nanomedicines is to create precise and highly efficient drugs that accumulate in targeted pathological tissues and not effect the healthy tissue. There are many nanomedicines that cannot avoid damage to healthy tissues because they do not specifically target the pathological tissues. Recently, smart drug delivery systems that possess active targeting and stimuli responsiveness are actively being developed. These smart drug delivery systems represent a promising strategy to eliminate pathological tissues while protecting normal tissues (Zhang J. et al., 2020).
Safety and Quality
It is imperative to understand the toxicology of the drugs, the physicochemical properties of NPs, and the properties of nanocarriers since safety is the crucial issues for nanomedicines, even after a nanomedicine is approved for clinical use, it can be withdrawn from the market due to safety issues. In human body, nanomedicines get exposed to biological environments such as the blood, extracellular matrix, cytoplasm, and cellular organelles, which may alter their biological performance, but also elicit undesired toxicities. Currently, there is a lack of standardized methods for evaluation of the safety of nanomedicines. It should be emphasized that the methods used for traditional drugs cannot accurately evaluate the safety of nanomedicines (Wolfram et al., 2015). For example, drugs such as paclitaxel or doxorubicin, which have been extensively studied, still facing the complex toxicity issues when used with novel nanomedicines and nanocarriers (Hua et al., 2018). To increase the safety and quality, many countries and organizations have enacted and implemented good laboratory and manufacturing practices. It is important to formulate good laboratory practices for nanomedicines to improve the success rate and to promote the development of safe and effective nanomedicines (Zhang J. et al., 2020).
Biodistribution of Drugs
Other challenges involve the drug biodistribution and biological barrier penetration which aim to enhance the accumulation of the drug at the target site and reduced the accumulation in healthy tissues (Lu et al., 2021). Biodistribution is affected by interactions with external and internal biological barriers, for which reason the interaction between nanomedicines and biological barriers must be well understood (Elsabahy and Wooley, 2012). Furthermore, nanomedicines often do not directly interact with living cells, but instead become coated with a protein corona, which alters the biological effects of the NPs and influences the cell uptake, biodistribution, clearance, toxicity, and immune response. Therefore, it is important to also focus on the protein coronae formed around NPs and the resulting biological responses to nanomedicines (Mahmoudi et al., 2016).
Scale up, Cost and Regulations
Additionally, nanomedicines may fail to enter the market because unmeet the requirements of scale-up synthesis and reproducibility. In both laboratory or preclinical investigations and in clinical studies, nanomedicines have been mainly synthesized in small batches (Anselmo and Mitragotri, 2019). Not only the manufacturing cost of nanomedicines scale-up high, but the cost of preclinical and clinical development is also increasing. Acquiring regulatory approvals is difficult for new nanomedicines, especially when existing products on the market have the same target indication (Ventola, 2017). The lack of regulation and standards for nanomedicines in manufacturing practices, quality control, safety, and efficacy evaluation are all barriers for the development of nanomedicines. There are currently no global regulatory standards specific to clinical translation of nanotherapeutics. Only initial guidance documents for nanotechnology products have been issued by regulatory authorities such as the FDA to provide guidance. There are also significant differences between different geographical regions in addressing the application of nanomedicines, so that nanomedicines approved in one country may not be approved in other countries. Strengthening of regulatory standards is a major challenge but must be overcome to achieve efficient nanomedicine development (Mühlebach, 2018).
Conclusion
This review has highlighted the existing use of diverse nanomedicines for the diagnosis and treatment of CRC. Despite the fact that the field is young and growing, a number of studies have reported on how combining metallic NPs and multiple targeting strategies establishes conditions for the improved treatment and diagnosis of CRC. Although considerable challenges and issues remain to be addressed, the potential impact of nanomedicine on the diagnosis and treatment of CRC is extensively recognized. As discussed through several examples in this review, careful modification and characterization of metallic NPs is needed in order to exploit the potential impact of nanomedicine on the clinical management of CRC and advance its use in the CRC treatment and early diagnosis.
Author Contributions
KP, SA, MM-Z, and WNWH wrote this paper. ZI, J-LS, KF, VU, JJ, and RM supervised this work and revised the manuscript. All authors contributed to the paper and approved the submitted version.
Funding
This work was supported by Universiti Sains Malaysia Research University Grant (Grant number: 1001/PPSP/8012294).
Conflict of Interest
Author VU was employed by the company TardigradeNano LLC.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Abu Hassan, M. R., Ismail, I., Mohd Suan, M. A., Ahmad, F., Wan Khazim, W. K., Othman, Z., et al. (2016). Incidence and Mortality Rates of Colorectal Cancer in Malaysia. Epidemiol. Health 38, e2016007. doi:10.4178/epih.e2016007
Agans, R. T., Gordon, A., Hussain, S., and Paliy, O. (2019). Titanium Dioxide Nanoparticles Elicit Lower Direct Inhibitory Effect on Human Gut Microbiota Than Silver Nanoparticles. Toxicol. Sci. 172 (2), 411–416. doi:10.1093/toxsci/kfz183
Allen-Vercoe, E., Strauss, J., and Chadee, K. (2011). Fusobacterium Nucleatum. Gut Microbes 2 (5), 294–298. doi:10.4161/gmic.2.5.18603
Anselmo, A. C., and Mitragotri, S. (2019). Nanoparticles in the Clinic: An Update. Bioeng. Transl Med. 4 (3), e10143. doi:10.1002/btm2.10143
Bawa, R. (2011). Regulating Nanomedicine - Can the FDA Handle it?. Cdd 8 (3), 227–234. doi:10.2174/156720111795256156
Bayford, R., Rademacher, T., Roitt, I., and Wang, S. X. (2017). Emerging Applications of Nanotechnology for Diagnosis and Therapy of Disease: a Review. Physiol. Meas. 38 (8), R183–r203. doi:10.1088/1361-6579/aa7182
Bharali, D. J., and Mousa, S. A. (2010). Emerging Nanomedicines for Early Cancer Detection and Improved Treatment: Current Perspective and Future Promise. Pharmacol. Ther. 128 (2), 324–335. doi:10.1016/j.pharmthera.2010.07.007
Brennan, C. A., and Garrett, W. S. (2019). Fusobacterium Nucleatum - Symbiont, Opportunist and Oncobacterium. Nat. Rev. Microbiol. 17 (3), 156–166. doi:10.1038/s41579-018-0129-6
Butcher, N. J., Mortimer, G. M., and Minchin, R. F. (2016). Unravelling the Stealth Effect. Nat. Nanotech 11 (4), 310–311. doi:10.1038/nnano.2016.6
Chow, E. K.-H., and Ho, D. (2013). Cancer Nanomedicine: from Drug Delivery to Imaging. Sci. Translational Med. 5 (216), 216rv4. doi:10.1126/scitranslmed.3005872
Dahiya, D. K., Renuka, , and Puniya, A. K. (2018). Impact of Nanosilver on Gut Microbiota: a Vulnerable Link. Future Microbiol. 13, 483–492. doi:10.2217/fmb-2017-0103
de Almeida, C. V., Taddei, A., and Amedei, A. (2018). The Controversial Role of Enterococcus faecalis in Colorectal Cancer. Ther. Adv. Gastroenterol. 11, 1756284818783606. doi:10.1177/1756284818783606
Delmas, J., and Bonnet, R. (2015). Escherichia coli: The Good, the Bad and the Ugly. J. Clin. Mirobiology 4 (2), 1–3. doi:10.4172/2327-5073.1000195
Desai, N. (2012). Challenges in Development of Nanoparticle-Based Therapeutics. Aaps j 14 (2), 282–295. doi:10.1208/s12248-012-9339-4
Dieterich, W., Schink, M., and Zopf, Y. (2018). Microbiota in the Gastrointestinal Tract. Med. Sci. 6 (4), 116. doi:10.3390/medsci6040116
Elsabahy, M., and Wooley, K. L. (2012). Design of Polymeric Nanoparticles for Biomedical Delivery Applications. Chem. Soc. Rev. 41 (7), 2545–2561. doi:10.1039/c2cs15327k
Etheridge, M. L., Campbell, S. A., Erdman, A. G., Haynes, C. L., Wolf, S. M., and McCullough, J. (2013). The Big Picture on Nanomedicine: the State of Investigational and Approved Nanomedicine Products. Nanomedicine: Nanotechnology, Biol. Med. 9 (1), 1–14. doi:10.1016/j.nano.2012.05.013
Fan, X., Yuan, Z., Shou, C., Fan, G., Wang, H., Gao, F., et al. (2019). cRGD-Conjugated Fe3O4@PDA-DOX Multifunctional Nanocomposites for MRI and Antitumor Chemo-Photothermal Therapy. Ijn 14, 9631–9645. doi:10.2147/ijn.s222797
Fiore, E., Van Tyne, D., and Gilmore, M. S. (2019). Pathogenicity of Enterococci. Microbiol. Spectr. 7 (4). doi:10.1128/microbiolspec.GPP1123-0053-201810.1128/microbiolspec.gpp3-0053-2018
Galvin, P., Thompson, D., Ryan, K. B., McCarthy, A., Moore, A. C., Burke, C. S., et al. (2012). Nanoparticle-based Drug Delivery: Case Studies for Cancer and Cardiovascular Applications. Cell. Mol. Life Sci. 69 (3), 389–404. doi:10.1007/s00018-011-0856-6
Gangadoo, S., Bauer, B. W., Bajagai, Y. S., Van, T. T. H., Moore, R. J., and Stanley, D. (2019). In Vitro growth of Gut Microbiota with Selenium Nanoparticles. Anim. Nutr. 5 (4), 424–431. doi:10.1016/j.aninu.2019.06.004
Germain, M., Caputo, F., Metcalfe, S., Tosi, G., Spring, K., Åslund, A. K. O., et al. (2020). Delivering the Power of Nanomedicine to Patients Today. J. Controlled Release 326, 164–171. doi:10.1016/j.jconrel.2020.07.007
Golan, T., Grenader, T., Ohana, P., Amitay, Y., Shmeeda, H., La‐Beck, N. M., et al. (2015). Pegylated Liposomal Mitomycin C Prodrug Enhances Tolerance of Mitomycin C: a Phase 1 Study in Advanced Solid Tumor Patients. Cancer Med. 4 (10), 1472–1483. doi:10.1002/cam4.491
Grenha, A. (2011). The Era of Nanomedicine. J. Pharm. Bioall Sci. 3 (2), 181. doi:10.4103/0975-7406.80757
Han, Y. W. (2015). Fusobacterium Nucleatum: a Commensal-Turned Pathogen. Curr. Opin. Microbiol. 23, 141–147. doi:10.1016/j.mib.2014.11.013
Hua, S., de Matos, M. B. C., Metselaar, J. M., and Storm, G. (2018). Current Trends and Challenges in the Clinical Translation of Nanoparticulate Nanomedicines: Pathways for Translational Development and Commercialization. Front. Pharmacol. 9, 790. doi:10.3389/fphar.2018.00790
Inamura, K. (2021). Gut Microbiota Contributes towards Immunomodulation against Cancer: New Frontiers in Precision Cancer Therapeutics. Semin. Cancer Biol. 70, 11–23. doi:10.1016/j.semcancer.2020.06.006
Iyadorai, T., Mariappan, V., Vellasamy, K. M., Wanyiri, J. W., Roslani, A. C., Lee, G. K., et al. (2020). Prevalence and Association of Pks+ Escherichia coli with Colorectal Cancer in Patients at the University Malaya Medical Centre, Malaysia. PLOS ONE 15 (1), e0228217. doi:10.1371/journal.pone.0228217
Jain, P., Patel, K., Jangid, A. K., Guleria, A., Patel, S., Pooja, D., et al. (2020). Modulating the Delivery of 5-Fluorouracil to Human Colon Cancer Cells Using Multifunctional Arginine-Coated Manganese Oxide Nanocuboids with MRI Properties. ACS Appl. Bio Mater. 3 (10), 6852–6864. doi:10.1021/acsabm.0c00780
Jandhyala, S. M., Talukdar, R., Subramanyam, C., Vuyyuru, H., Sasikala, M., and Nageshwar Reddy, D. (2015). Role of the normal Gut Microbiota. Wjg 21 (29), 8787–8803. doi:10.3748/wjg.v21.i29.8787
Jiang, Y., Guo, Z., Fang, J., Wang, B., Lin, Z., Chen, Z.-S., et al. (2020). A Multi-Functionalized Nanocomposite Constructed by Gold Nanorod Core with Triple-Layer Coating to Combat Multidrug Resistant Colorectal Cancer. Mater. Sci. Eng. C 107, 110224. doi:10.1016/j.msec.2019.110224
Juan, L., Yang, S., Lei, R., Gu, W., Qin, Y., Ma, S., et al. (2018). Oral Administration of Rutile and Anatase TiO2 Nanoparticles Shifts Mouse Gut Microbiota Structure. Nanoscale 10 (16), 7736–7745. doi:10.1039/c8nr00386f
Kaper, J. B., Nataro, J. P., and Mobley, H. L. T. (2004). Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2 (2), 123–140. doi:10.1038/nrmicro818
Kasprzak, A., and Szaflarski, W. (2020). Role of Alternatively Spliced Messenger RNA (mRNA) Isoforms of the Insulin-like Growth Factor 1 (IGF1) in Selected Human Tumors. Ijms 21 (19), 6995. doi:10.3390/ijms21196995
Kong, F., and Cai, Y. (2019). Study Insights into Gastrointestinal Cancer through the Gut Microbiota. Biomed. Res. Int. 2019, 8721503. doi:10.1155/2019/8721503
Kumagai, M., Sarma, T. K., Cabral, H., Kaida, S., Sekino, M., Herlambang, N., et al. (2010). Enhanced In Vivo Magnetic Resonance Imaging of Tumors by PEGylated Iron-Oxide-Gold Core-Shell Nanoparticles with Prolonged Blood Circulation Properties. Macromol. Rapid Commun. 31 (17), 1521–1528. doi:10.1002/marc.201000341
Lamas, B., Martins Breyner, N., and Houdeau, E. (2020). Impacts of Foodborne Inorganic Nanoparticles on the Gut Microbiota-Immune axis: Potential Consequences for Host Health. Part. Fibre Toxicol. 17 (1), 19. doi:10.1186/s12989-020-00349-z
Le Goas, M., Paquet, M., Paquirissamy, A., Guglielmi, J., Compin, C., Thariat, J., et al. (2019). Improving 131I Radioiodine Therapy by Hybrid Polymer-Grafted Gold Nanoparticles. Ijn 14, 7933–7946. doi:10.2147/ijn.s211496
Lee, H., Lee, Y., Song, C., Cho, H. R., Ghaffari, R., Choi, T. K., et al. (2015). An Endoscope with Integrated Transparent Bioelectronics and Theranostic Nanoparticles for colon Cancer Treatment. Nat. Commun. 6, 10059. doi:10.1038/ncomms10059
Li, J., Lei, R., Li, X., Xiong, F., Zhang, Q., Zhou, Y., et al. (2018). The Antihyperlipidemic Effects of Fullerenol Nanoparticles via Adjusting the Gut Microbiota In Vivo. Part. Fibre Toxicol. 15 (1), 5. doi:10.1186/s12989-018-0241-9
Liu, W., Yang, X.-L., and Winston Ho, W. S. (2011). Preparation of Uniform-Sized Multiple Emulsions and Micro/nano Particulates for Drug Delivery by Membrane Emulsification. J. Pharm. Sci. 100 (1), 75–93. doi:10.1002/jps.22272
Liu, Y., Luo, J., Chen, X., Liu, W., and Chen, T. (2019). Cell Membrane Coating Technology: A Promising Strategy for Biomedical Applications. Nano-Micro Lett. 11 (1), 100. doi:10.1007/s40820-019-0330-9
Lu, W., Yao, J., Zhu, X., and Qi, Y. (2021). Nanomedicines: Redefining Traditional Medicine. Biomed. Pharmacother. 134, 111103. doi:10.1016/j.biopha.2020.111103
Mahmoudi, M., Bertrand, N., Zope, H., and Farokhzad, O. C. (2016). Emerging Understanding of the Protein corona at the Nano-Bio Interfaces. Nano Today 11 (6), 817–832. doi:10.1016/j.nantod.2016.10.005
Mao, Z., Li, Y., Dong, T., Zhang, L., Zhang, Y., Li, S., et al. (2019). Exposure to Titanium Dioxide Nanoparticles during Pregnancy Changed Maternal Gut Microbiota and Increased Blood Glucose of Rat. Nanoscale Res. Lett. 14 (1), 26. doi:10.1186/s11671-018-2834-5
Matsumura, Y., and Maeda, H. (1986). A New Concept for Macromolecular Therapeutics in Cancer Chemotherapy: Mechanism of Tumoritropic Accumulation of Proteins and the Antitumor Agent Smancs. Cancer Res. 46 (12 Pt 1), 6387–6392.
Meijnikman, A. S., Gerdes, V. E., Nieuwdorp, M., and Herrema, H. (2018). Evaluating Causality of Gut Microbiota in Obesity and Diabetes in Humans. Endocr. Rev. 39 (2), 133–153. doi:10.1210/er.2017-00192
Moghimi, S. M., Hunter, A. C., and Murray, J. C. (2005). Nanomedicine: Current Status and Future Prospects. FASEB j. 19 (3), 311–330. doi:10.1096/fj.04-2747rev
Mühlebach, S. (2018). Regulatory Challenges of Nanomedicines and Their Follow-On Versions: A Generic or Similar Approach? Adv. Drug Deliv. Rev. 131, 122–131. doi:10.1016/j.addr.2018.06.024
Nerad, E., Lambregts, D. M. J., Kersten, E. L. J., Maas, M., Bakers, F. C. H., van den Bosch, H. C. M., et al. (2017). MRI for Local Staging of Colon Cancer: Can MRI Become the Optimal Staging Modality for Patients with Colon Cancer? Dis. Colon Rectum 60 (4), 385–392. doi:10.1097/dcr.0000000000000794
Ni, J., Wu, G. D., Albenberg, L., and Tomov, V. T. (2017). Gut Microbiota and IBD: Causation or Correlation? Nat. Rev. Gastroenterol. Hepatol. 14 (10), 573–584. doi:10.1038/nrgastro.2017.88
Norris, R. E., Shusterman, S., Gore, L., Muscal, J. A., Macy, M. E., Fox, E., et al. (2014). Phase 1 Evaluation of EZN-2208, a Polyethylene Glycol Conjugate of SN38, in Children Adolescents and Young Adults with Relapsed or Refractory Solid Tumors. Pediatr. Blood Cancer 61 (10), 1792–1797. doi:10.1002/pbc.25105
Ohno, M., Nishida, A., Sugitani, Y., Nishino, K., Inatomi, O., Sugimoto, M., et al. (2017). Nanoparticle Curcumin Ameliorates Experimental Colitis via Modulation of Gut Microbiota and Induction of Regulatory T Cells. PLoS One 12 (10), e0185999. doi:10.1371/journal.pone.0185999
Pearce, A., Haas, M., Viney, R., Pearson, S. A., Haywood, P., Brown, C., et al. (2017). Incidence and Severity of Self-Reported Chemotherapy Side Effects in Routine Care: A Prospective Cohort Study. PLoS One 12 (10), e0184360. doi:10.1371/journal.pone.0184360
Peng, G., Hakim, M., Broza, Y. Y., Billan, S., Abdah-Bortnyak, R., Kuten, A., et al. (2010). Detection of Lung, Breast, Colorectal, and Prostate Cancers from Exhaled Breath Using a Single Array of Nanosensors. Br. J. Cancer 103 (4), 542–551. doi:10.1038/sj.bjc.6605810
Purcell, R. V., Pearson, J., Aitchison, A., Dixon, L., Frizelle, F. A., and Keenan, J. I. (2017). Colonization with Enterotoxigenic Bacteroides Fragilis Is Associated with Early-Stage Colorectal Neoplasia. PLOS ONE 12 (2), e0171602. doi:10.1371/journal.pone.0171602
Quigley, E. M. (2013). Gut Bacteria in Health and Disease. Gastroenterol. Hepatol. (N Y) 9 (9), 560–569.
Riaz Rajoka, M. S., Mehwish, H. M., Xiong, Y., Song, X., Hussain, N., Zhu, Q., et al. (2021). Gut Microbiota Targeted Nanomedicine for Cancer Therapy: Challenges and Future Considerations. Trends Food Sci. Tech. 107, 240–251. doi:10.1016/j.tifs.2020.10.036
Richard, M. L., Liguori, G., Lamas, B., Brandi, G., da Costa, G., Hoffmann, T. W., et al. (2018). Mucosa-associated Microbiota Dysbiosis in Colitis Associated Cancer. Gut Microbes 9 (2), 131–142. doi:10.1080/19490976.2017.1379637
Saini, R., Saini, S., and Sharma, S. (2010). Nanotechnology: the Future Medicine. J. Cutan. Aesthet. Surg. 3 (1), 32–33. doi:10.4103/0974-2077.63301
Sajja, H., East, M., Mao, H., Wang, Y., Nie, S., and Yang, L. (2009). Development of Multifunctional Nanoparticles for Targeted Drug Delivery and Noninvasive Imaging of Therapeutic Effect. Cddt 6 (1), 43–51. doi:10.2174/157016309787581066
Saus, E., Iraola-Guzmán, S., Willis, J. R., Brunet-Vega, A., and Gabaldón, T. (2019). Microbiome and Colorectal Cancer: Roles in Carcinogenesis and Clinical Potential. Mol. Aspects Med. 69, 93–106. doi:10.1016/j.mam.2019.05.001
Sears, C. L., and Garrett, W. S. (2014). Microbes, Microbiota, and colon Cancer. Cell Host & Microbe 15 (3), 317–328. doi:10.1016/j.chom.2014.02.007
Sears, C. L., Geis, A. L., and Housseau, F. (2014). Bacteroides Fragilis Subverts Mucosal Biology: from Symbiont to colon Carcinogenesis. J. Clin. Invest. 124 (10), 4166–4172. doi:10.1172/jci72334
Sekirov, I., Russell, S. L., Antunes, L. C. M., and Finlay, B. B. (2010). Gut Microbiota in Health and Disease. Physiol. Rev. 90 (3), 859–904. doi:10.1152/physrev.00045.2009
Siegel, R. L., Miller, K. D., Goding Sauer, A., Fedewa, S. A., Butterly, L. F., Anderson, J. C., et al. (2020). Colorectal Cancer Statistics, 2020. CA A. Cancer J. Clina Cancer J. Clinicians 70 (3), 145–164. doi:10.3322/caac.21601
Sushnitha, M., Evangelopoulos, M., Tasciotti, E., and Taraballi, F. (2020). Cell Membrane-Based Biomimetic Nanoparticles and the Immune System: Immunomodulatory Interactions to Therapeutic Applications. Front. Bioeng. Biotechnol. 8, 627. doi:10.3389/fbioe.2020.00627
Teng, Y., Ren, Y., Sayed, M., Hu, X., Lei, C., Kumar, A., et al. (2018). Plant-Derived Exosomal MicroRNAs Shape the Gut Microbiota. Cell Host & Microbe 24 (5), 637–652. e638. doi:10.1016/j.chom.2018.10.001
Tian, X., Nguyen, M., Foote, H. P., Caster, J. M., Roche, K. C., Peters, C. G., et al. (2017). CRLX101, a Nanoparticle-Drug Conjugate Containing Camptothecin, Improves Rectal Cancer Chemoradiotherapy by Inhibiting DNA Repair and HIF1α. Cancer Res. 77 (1), 112–122. doi:10.1158/0008-5472.can-15-2951
Tong, L., Hao, H., Zhang, X., Zhang, Z., Lv, Y., Zhang, L., et al. (2020). Oral Administration of Bovine Milk‐Derived Extracellular Vesicles Alters the Gut Microbiota and Enhances Intestinal Immunity in Mice. Mol. Nutr. Food Res. 64 (8), 1901251. doi:10.1002/mnfr.201901251
Ventola, C. L. (2017). Progress in Nanomedicine: Approved and Investigational Nanodrugs. P T 42 (12), 742–755.
Wang, L., Yan, L., Liu, J., Chen, C., and Zhao, Y. (2018). Quantification of Nanomaterial/Nanomedicine Trafficking In Vivo. Anal. Chem. 90 (1), 589–614. doi:10.1021/acs.analchem.7b04765
Wang, Y., Zhang, Z., Xu, S., Wang, F., Shen, Y., Huang, S., et al. (2017). pH, Redox and Photothermal Tri-responsive DNA/polyethylenimine Conjugated Gold Nanorods as Nanocarriers for Specific Intracellular Co-release of Doxorubicin and Chemosensitizer Pyronaridine to Combat Multidrug Resistant Cancer. Nanomedicine: Nanotechnology, Biol. Med. 13 (5), 1785–1795. doi:10.1016/j.nano.2017.01.014
Wassenaar, T. M. (2018). E. coli and Colorectal Cancer: a Complex Relationship that Deserves a Critical Mindset. Crit. Rev. Microbiol. 44 (5), 619–632. doi:10.1080/1040841x.2018.1481013
Weiss, G. J., Chao, J., Neidhart, J. D., Ramanathan, R. K., Bassett, D., Neidhart, J. A., et al. (2013). First-in-human Phase 1/2a Trial of CRLX101, a Cyclodextrin-Containing Polymer-Camptothecin Nanopharmaceutical in Patients with Advanced Solid Tumor Malignancies. Invest. New Drugs 31 (4), 986–1000. doi:10.1007/s10637-012-9921-8
Werner, J., and Heinemann, V. (2016). Standards and Challenges of Care for Colorectal Cancer Today. Visc. Med. 32 (3), 156–157. doi:10.1159/000447070
Wexler, H. M. (2007). Bacteroides : the Good, the Bad, and the Nitty-Gritty. Clin. Microbiol. Rev. 20 (4), 593–621. doi:10.1128/cmr.00008-07
Wick, E. C., and Sears, C. L. (2010). Bacteroides Spp. And Diarrhea. Curr. Opin. Infect. Dis. 23 (5), 470–474. doi:10.1097/qco.0b013e32833da1eb
Wolfram, J., Zhu, M., Yang, Y., Shen, J., Gentile, E., Paolino, D., et al. (2015). Safety of Nanoparticles in Medicine. Cdt 16 (14), 1671–1681. doi:10.2174/1389450115666140804124808
Xu, Z.-q., Broza, Y. Y., Ionsecu, R., Tisch, U., Ding, L., Liu, H., et al. (2013). A Nanomaterial-Based Breath Test for Distinguishing Gastric Cancer from Benign Gastric Conditions. Br. J. Cancer 108 (4), 941–950. doi:10.1038/bjc.2013.44
Zhang, C., Yan, L., Wang, X., Zhu, S., Chen, C., Gu, Z., et al. (2020a). Progress, Challenges, and Future of Nanomedicine. Nano Today 35, 101008. doi:10.1016/j.nantod.2020.101008
Zhang, J., Chen, Z., Kong, J., Liang, Y., Chen, K., Chang, Y., et al. (2020b). Fullerenol Nanoparticles Eradicate Helicobacter pylori via pH-Responsive Peroxidase Activity. ACS Appl. Mater. Inter 12 (26), 29013–29023. doi:10.1021/acsami.0c05509
Zhang, Z., Qian, H., Yang, M., Li, R., Hu, J., Li, L., et al. (2017). Gambogic Acid-Loaded Biomimetic Nanoparticles in Colorectal Cancer Treatment. Ijn 12, 1593–1605. doi:10.2147/ijn.s127256
Zhangjian, C., Han, S., Zhou, D., and Jia, S. (2019). Effects of Oral Exposure to Titanium Dioxide Nanoparticles on Gut Microbiota and Gut-Associated Metabolism In Vivo. Nanoscale 11 (46), 22398–22412. doi:10.1039/c9nr07580a
Zheng, D., Liwinski, T., and Elinav, E. (2020). Interaction between Microbiota and Immunity in Health and Disease. Cell Res 30 (6), 492–506. doi:10.1038/s41422-020-0332-7
Keywords: dysbiosis, microbiota, nanoparticle, immunomodulation, colorectal cancer
Citation: Perumal K, Ahmad S, Mohd-Zahid MH, Wan Hanaffi WN, Z.A. I, Six J-L, Ferji K, Jaafar J, Boer JC, Plebanski M, Uskoković V and Mohamud R (2021) Nanoparticles and Gut Microbiota in Colorectal Cancer. Front. Nanotechnol. 3:681760. doi: 10.3389/fnano.2021.681760
Received: 17 March 2021; Accepted: 31 May 2021;
Published: 21 July 2021.
Edited by:
Zhi Ping Xu, The University of Queensland, AustraliaReviewed by:
Qin Jiang, Donghua University, ChinaBuddolla Viswanath, Dr. Buddolla's Institute of Life Sciences, India
Copyright © 2021 Perumal, Ahmad, Mohd-Zahid, Wan Hanaffi, Z.A., Six, Ferji, Jaafar, Boer, Plebanski, Uskoković and Mohamud. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rohimah Mohamud, cm9oaW1haG1AdXNtLm15
 Komathi Perumal
Komathi Perumal Suhana Ahmad
Suhana Ahmad Manali Haniti Mohd-Zahid
Manali Haniti Mohd-Zahid Wan Nurhidayah Wan Hanaffi
Wan Nurhidayah Wan Hanaffi Iskander Z.A.
Iskander Z.A. Jean-Luc Six3
Jean-Luc Six3 Khalid Ferji
Khalid Ferji Jennifer C. Boer
Jennifer C. Boer Vuk Uskoković
Vuk Uskoković Rohimah Mohamud
Rohimah Mohamud