- 1Department of Biophysics, Biological Faculty, Lomonosov Moscow State University, Moscow, Russia
- 2Department of Theoretical Physics, Kursk State University, Kursk, Russia
- 3Department of physics, Saratov State University, Saratov, Russia
Calcium transients in thin astrocytic processes can be important in synaptic plasticity, but their mechanism is not completely understood. Clearance of synaptic glutamate leads to increase in astrocytic sodium. This can electrochemically favor the reverse mode of the Na/Ca-exchanger (NCX) and allow calcium into the cell, accounting for activity-dependent calcium transients in perisynaptic astrocytic processes. However, cytosolic sodium and calcium are also allosteric regulators of the NCX, thus adding kinetic constraints on the NCX-mediated fluxes and providing for complexity of the system dynamics. Our modeling indicates that the calcium-dependent activation and also calcium-dependent escape from the sodium-mediated inactive state of the NCX in astrocytes can form a positive feedback loop and lead to regenerative calcium influx. This can result in sodium-dependent amplification of calcium transients from nearby locations or other membrane mechanisms. Prolonged conditions of elevated sodium, for example in ischemia, can also lead to bistability in cytosolic calcium levels, where a delayed transition to the high-calcium state can be triggered by a short calcium transient. These theoretical predictions call for a dedicated experimental estimation of the kinetic parameters of the astrocytic Na/Ca-exchanger.
1. Introduction
Astrocytes are tightly involved in the metabolic supply in the brain, life cycle of the synapse and neuronal activity (Prebil et al., 2011; Lee et al., 2014; Verkhratsky and Nedergaard, 2014; Rose and Chatton, 2016). Calcium transients, engulfing areas from single astrocyte processes to whole spatial domains occupied by a single astrocyte to intercellular waves of elevated Ca2+, play a central role in shaping astrocytic signals to the neural tissue (Khakh and McCarthy, 2015; Bazargani and Attwell, 2016; Verkhratsky et al., 2017). If Ca2+ transients are the keystone of the astrocyte-mediated regulatory pathways, it is crucial to understand the mechanisms of transient origination and expansion to neighboring regions, as well as interplay between multiple mechanisms. Because thin astrocytic processes (leaflets in terminology suggested by Tong et al., 2013; Khakh and Sofroniew, 2015) are the main “sensors” of the neuronal microenvironment and the most active Ca2+ signaling occurs there, the mechanisms underlying formation of this activity are of key importance, but are still not clear. So, how does astrocyte read out ambient synaptic activity and transform it into local Ca2+ transients? We argue that Na/Ca-exchanger (NCX)—a membrane transporter protein that exchanges three Na+ ions for one Ca2+ ion—and its modulation by cytosolic Na+ and Ca2+ have important and previously unanticipated effects on Ca2+ transient formation and spreading.
The most widely known mechanism for astrocytic Ca2+ signaling includes Ca2+-dependent activation of inositoltrisphosphate (IP3) receptors (IP3Rs) followed by Ca2+–dependent Ca2+ release from endoplasmic reticulum (ER). Initial IP3 production is attributed to activation of astrocytic G protein-coupled receptors to glutamate (mGluRs), with subsequent production of diacylglycerol and IP3 by phospholipase Cβ. The importance of this pathway has been challenged by questioning the existence of mGluRs in adult astrocytes (Sun et al., 2013) and the lack of effects of knocking out astrocytic IP3Rs on pyramidal neuron synaptic activity in hippocampus (Petravicz et al., 2008). On the other hand, contrary to somata and thick branches, spontaneous Ca2+ transients in the leaflets are independent of intracellular stores and IP3-mediated mechanisms. Transients in the leaflets remain even in IP3R–knockout animals (Srinivasan et al., 2015), and are not affected by a variety of blockers, while being sensitive to external Ca2+ concentration (Rungta et al., 2016), thus implying that Ca2+ must enter the leaflet cytoplasm from extracellular space. This is also supported by spatial constraints: the leaflets are so small they do not contain organelles (Patrushev et al., 2013). The dominating mechanism of Ca2+ transients in leaflets is debated, with tentative pathways encompassing several types of transient receptor potential (TRP) channels, astrocytic ionotropic receptors to glutamate, voltage-gated Ca2+ channels and NCX working in Ca2+ entry mode (Verkhratsky et al., 2017).
Here we focus on Ca2+ entry, mediated by NCX. In most conditions, the NCX utilizes the Na+ electrochemical gradient to expell Ca2+ from the cell (“forward mode”). The opposite exchange direction (“reverse mode”) becomes energetically favorable at high enough [Na+]i and depolarized membrane potential. For example, in the cardiomyocytes, the NCX switches between the modes during the contraction cycle (Shattock et al., 2015). NCX-mediated Ca2+ entry can be directly linked to local synaptic activity with the following causal chain of events (Rojas et al., 2007; Kirischuk et al., 2012; Reyes et al., 2012): neurotransmitter clearance is mediated by cotransporter proteins utilizing Na+ gradient, which leads to Na+ influx and synaptic activity-related Na+ transients in astrocytes (Langer and Rose, 2009; Langer et al., 2012). In the cortex, glutamate is the primary neurotransmitter, which is cotransporter with 3 Na+ ions per glutamate molecule. Increase in [Na+]i in turn reverses the NCX cycle direction leading to Ca2+ entry from the extracellular space in exchange for 3 Na+ ions, placing NCX as a major contributor to overall Ca2+ and Na+ homeostasis in astrocytes (Reyes et al., 2012).
This Na+-paved link from synaptic activity to cytosolic Ca2+ can partly explain why the membrane of astrocyte perisynaptic processes is enriched in NCX (Minelli et al., 2007). There is however an additional facet to this picture so far not discussed in the context of astrocytes. Cardiac NCX1 isoform is allosterically regulated by Na+ and Ca2+ from the cytoplasmic side (Hilgemann et al., 1992a,b; Matsuoka et al., 1996). Specifically, already at resting [Na+]i, the NCX is inhibited by Na+, but this Na+-block is relieved by intracellular Ca2+ at concentrations close to 1 μM. This can have important dynamical consequences for Ca2+ dynamics in leaflets, which we demonstrate in a simple model of NCX in terms of the Hodgkin–Huxley formalism. We conjecture that high [Na+]i and depolarization conditions allow Ca2+-induced Ca2+ entry through the NCX, making it a Ca2+-sensitive source or even mediate a Ca2+ wave expansion. Our results extend the current understanding of the role of NCX in Ca2+ dynamics as formulated by Kirischuk et al. (2012) that “NCX provides for rapid and short-lived Ca2+ microdomains” by a novel regenerative Ca2+ entry mechanism.
2. Simple Model of the NCX
NCX is a reversible transporter exchanging three Na+ ions for one Ca2+ ion. Its cycle is described by a kinetic scheme shown in Figure 1A (Matsuoka et al., 1996) (counter-clockwise, “forward mode”): a substrate-free protein with outward-facing binding cite (E2) binds Na+ ions (E2·3Na+) and changes its conformation to the inward-facing binding site (E1·3Na+), from where it can either release Na+ to acquire the substrate-free inward-facing conformation (E1) or undergo transition to Na+-bound inactive conformation I1 in a Na+-dependent manner; the protein in E1 state can bind a Ca2+ ion (E1·Ca2+) or switch to substrate-free inactive state I2; the inward-facing Ca2+-bound state (E1·Ca2+) flips to the outward-facing Ca2+-bound state (E2·Ca2+), and dissociation of Ca2+ closes the cycle. All the transitions are reversible and going rough the cycle in the clockwise direction corresponds to “reverse” (Ca2+-influx) mode. Transitions from I1 and I2 are facilitated by cytosolic Ca2+. Thus, NCX activity is allosterically regulated by cytosolic Na+ and Ca2+ (Hilgemann et al., 1992a,b); Ca2+ can bind to two regulatory sites with different affinities (Boyman et al., 2009): sub-micromolar Ca2+ binding to the high-affinity site rapidly facilitates transition from inactive state I2; Na+-dependent transition to I1 is slower and the deinactivation is mediated by Ca2+ binding to the low-affinity Ca2+ site (Matsuoka et al., 1996).
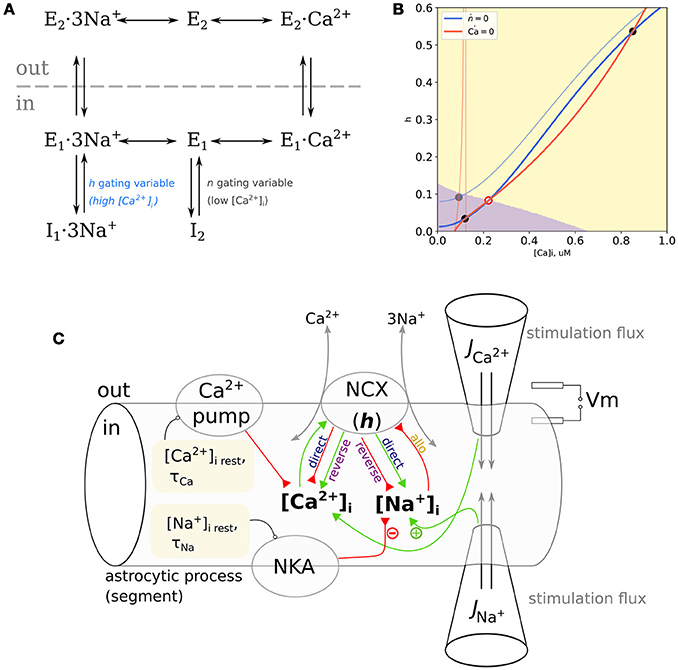
Figure 1. (A) Kinetic cycle of the NCX based on Matsuoka et al. (1996). The I2 inactivated state is Na+-independent and relieved by at ≈ 100 nM range with a fast kinetics, the I1 is Na+-dependent and relieved by at ≈ 1 μM level with a slower kinetics. (B) NCX as a trigger. Nullclines of the proposed model with clamped , blue: dh/dt = 0, red: ; pale curves: mM, bold curves: mM. Attraction domains corresponding to low-Ca2+ and high-Ca2+ states at mM are shown in purple and yellow, respectively. Stable equilibria are marked with gray filled circles, an unstable equilibrium is marked with a red open circle. (C) Scheme of the model space, representing a small section of the thin astrocytic process. Resting values for [Na+] and [Ca2+] are set by Na+/K+-pump (NKA) and Ca2+-pump, NCX can exert either positive or negative influence on and , depending on the mode. Probing external pulses of Ca2+ and Na+ are administered through virtual “pipettes” Positive and negative effects are indicated with green and red arrows.
We follow the general approach of earlier works by Weber et al. (2001) and Ginsburg et al. (2013) and model the NCX-mediated Ca2+ flow (1) as a product of maximal flow , electrochemical component JΔE (2)–(3), and gating variables n and h corresponding to deinactivation dependent on occupancy of the high- and low-affinity Ca2+ binding sites. The JΔE factor is described as a simplification of the normalized net reaction rate of the ping-pong bi-bi cyclic reaction scheme. In essence, JΔE models the main kinetic cycle shown in Figure 1A, while h and n correspond to the fraction of active enzyme in the exchange with I1 and I2 states, respectively.
Here KM· are the corresponding Michaelis constants, η ∈ 0 … 1 is the position of the energy barrier of the enzyme in the membrane electric field relative to membrane width, and ksat parameter controls the saturation of Jncx at negative potentials; these parameters are as in Weber et al. (2001) without change.
The allosteric factor of Weber et al. (2001) and Ginsburg et al. (2013) described binding to the high-affinity Ca2+-sensing site and deinactivation from I2. The authors assumed that [Na+]i in cardiomyocytes didn't reach levels required for the transition to I1, which was thus not considered in their model. In contrast, Matsuoka et al. (1996) reported that Na+-dependent inactivation saturates at 25–50 mM [Na+]i, which is near the physiological range of [Na+]i in astrocytes—15–20 mM (Verkhratsky et al., 2017), suggesting it to have a significant impact on astrocytic NCX activity. In a detailed experiment-based model developed by Fujioka et al. (2000) the kinetic rates of the E1 ⇌ I2 reaction are an order of magnitude higher than of the tranistion. This is also supported by Boyman et al. (2009), where the kinetics of the high-affinity Ca2+ binding site is also estimated to be much faster than of the low-affinity site. Correspondingly, as a simplification, we regard the n variable as instantaneous with the Ca2+-dependent steady state
and only describe kinetics of the h gating variable corresponding to Na+-dependent inactivation and Ca2+-dependent deinactivation mediated by the low-affinity site (5)–(7):
The steady-state value of h negatively depends on [Na+]i, but the extent of this influence is scaled by [Ca2+]i with cooperativity coefficients HNa and HCa, correspondingly (6). We also acknowledge the dependence of τh on [Ca2+]i reported in Matsuoka et al. (1996). This dependence is parameterized by the time scale at low [Ca2+]i, τ0, Hill coefficient Hτ and time scale at high [Ca2+]i, τmin = 0.25. Qualitative reproduction of tue key experimental kinetics from Matsuoka et al. by our model is shown in Supplementary Figure 1.
Below we demonstrate that the modulation of the NCX by Na+ and Ca2+ can account for nonlinear Ca2+-induced Ca2+-entry and in bistability between low- and high-Ca2+ states during the high Na+-conditions.
3. Non-Linear Amplification of Calcium Transients by the NCX
3.1. Phaseplane Analysis
We start with phaseplane analysis of a two-variable model where NCX is the only nonlinear mechanism responsible for Ca2+ dynamics and [Na+]i is a parameter:
where Jncx is defined in (1)–(3), and for simplicity, all Ca2+ equilibrating mechanisms, including Ca2+ buffering in the excessive buffer approximation, are lumped into a single linear term setting resting Ca2+ concentration and equilibration timescale τCa. The second equation of the model is the kinetics of the h gating variable (5).
Phase plane of this model is delineated by two nullclines, i.e., curves on the plane defined by the conditions dh/dt = 0 (h-nullcline), and (Ca2+-nullcline). Any point on this plane corresponds to some state of the model, a combination of specific [Ca2+]i and h values. Dynamics of the model starting from some initial conditions will be a line on this plane, called phase trajectory. Because in all points lying on the Ca2+-nullcline, all trajectories will cross it vertically. Correspondingly, all trajectories must cross the h-nullcline horizontally. Nullcline intersections are equilibrium points because here both variables don't change: . Equilibria can be stable if small perturbations from it relax back to the equilibrium or unstable if a small perturbation grows in time. When there are several stable equilibria on the plane, the system is multistable, and it's state space is delineated by basins of attraction of the stable equilibria. Change in parameters can shift and bend nullclines leading to changes in the system behavior.
While h-nullcline (defined by h = h∞) shows only subtle dependence on [Na+]i, the Ca2+-nullcline is more parameter-sensitive. First, it has a discontinuity when JΔE=0 which implies
Because when Jncx vanishes, the generally does not, this discontinuity does not affect the dynamics under study. Second, the curvature near the resting Ca2+ range and slope of the Ca2+-nullcline considerably depend on the , [Na+]i, and Vm.
Consequently, at low [Na+]i for any reasonable , as well as low and variable [Na+]i, there is a single and stable equilibrium point. However, the appropriate combination of high [Na+]i, depolarized Vm and result in three intersection points, thus indicating bistability and a trigger-like behavior. Both cases are illustrated in Figure 1B at resting [Na+]i (20 mM, pale lines) the Ca2+–nullcline shows a discontinuity within a physiological range of resting [Ca2+]i and a single equilibrium point. Increasing [Na+]i to 80 mM tilts the Ca2+–nullcline, and drives the discontinuity to very high values of [Ca2+]i. It leads to appearance of two stable equilibrium points: one at low [Ca2+]i and one at higher [Ca2+]i. All trajectories starting from the purple region of the phase plane will converge to the low–Ca2+ state, while all trajectories starting from the yellow region will converge to the high–Ca2+ state.
Because both Vm and [Na+]i shape the Ca2+-nullcline, a smaller increase in [Na+]i would lead to a similar nullcline configuration at a more depolarized Vm. Maps of the highest stable [Ca2+]i and regions of Vm–[Na+]i combinations leading to bistability for different values are shown in Supplementary Figure 2. In summary, phase plane analysis predicts that the Na+ and Ca2+ modulation of the NCX leads to bistability in the system with NCX shaping the Ca2+ exchange.
3.2. Sodium-Dependent Amplification of Calcium Transients by NCX
We next turn to a simple point model with both [Na+]i and [Ca2+]i as dynamical variables and an additional controlled Ca2+-flux thus extending (8) to look like:
and add a simple dynamics for Na+ also with a controlled Na influx :
Model space is summarized in Figure 1C and simulation of this model is shown in Figure 2. When only short Na+ pulses are given (Figure 2A), the system responds with small elevations of Ca2+ (yellow dashed line). When short Ca2+ pulses are given simultaneously with Na+ pulses, the resulting Ca2+ transients are amplified nonlinearly, more than can be explained by a simple summation of responses to separate Ca2+ and Na+ pulses (blue line). A smaller Na+ pulse (first) leads to a less pronounced effect than a larger one (second), the difference being primarily in the duration of the resulting Ca2+ transient. Short Na+ pulses cannot demonstrate bistability of the model, because most trajectories will have to pass near the nullclines (Figure 1B) resulting in slow system dynamics. The extent to which NCX can widen Ca2+ transients can be demonstrated with long Na+ pulses (Figure 2B). Here 200–s Na+ pulses lead to prolonged Na+ elevations to different levels, and the model responses (black lines) are compared to a model where allosteric modulation is turned off and is reduced to compensate for higher ratio of active transporters (blue lines). The situation of long-term Na+ elevation can also reflect a pathological condition, for example prolonged ischemia, when the Na/K–pump activity is challenged by shortage in ATP or epileptic activity of neurons, leading to a sustained influx of Na+ into astrocytes with glutamate. Astrocytic [Ca2+]i responds to the onset of with a small transient which returns to resting [Ca2+]i due to Na+-dependent drop in h. A short Ca2+ stimulating pulse is widened by NCX in the Na+-dependent manner due to a regenerative Ca+ influx, where rising [Ca2+]i deinactivates NCX (increases h) with a positive feedback. Eventually, a high enough increase in [Na+]i is sufficient to lead to a sustained high–Ca2+ state. Note,that activation of NCX by a Ca2+ pulse also leads to a drop in [Na+]i level. The same Ca2+ pulse given without Na+ leads only to a small Na+ transient due to NCX working in the direct mode.
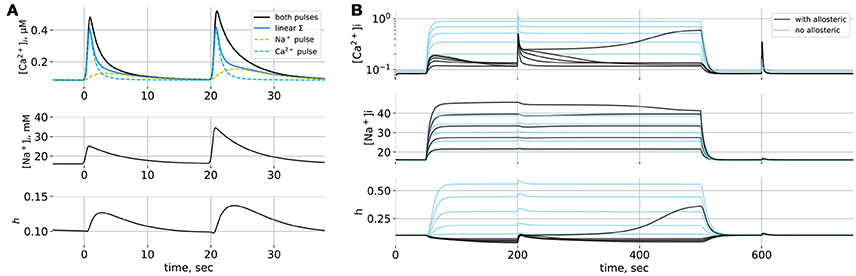
Figure 2. (A) Simulations of the point model. (A) Responses to short Na+-pulses. Solid black line: reponse to simultaneous Ca2+ and Na+ pulses, dashed lines: responses to separate Ca2+ and Na+ pulses, solid blue line: linear summation of independent responses. The two Na+ pulses differ in amplitude. (B) Responses to prolonged Na+ elevations of different amplitudes; Na+ influx lasts from t = 50 to t = 500 s; at t = 200 s and t = 600 s short Ca2+ pulses are given. Black lines: NCX model with allosteric regulation by Na+ and Ca2+, blue lines: NCX model without allosteric regulation.
Will the same mechanism allow for spreading waves of Ca2+ elevation in a spatially distributed system? We tested this conjecture in a minimalistic one-dimensional model describing a section of a thin astrocytic process lacking internal Ca2+ stores. One-dimensional space is discretized into elements 0.35 μm long. Each element contains the same local dynamics as in the point model with an additinal diffusion of Na+ and Ca2+ between the elements. Only the central (x = 0) element experienced an external Ca2+-pulse and elements from x = 0 to x = 10μm. on the one side experienced a short Na+ pulse. Ca2+ could diffuse in both directions from the central position, but [Na+]i profile was uneven, leading to anisotropic amplification and spreading of the initial Ca2+ pulse as compared to linear summation of the [Ca2+]i changes caused by Ca2+ and Na+ pulses given separately (Supplementary Figure 3).
4. Discussion
We argue that Na+ influx caused by neurotransmitter uptake, if happening under resting [Ca2+]i-conditions, beside a small elevation in [Ca2+]i, can “prime” the NCX by simultaneously electrochemically favoring Ca2+ influx via the reverse mode and inactivating NCX by Na+-dependent transition to I1. In this state, a localized Ca2+ surge, e.g., via spontaneous opening of a TRP channel, will be amplified nonlinearly by the NCX rescued form the inactive conformation by Ca2+. In contrast, under resting membrane potential and low [Na+]i, localized Ca2+ transients will be reduced by NCX working in the direct mode. This attenuation can be small if a large portion of the NCX is already inactivated at resting [Na+]i, but can be substantial in sub-normal [Na+]i conditions.
Our modeling predicts that NCX can increase amplitude and duration of Ca2+ transients, account for switching between low-Ca2+ and high-Ca2+ states and even spatially spreading zones of elevated Ca2+. The extent and duration of Na+ elevations are dependent on the timescale of Na+ clearance from the cytoplasm by the Na/K-pump. In hippocampal slices (Langer et al., 2012, 2016) activity-dependent Na+ elevations were observed on a similar timescale to the presented in simulations. On this timescale the NCX-mediated Ca2+ influx can prime IP3 receptors on ER membranes in the astrocytic branchlets and branches in a fashion, similar to that proposed in cardiomyocytes, where the reverse mode of NCX is supposed to participate in excitation–contraction coupling (Shattock et al., 2015). So, NCX can serve as a nonlinear link from synaptic activity to the complex Ca2+ dynamics of the astrocyte. This, especially in view of apparent colocalization between NCX and IP3-receptors in plasma membrane-ER junctions (Blaustein et al., 2002), calls for further theoretical investigation of interaction between the store-operated Ca2+ dynamics and the NCX-mediated Ca2+ influx. It is also expected that under a metabolically challenged state, when the Na/K-pump turnover is slowered, there will be higher Na+ elevations and correspondigly higher NCX-mediated Ca2+ influx. Corroborating this prediction, a recent study by Gerkau et al. (2017) demonstrated pronounced Na+ loading and increased Ca2+ transients in astrocytes and neurons under chemical ischemia; blocking the reverse mode of NCX increased Na+ loading and dramatically reduced Ca2+ activity. Pathologically high synaptic activity is also expected to both cause higher Na+ accumulation and locally depolarize perisynaptic astrocyte processes due to increased , caused by postsynaptic currents.
The proposed model is primarily based on experimental data for cardiac NCX1 isoform (Matsuoka et al., 1996; Ginsburg et al., 2013), because there is no detailed experimental data on kinetics and modulation available for astrocytic NCX. Astrocytes do express NCX1 (Minelli et al., 2007), but other NCX types are also present, and even for the same isoform, kinetic properties may be tissue dependent. Nevertheless, the predictions of the model look compelling enough in the context of astrocytic Ca2+ signaling to justify a dedicated experimental testing for the predicted nonlinear interaction between NCX and Ca2+ under high-Na+ and low-Na+ conditions.
Data Availability Statement
Model implementation and parameter values used in the simulations are supplemented as a Jupyter (Kluyver et al., 2016) notebook available online at https://zenodo.org/record/1218115.
Author Contributions
AB: model formulation, preparing the figures, writing the paper; AV: model simulations, preparing the figures; DV: model simulations, preparing the Supplementary Materials; DP: model simulations, phase-plane analysis, writing the paper.
Funding
This study was supported by Russian Science Foundation, grant 17-74-20089. DP acknowledges the personal support from the Ministry of Education and Science of the Russian Federation, contract 3.5507.2017/6.7, which covered phase-plane analysis.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fncel.2018.00250/full#supplementary-material
References
Bazargani, N., and Attwell, D. (2016). Astrocyte calcium signaling: the third wave. Nat. Neurosci. 19, 182–189. doi: 10.1038/nn.4201
Blaustein, M. P., Juhaszova, M., Golovina, V. A., Church, P. J., and Stanley, E. F. (2002). Na/ca exchanger and PMCA localization in neurons and astrocytes: functional implications. Ann. N.Y. Acad. Sci. 976, 356–366. doi: 10.1111/j.1749-6632.2002.tb04762.x
Boyman, L., Mikhasenko, H., Hiller, R., and Khananshvili, D. (2009). Kinetic and equilibrium properties of regulatory calcium sensors of NCX1 protein. J. Biol. Chem. 284, 6185–6191. doi: 10.1074/jbc.M809012200
Fujioka, Y., Hiroe, K., and Matsuoka, S. (2000). Regulation kinetics of Na+-Ca2+ exchange current in guinea-pig ventricular myocytes. J. Physiol. 529(Pt 3), 611–623. doi: 10.1111/j.1469-7793.2000.00611.x
Gerkau, N. J., Rakers, C., Durry, S., Petzold, G. C., and Rose, C. R. (2017). Reverse NCX attenuates cellular sodium loading in metabolically compromised cortex. Cereb. Cortex 9, 1–17. doi: 10.1093/cercor/bhx280
Ginsburg, K. S., Weber, C. R., and Bers, D. M. (2013). Cardiac Na+-Ca2+ exchanger: dynamics of ca2+-dependent activation and deactivation in intact myocytes. J. Physiol. 591, 2067–2086. doi: 10.1113/jphysiol.2013.252080
Hilgemann, D. W., Collins, A., and Matsuoka, S. (1992a). Steady-state and dynamic properties of cardiac sodium-calcium exchange secondary modulation by cytoplasmic calcium and ATP. J. Gen. Physiol. 100, 933–961.
Hilgemann, D. W., Matsuoka, S., Nagel, G. A., and Collins, A. (1992b). Steady-state and dynamic properties of cardiac sodium-calcium exchange. Sodium-dependent inactivation. J. Gen. Physiol. 100, 905–932.
Khakh, B. S., and McCarthy, K. D. (2015). Astrocyte calcium signaling: from observations to functions and the challenges therein. Cold Spring Harb. Perspect. Biol. 7:a020404. doi: 10.1101/cshperspect.a020404
Khakh, B. S., and Sofroniew, M. V. (2015). Diversity of astrocyte functions and phenotypes in neural circuits. Nat. Neurosci. 18, 942–952. doi: 10.1038/nn.4043
Kirischuk, S., Parpura, V., and Verkhratsky, A. (2012). Sodium dynamics: another key to astroglial excitability? Trends Neurosci. 35, 497–506. doi: 10.1016/j.tins.2012.04.003
Kluyver, T., Ragan-Kelley, B., Pérez, F., Granger, B., Bussonnier, M., Frederic, J., et al. (2016). “Jupyter notebooks – a publishing format for reproducible computational workflows,” in Positioning and Power in Academic Publishing: Players, Agents and Agendas, eds F. Loizides and B. Schmidt (Amsterdam: IOS Press), 87–90.
Langer, J., Gerkau, N. J., Derouiche, A., Kleinhans, C., Moshrefi-Ravasdjani, B., Fredrich, M., et al. (2016). Rapid sodium signaling couples glutamate uptake to breakdown of ATP in perivascular astrocyte endfeet. Glia 65, 293–308. doi: 10.1002/glia.23092
Langer, J., and Rose, C. R. (2009). Synaptically induced sodium signals in hippocampal astrocytes in situ. J. Physiol. 587(Pt 24), 5859–5877. doi: 10.1113/jphysiol.2009.182279
Langer, J., Stephan, J., Theis, M., and Rose, C. R. (2012). Gap junctions mediate intercellular spread of sodium between hippocampal astrocytes in situ. Glia 60, 239–252. doi: 10.1002/glia.21259
Lee, H. S., Ghetti, A., Pinto-Duarte, A., Wang, X., Dziewczapolski, G., Galimi, F., et al. (2014). Astrocytes contribute to gamma oscillations and recognition memory. Proc. Natl. Acad. Sci. U.S.A. 111, E3343–E3352. doi: 10.1073/pnas.1410893111
Matsuoka, S., Philipson, K. D., and Hilgemann, D. W. (1996). Multiple functional states of the cardiac Na+-Ca2+ exchanger. Whole-cell, native-excised, and cloned-excised properties. Ann. N.Y. Acad. Sci. 779, 159–170. doi: 10.1111/j.1749-6632.1996.tb44784.x
Minelli, A., Castaldo, P., Gobbi, P., Salucci, S., Magi, S., and Amoroso, S. (2007). Cellular and subcellular localization of Na+-Ca2+ exchanger protein isoforms, NCX1, NCX2, and NCX3 in cerebral cortex and hippocampus of adult rat. Cell Calcium 41, 221–234. doi: 10.1016/j.ceca.2006.06.004
Patrushev, I., Gavrilov, N., Turlapov, V., and Semyanov, A. (2013). Subcellular location of astrocytic calcium stores favors extrasynaptic neuron-astrocyte communication. Cell Calcium 54, 343–349. doi: 10.1016/j.ceca.2013.08.003
Petravicz, J., Fiacco, T. A., and McCarthy, K. D. (2008). Loss of ip3 receptor-dependent ca2+ increases in hippocampal astrocytes does not affect baseline ca1 pyramidal neuron synaptic activity. J. Neurosci. 28, 4967–4973. doi: 10.1523/JNEUROSCI.5572-07.2008
Prebil, M., Jensen, J., Zorec, R., and Kreft, M. (2011). Astrocytes and energy metabolism. Arch. Physiol. Biochem. 117, 64–69. doi: 10.3109/13813455.2010.539616
Reyes, R. C., Verkhratsky, A., and Parpura, V. (2012). Plasmalemmal Na+/Ca2+ exchanger modulates Ca2+-dependent exocytotic release of glutamate from rat cortical astrocytes. ASN Neuro 4:e00075. doi: 10.1042/AN20110059
Rojas, H., Colina, C., Ramos, M., Benaim, G., Jaffe, E. H., Caputo, C., et al. (2007). Na+ entry via glutamate transporter activates the reverse Na+/Ca2+ exchange and triggers Ca(i)2+-induced Ca2+ release in rat cerebellar type-1 astrocytes. J. Neurochem. 100, 1188–1202. doi: 10.1111/j.1471-4159.2006.04303.x
Rose, C. R., and Chatton, J. Y. (2016). Astrocyte sodium signaling and neuro-metabolic coupling in the brain. Neuroscience 323, 121–134. doi: 10.1016/j.neuroscience.2015.03.002
Rungta, R. L., Bernier, L. P., Dissing-Olesen, L., Groten, C. J., LeDue, J. M., Ko, R., et al. (2016). Ca2+ transients in astrocyte fine processes occur via Ca2+ influx in the adult mouse hippocampus. Glia 64, 2093–2103. doi: 10.1002/glia.23042
Shattock, M. J., Ottolia, M., Bers, D. M., Blaustein, M. P., Boguslavskyi, A., Bossuyt, J., et al. (2015). Na(+) /Ca(2+) exchange and Na(+) /K(+) -ATPase in the heart. J. Physiol. 593, 1361–1382. doi: 10.1113/jphysiol.2014.282319
Srinivasan, R., Huang, B. S., Venugopal, S., Johnston, A. D., Chai, H., Zeng, H., et al. (2015). Ca(2+) signaling in astrocytes from Ip3r2(-/-) mice in brain slices and during startle responses in vivo. Nat. Neurosci. 18, 708–717. doi: 10.1038/nn.4001
Sun, W., McConnell, E., Pare, J. F., Xu, Q., Chen, M., Peng, W., et al. (2013). Glutamate-dependent neuroglial calcium signaling differs between young and adult brain. Science 339, 197–200. doi: 10.1126/science.1226740
Tong, X., Shigetomi, E., Looger, L. L., and Khakh, B. S. (2013). Genetically encoded calcium indicators and astrocyte calcium microdomains. Neuroscientist 19, 274–291. doi: 10.1177/1073858412468794
Verkhratsky, A., and Nedergaard, M. (2014). Astroglial cradle in the life of the synapse. Philos. Trans. R. Soc. B Biol. Sci. 369, 20130595–20130595. doi: 10.1098/rstb.2013.0595
Verkhratsky, A., Rodríguez-Arellano, J. J., Parpura, V., and Zorec, R. (2017). Astroglial calcium signalling in Alzheimer's disease. Biochem. Biophys. Res. Commun. 483, 1005–1012. doi: 10.1016/j.bbrc.2016.08.088
Keywords: astrocytic calcium, sodium-calcium exchanger, NCX, calcium-induced calcium entry, calcium wave
Citation: Brazhe AR, Verisokin AY, Verveyko DV and Postnov DE (2018) Sodium–Calcium Exchanger Can Account for Regenerative Ca2+ Entry in Thin Astrocyte Processes. Front. Cell. Neurosci. 12:250. doi: 10.3389/fncel.2018.00250
Received: 14 April 2018; Accepted: 23 July 2018;
Published: 14 August 2018.
Edited by:
Christian Lohr, Universität Hamburg, GermanyReviewed by:
Christine R. Rose, Heinrich Heine Universität Düsseldorf, GermanyAsaph Zylbertal, University College London, United Kingdom
Copyright © 2018 Brazhe, Verisokin, Verveyko and Postnov. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alexey R. Brazhe, YnJhemhlQGJpb3BoeXMubXN1LnJ1
 Alexey R. Brazhe
Alexey R. Brazhe Andrey Y. Verisokin
Andrey Y. Verisokin Darya V. Verveyko
Darya V. Verveyko Dmitry E. Postnov
Dmitry E. Postnov