- 1Department of Biotechnology, St. Joseph’s College of Engineering, Chennai, India
- 2School of Chemical Engineering, Jeonbuk National University, Jeonju, South Korea
- 3School of Semiconductor and Chemical Engineering, Jeonbuk National University, Jeonju, South Korea
In the present work, the biodiesel was produced from waste cooking oil (WCO) using heterogeneous zinc doped iron nanocatalyst and tetrabutylammonium iodide (TBAI) as co-catalyst. The heterogeneous zinc doped iron nanocatalyst was synthesized and characterized. The functional group in the heterogeneous nanocatalyst was confirmed using FTIR analysis, the crystalline nature was studied by XRD analysis, and the size and structure of the nanocatalyst were analyzed by SEM. The optimization of transesterification parameters like oil to methanol molar ratio, zinc doped iron concentration, TBAI concentration, temperature, and time were carried out for the maximum conversion of biodiesel from WCO. At 50 min the maximum biodiesel conversion of 90% was achieved at 55°C with 12% catalyst, 30% co-catalyst, and 1:11 WCO to methanol ratio. The presence of functional groups and the methyl ester composition of the biodiesel from WCO were confirmed by FTIR and GC-MS analysis. The use of zinc doped iron nanocatalyst with TBAI showed good catalytic activity to produce biodiesel from WCO.
Introduction
The constant increase in the world energy demand along with the consumption of non-renewable energy sources resulted in the depletion of fossil fuel energy (Cavalcante et al., 2021). The use of fossil fuel sources causes more air pollution and global warming compared to others. So as a renewable energy source, biofuels were produced as an alternative to conventional petroleum. Renewable biofuel should be low cost, readily available, and eco-friendly (Ong et al., 2019). Compared to other biofuels, biodiesel and bioethanol are the alternative sources of renewable fuel for transportation sectors. Biodiesel can be directly used, or it can be blended and used as petrodiesel for diesel engines. The burning of biodiesel leads to less global warming compared to fossil fuels (Hosseinzadeh-Bandbafha et al., 2018). Advantages of biodiesel are high combustion efficiency, biodegradable, low sulfur content, and high cetane number and flash point. This has drawn the attention of researchers to produce in high quantity (Demirbas, 2007). The BD was considered as the clean energy source, which protects the environment by reducing the direct and indirect release of greenhouse gases like CO2, CO, SO2, and HC (Mathew et al., 2021).
Biodiesel can be produced from the feedstocks that contain triglycerides (TGs) (Ambat et al., 2018). The first-generation feedstocks are sunflower oil (Jalalmanesh et al., 2021), palm oil (Zulqarnain et al., 2021), coconut oil (Bambase et al., 2021), peanut oil (Shalmashi and Khodadadi, 2019), and soyabean oil (Huang et al., 2021). Second generation feedstocks are linseed oil (Balamurugan et al., 2021), rubber seed oil (Uzoh et al., 2021), Jatropha curcas oil (Yusuff et al., 2021), and castor oil (Naveenkumar and Baskar, 2021). Third generation feedstocks like waste cooking oil (Guo et al., 2021; Naeem et al., 2021), chicken fat (Kirubakaran and Arul Mozhi Selvan, 2021), vegetable oil refinery waste (Alishahi et al., 2021), and algal oil (Patle et al., 2021) are used for biodiesel production. Around 70% of the production cost of biodiesel is from feedstock (Zulqarnain et al., 2021). The feedstock selection based on free fatty acid is a significant role in biodiesel production (Ming et al., 2018). BD can be produced in four different methods such as blending vegetable oil and diesel, microemulsion, pyrolysis, esterification, and transesterification. The BD produced through esterification and transesterification reaction was low viscosity and of better quality compared to other methods of production (Liu et al., 2021).
The transesterification process is the reaction between feedstock oil and alcohol that results in the formation of BD and glycerol (Qu et al., 2021). The use of third-generation feedstock like WCO for biodiesel production is the eco-friendly recycling process because it reduces pollution during its improper disposal. For transesterification reaction short-chain alcohol and suitable base catalyst like homogeneous or heterogeneous catalysts are used. The use of the homogenous catalyst for biodiesel synthesis has some drawbacks. It reduces the purity of biodiesel, glycerol yield, generates more wastewater during washing, and the impossibility of catalyst reuse during the purification step (Degfie et al., 2019). Heterogenous solid catalysts were highly used by researchers for their advantages in biodiesel production (Rabie et al., 2019). Catalyst reusability, high stability, high-quality biodiesel, non-toxic, and easy separation of catalyst are the main advantages of heterogenous catalyst (Baskar and Aiswarya, 2016; Jamil et al., 2020). Methanol and ethanol are the commonly used alcohols, in this methanol is widely used because of its chemical advantage (Pugazhendhi et al., 2020).
The biodiesel was produced from WCO using diatomite supported by CaO/MgO nanocomposite and obtained the maximum biodiesel yield of 96.47% under the optimized conditions of 1:15 WCO to methanol molar ratio, 6% catalyst concentration at the reaction temperature of 90°C for time 120 min (Rabie et al., 2019). The heterogeneous catalyst, 12% (w/w) Cu doped ZnO nanocomposite was used for the maximum biodiesel conversion of 97.71% achieved from WCO under the optimized conditions such as oil to methanol ratio 1:8 for 50 min at 55°C. The high biodiesel yield can be achieved by the presence of a huge surface in the catalyst and reused up to five cycles without degradation (Gurunathan and Ravi, 2015).
Nowadays, ionic liquids (ILs) are used for eco-friendly biodiesel production (Piemonte et al., 2016). Compared to the conventional homogeneous and heterogeneous catalyst, the ILs catalyst has high dissolving capacity, immiscibility with organic solvents, high chemical stability, and recyclability (Maciel et al., 2019). The use of ILs reduces the reaction and purification steps and increases the economic feasibility (Muhammad et al., 2015). For transesterification reactions, ILs are used as a catalyst. The ILs are a heat stable, non-flammable organic salts solution that contains both cations and anions, so it is used for eco-friendly biodiesel production (Ullah et al., 2017). The physicochemical properties like viscosity, melting point, density, conductivity, and the refractive index can be indirectly affected by the cations and anions of ILs. Based on the physicochemical properties, the ILs are divided into two categories such as acid ionic liquids (AILs) and basic ionic liquids (BILs) (Deshmukh et al., 2019). Due to the high cost, difficulty in product separation, and problems in catalyst recovery, the ILs were used along with the conventional catalyst to improve its catalytic performance for the BD production (Ong et al., 2021). ILs can influence the catalytic activity in biodiesel conversion. Tetrabutylammonium iodide reagent (TBAI) is highly soluble in methanol and has low solubility in ethyl acetate so it can be completely recovered and reused (Casiello et al., 2019). The 96% of biodiesel conversion was obtained from WCO using solid acid catalyst SO4/Fe-Al-TiO2 of 3%, methanol to oil ratio 10:1 at 90°C for the reaction time of 2.5 h (Gardy et al., 2018). SO4/Fe-Al-TiO2 catalyst can be reused up to 10 cycles for transesterification reaction. The application of ZnO/TBAI as a catalyst was used for biodiesel production from various sources of oil. The ZnO/TBAI catalyst is efficient for high yield of biodiesel in one step from WCO (Casiello et al., 2019). The zinc doped iron nanocatalyst and TBAI as co-catalyst were efficient in converting maximum production of BD from WCO. The high purity glycerol was removed from the reaction mixture by water washing. TBAI can be easily recovered due to its low solubility in methanol. A novelty of the work is to prepare the zinc doped iron nanocatalyst and TBAI as co-catalyst, which is used for biodiesel production from waste cooking oil and optimize the best condition.
In the present work, the zinc doped iron nanocatalyst and TBAI as co-catalyst were successfully used for biodiesel synthesis from WCO. The influence of various parameters like oil to methanol molar ratio, amount of catalyst, co-catalyst loading, reaction temperature, and time were optimized for maximum biodiesel conversion through the transesterification process. Both the zinc doped iron nanocatalyst and biodiesel were characterized, and the reaction mechanisms were determined from the kinetic analysis.
Materials and Methods
WCO Collection and Pre-Treatment
The WCO was collected from the local restaurants in Chennai, India. Then the WCO was allowed to settle for 4–6 d under room temperature. The food particles and other residues in the WCO were removed by being filtered through a 100-nm size sieve. Then the WCO was heated at 110°C to remove the moisture (Degfie et al., 2019).
Zinc Doped Iron Nanocatalyst Preparation
The co-precipitation method was used for the preparation of the zinc doped iron oxide magnetic catalyst. The 50 ml of 0.5M FeSO4.7H2O was mixed with 50 ml of 0.5M ZnSO4 for 10 min. Then 0.5M NaOH was added drop wise until the pH was reached to 12 and mixed for 30 min in the stirrer. At room temperature, the mixture was settled for 18 h for precipitation. The precipitate was then centrifuged, and the pellet was washed with distilled water and ethanol several times. The washed pellets were resuspended in water and dehydrated by keeping them in a hot air oven for 1 h at 200°C. Then the dried powder was calcinated at 700°C for 30 min. The calcinated powder is the activated catalyst obtained from the precipitate. The activated catalyst is again washed in distilled water and then calcinated to remove the residual ash and reactivate, respectively. This results in the formation of zinc doped iron nanoparticles. The activated magnetic nanoparticle was prepared and used for the production of biodiesel (Baskar and Soumiya, 2016; Atla et al., 2018). To the prepared nanocatalyst, tetra-n-butylammonium iodide (TBAI) reagent was employed as a co-catalyst for the synthesis of biodiesel from WCO (Casiello et al., 2019).
Catalyst Characterization
The functional group present in the obtained zinc doped iron nanocatalyst was analyzed using FTIR spectra (Perkin Elmer Spectrum-1) in the range between 400 and 4000 cm−1. The phase and crystalline structure of the synthesized nanocatalyst was characterized by XRD (Bruker aXS KAPPA APEX-II). The morphology and topology of the catalyst were studied using SEM (FEI Quanta FEG 200F) (Mahmood Khan et al., 2020).
Biodiesel Production and Process Optimization
Transesterification of WCO was carried out using methanol in the presence of zinc doped iron nanocatalyst and TBAI as co-catalyst at the desired temperature. The reaction mixture was then centrifuged for 15 min at 5000 rpm. After centrifugation, the supernatant was transferred to a test tube and ethyl acetoacetate was added to precipitate the co-catalyst salt employed (TBAI). The supernatant contains biodiesel.
The transesterification conditions like oil to methanol ratio, catalyst loading, co-catalyst loading, reaction time, and temperature were varied to achieve the optimum condition for maximum biodiesel conversion from WCO (Baskar and Soumiya, 2016).
Biodiesel Characterization
The function group present on the biodiesel from WCO was analyzed using the spectra obtained from FTIR in the range between 400 and 4000 cm−1 and the peaks were recorded. The chemical composition of the synthesized biodiesel was identified by GC-MS (Baskar and Soumiya, 2016).
Kinetic Studies
The order of kinetics of the reaction was studied at different reaction temperatures at 40, 45, 50, 55, and 60°C. The kinetics of the reaction were defined from the following Eq. 1,
where P is methyl esters in terms of yield (%), t is the reaction time (min), and k is the rate of the reaction (min1). The “ln [P]” vs. “t” at different intervals of time and temperature was plotted. The yield tends to increase simultaneously with time and temperature and the reaction rate increases with temperature. The activation energy was studied using Arrhenius Eq. 2,
Ea is the activation energy (J/mol), R is the universal gas constant (8.314 J/mol.K), and T is the absolute temperature in Kelvin. A is the frequency factor (collision factor) and k is the reaction rate constant. The activation energy was calculated from the slope and intercept of the graph between ln(k) vs. 1/T (Gupta and Rathod, 2018).
Results and Discussion
Characterization of Zinc Doped Iron Nanocatalyst by FTIR Analysis
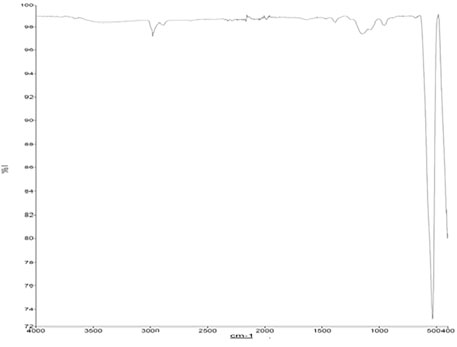
The functional group present in the zinc doped iron nanocatalyst was analyzed by Fourier transform infrared spectroscopy (FTIR). The FTIR spectra of zinc doped iron nanocatalyst were recorded in the range of 4000–400 cm−1 and is shown in Figure 1. The peaks obtained were compared to the vibrating mode. The major peaks were observed at 2950, 1450, 1250, 940, 550, and 490 cm−1. The peaks show the presence of CO bonds, OH bonds, and CH bonds. The small peaks are due to the presence of dopants over the lattice (Baskar and Soumiya, 2016).
Characterization of Zinc Doped Iron Nanocatalyst by XRD
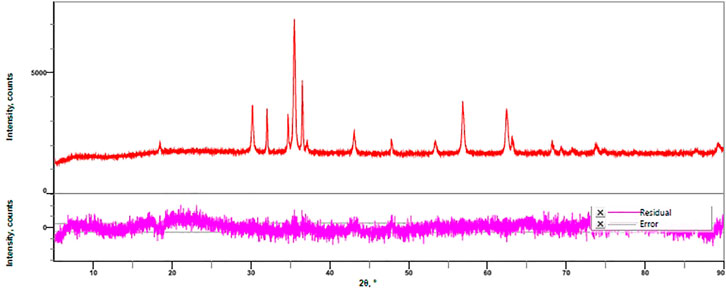
The crystalline form of nanocatalyst was identified using the XRD technique. The XRD profile of zinc doped iron nanocatalyst is shown in Figure 2. Figure 2 shows the zinc and iron in single-phase and the crystalline form of zinc doped iron nanocatalyst. There is a major shift in the 2θ value after doping was observed from the spectra. There are no impurity peaks in the XRD patterns indicating that the dopants are well integrated into the lattices (Joseph et al., 2021).
Characterization of Zinc Doped Iron Nanocatalyst by SEM
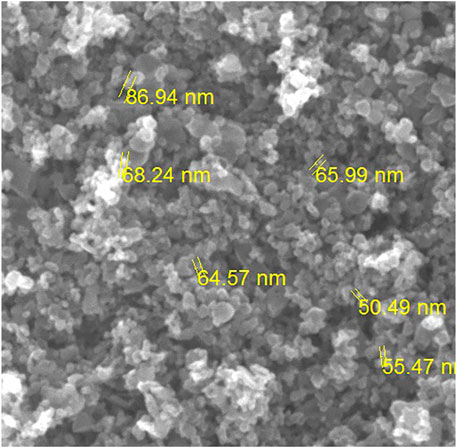
The morphology of zinc doped iron nanocatalyst is studied using scanning electron microscopy (SEM). Figure 3 shows the porous nature of the synthesized zinc doped iron nanocatalyst. The SEM image shows the agglomerated image of doped zinc and iron nanoparticles. The size of the nanoparticles was reduced after doping (Kumar et al., 2019). The size of the nanocatalyst was observed between 86.94 and 50.49 nm.
Effect of Nanocatalyst Concentration
The batch of experiments was carried out by varying the zinc doped iron nanocatalyst concentration 2–12% (w/w) with the WCO to methanol molar ratio of 1:11, co-catalyst concentration of 30% at 50°C for 45 min was carried out to obtain the optimum concentration of catalyst for maximum biodiesel production. Figure 4 shows the result of the experiment; the biodiesel yield increases as the catalyst quantity increases from 2 to 12% (w/w) and after 14–16% (w/w) of catalyst loading shows a reduction in the biodiesel yield. The excess catalyst amount leads to saponification reaction, the soap formation, and interrupts the biodiesel yield (Keera et al., 2018). Thus, 12% (w/w) catalyst concentration was the optimum catalyst concentration for biodiesel production from WCO. The zinc doped iron nanocatalyst concentration of 14% (w/w) shows 91% of biodiesel production from castor oil (Baskar and Soumiya, 2016).
Effect of Oil to Methanol Molar Ratio
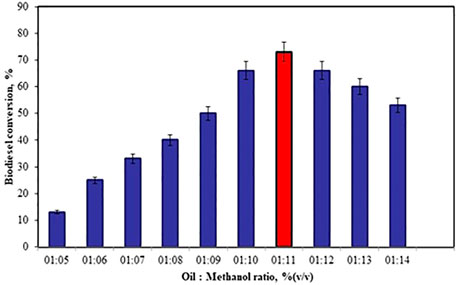
The oil to methanol molar ratio is the important parameter considered in biodiesel conversion. The experiments were carried out by changing the oil to methanol molar ratio from 1:5 to 1:14 under the other constant conditions 12% (w/w) catalyst concentration, 40% co-catalyst concentration at 50°C for 45 min. There is an increase in the biodiesel yield from 13 to 73% observed as the oil to methanol molar ratio was increased from 1:5 to 1:11 (Figure 5). The biodiesel conversion was decreased from 73 to 53% as the molar ratio was increased from 1:11 to 1:14. The reduction in the biodiesel conversion is due to the accumulation of methanol, which makes the reaction mixture more viscous. Excess oil to methanol ratio increases glycerol solubility and thus decreases the FAME yield (Sharma et al., 2019).
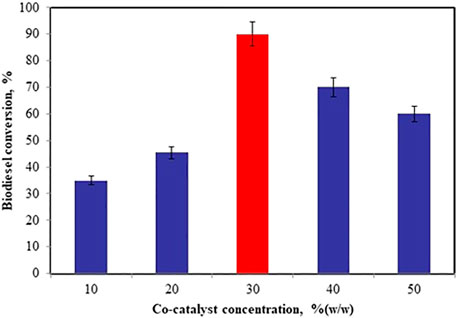
Effect of Co-Catalyst Concentration
The ionic liquid, TBAI was used as the co-catalyst along with zinc doped iron nanocatalyst for the conversion of biodiesel from WCO. The optimum co-catalyst concentration was achieved from the batch of experiments varying the co-catalyst amount from 10 to 50%, catalyst concentration of 12%, oil to methanol molar ratio 1:11 at 50°C for 45 min. At 30% (w/w) of co-catalyst concentration, 90% of FAME conversion was obtained. Ionic liquid TBAI as co-catalyst with zinc doped iron nanocatalyst increases the biodiesel yield. TBAI reagent can be completely recovered by adding ethyl acetate into the reaction mixture (Casiello et al., 2019). The optimal co-catalyst concentration was found to be 30% (w/w) and is shown in Figure 6.
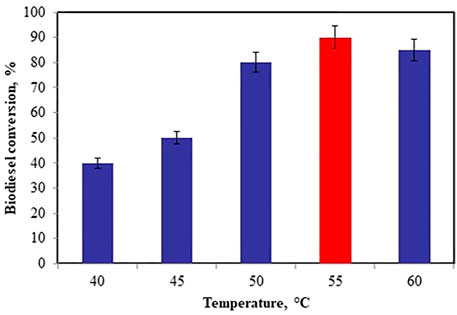
Effect of Reaction Temperature
For the optimization of reaction temperature, the temperature changed from 40 to 60°C while maintaining the other reaction parameters like catalyst concentration 12% (w/w), co-catalyst loading of 30% (w/w), and WCO to methanol molar ratio 1:11 for 45 min. As the temperature increases from 40 to 55°C, there is an increment in the biodiesel yield from 40 to 90% and after 55°C there is a decrement in biodiesel yield, due to the vaporization of methanol (Borah et al., 2019). Hence, 55°C was considered as the optimum reaction temperature as shown in Figure 7.
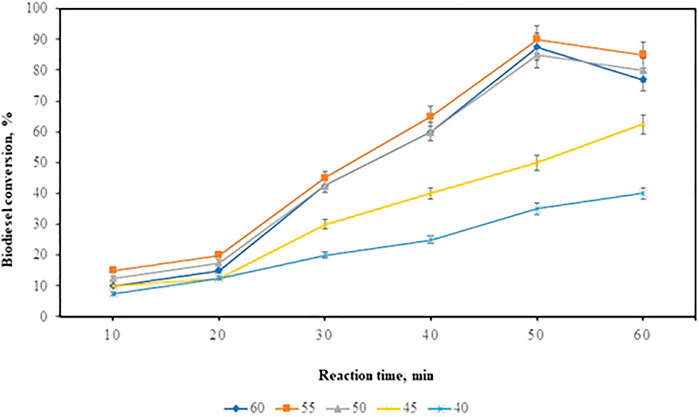
Effect of Reaction Time
For the reaction time optimization, the time was varied from 10 to 60 min, in the intervals of 10 min. The reaction was carried out at optimized 1:11 oil to methanol molar ratio, 12% catalyst concentration, 30% co-catalyst concentration, and the reaction temperature varying 40–60°C. When the reaction time increases to 50 min, there is an increase in the biodiesel conversion as shown in Figure 8. The optimum biodiesel yield of 90% was synthesized from the transesterification of WCO using a zinc doped iron nanocatalyst. The transesterification reaction takes place in the forward direction and when it reaches the optimal time it stands in equilibrium. Long reaction time shows a decrease in the biodiesel yield due to reverse reaction (Degfie et al., 2019). The results were obtained from the effect of time and temperature optimization used for kinetic studies.
Kinetic Studies
The kinetics of zinc doped iron-catalyzed transesterification reaction were studied from optimization of reaction time. WCO to methanol molar ratio of 1:11, zinc doped iron oxide nanocatalyst concentration of 12%, and co-catalyst concentration of 30% at different temperatures changing from 40 to 60°C. Transesterification reactions follow first-order kinetics as a function of time and is also involved in it for the methyl ester formation. The biodiesel yield increases with respect to time and temperature and the reaction rate also simultaneously increases with the temperature. The rate constant mainly depends on the reaction temperature with respect to time (Pugazhendhi et al., 2020). Based on the methyl ester formation, the rate constant (k) and activation energy (Ea) can be calculated (Verma and Sharma, 2016). The rate constant of biodiesel production at optimal temperature of 55°C and time 50 min was found as 0.0387 min−1.
The activation energy (Ea) was calculated using Eq. 3.
The biodiesel production from waste cooking WCO using zinc doped iron oxide nanocatalyst and TBAI as co-catalyst was found to follow the first-order kinetics. The activation energy required for the transesterification of WCO using zinc doped iron oxide nanocatalyst and TBAI as co-catalyst was found to be 9.494 J/mol.
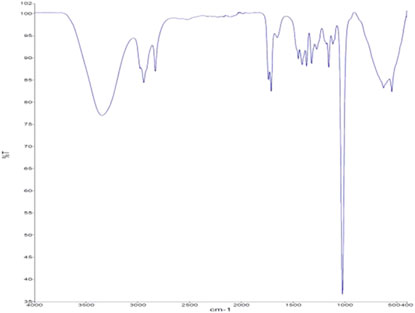
Biodiesel Characterization by FTIR
The FTIR spectra of biodiesel are represented in Figure 9. The peaks in 3000–3500 cm−1 show the presence of (−OH) stretching vibration of phenol, alcohol, or carboxylic acid. The absorption band of 2922 and 2855 cm−1 represents methyl group stretching. The peak at 1739 and 1734 cm−1 refers to the C=O in carboxyl, ketone, or aldehyde groups and shows the presence of ester in the FAME sample. The peak at 1455 and 1446 cm−1 refers to the stretching of–C–H–(alkane) in the synthesized biodiesel. The peaks from 1245 to 1033 cm−1 correspond to the presence of (C-O) and (C-O-C). The peak at 1193 and 1033 cm−1 represents O-CH3 stretching. The FTIR spectra analyze the functional group present in the biodiesel produced from WCO using zinc doped iron catalyst and TBAI co-catalyst (Elango et al., 2019).
Biodiesel Characterization by GC-MS Analysis
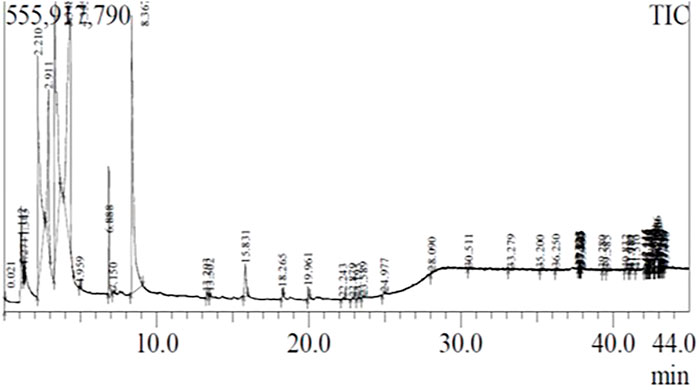
The methyl ester in the biodiesel synthesized from WCO was analyzed by gas chromatography-mass spectrometry (GC-MS). The mono, di, and triglycerides concentration in the biodiesel was analyzed. NIST 11 and WILEY 8 GC–MS libraries were used to compare the chromatogram peaks. Figure 10 shows different peaks that represent the presence of methyl esters. The maximum peak was obtained at the retention time of 3.348 min and the peak indicates 2,3-dihydroindole-4-ol-2-one, 5,7 dibromo-3,3 dimethyl ester (Naveenkumar and Baskar, 2019).
Conclusion
Using magnetic core catalyst, the catalyst can be isolated by an external magnetic power after the completion of the process. In addition, the ZnO coating over the surface of the core is not likely to be uniform and hence can offer an alike mesoporous structure due to the coating of zinc. This tends to increase the reactive site and can thereby show more yield. The presence of Zn does the function of esterification, thereby reducing the free fatty acid content and can aid in the increase in the yield of the product. The optimal conditions are found to be 1:11 WCO to methanol ratio, 12% w/w of catalyst loading (zinc doped iron), 30% w/w of co-catalyst amount (TBAI) for 50 min of reaction time at 55°C to produce 90% of biodiesel from WCO. The use of low-cost zinc doped iron/TBAI catalyst shows high catalytic activity and can be easily recovered and reused for further cycles without degradation in its activity. The kinetics of the reaction tends to fit in with first-order kinetics and the activation energy was found to be 3388.607 J/mol.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
AN and BG defined the problem and set the objectives. AN carried out the experimental work. NR prepared the draft manuscript. AN and GB carried out the data analysis. GB and JH revised the draft manuscript. GB supervised the entire work.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Alishahi, A., Golmakani, M. T., and Niakousari, M. (2021). Feasibility Study of Microwave‐Assisted Biodiesel Production from Vegetable Oil Refinery Waste. Eur. J. Lipid Sci. Technol. 123, 2000377. doi:10.1002/ejlt.202000377
Ambat, I., Srivastava, V., and Sillanpää, M. (2018). Recent Advancement in Biodiesel Production Methodologies Using Various Feedstock: A Review. Renew. Sustain. Energ. Rev. 90, 356–369. doi:10.1016/j.rser.2018.03.069
Atla, S. B., Lin, W.-R., Chien, T.-C., Tseng, M.-J., Shu, J.-C., Chen, C.-C., et al. (2018). Fabrication of Fe3O4/ZnO Magnetite Core Shell and its Application in Photocatalysis Using Sunlight. Mater. Chem. Phys. 216, 380–386. doi:10.1016/j.matchemphys.2018.06.020
Balamurugan, S., Gokul, C., Abith Tamil Dheen, S., Eashwar, S. J., and Arun Kumar, N. (2021). Application of Grey Relational Analysis in Biodiesel Production from Linseed Oil Using Novel Eggshell Catalyst. Mater. Today Proc. 45, 1962–1969. doi:10.1016/j.matpr.2020.09.255
Bambase, M. E., Almazan, R. A. R., Demafelis, R. B., Sobremisana, M. J., and Dizon, L. S. H. (2021). Biodiesel Production from Refined Coconut Oil Using Hydroxide-Impregnated Calcium Oxide by Cosolvent Method. Renew. Energ. 163, 571–578. doi:10.1016/j.renene.2020.08.115
Baskar, G., and Aiswarya, R. (2016). Trends in Catalytic Production of Biodiesel from Various Feedstocks. Renew. Sustain. Energ. Rev. 57, 496–504. doi:10.1016/j.rser.2015.12.101
Baskar, G., and Soumiya, S. (2016). Production of Biodiesel from castor Oil Using Iron (II) Doped Zinc Oxide Nanocatalyst. Renew. Energ. 98, 101–107. doi:10.1016/j.renene.2016.02.068
Borah, M. J., Devi, A., Borah, R., and Deka, D. (2019). Synthesis and Application of Co Doped ZnO as Heterogeneous Nanocatalyst for Biodiesel Production from Non-edible Oil. Renew. Energ. 133, 512–519. doi:10.1016/j.renene.2018.10.069
Casiello, M., Catucci, L., Fracassi, F., Fusco, C., Laurenza, A., di Bitonto, L., et al. (2019). ZnO/ionic Liquid Catalyzed Biodiesel Production from Renewable and Waste Lipids as Feedstocks. Catalysts 9, 71. doi:10.3390/catal9010071
Cavalcante, F. T. T., Neto, F. S., Rafael de Aguiar Falcão, I., Erick da Silva Souza, J., de Moura Junior, L. S., da Silva Sousa, P., et al. (2021). Opportunities for Improving Biodiesel Production via Lipase Catalysis. Fuel 288, 119577. doi:10.1016/j.fuel.2020.119577
Degfie, T. A., Mamo, T. T., and Mekonnen, Y. S. (2019). Optimized Biodiesel Production from Waste Cooking Oil (WCO) Using Calcium Oxide (CaO) Nano-Catalyst. Sci. Rep. 9, 18982. doi:10.1038/s41598-019-55403-4
Demirbas, A. (2007). Importance of Biodiesel as Transportation Fuel. Energy Policy 35, 4661–4670. doi:10.1016/j.enpol.2007.04.003
Deshmukh, S., Kumar, R., and Bala, K. (2019). Microalgae Biodiesel: A Review on Oil Extraction, Fatty Acid Composition, Properties and Effect on Engine Performance and Emissions. Fuel Process. Technol. 191, 232–247. doi:10.1016/j.fuproc.2019.03.013
Elango, R. K., Sathiasivan, K., Muthukumaran, C., Thangavelu, V., Rajesh, M., and Tamilarasan, K. (2019). Transesterification of castor Oil for Biodiesel Production: Process Optimization and Characterization. Microchem. J. 145, 1162–1168. doi:10.1016/j.microc.2018.12.039
Gardy, J., Osatiashtiani, A., Céspedes, O., Hassanpour, A., Lai, X., Lee, A. F., et al. (2018). A Magnetically Separable SO4/Fe-Al-TiO2 Solid Acid Catalyst for Biodiesel Production from Waste Cooking Oil. Appl. Catal. B: Environ. 234, 268–278. doi:10.1016/j.apcatb.2018.04.046
Guo, M., Jiang, W., Chen, C., Qu, S., Lu, J., Yi, W., et al. (2021). Process Optimization of Biodiesel Production from Waste Cooking Oil by Esterification of Free Fatty Acids Using La3+/ZnO-TiO2 Photocatalyst. Energ. Convers. Manage. 229, 113745. doi:10.1016/j.enconman.2020.113745
Gupta, A. R., and Rathod, V. K. (2018). Calcium Diglyceroxide Catalyzed Biodiesel Production from Waste Cooking Oil in the Presence of Microwave: Optimization and Kinetic Studies. Renew. Energ. 121, 757–767. doi:10.1016/j.renene.2017.11.027
Gurunathan, B., and Ravi, A. (2015). Biodiesel Production from Waste Cooking Oil Using Copper Doped Zinc Oxide Nanocomposite as Heterogeneous Catalyst. Bioresour. Technol. 188, 124–127. doi:10.1016/j.biortech.2015.01.012
Hosseinzadeh-Bandbafha, H., Tabatabaei, M., Aghbashlo, M., Khanali, M., and Demirbas, A. (2018). A Comprehensive Review on the Environmental Impacts of Diesel/biodiesel Additives. Energ. Convers. Manage. 174, 579–614. doi:10.1016/j.enconman.2018.08.050
Huang, J., Zou, Y., Yaseen, M., Qu, H., He, R., and Tong, Z. (2021). Fabrication of Hollow Cage-like CaO Catalyst for the Enhanced Biodiesel Production via Transesterification of Soybean Oil and Methanol. Fuel 290, 119799. doi:10.1016/j.fuel.2020.119799
Jalalmanesh, S., Kazemeini, M., Rahmani, M. H., and Zehtab Salmasi, M. (2021). Biodiesel Production from Sunflower Oil Using K2CO3 Impregnated Kaolin Novel Solid Base Catalyst. JAOCS. J. Am. Oil Chemists’ Soc. 98, 633–642. doi:10.1002/aocs.12486
Jamil, U., Husain Khoja, A., Liaquat, R., Raza Naqvi, S., Nor Nadyaini Wan Omar, W., and Aishah Saidina Amin, N. (2020). Copper and Calcium-Based Metal Organic Framework (MOF) Catalyst for Biodiesel Production from Waste Cooking Oil: A Process Optimization Study. Energ. Convers. Manage. 215, 112934. doi:10.1016/j.enconman.2020.112934
Joseph, J. A., Nair, S. B., John, S. S., Remillard, S. K., Shaji, S., and Philip, R. R. (2021). Zinc-doped Iron Oxide Nanostructures for Enhanced Photocatalytic and Antimicrobial Applications. J. Appl. Electrochem. 51, 521–538. doi:10.1007/s10800-020-01512-2
Keera, S. T., El Sabagh, S. M., and Taman, A. R. (2018). Castor Oil Biodiesel Production and Optimization. Egypt. J. Pet. 27, 979–984. doi:10.1016/j.ejpe.2018.02.007
Kirubakaran, M., and Arul Mozhi Selvan, V. (2021). Experimental Investigation on the Effects of Micro Eggshell and Nano-Eggshell Catalysts on Biodiesel Optimization from Waste Chicken Fat. Bioresour. Technol. Rep. 14, 100658. doi:10.1016/j.biteb.2021.100658
Kumar, M., Sharma, A., Maurya, I. K., Thakur, A., and Kumar, S. (2019). Synthesis of Ultra Small Iron Oxide and Doped Iron Oxide Nanostructures and Their Antimicrobial Activities. J. Taibah Univ. Sci. 13, 280–285. doi:10.1080/16583655.2019.1565437
Liu, X., Zhu, F., Zhang, R., Zhao, L., and Qi, J. (2021). Recent Progress on Biodiesel Production from Municipal Sewage Sludge. Renew. Sustain. Energ. Rev. 135, 110260. doi:10.1016/j.rser.2020.110260
Maciel, V. G., Wales, D. J., Seferin, M., Ugaya, C. M. L., and Sans, V. (2019). State-of-the-art and Limitations in the Life Cycle Assessment of Ionic Liquids. J. Clean. Prod. 217, 844–858. doi:10.1016/j.jclepro.2019.01.133
Mahmood Khan, H., Iqbal, T., Haider Ali, C., Javaid, A., and Iqbal Cheema, I. (2020). Sustainable Biodiesel Production from Waste Cooking Oil Utilizing Waste Ostrich (Struthio camelus) Bones Derived Heterogeneous Catalyst. Fuel 277, 118091. doi:10.1016/j.fuel.2020.118091
Mathew, G. M., Raina, D., Narisetty, V., Kumar, V., Saran, S., Pugazhendi, A., et al. (2021). Recent Advances in Biodiesel Production: Challenges and Solutions. Sci. Total Environ. 794, 148751. doi:10.1016/j.scitotenv.2021.148751
Ming, C., Rizwanul Fattah, I. M., Chan, Q. N., Pham, P. X., Medwell, P. R., Kook, S., et al. (2018). Combustion Characterization of Waste Cooking Oil and Canola Oil Based Biodiesels under Simulated Engine Conditions. Fuel 224, 167–177. doi:10.1016/j.fuel.2018.03.053
Muhammad, N., Elsheikh, Y. A., Mutalib, M. I. A., Bazmi, A. A., Khan, R. A., Khan, H., et al. (2015). An Overview of the Role of Ionic Liquids in Biodiesel Reactions. J. Ind. Eng. Chem. 21, 1–10. doi:10.1016/j.jiec.2014.01.046
Naeem, A., Wali Khan, I., Farooq, M., Mahmood, T., Ud Din, I., Ali Ghazi, Z., et al. (2021). Kinetic and Optimization Study of Sustainable Biodiesel Production from Waste Cooking Oil Using Novel Heterogeneous Solid Base Catalyst. Bioresour. Technol. 328, 124831. doi:10.1016/j.biortech.2021.124831
Naveenkumar, R., and Baskar, G. (2019). Biodiesel Production from Calophyllum inophyllum Oil Using Zinc Doped Calcium Oxide (Plaster of Paris) Nanocatalyst. Bioresour. Technol. 280, 493–496. doi:10.1016/j.biortech.2019.02.078
Naveenkumar, R., and Baskar, G. (2021). Process Optimization, green Chemistry Balance and Technoeconomic Analysis of Biodiesel Production from castor Oil Using Heterogeneous Nanocatalyst. Bioresour. Technol. 320, 124347. doi:10.1016/j.biortech.2020.124347
Ong, H. C., Chen, W.-H., Farooq, A., Gan, Y. Y., Lee, K. T., and Ashokkumar, V. (2019). Catalytic Thermochemical Conversion of Biomass for Biofuel Production: A Comprehensive Review. Renew. Sustain. Energ. Rev. 113, 109266. doi:10.1016/j.rser.2019.109266
Ong, H. C., Tiong, Y. W., Goh, B. H. H., Gan, Y. Y., Mofijur, M., Fattah, I. M. R., et al. (2021). Recent Advances in Biodiesel Production from Agricultural Products and Microalgae Using Ionic Liquids: Opportunities and Challenges. Energ. Convers. Manage. 228, 113647. doi:10.1016/j.enconman.2020.113647
Patle, D. S., Pandey, A., Srivastava, S., Sawarkar, A. N., and Kumar, S. (2021). Ultrasound-intensified Biodiesel Production from Algal Biomass: a Review. Environ. Chem. Lett. 19, 209–229. doi:10.1007/s10311-020-01080-z
Piemonte, V., di Paola, L., Iaquaniello, G., and Prisciandaro, M. (2016). Biodiesel Production from Microalgae: Ionic Liquid Process Simulation. J. Clean. Prod. 111, 62–68. doi:10.1016/j.jclepro.2015.07.089
Pugazhendhi, A., Alagumalai, A., Mathimani, T., and Atabani, A. E. (2020). Optimization, Kinetic and Thermodynamic Studies on Sustainable Biodiesel Production from Waste Cooking Oil: An Indian Perspective. Fuel 273, 117725. doi:10.1016/j.fuel.2020.117725
Qu, T., Niu, S., Zhang, X., Han, K., and Lu, C. (2021). Preparation of Calcium Modified Zn-Ce/Al2O3 Heterogeneous Catalyst for Biodiesel Production through Transesterification of palm Oil with Methanol Optimized by Response Surface Methodology. Fuel 284, 118986. doi:10.1016/j.fuel.2020.118986
Rabie, A. M., Shaban, M., Abukhadra, M. R., Hosny, R., Ahmed, S. A., and Negm, N. A. (2019). Diatomite Supported by CaO/MgO Nanocomposite as Heterogeneous Catalyst for Biodiesel Production from Waste Cooking Oil. J. Mol. Liquids 279, 224–231. doi:10.1016/j.molliq.2019.01.096
Shalmashi, A., and Khodadadi, F. (2019). Ultrasound-assisted Synthesis of Biodiesel from Peanut Oil by Using Response Surface Methodology. Energ. Environ. 30, 272–291. doi:10.1177/0958305X18790952
Sharma, A., Kodgire, P., and Kachhwaha, S. S. (2019). Biodiesel Production from Waste Cotton-Seed Cooking Oil Using Microwave-Assisted Transesterification: Optimization and Kinetic Modeling. Renew. Sustain. Energ. Rev. 116, 109394. doi:10.1016/j.rser.2019.109394
Ullah, Z., Bustam, M. A., Man, Z., Khan, A. S., Muhammad, N., and Sarwono, A. (2017). Preparation and Kinetics Study of Biodiesel Production from Waste Cooking Oil Using New Functionalized Ionic Liquids as Catalysts. Renew. Energ. 114, 755–765. doi:10.1016/j.renene.2017.07.085
Uzoh, C. F., Nnuekwe, A., Onukwuli, O., Ofochebe, S., and Ezekannagha, C. (2021). Optimal Route for Effective Conversion of Rubber Seed Oil to Biodiesel with Desired Key Fuel Properties. J. Clean. Prod. 280, 124563. doi:10.1016/j.jclepro.2020.124563
Verma, P., and Sharma, M. P. (2016). Comparative Analysis of Effect of Methanol and Ethanol on Karanja Biodiesel Production and its Optimisation. Fuel 180, 164–174. doi:10.1016/j.fuel.2016.04.035
Yusuff, A. S., Kumar, M., Obe, B. O., and Mudashiru, L. O. (2021). Calcium Oxide Supported on Coal Fly Ash (CaO/CFA) as an Efficient Catalyst for Biodiesel Production from Jatropha Curcas Oil. Top. Catal. doi:10.1007/s11244-021-01478-1
Keywords: biodiesel, waste cooking oil, transesterification, nanocatalyst, tetrabutylammonium iodide
Citation: Baskar G, Anita NT, Jeehoon H and Naveenkumar R (2022) Ionic Liquid Co-Catalyst Assisted Biodiesel Production From Waste Cooking Oil Using Heterogeneous Nanocatalyst: Optimization and Characterization. Front. Nanotechnol. 4:823759. doi: 10.3389/fnano.2022.823759
Received: 28 November 2021; Accepted: 18 February 2022;
Published: 30 March 2022.
Edited by:
Francis Verpoort, Wuhan University of Technology, ChinaReviewed by:
Krishnamurthi Tamilarasan, SRM Institute of Science and Technology, IndiaMelvin Samuel, University of Wisconsin–Milwaukee, United States
Copyright © 2022 Baskar, Anita, Jeehoon and Naveenkumar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gurunathan Baskar, YmFzZzIwMDRAZ21haWwuY29t
 Gurunathan Baskar
Gurunathan Baskar Nalathamalar T. Anita
Nalathamalar T. Anita Han Jeehoon
Han Jeehoon Rajendran Naveenkumar
Rajendran Naveenkumar